Abstract
The inhibitory neurotransmitter GABA may act as a trophic signal for developing monoamine neurons in embryonic rat brain, because GABA neurons and their receptors appear in brainstem during generation of monoamine neurons. To test this hypothesis, we used dissociated cell cultures from embryonic day 14 rat brainstem, which contains developing serotonin (5-HT), noradrenaline (tyrosine hydroxylase; TH), and GABA neurons. Immunocytochemistry and reverse transcription-PCR (RT-PCR) revealed the presence of multiple α, β, γ, and δ subunits in these cultures. Competitive RT-PCR demonstrated high levels of β3 subunit transcripts. Expression of functional GABAAreceptors was demonstrated using 36Cl− flux assays. To investigate GABAergic regulation of neuronal survival and growth, cultures were treated for 1–3 d in vitro with 10 μm GABA and/or GABAA antagonist (bicuculline or the pesticide dieldrin). The effects of treatments were quantified by analysis of immunoreactive 5-HT, TH, and GABA neurons. GABAA receptor ligands differentially regulated neuronal survival and growth depending on neurotransmitter phenotype. GABA exerted positive effects on monoamine neurons, which were countered by bicuculline (and dieldrin, 5-HT neurons only). By itself, bicuculline produced inhibitory effects on both 5-HT and TH neurons, whereas dieldrin potently inhibited 5-HT neurons only. GABA neurons responded positively to both antagonists, but more strongly to bicuculline. Taken together, these results demonstrate that the activation/inhibition of GABAA receptors produces opposite effects on the development of embryonic monoamine and GABA neurons. This suggests that these neurotransmitter phenotypes may express GABAAreceptors that differ in fundamental ways, and these differences determine the developmental responses of these cells to GABAergic stimuli.
Keywords: GABAA receptor, 5-HT, tyrosine hydroxylase, survival, neurite outgrowth, rat, bicuculline, organochlorine pesticides, dieldrin, RT-PCR, chloride influx, embryonic, brainstem
GABA is present in the mammalian brain during early stages of development, where it may act as a trophic signal for developing neurons. In the embryonic rat, GABA axons project through the brainstem when serotonergic (5-HT) and noradrenergic neurons are being generated (Lauder et al., 1986). This raises the possibility that GABA could exert trophic influences on developing monoamine neurons, if they express appropriate receptors. In adult rat brain, these neurons do express functional GABAA receptors (Smith and Gallager, 1987; Fritschy et al., 1992; Nicholson et al., 1992), but embryonic receptor expression has yet to be investigated. Trophic actions of GABA on other types of neurons are well documented (Spoerri and Wolff, 1981;Eins et al., 1983; Meier et al., 1984, 1991; Spoerri, 1988; Prasad and Barker, 1990; Hansen et al., 1991; Wolff et al., 1993; Abraham et al., 1994; Behar et al., 1994; Belhage et al., 1997) and seem to involve GABAA receptors coupled to Cl− (Meier et al., 1985, 1991; Mehta and Ticku, 1988) and Ca2+ channels (Connor et al., 1987; Reichling et al., 1994; Obrietan and van den Pol, 1995; Xian et al., 1995) .
GABAA receptors are pentomeric complexes consisting of several subunits (α1–6, β1–4, γ1–3, δ, ρ1–2). Molecular cloning has identified a family of GABAA isoreceptors (subtypes) formed by several subunits from these classes (for review, see MacDonald and Olsen, 1994). These receptors are targeted by benzodiazepines, barbiturates, neurosteroids (Schofield et al., 1987;Barnard, 1988; Olsen and Tobin, 1990; for review, see Morrow, 1995), and organochlorine pesticides (Abalis et al., 1986; Gant et al., 1987;Costa, 1988; Bloomquist, 1992).
GABAAreceptors develop in approximate spatiotemporal coincidence with GABAergic neurons and axons (Cobas et al., 1991; Schlumpf et al., 1989), suggesting that GABA may modulate expression of these receptors.In situ hybridization has revealed transient expression patterns of GABAA subunit transcripts in developing rat brain (Olsen and Tobin, 1990; Gambarana et al., 1991; Bovolin et al., 1992; Laurie et al., 1992; Poulter et al., 1992; Zheng et al., 1993; Ma and Barker, 1995). These subunits form functional GABAAreceptors that can be activated by specific agonists (Hebebrand et al., 1988; Kellogg and Pleger, 1989; Fiszman et al., 1990; Schlumpf et al., 1992; Ma et al., 1993).
GABAA receptors have been suggested to mediate trophic effects of GABA during neuroembryogenesis (Ma and Barker, 1995). In the present study, we have used dissociated cell cultures from embryonic rat brainstem to study the effects of GABA on the growth and survival of developing monoamine and GABA neurons and have used GABAA antagonists to assess the involvement of GABAA receptors.
MATERIALS AND METHODS
Cell cultures
Primary dissociated cell cultures were prepared from embryonic day 14 (E14) rat brainstem as described previously (Liu and Lauder, 1991). Cells were plated in 12-well plates in complete medium [DMEM + 10% fetal calf serum (FCS) + penicillin/streptomycin/dextrose] at a density of 106 cells/milliliter on polylysine-coated coverslips. At 1 d in vitro (1 DIV), cultures were switched to serum-free medium [DMEM + insulin, transferrin, selenium (ITS) + 0.1% bovine serum albumin (BSA)]. To determine the effects on neuronal survival and growth, cultures were treated with 10 μm GABA and/or GABAA antagonist (bicuculline or dieldrin) in serum-free medium (to avoid GABA from serum-containing medium; Loscher, 1979) daily for 48 hr beginning at 1 DIV.
Immunocytochemistry and cell counts
At 3 DIV, cultures were rinsed with HBSS and fixed with 4% paraformaldehyde in 70 mm phosphate buffer. Cultures then were rinsed in PBS, permeabilized with 0.2% Triton X-100, and immunostained using the avidin–biotin peroxidase method (Vector Labs, Burlingame, CA) with specific rabbit polyclonal antisera against 5-HT-hemocyanin conjugates (Wallace et al., 1982), tyrosine hydroxylase (TH) (Boehringer Mannheim, Indianapolis, IN), GABA-hemocyanin conjugates (Lauder et al., 1986), GABAA receptor α1 subunits (Sato and Neale, 1989; gift of Dr. Joseph Neale), or a monoclonal antibody to GABAA receptor β2–3 subunits (bd-17, Boehringer Mannheim). To determine the effects of treatments on neuronal survival, the number of 5-HT, TH, and GABA immunoreactive neurons was counted in three cultures/treatment group using an ocular grid. Twenty grid areas (4.2 mm2) were counted per culture. Data were expressed as the mean number of cells/mm2/culture, and then converted to percentage control by dividing individual data points by the overall control mean. Data were statistically analyzed using one-way ANOVA followed by thepost hoc Dunnet’s multiple comparison test (p < 0.05).
Morphometry
Cell size, shape, and complexity of neurite outgrowth were measured for 5-HT, TH, and GABA neurons as indices of morphological changes in these neurons in response to treatments. Measured neurites consisted mainly of dendrites, because long, thin processes (presumably axons) were eliminated from the analysis. Morphometry was performed using a custom-designed computer imaging system, which automatically measured soma area (SA), field area (FA; area covered by a neurite arbor), number of neurites (NN), and number of terminal neurite segments (NTS), and then stored the images for further analysis. Details of this system and the morphometric parameters measured have been described previously (Lieth et al., 1990; Liu and Lauder, 1991;Lieth, 1992). In this experiment, 20 randomly selected neurons were analyzed from each of three cultures per treatment group. Data from these three experiments (total of 60 cells per group) were averaged to obtain means for each parameter ± SEM. Data (n = 60) were analyzed using one-way ANOVA followed by post hocBonferroni multiple comparisons.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA purification. Total RNA was extracted as described previously (Morrow et al., 1992). Culture media was removed from each well, and the cells were washed with ice-cold, sterile PBS. Prechilled 4 m guanidine thiocyanate was added to each of the wells, and then the solution from the combined six wells was transferred to centrifuge tubes, followed by homogenization on ice using a Polytron. RNA was purified by ultracentrifugation over a 5.7m CsCl2 cushion and resolubilized in 0.2m sodium acetate (with 0.1% SDS). Extraneous protein was removed by consecutive extraction with equal volumes of Tris-buffered phenol and chloroform/isoamyl alcohol (49:1), followed by precipitation with 100% ethanol overnight. The yield of total RNA was determined by measuring the absorbance of an aliquot of the resuspended stock at 260/280 nm.
General RT-PCR. Aliquots of total RNA (1.0 μg) were reverse-transcribed in First Strand buffer (50 mm Tris, pH 8.3, 3 mm MgCl2, 75 mm KCl) with 10 mm dithiothreitol, 1 mm deoxynucleotide triphosphates (dNTPs), 25 μm random hexamers, and 200 U Moloney Murine Leukemia Virus reverse transcriptase (BRL, Bethesda, MD) at 37°C for 60 min. The resulting cDNA was heat-denatured at 95°C for 10 min, and then tubes were put on ice until they were ready for PCR. The PCR reaction was conducted in PCR buffer (50 mmTris, pH 9.0, 20 mm ammonium sulfate, 1.5 mmMgCl2) with 50 μm each of 5′ (sense) and 3′ (antisense) primers, 200 μm dNTP, and 1 U Hot Tub Polymerase (Amersham, Arlington Heights, IL). The PCR reaction was performed with 26 cycles using a DNA Thermal Cycler, each cycle consisting of 94°C for 45 sec, 60°C for 45 sec, and 72°C for 1 min, followed by a final elongation step (72°C for 15 min). Aliquots of PCR products were run on a 1.8% agarose gel in 0.5 × TBE buffer.
Competitive RT-PCR reaction using internal standards.Competitive RT-PCR reactions using internal standards specific for α1, β3, and γ1 subunits were conducted according to methods described previously (Bovolin et al., 1992; Grayson et al., 1993;Devaud et al., 1995). The cloned amplification products were sequenced to verify authenticity, and the primers were complementary to unique sequences within corresponding cDNAs (Bovolin et al., 1992). Various amounts of the internal standard cRNA (containing a BglII restriction site) were added to a constant amount of the total RNA of interest to generate a competitive PCR amplification curve. Each mixture was reverse-transcribed and run through PCR as in the general protocol, except for the addition of 1–2 μCi [32P]dCTP/tube and the magnesium concentration, which was optimized for each primer pair and determined to be 1.5 mm for the α subunit mRNAs, 2.0 mm for β subunit mRNAs, and 3.0 mm for γ subunit mRNAs (Devaud et al., 1995). Aliquots of PCR products were digested overnight withBglII and separated on a 1.8% agarose gel. The gels were dried and exposed to a phosphorimaging screen for 1–2 hr. The signal intensity for both the native RNA products (∼300 bp) and the cRNA products (∼150 bp after digestion) was quantified using a phosphorimager (Molecular Dynamics, Sunnyvale, CA). Data were presented as the ratio of amounts incorporated into the amplified cRNA internal standards to amounts incorporated into the corresponding subunit mRNA amplification product versus the known amounts of internal standard cRNA added to the test sample to generate a competitive PCR linear regression curve. The amount of target RNA was calculated directly from this curve (Grayson et al., 1993).
GABAA receptor-mediated 36Cl−influx into cultured cells
Chloride influx was measured in cultured brainstem cells by modification of a previously described method (Mehta and Ticku, 1988, 1992). Briefly, after culture of cells for 1 DIV in DMEM + 10% FCS followed by 2 DIV in DMEM + ITS + 0.1% BSA, coverslips were removed from culture wells and rinsed for 3–4 sec at room temperature in assay buffer (HEPES-buffered saline containing 20 mmHEPES, 118 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, and 2.5 mmCaCl2, adjusted to pH 7.4 with Tris-base, with 10 mm glucose added just before use). Immediately after this rinse, the coverslips were drained rapidly on tissue paper and transferred to 3 ml HEPES-buffered saline containing 2 μCi36Cl−/ml (specific activity 13.38 Ci/gm), with or without various concentrations of GABA (5–60 μm) ± bicuculline (10 μm). Influx was terminated after 5 sec by transfer of the coverslip to 1000 ml ice-cold HEPES-buffered saline (with 100 mg/l picrotoxin) and then rinsed for 7 sec in another beaker containing 1000 ml ice-cold HEPES-buffered saline. Coverslips were then drained and transferred to scintillation vials containing 1 ml 0.2N NaOH. The vials were vortexed and then allowed to sit at room temperature for 1 hr. Aliquots (100 μl) were removed for protein determination using the Bradford method (Bradford, 1976). Retained radioactivity was determined by liquid scintillation spectroscopy. Values for 36Cl−influx were expressed as nanomol/milligram protein. Background levels of36Cl− influx (without added GABA) were determined and subtracted from all values.
RESULTS
Embryonic brainstem cultures contain GABA and monoamine neurons, and express multiple GABAA receptor subunits
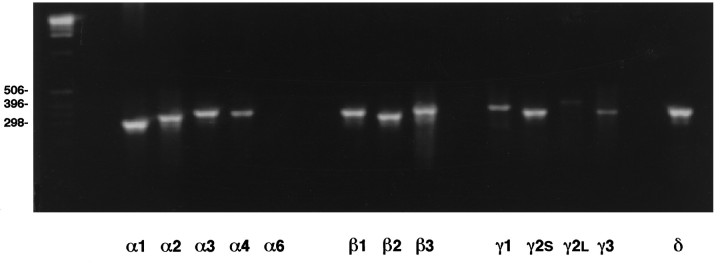
Immunocytochemistry demonstrated that 5-HT, TH, and GABA-immunoreactive neurons were present in E14 brainstem cultures (Fig. 1) and revealed extensive expression of α1 and β2–3 GABAA subunit proteins, where dense immunoreactivity was localized to the outer membranes of neuronal somata and neurites (Fig. 2). General RT-PCR demonstrated the presence of mRNA transcripts encoding multiple α (α1–4, but not α6), β (β1–3), γ (γ1, γ2S,γ2L, γ3), and δ subunits of GABAA receptors (Fig. 3). Many of these subunits may be expressed by glial cells as well as by neurons in these cultures, because detectable levels of α1–3, β2–3, γ1, γ2S, and δ transcripts have also been found in purified glial cultures from E14 brainstem (J. Liu, R. Haberman, and J. Lauder, unpublished results). Quantitative competitive RT-PCR was performed for α1 and β3 subunits, because α1 and β2–3 antibodies had been used to characterize cultures immunocytochemically. The γ1 subunit was chosen for comparison. Competitive RT-PCR revealed that β3 subunit transcripts were highly expressed (272.5 ± 26.1 pg/μg total RNA) compared with α1 (1.9 ± 0.06 pg/μg total RNA) and γ1 (6.9 ± 0.3 pg/μg total RNA) transcripts (Fig. 4).
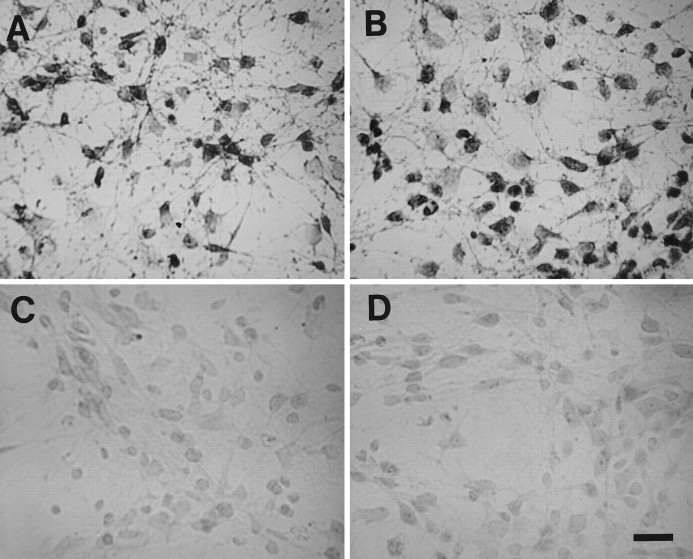
Fig. 1.
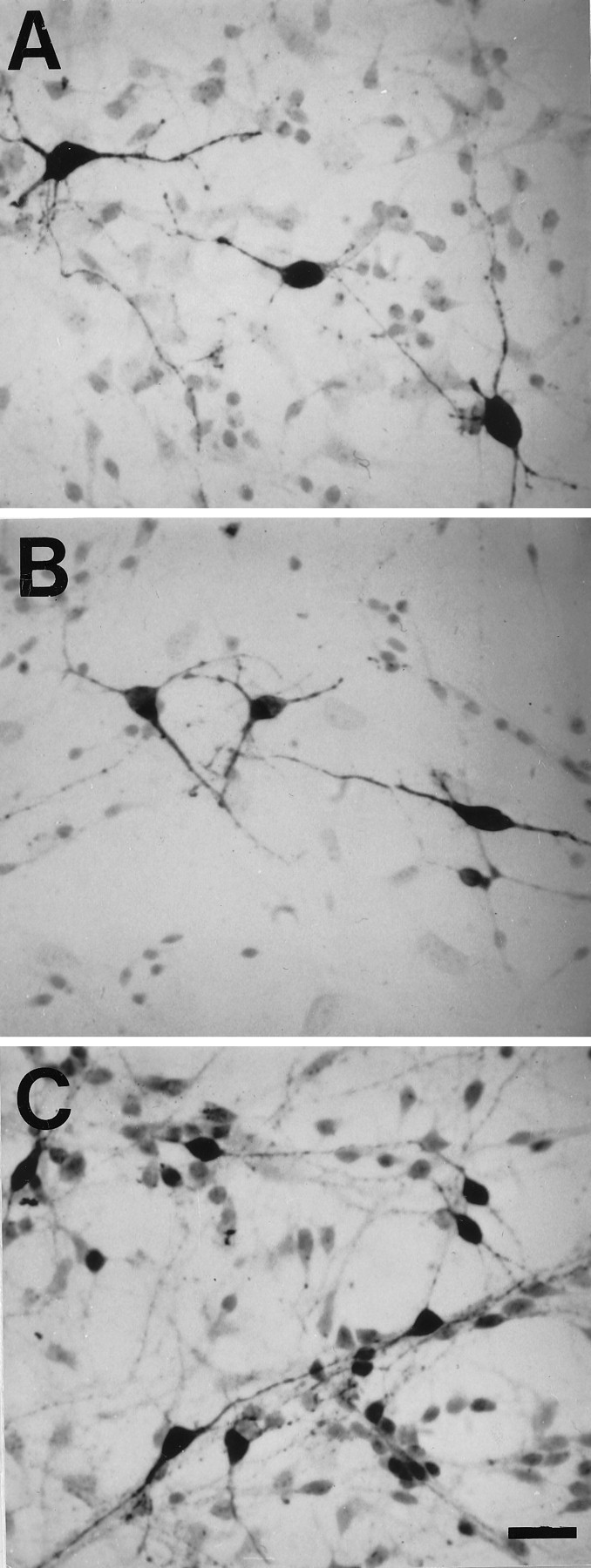
Representative 5-HT (A), TH (B), and GABA (C) immunoreactive neurons from E14 brainstem cultures. Cells were cultured for 1 d in DMEM + 10% FCS and then switched to serum-free medium (DMEM + ITS + 0.1% BSA) for 48 hr. Scale bar, 50 μm.
Fig. 2.
Expression of GABAA receptor subunit proteins in E14 brainstem cultures. Immunocytochemistry with anti-GABAA α1 rabbit polyclonal antibody (A) and anti-GABAA β2/3 monoclonal antibody (B). C, Background control with α1 primary antibody omitted. D, Background control with β2/3 primary antibody omitted. Cells were cultured for 1 d in DMEM + 10% FCS and then switched to serum-free medium (DMEM + ITS + 0.1% BSA) for 48 hr. Scale bar, 50 μm.
Fig. 3.
RT-PCR analysis of GABAA receptor subunit mRNAs in E14 rat brainstem cultures. Cells were cultured for 1 d in DMEM + 10% FCS and then for 2 d in serum-free medium (DMEM + ITS + 0.1% BSA). RT-PCR revealed expression of mRNAs encoding most of the known GABAA receptors, except α6.
Fig. 4.
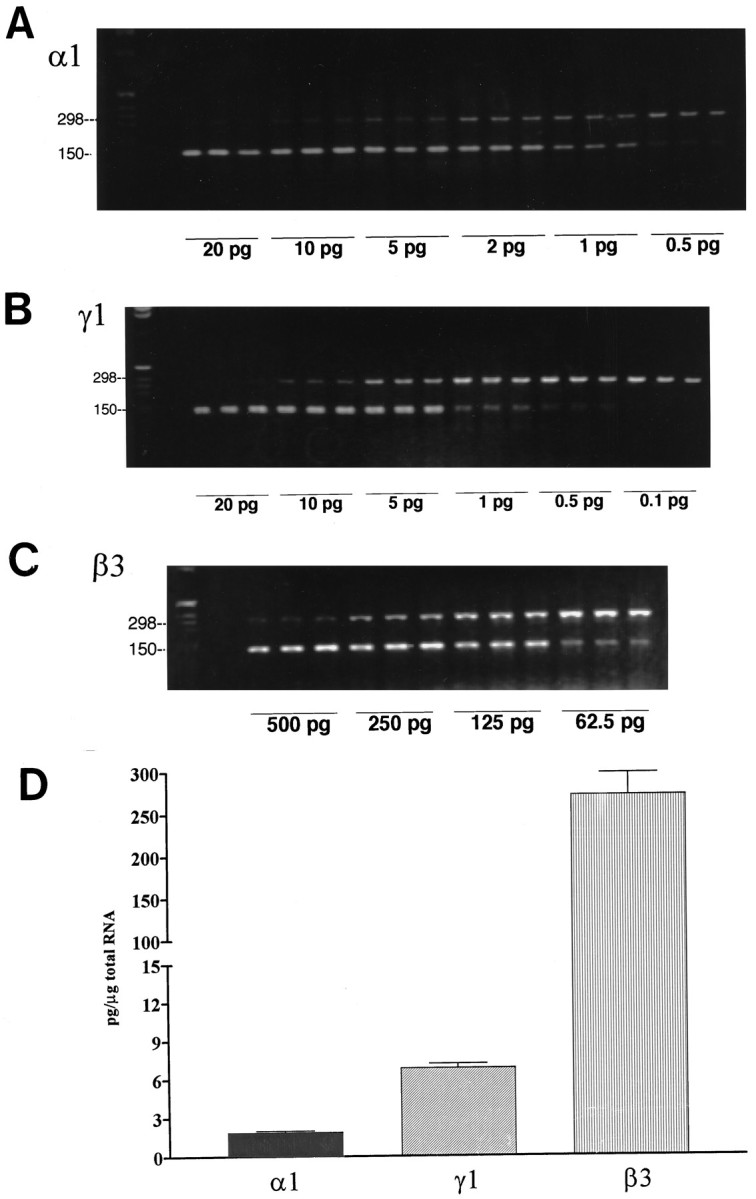
A–C, Representative gels for GABAA receptor subunit mRNAs from cultured E14 rat brainstem analyzed by competitive RT-PCR using internal standards. A series of concentrations of internal standard cRNAs were added to each tube containing 1 μg of total RNA. The PCR products from each tube are shown in triplicate for each subunit. Top bands, PCR products of target mRNA. Bottom bands,BglII-digested internal standard PCR products. Note that increasing concentrations of internal standards compete with target mRNA for amplification. The point of equivalence was determined by linear regression analysis of the ratio of counts incorporated into the target PCR product across the series of concentrations of internal standards. The point of equivalence (when the ratio is 1) is the absolute concentration of GABAA receptor subunit mRNA/microgram of total RNA. D, Quantification of GABAA receptor subunit mRNA levels assayed in this study. Note that β3 is the most abundant subunit compared with α1 and γ1. Cells were cultured for 1 d in DMEM + 10% fetal calf serum and then for 2 d in serum-free medium (DMEM + ITS + 0.1% BSA).
GABAA ligands regulate Cl− influx, indicating the presence of functional GABAA receptors
GABA produced concentration-dependent and saturable effects on Cl− influx in E14 brainstem cells at 3 DIV, with maximal enhancement at 10 μm GABA to ∼13.6 nmol/mg protein (Fig. 5), which increased Cl− influx over basal values by 400%. This effect was blocked by 10 μm bicuculline.
Fig. 5.
GABAA receptor-mediated Cl− uptake into cultured E14 brainstem cells. Addition of exogenous GABA enhanced Cl− uptake from basal levels of 3.3 nmol/mg protein in a dose-dependent manner to 13.6 nmol/mg protein (at 10 μm GABA). Addition of 10 μmbicuculline lowered Cl− uptake stimulated by 10 μm GABA to 4.3 nmol/mg protein.
Neuronal survival and growth are differentially affected by GABAA ligands
Survival
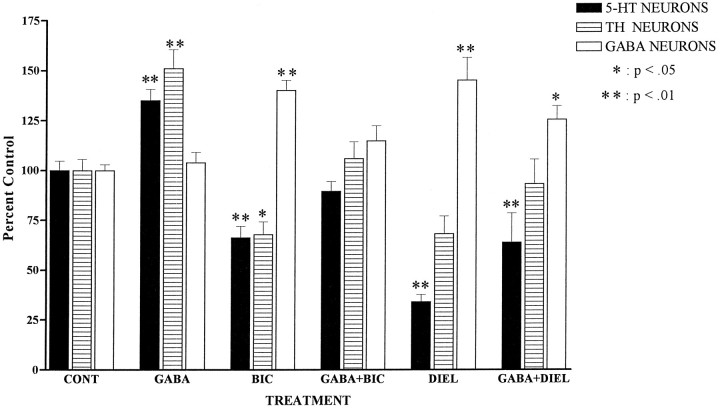
The effects of GABA agonist and antagonist treatments (10 μm GABA and/or bicuculline or dieldrin in serum-free medium for 48 hr) on the density of 5-HT, TH, and GABA neurons are shown in Figure 6. GABA significantly stimulated survival of 5-HT and TH neurons but did not affect the survival of GABA neurons. The classical GABAA receptor antagonist bicuculline significantly reduced the survival of 5-HT and TH neurons, whereas the pesticide dieldrin reduced the survival of 5-HT neurons only. On the contrary, both GABAA antagonists significantly enhanced survival of GABA neurons. GABA completely blocked the effects of bicuculline on all three neuronal phenotypes, but did not reverse the effects of dieldrin on 5-HT and GABA neurons.
Fig. 6.
Effects of GABA receptor ligands on survival of 5-HT, TH, and GABA neurons in E14 rat brainstem cultures (number of immunoreactive neurons/mm2, expressed as percentage control). Cells were cultured for 1 d in DMEM + 10% FCS and then switched to serum-free medium (DMEM + ITS + 0.1% BSA) plus ligand for 48 hr. Cultures were then fixed, stained with antibodies to 5-HT, TH, or GABA, and immunoreactive neurons were counted. Individual data from three separate experiments (n = 60 cells/treatment group) were converted to percentage control by dividing individual data points by the overall mean control value. Statistical analysis was performed by ANOVA followed by Dunnet’s multiple comparison test when ANOVA was significant (p < 0.05).
Growth
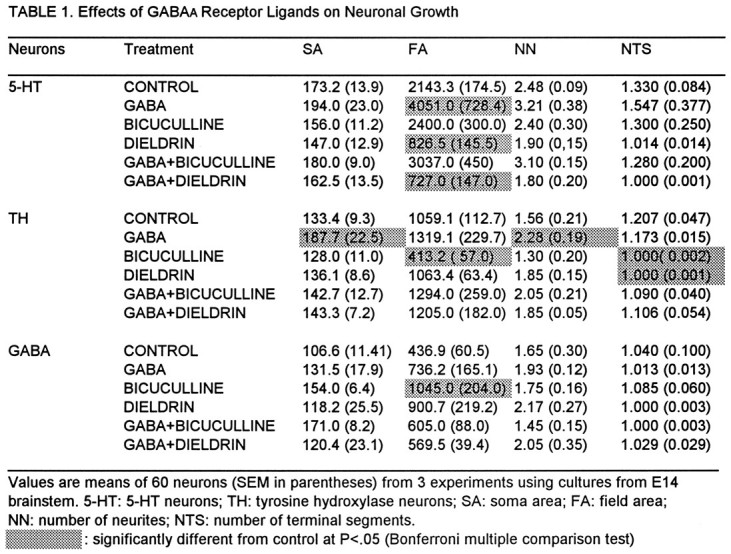
The effects of treatments (described above) on the growth of cell bodies and neurites are shown in Table 1.
SA: The size of cell somas (soma area) of TH neurons was significantly increased by GABA. When added together with GABA, bicuculline and dieldrin inhibited this growth effect but had no significant effect when given alone. Other treatments had no significant effects on cell somas of these neurons. Cell somas of GABA and 5-HT neurons were not affected significantly by any treatment.
FA: The surface area covered by neurites (field area) of 5-HT neurons was increased after treatment with GABA, whereas GABA had no significant effects on the FA of TH or GABA neurons. Dieldrin significantly reduced the FA in 5-HT neurons, an effect that was not overcome by co-administration of GABA, whereas bicuculline had no significant effect; however, bicuculline did decrease the FA in TH neurons, which was reversed by GABA. Although GABA itself had no significant effect on the FA of GABA neurons, bicuculline stimulated this parameter, which was blocked by co-administration of GABA. Dieldrin also seemed to increase the FA of these neurons to some extent, but this effect did not reach significance using the Bonferroni test (although it was significant by the less conservative post hoc t test).
NN: The number of primary neurites of TH neurons was increased significantly by GABA, which was reduced to nonsignificant levels by bicuculline. Although the one-way ANOVA for treatment effects on NN of 5-HT neurons was highly significant (p < 0.001), and post hoc ttests indicated significant growth-promoting effects of GABA and inhibitory effects of dieldrin, no treatment effect reached significance when it was analyzed by the more conservative Bonferroni test. No significant effects on the NN of GABA neurons were seen after any treatment.
NTS: GABA did not significantly affect the number of terminal neurite segments of 5-HT and TH neurons, but both bicuculline and dieldrin significantly decreased this parameter in TH neurons, effects that were reversed by GABA. The NTS of GABA neurons were unaffected by any treatment.
DISCUSSION
GABA acts as a trophic signal for monoamine neurons but negatively regulates development of GABA neurons
GABA stimulated survival and growth of 5-HT and TH neurons in embryonic brainstem cultures, and these effects were reversed by GABAA antagonists. These cultures expressed multiple GABAA receptor subunit mRNA transcripts, as measured by RT-PCR, and exhibited functional GABA-gated Cl− channels, as determined by 36Cl− influx. The amount of36Cl− influx stimulated by 10 μmGABA was comparable to values reported previously in whole fetal rat brain (Kellogg and Pleger, 1989), suggesting that GABA-gated channels are highly expressed in these cultures. Taken together, these results suggest that GABAA receptors mediate the trophic effects of GABA on brainstem monoamine neurons in these cultures. Although it could be argued that GABA-stimulated release of 5-HT from serotonin neurons (Becquet et al., 1993) could account for some of the positive effects of GABA on 5-HT and TH neurons, two points argue against this possibility. First, 5-HT does not promote survival of brainstem monoamine neurons, although it does stimulate their growth (Lauder, 1990; Liu and Lauder, 1991). Second, the GABAA antagonist bicuculline reversed the positive effects of GABA on monoamine neurons and inhibited growth and survival of these cells when added alone. For these reasons, we believe it is unlikely that released 5-HT played a significant role in the positive effects of GABA on monoamine neurons.
Although GABA itself did not have significant effects on GABA neurons, both bicuculline and dieldrin stimulated survival of these cells, and bicuculline promoted areal growth of neurites (FA). The positive effects of these GABAA antagonists suggest that GABA may negatively autoregulate development of the GABAergic neuronal population in embryonic brainstem in vivo. The lack of significant effects of GABA on GABA neurons in our cultures could be attributable to the presence of endogenous GABA (released by GABA neurons) (Barbin et al., 1993; Becquet et al., 1993), which produced maximal inhibitory effects on these cells before treatment. GABA breaks down within hours after being added to culture medium. Therefore, it is possible that if muscimol had been used instead of GABA, significant negative effects on GABA neurons would have been found; however, because GABA was added daily to the culture medium, and significantly affected growth and survival of monoamine neurons, we think it unlikely that degradation of GABA contributed significantly to the lack of effects on GABA neurons.
Differential effects of receptor ligands suggest heterogeneous expression of GABAA receptor subunits by monoamine and GABA neurons
The differential effects of GABAA receptor ligands on cultured monoamine and GABA neurons raise the interesting possibility that these neurotransmitter phenotypes express different amounts of GABAA receptors and/or distinct GABAA isoreceptors (subtypes) with differing pharmacological properties. These findings are consistent with evidence that (1) GABA promotes differentiation of different types of neurons to varying degrees in other culture systems (Hansen et al., 1984; Meier et al., 1985; Spoerri, 1988; Michler, 1990; Belhage et al., 1997) and (2) adult GABA neurons and 5-HT neurons express different complements of particular GABAA receptor subunits (Gao et al., 1993; Gao and Fritschy, 1994).
Differential effects of GABAA receptor ligands on growth of cell bodies and neurites (Table 1) raises the further possibility that GABAA receptors may be differentially distributed on cultured 5-HT, TH, and GABA neurons. This view is supported by previous evidence that (1) high affinity GABAAreceptors are situated on cell bodies of cerebellar granule cells, whereas low affinity GABAA receptors are preferentially located on cell processes (Hansen et al., 1991); (2) α2, α5, β3, and γ2 subunit transcripts are expressed by cell somas, neurites, and growth cones of cortical neurons, whereas α3 and β3 subunits are confined to cell somas (Poulter et al., 1994); and (3) GABAA subunit expression on cell bodies and neurites of developing cerebellar granule cells is differentially regulated by the GABAA agonist THIP (Gaboxadol), suggesting that sorting and targeting of newly synthesized receptors may undergo maturational changes that can be influenced by agonists (Elster et al., 1995).
Organochlorine pesticides affect neuronal growth and survival and alter trophic effects of GABA
Certain pesticides exert their neurotoxic actions by selectively blocking ion channels in the insect nervous system. Organochlorine pesticides such as dieldrin interact with specific sites on the GABAA–Cl− channel complex to block GABA-induced Cl− flux in both insect and mammalian cells (Abalis et al., 1986; Gant et al., 1987; Bloomquist, 1992; Pomés et al., 1994a,b). The GABA recognition site has been reported to be targeted by dieldrin (Eldefrawi and Eldefrawi, 1987; Ogata et al., 1988; Tokutomi et al., 1994). Other evidence, however, suggests that dieldrin binds to the picrotoxin site on the Cl− channel (Nagata and Narahashi, 1994) and suppresses GABA-induced Cl− currents in a noncompetitive manner (Nagata et al., 1994).
Data from the present study indicate that dieldrin, like bicuculline, has opposite effects on growth and survival of cultured monoamine and GABA neurons. This could be explained by different binding characteristics and sensitivity of these GABAA receptors to dieldrin. If this is the case, then it would suggest that GABAA receptors expressed by embryonic monoamine and GABA neurons may have different subunit compositions, because subunit composition is known to affect the binding characteristics and sensitivity of GABAA receptors to particular receptor ligands (Pritchett et al., 1989; Malherbe et al., 1990; Pritchett and Seeburg, 1990; Burt and Kamatchi, 1991).
Summary
The present study has revealed evidence that GABA acts as a trophic signal for embryonic brainstem monoamine neurons by activating GABAA receptors, but it may be a negative autoregulatory signal for developing GABA neurons. Consistent with this interpretation, bicuculline and dieldrin had negative effects on monoamine neurons but exerted positive effects on GABA neurons. These differential effects of GABAA receptor ligands on neurons of serotonergic, noradrenergic, and GABAergic phenotypes raise the possibility that prenatal exposure to pesticides or drugs acting as GABAA antagonists could interfere with the positive and negative regulatory influences of GABA, thereby producing imbalances in monoaminergic and GABAergic neurotransmission in the developing brain. If long-lasting, these effects could have functional and behavior consequences in offspring. The differential effects of GABAA receptor ligands on the growth of cell bodies and neurites suggest further that particular cellular compartments may express different levels and/or distinct populations of GABAA receptor subtypes.
Footnotes
This work was supported by National Institute of Environmental Health Sciences Grant R01 ES07017 to J.M.L. We are grateful to Dr. Joseph Neale for the gift of α1 antibodies, to Mary Beth Wilkie for editorial assistance, and to Drs. Cindy Lawler and Josephine Johns for consultation on the statistical analyses.
Correspondence should be addressed to Dr. Jean M. Lauder, Department of Cell Biology and Anatomy, CB 7090, University of North Carolina School of Medicine, Chapel Hill, NC 27599-7090.
REFERENCES
- 1.Abalis IM, Eldefrawi ME, Eldefrawi AT. Effects of insecticides on GABA-induced chloride influx into rat brain microsacs. J Toxicol Environ Health. 1986;18:13–23. doi: 10.1080/15287398609530844. [DOI] [PubMed] [Google Scholar]
- 2.Abraham JH, Seiler N, Schousboe A. Induction of low-affinity GABAA receptor by the GABA-agonist THIP in cultured rat cerebellar granule cells is prevented by inhibition of polyamine biosynthesis. J Neurosci Res. 1994;39:656–662. doi: 10.1002/jnr.490390605. [DOI] [PubMed] [Google Scholar]
- 3.Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993;152:150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 4.Barnard EA. The structure of the GABA/benzodiazepine receptor complex with its gated ion channel. In: Squires RP, editor. GABA and benzodiazepine receptors. CRC; Boca Raton, FL: 1988. pp. 103–122. [Google Scholar]
- 5.Becquet D, Hery M, Francois-Bellan AM, Giraud P, Deprez P, Faudon M, Fache MP, Hery F. Glutamate, GABA, glycine and taurine modulate serotonin synthesis and release in rostral and caudal rhombencephalic raphe cells in primary cultures. Neurochem Int. 1993;23:269–283. doi: 10.1016/0197-0186(93)90118-o. [DOI] [PubMed] [Google Scholar]
- 6.Behar TN, Schaffer AE, Colton CA, Somogy R, Olah Z, Lehel C, Barker JL. GABA-induced chemokinesis and NGF-induced chemotaxis of embryonic spinal cord neurons. J Neurosci. 1994;14:29–38. doi: 10.1523/JNEUROSCI.14-01-00029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhage B, Hansen GH, Elster L, Schousboe A (1997) Effects of α-aminobutyric acid (GABA) on synaptogenesis and synaptic function. Persp Dev Neurosci, in press. [PubMed]
- 8.Bloomquist JR. Intrinsic lethality of chloride-channel-directed insecticides and convulsants in mammals. Toxicol Lett. 1992;60:289–298. doi: 10.1016/0378-4274(92)90287-t. [DOI] [PubMed] [Google Scholar]
- 9.Bovolin P, Santi MR, Memo M, Costa E, Grayson DR. Distinct developmental patterns of rat α1, α5, γ2S and γ2L γ-aminobutyric acid receptor subunit mRNAs in vivo and in vitro. J Neurochem. 1992;59:62–72. doi: 10.1111/j.1471-4159.1992.tb08876.x. [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Burt DR, Kamatchi GL. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- 12.Cobas A, Fairen A, Alvarez-Bolado G, Sanchez MP. Prenatal development of the intrinsic neurons of the rat neocortex: a comparative study of the distribution of the GABA-immunoreactive cells and the GABAA receptor. Neuroscience. 1991;40:375–397. doi: 10.1016/0306-4522(91)90127-a. [DOI] [PubMed] [Google Scholar]
- 13.Connor JA, Tseng HY, Hockberger PE. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant culture. J Neurosci. 1987;7:1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa LG. Interactions of neurotoxicants with neurotransmitter systems. Toxicology. 1988;49:359–366. doi: 10.1016/0300-483x(88)90019-4. [DOI] [PubMed] [Google Scholar]
- 15.Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- 16.Eins S, Spoerri PE, Heyder E. GABA or sodium bromide-induced plasticity of neurites of mouse neuroblastoma cells in culture: a quantitative study in culture. Cell Tissue Res. 1983;229:457–460. doi: 10.1007/BF00214987. [DOI] [PubMed] [Google Scholar]
- 17.Eldefrawi AT, Eldefrawi ME. Receptors for γ-aminobutyric acid and voltage-dependent chloride channels as targets for drugs and toxicants. FASEB J. 1987;1:262–271. doi: 10.1096/fasebj.1.4.2443413. [DOI] [PubMed] [Google Scholar]
- 18.Elster L, Hansen GH, Belhage B, Fritschy JM, Möhler H, Schousboe A. Differential distribution of GABAA receptor subunits in soma and processes of cerebellar granule cells: effects of maturation and a GABA agonist. Int J Dev Neurosci. 1995;13:417–428. doi: 10.1016/0736-5748(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 19.Fiszman ML, Novotny EA, Lange GD, Barker JL. Embryonic and early postnatal hippocampal cells respond to nanomolar concentrations of muscimol. Dev Brain Res. 1990;53:186–193. doi: 10.1016/0165-3806(90)90005-j. [DOI] [PubMed] [Google Scholar]
- 20.Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gambarana C, Beattie CE, Rodriguez ZR, Siegel RE. Region-specific expression of messenger RNAs encoding GABAA receptor subunits in the developing rat brain. Neuroscience. 1991;45:423–432. doi: 10.1016/0306-4522(91)90238-j. [DOI] [PubMed] [Google Scholar]
- 22.Gant DB, Eldefrawi ME, Eldefrawi AT. Cyclodiene insecticides inhibit GABAA receptor-regulated chloride transport. Toxicol Appl Pharmacol. 1987;88:313–321. doi: 10.1016/0041-008x(87)90206-7. [DOI] [PubMed] [Google Scholar]
- 23.Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the α1 subunit to neurochemically distinct subpopulations of rat hippocampal neurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 24.Gao B, Fritschy JM, Benke D, Möhler H. Neuron-specific expression of GABAA receptor subtypes: differential association of the α1- and α3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- 25.Grayson DR, Bovolin P, Santi MR. Absolute quantitation of γ-aminobutyric acid A receptor subunit mRNAs by competitive polymerase chain reaction. Methods Neurosci. 1993;12:191–208. [Google Scholar]
- 26.Hansen GH, Meier E, Schousboe A. GABA influences the ultrastructure composition of cerebellar granule cells during development in culture. Int J Dev Neurosci. 1984;2:247–257. doi: 10.1016/0736-5748(84)90019-4. [DOI] [PubMed] [Google Scholar]
- 27.Hansen GH, Belhage B, Schousboe A. Effect of a GABA agonist on the expression and distribution of GABAA receptors in the plasma membrane of cultured cerebellar granule cells: an immunocytochemical study. Neurosci Lett. 1991;124:162–165. doi: 10.1016/0304-3940(91)90084-7. [DOI] [PubMed] [Google Scholar]
- 28.Hebebrand J, Hofmann D, Reichelt R, Scnarr S, Knapp M, Propping P, Födisch HJ. Early ontogeny of the central benzodiazepine receptor in human embryos and fetuses. Life Sci. 1988;43:2127–2136. doi: 10.1016/0024-3205(88)90363-3. [DOI] [PubMed] [Google Scholar]
- 29.Kellogg CK, Pleger GL. GABA-stimulated chloride uptake and enhancement by diazepam in synaptoneurosomes from rat brain during prenatal and postnatal development. Brain Res Dev Brain Res. 1989;49:87–95. doi: 10.1016/0165-3806(89)90061-8. [DOI] [PubMed] [Google Scholar]
- 30.Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann NY Acad Sci. 1990;600:296–314. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- 31.Lauder JM, Han VKM, Henderson P, Verdoorn T. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- 32.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Lauder JM. Serotonin and nialamide differentially regulate survival and growth of cultured serotonin and catecholamine neurons. Dev Brain Res. 1991;60:59–67. doi: 10.1016/0165-3806(91)90180-q. [DOI] [PubMed] [Google Scholar]
- 34.Lieth E. Computerized morphometric analysis of neurons in culture. Methods Neurosci. 1992;10:391–405. [Google Scholar]
- 35.Lieth E, McClay DR, Lauder JM. Neuronal-glial interactions: complexity of neurite outgrowth correlation with substrate adhesive of serotonergic neurons. Glia. 1990;3:169–170. doi: 10.1002/glia.440030304. [DOI] [PubMed] [Google Scholar]
- 36.Loscher W. GABA in plasma and cerebrospinal fluid of different species: effects of gamma-acetylenic GABA, gamma-vinyl GABA and sodium valproate. J Neurochem. 1979;32:1587–1591. doi: 10.1111/j.1471-4159.1979.tb11104.x. [DOI] [PubMed] [Google Scholar]
- 37.Ma W, Barker JL. Complementary expression of transcripts encoding GAD67 and GABAA receptor alpha 4, beta 1, and gamma 1 subunits in the proliferative zone of the embryonic rat central nervous system. J Neurosci. 1995;15:2547–2560. doi: 10.1523/JNEUROSCI.15-03-02547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1993;338:357–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 40.Malherbe P, Sigel E, Baur R, Persohn E, Richards JG, Möhler H. Functional expression and sites of gene transcription of a novel α subunit of the GABAA receptor in rat brain. FEBS Lett. 1990;260:261–265. doi: 10.1016/0014-5793(90)80118-3. [DOI] [PubMed] [Google Scholar]
- 41.Mehta AK, Ticku MK. Developmental aspects of benzodiazepine receptors and GABA-gated chloride channels in primary cultures of spinal cord neurons. Brain Res. 1988;454:156–163. doi: 10.1016/0006-8993(88)90814-1. [DOI] [PubMed] [Google Scholar]
- 42.Mehta AK, Ticku MK. Chronic GABA exposure down-regulates GABA- benzodiazepine receptor-ionophore complex in cultured cerebral cortical neurons. Mol Brain Res. 1992;16:29–36. doi: 10.1016/0169-328x(92)90190-m. [DOI] [PubMed] [Google Scholar]
- 43.Meier E, Drejer J, Schousboe A. GABA induces functionally active low-affinity GABA receptors on cultures cerebellar granule cells. J Neurochem. 1984;43:1737–1744. doi: 10.1111/j.1471-4159.1984.tb06102.x. [DOI] [PubMed] [Google Scholar]
- 44.Meier H, Hansen GH, Schousboe A. The trophic effect of GABA on cerebellar granule cells is mediated by GABA receptors. Int J Dev Neurosci. 1985;3:401–407. doi: 10.1016/0736-5748(85)90074-7. [DOI] [PubMed] [Google Scholar]
- 45.Meier H, Hertz L, Schousboe A. Neurotransmitters as developmental signals. Neurochem Int. 1991;19:1–15. [Google Scholar]
- 46.Michler A. Involvement of GABA receptors in the regulation of neurite growth in cultured embryonic chick tectum. Int J Dev Neurosci. 1990;8:463–472. doi: 10.1016/0736-5748(90)90078-g. [DOI] [PubMed] [Google Scholar]
- 47.Morrow AL. Regulation of GABAA receptor function and gene expression in the central nervous system. Int Rev Neurobiol. 1995;38:1–41. doi: 10.1016/s0074-7742(08)60523-1. [DOI] [PubMed] [Google Scholar]
- 48.Morrow AL, Herbert JS, Montpied P. Differential effects of chronic ethanol administration of GABAA receptor α1 and α6 subunit mRNA levels in rat cerebellum. J Mol Cell Neurosci. 1992;3:251–258. doi: 10.1016/1044-7431(92)90045-4. [DOI] [PubMed] [Google Scholar]
- 49.Nagata K, Narahashi T. Dual action of the cyclodiene insecticide dieldrin on the γ-aminobutyric acid receptor-chloride channel complex of rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1994;269:164–172. [PubMed] [Google Scholar]
- 50.Nagata K, Hamilton BJ, Carter DB, Narahashi T. Selective effects of dieldrin on the GABAA receptor-channel complex subunits expressed in human embryonic kidney cells. Brain Res. 1994;645:19–26. doi: 10.1016/0006-8993(94)91633-0. [DOI] [PubMed] [Google Scholar]
- 51.Nicholson LFB, Faull RLM, Waldvogel HJ, Dragunow M. The regional, cellular and subcellular location of GABAA/benzodiazepine receptors in the substantia nigra. Neuroscience. 1992;50:355–370. doi: 10.1016/0306-4522(92)90429-6. [DOI] [PubMed] [Google Scholar]
- 52.Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogata N, Vogel SM, Narahashi T. Lindane but not deltamethrin blocks a component of a GABA-activated chloride channel. FASEB J. 1988;2:2895–2900. doi: 10.1096/fasebj.2.13.2458984. [DOI] [PubMed] [Google Scholar]
- 54.Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB J. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- 55.Pomés A, Rodriguez-Farré E, Suñol C. Disruption of GABA-dependent chloride flux by cyclodienes and hexachlorocyclohexanes in primary cultures of cortical neurons. J Pharmacol Exp Ther. 1994a;271:1616–1623. [PubMed] [Google Scholar]
- 56.Pomés A, Frandsen AA, Suñol C, Sanfeliu E, Rodriguez-Farré E, Schousboe A. Lindane cytotoxicity in cultured neocortical neurons is ameliorated by GABA and flunitrazepam. J Neurosci Res. 1994b;39:663–668. doi: 10.1002/jnr.490390606. [DOI] [PubMed] [Google Scholar]
- 57.Poulter MO, Barker JL, O’Carroll A-M, Lolait SJ, Mahan LC. Transient and differential expression of GABA receptor subunit mRNAs during development of the rat CNS. J Neurosci. 1992;12:2888–2900. doi: 10.1523/JNEUROSCI.12-08-02888.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poulter MO, Larmet Y, Pruss R, Feltz P. Preferential and transient expression of GABAA receptor subunit mRNAs in growing neurites of early postnatal cortical cells. Soc Neurosci Abstr. 1994;20:659. [Google Scholar]
- 59.Prasad A, Barker JL. Functional GABAA receptor are critical for survival and process outgrowth of embryonic chick spinal cord cells. Soc Neurosci Abstr. 1990;16:647. [Google Scholar]
- 60.Pritchett DB, Seeburg PH. Gamma-aminobutyric acid A receptor α5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 61.Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- 62.Reichling DB, Kyrozis A, Wang J, Macdermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transient in rat dorsal horn neurons. J Physiol (Lond) 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato TN, Neale JH. Immunological identification of multiple α1-like subunits of the γ-aminobutyric acidA receptor complex purified from neonatal rat cortex. J Neurochem. 1989;53:1089–1095. doi: 10.1111/j.1471-4159.1989.tb07400.x. [DOI] [PubMed] [Google Scholar]
- 64.Schlumpf M, Ramseier H, Abriel H, Youmbi M, Baumann JB, Lichtensteiger W. Diazepam effects on the fetus. Neurotoxicology. 1989;10:501–516. [PubMed] [Google Scholar]
- 65.Schlumpf M, Parmar R, Schreiber A, Ramseier HR, Bütikofer E, Abriel H, Barth M, Rhyner T, Lichtensteiger W. Nervous and immune systems as targets for developmental effects of benzodiazepines. Dev Pharmacol Ther. 1992;18:145–158. [PubMed] [Google Scholar]
- 66.Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, Seeburg PH, Barnard EA. Sequence and function expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- 67.Smith D, Gallager D. GABA, benzodiazepine and serotonergic receptor development in the dorsal raphe nucleus: electrophysiological studies. Dev Brain Res. 1987;35:191–198. doi: 10.1016/0165-3806(87)90044-7. [DOI] [PubMed] [Google Scholar]
- 68.Spoerri PE. Neurotrophic effects of GABA in culture of embryonic chick brain and retina. Synapse. 1988;2:11–12. doi: 10.1002/syn.890020104. [DOI] [PubMed] [Google Scholar]
- 69.Spoerri PE, Wolff JR. Effect of GABA-administration on murine neuroblastoma cells in culture. Cell Tissue Res. 1981;218:567–579. doi: 10.1007/BF00210116. [DOI] [PubMed] [Google Scholar]
- 70.Tokutomi N, Ozoe Y, Katayama N, Akaike N. Effects of lindane (γ-BHC) and related convulsants on GABAA receptor-operated chloride channels in frog dorsal root ganglion neurons. Brain Res. 1994;643:66–73. doi: 10.1016/0006-8993(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 71.Wallace JA, Petrusz P, Lauder JM. Serotonin immunocytochemistry in the adult and developing rat brain: methodological and pharmacological considerations. Brain Res Bull. 1982;9:117–129. doi: 10.1016/0361-9230(82)90127-7. [DOI] [PubMed] [Google Scholar]
- 72.Wolff JR, Joo F, Kasa P. Modulation by GABA of neuroplasticity in the central and peripheral nervous system. Neurochem Res. 1993;18:453–461. doi: 10.1007/BF00967249. [DOI] [PubMed] [Google Scholar]
- 73.Xian H, Maric D, Maric I, Barker JL. GABA elevates cytoplasmic calcium in fractionated embryonic rat cortical cells. Soc Neurosci Abstr. 1995;21:1288. [Google Scholar]
- 74.Zheng T, Santi MR, Bovolin P, Marlier LNJ-L, Grayson DR. Developmental expression of the α6 GABAA receptor subunit mRNA occurs only after cerebellar granule cell migration. Dev Brain Res. 1993;75:92–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]