Abstract
The Hu proteins are a group of antigens targeted in an immune-mediated neurodegenerative disorder associated with cancer. We have cloned and characterized four members of the Hugene family from mouse. We find that the Hu genes encode a large number of alternatively spliced transcripts to produce a series of related neuron-specific RNA binding proteins. Despite this complexity, we have discerned several ordered features ofHu expression. In the embryo, specific Hugenes are expressed in a hierarchy during early neurogenesis. In the E16 developing cortex, mHuB is induced in very early postmitotic neurons exiting the ventricular zone, mHuDis expressed in migrating neurons of the intermediate zone, andmHuC is expressed in mature cortical plate neurons. Such a hierarchy suggests distinct functional roles for each gene in developing neurons. In the adult, all neurons express some set ofHu mRNA and protein. However, specific patterns are evident such that individual neuronal types in the hippocampus, cerebellum, olfactory cortex, neocortex, and elsewhere express from one to several Hu genes. The complexity of potential protein variants within a gene family and of different Hu family members within a neuron suggests a diverse array of function. Given the strong homologies among the Hu proteins, the Drosophilaneurogenic gene elav, and the Drosophilasplicing factor sxl, we predict that different combinations of Hu proteins determine different neuron-specific aspects of post-transcriptional RNA regulation. Our findings of specific developmental patterns of expression and the correlation between immune targeting of the Hu proteins and adult neurodegenerative disease suggest that the Hu proteins are critical in both the proper development and function of mature neurons.
Keywords: neuron-specific gene expression, paraneoplastic neurologic disease, RNA binding protein, Hu onconeural antigen, autoimmunity
RNA binding proteins specifically expressed in the nervous system have been described in several species, although their functions remain unknown. Two distinct families of mammalian neuron-specific RNA binding proteins (n-RBPs) have been identified as target antigens in the human paraneoplastic neurological disorders (for review, see Darnell, 1996). The Nova family of proteins, identified as target antigens in a paraneoplastic motor disorder (Buckanovich et al., 1993), harbor three KH-type RNA binding domains also found inFMR-1, the fragile X mental retardation gene, and a number of splicing factors (Burd and Dreyfuss, 1994; Arning et al., 1996; Min et al., 1996). Nova-1 functions as an RNA binding protein in vitro and is expressed only in CNS neurons in a restricted pattern during development and adulthood (Buckanovich et al., 1996). The Hu family of proteins was identified as target antigens in a paraneoplastic neurological syndrome (the Hu syndrome) consisting of a diverse set of neuronal degenerations associated with small-cell lung cancer. The defining feature of the Hu syndrome is the presence of antibodies present in patients’ serum and cerebrospinal fluid that recognize antigens present in small-cell lung tumors and in neurons (for review, see Posner, 1995; Darnell, 1996). These antisera have allowed the cloning of target antigens, which have been used as defined diagnostic reagents to identify patients with the Hu syndrome. As a result, it has become clear that many Hu patients develop neurological degenerations that initially affect discrete areas of the nervous system, including the dorsal root ganglia, the limbic system, cerebellum, brainstem, motor, or autonomic nervous system. Most patients subsequently develop a complex syndrome best characterized as a multifocal neuronal degeneration and die from neurological causes, on average, seven months from the time of their diagnosis (Dalmau et al., 1991).
Hu antisera recognize a nuclear antigen present in all neurons but not expressed in other tissues (Graus et al., 1985; Dalmau et al., 1992). Hu antisera also recognize a set of antigens ofMr 35–40 kDa on Western blots of brain or tumor extracts and were used to clone a brain cDNA, termed HuD, encoding a target antigen (Szabo et al., 1991). Subsequently, two additional genes encoding target antigens have been identified:HuC (Szabo et al., 1991) or ple21 (Sakai et al., 1993) andHel-N1 (Levine et al., 1993), termed here HuB; additional antigens are likely to exist (Good, 1995).
The Hu family of genes shares homology with theDrosophila elav and sex lethal(sxl) genes. These homologies are concentrated in three RNA recognition motifs present in all of the Hu antigens. The function that Hu proteins play as RNA binding proteins in the vertebrate nervous system is not known. elav is essential for neurogenesis in flies, whereas sxl regulates alternative splicing of its own and tra transcripts. These observations suggest possible developmental and functional roles for the Hu proteins. Several in vitro studies have demonstrated that the Hu proteins bind to RNA. HuB was found to bind to AU-rich RNA sequences in vitro (Levine et al., 1993; Gao et al., 1994). Similar findings subsequently were reported with HuD and a related protein HuR (Liu et al., 1995; Ma et al., 1996); recently, the mouse homolog of HuB has been reported to bind to a GA-rich sequence (Abe et al., 1996). The significance of the AU-rich sequences that Hu proteins bind could relate to mRNA turnover, although this is uncertain (seeLagnado et al., 1994; Zubiaga et al., 1995); similar AU-rich sequences may be involved in various aspects of RNA metabolism, including the regulation of RNA turnover (Shaw and Kamen, 1986; Zubiaga et al., 1995) and splicing (McMullough and Schuler, 1993).
It is unknown whether the clinical diversity seen in the Hu syndrome correlates in any way with the molecular diversity of the genes encoding target Hu antigens. In situ hybridization studies with a probe derived from conserved regions of the Hu coding sequences (King et al., 1994) are consistent with immunohistochemical observations that the Hu antibody binds to all neurons (Posner and Furneaux, 1990). However, no study has been made of the expression pattern of individual Hu family members by using specific reagents. The diverse number of Hu genes suggests that individual Hu transcripts might be expressed at either different developmental times or in specific subsets of neurons. Such studies are not only of importance in developing an understanding of the role that these RNA binding proteins play in the development and function of neurons but in addressing their role as target neuronal antigens in autoimmune neurological disease. For example, it is unknown whether the progression from unifocal to multifocal neurological disease in Hu patients might correlate with an autoimmune attack against a succession of Hu antigens.
To address these issues, we have developed gene-specific probes to study the expression and function of Hu gene family members in the mouse. We have identified four mouse Hu gene family members (termed mHuA through mHuD) that encode target antigens reactive with Hu antisera. Each gene has a complex pattern of alternative splicing, predicting at least 18 different protein variants that can be generated from the four genes. We have used gene-specific in situ hybridization probes to identify marked variability in the expression patterns of eachmHu gene within the developing and adult mouse nervous systems. We present evidence that there is a hierarchy of Huexpression in early postmitotic neurons, such thatmHuB is expressed in the earliest differentiated neurons, followed by mHuD and thenmHuC. In addition, in the adult CNS there are several neuronal cell types that demonstrate specific expression of individualHu family members. On the basis of our data, we conclude that the Hu proteins are likely to play specific roles in discrete sets of developing and adult neurons, and each may serve as a target antigen in the Hu neurological syndrome.
MATERIALS AND METHODS
Degenerate oligonucleotide PCR screening. The published Hu family member sequences show extremely high amino acid conservation between members. This sequence conservation is particularly strong (>95%) among each of the three 80 amino acid RNA recognition motifs (RRMs) present in each family member. Four sets of degenerate oligonucleotides centering around the conserved eight and six amino acid RNP1 and RNP2 submotifs within each RRM (FP1, 5′-GGI TAT/C GGI/C TTT/C GTI AAC/T TA; FP2, 5′-GGI TTC/T ATI/C C/AGI/C TTT/C GAT/C AA; RP1, 5′-AG/AG/A G/ATT G/ATA I/CAC/T A/GAA IAT; RP2, 5′-TTG/A TCA/G AAI/C CG/TI/C ATA/G AAI CC; I refers to inosine) were used in several combinations and conditions to amplify sequences from human fetal cDNA and human hippocampal cDNA library (Stratagene, La Jolla, CA) DNA. These products were subcloned (TA system, Invitrogen, San Diego, CA) and analyzed by exclusive colony hybridization by usingHuC, HuD, and HuB (Hel-N1) probes. Human and mouse cDNA libraries (Stratagene) were screened by using these clones to identify full-length sequences.
Developmental expression of alternatively processed Hu transcripts. Total RNA was isolated from E10, E16, P8, and adult mouse brain as described (Chomcynski and Sacchi, 1987). Single-stranded cDNA was synthesized with 5 μg of total RNA in a standard reverse transcriptase reaction primed by random hexamers (Boehringer Mannheim, Indianapolis, IN). PCR was performed with primers specific for cDNAs ofmHuA (P1, 5′-TCACAGTGAAGTTTGCAG; P2, 5′-ATTGACACCAGAAATCCC), mHuB (P1, 5′-TCACTGTAAAGTTTGCTA; P2, 5′-ATTAATTCCAGCCAGACT),mHuC (P1, 5′-TCAGCGTCAAGTTCGCAA; P2, 5′-GCCACTCATGCCATCGAT), and mHuD (P1, 5′-TTACTGTGAAGTTTGCCA; P2, 5′-GATGTTCATTCCCACAAG) surrounding the coding region between RRM 2 and RRM 3. PCR reactions containing 2.5 μCi of deoxycytidine 5′ [α-32P]triphosphate were run for 33 cycles (30 sec at 94°C, 30 sec at 58°C, and 45 sec at 72°C), and 10% of the reaction was run on 10% polyacrylamide gel electrophoresis and exposed to XAR-5 autoradiography film. PCR products were subcloned and sequenced to confirm their identity.
In situ hybridization. Protocols for hybridizations were essentially as described (Gibbs and Pfaff, 1994). Sense and antisense RNA probes (250–350 bp) from 3′-UTR of the mouseHu clones were transcribed in vitro with T7 RNA polymerase and [33P]-UTP. Slides were incubated at 50°C for 30 hr in a moist chamber with 1 × 106 cpm of labeled probe per 50 μl of hybridization solution. Slides were dipped in Kodak NTB2 emulsion, exposed in the dark for 7–10 d, developed, and counterstained with cresyl violet.
Fusion proteins. cDNAs encoding each Hu family member were cloned into pET21a (Novagen, Madison, WI) such that each was in an open reading frame encoding the T7 epitope at the N terminus with a histidine tag at the C terminus. After transformation of each construct into Escherichia coli BL21(DE3)pLysS, the bacteria were grown in LB broth at 37°C for a few hours and induced with 1 mm isopropyl thiogalactoside. Fusion proteins were purified by nickel-chelation chromatography.
Affinity purification of antibody. The full-length HuC fusion protein was coupled covalently to cyanogen bromide Sepharose 4B (Pharmacia, Uppsala, Sweden) according to the manufacturer’s instructions. Hu antiserum (10 ml) was spun at 40,000 ×g to remove precipitates, and the supernatant was incubated with 2 ml of Hu fusion protein–cyanogen bromide Sepharose overnight in RIPA buffer (150 mm NaCl, 50 mm Tris, pH 7.4, 0.1% SDS, 0.1% Nonidet P-40, and 0.5% deoxycholate). Sepharose was washed five times in 50 ml of RIPA, column-eluted with 4 ml of 0.2m glycine, pH 2.0, neutralized with 1 m Tris, pH 9.5, and dialyzed against PBS.
Northern blot analysis. Total RNA (15 μg) from the indicated mouse tissues was run on the 1% agarose-formaldehyde gel and transferred onto nylon filters (Amersham, Arlington Heights, IL). Probe was prepared by random prime labeling (Prime-It; Stratagene) of 3′ UTR fragments of mHuA or mHuC cDNA and hybridized in 50% formamide at 42°C for 24 hr. Stringent washes were in 0.1× SSC, 0.1% SDS at 55°C, and XAR film exposure was for 72 hr.
Immunohistochemistry. Adult female mice were anesthetized with ether and were perfused with saline, followed by 4% paraformaldehyde for 4 hr, and then placed in a 10% sucrose/PBS solution at 4°C overnight. Frozen sections (11 μm) were cut in the sagittal plane through the cerebellum and cortex, incubated in 0.3% H2O2, and washed with PBS. Subsequently, sections were incubated with primary antibody (affinity-purified Hu antiserum diluted 1:500 or rabbit anti-glial fibrillary acidic protein (GFAP) diluted 1:300 in 1% goat serum, PBS) for 48 hr at 4°C. Immunoreactivity was visualized via the avidin–biotin–HRP technique (Vectastain Elite Kit, Vector Laboratories, Burlingame, CA), with final incubation in 0.05% 3–3′-diaminobenzidine tetrahydrochloride with 0.01% H2O2 in 0.05 m Tris buffer and counterstained in cresyl violet. For immunofluorescence, sections were incubated with secondary antibodies (FITC anti-rabbit and rhodamine anti-human; Vector) for 1 hr and washed three times in PBS.
RESULTS
Cloning of mouse Hu gene family members
To obtain gene-specific probes for analysis of the expression ofHu family members during mouse development, we used degenerate oligonucleotide primers derived from a conserved region of the Hu genes for PCR amplification of cDNA, coupled with traditional low-stringency cDNA screening, to identify four full-length mouse genes (mHuA–D) encoding Hu family members. Figure 1A shows the alignment of the predicted proteins of the four mHu genes identified via this approach. These include mouse homologs of several mammalian and Xenopus Hu genes: mHuA[corresponding to the Xenopus elrA (Good, 1995) and human HuR (Ma et al., 1996) genes], mHuB(homologous to human Hel-N1; Levine et al., 1993),mHuC [corresponding to the human HuC gene (Szabo et al., 1991), also known as ple21 (Sakai et al., 1993)], andmHuD (corresponding to the human HuD gene;Szabo et al., 1991). In each case there is extremely high sequence homology between individual family members within and across species. This homology is greatest in the RNA recognition motifs (RRM), including RRM 1 and RRM 2, regions thought to encode the Hu antigenic epitopes (Manley et al., 1995). Moreover, the region of greatest variability between the predicted proteins, and thereby potentially a region related to gene-specific function, lies in the spacer region between RRM 2 and RRM 3. The mouse Hu genes are highly homologous to the Drosophila elav and sxlproteins, as noted for previously cloned Hu genes. An analysis of the similarity of these genes (Fig. 1B) indicates that, of the four mHu genes,mHuA is the most closely related to bothelav and sxl. Similarly, analysis of the evolutionary relationship among these genes (Fig. 1C) suggests that the ancestral Hu gene ismHuA, and the most recently diverged members aremHuB and mHuD.
Fig. 1.

A, Amino acid alignments of Hu-related proteins. The deduced amino acid sequence for four mouseHu genes (mHuA, mHuB, mHuC, mHuD) is compared with the Drosophila elav and sxlgenes. Residues identical to the consensus are shown in bold type, and conservative substitutions are showngray-shaded. The extent of RNA recognition motifs (RRM) 1–3 is indicated.B, Sequence distances between mHu proteins and theDrosophila elav and sxl proteins.Numbers shown represent percentage of similarity or percentage of divergence between the sequences shown inA. Amino acid sequences were aligned by using the Megalign program in the DNASTAR software package. C, Phylogenetic tree comparing the ancestral relationships between the mHu proteins and the Drosophila elav and sxlproteins. The scale beneath the tree measures sequence distances. The sequence relationships were determined by using the Megalign program to compare the sequences shown inA.
Alternative splicing in the Hugene family
A series of alternatively spliced transcripts was identified during the course of cDNA cloning, including splice variants in both the coding sequence and untranslated regions (UTRs). Numerous splice variants for the mHu genes were found to be restricted to the spacer region, including some previously identified splice variants (Szabo et al., 1991; Gao et al., 1994; Liu et al., 1995). To explore the splicing variation in this region systematically and to confirm the existence and distribution of each coding variant in the developing and adult nervous system, we performed RT-PCR analysis of brain RNAs, using PCR primers flanking this spacer region. Using primers specific to each of the four Hu genes, we demonstrated that amplified cDNA products were derived from RNA in an RT-dependent manner and confirmed their identity by cloning and sequencing the PCR products (Fig. 2A). The HuB gene produced a splice variant (previously reported as Hel-N2; Gao et al., 1994) for which the expression was restricted to mouse embryogenesis, whereas HuC andHuD produced splice variants expressed during development of the nervous system and in adulthood. Figure 2B shows the spectrum of coding region variants that were identified. In each case splicing variants conform to a specific pattern in which one splice donor (the coding sequences of which end in QRFR) is spliced alternatively to one of three different splice acceptors (Table1). The mHuC transcript uses one unique splice acceptor to generate splice variant 3 and shows the highest degree of complexity of spacer region alternative splice variants throughout development. We confirmed that the sequence variation corresponded to alternative splicing by sequencing genomicmHuB and mHuC clones (data not shown), which showed the same intron structure in this region as previously reported for the human HuD gene (Liu et al., 1995).
Fig. 2.
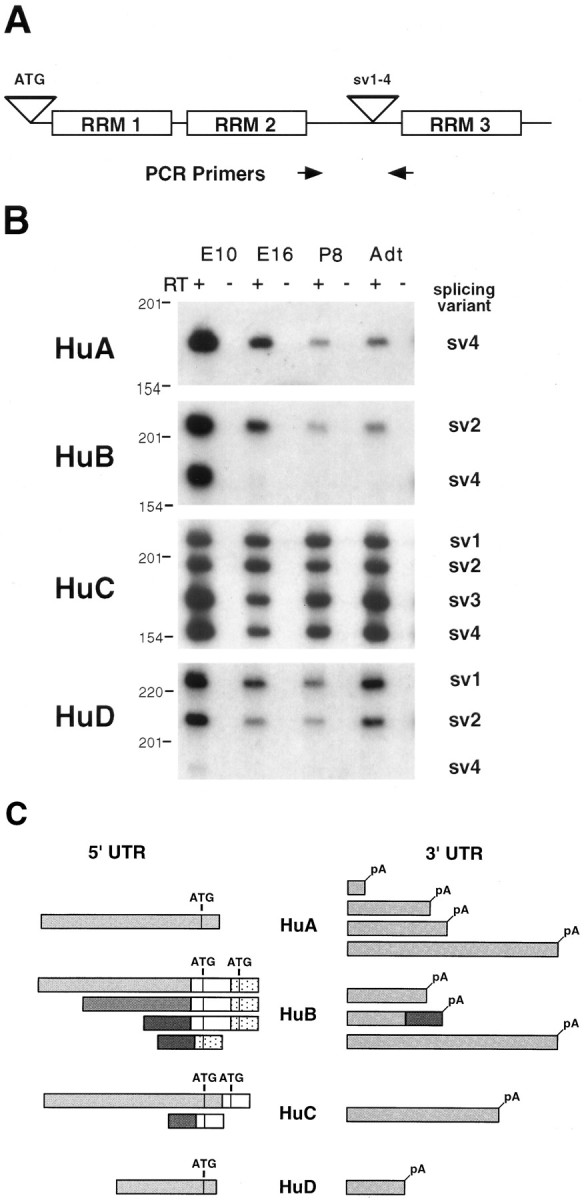
Alternative splicing of mouse Hutranscripts. A, Location of alternative splicing inHu transcripts and the origin of primers for PCR. Two alternative splicing sites are shown. The site labeledATG includes splice variants encoding alternative start codons, and the site labeled sv1–4 includes splice variants within the spacer region between RRM 2 andRRM 3. B, Developmental analysis of splice variants sv1–4 by RT-PCR. Total RNA from the mouse brain of indicated developmental stage was reverse-transcribed with (lanes marked +) or without (lanes marked −) RT and amplified by PCR with gene-specific primers, the locations of which are indicated byarrows in A. The name of each splicing variant is indicated and corresponds with the labels used in Table 1. Because sv2A and sv2B of HuB are not able to be distinguished by RT-PCR, both simply are named as sv2here. DNA size markers are shown on the left (in bp).C, Schematic drawing of Hu UTR alternative splice variants. ATG indicates putative initiation codons, and pA indicates polyadenylation sites found in cDNA clones. There is no apparent correlation between 5′ or 3′ UTR sequences between Hu families.
Table 1.
Summary of Hu coding region alternate splice variants
| Variant | Sequence |
|---|---|
| HuA | |
| sv4 | QRFR---------------------------FSP |
| HuB | |
| sv2A | QRFRLDNLLNMAYGVKR--------------FSP |
| sv2B | QRFRLDNLLNMAYGVKR--------------FSP |
| sv4 | QRFR---------------------------FSP |
| HuC | |
| sv1 | QRFRLDNLLNMAYGVKS-------PLSLIARFSP |
| sv2 | QRFRLDNLLNMAYGVKR--------------FSP |
| sv3 | QRFR--------------------PLSLIARFSP |
| sv4 | QRFR---------------------------FSP |
| HuD | |
| sv1 | QRFRLDNLLNMAYGVKRLMSGPVPPSACPPRFSP |
| sv2 | QRFRLDNLLNMAYGVKR--------------FSP |
| sv4 | QRFR---------------------------FSP |
Splice variants 1–4 from the spacer region between RRM 2 and RRM 3 (as illustrated in Fig. 2A) are shown. Variants from each gene are named on the left, and sequences are shown in single-letter amino acid code, with gaps as hyphens. Alternate splice junctions were confirmed by cloning and sequencing mouse genomic clones (data not shown).
Numerous splice variants also were found in the noncoding region ofHu gene family members (Fig. 2C). These include transcripts with alternative start codons in mHuB andmHuC yielding potential additional protein variants (Fig. 2C); such a variant 5′ end also was found to be encoded in human HuA transcripts but was not found in the mouse (data not shown). The 3′ UTR coding variants included alternative polyadenylation sites (mHuA and mHuB) and true alternative exon usage (mHuB). These observations suggest that the Hu noncoding regions may play specific roles in the post-transcriptional regulation of theHu mRNAs (see below). The complexity of the UTR splicing variation in the mHu genes increases in relation to the apparent evolutionary age of each gene family member. ThusHuB, the presumed ancestral mHu gene, shows the greatest complexity of UTR splice variants (4).
Hu expression
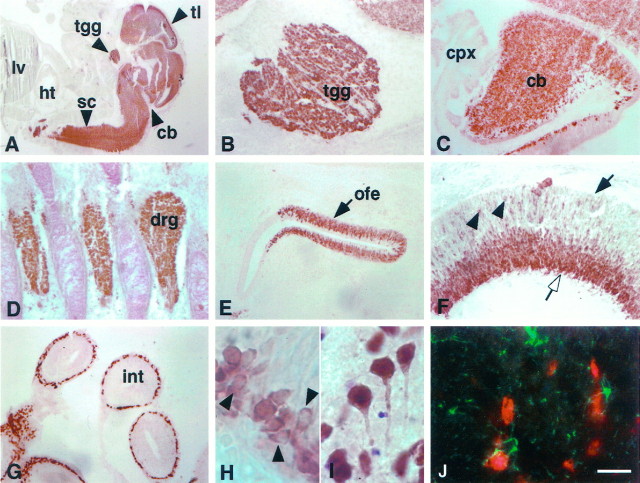
The expression of the human Hu antigen has been reported to be restricted to brain (Dalmau et al., 1992), and expression of several Hu genes has been reported to be neuron-specific, with the exception of mHuB mRNA, which also may be weakly expressed in testis (King et al., 1994), and mHuA homologs, which were reported to be ubiquitously expressed in human andXenopus tissues (Good, 1995; Ma et al., 1996). Because ubiquitous expression of HuA would seem incongruous with the reported neuron-specific expression of the Hu antigen, we evaluated both the expression of the Hu antigen by immunohistochemical and Western blot analysis and expression of the mHuA gene. Immunohistochemical analysis of E16 mouse embryos, including several neuronal and non-neuronal tissues, demonstrated intense reactivity with the Hu antigen in all neurons of the central and peripheral nervous systems but no reactivity with non-neuronal tissues, consistent with previous reports (Fig. 3; Posner and Furneaux, 1990;Dalmau et al., 1992; Marusich et al., 1994; Barami et al., 1995). Hu-positive cells had the morphology of neurons in the adult (Fig.3I); interestingly, newly born periventricular neurons present in the E16 cortex showed predominantly cytoplasmic staining (Fig. 3H), whereas adult neurons showed both strong nuclear and cytoplasmic staining (Fig. 3I). To demonstrate whether this reactivity was restricted to neurons, we double-stained sections with GFAP and Hu antisera. Hu and GFAP reactive cells were mutually exclusive (Fig. 3J).
Fig. 3.
Distribution of Hu immunoreactivity in the E14 and adult mouse. A, Sagittal section (11 μm) from E14 mouse stained with affinity-purified Hu antiserum. There is intense immunoreactivity in the central and peripheral nervous systems, including the telencephalon, cerebellum, and spinal cord, but no reactivity in other tissues, including liver and heart.B, Hu immunoreactivity in E14 trigeminal ganglia.C, Hu immunoreactivity in E14 cerebellum, demonstrating intense staining in the developing cerebellum with no staining of the choroid plexus. D, Hu immunoreactivity in E14 dorsal root ganglia. E, Hu immunoreactivity in E14 olfactory epithelium. F, Hu immunoreactivity in E14 retina; Hu staining is intense in ganglion cell layer (open arrow) but absent in the ventricular surface (solid arrow), except in some scattered cells (arrowheads).G, Hu expression in the ganglion cells in the small intestine. H, High-power magnification of Hu immunoreactivity in E14 cortex. Note the cytoplasmic staining in the developing cells (arrowheads). I, High-power magnification of Hu immunoreactivity in a horizontal section (11 μm) of adult cortex, demonstrating that Hu reactivity in the differentiated neuron is both nuclear and cytoplasmic (arrows). J, Immunofluorescence double exposure of GFAP (green) and Hu(red) immunoreactivity in a horizontal section of adult cortex, demonstrating that the two are mutually exclusive.tl, Telencephalon; tgg, trigeminal ganglia; sc, spinal cord; cb, cerebellum;ht, heart; lv, liver; cpx, choroid plexus; drg, dorsal root ganglia;ofe, olfactory epithelium; int, small intestine. Scale bars: 2 mm in A; 160 μm inB–E, G; 80 μm in F; 20 μm in H–J.
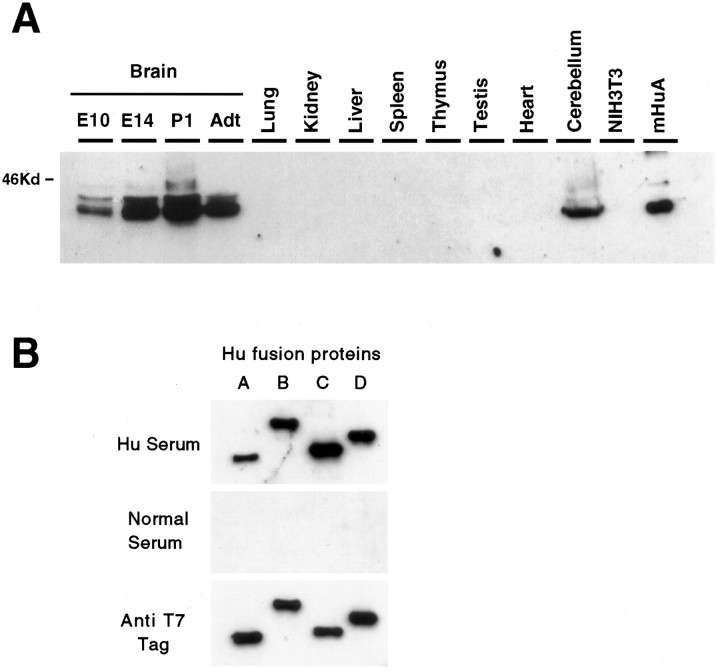
We also examined Hu antigen expression by Western blot analysis. When protein extracts from multiple mouse tissues were run on SDS-PAGE and probed with Hu antisera, immunoreactive proteins were detected only in brain extracts (Fig. 4A), consistent with a similar analysis of human tissues (Dalmau et al., 1992). One explanation for the observed restricted Hu antigen expression and the broad distribution of HuA homolog mRNAs is that the HuAgenes may encode a protein that is not reactive with Hu antisera (Ma et al., 1996). We examined this possibility by expressing full-length coding sequence for the mHu genes as bacterial fusion proteins and by probing Western blots with Hu antisera. The mHuA fusion protein was reactive at high titer with Hu antisera, but not with control antisera (Fig. 4A; data not shown). When we compared the reactivity of the four mHu fusion proteins, we found that each was reactive at high titer with Hu antisera obtained from three different Hu patients but showed no reactivity with control serum (Fig.4B; data not shown). Because we also found similar immunoreactivity with each of the four human Hu fusion proteins (data not shown), we conclude that each of the four Hu genes encodes potential autoantigens in the Hu paraneoplastic disorder. The apparent antigenicity of each gene product varies; whenHu immunoreactivity was normalized for the amount of fusion protein present in each assay (assessed with a T7 antibody directed against each Hu fusion protein; Fig. 4B), we found that the HuC gene encoded the most immunoreactive andmHuA the least immunoreactive epitope, differing by a factor of ∼5 (assessed by densitometry; data not shown). The greater sensitivity of our Western assay may account for the discrepancy between these results and a previous report in which 125I protein-A detection was used to assay the reactivity of Hu disease antisera with HuA fusion protein (Ma et al., 1996).
Fig. 4.
Western blot analysis of Hu antigen expressionin vivo and in vitro. A, Hu expression in the developing mouse brain and various adult tissues. Total cellular extracts from the indicated tissues, National Institutes of Health 3T3 cells, or purified mouse HuA fusion protein were run on 10% SDS-PAGE, transferred to nitrocellulose, and probed with Hu antiserum. The blot also was probed with an anti-tubulin antibody, which revealed that each of the nonbrain samples had at least as much (in most cases greater) protein loaded as in the lanes marked Brain orCerebellum (data not shown). B, Hu antiserum recognizes recombinant fusion proteins of all four Hu family members. Equal amounts of T7-tagged bacterial fusion proteins of mouseHuA, HuB, HuC, andHuD were probed with paraneoplastic Hu antiserum (Hu Serum). The blot also was probed with normal human serum (Normal Serum), which was not reactive with the Hu fusion proteins, and an anti-T7 tag monoclonal antibody (Anti T7 Tag) used as a positive control and used to normalize the quantity of fusion protein present in each sample. Identical results were obtained with three different Hu disease antisera and Hu antisera affinity-purified with HuC fusion protein (data not shown).
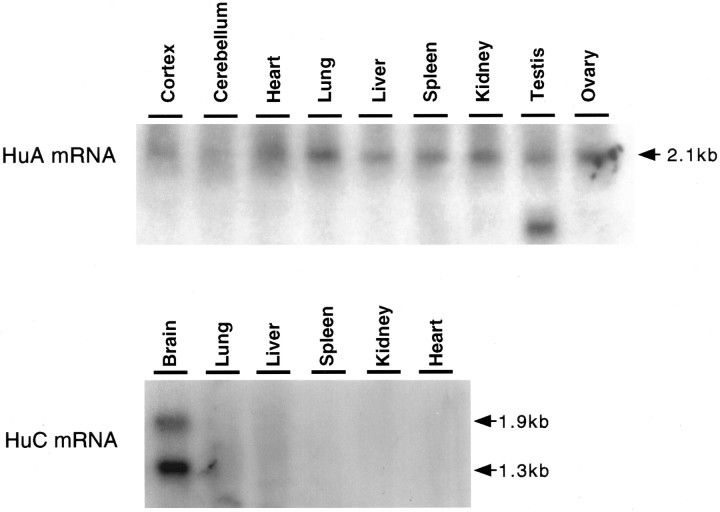
Because several reports indicated that mRNA-encoding HuA homologs were ubiquitously expressed, we evaluated the tissue distribution ofmHuA gene expression in the mouse. Northern blot analysis with an mHuA coding region probe to examine mRNA in multiple tissues (Fig. 5) confirmed that mHuA RNA expression could be detected ubiquitously, whereas mHuCexpression was restricted to brain. We next examined whether subtle differences in brain versus nonbrain mHuA mRNA, such as small alternatively spliced exons not detectable by Northern blot analysis, could account for the discrepancy between the restricted distribution of the Hu protein and mRNA. We cloned full-length cDNAs encoding mHuA from a nonbrain (spleen) tissue that does not express immunoreactive Hu antigen (Fig. 4A; data not shown) and compared the sequences with brain mHuA cDNA sequences. These data revealed that the brain and spleenmHuA mRNA were identical within the full coding region and regions of the UTR that were sequenced. We conclude that the discrepancy between the restricted expression of Hu protein and the widespread expression of the mHuA gene may be a result of post-transcriptional regulation of mHuA gene expression. Such regulation also may occur with HuB expression, because we and others detect no protein expression in ovary or testis (Fig.4A; Dalmau et al., 1992), whereas others have found expression of HuB homolog mRNA in these tissues (King et al., 1994; Good, 1995). Finally, we note that determination of the tissue pattern of mHuA protein expression will need to await the development of specific antibodies.
Fig. 5.
Northern blot analysis ofHuA and HuC in various mouse tissues. Fifteen micrograms of total RNA from the indicated adult mouse tissues were separated on an agarose-formaldehyde gel, transferred onto nylon membranes, and hybridized with 32P-labeled 3′ UTR cDNA probes specific for mHuA ormHuC. RNAs were visualized with ethidium bromide for examining the equivalent amount of each sample (data not shown).
Tissue and developmental expression of individual Hugene family members
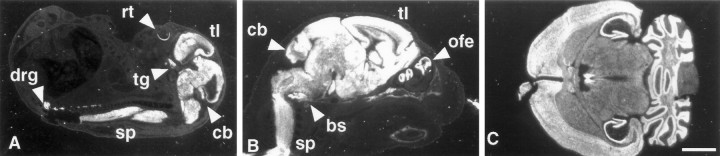
To examine the expression pattern of individual Hugenes, we performed in situ hybridization with gene-specific probes. Sense and antisense 3′ UTR sequences from the clonedmHu genes were transcribed and used to probe sections from developing and adult mice. Figure 6 shows a panel of mouse embryonic and adult sections probed with an antisense riboprobe specific for mHuC. In each section the hybridization pattern is restricted entirely to the nervous system. At E16 it is evident that neurons of the central and peripheral nervous systems expressHuC mRNA; there is robust reactivity in the retina, telencephalon, midbrain, hindbrain, and spinal cord and peripheral nervous system, including the trigeminal ganglia and dorsal root ganglia (Fig. 6A). At P0, mHuC expression remains tightly restricted to the nervous system (Fig.6B), and in the adult brain there is widespread reactivity restricted to cells of the central and peripheral nervous systems (Fig. 6C). Similar nervous system-specific expression was evident for all mHu probes (exceptmHuA) throughout embryogenesis and in adulthood (see below; data not shown).
Fig. 6.
Specific expression of mHuC in the nervous system; dark-field microscopy of HuC in situhybridization in the mouse. Sagittal sections (11 μm) of E16 (A) and P0 (B) mouse and a horizontal section (11 μm) of adult mouse brain (C) were hybridized with 33P-labeled antisensemHuC-specific cRNA probe. Mouse HuCexpression is observed in the telencephalon, cerebellum, spinal cord, dorsal root ganglia, and olfactory epithelium at E16 and P0 and is absent in non-neural tissues. Expression in the nervous system persists through adulthood (C) and remains absent in non-neural tissue (data not shown). No reactivity was observed with a sense riboprobe (data not shown). bs, Brain stem;tg, trigeminal ganglia; rt, retina;tl, telencephalon; cb, cerebellum;drg, dorsal root ganglia; ofe, olfactory epithelium; sp, spinal cord. Scale bar, 2 mm.
Immunohistochemical analysis has indicated previously that Hu protein expression is induced within hours of neurogenesis in the developing avian brain, when it is measured after 3H-thymidine or BrdU labeling of early postmitotic neurons (Marusich et al., 1994; Barami et al., 1995). We compared the early developmental expression of individual mHu genes with the expression of the Hu antigen. We assumed in these studies that the Hu immunohistochemical pattern is a measure of the sum of all Hu antigens expressed (see Fig. 3). At E16 in the developing mouse neocortex, immunohistochemical staining with affinity-purified anti-Hu antibody demonstrated robust reactivity in the postmitotic neurons of the cortical plate, in migrating neurons of the intermediate zone, and some reactivity in scattered neurons in the ventricular zone (Fig. 7A). Similarly, developing neurons of the P9 cerebellum showed Hu immunoreactivity in the inner cells of the external granular layer (EGL), Purkinje neurons, and neurons within the internal granular layer (IGL; Fig.7E). We interpret the Hu immunohistochemical reactivity of a subset of neurons within the EGL and ventricular zone as consistent with previous reports that Hu protein is induced at the time of neurogenesis.
Fig. 7.
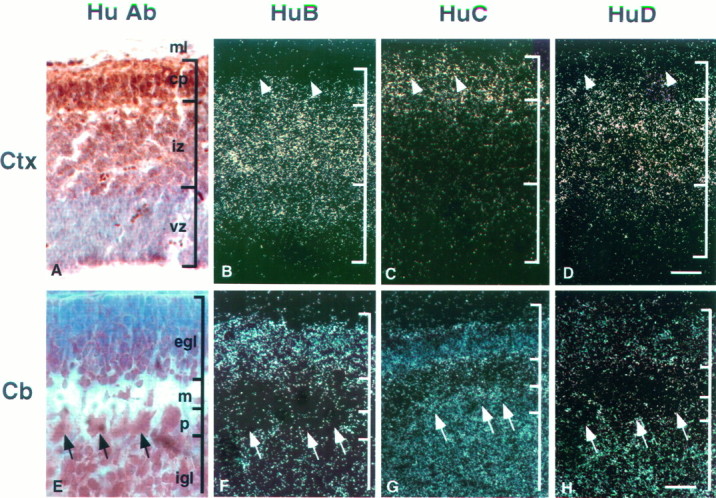
Expression patterns of Hu mRNAs in developing brain. Sagittal sections (11 μm) of E14 mouse cortex (Ctx; A–D) and P9 mouse cerebellum (Cb; E–H) were analyzed by immunohistochemistry (A, E) and in situhybridization (B–D, F–H). Affinity-purified Hu antibody was used for immunohistochemistry (A, E). Serial sections were hybridized with 33P-labeled antisenseHuB (B, F), HuC(C, G), and HuD (D, H) gene-specific 3′ UTR cRNA probes. In the developing cortex, mHuB is expressed in some cells of the ventricular zone and cells of the intermediate zone;mHuB diminishes in the cortical plate, with no expression evident in the outermost differentiated neurons (arrowheads). mHuC is detected only in the cortical plate, including the differentiated neurons (arrowheads). mHuD expression is intense in the intermediate zone, diminishes in the cortical plate, and is very weak or absent in the differentiated neurons (arrowheads). In developing cerebellum,mHuB is expressed primarily in the external granule cell layer, whereas the expression of mHuC andmHuD is distributed widely. Purkinje cells (arrows) express only mHuC. Sections were counterstained with cresyl violet. ml, Marginal layer;cp, cortical plate; iz, intermediate zone; vz, ventricular zone; egl, external germinal cell layer; m, molecular layer;p, Purkinje cell layer; igl, internal granule cell layer. Scale bars: 60 μm in A–D; 15 μm in E–H.
Hierarchies of mHu mRNA expression in developing neurons
Interestingly, the mHu mRNAs showed a hierarchy of expression in developing neocortical and cerebellar neurons.mHuB mRNA was expressed in the very earliest stages of neural development, with neocortical expression evident in the outer layer of cells in the ventricular zone, continuing into neurons of the intermediate zone, and diminishing in neurons of the cortical plate;mHuC mRNA expression was absent in neurons of the cortical ventricular zone and intermediate zone but was robustly expressed in neurons of the cortical plate (Fig. 7B,C). mHuDexpression showed an intermediate pattern of expression, with mRNA first evident in the intermediate zone neurons and diminishing in cortical plate neurons (Fig. 7D).
A similar pattern of mHu gene expression was evident in neurons of the developing cerebellum. Again, mHuBexpression was evident in the most immature neurons of the developing cerebellum, in the inner layer of the EGL, with some expression apparent in neurons migrating through the molecular layer, little or no expression in the IGL, and no expression in Purkinje neurons (Fig.7F). mHuC expression was robust in the mature Purkinje neurons and IGL neurons, but also it was evident in some neurons in the EGL (Fig. 7G). mHuDexpression also was expressed within the developing cerebellum; it clearly was absent from Purkinje neurons, showed only trace expression in the EGL, and was detected at an intermediate level in the IGL (Fig.7H). These data suggest that themHuB gene is unique among the mHugenes in being expressed extremely early in neurogenesis and suggest that the immunohistochemical reactivity previously observed in early postmitotic cortical neurons (Marusich et al., 1994; Barami et al., 1995) may have represented the HuB protein.
mHu mRNA expression in adult brain
Analysis of the adult neuronal expression of themHu genes revealed additional regional differences in expression patterns of mHuB, mHuC, and mHuD (Fig. 8 and Table 2). Within the hippocampus (Fig.8A–C) mHuB showed restricted expression limited to CA2–CA3–CA4 pyramidal neurons, with no staining evident in the dentate neurons or adjacent entorhinal cortex, whereasmHuC expression was present in all neurons throughout this region, and mHuD expression was absent in dentate neurons but concentrated within the pyramidal neurons and adjacent entorhinal cortex. Within the adult olfactory system (Fig.8D–F) mHuB was expressed predominantly within large neurons of the olfactory bulb (mitral neurons), with only scattered expression in small granule neurons; there was also robust and specific expression within the accessory olfactory bulb. mHuD showed an overlapping pattern of expression that included mRNA within both olfactory mitral and granule cells, whereas mHuC expression was robust throughout the olfactory system.
Fig. 8.
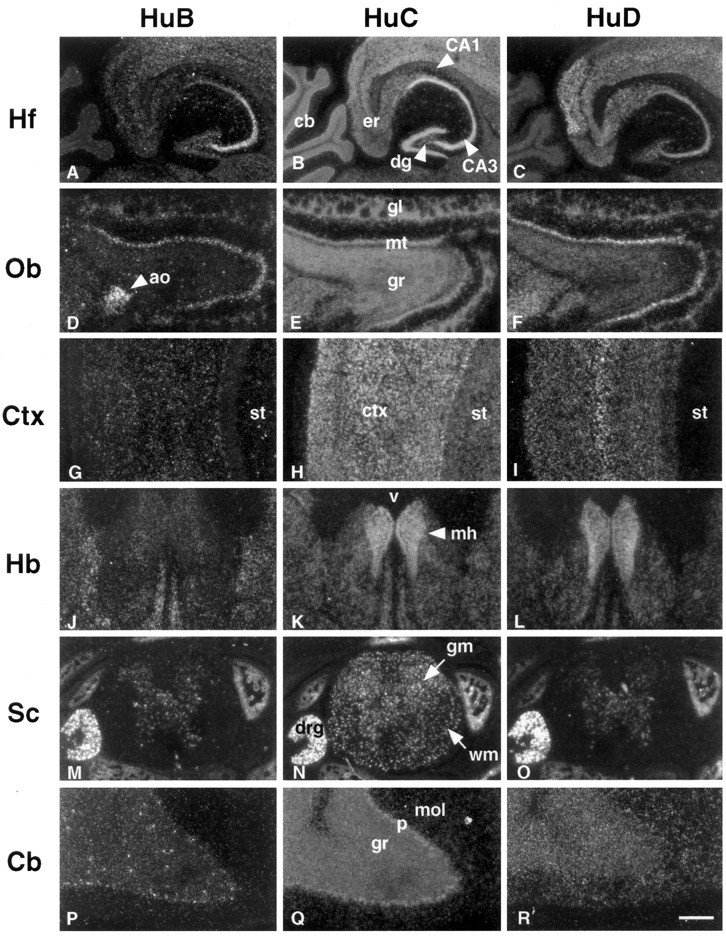
In situ hybridization ofHu mRNAs in adult mouse brain and spinal cord. Horizontal sections (11 μm) of adult mouse brain and spinal cord were hybridized with 33P-labeled antisense probes specific formHuB (A, D, G, J, M, P),mHuC (B, E, H, K, N, Q), andmHuD (C, F, I, L, O, R) cRNA probes. Hippocampal formation (Hf), olfactory bulb (Ob), cerebral cortex (Ctx), habenula (Hb), spinal cord (Sc), and cerebellum (Cb) are shown. Note that the hybridization signal of mHuB is intense in hippocampal pyramidal cells CA2, CA3, and CA4, mitral cell layer (mt), accessory olfactory bulb (ao), and dorsal root ganglia, but the signal is absent in the cerebellum except for some scattered cells in the granule cell layer (gr). The mRNA of mHuC is widely expressed in the adult nervous system, including hippocampal dentate gyrus (dg), pyramidal cells, glomerular (gl), mitral and granule cell layer (gr) of olfactory bulb, cortex, corpus striatum (st), medial habenula (mh), gray (gm) and white matter (wm) of spinal cord, dorsal root ganglia (drg), and Purkinje cells (p). mHuD expression is prominent in the entorhinal cortex (er), medial habenula, and dorsal root ganglia, but it is absent in dentate gyrus, corpus striatum, and Purkinje cells. No reactivity was observed with sense riboprobes (data not shown). v, Third ventricle;mol, molecular layer. Scale bars: 400 μm inA–C; 300 μm inD–F; 200 μm in G–I; 300 μm inJ–L; 400 μm in M–O; 100 μm inP–R.
Table 2.
Summary of differential expression of mouse Hugenes by in situ hybridization studies
| HuA | HuB | HuC | HuD | |
|---|---|---|---|---|
| E16: | ||||
| Retina | ++ | +++ | +++ | +++ |
| Neocortex | + | ++ | +++ | + |
| Corpus striatum | + | − | ++ | − |
| Thalamus | − | +++ | +++ | +++ |
| Hypothalamus | − | +++ | +++ | ++ |
| Midbrain | +/− | +++ | +++ | + |
| Cerebellum | ++ | + | +++ | +/− |
| Pons | + | +++ | +++ | +++ |
| Spinal cord–dorsal | + | +++ | +++ | +++ |
| ventral | + | ++ | +++ | +++ |
| Dorsal root ganglia | + | +++ | +++ | +++ |
| PO: | ||||
| Nasal epithelium | + | + | +++ | +++ |
| Olfactory bulb | + | ++ | +++ | +++ |
| Trigeminal ganglia | ++ | +++ | +++ | +++ |
| Retina | +/− | +/− | +++ | + |
| Cerebral cortex | + | +/− | +++ | + |
| Hippocampus–pyamidal cells | ++ | ++ | +++ | ++ |
| granule cells | ++ | − | ++ | − |
| Corpus striatum | + | − | +++ | − |
| Thalamus | − | ++ | +++ | + |
| Hypothalamus | + | ++ | ++ | ++ |
| Midbrain | +/− | ++ | +++ | + |
| Cerebellum–Purkinje cells | + | − | +++ | − |
| EGL | + | +++ | ++ | + |
| IGL | − | − | +++ | ++ |
| Pons | +/− | ++ | +++ | ++ |
| Spinal cord–dorsal | + | ++ | +++ | ++ |
| ventral | + | + | +++ | + |
| Dorsal root ganglia | ++ | +++ | +++ | +++ |
| Sympathetic ganglia | + | +++ | +++ | +++ |
| Adult: | ||||
| Olfactory bulb–mitral cells | +/− | ++ | ++ | ++ |
| granule cells | − | − | ++ | + |
| Cerebral cortex | + | +/− | +++ | ++ |
| Hippocampus–CA 1 | ++ | − | +++ | ++ |
| CA 2 | ++ | ++ | +++ | ++ |
| CA 3, 4 | ++ | +++ | +++ | ++ |
| granule cells | + | − | +++ | − |
| Entorhinal cortex | +/− | − | ++ | +++ |
| Corpus striatum | +/− | − | ++ | − |
| Thalamus | − | ++ | +++ | + |
| Hypothalamus | +/− | ++ | ++ | +/− |
| Habenula | ++ | − | +++ | +++ |
| Amygdala | − | + | +++ | ++ |
| Midbrain | +/− | + | ++ | +/− |
| Cerebellum–Purkinje cells | + | − | +++ | − |
| granule cells | − | − | +++ | + |
| Pons | +/− | ++ | +++ | ++ |
| Olivary nucleus | − | − | +++ | +++ |
| Spinal cord–dorsal | +/− | ++ | +++ | ++ |
| ventral | − | + | +++ | + |
| Dorsal root ganglia | ++ | +++ | +++ | +++ |
+++, Dark signal; ++, average signal; +, weak signal; +/−, very weak signal; −, undetectable signal.
Within the neocortex (Fig. 8G–I)mHuB was detectable only in scattered neurons,mHuC was strongly expressed in all neocortical neurons, andmHuD was expressed most prominently in the large projection neurons in layer 5. The expression of both mHuCand mHuD was evident within the medial habenula, whereasmHuB expression was absent (Fig. 8J–L). Dorsal root ganglia neurons expressed abundant amounts ofmHuB, mHuC, andmHuD, whereas neurons within the spinal cord predominantly expressed mHuC (Fig.8M–O). In addition, a population of cells present in the spinal cord white matter expresses mHuC mRNA; the identity of these cells is unclear; they do express Hu protein by immunohistochemical assay and are GFAP-negative (data not shown). In the adult cerebellum (Fig. 8P–R)mHuB expression is downregulated except for expression within scattered small cells in the granule layer; the identity of these cells is unclear. mHuD shows faint expression in the granule layer, as well as scattered expression in the molecular layer; again, the identity of the latter cells is unclear; they are GFAP-negative (data not shown) and may represent expression in scattered neurons present in the cerebellar molecular layer.mHuC expression is robust within adult Purkinje and granule neurons (Fig. 8Q). A summary of mHugene expression is presented in Table 2. The results support the conclusion that mHuC expression is nearly ubiquitous in mature postmitotic neurons, whereas the cellular distribution and levels of mHuB and mHuD vary widely during development and in adulthood.
DISCUSSION
The Hu gene family encodes a complex set of neuronal RNA binding proteins
We have used degenerate PCR and standard cDNA cloning to identify four genes encoding mouse homologs of target antigens in the humanHu paraneoplastic neurological degenerations. Each of these genes produces a complex set of mRNA variants caused by alternative splicing in the coding and noncoding regions and caused by the usage of alternative polyadenylation sites. Although alternative exon usage within the coding region of some Hu genes has been noted previously, we have found that the same pattern of alternative exon usage is conserved in three of the four Hu genes, suggesting that the regulation of splicing at this point is likely to be a significant point in the regulation of Hu protein function. Splicing variants are able to generate between one and four variant protein species from each of the Hu primary transcripts; some additional splice variants (mHuB andmHuC) add N-terminal coding sequence and alternative initiator methionines. In total, the four Hu genes encode at least 18 different potential protein variants.
Alternative splicing of neuronal mRNAs is a widespread phenomenon; in extreme examples, such as the neuronal neurexin receptor, it is believed that alternative splicing generates several thousand transcripts from three genes (Ullrich et al., 1995). The Hugenes are unique in this context in that they are RNA binding proteins highly related to sxl. sxl acts in Drosophila to regulate alternative splicing of its own pre-mRNA, as well as splicing of the tra pre-mRNA. The high degree of homology between theHu genes and sxl (Szabo et al., 1991), the complexity of Hu protein variants, the hierarchical pattern of Hu expression, and the predominant nuclear localization of the Hu antigens suggest that the Hu proteins might act to regulate alternative splicing of neuronal pre-mRNAs. Neuronal pre-mRNAs encoding complex sets of proteins, such as the neurexins or the Hu proteins themselves in which the expression of multiple mRNA splice variants are regulated within subsets of developing and mature neurons, are potential candidates for targets of Hu action. Such a function might coexist with a cytoplasmic role for Hu proteins, including binding of AU-rich elements implicated as Hu binding sites in vitro (Levine et al., 1993; Gao et al., 1994; Liu et al., 1995; Ma et al., 1996). Finally, different Hu antigens may serve entirely different functions, an issue that could be approached with antibodies that discriminate between family members.
The extensive variability in 5′ and 3′ UTR variants in theHu genes may relate to alternative usage ofcis-acting control elements, in particular, variation in elements regulating expression of individual Hu mRNAs. The strongest support for this possibility comes from analysis of themHuA gene in which expression of the protein and mRNA is uncoupled, suggesting that mHuA expression is regulated at a post-transcriptional level. One mechanism for restricting mHuA protein expression to neurons would be the use of a cis-acting element within the mHuA mRNA that participates in regulating neuron-specific translation of the mHuA mRNA; such a neuronal regulatory element has been found in the BTEB mRNA and suggested for the paraneoplastic cdr2 antigen mRNA, both of which are ubiquitously expressed but translated only in neurons and testis (Imataka et al., 1994; Corradi et al., 1997).
The role of Hu proteins in neural development
The wide variability in the developmental expression of individualHu genes suggests that the different Hu proteins function in different stages in the early development of postmitotic neurons. By using in situ probes capable of distinguishing eachHu family member, we are able to conclude that themHuB gene, and to a lesser extent the mHuC gene, functions specifically in the earliest postmitotic neurons. For example, we find that the mHuB gene is expressed in neurons migrating out of the periventricular zone into the intermediate zone. Similarly, mHuB is robustly expressed in the inner layers of the cerebellar EGL (see Fig. 6). Previous autoradiographic studies have shown that the small cells in the superficial layer of the EGL correspond to proliferating cells, whereas the deeper cells, corresponding to mHuB (and to a lesser extentmHuC)-positive cells, are undergoing initial steps of neuronal differentiation and axon extension (Miale and Sidman, 1961;Fujita, 1967). Because our immunohistochemical analysis also demonstrates Hu protein expression in these neurons, we propose that the mHuB protein is the earliest Hu family member protein expressed after cortical neurogenesis and that mHuB, perhaps together with mHuC, is the earliest Hu family member expressed in developing cerebellar granule neurons.
Although we did not perform double labeling with mitotic markers such as BrdU, previous studies have suggested that the Hu antigen is detectable at or near the time of cell cycle exit in the avian nervous system (Marusich et al., 1994; Barami et al., 1995). More generally, studies of neuronal differentiation in the chick retina demonstrate that neuronal differentiation markers may be induced within minutes of exit from the cell cycle (Waid and McLoon, 1995). Our data indicate that each of the three types of differentiating neurons—early postmitotic, migrating, and mature neurons—of the cortex and cerebellum express a unique combination of Hu genes, suggesting a correlation between neuronal development and the function of different sets of Hu proteins.
In contrast to the expression of mHuB, the mHuCgene is expressed nearly ubiquitously in mature postmitotic neurons during development and adulthood. In several regions of the nervous system, mHuC seems to be the only mHu gene family member that is expressed. For example, only mHuC is expressed in cerebellar Purkinje neurons or hippocampal dentate neurons, suggesting a specific role for the protein in these neurons. Similarly, some groups of neurons preferentially expressmHuB or mHuD, although typically they are coexpressed with mHuC. Thus, neurons of the accessory olfactory bulb express mHuB, whereas neurons of the habenula, entorhinal cortex, and layer 5 in the neocortex expressmHuD. It is possible that individual splice variants of any of the Hu genes may further subdivide the apparent patterns of expression of any one Hu family member into smaller domains. Taken together, these observations suggest that themHu genes perform nonredundant functions. Moreover, the expression of some Hu genes in specific domains of the nervous system suggests that they play a role in the development and function of such regions.
Neuronal RNA binding proteins
One remarkable feature of the current study is the exquisite tissue specificity with which the Hu genes are expressed within the nervous system. Most mammalian RNA binding proteins identified have been found to be ubiquitously expressed, including the great majority of RRM-containing proteins (see Birney et al., 1993;Burd and Dreyfuss, 1994). The most significant exceptions to date are three classes of neuron-specific RBPs. The first class, related to the hnRNP K protein (Siomi et al., 1993a; Burd and Dreyfuss, 1994), is exemplified by the paraneoplastic antigen Nova-1 (Buckanovich et al., 1993) and includes the fragile X gene, FMR-1(Burd and Dreyfuss, 1994). Both Nova-1 and FMR-1 are RNA binding proteins (Siomi et al., 1993b; Buckanovich et al., 1996) believed to be expressed in neurons. However, although FMR-1 is expressed within most neurons, with some regional variation, it also is expressed outside of the nervous system (Abitbol et al., 1993; Devys et al., 1993; Hinds et al., 1993). In contrast, Nova-1 expression is restricted strictly to a subset of CNS neurons (Buckanovich et al., 1993, 1996), indicating that its function is unique to neurons. A second class of RRM containing n-RBPs is related to the hnRNP A/B proteins (Dreyfuss et al., 1993) and includes the Drosophila (Nakamura et al., 1994) and mouse (Sakakibara et al., 1996) musashi proteins and the Xenopus laevis nrp-1 protein (Richter et al., 1990). The nrp-1 and mouse musashi n-RBPs are expressed in the ventricular zone of the developing neural tube, and musashi is required for the proper development of adult sensory organs (Nakamura et al., 1994).
A third class of n-RBPs consists of RRM-containing proteins related to the paraneoplastic Hu genes. The closest Hurelatives in this class are elav, which is required for neurogenesis in the early embryo (Robinow et al., 1988a,b; Yao et al., 1993), and rbp9, which is expressed at later developmental stages (Kim and Baker, 1993). A third more distantly related protein,cpo, is an RBP for which the expression is restricted primarily to the developing peripheral nervous system, although it also is found in some other tissues (Bellen et al., 1992). Althoughelav and rbp9 are ubiquitously expressed within the Drosophila nervous system, the Hu genes show marked variability in both developmental patterns of expression and tissue distribution within the nervous system. We observed a hierarchy of Hu gene expression in neuronal development and a great heterogeneity of Hu gene expression within individual neuronal types in the adult (see Fig. 8). These results suggest that, in contrast to ubiquitously expressed RBPs, the Hu genes perform cell-specific roles in individual stages of differentiating neurons and within specific neuronal types. Clarification of those roles awaits identification of the RNA targets of these proteins in neurons.
The Hu genes encode a diverse set of disease antigens
The complexity of expression of the mHu mRNAs not only suggests specific roles for individual family members but has implications for the paraneoplastic Hu syndrome. The Hugenes are the targets of autoimmune attack in the adult brain; antibodies to HuA (see Fig. 4B), HuB (Dropcho and King, 1994), HuC, and HuD (Szabo et al., 1991; Manley et al., 1995) fusion proteins now have been documented in the sera of Hu patients. The initiating antigen expressed in small-cell lung cancers is likely to be a subset of the neuronal Hu antigens. In an RT-PCR analysis of mRNA expression in three PND-associated small-cell lung tumors,HuD, but not HuB (Hel-N1) orHuC, gene expression was detected (Manley et al., 1995). It remains unclear, however, which Hu antigens serve as targets for the autoimmune neurological assault.
It is of interest that some of the patterns of Hu expression correlate with discrete syndromes of neurological dysfunction seen in many patients presenting with the Hu syndrome (Dalmau et al., 1991). Thus, some Hu patients suffer a pure cerebellar degeneration that cannot be differentiated from typical paraneoplastic cerebellar degeneration in which Purkinje neurons are targeted (Dalmau et al., 1991). Similarly, ∼5% of Hu patients develop a pure limbic encephalopathy referable to hippocampal dysfunction (Dalmau et al., 1991). Although the cellular level of these neurological disorders is uncertain, pure cerebellar symptoms correlate with the exclusive expression of mHuC in Purkinje neurons and the near exclusive expression of mHuCin adult granule cells; similarly, limbic symptoms correlate with specific expression of HuC in dentate neurons or to the subsets of hippocampal pyramidal neurons expressing only HuDand HuC (see Table 1). Such exclusive vulnerability of some neurons to autoimmune dysfunction might relate to a lack of redundancy of Hu antigen expression in these neurons. The most common symptom, suffered by >70% of Hu patients (Dalmau et al., 1991; Posner, 1995), is a dorsal root ganglionopathy, which may correlate with the vulnerability of neurons in the peripheral nervous system versus CNS and with the high levels of antigen expression in the adult DRG (see Fig. 8).
Taken together, our data suggest that finer analysis of the neuronal dysfunction in adults may be able to be correlated with targeting of discrete Hu gene family members in various regions of the nervous system. Conversely, the indiscriminate attack against all Hu proteins evident in Hu antisera correlates with the multifocal neurological degeneration that 75% of Hu patients ultimately develop (Posner, 1995). The progression of unifocal to multifocal neurological symptoms that occurs in most of these patients may correspond to a progression of autoimmune targeting of specific Hu epitopes to a targeting of common Hu epitopes.
Conclusions: complexity of the Hu RNA binding proteins
Our work illustrates several levels of complexity in the family of Hu RNA binding proteins. First, analysis of alternative splice variants suggests that at least 18 different Hu proteins are encoded by four genes. Second, the regulation of expression of each gene (and perhaps each subtype of spliced gene product) seems likely to be regulated at both the transcriptional and post-transcriptional levels on the basis of our finding multiple alternate 5′ and 3′ UTR variants as well as direct evidence of post-transcriptional regulation of mHuA expression. Third, within any one neuron it is apparent that different combinations of Hu genes are expressed. This is well illustrated in both developing neurons, such as those in the cortex and cerebellum, and in the adult, where there is differential expression of mHugenes in the many regions, including the hippocampus, cerebellum, neocortex, and olfactory bulb. Loss-of-function experiments may be able to test the prediction that such hierarchies of expression of differentHu gene products are responsible for some aspect of complexity within these different neuronal groups.
Footnotes
These studies were supported by grants to R.B.D. from the National Institute of Neurological Disorders and Stroke (RO1 NS34389) and the Irma T. Hirschl Trust. H.J.O. was supported by National Research Service Award Postdoctoral Training Grant CA 09673-18. We thank members of our laboratory for discussion and critical reading of this manuscript. We also thank Geoff Manley and Ron Buckanovich for useful discussions in the early stages of this work.
Correspondence should be addressed to Dr. Robert B. Darnell, Laboratory of Molecular Neuro-Oncology, The Rockefeller University, 1230 York Avenue, New York, NY 10021.
REFERENCES
- 1.Abe R, Yamamoto K, Sakamoto H. Target specificity of neuronal RNA-binding protein, Hel-N1: direct binding to the 3′ untranslated region of its own mRNA. Nucleic Acids Res. 1996;24:2011–2016. doi: 10.1093/nar/24.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abitbol M, Menini C, Delezoide A, Rhyner T, Vekemans M, Mallet J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat Genet. 1993;4:147–153. doi: 10.1038/ng0693-147. [DOI] [PubMed] [Google Scholar]
- 3.Arning S, Grüter P, Bilbe G, Kramer A. Mammalian splicing factor SF1 is encoded by variant cDNAs and binds to RNA. RNA. 1996;2:794–810. [PMC free article] [PubMed] [Google Scholar]
- 4.Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- 5.Bellen HJ, Kooyer S, D’Evelyn D, Pearlman J. The Drosophila couch potato protein is expressed in nuclei of peripheral neuronal precursors and shows homology to RNA-binding proteins. Genes Dev. 1992;6:2125–2136. doi: 10.1101/gad.6.11.2125. [DOI] [PubMed] [Google Scholar]
- 6.Birney E, Kuman S, Krainer AR. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckanovich RJ, Posner JB, Darnell RB. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron. 1993;11:657–672. doi: 10.1016/0896-6273(93)90077-5. [DOI] [PubMed] [Google Scholar]
- 8.Buckanovich RJ, Yang YY, Darnell RB. The onconeural antigen Nova-1 is a neuron-specific RNA binding protein, the activity of which is inhibited by paraneoplastic antibodies. J Neurosci. 1996;16:1114–1122. doi: 10.1523/JNEUROSCI.16-03-01114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 10.Chomcynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Corradi JP, Yang CW, Darnell JC, Dalmau J, Darnell RB. A post-transcriptional regulatory mechanism restricts expression of the paraneoplastic cerebellar degeneration antigen cdr2 to immune privileged tissues. J Neurosci. 1997;17:1406–1415. doi: 10.1523/JNEUROSCI.17-04-01406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuropathy: a clinical study of 71 patients. Medicine (Baltimore) 1991;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881–886. [PMC free article] [PubMed] [Google Scholar]
- 14.Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devys D, Lutz Y, Rouyer N, Bellocq J, Mandel J. The FMR-1 protein is cytoplasmic, most abundant in neurons, and appears normal in carriers of a fragile X permutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- 16.Dreyfuss G, Matunis MJ, Piñol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 17.Dropcho EJ, King PH. Autoantibodies against the Hel-N1 RNA-binding protein among patients with lung carcinoma: an association with type I anti-neuronal nuclear antibodies. Ann Neurol. 1994;36:200–205. doi: 10.1002/ana.410360212. [DOI] [PubMed] [Google Scholar]
- 18.Fujita S. Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol. 1967;32:277–287. doi: 10.1083/jcb.32.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao FB, Carson CC, Levine T, Keene JD. Selection of a subset of mRNAs from combinatorial 3′ untranslated region libraries using neuronal RNA-binding protein Hel-N1. Proc Natl Acad Sci USA. 1994;91:11207–11211. doi: 10.1073/pnas.91.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbs RB, Pfaff DW. In situ hybridization detection of trkA mRNA in brain: distribution, co-localization with p75NGFR, and up-regulation by nerve growth factor. J Comp Neurol. 1994;341:324–339. doi: 10.1002/cne.903410304. [DOI] [PubMed] [Google Scholar]
- 21.Good PJ. A conserved family of elav-like genes in vertebrates. Proc Natl Acad Sci USA. 1995;92:4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graus F, Cordon-Cardo C, Posner J. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology. 1985;35:538–543. doi: 10.1212/wnl.35.4.538. [DOI] [PubMed] [Google Scholar]
- 23.Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. Tissue-specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- 24.Imataka H, Nakayama K, Yasumoto K, Mizuno A, Fujii-Kuriyama Y, Hayami M. Cell-specific translational control of transcription factor BTEB expression. J Biol Chem. 1994;269:20668–20673. [PubMed] [Google Scholar]
- 25.Kim Y-J, Baker B. The Drosophila gene rbp9 encodes a protein that is a member of a conserved group of putative RNA binding proteins that are nervous system-specific in both flies and humans. J Neurosci. 1993;13:1045–1056. doi: 10.1523/JNEUROSCI.13-03-01045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King PH, Levine TD, Fremeau RT, Keene JD. Mammalian homologs of Drosophila ELAV localized to a neuronal subset can bind in vitro to the 3′ UTR of mRNA encoding the Id transcriptional repressor. J Neurosci. 1994;14:1943–1952. doi: 10.1523/JNEUROSCI.14-04-01943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagnado CA, Brown CY, Goodall GJ. AUUUA is not sufficient to promote poly(A+) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A). Mol Cell Biol. 1994;14:7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine TD, Gao F, King PH, Andrews LG, Keene JD. Hel-N1: an autoimmune RNA-binding protein with specificity for 3′ uridylate-rich untranslated regions of growth factor mRNAs. Mol Cell Biol. 1993;13:3494–3504. doi: 10.1128/mcb.13.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Dalmau J, Szabo A, Rosenfeld M, Huber J, Furneaux H. Paraneoplastic encephalomyelitis antigens bind to the AU-rich elements of mRNA. Neurology. 1995;45:544–550. doi: 10.1212/wnl.45.3.544. [DOI] [PubMed] [Google Scholar]
- 30.Ma W, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed elav-like protein. J Biol Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 31.Manley GT, Smitt PS, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol. 1995;38:102–110. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- 32.Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- 33.McMullough AJ, Schuler MA. AU-rich intronic elements affect pre-mRNA 5′ splice site selection in Drosophila melanogaster. Mol Cell Biol. 1993;13:7689–7697. doi: 10.1128/mcb.13.12.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miale I, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:227–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- 35.Min H, Turck CW, Black DL (1996) A new regulatory protein, KSR, mediates exon inclusion through an intronic splicing enhancer. Genes Dev, in press. [DOI] [PubMed]
- 36.Nakamura M, Okano H, Blendy JA, Montell C. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron. 1994;13:67–81. doi: 10.1016/0896-6273(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 37.Posner JB. Anti-Hu autoantibody-associated sensory neuropathy/encephalomyelitis: a model of paraneoplastic syndrome. Perspect Biol Med. 1995;38:167–181. doi: 10.1353/pbm.1995.0043. [DOI] [PubMed] [Google Scholar]
- 38.Posner JB, Furneaux HM. Paraneoplastic syndromes. In: Waksman BH, editor. Immunologic mechanisms in neurologic and psychiatric disease. Raven; New York: 1990. pp. 187–219. [PubMed] [Google Scholar]
- 39.Richter K, Good PJ, Dawid IB. A developmentally regulated, nervous system-specific gene in Xenopus encodes a putative RNA-binding protein. New Biol. 1990;2:556–565. [PubMed] [Google Scholar]
- 40.Robinow S, Campos A, Yao K, White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988a;242:1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- 41.Robinow S, Campos A, Yao K, White K. The locus Elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988b;126:294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- 42.Sakai K, Gofuku M, Kitagawa Y, Ogasawara T, Hirose G, Yamazaki M, Koh CS, Yanagisawa N, Steinman L. A hippocampal protein associated with paraneoplastic neurologic syndrome and small cell lung carcinoma. Biochem Biophys Res Commun. 1993;199:1200–1208. doi: 10.1006/bbrc.1994.1358. [DOI] [PubMed] [Google Scholar]
- 43.Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H. Mouse musashi-1, a neural RNA binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 44.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 45.Siomi H, Matunis MJ, Michael WM, Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993a;21:1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siomi H, Siomi M, Nussbaum R, Dreyfuss G. The protein product of the fragile X gene, FMR-1, has characteristics of an RNA-binding protein. Cell. 1993b;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- 47.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and sex lethal. Cell. 1991;67:325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 48.Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 49.Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;14:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 50.Yao K-M, Samson M-L, Reeves R, White K. Gene elav of Drosophila melanogaster: a prototype for neuronal-specific RNA binding protein gene family that is conserved in flies and humans. J Neurobiol. 1993;24:723–739. doi: 10.1002/neu.480240604. [DOI] [PubMed] [Google Scholar]
- 51.Zubiaga AM, Belasco JG, Greenberg ME. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol Cell Biol. 1995;15:2219–2230. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]