Abstract
The retinohypothalamic tract (RHT) relays photic information from the eyes to the suprachiasmatic nucleus (SCN). Activation of this pathway by light plays a role in adjusting circadian timing via a glutamatergic pathway at night. Here we report a new signaling pathway by which the RHT may regulate circadian timing in the daytime as well. We used dual immunocytochemistry for pituitary adenylate cyclase-activating peptide (PACAP) and the in vivotracer cholera toxin subunit B and observed intense PACAP-immunoreactivity (PACAP-IR) in retinal afferents in the rat SCN as well as in the intergeniculate leaflet (IGL) of the thalamus. This PACAP-IR in the SCN as well as in the IGL was nearly lost after bilateral eye enucleation. PACAP afferents originated from small ganglion cells distributed throughout the retina. The phase of circadian rhythm measured as SCN neuronal activity in vitro was significantly advanced (3.5 ± 0.4 hr) by application of 1 × 10−6m PACAP-38 during the subjective day [circadian time (CT)-6] but not at night (CT14 and CT19). The phase-shifting effect is channeled to the clock via a PACAP-R1 receptor, because mRNA from this receptor was demonstrated in the ventral SCN by in situhybridization. Furthermore, vasoactive intestinal peptide was nearly 1000-fold less potent in stimulating a phase advance at CT6. The signaling mechanism was through a cAMP-dependent pathway, which could be blocked by a specific cAMP antagonist, Rp-cAMPS. Thus, in addition to its role in nocturnal regulation by glutamatergic neurotransmission, the RHT may adjust the biological clock by a PACAP/cAMP-dependent mechanism during the daytime.
Keywords: suprachiasmatic nucleus, circadian, phase shifting, brain slice, PACAP receptor, cAMP, rat, intergeniculate leaflet, ganglion cells
Mammalian circadian rhythms are generated by an endogenous circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Turek, 1985; Klein et al., 1991; Harrington et al., 1994; Morin, 1994). Photic cues are transmitted to the SCN via the retinohypothalamic tract (RHT) (Moore and Lenn, 1972; Johnson et al., 1988). These inputs originate from a specific subset of retinal ganglion cells (Moore et al., 1995). Light-induced phase shifts involve excitatory neurotransmission in the SCN (Harrington et al., 1994;Morin, 1994). Glutamate is present in retinal afferents (Castel et al., 1993), and both NMDA and non-NMDA receptors are expressed in the SCN (van den Pol, 1994; Mikkelsen et al., 1995a; van den Pol et al., 1995). Furthermore, NMDA receptor activation, calcium influx, nitric oxide, and cGMP signaling lead to phase shifts in vivo and in vitro, and their effects are restricted to the subjective night (Prosser et al., 1989; Mikkelsen et al., 1993; Rea et al., 1993; Ding et al., 1994; Weber et al., 1995a,b).
Another system affecting the SCN is dependent on cAMP. Application of a cAMP analog in vitro produces prominent phase advances in the subjective day but not in the subjective night (Prosser and Gillette, 1989). The phase response curve is more similar to those produced by nonphotic arousal stimuli, such as injection of saline, forced activity in novelty, or exposure to a dark pulse in the subjective day (Mrosovsky and Salomon, 1987; Hastings, 1992; Sumova et al., 1994). Although neuropeptide Y (NPY) (Biello et al., 1994) and serotonin (Edgar et al., 1993) have been implicated in nonphotic shifts, the primary neurotransmitter that mediates daytime phase shifts through cAMP is not yet known.
One possible candidate is pituitary adenylate cyclase-activating peptide (PACAP), a powerful stimulator of adenylate cyclase activity (Miyata et al., 1989, 1990; Arimura, 1992). PACAP has been demonstrated in the CNS (Arimura et al., 1991; Ghatei et al., 1993; Masuo et al., 1993; Arimura and Shioda, 1995; Hannibal et al., 1995a,b; Mikkelsen et al., 1995b) as well as the peripheral nervous system of the rat (Arimura and Shioda, 1995; Frödin et al., 1995; Fahrenkrug and Hannibal, 1996). PACAP immunoreactivity (PACAP-IR) has been found in the SCN region, but no information is available regarding its role there (Masuo et al., 1993). PACAP stimulates three types of PACAP receptors (Ishihara et al., 1992; Hashimoto et al., 1993; Lutz et al., 1993; Pisegna and Wank, 1993; Spengler et al., 1993). Only the PACAP-R3 receptor mRNA has been demonstrated in the SCN (Lutz et al., 1993).
We used immunocytochemistry in combination with retrograde tracing from the retina and demonstrate here that PACAP is found in the RHT. By in situ hybridization we show that the PACAP-R1 receptor is expressed in the SCN. A possible role of PACAP in the circadian system was investigated in vitro using an SCN brain-slice preparation (Ding et al., 1994). The findings provide evidence that PACAP may be the primary transmitter that activates cAMP in the SCN during the daytime.
MATERIALS AND METHODS
Animals. Male Wistar rats (180–200 gm) housed in 12 hr light/dark cycle were used for the anatomical studies. All tracing experiments were conducted in the daytime. In one experiment, eight rats were bilaterally enucleated under tribromomethanol anesthesia. After 14 d, the brains of these animals and eight sham-operated animals were fixed as described below. The brains from the two groups were cut in 40-μm-thick sections on a freezing microtome and processed for immunocytochemistry as described below. In another experiment, six rats were given unilateral intraocular injections of cholera toxin subunit B (ChB), as described previously (Mikkelsen, 1992), and they survived for 8 d before fixation. The brains of animals injected with ChB were cut in 12-μm-thick sections on a cryostat and mounted on silane-coated glass slides for double immunofluorescence procedures (see below). In a third experiment, four animals received 1 gm/ml colchicine (Sigma, St. Louis, MO) in 10 μl of PBS in each vitreous body 24 hr before fixation to increase immunostaining. The retinas were removed as whole mounts after fixation and processed for immunocytochemistry.
Immunocytochemistry. On the day of fixation, the animals were anesthetized with tribromoethanol (20 mg/100 gm body weight) and perfused via the left ventricle with a room temperature solution of saline (0.9%) to which heparin (15,000 IU/l) was added (75–100 ml over 3 min). This perfusion was followed by 2% paraformaldehyde, 0.2% picric acid in 0.1 m sodium phosphate buffer, pH 7.2 (300 ml over 15 min). After fixation, the brains were removed rapidly and post-fixed in the same fixative for 24 hr. After post-fixation, the brains were equilibrated in PBS (0.05 m, pH 7.4) containing 30% sucrose for 48 hr at 4°C and then sectioned in 40-μm-thick sections in a freezing microtome. Whole mounts of the retina were processed like the free-floating brain sections, as described below.
Immunocytochemical visualization of PACAP-IR was carried out as described previously using the avidin–biotin bridge method (Hannibal et al., 1995a). The sections and the entire retina whole mounts were incubated for 24 hr with a monoclonal anti-PACAP antibody at 4°C. The specificity of the monoclonal antibody (code MabJHH1) has been characterized previously and displays equal affinity for PACAP-38 and PACAP-27, recognizing an epitope between amino acid 6 and 16, but it has no affinity for structurally related peptides such as vasoactive intestinal peptide (VIP) (Hannibal et al., 1995a). After incubation with the primary antibody, the sections were washed and then incubated for 60 min at room temperature in biotinylated rabbit anti-mouse diluted 1:600 (Dako, Glostrup, Denmark). The sections were then washed and finally incubated for 60 min at room temperature in ABC–streptavidin horseradish peroxidase complex diluted 1:125 (Dako). After the sections were washed, they were incubated for peroxidase activity in a solution of diaminobenzidine (DAB) for 15 min, and the reaction was terminated by washing the sections in excessive amounts of water. Finally, the sections and whole-mount retinas were mounted on gelatinized slides, dried, and embedded in Depex. Control sections for single antigen immunocytochemistry were processed routinely by either omitting or replacing the primary antibody with an equivalent concentration of either goat or rabbit preimmune serum or with antibody preabsorbed with PACAP-38 and PACAP-27 (20 μg/ml). All immunocytochemical staining was blocked by these procedures.
Immunocytochemical visualization of PACAP-IR and ChB was performed using the procedure described previously for visualization of two antigens (Hannibal et al., 1995b). Briefly, the sections were incubated in a mixture of monoclonal PACAP-antibody (supernatant diluted 1:2) and goat anti-ChB antiserum (List Biologicals, Campbell,CA) (diluted 1:750) for 24 hr at 4°C. After this incubation, sections were washed and incubated for 60 min in a mixture of biotinylated rabbit anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) diluted 1:50 and a fluorescein isothiocyanate-conjugated donkey anti-goat IgG (Nordic Immunology, Tilburg, The Netherlands) diluted 1:80. After they were washed, the sections were finally incubated for 60 min with a streptavidin–Texas Red-conjugated complex (Amersham, Birkerød, Denmark) diluted 1:50, washed, and mounted on gelatin-coated slides and embedded in Glycergel (Dako).
In situ hybridization histochemistry. In situ hybridization was performed using a slight modification of the previously described procedure (Fahrenkrug and Hannibal, 1996). Twelve-micrometer sections from three rats were used. The35S-UTP-labeled antisense and sense RNA probes were prepared by in vitro transcription using T7 (antisense) and SP6 (sense) RNA polymerase. The template containing a cDNA encoding the whole PACAP type I receptor sequence (nucleotide 20–1546) (Pisegna and Wank, 1993) was kindly given by Dr. Steven A. Wank. The plasmid (pGEM-3Z) was linearized with HindIII for antisense probe and with EcoR 1 for the sense probe. Transcription was performed at 37°C for 2 hr in 20 μl containing 5× TB buffer (Boehringer Mannheim, Mannheim, Germany), 25 mmdithiothreitol (DTT), 20 U RNasin (Amersham), 1.5 mmNTP-mix (Boehringer Mannheim), 40 U polymerase (T7, Stratagene, La Jolla, CA, or SP6, Boehringer Mannheim), and 2 μm35S-UTP (3000 mCi, Amersham). After the DNA template was removed by adding 1 μl of RNasin (30–40 U), 2 μl of tRNA (10 μg/μl), and 1 μl of DNase (Boehringer Mannheim), and incubation for an additional 15 min at 37°C, the probes were purified by water/phenol extraction followed by chloroform/isoamyl alcohol extraction, and finally NH4 acetate/ethanol precipitation. The labeled product was fragmented by incubation in hydrolysis buffer for 50 min at 60°C and used in a concentration of 1 × 107 cpm/ml. After hybridization overnight at 53°C, the sections were washed in 4× saline sodium citrate (4× SSC = 0.60m NaCl, 0.060 m sodium citrate), 4 mm DTT for a few minutes at room temperature followed by RNase treatment for 30 min (RNase A buffer, Sigma). After they were washed in 2× SSC, 2 mm DDT at room temperature for 60 min followed by washing in 0.01× SSC, 2 mm DDT at 60°C for 60 min and 1× SSC, 2 mm DDT for 10 min at room temperature, the sections were dehydrated through a series of alcohols. The slides were finally exposed to Amersham Hyperfilm for 3 weeks. For control purposes, hybridization was performed in parallel using an antisense and a sense probe on consecutive sections.
SCN brain slice and neurophysiological methods. These methods have been described in detail previously (Ding et al., 1994). Briefly, a 500 μm coronal hypothalamic slice containing the paired SCN was prepared at least 2 hr before the onset of the dark phase from 6- to 9-week-old inbred Long–Evans rats housed in a 12 hr light/dark lighting schedule. Brain slices survived for 3 d with continuous perfusion (34 ml/hr) by Earle’s balanced salt solution (EBSS) supplemented with 24.6 mm glucose, 26.2 mmsodium bicarbonate, and 5 mg/l of gentamicin and saturated with 95% O2/5% CO2 at 37°C (pH 7.4). The single-unit activity of SCN neurons was recorded extracellularly with a glass microelectrode, and running means were calculated to determine the peak of activity.
The effects of 10−6m PACAP-38 (Sigma), the dominant product of post-transcriptional processing of the PACAP precursor in the rat brain (Hannibal et al., 1995a), were examined at circadian time (CT)-6, -14, and -19. For treatments, the perfusion was stopped, and a 1.0 μl microdrop of test substance dissolved in EBSS was applied directly to the SCN. After 10 min, the SCN surface was washed with EBSS, perfusion was resumed, and the time of peak was assessed on the subsequent days. Extracellular single-unit activities were sampled throughout the SCN in brain slice in 10 sec intervals over 2 min and grouped into a 2 hr running average to determine the peak of firing activity (Ding et al., 1994). Because a PACAP-R3 (= VIP-R2) receptor has been demonstrated in the SCN (Lutz et al., 1993), we evaluated the effects of VIP at the time of maximal SCN sensitivity to PACAP. Dose–response curves were generated at CT6 by applying PACAP-38 at a dose from 10−10m to 10−5m or VIP from 10−7m to 10−4m for a 10 min pulse in a 1 μl droplet. For each dose, three to four experiments were performed. To investigate the specificity of the PACAP effect as well as the signaling pathway involved, a PACAP antagonist PACAP 6–38 (Robberecht et al., 1992) (10 μm) and a competitive inhibitor for cAMP-dependent processes, Rp-cAMPS (Rp) (Prosser et al., 1994) (10 μm), were added to EBSS 20 min before PACAP-38 was applied. Experiments were performed with the experimenter “blind” to the treatment protocol.
RESULTS
PACAP-IR is localized in the retina and in the retinorecipient SCN
With use of a specific antibody against PACAP, PACAP-IR was demonstrated within the RHT, in the retinal papilla (Fig.1A), in retinal ganglion cells (Fig.1B), and in nerve fibers and terminals primarily in the ventrolateral part of the SCN (Figs. 1C–E) in normal adult rats. The PACAP-IR retinal ganglion cells projecting to the circadian system belong to a homogeneous population of neurons spread throughout the retina and seemed to represent a population of small neurons resembling the type III, or W, cell type (Cooper et al., 1993;Moore et al., 1995). The individual neurons have an oval perikarya with two to four thin, sparsely branching processes (Fig.1B). The PACAP-IR ganglion cells were seen without colchicine, but the staining was less intense (data not shown).
Fig. 1.
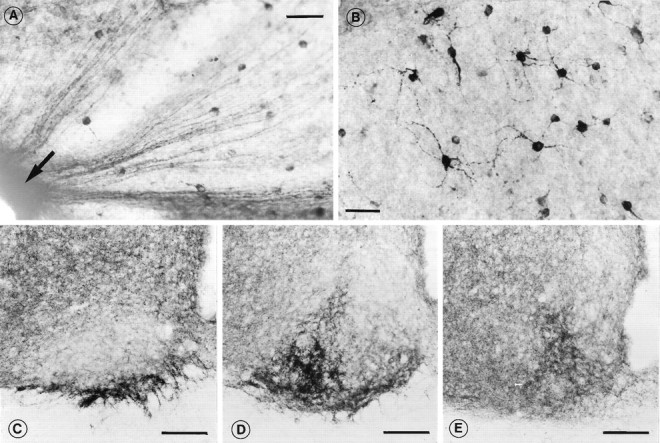
Demonstration of DAB-stained PACAP-IR ganglion cells of the retina (A, B) and PACAP-IR nerve fiber terminals in the SCN (C–E) using a monoclonal antibody against PACAP. A, Low-power photomicrograph of whole-mount retina demonstrating an accumulation of PACAP-IR fibers coursing into the retinal papilla; B, PACAP-IR retinal ganglion cells are distributed throughout the retina. The cells are mostly small with less extensive arborization. C–E, Three different levels (rostrocaudal) of the fibers in the retinorecipient area of the SCN. A dense accumulation of PACAP-IR nerve fibers is observed in the ventral and lateral part, overlapping with the retinal innervation. Scale bars: A, 100 μm;B, 50 μm; C–E, 100 μm.
Within the SCN, the exact position of PACAP terminals varied along the rostrocaudal axis of the nucleus and overlapped extensively with the retinorecipient area. In the rostral SCN, the PACAP-IR nerve fibers were located in the extreme ventral part of the nucleus, whereas in the middle and caudal SCN, the number of fibers increased and the location changed to more lateral and laterodorsal positions (Figs.1C–E).
PACAP innervation of the SCN and the intergeniculate leaflet (IGL)
To determine the extent of labeling attributable to retinal innervation, the distribution of PACAP was studied in normal and bilaterally enucleated rats. In the enucleated animals, the number of PACAP-IR nerve fibers in the SCN was markedly diminished. In particular, prominent reduction was observed in the retinorecipient area of enucleated animals (Fig.2A,B). Detectable levels of immunoreactivity were still found in the SCN inside and outside the retinorecipient area after enucleation. Simultaneous visualization of PACAP- and ChB-IR in the SCN using the anterograde tracer ChB revealed that the majority of PACAP-IR nerve fibers also exhibited ChB-IR (Fig.2C,D), giving further evidence that the nerve fibers originate from the ganglion cells in the retina. To determine whether PACAP-containing axons of retinal ganglion cells innervated the IGL, PACAP-IR in this structure was analyzed as well. A considerable plexus of PACAP-IR nerve fibers and varicose terminals, which overlapped extensively with the distribution of retinal afferents, was demonstrated in the IGL (Fig. 2E,F). After enucleation, fibers in the IGL almost completely disappeared (not shown).
Fig. 2.
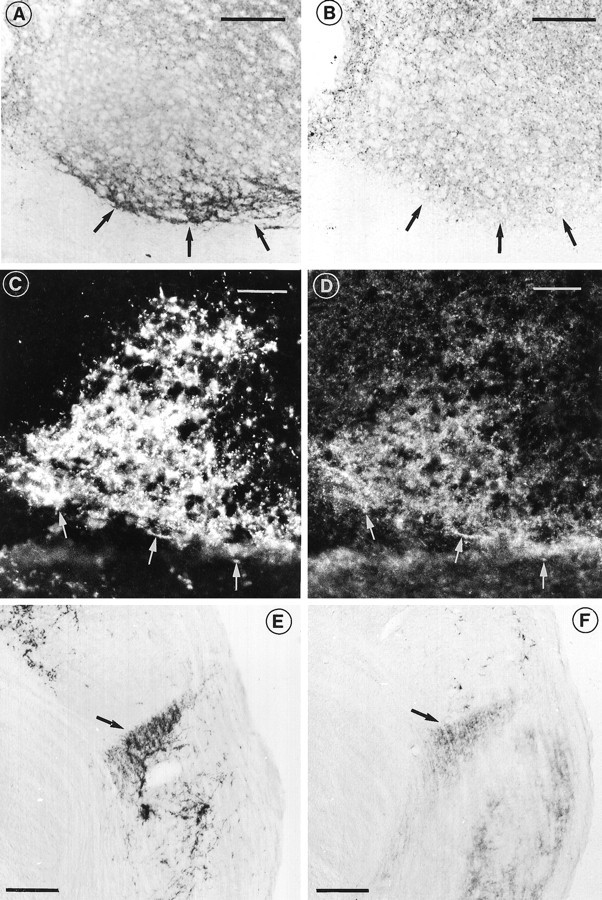
Demonstration of DAB-stained PACAP-IR nerve fibers in the ventral SCN of normal animals (A) and in enucleated animals (B). Arrows indicate the extent of the retinorecipient area, which showed a dramatic reduction of PACAP-IR fibers in this area after enucleation. Dual fluorescence immunocytochemistry (C, D) showing the distribution of ChB-IR (C) and PACAP-IR (D) in the SCN. The arrows point to positive elements that contain both ChB- and PACAP-IR. DAB-staining of the normal IGL showing a substantial number of fibers originating from the ipsilateral retina (E) and PACAP (F) observed in adjacent sections. Scale bars:A–D, 100 μm; E, F, 200 μm.
PACAP adjusts the phase of the circadian rhythm in the SCN
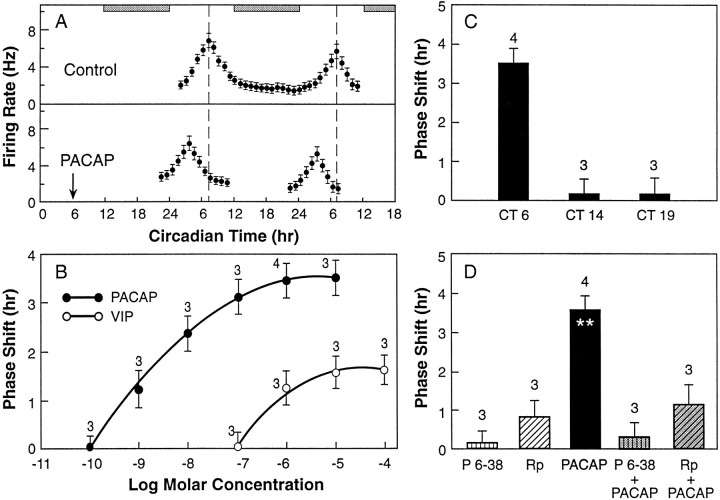
To determine the functional implications of PACAP innervation of the SCN, we assessed the effect of PACAP-38 on the phasing of the SCN rhythm of neuronal activity. Application of PACAP-38 altered the phase of the circadian rhythm of neuronal activity (Fig.3A). The phase shift occurred as a prominent advance of the activity peak by 3.5 ± 0.4 hr when PACAP-38 was applied in a 1 μl drop at CT6, mid-subjective daytime. (CT0 is defined as the time when light comes on in the donor colony.) No phase shift was demonstrated at CT14 or CT19 (Fig.3C). The phase-shifting effect was dose-dependent, with a half-maximal shift occurring in response to a microdrop of 3 × 10−9m of PACAP-38 (Fig. 3B). Notably, the 1 μl drop of PACAP-38 would be diluted significantly during diffusion into the SCN. Thus, the effective concentration is likely to be in the range seen in binding assays of PACAP receptors (Pisegna and Wank, 1993). The PACAP-induced phase shift was fully blocked by the specific peptide antagonist PACAP(6–38) (Fig. 3D), demonstrating the specificity of the PACAP signal.
Fig. 3.
A, PACAP directly resets the phase of the SCN circadian rhythm of neuronal activity. Top panel, Circadian rhythms of neuronal activity of the SCN in brain slice recorded from 112 units over 38 hr under constant conditions in vitro. The rhythm peaked in mid-subjective day at CT7, on both day 2 and day 3 in vitro.Bottom panel, Effect of PACAP applied at CT6 advanced the peak of the SCN activity rhythm by 3.5 hr. A 1 μl droplet of 1 × 10−6m PACAP-38 was applied directly to the SCN for 10 min, followed by a rinse in medium. Horizontal bars indicate subjective night. B, Dose–response curve for a 10 min pulse of 1 μl of PACAP-38 (closed circles) and VIP (open circles) to the SCN in vitro at CT6. Each data point represents the mean ± SD of three to four experiments, as indicated, measuring the time-of-peak as in Figure 3A. Half-maximal response was achieved at 3 × 10−9mPACAP and 7 × 10−7m VIP. Experiments were performed with the experimenter “blind” to the treatment protocol. C, Phase advance by PACAP depends on the circadian time of application to the SCN (dosage as in Fig.3A). Each data point represents three to four experiments as indicated. Phase advance is 3.5 ± 0.4 hr at CT6. No significant phase shift was detected at CT14 or CT19, points of maximal responsiveness to light and glutamate (Ding et al., 1994).D, The phase shift by PACAP was blocked by the PACAP receptor antagonist PACAP 6–38, and a competitive inhibitor for cAMP dependent processes, Rp-cAMPS. Brain slices were incubated for 20 min with 10 μm PACAP 6–38 or 10 μm Rp-cAMPS before PACAP application in a microdrop onto the SCN for 10 min. Each data point represents the mean ± SD of three to four experiments as indicated. Significant difference was found between PACAP- versus Rp-cAMPS-treated groups, and between PACAP and PACAP 6–38 + PACAP-treated and Rp-cAMPS + PACAP-treated groups, respectively. No significant difference was detected between PACAP 6–38, Rp-cAMPS, and antagonist + PACAP-treated groups. ** p ≤ 0.01.
The effects of PACAP on the SCN are channeled via a PACAP-R1 receptor and a cAMP-mediated pathway
By in situ hybridization histochemistry using an antisense cRNA probe, PACAP-R1 receptor mRNA was demonstrated in the ventral SCN (Fig. 4A). No signal was obtained with the sense probe (Fig. 4B). This demonstrates that the PACAP-selective type-1 receptor, which exhibits a 1000-fold lower affinity for VIP than for PACAP (Spengler et al., 1993), is expressed in the retinorecipient SCN. To examine whether this receptor mediates phase resetting of the biological clock, we investigated the effects of VIP in vitro. A PACAP-R3 receptor (= VIP type 2 receptor), which has equal affinities for either PACAP or VIP, had also been demonstrated in the SCN (Lutz et al., 1993). Therefore, we examined the response to VIP over a range of concentrations. VIP was 1000-fold less potent than PACAP at altering the phasing of the SCN circadian rhythm. The half-maximal response to VIP was calculated as a 0.75 hr phase advance to a microdrop containing 7 × 10−7m VIP. As can be seen in Figure3B, a shift of this magnitude would be produced by 7 × 10−10m PACAP.
Fig. 4.
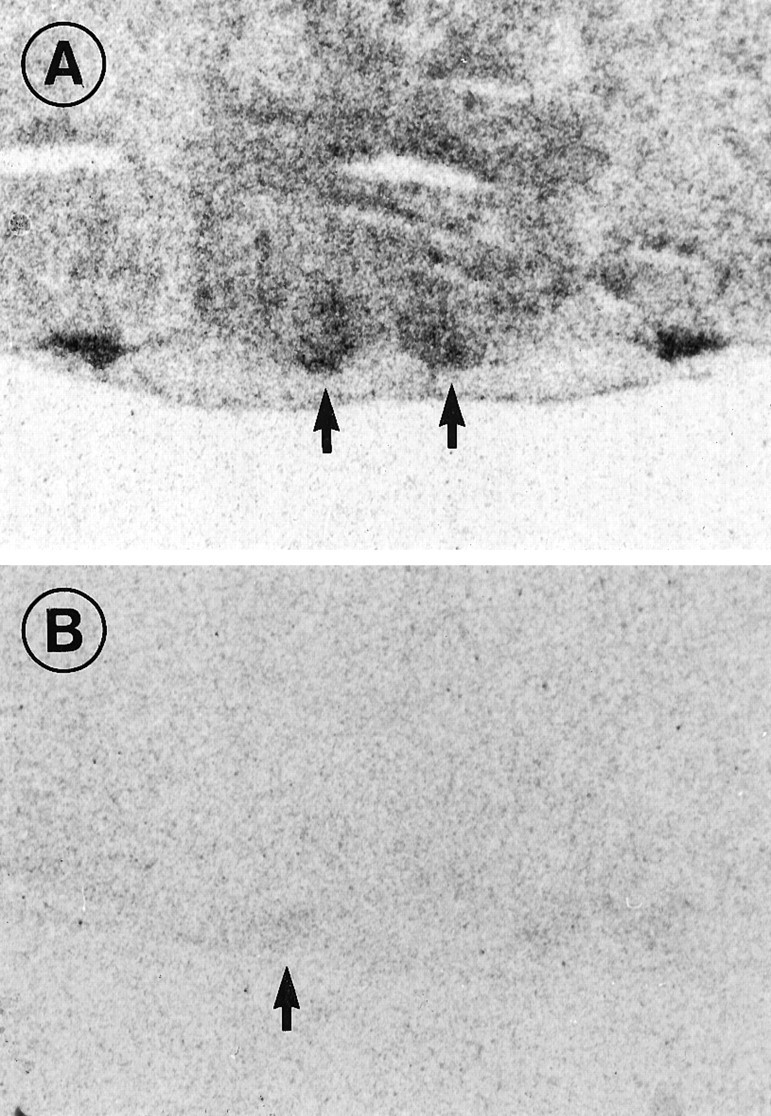
High accumulation of PACAP-R1 mRNA is present in the SCN (arrows) using in situhybridization with a cRNA-antisense probe (A). No signal could be obtained by the sense probes on consecutive sections containing the SCN (B).
To investigate the second messenger pathway activated by PACAP, we tested the effect of PACAP together with a competitive inhibitor for cAMP-dependent processes, Rp-cAMPS. Application of Rp-cAMPS before PACAP application completely blocked the phase advance of PACAP at CT6 (Fig. 3D), confirming that the PACAP-R1 receptor stimulates a second messenger pathway involving cAMP/protein kinase A.
Statistics
General linear regression for unbalanced ANOVA and post hoc test (Duncan) showed significant difference between the phase of SCN neuronal activity after PACAP treatment compared with either PACAP 6–38 peptide alone or in combination with PACAP-38 (p ≤ 0.01). The time-of-peak was not significantly different between PACAP 6–38 and PACAP 6–38 plus PACAP-treated groups. A significant difference was also found when the PACAP treatment group was compared with the Rp-cAMPS-treated group alone or in combination with PACAP (p ≤ 0.01). No significant difference was detected between Rp-cAMPS and Rp-cAMPS + PACAP-treated groups.
DISCUSSION
By combining both neuroanatomical and neurophysiological approaches, we have demonstrated that (1) PACAP-IR fibers project from diffuse retinal ganglion cells to two projection sites of the circadian system, the hypothalamic SCN and the thalamic IGL; (2) PACAP can reset the SCN circadian rhythm in daytime, but not at night; and (3) this effect is selective for PACAP over VIP and is channeled via a PACAP-R1 receptor through a cAMP-dependent pathway. These data suggest a prominent new role for PACAP in RHT signaling. The data support these findings, and their significance will be discussed in turn.
Localization of PACAP-IR to the RHT
The present experiments using a range of anatomical methods show that PACAP is localized in the RHT. First, PACAP-IR appears in diffusely distributed retinal ganglion cells of a stellate morphology, like those that give rise to the RHT (Moore et al., 1995). Second, the retinorecipient zone of the SCN comprises an area of the lateral and ventral parts in which the PACAP-IR fibers terminate. PACAP-IR is also localized in projections to another retinal recipient region of the thalamic lateral geniculate nucleus, the IGL, which is involved in circadian timing. Third, a substantial number of PACAP-IR fibers and terminal varicosities co-stored a neuronal tract tracer taken up by retinal ganglion cells. Finally, enucleation showed that nearly all PACAP-IR fibers disappear from the SCN and IGL; however, detectable levels of PACAP-IR were still found in the SCN inside and outside the retinorecipient area after enucleation, indicating that a minor afferent system originated from the brain. Furthermore, minor fibers containing PACAP-IR that was unaffected by enucleation were observed in other visual centers (not shown).
The pathway transmitting the light signal to the SCN consists of a subset of retinal photoreceptors and ganglion cells (Foster et al., 1991; Cooper et al., 1993; Moore et al., 1995), and it is considered to be a unique system in the retina related to regulation of circadian timing. These retinal ganglion cells are described as the type III, or W, cell type and are scattered within the retina. Whether all of the PACAP-positive ganglion cells are involved in circadian function remains to be established; however, their morphology with restricted dendritic arborization resembles the types identified by Moore et al. (1995), and the restricted loss of immunoreactivity in elements of the circadian timing system, such as the SCN and the IGL, after enucleation suggest that they belong exclusively to this function. On the other hand, whether there are more ganglion cells implicated in circadian function than these positive for PACAP cannot be determined from the present results. PACAP has been demonstrated previously only by immunochemical methods in extracts taken from the SCN (Masuo et al., 1993). These results correspond with the data presented in this study using immunocytochemistry. They differ from the findings presented byKöves et al. (1991), probably because of a lower avidity and/or different specificity of the antiserum used in those experiments. Our antibody has been characterized in detail previously (Hannibal et al., 1995a). A good correlation was found between the presence of PACAP-IR and PACAP mRNA in neurons of the paraventricular hypothalamic nucleus, demonstrating the specificity of the antibody used (Hannibal et al., 1995b).
Mechanism of PACAP signaling at the SCN
The present study provides evidence that PACAP adjusts the phase of the SCN through the PACAP-R1 receptor and a cAMP signaling pathway. First, neurons expressing PACAP-R1 mRNA are localized in the SCN. In addition to PACAP-R1, PACAP-R3 receptor (VIP-R2) mRNA is also expressed in the SCN (Lutz et al., 1993; Usdin et al., 1994). Notably, PACAP-R1 is more concentrated in the ventral than in the dorsomedial part of the SCN, whereas PACAP-R3 is primarily in dorsomedial aspects of the SCN (Lutz et al., 1993). Even though both receptors are coupled to cAMP cascades, PACAP-R1 has 1000-fold higher affinity for PACAP than for VIP, whereas the PACAP-R3 displays similar affinities for the two peptides (Hashimoto et al., 1993; Lutz et al., 1993; Pisegna and Wank, 1993; Spengler et al., 1993; Usdin et al., 1994). Accordingly, VIP was nearly 1000-fold less potent in stimulating a phase shift than PACAP, confirming that PACAP-R3 is not involved in this response.
This strongly implies that PACAP could stimulate cAMP production in SCN neurons after appropriate stimulation of the RHT. This is supported further by the findings that (1) Rp-cAMPS blocks the phase-shifting effect of PACAP and (2) the SCN exhibits similar phase-dependent sensitivity to PACAP and cAMP (Prosser and Gillette, 1989).
Phase-dependent sensitivity and relationship to rolesin vivo
The effects of PACAP on clock phasing could be produced at CT6, but not at CT14 or CT19. In other words, the receptivity of the clock to PACAP changes over the circadian cycle of the clock, even though the SCN is maintained in constant conditions in vitro. This restricted sensitivity to a signaling molecule follows the pattern of clock-controlled regulation of entrainment pathways now well documented for other regulators of circadian timing (Gillette et al., 1995;Gillette, 1996). The similarity between the phases of sensitivity to cAMP (Prosser and Gillette, 1989), serotonin (Medanic and Gillette, 1992), NPY (Medanic and Gillette, 1993), and PACAP in vitrosuggests that these sensitivities and neurotransmission may be coupled. Indeed, their sensitive periods appear in daytime. This pattern is in antiphase to the timing of clock sensitivity to acetylcholine and cGMP (Prosser and Gillette, 1989; Liu and Gillette, 1996) as well as to a pathway involving light, glutamate, NMDA receptor activation, NO donors, and transcriptional activation by CREB and Fos, which are effective at night (Rea et al., 1993; Ding et al., 1994, 1997).
Paradoxically, PACAP, which adjusts clock phasing in the daytime, is localized to types of cell and the pathway that mediates nocturnal phase resetting via glutamate. Our discovery of a peptide neurotransmitter within a photic sensory pathway whose primary neurotransmitter is an excitatory amino acid raises a question as to what stimulus conditions might regulate release of either or both of these neurotransmitters. Studies of pain sensory pathways, which also contain both classical and peptide neurotransmitters, provide a precedent for different stimulus conditions inducing different neurotransmitter release profiles (Hökfelt et al., 1980). We should not assume that PACAP and glutamate are necessarily colocalized, because two fiber tracts have been described within the hamster RHT (Treep et al., 1995). Presently, we do not know whether PACAP is released by retinal fibers in the SCN in response to light, in day and/or night, or whether it is co-released with glutamate. This should not exclude the possibility, however, that PACAP is released during subjective day by other photic changes (light to dark, dark to light, or changes in light intensity) and/or is released by other stimuli, or that PACAP is released during the subjective night by light to play a modulatory role in the context of other neurotransmitter activities.
Light by itself does not cause phase shifting in the daytime, whereas arousal stimuli, such as dark pulses and intense locomotor activity, produce prominent phase advances (Ellis et al., 1982; Mrosovsky, Salomon, 1987; Mrosovsky, 1995; Boulos and Rusak, 1996). It is considered that NPY released from the geniculohypothalamic tract is essential for generation of this type of phase shift (Biello et al., 1994) during subjective day, whereas NPY released at night has a modulatory function on light-induced phase shifts (Biello, 1995). Serotonin (5-hydroxytryptamine), which reaches the retinorecipient area of SCN mainly via projections from the median raphe nucleus (Meyer-Bernstein and Morin, 1996), induces significant phase advances during the subjective day at the time that PACAP is effective (Edgar et al., 1993), whereas serotonin seems to modulate light-induced phase shifts during the subjective night (Rea et al., 1994; Ying and Rusak, 1994). Localization of PACAP-IR in retinorecipient SCN, where NPY and serotonin projections terminate, suggests that an integration of these signals may occur in the SCN. PACAP in the RHT may either mediate or modulate the effect of signals initiated by these transmitters.
In summary, PACAP was demonstrated in projections from the retina to the SCN and IGL, two central sites that regulate the circadian system. In the SCN, PACAP was found to reset the phase of the biological clock through a PACAP-R1 receptor via a cAMP signaling pathway. The sensitivity of SCN to PACAP-induced phase shifting appeared in the subjective daytime. This implies strongly that PACAP could be a new regulator in the circadian system.
Footnotes
This study was supported by the Danish Medical Research Council, the Danish Biotechnology program for Cellular Communication, and Public Health Service (USA) Grant NS22155 from the National Institute of Neurological Disorders and Stroke (M.U.G.). J.D.M. is the recipient of a Hallas-Møller Research stipend from the NOVO Nordisk Foundation.
Correspondence should be addressed to Jens Hannibal, Department of Clinical Biochemistry, Bispebjerg Hospital, Bispebjerg Bakke 23, DK-2400 Copenhagen NV, Denmark.
REFERENCES
- 1.Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept. 1992;37:287–303. [PubMed] [Google Scholar]
- 2.Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 3.Arimura A, Somogyvari Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 4.Biello SM. Enhanced photic phase shifting after treatment with antiserum to neuropeptide Y. Brain Res. 1995;673:25–29. doi: 10.1016/0006-8993(94)01345-i. [DOI] [PubMed] [Google Scholar]
- 5.Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 6.Boulos Z, Rusak B. Circadian phase response curves for dark pulses in the hamster. J Comp Physiol. 1996;146:411–417. [Google Scholar]
- 7.Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper HM, Herbin M, Nevo E. Occular regression conceals adaptive progression of the visual system in a blind subterranean mammal. Nature. 1993;361:156–159. doi: 10.1038/361156a0. [DOI] [PubMed] [Google Scholar]
- 9.Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 10.Ding JM, Hurst WJ, Faiman LE, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation through light, glutamate and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgar DM, Miller JD, Prosser RA, Dean RR, Dement WC. Serotonin and the mammalian circadian system. II. Phase-shifting rat behavioral rhythms with serotonergic agonists. J Biol Rhythms. 1993;8:17–31. doi: 10.1177/074873049300800102. [DOI] [PubMed] [Google Scholar]
- 12.Ellis GB, Mcklveen RE, Turek FW. Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am J Physiol. 1982;242:R44–R50. doi: 10.1152/ajpregu.1982.242.1.R44. [DOI] [PubMed] [Google Scholar]
- 13.Fahrenkrug J, Hannibal J. PACAP in the rat female genital tract: effect of capsaicin. Neuroscience. 1996;73:1049–1060. doi: 10.1016/0306-4522(96)00082-6. [DOI] [PubMed] [Google Scholar]
- 14.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 15.Frödin M, Hannibal J, Wulff BS, Gammeltoft S, Fahrenkrug J. Neuronal localization of pituitary adenylate cyclase activating polypeptide 38 in the adrenal medulla and growth-inhibitory effect on chromaffin cells. Neuroscience. 1995;65:599–608. doi: 10.1016/0306-4522(94)00522-7. [DOI] [PubMed] [Google Scholar]
- 16.Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol. 1993;136:159–166. doi: 10.1677/joe.0.1360159. [DOI] [PubMed] [Google Scholar]
- 17.Gillette MU (1996) Regulation of entrainment pathways by the suprachiasmatic circadian clock: sensitivities to second messengers. In: Progress in brain research (Buijs R, Romijn H, Pennartz C, Mirmiran M, eds), pp 119–130. [DOI] [PubMed]
- 18.Gillette MU, Medanic M, McArthur AJ, Liu C, Ding JM, Faiman LE, Weber ET, Tcheng TK, Gallman EA. Circadian clocks and their adjustment (Ciba Symposium 183), pp 134–153. Wiley; Chichester, UK: 1995. Intrinsic neuronal rhythms in the suprachiasmatic nuclei and their adjustment. [DOI] [PubMed] [Google Scholar]
- 19.Hannibal J, Mikkelsen JD, Clausen H, Holst JJ, Wulff BS, Fahrenkrug J. Gene expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat hypothalamus. Regul Pept. 1995a;55:133–148. doi: 10.1016/0167-0115(94)00099-j. [DOI] [PubMed] [Google Scholar]
- 20.Hannibal J, Mikkelsen JD, Fahrenkrug J, Larsen PJ. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology. 1995b;136:4116–4124. doi: 10.1210/endo.136.9.7649120. [DOI] [PubMed] [Google Scholar]
- 21.Harrington ME, Rusak B, Mistlberger RE. Anatomy and physiology of the mammalian circadian system. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. W.B. Saunders; Philadelphia: 1994. pp. 286–301. [Google Scholar]
- 22.Hashimoto H, Ishihara T, Shigemoto R, Mori K, Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993;11:333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- 23.Hastings MH, Mead SM, Vindlacheruvu RR, Ebling FJ, Maywood ES, Grosse J. Non-photic phase shifting of the circadian activity rhythm of Syrian hamster: the relative potency of arousal and melatonin. Brain Res. 1992;591:20–26. doi: 10.1016/0006-8993(92)90973-d. [DOI] [PubMed] [Google Scholar]
- 24.Hökfelt T, Johansson O, Ljungdahl Å, Lundberg J, Schultzberg M. Peptidergic neurones. Nature. 1980;284:515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 27.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s Clock. Oxford UP; New York: 1991. [Google Scholar]
- 28.Köves K, Arimura A, Gorcs TG, Somogyvari Vigh A. Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology. 1991;54:159–169. doi: 10.1159/000125864. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Gillette MU. Cholinergic regulation of the SCN circadian rhythm through a muscarinic mechanism at night. J Neurosci. 1996;16:744–751. doi: 10.1523/JNEUROSCI.16-02-00744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 31.Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M. Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res. 1993;602:57–63. doi: 10.1016/0006-8993(93)90241-e. [DOI] [PubMed] [Google Scholar]
- 32.Medanic M, Gillette MU. Serotonin regulates the phase of the rat suprachiasmatic circadian pacemaker in vitro only during the subjective day. J Physiol (Lond) 1992;450:629–642. doi: 10.1113/jphysiol.1992.sp019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medanic M, Gillette MU. Suprachiasmatic circadian pacemaker of rat shows two windows of sensitivity to neuropeptide Y in vitro. Brain Res. 1993;620:281–286. doi: 10.1016/0006-8993(93)90166-k. [DOI] [PubMed] [Google Scholar]
- 34.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikkelsen JD. Visualization of efferent retinal projections by immunohistochemical identification of cholera toxin subunit B. Brain Res Bull. 1992;28:619–623. doi: 10.1016/0361-9230(92)90112-b. [DOI] [PubMed] [Google Scholar]
- 36.Mikkelsen JD, Larsen PJ, Ebling FJ. Distribution of N-methyl d-aspartate (NMDA) receptor mRNAs in the rat suprachiasmatic nucleus. Brain Res. 1993;632:329–333. doi: 10.1016/0006-8993(93)91171-n. [DOI] [PubMed] [Google Scholar]
- 37.Mikkelsen JD, Larsen PJ, Mick G, Vrang N, Ebling FJ, Maywood ES, Hastings MH, Moller M. Gating of retinal inputs through the suprachiasmatic nucleus: role of excitatory neurotransmission. Neurochem Int. 1995a;27:263–272. doi: 10.1016/0197-0186(95)00039-b. [DOI] [PubMed] [Google Scholar]
- 38.Mikkelsen JD, Hannibal J, Fahrenkrug J, Larsen PJ, Olcese J, McArdle C. Pituitary adenylate cyclase activating peptide-38 (PACAP-38), PACAP-27, and PACAP related peptide (PRP) in the rat eminence and pituitary. J Neuroendocrinol. 1995b;7:47–55. doi: 10.1111/j.1365-2826.1995.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 39.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 40.Miyata A, Jiang LJ, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- 41.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 42.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 43.Morin LP. The circadian visual system. Brain Res. 1994;19:102–127. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 44.Mrosovsky N. A non-photic gateway to the circadian clock of hamsters. Ciba Foundation Symposium. 1995;183:154–167. doi: 10.1002/9780470514597.ch9. [DOI] [PubMed] [Google Scholar]
- 45.Mrosovsky N, Salomon PA. A behavioral method for accelerating re-entrainment of rhythms to new light-day cycles. Nature. 1987;330:372–373. doi: 10.1038/330372a0. [DOI] [PubMed] [Google Scholar]
- 46.Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci USA. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prosser RA, McArthur AJ, Gillette MU. cGMP induces phaseshifts of a mammalian circadian pacemaker at night, in antiphase to cAMP effects. Proc Natl Acad Sci USA. 1989;86:6812–6815. doi: 10.1073/pnas.86.17.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prosser RA, Heller HC, Miller JD. Serotonergic phase advances of the mammalian circadian clock involve protein kinase A and K+ channel opening. Brain Res. 1994;644:67–73. doi: 10.1016/0006-8993(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 50.Rea MA, Buckley B, Lutton LM. Local administration of EAA antagonists blocks light-induced phase shifts and c-fos expression in hamster SCN. Am J Physiol. 1993;265:R1191–R1198. doi: 10.1152/ajpregu.1993.265.5.R1191. [DOI] [PubMed] [Google Scholar]
- 51.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robberecht P, Gourlet P, de Neef P, Woussen Colle MC, Vandermeers Piret MC, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes: discovery of PACAP (6–38) as a potent antagonist. Eur J Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- 53.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 54.Sumova A, Ebling FJ, Maywood ES, Herbert J, Hastings MH. Non-photic circadian entrainment in the Syrian hamster is not associated with phosphorylation of the transcriptional regulator CREB within the suprachiasmatic nucleus, but is associated with adrenocortical activation. Neuroendocrinology. 1994;59:579–589. doi: 10.1159/000126708. [DOI] [PubMed] [Google Scholar]
- 55.Treep JA, Abe H, Rusak B, Goguen DM. Two distinct retinal projections to the hamster suprachiasmatic nucleus. J Biol Rhythms. 1995;10:299–307. doi: 10.1177/074873049501000403. [DOI] [PubMed] [Google Scholar]
- 56.Turek FW. Circadian neural rhythms in mammals. Annu Rev Physiol. 1985;47:49–64. doi: 10.1146/annurev.ph.47.030185.000405. [DOI] [PubMed] [Google Scholar]
- 57.Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distribution. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- 58.van den Pol AN. Metabotropic glutamate receptor mGluR1 distribution and ultrastructural localization in hypothalamus. J Comp Neurol. 1994;349:615–632. doi: 10.1002/cne.903490409. [DOI] [PubMed] [Google Scholar]
- 59.van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995;362:134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- 60.Weber ET, Gannon RL, Rea MA. cGMP-dependent protein kinase inhibitor blocks light-induced phase advances of circadian rhythms in vivo. Neurosci Lett. 1995a;197:227–230. doi: 10.1016/0304-3940(95)11961-u. [DOI] [PubMed] [Google Scholar]
- 61.Weber ET, Gannon RL, Michel AM, Gillette MU, Rea MA. Nitric oxide inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res. 1995b;692:137–142. doi: 10.1016/0006-8993(95)00685-j. [DOI] [PubMed] [Google Scholar]
- 62.Ying SW, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]