Abstract
The rapid suppression of CNS function produced by cyanide (CN) was studied by field, intracellular, and whole-cell recording in hippocampal slices (at 33–34°C). Population spikes and field EPSPs were depressed by 4–5 min bath applications of 50–100 μm CN (IC50 was 18 μm for spikes and 72 μm for EPSPs). The actions of CN were reversibly suppressed by the adenosine antagonists 8-sulfophenyltheophylline (8-SPT; 10 μm) and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 0.2 μm), potentiated by the adenosine transport inhibitor dipyridamole (0.5 μm), but unaffected by the KATP channel blocker glyburide (10 μm). Therefore the CN-induced reductions of synaptic efficacy and postsynaptic excitability—demonstrated by synaptic input:output plots—are mediated mainly by adenosine. In whole-cell or intracellular recordings, CN depressed EPSCs and elicited an increase in input conductance and an outward current, the reversal potential of which was approximately −90 mV (indicating that K+ was the major carrier). These effects also were attenuated by 8-SPT. In the presence of 1 mm Ba, CN had no significant postsynaptic action; Cs (2 mm) also prevented CN-induced outward currents but only partly blocked the increase in conductance. Another 8-SPT-sensitive action of CN was to depress hyperpolarization-activated slow inward relaxations (Q current). At room temperature (22–24°C), although it did not change holding current and slow inward relaxations, CN raised the input conductance; this effect also was prevented by 8-SPT (10 μm), but not by glyburide (10 μm). Adenosine release thus appears to be the major link between acute CN poisoning and early depression of CNS synaptic function.
Keywords: adenosine antagonists, KATP channel blocker, synaptic transmission, outward current, input conductance, barium, cesium
Cyanide (CN) is a specific inhibitor of cytochrome oxidase, which is essential for mitochondrial ATP production (Keilin, 1930; Isom and Way, 1984). Its use as a suicidal, homicidal, chemical warfare, and genocidal agent is well known. CN has a characteristically rapid action, especially on the CNS. In cases in which a lethal dose has been administered, it frequently has been noted that the electrical activity of the brain has stopped although the heart is still beating (Bernthal et al., 1928; Dixon and Elliott, 1929; Barcroft, 1931).
CN quickly but reversibly depresses synaptic transmission in hippocampal slices (Aitken and Braitman, 1989). CN causes a hyperpolarization of some neurons (Biscoe and Duchen, 1989; Duchen, 1990; Cummins et al., 1991; Murphy and Greenfield, 1991; Latha et al., 1994) but sharply depolarizes others (Haddad and Jiang, 1993; Sun and Reis, 1994). The exact links between CN-induced metabolic inhibition and the almost immediate suppression of CNS function remain unclear.
Several authors have suggested that the opening of ATP-sensitive K channels (Noma, 1983) by a fall in [ATP]i may explain the depressant action of CN on various cells (Nichols and Lederer, 1990;Murphy and Greenfield, 1991; Findlay, 1993; Schwanstecher and Panten, 1993).
On the other hand, adenosine is known to be released in the brain by anoxia and ischemia (Berne et al., 1974; Van Wylen et al., 1986;Richardt et al., 1987, 1994); in some isolated neural preparations, CN suppresses adenosine reuptake and so causes its extracellular accumulation (Thampy and Barnes, 1983; Maire et al., 1984; Kurbat et al., 1993). Adenosine is one of the most potent neuromodulators (Phillis and Wu, 1981; Dunwiddie, 1985; Snyder, 1985; Greene and Haas, 1991): it inhibits neuronal activity by enhancing K+conductance, which causes hyperpolarization (Greene and Haas, 1985;Proctor and Dunwiddie, 1987; Trussell and Jackson, 1987), and by decreasing Ca2+ currents in nerve endings, it reduces transmitter release (Scholz and Miller, 1991; Mogul et al., 1993; Wu and Saggau, 1994). Therefore, adenosine release could be responsible for the nearly immediate loss of synaptic transmission produced by CN—in keeping with the demonstrated involvement of adenosine in the block of synaptic transmission in hippocampal slices produced by other types of energy deprivation, such as anoxia and hypoglycemia (Fowler, 1993; Zhu and Krnjević, 1993, 1997).
In the present experiments we examined the cellular mechanisms of the action of CN in hippocampal slices. The results provide clear evidence that adenosine is an important mediator of the action of CN on neuronal activity.
A preliminary report of some of these results has appeared in abstract form (Zhu and Krnjević, 1995).
MATERIALS AND METHODS
Hippocampal slices from male Sprague Dawley rats were prepared from halothane-anesthetized Sprague Dawley rats (Charles River, Québec, Canada) weighing 110–180 gm. After decapitation, the brain was removed quickly into cold artificial cerebrospinal fluid (ACSF) at ∼4°C, well oxygenated with 95% O2/5% CO2 (carbogen). Its composition was (in mm): NaCl 124, KCl 3, MgCl2 1.3, CaCl2 2.0, NaH2PO4 1.2, and glucose 11, pH 7.3. For all extracellular and some intracellular recordings, 450-μm-thick transverse slices were cut with a McIlwain tissue chopper. Especially for whole-cell recordings, 350–400 μm slices were cut with a Vibroslice (Campden Instruments, UK). The slices were allowed to recover in carbogenated ACSF at room temperature for >1 hr before recordings began. All experiments were performed on fully submerged slices kept at 33–34°C (Zhu and Krnjević, 1994).
Field recordings were made with 2 m NaCl electrodes from the CA1 pyramidal layer or stratum radiatum. To evoke synaptic responses, we applied stimuli at 0.1 Hz through insulated nickel–chromium wires placed in the stratum radiatum. Intracellular recordings were obtained with 3 m KCl electrodes (70–90 MΩ). Whole-cell recordings were done “blind” (Blanton et al., 1989), with patch pipettes (4–6 MΩ) filled with (in mm): 150 KMeSO4, 10 HEPES, 2 MgCl2, 0.1 CaCl2, 1.1 EGTA, 2 ATP, and 0.4 GTP. An Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) was used for all experiments, including voltage-clamping in the discontinuous mode, at a switching frequency of 3.5–6.0 kHz. The head stage output was monitored continuously to ensure adequate settling in each duty cycle.
Drugs were obtained as follows: kynurenate, bicuculline, and dipyridamole from Sigma (St. Louis, MO); KCN from J. T. Baker (Phillipsburg, NJ); 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) and 8-sulfophenyltheophylline (8-SPT) from Research Biochemicals (Natick, MA); tetrodotoxin (TTX) from the Qinhuangdao Trading Corporation (Qinhuangdao, China). Glyburide was a gift from Hoechst Canada (Montréal, Canada). The data are expressed as mean ± SEM; whenever possible, differences between means were examined by the paired t test.
RESULTS
Field recordings
Figure 1A illustrates the gradual disappearance of field potentials when 100 μm CN was applied for 5 min: first, population spikes and then fEPSPs. After washout of CN, fEPSPs returned before the population spikes. In 13 slices, 50–100 μm CN fully suppressed population spikes (down by 99 ± 1.3%).
Fig. 1.
CN-induced depression of CA1 excitatory synaptic transmission is blocked by an adenosine receptor antagonist.A, CA1 population spikes were elicited by stimulating stratum radiatum at 0.1 Hz. The initial positive slope reflects the rising phase of the EPSP, and the sharp downward (negative) deflection is the population spike (50% maximal). Bath application of 100 μm KCN for 5 min reversibly depressed transmission.B, Dot plots of CA1 population spike amplitude in another slice. Top, Time course of CN-induced depression. Middle, The action of CN was strongly attenuated by 8-SPT. Bottom, Partial recovery of the action of CN after washing slice for 60 min. CN superfusion is marked by arrows. C, D, Dose–response curves for depression of EPSP (C) and population spike (D); 8-SPT shifted both dose–response curves to the right. One hundred percent represents full suppression of fEPSP and population spikes. IC50 for CN action is indicated bydashed lines. Data from several experiments were pooled to obtain mean ± SEM (n = 5–9).
Adenosine antagonists
To detect a possible role of adenosine in the mechanism of action of CN, we applied the adenosine antagonist 8-SPT (Bruns et al., 1980). As shown in Figure 1B, the CN-induced depression was attenuated markedly (n = 25): in 10 μm8-SPT, 50 μm CN reduced population spikes by only 14 ± 8.4% (n = 5) and 100 μm CN by 41 ± 4.4% (n = 8); these effects of 8-SPT were readily reversible. The dose–response curves for CN thus were shifted to the right by 8-SPT (Fig. 1C,D): IC50 values for the action of CN on fEPSPs and population spikes were 72 and 18 μm, respectively, under control conditions and ∼4 times higher, 254 and 85 μm, in the presence of 10 μm 8-SPT. A more selective A1 adenosine antagonist, DPCPX (Bruns et al., 1987), had a similar action: in 200 nm DPCPX, 100 μm CN depressed population spikes by only 51 ± 2.5% (n = 5).
Adenosine transport block
We also looked at the effect of dipyridamole, which raises extracellular levels of adenosine by blocking adenosine transport (Young and Jarvis, 1983; Phillis et al., 1989). Bath applications of dipyridamole (0.5 μm) did not delay the block of population spikes (half-block time was 139 ± 6.4 sec in control runs and 138 ± 5.4 sec in the presence of 0.5 μmdipyridamole), but they delayed significantly the recovery of population spikes after the end of CN applications (by 47 ± 10%, for n = 6, p < 0.01, from the control value of 199 ± 1.6 sec), thus confirming a significant involvement of adenosine.
KATP channel block
Because CN blocks ATP synthesis, we considered whether CN might act by opening ATP-sensitive K (KATP) channels. This could depress synaptic transmission by hyperpolarizing either pre- or postsynaptic elements (or both). In six slices, however, the specific blocker of KATP channels, glyburide (10 μm), did not prevent the depression of EPSPs by CN (Fig. 2). The mean times to half-block (from the start of the application of CN) and half-recovery (after its end) were 127 ± 13.5 and 186 ± 14.6 sec, respectively, in control runs; in the presence of 10 μm glyburide, they were virtually identical, 133 ± 10.4 and 191 ± 14.0 sec, respectively.
Fig. 2.
KATP channel blocker did not attenuate CN-induced depression of synaptic transmission. CA1 population spike (50% maximal) amplitudes are expressed as dot plots. The time of CN application is indicated by thick horizontal line inA. Top, Control run.Bottom, In presence of KATP channel blocker glyburide (10 μm).
Synaptic sites of CN action
To identify the site of CN action, we looked for selective changes in the afferent volley (the compound action potential of afferent axons), EPSP and population spike. In Figure3A the peak-to-peak amplitude of the afferent volley is plotted as function of stimulus strength: the lack of effect of CN shows that CN did not alter axonal excitability.
Fig. 3.
Analysis of the site of CN action.A, Plots of afferent volley (peak-to-peak amplitude) as function of stimulus duration show that neither CN alone (top) nor CN in presence of 8-SPT (bottom) affects the afferent volley. B, The efficacy of synaptic transmission was examined by plotting the initial rate of rise of EPSPs as function of the size of the afferent volley. Top, CN reduces the slope of the plot and therefore the efficacy of synaptic transmission. Bottom, This effect was nearly abolished by 8-SPT. C, Plots of population spike as function of initial slope of EPSP (E–S coupling).Top, For a given EPSP, CN decreases corresponding population spike; reduced coupling indicates that CN also affects postsynaptic mechanisms. Bottom, 8-SPT also attenuated this CN action. A and B were recorded from CA1 stratum radiatum of same slice. Data in C were recorded from CA1 pyramidal layer of another slice.
The efficacy of synaptic transmission was assessed by plotting the initial rate of rise of fEPSPs as function of afferent volley size (Fig. 3B); 50 μm CN, applied for 5 min, reduced the slope of such plots by 18 ± 3.5% (forn = 5, p < 0.01). This effect was prevented by 8-SPT (in the bottom plots of Fig. 3B, CN changed the slopes by only 2 ± 1.4%, n = 5).
A possible postsynaptic action was investigated by plotting population spike amplitude as function of EPSP rate of rise (Fig. 3C). This “E–S relation” is an index of the ability of EPSPs to generate action potentials (Andersen et al., 1980). As shown by the top plots of Figure 3C, in the presence of CN EPSPs consistently generated smaller population spikes. In five slices, the mean reduction (estimated by comparing the areas under plots) was by 57 ± 10.9% (p < 0.01), but in the presence of 8-SPT CN produced no significant change (−1.9 ± 4.5%, n= 5; bottom plots in Fig. 3C). Judging by these data, the depression of transmission induced by a brief CN application—apparently affecting both synaptic efficacy and E–S coupling—is caused mainly by adenosine.
Whole-cell and intracellular recordings
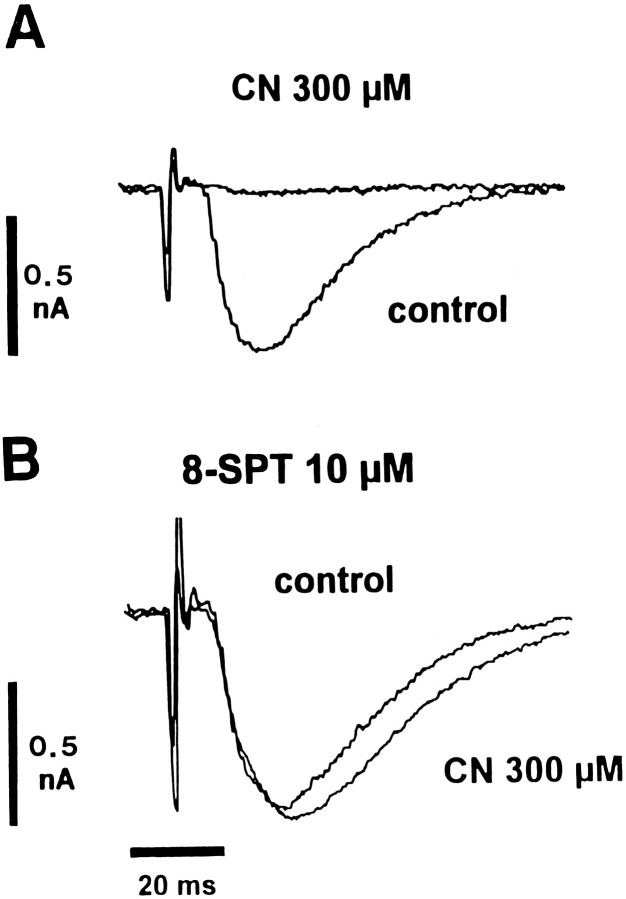
The effect of CN on synaptic transmission was examined further by whole-cell recording under voltage clamp. As shown in Figure4A, 300 μm CN nearly abolished EPSCs elicited by stimulation of stratum radiatum. In nine neurons, 3–4 min applications of CN (200–300 μm) reduced EPSCs by 94 ± 3.1% from control values of 0.39 ± 0.03 nA, at VH −70 mV. In the presence of 10 μm 8-SPT, CN was far less effective (Fig.4B), depressing EPSCs by only 21 ± 11.6% (p > 0.10) from control values of 0.44 ± 0.07 nA (n = 4).
Fig. 4.
8-SPT prevents CN-induced suppression of EPSCs. In whole-cell recording from a CA1 neuron, EPSCs were elicited by stimulation of stratum radiatum at 0.1 Hz. Membrane potential was held at −70 mV throughout. A, Control run.B, From same neuron in presence of 8-SPT (10 μm).
Postsynaptic changes
In voltage-clamp recordings with intracellular (3 mKCl) electrodes (Fig. 5A), atVH approximately −70 mV, CN elicited very substantial outward currents (176 ± 4.0 pA) and increased GN by 20 ± 2.6% (p < 0.001,n = 16) from control values of 20 ± 1.8 nS. Both outward current and conductance rise were reduced by 10 μm 8-SPT (Fig. 5B). In the presence of 8-SPT, CN elicited a nonsignificant inward current of 35 ± 18 pA, and GN rose by only 2.2 ± 1.3% (p > 0.10)—from 23 ± 2.9 nS (n = 6) (Table 1). The voltage dependence of CN action is shown by the current–voltage plots of Figure 6: note the reversal potential near −90 mV (arrow in Fig. 6A). The difference current evoked by CN is plotted in Figure 6C (open circles). Its reversal at −90 ± 2.2 mV indicates that CN probably activates a K conductance.
Fig. 5.
Adenosine mediates outward current and increase in input conductance elicited by CN. Intracellular recording (with 3m KCl electrode) was from a CA1 neuron under voltage clamp in the presence of 0.5 μm TTX. Transmitter-mediated responses were minimized further by 2 mm kynurenate and 10 μm bicuculline. Hyperpolarizing pulses (−20 mV, 500 msec—monitored on topmost trace) and corresponding currents are shown on expanded time scale before, during, and after CN application. Zero current levels are indicated by horizontal arrows, at left of traces. Note substantial outward current, as well as increased conductance (A), which was blocked by 8-SPT (B). C was recorded after 45 min wash.
Table 1.
8-SPT, Ba, and Cs block increase (Δ) in input conductance (nS) elicited by CN (300 μm)
| Before CN | Δ in CN | After wash | n | |
|---|---|---|---|---|
| Control | 20 ± 1.8 | 3.9 ± 0.521-165 | 19 ± 2.1 | 16 |
| 8-SPT (10 μm) | 23 ± 2.9 | 0.52 ± 0.29 | 22 ± 2.8 | 6 |
| Wash | 21 ± 9.9 | 3.2 ± 0.531-160 | 21 ± 2.5 | 6 |
| Control | 20 ± 5.4 | 3.4 ± 0.64* | 19 ± 5.1 | 3 |
| Ba (1 mm) | 12 ± 3.2 | 0.0 ± 0.003 | 13.5 ± 3.1 | 3 |
| Wash | 15.5 ± 4.4 | 2.5 ± 0.251-160 | 16 ± 5.0 | 3 |
| Control | 20 ± 2.6 | 4.1 ± 0.911-160 | 20 ± 2.6 | 9 |
| Cs (2 mm) | 15 ± 2.0 | 1.8 ± 0.76* | 14.5 ± 1.9 | 9 |
| Wash | 11 ± 0.74 | 2.6 ± 0.68* | 11.5 ± 0.67 | 4 |
Data were obtained by intracellular recording with 3m KCl electrodes at 34°C; n is number of cells tested.
F1-165: p < 0.001,
F1-160: p < 0.01, and
p < 0.05, all paired t tests.
Fig. 6.

Voltage dependence of CN-induced current, from cell illustrated in Figure 5. A, Data obtained before, during, and after CN application; note intersection of control and test plots near −90 mV (indicated by arrow).B, The action of CN was strongly attenuated by 8-SPT.C, Difference currents—obtained by subtracting control values of currents (before CN application) from currents recorded in presence of CN—are the currents activated by the action of CN. Note that relatively large CN difference current (open circles) was much reduced by 8-SPT (closed circles).
As observed with hypoxia (Zhang and Krnjević, 1993), the postsynaptic effects of CN were much smaller in whole-cell recordings: at VH −55 mV, CN elicited an outward current of only 80 ± 8.2 pA, accompanied by a just significant increase in GN (6 ± 2.9%; p = 0.05) from a low control level of 12 ± 1.0 nS (n = 15). In the presence of 8-SPT, CN evoked an inward current of 16 ± 3.9 nA, accompanied by a nonsignificant 2 ± 2.5%decrease in input conductance from control values of 13 ± 1.0 nS (for n = 12). All of these data suggest that adenosine release is a major component of the mechanism of action of CN, which reduces postsynaptic excitability by activating K channels.
In general, lowering the temperature greatly reduced the effects of CN (like those of hypoxia; Hochachka and Mommsen, 1983; Morris et al., 1991). Nevertheless, CN increased the input conductance (Fig.7a,b) even in slices kept at 22–24°C. In seven whole-cell recordings at VH −50 mV, applications of 300 μm CN raised GN by 3.7 ± 1.04 nS (p < 0.01; from 10 ± 2.1 nS), but the holding current did not change significantly (by 12 ± 12.1 pA, from the control level of 69 ± 16.1 pA)—mainly because of the marked positive shift in reversal potential for the action of CN (at arrow in Fig. 7a), presumably because of the corresponding shift inEK or the activation of other ionic channels. At room temperature, glyburide (Fig. 7c,d) again failed to prevent the CN-induced rise in GN (by 4.7 ± 0.85 nS,p < 0.005, n = 6; from a control value of 7.5 ± 0.70 nS). These data further support the conclusion that KATP channels do not contribute significantly to CN-induced responses of CA1 neurons. As before, 10 μm 8-SPT abolished the effect of CN (Fig. 7e,f), GN increasing by only 0.3 ± 1.65 nS (n = 6) from a control value of 5.5 ± 0.40 nS.
Fig. 7.
Current–voltage plots illustrate the action of CN at room temperature. Data are from whole-cell recordings. Linear portions of I–V relations are shown expanded inbottom panels. Open circles,squares, and triangles are data obtained before, during, and after CN application, respectively. Note relatively positive reversal potentials. CN increased input conductance (a, b); in 10 μm glyburide, the action of CN on conductance was even enhanced (c, d). In e and f, 8-SPT blocked the action of CN. For further details, see text.
Barium and cesium
Both Ba (1 mm) and Cs (2 mm) attenuated the postsynaptic action of CN. As a potent blocker of a variety of K channels, Ba can be expected to be an antagonist of CN. Indeed, Ba (1–2 mm) strongly suppressed CN-induced changes in holding current and GN, as shown in Figure 8. In three cells held at approximately −70 mV (intracellular recording), Ba (1 mm) abolished the outward currents elicited by CN (113 ± 37 pA), replacing them by small inward currents (1.3 ± 0.64 pA). The CN-induced increase in GN also was fully blocked by Ba (n = 3) (Table 1). Similar results were obtained in whole-cell recordings (with VHapproximately −55 mV): in 1 mm Ba, CN (300 μm) elicited no significant shift in base line current (−1.4 ± 5.14 pA, n = 8) from the control base line of −14 ± 6.8 pA and had no effect on GN(0.8 ± 5.4% increase from 8.6 ± 0.46 nS, n= 8).
Fig. 8.
Ba blocks CN-induced outward current and reduces input conductance change. Intracellular recording was from CA1 neuron under voltage clamp in presence of 0.5 μm TTX, 1 mm kynurenate, and 10 μm bicuculline. As in Figure 5, traces were accelerated at intervals for better display of currents evoked by hyperpolarizing pulses (monitored in topmost trace), and zero current levels are indicated byhorizontal arrows at left. CN-induced outward current and increase in input conductance (A) were blocked by 1 mm Ba (B).Trace in C shows partial recovery after wash.
More surprising was the finding that Cs (2 mm) also diminished the outward currents elicited by CN, both in whole-cell and intracellular recordings (Fig. 9). In nine control intracellular recordings, CN (300 μm) evoked outward currents of 80 ± 15.5 pA (p < 0.001); after adding 2 mm Cs, they were replaced by nonsignificant inward currents of 13 ± 8.1 pA. However, Cs did not fully suppress CN-induced increases in input conductance (Table 1). These results suggest that part of the action of CN is mediated by block of the hyperpolarization-activated inward cur-rent (IQ) (Maccaferri et al., 1993; Perkins and Wong, 1995). Indeed, 300 μm CN sharply reducedIQ-like slow inward relaxations seen during hyperpolarizing pulses (Fig. 10A). Such data from six neurons are plotted as a function of membrane potential in the left panel of Figure 10B. The suppression of this effect of CN by 8-SPT (Fig. 10, right panels) is in agreement with previous reports that adenosine reduces Ih in geniculocortical neurons (Pape, 1993) and mesopontine cholinergic neurons (Rainnie et al., 1994). It provides further evidence that adenosine is the principal mediator of the actions of CN on membrane currents. Cooling the slices to 22–24°C prevented the CN-induced depression of the inward relaxations (Fig. 11; n = 6), perhaps owing to reduction of adenosine release. Thus, the postsynaptic hyperpolarizing effect of CN appears to be mediated partly by an increase in GN (presumably mainly a K conductance) and partly by depression of an IQ-like inward current.
Fig. 9.
CN-induced outward current also is attenuated by Cs. Arrangement as in Figures 5 and 8: outward current (A) was blocked by 2 mm Cs (B). Trace in C shows partial recovery of the effects of CN after wash. As before, zero current levels are indicated by horizontal arrows atleft. Note that Cs did not fully prevent CN-induced increase in input conductance.
Fig. 10.
Adenosine antagonist 8-SPT attenuated CN-induced depression of Q-like current. A, Whole-cell recordings of slow inward relaxations during hyperpolarizing pulses (Q-like current) (a), their depression by 300 μm CN (b), and block of this effect by 8-SPT (c, d). Slow inward relaxations—evoked by 500 msec hyperpolarizing voltage steps (−10 to −60 mV, from VH −50 mV)—were measured by subtracting instantaneous current from steady-state current for each trace. B, Current–voltage relations of inward relaxations before (open circles) and during CN applications (closed circles). Left, Control runs (n = 6); right, CN had little effect in presence of 10 μm 8-SPT (n = 5).
Fig. 11.
CN did not significantly depress hyperpolarization-activated Q-like current at room temperature (22–24°C). Slow inward relaxations were evoked as in Figure 10. Shown is voltage dependence of inward relaxation before (open circles) and during CN application (closed circles); n = 6.
DISCUSSION
The present results confirm an earlier report that CN reversibly suppresses synaptic transmission in CA1 (Aitken and Braitman, 1989). In addition, they show that the action of CN is reduced greatly by DPCPX or 8-SPT and enhanced by dipyridamole and, therefore, principally caused by adenosine release. The adenosine-mediated depression of EPSPs is mainly attributable to the block of glutamate release (Phillis and Wu, 1981; Dunwiddie, 1985;Snyder, 1985; Greene and Haas, 1991). Precisely how adenosine acts on nerve endings is not yet certain. Are the terminal Ca2+currents affected directly or as a result of GK increase? There is also reason to believe that ATP is essential for glutamate release (Nicholls, 1989), which thus could be depressed by even minor depletions of ATP—such as those observed during hypoxia in areas rich in synapses (Lipton and Whittingham, 1982). However, in view of the striking protective effect of the adenosine antagonists, it seems that adenosine must be the principal agent responsible for the block of EPSPs.
There had been no previous reports of membrane effects of CN on CA1 neurons in slices. Studies on other neurons revealed a variety of effects, apparently not because of adenosine. Thus, both in the spinal cord and brainstem, CN has a sharp excitatory and depolarizing action (Godfraind et al., 1971; Haddad and Jiang, 1993; Sun and Reis, 1994)—explained by a rapid activation of Na or Ca currents and quite unlike the hyperpolarizing effect of adenosine on the same cells (Sun and Reis, 1994). By contrast, Murphy and Greenfield (1991) and Trapp and Ballanyi (1995) observed hyperpolarizing effects on neurons in substantia nigra and dorsal vagal nucleus, respectively, which they ascribed to activation of KATP channels, caused by depletion of ATP.
In the present experiments on slices, the hyperpolarizing effect was like that seen in acutely dissociated hippocampal neurons (Cummins et al., 1991). It proved to be quite insensitive to glyburide and so unlikely to be mediated by KATP channels, but it was very much reduced by adenosine A1 antagonists: the activation of A1 receptors must, therefore, play an essential role in the underlying mechanism. This should not be surprising, because CN is known to release adenosine from nerve cells (Maire et al., 1984; Kurbat et al., 1993), and adenosine has a well known hyperpolarizing effect on hippocampal neurons, produced by a relatively direct—only G-protein-mediated—enhancement of GK (Greene and Haas, 1985; Trussell and Jackson, 1987).
But why does CN not have the same effect on neurons in other parts of the brain? Moreover, one would expect the hyperpolarizing action of conventional hypoxia to have a similar mechanism. So far, there has been little evidence that the hyperpolarization (as opposed to the synaptic block) produced in slices by hypoxia is caused by adenosine (Leblond and Krnjević, 1989; Spuler and Grafe, 1989) (but cf. Zhu and Krnjević, 1997).
One must, therefore, consider some alternative hyperpolarizing mechanism. It is well known that CN raises cytoplasmic free Ca2+ in a variety of cells—including several types of neurons (Biscoe and Duchen, 1990; Duchen et al., 1990; Dubinsky and Rothman, 1991; Duchen and Biscoe, 1992; Kaplin et al., 1996), as well as glia (Brismar and Collins, 1993), vascular smooth muscle (Miller et al., 1993), and chromaffin cells (Latha et al., 1994). CN thus could produce hyperpolarization by activating a Ca2+-sensitive GK (GK(Ca)). There is, indeed, evidence that hypoxic hyperpolarizations are caused by Ca2+ release from an IP3-dependent store (Belousov et al., 1995), likely initiated by glycolytically produced nicotinamide adenine dinucleotide (NADH) (Kaplin et al., 1996).
Are these mechanisms mutually exclusive? Not necessarily. In several kinds of cells adenosine, acting via A1 receptors, triggers the formation of IP3 (Arend et al., 1988; Kohl et al., 1990; Gerwins and Fredholm, 1992). This IP3 may be essential for the action of NADH, because NADH acts bysensitizing IP3 receptors (Kaplin et al., 1996). Thus, a synergistic convergence of two effects of CN, enhanced glycolysis and adenosine release, may be necessary to raise [Ca2+] sufficiently to activate GK(Ca). In keeping with such a mechanism, adenosine increases slow afterhyperpolarizations of CA1 neurons (Greene and Haas, 1985).
Like the activation of GK by adenosine (Trussell and Jackson, 1987; Gerber et al., 1989), the adenosine-evoked formation of IP3 is mediated by a (perhaps the same) pertussis-sensitive G-protein (Arend et al., 1988). However, in their experiments on CA1 neurons pretreated with pertussis toxin, Spuler and Grafe (1989) found no suppression of anoxic (or hypoglycemic) hyperpolarizations, although adenosine applications were ineffective, and therefore concluded that adenosine could not be the essential mediator of these hyperpolarizations. One can suppose that, under some conditions, GK(Ca) (or some other GK) may be activated by ATP depletion or that another factor is released, which can substitute for adenosine. Whether adenosine or such an alternative mechanism is predominant presumably will vary with the general metabolic state, as well as with local conditions at different sites in the brain. With regard to CA1 neurons, adenosine clearly accounts for a major portion of the hyperpolarizing effects of CN.
The question remains whether this is mediated by the GKthat is independent of second messengers (Trussell and Jackson, 1987;Gerber et al., 1989) or by the more complex route via IP3and GK(Ca) suggested above. Our finding that Ba suppresses the effect of CN does not help to distinguish between these alternatives, because Ba blocks a variety of K currents, includingIAHP (Connor, 1979), IM(Constanti et al., 1981), and IK (Armstrong et al., 1982), as well as that elicited by adenosine (Trussell and Jackson, 1987; Gerber et al., 1989).
The present experiments show that Cs also attenuates the action of CN, in agreement with its comparable effect on hypoxic hyperpolarizations (Leblond and Krnjević, 1989). Bearing in mind the well known block of the hippocampal Q current by Cs (Maccaferri et al., 1993), it is likely that the CN-evoked outward current is caused at least partly by adenosine-induced suppression of ongoing Q current. Indeed, the adenosine antagonist 8-SPT attenuated the effect of CN on Q current, in keeping with previous studies in which adenosine decreased Q-like inward currents (Pape, 1993; Rainnie et al., 1994). On the other hand, there is evidence that Cs depresses the M current (Coggan et al., 1994), which also may be enhanced by a CN-induced rise in cytoplasmic [Ca] (Yu et al., 1994).
There is increasing interest in the effects of CN on CNS (Way, 1984;Jones et al., 1987; Baud et al., 1991; Dubinsky and Rothman, 1991;Krieglstein and Rischke, 1991; Brismar and Collins, 1993; Miller et al., 1993; Sturm et al., 1993). CN is a component of cigarette smoke, and it has been identified as a major cause of death from accidental smoke inhalation (Way, 1984; Jones et al., 1987; Baud et al., 1991). Adenosine attenuates neuronal damage induced by ischemia or CN (Krieglstein and Rischke, 1991; Sturm et al., 1993), presumably by reducing glutamate release (Patel et al., 1991, 1992; Cai and McCaslin, 1992). Therefore, adenosine release caused by CN has a neuroprotective effect in slices in vitro. However, CN-induced adenosine release also suppresses CNS function, which in turn leads to loss of consciousness and then to loss of respiratory function. The present results point to adenosine as a crucial element in the intrinsic mechanism that links acute CN poisoning with almost immediate depression of CNS function.
Footnotes
This research was supported financially by the Medical Research Council of Canada and partly by the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (Québec). We thank Murray Sweet for his photographic work.
Correspondence should be addressed to Dr. Kresimir Krnjević, McGill University, McIntyre Medical Sciences Building, Room 1208, 3655 Drummond Street, Montréal, Québec, Canada H3G 1Y6.
Dr. Zhu’s present address: Departments of Pharmacological and Physiological Sciences, University of St. Louis School of Medicine, 1402 South Grand Boulevard, St. Louis, MO 63104.
REFERENCES
- 1.Aitken PG, Braitman DJ. The effects of cyanide on neuronal and synaptic function in hippocampal slices. Neurotoxicology. 1989;10:239–247. [PubMed] [Google Scholar]
- 2.Andersen P, Sundberg SH, Sveen O, Swann JW, Wigstrom K. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol (Lond) 1980;320:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arend LJ, Burnatowska-Hledin MA, Spielman WS. Adenosine receptor-mediated calcium mobilization in cortical-collecting tubule cells. Am J Physiol. 1988;255:C581–C588. doi: 10.1152/ajpcell.1988.255.5.C581. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong CM, Swenson RP, Taylor SR. Block of squid axon K channels by internally and externally applied barium ions. J Gen Physiol. 1982;80:663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcroft J. The toxicity of atmospheres containing hydrocyanide acid gas. J Hyg. 1931;31:1–34. doi: 10.1017/s0022172400010664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baud FJ, Barriot P, Toffis V, Riou B, Vicaut E, Lecarpentier Y, Bourdon R, Astier A, Bismuth C. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761–1766. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 7.Belousov A, Godfraind JM, Krnjević K. Internal Ca2+ stores involved in anoxic responses of rat hippocampal neurons. J Physiol (Lond) 1995;486:547–556. doi: 10.1113/jphysiol.1995.sp020833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berne RM, Rubio R, Curnish RR. Release of adenosine from ischemic brain. Circ Res. 1974;35:262–271. [Google Scholar]
- 9.Bernthal TB, Bronk DW, Cordero N, Gesell R. The regulation of respiration. XVIII. The effects of low and high alveolar oxygen pressure and of sodium cyanide on the carotid and femoral flow of blood as studied with the continuous electrometric method. Am J Physiol. 1928;83:435–444. [Google Scholar]
- 10.Biscoe TJ, Duchen MR. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol (Lond) 1989;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biscoe TJ, Duchen MR. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol (Lond) 1990;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanton MG, Lo Turco JJ, Kriegstein AR. Whole-cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 13.Brismar T, Collins VP. Effect of external cation concentration and metabolic inhibitors on membrane potential of human glial cells. J Physiol (Lond) 1993;460:365–383. doi: 10.1113/jphysiol.1993.sp019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruns RF, Daly JW, Snyder SH. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H] adenosine and 1,3-diethyl8-[3H] phenylxanthine. Proc Natl Acad Sci USA. 1980;77:5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hartman JD, Hays SJ, Huang CC. Binding of A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, McCaslin PP. Selective effects of cyanide (100 μm) on the excitatory amino acid-induced elevation of intracellular calcium levels in neuronal culture. Neurochem Res. 1992;17:803–808. doi: 10.1007/BF00969016. [DOI] [PubMed] [Google Scholar]
- 17.Coggan JS, Purnyn SL, Knoper SR, Kreulen DL. Muscarinic inhibition of two potassium currents in guinea-pig prevertebral neurons: differentiation by extracellular cesium. Neuroscience. 1994;59:349–361. doi: 10.1016/0306-4522(94)90601-7. [DOI] [PubMed] [Google Scholar]
- 18.Connor JA. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol (Lond) 1979;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constanti A, Adams PR, Brown DA. Why do barium ions imitate acetylcholine? Brain Res. 1981;206:244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- 20.Cummins TR, Donnelly DF, Haddad GG. Effect of metabolic inhibition on the excitability of isolated hippocampal CA1 neurons: developmental aspects. J Neurophysiol. 1991;66:1471–1482. doi: 10.1152/jn.1991.66.5.1471. [DOI] [PubMed] [Google Scholar]
- 21.Dixon M, Elliott KAC. The effect of cyanide on respiration of animal tissues. Biochem J. 1929;23:812–830. doi: 10.1042/bj0230812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubinsky JM, Rothman SM. Intracellular calcium concentrations during “chemical hypoxia” and excitotoxic neuronal injury. J Neurosci. 1991;11:2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duchen MR. Effects of metabolic inhibition on the membrane properties of isolated mouse primary sensory neurons. J Physiol (Lond) 1990;424:378–409. doi: 10.1113/jphysiol.1990.sp018073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol (Lond) 1992;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duchen MR, Valdeolmillos M, O’Neill SC, Eisner DA. Effects of metabolic blockage on the regulation of intracellular calcium in dissociated mouse sensory neurons. J Physiol (Lond) 1990;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;14:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 27.Findlay I. Sulphonylurea drugs no longer inhibit ATP-sensitive K+ channels during metabolic stress in cardiac muscle. J Pharmacol Exp Ther. 1993;226:456–467. [PubMed] [Google Scholar]
- 28.Fowler JC. Purine release and inhibition of synaptic transmission during hypoxia and hypoglycaemia in rat hippocampal slices. Neurosci Lett. 1993;157:83–86. doi: 10.1016/0304-3940(93)90648-5. [DOI] [PubMed] [Google Scholar]
- 29.Gerber U, Greene RW, Haas HL, Stevens DR. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol (Lond) 1989;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerwins P, Fredholm BB. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem. 1992;267:16081–16087. [PubMed] [Google Scholar]
- 31.Godfraind JM, Kawamura H, Krnjević K, Pumain R. Actions of dinitrophenol and some other metabolic inhibitors on cortical neurones. J Physiol (Lond) 1971;215:199–222. doi: 10.1113/jphysiol.1971.sp009465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene RW, Haas HL. Adenosine actions on CA1 pyramidal neurones in rat hippocampal slices. J Physiol (Lond) 1985;366:119–127. doi: 10.1113/jphysiol.1985.sp015788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog Neurobiol. 1991;36:329–341. doi: 10.1016/0301-0082(91)90005-l. [DOI] [PubMed] [Google Scholar]
- 34.Haddad GG, Jiang C. Mechanisms of anoxia-induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res. 1993;625:261–268. doi: 10.1016/0006-8993(93)91067-3. [DOI] [PubMed] [Google Scholar]
- 35.Hochachka PW, Mommsen TP. Protons and anaerobiosis. Science. 1983;219:1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- 36.Isom GE, Way JL. Effects of oxygen on the antagonism of cyanide intoxication: cytochrome oxidase, in vitro. Toxicol Appl Pharmacol. 1984;74:57–62. doi: 10.1016/0041-008x(84)90269-2. [DOI] [PubMed] [Google Scholar]
- 37.Jones J, McMullen MJ, Dougherty J. Toxic smoke inhalation: cyanide poisoning in fire victims. Am J Emerg Med. 1987;5:317–321. doi: 10.1016/0735-6757(87)90360-3. [DOI] [PubMed] [Google Scholar]
- 38.Kaplin AI, Snyder SH, Linden DJ. Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-trisphosphate receptors mediates hypoxic mobilization of calcium. J Neurosci. 1996;16:2001–2011. doi: 10.1523/JNEUROSCI.16-06-02002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keilin D. Cytochrome and intracellular oxidase. Proc R Soc Lond [Biol] 1930;106:418–444. [Google Scholar]
- 40.Kohl C, Linck B, Schmitz W, Scholz H, Scholz J, Tóth M. Effects of carbachol and (−)-N6-phenylisopropyladenosine on myocardial inositol phosphate content and force of contraction. Br J Pharmacol. 1990;101:829–834. doi: 10.1111/j.1476-5381.1990.tb14165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieglstein J, Rischke R. Vinpocetine increases the neuroprotective effect of adenosine in vitro. Eur J Pharmacol. 1991;19:7–10. doi: 10.1016/0014-2999(91)90762-f. [DOI] [PubMed] [Google Scholar]
- 42.Kurbat JM, Buchanan RJ, Wulff SC, Yoon K-W. Cyanide-mediated adenosine release from rat hippocampal neurons. Soc Neurosci Abstr. 1993;19:1661. [Google Scholar]
- 43.Latha MV, Borowitz JL, Yim GK, Kanthasamy A, Isom GE. Plasma membrane hyperpolarization by cyanide in chromaffin cells: role of potassium channels. Arch Toxicol. 1994;68:370–374. doi: 10.1007/s002040050084. [DOI] [PubMed] [Google Scholar]
- 44.Leblond J, Krnjević K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989;62:1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Lipton P, Whittingham TS. Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea-pig hippocampus. J Physiol (Lond) 1982;325:51–65. doi: 10.1113/jphysiol.1982.sp014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- 47.Maire JC, Medilanski J, Straub RW. Release of adenosine, inosine, and hypoxanthine from rabbit non-myelinated nerve fibres at rest and during activity. J Physiol (Lond) 1984;357:67–77. doi: 10.1113/jphysiol.1984.sp015489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller AL, Morales E, Leblanc NR, Cole WC. Metabolic inhibition enhances Ca2+-activated K+ current in smooth muscle cells of rabbit portal vein. Am J Physiol. 1993;256:H2184–H2195. doi: 10.1152/ajpheart.1993.265.6.H2184. [DOI] [PubMed] [Google Scholar]
- 49.Mogul DJ, Adams ME, Fox AP. Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron. 1993;10:327–334. doi: 10.1016/0896-6273(93)90322-i. [DOI] [PubMed] [Google Scholar]
- 50.Morris ME, Leblond J, Agopyan N, Krnjević K. Temperature dependence of extracellular ionic changes evoked by anoxia in hippocampal slices. J Neurophysiol. 1991;65:157–167. doi: 10.1152/jn.1991.65.2.157. [DOI] [PubMed] [Google Scholar]
- 51.Murphy KP, Greenfield SA. ATP-sensitive potassium channels counteract anoxia in neurons of the substantia nigra. Exp Brain Res. 1991;84:355–358. doi: 10.1007/BF00231456. [DOI] [PubMed] [Google Scholar]
- 52.Nicholls DG. Release of glutamate, aspartate, and γ-aminobutyric acid from isolated nerve terminals. J Neurochem. 1989;52:331–341. doi: 10.1111/j.1471-4159.1989.tb09126.x. [DOI] [PubMed] [Google Scholar]
- 53.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol (Lond) 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noma A. ATP-regulated single K channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 55.Pape H-C. Adenosine promotes burst activity in guinea-pig geniculocortical neurons through two different ionic mechanisms. J Physiol (Lond) 1993;447:729–753. doi: 10.1113/jphysiol.1992.sp019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel MN, Ardelt BK, Yim GK, Isom GE. Cyanide induces Ca2+-dependent and -independent release of glutamate from mouse brain slices. Neurosci Lett. 1991;131:42–44. doi: 10.1016/0304-3940(91)90332-n. [DOI] [PubMed] [Google Scholar]
- 57.Patel MN, Yim GK, Isom GE. Blockage of N-methyl-d-aspartate receptors prevents cyanide-induced neuronal injury in primary hippocampal cultures. Toxicol Appl Pharmacol. 1992;115:124–129. doi: 10.1016/0041-008x(92)90375-3. [DOI] [PubMed] [Google Scholar]
- 58.Perkins KL, Wong RKS. Intracellular QX-314 blocks the hyperpolarization-activated inward current IQ in hippocampal CA1 pyramidal cells. J Neurophysiol. 1995;73:911–915. doi: 10.1152/jn.1995.73.2.911. [DOI] [PubMed] [Google Scholar]
- 59.Phillis JW, Wu PH. The role of adenosine and its nucleotides in central nervous system. Prog Neurobiol. 1981;16:187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- 60.Phillis JW, O’Regan MH, Walter GA. Effects of two nucleoside transport inhibitors, dipyridamole and soluflazine, on purine release from the rat cerebral cortex. Brain Res. 1989;481:309–316. doi: 10.1016/0006-8993(89)90808-1. [DOI] [PubMed] [Google Scholar]
- 61.Proctor WR, Dunwiddie TV. Pre- and postsynaptic actions of adenosine in the in vitro rat hippocampus. Brain Res. 1987;426:187–190. doi: 10.1016/0006-8993(87)90441-0. [DOI] [PubMed] [Google Scholar]
- 62.Rainnie DR, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardt G, Waas W, Kranzhöfer R, Mayer E, Schömig A. Adenosine inhibits exocytotic release of endogenous noradrenaline in rat heart: a protective mechanism in early myocardial ischemia. Circ Res. 1987;61:117–123. doi: 10.1161/01.res.61.1.117. [DOI] [PubMed] [Google Scholar]
- 64.Richardt G, Blessing R, Schömig A. Cardiac noradrenaline release accelerates adenosine formation in the ischemic rat heart: role of neuronal noradrenaline carrier and adrenergic receptors. J Mol Cell Cardiol. 1994;26:1321–1328. doi: 10.1006/jmcc.1994.1150. [DOI] [PubMed] [Google Scholar]
- 65.Scholz KP, Miller RJ. Analysis of adenosine actions on Ca2+ currents and synaptic transmission in cultured rat hippocampal pyramidal neurones. J Physiol (Lond) 1991;435:373–393. doi: 10.1113/jphysiol.1991.sp018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwanstecher C, Panten U. Tolbutamide- and diazoxide-sensitive K+ channels in neurons of substantia nigra pars reticulata. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:113–117. doi: 10.1007/BF00168546. [DOI] [PubMed] [Google Scholar]
- 67.Snyder SH. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- 68.Spuler A, Grafe P. Adenosine, “pertussis-sensitive” G-proteins, and K+ conductance in central mammalian neurones under energy deprivation. Neurosci Lett. 1989;98:280–284. doi: 10.1016/0304-3940(89)90414-x. [DOI] [PubMed] [Google Scholar]
- 69.Sturm CD, Frisella WA, Yoon KW. Attenuation of potassium cyanide-mediated neuronal cell death by adenosine. J Neurosurg. 1993;79:111–115. doi: 10.3171/jns.1993.79.1.0111. [DOI] [PubMed] [Google Scholar]
- 70.Sun M-K, Reis DJ. Hypoxia-activated Ca2+ currents in pacemaker neurones of rat rostral ventrolateral medulla in vitro. J Physiol (Lond) 1994;476:101–116. [PMC free article] [PubMed] [Google Scholar]
- 71.Thampy KG, Barnes EM., Jr Adenosine transport by primary cultures of neurons from chick embryo brain. J Neurochem. 1983;40:874–879. doi: 10.1111/j.1471-4159.1983.tb08061.x. [DOI] [PubMed] [Google Scholar]
- 72.Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol (Lond) 1995;487:37–50. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trussell LO, Jackson MB. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987;7:3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Wylen DGL, Park TS, Rubio R, Berne RM. Increases in cerebrospinal fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986;6:522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- 75.Way JL. Cyanide intoxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- 76.Wu L-G, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 77.Young JD, Jarvis SM. Nucleoside transport in animal cells. Biosci Rep. 1983;3:309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]
- 78.Yu SP, O’Malley MD, Adams PR. Regulation of M current by intracellular calcium in bullfrog sympathetic ganglion neurons. J Neurosci. 1994;14:3487–3499. doi: 10.1523/JNEUROSCI.14-06-03487.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Krnjević K. Whole-cell recording of anoxic effects on hippocampal neurons in slices. J Neurophysiol. 1993;69:118–127. doi: 10.1152/jn.1993.69.1.118. [DOI] [PubMed] [Google Scholar]
- 80.Zhu PJ, Krnjević K. Adenosine release is a major cause of failure of synaptic transmission during hypoglycaemia in rat hippocampal slices. Neurosci Lett. 1993;155:128–131. doi: 10.1016/0304-3940(93)90689-i. [DOI] [PubMed] [Google Scholar]
- 81.Zhu PJ, Krnjević K. Endogenous adenosine deaminase does not modulate synaptic transmission in rat hippocampal slices under normoxic or hypoxic condition. Neuroscience. 1994;63:489–497. doi: 10.1016/0306-4522(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 82.Zhu PJ, Krnjević K. Adenosine mediates cyanide (CN)-induced inhibition of hyperpolarization-activated inward current in whole-cell recorded hippocampal neurons in slices. Soc Neurosci Abstr. 1995;21:589. [Google Scholar]
- 83.Zhu PJ, Krnjević K (1997) Endogenous adenosine on membrane properties of CA1 neurons in rat hippocampal slices during normoxic and hypoxia. Neuropharmacology, in press. [DOI] [PubMed]