Abstract
Cerebellar granule neurons cultured with serum develop a mature neuronal phenotype, including stimulus-coupled release of glutamate, and depend on elevated potassium for survival. We find that cells cultured with serum undergo two phases of cell death. By 6 din vitro, 30–50% of the cells present are dead; after this time the remaining cells die. Elevated potassium prevents only this later phase of death, whereas neurotrophins protect these cells against the early phase of death. Factors that bind p75NTRor TNF-R, members of the same receptor family, exhibit voltage-sensitive calcium channel-dependent protection, whereas ligands of expressed Trk receptors show additional calcium channel-independent protection. The cells express TrkB protein and show elevated c-Fos and c-Jun levels in response to BDNF. No TrkA is detected, although p75NTR protein is expressed and NGF induces depolarization-dependent elevation of c-Jun levels. In the presence of the protein kinase C inhibitor bisindolylmaleimide, BDNF-induced survival promotion is reduced partially, whereas NGF-induced death is unmasked. Basal survival mechanisms are insensitive to inhibition of PK-C or PI-3 kinase. We conclude that BDNF promotes survival in part via its TrkB receptor, whereas there is an additional pathway promoting survival and elevating c-Jun evoked by both NGF and BDNF via a non-Trk receptor.
Keywords: NGF, BDNF, TNF, neurotrophins, p75NTR, TrkB, cell death, cerebellar granule neurons, PK-C
Cultured cerebellar granule neurons, a popular model CNS culture for the study of neuronal signaling and development, express a wide range of receptor subtypes and develop stimulus-coupled glutamate release when cultured in serum-based medium. Under these culture conditions the cells depend on chronic depolarization or other calcium-elevating stimuli for continued survival (Thangnipon et al., 1983; Gallo et al., 1987; Balazs et al., 1988b,c, 1990). Without such stimuli a delayed apoptotic death occurs (Copani et al., 1995). This dependence has been proposed to mimic a trophic requirement of cells in the cerebellar granule layer for innervation by the mossy fibers (Balazs et al., 1990). During the first 3–5 weeks of postnatal life, there is a well characterized major loss of granule neurons in the cerebellum (Landis and Sidman, 1978). However, considerable cell loss occurs at postnatal day 5–6 because of an early phase of apoptosis (Wood et al., 1993; Krueger et al., 1995).
The neurotrophins NGF, BDNF, and neurotrophin-3 (NT-3) are survival-promoting factors reported to act via their specific tyrosine kinase receptors TrkA, TrkB, and TrkC, respectively (for review, seeGlass and Yancopoulos, 1993), and via p75NTR, the nonselective neurotrophin receptor of the tumor necrosis factor (TNF)/Fas/CD40 family (Mahadeo et al., 1994). Activation of Trks initiates a number of signaling cascades, including Ras-dependent activation of the extracellular signal-regulated kinase/microtubule-associated protein (ERK/MAP) kinase cascade (Ohmichi et al., 1992), which induces c-fos transcription (Karin and Hunter, 1995). The signaling events subsequent to ligand binding to p75NTR, initially characterized as altering the specificity and binding of coexpressed Trks (for review, see Chao and Hempstead, 1995), include the activation of sphingomyelinase (Dobrowsky et al., 1994), NF-κB (Carter et al., 1996a), and Jun kinase (JNK;Casaccia-Bonnefil et al., 1996).
BDNF has been reported to reduce the death of cerebellar granule neurons in serum-free culture as early as 2 d in vitro (Gao et al., 1995) (see also Segal et al., 1992; Lindholm et al., 1993). However, in the absence of serum, delayed death does not occur (Kingsbury et al., 1985). Because cerebellar granule neurons in culture require serum-containing medium to acquire depolarization-dependent survival and a mature physiology, such as expression of amino acid carriers and stimulus-coupled neurotransmitter release (Balazs et al., 1988a), serum-based cultures may mimic more closely the cells in vivo. Thus we have investigated the requirements for survival promotion by neurotrophins under these more physiological conditions.
In this study we show that there is considerable death of cerebellar granule neurons developing in serum-based culture before depolarization-dependent survival. We find that NGF, BDNF, NT-3, and TNF all protect against basal cell death. Two components of protection by neurotrophins are resolved: (1) factors that bind p75NTRor TNF-R, members of the same receptor family, provide protection dependent on calcium channel activity; (2) those factors that are able to bind the Trk receptors present provide an additional calcium channel-independent component of protection. In addition, basal survival mechanisms exist that allow partial survival in the absence of neurotrophins.
MATERIALS AND METHODS
Cell culture. Cerebellar granule neurons were prepared from 7-d-old rats as previously described (Courtney and Nicholls, 1992), except that cells were plated at 250,000/cm2. Dissociated cells were plated onto either poly-l-lysine-coated coverslips (10.5 mm × 10.5 mm) and cultured in 24-well plates for cell death studies or plated on poly-l-lysine-coated 6-well plates for immunoblot analysis. Cells were cultured in Minimum Essential Media (MEM; Life Technologies, Paisley, Scotland) supplemented with 10% (v/v) fetal calf serum (Life Technologies), 33 mm glucose, 2 mm glutamine, 50 U/ml penicillin, 50 μm streptomycin, and 20 mm supplementary KCl, as indicated (i.e., low KCl = 5.4 mm KCl; elevated KCl = 25.4 mm KCl). Culture medium was replaced at 1 d in vitro (DIV). When experiments were performed on cells older than 6 DIV, medium also was replaced at 7 DIV. However, the depolarization dependence of survival observed also was observed in preliminary experiments without this medium change and thus cannot be attributed to this medium change. Cytosine arabinofuranoside (10 μm; Sigma, St. Louis, MO) was included in the culture medium change at 1 d to inhibit non-neuronal cell proliferation. At this time 1 μmnifedipine (Sigma), 10 ng/ml of NGF (>98%; Alomone, Jerusalem, Israel), BDNF (>96%; Peprotech, Princeton, NJ), NT-3 (>98%; Peprotech), TNF-α (>97%; Peprotech), and 10 nm12-O-tetradecanoyl phorbol-13-acetate (TPA; Sigma) plus the inhibitors wortmannin (100 nm; Calbiochem, La Jolla, CA), bisindolylmaleimide GF 109203X (1 μm; Calbiochem), curcumin (10 μm; Sigma), or C2 ceramide (Sigma) were added, as indicated.
Purity of the preparation. The preparation used in this study is a well characterized one, the purity of which has been the subject of several studies. The cultures have been reported to contain ∼95% small interneurons, predominantly granule neurons (Thangnipon et al., 1983), and 4–6% GABAergic neurons (Resink et al., 1994). This is achieved in part by the huge abundance of granule neurons in the cerebellum and in part by the isolation procedure that does not allow the survival of larger, earlier differentiating neurons such as Purkinje cells (Weber and Schachner, 1984). Culturing in serum-containing medium supplemented with the antimitotic agent arabinosyl cytosine is more effective than culturing without serum at reducing the proportion of contaminating cells. Under the former conditions the major contaminants are GFAP+ astrocytes, accounting for 1–3% of cells (Kingsbury et al., 1985). Anti-GFAP staining of parallel cultures to those used in this study reveals GFAP+ contamination within this range. These GFAP+ cells are morphologically distinguishable, having either very large nuclei or a large number of thick processes. At the wavelengths used, propidium iodide stains protein to a minor extent, and the Hoechst stain gives the background a slight green fluorescence, allowing morphological identification of both characteristics of contaminating cells during the counting of nuclei. Thus these contaminants are excluded readily from the statistics. This leaves the possibility that some of the dead cells counted might be non-neuronal cells and that this might interfere with the measurements. However, a minor contaminant of 3% of living cells in control cultures would have to become over fourfold more prevalent in treated cultures to account for a 10% measured reduction in cell death. No such increases of these morphologically distinguishable cells were detected. Contaminating neurons from the cerebellum are also distinguishable by their large nuclei; the same argument is valid for these.
Cell death measurements. At the times indicated in the figures, Hoechst 33342/propidium iodide in MEM was added to a final concentration of 5 μg/ml Hoechst 33342 (Molecular Probes, Eugene, OR) and 20 μg/ml propidium iodide (Sigma). After 25 min, cells were washed in PBS, fixed for 30 min in 4% paraformaldehyde, washed again, and mounted in Mowiol 4-88 (Hoechst AG, Frankfurt am Main, Germany) mounting medium (Harlow and Lane, 1988) containing 2.5% (w/v) 1,4-diazabicyclo[2.2.2]octane (DABCO). Slides were examined with a fluorescence microscope (Leica, Heerbrugg, Switzerland) equipped with a 1000U CCD Camera (Electrim, Princeton, NJ) under control of software developed by the authors. An image at 7-amino-4-methylcoumarin-3-acetic acid-N′-hydroxysuccinimide ester wavelengths (excitation bandpass 340–380 nm, dichroic 400 nm, emission long-pass 425 nm) and an image at rhodamine B wavelengths (excitation bandpass 515–560 nm, dichroic 580 nm, emission long-pass 590 nm) were captured on cyan blue and red channels, respectively, of a true color image for each of nine evenly spaced fields per coverslip, and the proportion of total stained nuclei that did not stain with cell-impermeant propidium iodide was taken as the proportion of cells surviving. The method has the advantage (over the propidium iodide/fluorescein diacetate method) that relatively dense cells may be counted. At the excitation wavelengths used, propidium iodide also stains protein to a small extent. This results in an occasional red rim around the blue nuclei of living cells and is resolved easily from double-stained magenta nuclei scored as dead. Data shown were obtained from between three and nine coverslips per condition. These coverslips were obtained, in each case, from two to three separate cell preparations.
In the graphs the term “% dead cells” is the percentage of cells present scored as dead under basal conditions. “% Protection” is defined as the increase in survival above control (i.e., 0% protection = control survival), where 100% protection = 100% survival. This allows direct comparison of survival effects of compounds under different conditions for which the control survival is not constant.
Calcium measurements. Cells were loaded for 40′ in Modified Elliot’s Medium (Courtney et al., 1990) containing 5 μmFluo-3/AM (Molecular Probes), 33 μg/ml BSA (Sigma), and 0.02% pluronic acid (Molecular Probes), washed five times in Mg2+-free Elliot’s, and placed in a chamber heated to 37°C on the stage of a Nikon Diaphot microscope. Cells were excited at 480 nm through a 40×/1.3 N.A. objective, and emission light was passed through a DM 510 dichroic mirror and 520 nm barrier filter onto a 1000TE cooled CCD (Electrim). Images were collected once per second under the control of software developed by the authors. Data were analyzed with analysis software described previously (Lindqvist et al., 1995). Spontaneous calcium channel activity was recorded and then terminated by addition of 1 μm nifedipine to the incubating medium where indicated in the figure. Single wavelength fluorescence values (F) were converted to cytoplasmic-free [Ca2+], as previously reported, with the formula [Ca2+] = KD × (F −Fmin)/(Fmax −F), using published KD values (385 nm; Kao et al., 1989). Calibration values were obtained by using ionomycin in parallel experiments. The data shown were obtained from 7 DIV cells cultured in low KCl; nifedipine-blocked spontaneous activity also was observed at earlier stages in development, although this activity developed from smaller, more sustained elevations to larger, transient elevations with increasing age in culture. Cells cultured in elevated KCl exhibited no detectable nifedipine-sensitive spontaneous calcium elevations.
Protein detection and quantitation. Levels of c-Fos and c-Jun were assessed by exposing cells for 2 hr to 10 ng/ml NGF, BDNF, NT-3, or TNF or 10 nm TPA and blotting the cell lysates with polyclonal anti-Fos antibody (Oncogene Science, Uniondale, NY) or monoclonal anti-c-Jun antibody (Transduction Labs, Lexington, KY), and film was exposed over the linear range by the ECL method, according to the manufacturer’s instructions (Amersham International, Amersham, UK). Exposed films were digitized by flatbed scanning and quantitated by imaging software developed by the authors (Lindqvist et al., 1995). Changes in c-Fos immunoreactivity in elevated KCl were detected in a band ∼62 kDa; this band was quantitated. In low KCl, changes were detected in a band ∼55 kDa, and a 62 kDa band could not be detected. Therefore, under these conditions the 55 kDa band was quantitated. Rabbit polyclonal subtype-specific Trk 763, TrkB 794, and TrkC 798 from Santa Cruz Biotech (Santa Cruz, CA) were used for immunodetection of TrkA, TrkB, and TrkC, respectively. A TrkA-specific antiserum generously provided by Dr. Louis Reichardt (University of California, San Francisco) also was used. Rabbit antiserum 9651 raised to the ligand-binding sequences of p75 sequences, generously provided by Dr. Moses Chao (Cornell University Medical College, New York), was used for immunodetection of p75NTR and for blocking NGF action via p75NTR.
Immunocytochemistry. Immunocytochemical staining for NF-κB p65 subunit was performed as described in Menon et al. (1993) by using 1:500 biotin anti-rabbit (Sigma) and 1:50 ExtrAvidin fluorescein (Sigma). Slides were examined under a Leica confocal microscope with a 100× objective.
RESULTS
Cell death occurs in two phases
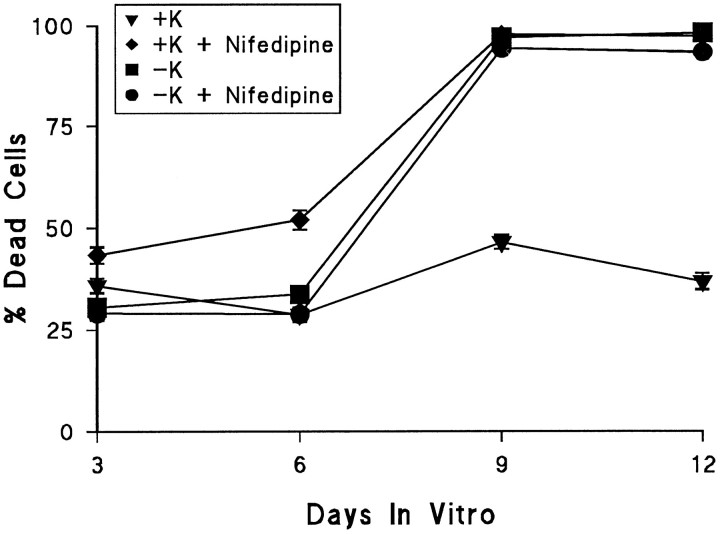
We first determined the time course of cell death of cerebellar granule neurons in serum-containing culture. Hoechst 33342 is membrane-permeant and stains all nuclei, whereas propidium iodide is membrane-impermeant. Cell death is calculated as the proportion of nuclei present that stains with propidium iodide. This technique avoids many artifacts to which other methods are susceptible (Juurlink and Hertz, 1993). It detects damage to the plasma membrane and thus measures both necrotic and late apoptotic cells. Almost all of the cells scored as dead in the results that follow had pyknotic nuclei; the predominant mode of death described here, therefore, may be of an apoptotic nature. Figure 1 shows the percentage of dead cells present over the first 12 DIV. Cells were cultured in the absence or presence of 20 mm supplementary KCl and 1 μm of the L-type voltage-sensitive calcium channel (VSCC) inhibitor nifedipine, as indicated. In agreement with previous results (Gallo et al., 1987), there is a requirement for depolarization for prolonged survival involving L-type VSCCs. However, a significant proportion of cells present even during the first 6 DIV are dead, and depolarization does not prevent this death.
Fig. 1.
Cell death occurs in two phases: L-type voltage-sensitive calcium channels prevent only the late phase. Of the cells present at 3–6 d in vitro (DIV), 30–50% were dead; the remaining cells died between 6 and 9 DIV. The early phase was not prevented by elevated KCl (+K). Elevated KCl in the absence of nifedipine prevented only the late phase of cell death.
Protection from basal cell death by neurotrophins, TNF, and TPA
BDNF enhances survival of cerebellar granule neurons in serum-free culture (Segal et al., 1992; Lindholm et al., 1993; Gao et al., 1995). However, depolarization has been reported not to enhance survival under these conditions (Kingsbury et al., 1985), and maturation is abrogated to the extent that amino acid carriers are not expressed and stimulus-coupled transmitter release does not occur or is delayed (Balazs et al., 1988a). As depolarization dependence begins after 6 DIV, we asked whether activation of neurotrophin receptors regulates the early phase of cell survival.
Figure 2 shows the percentage of cells present scored as dead at 6 DIV in cultures incubated with the dimeric neurotrophins NGF or BDNF from 1 DIV. In cultures incubated with neurotrophin-supplemented elevated KCl medium alone (leftset of columns), ∼38% of the cells are dead in controls. NGF moderately but significantly reduces this proportion, and BDNF more strongly reduces cell death. In parallel experiments increasing amounts of 9651 rabbit anti-p75, an antiserum that binds the ligand-binding site of p75NTR (Huber and Chao, 1995), were included. At 1:200 dilution (right set of columns), NGF no longer reduces cell death, whereas BDNF is still protective. This suggests that binding of NGF to p75NTR is essential for NGF-evoked reduction of cell death, whereas BDNF is able to reduce death independent of binding to p75NTR. The p75NTRantiserum alone reduces cell death. This would be consistent with the divalent p75NTR antibodies mimicking the NGF-evoked reduction in death and a component of the BDNF-evoked reduction in death. Thus NGF has no further effect above that of the antiserum, and BDNF has a smaller effect than in the absence of antiserum.
Fig. 2.

Neurotrophic factors protect from cell death at 6 DIV. Antiserum to p75NTR ligand-binding domain prevents NGF-evoked protection. Shown is quantitation of the protective effects of the neurotrophins NGF and BDNF on cerebellar granule neuron cultures. NGF and BDNF both reduce cell death. Increasing amounts of antiserum raised against the ligand-binding domain of p75NTR prevent NGF-evoked survival promotion and reduce basal death without effect on survival in the presence of BDNF.
The neurotrophic factors NGF, BDNF, and NT-3 would be expected to activate their respective Trk receptors, if present, and the nonselective p75NTR. p75NTR is a member of the TNF receptor family (Mahadeo et al., 1994) that exhibits common structural and signaling features. Trks activate a number of signaling cascades, including protein kinase C (PK-C) via PL-Cγ (Vetter et al., 1991) and the MAP kinase cascade via Ras (Ohmichi et al., 1992). TPA also activates PK-C, which has been reported to activate the MAP kinase cascade via Raf (Kolch et al., 1993). Therefore, we tested NGF, BDNF, and NT-3 while attempting to mimic the actions of p75NTRactivation with TNF and to activate specific components of Trk signaling with TPA.
Cells were cultured in the absence or presence of 20 mmsupplementary KCl. Then neurotrophins, TNF-α, TPA, and 1 μm nifedipine were introduced during the medium change at 1 DIV. Because the basal level of death in the absence of neurotrophins was not identical in the different conditions of depolarization and nifedipine (see Fig. 1), protection from death by neurotrophins was normalized to the maximum possible reduction in death in each condition of depolarization and nifedipine (see Materials and Methods).
NGF, BDNF, NT-3, and TNF protected against cell death (Fig.3A). The modest protective effects of NGF and TNF were eliminated by culturing the cells in low KCl in the presence of nifedipine, conditions expected to reduce VSCC activity to a minimum. The greater protection evoked by BDNF and NT-3 was reduced only partially under such conditions. Ceramide is a putative mediator of p75NTR and TNF-R signaling (Yanaga and Watson, 1992;Dobrowsky et al., 1995). Thus we investigated the protective effects of the water-soluble analog C2 ceramide on cells cultured in elevated KCl, which are sensitive to protection by NGF and TNF. Low concentrations of C2 ceramide protected cells against death to a similar extent to NGF and TNF (Fig. 3B). At 10 μm, ceramide had no effect, whereas 100 μmcaused the death of all the cells in the culture (data not shown).
Fig. 3.
Neurotrophic factors, TNF, TPA, and ceramide protect from cell death at 6 DIV. A, Quantitation of the protective effects of neurotrophins, TNF, and TPA on cerebellar granule neuron cultures. NGF, BDNF, NT-3, and TNF all evoke protection against cell death. In elevated KCl (+K), 1 μm nifedipine (+Nif) reduces the protective effects, and 10 nm TPA behaves like a neurotrophin. In low KCl (−K), nifedipine eliminates protection by NGF and TNF, but not BDNF or NT-3. Under these conditions 10 nm TPA is toxic. All other conditions significantly (p < 0.05) protect against cell death. B, C2 ceramide at 0.1–3.0 μm protects cells cultured in elevated KCl against cell death to a similar extent to NGF and TNF. Higher concentrations do not protect against cell death. C, Spontaneous cytoplasmic-free calcium elevations exist in cells cultured in low KCl. These elevations are blocked by addition of 1 μm nifedipine.
Although nifedipine might be expected to be effective only when elevated KCl is present to depolarize the neurons, cerebellar granule neurons cultured in low KCl can be observed to undergo spontaneous VSCC activity that is blocked by nifedipine (Fig. 3C); such activity is not observed in cells cultured in elevated KCl (Courtney et al., 1990). Furthermore, these neurons undergo spontaneous L-type and N-type VSCC activity in culture medium (Doherty et al., 1991). Thus it is likely that nifedipine modulates the actions of neurotrophins via L-type VSCCs, even in low KCl conditions. These results suggest that NGF and TNF act by mechanisms dependent on L-type VSCC activity, whereas BDNF and NT-3 act via an additional mechanism that is independent of VSCC activity. TPA was protective to cells cultured in the presence of elevated KCl, but, unlike the other factors tested, it was toxic to cells cultured in low KCl.
Figure 4 shows representative fields of cells used in these studies. Figure 4A shows phase-contrast images of cells in the absence or presence of elevated KCl at 6 DIV. In Figure4B, cells stained by the cell death quantitation technique that was used are shown. The proportion of dead cells (with double-stained nuclei) at 6 DIV was not altered significantly by the presence of elevated KCl; however, at 9 DIV virtually all cells were dead unless the culture medium was supplemented with elevated KCl. It should be noted that the proportion of dead cells at any time point is an underestimation of the total death of cells up to that time, because cells that had died at earlier time points continue to degenerate and are lost from the culture surface (Xia et al., 1995). Representative fields of cells at 6 DIV cultured with BDNF or TPA in low KCl are shown in Figure 4C. Under these conditions BDNF reduced cell death, whereas TPA notably increased it.
Fig. 4.

Representative fields of cerebellar granule neuron cultures in phase-contrast mode and in fluorescence mode after exposure to Hoechst 33342 and propidium iodide. A, Phase-contrast images of cerebellar granule neuron cultures at low magnification are shown. Left, Cells cultured in low KCl (−K) are shown. Right, Cells are cultured in elevated KCl (+K). B, The presence of elevated KCl (+K) in the culture medium does not alter significantly the proportion of cells with double-stained nuclei at 6 DIV (upper pair). However, by 9 DIV virtually all nuclei double stain, i.e., are dead, unless supplementary KCl is present (lower pair).C, Representative fields of cells cultured in low KCl (−K) stained at 6 DIV. Under these conditions 10 ng/ml BDNF protects from cell death, whereas 10 nm TPA is toxic.
Cerebellar granule neurons express TrkB and p75NTR, but not TrkA, receptors
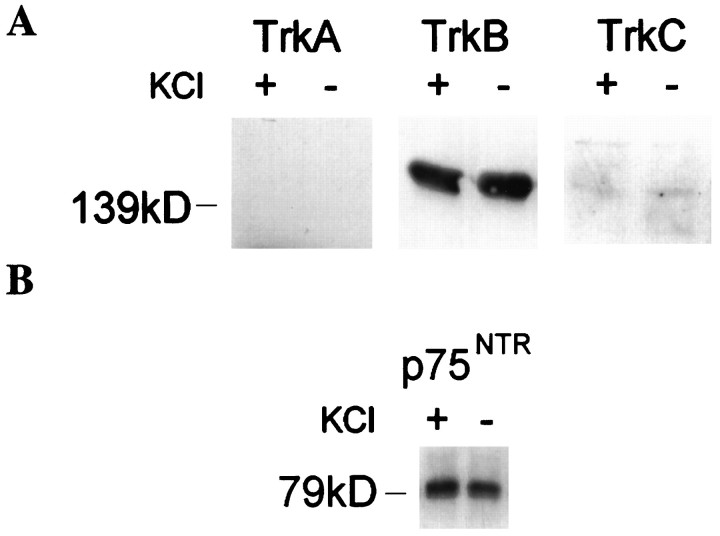
To understand the mechanism of action of the neurotrophins, we determined which neurotrophin receptor subtypes were expressed in cerebellar granule neurons under the conditions in which neurotrophins had been added. Lysates of cells cultured in low or elevated KCl were probed with specific polyclonal antibodies (Fig.5A). Intense staining at ∼145 kDa was obtained with the anti-TrkB antibody. A faint band could be detected with prolonged exposure to the anti-TrkC immunoblot. However, no band was detectable with the anti-TrkA. A polyclonal antiserum raised to the extracellular domain of TrkA (Clary et al., 1994) also was tested. This antiserum detected TrkA (140 kDa) in PC12 extracts, but no comigrating band was detected in granule neuron extracts (data not shown). Slight cross-reactivity was observed with a more slowly migrating band, probably 145 kDa TrkB. We also detected p75NTR protein in cerebellar granule lysates (Fig. 5B) with the polyclonal antibody [9651] used in Figure 2, consistent with previous reports of mRNA for p75NTR in these neurons in culture (Lindholm et al., 1993; Segal et al., 1995) and early during development in vivo (Huber and Chao, 1995).
Fig. 5.
Neurotrophin receptors in cerebellar granule neuron cultures. A, Cerebellar granule neurons at 1 DIV express TrkB, the selective receptor for BDNF, but not TrkA. Slight immunoreactivity to anti-TrkC at the appropriate molecular weight is detectable after prolonged exposure of film. B, Cerebellar granule neurons at 1 DIV express p75NTR, the nonselective neurotrophin receptor.
Regulation of immediate early gene product levels and NF-κB localization by neurotrophins, TNF, and TPA
Trks induce Ras-mediated activation of the MAP kinase cascade (Ohmichi et al., 1992), increasing transcription of the immediate early gene (IEG) c-fos (Cordon-Cardo et al., 1991). On the other hand, in some cells p75NTR and TNF receptors activate JNK (Kyriakis et al., 1994; Casaccia-Bonnefil et al., 1996), a kinase that leads to increased c-jun transcription and c-Jun stability (for review, see Kyriakis et al., 1995; Musti et al., 1997).
To investigate whether these cascades might be activated as a result of exposure to neurotrophins at 1 DIV, we measured the levels of c-Fos and c-Jun in cells stimulated with NGF, BDNF, NT-3, TNF, or TPA by immunoblot densitometry. A 2 hr exposure was chosen because the levels of these proteins clearly are elevated after this time (our unpublished observations). In elevated KCl, BDNF, NT-3, and TPA elevated c-Fos levels, whereas neither NGF nor TNF increased c-Fos (Fig.6A). In low KCl, BDNF still detectably increased c-Fos levels, but the other treatments did not. Given the presence of TrkB protein and the absence of TrkA, these results are consistent with BDNF elevating c-Fos levels via TrkB and presumably the MAP kinase cascade. TPA also may be acting via this pathway (Kolch et al., 1993).
Fig. 6.
Intracellular signaling in response to neurotrophins, TNF, and TPA in cerebellar granule neuron cultures.A, Addition of BDNF, NT-3 (10 ng/ml), or TPA (10 nm) for 2 hr to cells 1 DIV elevates c-Fos levels (*p < 0.05); NGF or TNF (10 ng/ml) does not elevate c-Fos levels. In low KCl (−K) the BDNF-evoked elevation is reduced, and the NT-3-evoked and TPA-evoked elevations are undetectable. B, Addition of NGF, BDNF (10 ng/ml), or TPA (10 nm) for 2 hr elevates c-Jun levels (*p < 0.05, **p < 0.025); NT-3 or TNF (10 ng/ml) does not elevate c-Jun levels. In low KCl (−K) the BDNF-evoked elevation is reduced, and the NGF-evoked elevation is eliminated; the TPA response is maintained under these conditions. C, Immunofluorescently stained 1 DIV cells stimulated for 2 hr with neurotrophins or TNF (100 ng/ml), TPA (100 nm), or C2 ceramide (10 μm), as indicated, were scanned with a confocal microscope. No induction of translocation of cytosolic NF-κB was detected in any condition. −1° denotes control staining omitting 1° antibody.
In elevated KCl, NGF, BDNF, and TPA also increased c-Jun levels; neither NT-3 nor TNF detectably changed c-Jun levels (Fig.6B). The absence of elevated KCl eliminated the response to NGF and partially reduced the response to BDNF, whereas the response to TPA was unchanged. Although TNF did not alter IEG levels detectably at 1 DIV, we have observed c-Jun elevations in response to TNF in 3 DIV cells (data not shown). These results are consistent with NGF acting via a non-Trk receptor, presumably p75NTR, whereas BDNF acts at least in part via a separate, presumably TrkB-mediated, pathway.
p75NTR and TNF-R activation have been reported to evoke nuclear translocation of NF-κB (Yang et al., 1993; Carter et al., 1996a), which recently has been shown to have an antiapoptotic role (Liu et al., 1996). Therefore, we investigated whether the survival-promoting factors that were used evoked translocation of the p65 subunit of NF-κB. Confocal microscopy of immunostained cerebellar granule neurons revealed no bulk translocation of the NF-κB p65 subunit into the nucleus on stimulation with the survival-promoting factors tested (Fig. 6C). However, some constitutive nuclear staining, consistent with a previous report (Guerrini et al., 1995), could be detected under all conditions.
Inhibitor studies
These data suggest that two different mechanisms may mediate the signaling and survival-promoting effects of NGF and BDNF. Because distinct protein kinases may be involved, the effects of kinase inhibitors on the actions of these two factors on cells cultured in elevated KCl were compared.
Wortmannin does not affect basal survival or BDNF-evoked survival promotion
Phosphatidylinositol-3 kinase (PI-3K) is a prominent kinase activated by tyrosine kinase receptors, including Trks, and has been proposed to mediate NGF-evoked survival of neuronally differentiated PC12 cells (Yao and Cooper, 1995). Therefore, we investigated whether inhibition of PI-3K with the specific inhibitor wortmannin (Okada et al., 1994) altered basal or neurotrophin-promoted survival. Wortmannin (100 nm) at 1 DIV had no significant effect on basal cell death at 6 DIV, nor did it prevent survival promotion by BDNF (Fig.7). However, NGF did not improve survival significantly in the presence of this inhibitor. Thus it is unlikely that wortmannin does not inhibit BDNF-evoked survival as a result of low stability. Furthermore, BDNF has a clear survival-promoting effect within 24 hr of addition (data not shown), and TrkB may, in fact, desensitize within this time period (Carter et al., 1995). Thus BDNF-induced survival does not depend on wortmannin-sensitive PI-3Ks.
Fig. 7.
Effects of specific inhibitors on basal and neurotrophin-enhanced survival. Cells were cultured in the presence of elevated potassium and either no neurotrophin, NGF, or BDNF (10 ng/ml) and no inhibitor (Control), wortmannin (100 nm), bisindolylmaleimide (1 μm), or curcumin (10 μm). Neither wortmannin nor bisindolylmaleimide altered basal cell death, but in the presence of bisindolylmaleimide NGF became toxic and BDNF-evoked protection was reduced. Curcumin increased basal cell death, revealing enhanced protection by the neurotrophins. *p < 0.02 and ***p < 0.001 indicate significant difference, as compared with death with the same inhibitor and no neurotrophin; **p < 0.01 indicates significant difference, as compared with the corresponding inhibitor-free control.
PK-C activity is absolutely required for NGF-evoked survival promotion and partly required for full BDNF-evoked survival promotion
PK-C previously has been attributed a mediator role in BDNF-evoked survival in cerebellar granule neurons cultured in serum-free medium (Zirrgiebel et al., 1995). Using the specific PK-C inhibitor bisindolylmaleimide GF 109203X (Toullec et al., 1991), we investigated the role of PK-C in basal survival and neurotrophin-evoked survival promotion in neurons cultured with serum (Fig. 7). Survival under basal conditions was not altered by PK-C inhibition. This suggests that the responses observed to 10 nm TPA were attributable to activation and not to downregulation of PK-C. In further support of this, survival promotion in elevated KCl also was observed with 1 nm TPA (data not shown). In the presence of the PK-C inhibitor, NGF significantly reduces survival, suggesting, first, that the protective effect of NGF is dependent on PK-C and, second, that NGF induces some event that is toxic to the cells, at least in the absence of PK-C activity. The protective effect of BDNF is reduced only partially by the PK-C inhibitor.
The nonselective kinase inhibitor K252a is used frequently to suggest that neurotrophin responses are Trk-mediated. However, this inhibitor is known to have nanomolar IC50 values against PK-A, PK-G, and PK-C (Kase et al., 1987) and is even more potent against phosphorylase kinase and CaM kinase II than it is against Trks (Elliott et al., 1990; Hashimoto et al., 1991; Tapley et al., 1992). Another inhibitor of CaM kinase II potently reduces basal survival in these cells (our unpublished observations); consistent with this, K252a compromised basal survival at concentrations as low as 10 nm, under which conditions BDNF still clearly promoted survival (data not shown). This inhibitor thus is not useful in dissecting the survival responses reported here.
The survival promotion evoked by NGF as a proportion of the cell population is rather modest. Thus we sought conditions under which the signaling evoked by NGF might be more limiting under basal conditions to potentiate survival promotion by NGF. The tumor suppressor curcumin antagonizes the DNA-binding activity of c-Jun (IC50 ∼20 μm; Huang et al., 1991) and inhibits NF-κB translocation (IC50 <40 μm; Singh and Aggarwal, 1995). Both NGF and BDNF increased the levels of c-Jun, and p75NTR activation by NGF has been reported to translocate NF-κB (Carter et al., 1996a), which has been attributed with an antiapoptotic function. Because we detected constitutive levels of c-Jun and of nuclear NF-κB immunoreactivity (Fig.6B,C), we investigated whether partialAP-1/NF-κB antagonism with curcumin might cause the basal levels of c-Jun and translocated NF-κB to be more limiting, thus potentiating survival–promotion via these pathways. The addition of 100 μm curcumin completely eliminated survival of cerebellar granule neurons in the presence or absence of NGF or BDNF (data not shown). However, 10 μm curcumin increased basal death to only 50% (Fig. 7). Under these conditions both NGF and BDNF evoked clear reductions in death, amounting to >20 and 35% of the cells present, respectively.
DISCUSSION
Our study investigates specific signaling mechanisms in neurotrophin-evoked survival–promotion in cerebellar granule neurons during the first week in culture. Considerable cell death occurs during this period. Unlike the delayed phase of cell death, this death is not reduced by inhibition of VSCC activity (Fig. 1). This allowed us to investigate alternative signaling mechanisms, such as those evoked by the neurotrophins, in the survival of these CNS neurons.
NGF and BDNF promote survival of cerebellar granule neurons via two distinct signaling mechanisms
The NGF-evoked and TNF-evoked protection against basal death can be eliminated by VSCC inhibition. In contrast, part of the protection evoked by BDNF and NT-3 persists during VSCC inhibition (Fig.3A). The simplest explanation is that NGF and TNF act via the same pathway, whereas BDNF and NT-3 act in part via this mechanism and in part by an independent pathway. Neurotrophins act via specific Trk receptors and the nonselective p75NTR. The model proposed corresponds to NGF acting exclusively via p75NTR, whereas BDNF and NT-3 act both via this receptor and via a Trk receptor; hence, there are two components of protection resolved by VSCC modulation. TNF would act via its own receptor, known to be structurally and functionally homologous to p75NTR. The finding that anti-p75NTR antiserum, which blocks NGF binding, prevents and even mimics NGF-evoked survival promotion (Fig.2) supports this interpretation.
The presence of an antimitotic ensured that cell counts reflected changes in survival and not proliferation. The cultures used consist of >90% granule neurons, and no increases in contaminating cell types were detected that could account for the modest protection evoked by NGF and TNF (see Materials and Methods). Furthermore, the increased effectiveness of NGF in the presence of curcumin (Fig. 7) strongly suggests that the major cell type, i.e., the cerebellar granule neuron, is responsible for the survival promotion that was observed.
Testing the two-component model
TrkB protein and p75NTR are present, but TrkA is undetectable
We detected TrkB, the specific receptor for BDNF, in cerebellar granule neuron lysates, whereas TrkA, the specific receptor for NGF, was undetectable (Fig. 5). This is consistent with the detection oftrkB, but not trkA, mRNA reported previously (Segal et al., 1992; Lindholm et al., 1993). The faint TrkC immunoreactivity detected may represent cross-reactivity with TrkB; however, TrkC protein probably is present because NT-3 induces tyrosine phosphorylation in these cells of a pan-Trk-immunoprecipitable band migrating more slowly than TrkB (Segal et al., 1995). NT-3 binds to and activates TrkB at the concentrations used here (Soppet et al., 1991) and thus may be expected to mimic BDNF even in the absence of TrkC. Because p75NTR was detected, consistent with the reported detection of mRNA for p75NTR (Segal et al., 1995), NGF presumably is acting via this receptor. This suggests that the actions of NGF are mediated entirely by p75NTR, hence the similarity in survival promotion to that evoked by TNF. BDNF would be expected to act via both TrkB and p75NTR.
Regulation of c-Fos and c-Jun
Trks, unlike p75NTR, increase the expression ofc-fos mRNA via the MAP kinase cascade (Cordon-Cardo et al., 1991; Ohmichi et al., 1992). Consistent with the presence of TrkB, but not TrkA, BDNF elevates c-Fos levels, whereas NGF does not (Fig.6A). p75NTR has been reported to activate JNK (Casaccia-Bonnefil et al., 1996), which increases c-juntranscription and c-Jun stability (for review, see Kyriakis et al., 1994; Musti et al., 1997). The role of Ras activators such as Trks in JNK activation is controversial (Minden et al., 1994; Xia et al., 1995) (see also Masuelli and Cutler, 1996). Thus both NGF and BDNF might be expected to activate JNK via p75NTR, whereas BDNF might induce additional activation via TrkB/Ras. Our results are consistent with these predictions, increases in c-Jun being induced by NGF and BDNF. NGF does not elevate c-Jun levels in low KCl, whereas BDNF again is only partially sensitive to this condition that eliminates the NGF response, supporting our interpretation that BDNF acts via two independent pathways.
Mediators of p75NTR/TNF-R regulation of survival
Ligand binding to p75NTR may promote cell death (Casaccia-Bonnefil et al., 1996; Frade et al., 1996) or survival (Rabizadeh et al., 1993; Cortazzo et al., 1996). This is also the case for TNF-R. Both receptors evoke similar signaling events, including JNK activation (Kyriakis et al., 1994; Casaccia-Bonnefil et al., 1996) and the NF-κB pathway (Yang et al., 1993; Carter et al., 1996a). TNF-evoked death occurs via a TRAF2-independent TRADD–FADD pathway (Hsu et al., 1996). Similar zinc finger proteins may associate with p75NTR (Carter et al., 1996b). The potentiation of NGF survival promotion by curcumin is consistent with an essential requirement for some c-Jun and/or NF-κB for survival, although curcumin also may be acting on other targets, especially when present at 100 μm. TNF-R and p75NTR also activate sphingomyelinase/ceramide signaling (Yanaga and Watson, 1992; Dobrowsky et al., 1995), which may activate JNK (Westwick et al., 1995). Ceramide may be responsible for the NGF-evoked and TNF-evoked survival promotion, because exogenous C2 ceramide also promotes survival of cerebellar granule neurons (Fig. 3B), as it does in sympathetic neurons (Ito and Horigome, 1995).
JNK and c-Jun activation have been proposed to mediate apoptosis (Ham et al., 1995; Xia et al., 1995; Cuvillier et al., 1996; Verheij et al., 1996). However, a noncytotoxic TRAF2-mediated pathway activates JNK and NF-κB in response to TNF (Natoli et al., 1997). In many cases, activation of c-Jun and the JNK pathway correlates with cell maturation (Pulverer et al., 1993; Su et al., 1994; Berberich et al., 1996;Heasley et al., 1996) and protection from apoptosis (Hallahan et al., 1995; Sakata et al., 1995; Johnson et al., 1996) Indeed, the JNK activator SEK1 mediates survival in T-cell development (Nishina et al., 1997). The elevation of c-Jun levels correlates with protection against early cell death in cerebellar granule neurons.
NF-κB may be antiapoptotic (Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996), although it has been proposed to mediate glutamate-evoked death of mature cerebellar granule neurons (Grilli et al., 1996). We did not observe translocation of the p65 subunit of NF-κB on activation of survival-promoting pathways in cerebellar granule neurons (Fig. 6C). However, we cannot exclude NF-κB as a mediator of neuroprotection in cerebellar granule neurons, because multiple NF-κB isoforms exist. Furthermore, it may be that only a small proportion, below our detection limit, of cellular NF-κB need translocate to the nucleus (Gilmore, 1996) for protection to occur.
Measurements of IEG regulation indicate which signaling events can be evoked by the factors and their dependence on depolarization. However the rapid responses at 1 DIV should not be expected to explain all the survival-promoting effects observed 5 d later. For example, not NGF nor TNF nor NT-3 has any detectable effect at 2 hr in low KCl, although each still evokes survival–promotion by 6 DIV. It may be possible that gradual changes not detectable within 2 hr at 1 DIV are evoked, perhaps as a result of developmental changes in receptor expression or spontaneous calcium channel activity. Thus some of the survival promotion does not seem to require increases in c-Fos or c-Jun at such early time points. Although c-Fos is reported to have a short half-life and rapidly downregulate its own expression (Sassone-Corsi et al., 1988) (see also Thompson et al., 1995), we have observed that BDNF addition to cerebellar granule neuron cultures at 1 DIV causes a prolonged elevation of c-Fos protein levels (Coffey et al., 1995).
The role of PK-C in survival
PK-C activity is required for both NGF-evoked survival and a component of BDNF-evoked survival (Fig. 7). This is again consistent with BDNF acting in part via a pathway that also is activated by NGF and in part by an independent pathway. Basal survival is unaffected, suggesting that basal and neurotrophin-evoked survival mechanisms are distinct, and furthermore that any endogenous neurotrophins present do not enhance basal survival. However, this requirement for PK-C activity does not indicate necessarily that NGF activates PK-C in these cells or that this is the mechanism of survival promotion; this is unlikely, given the toxicity of TPA in low KCl. In the presence of the PK-C inhibitor the addition of NGF increases cell death (Fig. 7). PK-C prevents apoptosis-associated DNA fragmentation evoked by ceramide (Obeid et al., 1993), which may accumulate on activation of p75NTR (Dobrowsky et al., 1994, 1995). Thus basal PK-C activity protects against such an NGF/p75NTR-evoked signal that is otherwise toxic, as proposed for TNF receptors (Cuvillier et al., 1996). Because BDNF also seems to act via p75NTR, this would explain the sensitivity of BDNF-evoked survival promotion to PK-C inhibitors we and others (Zirrgiebel et al., 1995) observed. Components of the serum-based medium might be responsible for elevating basal PK-C activity sufficiently to allow NGF to be survival-promoting, explaining why NGF-promoted survival has not been detected in this cell type in serum-free conditions (Segal et al., 1992; Lindholm et al., 1993; Gao et al., 1995).
In summary, NGF activates signal cascades in cerebellar granule neuronal cultures, leading to the elevation of c-Jun levels, and enhances survival in the absence of TrkA protein in a manner blocked by anti-p75NTR antiserum, suggesting a trophic role for p75NTR. BDNF is also protective, acting via an additional independent pathway likely to be via TrkB, which is expressed by the cells. PK-C inhibition reduces BDNF-evoked survival promotion and unmasks NGF/p75NTR-evoked cell death without affecting basal survival in the absence of neurotrophins. Thus three distinct survival mechanisms in these cells are resolved: a basal neurotrophin-independent mechanism, an NGF/BDNF-evoked calcium-sensitive mechanism, and a further mechanism evoked by BDNF, but not NGF.
Footnotes
This work was supported by the Academy of Finland, the Wellcome Trust, the Sigrid Jusélius Stiftelse, the Magnus Ehrnrooth Stiftelse, the Oskar Öflund Stiftelse, and the Finska Vetenskaps-Societeten. We thank Heiti Paves (Estonian Academy of Sciences, Tallinn, Estonia) for providing some neurotrophins for pilot experiments, Louis Reichardt (University of California, San Francisco) for providing us with TrkA-specific antiserum, and Moses Chao (Cornell University Medical College, New York) for providing us with anti-p75NTRantiserum 9651.
Correspondence should be addressed to Dr. Michael J. Courtney, Department of Biochemistry and Pharmacy, Åbo Akademi University, BioCity, P.O. Box 66, FIN-20521 Turku, Finland.
Dr. Åkerman’s present address: Department of Physiology and Medical Biophysics, Uppsala University, BMC Box 572, S-75123 Uppsala, Sweden.
REFERENCES
- 1.Balazs R, Gallo V, Kingsbury A. Effect of depolarization on the maturation of cerebellar granule cells in culture. Brain Res. 1988a;468:269–276. doi: 10.1016/0165-3806(88)90139-3. [DOI] [PubMed] [Google Scholar]
- 2.Balazs R, Hack N, Jorgensen OS. Stimulation of the N-methyl-d-aspartate receptor has a trophic effect on differentiating cerebellar granule cells. Neurosci Lett. 1988b;87:80–86. doi: 10.1016/0304-3940(88)90149-8. [DOI] [PubMed] [Google Scholar]
- 3.Balazs R, Jorgensen OS, Hack N. N-methyl-d-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988c;27:437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- 4.Balazs R, Hack N, Jorgensen OS. Interactive effects involving different classes of excitatory amino acid receptors and the survival of cerebellar granule cells in culture. Int J Dev Neurosci. 1990;8:347–359. doi: 10.1016/0736-5748(90)90068-d. [DOI] [PubMed] [Google Scholar]
- 5.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 6.Berberich I, Shu G, Siebelt F, Woodgett JR, Kyriakis JM, Clark EA. Cross-linking CD40 on B cells preferentially induces stress-activated protein kinases rather than mitogen-activated protein kinases. EMBO J. 1996;15:92–101. [PMC free article] [PubMed] [Google Scholar]
- 7.Carter BD, Zirrgiebel U, Barde YA. Differential regulation of p21ras activation in neurons by nerve growth factor and brain-derived neurotrophic factor. J Biol Chem. 1995;270:21751–21757. doi: 10.1074/jbc.270.37.21751. [DOI] [PubMed] [Google Scholar]
- 8.Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhäuser N, Böhm-Mattaei R, Baeuerle PA, Barde YA. Selective activation of NF-κB by nerve growth factor through the neurotrophin receptor p75. Nature. 1996a;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 9.Carter BD, Kaltschmidt C, Dechant G, Casademunt E, Chao MV, Barde YA. Mechanisms of signal transduction through the p75 neurotrophin receptor. Soc Neurosci Abstr. 1996b;22:482.10. [Google Scholar]
- 10.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;282:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 11.Chao MV, Hempstead BL. p75 and Trk: a two receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 12.Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffey ET, Åkerman KEO, Courtney MJ. Regulation of cerebellar granule cell development by BDNF and depolarization. Soc Neurosci Abstr. 1995;21:422.5. [Google Scholar]
- 14.Copani A, Bruno VMG, Barresi V, Battaglia G, Condorelli DF, Nicoletti F. Activation of metabotropic glutamate receptors prevents neuronal apoptosis in culture. J Neurochem. 1995;64:101–108. doi: 10.1046/j.1471-4159.1995.64010101.x. [DOI] [PubMed] [Google Scholar]
- 15.Cordon-Cardo C, Tapley P, Jing SQ, Nanduri V, O’Rourke E, Lamballe F, Kovary K, Klein R, Jones KR, Reichardt LF, Barbacid M. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991;66:173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortazzo MH, Kassis ES, Sproul KA, Schor NF. Nerve growth factor (NGF)-mediated protection of neural crest cells from antimitotic agent-induced apoptosis: the role of the low-affinity NGF receptor. J Neurosci. 1996;16:3895–3899. doi: 10.1523/JNEUROSCI.16-12-03895.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtney MJ, Nicholls DG. Interactions between phospholipase C-coupled and NMDA receptor in cultured cerebellar granule cells: protein kinase C-mediated inhibition of NMDA responses. J Neurochem. 1992;59:983–992. doi: 10.1111/j.1471-4159.1992.tb08339.x. [DOI] [PubMed] [Google Scholar]
- 18.Courtney MJ, Lambert JJ, Nicholls DG. The interactions between plasma membrane depolarization and glutamate receptor activation in the regulation of cytoplasmic-free calcium in cultured cerebellar granule cells. J Neurosci. 1990;10:3873–3879. doi: 10.1523/JNEUROSCI.10-12-03873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 20.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 21.Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis. J Biol Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- 22.Doherty P, Ashton SV, Moore SE, Walsh FS. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G-protein-dependent activation of L and N-type neuronal Ca+ channels. Cell. 1991;67:21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- 23.Elliott LH, Wilkinson SE, Sedgwick AD, Hill CH, Lawton G, Davis PD, Nixon JS. K252a is a potent and selective inhibitor of phosphorylase kinase. Biochem Biophys Res Commun. 1990;171:148–154. doi: 10.1016/0006-291x(90)91369-4. [DOI] [PubMed] [Google Scholar]
- 24.Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 25.Gallo V, Kingsbury A, Balazs R, Jorgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao WQ, Zheng JL, Karihaloo M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J Neurosci. 1995;15:2656–2667. doi: 10.1523/JNEUROSCI.15-04-02656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilmore TD. Regulation of Rel transcription complexes. In: Goodbourne S, editor. Eukaryotic gene transcription. IRL, Oxford UP; Oxford: 1996. pp. 102–131. [Google Scholar]
- 28.Glass DJ, Yancopoulos GD. The neurotrophins and their receptors. Trends Cell Biol. 1993;3:262–267. doi: 10.1016/0962-8924(93)90054-5. [DOI] [PubMed] [Google Scholar]
- 29.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 30.Guerrini L, Blasi F, Denis-Donini S. Synaptic activation of NF-κB by glutamate in cerebellar granule neurons in vitro. Proc Natl Acad Sci USA. 1995;92:9077–9081. doi: 10.1073/pnas.92.20.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallahan DE, Dunphy E, Virudachalam S, Sukhatme VP, Kufe DW, Weichselbaum RR. c-Jun and Egr-1 participate in DNA synthesis and cell survival in response to ionizing radiation exposure. J Biol Chem. 1995;270:30303–30309. doi: 10.1074/jbc.270.51.30303. [DOI] [PubMed] [Google Scholar]
- 32.Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, Rubin LL. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 33.Harlow E, Lane D. Antibodies. Cold Spring Harbor Laboratory; A laboratory manual. Cold Spring Harbor, NY: 1988. [Google Scholar]
- 34.Hashimoto Y, Nakayama T, Teramoto T, Kato H, Watanabe T, Kinoshita M, Tsukamoto K, Tokunaga K, Kurokawa K, Nakanishi S, Matsuda Y, Nomura Y. Potent and preferential inhibition of Ca2+/calmodulin-dependent protein kinase II by K252a and its derivative, KT5926. Biochem Biophys Res Commun. 1991;181:423–429. doi: 10.1016/s0006-291x(05)81436-6. [DOI] [PubMed] [Google Scholar]
- 35.Heasley LE, Storey B, Fanger GR, Butterfield L, Zamarripa J, Blumberg D, Maue RA. GTPase-deficient G alpha 16 and G alpha q induce PC12 cell differentiation and persistent activation of c-Jun NH2 terminal kinases. Mol Cell Biol. 1996;16:648–656. doi: 10.1128/mcb.16.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD–TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 37.Huang T-S, Lee S-C, Lin J-K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci USA. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol. 1995;167:227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- 39.Ito A, Horigome K. Ceramide prevents neuronal programmed cell death induced by nerve growth factor deprivation. J Neurochem. 1995;65:463–466. doi: 10.1046/j.1471-4159.1995.65010463.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson NL, Gardner AM, Diener KM, Lange-Carter CA, Gleavy J, Jarpe MB, Minden A, Karin M, Zon LI, Johnson GL. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 41.Juurlink BH, Hertz L. Ischemia-induced death of astrocytes and neurons in primary culture: pitfalls in quantifying neuronal cell death. Dev Brain Res. 1993;71:239–246. doi: 10.1016/0165-3806(93)90175-a. [DOI] [PubMed] [Google Scholar]
- 42.Kao JPY, Harootunian AT, Tsien RY. Photochemically generated cytosolic calcium pulses and their detection by Fluo-3. J Biol Chem. 1989;264:8179–8184. [PubMed] [Google Scholar]
- 43.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 44.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 45.Kingsbury AE, Gallo V, Woodhams PL, Balazs R. Survival, morphology, and adhesion properties of cerebral interneurones cultured in chemically defined and serum-supplemented medium. Dev Brain Res. 1985;17:17–25. doi: 10.1016/0165-3806(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 46.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marmé D, Rapp UR. Protein kinase C α activates Raf-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 47.Krueger BK, Burne JF, Raff MC. Evidence for large-scale astrocyte death in the developing cerebellum. J Neurosci. 1995;15:3366–3374. doi: 10.1523/JNEUROSCI.15-05-03366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 49.Kyriakis JM, Woodgett JR, Avruch J. The stress-activated protein kinases. A novel ERK subfamily responsive to cellular stress and inflammatory cytokines. Ann NY Acad Sci. 1995;766:303–319. doi: 10.1111/j.1749-6632.1995.tb26683.x. [DOI] [PubMed] [Google Scholar]
- 50.Landis DMD, Sidman RL. Electron microscopic analysis of postnatal histogenesis in the cerebellar cortex of staggerer mutant mice. J Comp Neurol. 1978;179:831–864. doi: 10.1002/cne.901790408. [DOI] [PubMed] [Google Scholar]
- 51.Lindholm D, Dechant G, Heisenberg CP, Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993;5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 52.Lindqvist C, Holmberg C, Oetken C, Courtney M, Ståhls A, Åkerman KEO. Rapid Ca mobilization in single LGL cells upon interaction with K562 target cells—role of the CD18 and CD16 molecules. Cell Immunol. 1995;165:71–76. doi: 10.1006/cimm.1995.1188. [DOI] [PubMed] [Google Scholar]
- 53.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis, while NF-κB activation prevents cell death. Cell. 1996;87:565–575. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 54.Mahadeo D, Kaplan L, Chao MV, Hempstead BL. High-affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J Biol Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- 55.Masuelli L, Cutler ML. Increased expression of the ras suppressor rsu-1 enhances erk-2 activation and inhibits jun kinase activation. Mol Cell Biol. 1996;16:5466–5476. doi: 10.1128/mcb.16.10.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menon SD, Qin S, Guy GR, Tan YH. Differential induction of nuclear NF-κB by protein phosphatase inhibitors in primary and transformed human cells. Requirement for both oxidation and phosphorylation in nuclear translocation. J Biol Chem. 1993;268:26805–26812. [PubMed] [Google Scholar]
- 57.Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 58.Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 59.Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 60.Nishina H, Fischer KD, Radvanyi L, Shahinian A, Hakem R, Rubie EA, Bernstein A, Mak TW, Woodgett JR, Penninger JM. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 61.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 62.Ohmichi M, Pang L, Decker SJ, Saltiel AR. Nerve growth factor stimulates the activities of the raf-1 and the mitogen-activated protein kinases via the trk protooncogene. J Biol Chem. 1992;267:14604–14610. [PubMed] [Google Scholar]
- 63.Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol-3 kinase. J Biol Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- 64.Pulverer BJ, Hughes K, Franklin CF, Kraft CS, Leevers SJ, Woodgett JR. Co-purification of mitogen-activated protein kinases with phorbol ester-induced c-Jun kinase activity in U937 leukemic cells. Nature. 1993;353:670–674. [PubMed] [Google Scholar]
- 65.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 66.Resink A, Hack N, Boer GJ, Balazs R. Growth conditions differentially modulate the vulnerability of developing cerebellar granule cells to excitatory amino acids. Brain Res. 1994;655:222–232. doi: 10.1016/0006-8993(94)91617-9. [DOI] [PubMed] [Google Scholar]
- 67.Sakata N, Patel HR, Terada N, Aruffo A, Johnson GL, Gelfand EW. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J Biol Chem. 1995;270:30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- 68.Sassone-Corsi P, Sisson JC, Verma IM. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 69.Segal RA, Takahashi H, McKay RD. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992;9:1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- 70.Segal RA, Pomeroy SL, Stiles CD. Axonal growth and fasciculation linked to differential expression of BDNF and NT-3 in developing cerebellar granule cells. J Neurosci. 1995;15:4970–4981. doi: 10.1523/JNEUROSCI.15-07-04970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloymethane). J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 72.Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, Nikilics K, Parada LF. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 73.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 74.Thangnipon W, Kingsbury A, Webb M, Balazs R. Observations on rat cerebellar granule cells in vitro: influence of substratum, potassium concentration, and relationship between neurones and astrocytes. Eur J Pharmacol. 1983;140:275–283. doi: 10.1016/0165-3806(83)90215-8. [DOI] [PubMed] [Google Scholar]
- 75.Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- 76.Thompson MA, Ginty DD, Bonni A, Greenberg ME. L-type voltage-sensitive Ca2+ channel activation regulates c-fos transcription at multiple levels. J Biol Chem. 1995;270:4224–4235. doi: 10.1074/jbc.270.9.4224. [DOI] [PubMed] [Google Scholar]
- 77.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Biossin E, Boursier E, Loriolle F, Duhame L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 78.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 79.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 80.Vetter ML, Martin-Zanca D, Parada LF, Bishop JM, Kaplan DR. Nerve growth factor rapidly stimulates tyrosine phosphorylation of phospholipase C gamma 1 by a kinase activity associated with the product of the trk protooncogene. Proc Natl Acad Sci USA. 1991;88:5650–5654. doi: 10.1073/pnas.88.13.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C-Y, Mayo MW, Baldwin AS., Jr TNF-α and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 82.Weber A, Schachner M. Maintenance of immunocytologically identified Purkinje cells from mouse cerebellum in monolayer culture. Brain Res. 1984;311:119–130. doi: 10.1016/0006-8993(84)91404-5. [DOI] [PubMed] [Google Scholar]
- 83.Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 84.Wood KA, Dipasquale B, Youle RJ. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- 85.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 86.Yanaga F, Watson SP. Tumor necrosis factor alpha stimulates sphingomyelinase through the 55 kDa receptor in HL-60 cells. FEBS Lett. 1992;314:297–300. doi: 10.1016/0014-5793(92)81493-6. [DOI] [PubMed] [Google Scholar]
- 87.Yang Z, Costanzo M, Golde DW, Kolesnick RN. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor κB translocation in intact HL-60 cells. J Biol Chem. 1993;268:20520–20523. [PubMed] [Google Scholar]
- 88.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 89.Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D. Characterisation of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]