Abstract
The type 3 serotonin receptor (5-HT3R) is a ligand-gated ion channel whose presence in the CNS has been established by radioligand binding, in situ hybridization, and immunohistochemical analysis. To analyze further the role of the 5-HT3R in the CNS, we used in situhybridization and immunocytochemistry to determine that 5-HT3R-expressing neurons are mainly GABA-containing cells in the rat telencephalon. We determined that 5-HT3R/GABA-containing neurons do not exhibit somatostatin immunoreactivity but often contain cholecystokinin (CCK) immunoreactivity. 5-HT3R-expressing cells with CCK immunoreactivity were observed in the neocortex, olfactory cortex, hippocampus, and amygdala. The 5-HT3R/CCK interneurons represent between 35 and 66% of the total population of CCK-containing cells in the neocortex.
Further characterization of the 5-HT3R/GABAergic neurons was based on their calcium-binding protein immunoreactivity and showed that these neurons lack parvalbumin (PV) and represent a subpopulation of calbindin (CB)-containing interneurons that were preferentially present in the CA1–CA3 subfield of the hippocampus. Although some 5-HT3R/GABAergic neurons with calretinin (CR) were found in the neocortex, olfactory cortex, hippocampus, and amygdala, these neurons were more often present in the agranular insular and piriform cortices.
We conclude that the neuronal expression of the 5-HT3R is selective within the GABA neuron population in the rat telencephalon. These 5-HT3R-expressing interneurons might contain CCK, CB, and CR. We suggest that serotonin through the 5-HT3R may regulate GABA and CCK neurotransmission in the telencephalon.
Keywords: 5-HT3R, serotonin, GABA, interneurons, calcium-binding proteins, cholecystokinin
The neurotransmitter serotonin [5-hydroxytryptamine (5-HT)] interacts with several receptors (Hoyer et al., 1994), all of which are G-protein-coupled (Hoyer et al., 1994), with the exception of the 5-HT3 receptor (5-HT3R), which is a ligand-gated ion channel (Derkach et al., 1989). Electrophysiological studies on neurons and neuronal cell lines indicate that stimulation of the 5-HT3R causes a rapid depolarization produced by an increased membrane permeability to monovalent cations (Peters and Lambert, 1989). The cloning of a functional subunit of the 5-HT3R (subunit A) from the neuroblastoma cell line NCB-20 has confirmed that the 5-HT3R is a member of the superfamily of ligand-gated ion channels and is structurally related to the nicotinic, GABAA, and NMDA receptors (Manricq et al., 1991).
Radioligand binding studies have detected 5-HT3R binding sites in the CNS of rodents, primates, and humans (Kilpatrick et al., 1987, 1988, 1989; Waeber et al., 1988, 1989, 1990; Barnes et al., 1989a, 1990; Pratt et al., 1990; Gehlert et al., 1991; Jones et al., 1992; Laporte et al., 1992). High-density 5-HT3R binding sites have been found in the hindbrain: the nucleus of the tractus solitarius, area postrema, nucleus of the spinal tract of the trigeminal nerve, and dorsal motor nucleus of the vagus (Waeber et al., 1988, 1989, 1990; Barnes et al., 1990; Pratt et al., 1990; Gehlert et al., 1991; Jones et al., 1992; Laporte et al., 1992). Moderately dense 5-HT3R binding sites have been found consistently in cortex, amygdala, and hippocampus (Kilpatrick et al., 1987, 1988;Waeber et al., 1988; 1989; 1990; Barnes et al., 1989a; Gehlert et al., 1991; Jones et al., 1992; Laporte et al., 1992). In contrast, only a few studies have reported specific binding sites in the nucleus accumbens, striatum, or substantia nigra (Kilpatrick et al., 1987;Waeber et al., 1988; Gehlert et al., 1991; Laporte et al., 1992). The presence of neurons containing 5-HT3R has been confirmed in some of these brain areas by in situ hybridization (Tecott et al., 1993; D. Johnson and S. Heinemann, personal communication) and immunocytochemical analysis (Morales et al., 1996a).
The function of the 5-HT3R in the CNS as well as in the neuronal circuits in which this receptor might participate remains to be established. The use of 5-HT3R antagonists in behavioral studies, however, has led to the view that this receptor participates in several pharmacological events, such as anxiolytic, antipsychotic, and cognitive-enhancing actions and facilitation of the withdrawal from drugs of abuse (Carboni et al., 1989; Costall et al., 1990, 1993;Nevins and Anthony, 1994). In addition, activation of 5-HT3R has been demonstrated to modulate the release of various neurotransmitters in the brain (Barnes et al., 1989b; Blandina et al., 1989; Chen et al., 1991, 1992; Paudice and Raiteri, 1991; Maura et al., 1992).
To obtain information on the functional significance of the 5-HT3R-containing neurons, we sought to determine additional neurochemical features of the neurons that express this receptor in the telencephalon.
MATERIALS AND METHODS
Tissue preparation. Twenty adult male Sprague Dawley 20 rats (100–120 gm body weight) were anesthetized with choral hydrate (3.5 mg/100 gm body weight) and perfused transcardially with a solution of 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.3. Brains were post-fixed overnight, rinsed with PB, and sequentially transferred to 12%, 14%, and 16% sucrose solutions. Brains were then frozen on dry ice, and sections of 30–40 μm thickness were obtained on a cryostat.
Probe preparation. [35S]- and [33P]-labeled sense and antisense RNA probes were generated from a 730 basepair (bp) PstI insert (corresponding to nucleotides 1500–2230 of the rat 5HT3R cDNA), using the TransProbe T Kit (Pharmacia, Piscataway, NJ). The [35S]- and [33P]-labeled antisense probes were synthesized separately.
In situ hybridization–immunocytochemistry labeling. In situ hybridization combined with immunolabeling was performed as described previously (Morales et al., 1996a). Free-floating cryosections were incubated in PB supplemented with 0.5% Triton X-100 for 10 min, rinsed 2 × 5 min with PB, treated with 0.2N HCl for 10 min, rinsed 2 × 5 min with PB, and then acetylated in 0.25% acetic anhydride in 0.1 mtriethanolamine, pH 8.0, for 10 min. Sections were rinsed 2 × 5 min with PB and post-fixed with 4% paraformaldehyde for 10 min, and after a final rinse with PB, sections were prehybridized for 3 hr at 55°C in hybridization buffer (50% formamide, 10% dextran sulfate, 5× Denhardt’s solution, 0.62 m NaCl, 50 mmdithiothreitol, 10 mm EDTA, 20 mm PIPES, pH 6.8, 0.2% SDS, 250 μg/ml ssDNA, 250 μg/ml tRNA). After prehybridization, sections were hybridized at 55°C for 16 hr in hybridization buffer containing [35S]- and [33P]-labeled single-stranded RNA probes at 107 cpm/ml. Sections were treated with RNase A at 4 μg/ml at 37°C for 1 hr, washed in 1 × SSC, 50% formamide at 55°C for 2 hr, and in 0.1 × SSC at 68°C for 1 hr. Sections were rinsed with PB, incubated in 1% bovine serum albumin (BSA) supplemented with 0.3% Triton X-100 in PB for 1 hr, and then incubated with the corresponding primary antibody for 24 hr at 4°C. A previously well characterized rabbit polyclonal antibody against SS, SS-320 (Morrison et al., 1983), was used at dilution 1:3000 (antibody was provided by Dr. R. Benoit, Montreal, Quebec, Canada); anti-GABA rabbit antibody (Sigma, St. Louis, MO) was used at dilution 1:2000. A specific anti-cholecystokinin (CCK) monoclonal antibody raised in mouse was used at dilution 1:2000 (provided by Dr. D. Goodwillie, Farmitalia Carlo Erba, Nerviano, Italy), and anti-parvalbumin (PV), anti-calbindin (CB) mouse monoclonal, and anti-calretinin (CR) rabbit polyclonal antibodies were used at dilution 1:2000 (Swant).
After sections were rinsed 3 × 10 min in PB, they were processed with an ABC kit (Vector, Burlingame, CA). Material was incubated in a 1:200 dilution of the corresponding biotinylated secondary antibody. After they were rinsed with PB, sections were incubated with avidin–biotinylated horseradish peroxidase for 2 hr. Samples were rinsed, and the peroxidase reaction was developed with 0.05% 3,3-diaminobenzidine-4 HCl (DAB) and 0.003% hydrogen peroxide (H2O2). All antibody dilutions were performed in PB supplemented with 1% BSA and 0.3% Triton X-100. Sections were mounted on coated slides, air-dried, dipped in nuclear track emulsion, and exposed for several weeks before development. Material was photographed under bright-field or epiluminescence microscopy.
When hybridization was performed with sense probes, few silver grains were scattered on the sections. This level of signal was very low and was considered as unspecific background. In contrast, hybridization with antisense probes resulted in a localized signal distribution.
Double immunocytochemistry. Free-floating cryosections were processed as described under immunocytochemistry. Sections were incubated with a specific rabbit polyclonal anti-5-HT3R antibody (0165) at 1:4000 dilution for 24–48 hr at 4°C. We have established previously the specificity of the anti-5-HT3R antibody used in this study (Morales et al., 1996a). After the peroxidase reaction was developed with DAB and H2O2, the sections were rinsed with PB, and the remaining hydrogen peroxidase activity was inactivated by incubating sections in methanol containing 0.3% H2O2 for 15 min at room temperature. After several rinses with PB, sections were treated with an avidin solution (Vector) to block biotin groups for 1 hr at room temperature. Sections were incubated in the second primary antibody (anti-CCK at 1:2000 dilution) supplemented with biotin-blocking solution (Vector) and incubated for 24 hr at 4°C. After they were rinsed 3 × 10 min in PB, sections were processed with an ABC kit (Vector). Material was incubated in a 1:200 dilution of anti-mouse biotinylated secondary antibody. After sections were rinsed with PB, they were incubated with avidin–biotinylated horseradish peroxidase for 2 hr. Samples were rinsed with 0.1 m sodium phosphate buffer (SPB), pH 6.8, and the peroxidase reaction was developed in 0.01% benzidine dihydrochloride, 0.025% sodium nitroferricyanide, and 0.005% H2O2 in SPB (Levey et al., 1986), which results in a granular blue-black reaction product.
Data analysis. Sections processed for in situhybridization and immunocytochemistry were analyzed and photographed with bright-field or epiluminescence microscopy. We performed a semiquantitative analysis of double-labeled cells in the neocortex. A neuron was considered double-labeled when its soma was brown and contained more than 15 silver grains. In pilot experiments we found that 15–20 silver grains represent weak label above background. Double-labeled cells were counted in three random sections of three different experiments, and the percentage of neurons containing both 5-HT3R mRNA and immunoreactivity was calculated from the total population of immunolabeled cells.
RESULTS
We have previously used in situ hybridization and immunocytochemistry to show the presence of GABA in neurons that express the 5-HT3R in the neocortex and hippocampus (Morales et al., 1996b). In the present study, we investigated further the neurochemical composition of 5-HT3R-expressing neurons in the telencephalon.
Colocalization of 5-HT3R transcripts and GABA
Coexistence of 5-HT3R transcripts with GABA immunoreactivity was observed in several areas of the telencephalon. These 5-HT3R/GABA neurons were often found in those brain areas that contained cells with high levels of 5-HT3R expression, such as the neocortex (Fig.1A,B), olfactory regions, hippocampal formation, amygdaloid complex, and septal region (Fig.2A,B). Double-labeled cells were also detected occasionally in areas in which neurons contained low levels of 5-HT3R mRNA, such as the corpus callosum, corpus striatum, thalamus, and hypothalamus. A semiquantitative analysis performed from three random sections of three different experiments indicated that between 70 and 95% of the 5-HT3R-expressing cells were GABAergic throughout the telencephalon (n = 550 cells).
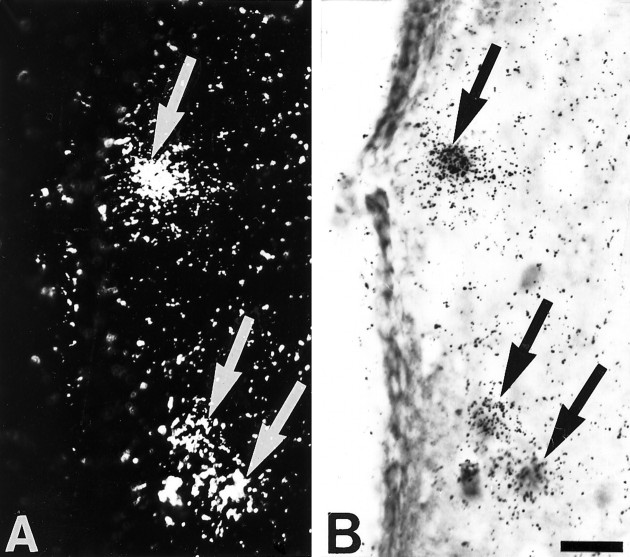
Fig. 1.
Simultaneous detection of 5-HT3R transcripts and GABA immunoreactivity in layer II of parietal cortex.A, Observation of 5-HT3R-expressing cells under epiluminescence microscopy. B, Observation of GABA-immunoreactive neurons under bright-field microscopy. In this section, all 5-HT3R-expressing cells showed GABA immunoreactivity (filled arrows inA and B), but not all GABA-immunoreactive neurons contained 5-HT3R transcripts (open arrows in B). Scale bar, 25 μm.
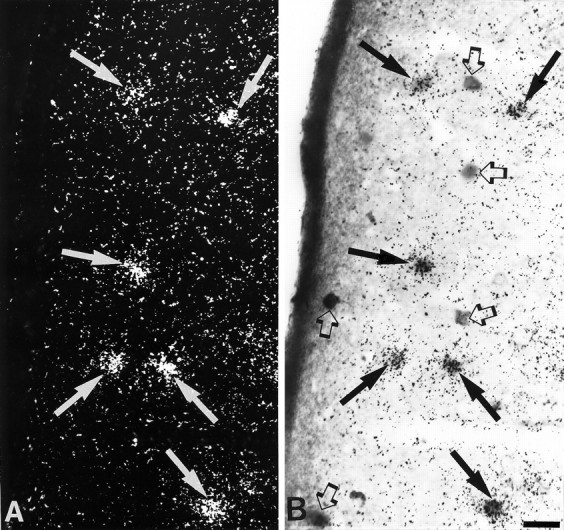
Fig. 2.
Simultaneous detection of 5-HT3R transcripts and GABA immunoreactivity in the medial septal nucleus.A, Observation of 5-HT3R-expressing cells under epiluminescence microscopy. B, Observation of GABA-immunoreactive neurons under bright-field microscopy. Double-labeled cells are indicated by arrows. Scale bar, 25 μm.
The 5-HT3R/GABAergic neurons were found throughout the different layers of the neocortex but were preferentially concentrated in layers II–III and V–VI. This pattern of distribution was observed from the frontal to the occipital regions of the neocortex. Within the olfactory regions, 5-HT3R/GABAergic neurons were detected in the olfactory tubercle, piriform cortex, endopiriform nucleus, anterior olfactory nucleus, and taenia tecta. The 5-HT3R/GABAergic neurons of the hippocampal formation were distributed in all layers of the CA1–CA3 subfields of the hippocampus, dentate gyrus, hilus, and subiculum. The 5-HT3R/GABAergic cells of the amygdaloid complex were found mainly in the lateral, basolateral, basomedial, and cortical amygdaloid nuclei.
Colocalization of 5-HT3R transcripts and neuropeptides
Somatostatin (SS) and CCK are neuropeptides known to coexist with GABA in nonoverlapping neuronal populations in the cortex and hippocampus (Somogyi et al., 1984; Sloviter and Nilaver, 1987). Thus, we investigated whether either of these peptides was present in the 5-HT3R-expressing neurons. No SS immunoreactivity was found in any 5-HT3R-expressing neurons, although single SS-immunopositive or 5-HT3R-expressing neurons were identified readily in cortex and hippocampus. In contrast, CCK immunoreactivity was often observed in 5-HT3R-expressing neurons of the neocortex (Fig. 3A,B), olfactory regions (Fig. 3C,D), hippocampal formation, and amygdaloid complex.
Fig. 3.
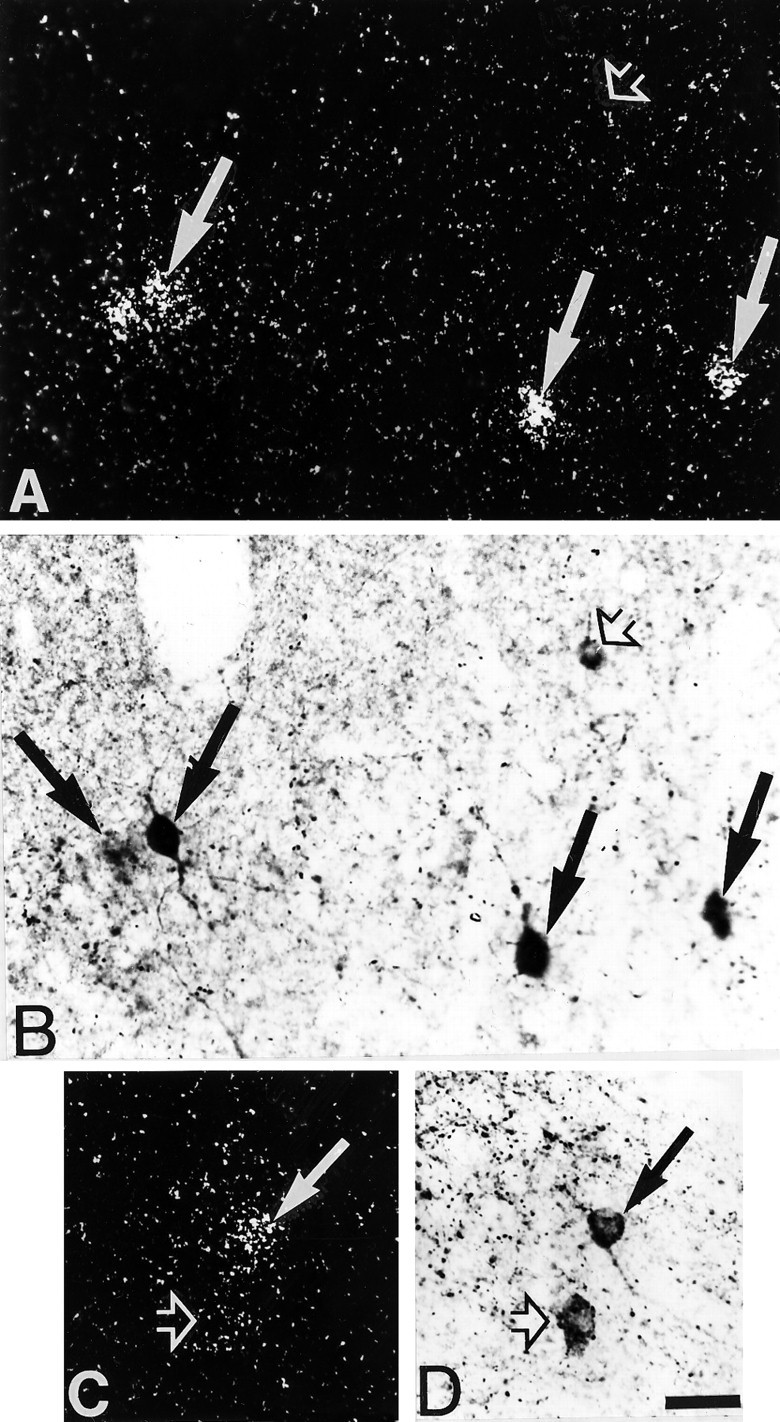
Simultaneous detection of 5-HT3R transcripts and CCK immunoreactivity in the temporal (A, B) and piriform (C, D) cortices. A, C, Observation of 5-HT3R-expressing cells under epiluminescence microscopy. B, D, Observation of CCK-immunoreactive neurons under bright-field microscopy. In these sections, several 5-HT3R-expressing cells showed CCK immunoreactivity (filled arrows), but not all CCK-immunoreactive neurons contained 5-HT3R transcripts (open arrows). Scale bar, 25 μm.
Within the neocortex, 5-HT3R/CCK-labeled cells were distributed mainly in layers II–III, although colocalization was also observed in cells of deeper layers. These double-labeled neurons were found frequently in the motor, prelimbic, and visual cortices (seen in Table 1). A semiquantitative analysis indicated that between 35 and 65% of the CCK-containing cells in layers II–III of the neocortex (n = 726 cells) express the 5-HT3R (Table 1). We frequently observed 5-HT3R/CCK-labeled cells in the anterior olfactory nucleus and piriform cortex, and a semiquantitative evaluation of these cells showed that 55–65% of the CCK-immunoreactive cells (n= 147 cells) contained 5-HT3R transcripts (Table 1). These results indicate that the percentage of 5-HT3R/CCK-labeled neurons is not homogenous within the different cortical areas.
Table 1.
Percentage of CCK-immunoreactive neurons expressing the 5-HT3R
| Region | Percentage of double-labled neurons1-a |
|---|---|
| Neocortex | |
| Motor cortex | 49 ± 0.47 (n = 119) |
| Agranular insular cortex | 35 ± 0.47 (n = 25) |
| Anterior cingulate cortex | 60 ± 0.47 (n = 62) |
| Auditory cortex | 36 ± 0.47 (n = 113) |
| Posterior parietal association cortex | 66 ± 0.47 (n = 55) |
| Prelimbic cortex | 55 ± 0.47 (n = 89) |
| Somatosensory cortex | 35 ± 0.47 (n = 70) |
| Visual cortex | 46 ± 0.47 (n = 193) |
| Olfactory system | |
| Anterior olfactory nucleus | 65 ± 0.47 (n = 45) |
| Piriform cortex | 53 ± 0.47 (n = 102) |
| Entorhinal cortex | 55 ± 0.47 (n = 75) |
Total number of CCK-immunopositive cells (n) was counted in three sections each from three different experiments, and the percentage of CCK-immunoreactive cells expressing the 5-HT3R was calculated.
5-HT3R/CCK-labeled cells were found scattered throughout the hippocampus in the stratum (st.) oriens, st. pyramidale, and st. radiatum of the CA1–CA3. Within the st. oriens, double-labeled cells were sometimes located near the alveus or close to the st. pyramidale. The 5-HT3R/CCK-labeled interneurons were located at the border of the granular cell layer in the dentate gyrus. Several nuclei of the amygdaloid complex also have 5-HT3R/CCK neurons, mainly the lateral, basolateral, basomedial, and cortical nuclei.
Colocalization of 5-HT3R and CCK
In another set of experiments, we used an anti-5-HT3R antibody (Morales et al., 1996a) for the simultaneous detection of 5-HT3R and CCK immunoreactivity. We found that some 5-HT3R-immunolabeled neurons were also positive for CCK immunostaining. The pattern of distribution of these double-immunostained cells parallels that observed for CCK-immunoreactive/5-HT3R mRNA-hybridized neurons in the neocortex and the hippocampal formation. Double-immunolabeled cells, however, were difficult to evaluate when the CCK immunoproduct appeared as sparse grains or dense aggregates in the cell bodies, limiting accurate quantitation of double-labeled cells.
Double 5-HT3R/CCK-immunoreactive neurons were scattered in all layers of the neocortex and occasionally were found in the white matter. Layers II–III contained the highest density of 5-HT3R/CCK-immunoreactive neurons, and immunoreactive CCK was observed in the perikarya and processes; however, the 5-HT3R immunoproduct was restricted mainly to the cell bodies.
Double 5-HT3R/CCK-immunolabeled perikarya were found in the st. oriens, st. pyramidale, st. radiatum, and st. lacunosum moleculare of CA1 (Figs. 4A,5A,B) and CA3 (Fig. 4B) subfields. The 5-HT3R/CCK-labeled interneurons contained CCK-immunopositive processes extending from the st. oriens to the st. pyramidale and from the st. radiatum to the st. lacunosum moleculare (Fig. 5A). In addition, double 5-HT3R/CCK cell bodies located in the st. pyramidale of the CA1 subfield showed CCK-labeled processes extending into the st. pyramidale (Fig. 5B). Double 5-HT3R/CCK-immunolabeled perikarya were observed immediately below the granule cell layer of the dentate layer, with CCK-immunopositive processes entering the granule cell layer (Fig.5C).
Fig. 4.
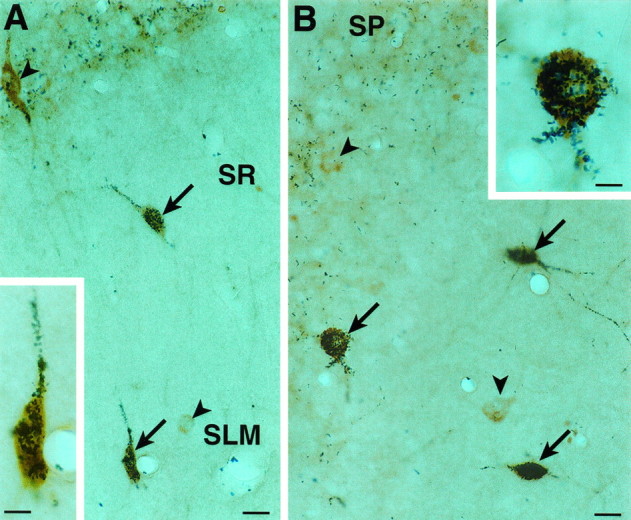
Simultaneous detection of 5-HT3R and CCK immunoreactivity in the CA1 (A) and CA3 (B) subfields of the hippocampus. Colocalization of 5-HT3R (brown) and CCK (granular blue-black) immunoproducts was found in several neurons (arrows), but not all 5-HT3R-immunoreactive cells contained CCK immunoreactivity (arrowheads). Insets in Aand B show double-labeled neurons at high magnification.SR, St. radiatum; SLM, st. lacunosum moleculare. Scale bar, 12.5 μm; for inset, 6 μm.
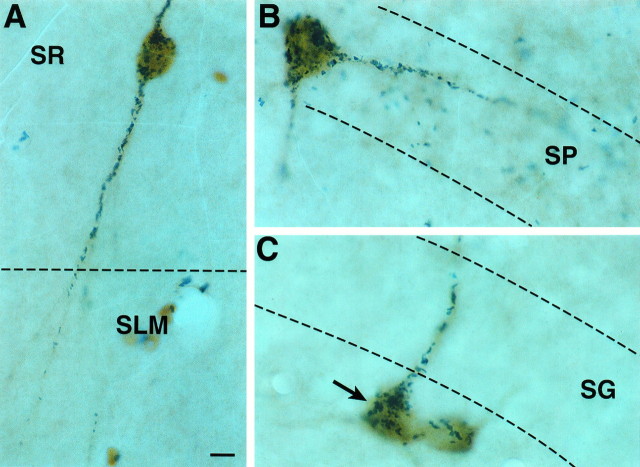
Fig. 5.
Simultaneous detection of 5-HT3R and CCK immunoreactivity in CA1 (A, B) and dentate gyrus (C). A, Note a 5-HT3R/CCK double-labeled cell in the SR extending into theSLM. B, A 5-HT3R/CCK double-labeled cell in the st. pyramidale (SP) extending into the cell layer. C, Two 5-HT3R/CCK double-labeled neurons immediately below the st. granulare (SG); one of them projects into the granule cell layer (arrow). SR, St. radiatum;SLM, st. lacunosum moleculare. Scale bar, 6 μm.
Colocalization of 5-HT3R transcripts and Ca2+-binding proteins
GABAergic cells are known to contain different Ca2+-binding proteins, PV, CB, and CR (Baimbridge et al., 1992, for review). Thus, we sought to determine whether any of these proteins were present in 5-HT3R-expressing neurons. Although no PV immunoreactivity was found in 5-HT3R-expressing neurons, both CB and CR did colocalize with 5-HT3R-expressing neurons.
5-HT3R/CB double-labeled neurons were found occasionally in the entorhinal cortex and basomedial nucleus of the amygdala. In contrast, this type of neuron was often observed in basket cells of the dentate gyrus and the different strata of the hippocampus, preferentially in the st. radiatum of the ventral portion of the CA1 and CA3 subfields (Figs. 6), and a semiquantitative analysis of double-labeled cells indicated that between 33 and 63% of the CB-immunoreactive cells (n = 267 cells) expressed the 5-HT3R in these regions.
Fig. 6.
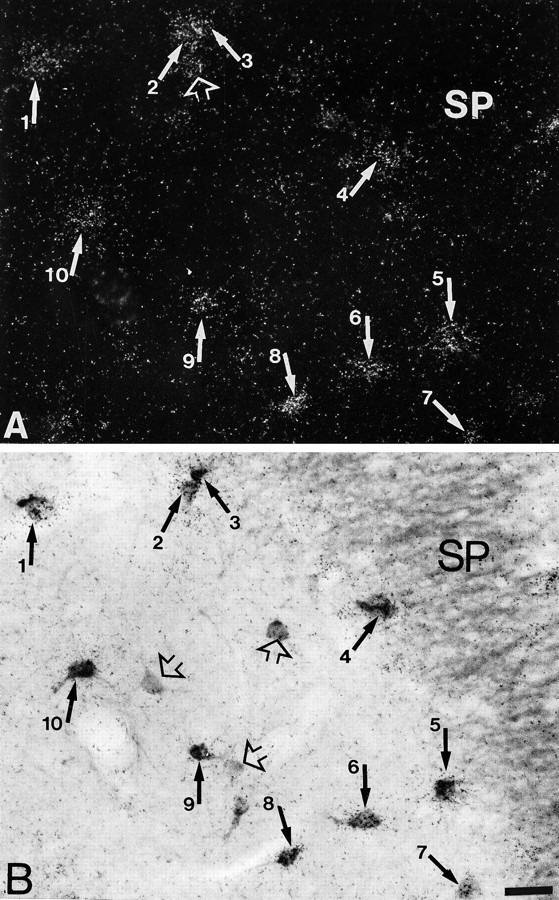
Simultaneous detection of 5-HT3R transcripts and CB immunoreactivity in CA3. A, Observation of 5-HT3R-expressing cells under epiluminescence microscopy. B, Observation of CB-immunoreactive neurons under bright-field microscopy. In this section, the majority of 5-HT3R-expressing cells showed CB immunoreactivity (filled arrows inA and B), but a few CB-reactive cells were single-labeled for the protein (open arrows inB). Note a 5-HT3R-expressing cell without CB immunoreactivity (open arrow in A). Scale bar, 22 μm.
5-HT3R/CR double-labeled neurons showed a wider distribution than those for CB. The 5-HT3R/CR-containing neurons were scattered throughout the forebrain: neocortex, olfactory tubercle, endopiriform nucleus, anterior olfactory nucleus, hippocampal formation, posteromedial cortical, and dorsolateral nuclei of the amygdala. 5-HT3R/CR double-labeled neurons, however, were concentrated preferentially in the motor (Figs.7A,B) agranular, perirhinal, insular, and piriform cortices. Within the hippocampal formation, these neurons were distributed mainly in the st. lacunosum moleculare of the subiculum (Fig. 8A,B) and CA1–CA3 areas.
Fig. 7.
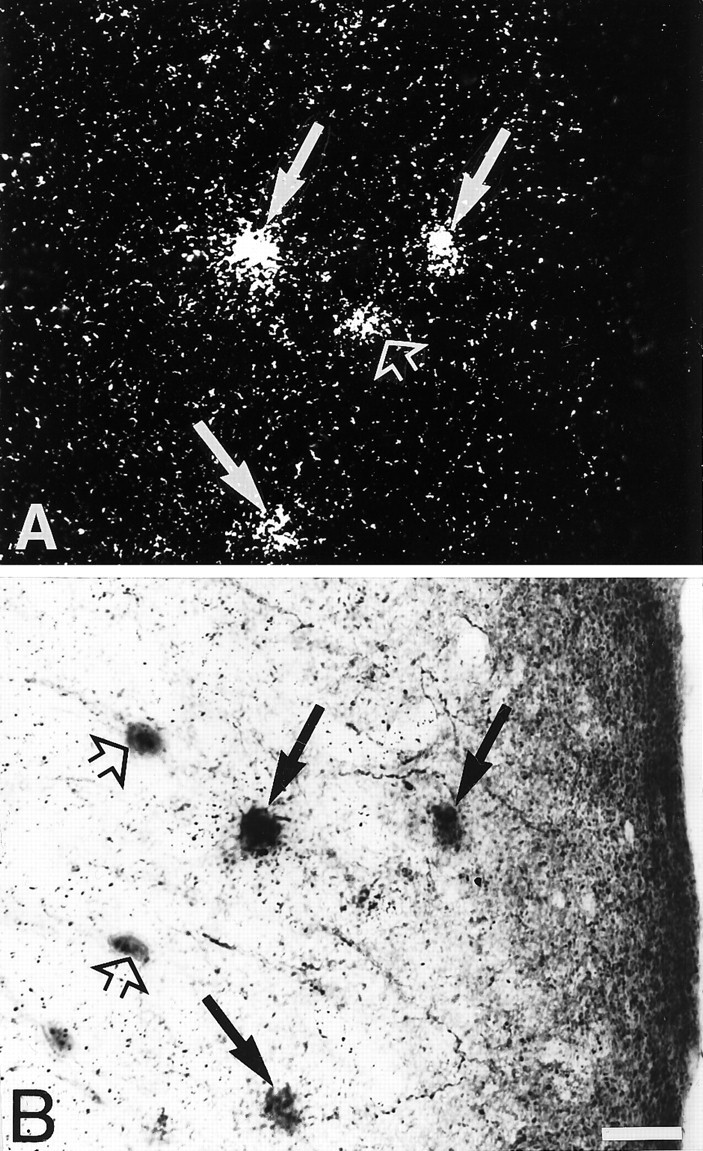
Simultaneous detection of 5-HT3R transcripts and CR immunoreactivity in the occipital cortex.A, Observation of 5-HT3R-expressing cells under epiluminescence microscopy. B, Observation of CR-immunoreactive neurons under bright field. In this section, several of the 5-HT3R-expressing cells showed CR immunoreactivity (filled arrows in A andB), and few were single-labeled (open arrows in A and B). Scale bar, 25 μm.
Fig. 8.
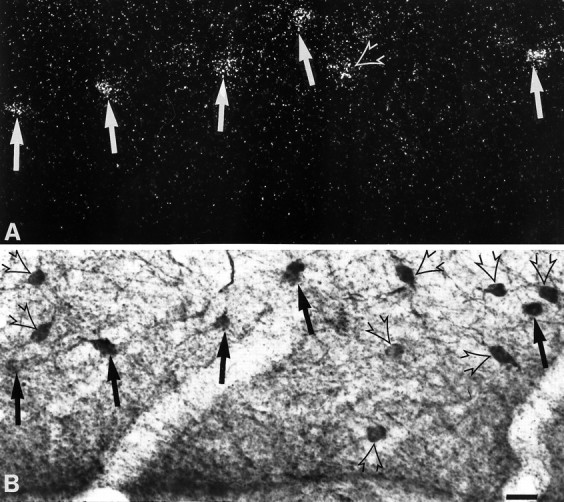
Simultaneous detection of 5-HT3R transcripts and CR immunoreactivity field in the st. lacunosum moleculare of the subiculum. A, Observation of 5-HT3R-expressing cells under epiluminescence microscopy.B, Observation of CR-immunoreactive neurons under bright field. In this section, the majority of 5-HT3R-expressing cells showed CR immunoreactivity (filled arrowsin A and B), and few were single-labeled (open arrows in A and B). Scale bar, 18 μm.
DISCUSSION
Characterization OF 5-HT3R/GABA-expressing cells
It has been demonstrated that cortical and hippocampal GABAergic interneurons are a heterogeneous population with regard to their morphology, biochemical composition, and synaptic connections (Wolff and Chronwall, 1982; Freund et al., 1983, 1990; Hendry et al., 1984;Somogyi et al., 1984; Kosaka et al., 1985; Celio, 1986; Sloviter and Nilaver, 1987; Katsumaru et al., 1988). Various biochemical properties of the GABAergic interneurons have been widely used to distinguish subpopulations of GABAergic neurons in cortex and hippocampus (Hendry et al., 1984; Somogyi et al., 1984; Kosaka et al., 1985; Celio, 1986;Sloviter and Nilaver, 1987; Katsumaru et al., 1988; Freund et al., 1990). Subpopulations of GABAergic neurons can be identified according to their calcium-binding protein (i.e., PV, CB, and CR) or neuropeptide content (i.e., SS and CCK). Our data indicate that the 5-HT3R/GABA-expressing neurons belong to a distinct group of GABAergic cells that lack SS and PV but may contain CCK, CB, and CR. These three subgroups of 5-HT3R/GABAergic interneurons have a distinct regional distribution, which is consistent with observations from cortical and hippocampal interneurons showing that PV- and CB-containing neurons form two different populations of GABAergic neurons that do not coexist with CCK (Hendry et al., 1984; Hendry and Jones, 1985; Gulyás et al., 1991). These data support the view that the 5-HT3R-containing neurons are composed of a biochemically heterogeneous subpopulation of neurons that may be involved in different inhibitory circuits.
Coexistence of 5-HT3R and CCK within the same cells
Previous studies have shown that the vast majority of cortical and hippocampal CCK-containing neurons are GABAergic in the rat brain (Hendry et al., 1984; Somogyi et al., 1984; Kosaka et al., 1985). We found that the 5-HT3R, which is expressed in some interneurons, is present in a subpopulation of CCK-immunoreactive neurons in the neocortex, olfactory system, and hippocampal formation.
The colocalization of 5-HT3R and CCK immunoreactivity within some interneurons implies functional interactions between serotonergic terminals and CCK-containing cells. In addition, the findings that 35–66% of the CCK-containing cells in layers II–III of the neocortex and 55–65% of neurons of the anterior olfactory nucleus and piriform cortex express the 5-HT3R suggest that CCK neurotransmission is highly regulated by serotonin in the rat telencephalon. The presence, however, of putative serotonergic contacts on CCK cells has not yet been reported. In contrast, some neurochemical studies indicate that 5-HT3R activation is capable of mediating the release of CCK. Paudice and Raiteri (1991) demonstrated that serotonin or 1-phenylbiguanide, a selective 5-HT3R agonist, enhances the depolarization-evoked release of CCK from synaptosomes prepared from rat cerebral cortex, and this effect was prevented by 5-HT3R antagonists. The 5-HT3R antagonists also prevent the veratrine-evoked release of CCK in frontal cortex of freely moving rats (Raiteri et al., 1993), suggesting that activation of 5-HT3R on CCK-releasing nerve endings might mediate the release of CCK. These pharmacological studies did not show which of the two possible sources of CCK in cortex, an intrinsic source from local CCK-containing cells or an extrinsic one from projections of the mesencephalon, were the source of released CCK. Our data on the presence of 5-HT3R/CCK-containing neurons in cortex and hippocampus suggest that activation of local 5-HT3R/CCK-containing cells might participate in the release of CCK in cortex and hippocampus.
The activity of hippocampal 5-HT3R/CCK-containing neurons is likely to be regulated by the raphe nucleus. In addition, another source of regulation might be provided by the septum, because it has been reported that hippocampal CCK-containing neurons are innervated by GABAergic septal fibers (Gulyás et al., 1990), thus making the 5-HT3R/CCK-containing neurons the target of subcortical pathways such as those originating in the septum and raphe nucleus.
We also detected 5-HT3R/CCK double-immunolabeled neurons extending into the pyramidal and granule layers of the hippocampus and dentate gyrus. These results suggest that some of the 5-HT3R/GABA/CCK-containing neurons might inhibit principal neurons in these regions. In agreement with this suggestion, it is known that CCK-positive boutons establish symmetrical synaptic contacts with perikarya and dendrites of pyramidal and nonpyramidal neurons in hippocampus (Harris et al., 1985; Hendry and Jones, 1985; Nunzi et al., 1985; Totterdell and Smith, 1986), and that CCK immunoreactivity is found in axonal terminals contacting perikarya of neurons in the cortex (Köhler and Chan-Palay, 1982). The activation of 5-HT3R in CCK-containing interneurons might result in the direct inhibition of principal neurons, thus affecting areas to which these principal neurons project (Fig. 9). This inhibition might occur in pyramidal neurons of the subiculum that project to the nucleus accumbens and are innervated by CCK-immunoreactive terminals (Totterdell and Smith, 1986).
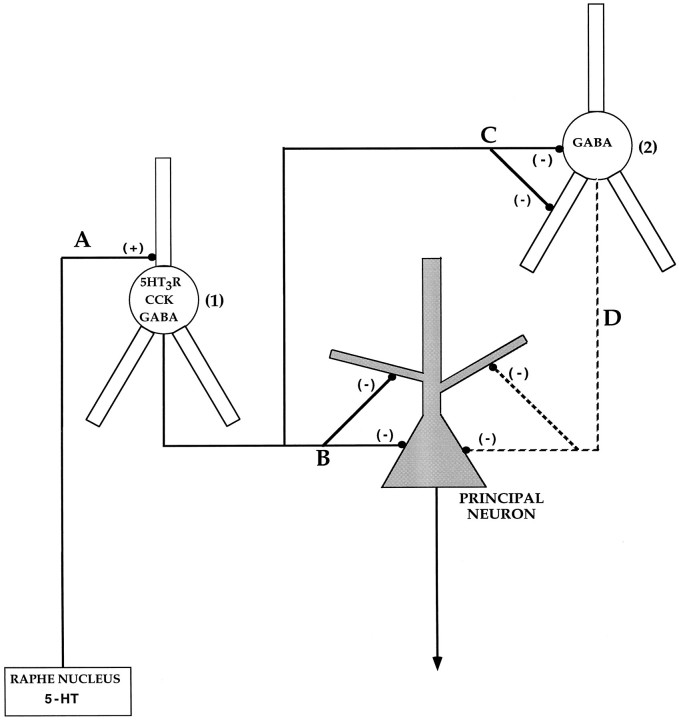
Fig. 9.
Hypothetical circuitry involving serotonergic activation of 5-HT3R on inhibitory interneurons. Serotonin-immunoreactive terminals make asymmetrical synapses on dendrites of hippocampal and cortical interneurons (Freund et al., 1990; Smiley and Goldman-Rakic, 1996); some of these interneurons containing 5-HT3R and CCK (1) might be activated by 5-HT, resulting in inactivation of other interneurons (2) and principal neurons. It is known that CCK-positive boutons establish symmetrical synaptic contacts with perikarya and dendrites of pyramidal and nonpyramidal neurons in hippocampus (Harris et al., 1985; Hendry and Jones, 1985; Nunzi et al., 1985; Totterdell and Smith, 1986), and that CCK immunoreactivity is found in axonal terminals innervating the perikarya of neurons in the cortex (Köhler and Chan-Palay, 1982). The activation of 5-HT3R in CCK-containing interneurons (1) might result in the direct inhibition of principal neurons, thus affecting areas to which these principal neurons project.
Coexistence of 5-HT3R with Ca2+-binding proteins
The characterization of 5-HT3R/GABAergic neurons on the basis of the content of their calcium-binding proteins suggests that these neurons lack PV but may contain CR and CB.
Some 5-HT3R/CR-containing cells were found in the neocortex, olfactory cortex, hippocampus, and amygdala, but these neurons were more often present in the agranular insular and piriform cortices. In contrast, the 5-HT3R/CB double-labeled neurons were found mainly in the CA1–CA3 subfields of the hippocampus. These results are consistent with previous observations showing that serotonergic median raphe axons selectively innervate the somata and dendritic trees of CB-containing GABAergic interneurons in the CA1 and CA3 subfields of the rat hippocampus, but never those that contain PV (Freund et al., 1990; Halasy et al., 1992; Hornung and Celio, 1992;Miettinen et al., 1992). Likewise, CR-immunoreactive neurons of the hippocampus also receive serotonergic synaptic contacts from the median raphe nuclei (Acsády et al., 1993). In addition, the hippocampal CB- and CR-immunoreactive neurons receive innervations from the medial septum (Freund and Antal, 1988; Freund et al., 1990; Gulyás et al., 1990; Acsády et al., 1993). The CB-containing interneurons are mainly basket and axo-axonic cells that make symmetrical synapses on the soma of the principal cells but also contact the axon initial segments, proximal dendrites, and dendritic spines of the pyramidal cells (Katsumaru et al., 1988; DeFelipe et al., 1989). The presence of CB and CR in 5-HT3R-expressing interneurons suggests that these interneurons might belong to those hippocampal CB- and CR-immunoreactive neurons shown previously to be innervated by subcortical inputs from the medial septal and median raphe nuclei. In addition, these 5-HT3R/CB and 5-HT3R/CR interneurons might modulate neuronal transmission of interneurons and principal neurons.
In the present study we demonstrated that the neuronal expression of the 5-HT3R is selective within the GABA neuron population in the rat telencephalon, extending preliminary observations showing that 5-HT3R transcripts coexist with GABA in the neocortex and hippocampus (Morales et al., 1996b). Despite the extensive colocalization of 5-HT3R transcripts and GABA in the same neurons throughout the telencephalon, the expression of the 5-HT3R is not restricted to GABAergic cells in the CNS; for instance, we have detected 5-HT3R mRNA in some dopaminergic neurons of the mesencephalon (M. Morales and F. E. Bloom, unpublished observations) and in motoneurons of the dorsal horn of the spinal cord (Morales et al., 1996a).
The widespread colocalization of 5-HT3R transcripts and GABA in the same neurons suggests the participation of 5-HT3R in the excitation of inhibitory neurons in several brain regions. Consistent with these observations, previous immunohistochemical studies have shown that serotonin-immunoreactive terminals make asymmetrical synapses on dendrites of hippocampal and cortical interneurons (Freund et al., 1990; Smiley and Goldman-Rakic, 1996). Furthermore, 5-HT directly excites GABAergic interneurons via 5-HT3R and consequently increases the frequency of inhibitory synaptic events recorded in CA1 pyramidal cells of rat hippocampal slices (Ropert and Guy, 1991). Although no similar electrophysiological findings have been reported in cortical areas, it is likely that some of the 5-HT3R/GABAergic cortical neurons will also inhibit cortical pyramidal neurons or interneurons. The enhancement of GABAergic inhibition by activation of 5-HT3R might be relevant for brain regulatory events, because 5-HT3R agonists will inhibit the induction of long-term potentiation (LTP) in CA1 and CA3 (Corradetti et al., 1992;Maeda et al., 1994) through the facilitation of GABAergic neurons (via GABAA receptors). In addition, intraperitoneal administration of the 5-HT3R antagonist ondansetron increased the frequency of the hippocampal theta rhythm, the induction of LTP in CA1, and the retention of olfactory and spatial memory in freely moving rats, suggesting that 5-HT3R blockage of GABAergic cells results in the disinhibition of pyramidal cells (Stäubli and Xu, 1995). These observations suggest that the 5-HT3R might participate in inhibitory and disinhibitory circuits in the rat telencephalon.
Relations to other 5-HT receptor localizations
The functional role played by the 5-HT3R we have localized to GABA-containing interneurons provides only one facet of what may be viewed as serotonergic synaptic functions in the cerebral cortex and hippocampal formation. Clearly, the 5-HT3R is only one of several 5-HT receptor subclasses whose cellular locations have already been assessed. In several recent studies on the cellular distribution of other 5-HT receptors, only the 5-HT2R (Molineaux et al., 1989; Morilak et al., 1993; Freund and Buzsaki, 1996) has been detected on interneurons in addition to the 5-HT3R; however, the 5-HT2R is also present in principal neurons (Morilak et al., 1993; Hamada et al., 1996). The other major subtypes of 5-HT receptors, such as the 5HT1AR (Gerard et al., 1994) and the 5HT6R (Miquel et al., 1996) have been attributed only to the pyramidal neurons of the hippocampus but were not detected in interneurons. Although such studies cannot define precise roles for the different 5-HT-transducing target neurons, the contrasting localization may provide the basis for developing hypotheses for future pharmacological analyses.
In conclusion, we demonstrated that the 5-HT3R-expressing neurons are mainly GABAergic in the rat telencephalon, suggesting that depolarization of these cells by serotonin might regulate inhibitory and disinhibitory circuits in the rat telencephalon. The 5-HT3R-expressing cells might contain CCK, CB, and CR. This biochemical heterogeneity may reflect the participation of these neurons in different inhibitory circuits. In addition, the high degree of coexistence between the 5-HT3R and CCK is indicative of the importance that the 5-HT3R has in regulating CCK neurotransmission in the neocortex.
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA 06420. We thank Drs. David Johnson and Steve Heinemann for the rat 5-HT3R-A cDNA clone, and Elena Battenberg for her role in early experiments.
Correspondence should be addressed to Marisela Morales, The Scripps Research Institute, Department of Neuropharmacology, SBR-1, 10550 North Torrey Pines Road, La Jolla, CA 92037.
REFERENCES
- 1.Acsády L, Halasy K, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus-III: their inputs from the median raphe and medial septal nuclei. Neuroscience. 1993;52:829–841. doi: 10.1016/0306-4522(93)90532-k. [DOI] [PubMed] [Google Scholar]
- 2.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 3.Barnes JM, Barnes NM, Costall B, Ironside JW, Naylor RJ. Identification and characterization of 5-hydroxytryptamine3 recognition sites in human brain tissue. J Neurochem. 1989a;53:1787–1793. doi: 10.1111/j.1471-4159.1989.tb09244.x. [DOI] [PubMed] [Google Scholar]
- 4.Barnes JM, Barnes NM, Costall B, Naylor RJ, Tyers MB. 5-HT3 receptors mediate inhibition of acetylcholine release in cortical tissue. Nature. 1989b;338:762–763. doi: 10.1038/338762a0. [DOI] [PubMed] [Google Scholar]
- 5.Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ. Characterization and autoradiographic localization of 5-HT3 receptor recognition sites identified with [3H]-(S)-zacopride in the forebrain of the rat. Neuropharmacology. 1990;29:1037–1045. doi: 10.1016/0028-3908(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 6.Blandina P, Goldfarb J, Craddock-Royal B, Green JP. Release of endogenous dopamine by stimulation of 5-hydroxytryptamine3 receptors in rat striatum. J Pharmacol Exp Ther. 1989;251:803–809. [PubMed] [Google Scholar]
- 7.Carboni E, Acquas E, Leone P, Di Chiara G. 5-HT3 receptor antagonists block morphine- and nicotine- but not amphetamine-induced reward. Psychopharmacology. 1989;97:175–178. doi: 10.1007/BF00442245. [DOI] [PubMed] [Google Scholar]
- 8.Celio MR. Parvalbumin in most γ-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231:995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, van Praag HM, Gardner EL. Activation of 5-HT3 receptor by 1-phenylbiguanide increases dopamine release in the rat nucleus accumbens. Brain Res. 1991;543:354–357. doi: 10.1016/0006-8993(91)90050-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Paredes W, van Praag HM, Lowinson JH, Gardner EL. Presynaptic dopamine release is enhanced by 5-HT3 receptor activation in medial prefrontal cortex of freely moving rats. Synapse. 1992;10:264–266. doi: 10.1002/syn.890100308. [DOI] [PubMed] [Google Scholar]
- 11.Corradetti R, Ballerini L, Pugliese AM, Pepeu G. Serotonin blocks the long-term potentiation induced by primed burst stimulation in the CA1 region of rat hippocampal slices. Neuroscience. 1992;46:511–518. doi: 10.1016/0306-4522(92)90140-w. [DOI] [PubMed] [Google Scholar]
- 12.Costall B, Naylor RJ, Tyers MB. The psychopharmacology of 5-HT3 receptors. J Pharmacol Ther. 1990;47:181–202. doi: 10.1016/0163-7258(90)90086-h. [DOI] [PubMed] [Google Scholar]
- 13.Costall B, Domeney AM, Kelly ME, Tomkins DM, Naylor RJ, Wong EH, Smith WL, Whiting RL, Eglen RM. The effect of the 5-HT3 receptor antagonist, RS-42358–197, in animal models of anxiety. Eur J Pharmacol. 1993;234:91–99. doi: 10.1016/0014-2999(93)90710-y. [DOI] [PubMed] [Google Scholar]
- 14.DeFelipe J, Hendry SHC, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989;503:49–54. doi: 10.1016/0006-8993(89)91702-2. [DOI] [PubMed] [Google Scholar]
- 15.Derkach V, Suprenant A, Northand RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;33:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- 16.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 17.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Freund TF, Martin KAC, Smith AD, Somogyi P. Glutamate decarboxylase-immunoreactive terminals of Golgi-impregnated axo-axonic cells and of presumed basket cells in synaptic contact with pyramidal cells of the cat’s visual cortex. J Comp Neurol. 1983;221:263–278. doi: 10.1002/cne.902210303. [DOI] [PubMed] [Google Scholar]
- 19.Freund TF, Gulyás AI, Acsády L, Görcs T, Tóth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proc Natl Acad Sci USA. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehlert DR, Gackenheimer SL, Wong DT, Robertson DW. Localization of 5-HT3 receptors in the rat brain using [3H]LY278584. Brain Res. 1991;553:149–154. doi: 10.1016/0006-8993(91)90242-n. [DOI] [PubMed] [Google Scholar]
- 21.Gerard C, Langlois X, Gingrich J, Doucet E, Verge D, Kia HK, Raisman R, Gozlan H, Mestikawy SEL, Hamon M. Production and characterization of polyclonal antibodies recognizing the intracytoplasmic third loop of the 5 hydroxytryptamine 1A receptor. Neuroscience. 1994;62:721–739. doi: 10.1016/0306-4522(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 22.Gulyás AI, Görcs TJ, Freund TF. Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents. Neuroscience. 1990;37:31–44. doi: 10.1016/0306-4522(90)90189-b. [DOI] [PubMed] [Google Scholar]
- 23.Gulyás AI, Tóth K, Dános P, Freund TF. Subpopulation of GABAergic neurons containing parvalbumin, calbindin D28k, and cholecystokinin in the rat hippocampus. J Comp Neurol. 1991;312:371–378. doi: 10.1002/cne.903120305. [DOI] [PubMed] [Google Scholar]
- 24.Halasy K, Miettinen R, Szabat E, Freund TF. GABAergic interneurons are the major postsynaptic targets of median raphe afferents in the rat dentate gyrus. Eur J Neurosci. 1992;4:144–153. doi: 10.1111/j.1460-9568.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamada S, Senzaki K, Tabuchi K, Yamamoto H, Yamamoto T, Yoshikawa S, Okano H, Okado N. Localization of 5-HT2A receptor in rat cerebral cortex by immunohistochemistry. Soc Neurosci Abstr. 1996;22:1774. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- 26.Harris KM, Marshall PE, Landis DMD. Ultrastructural study of cholecystokinin-immunoreactive cells and processes in area CA1 of the rat hippocampus. J Comp Neurol. 1985;233:147–158. doi: 10.1002/cne.902330202. [DOI] [PubMed] [Google Scholar]
- 27.Hendry SHC, Jones EG. Morphology of synapses formed by cholecystokinin-immunoreactive axon terminals in regio superior of rat hippocampus. Neuroscience. 1985;16:57–68. doi: 10.1016/0306-4522(85)90047-8. [DOI] [PubMed] [Google Scholar]
- 28.Hendry SHC, Jones EG, DeFelipe J, Schmechel D, Brandon C, Emson PC. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc Natl Acad Sci USA. 1984;81:6526–6530. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornung JP, Celio MR. The selective innervation by serotonergic axons of calbindin-containing interneurons in the neocortex and hippocampus of the marmoset. J Comp Neurol. 1992;320:457–467. doi: 10.1002/cne.903200404. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 31.Jones DN, Barnes NM, Costall B, Domeney AM, Kilpatrick GJ, Naylor RJ, Tyers MB. The distribution of 5-HT3 recognition sites in the marmoset brain. Eur J Pharmacol. 1992;215:63–67. doi: 10.1016/0014-2999(92)90609-8. [DOI] [PubMed] [Google Scholar]
- 32.Katsumaru H, Kosaka T, Heizmann CW, Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988;72:347–362. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- 34.Kilpatrick GJ, Jones BJ, Tyers MB. The distribution of specific binding of the 5-HT3 receptor ligand [3H]GR65630 in rat brain using quantitative autoradiography. Neurosci Lett. 1988;94:156–160. doi: 10.1016/0304-3940(88)90287-x. [DOI] [PubMed] [Google Scholar]
- 35.Kilpatrick GJ, Jones BJ, Tyers MB. Binding of the 5-HT3 ligand, [3H]GR65630, to rat area postrema, vagus nerve and the brains of several species. Eur J Pharmacol. 1989;159:157–164. doi: 10.1016/0014-2999(89)90700-0. [DOI] [PubMed] [Google Scholar]
- 36.Köhler C, Chan-Palay V. The distribution of cholecystokinin-like immunoreactive neurons and nerve terminals in the retrohippocampal region in the rat and guinea-pig. J Comp Neurol. 1982;210:136–146. doi: 10.1002/cne.902100204. [DOI] [PubMed] [Google Scholar]
- 37.Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yanaihara N, Wu J-Y, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J Comp Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- 38.Laporte AM, Koscielniak T, Ponchant M, Verge D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I]iodo-zacopride and [3H]zacopride as radioligands. Synapse. 1992;10:271–281. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- 39.Levey AI, Bolam JP, Rye DB, Hallenger AE, Demuth RM, Mesulum M-M, Wainer BH. A light and electron microscopic procedure for sequential double antigen localization using diamine benzidine and benzidine dihydrochloride. J Histochem Cytochem. 1986;34:1449–1457. doi: 10.1177/34.11.2430010. [DOI] [PubMed] [Google Scholar]
- 40.Maeda T, Kaneko S, Satoh M. Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slices. Neurosci Res. 1994;18:277–282. doi: 10.1016/0168-0102(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 41.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 42.Maura G, Andrioli GC, Cavazzani P, Raiteri M. 5-Hydroxytryptamine3 receptors sited on cholinergic axon terminals of human cerebral cortex mediate inhibition of acetylcholine release. J Neurochem. 1992;58:2334–2336. doi: 10.1111/j.1471-4159.1992.tb10983.x. [DOI] [PubMed] [Google Scholar]
- 43.Miettinen R, Gulyás AI, Baimbridge KG, Jacobowitz DM, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus-II: co-existence with other calcium binding proteins and GABA. Neuroscience. 1992;48:29–43. doi: 10.1016/0306-4522(92)90335-y. [DOI] [PubMed] [Google Scholar]
- 44.Miquel MC, Lefevre K, Sari Y, Brisorgueil MJ, Calas A, Gerard C, Hamon M, Verge D. Immunocytochemical visualization of 5HT6 receptors in the rat brain. Soc Neurosci Abstr. 1996;22:1782. [Google Scholar]
- 45.Molineaux SM, Jessell TM, Axel R, Julius D. 5HT1C receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Mol Brain Res. 1996a;36:251–260. doi: 10.1016/0169-328x(96)88406-3. [DOI] [PubMed] [Google Scholar]
- 47.Morales M, Battenberg E, de Lecea L, Bloom FE. The type 3 serotonin receptor is expressed in a subpopulation of GABAergic neurons in the rat neocortex and hippocampus. Brain Res. 1996b;731:199–202. doi: 10.1016/0006-8993(96)00557-4. [DOI] [PubMed] [Google Scholar]
- 48.Morilak DA, Garlow SJ, Ciaranello RD. Immunocytochemical localization and description of neurons expressing serotonin 2 receptors in the rat brain. Neuroscience. 1993;54:701–717. doi: 10.1016/0306-4522(93)90241-7. [DOI] [PubMed] [Google Scholar]
- 49.Morrison JH, Benoit R, Magistretti PJ, Bloom FE. Immunohistochemical distribution of pro-somatostatin-related peptides in cerebral cortex. Brain Res. 1983;262:344–351. doi: 10.1016/0006-8993(83)91031-4. [DOI] [PubMed] [Google Scholar]
- 50.Nevins ME, Anthony EW. Antagonists at the serotonin-3 receptor can reduce the fear-potentiated startle response in the rat: evidence for different types of anxiolytic activity? J Pharmacol Exp Ther. 1994;268:248–254. [PubMed] [Google Scholar]
- 51.Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and nonpyramidal neurons in the hippocampus. J Comp Neurol. 1985;237:485–505. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- 52.Paudice P, Raiteri M. Cholecystokinin release mediated by 5-HT3 receptors in rat cerebral cortex and nucleus accumbens. Br J Pharmacol. 1991;103:1790–1794. doi: 10.1111/j.1476-5381.1991.tb09864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters JA, Lambert JJ. Electrophysiology of 5-HT3 receptors in neuronal cell lines. Trends Pharmacol Sci. 1989;10:172–174. doi: 10.1016/0165-6147(89)90230-7. [DOI] [PubMed] [Google Scholar]
- 54.Pratt GD, Bowery NG, Kilpatrick GJ, Leslie RA, Barnes NM, Naylor RJ, Jones BJ, Nelson DR, Palacios JM, Slater P, Reynolds DJM. Consensus meeting agrees distribution of 5-HT3 receptors in mammalian hindbrain. Trends Pharmacol Sci. 1990;11:135–137. doi: 10.1016/0165-6147(90)90058-g. [DOI] [PubMed] [Google Scholar]
- 55.Raiteri M, Paudice P, Vallebuona F. Inhibition by 5-HT3 receptor antagonists of release of cholecystokinin-like immunoreactivity from the frontal cortex of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:111–114. doi: 10.1007/BF00168781. [DOI] [PubMed] [Google Scholar]
- 56.Ropert N, Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol (Lond) 1991;441:121–136. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sloviter R, Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentate and hippocampus of the rat. J Comp Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- 58.Smiley JF, Goldman-Rakic PS. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 1996;367:431–443. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 59.Somogyi P, Hodgson AJ, Smith AD, Nunzi MG, Gorio A, Wu J-Y. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984;10:2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stäubli U, Xu FB. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Totterdell S, Smith AD. Cholecystokinin-immunoreactive boutons in synaptic contact with hippocampal pyramidal neurons that project to the nucleus accumbens. Neuroscience. 1986;19:181–192. doi: 10.1016/0306-4522(86)90014-x. [DOI] [PubMed] [Google Scholar]
- 63.Waeber C, Dixon K, Hoyer D, Palacios JM. Localization by autoradiography of neuronal 5-HT3 receptors in the mouse CNS. Eur J Pharmacol. 1988;151:351–352. doi: 10.1016/0014-2999(88)90825-4. [DOI] [PubMed] [Google Scholar]
- 64.Waeber C, Hoyer D, Palacios JM. 5-hydroxytryptamine3 receptors in the human brain: autoradiographic visualization using [3H]ICS 205–930. Neuroscience. 1989;31:393–400. doi: 10.1016/0306-4522(89)90382-5. [DOI] [PubMed] [Google Scholar]
- 65.Waeber C, Pinkus LM, Palacios JM. The (S)-isomer of [3H]zacopride labels 5-HT3 receptors with high affinity in rat brain. Eur J Pharmacol. 1990;181:283–287. doi: 10.1016/0014-2999(90)90090-s. [DOI] [PubMed] [Google Scholar]
- 66.Wolff JR, Chronwall BM. Axosomatic synapses in the visual cortex of adult rat: a comparison between GABA-accumulating and other neurons. J Neurocytol. 1982;11:409–425. doi: 10.1007/BF01257986. [DOI] [PubMed] [Google Scholar]