Abstract
Both serotonergic dysfunction and glucocorticoid hypersecretion are implicated in affective and eating disorders. The adverse effects of serotonergic (5-HT)2C receptor activation on mood and food intake, the antidepressant efficacy of 5-HT2 receptor antagonists, and the hyperphagia observed in 5-HT2Creceptor knockout mice all suggest a key role for increased 5-HT2C receptor-mediated neurotransmission. Glucocorticoids, however, downregulate 5-HT2Creceptor mRNA in the hippocampus, and it is unclear how increased 5-HT2C receptor sensitivity is achieved in the presence of elevated glucocorticoid levels in depression. Here we show a monophasic diurnal rhythm of 5-HT2C receptor mRNA expression in the rat hippocampus that parallels time-dependent variations in 5-HT2C receptor agonist-induced behaviors in open field tests. Rats entrained to chronic food restriction show marked but intermittent corticosterone hypersecretion and maintain an unaltered 5-HT2C receptor mRNA rhythm. The 5-HT2Creceptor mRNA rhythm, however, is suppressed by even modest constant elevations of corticosterone (adrenalectomy + pellet) or with elevated corticosterone during the daytime (8 A.M.), whereas a normal rhythm exists in animals that have the same dose of corticosterone in the evening (6 P.M.). Thus, animals showing even a transient daytime corticosterone nadir exhibit normal hippocampal 5-HT2Creceptor mRNA rhythms, even in the presence of overt corticosterone hypersecretion. Chronic food restriction also abolishes the normal diurnal variation in hippocampal glucocorticoid receptor (GR) and mineralocorticoid receptor mRNAs and produces, unusually, both elevated corticosterone and increased GR. The mismatch between elevated glucocorticoids and maintained 5-HT2C receptor and increased GR gene expression in the hippocampus provides a new model to dissect mechanisms that may underlie affective and eating disorders.
Keywords: 5-HT2C receptor, serotonin, corticosterone, diurnal rhythm, food restriction, depression, 5-HT1Areceptor, glucocorticoid receptor, mineralocorticoid receptor
Abnormalities of both serotonergic (5-HT) neurotransmission and hypothalamic-pituitary-adrenal (HPA) axis activity are found in depression and eating disorders and may play important pathogenic roles. Both conditions are frequently associated with elevated plasma cortisol levels (Carroll et al., 1976) and insensitivity to glucocorticoid (dexamethasone) feedback (Carroll, 1982), presumed to be caused by increased central drive on the HPA axis. Abnormal 5-HT neurotransmission is also believed to be a key factor in depression (Meltzer and Lowy, 1987). Many clinically efficacious antidepressants alter 5-HT neurotransmission, improving both mood and hypercortisolemia (Ogren et al., 1979; Linkowski et al., 1987).
Glucocorticoids act via intracellular high-affinity mineralocorticoid receptors (MRs) and lower-affinity glucocorticoid receptors (GRs) (Reul and de Kloet, 1985; McEwen et al., 1986). In contrast, 5-HT binds to a number of distinct cell membrane sites (Peroutka, 1993). The hippocampus has a dense 5-HT innervation, highly expresses both corticosteroid and 5-HT receptors (Palacios et al., 1990; de Kloet, 1991; Wright et al., 1995), and is an important locus for the interaction of the two systems. Serotonin is important for the maintenance of corticosteroid receptor gene expression in the hippocampus (Seckl et al., 1990; Yau et al., 1994). Similarly, glucocorticoids alter hippocampal expression of at least two 5-HT receptor subtypes, 5-HT1A (Chalmers et al., 1993; Zhong and Ciaranello, 1995) and 5-HT2C (Holmes et al., 1995b). 5-HT1A and 5-HT2C receptors are excellent candidates to mediate functional abnormalities in depression and both are highly expressed in the hippocampus (Mengod et al., 1990; Chalmers and Watson, 1991). Sets of antidepressant drugs act on each receptor (Robinson, 1993), and both are implicated in HPA regulation (Fuller, 1992). m-Chlorophenylpiperazine (mCPP), which binds to 5-HT2C receptors with high affinity, increases plasma glucocorticoid levels, reduces food intake (Blundell, 1992; Clifton et al., 1993), attenuates locomotion, and exerts dysphoric effects in some depressive illnesses (JosephVanderpool et al., 1993; Jacobsen et al., 1994), whereas 5-HT2C (and -2A) receptor antagonists (e.g., ketanserin and ritanserin) are clinically efficacious antidepressants (Robinson, 1993).
The mRNAs encoding 5-HT2C receptors, GR, and MR, but not 5-HT1A receptors, exhibit a circadian rhythm in the hippocampus (Herman et al., 1993; Holmes et al., 1995a,b). Affective disorders are frequently associated with blunting (elevation) of the normal afternoon/evening nadir of the cortisol circadian rhythm (Linkowski et al., 1987). Central 5-HT activity also shows a diurnal rhythm, with higher 5-HT release in the hippocampus during the active period (darkness in rats) (Kalén et al., 1989). Other diurnal functions (e.g., sleep, psychological performance) are also disrupted in depression (Healy, 1987), and clearly, diurnally varying processes underlying the interaction between 5-HT and cortisol may be important to understanding abnormal central mechanisms in affective disorders (Moffoot et al., 1994). In previous work we found that the rhythm of hippocampal 5-HT2C receptor gene expression was maintained in adrenalectomized rats (Holmes et al., 1995b) but suppressed by chronic stress-mediated elevations of plasma corticosterone (Holmes et al., 1995a), presumably an adaptive process to attenuate sensitivity of this receptor with chronic stress. To investigate further the diurnal cues regulating hippocampal receptor expression, a food restriction paradigm (Krieger and Hauser, 1978) was used that alters the plasma corticosterone rhythm as well as feeding and locomotor behavior, leaving only the light–dark cues unchanged.
In this study we wished to determine (1) the control of corticosteroid and serotonin receptor mRNA expression in the hippocampus throughout the 24 hr period, (2) any link between diurnal hippocampal 5-HT2C receptor mRNA changes and possible diurnal rhythms in 5-HT2C receptor-mediated behaviors, and (3) the role of diurnal glucocorticoid effects and food restriction (a chronic intermittent and perhaps pathophysiologically relevant stressor) on the diurnal patterns of receptor gene expression.
MATERIALS AND METHODS
Behavioral experiments
Open field behavior in response to mCPP. Male Han–Wistar rats were given mCPP (1 mg/kg, i.p.; Sigma, St. Louis, MO) or saline 30 min before testing in the open field (novel environment). Animals were placed in a large Perspex box (60 × 40 cm) divided into eight zones for 5 min. The number of times the rat crossed into a new zone and the number of rearings were recorded. Animals (six to eight per group) were tested at 9 A.M., 1 P.M., or 5 P.M.
Inhibition of food intake in response to mCPP.Sixteen rats (∼300 gm) were housed singly in a light-controlled environment (lights on from 7 A.M. to 7 P.M.). To test the feeding response to the 5-HT2C receptor agonist, animals were fasted overnight, and then 30 min after an intraperitoneal injection of saline or mCPP (1 mg/kg), the amount of food ingested over a 1 hr period was determined. mCPP or saline were given in a random design, spaced 1 week apart at 9 A.M. and 1, 5, and 9 P.M.
Food restriction
Han–Wistar rats (∼250 gm) were housed in pairs and handled daily for 1 week before the study. Lights were on from 7 A.M. to 7 P.M. For food restriction studies, rats were allowed access to food pellets only between 10 A.M. and noon. Water was available ad libitum. The animals were entrained to the food restriction regimen for 3 weeks before they were killed. Controls had tap water and rat chow available ad libitum. On the day of the experiment, four control and four food-restricted animals were decapitated every 4 hr throughout a 24 hr period (n = 8 at 8 P.M. and midnight). Care was taken to cause minimal stress to the rats before decapitation, which was completed within 1 min of disturbing a cage. Trunk blood was collected, and brains were removed, frozen on dry ice, and stored at −80°C. Cryostat sections (10 μm) were cut at the level of the posterior hippocampus (approximately Bregma −4.80 mm;Paxinos and Watson, 1986). Sections were thaw-mounted onto gelatin-subbed, poly-l-lysine-coated slides and stored at −80°C, ready for in situ hybridization histochemistry.
Adrenalectomy and constant corticosterone replacement
Food restriction caused two peaks in plasma corticosterone (the normal diurnal evening peak and an additional morning peak at the normal nadir). To determine whether these changes in corticosterone were responsible for alterations in gene expression, animals were bilaterally adrenalectomized by the dorsal approach under halothane anesthesia, and a continuous-release corticosterone pellet (100 mg, 60-day release pellet; Innovative Research of America, Toledo, OH) was inserted subcutaneously to produce constant corticosterone levels approximating the 24 hr mean. Sham-operated animals were given a placebo pellet. Animals were allowed to recover from anesthesia and placed in cages in pairs (with 0.9% saline to drink for the adrenalectomized rats) for 7 d before they were killed at either 8 A.M. or 8 P.M. Groups contained five to six animals.
Adrenalectomy and pulsatile corticosterone replacement
To determine whether the timing of the corticosterone peak was important in the regulation of hippocampal receptor gene expression, rats were adrenalectomized and given corticosterone injections (20 mg/kg, s.c., in 200 μl of corn oil for 7 d) at various times of day. One group received corticosterone injections at 8 A.M., the normal diurnal nadir of corticosterone. Another group received corticosterone at 6 P.M. to reproduce the normal elevation of plasma corticosterone levels at the onset of the dark phase. Controls were sham adrenalectomized and received 200 μl of corn oil daily. Rats (four per group) were killed at 8–10 A.M., 2–4 P.M., and 8–10 P.M.; the brains were removed and processed for in situ hybridization histochemistry, as above.
5-HT and GR subtype in situhybridization histochemistry
In situ hybridization histochemistry was performed as described previously (Seckl et al., 1990; Yau et al., 1994). In brief, sections were post-fixed in 4% paraformaldehyde and washed in 2× SSC. For 5-HT receptor subtype mRNA detection, sections were prehybridized with buffer for 2 hr at 50°C; sections for GR and MR mRNA detection were hybridized directly. [35S]UTP-labeled mRNA antisense probes were transcribed in vitro from linearized plasmids of rat 5-HT1A receptor cDNA (Albert et al., 1990) and 5-HT2C receptor cDNA (Julius et al., 1988), GR, and MR cDNA as described previously (Seckl et al., 1990). Probes (10–20 × 106 counts/ml) were denatured, added to hybridization buffer, applied to sections, hybridized, and washed under stringent conditions, as reported previously (Seckl et al., 1990). Slides were dipped in Kodak NTB2 emulsion, exposed at 4°C for 4 weeks, developed, and counterstained (1% pyronin). Expression was quantified by counting silver grains overlying identified neurons under bright-field illumination using a computer-driven image analysis system (Seescan, Cambridge, UK) and rate-of-change filters (Aldridge and Seckl, 1993). Expression was estimated over at least 15 cells per subfield for each animal. Specificity was demonstrated using 35S-labeled “sense” RNA probes of similar specific activity, hybridized under identical conditions. No specific cellular hybridization signal was seen with any sense probe (data not shown; but see Seckl et al., 1990).
Plasma corticosterone measurements
Corticosterone was measured in trunk blood samples by specific radioimmunoassay, as described previously (MacPhee et al., 1989) with antiserum donated by Dr. C. Kenyon, Edinburgh. The detection limit of the assay was 6 nmol/l.
Statistical analysis
For the in situ hybridization results, the average number of grains counted over each hippocampal area was standardized for each experiment as percentage grains of the 8 P.M. control group (n = 4–6 per group). All results were analyzed by a one- or two-way ANOVA, followed by Dunnett’s post hoc test. Significance levels were taken as p < 0.05. For the mCPP-induced effects, the drug group was compared with the control group at each time of day using a t test (n= 6–8).
RESULTS
Circadian rhythm of hippocampal 5-HT2C receptor mRNA expression and 5-HT2C receptor agonist-induced behavior
A diurnal rhythm of 5-HT2C receptor mRNA expression was seen in the ventral CA1 region of the hippocampus (Fig.1A) as well as dorsal CA1 and subiculum. When 5-HT2C receptor agonist-induced behavior was compared at different times of day, a diurnal rhythm in agonist effectiveness was apparent (Fig. 1B,C). In the open field test, mCPP significantly inhibited the locomotor activity of the rats, as determined by the number of crossings of the open field zones, at 9 A.M. but not at 1 or 5 P.M. (Fig. 1B). Similarly, the number of rearings was significantly reduced in the mCPP-treated animals at 9 A.M. and 1 P.M., but not at 5 P.M. (Fig.1C); however, there was no effect of time of day on the efficacy of mCPP to inhibit feeding (Fig. 1D).
Fig. 1.
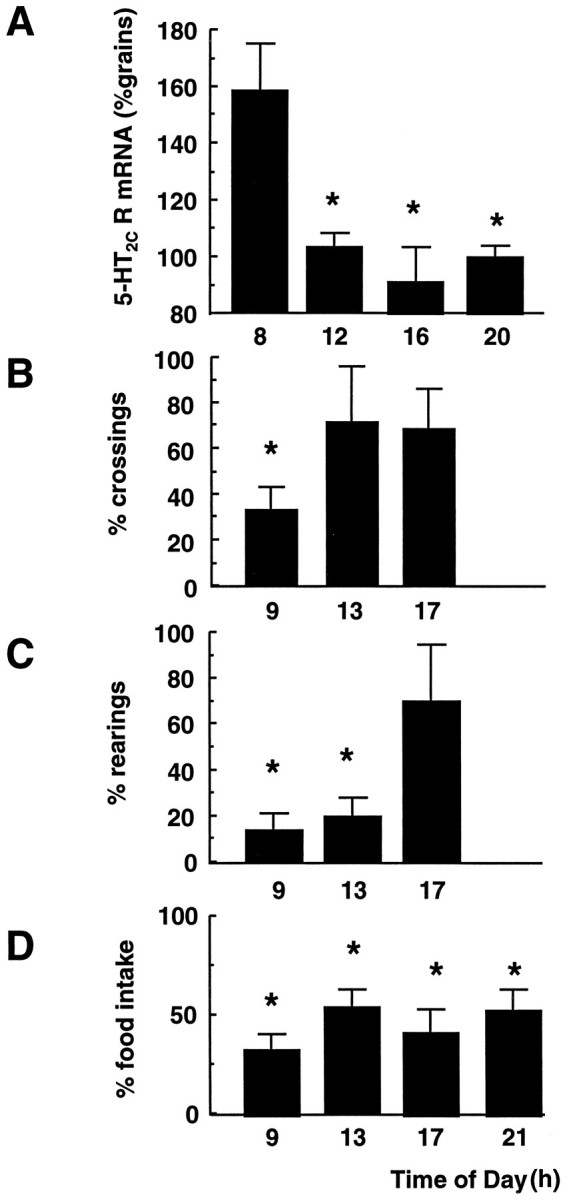
Diurnal variation of 5-HT2C receptor mRNA expression and mCPP-induced behaviors. A, 5-HT2C receptor mRNA expression in the CA1 subregion of the hippocampus over a 24 hr period. Receptor mRNA levels were determined by in situ hybridization histochemistry. The mean number of grains/subregion/rat was standardized to the expression observed in the control rats at 8 P.M. (20) (100%). Values represent mean ± SEM; n = 4. *p < 0.05 compared with 8 A.M. (8).B–D, Sensitivity to mCPP-induced inhibition;B, locomotor behavior; C, rearings in an open field. The % number of crossings of open field zones and % number of rearings in a 5 min period after mCPP (1 mg/kg, i.p.) 30 min before testing, compared with controls (saline injected) tested at the same time of day. Numbers per group = 6–8. D,The % inhibition of food intake 30 min after mCPP (1 mg/kg, i.p.) compared with control animals tested at the same time of day. Animals were fasted overnight before testing, and the test period was for 1 hr. Number of animals per group = 8. All columns represent mean results per group ±SEM. *p < 0.05 compared with controls tested at the same time of day. Time of day: 9 = 9 A.M.; 13 = 1 P.M.; 17 = 5 P.M.; 21 = 9 P.M.
The effect of food restriction on plasma corticosterone and hippocampal receptor gene expression
Body weight and food intake
During the 3 week period of the food restriction experiment, the control rats increased their weight by 16%, whereas the animals fed for only 2 hr/d maintained their initial body weight. The food intake per day was 14 ± 1 gm for the animals on food restriction compared with 22 ± 1 gm for the control rats fed ad libitum.
Plasma corticosterone
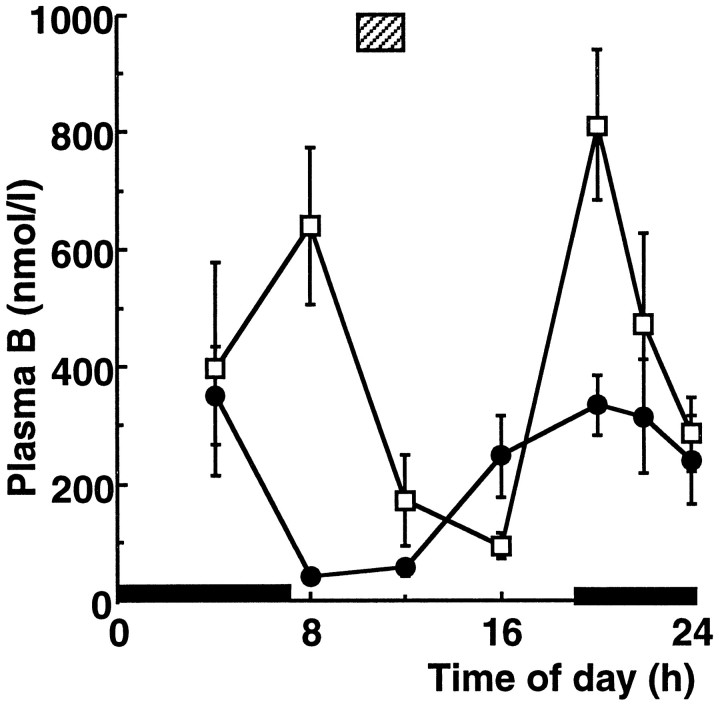
In control animals, plasma corticosterone showed a well defined diurnal rhythm, with a nadir at 8 A.M.-noon and a peak at 8 P.M. (Fig.2). Chronic exposure to food restriction (food available only from 10 A.M.-noon) produced a biphasic rhythm in plasma corticosterone, with a novel peak at 8 A.M. (just before food availability) as well as the usual diurnal peak at 8 P.M. (just after lights off and at the beginning of the activity period). Food-restricted rats, however, showed a plasma corticosterone nadir, although delayed, at 4 P.M. (Fig. 2). The total 24 hr secretion of corticosterone in food-restricted rats was significantly greater (94% higher) than in controls with access to food ad libitum.
Fig. 2.
Twenty-four hour profile of plasma corticosterone (Plasma B) levels in control (•) and in animals after 3 week food restriction (□). Values represent mean ± SEM;n = 4–8. ▨ represents period of food availability for food-restricted animals, and black barsrepresent period of darkness. Time notation as in Figure 1legend.
5-HT2C receptor mRNA expression
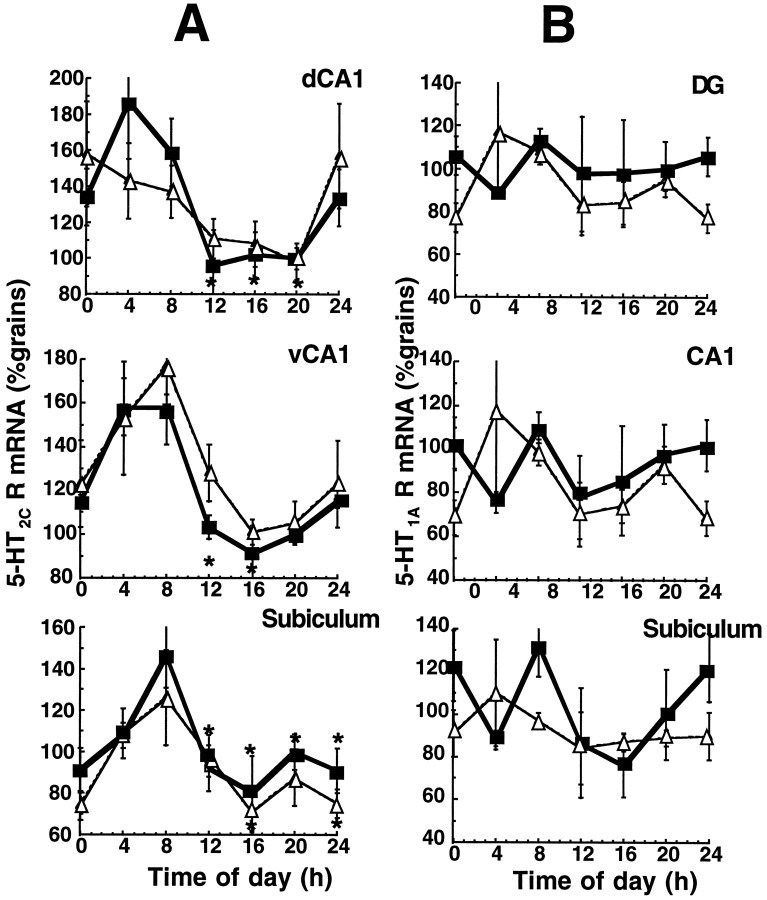
5-HT2C receptor mRNA expression, measured in various subregions of the hippocampus at 4 hr intervals, exhibited a monophasic rhythm in dorsal CA1, ventral CA1, and subiculum, peaking at 4–8 A.M. and falling to a nadir at 4 P.M. (Fig. 3A). The rhythm in receptor expression is similar to the rhythm in plasma corticosterone; however, it is shifted to the right (delayed) by 4–8 hr. In contrast to plasma corticosterone, the rhythm in 5-HT2C receptor gene expression in dorsal CA1, ventral CA1, and subiculum were unaltered in food-restricted animals (Fig.3A). No circadian changes were seen in 5-HT2Creceptor mRNA expression in CA3 in either group (data not shown).
Fig. 3.
Twenty-four hour profile of (A) 5-HT2C and (B) 5-HT1A receptor mRNA expression in control (▪) and food-restricted animals (▵) in various hippocampal subregions. Receptor mRNA levels were determined byin situ hybridization histochemistry. The mean number of grains/subregion/rat was standardized to the expression observed in the control rats at 8 P.M. (20) (100%). Values represent mean ± SEM; n = 4. *p < 0.05 compared with peak value. Time notation as in Figure 1legend.
5-HT1A receptor mRNA expression
No changes in 5-HT1A receptor mRNA expression were observed over the 24 hr period in any region of the hippocampus measured (Fig. 3B). Furthermore, food restriction exerted no effect on 5-HT1A receptor mRNA expression in the hippocampus (DG, CA1, and subiculum) at any time of day (Fig.3B).
MR mRNA expression
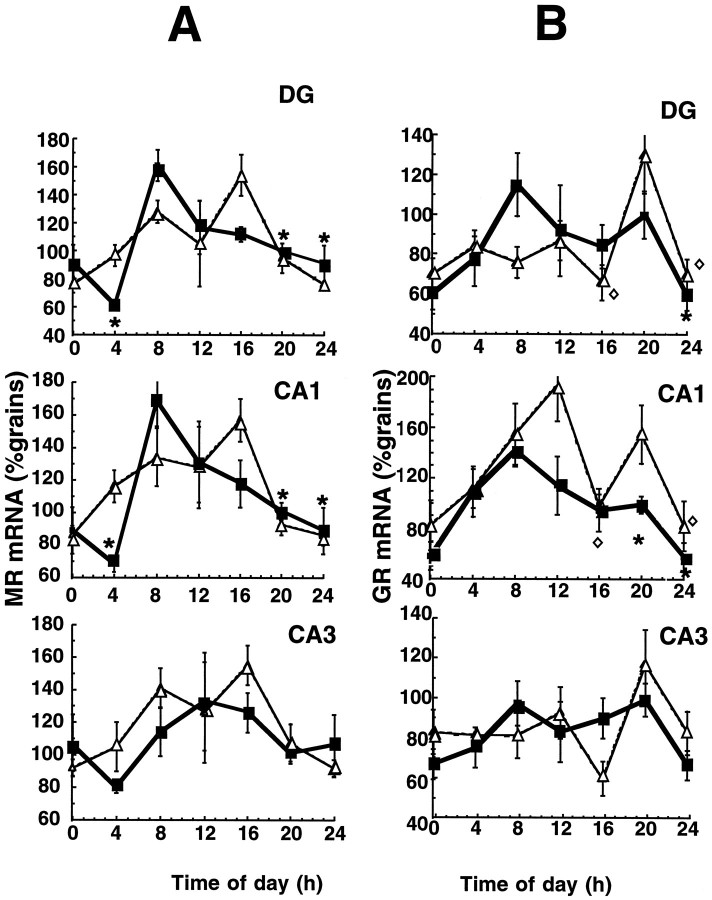
In control rats, hippocampal MR gene expression exhibited a clear significant circadian rhythm, with a peak at 8 A.M. and a nadir between midnight and 4 A.M., in both dentate gyrus and CA1 neurons (Fig.4A). No diurnal variation in MR mRNA was found in CA3. After food restriction the rhythm of MR gene expression was disrupted with a nonsignificant variance with time. Within this, the peak MR mRNA expression was delayed until 4 P.M., although the nadir remained at midnight.
Fig. 4.
Twenty-four hour profile of (A) MR and (B) GR mRNA expression in control (▪) and food-restricted (▵) animals in various hippocampal subregions. Receptor mRNA levels were determined by in situhybridization histochemistry. The mean number of grains/subregion/rat was standardized to the expression observed in the control rats at 8 P.M. (20) (100%). Values represent mean ± SEM;n = 4. *p < 0.05 compared with peak value. Time notation as in Figure 1 legend.
GR mRNA expression
There was also a clear significant diurnal rhythm of GR mRNA expression in controls, again confined to the dentate gyrus and CA1. The diurnal peak was at 8 A.M. and the nadir at midnight (Fig.4B). Food restriction altered the rhythms of GR mRNA in both dentate gyrus and CA1, although significant variance with respect to time of day persisted. In the dentate gyrus, the GR mRNA peak shifted to 8 P.M., whereas in CA1 peaks at noon and 8 P.M. occurred (Fig. 4B), a biphasic pattern similar to plasma corticosterone levels (correlation of GR mRNA changes with plasma B changes; p = 0.03). No significant changes in GR gene expression were observed over time in the CA3 region of the hippocampus in either group.
The effect of constant corticosterone levels on circadian variations in hippocampal receptor gene expression
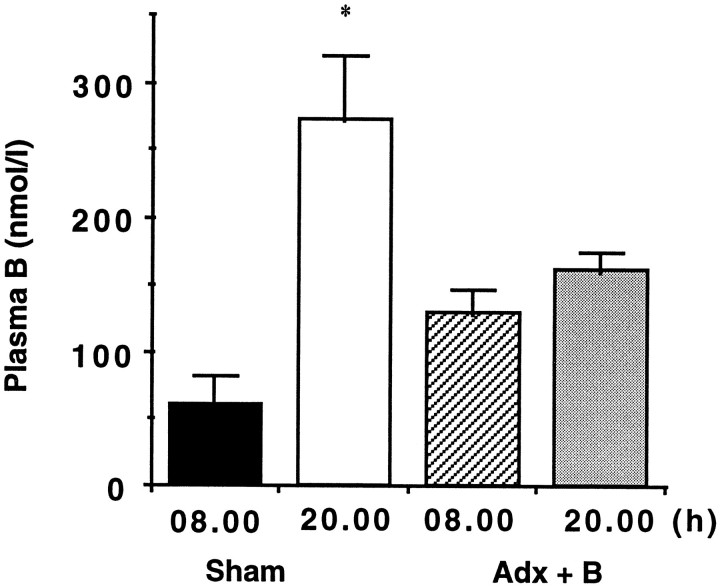
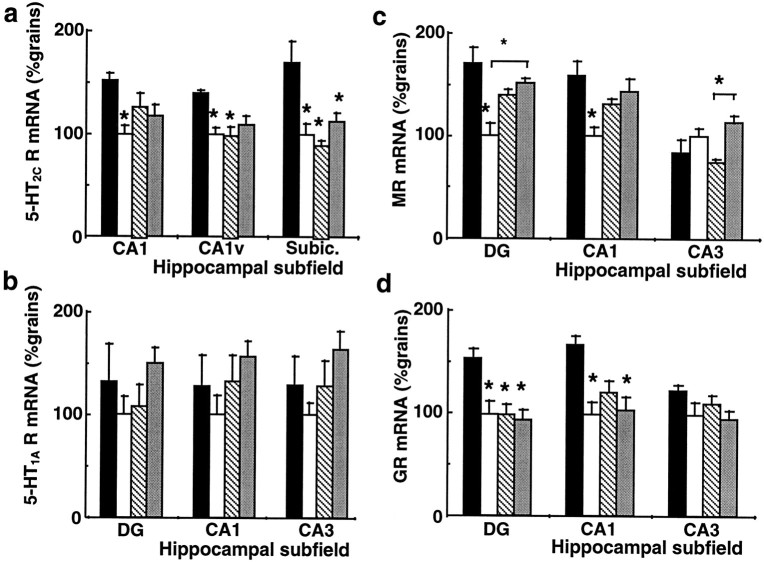
In rats sham-adrenalectomized with placebo pellet, plasma corticosterone levels showed a normal circadian variation (low morning and high evening levels) (Fig. 5). Adrenalectomized rats with 100 mg corticosterone pellets had plasma corticosterone levels modestly elevated over the control morning nadir but fixed throughout the day (Fig. 5). Sham-adrenalectomized controls had higher hippocampal 5-HT2C receptor mRNA expression at 8 A.M. than at 8 P.M. (Fig. 6a), as seen in the (unoperated) controls in the first study; however, constant corticosterone levels abolished the diurnal rhythm. Expression was fixed at levels similar to the 5-HT2C receptor mRNA nadir at 8 P.M., most notably in ventral CA1 and the subiculum. Similarly, the normal diurnal variation in MR and GR gene expression in the hippocampus was lost in animals with constant corticosterone levels (Fig. 6c,d). GR mRNA levels approximated the evening diurnal nadir with constant corticosterone, whereas MR mRNA expression was not clearly repressed, although diurnal variation was absent. No differences were observed in 5-HT1A receptor mRNA expression in the hippocampus of any group (Fig. 6b).
Fig. 5.
Plasma corticosterone levels obtained at 8 A.M. (08.00) and 8 P.M. (20.00) in sham-adrenalectomized (black and open columns) rats or rats adrenalectomized with a 100 mg corticosterone slow-release pellet subcutaneously (striped and stipled columns). *p < 0.05 compared with 8 A.M.
Fig. 6.
The effect of constant corticosterone levels on the diurnal rhythm of (a) 5-HT2C receptor (5-HT2C R mRNA), (b) 5-HT1A receptor (5-HT1AR mRNA), (c) MR (MR mRNA), and (d) GR (GR mRNA) mRNA expression in hippocampal subfields. Expression was measured in sham-operated animals at 8 A.M. (black columns) and 8 P.M. (open columns) and compared with adrenalectomized rats with a corticosterone pellet at 8 A.M. (striped column) and 8 P.M. (stipled column). Receptor mRNA levels were determined by in situhybridization histochemistry. The mean number of grains/subregion/rat was standardized to the expression observed in the control rats at 8 P.M. (100%). Values represent mean ± SEM; n= 5–6. *p < 0.05 compared with value at 8 A.M. in sham-operated rats.
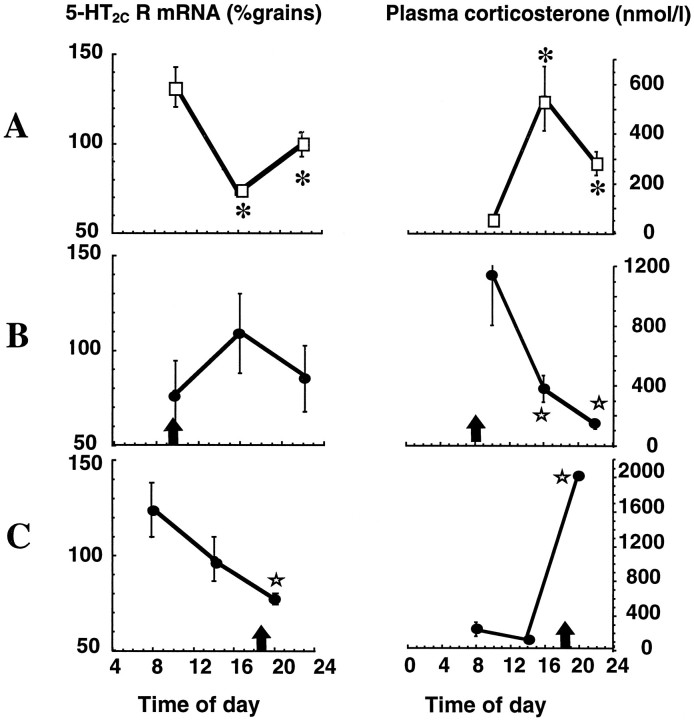
The effect of pulsatile corticosterone on hippocampal 5-HT2C receptor mRNA expression
To examine the possibility that the absence of a double diurnal peak in hippocampal 5-HT2C receptor gene expression in parallel to plasma corticosterone in food-restricted rats might reflect anergy of the response to elevated glucocorticoids in the morning, corticosterone was administered by injection in the morning and at the normal diurnal peak (evening). Injection of corticosterone produced elevated plasma corticosterone for 12 hr (Fig. 7). Corticosterone injection at 8 A.M. resulted in a constant level of 5-HT2C receptor gene expression throughout the day in all hippocampal subfields (CA1 data shown in Fig. 7B). In contrast, injection of corticosterone at 6 P.M., just before lights off, produced a rise in 5-HT2C receptor mRNA expression in CA1 (Fig. 7C) and subiculum (not shown) at 8 A.M., similar to that in sham-adrenalectomized (Fig. 7A) and other control animals.
Fig. 7.
The effect of pulsatile corticosterone replacement on the diurnal rhythm of 5-HT2C receptor mRNA expression in ventral CA1 of the hippocampus and plasma corticosterone levels. Sham-operated controls (□) compared with adrenalectomized rats with corticosterone replacement (•). A, Sham-operated controls; B, corticosterone injection (20 mg/kg, s.c.) at 8 A.M. (8); C, corticosterone injection (20 mg/kg, s.c.) at 6 P.M. The arrowrepresents time of corticosterone injection. Receptor mRNA levels were determined by in situ hybridization histochemistry. The mean number of grains/subregion/rat was standardized to the expression observed in the control rats at 8 P.M. (20) (100%). Values represent mean ± SEM; n = 4. *p < 0.05 compared with 8–10 A.M. control value. ⋆ p < 0.05 compared with 8–10 A.M. corticosterone replacement value. Time notation as in Figure 1legend.
Variations of 5-HT2C receptor mRNA expression at extra-hippocampal sites
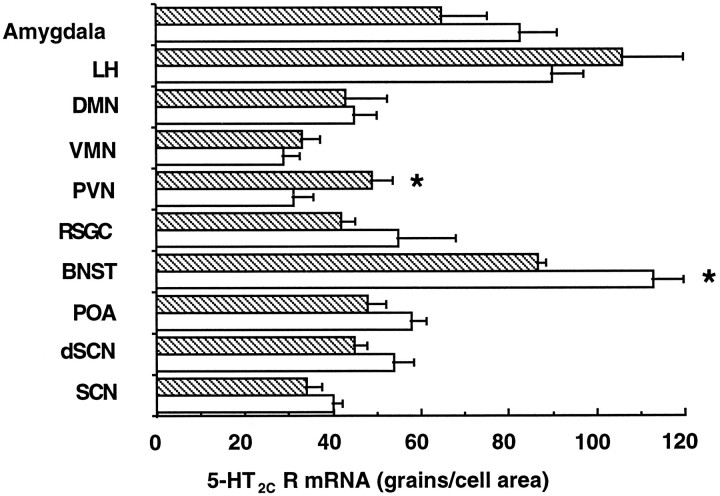
To determine whether the circadian variation is specific to the hippocampus, 5-HT2C receptor gene expression was also determined in other areas of the brain. 5-HT2C receptor mRNA expression was measured at 8 A.M. and 8 P.M. in the suprachiasmatic nucleus (SCN), preoptic area, bed nucleus of the stria terminalis (BNST), retrosplenic granule cortex, paraventricular nucleus of the hypothalamus (PVN), ventromedial nucleus, dorsomedial nucleus, lateral hypothalamus, and amygdala. Most areas did not show any variation of expression with time of day. The two exceptions were the BNST, which like the hippocampus had higher 5-HT2C receptor gene expression at 8 A.M., and the PVN, which had an opposite rhythm, showing higher expression at 8 P.M. (Fig. 8).
Fig. 8.
5-HT2C receptor mRNA expression in other brain regions at 8 A.M. (open columns) and 8 P.M. (striped columns). Values represent mean ± SEM;n = 4–6. *p < 0.05 compared with alternate time point. LH, Lateral hypothalamus;DMN, dorsomedial nucleus; VMN, ventromedial nucleus; PVN, paraventricular nucleus;RSGC, retrosplenic granular cortex; BNST, bed nucleus of the stria terminalis; POA, preoptic area;dSCN, dorsal suprachiasmatic nucleus;SCN, suprachiasmatic nucleus.
DISCUSSION
Circadian expression of hippocampal 5-HT2C receptor mRNA and 5-HT2C receptor agonist-mediated behavior
This study confirms and extends our previous observation of higher 5-HT2C receptor mRNA expression in discrete subregions of the hippocampus in the morning (Holmes et al., 1995a) to show a clear monophasic diurnal rhythm of 5-HT2C receptor gene expression. To determine whether the diurnal changes in hippocampal 5-HT2C receptor mRNA expression reflect changes in the number of binding sites available and hence 5-HT2Creceptor-mediated neurotransmission, two behavioral tests for 5-HT2C receptor agonist-mediated behavior were used (there are no radioligands that are sufficiently selective to discriminate 5-HT2A and -2C receptor binding sites). It has been shown previously that the 5-HT2C agonist mCPP inhibits locomotor activity in the open field test (Kennett and Curzon, 1988a) and induces hypophagia (Kennett and Curzon, 1988b). We found that mCPP-induced inhibition of locomotor behavior and rearing in the open field test exhibits a circadian periodicity, with inhibition more apparent at 9 A.M., which correlates well with the rhythm of hippocampal 5-HT2C receptor mRNA expression. mCPP inhibition of feeding, however, an effect likely to be mediated by 5-HT2C receptors outwith the hippocampus, showed no circadian rhythmicity, data that concur with the lack of diurnal changes in 5-HT2C receptor gene expression at most other brain sites and also suggest that the diurnal rhythm observed in open field behavior is not attributable to any diurnal variance in metabolism of the drug (which would produce generalized effects). Thus it seems probable that the diurnal rhythm in hippocampal 5-HT2C receptor mRNA expression is translated into a rhythm of 5-HT2C receptor protein and hence 5-HT2Creceptor-mediated behaviors.
Corticosterone
Chronic food restriction altered the normal diurnal profile of plasma corticosterone, producing a biphasic rhythm with an extra peak at 8 A.M. just before food availability, as well as the expected rise at the beginning of the dark phase. Krieger and Hauser (1978) have shown previously that food restriction has a potent influence on the diurnal rhythm of plasma corticosterone. In this study, plasma corticosterone levels over 24 hr were considerably greater in food-restricted animals than in controls. Thus, the previously reported HPA activation with short-term food restriction (Akana et al., 1994) persists for several weeks. The marked if episodic chronic hypersecretion of corticosterone suggests that long-term food restriction is a model of chronic intermittent stress and hence may be relevant for the study of molecular and other processes in some affective and eating disorders.
5-HT2C receptors
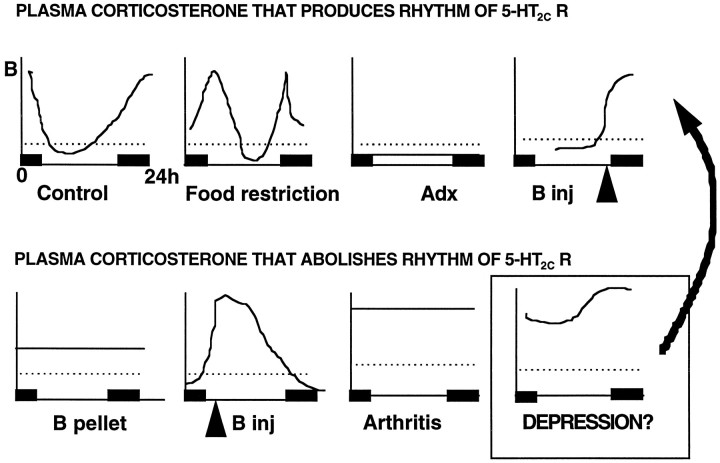
We have demonstrated previously that glucocorticoids regulate hippocampal 5-HT2C receptor mRNA expression (Holmes et al., 1995a,b); however, the relationships are not straightforward. Thus, the normal diurnal rhythm of hippocampal 5-HT2C receptor gene expression persists in adrenalectomized rats (Holmes et al., 1995b), implying that this is determined by factor(s) other than the glucocorticoid rhythm, possibly associated with the light–dark cycle cues. 5-HT2C receptor mRNA, however, is suppressed in the hippocampus by continuously elevated glucocorticoids [at diurnal maximum levels (Donaldson et al., 1993)] in chronic arthritis stress (Holmes et al., 1995a). This suppression is also seen with continuously but very modestly elevated corticosterone levels using low-dose pellets in this study, suggesting that whenever glucocorticoids are persistently elevated above low basal levels they suppress the rhythm of hippocampal 5-HT2C receptor mRNA (a summary of the correlation of 5-HT2C receptor mRNA expression in the hippocampus with plasma corticosterone profiles is presented in Fig.9).
Fig. 9.
Correlation of 24 hr profiles of plasma corticosterone with hippocampal 5-HT2C receptor mRNA diurnal rhythmicity. Dotted line represents normal nadir levels of corticosterone.
These effects of glucocorticoids, however, are clearly dependent on the manner and timing of exposure. When corticosterone levels were increased during the dark phase (mimicking the normal circadian rhythm), a normal rhythm in 5-HT2C receptor mRNA was observed. This rhythm was abolished, with suppressed receptor gene expression, if the same dose of corticosterone was given with a peak in the daylight hours. These data suggest that a nadir of corticosterone coinciding with the light-phase period of inactivity is required for the normal nocturnal increase in hippocampal 5-HT2Creceptor mRNA. This contention is supported by the effects of food restriction. This procedure did not suppress the rhythm of 5-HT2C receptor mRNA expression in the hippocampus, despite markedly elevated mean plasma corticosterone levels. There was a clear diurnal nadir of corticosterone (∼100 nmol/l) during the day, however, which thus may allow the later rise of 5-HT2Creceptor transcripts. Of course, providing the mRNA is translated (data from the behavioral studies suggest that this hypothesis is likely), then food restriction may inappropriately maintain 5-HT2C receptor sensitivity in the face of chronic intermittent stress and increased 5-HT transmission, perhaps amplifying the deleterious actions on mood and behavior mediated by this receptor subtype (Kennett et al., 1989, 1994). In contrast, continuously elevated glucocorticoids or loss of the association between the light–dark cycle and corticosterone suppresses the 5-HT2Creceptor rhythm and therefore presumably reduces the overall sensitivity to activation of 5-HT2C receptors. Perhaps much greater levels of stress or glucocorticoids are required under such circumstances to overcome the “compensatory” decrease in receptor expression before adverse affective events may occur. The food restriction protocol thus may be useful to investigate intermittent stress or mismatch effects, perhaps as occur in depression and eating disorders (Fig. 9).
The implications of the different effects of chronic intermittent versus continuous glucocorticoid excess (i.e., stress vs Cushing’s disease or pharmacotherapy) on mood pathology remain to be explored; however, increased 5-HT2C binding sites occur in animals exposed to chronic unpredictable stress (an animal model of depression) when plasma corticosterone levels are allowed to reach nadir levels between stresses (Moreau et al., 1993). Furthermore, animals reared in isolation have increased sensitivity to 5-HT2C agonists (Fone et al., 1996), confirming in another model of depression the importance of 5-HT2C receptor sensitivity. Patients with depression exhibit abnormal circadian rhythms of plasma cortisol, with a prolonged peak and early timing of the nadir, although a transient (or elevated) nadir usually occurs (Linkowski et al., 1987). This nadir, however, may be sufficient to maintain the diurnal rhythmicity and sensitivity of 5-HT2C receptors in depression, even in the presence of elevated glucocorticoids. Moreover, there is a diurnal variation in performance of various neuropsychological tasks in normal and depressed individuals; however, the variations are often reversed in patients with depression (Moffoot et al., 1994). Some tasks, particularly those involving short-term memory, are dependent on hippocampal processing, and hence the diurnal expression of the 5-HT2C receptor may be an important factor in the production of these diurnal differences.
5-HT2C receptor mRNA is also expressed in extra-hippocampal sites, including the SCN, which generates/regulates diurnal rhythms; however, we found high expression limited to a few cells around the dorsal cap of the SCN, along with lower expression in a subgroup of cells within the body of the nucleus. This distribution contrasts with very high expression throughout the SCN reported by others (Roca et al., 1993). Whatever the cause of the discrepancy, no circadian rhythm of receptor gene expression was observed in any SCN subfield, and thus 5-HT2C receptors seem unlikely to be directly involved in or responsive to SCN rhythms. Most other loci of 5-HT2Creceptor mRNA showed no diurnal changes; the exceptions of the BNST and the PVN are discussed below. The rhythm of 5-HT2C was selective in as far as no circadian changes in 5-HT1Areceptor mRNA expression in the hippocampus were documented.
GR and MR rhythms
Both MR and GR mRNA expression in the hippocampus show circadian variation (Herman et al., 1993; Holmes et al., 1995a), findings confirmed here. A monophasic rhythm of both transcripts was seen, with the data fitting better with other diurnal processes (and the corticosterone rhythm) than the biphasic rhythm of MR reported previously by Herman et al. (1993). GR mRNA was clearly suppressed by the continuously if very modestly elevated corticosterone levels with fixed-replacement, suggesting sensitive autoregulation (Burnstein et al., 1991). In food-restricted rats, however, there was clear divergence from the expected inverse relationship between corticosterone levels and GR gene expression, with overall hypersecretion of corticosterone associated with increasedGR mRNA in CA1 at some time points and maintained expression at all others. These data suggest that the increase in GR gene expression reflects other factors (stress, neurotransmitter release) in food restriction, again producing a mismatch, this time between corticosteroid receptors and their ligand. The MR mRNA rhythm was also abolished by fixed-level corticosterone replacement, suggesting that glucocorticoids are important; however, MR levels were not reduced to the usual diurnal nadir and clearly other cues are important in MR mRNA control. Again, food restriction disrupted MR diurnal variation, effects that may interfere with the normal MR-associated facilitation of hippocampal neuronal activation (Joëls and de Kloet, 1991). Why GR and MR (and 5-HT2C receptors) in CA3 should be exempt from diurnal variation and other regulatory influences in this study and others remains an unexplored anomaly. The various promoters of the MR gene show some site-specific expression and may underlie such effects (Kwak et al., 1993).
HPA axis regulation
The highest hippocampal 5-HT2C receptor mRNA expression is in ventral CA1 and the subiculum, areas showing circadian changes. Efferents from the subiculum project to the PVN, either directly (Kiss et al., 1983) or via the BNST (Herman et al., 1994). Lesioning of the fimbria-fornix pathway to the BNST has been reported to abolish the circadian rhythm of glucocorticoids (Fischette et al., 1980), although this has been contested (Bradbury et al., 1993). It is therefore possible that changes in subicular 5-HT2Creceptor activity could alter the recognized hippocampal regulation of HPA axis activity (Jacobson and Sapolsky, 1991). Interestingly, the BNST relay is also under serotonergic control, and here too 5-HT2C receptor mRNA is highly expressed and shows a diurnal cycle. Moreover, the PVN expresses 5-HT2C receptor mRNA, again with a circadian variation, although this is opposite (highest levels in the evening) to the hippocampus/subiculum and BNST (highest in the morning). This may be relevant to the negative influence of the hippocampus on the PVN. The pathway from the BNST to the PVN is GABAergic and inhibitory (Herman et al., 1994). Therefore, an increase in receptor number in the hippocampus and BNST may reinforce a decrease in receptor number at the PVN.
Footnotes
This work was supported by the Wellcome Trust as a Career Development Fellowship (M.C.H.) and a Senior Clinical Fellowship (J.R.S.). We thank Keith Chalmers for his technical assistance.
Correspondence should be addressed to Dr. M. C. Holmes, Molecular Endocrinology, Molecular Medicine Centre, Western General Hospital, Edinburgh EH4 2XU, Scotland, UK.
REFERENCES
- 1.Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- 2.Albert PR, Zhou Q-Y, van Tol HHM, Bunzow JR, Civelli O. Cloning, functional expression and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–5832. [PubMed] [Google Scholar]
- 3.Aldridge J, Seckl JR. Quantitation of emulsion autoradiography in situ hybridization using image analysis and rate of change filters. J Anat. 1993;183:183. [Google Scholar]
- 4.Blundell JE. Serotonin and the biology of feeding. Am J Clin Nutr. 1992;55:155S–159S. doi: 10.1093/ajcn/55.1.155s. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury MJ, Strack AM, Dallman MF. Lesions of the hippocampal efferent pathway (fimbria-fornix) do not alter sensitivity of adrenocorticotropin to feedback inhibition by corticosterone in rats. Neuroendocrinology. 1993;58:396–407. doi: 10.1159/000126569. [DOI] [PubMed] [Google Scholar]
- 6.Burnstein K, Bellingham D, Jewell C, Powell-Oliver F, Cidllowski J. Autoregulation of glucocorticoid receptor gene expression. Steroids. 1991;56:52–58. doi: 10.1016/0039-128x(91)90124-e. [DOI] [PubMed] [Google Scholar]
- 7.Carroll BJ. The dexamethasone suppression test for melancholia. Br J Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- 8.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from non-depressed patients. Arch Gen Psychiatry. 1976;138:1218–1221. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers D, Watson S. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain: a combined in situ hybridization/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers D, Kwak S, Mansour A, Akil H, Watson S. Corticosteroids regulate brain hippocampal 5-HT1A receptor mRNA expression. J Neurosci. 1993;13:914–923. doi: 10.1523/JNEUROSCI.13-03-00914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifton PG, Barnfield AM, Curzon G. Effects of food-deprivation and mCPP treatment on the microstructure of ingestive behavior of male and female rats. J Psychopharmacol. 1993;7:257–264. doi: 10.1177/026988119300700304. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson LF, Seckl JR, McQueen DS. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J Neurosci Methods. 1993;49:5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- 14.Fischette CT, Komisaruk BR, Edinger HM, Feder HH, Siegel A. Differential fornix ablations and the circadian rhythmicity of adrenal corticosteroid secretion. Brain Res. 1980;195:373–387. doi: 10.1016/0006-8993(80)90073-6. [DOI] [PubMed] [Google Scholar]
- 15.Fone KCF, Shalders K, Fox ZD, Arthur R, Marsden CA. Increased 5-HT(2C) receptor responsiveness occurs on rearing rats in social isolation. Psychopharmacology. 1996;123:346–352. doi: 10.1007/BF02246645. [DOI] [PubMed] [Google Scholar]
- 16.Fuller R. The involvement of serotonin in regulation of pituitary-adrenocortical function. Front Neuroendocrinol. 1992;13:250–270. [PubMed] [Google Scholar]
- 17.Healy D. Rhythm and blues: neurochemical, neuropharmacological and neuropsychological implications of a hypothesis of circadian rhythm dysfunction in the affective disorders. Psychopharmacology. 1987;93:271–285. doi: 10.1007/BF00187243. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Watson SJ, Chao HM, Coirini H, McEwen BS. Diurnal regulation of glucocorticoid receptor and mineralocorticoid receptor mRNAs in rat hippocampus. Mol Cell Neurosci. 1993;4:181–190. doi: 10.1006/mcne.1993.1022. [DOI] [PubMed] [Google Scholar]
- 19.Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 20.Holmes MC, French KL, Seckl JR. Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Mol Brain Res. 1995a;28:186–192. doi: 10.1016/0169-328x(94)00207-u. [DOI] [PubMed] [Google Scholar]
- 21.Holmes MC, Yau JLW, French KL, Seckl JR. The effect of adrenalectomy on 5HT and corticosteroid receptor subtype mRNA expression in rat hippocampus. Neuroscience. 1995b;64:327–337. doi: 10.1016/0306-4522(94)00407-v. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen FM, Mueller EA, Rosenthal NE, Rogers S, Hill JL, Murphy DL. Behavioural responses to intravenous meta-chlorophenylpiperazine in patients with seasonal affective disorder and control subjects before and after phototherapy. Psychiatry Res. 1994;52:181–197. doi: 10.1016/0165-1781(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenal axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 24.Joëls M, de Kloet ER. Mineralocorticoid effects on electrical activity in the rat brain. In: Bonvalet J-P, editor. Aldosterone: fundamental aspects. Colloque INSERM. Vol. 215. John Libbey; Paris: 1991. pp. 239–248. [Google Scholar]
- 25.Joseph Vanderpool JR, Jacobsen FM, Murphy DL, Hill JL, Rosenthal NE. Seasonal variation in behavioral responses to m-CPP in patients with seasonal affective disorder and controls. Biol Psychiatry. 1993;33:496–504. doi: 10.1016/0006-3223(93)90003-v. [DOI] [PubMed] [Google Scholar]
- 26.Julius D, MacDermott AB, Axel R, Jessel T. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988;241:558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- 27.Kalén P, Rosegren E, Lindvall OA. Hippocampal noradrenaline and serotonin release over 24 hours as measured by the dialysis technique in freely moving rats: correlation to behavioural activity state, effect of handling and tail-pinch. Eur J Neurosci. 1989;1:181–188. doi: 10.1111/j.1460-9568.1989.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 28.Kennett GA, Curzon G. Evidence that mCPP may have behavioural effects mediated by central 5-HT1C receptors. Br J Pharmacol. 1988a;94:137–147. doi: 10.1111/j.1476-5381.1988.tb11508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors: hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology. 1988b;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 30.Kennett GA, Whitton P, Shah K, Curzon G. Anxiogenic-like effects of mCPP and TFMPP in animal models are opposed by 5-HT1C receptor antagonists. Eur J Pharmacol. 1989;164:445–454. doi: 10.1016/0014-2999(89)90252-5. [DOI] [PubMed] [Google Scholar]
- 31.Kennett GA, Lightowler S, De Biasi V, Stevens NC, Wood MD, Tulloch IF, Blackburn TP. Effect of chronic administration of selective 5-hydroxytryptamine and noradrenaline uptake inhibitors on a putative indes of 5-HT(2C/2B) receptor. Neuropharmacology. 1994;33:1581–1588. doi: 10.1016/0028-3908(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 32.Kiss JZ, Palkovits M, Záborszky L, Tribollet E, Szabó D, Makara GB. Quantitative histological studies on the hypothalamic paraventricular nucleus in rats. II. Number of local and certain afferent nerve terminals. Brain Res. 1983;265:11–20. doi: 10.1016/0006-8993(83)91328-8. [DOI] [PubMed] [Google Scholar]
- 33.Krieger DT, Hauser H. Comparison of synchronization of circadian corticosteroid rhythms by photoperiod and food. Proc Natl Acad Sci USA. 1978;75:1577–1581. doi: 10.1073/pnas.75.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak SP, Patel PD, Thompson RC, Akil H, Watson SJ. 5′-Heterogeneity of the mineralocorticoid receptor messenger ribonucleic acid: differential expression and regulation of splice variants within the rat hippocampus. Endocrinology. 1993;133:2344–2350. doi: 10.1210/endo.133.5.8404687. [DOI] [PubMed] [Google Scholar]
- 35.Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, Copinschi G, Van Cauter E. 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab. 1987;65:141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- 36.MacPhee IAM, Antoni FA, Mason DW. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989;169:431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, de Kloet ER, Rostene W. Adrenal steroid receptors and action in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 38.Meltzer HY, Lowy MT. The serotonin hypothesis of depresion. In: Meltzer HY, editor. Psychopharmacology: the third generation of progress. Raven; New York: 1987. pp. 513–526. [Google Scholar]
- 39.Mengod G, Pompeiano M, Martinez-Mir M, Palacios J. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry: correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- 40.Moffoot APR, O’Carroll RE, Bennie J, Carroll S, Dick H, Ebmeier KP, Goodwin GM. Diurnal variation of mood and neuropsychological function in major depression with melancholia. J Affective Disord. 1994;32:257–269. doi: 10.1016/0165-0327(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 41.Moreau JL, Jenck F, Martin JR, Perrin S, Haefely WE. Effects of repeated mild stress and two antidepressant treatments on the behavioral response to 5HT(1C) receptor activation in rats. Psychopharmacology. 1993;110:140–144. doi: 10.1007/BF02246963. [DOI] [PubMed] [Google Scholar]
- 42.Ogren SO, Fuxe K, Agnati L, Gustafsson JA, Jonsson G, Holm A. Reevaluation of the indoleamine hypothesis of depression, evidence for a reduction of functional activity of central 5-HT systems by antidepressant drugs. J Neural Transm. 1979;46:85–103. doi: 10.1007/BF01250331. [DOI] [PubMed] [Google Scholar]
- 43.Palacios J, Waeber C, Hoyer D, Mengod G. Distribution of serotonin receptors. Ann NY Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 45.Peroutka S. 5-Hydroxytryptamine receptors. J Neurochem. 1993;60:408–416. doi: 10.1111/j.1471-4159.1993.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 46.Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdissection and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 47.Robinson DS. Serotonin receptor subtypes and affective disorders. Clin Neuropharmacol. 1993;16:S1–S5. [PubMed] [Google Scholar]
- 48.Roca AL, Weaver DR, Reppert SM. Serotonin receptor gene expression in the rat suprachiasmatic nuclei. Brain Res. 1993;608:159–165. doi: 10.1016/0006-8993(93)90789-p. [DOI] [PubMed] [Google Scholar]
- 49.Seckl JR, Dickson K, Fink G. Central 5,7-dihydroxytryptamine lesions decrease hippocampal glucocorticoid and mineralocorticoid receptor messenger ribonucleic acid expression. J Neuroendocrinol. 1990;2:911–916. doi: 10.1111/j.1365-2826.1990.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 50.Wright D, Seroogy K, Lundgren K, Davis B, Jennes L. Comparative localization of serotonin1A,1C and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 51.Yau JLW, Kelly PAT, Sharkey J, Seckl JR. Chronic 3,4-methylenedioxymethamphetamine (MDMA) administration decreases glucocorticoid and mineralocorticoid receptor, but increases 5-HT1C receptor gene expression in the rat hippocampus. Neuroscience. 1994;61:31–40. doi: 10.1016/0306-4522(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 52.Zhong P, Ciaranello RD. Transcriptional regulation of hippocampal 5-HT1A receptors by corticosteroid hormones. Mol Brain Res. 1995;29:23–34. doi: 10.1016/0169-328x(94)00225-4. [DOI] [PubMed] [Google Scholar]