Abstract
The mechanisms underlying the subcellular localization of neurotrophins and their receptors are poorly understood. We show that in cultured hippocampal neurons, the mRNAs for BDNF and TrkB have a somatodendritic localization, and we quantify the extent of their dendritic mRNA localization. In the dendrites the labeling covers on average the proximal 30% of the total dendritic length. On high potassium depolarization, the labeling of BDNF and TrkB mRNA extends on average to 68% of the dendritic length. This increase does not depend on new RNA synthesis, is inhibited by the Na+channel blocker tetrodotoxin, and involves the activation of glutamate receptors. Extracellular Ca2+, partly flowing through L-type Ca2+ channels, is absolutely required for this process to occur. At the protein level, a brief stimulation of hippocampal neurons with 10 mm KCl leads to a marked increase of BDNF and TrkB immunofluorescence density in the distal portion of dendrites, which also occurs, even if at lower levels, when transport is inhibited by nocodazole. The protein synthesis inhibitor cycloheximide abolishes this increase. The activity-dependent modulation of mRNA targeting and protein accumulation in the dendrites may provide a mechanism for achieving a selective local regulation of the activity of neurotrophins and their receptors, close to their sites of action.
Keywords: neurotrophins, dendritic mRNA, BDNF, TrkB, synaptic plasticity, hippocampal neurons
The neurotrophin BDNF (Leibrock et al., 1989; Barde, 1990) has been involved in modulating synaptic plasticity (Thoenen, 1995; Bonhoeffer, 1996). In particular, BDNF can increase synaptic transmission (Lohof et al., 1993; Knipper et al., 1994a,b; Leßmann et al., 1994; Kang and Schumann, 1995; Levine et al., 1995; Carmignoto et al., 1997) and has been implicated in hippocampal LTP (Korte et al., 1995, 1996; Patterson et al., 1996). Furthermore, BDNF enhances synaptic transmission in the rat hippocampus requiring local dendritic synthesis of proteins whose identity was not determined (Kang and Schumann, 1996).
BDNF and its receptor TrkB are stored in the dendrites (Wetmore et al., 1994; Dugich-Djordjevic et al., 1995; Cabelli et al., 1996; Cellerino et al., 1996; Goodman et al., 1996), but the mechanisms of targeting of these two proteins are largely unknown.
The specific sorting of mRNAs is a possible mechanism to localize proteins to the dendrites (Steward, 1994, 1997; Johnston, 1995). An increasing number of dendritic mRNAs have been identified, including those encoding MAP2, ARC/arg 3.1, α-CaMKinase II α-subunit, IP3-receptor, NMDAR1 subunit, glycine receptor α-subunit, vasopressin, and dendrin (Garner et al., 1988; Burgin et al., 1990;Furuichi et al., 1993; Link et al., 1995; Lyford et al., 1995; Gazzaley et al., 1997; Herb et al., 1997; Prakash et al., 1997; Racca et al., 1997). Recent studies demonstrated the existence of a protein synthesis machinery in dendrites, including ribosomes, tRNA, translation factors (Steward and Levy, 1982; Tiedge and Brosius, 1996), endoplasmic reticulum, and Golgi-like apparatus (Torre and Steward, 1996; Spacek and Harris, 1997). Moreover, the local protein synthesis in fully developed dendrites (Torre and Steward, 1992) and in isolated growth cones (Crino and Eberwine, 1996) has been demonstrated unequivocally.
TrkB and BDNF mRNAs have been amplified from dendritic growth cones (Crino and Eberwine, 1996); however, the localization of these two mRNAs in the dendrites of mature neurons is controversial. Most studies did not describe a BDNF mRNA dendritic localization (Ernfors et al., 1990, 1992; Isackson et al., 1991; Kokaia et al., 1993; Miranda et al., 1993; Ringstedt et al., 1993; Tinmusk et al., 1993; Castrén et al., 1995; Lauterborn et al., 1996; Conner et al., 1997), whereas a few others were suggestive of a very proximal dendritic localization (Wetmore et al., 1990, 1994; Dugich-Djordjevich et al., 1992;Schmidt-Kastner et al., 1996a,b). Recently, the localization of TrkB mRNA in the dendritic compartment of retinal neurons at the end of their development was shown (Ugolini et al., 1995). However, other studies did not report a similar localization (Ringstedt et al., 1993;Schmidt-Kastner et al., 1996a,b).
In view of the role of BDNF and TrkB in synaptic plasticity (Thoenen, 1995; Bonhoeffer, 1996), the clarification of this issue is crucial. This study demonstrates that BDNF and TrkB mRNAs are localized in the dendrites of hippocampal neurons in culture, that depolarization extends these mRNAs to the distal dendrites, and that a brief depolarization after blockade of dendritic transport increases the levels of BDNF and TrkB proteins in the distal dendritic compartment.
MATERIALS AND METHODS
Cell cultures. Primary cell cultures were made from rat hippocampal neurons according to Malgaroli and Tsien (1992), with slight modifications. Hippocampi were dissected from 2- to 4-d-old animals. Isolation and slicing were performed in 200 μmkinurenic acid (Sigma, St. Louis, MO) and 25 μm2-amino-5-phosphonovalerate (Tocris Neuramin, Bristol, UK). Tissue slices were digested with trypsin in the presence of DNase, blocked with trypsin inhibitor on ice, and dissociated in medium containing DNase. Cells were recovered and washed by two successive centrifugations at 500 rpm and plated on glass coverslips coated with 50 μg/ml polyornithine and 2% Matrigel (Collaborative Research, Bedford, MA) in 35 mm Nunc petri dishes. Cells were cultured for 7 d in a 5% CO2 humidified incubator, in minimum essential medium with Earle’s salts and Glutamax I (Life Technologies, Gaithersburg, MD) to which 5–10% fetal bovine serum, 6 mg/mld-glucose, 3.6 mg/ml HEPES, 0.1 μg/ml biotin, 1.5 μg/ml vitamin B12, 30 μg/ml insulin, and 100 μg/ml bovine transferrin were added. Proliferation of non-neural cells was prevented by the addition of 2.5–5.0 μm cytosine β-d-arabinofuranoside from the second day in culture onward.
Electrophysiology and KCl stimulation of cultured hippocampal neurons. Whole-cell recordings were performed at room temperature (rt) (23–25°C) on large pyramidal cells with an EPC 7 patch-clamp amplifier. Patch pipettes were made from thin-wall glass (outside diameter 1.5 μm) with 6–8 MΩ resistance and were filled with 110 mm potassium gluconate, 10 mm NaCl, 5 mm MgCl2, 0.6 mm EGTA, 2 mm Na2-ATP, 49 mm HEPES, pH 7.2. Extracellular oxygenated control solution contained 3.5 mmKCl, 132 mm NaCl, 1 mmMgCl2, 2 mm CaCl2, 20 mmd-glucose, 10 mm HEPES, pH 7.4. Cells were depolarized for 30 min at rt with oxygenated K-medium (10 mm KCl, 1.8 mmCaCl2·2H2O, 0.8 mmMgSO4·7H2O, 101 mm NaCl, 26 mm NaHCO3, 1 mmNaH2PO4·2H2O, 0.7%d-glucose, 15 mm HEPES, pH 7.4, or KK-medium (20 mm KCl, 1.8 mmCaCl2·2H2O, 0.8 mmMgSO4·7H2O, 110 mm NaCl, 26 mm NaHCO3, 1 mmNaH2PO4·2H2O, 0.7%d-glucose, 15 mm HEPES, pH 7.4.
For mRNA or protein localization experiments, cells were depolarized for the indicated times, at 37°C, with the K or the KK high potassium media described above. For pharmacological blockade experiments, cells were incubated in normal culture medium or in K- or KK-medium, supplemented with drugs, for the indicated times at 37°C. Drug concentrations were 1 mm kinurenic acid (Sigma), 1 μm nifedipine (Sigma), 0.5 μm tetrodotoxin (TTX) (Sigma), 5 μg/ml actinomycin-d (Sigma), 1 μm cycloheximide (Sigma), and 1 μg/ml nocodazole (Sigma). When cycloheximide or actinomycin-d were used, preincubation before depolarization was performed as described above for 30 min, whereas in the case of nocodazole, preincubation at 37°C was 6 hr long. Ca2+-free experiments were performed in Ca2+-free control medium containing 5 mm KCl, 1.8 mm MgCl2, 0.8 mm MgSO4·7H2O, 116 mmNaCl, 26 mm NaHCO3, 1 mmNaH2PO4·2H2O, 0.7%d-glucose, 15 mm HEPES, pH 7.4, or in Ca2+-free K-medium containing 10 mm KCl, 1.8 mm MgCl2, 0.8 mmMgSO4·7H2O, 101 mm NaCl, 26 mm NaHCO3, 1 mmNaH2PO4·2H2O, 0.7%d-glucose, 15 mm HEPES, pH 7.4, supplemented with 10 μm EGTA or BAPTA-AM.
Riboprobes and oligonucleotides. The 700-bp-long rat β-actin cDNA (Nudel et al., 1983) cloned into Bluescript was kindly provided by Dr. R. Possenti [Institute of Neurobiology, Consiglio Nazionale delle Ricerche (CNR), Rome]. The rat BDNF cDNA pBCDPst (nucleotides 74–525) (Maisonpierre et al., 1991) was kindly provided by Dr. A. Negro (Fidia Research Laboratory, Padova). The rat TrkB cDNA clone was kindly provided by Dr. Y. Bozzi (Institute of Neurophysiology, CNR, Pisa) (Bozzi et al., 1995) and contained the first 238 bp of the region coding for the tyrosine-kinase domain (nucleotides 2163–2401) (Middlemas et al., 1991). The 480-nucleotides-long mouse TrkA clone pDM97 (Holtzman et al., 1992) coded for part of the extracellular portion of the receptor (kindly provided by Dr. C. K. Chen, Johns Hopkins University School of Medicine, Baltimore, MD). After linearization of the plasmids, the digoxigenin-labeled riboprobes were synthesized with a SP6/T7 DIG-RNA labeling kit (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer’s instructions. To acquire an independent confirmation of the specificity of the labeling pattern obtained with the riboprobes, oligonucleotides were designed from regions not overlapping with the riboprobe sequences. The TrkB oligonucleotide probe was complementary to the nucleotides 1360–1407 in the region encoding the juxtamembrane cytoplasmic domain of the TrkB full length receptor mRNA (Middlemas et al., 1991). The BDNF oligonucleotide probe was complementary to the nucleotides 649–694 of the coding region of the rat BDNF mRNA sequence (Maisonpierre et al., 1991). Both oligonucleotide sequences have been used in previous studies for in situ hybridization (Ernfors et al., 1990, 1992; Merlio et al., 1993). To avoid any risk of unspecific hybridization caused by the labeled tail, only a single digoxigenin-labeled ddUTP was added with a terminal transferase at the 3′ end of the oligonucleotides by means of a DIG-oligonucleotide 3′-end labeling kit (Boehringer Mannheim), according to manufacturer’s instructions.
In situ hybridization on cultured hippocampal neurons.For in situ hybridization with riboprobes, cells were fixed for 10 min at room temperature in 4% paraformaldehyde in PBS, washed in PBS, and permeabilized in ethanol for 15 min at −20°C. After rehydration with decreasing ethanol concentrations in PBS at rt, cells were prehybridized at 55°C for 90 min in the hybridization mix containing 20 mm Tris/HCl, pH 7.5, 1 mm EDTA, 1× Denhardt’s solution, 300 mm NaCl, 100 mmdithiothreitol, 0.5 mg/ml salmon sperm DNA, 0.5 mg/ml polyadenylic acid, and 50% formamide. In situ hybridization was performed overnight at 55°C in the hybridization mix to which 10% dextrane sulfate and the riboprobes (50–100 ng/ml) were added. High-stringency washes were performed in 0.1% SSC/0.1% Tween-20 at 60°C. Cells hybridized with digoxigenin-labeled riboprobes were incubated overnight at 4°C with anti-DIG Fab fragments coupled to alkaline phosphatase (Boehringer Mannheim), diluted 1:500 in 10% fetal calf serum in PBS + 0.1% Tween 20 (PBST). After a thorough wash in PBST, cells were reacted with 4-nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate in 100 mm Tris/HCl, pH 9.5, 50 mm MgCl2, 100 mmNaCl, and 1 mm Levamisol. Alkaline phosphatase development was performed for 16 hr at 4°C to obtain reproducible results and to avoid saturation of the reaction (Augood et al., 1991). In situ hybridization with digoxigenin-labeled oligonucleotides was performed essentially as described above, but the hybridization temperature and washing conditions were modified. Briefly, hybridization was performed for 24 hr at 42°C, and then cells were washed 15 min with 2× SSC at room temperature and 15 min in 0.2× SSC at 37°C. Anti-Dig antibody incubation and staining development were performed as described above.
Antibody staining of cultured hippocampal neurons. Double staining of cultured hippocampal neurons was performed with the samein situ hybridization procedure described above, followed by anti-MAP2 immunostaining. Cells were coincubated overnight at 4°C with the anti-MAP2 monoclonal antibodies (Boehringer Mannheim) diluted 1:2000 and the anti DIG-alkaline phosphatase-coupled Fab fragments (Boehringer Mannheim) diluted 1:500 in 10% fetal calf serum in PBST. After washes in PBST, cells were incubated 1 hr at rt with biotinylated anti-mouse IgG antibodies (Vector, Burlingame, CA) diluted 1:100 in 10% fetal calf serum in PBST. Subsequently, cells were washed in PBST and reacted with 4-nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate in 100 mm Tris/HCl, pH 9.5, 50 mm MgCl2, 100 mmNaCl, and 1 mm Levamisol overnight at 4°C. Finally, cells were incubated 20 min at rt in streptavidin–FITC (Sigma) diluted 1:500 in 10 mm HEPES, pH 8.2, 150 mm NaCl buffer, washed in PBS twice, and mounted in Vectashield (Vector). Fluorescence was analyzed with a fluorescein filter under dark field, on a Zeiss Axiophot microscope.
Immunohistochemistry on cultured hippocampal neurons was preceded by the same fixation and permeabilization steps used for in situ hybridization described above. Fixed and permeabilized cells were preincubated for 30 min at rt in 3% BSA in PBS, incubated 3 hr at rt with an antibody recognizing the TrkB full length isoform (Santa Cruz Ab794, made in rabbit, diluted 1:100 in 3% BSA in PBS), or anti-BDNF [(Promega, Madison, WI) made in chicken, diluted 1:100 in 3% BSA in PBS]. After they were washed in PBS, cells were incubated for 1 hr at rt with biotinylated anti-rabbit IgG antibody (Vector) or biotinylated anti-chicken IgG antibody (Promega) diluted 1:200 in 3% BSA in PBS, then washed in PBS, incubated in streptavidin–FITC as described above, and mounted in Vectashield (Vector).
Quantitative imaging analysis and statistics. Nonradioactivein situ hybridization was analyzed by viewing stained cultures under bright-field illumination with an Olympus microscope with DIC (Differential Interference Contrast) equipped lens (40× magnification). Stained neurons were acquired with a Hitachi CCD camera and digitized with the image analysis program Imageplus (Microsoft). The function “Trace” was used to measure, starting from the base of the dendrites, the maximal distance of dendritic labeling (MDDL). Dendrites were traced, in a conservative manner, up to the point at which the in situ labeling was clearly distinguishable from the background. The background level obtained in sister cell cultures hybridized with the sense probes was used as a reference to distinguish the actual labeling obtained with the antisense probes from the background. The number of dendrites measured is indicated in Table 1. The individual preparations were coded and analyzed in a blind manner. The data of the MDDL were normalized by dividing each single measurement obtained in the different experimental conditions by the mean of the controls, and they were statistically analyzed with unpaired Student’s t tests.
Table 1.
Maximal distance of dendritic staining
| Mean (μm) | SE | p | n | |
|---|---|---|---|---|
| BDNF | ||||
| Control | 31.5 | ±0.7 | 403 | |
| 30′ K | 30.9 | ±0.8 | ≤0.000011-a | 310 |
| 3h K | 55.1 | ±1.0 | ≤0.000011-b | 310 |
| 30′ KK | 33.6 | ±0.8 | ≤0.000011-c | 386 |
| 3h KK | 46.4 | ±1.0 | ≤0.00001a,b | 350 |
| 3h K kinu | 41.7 | ±1.0 | ≤0.00001a,b | 276 |
| 3h K nife | 43.4 | ±0.9 | ≤0.00001a,b | 389 |
| 3h KK kinu | 39.6 | ±0.9 | ≤0.00001b,c | 324 |
| 3h KK nife | 32.3 | ±0.9 | ≤0.000011-c | 273 |
| 3h K TTX | 35.7 | ±1.1 | ≤0.01; 0.00001a,b | 221 |
| C act.D | 23.6 | ±1.5 | ≤0.011-b | 74 |
| 3h K act.D | 46.6 | ±1.3 | ≤0.000011-b | 146 |
| TrkB | ||||
| Control | 24.5 | ±0.5 | 451 | |
| 30′ K | 34.7 | ±0.8 | ≤0.00001a,b | 306 |
| 3h K | 52.5 | ±1.2 | ≤0.000011-b | 301 |
| 30′ KK | 33.6 | ±0.8 | ≤0.00001b,c | 313 |
| 3h KK | 49.8 | ±1.3 | ≤0.000011-b | 318 |
| 3h K kinu | 30.3 | ±0.8 | ≤0.00001a,b | 321 |
| 3h K nife | 41.1 | ±1.0 | ≤0.00001a,b | 305 |
| 3h KK kinu | 36.5 | ±1.2 | ≤0.00001b,c | 176 |
| 3h KK nife | 28.8 | ±0.7 | ≤0.00001b,c | 304 |
| 3h K TTX | 31.6 | ±1.3 | ≤0.00001a,b | 72 |
| C act.D | 18.8 | ±1.2 | ≤0.011-b | 86 |
| 3h K act.D | 35.6 | ±1.2 | ≤0.000011-b | 147 |
SE, Standard error; n, number of dendrites measured.
Respect to 3h K; t test.
Respect to control; t test.
Respect to 3h KK; t test.
Fluorescent immunocytochemistry was analyzed by confocal microscopy with a Molecular Dynamics MultiProbe 2001 (Sunnyvale, CA) confocal device mounted on a Nikon microscope. The individual preparations were coded and analyzed in a blind manner. Individual neurons whose entire dendritic domains were distinguishable from those of neighboring neurons were selected. For each neuron, five optical sections 0.7 μm thick were acquired and then were integrated in a single projection with the function “look-through” to ensure the visualization of the entire width of the dendrites on the z axis. This function gives the same results as the function “maximum intensity” used in similar studies (Blöchl and Thoenen, 1996), but is more conservative because it is less sensitive to background. Quantification of the fluorescence intensity and area measurements was performed by using the program “Area” of the Molecular Dynamics software package. Dendrites, longer than 100 μm, were subdivided into two segments and contoured manually. The proximal segment included the region up to 30 μm from the base of the dendrites. The distal segment comprised the distal region of the dendrites extending from 30 to 90 μm from the base of the dendrites. The sum of the total pixel intensities for each segment was divided by the area of the segment measured (fluorescence density). The data of the fluorescence density were normalized by dividing each single measurement by the mean of controls and were statistically analyzed with unpaired Student’st tests.
Differential extraction of tubulin. Soluble and polymerized tubulin was extracted from control cell cultures or treated for 6 hr with 1 μg/ml nocodazole, essentially as described (Fasulo et al., 1996). Briefly, soluble proteins were extracted in microtubule stabilizing buffer M1 (80 mm PIPES, 1 mmMgCl2, 2 mm EGTA, 2 mglycerol, 1 mm GTP, 0.1 mm PMSF, pH 6.9) for 12 min at 37°C. Thereafter, the same cells were extracted under microtubule depolymerizing conditions in ice-cold M2 buffer (80 mm PIPES, 1 mm MgCl2, 5 mm CaCl2, 2 m glycerol, 0.1 mm PMSF, pH 6.9) for 5 min. Cell extracts M1 and M2 were analyzed by SDS-PAGE and Western blotting with the monoclonal anti-tubulin antibody YOL1 (Kilmartin et al., 1982) diluted 1:2000. Bands were scanned with an APPLE scanner and quantified by the National Institutes of Health-Image software.
RESULTS
BDNF and TrkB mRNAs are localized in the dendrites
The subcellular localization of the mRNA for BDNF and TrkB was studied by nonradioactive in situ hybridization in rat hippocampal neurons in culture. An increasing number of laboratories have published studies using nonradioactive probes for the analysis of the subcellular localization of the mRNAs (Ainger, 1993; Hannan et al., 1995; Bian et al., 1996; Knowles et al., 1996). This technique, although comparably or only slightly less sensitive than radioactivein situ hybridization with 35S-labeled probes, allows a better spatial resolution because the morphology of the cell processes is more clearly distinguishable (for review, see Emson, 1993).
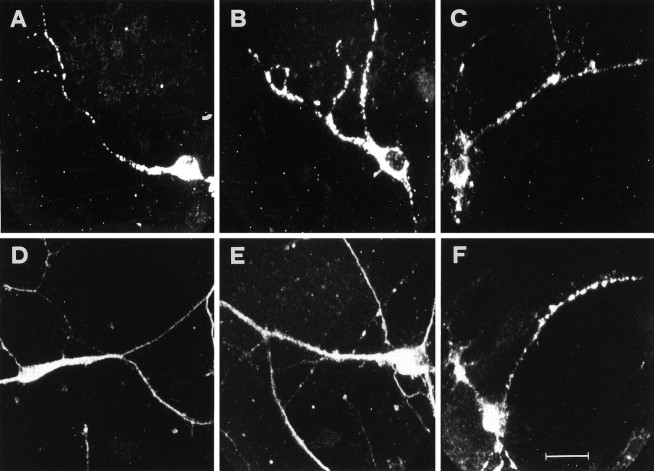
Almost all of the neurons in the culture are labeled with the BDNF and the TrkB riboprobes, with a prominent staining of the cell body and proximal dendrites (Figs.1A,2B for BDNF; 1E, 3A for TrkB). Interestingly, several cells show a strong labeling corresponding to branching points, and a subpopulation of 3% of the cells exhibit labeling corresponding to dendritic varicosities (Fig.1A,D,H, arrowheads). In double-labeling experiments, dendritic processes were identified with a monoclonal antibody against the microtubule-associated protein MAP2, a specific marker for the dendritic compartment (Caceres et al., 1984). These experiments demonstrate that the processes labeled by the BDNF and TrkB probes are dendrites (Fig. 1B,F). Axons, identified as cellular processes negative for MAP2, are never found to be stained with the BDNF or the TrkB probes. Neither cell bodies nor processes are stained with corresponding sense riboprobes (Fig. 1C for BDNF; G for TrkB). The specificity of the dendritic staining observed in hippocampal cultures was independently confirmed by using digoxigenin-labeled oligonucleotide probes, complementary to mRNA sequences nonoverlapping with those recognized by the riboprobes (Fig.1D for BDNF; H for TrkB). These oligonucleotides were designed in accordance with previous studies (Ernfors et al., 1990, 1992; Merlio et al., 1993) and were specific for the full length isoform of TrkB and for the exon 5 of BDNF, respectively (see Materials and Methods). Thus, under our experimental conditions, the mRNA for BDNF and TrkB can be detected unequivocally in the proximal portion of dendrites in cultured rat hippocampal neurons.
Fig. 1.
Subcellular distribution of BDNF and TrkB mRNAs in cultured hippocampal neurons. Staining by nonradioactive in situ hybridization with digoxigenin-labeled riboprobes and oligonucleotides. Fields are viewed with Nomarski optics, except immunofluorescence in B and F.A, The BDNF riboprobe labels the cell soma and a process identified as a dendrite in B by a double-labeling with an anti-MAP2 monoclonal antibody. C, No staining is observed with a BDNF sense riboprobe. D, The BDNF antisense oligonucleotide probe shows somatodendritic labeling.E, The TrkB riboprobe stains the cell soma and a dendritic process identified as a dendrite, in F, by the anti-MAP2 antibody. G, No labeling is detected with the TrkB sense riboprobe. H, The TrkB antisense oligonucleotide probe labels the cell soma and a dendrite.Arrowheads indicate labeling at dendritic branchings or varicosities. Scale bar (shown in H): 20 μm forA–H.
Fig. 2.
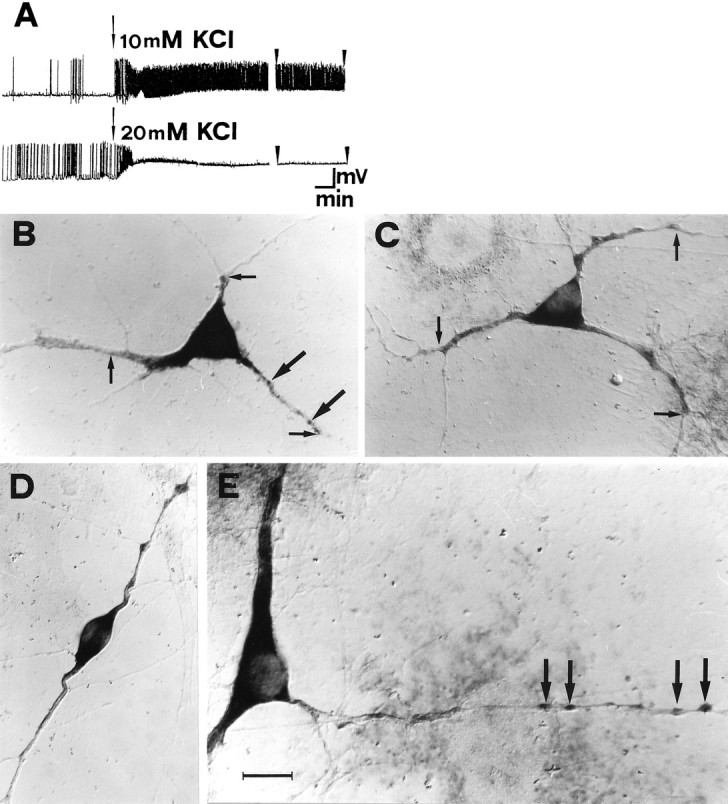
High potassium increases the dendritic localization of BDNF mRNA in cultured hippocampal neurons.A, Whole-cell patch-clamp recordings, under current-clamp conditions, after isotonic depolarization with 10 mm (top trace) and 20 mm KCl (bottom trace). The arrows mark the onset of the depolarization. The arrowheads mark the last 3 min of a 30 min recording time. Calibration: 45 mV, 1 min.B, In situ hybridization on cultured hippocampal neurons with BDNF riboprobe, viewed with Nomarski optics.Small arrows mark the maximal distance at which a dendritic labeling was scored (MDDL). In the dendrites, staining appears granular (large arrows). C, D, Depolarization with 10 mm KCl for 3 hr increases the dendritic localization of BDNF mRNA. E, At higher magnification, after 3 hr depolarization in 10 mm KCl, intensely labeled granules are detected within varicosities at great distance from the cell body (large arrows). Scale bar (shown in E): 20 μm for B, C, D; 14 μm for E.
KCl depolarization extends BDNF and TrkB mRNA dendritic labeling
BDNF mRNA expression is upregulated by physiological stimuli (Castrén et al., 1992) and by stimuli leading to hippocampal long-term potentiation (Patterson et al., 1992; Castrén et al., 1993; Dragunow et al., 1993; Lindholm et al., 1994). Moreover, kainic acid- or pilocarpine-induced seizures lead to an upregulation of the mRNAs for both BDNF and its receptor TrkB (Dugich-Djordjevich et al., 1992; Kokaia et al., 1993; Merlio et al., 1993; Wetmore et al., 1994;Schmidt-Kastner et al., 1996a; for review, see Lindvall et al., 1994). To investigate whether depolarization would also influence the localization of BDNF and TrkB mRNAs within the cells, cultures were gently depolarized by increasing the extracellular KCl concentration from 3.5 mm to either 10 or 20 mm. The electrical activity of the cultures was verified electrophysiologically, by measuring the membrane potential of pyramidal neurons under current clamp in a whole-cell patch-clamp configuration (Fig.2A). The resting potential in control conditions is −65 mV. In 10 mm KCl, the membrane potential is shifted to −55 mV, and an intense activity of action potentials, persisting throughout the recording time, is elicited in the cells (Fig. 2A, top). At 20 mm KCl, the membrane potential further depolarized to −13 mV (Fig. 2A, bottom). The onset of depolarization was initially associated with an increase in spike frequency, after which action potentials disappeared, most likely because of inactivation of voltage-dependent Na+channels.
The depolarization of hippocampal cells with KCl affects the subcellular localization of both BDNF and TrkB mRNAs. The in situ staining for BDNF mRNA is found at much greater distances from the cell body than in control cells after a 3 hr incubation with 10 mm KCl (compare Fig. 2, B, control, andC, D, and E, 10 mm KCl) and 20 mm KCl (not shown). The increase in the dendritic localization of BDNF mRNA was observed in cells of different morphologies (compare Fig. 2, C and D). The mRNA for BDNF is found in both large and thin caliber dendrites (Fig.2E). In the majority of the dendrites, regardless of their caliber, the staining appears to be discontinuous, in the form of granules localized, in some cases, to varicosities (Fig.2B,E, large arrows). These granules may correspond to the RNA-containing granules described in living neurons (Knowles et al., 1996). It is undetermined at present whether the absence of staining in some dendritic regions reflects a concentration of mRNA below the sensitivity threshold of the method or is caused by an absence of mRNA in these regions.
Similar results were obtained with hippocampal cultures labeled with the probe for the full length isoform of TrkB. Control cells show labeling of the cell body and of proximal dendrites (Fig.3A). Similar to what is observed for BDNF, a strong increase is also observed for TrkB in the extent of mRNA dendritic staining on depolarization for 3 hr in 10 mm KCl (compare Fig. 3, A and B) and 20 mm KCl (not shown). In Figure 3B, one of the two neurons shown displays TrkB mRNA labeling in the distal regions of the dendrites, whereas the other does not. These experiments demonstrate that neuronal depolarization is able to increase the extent of dendritic localization of both BDNF and TrkB mRNAs.
Fig. 3.
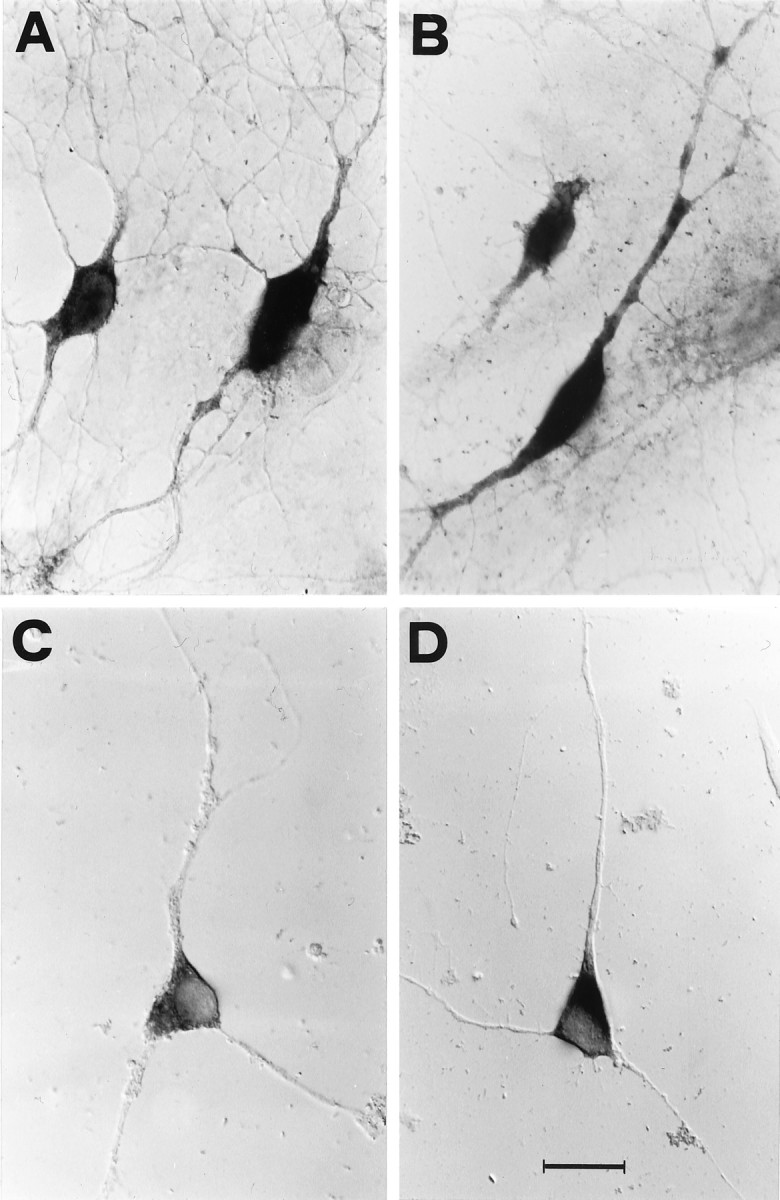
High potassium increases the dendritic localization of TrkB, but not of β-actin, mRNAs in cultured hippocampal neurons. Nonradioactive in situhybridization on cultured hippocampal neurons viewed with Nomarski optics. A, The TrkB riboprobe stains both cell bodies and dendrites. B, On depolarization with 10 mm KCl for 3 hr, the dendritic labeling is detectable at a much greater distance from the cell body. C, The β-actin probe labels the cell bodies under control conditions and (D) after 3 hr depolarization in 10 mm KCl. Scale bar (shown in D): 20 μm forA–D.
To determine whether the effect of KCl reflects an overall change in the subcellular distribution of the mRNAs, rather than a specific effect on the BDNF and TrkB mRNAs, we examined the distribution of the β-actin mRNA, which in hippocampal neurons is almost exclusively localized to the cell soma (Kleiman et al., 1990, 1994), with the exception of occasional labeling of proximal dendrites (Knowles et al., 1996). In control conditions, the subcellular localization of the β-actin mRNA is restricted to the cell body (Fig. 3C). Incubation for 3 hr in depolarizing medium leads to a slight increase in the labeling intensity of the soma but does not lead to the appearance of stained dendrites in the culture (Fig. 3D). In agreement with the findings of Knowles et al. (1996), some proximal dendrites occasionally display a weak labeling, both in control and in depolarized cultures (not shown). Thus, in contrast to BDNF and TrkB, no difference in the subcellular localization of β-actin mRNA was induced by KCl depolarization. Similar results were obtained when the subcellular distribution of the mRNA for the TrkA neurotrophin receptor was analyzed. At variance with the TrkB mRNA, only a subpopulation of the cultured hippocampal neurons expresses the TrkA mRNA. In control conditions, the TrkA mRNA is localized exclusively in the cell soma, extending only to the very proximal portion of the dendrites, in some cells. Depolarization for 3 hr in 10 mm KCl does not change this: the mRNA remain restricted to the cell soma (not shown) (our unpublished results). These results demonstrate that during the KCl depolarization, the increase in dendritic localization observed for BDNF and TrkB mRNAs does not reflect a generalized subcellular rearrangement of the mRNAs.
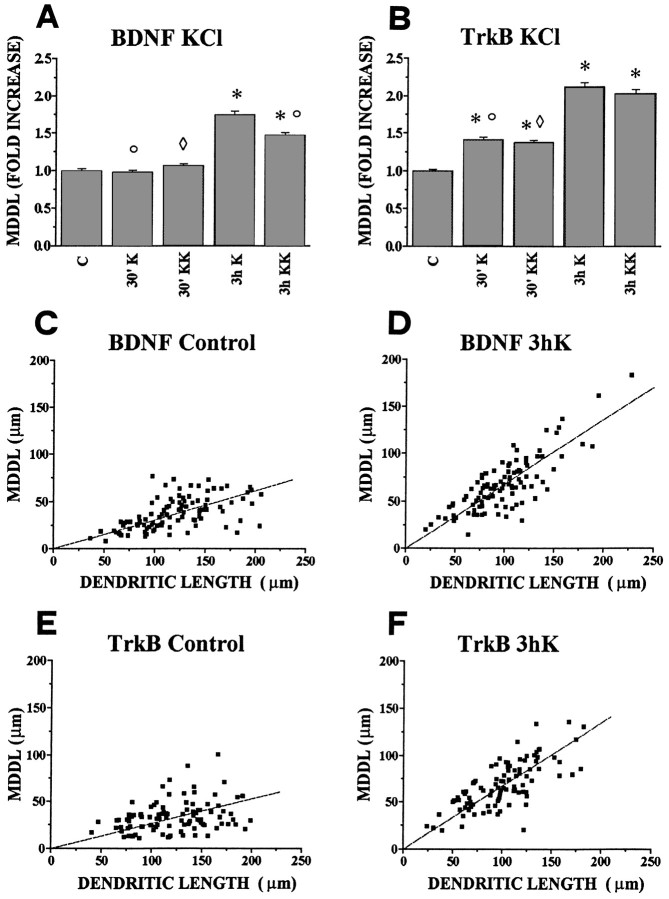
After this first qualitative evaluation, a quantitative statistical analysis was performed. The maximum distance from the cell body at which labeled BDNF and TrkB mRNA could be detected in individual dendrites (MDDL) was determined. An example of this procedure is marked by the small arrows in Figure 2B,C. For these measurements, cells with a well identified dendritic tree were randomly chosen within the cell population. In control cultures, the average MDDL for BDNF mRNA is 31.5 μm (Table1). Incubation with 10 mm or 20 mm KCl for 3 hr leads to a 1.75- and 1.47-fold increase of the MDDL, respectively (Fig.4A, Table 1). After shorter incubation times in 10 or 20 mm KCl (30 min), no significant variation could be observed. The quantitative analysis also confirmed that incubation for 3 hr in depolarizing media leads to a great increase of the extent of TrkB mRNA dendritic labeling (Fig.4B). In control cultures the average MDDL for TrkB mRNA was 24.5 μm (Table 1). After 3 hr stimulation in 10 or 20 mm KCl the MDDL increases by approximately twofold (Fig.4B, Table 1). At variance with BDNF, incubation in high potassium for shorter times (30 min) leads to a small but statistically significant (p ≤ 0.00001) increase of MDDL for TrkB mRNA: 1.42-fold and 1.37-fold for 10 or 20 mm KCl, respectively (Fig. 4B, Table 1). Thus, the subcellular localization of BDNF and TrkB mRNAs is differentially regulated by KCl.
Fig. 4.
Quantitative analysis of dendritic localization of BDNF and TrkB mRNAs after depolarization of hippocampal neurons in culture. Bars in A and Bindicate the fold increase, with respect to controls, of the mean maximal distance at which the in situ labeling in dendrites was detectable (MDDL). A, BDNF mRNA. Depolarization with either 10 (K) or 20 mm KCl (KK) increases the mean maximal distance of dendritic labeling (MDDL) at 3 hr (3 h K and 3 h KK) but not at 30 min (30′ K and 30′ KK). The average MDDL measured with 10 mm KCl (3 h K) is larger than with 20 mm KCl (3 h KK). B, TrkB mRNA. Both KCl concentrations induce a significant increase in MDDL of TrkB mRNA after 30 min (30′ K, 30′ KK) and a stronger increase at 3 hr (3 h K, 3 h KK). ○, Significantly different with respect to the 3 h K-stimulated; ⋄, significantly different with respect to the 3 h KK stimulated; and *, significantly different with respect to the control (no stimulation). Error bars represent SE. The corresponding numerical values, number of dendrites measured, and significance values are shown in Table 1.C, D, E, F, Correlation plots of the MDDL versus dendritic length. Each point refers to one MDDL determination, together with the length of the corresponding dendrite, under the experimental conditions indicated. The scatter plots were fitted by linear regression lines through the origin. C, Slope = 0.30, correlation coefficient r = 0.57; D, slope = 0.68; r = 0.80; E, slope = 0.26; r = 0.31; F, slope = 0.67; r = 0.72.
The previous results represent the average MDDL calculated on the total population of dendrites, including dendrites with very different length. To put the extent of the dendritic labeling into relationship with the length of the dendrites analyzed, each individual MDDL measurement was plotted as a function of the length of the corresponding dendrite. For this set of measurements we selected the most isolated cells in the culture, in which individual processes could be followed for their entire length. The results of the correlation plots for BDNF and TrkB are reported in Figure 4C–F. In control conditions, the correlation between the MDDL and the length of the dendrites is 0.57 for BDNF and 0.31 for TrkB. The regression lines have slopes of 0.30 and 0.26 for BDNF and TrkB, respectively. This slope corresponds to the actual average value of dendritic “filling” with BDNF and TrkB mRNA, ∼30 and 26% of the entire dendritic length, respectively. Depolarization of the cultures with 10 mm KCl for 3 hr induces an increase in the slope as well as in the correlation coefficient of the regression lines, for both BDNF (slope = 0.68; r = 0.80) and TrkB (slope = 0.67; r = 0.72). On average, BDNF and TrkB mRNAs label 68 and 67% of the total dendritic length, respectively. It is worth noting here that under depolarizing conditions several dendrites are stained for 100% of their length (Fig. 4D,F). In general, under depolarizing conditions staining extends to secondary and tertiary branchings, especially of large-caliber dendrites, but similar to MAP2 mRNA, was rarely seen in the very fine, distal dendritic processes (Kleiman et al., 1990). Thus, under depolarizing conditions, the mRNAs for both BDNF and TrkB occupy a much greater proportion of the total dendritic length than in control conditions, with several dendrites being labeled throughout their total length.
The effects of KCl depolarization do not depend on new mRNA synthesis
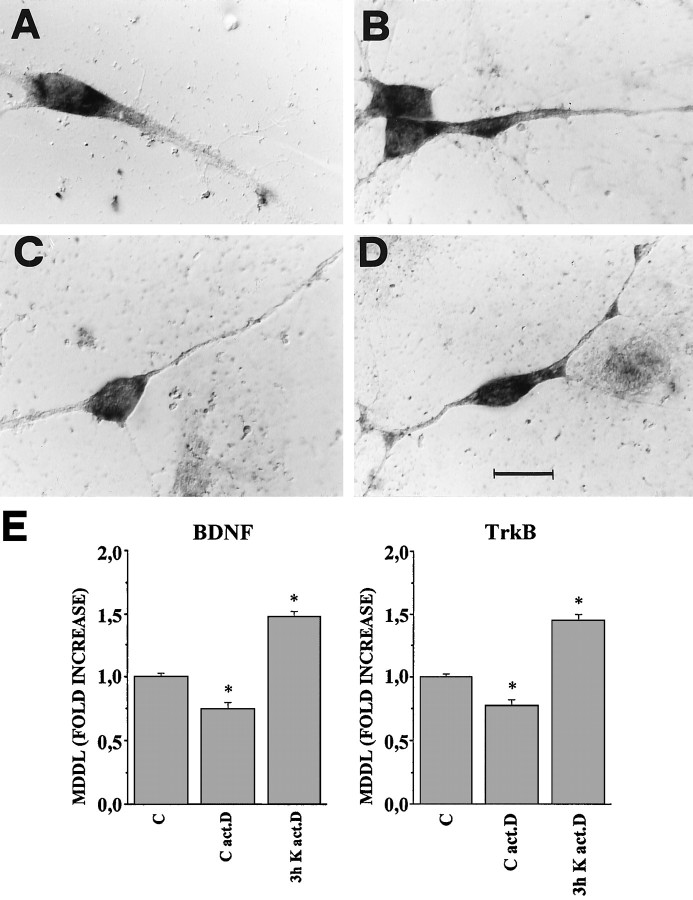
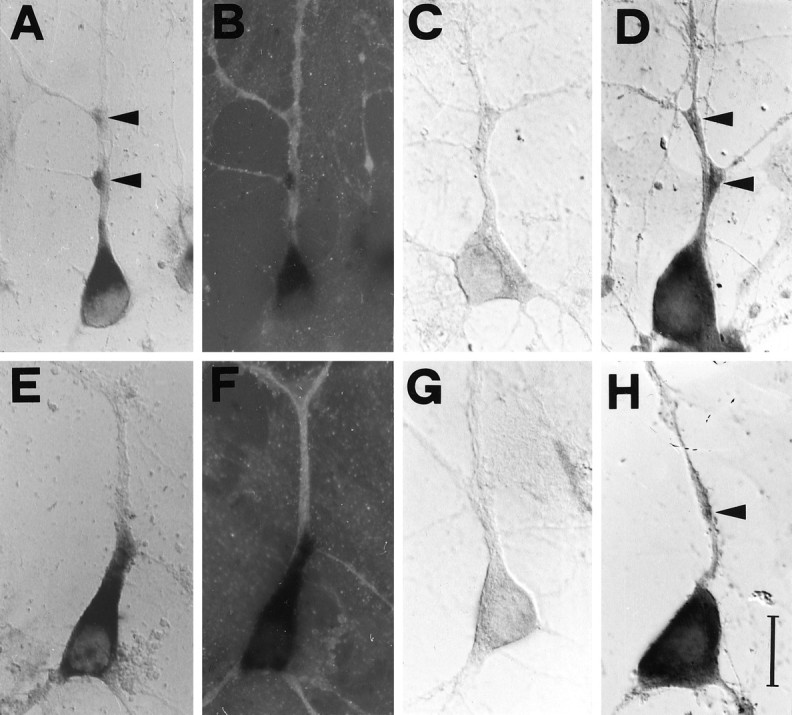
Previous studies have shown that depolarization of hippocampal neurons in culture with high potassium concentrations (50 mm) results in an increase of the total amount of BDNF mRNA that peaks after 6 hr (Zafra et al., 1990, 1992). To determine whether the increase of MDDL was secondary to an increase in mRNA levels, cultures were depolarized in the presence of 5 μg/ml of actinomycin-d to inhibit the synthesis of mRNA. At a qualitative level, treatment of unstimulated cultures for 3.5 hr with actinomycin-d results in only a small reduction of the staining intensity for both BDNF and TrkB mRNAs with respect to normal untreated cultures (Fig.5A,C), showing that the mRNAs for BDNF and TrkB do not turn over significantly during the time of the experiment. The quantitative analysis shows that the extent of MDDL in these conditions is also reduced, being 0.75- and 0.77-fold with respect to the controls for BDNF and TrkB mRNAs, respectively (Fig.5E). However, when cells pretreated with actinomycin-d for 30 min are depolarized in 10 mm KCl for 3 hr in the continuous presence of actinomycin-d, the staining for both the BDNF and TrkB mRNAs extends for a greater distance than in the controls (Fig.5B,D). The statistical analysis confirmed this result to be highly significant (BDNF = 1.48-fold; TrkB = 1.45-fold;p ≤ 0.00001) (Fig. 5E). These results show that the increase in the extension of the dendritic localization for the BDNF and TrkB mRNAs does not require the ongoing synthesis of new mRNA.
Fig. 5.
The effects of KCl depolarization do not depend on new mRNA synthesis. Nonradioactive in situ hybridization on cultured hippocampal neurons viewed with Nomarski optics. In control conditions, cells were kept in the presence of actinomycin-d for 3.5 hr and then stained with (A) BDNF or (C) TrkB riboprobes. After a pretreatment of 30 min with actinomycin-d, cultures were depolarized for 3 hr in 10 mm KCl in the continuous presence of actinomycin-d and stained with (B) BDNF or (D) TrkB riboprobes. Scale bar (shown inD): 20 μm for A–D. E, Quantitative analysis of the MDDL for BDNF and TrkB mRNAs with actinomycin-d. Bars indicate the fold increase, with respect to controls, of the mean maximal distance at which the in situ labeling was detectable (MDDL). Results are similar for both BDNF and TrkB mRNAs: treatment of control cultures with actinomycin-d for 3.5 hr (C act.D) reduces the MDDL with respect to untreated controls (C), whereas a strong increase of MDDL induced by 3 hr in 10 mm KCl also occurs in the presence of actinomycin-d (3h K act.D). Error bars represent SE. *, Significantly different from controls. The corresponding numerical values, the number of dendrites measured, and the significance values are shown in Table 1.
Role of Ca2+ in the activity-dependent increase of dendritic localization of BDNF and TrkB mRNAs
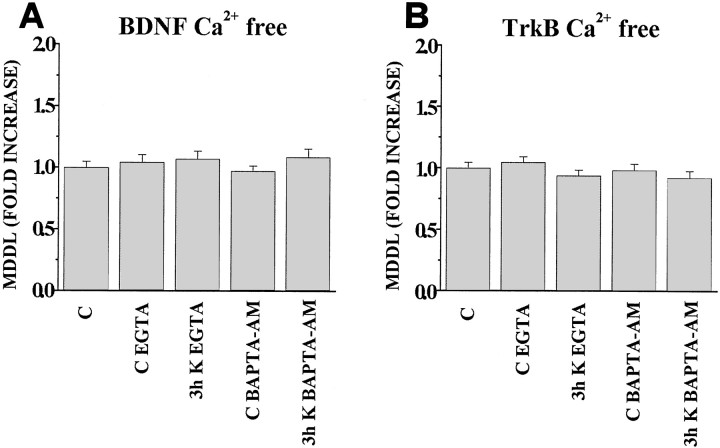
Ca2+ is a fundamental second messenger through which electrical activity can influence intracellular processes. To study the Ca2+ dependence of the activity-dependent targeting of BDNF and TrkB mRNAs, cells were incubated with 10 mm KCl in Ca2+-free medium supplemented with EGTA to block the external Ca2+ or with BAPTA-AM to block the internal Ca2+. In these Ca2+-free conditions, the general levels of staining are much reduced in comparison with control cultures, and several neurons appeared flattened and partially swollen (not shown). Measurement of the MDDL in neurons having a normal morphology shows that on depolarization with 10 mm KCl for 3 hr, the MDDL for BDNF mRNA (Fig. 6A) or for TrkB mRNA (Fig. 6B) does not change with respect to controls. Interestingly, no statistically significant difference can be observed when either EGTA or BAPTA-AM are used. These results demonstrate that extracellular Ca2+ is absolutely required for the KCl-induced increase in the dendritic targeting of BDNF and TrkB mRNA.
Fig. 6.
Effects of Ca2+-free medium on the KCl-induced increase in dendritic localization of BDNF and TrkB mRNAs. Quantitative analysis of the MDDL for BDNF and TrkB mRNAs in nominally Ca2+-free medium supplemented with 10 μm EGTA or BAPTA-AM. Bars indicate the fold increase, with respect to controls, of the mean maximal distance at which the in situ labeling was detectable (MDDL) for 80–150 dendrites. A, In nominally Ca2+-free medium the MDDL for BDNF mRNA, induced by depolarization with 10 mm KCl for 3 hr, does not change with respect to controls. The effects are identical in the presence of either EGTA or BAPTA-AM. B, MDDL for TrkB mRNA. At the level of p ≤ 0.05 (Student’st test), no significant variation in MDDL with respect to controls can be observed when cultures are depolarized under Ca2+-free conditions. Identical effects are seen with either EGTA or BAPTA-AM. C, Unstimulated controls in normal medium. CEGTA, Unstimulated controls maintained 3 hr in Ca2+-free medium containing EGTA;CBAPTA-AM, unstimulated controls maintained 3 hr in Ca2+-free medium containing BAPTA-AM; 3h K EGTA, neurons stimulated with 10 mm KCl in Ca2+-free medium containing EGTA; 3h K BAPTA-AM, neurons stimulated with 10 mm KCl in Ca2+-free medium containing BAPTA-AM. Error bars represent SE.
TTX, kinurenic acid, and nifedipine counteract the KCl-induced increase in dendritic localization of BDNF and TrkB mRNA
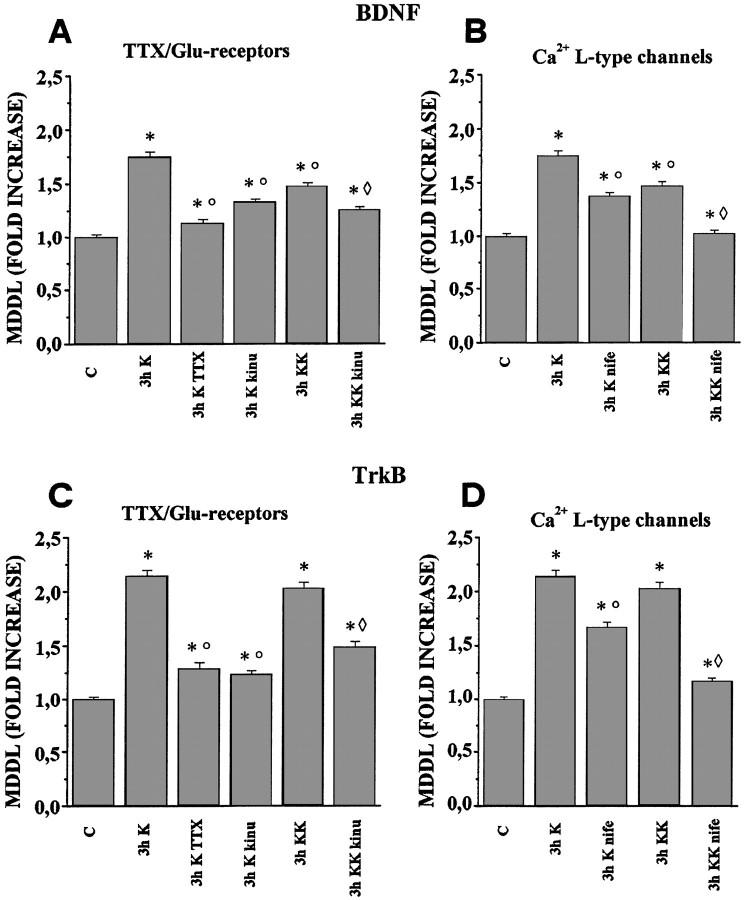
To further characterize the activity-dependent increase in MDDL for the BDNF and TrkB mRNAs, the Na+ channel blocker TTX was used. Depolarization with 10 mm KCl in the presence of 0.5 μm TTX for 3 hr results in a strong inhibition of the KCl-induced increase of MDDL, for both BDNF (81% inhibition) and TrkB mRNA (75% inhibition) (Fig.7A,C, Table 1). These results indicate that the increase in the dendritic localization of BDNF and TrkB mRNAs observed in 10 mm KCl requires Na+-dependent action potentials to occur.
Fig. 7.
Effects of TTX, kinurenic acid, and nifedipine on the KCl-induced increase in dendritic localization of BDNF and TrkB mRNAs. Quantitative analysis of the MDDL for BDNF and TrkB mRNAs in the presence of TTX, kinurenic acid, and nifedipine. Barsindicate the fold increase, with respect to controls, of the mean maximal distance at which the in situ labeling was detectable (MDDL). A, Continuous presence of tetrodotoxin (TTX) inhibits the increase in MDDL for BDNF mRNA, induced by depolarization with 10 mmKCl for 3 hr (3h K TTX). The glutamate receptor antagonist kinurenic acid partially counteracts the KCl-induced increase by either 10 (3h K kinu) or 20 mmKCl (3h KK kinu). B, The L-type Ca2+ channel blocker nifedipine has distinct effects at different KCl concentrations, with a partial inhibition at 10 mm KCl (3h K nife) and an almost complete inhibition at 20 mm KCl (3h KK nife).C, TTX strongly inhibits the MDDL increase for TrkB mRNA in 10 mm KCl (3h K TTX). The glutamate receptor antagonist kinurenic acid reduced the 10 mm KCl depolarization effects (3h K kinu) more effectively than at 20 mm KCl (3h KK kinu). D, In contrast, nifedipine almost completely abolishes the effects induced by 20 mm KCl (3h KK nife) and only partially inhibits those induced by 10 mm KCl depolarization (3h K nife). Error bars represent SE. ○, Significantly different with respect to the 3 h K-stimulated; ⋄, significantly different with respect to the 3 h KK stimulated; *, significantly different with respect to the control (no stimulation). Also see Table 1.
Hippocampal neurons express glutamate receptors of the NMDA and AMPA types. To ascertain the contribution of glutamatergic synaptic transmission to the KCl-induced increase in dendritic localization of BDNF and TrkB mRNA, kinurenic acid (1 mm), which blocks both NMDA and AMPA glutamate receptors, was added to cell cultures. The addition of kinurenic acid to either the 10 or 20 mm KCl medium partially counteracts the increase in MDDL for BDNF mRNA: the resulting MDDL increase is 1.32-fold (57% inhibition) for 10 mm KCl and 1.26-fold (55% inhibition) for 20 mm KCl (Fig. 7A, Table 1). In contrast, for TrkB mRNA, the inhibitory effect of kinurenic acid is more effective at 10 mm KCl, resulting in an MDDL increase of only 1.23-fold (80% inhibition), whereas at 20 mm KCl it has an effect similar to that observed for BDNF mRNA (1.48-fold increase; 53% inhibition) (Fig. 7C, Table 1). Thus, glutamatergic synaptic transmission is involved in the KCl-induced increase in MDDL at the two tested concentrations in a similar way for the BDNF mRNA, and the TrkB mRNA is more relevant at 10 than at 20 mm KCl.
Glutamate receptors of the NMDA type are known to be important for the Ca2+ influx in the neurons during synaptic activity. Another important Ca2+ entry route is represented by the voltage-sensitive Ca2+ channels, in particular those of the L-type. To test the contribution of these channels to the regulation of mRNA targeting, nifedipine (1 μm), a specific blocker of the L-type voltage-dependent Ca2+ channel, was used. When nifedipine is added to the 10 mm KCl medium, the MDDL increase is 1.38-fold for BDNF mRNA (50% inhibition) (Fig. 7B, Table 1) and 1.67-fold (58% inhibition) (Fig. 7D, Table 1) for TrkB mRNA. In contrast, the MDDL increase observed in 20 mm KCl for both mRNAs is almost completely abolished by nifedipine (96% and 83% inhibition for BDNF and TrkB, respectively) (Fig. 7B,D, Table 1). Taken together, these data suggest that the MDDL increase of BDNF and TrkB mRNAs observed in 20 mm KCl is almost totally dependent on Ca2+ entry through the L-type voltage-sensitive Ca2+ channels, whereas in 10 mm KCl the MDDL increase appears to depend only partially on the activation of these channels.
Dendritic TrkB and BDNF immunoreactivity is rapidly increased by electrical activity
The finding that the dendritic targeting of TrkB and BDNF mRNA is increased in hippocampal cultured neurons after KCl stimulation leads naturally to the question of whether the dendritic content of the corresponding proteins also would be subjected to modulation by electrical activity. To answer this question, the distribution of the BDNF and TrkB proteins in hippocampal cells was studied by immunofluorescence and confocal microscopy analysis with anti-BDNF and anti-TrkB antibodies, in control cultures and in cultures treated for 10 min with 10 mm KCl. For these experiments we used antibodies that were shown in previous studies to detect BDNF (Goodman et al., 1996) and TrkB (Cellerino et al., 1996) in the somatodendritic compartment of neurons. In control cultures, BDNF immunoreactivity appears to be concentrated mainly in small spots distributed along the dendrites, with a prominent localization to the proximal dendrites and the cell soma (Fig.8A). When cells were depolarized for 10 min with 10 mm KCl, larger and brighter spots could be observed in the distal dendrites, whereas the cell soma appeared to be stained similarly to controls (Fig.8B). TrkB immunoreactivity in control conditions appears to be distributed more evenly along the whole cell (Fig.8D). After incubation in 10 mm KCl for 10 min, a strong increase in the TrkB immunoreactivity, restricted to the dendrites, is observed, whereas the cell soma staining remains similar to that of controls (Fig. 8E).
Fig. 8.
A short incubation in high potassium increases BDNF and TrkB protein levels in the dendritic compartment. Immunohistochemistry on cultured hippocampal neurons. Each picture represents the integration in a single projection of a series of five optical sections obtained with a confocal microscope, as described in Materials and Methods. A, Anti-BDNF immunostaining of control cultures. B, Anti-BDNF immunostaining after 10 mm KCl depolarization for 10 min. C, Anti-BDNF staining after a preincubation with nocodazole for 6 hr followed by 10 min of 10 mm KCl depolarization in the continuous presence of nocodazole. D, Staining with anti-TrkB antibody in control conditions. E, Staining with anti-TrkB antibodies after 10 min depolarization in 10 mm KCl. F, Anti-TrkB staining after a preincubation with nocodazole for 6 hr followed by 10 min of 10 mm KCl depolarization in the continuous presence of nocodazole. Scale bar (shown in F): 20 μm forA–F.
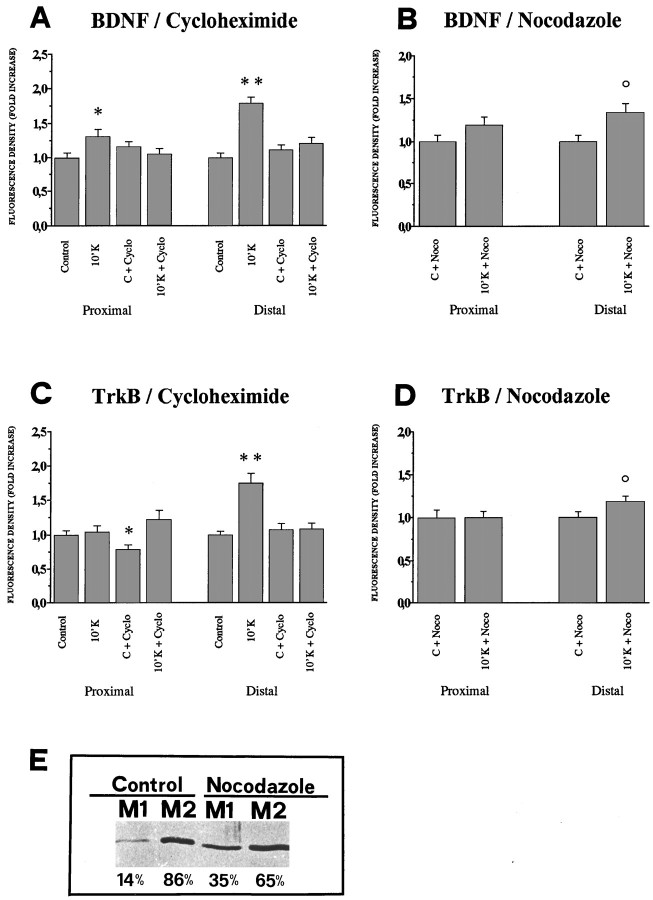
A quantification of these results was performed with confocal microscopy by measuring the immunofluorescence signal within the dendrites. For these measurements only the principal dendrites of the cells (i.e., >100 μm in length) were chosen. The immunofluorescence signal was integrated over five different confocal sections taken across the entire width of the dendrites. The integrated images of the dendrites were longitudinally subdivided into two regions, which were contoured manually: a proximal region (from the base of the dendrite up to 30 μm from the cell soma) and a distal region (from 30 μm up to 90 μm from the cell soma). In control conditions, the majority of dendrites >100 μm in length contained BDNF and TrkB mRNAs in both the proximal and distal regions described above (Fig. 4C,E). The fluorescence density (see Materials and Methods) over the proximal and distal regions was quantified under different experimental conditions. The 10 mm KCl depolarization induces in the proximal region a 1.30-fold increase of the BDNF fluorescence density and a stronger increase of 1.78-fold in the distal region (Fig.9A). Also for TrkB, after 10 min in 10 mm KCl an increase in fluorescence density in the distal dendrites is found (1.75-fold), whereas no significant variation is found in the proximal region (Fig. 9C). These experiments demonstrate that electrical activity affects BDNF and TrkB protein levels by rapidly increasing their amount in the dendrites.
Fig. 9.
Quantitative analysis of BDNF and TrkB protein levels in proximal and distal regions of the dendrites. Immunofluorescence for BDNF or TrkB was acquired by five confocal sections and integrated in a single projection, in control conditions and after 10 min depolarization with 10 mm KCl, in the presence or absence of cycloheximide (A, C) and of nocodazole (B, D). Fluorescent density of BDNF (A, B) and TrkB (C, D) was determined in proximal and distal regions of the projections of labeled dendrites as described in Materials and Methods. Bars represent the mean fold increase of the fluorescence density of 45 dendrites, with respect to the controls (=1.0). Error bars represent SE.A, Incubation of cells in 10 mm KCl for 10 min leads to a strong increase of BDNF fluorescence density in both proximal and distal regions (10′K). Incubation of control cells with the protein synthesis inhibitor cycloheximide does not alter the basal levels of fluorescence density for BDNF in proximal and distal regions (C + Cyclo). Cycloheximide completely inhibits the increase in fluorescence density induced by the 10 mm KCl stimulus (10′K + Cyclo).B, After pretreatment of cells with nocodazole for 6 hr the levels of BDNF fluorescence density in the proximal dendrites depolarized for 10 min with 10 mm KCl in the continuous presence of nocodazole (10′K + Noco) were comparable to control (C + Noco), whereas a significant fluorescence density increase could be detected in the distal dendrites (10′K + Noco). C, Incubation of cells in 10 mm KCl for 10 min led to a strong increase of fluorescence density for TrkB in the distal region (10′K) but not in the proximal region, and this effect was abolished by cycloheximide (10′K + Cyclo). Incubation of control cells with cycloheximide reduces the basal levels of TrkB fluorescence density in the proximal but not in the distal regions (C + Cyclo). D, After pretreatment of cells with nocodazole for 6 hr, followed by a depolarization for 10 min with 10 mm KCl in continuous presence of nocodazole, the TrkB fluorescence density in the proximal dendrites (10′K + Noco) was comparable to the controls (C + Noco), whereas a fluorescence density increase could be detected in the distal dendrites (10′K + Noco). Significance with respect to controls: op ≤ 0.05; *p ≤ 0.01; **p ≤ 0.001.E, Western blot for the soluble and microtubule cellular pools of tubulin in hippocampal neurons in culture. M1, Soluble tubulin fraction; M2, polymerized tubulin fraction. After 6 hr incubation with nocodazole, the soluble, unpolymerized tubulin fraction is doubled.
The activity-dependent increase in dendritic BDNF and TrkB immunoreactivity requires protein synthesis
To determine whether new protein synthesis is involved in the KCl-induced increase of BDNF and TrkB dendritic immunostaining, cultures were preincubated for 30 min with the protein synthesis inhibitor cycloheximide, before the depolarizing stimulus. Cultures were then incubated for 10 min either in 10 mm KCl or in control medium, in the continuous presence of cycloheximide. In unstimulated cultures, cycloheximide does not change the basal levels of fluorescence density of BDNF or TrkB, with respect to those of control conditions; the only exception is a slight decrease of TrkB in the proximal dendrites (Fig. 9A,C). This shows that under basal conditions the half-life of the BDNF and TrkB proteins, in all compartments for BDNF and at least in the distal dendrites for TrkB, is >40 min. In KCl-stimulated cultures, cycloheximide completely prevents the increase in BDNF and TrkB immunoreactivity in the distal dendritic compartment (Fig. 9A,C). For BDNF, the cycloheximide treatment also fully inhibits the increase found in the proximal region. Taken together, these results demonstrate that the electrical activity-dependent increase in BDNF and TrkB immunostaining observed in distal and proximal dendrites requires new protein synthesis.
The activity-dependent increase in dendritic BDNF and TrkB immunoreactivity occurs also under dendritic transport blockade
The kinetics of fast protein transport in dendrites (Kiss et al., 1977; Feig and Lipton, 1993) would allow, in principle, proteins newly synthesized in the perikaryon to be transported into the distal dendrites during the 10 min depolarization, leading to their accumulation.
To ascertain whether the immunofluorescence increase observed in the distal dendrites would reflect an accumulation caused by transport of newly synthesized BDNF and TrkB protein from the perikaryon, cells were depolarized in the presence of nocodazole (1 μg/ml), which inhibits the protein transport in dendrites, by affecting the amount of polymerized tubulin (Dotti and Banker, 1991; Cid-Arregui et al., 1995). In control experiments, to confirm the effectiveness of the nocodazole treatment, cells were preincubated with the drug for 6 hr. Thereafter, differential extractions of soluble and microtubule fractions were performed, and the relative amount of tubulin in the polymerized and unpolymerized fractions was quantified by optical scanning of the Western blot stained with anti-tubulin antibodies. Nocodazole treatment leads to a decrease of polymerized tubulin (from 86% of the total tubulin amount in control conditions to 65%) and a corresponding 2.5-fold increase of the unpolymerized tubulin pool (from 14 to 35% of the total tubulin amount) (Fig. 9E). Nocodazole-treated cells show an immunofluorescence signal for both BDNF and TrkB localized in the cell body and in dendrites, especially in dendritic varicosities. This beaded immunofluorescence distribution is even more evident in cultures stimulated with 10 mm KCl for 10 min, in the continuous presence of nocodazole (Fig. 8C,F). The quantitative analysis of the fluorescent density of cell cultures pretreated for 6 hr with nocodazole shows that after 10 min stimulation in 10 mm KCl the BDNF and TrkB fluorescence density is significantly increased in the distal dendrites (1.34-fold for BDNF and 1.19-fold for TrkB) (Fig. 9B,D). No significant increase was seen in the proximal dendrites. Thus, a local increase in the BDNF and TrkB protein amount can be triggered by a brief depolarization independently of macromolecule transport from adjacent regions.
DISCUSSION
Neurotrophins are localized to the dendritic processes of neurons, from which they can be secreted in an activity-dependent way (Blöchl and Thoenen, 1996; Goodman et al., 1996). The BDNF-receptor TrkB is also found in dendrites (Cabelli et al., 1996;Cellerino et al., 1996). This study investigated whether the mRNAs coding for BDNF and for its receptor TrkB are targeted to dendrites, contributing to the final destination of the corresponding proteins. Herein we demonstrated the following: (1) BDNF and TrkB mRNAs are localized to the somatodendritic compartment of cultured hippocampal neurons; (2) the KCl depolarization extends the localization of BDNF and TrkB mRNAs to the distal portion of dendrites up to their end; (3) the enhancement of the dendritic extent of BDNF and TrkB mRNAs by KCl is not secondary to an increase in mRNA synthesis; (4) the KCl effect depends on extracellular Ca2+; (5) a short KCl depolarization stimulates the synthesis of BDNF and TrkB proteins, which are accumulated in the distal portion of dendrites; and (6) this accumulation still occurs, although at lower levels, when the dendritic transport is inhibited. These results support the view that synaptic activity can regulate the amount of dendritic proteins by modulating the local translation of the corresponding mRNA (Steward, 1994, 1997;Schumann, 1997). In addition, our results, together with the results obtained by others for the ARC mRNA (Lyford et al., 1995), extend this concept by suggesting that electrical activity may also regulate the composition of the dendritic pool of mRNAs, through a modulation of their transport and/or of their half-life. The demonstration that BDNF and TrkB mRNAs are subject to this form of regulation has implications for the proposed mode of action of the encoded proteins in synaptic plasticity.
BDNF and TrkB mRNAs are widely expressed in the rat brain. Notably, the majority of the previous studies did not specifically examine the issue of the subcellular localization of these two mRNAs, because they were focused on their overall expression pattern in the brain, showing pictures at magnification too low to detect the dendrites or lacking a clear identification of the dendritic processes. Only one study reported a dendritic localization for TrkB mRNA (Ugolini et al., 1995), and a few others were suggestive of a proximal dendritic localization of BDNF or TrkB mRNAs (Wetmore et al., 1990, 1994; Dugich-Djordjevich et al., 1992; Schmidt-Kastner et al., 1996a,b). Here, the application onto isolated neurons in culture of a nonradioactive in situhybridization technique, having high resolution of the cell morphology, has allowed us to detect unequivocally the presence of BDNF and TrkB mRNAs in the dendrites. In control conditions the staining extends on average to 31.5 and 24.5 μm for BDNF and TrkB mRNAs, respectively. This staining has to be considered dendritic, similar to results of the detailed study by Martone et al. (1996) showing that the α-Ca/Calmodulin kinase II mRNA, which is dendritic, “could be followed for a distance of 30–40 μm from the cell body,” whereas the nondendritic β-subunit mRNA was restricted within the first 15 μm. Remarkably, in our study electrical activity extends the localization of BDNF and TrkB mRNAs to almost the entire dendritic length. In agreement with this in vitro study, the mRNA for BDNF and TrkB, but not for TrkA, is dendritically localized in vivo as well (Tongiorgi et al., 1996a,b). This may reflect a basal level of electrical stimulation of the labeled cells.
Theoretically, the KCl treatment might induce a general intracellular reorganization, attributable to cytotoxic effects. Previous studies on BDNF expression in cultured hippocampal neurons (Zafra et al., 1990,1992; Elliott et al., 1994) used a higher concentration of KCl (50 mm), with respect to our study (10 or 20 mm), for a much longer time (up to 48 hr) than in our experiments, without describing cytotoxic effects. The presence of dendritic varicosities is not a sign of cell suffering per se: in cortical neurons, cytotoxic concentrations of the glutamate analog NMDA induce a dramatic increase in the number of dendritic varicosities, but a background level of varicosities was present in control conditions as well (Faddis et al., 1997). Furthermore, in spinal neurons, a physiological stimulation also can induce formation of varicosities (Mantyh et al., 1995). In our experimental conditions, the minor increase in the number of cells with stained dendritic varicosities that was observed (from 3% for controls to 5% for stimulated) argues against a general cytotoxic effect of KCl. The significance of the accumulation of grains of mRNA staining in the varicosities is unclear, but it might reflect the existence of preferential sites for protein synthesis, as suggested by the accumulation of BDNF and TrkB proteins in varicosities of nocodazole-treated cells.
Electrical activity increases the extent of dendritic BDNF and TrkB mRNA localization, by and large, in a similar way. At both KCl concentrations tested, the increase of the intracellular concentration of Ca2+ emerges as one of the triggering events of the cascade that modulates localization of both mRNAs. However, the regulation of TrkB mRNA localization differs from that of BDNF in some respects. First, for BDNF mRNA the effect of 10 mm KCl is stronger than that of 20 mm KCl, whereas for TrkB the two KCl concentrations have a comparable effect. Second, the increase in dendritic targeting is more rapid for TrkB than for BDNF mRNA. Third, the glutamatergic synaptic transmission seems to be more important in regulating the dendritic localization of TrkB than of BDNF mRNA. The increased localization of BDNF and TrkB mRNAs induced by depolarization is a slow process. It is not clear at present whether the limiting time step is the transport process itself [the rate of mRNA transport in the dendrites is on average 11 μm/hr, (Davis et al., 1990)] or the signal transduction processes leading to the observed phenomena. A precise analysis of the factors involved in the regulation of the localization of mRNAs is beyond the scope of the present study but is of high interest. Similar to results in our study, kainic acid-induced seizures increase the dendritic localization of ARC mRNA (Lyford et al., 1995), suggesting that similar mechanisms also may operatein vivo. Whether the increased targeting of BDNF and TrkB mRNAs, ensuing membrane depolarization, is attributable to a stimulation of a specific transport mechanism or to an increased half-life of the two mRNAs, is at the moment an open question. In any event, the experiments with actinomycin-d demonstrate that the extension of the BDNF and TrkB mRNAs to the distal dendrites is not secondary to an mRNA synthesis stimulation by KCl.
The modulation of BDNF and TrkB protein levels was studied by using shorter KCl stimulation protocols (10 min). A consistent picture emerges from these experiments: (1) the levels of BDNF and TrkB protein in the distal dendritic regions increase as a result of KCl-induced depolarization; (2) the basal levels of BDNF and TrkB protein in control conditions are not affected by a 40 min cycloheximide incubation; and (3) the KCl-induced increase is totally inhibited by cycloheximide and thus protein synthesis dependent. The increase in distal BDNF and TrkB proteins may be caused, therefore, by an increased translation of a preexisting pool of mRNAs and/or to a reduced degradation of these proteins. The cycloheximide dependence of the observed increase argues in favor of the first possibility, i.e., a translational control. During the 10 min depolarization, the transport of BDNF or TrkB proteins from the cell body may contribute to the observed phenomenon, because the most rapid dendritic transport of newly formed proteins in rat hippocampal cells is 50 μm/min (Kiss et al., 1977). However, after incubation of hippocampal cells for 6 hr with nocodazole that fully inhibits, in a reversible manner, the dendritic transport in hippocampal neurons (Dotti and Banker, 1991;Cid-Arregui et al., 1995), a significant increase in dendritic proteins on depolarization could still be detected. Taken together, these data suggest that BDNF and TrkB protein might be synthesized in the periphery of the hippocampal neurons, after KCl depolarization. The existence of the complete machinery for protein synthesis in the dendritic compartment of neurons is now well established (Steward, 1997). Also, the requirement for dendritic protein synthesis in synaptic plasticity phenomena has been postulated to form one of the mechanisms whereby synapse specificity of potentiated synapses is achieved (Kang and Schumann, 1996; Schumann, 1997).
Of note is the fact that in the culture system studied, the large majority of the neurons express both BDNF and TrkB. In accordance with previous studies in vivo (Kokaia et al., 1993; Miranda et al., 1993), this has suggested the potential for an autocrine loop at the cellular level (Miranda et al., 1993). Our results extend this concept, suggesting the theoretical possibility for local dendritic autocrine loops.
In conclusion, our data suggest that the dendritic transport and/or half-life of the BDNF and TrkB mRNAs is selectively enhanced by the electrical activity and that the mRNA localization can contribute to the local synthesis of these two proteins in response to enhanced synaptic activity.
Footnotes
This work was supported by a research grant from the Human Frontier Science Project Organization (HFSPO) (RG93-93) to A.C. E.T. was supported by a postdoctoral fellowship from the International Centre for Genetic Engineering and Biotechnology (ICGEB) of Trieste. We thank Professor E. Cherubini and Dr. L. Domenici (SISSA, Trieste) for helpful suggestions and discussions; Professor E. Ferrero and Dr. P. Giulianini (University of Trieste) for help with the imaging system; and Drs. S. Hunt and K. Smith (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK) for advice on confocal analysis. We thank Dr. L. Fasulo for precious advice and I. Masi for excellent technical help with cell cultures. We are greatly indebted to Dr. P. Andjus (SISSA, Trieste) for the electrophysiological recordings and to those cited in the text for providing plasmids.
Correspondence should be addressed to Dr. A. Cattaneo, International School for Advanced Studies (SISSA), Neuroscience Program, Via Beirut 2/4-34014 Trieste, Italy.
REFERENCES
- 1.Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993;123:431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augood SJ, Kiyama H, Faull RLM, Emson PC. Dopaminergic D1 and D2 receptor antagonists decrease prosomatostatin mRNA expression in rat striatum. Neuroscience. 1991;44:35–44. doi: 10.1016/0306-4522(91)90249-n. [DOI] [PubMed] [Google Scholar]
- 3.Barde Y-A. The nerve growth factor family. Prog Growth Factor Res. 1990;2:237–248. doi: 10.1016/0955-2235(90)90021-b. [DOI] [PubMed] [Google Scholar]
- 4.Bian F, Chu T, Schilling K, Oberdick J. Differential mRNA transport and the regulation of protein synthesis: selective sensitivity of Purkinje cell dendritic mRNAs to translational inhibition. Mol Cell Neurosci. 1996;7:116–133. doi: 10.1006/mcne.1996.0009. [DOI] [PubMed] [Google Scholar]
- 5.Blöchl A, Thoenen H. Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 6.Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Bozzi Y, Pizzorusso T, Cremisi F, Rossi FM, Barsacchi G, Maffei L. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience. 1995;69:1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- 8.Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabelli RJ, Allendorfer KL, Radeke MJ, Welcher AA, Feinstein SC, Shatz CJ. Changing patterns of expression and subcellular localization of TrkB in the developing visual system. J Neurosci. 1996;16:7965–7980. doi: 10.1523/JNEUROSCI.16-24-07965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons in culture. Dev Brain Res. 1984;13:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 11.Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol (Lond) 1997;498:153–164. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castrén E, Pitkänen M, Sirviö J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. NeuroReport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Castrén E, Thoenen H, Lindholm D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience. 1995;64:71–80. doi: 10.1016/0306-4522(94)00386-j. [DOI] [PubMed] [Google Scholar]
- 15.Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor TrkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 16.Cid-Arregui A, Parton RG, Simons K, Dotti CG. Nocodazole-dependent transport, and Brefeldin A-sensitive processing and sorting, of newly synthesized membrane proteins in cultured neurons. J Neurosci. 1995;15:4259–4269. doi: 10.1523/JNEUROSCI.15-06-04259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 19.Davis L, Burger B, Banker GA, Steward O. Dendritic transport: quantitative analysis of the time course of somatodendritic transport of recently synthesized RNA. J Neurosci. 1990;10:3056–3068. doi: 10.1523/JNEUROSCI.10-09-03056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dotti CG, Banker G. Intracellular organization of hippocampal neurons during the development of neuronal polarity. J Cell Sci. 1991;15:75–84. doi: 10.1242/jcs.1991.supplement_15.11. [DOI] [PubMed] [Google Scholar]
- 21.Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Brain-derived neurotrophic factor expression after long-term potentiation. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- 22.Dugich-Djordjevic MM, Tocco G, Willoughby DA, Najm I, Pasinetti G, Thompson RF, Baudry M, Lapchak PA, Hefti F. BDNF mRNA expression in the developing rat brain following kainic acid-induced seizure activity. Neuron. 1992;8:1127–1138. doi: 10.1016/0896-6273(92)90133-x. [DOI] [PubMed] [Google Scholar]
- 23.Dugich-Djordjevic MM, Peterson C, Isono F, Ohsawa F, Widmer HR, Denton TL, Bennett GL, Hefti F. Immunohistochemical visualization of brain-derived neurotrophic factor in the rat brain. Eur J Neurosci. 1995;7:1831–1839. doi: 10.1111/j.1460-9568.1995.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 24.Elliott RC, Inturrisi CE, Black IB, Dreyfus CF. An improved method detects differential NGF and BDNF gene expression in response to depolarization in cultured hippocampal neurons. Mol Brain Res. 1994;26:81–88. doi: 10.1016/0169-328x(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 25.Emson PC. In-situ hybridization as a methodological tool for the neuroscientist. Trends Neurosci. 1993;16:9–16. doi: 10.1016/0166-2236(93)90041-j. [DOI] [PubMed] [Google Scholar]
- 26.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 27.Ernfors P, Merlio J-P, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 28.Faddis BT, Hasbani MJ, Goldberg MP. Calpain activation contributes to dendritic remodeling after brief excitotoxic injury in vitro. J Neurosci. 1997;17:951–959. doi: 10.1523/JNEUROSCI.17-03-00951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fasulo L, Ovecka M, Kabat J, Bradbury A, Novak M, Cattaneo A. Overexpression of Alzheimer’s PHF core tau fragments: implications for the tau truncation hypothesis. Alzheimer Res. 1996;2:195–200. [Google Scholar]
- 30.Feig S, Lipton P. Pairing the cholinergic agonist carbachol with patterned Scheffer collateral stimulation initiates protein synthesis in hippocampal CA1 pyramidal cell dendrites via a muscarinic, NMDA-dependent mechanism. J Neurosci. 1993;13:1010–1021. doi: 10.1523/JNEUROSCI.13-03-01010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuichi T, Samon-Chazottes D, Fujino I, Yamada N, Hasegawa M, Miyaki A, Yoshikawa S, Guenet J-L, Mikoshiba K. Widespread expression of inositol 1,4,5-triphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Receptors Channels. 1993;1:11–24. [PubMed] [Google Scholar]
- 32.Garner CC, Tucker RP, Matus A. Selective localization of messenger RNA for the cytoskeletal protein MAP2 in dendrites. Nature. 1988;336:674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- 33.Gazzaley AH, Benson DL, Huntley GW, Morrison JH. Differential subcellular regulation of NMDAR1 protein and mRNA in dendrites of dentate gyrus granule cells after perforant path transection. J Neurosci. 1997;17:2006–2017. doi: 10.1523/JNEUROSCI.17-06-02006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman LJ, Valverde J, Lim F, Geschwind MD, Federhoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 35.Hannan AJ, Schevzov G, Gunning P, Jeffrey PL, Weinberger RP. Intracellular localization of tropomysin mRNA and protein is associated with development of neuronal polarity. Mol Cell Neurosci. 1995;6:397–412. doi: 10.1006/mcne.1995.1030. [DOI] [PubMed] [Google Scholar]
- 36.Herb A, Wisden W, Catania MV, Maréchal D, Dresse A, Seeburg PH. Prominent dendritic localization in forebrain neurons of a novel mRNA and its product, dendrin. Mol Cell Neurosci. 1997;8:367–374. doi: 10.1006/mcne.1996.0594. [DOI] [PubMed] [Google Scholar]
- 37.Holtzman DM, Li Y, Parada LF, Kinsman S, Chen CK, Valletta JS, Zhou J, Long JB, Mobley WC. p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron. 1992;9:465–478. doi: 10.1016/0896-6273(92)90184-f. [DOI] [PubMed] [Google Scholar]
- 38.Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 39.Johnston DS. The intracellular localization of messenger RNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 40.Kang H, Schumann EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 41.Kang H, Schumann EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 42.Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiss J. Synthesis and transport of newly formed proteins in dendrites of rat hippocampal pyramid cells, an electron microscope autoradiographic study. Brain Res. 1977;124:237–250. doi: 10.1016/0006-8993(77)90882-4. [DOI] [PubMed] [Google Scholar]
- 44.Kleiman R, Banker G, Steward O. Differential subcellular localization of particular RNAs in hippocampal neurons in culture. Neuron. 1990;5:821–830. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- 45.Kleiman R, Banker G, Steward O. Development of subcellular mRNA compartmentation in hippocampal neurons in culture. J Neurosci. 1994;14:1130–1140. doi: 10.1523/JNEUROSCI.14-03-01130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knipper M, Leung LS, Zhao D, Rylett RJ. Short-term modulation of glutamatergic synapses in adult rat hippocampus by NGF. NeuroReport. 1994a;5:2433–2436. doi: 10.1097/00001756-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Knipper M, da-Penha-Berzaghi M, Blöchl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994b;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 48.Knowles RB, Sabry SH, Martone ME, Deerinck TJ, Ellisman MH, Bassel GJ, Kosik S. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci USA. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korte M, Carrol P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korte M, Griesbeck O, Gravel C, Carrol P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauterborn JC, Rivera S, Stinis CT, Hayes VY, Isackson PJ, Gall CM. Differential effects of protein synthesis inhibition on the activity-dependent expression of BDNF transcripts: evidence for immediate-early gene responses from specific promoters. J Neurosci. 1996;16:7428–7436. doi: 10.1523/JNEUROSCI.16-23-07428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y-A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 54.Leßmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 55.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindholm D, Castrén E, Berzaghi M, Blöchl A, Thoenen H. Activity dependent and hormonal regulation of neurotrophin mRNA levels in the brain: implications for neuronal plasticity. J Neurobiol. 1994;25:136–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- 57.Lindvall O, Kokaia Z, Bengzon J, Elmér E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 58.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 60.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;15:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 61.Maisonpierre PC, Le Beau MM, Espinosa RI, Ip NY, Belluscio L, La Monte S, Squinto S, Furth ME, Yancopulos GD. Human and rat BDNF and NT3: gene structures, distributions and chromosomal localization. Genomics. 1991;10:558–568. doi: 10.1016/0888-7543(91)90436-i. [DOI] [PubMed] [Google Scholar]
- 62.Malgaroli A, Tsien RW. Glutamate-induced long term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature. 1992;357:134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- 63.Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, Simone DA. Receptor andocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 64.Martone ME, Pollock JA, Jones YZ, Ellisman MH. Ultrastructural localization of dendritic messenger mRNA in adult rat hippocampus. J Neurosci. 1996;16:7428–7436. doi: 10.1523/JNEUROSCI.16-23-07437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merlio J-P, Ernfors P, Kokaia Z, Middlemas DS, Bengzon J, Kokaia M, Smith M-L, Siesjö BK, Hunter T, Lindvall O, Persson H. Increased production of TrkB protein kinase receptors after brain insults. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- 66.Middlemas DS, Lindberg RA, Hunter T. TrkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNA for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nudel U, Zakut R, Shani M, Neuman S, Levy Z, Yaffe D. The nucleotide sequence of the rat cytoplasmatic β-actin gene. Nucleic Acids Res. 1983;11:1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 70.Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 71.Prakash N, Fehr S, Mohr E, Richter D. Dendritic localization of rat vasopressin mRNA: ultrastructural analysis and mapping of targeting elements. Eur J Neurosci. 1997;9:523–532. doi: 10.1111/j.1460-9568.1997.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 72.Racca C, Gardiol A, Triller A. Dendritic and postsynaptic localizations of glycine receptor α subunit mRNAs. J Neurosci. 1997;17:1691–1700. doi: 10.1523/JNEUROSCI.17-05-01691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ringstedt T, Lagercrantz H, Persson H. Expression of the members of the trk family in the developing postnatal rat brain. Dev Brain Res. 1993;72:119–131. doi: 10.1016/0165-3806(93)90165-7. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt-Kastner R, Humpel C, Wetmore C, Olson L. Cellular hybridization for BDNF, trkB, and NGF mRNAs and BDNF-immunoreactivity in rat forebrain after pilocarpine-induced status epilepticus. Exp Brain Res. 1996a;107:331–347. doi: 10.1007/BF00230416. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt-Kastner R, Wetmore C, Olson L. Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampal, striatum and cortex. Neuroscience. 1996b;74:161–183. doi: 10.1016/0306-4522(96)00093-0. [DOI] [PubMed] [Google Scholar]
- 76.Schumann E. Synapse specificity and long-term information storage. Neuron. 1997;18:339–342. doi: 10.1016/s0896-6273(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 77.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines in the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steward O. Dendrites as a compartment for macromolecular synthesis. Proc Natl Acad Sci USA. 1994;91:10766–10768. doi: 10.1073/pnas.91.23.10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steward O. mRNA localization in neurons: a multipurpose mechanism. Neuron. 1997;18:9–12. doi: 10.1016/s0896-6273(01)80041-6. [DOI] [PubMed] [Google Scholar]
- 80.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 82.Tiedge H, Brosius J. Translational machinery in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16:7171–7181. doi: 10.1523/JNEUROSCI.16-22-07171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tinmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 84.Tongiorgi E, Righi M, Cattaneo A. NGF, BDNF and TrkB but not TrkA mRNAs are localized to the dendrites of rat neurons. Soc Neurosci Abstr. 1996a;22:125.17. [Google Scholar]
- 85.Tongiorgi E, Righi M, Cattaneo A. Subcellular localization of neurotrophins and neurotrophin receptors: implications for synaptic plasticity. Proceedings of the International Symposium of Neuroscience, Belém, Brasil. Rev Brasil Biol. 1996b;56:175–182. [PubMed] [Google Scholar]
- 86.Torre ER, Steward O. Demonstration of local protein synthesis within dendrites using a new cell culture system that permits the isolation of living axons and dendrites from their cell bodies. J Neurosci. 1992;12:762–772. doi: 10.1523/JNEUROSCI.12-03-00762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Torre ER, Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized protein in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16:5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ugolini G, Cremisi F, Maffei L. TrkA, TrkB and p75 mRNA expression is developmentally regulated in the rat retina. Brain Res. 1995;704:121–124. doi: 10.1016/0006-8993(95)01191-9. [DOI] [PubMed] [Google Scholar]
- 89.Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 90.Wetmore C, Olson L, Bean AJ. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J Neurosci. 1994;14:1688–1700. doi: 10.1523/JNEUROSCI.14-03-01688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity-dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3345–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zafra F, Lindholm D, Castren E, Hatikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]