Abstract
Most autosomal dominant inherited forms of early onset Alzheimer’s disease (AD) are caused by mutations in the presenilin-1 (PS-1) gene on chromosome 14. PS-1 is an integral membrane protein with six to nine membrane-spanning domains and is expressed in neurons throughout the brain wherein it is localized mainly in endoplasmic reticulum (ER). The mechanism or mechanisms whereby PS-1 mutations promote neuron degeneration in AD are unknown. Recent findings suggest links among deposition of amyloid β-peptide (Aβ), oxidative stress, disruption of ion homeostasis, and an apoptotic form of neuron death in AD. We now report that expression of the human PS-1 L286V mutation in PC12 cells increases their susceptibility to apoptosis induced by trophic factor withdrawal and Aβ. Increases in oxidative stress and intracellular calcium levels induced by the apoptotic stimuli were exacerbated greatly in cells expressing the PS-1 mutation, as compared with control cell lines and lines overexpressing wild-type PS-1. The antiapoptotic gene product Bcl-2 prevented apoptosis after NGF withdrawal from differentiated PC12 cells expressing mutant PS-1. Elevations of [Ca2+]i in response to thapsigargin, an inhibitor of the ER Ca2+-ATPase, were increased in cells expressing mutant PS-1, and this adverse effect was abolished in cells expressing Bcl-2. Antioxidants and blockers of calcium influx and release from ER protected cells against the adverse consequences of the PS-1 mutation. By perturbing cellular calcium regulation and promoting oxidative stress, PS-1 mutations may sensitize neurons to apoptotic death in AD.
Keywords: Alzheimer’s disease, antioxidant, bcl-2, dantrolene, endoplasmic reticulum, fura-2, nerve growth factor
Alzheimer’s disease (AD) is a progressive and always fatal neurodegenerative disorder characterized by the death of neurons in brain regions involved in learning and memory processes (for review, see Selkoe, 1989). Accumulations of insoluble fibrillar aggregates of a protein called amyloid β-peptide (Aβ) are implicated in the pathogenesis of AD. Aβ is associated with degenerating neurons in AD brain, and mutations in the gene for the amyloid precursor protein (the source of Aβ) cause a small percentage of cases of inherited familial AD (for review, see Mullan and Crawford, 1993). Moreover, mice genetically engineered to produce mutated human amyloid precursor protein exhibit Aβ deposition and cognitive impairments (Games et al., 1995; Hsaio et al., 1996). Aβ damages and kills cultured neurons by a mechanism involving oxidative stress and disruption of cellular calcium homeostasis (Mattson et al., 1992; Behl et al., 1994; Fukuyama et al., 1994; Goodman and Mattson, 1994; Mark et al., 1995, 1997a). Apoptosis is a form of “programmed” cell death characterized by plasma membrane phospholipid alterations, cell shrinkage, and nuclear DNA condensation and fragmentation (Bredesen, 1995; Thompson, 1995). Aβ induces apoptosis in cultured neurons (Forloni et al., 1993; Loo et al., 1993), and studies of postmortem brain tissue suggest that neuronal apoptosis occurs in AD (Su et al., 1994; Smale et al., 1995). The molecular and cellular mechanisms that predispose neurons to apoptotic death in AD are unknown.
An important step toward elucidating the cause or causes of neuron degeneration in AD was made with the identification of the genes responsible for the majority of cases of autosomal dominant inherited forms of early onset AD. The genes, called presenilin-1 (PS-1; chromosome 14) and presenilin-2 (PS-2; chromosome 1), encode proteins predicted to be integral membrane proteins with six to nine membrane-spanning domains (Levy-Lahad et al., 1995; Rogaev et al., 1995; Sherrington et al., 1995; Doan et al., 1996; Li and Greenwald, 1996). Immunohistochemical analyses indicate that PS-1 is expressed in neurons throughout the brain (Cook et al., 1996; Cribbs et al., 1996;Elder et al., 1996); PS-1 has been localized to both degenerating (Murphy et al., 1996) and nondegenerating (Giannakopoulos et al., 1997) neurons in AD brain. In cultured cells PS-1 localizes to subcellular compartments and seems to be at particularly high levels in the endoplasmic reticulum (ER) (Kovacs et al., 1996; Walter et al., 1996). PS-1 can be processed endoproteolytically (Thinakaran et al., 1996), although the significance of such processing is unknown. Because PS-1 mutations account for the majority of cases of inherited early onset forms of AD, understanding the normal functions of PS-1 and how PS-1 mutations promote neuron degeneration are central issues in the AD field. We report that expression of a human PS-1 mutation in PC12 cells increases their vulnerability to apoptosis induced by trophic factor withdrawal and Aβ. The mechanism whereby mutant PS-1 promotes apoptosis seems to involve disruption of calcium homeostasis and increased oxidative stress.
MATERIALS AND METHODS
Expression of wild-type and mutant PS-1 in PC12 cells.Rat pheochromocytoma (PC12) cells (Black and Greene, 1982) were maintained at 37°C (5% CO2 atmosphere) in RPMI-1640 medium supplemented 10% with heat-inactivated horse serum and 5% with heat-inactivated fetal bovine serum. A full-length human PS-1 cDNA and a PS-1 cDNA containing the L286V mutation were cloned into either the expression vector pTRE in the Tet-off expression system (Clontech, Cambridge, UK) or pRc/CMV to produce pTRE-PS1 and pTRE-PS1L286V or pCMV-PS1 and pCMV-PS1L286V, where, in both cases, the expression of PS-1 and PS-1 L286V cDNAs is under the control of CMV promoter. PC12 cells were transfected with Lipofectamine (Life Technologies, Gaithersburg, MD). Stable expression of PS-1 L286V in PC12 cells with the pRc/CMV vector did not affect cell viability significantly during the G418 selection procedure. Double-stable PC12 cell lines in which PS-1 and PS-1 L286V expression could be suppressed or induced were established by first generating stable lines expressing the Tet-off system (cells were selected for 4 weeks in the presence of 0.8 mg/ml of G418). G418-resistant clones were isolated and cotransfected with the response plasmids pTRE-PS1 or pTRE-PS1L286V and pTK-Hyg. Cells were grown in selection medium containing 0.4 mg/ml hygromycin, and stable clones were isolated after 4 weeks and screened for PS-1 expression in the presence or absence of 2 μg/ml Tet by Western blot or RT-PCR. Resulting double-stable cell lines that exhibited high levels of expression after removal of tetracycline were used for subsequent experiments. A PC12 cell line overexpressing Bcl-2 (a generous gift from D. Bredesen, The Burnaham Institute, La Jolla, CA; Kane et al., 1993) was used to generate stable lines expressing the Tet-off system as described above. The latter lines then were cotransfected with the response plasmids pTRE-PS1 or pTRE-PS1L286V and pTK-Hyg, and stable lines exhibiting high levels of expression of wild-type and mutant PS-1 were used for experiments.
RT-PCR analysis was performed as described previously (Guo et al., 1996). Briefly, mRNA from the cultured cells was isolated and reverse-transcribed via the reverse transcription system (Promega, Madison, WI) and the 3′ primer 5′-GCTTCCCATTCCTCACTGAA-3′. cDNA (2.5 μl) was used as a template in a 50 μl PCR, using 15–40 cycles of 94°C (1 min), 60°C (2 min), and 72°C (2 min) with a final extension time of 10 min at 72°C. Reaction mixtures were as recommended for Taq polymerase (Perkin-Elmer Cetus, Oak Brook, IL), except that Taq was added after the mixtures were heated to 95°C for 7 min. The 3′ primer used was the oligonucleotide used to prime the cDNA synthesis; the 5′ primer was 5′- GTGGCTGTTTTGTGTCCGAA-3′. The PCR products were resolved and visualized by electrophoresis in 3% agarose gel stained with ethidium bromide. Because the Leu to Val mutation at codon 286 creates a PvuII site, the wild-type RT-PCR product could not be cut by PvuII and generated a single 251-bp fragment, whereas the mutation resulted in PvuII cleavage of the product into 79 and 172 bp fragments.
Experimental treatments. Cells were differentiated to a neuron-like phenotype by incubation in medium with reduced serum concentration (2% fetal bovine serum) and containing 50 ng/ml nerve growth factor (NGF) (Black et al., 1982). Immediately before experimental treatment the medium was replaced with Locke’s solution containing (in mm): NaCl 154, KCl 5.6, CaCl22.3, MgCl2 1.0, NaHCO3 3.6, glucose 5, and HEPES 5, pH 7.2. Serum withdrawal from undifferentiated PC12 cells and NGF withdrawal from differentiated PC12 cells were accomplished by repeated washing of cells with Locke’s solution. Synthetic Aβ25–35 was purchased from Bachem (Torrence, CA), and stocks were prepared at a concentration of 1 mm in water and allowed to incubate overnight at 37°C before addition to cultures. Nifedipine, sodium dantrolene, thapsigargin, vitamin E, and propyl gallate were purchased from Sigma (St. Louis, MO) and prepared as 500× stocks in ethanol.
Generation of PS-1 antibodies and Western blot analysis.Affinity-purified polyclonal antibody was isolated from serum of rabbits injected with a synthetic peptide with a sequence (NH2-NDDGGFSEEWEAQRD-COOH) corresponding to amino acids 331–345 of the loop region of human PS-1. Preliminary studies showed that this antibody recognizes both wild-type and PS-1 L286V. For Western blot analysis solubilized cell proteins were separated by electrophoresis in a 12% polyacrylamide gel, transferred to a nitrocellulose sheet, and immunoreacted with PS-1 antibody (1:100). The nitrocellulose sheet was processed further with HRP-conjugated anti-mouse secondary antibody and a chemiluminescence detection method (Amersham, Arlington Heights, IL).
Analyses of cell death and apoptosis. Quantification of LDH levels in culture medium was done as described previously (Bruce et al., 1996). Levels of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction, a measure of mitochondrial redox status and function (Shearman et al., 1994), were quantified as described previously (Mattson et al., 1995). Briefly, MTT solution (5 mg/ml PBS) was added to cultures (1:10, v:v; MTT solution/culture medium) and allowed to incubate for 3 hr. The cells were washed three times with Locke’s solution and solubilized in dimethylsulfoxide; the absorbance in each culture well was quantified with a plate reader. Methods used to establish apoptotic cell death included Hoescht and propidium iodide staining of DNA and suppression of cell death by macromolecular synthesis inhibitors. For Hoescht and propidium iodide staining, cells were fixed in 4% paraformaldehyde, membranes were permeabilized with 0.2% Triton X-100, and cells were stained with the fluorescent DNA-binding dyes Hoescht 33342 or propidium iodide, as described previously (Mark et al., 1995; Kruman et al., 1996). Hoescht-stained cultures were used to quantify the percentage of “apoptotic” cells; cells with condensed and fragmented DNA were considered apoptotic, whereas cells in which the DNA was distributed diffusely and uniformly throughout the nucleus were considered not apoptotic. Cells were visualized under epifluorescence illumination (340 nm excitation and 510 nm barrier filter) with a 40× oil immersion objective. Cells were counted in four random 40× fields per culture; counts were made without knowledge of cell type or treatment history. Images of propidium iodide-stained cells were acquired with a confocal laser scanning microscope (488 nm excitation and 510 nm barrier filter; Molecular Dynamics, Sunnyvale, CA) with a 60× oil immersion objective.
Measurements of intracellular peroxide and calcium levels.Peroxide levels were measured by using the dye 2,7-dichlorofluorescein diacetate (DCF) as described previously (Goodman and Mattson, 1994; Mattson et al., 1995). Ratiometric imaging of the calcium indicator dye fura-2 was performed as described previously (Mattson et al., 1992, 1993a). For measurements of DCF fluorescence and [Ca2+]i after exposure to Aβ, cells were loaded with DCF or fura-2 and maintained in the presence of Aβ during imaging.
RESULTS
PC12 cells expressing mutant PS-1 exhibit increased vulnerability to apoptosis induced by serum withdrawal and amyloid β-peptide
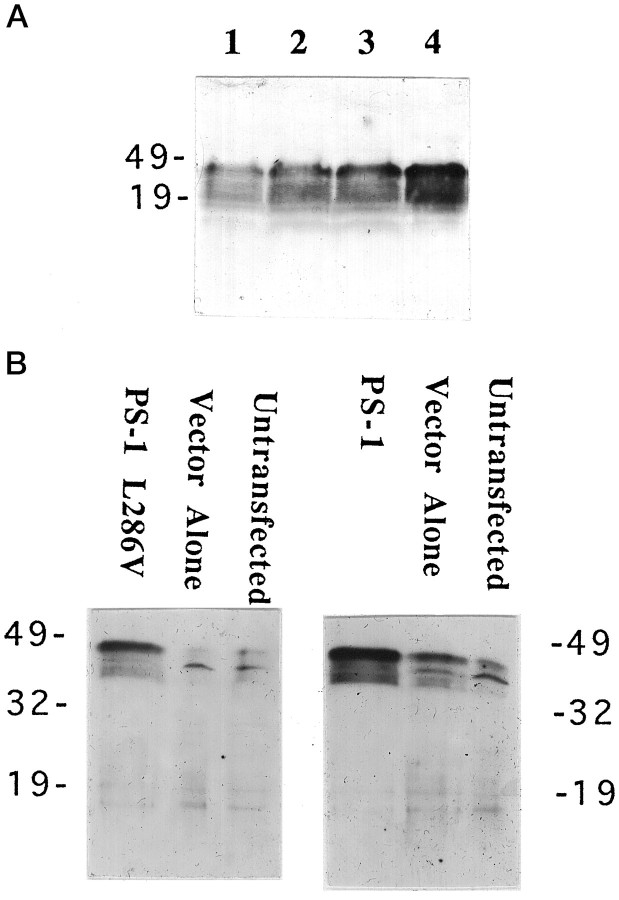
Rat neural (PC12) cells were stably transfected with a DNA construct encoding PS-1 containing the 286 leu–val mutation (L286V), and additional lines were transfected with wild-type PS-1 or vector alone. RT-PCR analysis showed that PS-1 and PS-1 L286V mRNAs were expressed at variable levels in each of the transfected cell lines isolated (data not shown) (cf. Guo et al., 1996). Levels of PS-1 expression were established in Western blot analyses (Fig.1); clonal lines expressing moderately high levels of wild-type PS-1 (n = 3) or PS1L286V (n = 3), one vector-transfected line, and the untransfected parent cell line were chosen for use in the present study. Densitometric analyses of Western blots showed that the levels of expression of wild-type and mutant PS-1 in the clones chosen were very similar and were at least five times greater than the background level of endogenous PS-1 (Fig.1B). Under the normal culture maintenance conditions, overexpression of wild-type or mutant PS-1 at the levels of the lines used in the present study did not seem to affect cell survival or growth.
Fig. 1.
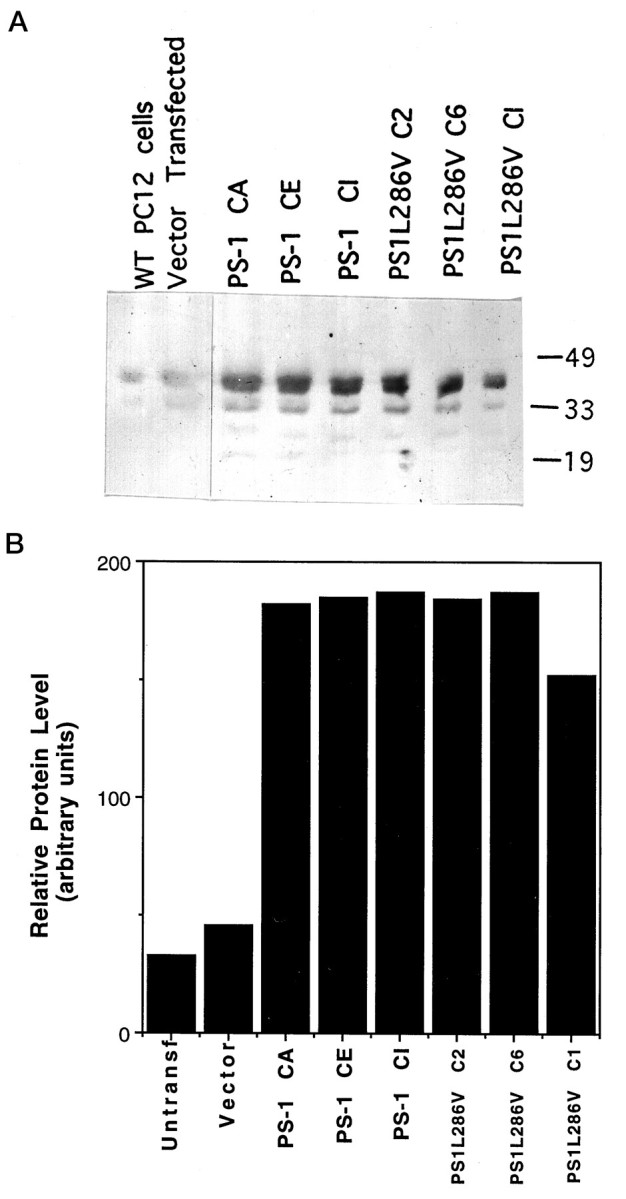
Expression of wild-type and mutant PS-1 in PC12 cells. A, Western blot showing PS-1 protein in untransfected and vector-transfected PC12 cells and in three different lines of cells expressing wild-type (PS-1) and mutant (PS1L286V) PS-1. Equivalent amounts of protein (50 μg/lane) from cell homogenates of the indicated cell lines (untransfected cells, vector-transfected cells, PS-1, and PS1L286V) were separated by SDS-PAGE, transferred to nitrocellulose, and probed with PS-1 antibody. Similar to results of other investigators (Thinakaran et al., 1996), in addition to recognizing full-length PS-1 (46 kDa band), our anti-loop PS-1 antibody also recognized presumptive proteolytic products of PS-1 ∼32 and 19 kDa. B, Densitometric analysis of relative levels of PS-1 protein in PC12 cell lines stably expressing wild-type (PS-1) and mutant (PS1L286V) PS-1. Note that basal PS-1 levels are relatively low in PC12 cells and that levels of expression of wild-type and mutant PS-1 were similar among the lines shown.
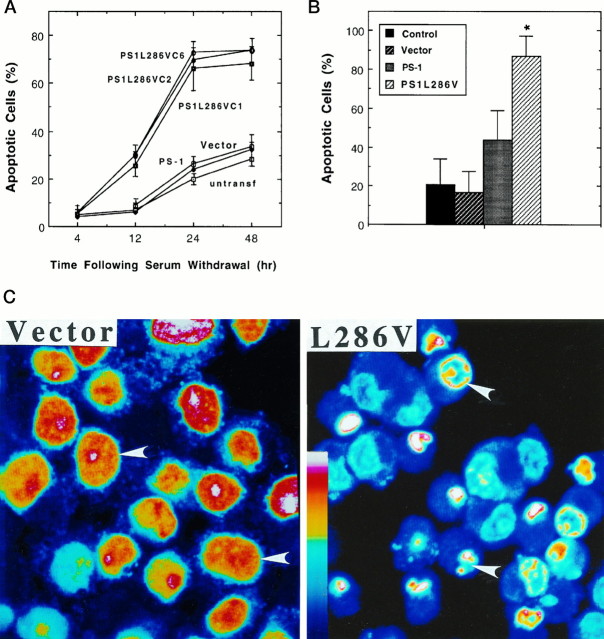
To determine whether the expression of PS-1 L286V affected cell death after trophic factor withdrawal, we deprived various lines of undifferentiated PC12 cells of serum, an insult previously shown to induce apoptosis (Bastitatou and Greene, 1991; Rukenstein et al., 1991). In untransfected and vector-transfected PC12 cells there was a progressive appearance of cells with apoptotic nuclei that occurred between 12 and 48 hr after serum withdrawal (Fig.2A). In PC12 cells expressing PS-1 L286V there was a dramatic increase in the numbers of apoptotic cells present at 12, 24, and 48 hr after serum withdrawal (Fig.2A,C). In another set of experiments cells were differentiated into a neuron-like phenotype by chronic exposure to NGF, and then the NGF was withdrawn. Apoptosis induced by NGF withdrawal in cell lines expressing mutant PS-1 was significantly greater than in untransfected cells, vector-transfected cells, and cells overexpressing wild-type PS-1 (Fig. 2B). NGF withdrawal induced apoptosis in ∼20% of cells in untransfected and vector-transfected cell lines and in ∼80% of the cells in lines expressing mutant PS-1. The level of apoptosis after NGF withdrawal in cells overexpressing wild-type PS-1 was somewhat higher than that in vector-transfected and untransfected lines, although the difference did not reach statistical significance (Fig. 2B).
Fig. 2.
PC12 cells expressing PS-1 L286V mutation exhibit increased vulnerability to apoptosis induced by trophic factor withdrawal. A, The percentages of cells exhibiting DNA condensation and fragmentation (apoptotic cells) were determined in Hoescht-stained cultures at the indicated times after serum withdrawal from undifferentiated PC12 cell lines: untransf, untransfected cells; Vector, cells transfected with empty vector; PS-1, cells transfected with wild-type PS-1 (pooled data from three different lines; C1, C2, and C6); and three different lines expressing mutant PS-1 (PS1L286V). Values represent the mean and SD of determinations made in four separate cultures (200 cells counted/culture). Values for each of the three cell lines expressing L286V were greater than corresponding values for untransfected cultures and cultures transfected with vector or wild-type PS-1 (12 hr time point, p < 0.05; 24 and 48 hr time points,p < 0.001); ANOVA with Scheffé’spost hoc tests. B, Control untransfected PC12 cells (Control) and PC12 cells transfected with empty vector (Vector), wild-type PS-1 (PS-1), or mutant PS-1 (PS1L286V) were differentiated in the presence of NGF (data pooled from analyses on all three lines expressing wild-type PS-1 and all three lines expressing PS-1 L286V; compare with Fig. 1). NGF was withdrawn, and 48 hr later the percentages of apoptotic cells in each culture were determined. Values represent the mean and SD of determinations made in four separate cultures. *p < 0.01 compared with each of the other values; ANOVA with Scheffé’s post hoc tests. C, Confocal images of propidium iodide fluorescence in vector-transfected and L286V-transfected PC12 cells 24 hr after serum withdrawal. Fluorescence intensity is depicted in pseudocolor according to the color scale bar. Note that DNA is distributed diffusely throughout the nuclei of most vector-transfected cells, whereas DNA is fragmented to varying extents in most cells expressing PS-1 L286V (arrowheads).
Previous studies showed that Aβ can induce apoptosis in cultured primary neurons (Loo et al., 1993) and PC12 cells (Gschwind and Huber, 1995). Exposure of undifferentiated PC12 cells to Aβ for 24 hr induced apoptotic nuclear changes, and the percentage of cells undergoing apoptosis was significantly greater in cells expressing PS-1 L286V than in untransfected cells, vector-transfected cells, and cells overexpressing wild-type PS-1 (Fig. 3A). Nuclear condensation and fragmentation induced by Aβ (Fig.3A) and serum withdrawal (data not shown) were prevented by the protein synthesis inhibitor cycloheximide, consistent with an apoptotic mechanism of cell death. It was reported previously that Aβ causes a relatively rapid (minutes to hours) impairment of mitochondrial function as measured by MTT reduction that occurs very early in the apoptotic process (Shearman et al., 1994; Kruman et al., 1996). Exposure of PC12 cells to Aβ resulted in a decrease in levels of MTT reduction in all cell lines examined, and the magnitude of the decrease in MTT reduction was significantly greater in each line expressing PS-1 L286V than in untransfected cells, cells transfected with empty vector, and lines overexpressing wild-type PS-1 (Fig.3B).
Fig. 3.
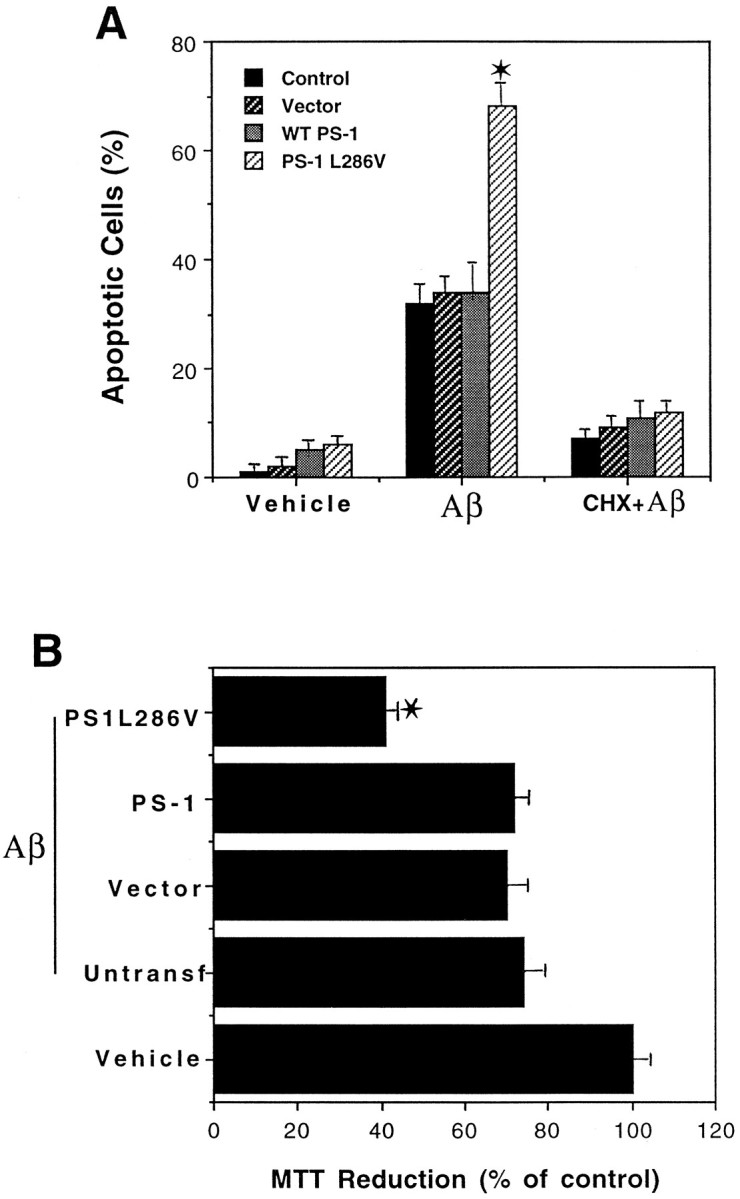
Mutant PS-1 increases PC12 cell vulnerability to apoptosis and mitochondrial dysfunction induced by amyloid β-peptide. A, The indicated cell lines were exposed to vehicle (Vehicle), 50 μm Aβ (Aβ), or 10 μm cycloheximide plus 50 μm Aβ (CHX + Aβ) for 24 hr. Then cells were stained with Hoescht dye, and the percentages of cells exhibiting DNA condensation and fragmentation were determined. Values represent the mean and SD of determinations made in four separate cultures (data pooled from analyses on all three lines expressing wild-type PS-1 and all three lines expressing PS-1 L286V; compare with Fig. 1). For all cell lines the values for cells exposed to Aβ were significantly greater than values for vehicle or CHX plus Aβ-treated cell lines (p < 0.01). *p < 0.01 compared with each of the other cell lines exposed to Aβ; ANOVA with Scheffé’s post hoc tests. B, Parallel cultures of untransfected control cells (Untransf), vector-transfected cells (Vector), three lines of cells transfected with wild-type PS-1 (PS-1; pooled data), and three lines of mutant PS-1 cells (PS1L286V; pooled data) were exposed for 4 hr to 50 μm Aβ, and relative levels of MTT reduction (a measure of mitochondrial function) were quantified. Values are the mean and SD of determinations made in four separate cultures and are expressed as a percentage of vehicle-treated control (vector-transfected) cells (data pooled from analyses on all three lines expressing wild-type PS-1 and all three lines expressing PS-1 L286V; compare with Fig. 1). There were no differences in basal levels of MTT reduction among the various control, wild-type PS-1-expressing, and mutant PS-1-expressing lines (data not shown). *p < 0.01 compared with corresponding values for untransfected, vector-transfected, and WT PS-1-transfected lines exposed to Aβ; ANOVA with Scheffé’s post hoctests.
Proapoptotic action of PS-1 mutation involves disruption of calcium homeostasis and induction of oxidative stress
To test the hypothesis that PS-1 mutations promote cell death by increasing oxidative stress, we determined whether antioxidants would protect cells against death induced by Aβ and measured levels of peroxides in the different cell lines after exposure to Aβ. When cultures were pretreated with the antioxidants propyl gallate or vitamin E before exposure to Aβ, cell death 24 hr later was reduced significantly, and the death-enhancing effect of L286V was prevented (Fig. 4A). In light of previous data implicating disruption of cellular calcium homeostasis in the mechanism of Aβ cytotoxicity (Mattson et al., 1992, 1993a; Mark et al., 1995) and apoptosis (Takei and Endo, 1994; Ciutat et al., 1995), we determined whether cells expressing PS-1 L286V exhibit increased sensitivity to Aβ-induced elevation of [Ca2+]i and whether agents that suppress calcium influx would protect cells against the adverse effects of PS-1 L286V. Aβ induced an increase in [Ca2+]iduring a 4 hr exposure period; the elevation of [Ca2+]i was significantly greater in cells expressing PS-1 L286V, as compared with control cell lines (Fig.4B). Aβ caused [Ca2+]i to increase to 150–170 nm in control cell lines and to >300 nm in L286V-expressing cells. Cell death induced by Aβ, and the death-enhancing effect of PS-1 L286V was attenuated significantly in cultures pretreated with nifedipine, a blocker of L-type voltage-dependent calcium channels (Fig. 4A). Dantrolene, an inhibitor of calcium release from ER stores, also in large part prevented Aβ-induced cell death in cells expressing L286V, suggesting a role for altered calcium release in the proapoptotic action of PS-1 L286V (Fig. 4A). Nifedipine and dantrolene also afforded partial protection against the adverse effects of mutant PS-1 on [Ca2+]i(Fig. 4B) and cellular peroxide levels (Fig.4C). As expected from previous studies (Behl et al., 1994;Goodman and Mattson, 1994), exposure of PC12 cells to Aβ for 4 hr caused an increase in cellular peroxide levels; the increase was significantly greater in cells expressing PS-1 L286V than in control cell lines (Fig. 4C). Pretreatment with either propyl gallate or vitamin E in large part abolished the Aβ-induced increase in peroxide levels (Fig. 4C). Collectively, these data indicated that mutant PS-1 may sensitize neural cells to apoptosis by perturbing calcium homeostasis and free radical metabolism.
Fig. 4.
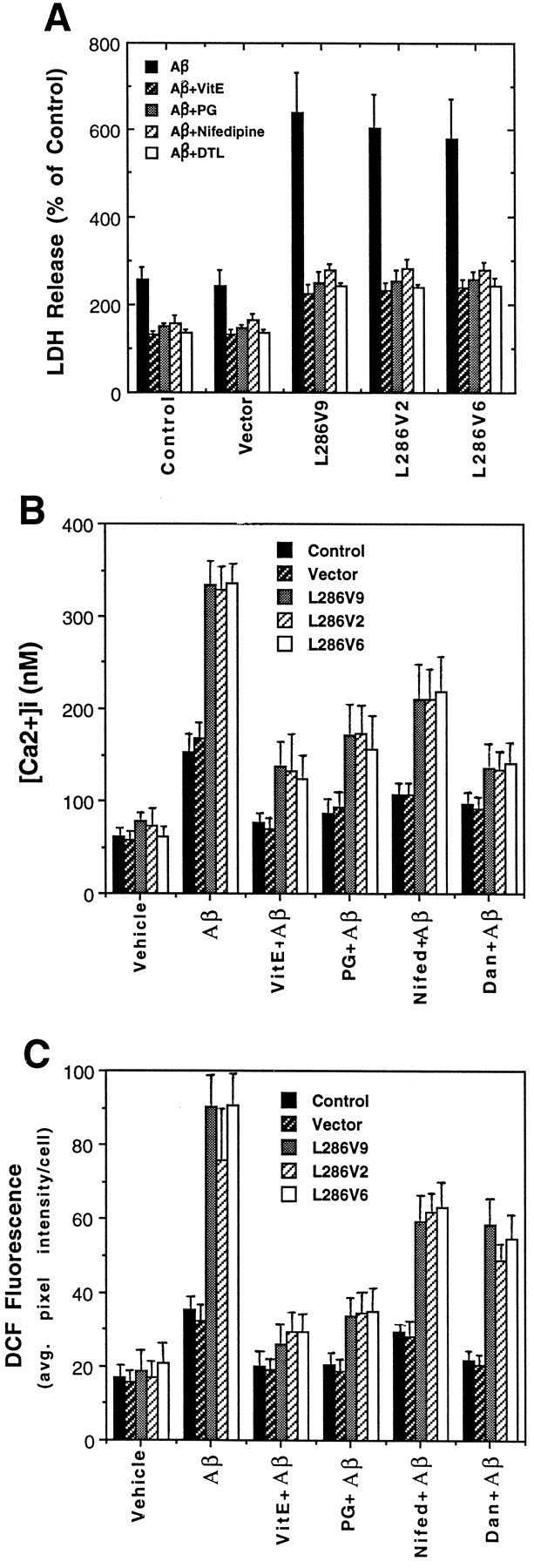
PC12 cells expressing PS-1 L286V mutation exhibit increased levels of oxidative stress and intracellular calcium after exposure to Aβ: attenuation by antioxidants and blockers of calcium influx and release from intracellular stores. A, Cultures were pretreated for 24 hr with 50 μm vitamin E (VitE) or for 2 hr with 5 μm propyl gallate (PG), 1 μm nifedipine (Nifedipine), or 1 μm dantrolene (DTL). Then cultures were exposed to 50 μm Aβ for 24 hr, and the medium was removed for LDH assay (cells exposed to Aβ first undergo apoptosis, followed by secondary necrosis, the latter being detected by LDH release assay). Values are expressed as a percentage of the maximal LDH release (mean and SD of 6–8 cultures); maximal LDH release was determined in parallel cultures (of each cell line) subjected to freeze–thaw. Values for each of the Aβ-treated cell lines expressing PS-1 L286V were significantly greater than each of the values for control (untransfected) and vector-transfected cell lines (p < 0.01) and for each of the values in PS-1 L286V cultures pretreated with vitamin E, propyl gallate, nifedipine, or dantrolene (p < 0.01 in each case); ANOVA with Scheffé’s post hoc tests.B, C, Cultures were pretreated with antioxidants or Ca2+ flux blockers as described forA and then exposed to vehicle or Aβ for 4 hr. Then the relative [Ca2+]i (fura-2 imaging) (B) and levels of peroxides (DCF Fluorescence) (C) in individual cells were quantified. Values are the mean and SD of determinations made in three to four cultures (15–25 cells for [Ca2+]imeasurements and 40–60 cells/culture for DCF measurements). Values for each of the Aβ-treated cell lines expressing L286V were significantly greater than each of the values in the vehicle-treated cultures (p < 0.01), each of the values in the cultures pretreated with vitamin E or propyl gallate (p < 0.01), and each of the values for L286V cells in the cultures pretreated with nifedipine or dantrolene (p < 0.05); ANOVA with Scheffé’spost hoc tests.
Apoptosis in differentiated PC12 cells induced by NGF withdrawal is enhanced by mutant PS-1
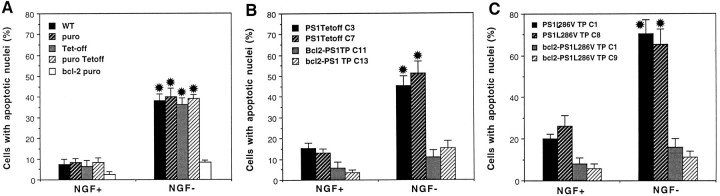
The experiments described above used PC12 cell lines stably expressing high levels of wild-type or mutant PS-1, a situation that could perturb metabolic pathways nonspecifically in the cells. Moreover, because wild-type and mutant PS-1 were expressed throughout the period of NGF-induced cell differentiation, we could not rule out the possibility that mutant PS-1 affects the differentiation process. To examine further the proapoptotic actions of PS-1 mutations, we therefore established PC12 lines expressing wild-type and mutant PS-1 under the control of a tetracycline-suppressible (Tet-off) promoter. For these experiments cells were maintained in the presence of tetracycline during the period of differentiation with NGF. After differentiation and 48 hr before NGF withdrawal, tetracycline concentration was reduced to induce PS-1 wild-type or mutant expression. As expected, levels of PS-1 expression increased with decreasing concentrations of tetracycline in the culture medium (Fig. 5A); subsequent experiments were performed in cultures induced to express wild-type or mutant PS-1 at comparable levels (Fig. 5B). Withdrawal of NGF from differentiated untransfected, vector-transfected, and Tet-off-transfected PC12 cells resulted in apoptosis of ∼40% of the cells during a 48 hr period (Fig. 6A). In contrast, apoptosis did not occur after NGF withdrawal in PC12 cells expressing Bcl-2. NGF withdrawal-induced apoptosis was enhanced significantly in PC12 cells expressing mutant PS-1 L286V (Fig.6C), as compared with cells expressing wild-type PS-1 (Fig.6B). Cells expressing PS-1 L286V also exhibited a modest increase in basal levels of apoptosis in the presence of NGF. PC12 cells expressing Bcl-2 in combination with wild-type or mutant PS-1 were completely resistant to apoptosis induced by NGF withdrawal (Fig. 6C). Collectively, these data indicate that mutant PS-1 possesses an adverse property not present in wild-type PS-1 that sensitizes neurons to apoptosis.
Fig. 5.
Controlled expression of PS-1 in PC12 cells with the use of a tetracyline-responsive transactivator. A, A PC12 cell line expressing the “Tet-off” construct was stably transfected with a pTRE-derived plasmid expressing PS-1 L286V gene. Cells were incubated for 48 hr in the presence of 2.0, 0.004, 0.002, and 0 μg/ml tetracycline (lanes 1–4, respectively). Cell proteins were separated by SDS-PAGE (100 μg/lane), transferred to a nitrocellulose sheet, and immunoreacted with PS-1 antibody. Note that, as the concentration of tetracycline was decreased, the levels of mutant PS-1 expression increased. B, Western blot showing that in the absence of tetracycline a double-stable PS-1 L286V cell line (C1) and a double-stable PS-1 cell line (C3) show significantly higher levels of PS-1 expression than do vector-transfected or untransfected cell lines.
Fig. 6.
Mutant PS-1 increases the vulnerability of differentiated PC12 cells to NGF withdrawal-induced apoptosis. Cultures of differentiated PC12 cells were incubated for 48 hr in serum-free medium containing or lacking NGF, and the percentage of cells exhibiting nuclear condensation and fragmentation was quantified. Values are the mean and SEM of determinations made in at least four separate cultures. A, Analyses in various control PC12 cell lines: WT, untransfected wild-type cells;puro, vector-transfected cells (pBabe-puro vector used for Bcl-2 expression); Tet-off, cells transfected with the Tet-off plasmid; puro Tet-off, cells doubly transfected with pBabe-puro vector and Tet-off plasmid; bcl-2 puro, cells expressing Bcl-2. Note that NGF withdrawal induced a similar level of apoptosis in all control lines, whereas cells expressing Bcl-2 were resistant to NGF withdrawal-induced apoptosis. *p < 0.01 compared with corresponding values for NGF+ cultures and the value for Bcl-2 NGF−cells. B, Bcl-2 protects PC12 cells overexpressing wild-type PS-1 against NGF withdrawal-induced apoptosis. Two lines of control cells expressing wild-type PS-1 (C3 andC7) and two different lines of cells expressing both Bcl-2 and PS-1 (C11 and C13) were analyzed. *p < 0.01 compared with corresponding values for NGF+ cells and compared with the value for the NGF− line expressing Bcl-2. C, Mutant PS-1 enhances vulnerability of PC12 cells to apoptosis induced by NGF withdrawal: protection by Bcl-2. Two lines of control cells expressing PS-1 L286V and two lines of cells expressing both Bcl-2 and PS-1 L286V were analyzed. *p < 0.05 compared with corresponding values for each line expressing wild-type PS-1 (B), p < 0.01 compared with corresponding values for cells maintained in the presence of NGF, andp < 0.01 compared with NGF−lines coexpressing Bcl-2; ANOVA with Scheffé’s post hoc tests for pair-wise comparisons.
Enhanced oxidative stress after NGF withdrawal and perturbed calcium homeostasis in differentiated PC12 cells expressing mutant PS-1
Withdrawal of NGF from differentiated untransfected and vector-transfected PC12 cells resulted in an increase in levels of cellular peroxides that occurred within 3 hr (Fig.7A). An increase in cellular peroxides also occurred after NGF withdrawal from PC12 cells expressing Bcl-2, suggesting that the antiapoptotic action of Bcl-2 occurred downstream of the oxidative stress (cf. Greenlund et al., 1995). NGF withdrawal-induced peroxide accumulation was enhanced greatly in PC12 cells expressing the mutant PS-1 L286V, as compared with cells expressing wild-type PS-1 (Fig. 7B). Basal levels of peroxides in cells expressing PS-1 L286V were somewhat higher than in control cells, although the difference did not reach statistical significance. PC12 cells expressing Bcl-2 in combination with mutant PS-1 exhibited peroxide levels after NGF withdrawal that were lower than in cells lacking Bcl-2, but the difference did not reach statistical significance (Fig. 7C).
Fig. 7.
Oxidative stress induced by NGF withdrawal is enhanced in PC12 cells expressing mutant PS-1. Cultures of differentiated PC12 cells were incubated for 3 hr in serum-free medium containing or lacking NGF, and levels of cellular peroxides were quantified by confocal laser scanning microscope image analysis of DCF fluorescence. Values are the mean and SEM of determinations made in at least four separate cultures. A, Analyses in various control PC12 cell lines: WT, untransfected wild-type cells; puro, vector-transfected cells;Tet-off, cells transfected with the Tet-off plasmid;puro Tet-off, cells doubly transfected with empty vector and Tet-off plasmid; bcl-2 puro, cells expressing Bcl-2. Note that NGF withdrawal induced a similar level of peroxide accumulation in all control lines and in cells overexpressing Bcl-2. Each value for NGF− cultures was significantly greater than the corresponding value for NGF+ cells (p < 0.01). B, Bcl-2 does not prevent NGF withdrawal-induced accumulation of peroxides in PC12 cells overexpressing wild-type PS-1. Two lines of control cells expressing wild-type PS-1 (C3 andC7) and two different lines of cells expressing both Bcl-2 and PS-1 (C11 and C13) were analyzed. Each value for NGF− cultures was significantly greater than the corresponding value for NGF+ cells (p < 0.01). C, Mutant PS-1 enhances accumulation of peroxides in PC12 cells deprived of NGF. Two lines of control cells expressing PS-1 L286V and two lines of cells expressing both Bcl-2 and PS-1 L286V were analyzed. *p < 0.05 compared with corresponding values for each line expressing wild-type PS-1 (B) andp < 0.01 compared with corresponding values for cells maintained in the presence of NGF; ANOVA with Scheffé’spost hoc tests for pair-wise comparisons).
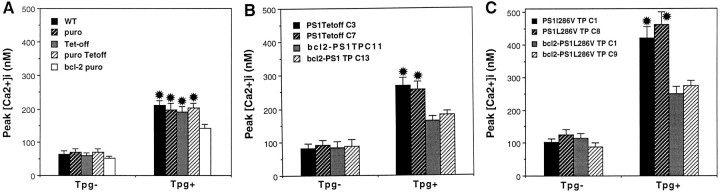
PS-1 is localized to the ER, and a recent study showed that PC12 cells expressing mutant PS-1 exhibit altered ER calcium regulation (Guo et al., 1996). We therefore examined calcium responses to thapsigargin, an inhibitor of the ER Ca2+–ATPase in differentiated PC12 cells expressing wild-type or mutant PS-1, with or without Bcl-2. Rest [Ca2+]i was ∼60 nm in control cells and was elevated to 80–110 nm in cells expressing PS-1 L286V (Fig. 8). In PC12 cells not expressing PS-1, thapsigargin caused an increase in [Ca2+]i to 160–180 nm, and the response was attenuated by ∼50% in cells expressing Bcl-2 (Fig. 8A). PC12 cells expressing PS-1 L286V exhibited a markedly en- hanced peak [Ca2+]i response to thapsigargin, with levels rising to ∼400 nm (Fig. 8C). The enhanced [Ca2+]i response to thapsigargin was abolished completely in PC12 cells expressing Bcl-2 (Fig.8C). Taken together with the data above showing that dantrolene protects PC12 cells against the proapoptotic actions of PS-1 L286V, these findings suggest that altered ER calcium regulation is involved mechanistically in the apoptotic action of mutant PS-1.
Fig. 8.
Elevations of [Ca2+]i induced by thapsigargin are enhanced significantly in PC12 cells expressing mutant PS-1: attenuation by Bcl-2. PC12 cells were incubated in serum-free medium and basal [Ca2+]i (Tpg−), and the peak [Ca2+]i after exposure to 1 μm thapsigargin (Tpg+) was quantified (cf. Guo et al., 1996). Values are the mean and SEM of determinations made in at least four separate cultures (15–20 cells/culture). A, Analyses in various control PC12 cell lines: WT, untransfected wild-type cells;puro, vector-transfected cells (vector for Bcl-2-expressing line); Tet-off, cells transfected with the Tet-off plasmid; puro Tet-off, cells doubly transfected with pBabe-puro and Tet-off plasmid; bcl-2 puro, cells expressing Bcl-2. *p < 0.01 compared with corresponding values for Tpg− cultures, andp < 0.05 compared with the Tpg+ value in cells expressing Bcl-2. B, The [Ca2+]i response to thapsigargin in cells expressing wild-type PS-1 is attenuated in cells coexpressing Bcl-2. *p < 0.01 compared with corresponding values for Tpg−, and p < 0.05 compared with Tpg+ values in cells expressing Bcl-2. C, Mutant PS-1 enhances [Ca2+]i responses to thapsigargin: attenuation by Bcl-2. *p < 0.001 compared with corresponding values for Tpg−,p < 0.01 compared with Tpg+ values in cells expressing Bcl-2, and p < 0.01 compared with Tpg+– PS1 Tet-off values (B); ANOVA with Scheffé’s post hoc tests for pair-wise comparisons.
DISCUSSION
Previous studies showed that both Aβ (Rabizadeh et al., 1994;Gschwind and Huber, 1995) and trophic factor withdrawal (Bastitatou and Greene, 1991; Rukenstein et al., 1991) induce apoptosis in PC12 cells. Similarly, Aβ (Forloni et al., 1993; Loo et al., 1993) and trophic factor deprivation (Prehn et al., 1994) induce apoptosis in neurons in primary cultures established from brain regions (e.g., hippocampus, neocortex, and basal forebrain) that are affected in AD. We found that both undifferentiated and differentiated PC12 cells expressing PS-1 L286V were extremely sensitive to apoptotic cell death when compared with various control cell lines. These findings suggest that the mutated PS-1 protein possesses an adverse proapoptotic property. Overexpression of wild-type PS-1, at levels similar to or greater than mutant PS-1 levels, did not result in increased vulnerability of PC12 cells to apoptosis, indicating that the proapoptotic action of PS-1 L286V was not simply the consequence of increased levels of PS-1 protein. Although the specific nature of that adverse property of the PS-1 mutation was not established in the present study, the data suggest an action on systems that regulate free radical metabolism and/or calcium homeostasis. Thus, levels of cellular peroxides induced by Aβ were increased greatly in cells expressing the PS-1 mutation, as compared with control lines, and two different antioxidants (vitamin E and propyl gallate) protected PC12 cells against cell death induced by Aβ. The antioxidants also suppressed Aβ-induced increases in intracellular peroxide and calcium levels, consistent with previous data suggesting that the mechanism of Aβ neurotoxicity involves membrane lipid peroxidation and impairment of membrane ion transport systems and calcium influx (Mattson et al., 1992; Behl et al., 1994;Goodman and Mattson, 1994; Mark et al., 1995, 1997a,b).
Previous studies of mechanisms of neuron death induced by trophic factor withdrawal and Aβ have implicated reactive oxygen species (ROS) (Hockenbery et al., 1993; Kane et al., 1993; Greenlund et al., 1995). We found that levels of oxidative stress and apoptosis after NGF withdrawal from differentiated PC12 cells were enhanced in cells expressing mutant PS-1, but not in cells overexpressing wild-type PS-1. These effects of mutant PS-1 were not attributable to changes that occurred during the process of cellular differentiation, because we allowed the cells to differentiate before induction of PS-1 expression by using the Tet-off system. Considerable data indicate that levels of oxidative stress are increased in AD brain, particularly in the environment of neuritic plaques and in neurofibrillary tangles (for review, see Benzi and Moretti, 1995; Smith et al., 1995); levels of oxidative stress also are increased in the brain during normal aging (Stadtman, 1992). The present findings suggest the possibility that PS-1 mutations may promote oxidative stress and thereby sensitize neurons to decrements in trophic factor support and increased accumulations of Aβ that occur in the aging brain. The data also may provide at least a partial explanation for the increased production of Aβ documented in blood and other tissues from human carriers of PS-1 mutations (Scheuner et al., 1996), because studies have shown that manipulations that promote metabolic stress and increase [Ca2+]i in cultured neurons can alter proteolytic processing of β-amyloid precursor protein (βAPP) in cultured cells in favor of increased Aβ production (Gabuzda et al., 1994; Querfurth and Selkoe, 1994).
PS-1 seems to be localized to the ER in several cell types, including neurons (Guo et al., 1996; Kovacs et al., 1996; Walter et al., 1996). Calcium imaging studies of cultured PC12 expressing PS-1 L286V have shown that this mutation alters calcium release from ER stores such that calcium responses to agonists that activate the IP3pathway (e.g., muscarinic cholinergic agonists and bradykinin) are enhanced greatly (Guo et al., 1996). The perturbed calcium homeostasis observed in PC12 cells expressing mutant PS-1 is consistent with reports that calcium signaling is altered in cultured fibroblasts taken from carriers of PS-1 mutations (McCoy et al., 1993; Ito et al., 1994). Our data suggest that disruption of calcium homeostasis by mutant PS-1 could be linked mechanistically to its proapoptotic action because dantrolene, an agent that blocks calcium release from ER, protected cells against the death-promoting effect of the PS-1 mutation. Recent findings in studies of non-neuronal cells have linked ER calcium regulation to apoptosis. For example, Lam et al. (1993) showed that glucocorticoids induce release of calcium from ER, which is correlated with subsequent DNA fragmentation and apoptosis, and Khan et al. (1996) provided evidence that lymphocyte apoptosis is mediated by increased expression of IP3 receptors. We found that thapsigargin-induced increases of [Ca2+]iwere enhanced in PC12 cells expressing mutant PS-1 and that Bcl-2 prevented the enhanced response in cells expressing mutant PS-1. Lam et al. (1994) showed that Bcl-2 protects lymphoma cells against apoptosis induced by thapsigargin and suppresses calcium release from ER. The localization of mutant PS-1 to ER (Guo et al., 1996) suggests that its effects on ER calcium homeostasis and apoptosis may be linked mechanistically, a possibility supported by our data. The ability of dantrolene to suppress the increased peroxide accumulation induced by Aβ in PC12 cells expressing PS-1 L286V suggests that calcium release from ER contributes to the enhanced oxidative stress associated with mutant PS-1 expression. However, in addition to calcium release from ER, calcium influx through voltage-dependent plasma membrane channels also may be involved, because nifedipine protected cells against Aβ toxicity and suppressed elevation of [Ca2+]iand peroxide accumulation induced by Aβ. These data are consistent with previous studies of primary neurons and PC12 cells showing that removal of extracellular calcium or treatment with nifedipine attenuates Aβ toxicity (Mattson et al., 1993a; Weiss et al., 1994).
The observation that both antioxidants and calcium channel blockers protected neurons against the adverse effects of the PS-1 mutation are consistent with a scenario in which Aβ induces a vicious cycle in which oxidative stress disrupts ion homeostasis, which, in turn, promotes further oxidative stress. There is now abundant evidence to support the involvement of such reciprocating cytotoxic cascades in many different neurodegenerative conditions (Mattson et al., 1992;Zhang et al., 1993; Goodman et al., 1996). Thus, although both the normal function of PS-1 and the exact alteration that results from PS-1 mutations are unknown, our data indicate that PS-1 mutations promote neurodegenerative apoptotic cascades involving perturbed ion homeostasis and oxidative stress.
Recent data from studies of brain tissue, blood, and cultured fibroblasts from carriers of PS mutations and studies of transfected cell lines indicate that cells expressing PS mutations produce greater than normal levels of Aβ1–42 (Borchelt et al., 1996; Lemere et al., 1996; Scheuner et al., 1996). In addition, studies of transgenic mice that express mutant PS-1 suggest that levels of Aβ1–42 are increased in brain tissue (Duff et al., 1996). The latter data suggest that PS-1 mutations may cause early onset AD by altering βAPP processing in ways that lead to increased Aβ production. The increased vulnerability of neuronal cells expressing PS-1 L286V to Aβ toxicity and trophic factor withdrawal documented in the present study is unlikely to result from increased Aβ production because PC12 cells are a rat cell line and, in contrast to human Aβ, rat Aβ is neither amyloidogenic nor neurotoxic (Otvos et al., 1993). However, it is conceivable that altered processing of βAPP could reduce levels of neuroprotective secreted forms of APP, which have been shown to protect neurons against oxidative apoptotic insults, including Aβ toxicity and glucose withdrawal (Mattson et al., 1993b; Goodman and Mattson, 1994; Furukawa et al., 1996).
Collectively, our data suggest that mutant PS-1 protein possesses an adverse proapoptotic property. Wolozin et al. (1996) recently reported that PC12 cells overexpressing wild-type PS-2 exhibit increased apoptosis after trophic factor withdrawal and that a PS-2 mutant enhanced basal levels of apoptosis. Deng et al. (1996) reported that overexpression of wild-type PS-2 increased vulnerability of PC12 cells to apoptosis induced by staurosporine or hydrogen peroxide. ALG-3, the mouse homolog of PS-2, was shown to modulate apoptosis in T lymphocytes (Vito et al., 1996), although in the latter case ALG-3 prevented apoptosis. We observed neither increased nor reduced apoptosis in PC12 cells overexpressing wild-type PS-1, suggesting that PS-1 does not function directly in an apoptotic pathway and that mutant PS-1 acquires a novel adverse property. However, we cannot rule out the possibility that mutant PS-1 interferes with a normal trophic property of endogenous PS-1 in a loss-of-function scenario.
Footnotes
This work was supported by Grants to M.P.M. from National Institutes of Health (NS30583 and AG10836) and the Alzheimer’s Association, to G.M.M. from National Institutes of Health (AG10917), and to B.L.S. from the University of Washington Nathan Shock Center for Excellence in the Basic Biology of Aging. We thank J. Begley, S. Bose, R. Pelfrey, and J. Xie for technical assistance.
Correspondence should be addressed to Dr. Mark P. Mattson, 211 Sanders-Brown Building, University of Kentucky, Lexington, KY 40536-0230.
REFERENCES
- 1.Bastitatou A, Greene LA. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991;115:461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β-protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 3.Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging. 1995;16:661–674. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- 4.Black MM, Greene LA. Changes in the colchicine susceptibility of microtubules associated with neurite outgrowth: studies with nerve growth factor-responsive PC12 pheochromocytoma cells. J Cell Biol. 1982;95:379–386. doi: 10.1083/jcb.95.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada C-M, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin-1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 6.Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 7.Bruce AJ, Malfroy B, Baudry M. β-amyloid toxicity in organotypic cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci USA. 1996;88:3633–3636. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciutat D, Esquerda JE, Caldero J. Evidence for calcium regulation of spinal cord motoneuron death in the chick embryo in vivo. Dev Brain Res. 1995;86:167–179. doi: 10.1016/0165-3806(95)00027-b. [DOI] [PubMed] [Google Scholar]
- 9.Cook DB, Sung JC, Golde TE, Felsenstein KM, Wojczyk BS, Tanzi RE, Trojanowski JQ, Lee VMY, Doms RW. Expression and analysis of presenilin-1 in a human neuronal system: localization in cell bodies and dendrites. Proc Natl Acad Sci USA. 1996;93:9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cribbs DH, Chen L, Bendle SM, La Ferla FM. Widespread neuronal expression of the presenilin-1 early-onset Alzheimer’s disease in the murine brain. Am J Pathol. 1996;148:1797–1806. [PMC free article] [PubMed] [Google Scholar]
- 11.Deng G, Pike CJ, Cotman CW. Alzheimer-associated presenilin-2 confers increased sensitivity to apoptosis in PC12 cells. FEBS Lett. 1996;397:50–54. doi: 10.1016/s0014-5793(96)01142-8. [DOI] [PubMed] [Google Scholar]
- 12.Doan A, Thinakaran G, Borchelt DR, Slunt HH, Ratovitsky T, Podlisny M, Selkoe DJ, Seeger M, Gandy SE, Price DL, Sisodia SS. Protein topology of presenilin-1. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 13.Duff K, Eckman C, Zehr C, Yu X, Prada C-M, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-β42(43) in brains of mice expressing mutant presenilin-1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 14.Elder GA, Tezapsidis N, Carter J, Shioi J, Bouras C, Li D, Johnston JM, Efthimiopoulos S, Friedrich VL, Jr, Robakis NK. Identification and neuron-specific expression of the S182/presenilin-1 protein in human and rodent brains. J Neurosci Res. 1996;45:308–320. doi: 10.1002/(SICI)1097-4547(19960801)45:3<308::AID-JNR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Forloni G, Chiesa R, Smiroldo S, Verga L. Apoptosis-mediated neurotoxicity induced by chronic application of β-amyloid fragment 25–35. NeuroReport. 1993;4:523–526. doi: 10.1097/00001756-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Fukuyama R, Wadhwani KC, Galdzicki Z, Rapoport SI, Ehrenstein G. β-Amyloid polypeptide increases calcium uptake in PC12 cells: a possible mechanism for its cellular toxicity in Alzheimer’s disease. Brain Res. 1994;667:269–272. doi: 10.1016/0006-8993(94)91505-9. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa K, Sopher B, Rydel RE, Begley JG, Martin GM, Mattson MP. Increased activity-regulating and neuroprotective efficacy of α-secretase-derived secreted APP is conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 18.Gabuzda D, Busciglio J, Chen L, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269:13623–13628. [PubMed] [Google Scholar]
- 19.Games D, Adams D, Alessandrinl R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagoplan S, Johnson-Wood K, Khan K, Lee M, Lelbowitz E, McConlogue S, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 20.Giannakopoulos P, Bouras C, Kovari E, Shioi J, Tezapsidis N, Hof PR, Robakis NK. Presenilin-1 immunoreactive neurons are preserved in late-onset Alzheimer’s disease. Am J Pathol. 1997;150:429–436. [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman Y, Mattson MP. Secreted forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptide-induce oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 22.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 23.Greenlund LJ, Deckwerth TL, Johnson EM. Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 24.Gschwind M, Huber G. Apoptotic cell death induced by β-amyloid 1–42 peptide is cell type-dependent. J Neurochem. 1995;65:292–300. doi: 10.1046/j.1471-4159.1995.65010292.x. [DOI] [PubMed] [Google Scholar]
- 25.Guo Q, Furukawa K, Sopher BL, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid β-peptide. NeuroReport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 26.Hockenbery D, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SH. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 27.Hsaio K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 28.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs DM, Fausett HJ, Page KJ, Kim T-W, Moir RD, Merriam DE, Hollister RD, Hallmark OG, Mancini R, Felsenstein KM, Hyman BT, Tanzi RE, Wasco W. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- 31.Kruman I, Guo Q, Bruce AJ, Bredesen DE, Mattson MP. Hydroxynonenal may mediate apoptotic neuronal death induced by trophic factor withdrawal and oxidative insults. Soc Neurosci Abstr. 1996;22:1481. [Google Scholar]
- 32.Lam M, Dubyak G, Distelhorst CW. Effect of glucocorticosteroid treatment on intracellular calcium homeostasis in mouse lymphoma cells. Mol Endocrinol. 1993;7:686–693. doi: 10.1210/mend.7.5.8316252. [DOI] [PubMed] [Google Scholar]
- 33.Lemere CA, Lopera F, Kosik KS, Lendon CL, Ossa J, Saido TC, Yamaguchi H, Ruiz A, Martinez A, Madrigal L, Hincapie L, Arango JC, Anthony DC, Koo EH, Goate AM, Selkoe DJ, Arango VJC. The E280A presenilin-1 Alzheimer mutation produces increased Aβ42 deposition and severe cerebellar pathology. Nat Med. 1996;2:1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- 34.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu C-E, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Greenwald I. Membrane topology of the C. elegans SEL-12 presenilin. Neuron. 1996;17:1015–1021. doi: 10.1016/s0896-6273(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 36.Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mark RJ, Hensley K, Butterfield DA, Mattson MP. Amyloid β-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal in disruption of ion homeostasis and neuronal death induced by amyloid β-peptide. J Neurochem. 1997a;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 39.Mark RJ, Pang Z, Geddes JW, Mattson MP. Amyloid β-peptide impairs glucose uptake in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997b;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattson MP, Tomaselli K, Rydel RE. Calcium-destabilizing and neurodegenerative effects of aggregated β-amyloid peptide are attenuated by basic FGF. Brain Res. 1993a;621:35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- 42.Mattson MP, Cheng B, Culwell A, Esch F, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of β-amyloid precursor protein. Neuron. 1993b;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 43.Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- 44.McCoy KR, Mullins RD, Newcomb TG, Ng GM, Pavlinkova G, Polinsky RJ, Nee LE, Sisken JE. Serum- and bradykinin-induced calcium transients in familial Alzheimer’s fibroblasts. Neurobiol Aging. 1993;14:447–455. doi: 10.1016/0197-4580(93)90103-i. [DOI] [PubMed] [Google Scholar]
- 45.Mullan M, Crawford F. Genetic and molecular advances in Alzheimer’s disease. Trends Neurosci. 1993;16:398–403. doi: 10.1016/0166-2236(93)90007-9. [DOI] [PubMed] [Google Scholar]
- 46.Murphy GM, Forno LS, Ellis WG, Nochlin D, Levy-Lahad E, Poorkaj P, Bird TD, Jiang Z, Cordell B. Antibodies to presenilin proteins detect neurofibrillary tangles in Alzheimer’s disease. Am J Pathol. 1996;149:1839–1846. [PMC free article] [PubMed] [Google Scholar]
- 47.Otvos L, Szendrei GI, Lee VM, Mantsch HH. Human and rodent Alzheimer β-amyloid peptides acquire distinct conformations in membrane-mimicking solvents. Eur J Biochem. 1993;211:249–257. doi: 10.1111/j.1432-1033.1993.tb19893.x. [DOI] [PubMed] [Google Scholar]
- 48.Prehn JH, Bindokas VP, Marcuccilli CJ, Krajewski S, Reed JC, Miller RJ. Regulation of neuronal Bcl2 protein expression and calcium homeostasis by transforming growth factor type beta confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci USA. 1994;91:12599–12603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid β-peptide production by cultured cells. Biochemistry. 1994;33:4550–4561. doi: 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- 50.Rabizadeh S, Bitler CM, Butcher LL, Bredesen DE. Expression of the low-affinity nerve growth factor receptor enhances β-amyloid peptide toxicity. Proc Natl Acad Sci USA. 1994;91:10703–10706. doi: 10.1073/pnas.91.22.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St. George-Hyslop PH. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 52.Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci. 1991;11:2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. The amyloid β-protein deposited in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 54.Selkoe DJ. Biochemistry of altered brain proteins in Alzheimer’s disease. Annu Rev Neurosci. 1989;12:463–490. doi: 10.1146/annurev.ne.12.030189.002335. [DOI] [PubMed] [Google Scholar]
- 55.Shearman MS, Ragan CI, Iversen LL. Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of β-amyloid-mediated cell death. Proc Natl Acad Sci USA. 1994;91:1470–1474. doi: 10.1073/pnas.91.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sansequ P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St. George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 57.Smale G, Nichols NR, Brady DR. Evidence for apoptotic cell death in Alzheimer’s disease. Exp Neurol. 1995;133:225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 58.Smith MA, Sayre LM, Monnier VM, Perry G. Radical aging in Alzheimer’s disease. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- 59.Stadtman E. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 60.Su JH, Anderson AJ, Cummings B, Cotman CW. Immunocytochemical evidence for apoptosis in Alzheimer’s disease. NeuroReport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 61.Takei N, Endo Y. Ca2+ ionophore-induced apoptosis on cultured embryonic rat cortical neurons. Brain Res. 1994;652:65–70. doi: 10.1016/0006-8993(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 62.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Norstedt C, Seeger M, Hardy J, Levey A, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SA. Endoproteolysis of presenilin-1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 63.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 64.Vito P, Lacan E, D’Adamio L. Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer’s disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- 65.Walter J, Capell A, Grunberg J, Pesold B, Schindzielorz A, Prior R, Podlisny MB, Fraser P, St. George-Hyslop PH, Selkoe DJ, Haass C. The Alzheimer’s disease-associated presenilins are differentially phosphorylated proteins located predominantly within the endoplasmic reticulum. Mol Med. 1996;2:673–691. [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss JH, Pike CJ, Cotman CW. Ca2+ channel blockers attenuate β-amyloid peptide toxicity to cortical neurons in culture. J Neurochem. 1994;62:372–375. doi: 10.1046/j.1471-4159.1994.62010372.x. [DOI] [PubMed] [Google Scholar]
- 67.Wolozin B, Iwasaki K, Vito P, Ganjei JK, Lacana E, Sunderland T, Zhao B, Kusiak JW, Wasco W, D’Adamio L. Participation of presenilin-2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science. 1996;274:1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Tatsuno T, Carney J, Mattson MP. Basic FGF, NGF, and IGFs protect hippocampal neurons against iron-induced degeneration. J Cereb Blood Flow Metab. 1993;13:378–388. doi: 10.1038/jcbfm.1993.51. [DOI] [PubMed] [Google Scholar]