Abstract
This study was designed to validate an in vivomeasurement of the functional sensitivity of basal ganglia neuronal circuits containing dopamine D2 receptors. We hypothesized that a D2 agonist would decrease striatopallidal neuronal activity, and hence regional cerebral blood flow (rCBF) over the axon terminals in the globus pallidus. Quantitative pallidal blood flow was measured using positron emission tomography (PET) with bolus injections of H215O and arterial sampling in six baboons before and after intravenous administration of the selective D2 agonist U91356a. We also tested whether the response to U91356a was modified by previous acute administration of various antagonists. Another baboon had serial measurements of blood flow under identical conditions, but received no dopaminergic drugs. In all animals that received U91356a, pallidal flow decreased in a dose-related manner. Global CBF had a similar response, but the decline in pallidal flow was greater in magnitude and remained significant after accounting for the global effect. A D2 antagonist, but not antagonists of D1, serotonin-2, or peripheral D2 receptors, prevented this decrease. This work demonstrates and validates an in vivo measure of the sensitivity of D2-mediated basal ganglia pathways. It also supports the hypothesis that activation of the indirect striatopallidal pathway, previously demonstrated using nonselective D2-like agonists, can be mediated specifically by D2 receptors. We speculate that the U91356a-PET technique may prove useful in detecting functional abnormalities of D2-mediated dopaminergic function in diseases such as parkinsonism, dystonia, Tourette syndrome, or schizophrenia.
Keywords: globus pallidus, dopamine D2 receptor, positron emission tomography, pharmacological activation, baboon, U91356a
Functional abnormalities of dopaminergic pathways are implicated in a number of syndromes, including parkinsonism, dystonia, chorea, tics, psychosis, and drug addiction (Jankovic and Tolosa, 1993; Knable et al., 1995; Nestler, 1995). However, the pathophysiology and pharmacology of these syndromes remain primarily unknown.
Longitudinal or human studies require an in vivo technique for studying these pathways. An in vivo method also may prove more relevant than in vitro techniques (Perlmutter and Raichle, 1986; Bloch and LeMoine, 1994; Gerfen and Keefe, 1994).
One approach to assess changes in neuronal function is to measure the effects of dopamimetics on regional cerebral metabolism or blood flow (rCBF). Ex vivo autoradiography gives precise anatomic detail (von Essen et al., 1980; Jauzac et al., 1982; McCulloch, 1982;McCulloch et al., 1982a,b,c; Palacios and Wiederhold, 1984, 1985; Pizzolato et al., 1984, 1985a,b, 1987; Trugman and Wooten, 1986, 1987; Mori et al., 1989; Engber et al., 1990; Russo et al., 1991;Stein and Fuller, 1992; Tarazi et al., 1993), whereas PET and other imaging techniques allow in vivo studies using stimulants (Mathew and Wilson, 1989; Daniel et al., 1991; Pearlson et al., 1993),lDOPA (Rougemont et al., 1984; Henriksen and Boas, 1985;Leenders et al., 1985; Perlmutter and Raichle, 1985; Melamed et al., 1986; Montastruc et al., 1987; Rotrosen 1987; Kobari et al., 1992,1995), nonspecific dopamine agonists (Cleghorn et al., 1991; Sabatini et al., 1991; Grasby et al., 1993; Rascol et al., 1993; Kapur et al., 1994), and nonselective D2-like agonists (Celsis et al., 1988; Perlmutter, 1995). D2-like antagonists, although less relevant to this study, also have been studied extensively (Holcomb et al., 1996).
The main rCBF response to the nonselective D2-like agonist quinpirole was a decrease in globus pallidus (GP) (Perlmutter et al., 1993). Because neuronal activity is highly correlated with metabolism and rCBF at axonal termini (Raichle, 1987; Jueptner and Weiller, 1995), we interpret this as reflecting decreased activity of striatopallidal neurons mediated by D2-like receptors (Pizzolato et al., 1985c; Strange, 1990; Harrison et al., 1992; Robertson et al., 1992;Levey et al., 1993; Young and Penney, 1993; Gerfen et al., 1995). This fits other data that D1-like receptors activate inhibitory striatal neurons that project to internal globus pallidus (GPi) or substantia nigra pars reticulata (SNr), and D2-like receptors decrease GPi/SNr activity indirectly via decreased striatal inhibition of the external globus pallidus (GPe) (Gerfen et al., 1990;Levey et al., 1993). The two pathways have important behavioral correlates (Young and Penney, 1993) and complex interactions (Bédard et al., 1992; Jackson and Westlind-Danielsson, 1994;Gerfen et al., 1995).
The discovery of numerous dopamine receptor subtypes and selective drugs (Jackson and Westlind-Danielsson, 1994) makes possible more precise characterization of pharmacological responses. Because D2 receptors are the most numerous D2-like receptor in striatum, we hypothesized that rCBF in the GP would decline after systemic administration of a specific D2 agonist, as it had after quinpirole, and that the dose–response would be biologically meaningful and pharmacologically specific.
MATERIALS AND METHODS
Subjects. These studies conformed to the Society for Neuroscience’s Policy on the Use of Animals in Neuroscience Research, including previous approval by the Washington University Animal Studies Committee. Subjects were five normal baboons (three male, two female; ages 3–10 years; 10.5–25.9 kg), and two male baboons (ages 4–8 years; 14.1–28.5 kg) that had had previous unilateral intracarotid administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), in one case resulting in marked hemiparkinsonism and in the other case producing only mild rigidity of the contralateral upper extremity. The normal baboons were used to establish the presence of the hypothesized rCBF response. To avoid exposing the normal baboons to dopamine antagonists, the untreated control hemispheres of the MPTP-treated baboons were used to test the pharmacological specificity of the hypothesized response (except for one experiment using domperidone pretreatment in a normal animal).
PET scan conditions. Subjects were given intramuscular atropine (in 4 cases; 0.2 mg, median 13 μg/kg) or glycopyrrolate (in 10 cases; 0.07–0.10 mg, median 0.08 mg or 5 μg/kg) to control pharyngeal secretions, and intramuscular ketamine (225–500 mg, median 250 mg or 17.2 mg/kg) for sedation. We placed a 20 gauge catheter into a limb vein for drug and radiopharmaceutical administration and a soft-cuffed endotracheal tube to permit ventilation with 70% nitrous oxide and 30% oxygen to maintain sedation throughout the study. The animal was paralyzed with gallamine or pancuronium to control pCO2 throughout the studies. The choice of chemical sedation is complex, but our experience and review of the literature suggests that N2O has less effects on rCBF responses than other general anesthetics and provides adequate sedation for the neuromuscular blockade. A similar anesthetic technique has been used for comparable studies (McCulloch and Teasdale, 1979; Ingvar et al., 1983; Akeson et al., 1993).
Arterial access was established with a 20 gauge catheter placed in the femoral artery by direct puncture after local anesthesia with 1% lidocaine. Arterial samples were obtained at intervals throughout the study, and pO2 and pCO2 were measured using a pH/blood gas analyzer and a CO-oximeter (System 1306 and IL-482, Instrumentation Laboratory, Lexington, MA). Arterial blood pressure, pulse, ventilatory rate, and temperature were also monitored continually throughout the study.
All PET scans were performed at least 3 hr after the ketamine and atropine or glycopyrrolate administration, to allow the acute effects of these agents on rCBF to dissipate. A 3 hr interval was chosen for the following reasons. On the one hand, NMDA antagonists clearly affect D2-like neuronal function 5–15 min after administration (Engber et al., 1993), ketamine affects accumulation of a D2-5HT2 ligand measured 30–80 min after intramuscular injection (Onoe et al., 1994), and ketamine alters regional cerebral metabolism and blood flow for 5–90 min (Hougaard et al., 1974; Nelson et al., 1980; Crosby et al., 1982; Oguchi et al., 1982; Hammer and Herkenham, 1983; Dhasmana et al., 1984; Cavazzuti et al., 1987; Lahti et al., 1995). On the other hand, some studies have detected no effects of ketamine on dopamine metabolism (Bacopoulos et al., 1979; Koshikawa et al., 1988). Ketamine-specific binding in the brain is virtually gone 30 min after intravenous administration (Björkman et al., 1992), and changes in CBF attributable to intravenous ketamine are gone at 90 min (the metabolite norketamine has negligible effects on global CBF) (Akeson et al., 1993; Hartvig et al., 1995; Lahti et al., 1996). The peak clinical effect of intramuscular ketamine is at 20 min, compared with 1 min after intravenous ketamine (Reves and Glass, 1990). To test the duration of ketamine effects using our protocol, we had previously measured CBF repeatedly in baboons given intramuscular ketamine and then kept sedated with N2O for up to 6 hr without other interventions. Ketamine caused an initial rapid decline in global CBF, which then gradually increased ∼25% during the first 1–2 hr followed by either a stable baseline or a slight further increase in CBF over the next 2 hr. The only notable regional effect on CBF was in the cortex (J. S. Perlmutter, unpublished observations).
After the 3 hr had elapsed, several baseline measurements of rCBF were obtained (described below). In some experiments, an antagonist was given intravenously at this point and several rCBF scans were repeated (described below). Then at approximately hourly intervals, we gave various doses of U91356a intravenously over 2–5 min, while continuously monitoring pulse and blood pressure. After each dose, several rCBF PET scans were obtained at 15 min intervals. Each successive dose of U91356a was ∼10-fold higher than the last. The number of doses used in a given study varied from one to three. PET scans were performed in a quiet room, with lights on to facilitate observation of the animal. The animal’s eyes were closed and covered to prevent drying. The data reported represent 179 PET rCBF scans.
PET methods. All PET images were acquired on a Siemens/CTI 953B scanner [Erlangen, Germany (Mazoyer et al., 1991; Spinks et al., 1993)] using the two-dimensional wobble sampling mode yielding 31 slices with a center-to-center spacing of 3.375 mm and a reconstructed resolution of 5 mm (full-width, half-maximum). CBF images were acquired using a 40 sec acquisition beginning with the arrival of isotope in the head, after a bolus injection of 30–50 mCi of H215O. Scans were reconstructed using a ramp filter and a measured attenuation factors for each subject. Continuous arterial sampling during each scan with an automated blood sampler and scintillation counter, calibrated daily against a well counter, allowed quantitative determination of rCBF. This method has been validated in our laboratory using baboons (Raichle et al., 1983; Videen et al., 1987).
Pharmacological agents. U91356a is a specific dopamine D2-receptor agonist. Its affinity (Ki) for various receptors has been reported as dopamine D1, >1000 nm; D2, 1.3 nm; D3, 32 nm; D4, 195 nm; and serotonin 5HT1A, 58 nm; with no submicromolar affinities for any other adrenergic, cholinergic, or serotonergic receptors tested (Piercey et al., 1995). U91356a was effective in treating MPTP-induced parkinsonism, but consistent with the in vitro specificity data, it did not affect striatal D1-like receptor density, as l-DOPA did (Martel et al., 1993). Some evidence suggests that 10–30 μg−1 · kg−1 · d might be a clinically relevant subcutaneous dose for treatment of MPTP-induced parkinsonism in monkeys (M. F. Piercey et al., unpublished observations). Additionally, the ED50 in rats for U91356a-induced inhibition of substantia nigra pars compacta neuronal firing is 32 μg/kg (Piercey et al., 1995). This evidence guided selection of the doses used in this study, which were grouped as low (1–2 μg/kg), medium (10–22 μg/kg), and high (100–220 μg/kg) doses.
Antagonists included SCH23390 (a dopamine D1 antagonist), 1 mg/kg; eticlopride (a dopamine D2 antagonist), 4 mg/kg; ketanserin (serotonin 5HT2 antagonist), 0.6 mg/kg; and domperidone (a dopamine D2 antagonist that penetrates the blood-brain barrier relatively poorly), 0.1 mg/kg.
Identification of anatomic locations in PET images. The head of the sedated animal was fixed in position with a headholder system that bolted to a acrylic “cap” surgically implanted in the skull and also to the PET scanning table (Perlmutter et al., 1991a). This ensured no movement of the head throughout the scan (Black et al., 1996a).
Anatomic regions were identified on a magnetic resonance image (MRI) of each animal’s brain, acquired on a different day from the PET studies, and then transferred to the PET image using an automated routine. The steps used in this process were as follows.
Three-dimensional MPRAGE images were acquired sagittally with a 1.5T Siemens Magnetom scanner using the same headholder as for PET studies. The magnet was shimmed before each study. A three-dimensional acquisition (TR = 9.7 msec, TE = 4 msec, and flip angle = 12°) used a slab thickness of 200 mm with 160 partitions and a 200 mm square field of view in a 256 × 256 matrix, giving 0.78 × 0.78 × 1.25 mm voxels. For convenience, a single interpolation step then resampled the MR images to 0.5 mm cubic voxels and rotated the brain to the same orientation as that of the Davis and Huffman (1968)stereotactic atlas of baboon brain, under visual guidance. A histogram method allowed uniform scaling of signal intensity. Specifically, the mean signal from a volume of interest (VOI) placed in air in the original image was mapped to 0 in the new image, the mean signal plus two standard deviations from pixels in a brain VOI centered on the splenium of the corpus callosum was mapped to 255, and pixels of intermediate signal intensity were linearly scaled between 0 and 255.
Anatomic locations in the MRI were identified as follows. The center of the anterior commissure (AC) and the superior border of the posterior commissure (PC) defined a transverse reference plane in the orientation of the Davis and Huffman atlas (1968). For a given atlas point, corresponding points in each animal’s MRI were computed by linear scaling in three orthogonal dimensions. The scaling in each dimension was determined by the distance between the AC and the PC (anterior-posterior, y), and two distances measured on the coronal plane passing through the AC: namely, the interputaminal distance (left-right, x), and the average vertical distance between the superior border of the caudate nuclei and the inferior border of the optic tracts (superior-inferior, z). All anatomical landmarks were chosen by a single observer (K.J.B.), blind to PET data, after review with a neuroradiologist with extensive experience in primate neuroanatomy (M.H.G.).
The atlas “center” of each GP was taken as (x,y, z) = (±10.5, A17.0, +6.6). A midline point in the cerebellum (0.0, P4.5, +1.0) was also chosen. The cerebellum has negligible D2 receptor density (Palacios and Pazos, 1987;Levey et al., 1993), but unfortunately remains susceptible to indirect dopaminergic effects on metabolism and blood flow (Jauzac et al., 1982;McCulloch et al., 1982c; Ingvar et al., 1983; Azuma et al., 1988;Celsis et al., 1988).
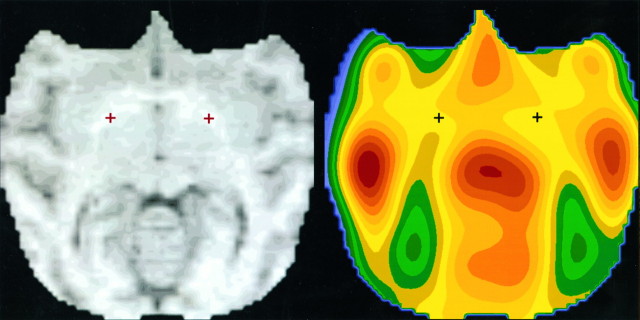
These three points on each animal’s MRI were transferred to the corresponding PET images using the Automated Image Registration (AIR) software of Woods et al. (1993) (Fig. 1). We have previously validated this method in baboons by reference to external fiducials attached to the headholder system and visible in both modalities; the maximum residual error at the GP after AIR alignment was 2.4 mm (mean error, 2.0 mm) (Black et al., 1996a).
Fig. 1.
The Davis and Huffman (1968) atlas points used for GP, as transferred to a representative MRI and matching PET image. Each PET image was sampled using a ball-shaped volume of interest with radius 5 mm, centered on the points indicated. (See Materials and Methods).
Sampling of PET images. Ball-shaped VOIs 10 mm in diameter (67 PET voxels, or 0.86 cm3) were centered on the left and right globus pallidus and midline cerebellum as described above, and the average rCBF within each of these VOIs was computed for each rCBF scan. Global CBF for each scan was computed as the average CBF within brain over the middle 5 axial PET planes showing the brain. “Within brain” was defined by a 40% intensity threshold on a baseline rCBF image after filtering to a resolution of 11 mm using a three-dimensional Gaussian filter.
Data analysis: dose–response studies. To minimize statistical noise, the rCBF from left and right GP were averaged before further analysis. The results were analyzed using an ANCOVA with GP rCBF as the dependent variable. This variable was hypothesized to depend primarily on three independent variables: dose, animal, and global flow. Specifically, similar doses were lumped together to minimize the number of dose levels, as described above. A categorical animal variable, each subject being a different “level,” was included to account for differences in rCBF between animals (an approach advocated by McCulloch et al., 1982a). Global blood flow was included as a covariate to test whether the effect in the GP was simply a reflection of global shifts in CBF. No other main or interaction effects were added because this model explained >99% of the variance in observed GP rCBF (model,R2 = 0.997; F(8,45)= 1991.8; p < 0.0001). The p values reported below correspond to the partial F statistic for the independent variable named.
Effects of U91356a on global or cerebellar CBF were tested with an ANOVA using dose and animal effects and a dose by animal interaction. To rule out systematic differences in pCO2 across different doses of U91356a, we computed a one-factor ANOVA with pCO2as the dependent variable and dose as the grouping factor.
Data analysis: antagonist pretreatment studies. In the MPTP-treated animals used for these studies, only the rCBF in the control hemisphere GP was analyzed. For each antagonist, an ANCOVA was performed as for the dose–response studies, with the addition to the model of a categorical pretreatment drug variable and a pretreatment drug by dose level interaction. This interaction term, which reflects differences in the response to U91356a depending on whether the antagonist was administered, is the variable of interest. (A similar approach to statistical analysis of drug effects was taken by Grasby et al., 1993.) The control experiments included in each ANCOVA overlapped, but were not identical, because for some antagonists only one MPTP-treated animal was studied.
RESULTS
Dose–response experiments
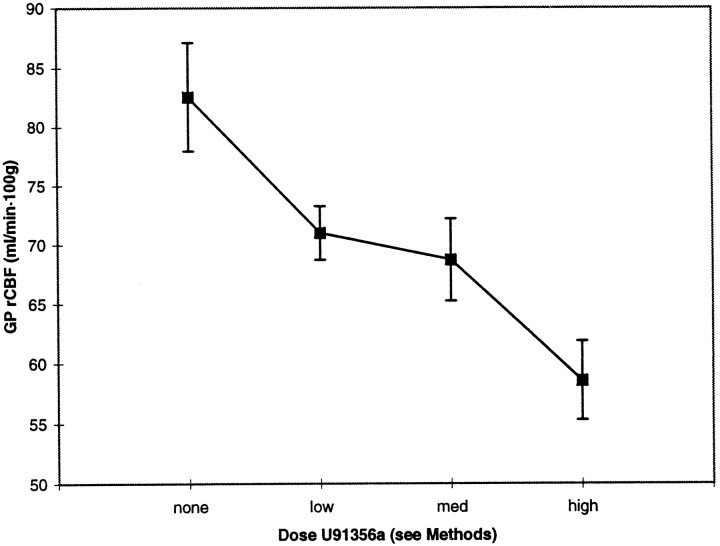
Pallidal rCBF decreased substantially after U91356a, in a dose-related manner (Fig. 2). This decrease remained significant after accounting for differences across animals and global changes in CBF (ANCOVA dose effect, p < 0.01). As expected, between-animal differences and global CBF also contributed significantly to total variance in this model (p< 0.0001 for each variable).
Fig. 2.
U91356a causes a dose-dependent decrease in GP rCBF. Mean (± SEM) values for blood flow in the GP are shown at baseline and after acute intravenous administration of low, medium, and high doses of the D2 agonist U91356a. Key to doses: low = 1 to 2 μg/kg, medium = 10 to 22 μg/kg, and high = 100 to 220 μg/kg.
There was also a dose-dependent decrease in global blood flow, with a substantial decrease of 29% at the highest dose (mean flows 80.8, 74.3, 71.2, and 57.1 ml/100 mg·min at baseline, low, medium, and high doses, respectively; dose effect, p < 0.0001; high dose significantly different from all other doses by post hoc Scheffé test). This was not attributable to systematic changes in arterial pCO2, as pCO2 did not vary with dose of U91356a (dose effect, p = 0.78).
After noting the marked fall in global flow in this planned analysis, we reconsidered our use of global blood flow as a covariate, because our “global” region was largely composed of cerebral cortex, which contains D2-like and 5HT receptors and also receives thalamocortical output influenced by the basal ganglia. We compared “global” blood flow with flow in the cerebellum (see Materials and Methods). Cerebellar flow did decline after U91356a (mean flows, 96.4, 89.6, 85.9, and 74.9 ml/100 mg·min at baseline, low, medium, and high doses, respectively; dose effect, p = 0.001; high dose significantly different from baseline by post hocScheffé test). However, at the highest dose of U91356a, rCBF declined more in the “global,” primarily cortical region, than in cerebellum (one-factor ANOVA, global:cblm ratio as dependent variable; dose effect p = 0.03). When cerebellar rCBF was substituted for “global” CBF as the covariate in the original ANCOVA, GP rCBF was even more strongly related to doses of U91356a (dose effect, p = 0.0001).
In the control animal that received no U91356a, there was no decline at corresponding time points. GP rCBF increased from 73.1 (3–4 hr after ketamine) to 87.0 ml−1 · 100 mg−1 · min (6–7 hr after ketamine), with similar increases in global and cerebellar flow and an increase in pCO2 (35.5 to 39.9). When normalized to global or cerebellar flow (Fig. 3), GP changed from 56.6 to 57.3 (global; dimensionless) and from 44.1 to 44.6 (cerebellar) during the same intervals.
Fig. 3.
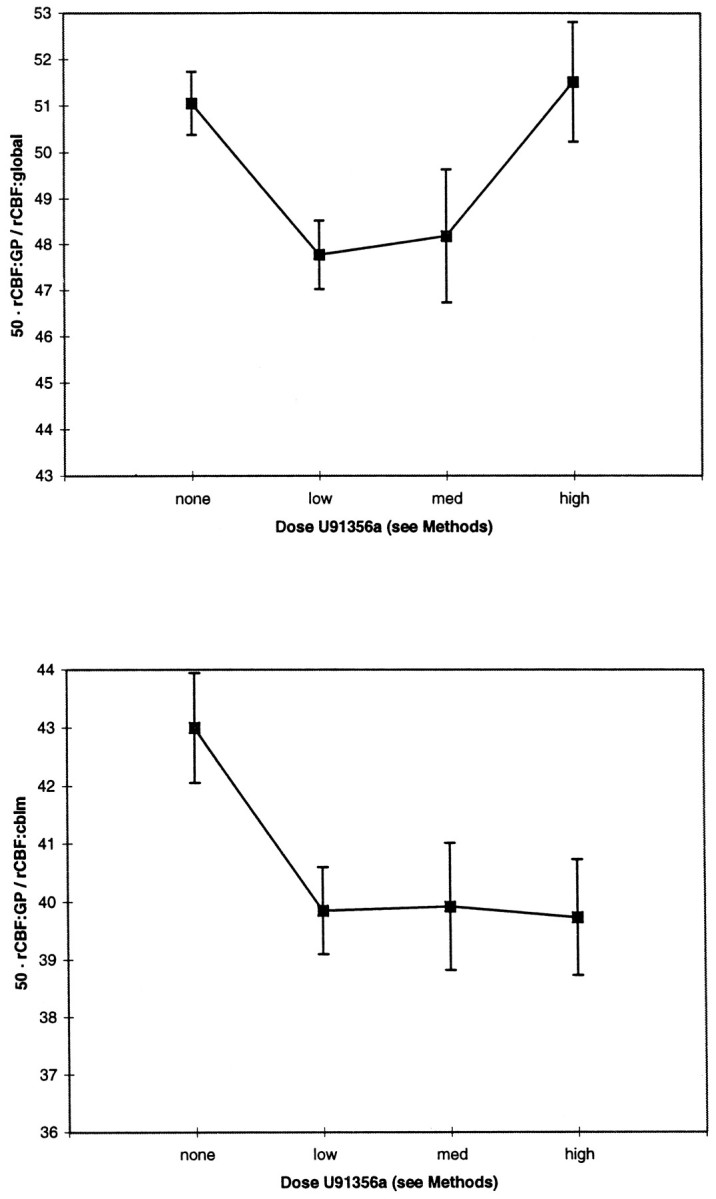
GP rCBF response to U91356a relative to global or cerebellar rCBF. Mean values for relative GP blood flow are graphed at baseline and after different doses of the D2agonist U91356a. In the top graph, absolute GP rCBF was normalized by setting global CBF to 50. In the bottom graph, cerebellum CBF was used rather than global CBF.
For practical reasons, lower doses of U91356a always preceded higher doses during a single study, raising the possibility that our results could be attributable to “priming” effects of lower doses, the passage of time, or diminishing effects of the preanesthetic ketamine. To examine these concerns, we administered a single dose of U91356a (200 μg/kg) after baseline rCBF measurements to one animal not previously exposed to dopamimetics (i.e., bypassing “low” and “medium” doses). Pallidal rCBF declined by 27.4%, the ratio (GP rCBF:global CBF) declined by 2.5%, and the ratio (GP rCBF:cblm CBF) declined by 5.6%, all similar to the overall results. Thus, these possible effects do not explain our results.
Because atropine, which might be expected to affect dopaminergic neurons, was used in a few cases as a preanesthetic, we compared results from two U91356a challenge experiments performed in the same animal, one using atropine and the other using glycopyrrolate (which is essentially devoid of central anticholinergic activity). The maximal decrease in relative GP flow normalized to cerebellum occurred at the medium dose of U91356a and was 9.4% (atropine) and 9.8% (glycopyrrolate). This suggests that atropine’s effect on our results was negligible.
Pharmacological specificity experiments
In the control hemisphere of the unilaterally MPTP-lesioned animals, we were able to replicate the dose-related effect of systemic U91356a on GP rCBF, with decreases of 15%, 18%, and 27% at low, medium, and high doses, respectively (dose effect,p = 0.0001; post hoc t tests,p < 0.02, 0.06, 0.0005, respectively; see filled circles, Fig. 3, top left graph). This suggests that the control hemisphere of these animals is a reasonable model for testing the pharmacological specificity of the nearly identical response seen in normal animals.
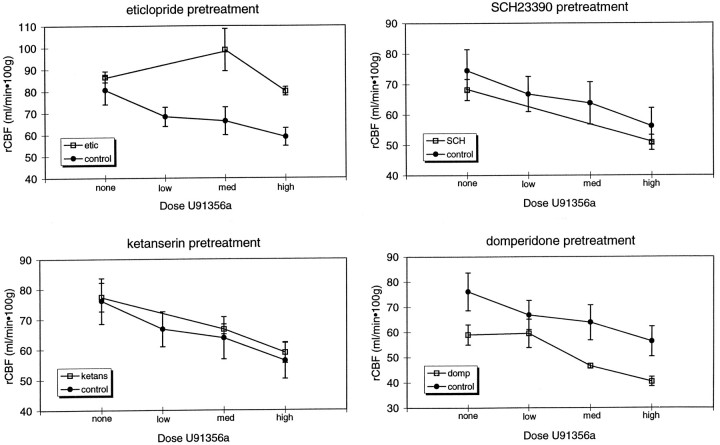
Eticlopride prevented this response to U91356a (pretreatment drug by dose interaction, p < 0.003), with post hoc t tests showing significant effects of eticlopride at each dose of U91356a (open squares, Fig. 4, top left graph).
Fig. 4.
Effect of pretreatment with various antagonists on the GP rCBF response to U91356a. In each graph, control experiments (mean ± SEM) are represented by filled circles and antagonist pretreatment experiments are represented by hollow squares. Top left, eticlopride (D2antagonist, 4 mg/kg); top right, SCH23390 (D1 antagonist, 1 mg/kg); bottom left, ketanserin (5HT2 antagonist, 0.6 mg/kg); bottom right, domperidone (peripheral D2 antagonist, 0.1 mg/kg).
The other antagonists tested, however, did not prevent the response to U91356a. The significance of the pretreatment drug by dose interaction was: domperidone, p = 0.56; ketanserin,p = 0.89; and SCH23390, p < 0.03. The comparison using SCH23390 reached significance because this D1 antagonist slightly augmented, rather than blocked, the U91356a-induced decrease in GP rCBF by least-squared means. The GP rCBF response to U91356a after pretreatment with various antagonists is depicted in Figure 4.
DISCUSSION
The selective dopamine D2 agonist U91356a induced a pharmacologically specific decline in pallidal rCBF at biologically relevant doses. Although global CBF also declined, the decrease in GP rCBF remained significant after accounting for this effect. We first examine technical concerns about our results and then their implications.
The comparison of atropine and glycopyrrolate as preanesthetics shows that atropine does not explain our findings. We minimized ketamine effects by waiting 3 hr after its administration before the first CBF measurement. Ketamine did not produce our results (see Results), although we cannot exclude a long-lasting synergistic effect on the response to dopamine agonists. Importantly, ketamine does not seem to disrupt coupling of metabolism to flow (Cavazzuti et al., 1987).
Inhaled N2O may affect rCBF directly but does not affect our results, because N2O concentration was constant. Under N2O anesthesia, rCBF responses to pCO2 changes or behavioral or pharmacological stimuli remain intact (Fox et al., 1992; Yaster et al., 1994). N2O was chosen because other anesthetics affect metabolic or flow responses to dopaminergics (Grome and McCulloch, 1981, 1983). However, in analogous studies with the D2-like agonist quinpirole, we showed identical pallidal rCBF responses in N2O-sedated baboons and in awake nemestrina monkeys (Perlmutter et al., 1993). Studying sedated animals has the advantage that rCBF changes in awake animals could reflect not only direct drug effects, but also secondary effects from drug-altered behavior.
For practical reasons, the pharmacological specificity studies were performed in animals given intracerebroventricular MPTP. Although our observations were confined to the “control” hemisphere, that hemisphere is not normal (Trugman and Wooten, 1986; Robinson, 1991;Todd et al., 1996). However, the response we found in normal animals was replicated in the control GP of the MPTP animals, so this concern is moot.
U91356a has only a 25- to 150-fold preference for D2 over D3, D4, and serotonin 1A receptors. Although U91356a has little efficacy at central 5HT1A receptors (Piercey et al., 1992), it is possible that some of the effects at higher doses may be attributable to these receptors. We are investigating a D3-preferring agonist for comparison (Black et al., 1996b).
PET images suffer from volume averaging, so that the apparent rCBF in GP includes contributions from adjacent regions. We minimized this by centering the VOI on the GP, using a small VOI (similar to the dimensions of GP, with a radius similar to the image resolution), and using a high-resolution PET and reconstruction filter.
Dopamine agonists modify cerebral vascular resistance, raising the question of whether U91356a uncouples rCBF and metabolism. rCBF remains proportional to regional cerebral metabolic rate (rCMR) under various conditions (McCulloch et al., 1982a; Beck et al., 1986; Tuor et al., 1986); thus, quantitative images of rCBF only need to be scaled to reflect rCMR. Dopaminergics usually do not affect the global CBF/CMR ratio (Berntman et al., 1976, 1978; McCulloch and Harper, 1977;McCulloch et al., 1978, 1982a; McCulloch and Edvinsson, 1980; Azuma et al., 1988; Sharkey et al., 1991), but certain drugs can change it (Ingvar et al., 1983; Leenders et al., 1985; Tuor et al., 1986; Beck et al., 1988; Sabatini et al., 1991). This raises the question of whether U91356a alters the vasculature to decrease global CBF or whether it decreases neuronal activity in large areas of brain. Several lines of evidence suggest the latter. The most direct evidence is that U91356a globally reduces cerebral metabolism in awake rats (Piercey et al., 1995). Also, in our study domperidone did not prevent the global fall in CBF. Domperidone does not affect central metabolism (Palacios and Wiederhold, 1987), but can block a disproportionate, presumably vascular, change in rCBF after apomorphine (Sabatini et al., 1991).
Qualitative PET images of relative flow often are normalized to global counts because many such studies are not performed quantitatively. This may be reasonable in behavioral activation studies in normals because activation does not produce appreciable global effects. However, this assumption may not hold for pharmacological studies, as we demonstrate. Because the purpose of the rCBF measurements is to indicate local neuronal activity that may lead to behavorial responses, it is important to consider whether neuronal activity corresponds best to absolute changes in rCBF or to relative changes compared with the rest of the brain. The definitive answer to this question is unknown. However, we assume that neuronal activity correlates with absolute flow or metabolism and demonstrate a dose–response curve for absolute flow in GP (Fig. 2). This dose response corresponds reasonably to previous physiological studies with U91356a (see Materials and Methods). Absolute flow also changes in other regions, so the effect is not limited to pallidum. However, we demonstrate (Fig. 3 and ANCOVA) that lower doses preferentially affect the pallidum. This does not imply that these lower doses must correspond to the maximal behavioral effect, because a further decrease in absolute pallidal activity at higher doses of U91356a may produce additional behavioral responses despite changes in other brain regions. This local versus global issue could not be addressed without our absolute flow measurements.
We now turn to the implications of our findings. U91356a decreased pallidal rCBF, consistent with the prediction that D2agonists inhibit the activity of striatopallidal neurons. Although this finding is consistent with previous studies of D2-like agonists, we extend this to a D2-selective agonist. In the only previous metabolic study of U91356a, regional effects were not reported (Piercey et al., 1995).
GP was more affected than other regions of the brain. The data are consistent with a relatively specific striatal effect at low-to-intermediate doses equaled by effects on cortex at higher doses that did not cause equivalent effects on cerebellum. These results may be caused by cortical D3, D4, or 5HT1 receptors at the highest dose.
We centered our volume of interest on the entire GP, rather just GPe, to which D2-receptor-bearing striatopallidal neurons preferentially project. A D2 agonist might be expected to decrease rCBF in GPe and GPi because of a decrease in the firing rate of subthalamic neurons projecting to GPi (via disinhibition of inhibitory pallidosubthalamic neurons). Alternatively, U91356a may affect pallidal rCBF via dopaminergic nigropallidal neurons (Parent et al., 1990). Although innervation of GPi by GPe might counteract this effect, the hypothesized decrease in GP rCBF was still observed.
An acute dose of lDOPA produced a nonsignificant 15% decrease in 2DG uptake in GP and enteropeduncular nucleus (EPN) (corresponding to primate GPe and GPi, respectively) in normal rats (Trugman and Wooten, 1986). On the other hand, in unilateral 6OHDA-lesioned rats a D2-like agonist caused bilateral metabolic increases in GP and EPN (Trugman and Wooten, 1987). However, these rats were circling rapidly during the time of 2DG uptake, and the increased metabolism may reflect this dramatic change in motor behavior.
The synergistic decrease in GP rCBF after the D1 antagonist SCH23390 is also consistent with the proposed model of dopaminergic pathways in basal ganglia. The lack of effect of the serotonin 5HT2 antagonist ketanserin on GP rCBF or on the pallidal response to U91356a suggests that blockade of these receptors produces minimal change in pallidal activity despite the known interactions between 5HT2 receptors and dopaminergic neurons (Kapur and Remington, 1996).
The methods used here have several potential advantages. First, we show that an in vivo PET technique is sensitive to the effects of U91356a. Advantages of PET over ex vivo studies include its minimally invasive nature and the possibility of repeated measurements in the same subject, including within-subject studies before and after lesions or treatment. Second, the D2 selectivity of U91356a allows for more specific conclusions regarding D2receptors. This may provide better specificity for a given effect and may also better clarify the effects of disease or interventions on D2-specific pathways. Third, pharmacological activation of rCBF provides complementary information to other PET methods for studying dopaminergic pathways. This provides less specific information about receptor binding than radioligand studies (Perlmutter et al., 1986, 1989, 1991b, 1997; Farde et al., 1989; Sadzot et al., 1991), but may be more sensitive to changes in postsynaptic neurons that affect D2 signal transduction (Breece et al., 1987). Another complementary technique is to study the pharmacological effects of dopaminergic agents on other neurotransmitter receptors (Dewey et al., 1990, 1993), a method that may provide more detail on dopaminergic output to neurons with cholinergic receptors, for example, but may miss effects on other circuits.
We are developing a baboon PET atlas and methods for combining data across animals. This will permit us to analyze the response to U91356a in other brain regions.
With a tool for measuring specific effects of D2-mediated pathways, we can explore several interesting questions. For instance, combined with our analogous study with a D1 agonist (Black et al., 1997), we can investigate in vivo how chroniclDOPA affects D1- and D2-mediated neuronal pathways in MPTP-hemiparkinsonsian monkeys, or inpatients with Parkinson’s disease. We also can investigate the sensitivity of D2 (specific)-influenced pathways in diseases such as dystonia, Tourette syndrome, schizophrenia, and drug addiction.
Footnotes
This work was supported by National Institutes of Health Grants AA07466, NS01898, NS32318, and NS31001; the Charles A. Dana Foundation (The Dana Clinical Hypotheses Research Program); the American Parkinson Disease Association; National Alliance for Research on Schizophrenia and Depression; and the McDonnell Center for Higher Brain Function. We gratefully acknowledge helpful discussions with Professor P. J. Bédard (Centre de Recherche en Neurobiologie, Hôpital de l’Enfant-Jésus, Québec, Canada), and Drs. M. F. Piercey and M. W. Moon (CNS Research, Pharmacía-Upjohn). U91356a was a generous gift of the Upjohn Company.
Correspondence should be addressed to Dr. Kevin John Black, Campus Box 8134, 4940 Children’s Place, St. Louis, MO 63110-1093.
REFERENCES
- 1.Åkeson J, Björkman S, Messeter K, Rosen I, Helfer M. Cerebral pharmacodynamics of anaesthetic and subanaesthetic doses of ketamine in the normoventilated pig. Acta Anaesthesiol Scand. 1993;37:211–218. doi: 10.1111/j.1399-6576.1993.tb03703.x. [DOI] [PubMed] [Google Scholar]
- 2.Azuma H, Miyazawa T, Mizokawa T, Magota A, Hara K. Stimulatory effects of lisuride on local cerebral blood flow and local cerebral glucose utilization in rats. Nippon Yakurigaku Zasshi. 1988;91:341–349. doi: 10.1254/fpj.91.341. [DOI] [PubMed] [Google Scholar]
- 3.Bacopoulos NG, Redmond DE, Roth RH. Serotonin and dopamine metabolites in brain regions and cerebrospinal fluid of a primate species: effects of ketamine and fluphenazine. J Neurochem. 1979;32:1215–1218. doi: 10.1111/j.1471-4159.1979.tb11048.x. [DOI] [PubMed] [Google Scholar]
- 4.Beck T, Vogg P, Krieglstein J. Effects of the indirect dopaminomimetic diethylpemoline on local cerebral glucose utilization and local cerebral blood flow in the conscious rat. Eur J Pharmacol. 1986;125:437–447. doi: 10.1016/0014-2999(86)90800-9. [DOI] [PubMed] [Google Scholar]
- 5.Beck T, Vogg P, Krieglstein J. Uncoupling of cerebral blood flow and glucose utilization by dihydroergocristine in the conscious rat. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:82–87. doi: 10.1007/BF00168816. [DOI] [PubMed] [Google Scholar]
- 6.Bédard PJ, Gomez Mancilla B, Blanchette P, Gagnon C, Di Paolo T. Levodopa-induced dyskinesia: facts and fancy. What does the MPTP monkey model tell us? Can J Neurol Sci. 1992;19:134–137. [PubMed] [Google Scholar]
- 7.Berntman L, Carlsson C, Hagerdal M, Siesjo BK. Excessive increase in oxygen uptake and blood flow in the brain during amphet- amine intoxication. Acta Physiol Scand. 1976;97:264–266. doi: 10.1111/j.1748-1716.1976.tb10261.x. [DOI] [PubMed] [Google Scholar]
- 8.Berntman L, Carlsson C, Hagerdal M, Siesjo BK. Circulatory and metabolic effects in the brain induced by amphetamine sulphate. Acta Physiol Scand. 1978;102:310–323. doi: 10.1111/j.1748-1716.1978.tb06078.x. [DOI] [PubMed] [Google Scholar]
- 9.Björkman S, Åkeson J, Nilsson F, Messeter K, Roth B. Ketamine and midazolam decrease cerebral blood flow and consequently their own rate of transport to the brain: an application of mass balance pharmacokinetics with a changing regional blood flow. J Pharmacokinet Biopharm. 1992;20:637–652. doi: 10.1007/BF01064423. [DOI] [PubMed] [Google Scholar]
- 10.Black KJ, Videen TO, Perlmutter JS (1996a) A metric for testing the accuracy of cross-modality image registration: validation and application. J Comp Assist Tomogr 1996:20:855–861. [DOI] [PubMed]
- 11.Black KJ, Videen TO, Perlmutter JS (1996b) Regional effects of a D3-preferring agonist on cerebral blood flow. Soc Neurosci Abstr 22(Part 1):498.
- 12.Black KJ, Videen TO, Perlmutter JS. In vivo regional cerebral blood flow responses to a dopamine D1 agonist. J Neuropsychiatry Clin Neurosci. 1997;9:132. [Google Scholar]
- 13.Bloch B, LeMoine C. Neostriatal dopamine receptors. Trends Neurosci. 1994;17:3–4. doi: 10.1016/0166-2236(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 14.Breece GR, Duncan GE, Napier TC, Bondy SC, Iorio LC, Mueller RA. 6-hydroxydopamine treatments enhance behavioral responses to intracerebral microinjection of D1- and D2-dopamine agonists into nucleus accumbens and striatum without changing dopamine antagonist binding. J Pharmacol Exp Ther. 1987;240:167–176. [PMC free article] [PubMed] [Google Scholar]
- 15.Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC. Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab. 1987;7:806–811. doi: 10.1038/jcbfm.1987.138. [DOI] [PubMed] [Google Scholar]
- 16.Celsis P, Rascol O, Demonet JF, Agniel A, Montastruc JL, Marc-Vergnes JP, Rascol A. Effet de la bromocriptine sur le debit sanguin cerebral dans la maladie de Parkinson. Rev Neurol (Paris) 1988;144:367–371. [PubMed] [Google Scholar]
- 17.Cleghorn JM, Szechtman H, Garnett ES, Nahmias C, Brown GM, Kaplan RD, Szechtman B, Franco S. Apomorphine effects on brain metabolism in neuroleptic-naive schizophrenic patients. Psychiatry Res. 1991;40:135–153. doi: 10.1016/0925-4927(91)90005-b. [DOI] [PubMed] [Google Scholar]
- 18.Crosby G, Crane AM, Sokoloff L. Local changes in cerebral glucose utilization during ketamine anesthesia. Anesthesiology. 1982;56:437–443. doi: 10.1097/00000542-198206000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis R, Huffman RD. A stereotaxic atlas of the brain of the baboon (Papio). University of Texas; Austin, TX: 1968. [Google Scholar]
- 21.Dewey SL, Brodie JD, Fowler JS, MacGregor RR, Schlyer DJ, King PT, Alexoff DL, Volkow ND, Shiue CY, Wolf AP, Bendriem B. Positron emission tomography (PET) studies of dopaminergic/cholinergic interactions in the baboon brain. Synapse. 1990;6:321–327. doi: 10.1002/syn.890060403. [DOI] [PubMed] [Google Scholar]
- 22.Dewey SL, Smith GS, Logan J, Brodie JD, Simkowitz P, MacGregor RR, Fowler JS, Volkow ND, Wolf AP. Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci USA. 1993;90:11816–11820. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhasmana KM, Saxena PR, Prakash O, Van Der Zee HT. A study on the influence of ketamine on systemic and regional haemodynamics in conscious rabbits. Arch Int Pharmacodyn. 1984;269:323–334. [PubMed] [Google Scholar]
- 24.Engber TM, Susel Z, Kuo S, Chase TN. Chronic levodopa treatment alters basal and dopamine agonist-stimulated cerebral glucose utilization. J Neurosci. 1990;10:3889–3895. doi: 10.1523/JNEUROSCI.10-12-03889.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engber TM, Anderson JJ, Boldry RC, Kuo S, Chase TN. N-methyl-D-aspartate receptor blockade differentially modifies regional cerebral metabolic responses to D1 and D2 dopamine agonists in rats with a unilateral 6-hydroxydopamine lesion. Neuroscience. 1993;54:1051–1061. doi: 10.1016/0306-4522(93)90595-7. [DOI] [PubMed] [Google Scholar]
- 26.Farde L, Eriksson L, Blomquist G, Halldin C. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET: a comparison to the equilibrium analysis. J Cereb Blood Flow Metab. 1989;9:696–708. doi: 10.1038/jcbfm.1989.98. [DOI] [PubMed] [Google Scholar]
- 27.Fox J, Gelb AW, Enns J, Murkin JM, Farrar JK, Manninen PH. The responsiveness of cerebral blood flow to changes in arterial carbon dioxide is maintained during propofol-nitrous oxide anesthesia in humans. Anesthesiology. 1992;77:453–456. doi: 10.1097/00000542-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Gerfen CR, Keefe KA. Neostriatal dopamine receptors. Trends Neurosci. 1994;17:2–3. doi: 10.1016/0166-2236(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 29.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 30.Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasby PM, Friston KJ, Bench CJ, Cowen PJ, Frith CD, Liddle PF, Frackowiak RS, Dolan RJ. The effect of the dopamine agonist, apomorphine, on regional cerebral blood flow in normal volunteers. Psychol Med. 1993;23:605–612. doi: 10.1017/s0033291700025381. [DOI] [PubMed] [Google Scholar]
- 32.Grome JJ, McCulloch J. The effects of chloral hydrate anaesthesia on the metabolic response in the substantia nigra to apomorphine. Brain Res. 1981;214:223–228. doi: 10.1016/0006-8993(81)90460-1. [DOI] [PubMed] [Google Scholar]
- 33.Grome JJ, McCulloch J. The effects of apomorphine upon local cerebral glucose utilization in conscious rats, and rats anaesthetized with chloral hydrate. J Neurochem. 1983;40:569–576. doi: 10.1111/j.1471-4159.1983.tb11320.x. [DOI] [PubMed] [Google Scholar]
- 34.Hammer RP, Jr, Herkenham M. Altered metabolic activity in the cerebral cortex of rats exposed to ketamine. J Comp Neurol. 1983;220:396–404. doi: 10.1002/cne.902200404. [DOI] [PubMed] [Google Scholar]
- 35.Harrison MB, Wiley RG, Wooten GF. Changes in D2 but not D1 receptor binding in the striatum following a selective lesion of striatopallidal neurons. Brain Res. 1992;590:305–310. doi: 10.1016/0006-8993(92)91111-q. [DOI] [PubMed] [Google Scholar]
- 36.Hartvig P, Valtysson J, Lindner K-J, Kristensen J, Karlsten R, Gustafsson LL, Persson J, Svensson JO, Øye I, Antoni G, Westerberg G, Långström B. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58:165–173. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 37.Henriksen L, Boas J. Regional cerebral blood flow in hemiparkinsonian patients: emission computerized tomography of inhaled 133Xenon before and after levodopa. Acta Neurol Scand. 1985;71:257–266. doi: 10.1111/j.1600-0404.1985.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 38.Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153:41–49. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- 39.Hougaard K, Hansen A, Brodersen P. The effect of ketamine on regional cerebral blood flow in man. Anesthesiology. 1974;41:562–567. doi: 10.1097/00000542-197412000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Ingvar M, Lindvall O, Stenevi U. Apomorphine-induced changes in local cerebral blood flow in normal rats and after lesions of the dopaminergic nigrostriatal bundle. Brain Res. 1983;262:259–265. doi: 10.1016/0006-8993(83)91016-8. [DOI] [PubMed] [Google Scholar]
- 41.Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther. 1994;64:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 42.Jankovic J, Tolosa E. Parkinson’s disease and movement disorders, 2nd Ed. Williams & Wilkins; Baltimore: 1993. [Google Scholar]
- 43.Jauzac P, Blasco A, Vigoni F, Valdiguie P, Bes A. Cerebral circulatory effects of a dopaminergic agonist (apomorphine) in the dog. J Cereb Blood Flow Metab. 1982;2:369–372. doi: 10.1038/jcbfm.1982.38. [DOI] [PubMed] [Google Scholar]
- 44.Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity?—implications for PET and fMRI. NeuroImage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- 45.Kapur S, Meyer J, Wilson AA, Houle S, Brown GM. Activation of specific cortical regions by apomorphine: an [15O]H2O PET study in humans. Neurosci Lett. 1994;176:21–24. doi: 10.1016/0304-3940(94)90861-3. [DOI] [PubMed] [Google Scholar]
- 46.Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- 47.Knable MB, Kleinman JE, Weinberger DR. Neurobiology of schizophrenia. In: Shatzberg AF, Nemeroff CB, editors. The American Psychiatric Press textbook of psychopharmacology. American Psychiatric; Washington, DC: 1995. pp. 479–499. [Google Scholar]
- 48.Kobari M, Fukuuchi Y, Shinohara T, Nogawa S, Takahashi K. Local cerebral blood flow and its response to intravenous levodopa in progressive supranuclear palsy: comparison with Parkinson’s disease. Arch Neurol. 1992;49:725–730. doi: 10.1001/archneur.1992.00530310071014. [DOI] [PubMed] [Google Scholar]
- 49.Kobari M, Fukuuchi Y, Shinohara T, Obara K, Nogawa S. Levodopa-induced local cerebral blood flow changes in Parkinson’s disease and related disorders. J Neurol Sci. 1995;128:212–218. doi: 10.1016/0022-510x(94)00237-i. [DOI] [PubMed] [Google Scholar]
- 50.Koshikawa N, Tomiyama K, Omiya K, Kobayashi M. Ketamine anaesthesia has no effect on striatal dopamine metabolism in rats. Brain Res. 1988;444:394–396. doi: 10.1016/0006-8993(88)90954-7. [DOI] [PubMed] [Google Scholar]
- 51.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. NeuroReport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 52.Lahti AC, Holcomb HH, Weiler MA, Corey PK, Zhao M, Medoff D, Tamminga CA (1996) Ketamine-induced effects on behavior and rCBF is enhanced in schizophrenic compared to normal individuals. Soc Neurosci Abstr 22(Part 2):1192.
- 53.Leenders KL, Wolfson L, Gibbs JM, Wise RJ, Causon R, Jones T, Legg NJ. The effects of L-DOPA on regional cerebral blood flow and oxygen metabolism in patients with Parkinson’s disease. Brain. 1985;108:171–191. doi: 10.1093/brain/108.1.171. [DOI] [PubMed] [Google Scholar]
- 54.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martel JC, Calon F, Blanchet P, Piercey M, Bedard PJ, DiPaolo T. Chronic treatment of MPTP monkeys with lDOPA compared to a D2 agonist: behavioral and biochemical evaluation. Soc Neurosci Abstr. 1993;19:403. [Google Scholar]
- 56.Mathew RJ, Wilson WH. Changes in cerebral blood flow and mental state after amphetamine challenge in schizophrenic patients. Neuropsychobiology. 1989;21:117–123. doi: 10.1159/000118564. [DOI] [PubMed] [Google Scholar]
- 57.Mazoyer B, Trebossen R, Deutch R, Casey M, Blohm K. Physical characteristics of the ECAT 953B/31: a new high resolution brain positron tomograph. IEEE Trans Med Imaging. 1991;10:499–504. doi: 10.1109/42.108583. [DOI] [PubMed] [Google Scholar]
- 58.McCulloch J. Mapping functional alterations in the CNS with [14C]deoxyglucose. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of psychopharmacology: new techniques in psychopharmacology, Vol 15. Plenum; New York: 1982. pp. 321–410. [Google Scholar]
- 59.McCulloch J, Edvinsson L. Cerebral circulatory and metabolic effects of piribedil. Eur J Pharmacol. 1980;66:327–337. doi: 10.1016/0014-2999(80)90465-3. [DOI] [PubMed] [Google Scholar]
- 60.McCulloch J, Harper AM. Cerebral circulation: effect of stimulation and blockade of dopamine receptors. Am J Physiol. 1977;233:H222–H227. doi: 10.1152/ajpheart.1977.233.2.H222. [DOI] [PubMed] [Google Scholar]
- 61.McCulloch J, Teasdale G. Effects of apomorphine upon local cerebral blood flow. Eur J Pharmacol. 1979;55:99–102. doi: 10.1016/0014-2999(79)90152-3. [DOI] [PubMed] [Google Scholar]
- 62.McCulloch J, Deshmukh VD, Harper AM. Indirect sympathomimetic agents and cerebral blood flow and metabolism. Eur J Pharmacol. 1978;47:11–18. doi: 10.1016/0014-2999(78)90368-0. [DOI] [PubMed] [Google Scholar]
- 63.McCulloch J, Kelly PA, Ford I. Effect of apomorphine on the relationship between local cerebral glucose utilization and local cerebral blood flow (with an appendix on its statistical analysis). J Cereb Blood Flow Metab. 1982a;2:487–499. doi: 10.1038/jcbfm.1982.56. [DOI] [PubMed] [Google Scholar]
- 64.McCulloch J, Savaki HE, Sokoloff L. Distribution of effects of haloperidol on energy metabolism in the rat brain. Brain Res. 1982b;234:81–90. doi: 10.1016/0006-8993(82)91122-2. [DOI] [PubMed] [Google Scholar]
- 65.McCulloch J, Savaki HE, McCulloch MC, Jehle J, Sokoloff L. The distribution of alterations in energy metabolism in the rat brain produced by apomorphine. Brain Res. 1982c;243:67–80. doi: 10.1016/0006-8993(82)91121-0. [DOI] [PubMed] [Google Scholar]
- 66.Melamed E, Globus M, Mildworf B. Regional cerebral blood flow in patients with Parkinson’s disease under chronic levodopa therapy: measurements during “on” and “off” response fluctuations. J Neurol Neurosurg Psychiatry. 1986;49:1301–1304. doi: 10.1136/jnnp.49.11.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montastruc JL, Celsis P, Agniel A, Demonet JF, Doyon B, Puel M, Marc-Vergnes JP, Rascol A. Levodopa-induced regional cerebral blood flow changes in normal volunteers and patients with Parkinson’s disease: lack of correlation with clinical or neuropsychological improvements. Mov Disord. 1987;2:279–289. doi: 10.1002/mds.870020405. [DOI] [PubMed] [Google Scholar]
- 68.Mori H, Suzuki M, Kawasaki Y, Yamaguchi N, Shiba K, Matsuda M, Hisada K, Kurachi M (1989) Distribution of effects of haloperidol on regional cerebral blood flow and D2receptor in the rat brain. J Cereb Blood Flow Metab 9[Suppl 1]:S138.
- 69.Nelson SR, Howard RB, Cross RS, Samson F. Ketamine-induced changes in regional glucose utilization in the rat brain. Anesthesiology. 1980;52:330–334. doi: 10.1097/00000542-198004000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Nestler EJ. Molecular basis of addictive states. Neuroscientist. 1995;1:212–220. [Google Scholar]
- 71.Oguchi K, Arakawa K, Nelson SR, Samson F. The influence of droperidol, diazepam, and physostigmine on ketamine-induced behavior and brain regional glucose utilization in rat. Anesthesiology. 1982;57:353–358. doi: 10.1097/00000542-198211000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Onoe H, Inoue O, Suzuki K, Tsukada H, Itoh T, Mataga N, Watanabe Y. Ketamine increases the striatal N-[11C]methylspiperone binding in vivo: positron emission tomography study using conscious rhesus monkey. Brain Res. 1994;663:191–198. doi: 10.1016/0006-8993(94)91263-7. [DOI] [PubMed] [Google Scholar]
- 73.Palacios JM, Pazos A. Visualization of dopamine receptors: a progress review. In: Creese I, Fraser CM, editors. Dopamine receptors. Alan R. Liss; New York: 1987. pp. 175–197. [Google Scholar]
- 74.Palacios JM, Wiederhold K-H. Presynaptic dopaminergic agonists modify brain glucose metabolism in a way similar to the neuroleptics. Neurosci Lett. 1984;50:223–229. doi: 10.1016/0304-3940(84)90490-7. [DOI] [PubMed] [Google Scholar]
- 75.Palacios JM, Wiederhold K-H. Dopamine D2 receptor agents, but not dopamine D1, modify brain glucose metabolism. Brain Res. 1985;327:390–394. doi: 10.1016/0006-8993(85)91543-4. [DOI] [PubMed] [Google Scholar]
- 76.Palacios JM, Wiederhold KH. Pharmacological investigation of neurotransmitter mechanisms in the basal ganglia using the 2-deoxyglucose technique: focus on the dopaminergic system. In: Sandler M, Feuerstein C, Scatton B, editors. Neurotransmitter interactions in the basal ganglia. Raven; New York: 1987. pp. 183–192. [Google Scholar]
- 77.Parent A, Lavoie B, Smith Y, Bedard P. The dopaminergic nigropallidal projection in primates: distinct cellular origin and relative sparing in MPTP-treated monkeys. Adv Neurol. 1990;53:111–116. [PubMed] [Google Scholar]
- 78.Pearlson GD, Jeffery PJ, Harris GJ, Ross CA, Fischman MW, Camargo EE. Correlation of acute cocaine-induced changes in local cerebral blood flow with subjective effects. Am J Psychiatry. 1993;150:495–497. doi: 10.1176/ajp.150.3.495. [DOI] [PubMed] [Google Scholar]
- 79.Perlmutter JS. PET evaluation of dopaminergic pathways. Clin Neuropharmacol. 1995;18:S188–S194. [Google Scholar]
- 80.Perlmutter JS, Raichle ME. Regional cerebral blood flow in hemiparkinsonism. Neurology. 1985;35:1127–1134. doi: 10.1212/wnl.35.8.1127. [DOI] [PubMed] [Google Scholar]
- 81.Perlmutter JS, Raichle ME. In vitro or in vivo receptor binding: where does the truth lie? Ann Neurol. 1986;19:384–385. doi: 10.1002/ana.410190413. [DOI] [PubMed] [Google Scholar]
- 82.Perlmutter JS, Larson KB, Raichle ME, Markham J, Mintun MA, Kilbourn MR, Welch MJ. Strategies for the in vivo measurement of receptor binding using positron emission tomography. J Cereb Blood Flow Metab. 1986;6:154–169. doi: 10.1038/jcbfm.1986.29. [DOI] [PubMed] [Google Scholar]
- 83.Perlmutter JS, Kilbourn MR, Welch MJ, Raichle ME. Nonsteady-state measurement of in vivo receptor binding with positron emission tomography: “dose–response” analysis. J Neurosci. 1989;9:2344–2352. doi: 10.1523/JNEUROSCI.09-07-02344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perlmutter JS, Lich LL, Margenau W, Buchholz S. PET measured evoked cerebral blood flow responses in an awake monkey. J Cereb Blood Flow Metab. 1991a;11:229–235. doi: 10.1038/jcbfm.1991.54. [DOI] [PubMed] [Google Scholar]
- 85.Perlmutter JS, Moerlein SM, Huang D-R, Todd R. Non-steady-state measurement of in vivo radioligand binding with positron emission tomography: specificity analysis and comparison with in vitro binding. J Neurosci. 1991b;11:1381–1389. doi: 10.1523/JNEUROSCI.11-05-01381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perlmutter JS, Rowe CC, Lich LL (1993) In vivo pharmacological activation of dopaminergic pathways in primates studied with PET. J Cereb Blood Flow Metab 13[Suppl 1]:S286.
- 87.Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, Moerlein SM. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17:843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piercey MF, Moon MW, Hoffman WE, Walters R, Blanchette P, Bédard P. U-91356A, a potent, effective, orally active D2 agonist useful in treating Parkinson’s Disease. Soc Neurosci Abstr. 1992;18:1081. [Google Scholar]
- 89.Piercey MF, Camacho-Ochoa M, Smith MW (1995) Functional roles for dopamine-receptor subtypes. Clin Neuropharm 18[Suppl 1]:S34–S42.
- 90.Pizzolato G, Soncrant TT, Rapoport SI. Haloperidol and cerebral metabolism in the conscious rat: relation to pharmacokinetics. J Neurochem. 1984;43:724–732. doi: 10.1111/j.1471-4159.1984.tb12792.x. [DOI] [PubMed] [Google Scholar]
- 91.Pizzolato G, Soncrant TT, Holloway HW, Rapoport SI. Reduced metabolic response of the aged rat brain to haloperidol. J Neurosci. 1985a;5:2831–2838. doi: 10.1523/JNEUROSCI.05-11-02831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pizzolato G, Soncrant TT, Larson DM, Rapoport SI. Reduced metabolic response of the rat brain to haloperidol after chronic treatment. Brain Res. 1985b;337:1–9. doi: 10.1016/0006-8993(85)91604-x. [DOI] [PubMed] [Google Scholar]
- 93.Pizzolato G, Soncrant TT, Rapoport SI. Time-course and regional distribution of the metabolic effects of bromocriptine in the rat brain. Brain Res. 1985c;341:303–312. doi: 10.1016/0006-8993(85)91069-8. [DOI] [PubMed] [Google Scholar]
- 94.Pizzolato G, Soncrant TT, Larson DM, Rapoport SI. Stimulatory effect of the D2 antagonist sulpiride on glucose utilization in dopaminergic regions of rat brain. J Neurochem. 1987;49:631–638. doi: 10.1111/j.1471-4159.1987.tb02910.x. [DOI] [PubMed] [Google Scholar]
- 95.Raichle ME. Circulatory and metabolic correlates of brain function in normal humans. In: Mountcastle VB, editor. Handbook of physiology: the nervous system V, Part 2. American Physiological Society; Bethesda, MD: 1987. pp. 643–674. [Google Scholar]
- 96.Raichle ME, Martin WRW, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H215O. II. Implementation and validation. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 97.Rascol OJ, Sabatini U, Chollet F, Montastruc JL, Marc-Vergnes JP, Rascol A. Impaired activity of the supplementary motor area in akinetic patients with Parkinson’s disease: improvement by the dopamine agonist apomorphine. Adv Neurol. 1993;60:419–421. [PubMed] [Google Scholar]
- 98.Reves JG, Glass PS. Nonbarbiturate intravenous anesthetics. In: Miller R, editor. Anesthesia, 3rd Ed. Churchill Livingstone; New York: 1990. pp. 243–279. [Google Scholar]
- 99.Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 100.Robinson T. Controls for lesions of the nigrostriatal dopamine system. Science. 1991;253:332. doi: 10.1126/science.1907023. [DOI] [PubMed] [Google Scholar]
- 101.Rotrosen J. Effects of amphetamine on local cerebral metabolism in normal and schizophrenic subjects as determined by positron emission tomography. Psychopharmacology (Berl) 1987;92:241–246. doi: 10.1007/BF00177923. [DOI] [PubMed] [Google Scholar]
- 102.Rougemont D, Baron JC, Collard P, Bustany P, Comar D, Agid Y. Local cerebral glucose utilisation in treated and untreated patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1984;47:824–830. doi: 10.1136/jnnp.47.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Russo KE, Hall W, Chi OZ, Sinha AK, Weiss HR. Effect of amphetamine on cerebral blood flow and capillary perfusion. Brain Res. 1991;542:43–48. doi: 10.1016/0006-8993(91)90995-8. [DOI] [PubMed] [Google Scholar]
- 104.Sabatini U, Rascol O, Celsis P, Houin G, Rascol A, Marc-Vergnes JP, Montastruc JL. Subcutaneous apomorphine increases regional cerebral blood flow in parkinsonian patients via peripheral mechanisms. Br J Clin Pharmacol. 1991;32:229–234. doi: 10.1111/j.1365-2125.1991.tb03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sadzot B, Price J, Mayberg H, Douglass K, Dannals R, Lever J, Ravert H, Wilson A, Wagner H, Feldman M, Frost J. Quantification of human opiate receptor concentration and affinity using high and low specific activity [11C]diprenorphine and positron emission tomography. J Cereb Blood Flow Metab. 1991;11:204–219. doi: 10.1038/jcbfm.1991.52. [DOI] [PubMed] [Google Scholar]
- 106.Sharkey J, McBean DE, Kelly PA. Acute cocaine administration: effects on local cerebral blood flow and metabolic demand in the rat. Brain Res. 1991;548:310–314. doi: 10.1016/0006-8993(91)91138-q. [DOI] [PubMed] [Google Scholar]
- 107.Spinks TJ, Jones T, Bailey DL, Townsend DW, Grootoonk S, Bloomfield PM, Gilardi M-C, Casey ME, Sipe B, Reed J. Physical performance of positron tomograph for brain imaging with retractable septa. Phys Med Biol. 1993;37:1637–1655. doi: 10.1088/0031-9155/37/8/002. [DOI] [PubMed] [Google Scholar]
- 108.Stein EA, Fuller SA. Selective effects of cocaine on regional cerebral blood flow in the rat. J Pharmacol Exp Ther. 1992;262:327–334. [PubMed] [Google Scholar]
- 109.Strange PG. Neostriatal dopamine receptors. Trends Neurosci. 1990;13:324–325. doi: 10.1016/0166-2236(90)90138-z. [DOI] [PubMed] [Google Scholar]
- 110.Tarazi FI, Shirakawa O, Tamminga CA. Low-dose raclopride spares the extrapyramidal system in rat brain from metabolic effects. Eur J Pharmacol. 1993;232:71–77. doi: 10.1016/0014-2999(93)90730-6. [DOI] [PubMed] [Google Scholar]
- 111.Todd RD, Carl J, Harmon S, O’Malley KL, Perlmutter JS. Dynamic changes in striatal dopamine D2 and D3 receptor protein and mRNA in response to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) denervation in baboons. J Neurosci. 1996;16:7776–7782. doi: 10.1523/JNEUROSCI.16-23-07776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trugman JM, Wooten GF. The effects of l-DOPA on regional cerebral glucose utilization in rats with unilateral lesions of the substan- tia nigra. Brain Res. 1986;379:264–274. doi: 10.1016/0006-8993(86)90780-8. [DOI] [PubMed] [Google Scholar]
- 113.Trugman JM, Wooten GF. Selective D1 and D2 dopamine agonists differentially alter basal ganglia glucose utilization in rats with unilateral 6-hydroxydopamine substantia nigra lesions. J Neurosci. 1987;7:2927–2935. doi: 10.1523/JNEUROSCI.07-09-02927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tuor UI, Edvinsson L, McCulloch J. Catecholamines and the relationship between cerebral blood flow and glucose use. Am J Physiol. 1986;251:H824–H833. doi: 10.1152/ajpheart.1986.251.4.H824. [DOI] [PubMed] [Google Scholar]
- 115.von Essen C, Zervas NT, Brown DR, Koltun WA, Pickren KS. Local cerebral blood flow in the dog during intravenous infusion of dopamine. Surg Neurol. 1980;13:181–188. [PubMed] [Google Scholar]
- 116.Videen TO, Perlmutter JS, Herscovitch P, Raichle ME. Brain blood volume, flow and oxygen utilization measured with O-15 radiotracers and positron emission tomography: revised metabolic computations. J Cereb Blood Flow Metab. 1987;7:513–516. doi: 10.1038/jcbfm.1987.97. [DOI] [PubMed] [Google Scholar]
- 117.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comp Assist Tomogr. 1993;14:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 118.Yaster M, Koehler RC, Traystman RJ. Interaction of fentanyl and nitrous oxide on peripheral and cerebral hemodynamics in newborn lambs. Anesthesiology. 1994;80:364–371. doi: 10.1097/00000542-199402000-00016. [DOI] [PubMed] [Google Scholar]
- 119.Young AB, Penney JB. Biochemical and functional organization of the basal ganglia. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders, 2nd Ed. Williams & Wilkins; Baltimore: 1993. pp. 1–11. [Google Scholar]