Abstract
We compared the electrophysiological responses to serotonin (5-HT) of neonatal and juvenile rat hypoglossal motoneurons (HMs) by using intracellular recording techniques in a brainstem slice preparation. In neonatal HMs (≤P8), 5-HT caused a substantial decrease in the amplitude of spike afterhyperpolarization (AHP) that was associated with an increase in the minimal repetitive firing frequency (Fmin). Previous work has shown that this effect of 5-HT was mediated by the 5-HT1A receptor and may be secondary to inhibition of N- and P/Q-type calcium channels. In contrast to results from neonates, we found that 5-HT did not inhibit the AHP in juvenile HMs (≥ P20). Application of a cocktail of calcium channel toxins (ω-Conotoxin-GVIA and ω-Agatoxin-IVA) to juvenile HMs substantially inhibited the AHP, indicating that calcium entry through N- and P/Q-type channels supports the AHP in juvenile HMs, as it does in neonates. In addition, intracellular injection of the long-lasting GTP analog GTPγS induced an agonist-independent increase in Fmin similar to that seen in neonates in the presence of 5-HT. Together, these results suggested that intracellular mechanisms downstream of the 5-HT1A receptor capable of inhibiting the AHP were intact in juvenile HMs. Therefore, we investigated the possibility that age-related changes in effects of 5-HT on the AHP resulted from altered expression of the 5-HT1A receptor. To this end, we performed ligand-binding autoradiography using [3H]8-OH-DPAT, a 5-HT1Aagonist, and in situ hybridization using radiolabeled oligonucleotide probes specific for the 5-HT1A receptor. The two approaches gave remarkably similar results. The highest levels of 5-HT1A receptor expression were found in neonatal HMs, with maximal binding and hybridization at approximately postnatal day 7 (P7) and only low levels of receptor expression by P28. Finally, immunohistochemistry for 5-HT revealed that these developmental changes in 5-HT1A receptor expression occurred coincident with a postnatal increase in serotonergic innervation of the hypoglossal nucleus (nXII). Together, these findings indicate that developmental changes occur in the serotonergic innervation of nXII and in the expression of 5-HT1A receptors in HMs during the early postnatal period, resulting in markedly different effects of 5-HT on firing behavior in neonatal and juvenile HMs.
Keywords: ontogeny, hypoglossal, motoneuron, raphe, electrophysiology, in situ hybridization, radioligand binding, immunohistochemistry
The first few weeks of postnatal life in the rat are a critical developmental period for motor systems when numerous structural and functional changes are occurring, including increased behavioral capabilities, maturation of the neuromuscular junction, and changing phenotype and target dependence of motoneurons (Jansen and Fladby, 1990; Kiyama et al., 1991; Seroogy et al., 1991; Chen and Chiu, 1992; Lowrie and Vrbová, 1992; Walton et al., 1992; Chiu et al., 1993). As the final common pathway in motor systems, motoneurons receive and integrate convergent inputs and transduce those inputs into an output pattern that ultimately directs motor behavior. It is becoming increasingly apparent that integrative properties of motoneurons, as well as the neurotransmitter mechanisms by which those properties are modulated, also undergo significant changes during the early postnatal period (for review, see Berger et al., 1996). This plasticity reflects differential expression of ion channels during development (Núñez-Abades et al., 1993; Bayliss et al., 1994a; Viana et al., 1994) as well as the modulation of those ion channels by neurotransmitters at any given developmental stage (Ziskind-Conhaim et al., 1993; Bayliss et al., 1994c; Funk et al., 1995). Moreover, the modulatory neurochemical systems that impinge on motoneurons are not static; their chemical phenotype and projection patterns also can change throughout development (Ziskind-Conhaim et al., 1993; Bayliss et al., 1994c). Thus, a single modulatory system potentially can exert a variety of effects on motoneurons during development that reflect a complex interplay between maturational changes occurring presynaptically in the modulatory system and postsynaptically in the receptor–effector systems expressed by the motoneurons.
The serotonergic raphe neuronal system is believed to provide important modulatory effects on motor output systems, including a direct excitatory influence on motoneurons. The excitatory effects of serotonin (5-HT) on adult rat motoneurons have been studied, and a number of ionic mechanisms underlying those effects have been described consistently, including a 5-HT2 receptor-mediated inhibition of a resting “leak” K+ current (IK,L) and a 5-HT1-like receptor-mediated augmentation of the hyperpolarization-activated mixed cationic current, Ih (Aghajanian and Rasmussen, 1989; Larkman et al., 1989; Anwyl, 1990; Rasmussen and Aghajanian, 1990; Larkman and Kelly, 1992; Hsiao et al., 1997).
In neonatal rat motoneurons the cellular mechanisms underlying excitatory effects of 5-HT are less clear. For example, variable effects of 5-HT were seen during development in embryonic and neonatal spinal motoneurons that became more consistently like those in adult motoneurons as the serotonergic innervation of the spinal ventral horn increased (Ziskind-Conhaim et al., 1993). Further variability in reported effects of 5-HT in the neonate may reflect differences in specific motoneuronal populations. For instance, 5-HT modulatedIh and/or IK,L in neonatal spinal motoneurons (Takahashi and Berger, 1990; Wang and Dun, 1990; Larkman et al., 1995), whereas in neonatal hypoglossal motoneurons (HMs) the 5-HT-current was not associated with any measurable change in Ih orIK,L (Berger et al., 1992). In addition, 5-HT enhanced a low-voltage-activated (LVA) calcium current in neonatal spinal motoneurons with no appreciable effect on high-voltage-activated (HVA) calcium current (Berger and Takahashi, 1990), whereas it inhibited N- and P/Q-type HVA calcium current in neonatal HMs via a 5-HT1A receptor-mediated mechanism (Bayliss et al., 1995). It was suggested that this inhibition of N- and P/Q-type voltage-dependent calcium channels—by decreasing the calcium entry required to support the calcium-activated K+ conductance that underlies the spike afterhyperpolarization (AHP) (Viana et al., 1993; Umemiya and Berger, 1994)—was responsible for the 5-HT1A-mediated inhibition of the AHP in neonatal HMs (Berger et al., 1992; Bayliss et al., 1995) and the increased current-induced spike firing behavior caused by 5-HT in those cells (Berger et al., 1992).
The 5-HT1A-mediated inhibition of the AHP that we observed in neonatal HMs has not been reported in any population of adult rat motoneurons (although 5-HT1A-induced inhibition of the AHP has been observed in adult lamprey motoneurons; Wikström et al., 1995). We hypothesized, therefore, that 5-HT1A-mediated inhibition of the AHP is regulated developmentally in HMs. Accordingly, we found that, whereas neonatal HMs recorded in a slice preparation responded to 5-HT with an inhibition of the AHP, juvenile HMs did not. We performed electrophysiological and histochemical experiments to determine the cause of this postnatal change in effects of 5-HT on the AHP. In short, our results suggest that a decrease in 5-HT1A receptor expression accounts for the postnatal changes in effects of 5-HT on the AHP in HMs. Preliminary accounts of these findings have been presented (Bayliss et al., 1992a; Talley et al., 1996).
MATERIALS AND METHODS
Intracellular recording. Developmental changes in effects of 5-HT were determined in electrophysiological experiments, using brainstem slices from rats obtained at different postnatal ages. Throughout this report we refer to animals ≤P8 as neonates and animals ≥P20 as juveniles. Previous reports have indicated that many of the electrophysiological properties of HMs are adult-like by this stage (Núñez-Abades et al., 1993; Bayliss et al., 1994a,c; Viana et al., 1994; Berger et al., 1996), and we consider it likely that data from these juvenile animals are representative of adults.
Slices were prepared essentially as previously described (Bayliss et al., 1994a,c; Viana et al., 1994). Animals either were decapitated rapidly (≤P8) or were anesthetized (ketamine/xylazine), artificially ventilated (95% 02/5% CO2, carbogen), and decapitated. The brainstem was exposed, blocked, and removed under a steady stream of ice-cold Ringer’s or sucrose-containing Ringer’s solution (see below for composition). The tissue block was glued to an agar support with cyanoacrylate glue, submerged in ice-cold Ringer’s, and cut at 400–500 μm with a Vibroslice (Campden Instruments, Berlin, Germany) or a Microslicer (DSK, Dosaka, Japan). Slices were transferred to an interface-type tissue chamber perfused with oxygenated Ringer’s and gassed with a humidified oxygen/carbon dioxide mix (95%/5%) at 33 ± 1°C.
The Ringer’s solution contained (in mm): NaCl 130, KCl 3, MgCl2 2, CaCl2 2, NaH2PO4 1.25, NaHCO3 26, and glucose 10. Sucrose-containing Ringer’s, made by substituting 260 mm sucrose for NaCl, was used in experiments with older animals to improve viability of the slices (Aghajanian and Rasmussen, 1989). 5-HT was prepared as a 10 mm frozen stock solution and added to the perfusate at a final concentration of 10–100 μm. Stock solutions (100 μm) of ω-Conotoxin GVIA (Bachem, Torrence, CA) and ω-Agatoxin-IVA (a generous gift from Pfizer, Groton, CT) were diluted in Ringer’s solution containing 0.1% cytochrome C and applied from a broken-tipped pipette to the surface of the slice near nXII.
HMs were identified as described previously (Bayliss et al., 1994a,c;Viana et al., 1994). Neurons included in this study were located within the boundaries of the hypoglossal nucleus, had a stable resting potential less than or equal to −60 mV and an overshooting action potential, and fired repetitively in response to depolarizing current pulses. These properties are characteristic of identified HMs (Viana et al., 1994). Single action potentials were evoked by intracellular injection of brief (2 msec) current pulses. Repetitive firing behavior was assessed by long rectangular current pulses or slow depolarizing ramp current injections. The minimal repetitive firing frequency (Fmin) was taken as the steady-state firing rate evoked at the lowest current (50–100 pA increments) that would support repetitive firing throughout the current pulse (Viana et al., 1995).
Intracellular recordings were obtained with microelectrodes (10–100 MΩ) filled with 3 m KCl. Electrodes containing the tetralithium salts of GTPγS and GTP (10 and 30 mm, respectively) were used in some experiments (Bayliss et al., 1994b). Electrical recordings were made with an Axoclamp 2A (Axon Instruments, Foster City, CA) amplifier, using active bridge and discontinuous current-clamp (DCC) modes. The headstage output was monitored continuously on a separate oscilloscope to set capacitance compensation and maximize sampling frequency according to published procedures (Finkel and Redman, 1985). Membrane current and voltage were monitored on a storage oscilloscope and a pen recorder and stored on an FM tape recorder for off-line analysis either with a digital oscilloscope (Gould, Glen Burnie, MD) and a microcomputer by using laboratory-developed software or a digitizer (Labmaster TL-1 or Digidata 1200A; Axon Instruments) interfaced with a microcomputer running pClamp software (Axon Instruments). Data were compared statistically by t test, with differences considered significant if p < 0.05.
In situ hybridization—5HT1A receptor mRNA.Localization and relative quantitation of 5-HT1Areceptor expression were performed by in situ hybridization with radiolabeled oligonucleotide probes complementary to 5-HT1A receptor mRNA. Sprague Dawley rats of either sex were taken at postnatal day (P) 0 (day of birth), P7, P14, P21, and P28–P32, and rapidly decapitated. Brains were removed quickly, and the brainstem was blocked and frozen onto cryostat chucks over dry ice. Coronal sections (10 μm) were cut on a microtome in a cryostat, thaw-mounted onto twice gelatin-coated slides, and stored at −80°C. Tissue sections from each age group were processed concurrently under identical conditions for each experiment (n = 4). Sections were processed for in situ hybridization essentially as described previously (Bayliss et al., 1994c). Briefly, slide-mounted sections were allowed to equilibrate at room temperature, fixed for 5 min in 4% paraformaldehyde in 0.1 m phosphate buffer (PB), rinsed extensively in 0.1 m PBS (once with 2 mg/ml glycine), and placed in 0.25% acetic anhydride in 0.1m triethanolamine and 0.9% saline, pH 8, before being transferred through a graded series of alcohols and chloroform. The sections were air-dried before incubation overnight at 37°C with 50–100 μl of hybridization buffer [50% formamide; 4× SSC (1× SSC = 0.15 m NaCl/0.015 m sodium citrate, pH 7.0); 10% dextran sulfate; 0.02% each of Ficoll, polyvinylpyrrolidone, and bovine serum albumin; 100 mmdithiothreitol; 500 μg/ml denatured salmon sperm DNA; and 250 μg/ml yeast tRNA] containing a cocktail of oligodeoxyribonucleotide probes complementary to nucleotides 1–32 and 1234–1266 of the published sequence of the rat 5-HT1A receptor (Albert et al., 1990). A third probe corresponding to the putative third intracellular loop of the receptor (nucleotides 778–810) also was tested. Although this third probe gave similar results, it was not used in quantitative studies because of the high background that it induced. The probes were labeled by using terminal deoxynucleotidyl transferase (Bethesda Research Labs, Bethesda, MD) and α-[35S] or α-[33P] dATP (New England Nuclear, Boston, MA), purified by gel filtration, and added to the hybridization buffer (40 × 106 cpm/ml). The specificity of the hybridization was assessed by demonstrating that each of the probes directed against different regions of the 5-HT1A receptor mRNA gave an identical anatomic distribution in adult brain that was entirely consistent with previously published results (Chalmers and Watson, 1991; Pompeiano et al., 1992; Wright et al., 1995).
After hybridization the buffer was decanted; the sections were dipped twice in 1× SSC at 55°C and washed in 1× SSC at 55°C (4 × 15 min) and at room temperature for 1 hr each. The sections were dipped briefly in distilled water and 95% ethanol, air-dried, and either apposed to Hyperfilm β-max x-ray film (Amersham, Arlington Heights, IL) in cassettes for periods of 7–14 d or dipped in liquid emulsion (Ilford K5-D) and exposed for 2–4 weeks at 4°C. The emulsion was developed in D19 (Kodak, Rochester, NY) and fixed with Kodak fixer. Sections were counterstained with toluidine blue (0.25%), coverslipped, and analyzed with a Leitz Diaplan microscope equipped with bright-field and dark-field condensers.
A computerized image analysis system (MCID, Imaging Research, St. Catherines, Ontario, Canada) was used for quantitation of hybridization signal. One or two sections from each animal were taken from each of four representative experiments and analyzed for silver grain density over individual cells. Sections chosen for analysis were from approximately the same rostrocaudal position in the medulla oblongata (region just caudal to transition from fourth ventricle to central canal). The channel-linking feature of the software was used to circumscribe HMs imaged in bright-field mode while grains were counted from the same cells imaged with dark-field optics. Every neuron within the hypoglossal nucleus of the section was included; the averaged silver grain density from each animal was used as a single data point for further statistical analysis. Age-related differences in silver grain density were analyzed statistically by ANOVA, with a Bonferroni modification of the t test used for pair-wise comparison between groups. Differences were considered significant ifp < 0.05.
Photomicrographic images were digitized with MCID, and autoradiographic images were obtained with a slide scanner (Nikon, Tokyo, Japan). The digitized images were imported into Adobe Photoshop, where contrast adjustment was performed on images from all sections together; final lettered figures were created in CorelDraw.
Receptor autoradiography—[3H]8-OH-DPAT binding.Developmental changes in 5-HT1A receptor binding were determined from autoradiograms generated from the binding of [3H]8-OH-DPAT, a 5-HT1A receptor-specific radioligand (Hoyer et al., 1994) to brainstem slices from rats of different ages (P0, P7, P14, P21, and P28). Rats were decapitated rapidly, brains were removed quickly, and the brainstem was blocked and frozen onto cryostat chucks over dry ice. Coronal sections (25 μm) were cut in a cryostat, thaw-mounted onto twice gelatin-coated slides, and stored at −80°C. Sections were processed by following published protocols (Manaker and Verderame, 1990). They were warmed to room temperature, allowed to dry for 1 hr, and equilibrated in 0.17m Tris-HCl, 4 mm CaCl2, and 0.1% ascorbate, pH 7.6, at room temperature for 30 min. Sections were incubated in the same buffer containing 10 μm pargyline and 2 nm [3H]8-OH-DPAT (137 Ci/mmol; Amersham) for 1 hr. Nonspecific binding was determined in the presence of 1 μm 5-HT. Sections were washed twice in ice-cold equilibration buffer for 5 min each, dipped in distilled water (also ice-cold), air dried, and placed in a cassette apposed to [3H]-sensitive x-ray film (Amersham) for 6–9 weeks, together with autoradiographic standards (Amersham).
After the film was developed, the sections were counterstained (cresyl violet), and the extent of specific binding in nXII was determined densitometrically from the film autoradiographs. Images of Nissl-stained sections were aligned digitally with autoradiographs from the same sections (or adjacent sections from the same animal) by using the channel-linking feature of the image analysis system (MCID); nXII was circumscribed by using the Nissl image, and the corresponding area of the autoradiographs was quantified densitometrically. Optical density measurements for each animal were made from three to six sections that included the same rostrocaudal portion of the nucleus (as above for in situ hybridization). The tissue equivalent values provided with the autoradiographic standards were used together with the specific activity of the ligand to convert optical density measurements into receptor concentration (fmol/mg), and the averaged value from each animal was treated as a single data point for subsequent statistical analysis. Age-related differences in binding were analyzed statistically by ANOVA, with a Bonferroni modification of the t test used for pair-wise comparison between groups. Differences were considered significant if p < 0.05.
Images of autoradiographs and Nissl-stained sections were obtained with a slide scanner (Nikon). The images were imported into Adobe Photoshop, where contrast adjustment on the autoradiographs was performed as a group; final lettered figures were created in CorelDraw.
Immunohistochemical localization of serotonin in the hypoglossal motor nucleus. Sprague Dawley rats were taken at each of the postnatal ages listed above (2 at each age), anesthetized [by hypothermia (<P14) or with ketamine/xylazine], and perfused transcardially with 4% paraformaldehyde containing 0.2% picric acid. The brain was removed, immersed in the same fixative for 1 hr, and then placed in 10% sucrose in PB overnight at 4°C. Coronal sections (14 μm) of the medulla oblongata through the hypoglossal nucleus were cut in a cryostat, thaw-mounted onto twice gelatin-coated glass slides, and rinsed in Tris-saline (TS; 50 mm Tris and 150 mm NaCl, pH 7.4). The sections were preincubated with TS containing 3% normal goat serum and then 1% H2O2, rinsed with TS, and then incubated overnight at 4°C with a rabbit polyclonal antisera to 5-HT (1:1000; Eugene Tech, Ridgefield Park, NJ) in TS containing 0.1% Triton X-100 in a humidified chamber. This 5-HT antisera has been characterized extensively (Towle et al., 1984; Volpe et al., 1992); in our preliminary experiments it stained neuronal somata in the brainstem with a restricted distribution, as expected for serotonergic neurons (i.e., in the raphe nuclei), whereas relative densities of immunoreactive fibers matched those of previous reports (Halliday et al., 1995). After being rinsed extensively in TS, sections were incubated with biotinylated goat anti-rabbit IgG (1:200) for 1 hr at room temperature and then with HRP-conjugated avidin for 45 min (ABC kit; Vector Laboratories, Burlingame, CA). They were reacted with diaminobenzidine and rinsed in TS before being coverslipped in DPX and examined with a Leitz Diaplan microscope equipped with a dark-field condenser.
RESULTS
Inhibition of the AHP by 5-HT—postnatal changes
The effect of 5-HT on the firing behavior of a neonatal HM is shown in Figure 1. Intracellular injections of brief (2 msec) depolarizing current pulses were used to evoke single action potentials under control conditions and in the presence of 5-HT (after returning membrane potential to control levels with current injection; Fig. 1A). As is clear from the records obtained in this representative neonatal HM and consistent with our previous results (Berger et al., 1992; Bayliss et al., 1995), 5-HT caused a substantial inhibition of the AHP that followed the action potential. The AHP is a primary determinant of the minimal repetitive firing frequency (Fmin) (Viana et al., 1995) and is involved in establishing the sensitivity of the spike firing response to injected current (Berger et al., 1992; Viana et al., 1993). Accordingly, as shown in Figure 1B, we found that 5-HT markedly increased the minimal repetitive firing frequency response to long (500 msec) depolarizing current pulses.
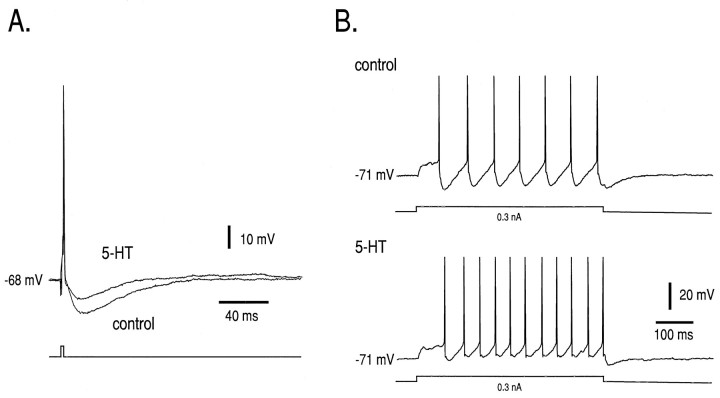
Fig. 1.
Effects of 5-HT on firing behavior in neonatal HMs. Intracellular recordings were made from a neonatal HM (P4).A, Intracellular injection of a brief depolarizing current pulse (1.3 nA; 2 msec) was used to evoke single action potentials under control conditions and during bath application of 5-HT (100 μm). The AHP was reduced substantially in the presence of 5-HT. Membrane potential was adjusted to −68 mV in both cases by current injection; 5-HT caused ∼10 mV membrane depolarization in this neuron. B, In the same cell long-duration current pulses (500 msec) were used to induce firing at the current threshold for repetitive firing before and during 5-HT application (0.3 nA). The minimal repetitive firing frequency (Fmin) was increased substantially by 5-HT. The neuron was held at −71 mV in both cases by current injection.
We compared the effect of 5-HT on neonatal and juvenile HMs, as shown in Figure 2 and Table 1. In both neonatal and juvenile HMs, 5-HT caused a membrane depolarization (Fig. 2,left), with the notable difference that the depolarization was not associated with increased input resistance (RN) in the neonate (see also Berger et al., 1992), whereas 5-HT increased RN by ∼20% in the juvenile HM. This effect of 5-HT has not been reported previously in juvenile HMs but commonly has been observed in other populations of adult motoneurons, in which 5-HT2 receptors were implicated (Aghajanian and Rasmussen, 1989; Larkman et al., 1989; Anwyl, 1990;Rasmussen and Aghajanian, 1990; Larkman and Kelly, 1992; Hsiao et al., 1997). The reason for age-related differences in effects of 5-HT onRN remains to be clarified. Of particular relevance to this study, however, we found that the AHP was decreased markedly by 5-HT in the neonatal HM (Fig. 2A,right), whereas in the juvenile HM 5-HT had little effect on the AHP (Fig. 2B, right). In neonates 5-HT decreased the AHP by >20% in 14 of 18 HMs tested (78%); in those responsive neurons the AHP was reduced from −11.4 ± 0.7 to −6.0 ± 0.7 mV (n = 14; p < 0.0001). By contrast, 5-HT did not reduce the AHP by >20% in any of the juvenile HMs tested (n = 6). The difference in percentage of reduction of the AHP by 5-HT between neonatal and juvenile HMs was highly significant (to ∼53 and 89% of control, respectively; p < 0.0005).
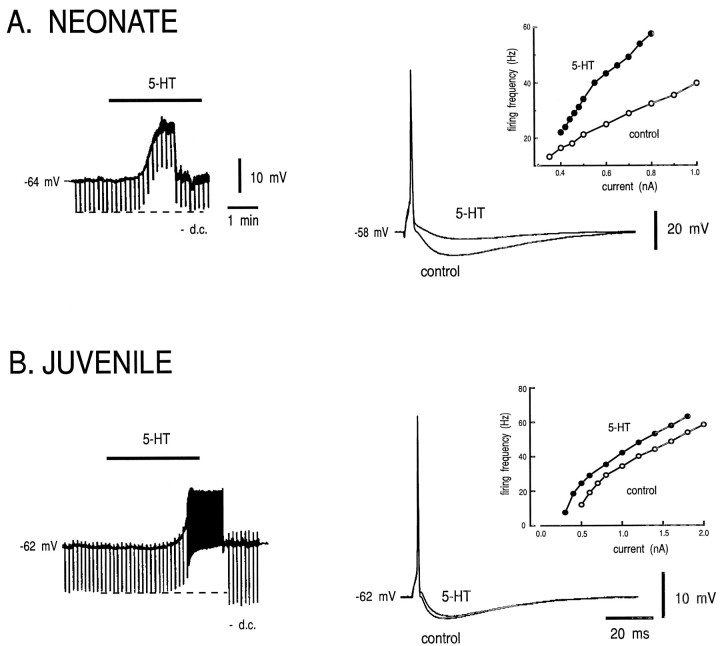
Fig. 2.
5-HT inhibits the AHP in neonatal HMs, but not in juvenile HMs. The effect of 5-HT on membrane potential and firing behavior was tested in neonatal (A) and juvenile (B) HMs. Left, Membrane potential was recorded before and during application of 5-HT (100 μm) via the perfusate; downward deflections in the trace represent membrane voltage responses to constant amplitude current pulses used to monitor input resistance (RN). During the response to 5-HT, current was injected to return the membrane potential to control levels (−DC). In the neonate (P5), 5-HT caused membrane depolarization with little change in RN. In the juvenile (P21), 5-HT caused membrane depolarization that was associated with a substantial increase in RN. Right, Action potentials were evoked with a brief (2 msec) current pulse before and during bath application of 5-HT. Membrane potential preceding the current pulse was adjusted to the same value in control and 5-HT by current injection. In the neonate, 5-HT caused a marked reduction in the amplitude of the AHP. By contrast, 5-HT had little effect on the AHP in the juvenile HM. Insets, The steady-state firing frequency-injected current relationship (f–Icurve) was obtained in control and then in the presence of 5-HT by using rectangular current pulses of increasing amplitude. In the neonate, 5-HT caused a clear increase in the slope of thef–I curve with an increase inFmin. In the juvenile, however, 5-HT caused a parallel leftward shift in the f–I curve. All records are from the same neonatal and juvenile HM; action potentials are truncated.
Table 1.
Effects of 5-HT on hypoglossal motoneurons
| Input resistance (% of control) | ΔIthreshold (nA) | AHP amplitude (% of control) | Δf–Islope (Hz/nA) | |
|---|---|---|---|---|
| Neonates1-a | 98.4 ± 4.1 (5) | −0.01 ± 0.04 (13) | 52.7 ± 5.5 (14) | 20.9 ± 4.1 (13) |
| Juvenile | 124.0 ± 9.71-b (6) | −0.4 ± 0.11-c (6) | 88.6 ± 2.91-c (6) | 2.6 ± 0.91-c (6) |
Includes cells in which 5-HT inhibited AHP by >20%.
Significantly different from neonates (p < 0.05).
Significantly different from neonates (p < 0.005).
These different effects of 5-HT were reflected in the different steady-state repetitive firing responses to injected current in neonatal and juvenile HMs (f–I curves; Fig. 2,insets). The decreased AHP in neonates was associated with an increased slope of the f–I curve and little change in the threshold current (Fig. 2A, inset). In those responsive neonatal HMs tested, 5-HT increased thef–I slope by 20.9 ± 4.1 Hz/nA (from 38.0 ± 2.8 to 58.9 ± 5.5 Hz/nA; n = 13; p < 0.0005) but had no effect on RN or the current threshold for repetitive firing. In juvenile HMs, by contrast, 5-HT caused a parallel, leftward shift in the steady-state f–Icurve (Fig. 2B; inset); accompanying a 24 ± 9.7% increase in RN, the threshold was shifted by −0.4 ± 0.1 nA with no significant change in thef–I slope (24.0 ± 3.4 vs 26.6 ± 4 Hz/nA;n = 6). Thus, the different effects of 5-HT on the properties of neonatal and juvenile HMs resulted in strikingly different firing behaviors. The remainder of this report focuses on mechanisms responsible for the differential effect of 5-HT on AHPs in neonatal and juvenile HMs.
Receptor-independent inhibition of the AHP in juvenile HMs
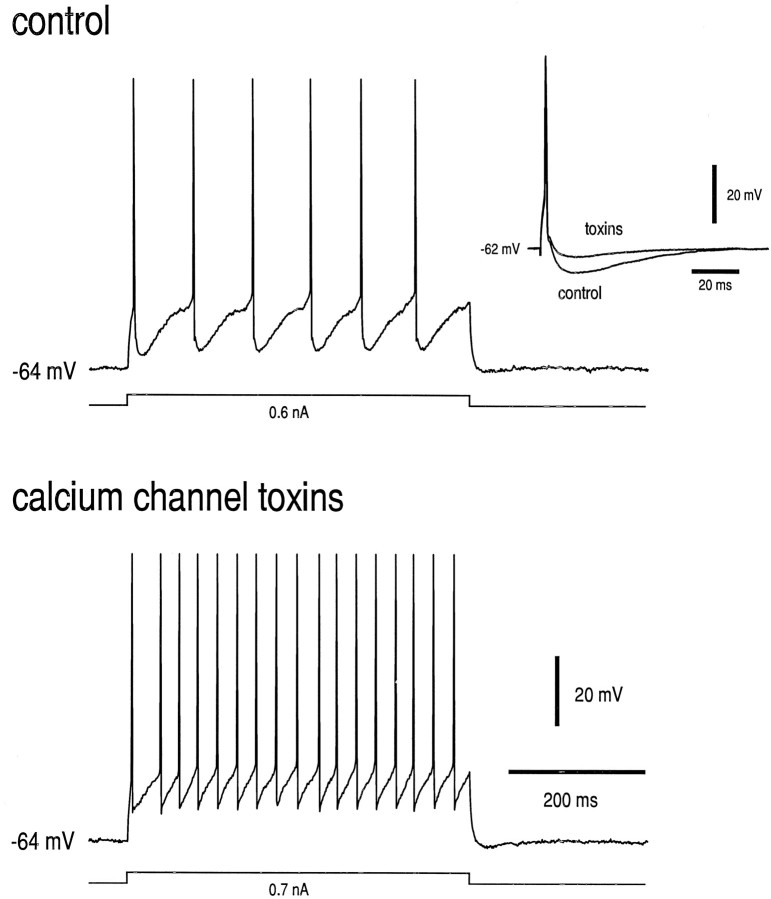
The AHP in neonatal HMs is mediated by a calcium-dependent K+ conductance (Viana et al., 1993), and the calcium that supports the AHP enters the motoneuron through N- and P/Q-type calcium channels (Viana et al., 1993; Umemiya and Berger, 1994). Furthermore, inhibition of the AHP by 5-HT is attributable, at least in part, to inhibition of N- and P/Q-type channels by 5-HT1A receptors via a G-protein-mediated mechanism (Bayliss et al., 1995). The postnatal change in effects of 5-HT on the AHP described above (Fig. 2) conceivably could result from altered expression of N- and P/Q-type calcium channels in juvenile HMs or in their support of the AHP. We tested this possibility by determining the effect of toxins that block N- (ω-CgTx-GVIA) and P/Q-type channels (ω-AgaTx-IVA) on the AHP in juvenile HMs. Data from a representative neuron are shown in Figure3. Microdroplet application of a cocktail of ω-CgTx-GVIA and ω-AgaTx-IVA (10 and 1.0 μm, respectively) to the surface of the slice caused a rapid and nearly complete abolition of the AHP in this motoneuron (see Fig. 3,inset); this effect was accompanied by a marked increase in the Fmin. Calcium channel toxins diminished the AHP in all juvenile HMs tested (n = 4); the AHP was reduced to 33.7 ± 2.5% of control (from −6.7 ± 0.9 to −2.3 ± 0.4 mV; p < 0.001). These data suggest that, as was the case for neonatal HMs (Viana et al., 1993; Umemiya and Berger, 1994), juvenile HMs express N- and/or P/Q-type calcium channels and that calcium entry via those channels is critical to the AHP.
Fig. 3.
Calcium channel toxins inhibit the AHP and increase Fmin in juvenile HMs. Effects on single action potentials and repetitive firing in a juvenile HM (P27) were determined before and after microdroplet application of a cocktail of calcium channel toxins (ω-CgTx-GVIA, 10 μm; ω-AgaTx-IVA, 1 μm). The motoneuron was held at −64 mV throughout the experiment. The minimum repetitive firing frequency was increased substantially by the calcium channel toxins.Inset, Calcium channel toxins decreased the amplitude of the AHP after single action potentials. Thus, N- and/or P/Q-type channels provide calcium to support the AHP in juvenile HMs.
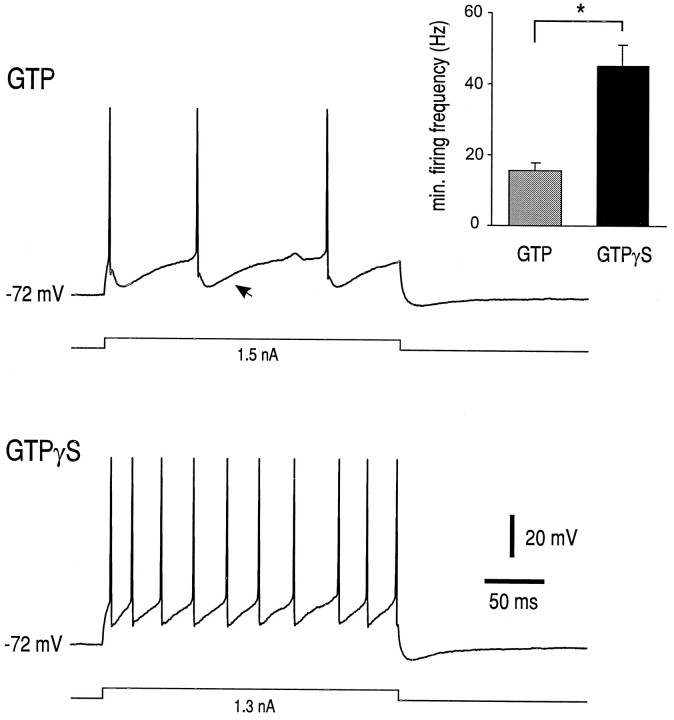
To determine whether transduction mechanisms downstream of the receptor remained intact in juvenile HMs, we recorded from cells with electrodes containing GTPγS. This nonhydrolyzable GTP analog activates intracellular transduction pathways, such as those leading to calcium current inhibition (see Swartz, 1993), in a ligand-independent manner. As a control, interleaved recordings were made from other juvenile HMs in the slices with GTP-containing electrodes. Representative records of repetitive firing behavior at Fmin in juvenile HMs recorded with GTP- and GTPγS-containing electrodes are presented in Figure 4. Note the prominent AHP in the cell recorded with a GTP-containing electrode that is not apparent in the cell recorded with GTPγS-containing electrode. Also, note that the cell impaled with the GTPγS-containing electrode had a substantially higher Fmin. In fact, averaged data shown in Figure 4 (inset) indicated that Fminin cells recorded with GTPγS-containing electrodes was 45.2 ± 5.7 Hz (n = 6), significantly higher than that in cells recorded with GTP-containing electrodes (15.6 ± 2.2 Hz;n = 6; p < 0.0005). These data suggest that juvenile HMs retain G-protein-mediated mechanisms that can be activated in a receptor-independent manner and that lead ultimately to inhibition of the AHP.
Fig. 4.
GTPγS increases the minimal repetitive firing frequency in juvenile HMs. Repetitive firing behavior was recorded in juvenile HMs (both P21) with electrodes containing either GTP or its nonhydrolyzable analog GTPγS; firing was evoked at the current threshold for minimum repetitive firing in the two cells (1.5 and 1.3 nA, respectively). Fmin was higher in the cell recorded with the GTPγS-containing electrode (∼39 Hz) than in the cell recorded with the GTP-containing electrode (∼11 Hz). Note also the prominent AHP in the cell recorded with the GTP-containing electrode (arrow); the AHP was substantially smaller in the cell recorded with the GTPγS-containing electrode.Inset, Averaged minimal repetitive firing frequency (Fmin) was significantly higher in cells recorded with GTPγS-containing electrodes (n = 6) than in cells recorded with GTP-containing electrodes (n = 6). *p < 0.0005.
Postnatal changes in 5-HT1A receptor expression
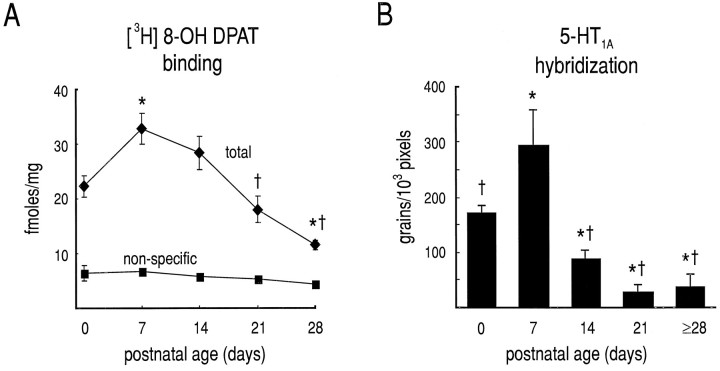
Our demonstration that mechanisms downstream of the receptor capable of causing AHP inhibition remained intact in juvenile HMs suggested that a postnatal change in 5-HT1A receptor expression might account for the inability of 5-HT to inhibit the AHP. To test that possibility, we performed radioligand binding and in situ hybridization experiments with 5-HT1Areceptor-specific probes. Data from representative experiments are shown in Figure 5. Radioligand-binding experiments that used saturating concentrations of [3H]8-OH-DPAT (2 nm), a ligand with relative binding specificity for 5-HT1A receptors, revealed high levels of binding in the hypoglossal nucleus in the early neonatal period (P0 and P7), with lower levels at later times (Fig. 5, left panels). Uniformly low levels of binding were detected in sections incubated with excess unlabeled 5-HT at all ages (see Fig. 7A,squares). Similar results were obtained in each of four independent experiments, suggesting that the number of 5-HT1A receptors (i.e., Bmax) decreases postnatally. The extremely low maximal binding in nXII of juvenile animals precluded accurate determination of receptor affinity. However, we obtained similar results by using a fourfold higher concentration of radioligand, indicating that the lack of binding in juvenile nXII was not attributable simply to a change in receptor affinity. Furthermore, these results were supported by in situ hybridization experiments with oligonucleotide probes directed against the 5-HT1A receptor mRNA (Fig. 5,right panels). Thus, hybridization signal was strong in nXII of young animals (P0 and P7) but markedly reduced by P14; the decrease in 5-HT1A receptor mRNA expression was sustained through P28. Photomicrographs from emulsion-dipped slides, presented in Figure6, show the cellular localization and density of hybridization signal within nXII from representative sections of a P0 and P28 animal. In sections from P0 animals there was a relatively high density of silver grains overlying individual cells; the strongest labeling was in the ventral aspects of nXII. In the juvenile nXII fewer labeled cells were apparent, and the density of silver grains overlying individual juvenile HMs was reduced.
Fig. 5.
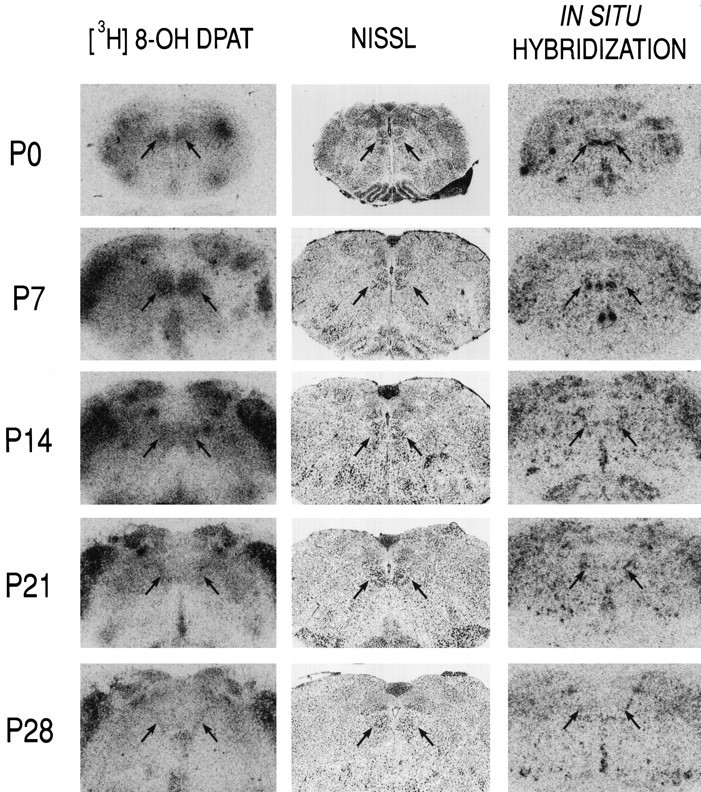
5-HT1A receptor expression decreases in the hypoglossal motor nucleus during postnatal development. Transverse slices of rat brainstem were taken at different postnatal ages (P0–P28) and incubated with [3H]8-OH-DPAT, a 5-HT1A receptor-specific ligand (left), or with a cocktail of two [33P]-labeled oligonucleotides complementary to 5-HT1A receptor mRNA (right) and apposed to autoradiographic film. Photomicrographs of autoradiograms show the intermediate portion of the hypoglossal nucleus (arrows), which also is evident in adjacent Nissl-stained sections (middle). The levels of [3H]8-OH-DPAT binding and of 5-HT1A receptor mRNA in the hypoglossal nucleus decrease dramatically during the postnatal period.
Fig. 7.
Quantification of postnatal changes in 5-HT1A receptor expression. Expression of 5-HT1A receptor was evaluated by quantitative ligand-binding autoradiography and in situhybridization. A, Film autoradiograms were generated from sections incubated in [3H]8-OH DPAT and analyzed densitometrically (see Materials and Methods); total andnon-specific binding were determined in adjacent sections incubated in the absence and presence of 1 μm5-HT, respectively. B, Sections were hybridized with antisense oligonucleotides and exposed to liquid emulsion. Silver grains were counted over individual motoneurons, and grain densities (grains/1000 pixels) were calculated; background was determined by measuring grain density over tissue where no cells were evident and subtracted. Both 5-HT1A receptor binding (A) and mRNA levels (B) peak near postnatalday 7 (P7) before dimin ishing to adult levels. In A and B, all values from a single animal were averaged, and the average was treated as a single data point; error bars represent SEM. Developmental changes in [3H]8-OH DPAT binding and in silver grain density were highly significant (F4,11 = 18.0,p < 0.0001 and F4,11 = 15.8, p < 0.0005, respectively); there was no effect of development on nonspecific binding shown in A(F4,8 = 1.8, p > 0.20). *, Different from P0; †, different from P7 (at p< 0.01).
Fig. 6.
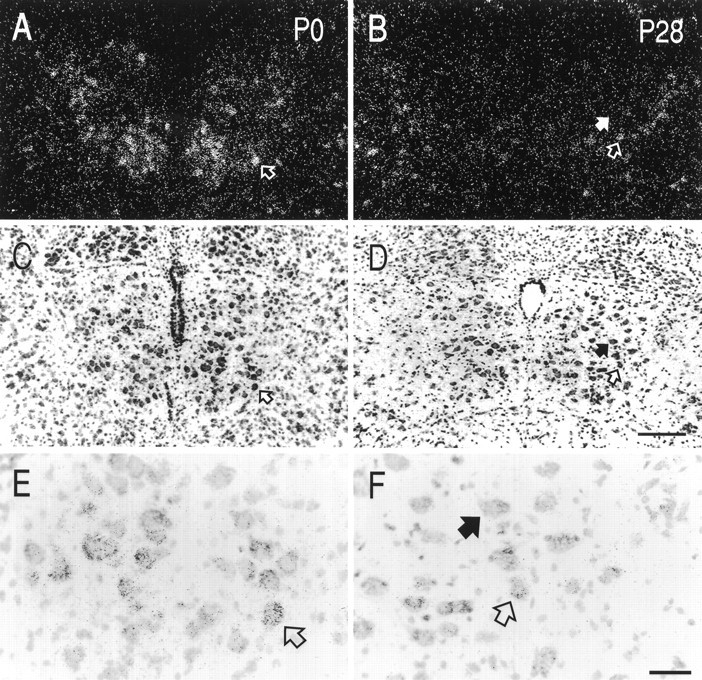
Distribution of 5-HT1A receptor mRNA in neonatal and juvenile hypoglossal nuclei. Sections from neonatal (P0; A, C, E) and juvenile (P28; B, D, F) brainstem were hybridized with [33P]-labeled oligonucleotides and exposed to liquid emulsion. Individual sections were photographed with dark-field (A, B) and bright-field (C–F) microscopy. Arrows indicate the same neurons in each of the photomicrographs. Dark-field images reveal a greater density of silver grains in the neonatal hypoglossal nucleus, as compared with that of the adult, indicating a higher concentration of 5-HT1A receptor mRNA in neonatal HMs. Labeling in the neonate was found predominantly in the ventral portion of the nucleus. Most juvenile HMs were unlabeled (filled arrows in B, D, F); scattered motoneurons were labeled in the juvenile (open arrows in B, D, F), but they were observed less frequently than in the neonate and contained a lower density of silver grains. Labeled cells, presumably interneurons, were seen at the border of the nucleus throughout development. Scale bars: 200 μm, A–D; 50 μm, E, F.
To quantify postnatal changes in 5-HT1A receptor expression in HMs, we measured optical density of the region of nXII in film autoradiographs from radioligand-binding experiments and the density of silver grains overlying individually labeled HMs in emulsion autoradiographs from in situ hybridization experiments (Fig.7). Results from each type of experiment were strikingly similar, and developmental changes were highly significant. Radioligand binding was high at P0 and peaked at P7 before declining to levels just above background by P28; nonspecific binding was consistently low at all ages (Fig. 7A). Likewise, 5-HT1A receptor mRNA accumulation peaked at P7 from initially high levels at P0 before decreasing to sustained low levels by P14 (Fig. 7B). Thus, these data indicate that levels of 5-HT1A receptor expression in nXII decrease postnatally, from high levels at <P8 to extremely low levels in older animals (>P20).
We also investigated the postnatal development of 5-HT1Areceptor mRNA expression in three other populations of motoneurons (cervical, lumbar, facial). In all three groups of motoneurons, higher levels of 5-HT1A receptor expression were observed in neonates (P5–P7) than in older animals (P28), in which expression was uniformly low (n = 4; data not shown). Thus, the transient high level of 5-HT1A receptor expression that we observed in neonatal HMs may be a general property of motoneurons.
Postnatal changes in 5-HT innervation of the hypoglossal nucleus
To determine whether the postnatal changes in 5-HT1Areceptor expression that we observed in HMs were correlated with changes in the 5-HT innervation of the hypoglossal nucleus, we performed immunohistochemical experiments on brainstem sections from perfusion-fixed rats of different postnatal ages. The distribution of 5-HT-immunoreactive fibers in nXII is shown in the dark-field photomicrographs of immunoperoxidase-stained sections of Figure8. There were few 5-HT-immunoreactive fibers in the hypoglossal nucleus (demarcated by dashed lines) from the P0 animal, more at P7, and a high fiber density by P28. The developmental change in the density of the 5-HT-immunoreactive fibers in nXII is also clear in the higher power bright-field photomicrographs, which illustrate the postnatal increase in 5-HT-immunoreactive varicosities. Thus, the decrease in expression of 5-HT1A receptors in HMs occurs at the same time as an increase in the density of the serotonergic innervation of the hypoglossal nucleus. It is important to point out, however, that there is a substantial 5-HT innervation of the hypoglossal nucleus at P7, when the levels of 5-HT1Areceptor binding and mRNA accumulation were maximal.
Fig. 8.
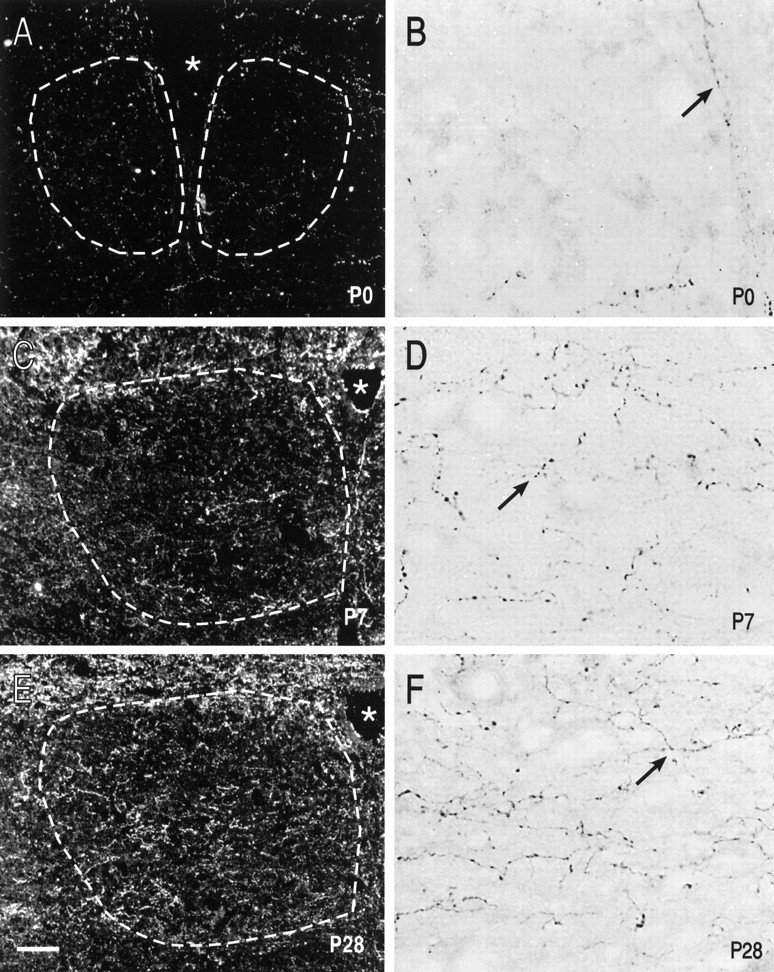
Postnatal changes in 5-HT innervation of nXII. Sections from neonatal (P0, A, B;P7, C, D) and juvenile (P28, E, F) rat brainstem were treated for immunohistochemical detection of 5-HT. Sections were photographed at lower power with dark-field microscopy (A, C, E) and at higher power with bright-field microscopy (B, D, F). There were few immunoreactive fibers in nXII at P0 (A, B), but by P7 a substantial 5-HT innervation already was apparent (C, D). The density of 5-HT-immunoreactive fibers was increased further in nXII of the P28 animal (E, F). Arrows indicate 5-HT-immunoreactive varicosities in nXII at each of the ages. Scale bars: 100 μm, A, C, E; 15 μm, B, D, F. Dashed white lines demarcate the approximate boundaries of nXII.
DISCUSSION
We found developmental increases in the serotonergic innervation of the rat hypoglossal nucleus that occurred coincident with decreased expression of 5-HT1A receptors by HMs. Importantly, a major effect of 5-HT on neonatal HMs—inhibition of the AHP—shown previously to be mediated by 5-HT1A receptors (Bayliss et al., 1995) was lost in juvenile HMs. The lack of effect of 5-HT on the AHP in juvenile HMs resulted from decreased 5-HT1A receptor expression, because juvenile HMs retained the N- and P/Q-type channels targeted by 5-HT1A receptors (Bayliss et al., 1995), and a decrease in the AHP could be demonstrated by using intracellular injection of GTPγS to activate G-protein-coupled mechanisms downstream of the 5-HT1A receptor. Inhibition of the AHP by 5-HT has been associated in neonatal HMs with an increased slope of the relationship between firing frequency and injected current (i.e., a higher input–output gain) (Berger et al., 1992); this effect on repetitive firing was absent in juvenile HMs, consistent with our finding that those juvenile HMs did not express 5-HT1Areceptors and showed no 5-HT-induced inhibition of the AHP. Rather, 5-HT caused a leftward, parallel shift in the f–I curve of juvenile HMs, probably because of the 5-HT-induced increase inRN in those cells (Bayliss et al., 1992b; Hsiao et al., 1997). These results indicate that significant postnatal changes occur in both the pre- and postsynaptic elements of the serotonergic raphe–hypoglossal motoneuronal system and predict that the functional consequence of raphe activity and 5-HT on HM firing behavior will be substantially different in neonates and adults.
Postnatal changes in 5-HT innervation of nXII and expression of 5-HT1A receptor by HMs
Ephemeral expression of 5-HT1A receptors, as we reported here for HMs, has been observed in other regions of the CNS. Thus, high levels of 5-HT1A receptor expression have been reported to occur transiently in the rat thalamus, inferior colliculus, and cerebellum and in the human cortex and cerebellum just before or approximately at the time of birth (Barpeled et al., 1991; Del Olmo et al., 1994; Miquel et al., 1994). On the other hand, steadily augmenting levels of 5-HT1A expression have been reported during the first 4 weeks of postnatal development in other regions (e.g., rat hippocampus and cortex) (Miquel et al., 1994). There have been no previous studies of 5-HT1A receptor expression in the caudal brainstem of neonatal rats. However, consistent with our results, only low levels of 5-HT1A receptor expression have been reported in the hypoglossal nucleus of adult rats by using a variety of techniques, including in situ hybridization, receptor binding, and immunohistochemistry (Manaker and Verderame, 1990; Chalmers and Watson, 1991; Pompeiano et al., 1992; Wright et al., 1995; Kia et al., 1996b). We showed that the 5-HT1Areceptor mRNA accumulation and 8-OH-DPAT binding paralleled each other, peaking at P7 from initially elevated levels at P0 before decreasing to low levels by P28; the decrease in receptor binding lagged somewhat the decrease in receptor mRNA levels. The parallel changes in receptor mRNA in HMs (which is primarily somatic) and 8-OH-DPAT binding in nXII are consistent with a predominantly postsynaptic and somatodendritic localization of 5-HT1A receptor on HMs (Kia et al., 1996a). Moreover, our data suggest that very little of the 5-HT1Abinding in nXII is located presynaptically on 5-HT fibers, because the developmental decrease in 5-HT1A binding occurred despite a marked increase in 5-HT innervation.
It is possible that the decrease in 5-HT1A expression we noted in HMs resulted from a selective cell death that occurred specifically in the subpopulation of HMs that express 5-HT1A receptor mRNA. Although our experiments do not rule out this possibility directly, we feel that this is unlikely because natural cell death in rat HMs has been shown to be entirely prenatal (Friedland et al., 1995). Thus, we favor the alternative interpretation that the decrease in 5-HT1A receptor expression by HMs reflects a change in regulatory mechanisms controlling 5-HT1A receptor gene expression in HMs (i.e., decreased transcription or mRNA stability). Interestingly, a similar transient neonatal pattern of expression has been reported for a number of other proteins in HMs (e.g., somatostatin, neurotensin, nerve growth factor receptor), perhaps reflecting a common gene regulatory mechanism (Kiyama et al., 1991; Seroogy et al., 1991; Chen and Chiu, 1992; Chiu et al., 1993).
The function served by high levels of 5-HT1A receptor expression in HMs (and other brain regions) during the early postnatal period is not clear, nor are the gene regulatory mechanisms responsible for the transient elevated receptor expression well understood. With regard to function, in addition to effects on HM electrical properties (discussed below), high levels of expression of 5-HT1Areceptor in areas of the neonate brain that are devoid of receptor in the adult and at times preceding substantial serotonergic innervation intimate that the receptors might be involved in neural development (Whitaker-Azmitia, 1991; Lauder, 1993). Indeed, activation of 5-HT1A receptors has been shown to influence neurite growth and synapse formation and to cause release of factors that promote neuronal survival (e.g., S100β) (Whitaker-Azmitia et al., 1990; Whitaker-Azmitia, 1991; Lauder, 1993). In this respect it is interesting to note that, coincident with the time of their maximal 5-HT1A receptor expression, HMs undergo a period of dendritic reshaping in which the total number of dendritic branches is reduced markedly (Núñez-Abades et al., 1994;Núñez-Abades and Cameron, 1995). It remains to be determined whether the transient expression of 5-HT1Areceptors by HMs participates in some way to orchestrating normal postnatal development in the rat brainstem. The gene regulatory mechanisms potentially responsible for controlling the transient 5-HT1A receptor expression in HMs may include factors extrinsic and/or intrinsic to the motoneurons. Our demonstration that 5-HT1A receptor expression decreased as the serotonergic innervation increased suggests that increased levels of 5-HT in the hypoglossal nucleus could provide the signal for decreased receptor expression. Certainly, agonist-dependent receptor downregulation is a common mechanism for controlling receptor expression. However, our preliminary observations suggest that this may not be the mechanism controlling 5-HT1A receptor downregulation in HMs; we found no evidence for maintained 5-HT1A receptor expression in HMs after chemotoxic lesion of serotonergic raphe neurons by treatment of neonatal rats with 5,7-dihydroxytryptamine (E. M. Talley and D. A. Bayliss, unpublished observations). Thus, blocking serotonergic innervation of nXII did not preserve 5-HT1A receptor expression by HMs.
Postnatal changes in effects of 5-HT on motoneurons
There is little information to date on developmental changes in effects of 5-HT on central neurons, including motoneurons (but seeZiskind-Conhaim et al., 1993; Muramoto et al., 1996). We have shown that inhibition of the AHP by 5-HT is a property of neonatal but not juvenile rat HMs, apparently by virtue of the expression of 5-HT1A receptors only during the early postnatal period. It remains to be determined whether the postnatal change in the effect of 5-HT on the AHP that we have described is limited to HMs or is a property common to other motoneurons. Although there have been numerous studies in which effects of 5-HT on neonatal and adult motoneurons have been investigated independently, it is difficult to ascribe differences in reported effects of 5-HT in neonates and adults to development per se, because the studies often used different recording methods or investigated different populations of motoneurons. Such concerns notwithstanding, 5-HT-induced decreases in the AHP generally have not been reported in adult rat motoneurons (Aghajanian and Rasmussen, 1989;Larkman et al., 1989; Anwyl, 1990; Larkman and Kelly, 1992) (but seeHsiao et al., 1997) but have been observed in spinal motoneurons from 2–3 week old rats (Wu et al., 1991), consistent with the idea that 5-HT-induced inhibition of the AHP may be a property of young neonatal motoneurons. Consistent with this, we also observed transiently higher levels of 5-HT1A receptor mRNA expression during the early neonatal period (∼P7) in other motoneuronal groups (i.e., facial, spinal). On the other hand, 5-HT1A receptors have been detected immunohistochemically at low levels in adult rat spinal motoneurons (Kia et al., 1996b), leaving open the possibility that 5-HT1A receptor-mediated mechanisms may persist throughout development in some populations of motoneurons.
It is also noteworthy that, whereas the 5-HT-induced depolarization was not associated with any measurable change in RNin neonatal HMs (see also Berger et al., 1992), the depolarization we observed in response to 5-HT in juvenile HMs was associated with increased RN. Where studied, 5-HT-induced depolarizations associated with increased RNhave been attributed to 5-HT2 receptor-mediated inhibition of leak potassium current (IK,L) (Aghajanian and Rasmussen, 1989; Larkman et al., 1989; Anwyl, 1990; Rasmussen and Aghajanian, 1990; Larkman and Kelly, 1992). Despite the apparent difference in ionic mechanism, the depolarization in neonatal HMs also may involve 5-HT2 receptors (Umemiya and Berger, 1995). The precise mechanism mediating depolarization in neonatal HMs has not been determined. In neonatal rat spinal motoneurons 5-HT caused depolarization via activation of Ih (Berger et al., 1992; Larkman et al., 1995) or via inhibition ofIK,L (Wang and Dun, 1990; Ziskind-Conhaim et al., 1993), but this did not seem to be the case for neonatal HMs (Berger et al., 1992). The reason for the developmental change in the effects of 5-HT2 receptors on RN is also unclear but may reflect differences in expression ofIK,L or receptor–channel coupling. In any case, the 5-HT depolarization in juvenile HMs is apparently similar to that observed in other adult motoneuronal populations, but it is distinctly different from that found in neonatal HMs.
Functional consequences
The early postnatal period is a critical period for maturation of the neuromuscular system (Walton et al., 1992) and a time during which many of the electrophysiological properties of HMs are changing dramatically (for review, see Berger et al., 1996). In addition to changes in intrinsic properties, developmental changes in the modulatory systems that impinge on HMs also have been demonstrated. For example, it was shown that the TRH innervation of HMs, which is also derived from caudal raphe neurons, increases postnatally, concomitant with increases in the motoneuronal response to TRH (Bayliss et al., 1994c; Funk et al., 1995). In that case, the developmental change in the response of HMs to TRH was attributed to an increase in TRH receptor expression (Bayliss et al., 1994c), whereas the change in effects of 5-HT on the AHP described here are attributable to a developmental decrease in 5-HT1A receptor expression. Factors necessitating these patterns of receptor expression in HMs are not known. One possibility suggested by our electrophysiological data is that 5-HT1A receptor-mediated inhibition of the AHP may act to enhance HM excitability in the neonate at a time when other excitatory mechanisms, such as those activated by TRH and 5-HT, in the adult are not yet functional (e.g., inhibition ofIK,L). It is notable that the distinct excitatory mechanisms used by raphe transmitters in neonatal and adult HMs (i.e., inhibition of the AHP and IK,L, respectively) have different consequences on subthreshold and firing behavior of HMs. Inhibition of IK,L by 5-HT and TRH in adult HMs, by increasing RN, will enhance both inhibitory and excitatory synaptic inputs and potentially allow previously subthreshold inputs to trigger action potentials and repetitive firing (see leftward shift of f–I curve in Fig.2B, inset). By contrast, in neonates in which 5-HT inhibits the AHP with no effect onRN, one would predict that subthreshold inputs (excitatory or inhibitory) would remain unaltered and that only suprathreshold inputs would be enhanced (see increased slope off–I curve in Fig. 2A,inset).
HMs innervate the tongue musculature, and their activity is important in a number of behaviors involving the tongue, including mastication, deglutition, and suckling (Krammer et al., 1979; Bartlett et al., 1990). In addition, HMs contribute significantly to the maintenance of upper airway patency during respiration, an effect that can be disrupted during sleep, leading to obstructive apneas (Remmers et al., 1978; Wiegand et al., 1991). HMs receive a serotonergic innervation from caudal medullary raphe neurons (Manaker and Tischler, 1993), the activity of which is correlated with both sleep–wake states and rhythmic motor activities such as breathing (Jacobs and Azmitia, 1992;Jacobs and Fornal, 1993; Veasey et al., 1995; Fornal et al., 1996). Moreover, Kubin and colleagues have shown in vivo that 5-HT has excitatory effects on HMs (Kubin et al., 1992) and that diminished release of 5-HT and depression of hypoglossal activity are tightly coupled during experimentally induced REM-like sleep (Kubin et al., 1994). Thus, increased levels of 5-HT released during waking states (when raphe neurons are active) may confer an excitatory bias to HMs; the withdrawal of 5-HT during REM sleep states (when raphe neurons are silent) can lead to decreased HM excitability via disfacilitation, potentially contributing to the loss of tone in tongue muscles and obstructive apneas that occur during REM sleep (Remmers et al., 1978;Wiegand et al., 1991). The data presented here indicate that the mechanism by which 5-HT provides this important excitatory bias to HMs is different in neonates and adults as a result of altered 5-HT receptor expression.
Footnotes
This work was supported by National Institutes of Health Grant NS33583. We thank Joshua Singer for his comments on this manuscript and Drs. Félix Viana and Albert J. Berger for their contributions to and support of this work in its early stages. We also thank Dr. Madelin Harrison for providing equipment and expertise for the use of MCID. We gratefully acknowledge Pfizer Research (Groton, CT) for the gift of ω-Agatoxin IVA.
Correspondence should be addressed to Dr. Douglas A. Bayliss, Department of Pharmacology, Box 448, 5017 Jordan Hall, University of Virginia, Charlottesville, VA 22908.
REFERENCES
- 1.Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- 2.Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–5832. [PubMed] [Google Scholar]
- 3.Anwyl R. Neurophysiological actions of 5-hydroxytryptamine in the vertebrate nervous system. Prog Neurobiol. 1990;35:451–468. doi: 10.1016/0301-0082(90)90031-b. [DOI] [PubMed] [Google Scholar]
- 4.Barpeled O, Grossisseroff R, Benhur H, Hoskins I, Groner Y, Biegon A. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neurosci Lett. 1991;127:173–176. doi: 10.1016/0304-3940(91)90787-t. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett D, Jr, Leiter JC, Knuth SL. Control and actions of the genioglossus muscle. In: Issa FG, Suratt PM, Remmers JE, editors. Sleep and respiration. Wiley-Liss; New York: 1990. pp. 99–108. [PubMed] [Google Scholar]
- 6.Bayliss DA, Viana F, Berger AJ. Early postnatal changes in rat hypoglossal motoneuron responses to thyrotropin-releasing hormone (TRH) and serotonin (5-HT) in vitro. Soc Neurosci Abstr. 1992a;18:511. [Google Scholar]
- 7.Bayliss DA, Viana F, Berger AJ. Mechanisms underlying excitatory effects of thyrotropin-releasing hormone on rat hypoglossal motoneurons in vitro. J Neurophysiol. 1992b;68:1733–1745. doi: 10.1152/jn.1992.68.5.1733. [DOI] [PubMed] [Google Scholar]
- 8.Bayliss DA, Viana F, Bellingham MC, Berger AJ. Characteristics and postnatal development of a hyperpolarization-activated inward current in rat hypoglossal motoneurons in vitro. J Neurophysiol. 1994a;71:119–128. doi: 10.1152/jn.1994.71.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Bayliss DA, Viana F, Berger AJ. Effects of thyrotropin-releasing hormone on rat motoneurons are mediated by G-proteins. Brain Res. 1994b;668:220–229. doi: 10.1016/0006-8993(94)90527-4. [DOI] [PubMed] [Google Scholar]
- 10.Bayliss DA, Viana F, Kanter RK, Szymeczek-Seay CL, Berger AJ, Millhorn DE. Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J Neurosci. 1994c;14:821–833. doi: 10.1523/JNEUROSCI.14-02-00821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayliss DA, Umemiya M, Berger AJ. Inhibition of N- and P-type calcium channels and the afterhyperpolarization in rat motoneurones by serotonin. J Physiol (Lond) 1995;485:635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 14.Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl Physiol. 1996;81:1039–1048. doi: 10.1152/jappl.1996.81.3.1039. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor messenger RNA and 5-HT1A binding in rat brain—a combined in situ hybridization/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 16.Chen EW, Chiu AY. Early stages in the development of spinal motor neurons. J Comp Neurol. 1992;320:291–303. doi: 10.1002/cne.903200303. [DOI] [PubMed] [Google Scholar]
- 17.Chiu AY, Chen EW, Loera S. A motor neuron-specific epitope and the low-affinity nerve growth factor receptor display reciprocal patterns of expression during development, axotomy, and regeneration. J Comp Neurol. 1993;328:351–363. doi: 10.1002/cne.903280303. [DOI] [PubMed] [Google Scholar]
- 18.Del Olmo E, Díaz A, Guirao-Piñeyro M, Del Arco C, Pascual J, Pazos A. Transient localization of 5-HT1A receptors in human cerebellum during development. Neurosci Lett. 1994;166:149–152. doi: 10.1016/0304-3940(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 19.Finkel AS, Redman SJ. Optimal voltage clamping with single microelectrodes. In: Smith TJ Jr, Lecar H, Redman SJ, Gage PW, editors. Voltage and patch clamping with microelectrodes. American Physiological Society; Bethesda, MD: 1985. pp. 95–120. [Google Scholar]
- 20.Fornal CA, Metzler CW, Marrosu F, Ribiero-do-Valle LE, Jacobs BL. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral–buccal movements. Brain Res. 1996;716:123–133. doi: 10.1016/0006-8993(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 21.Friedland DR, Eden AR, Laitman JT. Naturally occurring motoneuron cell death in rat upper respiratory tract motor nuclei: a histological, fast DiI, and immunocytochemical study in the hypoglossal nucleus. J Neurobiol. 1995;27:520–534. doi: 10.1002/neu.480270407. [DOI] [PubMed] [Google Scholar]
- 22.Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1995;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- 23.Halliday G, Harding A, Paxinos G. Serotonin and tachykinin systems. In: Paxinos G, editor. The rat nervous system, 2nd Ed. Academic; San Diego: 1995. pp. 929–974. [Google Scholar]
- 24.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–204. [PubMed] [Google Scholar]
- 25.Hsiao CF, Trueblood PR, Levine MS, Chandler SH (1997) Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol, in press. [DOI] [PubMed]
- 26.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- 28.Jansen JKS, Fladby T. The perinatal reorganization of the innervation of skeletal muscle in mammals. Prog Neurobiol. 1990;34:39–90. doi: 10.1016/0301-0082(90)90025-c. [DOI] [PubMed] [Google Scholar]
- 29.Kia HK, Brisorgueil MJ, Hamon M, Calas A, Verge D. Ultrastructural localization of 5-hydroxytryptamine (1A) receptors in the rat brain. J Neurosci Res. 1996a;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Vergé D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996b;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Kiyama H, Emson PC, Sato M, Tohyama M. The transient appearance of proneurotensin messenger RNA in the rat hypoglossal nucleus during development. Dev Brain Res. 1991;58:293–296. doi: 10.1016/0165-3806(91)90018-e. [DOI] [PubMed] [Google Scholar]
- 32.Krammer EB, Rath T, Lischka MF. Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res. 1979;170:533–537. doi: 10.1016/0006-8993(79)90970-3. [DOI] [PubMed] [Google Scholar]
- 33.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 34.Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- 35.Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol (Lond) 1992;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larkman PM, Penington NJ, Kelly JS. Electrophysiology of adult rat facial motoneurones: the effects of serotonin (5-HT) in a novel in vitro brainstem slice. J Neurosci Methods. 1989;28:133–146. doi: 10.1016/0165-0270(89)90018-6. [DOI] [PubMed] [Google Scholar]
- 37.Larkman PM, Kelly JS, Takahashi T. Adenosine 3′:5′-cyclic monophosphate mediates a 5-hydroxytryptamine-induced response in neonatal rat motoneurones. Pflügers Arch. 1995;430:763–769. doi: 10.1007/BF00386174. [DOI] [PubMed] [Google Scholar]
- 38.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 39.Lowrie MB, Vrbová G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992;15:80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- 40.Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- 41.Manaker S, Verderame HM. Organization of serotonin 1A and 1B receptors in the nucleus of the solitary tract. J Comp Neurol. 1990;301:535–553. doi: 10.1002/cne.903010405. [DOI] [PubMed] [Google Scholar]
- 42.Miquel MC, Kia HK, Boni C, Doucet E, Daval G, Mathiessen L, Hamon M, Vergé D. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Dev Brain Res. 1994;80:149–157. doi: 10.1016/0165-3806(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 43.Muramoto T, Mendelson B, Phelan KD, Garcia-Rill E, Skinner RD, Puskarich-May C. Developmental changes in the effects of serotonin and N-methyl-d-aspartate on intrinsic properties of embryonic chick motoneurons. Neuroscience. 1996;75:607–618. doi: 10.1016/0306-4522(96)00185-6. [DOI] [PubMed] [Google Scholar]
- 44.Núñez-Abades PA, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: relative changes in diameter and surface area of somata and dendrites. J Comp Neurol. 1995;353:129–142. doi: 10.1002/cne.903530112. [DOI] [PubMed] [Google Scholar]
- 45.Núñez-Abades PA, Spielmann JM, Barrionuevo G, Cameron WE. In vitro electrophysiology of developing genioglossal motoneurons in the rat. J Neurophysiol. 1993;70:1401–1411. doi: 10.1152/jn.1993.70.4.1401. [DOI] [PubMed] [Google Scholar]
- 46.Núñez-Abades PA, He F, Barrionuevo G, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: changes in length, branching pattern, and spatial distribution of dendrites. J Comp Neurol. 1994;339:401–420. doi: 10.1002/cne.903390308. [DOI] [PubMed] [Google Scholar]
- 47.Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen K, Aghajanian GK. Serotonin excitation of facial motoneurons: receptor subtype characterization. Synapse. 1990;5:324–332. doi: 10.1002/syn.890050409. [DOI] [PubMed] [Google Scholar]
- 49.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 50.Seroogy KB, Bayliss DA, Szymeczek CL, Hokfelt T, Millhorn DE. Transient expression of somatostatin messenger RNA and peptide in the hypoglossal nucleus of the neonatal rat. Dev Brain Res. 1991;60:241–252. doi: 10.1016/0165-3806(91)90053-l. [DOI] [PubMed] [Google Scholar]
- 51.Swartz KJ. Modulation of Ca2+ channels by protein kinase C in rat central and peripheral neurons: disruption of G-protein-mediated inhibition. Neuron. 1993;11:305–320. doi: 10.1016/0896-6273(93)90186-u. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol (Lond) 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talley EM, Sadr N, Bayliss DA. Postnatal development of 5-HT1A receptor-mediated responses and 5-HT1A receptor expression in rat hypoglossal motor neurons. Soc Neurosci Abstr. 1996;22:1847. [Google Scholar]
- 54.Towle AC, Breese GR, Mueller RA, Coyle S, Lauder JM. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310:67–75. doi: 10.1016/0006-8993(84)90010-6. [DOI] [PubMed] [Google Scholar]
- 55.Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brainstem. J Neurophysiol. 1995;73:1192–1200. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- 57.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- 59.Viana F, Bayliss DA, Berger AJ. Postnatal changes in rat hypoglossal motoneuron membrane properties. Neuroscience. 1994;59:131–148. doi: 10.1016/0306-4522(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 60.Viana F, Bayliss DA, Berger AJ. Repetitive firing properties of developing rat brainstem motoneurones. J Physiol (Lond) 1995;486:745–761. doi: 10.1113/jphysiol.1995.sp020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volpe BT, Hendrix CS, Park DH, Towle AC, Davis HP. Early postnatal administration of 5,7-dihydroxytryptamine destroys 5-HT neurons but does not affect spatial memory. Brain Res. 1992;589:262–267. doi: 10.1016/0006-8993(92)91285-m. [DOI] [PubMed] [Google Scholar]
- 62.Walton KD, Lieberman D, Llinás A, Begin M, Llinás RR. Identification of a critical period for motor development in neonatal rats. Neuroscience. 1992;51:763–767. doi: 10.1016/0306-4522(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 63.Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol (Lond) 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitaker-Azmitia PM. Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev. 1991;43:553–561. [PubMed] [Google Scholar]
- 65.Whitaker-Azmitia PM, Murphy R, Azmitia EC. Stimulation of astroglial 5-HT1A receptors releases the serotonergic growth factor, protein S-100, and alters astroglial morphology. Brain Res. 1990;528:155–158. doi: 10.1016/0006-8993(90)90210-3. [DOI] [PubMed] [Google Scholar]
- 66.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- 67.Wikström M, Hill R, Hellgren J, Grillner S. The action of 5-HT on calcium-dependent potassium channels and on the spinal locomotor network in lamprey is mediated by 5-HT1A-like receptors. Brain Res. 1995;678:191–199. doi: 10.1016/0006-8993(95)00183-q. [DOI] [PubMed] [Google Scholar]
- 68.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 69.Wu SY, Wang MY, Dun NJ. Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res. 1991;554:111–121. doi: 10.1016/0006-8993(91)90178-x. [DOI] [PubMed] [Google Scholar]
- 70.Ziskind-Conhaim L, Seebach BS, Gao B-X. Changes in serotonin-induced potentials during spinal cord development. J Neurophysiol. 1993;69:1338–1349. doi: 10.1152/jn.1993.69.4.1338. [DOI] [PubMed] [Google Scholar]