Abstract
Fast applications of GABA (1 mm) to nucleated and outside-out patches excised from granule neurons in cerebellar slices from developing rats evoked currents with a double exponential time course reminiscent of that of IPSCs. A neurosteroid 3α, 21dihydroxy-5α-pregnan-20-one (THDOC) remarkably increased the slow deactivation time constant and slowed down recovery from desensitization, as estimated by paired-pulse GABA applications. THDOC also reduced the amplitude of GABA currents, whereas it failed to affect the fast deactivation component and its relative contribution to peak amplitude. The effects of THDOC on slow deactivation were greater in rats younger than postnatal day 13 (P13) as compared with rats at P30–P35. THDOC failed to alter deactivation of short responses induced by a less-potent agonist taurine at saturating doses. These responses had deactivation kinetics described by a fast single exponential decay, little desensitization, and quick recovery. However, THDOC slowed deactivation if taurine responses were long enough to allow consistent desensitization, suggesting that desensitized states are required for the neurosteroid to modulate GABA responses. In outside-out patches, just as desensitized states prolonged GABA responses by producing reopening of channels activated by brief GABA pulses, THDOC increased the channel open probability by further increasing the number of late channel openings, resulting in a prolongation of the slow deactivation. Our data suggest that neurosteroid potentiates the inhibitory postsynaptic transmission via the prolongation of the slow deactivation and that the alteration of kinetics of entry and exit from desensitized states underlies the allosteric modification of GABAAreceptors by neurosteroids.
Keywords: GABAA receptor, desensitization, THDOC, IPSC, taurine, patch clamp
Neurosteroids, which may be synthesized in the nervous system, have been demonstrated to have anxiolytic, hypnotic, anesthetic, and anticonvulsant activities (for review, see Paul and Purdy, 1992; MacDonald and Olsen, 1994; Lambert et al., 1995). More recent studies have shown that neurosteroids may be involved in LTP, memory enhancement, behavioral actions, and neuroprotective effects (Frye, 1995; Steffensen, 1995; Yoo et al., 1996). GABAAreceptors have been established as a prime target for neurosteroid actions, and it has been proposed that there are neurosteroid binding sites in the GABAA receptor channel complex (Lambert et al., 1995). Indeed, neurosteroids have been demonstrated either to potentiate or to inhibit GABA-gated currents in transfected cells and primary cell cultures via allosteric modulations (Majewska et al., 1986; Callachan et al., 1987; Harrison et al., 1987; Turner et al., 1989; Puia et al., 1990; Gee et al., 1991; Zhu et al., 1996). Neurosteroids induce increases in channel open probability when steady-state activation is induced by low agonist concentrations (Mistry and Cottrell, 1990; Puia et al., 1990; Twyman and MacDonald, 1992).
The decay constants of GABA-mediated IPSCs in cerebellar granule neurons are characterized by fast and slow decay time components (Maconochie et al., 1994; Puia et al., 1994; Kaneda et al., 1995; Tia et al., 1996). Fast entry and recovery from GABAA receptor desensitization may contribute to the IPSC kinetics in hippocampal neurons and cerebellar granule cells (Jones and Westbrook, 1995, 1996;Tia et al., 1996). Several studies have demonstrated that neurosteroids potentiate inhibitory synaptic transmission by increasing the duration of the IPSC (Majewska et al., 1986; Harrison et al., 1987). However, the mechanisms of neurosteroid modulation of GABAergic synaptic transmission cannot be predicted easily from studies of neurosteroid effects on steady-state, low agonist-activated currents, because synaptic currents are produced by transient jumps in high GABA concentrations that induce desensitizing responses (Jones and Westbrook, 1995, 1996). Furthermore, presynaptic factors such as synchronization of transmitter release complicate the interpretation of the mechanism for the effects of allosteric modulators of GABAA receptor on inhibitory synaptic transmission (Mody et al., 1994).
To characterize the modulation of GABA-gated currents by neurosteroids as it may happen at the synapse, we report here the effects of neurosteroids on GABAA receptors under nonequilibrium recording conditions, using fast agonist applications to nucleated patches excised from granule cells of rat cerebellar slices. We investigate the neurosteroid-induced modulation of deactivation time constants of GABA-gated currents as well as entry into and recovery from desensitization.
Our previous studies in primary culture of cerebellar granule neurons have demonstrated that the GABAA receptor sensitivity to THDOC is reduced with development in culture (Zhu et al., 1996). The evidence from single-cell RT-PCR and recombinant cDNA transfection suggested that the increased expression of δ subunits during development in culture contributed to the decreased effect of neurosteroids (Zhu et al., 1996). In the present study we examine the effects of THDOC on GABA responses in granule cells of cerebellar slices at postnatal days 10–13 and 30–35, before and after the increased expression of the δ subunit in cerebellar granule neurons, as demonstrated with δ subunit-specific antibodies (Muller et al., 1994). We also study the neurosteroid-induced modulation with a less potent GABAA receptor agonist, taurine (Huxtable, 1992), and, finally, we investigate neurosteroid regulation of single channel currents activated by pulses of saturating GABA to outside-out patches.
MATERIALS AND METHODS
Cerebellar slices. Sagittal slices of cerebellum (150–200 μm) were prepared from 10 to 35-d-old Sprague Dawley rats as previously described (Puia et al., 1994). Electrodes were pulled from borosilicate glass capillaries (Wiretrol II, Drummond, Broomall, PA). Nucleated patches and outside-out patches of cerebellar granule neurons were obtained under visual control with an Axioskop FS microscope (Zeiss, Oberkochen, Germany) equipped with Nomarski optics and an electrically insulated water immersion 63× objective with a long working distance.
Solutions and drugs. Experiments were performed at room temperature (22–24°C), using an extracellular medium composed of (in mm): NaCl 120, KCl 3.1, K2HPO41.25, NaHCO3 26, dextrose 5.0, MgCl2 1.0, and CaCl2 2.0 with the addition of 1 μmtetrodotoxin (Sigma, St. Louis, MO). The solution was maintained at pH 7.4 by bubbling with 5% CO2/95% O2. Intracellular pipette solution contained (in mm): CsCl 145, 1,1 ethylene glycol-bis-(β-aminoethyl ether)N,N,N′,N′-tetra-acetic acid 5.0, MgATP 5.0, and HEPES 10, adjusted to pH 7.2 with CsOH. The slice was perfused continuously at a rate of 5 ml/min and completely submerged in a total volume of 500 μl. 3α, 21Dihydroxy-5α-pregnan-20-one (THDOC; RBI, Natick, MA) was dissolved in dimethyl sulfoxide (DMSO, <0.01% final concentration; Sigma). Taurine, strychnine, and bicuculline methiodide were obtained from Sigma.
Rapid application of agonists. We used a piezoelectric translator (P-245.30 Stacked Translator, Physik Instrument, Germany) to quickly move a double-barreled theta tubing positioned in front of the excised patch. One barrel of the applicator contained extracellular medium and the other with this solution contained GABAAreceptor agonists. For fast application of GABAA receptor agonists with THDOC, the solutions in the double-barreled pipette were exchanged by means of solenoid valves connected to a vacuum. THDOC was added to both control and GABA agonist-containing solutions. When taurine was used, strychnine (5 μm) was added to the solution in both barrels to block the activation of glycine receptors (Huxtable et al., 1992). After each patch recording on and off rates as well as pulse duration were estimated from currents generated by the liquid junction potential because of a 50:1 dilution of the GABA-containing solution after “blowing out” the patch. On and off rates were typically less than 0.2 msec, and 1 msec was the minimal duration of the liquid junction currents measured. Amplitude and deactivation kinetics were within 5% of the initial values in most experiments. However, in some recordings considerable rundown of initial peak amplitude was observed for unknown reasons. These recordings were discarded.
Data acquisition and analysis. Voltage-clamp recordings of GABA-evoked currents from nucleated and outside-out patches were made with an Axopatch-1D amplifier (Axon Instruments, Foster City, CA) after capacitance and series resistance compensation. Currents were filtered at 2 kHz with an 8-pole low-pass Bessel filter (Frequency Devices, Haverhill, MA) (Hamill et al., 1981) and digitized at 10 kHz by using a PC-compatible microcomputer equipped with a Digidata 1200 data acquisition board (Axon Instruments) and pClamp 6.03 software (Axon Instruments). Pulse duration and paired pulse protocols were controlled with pClamp 6.03 software. Off-line data analysis, curve fitting, and figure preparation were performed with Origin (MicroCal Software, Northampton, MA) and pClamp 6.0 (Axon Instruments) software. Peak amplitudes were measured at the absolute maximum of the currents, taking into account the noise of the baseline and noise around the peak. Rise times were measured as the time elapsed from 20 to 80% of the peak amplitude of the response. Curve fitting was performed by using simplex algorithm least-squares exponential fitting routines with single or double exponential equations of the form I(t) =If exp(−t/τf) +Is exp(−t/τs), whereIf and Is are the amplitudes of the fast and slow decay components, and τfand τs are their respective decay time constants. For 200 msec and 5 sec applications a constant term describing the response amplitude after this time was added. Single channel current records were low-pass-filtered at 10 kHz and stored on magnetic tape by pulse code modulation (sampling frequency, 94 kHz). For analysis, analog signals were sampled at 5 kHz and filtered at 1 kHz (8-pole Bessel filter). Events lists, open probability, and mean open time estimates were prepared with the pClamp 6.03 software suite. Rare openings at subconductance levels and superimposed openings were excluded from the dwell time analysis. Unless otherwise indicated, data are expressed as mean ± SEM; statistical analysis was performed with independent Student t tests, p < 0.05.
RESULTS
THDOC increases the slow deactivation of GABA responses
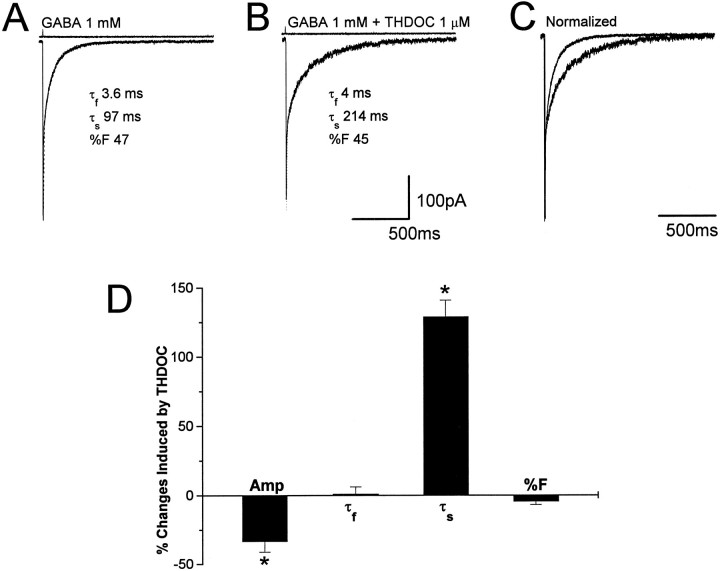
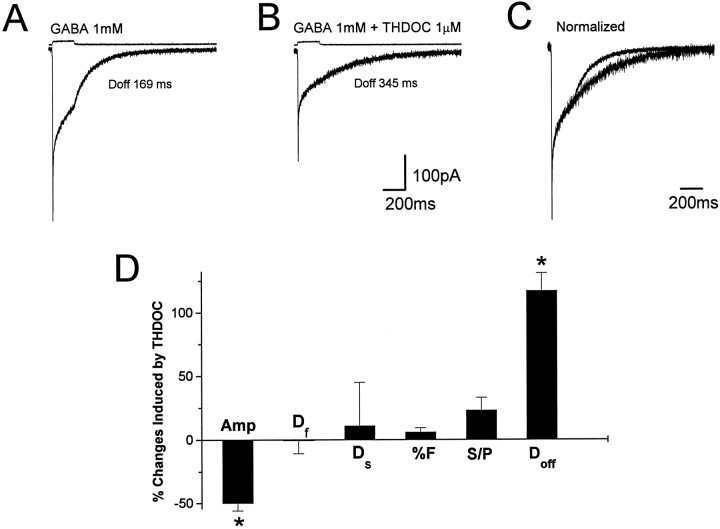
Nucleated patches were isolated from granule neurons visually identified by their location and morphological characteristics in rat cerebellar slices and briefly exposed to GABA by a piezoelectric-driven double-barreled application pipette at a holding potential of −60 mV. Typically, the patches were positioned at ∼100 μm from the tip of the double-barreled application pipette. As shown in Figure1, pulses of GABA (1 mm, 1 msec) evoked a fast-gated inward current. The response rapidly reached peak, and the rise time of 20–80% ranged from 0.25 to 0.6 msec at −60 mV. Patch responses to brief pulses of a high concentration of GABA in 17 granule neurons from postnatal day 10 (P10) to P13 had double exponential deactivation with a rapidly decaying component (τf = 4.2 ± 0.3 msec; fractional contribution to peak, %F = 58.3 ± 14.3) and a component with slow decay (τs = 121.9 ± 6.8 msec). We investigated the modification of GABA-gated current kinetics by the neurosteroid THDOC (1 μm). An example is shown in Figure 1, A andB, in which GABA currents are shown with and without THDOC coapplication. When the currents were normalized and superimposed, the prolongation of the slow deactivation component by THDOC could be observed (Fig. 1C). A complete wash-out from the THDOC effects was observed only in those few recordings in which the patch could be maintained for a long time after THDOC application. However, trend to recovery was observed in all patches tested. In Figure1D, we report the summary of the THDOC-induced variations of amplitude, fast and slow decay time constants, and the relative contribution to peak amplitude of the fast decay component (%F) for currents evoked by brief GABA pulses. Both the decrease in amplitude (−33 ± 8%) and the increase of slow decay time (129 ± 12%) were statistically significant (p < 0.05), whereas the variations of either the fast deactivation component or %F were not (1.2 ± 5 and −4.5 ± 2.3%, respectively). THDOC more than doubled the average charge transfer (from 22 ± 5 to 55 ± 12 pC), as calculated from the integral of the GABA responses. To study THDOC effects on agonist-induced desensitization, we exposed 12 nucleated patches to 200 msec applications of GABA (1 mm). As illustrated in Figure 2A, these responses have a fast rise time and a peak amplitude similar to that obtained by the application of a 1 msec pulse of GABA. However, the continuous presence of GABA evoked desensitization of the response, with a time course fit best with a double exponential function (τf = 2.9 ± 0.2 msec, τs = 76.7 ± 4.3 msec, %F = 51.6 ± 13.4). The response amplitude at the end of the 200 msec step was 35 ± 15% of the peak, and the offset deactivation at the end of the step (Doff) was best fit by a single exponential curve with an average time constant of 129 ± 10 msec. An example of the 1 μm THDOC effect is shown in Figure 2, where GABA currents are shown without and with THDOC coapplication (Fig.2A,B) and after normalization and superimposition (Fig. 2C). The summary of results in Figure2D shows that THDOC significantly reduced the peak amplitude (50.1 ± 6.1%) and increased the offset decay time constant (117 ± 14.5%), whereas it failed to alter significantly the desensitization time constants and the ratio of the amplitude at the end of the 200 msec pulse to the peak amplitude.
Fig. 1.
THDOC prolongs slow deactivation of currents elicited by brief GABA pulses. A, Average of 10 responses induced by a 1 msec application of 1 mm GABA to a nucleated patch excised from a granule neuron in a rat cerebellar slice (holding potential, −60 mV) with an indication of the double exponential fit.B, Averaged response of the same patch as in A to 1 mm GABA in the presence of THDOC (1 μm).C, Superimposed average currents recorded before and after the coapplication of GABA and THDOC. The average current recorded in the presence of THDOC has been normalized to the peak amplitude of the response in A. D, Summary of the percentage of changes produced by THDOC on GABA-gated currents elicited by 1 msec applications. Amp, Peak amplitude; τf, fast decay time constant; τs, slow decay time constant; %F, relative contribution of the fast component to peak amplitude. Each bar represents the mean ± SEM of 17 patches studied. Above each trace are shown the currents generated by the liquid junction potential because of a 50:1 dilution of the GABA-containing solution measured after “blowing out” the patch to give an indication of the duration of the pulse application. Vertical calibration does not apply to these traces.
Fig. 2.
THDOC fails to alter desensitization of currents elicited by 200 msec GABA steps. A, Average of 10 responses induced by 200 msec application of 1 mm GABA to a nucleated patch excised from a granule neuron in a rat cerebellar slice (holding potential, −60 mV) with an indication of the single exponential fit of the offset decay at the end of the 200 msec application. B, Averaged response of the same patch as in A to 1 mm GABA in the presence of THDOC (1 μm).C, Superimposed average currents recorded before and after the coapplication of GABA and THDOC. The averaged current inB has been normalized to the peak amplitude of the response in A. D, Summary of the percentage of changes produced by THDOC on GABA-gated currents induced by 200 msec applications. Amp, Peak amplitude;Df, fast time constant of desensitization;Ds, slow time constant of desensitization; %F, relative contribution of the fast desensitization component to peak amplitude; S/P, ratio between the amplitude at 200 msec (S) and peak amplitude (P);Doff, offset decay time constant at the end of the 200 msec application. Each bar represents the mean ± SEM of 12 patches studied. Above each traceare shown currents generated to give an indication of the duration of the GABA application. Vertical calibration does not apply to these traces.
In a set of parallel experiments in five granule cells at P12, we investigated the effects of preperfusion with 0.3 μmGABA, followed by rapid application of GABA (1 mm, 1 and 200 msec). We failed to observe significant changes in all of the kinetic parameters studied, although we observed a significant reduction of the peak amplitude with both 1 and 200 msec GABA applications (data not shown).
Recovery of GABA response from desensitization in the absence and presence of THDOC
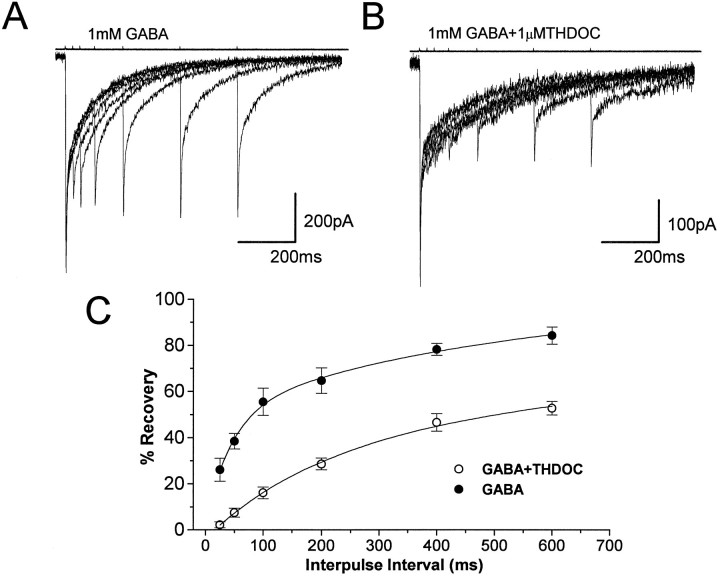
To characterize the recovery of GABA-gated channels from desensitization, we used paired applications of GABA (1 mm, 1 msec) on eight nucleated patches excised from granule neurons in P10–P13 rats. As previously reported (Jones and Westbrook, 1995; Tia et al., 1996), the response after the second application of GABA had an obvious reduction of peak currents (Fig. 3A), suggesting that a fraction of channels may be desensitized during the first 1 msec application. The recovery of channels from desensitization gradually increased with the interval between GABA applications, and the recovery time course of desensitization extended over several hundred milliseconds. Figure 3B shows that the coapplication of THDOC with GABA prolonged the interval of paired pulses required for the recovery of desensitized receptor channels. In Figure3C, the average recovery of the response from desensitization plotted against the paired pulse interval illustrates that the resensitization of GABAA receptor channels was slowed significantly by THDOC. Double exponential fitting of the recovery showed an increase from 44 to 195 msec for the fast recovery time constant and from 510 msec to 1.8 sec for the slow recovery time constant. We also investigated the effects of preperfusion with 0.3 μm GABA on recovery from desensitization caused by rapid application of GABA (1 mm, 1 msec; n = 5). In this case no significant differences were observed in the biexponential recovery from desensitization (from 56 to 49 msec for the fast time constant and from 691 to 545 msec for the slow recovery time constant).
Fig. 3.
THDOC slows recovery from desensitization induced by brief GABA pulses. Superimposed averages of five traces evoked by two successive applications of 1 msec GABA (1 mm) pulses separated by 25, 50, 100, 200, 400, and 600 msec intervals in a nucleated patch excised from a granule neuron at P11 in the absence (A) and the presence (B) of THDOC (1 μm) (holding potential, −60 mV). C, Comparison of the recovery time course of the second response from desensitization. The percentage of recovery from desensitization at each designated separation of two brief GABA pulses is calculated according to the formula ([Peak2 − onset]/[peak1 − onset] × 100) and plotted against the interpulse interval similar to that described by Jones and Westbrook (1995). Each data group represents the mean ± SEM of eight patches studied. Double exponential fitting of the recovery in the presence of THDOC showed an increase from 44 to 195 msec for the fast time constant and from 510 msec to 1.8 sec for the slow time constant. Above eachtrace are shown currents to give an indication of the duration of the pulse applications. Vertical calibration does not apply to these traces.
Developmental changes in neurosteroid modulation of GABA channel deactivation
Nucleated patches from granule neurons in cerebellar slices were used to compare the effects of THDOC on GABAA receptors in rats at P30–P35. As observed in younger rats (<P14), GABA-gated currents from nine patches at P30–P35 showed fast rise times and a quick deactivation with a bioexponential time constant. As previously reported (Tia et al., 1996), no obvious differences in the decay time course were seen when GABA-gated currents in patches from rats younger than P14 were compared with those older than P30. For granule cells from older rats, the mean decay time constants were τf = 2.7 ± 0.2 msec and τs = 128 ± 14 msec for the 1 msec pulse. The mean desensitization time constants for the 200 msec step pulse were Df = 2.6 ± 0.16 msec,Ds = 85 ± 7 msec; the response amplitude at the end of the 200 msec step was 26 ± 12% of the peak, and the decay time constant at the end of the 200 msec applicationDoff was 157 ± 12 msec. Similar with what was observed in younger rats, THDOC decreased the peak amplitude of the response and increased the decay time of GABA-gated currents after the removal of agonist for both 1 and 200 msec applications (Fig.4). However, as summarized in Figure 4, the increases of τs and Doff by THDOC were reduced significantly with development.
Fig. 4.
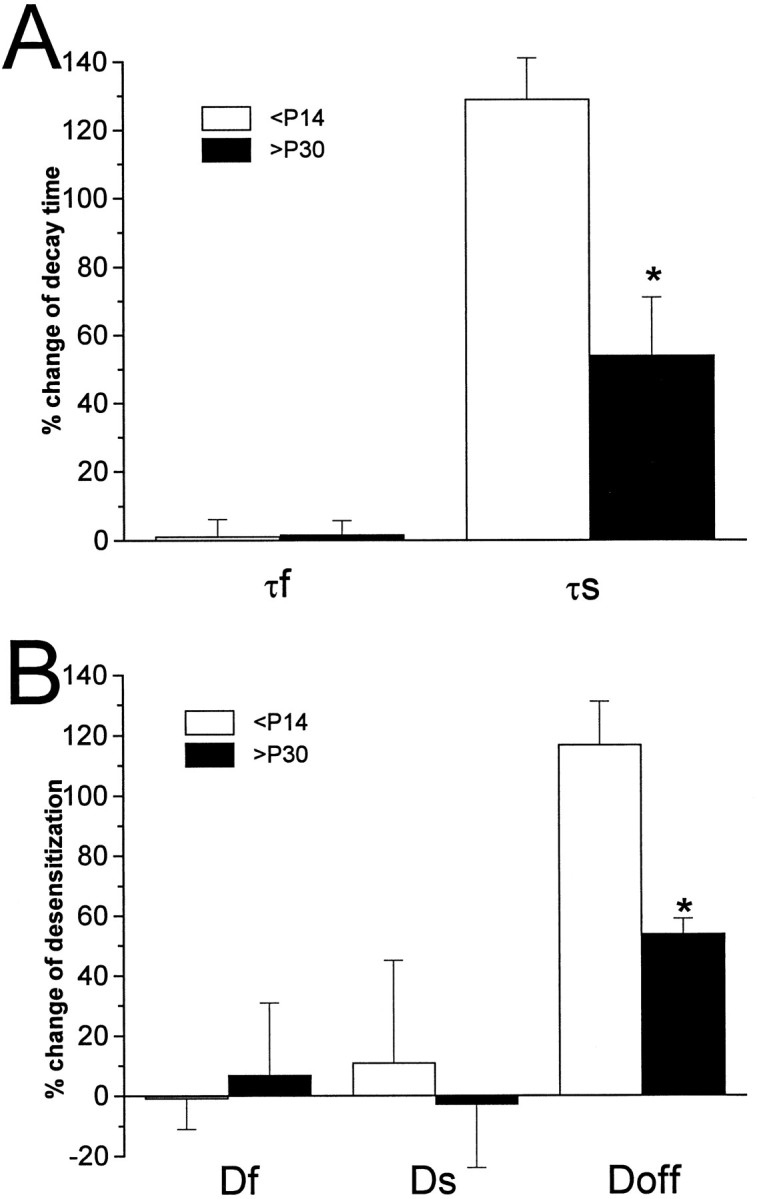
Developmental reduction of THDOC effects on GABA current deactivation. GABA responses were compared in nucleated patches isolated from cerebellar granule neurons of rats at postnatal day 10–13 and 30–35. Summary of the percentage of changes produced by THDOC on GABA-gated currents induced by 1 (A) or 200 msec (B) applications. Amp, Peak amplitude;Df, fast decay time constant;Ds, slow decay time constant; %F, relative contribution of the fast component to peak amplitude;S/P, ratio between the amplitude at 200 msec (S) and peak amplitude (P); Doff, offset decay time constant at the end of the 200 msec application.
Deactivation, desensitization, and the neurosteroid-induced modulation with taurine as an agonist of GABAAreceptors
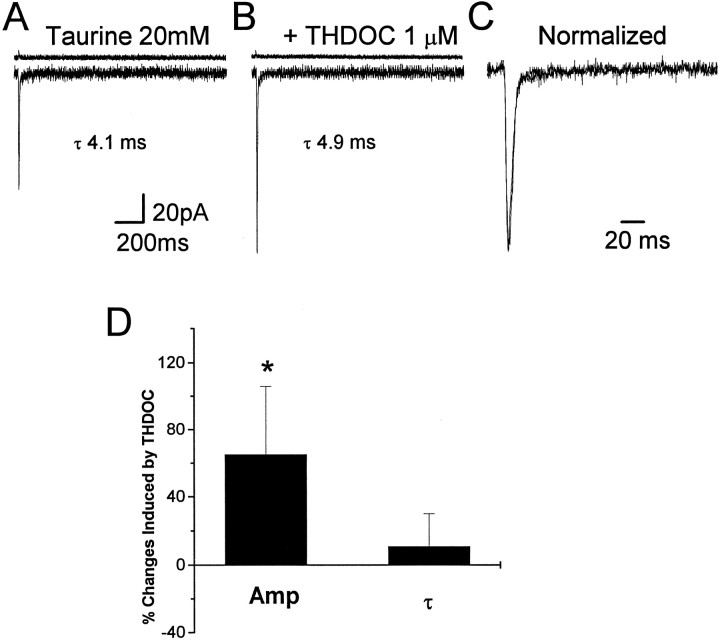
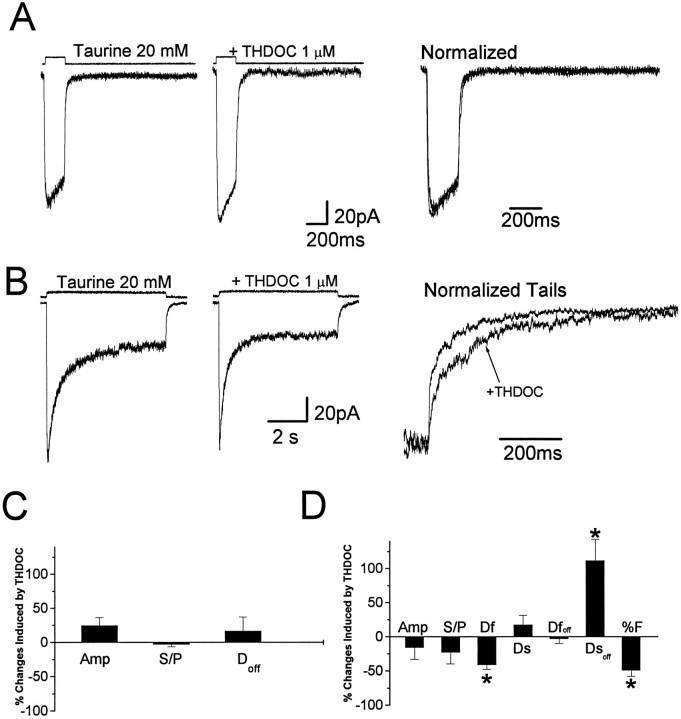
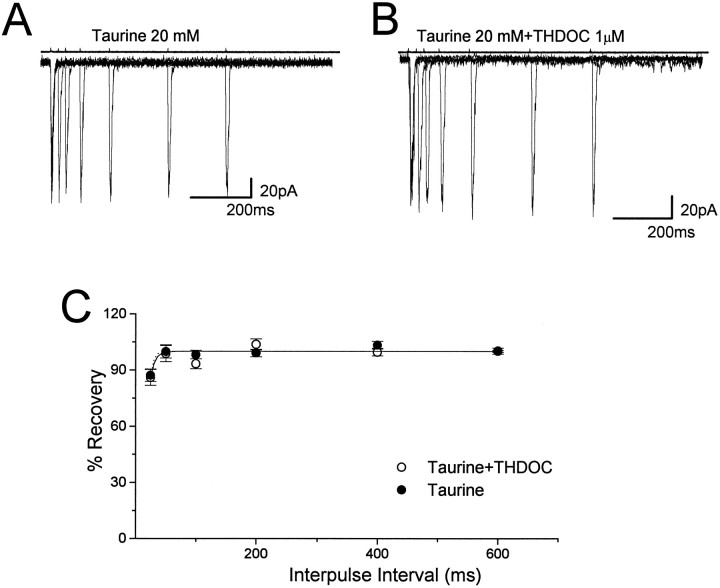
To investigate further the role of desensitization in neurosteroid-induced modification of GABA-gated decay kinetics, we applied taurine with brief pulses to outside-out nucleated patches excised from 11 granule neurons in rats at P10–P13. If the glycine receptor antagonist strychnine was not added, activation of large conductance channels sometimes was observed (Kaneda et al., 1995), consistent with the reported action of taurine as an agonist of glycine receptors (Huxtable et al., 1992). As shown in Figure 5, applications (2 msec) of taurine at a 20 mm concentration in an extracellular solution containing strychnine (5 μm) elicited Cl− currents with a fast rise time (typically less than 0.6 msec), followed by a rapid single exponential decay (τf = 6.2 ± 0.6 msec), which could be blocked by the GABAA receptor antagonist bicuculline methiodide (10 μm; data not shown, n = 4). Furthermore, taurine activates current with similar amplitude and decay in patches excised from human embryonic kidney cells (HEK293) transiently transfected with cDNAs encoding α1β2γ2 subunits of the GABAA receptor (W. J. Zhu, unpublished observations) (Dominguez-Perrot et al., 1996). These results made us confident that taurine was activating GABAA receptors, although it required much higher concentrations than GABA to obtain currents close to saturation (20 mm taurine). Coapplication of taurine with 1 μm THDOC (Fig. 5) in 11 nucleated patches excised from granule neurons showed no significant increase of the decay time constant (10.6 ± 19.2%), but THDOC increased the peak amplitude of taurine-activated currents by 65 ± 41%. Application of taurine (200 msec, 20 mm; n = 9 patches) evoked fast currents, which showed little desensitization (ratio of amplitude at the end of the 200 msec step to peak amplitude 79 ± 6%) when compared with GABA-induced responses (Fig.6A,C). THDOC (1 μm) did not increase the peak taurine-gated current (24 ± 12%) and the offset time constant at the end of the 200 msec application significantly (16 ± 21%) (Fig. 6A,C). Prolonged application of taurine (5 sec, 20 mm;n = 8 patches) evoked currents with consistent desensitization (ratio of amplitude at the end of the 5 sec step to peak amplitude 22 ± 10%; Fig. 6B) and double exponential deactivation kinetics at the end of the 5 sec application as previously reported for β-alanine, another less-potent agonist (Jones and Westbrook, 1995). THDOC (1 μm) did not decrease the peak taurine-gated current significantly (−16 ± 17%), but it accelerated the fast desensitization (−41 ± 7%) and it slowed down the deactivation kinetics at the end of the 5 sec application (Fig. 6B,D). We also investigated recovery from desensitization with paired pulses of taurine (20 mm, 2 msec) applied at variable intervals to eight nucleated patches excised from granule neurons. Figure 7shows one typical recording of such an experiment. The taurine-induced second response showed little inhibition by the first brief pulse at an interval of 25 msec, and an almost complete recovery of desensitized channels was observed by a 50 msec separation of the paired pulse. This was significantly different from the reduction of peak currents evoked by paired GABA pulses (Figs. 3A, 7A). These results indicate that taurine induced much less desensitization than GABA and has a prompt recovery. Figure 7C shows that when taurine (20 mm) and THDOC (1 μm) were applied together on nucleated patches, the recovery of desensitization of the taurine-induced response was very fast and had similar values to those obtained from averaged responses induced by taurine alone.
Fig. 5.
THDOC fails to prolong deactivation of responses evoked by brief taurine pulses. A, Averaged response of channel openings induced by 10 applications of taurine (2 msec, 20 mm) to a nucleated patch of a granule neuron excised from a rat cerebellar slice (holding potential, −60 mV). B, Averaged response of the same patch as in A to 20 mm taurine in the presence of THDOC (1 μm).C, Superimposed averaged currents recorded before and after the coapplication of taurine and THDOC. The averaged current inB has been normalized to the peak amplitude of the response in A. D, Summary of the percentage of changes produced by THDOC on taurine-gated currents. Amp, Peak amplitude; τ, decay time constant. Each bar represents the mean ± SEM of 11 patches studied. Above eachtrace are shown the currents indicating the duration of the pulse application. Vertical calibration does not apply to these traces.
Fig. 6.
THDOC alters desensitization of currents elicited by 5 sec but not 200 msec taurine applications. A, Left, Averaged response of 10 applications of taurine (200 msec, 20 mm) to a granule neuron nucleated patch excised from a rat cerebellar slice (holding potential, −60 mV). Middle, Averaged response of the same patch as in the left panel to 20 mm taurine in the presence of THDOC (1 μm). Right, Superimposed averaged currents recorded before and after the coapplication of taurine and THDOC. The averaged current in the middle panel has been normalized to the peak amplitude of the response in the left panel. Aboveeach trace in the right and middle panels are shown the currents indicating the duration of the taurine applications. Vertical calibration bars do not apply to these currents. B, Left, Averaged response of 10 applications of taurine (5 sec, 20 mm) to a nucleated patch.Middle, Averaged response of the same patch as in theleft panel to 20 mm taurine in the presence of THDOC (1 μm). Right, Superimposed normalized tail currents recorded before and after THDOC. C, Summary of the percentage of changes produced by THDOC on taurine-gated currents (200 msec applications). Amp, Peak amplitude;S/P, ratio between the amplitude at 200 msec (S) and peak amplitude (P); Doff, decay time constant at the end of the 200 msec application. Eachbar represents the mean ± SEM of nine patches studied.D, Summary of the percentage of changes produced by THDOC on taurine-gated currents (5 sec applications). Amp, Peak amplitude; S/P, ratio between the amplitude at 5 sec (S) and peak amplitude (P); Df andDs are the fast and slow desensitization time constants.Dfoff and Dsoff are the fast and slow decay time constant of deactivation at the end of the 5 sec application, and %F is the relative contribution of the fast component to peak amplitude. Each bar represents the mean ± SEM of eight patches studied.
Fig. 7.
THDOC fails to alter responses generated by paired pulses of taurine. Superimposed traces evoked by two successive applications of 2 msec pulses of 20 mm taurine (A) and taurine plus THDOC (1 μm) (B) separated by 25, 50, 100, 200, 400, and 600 msec intervals. C, Comparison of the recovery time course of the second response from desensitization. The percentage of recovery from desensitization at each designated separation of two brief taurine pulses is calculated as described in Figure 3. Exponential fitting of the recovery from desensitization yielded first order kinetics with a time constant of 5 msec for taurine and 7 msec for taurine plus THDOC. Data are expressed as mean ± SEM of eight patches studied.Above each trace are shown the currents used to measure the duration of the application. Vertical calibration does not apply to these traces.
Channel kinetics and GABAA receptor deactivation in the absence and presence of THDOC
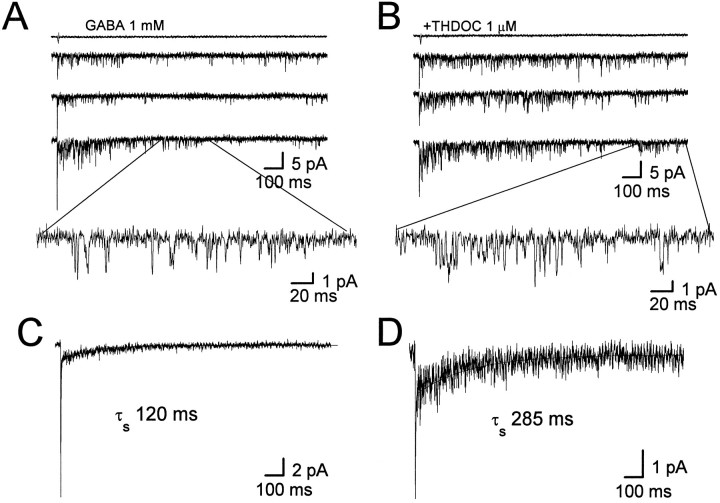
The reopening of GABA channels after desensitization has been proposed to shape the synaptic responses and modulate neuronal excitability (Jones and Westbrook, 1996). To elucidate the changes in channel gating caused by neurosteroids, we isolated outside-out patches from cerebellar granule neurons in the rat brain slices, and we recorded channel openings elicited by 1 msec GABA (1 mm) pulses at a holding potential of −70 mV, as illustrated in Figure8, A and B. Ensemble averages of patch currents (n = 5 patches) evoked by the fast application of GABA had a similar decay time course to GABA-evoked currents in nucleated patches and also were fit with double exponential functions (τf = 3.9 ± 0.5 msec, τs = 120 ± 7.9 msec; %F = 81 ± 7). However, the %F observed in outside-out patches was significantly greater than that in nucleated patches. We do not know at the present the reason for this discrepancy, which might be related to alterations of important cytoskeletal elements occurring in the small excised patches. In the presence of THDOC (1 μm), the slow decay time constant became 285 ± 10 msec, but the fast time constant remained 4.1 ± 0.7 msec, consistent with what was observed in nucleated patches. The fractional contribution to peak of the fast component was, however, slightly decreased to 63 ± 9%. As previously reported (Puia et al., 1990), the channel amplitude was not altered by THDOC (1.9 ± 0.3 vs 2.0 ± 0.7 pA). Because of the high level of channel activity as a consequence of the GABA applications, it was impossible to perform a conventional analysis of channel current kinetics. However, we were able to assess the channel current amplitude and average open time distribution by selecting the second half of each sweep (Fig. 8A,B). This analysis gave a mean open time of 1.09 ± 0.1 msec (GABA alone) and 1.1 ± 0.2 msec (GABA + THDOC), respectively (p > 0.05). In contrast, as can be observed in Figure 8, the number of late openings per sweep was increased remarkably in the presence of the neurosteroid, resulting in longer-lasting slow deactivation. We measured the average open probability in each sweep, demonstrating a significant increase from 0.15 ± 0.01 to 0.28 ± 0.01 (p < 0.01). These results suggest that THDOC increased the channel opening probability even in the absence of free GABA via the increased reopening of desensitized GABAA receptors after a brief GABA pulse.
Fig. 8.
THDOC increases late openings of single channel currents gated by a brief pulse of GABA. A, Channel activity evoked by a 1 msec pulse of 1 mm GABA as indicated by the current above each trace in an outside-out patch excised from a granule neuron in a cerebellar slice. The inset shows a fraction of the single channel current activity on an expanded time scale. B, Same as in A except that GABA was coapplied with THDOC (1 μm). C, D, Illustrated are the ensemble averages derived from 30 applications as inA and B, respectively. Ensemble responses are shown fit to biexponential functions with an indication of the slow decay time constant. Corresponding calibration bars are shownunder the right corner of the traces.
DISCUSSION
Neurosteroid prolongs GABAA receptor channel deactivation
Evidence from in vitro neuropharmacological studies has indicated that GABAA receptor function is modulated allosterically by neurosteroids (Majewska et al., 1986; Callachan et al., 1987; Harrison et al., 1987; Turner et al., 1989; Mistry and Cottrell, 1990; Puia et al., 1990, 1993; Gee et al., 1991; Twyman and MacDonald, 1992; Zhu et al., 1996). It is reasonable to propose that neurosteroids, synthesized and metabolized in the CNS (Paul and Purdy, 1992; Lambert et al., 1995), are potent endogenous modulators of IPSCs. Indeed, some reports have shown that neurosteroids and related compounds potentiate IPSCs by prolonging the decay time constant and thereby increasing the charge transfer and the strength of inhibition (Harrison et al., 1987; Cooper et al., 1995, 1996). Because IPSCs are mediated by a brief transient of saturating GABA concentration in the synaptic cleft (Edwards et al., 1990; Pearce, 1993; Celentano and Wong, 1994; Maconochie et al., 1994; Mody et al., 1994; Jones and Westbrook, 1995, 1996), it is difficult to extend directly the results of whole-cell recording and single channel current analysis performed under stationary agonist application to the synaptic modification of IPSCs by neurosteroids and other allosteric modulators. Therefore, in our study we investigate neurosteroid effects with fast (1 msec) applications of saturating concentrations of GABA (1 mm) onto excised nucleated patches to mimic the postsynaptic component of IPSCs (Maconochie et al., 1994; Puia et al., 1994; Jones and Westbrook, 1995; Tia et al., 1996). With the limitations that in nucleated patches from granule neurons the GABAA receptors studied are extrasynaptic, our studies of GABA-activated currents allow one to bypass possible presynaptic effects of neurosteroids on voltage-gated calcium channels that would influence neurotransmitter release. Furthermore, alteration of excitability by neurosteroids may lead to asynchrony of synaptic GABA release, an alternative mechanism for the prolongation of IPSC (Mody et al., 1994). In contrast to neurosteroid-induced potentiation of currents gated by low GABA concentrations (MacDonald and Olsen, 1994; Lambert et al., 1995), THDOC reduced the peak of GABA responses evoked by brief pulses of saturating concentration of GABA. The reduction of peak GABA response also was observed with preperfusion with low concentrations of GABA. This result is expected as a consequence of the reported direct agonist effect of THDOC and other neurosteroids (Majewska et al., 1986; Puia et al., 1990). In fact, similar to the action of GABA, when steroids are preperfused, they induce desensitization of a part of the available receptor population, thereby lowering the peak amplitude (Celentano and Wong, 1994; Mody et al., 1994; Jones and Westbrook, 1995). Despite the reduction of peak current, the THDOC effect was to increase the efficacy of inhibition, as seen by the increase of the charge transfer.
GABA-gated currents in patches from cerebellar granule cells are characterized by a double exponential decay (Maconochie et al., 1994;Puia et al., 1994; Tia et al., 1996). Previous studies reported multiexponential decay kinetics of spontaneous inhibitory postsynaptic currents (sIPSCs) of cerebellar granule neurons (Maconochie et al., 1994; Puia et al., 1994; Kaneda et al., 1995; Brickley et al., 1996;Tia et al., 1996) in the range of GABA-gated currents decay kinetics, although with several discrepancies. Our results show that the neurosteroid THDOC prolongs the slow decay component of GABA-gated currents, leaving the fast decay component and its relative contribution to peak amplitude unaffected. We also observed that the THDOC-induced increase of the slow decay time constant is reduced with development. This result, consistent with our previous studies in primary culture of rat cerebellar granule neurons (Zhu et al., 1996), likely relates to a decreased sensitivity to allosteric modulation by neurosteroids as a consequence of the increased expression of δ and/or α6 subunits in GABAA receptors of cerebellar granule cells during development (Laurie et al., 1992; Zheng et al., 1993; Muller et al., 1994).
Desensitization and neurosteroid-induced modification
Several possible mechanisms have been proposed to explain the biphasic decay of the IPSC (Edwards et al., 1990; Pearce, 1993). Recent evidence demonstrates that the balance of unbinding, desensitization, and reopening of desensitized receptors underlies the IPSC decay (Jones and Westbrook, 1995, 1996), indicating a major role for desensitization as a determinant of inhibitory synaptic transmission. Taurine, a putative agonist of GABAA and glycine receptors (for review, see Huxtable, 1992), directly gated Cl− channels sensitive to bicuculline with a much lower potency when compared with GABA, similar to what was reported for β-alanine (Jones and Westbrook, 1995). Taurine application studies of deactivation, desensitization, and recovery from desensitization led to the conclusion that the unbinding rate of taurine is very fast and that there is little entry into desensitized states, accompanied by an extremely rapid recovery. Brief pulses of taurine onto outside-out patches evoked responses lacking a second slow exponential decay, as is expected from the reduced desensitization and decreased reopening. A similar mechanism was described for l-cysteate and β-alanine in gating NMDA and GABAA receptors, respectively (Lester and Jahr, 1992; Jones and Westbrook, 1995). THDOC fails to alter deactivation of short-lasting taurine-gated currents although it increases the peak amplitude of these responses. The peak amplitude increase is probably attributable to the lack of complete receptor saturation with brief application, even at the high taurine concentrations used. The lack of THDOC effect on deactivation of these short taurine-gated responses demonstrates that neurosteroid effects are expressed when the slow component of deactivation is present. When compared with taurine, GABA-induced responses had slower deactivation, greater desensitization, and higher sensitivity to THDOC, suggesting that desensitization of GABAA receptors underlies neurosteroid modulation of GABA-gated current deactivation. Prolonged application of taurine-evoked currents with consistent desensitization was followed by double exponential deactivation kinetics, as previously reported for another less potent agonist, β-alanine (Jones and Westbrook, 1995). Our results show that THDOC slowed deactivation only after desensitizing taurine responses, further demonstrating that desensitized states are required for the neurosteroid to modulate GABA responses. THDOC also increased the fast desensitization rate of prolonged taurine responses but failed to affect the fast desensitization observed with 200 msec GABA applications. It is possible that because fast desensitization of GABA responses is so rapid, THDOC-induced increases of desensitization are difficult to measure. The suggestion that desensitization is crucial to neurosteroid modulation is supported further by the result that the neurosteroid markedly slowed the recovery from desensitization of GABAAreceptors, as estimated with paired pulse GABA application. Taken together, these data indicate that the main effects of the allosteric regulation of GABAA receptor by THDOC are to slow the rate of recovery from desensitization and possibly to increase the rate of entry into fast desensitized states. The lack of effect on fast deactivation of the GABA-activated current by the neurosteroid is consistent with the lack of observable effects on fast desensitization produced by GABA and with the hypothesis that fast deactivation is determined by the unbinding and desensitization, but not resensitization (Lester and Jahr, 1992; Jones and Westbrook, 1995,1996).
Neurosteroids have been reported to increase opening frequency for single channel currents activated at low GABA concentrations in stationary recording conditions, although there is disagreement on neurosteroid effects on burst duration (Puia et al., 1990; Twyman and MacDonald, 1992). It is possible that distinct effects may relate to distinct molecular structures of the GABAA receptors investigated in these studies. In the present study we report for the first time the effects of THDOC on GABA-activated channel currents in nonequilibrium conditions. We observed a significant increase in the number of late openings of GABA-gated channels outlasting the removal of the agonist, which resulted in a prolonged slow decay component of the ensemble average. We propose that the increase in the late opening results from particular combinations of microscopic kinetic parameters of entry and exit from desensitized states.
Neurosteroid and GABAergic synaptic transmissions
Our results indicate that the extent of desensitization induced by a brief pulse of GABA directly relates to the prolongation of the slow decay component of GABA-activated currents by neurosteroid. This result suggests that the regulation of desensitization and resensitization could be crucially important not only in shaping the IPSCs (Jones and Westbrook, 1995, 1996) but also in the allosteric regulation by neurosteroid. Prolongation of decay kinetics of IPSCs has been observed with neuroactive steroids (Harrison et al., 1987; Cooper et al., 1995,1996) but also has been observed with other allosteric regulators such as benzodiazepines and barbiturates (Segal and Barker, 1984; Vicini et al., 1986). Further studies are required to assess the intriguing possibility that regulation of desensitization and resensitization also might underlie the allosteric regulation of GABAA receptors by barbiturates and benzodiazepines.
It has been proposed that the potentiation of inhibitory synaptic transmission could be achieved by modulation of the frequency, time course, and amplitude of spontaneous and evoked IPSCs (Mody et al., 1994; Jones and Westbrook, 1996). The prolongation of decay time of postsynaptic responses increases the time course of cell hyperpolarization, leading to increased inhibition of neuronal firing (Jonas and Spruston, 1994; Mody et al., 1994; Jones and Westbrook, 1996). However, the result of slowed recovery from desensitization also predicts a reduction of the efficacy of inhibitory synapses during high frequency activity. These hypotheses will require verification within vitro and in vivo studies of steroid effects on inhibitory synaptic activities.
Our findings suggest the possibility that several naturally occurring steroids function as endogenous modulators to regulate inhibitory synaptic transmission by altering desensitization. The dependence of neurosteroid modulation on development and GABAA receptor subunit composition also may relate to developmental plasticity (Laurie et al., 1992; Zheng et al., 1993; Zhu et al., 1996). Last, taurine synthesized in several brain regions and localized in synaptic vesicles (Huxtable, 1992) weakly binds to GABAA receptors and unbinds before desensitizing. If synaptically released, taurine may produce short-lasting synaptic responses relatively insensitive to neurosteroid modulation and contribute further to the diversity of inhibitory postsynaptic transmission.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS32759 and K04 NS01680. We are grateful to Dr. Karl E. Krueger for his critical reading of this manuscript.
Correspondence should be addressed to Dr. Stefano Vicini, Department of Physiology and Biophysics, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Washington, DC 20007.
REFERENCES
- 1.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of GABAA receptor by progesterone metabolites. Proc R Soc Lond [Biol] 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- 3.Celentano J, Wong RKS. Multiphasic desensitization of the GABAA receptor in outside-out patches. Biophys J. 1994;66:1039–1050. doi: 10.1016/S0006-3495(94)80885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper EJ, Johnston GAR, Edwards FA. Differential sensitivity of synaptic GABAergic currents to a neuroactive steroid in brain slices from male rats. Soc Neurosci Abstr. 1995;21:1345. [Google Scholar]
- 5.Cooper EJ, Johnston GAR, Edwards FA. Developmental differences in synaptic GABAergic currents in hippocampal and cerebellar cells of male rats. Soc Neurosci Abstr. 1996;22:810. [Google Scholar]
- 6.Dominguez-Perrot C, Feltz P, Poulter MO. Recombinant GABAA receptor desensitization: the role of the γ2 subunit and its physiological significance. J Physiol (Lond) 1996;497:145–159. doi: 10.1113/jphysiol.1996.sp021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol (Lond) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frye CA. The neurosteroid 3 alpha, 5 apha-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- 9.Gee KW, Lan NC. γ-Aminobutyric acidA receptor complexes in rat cortex and spinal cord show differential responses to steroid modulation. Mol Pharmacol. 1991;40:995–999. [PubMed] [Google Scholar]
- 10.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high-resolution current recording from cell and cell-free membrane patches. Pflügers Arch. 1981;391:85–91. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 11.Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Jonas P, Spruston N. Mechanisms shaping glutamate-medicated excitatory postsynaptic currents in the CNS. Curr Opin Neurobiol. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 14.Jones MV, Westbrook GL. Desensitization states prolong GABAA channels’ response to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 15.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- 16.Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol (Lond) 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Neurosci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 18.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lester RA, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 21.Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 22.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 23.Mistry DK, Cottrell GA. Actions of steroid and bemegride on the GABAA receptor of mouse spinal neurons in culture. Exp Physiol. 1990;75:199–209. doi: 10.1113/expphysiol.1990.sp003394. [DOI] [PubMed] [Google Scholar]
- 24.Mody I, Koninck DE, Otis Y, Slotesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 25.Muller T, Fritschy JM, Grosche J, Pratt GD, Mohler H, Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci. 1994;14:2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 27.Pearce RA. Physiological evidence for two distinct GABAA responses in hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- 28.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptor. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 29.Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 30.Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 31.Segal M, Barker JL. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory connections. J Neurophysiol. 1984;52:469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- 32.Steffensen SC. Dehydroepiandrosterone sulfate suppresses hippocampal recurrent inhibition and synchronizes neuronal activity to theta rhythm. Hippocampus. 1995;5:320–328. doi: 10.1002/hipo.450050405. [DOI] [PubMed] [Google Scholar]
- 33.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental change of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner DM, Ransom RW, Yang JS-J, Olsen EW. Steroid anesthetics and naturally occurring analogs modulate the γ-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- 35.Twyman RE, MacDonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicini S, Alho H, Costa E, Mienville JM, Santi MR, Vaccarino FM. Modulation of γ-aminobutyric acid-mediated inhibitory synaptic currents in dissociated cortical cell cultures. Proc Natl Acad Sci USA. 1986;83:9269–9273. doi: 10.1073/pnas.83.23.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo A, Harris J, Dubrovsky B. Dose–response study of dehydroepiandrosterone sulfate on dentate gyrus long-term potentiation. Exp Neurol. 1996;137:151–156. doi: 10.1006/exnr.1996.0015. [DOI] [PubMed] [Google Scholar]
- 38.Zheng T, Santi MR, Bovolin P, Marlier LNJ-L, Grayson DR. Developmental expression of the α6 GABAA receptor occurs only after cerebellar granule cell migration. Dev Brain Res. 1993;75:91–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]
- 39.Zhu WJ, Wang JF, Krueger KE, Vicini S. δ subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]