Abstract
We studied by in situ hybridization histochemistry the expression of D3 receptor (D3R) mRNA at various stages of rat brain development. The first expression of D3R mRNA was detected at embryonic day 14 (E14) in the striatal and rhinencephalic neuroepithelia and throughout the tectal neuroepithelium. From E16 to E19 D3R mRNA expression extended along a rostrocaudal axis to additional proliferative ventricular zones of the basal forebrain, including the neuroepithelia of the olfactory bulb, nucleus accumbens, septum, and amygdala, whereas D1 and D2 receptor (D1R and D2R) mRNAs were expressed predominantly by migrating neuroblasts and/or differentiating striatal neurons. Only a few neuroblasts, migrating in the lateral cortical stream or developing as cerebellar Purkinje cells, expressed D3R mRNA from E18. At birth D3R expression mRNA appeared in differentiating neuronal fields of the nucleus accumbens and medial mamillary body primordia and on P5 reached a distribution similar to that found in adult. In addition, a transient upregulation was detected on P5 in the medial mamillary bodies, parietofrontal cortex, and olfactory tubercle. In the adult brain D3R gene expression continued in the striatal proliferative subventricular zone. The late expression D3R mRNA in neurons, after achievement of dopamine innervation, supports the existence of a regulating factor released from dopamine neurons, as suggested by denervation studies in the adult. The sustained and abundant D3R gene expression, predominantly in germinative neuroepithelial zones actively involved in neurogenesis of most basal forebrain structures, supports the hypothesis of a neurogenetic but minor morphogenetic modulatory role for the D3R during CNS development.
Keywords: in situ hybridization, neuroepithelium, neurogenesis, forebrain development, dopamines, D3R mRNA
Catecholamines seem to affect a number of developmental processes in primitive organisms, including growth, regeneration, and morphogenesis (Lauder, 1993). The dopaminergic system appears early in brain development of higher species (Voorn et al., 1988), and a neurodevelopmental role for dopamine has been suggested (Rosengarten and Friedhoff, 1979; Miller and Friedhoff, 1986).
Dopamine interacts with five dopamine receptor subtypes (D1R–D5R) having distinct localization and intracellular signaling, allowing this neurotransmitter to exert pleiotropic influences on target cells (Sibley and Monsma, 1992;Gingrich and Caron, 1993; Sokoloff and Schwartz, 1995). The D3R (Sokoloff et al., 1990) has a density in the adult rat brain two orders of magnitude lower than that of the D2R and is expressed in discrete brain regions, including the ventral striatum, mamillary bodies, and archicerebellum (Bouthenet et al., 1991; Lévesque et al., 1992; Diaz et al., 1995).
D1R and D3R both occur in the islands of Calleja and nucleus accumbens (Le Moine and Bloch, 1996), whereas colocalization of D2R and D3R seems exceptional (Bouthenet et al., 1991). It is not known whether these respective coexpression and segregation originate early in development. Previous works have detailed ontogeny of D1R and D2R in the rat brain (Guennoun and Bloch, 1992; Schambra et al., 1994). D1R and D2R messages are present from gestational day 14 in low amounts and increase slowly during development. These receptors are expressed mainly by migrating and maturing neurons, similar in location to those found in the adult brain, suggesting that respective receptor expression is an intrinsic property of these neurons (Schambra et al., 1994).
The regulation of D3R gene expression in adult is highly dependent on dopamine innervation. Thus, a lesion of dopamine neurons, impairment of axonal transport, or reduction of dopamine neuron firing, but not removal of dopamine or its known cotransmitter transmission, decreases D3R gene expression (Lévesque et al., 1995). This has led to the proposal that D3R gene regulation is under the positive influence of a yet unidentified anterograde factor released from dopamine neurons.
The prenatal development of D3R expression is documented very poorly. With the use of the highly sensitive reverse PCR, D3R transcripts were detected faintly in rodent embryos from gestational days 10–11 and clearly on day 14 (Cadoret et al., 1993; Fishburn et al., 1996). These studies, however, did not reveal the localization of D3R transcripts in embryonic brain. Autoradiographic analysis of rat brain during prenatal ontogeny with the use of the D2R/D3R ligand [125I]iodosulpride (Sales et al., 1988) revealed transient expressions of binding sites in the ventral part of the spinal cord and in the lateral ventricle lining that did not match D2R mRNA distribution and, therefore, possibly that were related to the D3R. In addition, a transient expression of D3R protein was observed in the parietal cortex during postnatal development of mouse brain (Demotes-Mainard et al., 1996).
Using in situ hybridization, we mainly show here that D3R mRNA expression is restricted almost entirely to the ventricular neuropithelium during the whole prenatal ontogeny and that the neuronal expression of the D3R appears later, after the settling of dopamine innervation.
MATERIALS AND METHODS
Animals and dissection. Timed pregnant female Wistar rats (Iffa-Credo) were used. Gestational age was determined from the mating time, and embryonic day 1 (E1) was designated as the day after insemination. After a 21 d gestation the day of birth was designed as postnatal day 0 (P0), and subsequent age was defined relative to this. For studies involving prenatal series, pregnant rats were decapitated, and embryos and fetuses were removed by cesarean section on E12, E14, E16, E17, E18, and E19. Postnatal studies were made with animals at ages P0 and P5.
Tissue preparation. Embryos and fetuses (E12–E19) removed free from the amniotic membranes were whole-frozen in liquid monochlorodifluoromethane (−30°C) and stored at −70°C. The postnatal animals (P0 and P5 pups) were decapitated, and their heads were collected and frozen. Tissue sections (8 and 10 μm for pre- and postnatal, respectively) made in coronal and sagittal planes on a cryostat were thaw-mounted at room temperature on SuperFrost Plus slides (Menzel-Glasser), fixed for 40 min at 4°C in 4% paraformaldehyde made up in 0.1 m PBS, and rinsed twice (5 min each) in PBS at 4°C and then for 5 min in PBS at room temperature. The sections were rinsed briefly in distilled water, dehydrated via graded alcohols, dried under a stream of cold air, and stored at −70°C until they were processed for in situhybridization.
Probes. The probes used were 33P-labeled riboprobes. Probes for rat D2R and D3R mRNAs corresponded to respective sequences of the third intracellular loop of the receptor and are described elsewhere (Sokoloff et al., 1990;Bouthenet et al., 1991). The probe for rat D1R corresponded to a C-terminal fragment (nucleotides 1382–1708) obtained by PCR and subcloned into pGEM-4Z. Riboprobes were synthesized with the Riboprobe Gemini System (Promega, Madison, WI), treated by RNase-free DNase (Boehringer Mannheim, Mannheim, Germany), and recovered from Chroma Spin −30 columns (Clontech, Cambridge, UK).
In situ hybridization. Thawed sections were treated with proteinase K (1 mg/ml) for 10 min at 37°C, acetylated in 0.1 m triethanolamine, pH 8, and 0.25% acetic anhydride for 10 min, rinsed in 2× SSC (20× SSC is 0.3 mTris-citrate and 3 m NaCl), dehydrated in increased concentrations of graded ethanol, and air-dried. Sections were covered with 50 μl of a hybridization buffer containing 70% formamide (for D2R and D3R) or 50% formamide (for D1R), 10% dextran sulfate, 1× Denhardt’s solution, 4× SSC (for D2R and D3R) or 2× SSC (for D1R), 0.1% Na-pyrophosphate, 100 μg/ml yeast-tRNA, 100 μg/ml denatured salmon sperm-DNA, 50 mm Tris-HCl, pH 7.4, 1 mm EDTA, and 2 × 106 dpm of a 33P-labeled antisense or sense probe. Slides were covered with squares of Nescofilm (Roth) and incubated overnight (12–14 hr) at 55°C. Then sections were cooled at room temperature in 2× SSC, treated with RNase A (200 μg/ml) for 1 hr at 37°C, and rinsed twice in 2× SSC for 15 min at room temperature, in 0.5× SSC for 30 min at 55°C, and in 0.1× SSC for 30 min at 60°C. Sections were dehydrated in graded alcohols containing 300 mm ammonium acetate and were dried under a stream of cold air. Autoradiograms were generated by apposing the radiolabeled tissue sections to β-Max Hyperfilms (Amersham, Braunschweig, Germany) for 1–2 weeks for D1R and D2R and 3–4 weeks for D3R.
Some sections from animals at each stage of development studied were dipped in a photographic emulsion (LM-1, Amersham) and exposed for 30–40 d. Dipped sections were counterstained with Mayer’s hemalum solution and examined with a photomicroscope Axiophot (Zeiss, Oberkochen, Germany).
RESULTS
We performed In situ hybridization using a33P-labeled D3R cRNA probe on brain sections of embryos, heads of fetuses and pups, from the 12th gestation day (13 d after insemination, E12) until the 5th day after birth (P5). Two to four animals from at least two different offspring were used at each stage. Structure identification, name, and abbreviations (Table1) are in agreement with the recommendations of theBoulder Committee (1970) and the atlas of the rat developing brain byBayer and Altman (1995). The regional and cellular distribution of D3R mRNA expression was determined with both film autoradiograms and emulsion-coated microautoradiographies on coronal and sagittal sections taken at various levels throughout the whole developing brain, but only relevant levels are illustrated on Figures1, 2, 3, 4, 5, 6, 7, 8, 9. In addition, cRNA probes for D1R and D2R subtypes were used in some experiments for comparison. Control hybridization experiments with sense probes resulted in autoradiograms devoid of signal (data not shown).
Table 1.
Abbreviations used in figures
| 4V | Fourth ventricle |
| IX | Cerebellum, lobule 9 |
| X | Cerebellum, lobule 10 |
| Acc | Nucleus accumbens |
| AccSh | Nucleus accumbens, shell part |
| AccC | Nucleus accumbens, core part |
| Amy | Amygdala |
| CC | Corpus callosum |
| Ci | Cingulate cortex |
| Cx | Cortex |
| Fr | Frontal cortex |
| ICj | Islands of Calleja |
| ICjM | Island of Calleja, major |
| In | Insulate cortex |
| Lcs | Lateral cortical stream |
| LV | Lateral ventricle |
| M | Mitotic zone of the neuroepithelium |
| MB | Medial mamillary bodies |
| Ne | Neuroepithelium |
| OB | Olfactory bulb |
| Pa | Pallidum |
| PFCx | Parietofrontal cortex |
| Pu | Purkinje cell layer |
| Re | Reservoir of lateral cortical stream |
| Rh | Rhinencephalon |
| S | Synthetic zone of the neuroepithelium |
| Sp | Septum |
| St | Striatum |
| SVZ | Subventricular zone |
| TNe | Tectal neuroepithelium |
| Tu | Pyramidal layer of olfactory tubercle |
Fig. 1.
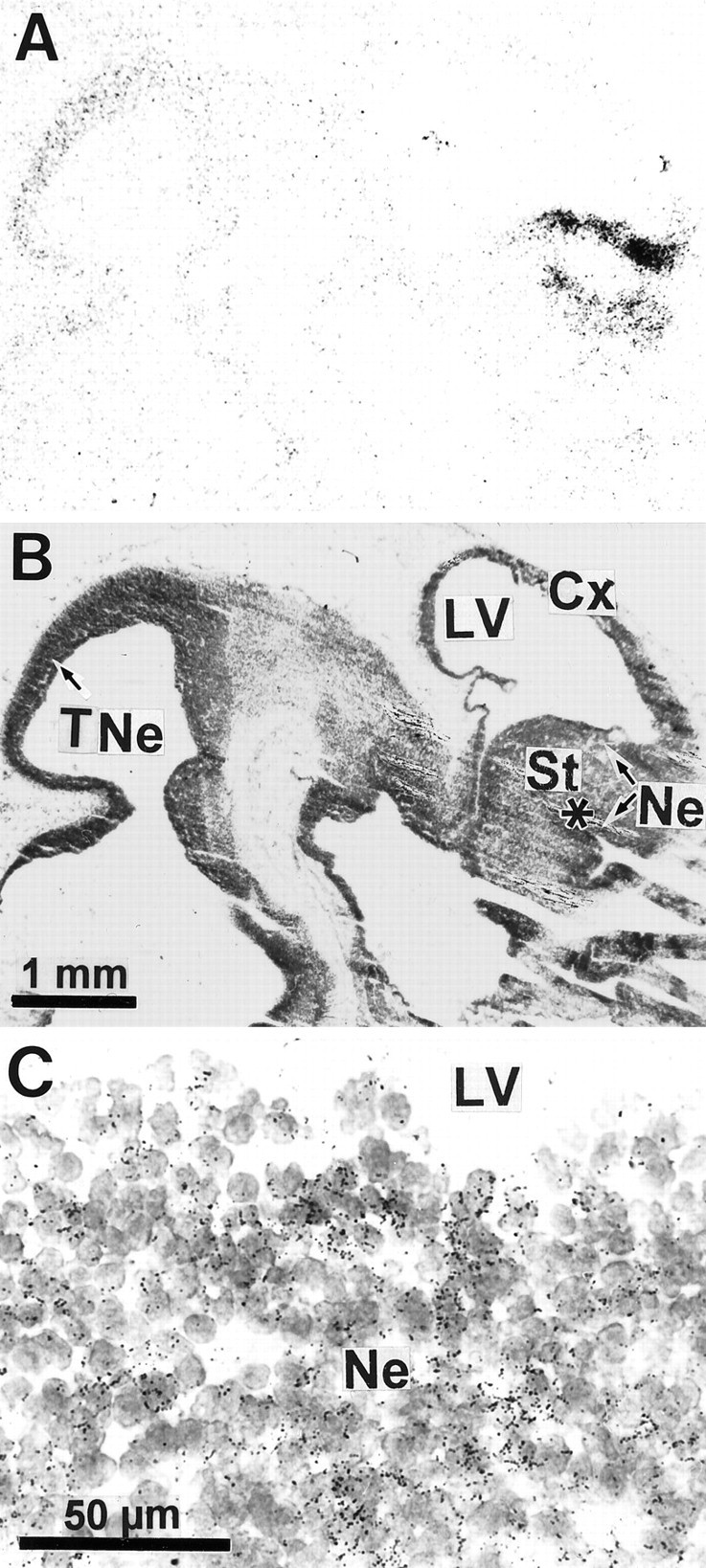
Expression of D3R mRNA in the brain of the 14 d embryo. A, Film autoradiographic hybridization signals from a parasagittal section hybridized with a D3R mRNA antisense probe. Hybridization signals are present only in neuroepithelia of striatum (St), rhinencephalon (indicated by asterisk), and tectum (TNe). Ct, Cortex. B, Structure locations are shown on the same section counterstained with Mayer’s hemalum solution and photographed with bright-field illumination. C, Photomicrograph of an emulsion-dipped section with bright-field illumination at the level of striatal neuroepithelium lining the lateral ventricle (LV), showing groups of labeled cells identified by clusters of dark silver grains overlying individual nuclei in the neuroepithelium (Ne).
Fig. 2.
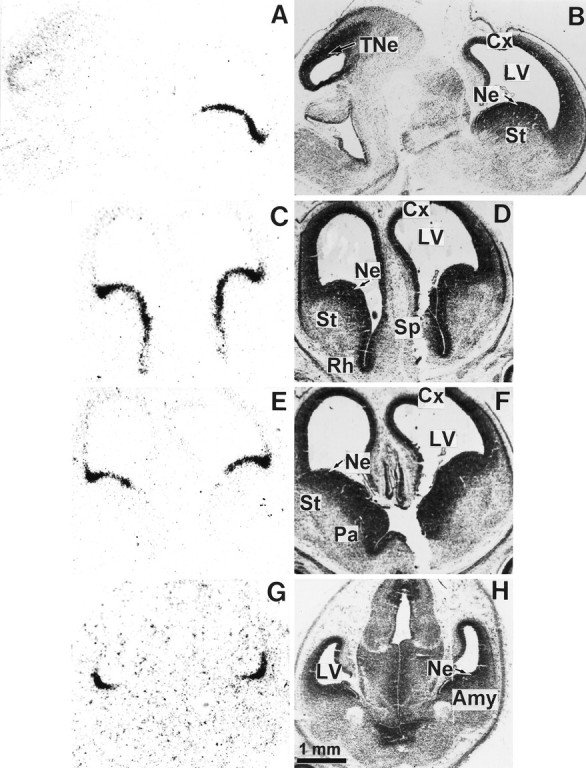
Expression of D3R mRNA in the brain of the 16 d embryo. The left panel shows film autoradiograms from a parasagittal section (A) and three coronal serial sections (C, E,G) hybridized with the D3R mRNA probe. TheC, E, and G sections are ordered from rostral to caudal forebrain levels. The right panel shows the same sections counterstained with Mayer’s hemalum and photographed in bright-field illumination. The expression of D3R gene transcripts in the basal forebrain neuroepithelium, continuous from rostral striatum (St) to amygdala (Amy), is interrupted remarkably at the pallidum level (Pa). Cx, Cortex;LV, lateral ventricle; Ne, neuroepithelium; Rh, rhinencephalon; Sp, septum; TNe, tectal neuroepithelium.
Fig. 3.
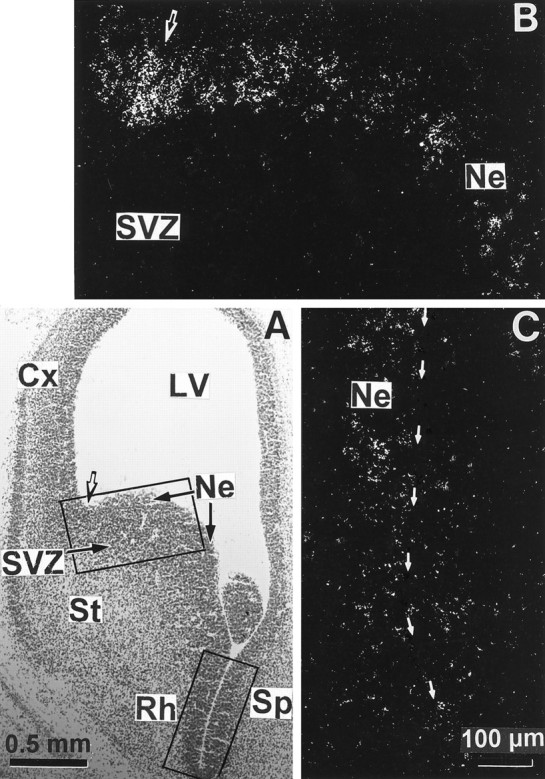
High magnifications of D3R mRNA hybridization signals in basal forebrain neuroepithelium of the 16 d embryo. The top and bottom rectangles in a bright-field photomicrograph from a coronal section counterstained with Mayer’s hemalum (A) delimit the fields shown inB and C, respectively. B,C, Dark-field photomicrographs taken from an emulsion-dipped section through the rostral striatal anlage that show the labeling in the striatal (St), rhinencephalic (Rh), and septal (Sp) neuroepithelia bordering the ventral horn of the lateral ventricle (LV), indicated by thin arrows inC, whereas the subventricular zone (SVZ) and the differentiating field of striatum are labeled only scarcely. The strongest signal is present approximately at the limit between striatal and cortical neuroepithelia in A, as determined by the dorsolateral corner of the lateral ventricle (indicated bythick arrows in A and B).Cx, Cortex; Ne, neuroepithelium.
Fig. 4.
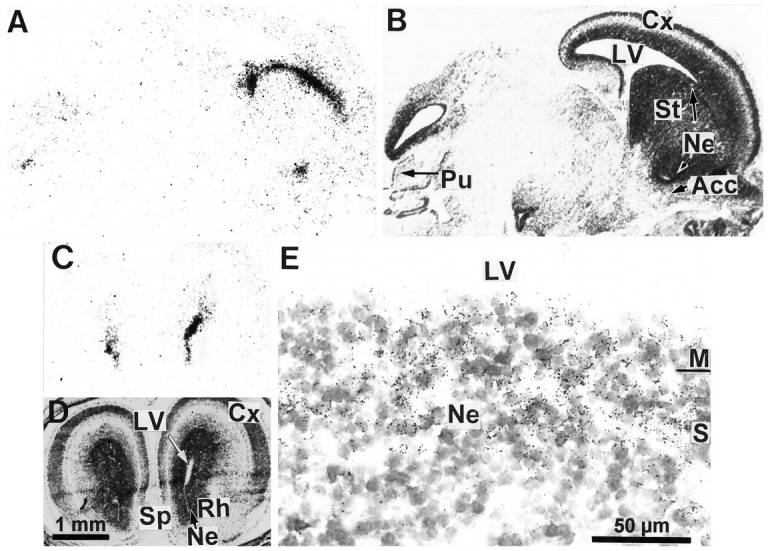
Expression of D3R mRNA in the basal forebrain and cerebellar primordia of the 18 d embryo. Autoradiograms of parasagittal (A) and coronal (C) sections hybridized with the D3R mRNA probe show signals in neuroepithelia (Ne) of striatum (St), nucleus accumbens (Acc), rhinencephalon (Rh), septum (Sp), and in the more caudal Purkinje cell layer (Pu) of cerebellar primordium. B, D, Corresponding sections counterstained with Mayer’s hemalum. High magnification of the striatal ventricular zone under bright-field illumination (E) illustrates that labeling is restricted to clusters of labeled cells present through the synthetic (S) and mitotic (M) neuroepithelial zones, delimiting a continuous periventricular band 100 μm thick. Cx, Cortex; LV, lateral ventricle; SVZ, subventricular zone.
Fig. 5.
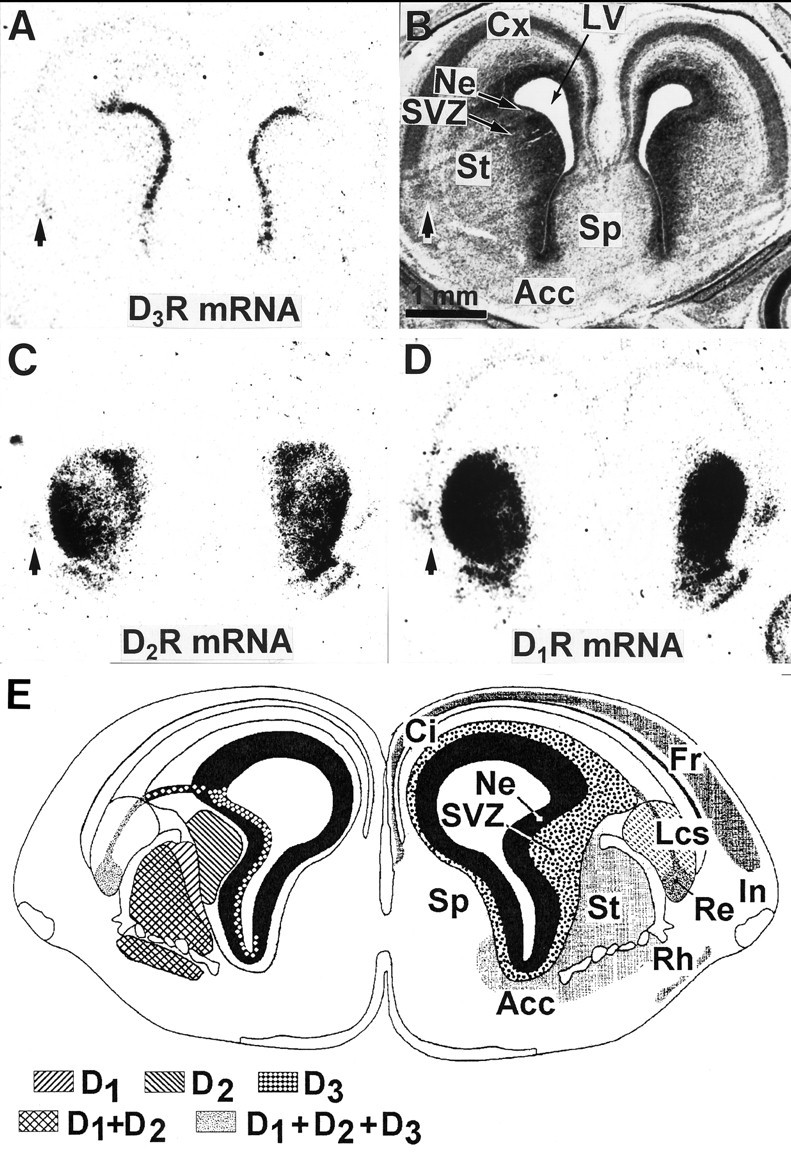
Compared expressions of D1R, D2R, and D3R mRNAs in the forebrain of the 18 d embryo. Shown are film autoradiograms of adjacent coronal sections at a rostral level of the developing striatum, hybridized with probes specific for either D3R (A), D2R (C), or D1R (D) mRNAs. B, Bright-field photomicrograph of section A, counterstained with the Mayer’s hemalum. Cells in the reservoir of lateral cortical stream (indicated by arrows) express the three receptor subtype mRNAs (A, C, D). The cortical neuroepithelium and cortical plate (Cx) are labeled by the D1R and D3R, but not D2R, mRNA probes. D1R, D2R, and D3R mRNAs are expressed in different striatal compartments, as shown inE, which represents a schematic coronal section at the same level and developmental stage, taken from the atlas by Bayer and Altman (1995) in which the right cerebral hemisphere displays the structures (Acc, nucleus accumbens; Ci, cingulate cortex; Fr, frontal cortex; In, insulate cortex; Lcs, lateral cortical stream;Re, reservoir; Rh, rhinencephalon;Sp, septum; St, striatum) and the left hemisphere displays the respective distributions of D1R, D2R, and D3R mRNAs. In the striatum D1R mRNA is present in the differentiation field (St), D2R mRNA in the subventricular zone (SVZ) and in the differentiation field, and D3R mRNA in the neuroepithelium (Ne).
Fig. 6.
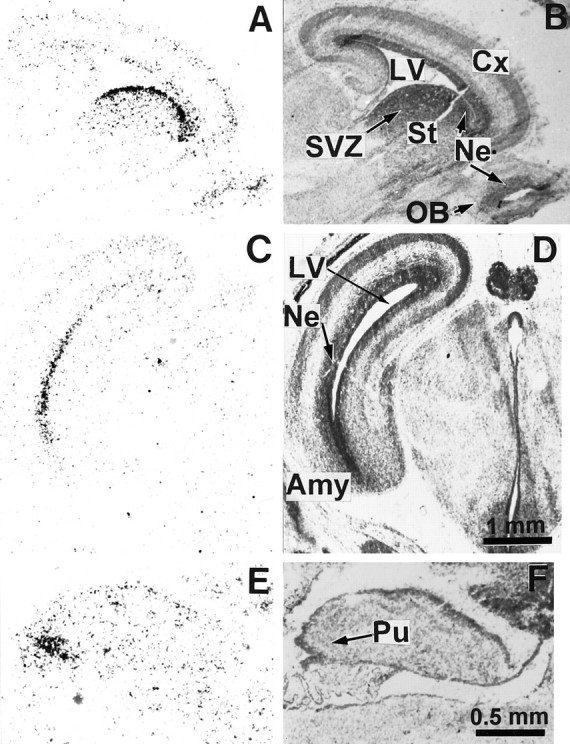
Expression of D3R mRNA in the brain of the 19 d embryo. The left panel shows film autoradiograms from parasagittal (A, E) and coronal (C) sections hybridized with the D3R mRNA probe. The right panels show corresponding sections counterstained with Mayer’s hemalum and photographed in bright-field illumination. Hybridization signals are detected at high level in several periventricular neuroepithelial regions (Ne) of the basal forebrain, including the striatum (St in A), amygdala (Amy in C), and in a group of Purkinje cells (Pu in E). Low expression of D3R is seen in the ventricular zone of the cortex and cortical plate (Cx), olfactory bulb (OB), and subventricular zone (SVZ) of the striatum inA. LV, Lateral ventricle.
Fig. 7.
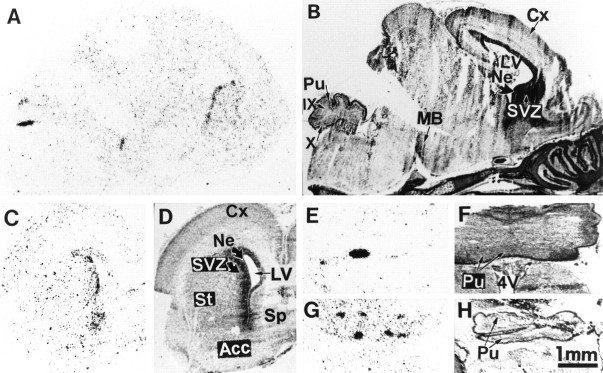
In situ hybridization of D3R mRNA in the brain of a newborn pup. A,C, E, and G are autoradiograms of parasagittal (A) and coronal (C, E, G) sections hybridized with the D3R probe; B,D, F, and H are corresponding sections counterstained with Mayer’s hemalum. D3R mRNA is seen in the residual neuroepithelium (Ne) and the adjacent subventricular zone (SVZ) of the striatum (St), nucleus accumbens (Acc), and septum (Sp) as well as in the medial mamillary body (MB) and as patches in the Purkinje cell layer (Pu) of lobules 9 (IX) and 10 (X) in the cerebellar cortex. Cx, Cerebral cortex;LV, lateral ventricle; 4V, fourth ventricle.
Fig. 8.
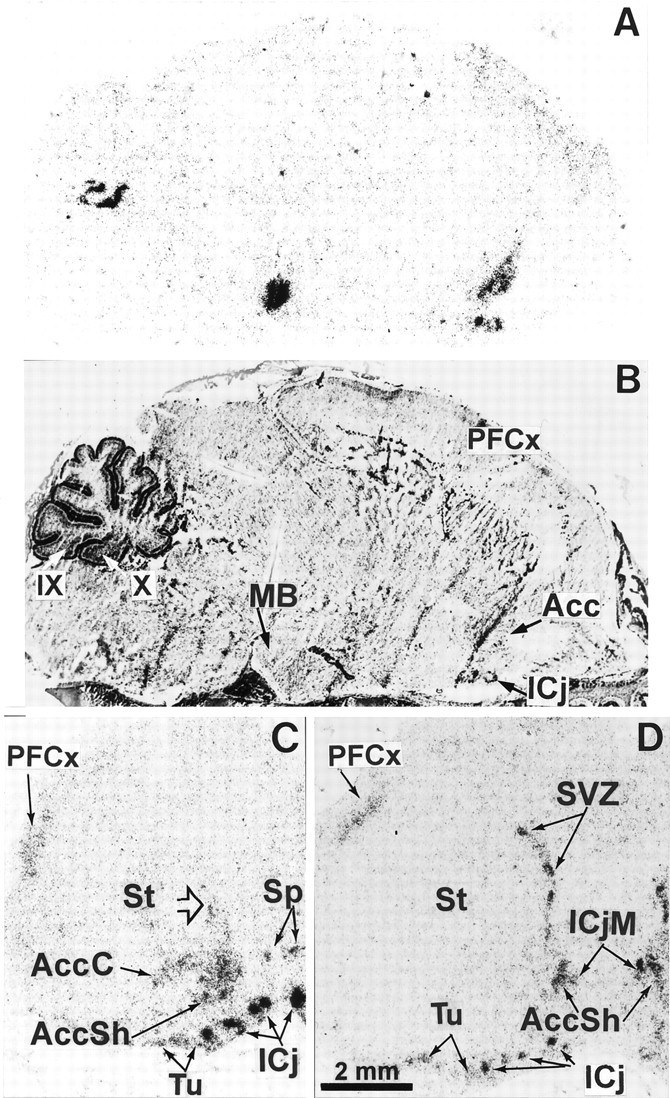
Expression of D3R mRNA in the brain of the 5 d pup. Shown are autoradiograms of parasagittal (A) and coronal [rostral (C) and caudal (D) level of nucleus accumbens] sections hybridized with the D3R probe. B corresponds to sectionA, counterstained with Mayer’s hemalum. High expression of transcripts is observed in the major (ICjM) and minor (ICj) islands of Calleja, nucleus accumbens shell (AccSh), medial mamillary body (MB), patches in the subventricular zone (SVZ) of striatum (St), and Purkinje cell layer of the cerebellar lobules 9 (IX) and 10 (X), whereas moderate signals are found in the core of nucleus accumbens (AccC), ventromedial region of the striatum (arrowhead), septum (Sp), parietofrontal cortex (PFCx), and olfactory tubercle (Tu).
Fig. 9.
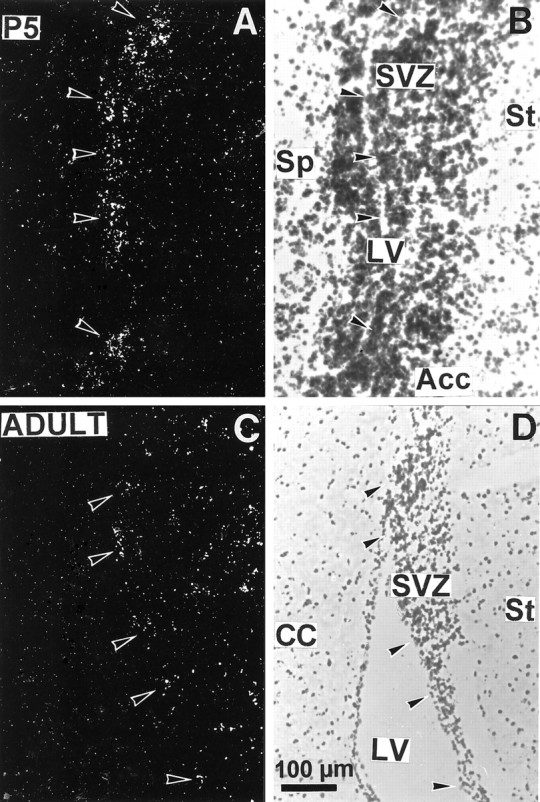
Expression of D3R gene transcripts in the subventricular zone of striatum in brains from a 5 d pup (A, B) or adult (C,D). Photomicrographs under dark-field illumination (A, C) show labeling in the striatal subventricular zone (SVZ) lining the lateral ventricle (LV), as indicated by arrowheads. B, D, Corresponding sections counterstained with Mayer’s hemalum. Acc, Nucleus accumbens;CC, corpus callosum; Sp, septum;St, striatum.
Midgestational development (E12–E16)
No hybridization signal was seen in any part of the developing brain at E12 (data not shown). Specific hybridization signal for D3R message was detected on both autoradiograms and emulsion-coated sections from E14 (Fig. 1). At this stage discrete germinative ventricular zones of the basal forebrain and dorsal midbrain exhibited distinct autoradiographic signals. In the forebrain, hybridization labeling was detected at high levels in the neuroepithelium of striatum (also referred to as lateral ganglionic eminence) and at moderate levels in rhinencephalic and septal neuroepithelia (Fig. 1A,B). D3R mRNA expression in the forebrain neuroepithelium abruptly ended at the rostral tip of the lateral ventricle, approximately corresponding to the limit between striatal and cortical neuroepithelia or dorsal and basal forebrain (Fig. 1A,B). In the midbrain, hybridization signals were detected at moderate levels in the tectal neuroepithelium. Examination of microautoradiographic hybridization labeling showed that D3R mRNA-labeled cells appeared as clusters of neuroepithelial cells at the vicinity of the lateral ventricle (Fig. 1C). Within the clusters a large majority of cells were labeled.
At E16 high levels of hybridization signals appeared as a narrow band all along the basal forebrain neuroepithelium from the striatum up to the amygdala. However, this continuity of D3R mRNA expression was interrupted remarkably at the level of the neuroepithelium of pallidum, which did not express transcripts at all (Fig. 2E). The hybridization signals were more pronounced at the limit between the striatal and cortical neuroepithelia (Figs. 2A,C,E, 3A). Labeling at a moderate level was still apparent in the tectal neuroepithelium, whereas faint signals were present in the cortical neuroepithelium.
Photomicrographs (Fig. 3) showed that at E16 D3R mRNA was expressed by cells lining the lateral ventricle at the level of ventricular zones of striatum, rhinencephalon, septum, and amygdala and appeared mostly in clustering cells rather than in a homogenous layer.
Late gestational development (E17–E19)
The distribution of D3R mRNA in brain embryo at E17 did not differ greatly from that at E16 (data not shown). At E18 most of the D3R mRNA still was expressed in the basal forebrain neuroepithelium, spanning the entire striatal border of the lateral ventricle and extending ventrally up to the ventral tip surrounded by primordia of nucleus accumbens and septum. In addition, hybridization signals were present in tectal neuroepithelium but at much lower levels, as compared with previous stages of gestation and in cortical neuroepithelium (Figs. 4A,C,5A). Labeled cells appeared as clusters in a 100-μm-thick band lining the ventricle (Fig. 4E), corresponding to the synthetic and mitotic zones of the neuroepithelium, as evidenced by Bayer and Altman (1991) with the use of [3H]thymidine labeling, whereas the differentiating field was labeled only very scarcely. This shows that D3R-expressing cells are proliferative and not postmitotic differentiating cells.
The first D3R mRNA-expressing neurons appeared at E18. Scattered labeled migrating neuronblasts were found to leave the junction between striatal and cortical ventricular zones, join the lateral cortical stream, and accumulate in the reservoir (Fig.5A,E), a region in which differentiated neurons sojourn before migrating to piriform and primary olfactory cortice and as yet unidentified areas of the basal telencephalon (Bayer and Altman, 1991,1995). A labeled cluster of settling Purkinje cells also was seen in the caudal part of the cerebellar anlage (Fig. 4A). These migrating Purkinje cells probably correspond to those destined to settle in cerebellar cortex in lobule 10 rather than in lobule 9, in agreement with the caudorostral cytogenetic gradient observed in the neurogenesis of Purkinje cells (Altman and Bayer, 1985).
The distribution of D3R mRNA was compared with that of D1R and D2R mRNAs on adjacent sections of the forebrain. At E18 (Fig. 5) the distributions of the three receptor subtypes were overlapping partially, but distinct. The three receptor mRNAs were found in the reservoir of the lateral cortical stream; this is the unique area in which we found coexpression of D1R, D2R, and D3R mRNAs. D1R and, to a lesser, extent D3R, but not D2R, mRNAs were expressed in the cortical neuroepithelium. In the striatal primordium D1R, D2R, and D3R mRNAs were expressed in distinct compartments: the neuroepithelium expressed exclusively D3R mRNA; the medial subventricular zone expressed almost exclusively the D2R mRNA (very scarce D3R-expressing cells were, however, found in this area); differentiating fields of striatum, nucleus accumbens, and rhinencephalon expressed D1R and D2R, but not D3R, mRNAs (Fig. 5).
At E19 (Fig. 6) the D3R mRNA was still prominent in the neuroepithelium, extending from rostral striatum to amygdala. The neuroepithelium of olfactory bulb also was labeled. At this stage labeling appeared as a band becoming thinner than previously, because the cortical ventricular zone of the neuroepithelium also had become thinner (Bayer and Altman, 1991). Labeling in Purkinje cell plate also appeared stronger and more extended.
Postnatal development (P0–P5)
At birth the D3R mRNA distribution still resembled that of late embryos, with only a few distinct characteristics found in adults (Fig. 7). The striatal neuroepithelium still was labeled heavily, although to a lesser level than in embryos. Labeling of subventricular zones of striatum and nucleus accumbens became stronger but did not appear in differentiating fields (Fig. 7A,C). Strong labeling also appeared in medial mamillary body (Fig. 7A), a region of high D3R expression in adults (Bouthenet et al., 1991). Also as in adults, labeled Purkinje cells were found within the cerebellum, in the entire lobule 10, and as patches in lobule 9 (Fig.7A,E–H).
More prominent changes occurred between P0 and P5. At this latter stage the distribution was already almost identical to that in the adult brain, with a few exceptions. Labeling along the striatal subventricular zone got thinner and was distributed as patches (Figs.8D, 9A). In adults D3R-expressing cells also were found in the striatal subventricular (also referred to as subependymal) zone (Fig.9C,D); a labeling of ependymal cells could not be excluded. At P5 major localizations found in adults were settled, as in lobules 9 and 10 of cerebellum, medial mamillary body, and ventral striatum (Fig. 8A). In the ventral striatum, although labeling was identical at P5 and in adults in the shell part of nucleus accumbens and in the islands of Calleja, there was D3R mRNA in the core of nucleus accumbens and in the olfactory tubercle (Fig. 8C,D), which were strongly reduced in adults (Diaz et al., 1995). A moderate labeling was detected at P5 in the parietofrontal cortex (Fig. 8A,C,D), which remained in adults as a laminated band of scarce positive cells in layer IV (data not shown).
DISCUSSION
We detected the first appearance of D3R mRNA at E14, in agreement with results obtained by PCR (Cadoret et al., 1993), and autoradiography by using the highly sensitive D2R/D3R radioligand [125I]iodosulpride (Sales et al., 1988). Our results suggest that D3R mRNA is more abundant during pre- and early postnatal ontogeny than in adult. The striatal neuroepithelium expresses strong signals from E14, culminating at E18, and progressively declines after birth. These signals seem to be of similar amplitude to those generated by a D2R mRNA probe, although the D2R is one to two orders of magnitude more abundant than the D3R in adult brain (Lévesque et al., 1992). The high abundance of D3R protein in the striatal neuroepithelium also is indicated by the strongest [125I]iodosulpride binding in this region, which exceeds that of D2R binding in differentiating striatum (Sales et al., 1988). Moreover, labeled neuroepithelium extends through almost the whole basal forebrain, whereas the D3R mRNA is expressed in the derived structures at a very low level and/or in discrete areas. In addition, transient expressions in neocortical and paleocortical regions, medial mamillary body, and the core part of nucleus accumbens were found in newborns.
Comparisons among the expression patterns of the D1R, D2R, and D3R mRNAs revealed marked differences, suggesting distinct expression regulation and roles (Table2). Whereas the D3R mRNA is expressed almost exclusively in the proliferative neuroepithelium during prenatal ontogeny, D1R and D2R mRNAs are expressed only transiently and sparingly in this structure and appear mostly in differentiating neurons, before dopamine innervation (Table 2). In addition, there is no overlap of D1R, D2R, and D3R mRNAs, except in the reservoir of the lateral cortical stream. In many instances the distribution of D3R mRNA markedly differs during prenatal and postnatal developments. According to the fate of D3R mRNA-expressing cells, three distinct patterns can be distinguished.
Table 2.
Comparison among appearances of D1R, D2R, and D3R mRNAs, [125I]iodosulpride binding, and dopamine immunoreactivity
| Structures | Figure numbersa | Dopamine receptor mRNA | [125I]IS bindingb | DA-IRc | ||
|---|---|---|---|---|---|---|
| D1Rd | D2Rd | D3R | ||||
| Olfactory bulb (Ne) | 6A | E18 | 0 | E16→P0 | E17 | nd |
| Rhinencephalon (Ne) | 2C, 3C, 4C | 0 | 0 | E14 | E15 | 0 |
| Islands of Calleja | 8A,C,D | nd | nd | [P0–P5] | nd | E19 |
| Septum (Ne) | 2C, 3C, 5A, 7C | 0 | 0 | E16 | E15 | 0 |
| Septum (DF) | 8C | E18 | nd | [P0–P5]h | [P0–P7] | [P0–P5] |
| Nucleus accumbens | ||||||
| Ne | 4A, 5A, 7C | 0 | 0 | E18 | E20 | 0 |
| SVZ | 7C, 8D, 9D | 0 | 0 | E19 | nd | 0 |
| DF (shell) | 8C,D | E18e | E18e | Pg5 f | P0 | P2 |
| DF (core) | 8C,D | E18 | E18 | [P0–P5]h | E19 | E19e |
| Striatum | ||||||
| Ne | 1–7 | E14→E17g | E14→E17g | E14 | E14 | E18g |
| SVZ | 6A, 7A,B, 8D, 9A | E14 | E14 | E19g | E14 | E16g |
| DF | 8C,9D | E17 | E16 | [P0–P5]f | E15 | E14 |
| Pallidum (Ne) | 2E | 0 | 0 | 0 | 0 | 0 |
| Amygdala (Ne) | 2G, 6C | nd | nd | E16 | 0 | 0 |
| Olfactory tubercle | 8C,D | E18 | E18 | [P0–P5] | E18 | E21 |
| Lateral cortical stream (Re) | 5 | nd | nd | E18 | nd | nd |
| Cortex (Ne) | 2E, 6A,C, 7C | E14→E17 | 0 | E16g | E13 | 0 |
| Cortical plate | 6A,C | E16 | E18 | E16g | E16 | E17 |
| Fronto-parietal cortex (DF) | 8C,D | E18 | E18 | [P0–P5]h | P7 | P17 |
| Median mammillary bodies | 7A, 8A | nd | nd | P0 | P14 | nd |
| Tectum (Ne) | 1A, 2A | E14 | 0 | E14→E18 | P5 | 0 |
| Cerebellum lobule 9 | 4A, 6E, 7A,E,8A | 0 | 0 | E18 | E20 | 0 |
| lobule 10 | 7A, 8A | 0 | 0 | [E19–P0] | E20 | 0 |
DF, Differentiation field; Ne, neuroepithelium; Re, reservoir; SVZ, subventricular zone; E→E or E→P, transient expression; [E-E or P-P], the exact time of appearance could not be determined; 0, undetectable; nd, not determined from available data.aFigure number in the present study illustrating the presence or absence of D3R mRNA;b[125I]iodosulpride binding, fromSales et al. (1988); cDopamine immunoreactivity, from Voorn et al. (1988) and Kalsbeek et al. (1992);dfrom Guennoun and Bloch (1992), Schambra et al. (1994), and present study; elateral part;fmedial part; gvery sparse labeling; hlabeling very sparse in adult—the exact date of disappearance is not known.
The first and most documented pattern corresponds to D3R mRNA-expressing neuroepithelial cells, generating neurons in which this marker thereafter becomes undetectable. Thus, prenatal D3R mRNA is confined almost entirely in neuroepithelial cells of striatum, amygdala, olfactory bulb, and tectum, most of the progeny of which rapidly lose the capacity to transcribe the D3R gene during migration and differentiation. D3R mRNA labeling in the striatal neuroepithelium overlaps the synthetic and mitotic zones, which are restricted to a thin layer close to the ventricle at early stages (E14–E16) but which spread in the subventricular zone at later stages (Bayer and Altman, 1995). At birth and during postnatal development the synthetic zone gets thinner along the lateral ventricle, just as the D3R mRNA labeling does. Additionally, cells continue to proliferate in the subependymal zone, corresponding to a remnant of neuroepithelium (Privat and Leblond, 1972; Jacobson, 1991), in which D3R mRNA occurs in adults.
The restricted expression of D3R mRNA in the neuroepithelium may be linked to the cell proliferative activity via other negative regulation by factors arresting cell division and triggering cell differentiation or may be linked to positive regulation by transcription factors (Arenander and De Vellis, 1994). In this latter respect, it is noteworthy that expressions of Dlx-2and Mash-1 homeobox genes (Porteus et al., 1991, 1994;Robinson et al., 1991), candidates for regulating patterning and differentiation of the forebrain, match, to some extent, D3R mRNA distribution. Alternatively, D3R gene expression may be activated by unknown factors, either released from neuroepithelium or circulating in the lateral ventricle and poorly diffusing through the parenchyma.
Both microheterogeneity and compartmentation are observed in the distribution of D3R gene transcripts within the neuroepithelium. Instead of forming a continuous sheet of neuroepithelial cells, D3R mRNA-expressing cells, rather, are organized in clusters evident throughout the entire development. Similar alternation of [3H]thymidine-labeled neuroepithelial cells has suggested different migration kinetic properties among these cells (Altman and Bayer, 1990). Distinct properties among neuroepithelial cells also may be related to their distinct fate. Lineage studies with retroviral markers suggest that certain germinal cells become restricted only to generating neurons (Luskin et al., 1988). Most adult medium-sized striatal neurons are distributed among dynorphin/substance P and D1R-expressing neurons and enkephalin and D2R-expressing neurons (Gerfen et al., 1990; Curran and Watson, 1995; Le Moine and Bloch, 1995). Adult striatal neurons also belong to either striosomes or matrix, making different outputs (Graybiel, 1984). Birth-dating studies have shown that striosome cells become postmitotic at a distinctly earlier time than matrix cells (Van der Kooy and Fishell, 1987). In adults, whereas colocalization of D2R and D3R seems exceptional if it exists (Bouthenet et al., 1991; Diaz et al., 1994), there are examples of D1R and D3R colocalization (Le Moine and Bloch, 1996; Bordet et al., 1997) (S. Ridray, N. Griffon, E. Souil, V. Mignon, S. Carboni, J. Diaz, J.-C. Schwartz, P. Sokoloff, unpublished observation) suggesting that D3R mRNA-expressing cells in the neuroepithelium may correspond to a distinct subpopulation.
In addition, the continuous distribution of D3R mRNA within the basal forebrain neuroepithelium is interrupted remarkably at the level of the pallidal neuroepithelium while persisting at more ventral or caudal levels. It is noteworthy that cell fate is distinct in the striatal and pallidal neuroepithelium, the latter presumably giving rise mostly to pallidal cholinergic magnocellular cells in no or only very low levels of D3R mRNA expressed in adults (Diaz et al., 1995).
The second and less documented pattern corresponds to cells that express D3R mRNA in both the neuroepithelium and its progeny. D3R mRNA is abundant in the neuroepithelium of nucleus accumbens at its appearance on E18 and in the shell subdivision after the appearance of the secondary germinal matrix (at P5; see Table2). Our data indicate that the expression of D3R mRNA is not continuous during cell differentiation and migration from the neuroepithelium but, rather, is triggered after the settling of dopamine innervation, which might exert a dual trophic influence. This is consistent with our hypothesis that regulation of D3R gene expression in the shell of nucleus accumbens is under the positive influence of (1) a trophic factor, synthesized by dopamine neurons, distinct from dopamine and its cotransmitters neurotensin and cholecystokinin, and conveyed by axonal transport, the release of which seems dependent on dopamine neuron activity (Lévesque et al., 1995); (2) activation of the D1R by dopamine or agonists (Bordet et al., 1997).
Nevertheless, a continuous expression of D3R mRNA occurs in cells originating approximately from the junction between striatal and cortical neuroepithelia, migrating through the lateral cortical stream, and sojourning in the reservoir (Bayer and Altman, 1995). Some cells arising from the cortical neuroepithelium differentiate in the parietofrontal cortex, which transiently expresses high levels of D3R mRNA (present study) and binding (Demotes-Mainard et al., 1996), and these cells remain in adult rats in layer IV. D3R mRNA also persists in human parietal cortex, where it could be detected by PCR in brain from normal subjects but not from psychiatric patients who may express an abnormal shortened transcript (Schmauss et al., 1993).
The third pattern corresponds to D3R mRNA-expressing neurons that differentiate from a neuroepithelium in which this marker is undetectable. D3R mRNA-expressing Purkinje cells, destined to settle in the archicerebellum (lobules 9 and 10) after birth (Bouthenet et al., 1991; Diaz et al., 1995), occur from their differentiation at E18, but not in Purkinje cell progenitors. A strong labeling also appears at P0 in the medial mamillary bodies, which derive from unlabeled hypothalamic neuroepithelium. Thus, in these structures, expression of the D3R seems to follow a more classical pattern, being triggered after the neuronal differentiation, possibly in relation to a functional specialization.
A specific role of the D3R in neurogenesis, but not in morphogenesis, can be inferred from its highest level of expression in prenatal ontogeny, as compared with adulthood and its selective localization in the proliferative zone of the neuroepithelium during ontogeny. Such a role is supported by the mitogenic response induced by recombinant D3R stimulation in transfected cells (Chio et al., 1994; Pilon et al., 1994). However, the origin of dopamine available for D3R stimulation is unclear in the neuroepithelium, where no dopamine fibers are present. One study (Specht et al., 1978) reported a few cells positive for tyrosine hydroxylase immunoreactivity at E12.5–E15 at the surface of striatal neuroepithelium. Nevertheless, there is no indication that these cells indeed produce dopamine. Another possibility is that the D3R receives dopamine diffusing through the cerebrospinal fluid from dopamine neurons in diencephalon and mesencephalon. Dopamine has a higher affinity at the D3R than at any other receptor subtype, allowing this receptor to respond to nanomolar dopamine concentrations. In adult rat brain examples of D3R localizations at some distance from dopaminergic fibers have suggested nonsynaptic actions of diffusing dopamine through this receptor (Diaz et al., 1995). Finally, a constitutive, i.e., agonist-independent, activity of the D3R has been suggested from studies that use transfected cells (Griffon et al., 1996b).
Schizophrenia, sometimes assumed to result from a neurodevelopmental disorder, is marked namely by neuroanatomical abnormalities such as ventricle enlargement (Weinberger, 1987; Waddington, 1993), possibly related to a defect in neuroepithelium proliferation. A role for the D3R in this pathological process is supported by some (but not all) genetic studies (Crocq et al., 1992; Nimgaonkar et al., 1993;Mant et al., 1994; Griffon et al., 1996a).
Footnotes
This work was supported by a Biomed 2 Grant from the European Commission (BMH4-CT96-0203). We are grateful to C. Sotelo for critical reading and helpful discussion of this manuscript.
Correspondence should be addressed to Dr. Pierre Sokoloff, Unitéde Neurobiologie et Pharmacologie, 2ter Rue d’Alésia, 75014 Paris, France.
REFERENCES
- 1.Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231:42–65. doi: 10.1002/cne.902310105. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Bayer SA. Vertical compartmentation and cellular transformations in the germinal matrices of the embryonic rat cerebral cortex. Exp Neurol. 1990;107:23–35. doi: 10.1016/0014-4886(90)90060-6. [DOI] [PubMed] [Google Scholar]
- 3.Arenander AT, De Vellis J. Development of the nervous system. In: Siegel GJ, editor. Basic neurochemistry: molecular, cellular, and medical aspects. Raven; New York: 1994. pp. 573–606. [Google Scholar]
- 4.Bayer SA, Altman J. Cell migration in the developing neocortex. In: Bayer SA, Altman J, editors. Neocortical development. Raven; New York: 1991. pp. 116–127. [Google Scholar]
- 5.Bayer SA, Altman J. Principles of neurogenesis, neuronal migration, and neuronal circuit formation. In: Paxinos G, editor. The rat nervous system. Academic; New York: 1995. pp. 1079–1098. [Google Scholar]
- 6.Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz J-C. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci USA. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–262. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- 8.Bouthenet M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 9.Cadoret MA, Jaber M, Bloch B. Prenatal D1, D1b, and D3 dopamine receptor gene expression in the rat forebrain: detection by reverse polymerase chain reaction. Neurosci Lett. 1993;155:92–95. doi: 10.1016/0304-3940(93)90680-j. [DOI] [PubMed] [Google Scholar]
- 10.Chio CL, Lajiness ME, Huff RM. Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Mol Pharmacol. 1994;45:51–60. [PubMed] [Google Scholar]
- 11.Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, Collier D, Lannfelt L, Sokoloff P, Schwartz J-C, Gill M, Nacher J-P, McGuffin P, Owen NJ. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. J Med Genet. 1992;29:858–860. doi: 10.1136/jmg.29.12.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran EJ, Watson SJ., Jr Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. J Comp Neurol. 1995;361:57–76. doi: 10.1002/cne.903610106. [DOI] [PubMed] [Google Scholar]
- 13.Demotes-Mainard J, Henry C, Jeantet Y, Arsaut J, Arnauld E. Postnatal ontogeny of dopamine D3 receptors in the mouse brain: autoradiographic evidence for a transient cortical expression. Dev Brain Res. 1996;94:166–174. doi: 10.1016/0165-3806(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 14.Diaz J, Lévesque D, Griffon N, Lammers C, Martres M-P, Sokoloff P, Schwartz J-C. Opposing roles for dopamine D2 and D3 receptors on neurotensin mRNA expression in nucleus accumbens. Eur J Neurosci. 1994;6:1384–1387. doi: 10.1111/j.1460-9568.1994.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 15.Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- 16.Fishburn CS, Bedford M, Lonai P, Fuchs S. Early expression of D3 dopamine receptors in murine embryonic development. FEBS Lett. 1996;381:257–261. doi: 10.1016/0014-5793(96)00119-6. [DOI] [PubMed] [Google Scholar]
- 17.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 18.Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- 19.Graybiel AM. Correspondence between the dopamine islands and striosomes of the mammalian striatum. Neuroscience. 1984;13:1157–1187. doi: 10.1016/0306-4522(84)90293-8. [DOI] [PubMed] [Google Scholar]
- 20.Griffon N, Crocq MA, Pilon C, Martres M-P, Mayerova A, Uyanik G, Burgert E, Duval F, Nacher J-P, Javoy-Agid F, Tamminga CA, Schwartz J-C, Sokoloff P. Dopamine D3 receptor gene: organization, transcript variants, and polymorphism associated with schizophrenia. Am J Med Genet Neuropsychiatr Genet. 1996a;67:58–70. doi: 10.1002/(SICI)1096-8628(19960216)67:1<63::AID-AJMG11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Griffon N, Pilon C, Sautel F, Schwartz J-C, Sokoloff P. Antipsychotics with inverse agonist activity at the dopamine D3 receptor. J Neural Transm. 1996b;103:1166–1175. doi: 10.1007/BF01271201. [DOI] [PubMed] [Google Scholar]
- 22.Guennoun R, Bloch B. Ontogeny of D1 and DARPP-32 gene expression in the rat striatum: an in situ hybridization study. Mol Brain Res. 1992;12:131–139. doi: 10.1016/0169-328x(92)90076-n. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson M. Developmental neurobiology, 3rd Ed, pp 41–93. Plenum; New York: 1991. The germinal cell, histogenesis, and lineage of nerve cells. [Google Scholar]
- 24.Kalsbeek A, Voorn P, Buijs M. Development of dopamine-containing systems in the CNS. In: Bjorklund A, Hökfelt T, Tohyama M, editors. Handbook of chemical neuroanatomy: ontogeny of transmitters and peptides in the CNS. Elsevier; New York: 1992. pp. 63–112. [Google Scholar]
- 25.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 26.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 27.Le Moine C, Bloch B. Expression of D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with D1 and D2 dopamine receptors. Neuroscience. 1996;73:131–143. doi: 10.1016/0306-4522(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 28.Lévesque D, Diaz J, Pilon C, Martres M-P, Giros B, Souil E, Schott D, Morgat J-L, Schwartz J-C, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]-hydroxy-N,N di-N-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lévesque D, Martres M-P, Diaz J, Griffon N, Lammers CH, Sokoloff P, Schwartz J-C. A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci USA. 1995;92:1719–1723. doi: 10.1073/pnas.92.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 31.Mant R, Williams J, Asherson P, Parfitt E, McGuffin P, Owen MJ. The relationship between homozygosity at the dopamine D3 receptor gene and schizophrenia. Am J Med Genet. 1994;54:21–26. doi: 10.1002/ajmg.1320540106. [DOI] [PubMed] [Google Scholar]
- 32.Miller JC, Friedhoff AJ. Prenatal neuroleptic exposure alters postnatal striatal cholinergic activity in the rat. Dev Neurosci. 1986;8:111–116. doi: 10.1159/000112246. [DOI] [PubMed] [Google Scholar]
- 33.Nimgaonkar VL, Zhang XR, Caldwell JG, Ganguli R, Chakravarti A. Association study of schizophrenia with dopamine D3 receptor gene polymorphisms: probable effects of family history of schizophrenia? Am J Med Genet Neuropsychiatr Genet. 1993;48:214–217. doi: 10.1002/ajmg.1320480408. [DOI] [PubMed] [Google Scholar]
- 34.Pilon C, Lévesque D, Dimitriadou V, Griffon N, Martres MP, Schwartz J-C, Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108-15 neuroblastoma–glioma hybrid cell line. Eur J Pharmacol Mol Pharmacol Sect. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 35.Porteus MH, Bulfone A, Ciaranello RD, Rubenstein JLR. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991;7:221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- 36.Porteus MH, Bulfone A, Liu J-K, Puelles L, Lo L-C, Rubenstein JLR. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 1994;14:6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Privat A, Leblond CP. The subependymal layer and neighboring region in the brain of the young rat. J Comp Neurol. 1972;146:277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- 38.Robinson GW, Wray S, Mahon KA. Spatially restricted expression of a member of a new family of murine Distal-less homeobox genes in the developing forebrain. New Biol. 1991;3:1183–1194. [PubMed] [Google Scholar]
- 39.Rosengarten H, Friedhoff AJ. Enduring changes in dopamine receptor cells of pups from drug administration to pregnant and nursing rats. Science. 1979;203:1133–1135. doi: 10.1126/science.570724. [DOI] [PubMed] [Google Scholar]
- 40.Sales N, Martres MP, Bouthenet ML, Schwartz JC. Ontogeny of dopaminergic D2 receptors in the rat nervous system: characterization and detailed autoradiographic mapping with [125I]iodosulpride. Neuroscience. 1988;28:673–700. doi: 10.1016/0306-4522(89)90014-6. [DOI] [PubMed] [Google Scholar]
- 41.Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau JR. Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 42.Schmauss C, Haroutunian V, Davis KL, Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc Natl Acad Sci USA. 1993;90:8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–65. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 44.Sokoloff P, Schwartz J-C. The novel dopamine receptors, half a decade later. Trends Pharmacol. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 45.Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 46.Specht LA, Pickel VM, Joh TH, Reis DJ. Immunocytochemical localization of tyrosine hydroxylase in processes within the ventricular zone of prenatal rat brain. Brain Res. 1978;156:315–321. doi: 10.1016/0006-8993(78)90511-5. [DOI] [PubMed] [Google Scholar]
- 47.Van der Kooy D, Fishell G. Neuronal birthdate underlies the development of striatal compartments. Brain Res. 1987;401:155–161. doi: 10.1016/0006-8993(87)91176-0. [DOI] [PubMed] [Google Scholar]
- 48.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. The pre- and postnatal development of the dopaminergic cell group in the developmental mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25:857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 49.Waddington J. Schizophrenia: developmental neuroscience and pathobiology. Lancet. 1993;341:531–536. doi: 10.1016/0140-6736(93)90288-r. [DOI] [PubMed] [Google Scholar]
- 50.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]