Abstract
Most in vitro studies of D1 dopaminergic modulation of excitability in neostriatal medium spiny neurons have revealed inhibitory effects. Yet studies made in more intact preparations have shown that D1 receptors can enhance or inhibit the responses to excitatory stimuli. One explanation for these differences is that the effects of D1 receptors on excitability are dependent on changes in the membrane potential occurring in response to cortical inputs that are seen only in intact preparations. To test this hypothesis, we obtained voltage recordings from medium spiny neurons in slices and examined the impact of D1 receptor stimulation at depolarized and hyperpolarized membrane potentials. As previously reported, evoked discharge was inhibited by D1 agonists when holding at negative membrane potentials (approximately −80 mV). However, at more depolarized potentials (approximately −55 mV), D1 agonists enhanced evoked activity. At these potentials, D1 agonists or cAMP analogs prolonged or induced slow subthreshold depolarizations and increased the duration of barium- or TEA-induced Ca2+-dependent action potentials. Both effects were blocked by L-type Ca2+ channel antagonists (nicardipine, calciseptine) and were occluded by the L-type channel agonist BayK 8644—arguing that the D1 receptor-mediated effects on evoked activity at depolarized membrane potential were mediated by enhancement of L-type Ca2+ currents. These results reconcile previous in vitro and in vivostudies by showing that D1 dopamine receptor activation can either inhibit or enhance evoked activity, depending on the level of membrane depolarization.
Keywords: dopamine; neuromodulation; firing patterns; calcium; neostriatum, basal ganglia
Neostriatal projection neurons are a major target of dopaminergic afferents. Despite the importance of this innervation, the impact of dopamine on the excitability of spiny neurons is controversial. One of the factors underlying the disagreement is receptor heterogeneity (Civelli et al., 1991; Sibley and Monsma, 1992;Surmeier et al., 1992). There are at least five dopamine receptor genes coding for D1 (D1a, D1b) and D2 (D2, D3, D4) receptor classes (Sibley, 1995), all of which are expressed in the neostriatum. To some extent, heterogeneity in physiological responses may be a reflection of receptor heterogeneity. However, this does not seem to be the case for D1 class responses, because medium spiny neurons express predominantly D1a mRNA (Gerfen, 1992;Bergson et al., 1995; Hersch et al., 1995; Surmeier et al., 1996).In vitro, D1 class agonists generally have been found to inhibit evoked discharge (Uchimura et al., 1986; Akaike et al., 1987; Calabresi et al., 1987; Hernández-López et al., 1996a; Pacheco-Cano et al., 1996) (cf. Rutherford et al., 1988). However, in vivo, dopamine and D1 receptor activation can either inhibit or excite evoked or spontaneous discharge (Gonzalez-Vegas, 1974; Kitai et al., 1976; Norcross and Spehlmann, 1978; Herrling and Hull, 1980; Mercuri et al., 1985; Hu et al., 1990;Kiyatkin and Rebec, 1996). Indirect evidence for an excitatory action of D1 agonists comes from their ability to induce immediate early gene expression (Berretta et al., 1992; Cenci et al., 1992; Cole et al., 1992; Steiner and Gerfen, 1993). These observations have been used (Gerfen, 1992; Alexander, 1995) to argue that D1receptor activation promotes discharge in spiny neurons—in clear contradiction to most physiological studies in vitro as well as many studies in vivo.
The difficulty in the interpretation of these results is that the activation of G-protein-coupled receptors exerts its effects in large measure by modulating the properties of voltage-dependent ionic conductances (Nicoll, 1988; Nicoll et al., 1990). However, activity of ionic conductances and neuronal firing patterns depends on membrane potential (Llinás, 1988; Bargas and Galarraga, 1995). As a consequence, the impact of receptor activation may be different at different membrane potentials.
Voltage-clamp techniques have provided a biophysical underpinning for some of the inhibitory effects of D1 receptor activation seen in current-clamp recordings: activation of D1receptors leads to suppression of Na+ currents, as well as N- and P-type Ca2+ currents (Surmeier et al., 1992, 1995;Cepeda et al., 1995; Schiffmann et al., 1995). However, D1receptor activation also has been shown to enhance L-type Ca2+ currents in spiny neurons (Surmeier et al., 1995). These currents are activated at relatively negative membrane potentials in neostriatal neurons (Bargas et al., 1994), suggesting that they may play a role in the maintenance of repetitive firing (Hounsgaard and Mintz, 1988). Enhancement of L-type Ca2+ currents could lead to increased discharge at depolarized membrane potentials.
Can this pattern of effects explain the discrepancy? In vivo, neostriatal neurons move between “up” and “down” states (Wilson, 1993; Wilson and Kawaguchi, 1996). In the absence of cortical input, spiny neurons are quiescent, with resting membrane potentials near −80 mV (“down-state”). This is the state seenin vitro. In response to cortical input, medium spiny neurons depolarize, with a mean membrane potential more positive than −60 mV (“up-state”). It was our working hypothesis that the voltage-dependent conductances known to be reduced by D1agonists play a role in the up-state transition and the integration of synaptic inputs but that the D1 receptor-mediated enhancement of L-type Ca2+ channels would lead to an elevation of evoked discharge once the up-state had been achieved.
A portion of this work has been presented in abstract form (Hernández-López et al., 1996b).
MATERIALS AND METHODS
Rat neostriatal slices were prepared as described previously (Bargas et al., 1988). In brief, male adult albino Wistar rats (200–300 gm) were anesthetized, and their brains were removed into ice-cold control saline (see below). Brain slices 400 μm thick were cut on a vibratome and placed in artificial cerebrospinal solution at 25°C. After 1 hr, slices were placed in a submerged recording chamber. Slices were superfused with saline containing (in mm): 120 NaCl, 3 KCl, 25 NaHCO3, 2 CaCl2, 1 MgCl2, and 11 glucose (290 mOsm/l with glucose, pH 7.4 after bubbling with 95% O2/5% CO2, at 32–34°C). Tetraethylammonium chloride (TEA), cesium chloride (Cs), barium chloride (Ba), and dibutyryl cAMP (db-cAMP) were obtained from Sigma (St. Louis, MO); dopamine, SKF 38393, SKF 81297, SKF 82958, SCH 23390, sulpiride, quinpirole, and BayK 8644 were obtained from Research Biochemicals (Natick, MA); calciseptine was obtained from Peptides International (Louisville, KY); nicardipine was obtained from Alomone Labs (Jerusalem, Israel). All reagents were added from freshly prepared stock solutions to the bath saline. Intracellular recordings were performed with microelectrodes filled with 3–4 m K-acetate (80–120 MΩ). Records were obtained with an active bridge electrometer (Neurodata Instruments), digitized, and saved on VHS tapes at 40 kHz to be analyzed off-line with the help of a PC-clone computer and programs designed using the LabView environment (National Instruments, Austin, TX). Recordings were always made at the head of the caudate–putamen nucleus (see Fig. 1 inSurmeier et al., 1995). After recording, some neurons were injected with biocytin as previously described (Horikawa and Armstrong, 1988;Flores-Hernández et al., 1994). Approximately 140 neurons were recorded, and 40 of them were filled and reconstructed. All of them were medium-sized spiny projection neurons. Stimulation consisted in intracellular depolarizing current steps that, once chosen, were maintained during test observations.
RESULTS
D1 agonists enhance the evoked response from depolarized membrane potentials
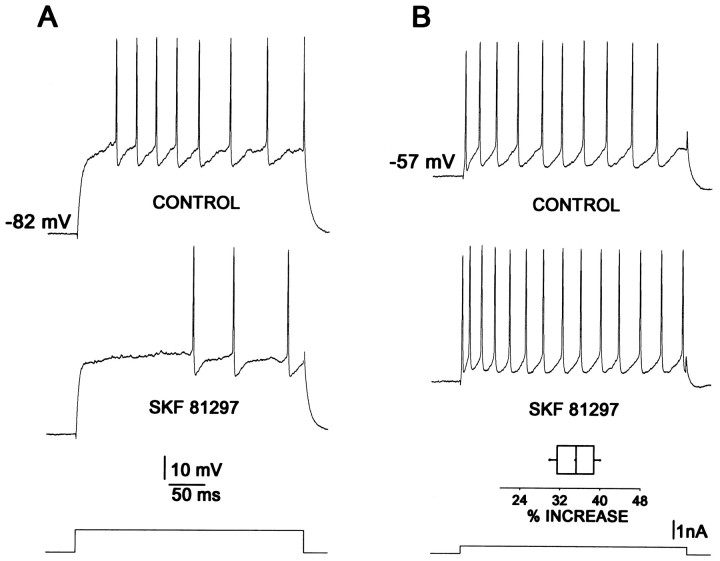
The most reproducible action of dopamine and D1receptor agonists is to decrease discharge evoked by intracellular current injection. For example, Figure1A shows the response of a medium spiny neuron to a current step in the absence (top) and presence (bottom) of a D1 receptor agonist (SKF 81297, 1 μm) when the resting membrane potential was near −80 mV. Similar effects of D1 class agonists have been reported previously by a number of groups (Uchimura et al., 1986;Akaike et al., 1987; Calabresi et al., 1987; Pacheco-Cano et al., 1996). This reduction in evoked discharge has been attributed to the modulation of Na+ channels (Calabresi et al., 1987;Surmeier et al., 1992; Cepeda et al., 1995), subthreshold K+ channels (Pacheco-Cano et al., 1996), and channels participating in the early afterhyperpolarization (AHP;Hernández-López et al., 1996a).
Fig. 1.
Actions of dopaminergic D1 receptor agonists are inhibitory or excitatory, depending on membrane potential.A, The firing response to a step depolarization at a resting membrane potential of approximately −82 mV (top, 7 action potentials) is reduced in the presence of 1 μm SKF 81297 (middle, 3 action potentials). Stimulus current is at the bottom. Stimulus and membrane potential are maintained for both conditions.B, In the same neuron the firing evoked from a membrane potential of approximately −57 mV (top, 10 action potentials) is increased by 1 μm SKF 81297 (middle, 14 action potentials). Stimulus at thebottom was maintained constant in B.Box plot in B shows mean firing increase for a sample of neurons (n = 6) in the presence of 1 μm SKF 81297.
When medium spiny neurons are held at depolarized membrane potentials similar to those found in the up-state (approximately −55 mV), the response to D1 receptor agonists changes dramatically. An example is shown in Figure 1B. Here, the neuron has been depolarized by intracellular current injection to −57 mV. Current steps from this potential evoked repetitive discharge (Fig.1B, top). The addition of SKF 81297 (1 μm) led to an enhancement in the evoked discharge (Fig.1B, bottom), in contrast to the effect at negative membrane potentials. This change in evoked firing was blocked by the D1 class antagonist SCH 23390 (1 μm,n = 4), arguing that the effect was mediated by D1a or D1b receptors. The D1receptor agonist increased the number of spikes evoked by a 200–300 msec current step given at relatively low frequency (0.1–0.2 Hz) by 34% (n = 6; p < 0.01 by Wilcoxon’st test). Similar facilitatory effects were produced by two other D1 receptor agonists (1–5 μm SKF 82958, n = 4; 1–5 μm SKF38393,n = 6). This facilitatory effect was clearly evident in 16 of 20 neurons tested.
How might changing the resting membrane potential produce such a qualitative alteration in the effects of D1 class agonists? One possibility is that membrane depolarization inactivates or closes ionic conductances that are important determinants of responsiveness from hyperpolarized potentials. Several ionic conductances that are prominent in medium spiny neurons fall into this category, including inward rectifiers and slowly inactivating K+ conductances (Surmeier et al., 1991; Galarraga et al., 1994; Nisenbaum and Wilson, 1995; Nisenbaum et al., 1996; Pacheco-Cano et al., 1996). Some of these conductances are the subject of inactivation on depolarization (Nisenbaum et al., 1996) or are blocked by extracellular Cs+ (Galarraga et al., 1994).
The inactivation of these outward currents then could unmask a D1 receptor-mediated enhancement of the L-type Ca2+ current, allowing it to depolarize the neuron further and augment the evoked discharge. So that this hypothesis could be tested, medium spiny neurons were driven by long current pulses (2 sec) at relatively high frequency (0.33 Hz). In this situation, D1 receptor agonists first inhibited and then enhanced the evoked firing. At the top of Figure 2 is shown the response to the long current step before superfusion of the D1 agonist. After beginning superfusion, the D1agonist suppressed the initial component of the response (as seen with the short steps), but the evoked discharge late in the step was enhanced (Fig. 2, middle). With more prolonged repetitive stimulation (and presumably greater inactivation of depolarization activated K+ currents), the response was enhanced throughout the current step (Fig. 2, bottom), just as when the cell was held at the depolarized potential. Similar effects were seen in all five of the other neurons tested.
Fig. 2.
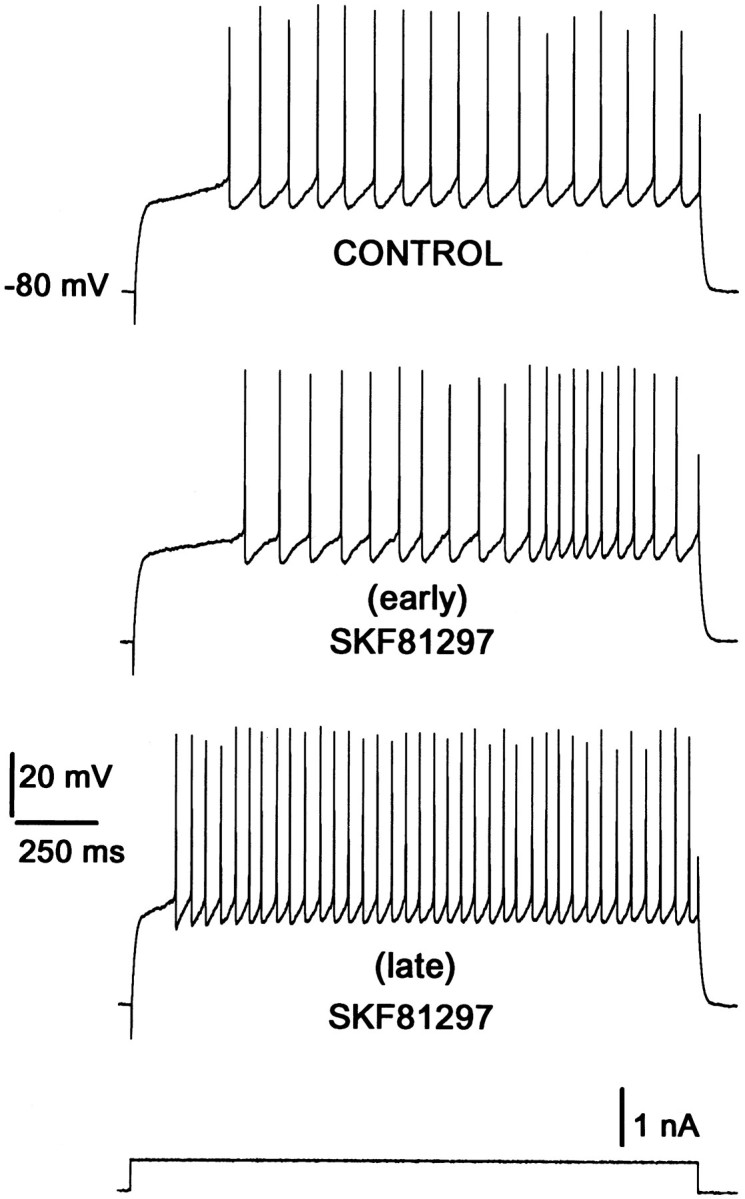
Repetitive step current pulses of long duration facilitate the excitatory action of dopaminergic D1 agonists. The control (top) shows tonic firing elicited by a 2 sec current step (bottom trace) from −80 mV. Superfusion with 1 μm SKF81297 induced a decrease in firing frequency initially. However, after a few minutes (≥5 min) some records had both the inhibitory and the facilitatory responses in the same trace (early). At longer times the only response remaining was facilitatory (late). [See also Fig. 4 and Surmeier et al. (1995); Fig. 6 and Uchimura et al. (1986); Fig. 1.]
Extracellular Cs+ mimics the effects of depolarization
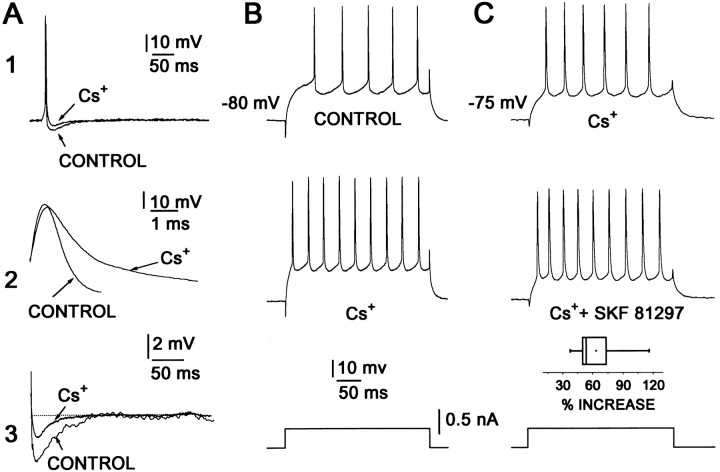
To examine further the role of K+ currents in shaping the response to D1 agonists, we studied the impact of Cs+ on the modulation. Extracellular application of Cs+ (2 mm) broadens action potentials, diminishes the spike afterhyperpolarization (Fig.3A; n = 9), and reduces inward rectification (Galarraga et al., 1994). The spike discharge evoked by current injection from hyperpolarized membrane potentials also is enhanced by Cs+ (Fig. 3B). To test whether Cs+-sensitive conductances were responsible for shaping the qualitative features of the response to D1receptor agonists from hyperpolarized membrane potentials, we applied agonists in the presence of Cs+ (2 mm). In accord with the inferred importance of K+ currents, D1 receptor agonists enhanced the response to current steps from hyperpolarized membrane potentials (between −75 and −80 mV) when Cs+ was present (Fig. 3C; see also Pacheco-Cano et al., 1996). On average, D1 receptor agonists increased the number of evoked spikes by nearly 60% in the presence of Cs+ (n = 6; p < 0.01 by Wilcoxon’s t test). These results argue that alterations in the complement of K+ conductances that govern the transition from the down- to the up-state or maintain the down-state qualitatively change the impact of D1 receptor stimulation.
Fig. 3.
Outward current blockage facilitates excitatory action of dopaminergic D1 agonists. A,Top records show a superimposition of single action potentials evoked by a brief current step (omitted) before and during superfusion with a 2 mm Cs+- containing solution (1). Cs+ (2 mm) slows down action potential repolarization (2) and reduces the afterhyperpolarization (AHP) that follows a single action potential (3). Parts 2 and 3 show events taken from Part 1 but displayed at different sweep speeds. B, Outward current blockage by Cs+ induces an increase in firing response to the same stimulus at a relatively hyperpolarized membrane potential. Current stimulus is shown at the bottom. C, The presence of Cs+ effects does not block a further increase in firing frequency by the addition of 1 μm SKF 81297.Box plot shows percentage of increase in firing during SKF 81297 in a sample of neurons in the presence of Cs+. Stimulus is shown at the bottom. B andC illustrate records from different cells.
D1 receptor agonists lengthen calcium-dependent action potentials
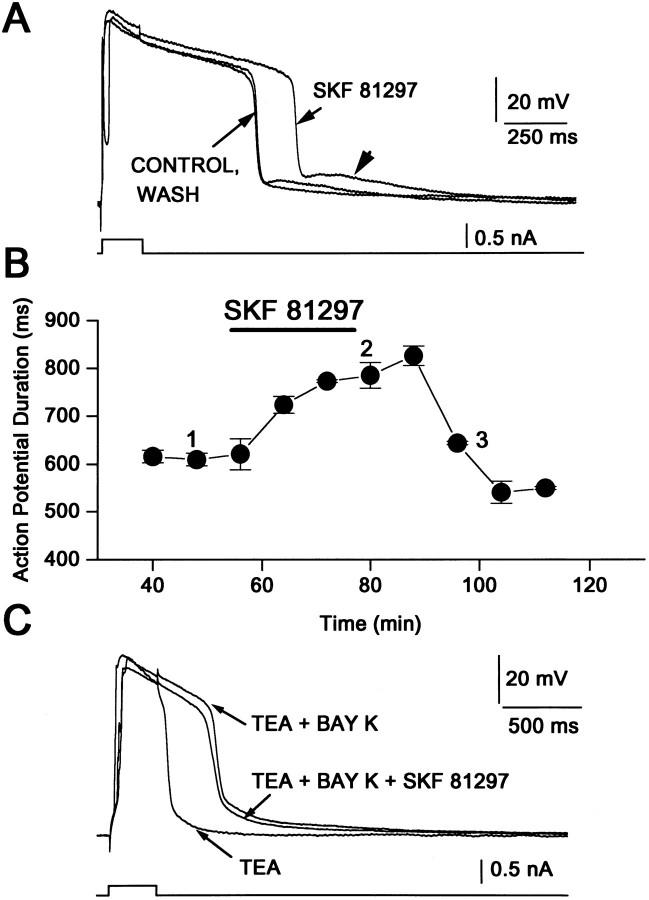
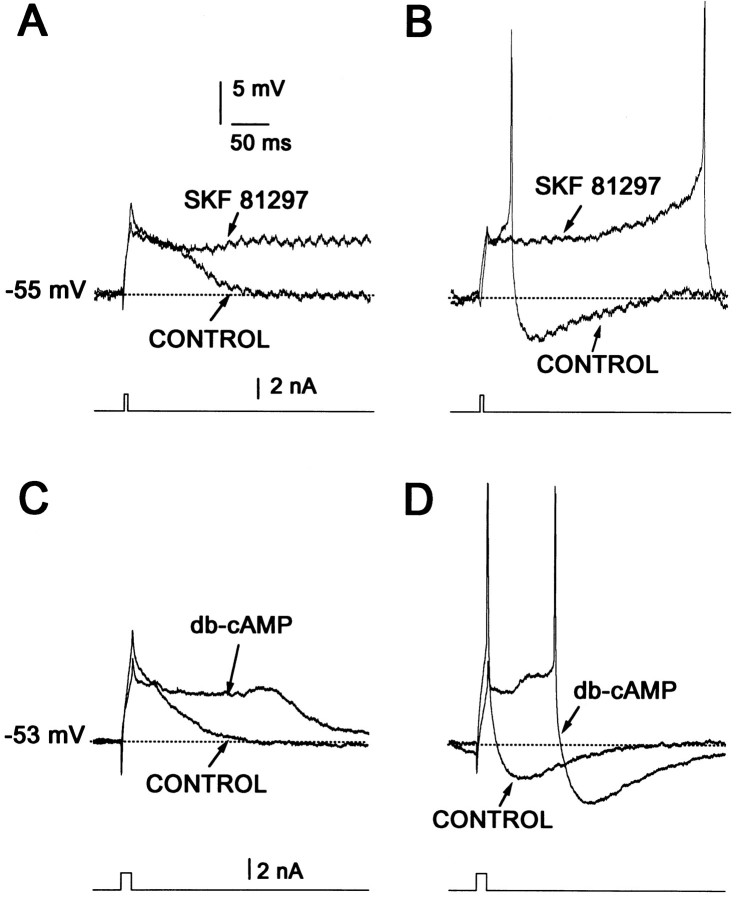
How does D1 receptor activation lead to an enhanced response to excitatory inputs? In some cells persistent Na+currents may induce or sustain repetitive firing (Llinás and Sugimori, 1980; Llinás, 1988; Bargas and Galarraga, 1995). However, Na+ currents are inhibited by D1agonists in spiny neurons (Surmeier et al., 1992; Cepeda et al., 1995;Schiffmann et al., 1995). In motoneurons, L-type Ca2+currents are responsible for sustained depolarizations and repetitive firing (Hounsgaard and Mintz, 1988). In medium spiny neurons, L-type Ca2+ currents are enhanced by D1 receptor activation (Surmeier et al., 1995), suggesting that this conductance may be responsible for the excitatory action at depolarized potentials. As a first test of this hypothesis, the impact of D1 class agonists on Ca2+-mediated action potentials (Ca2+ APs) was examined (see also Fig. 6 in Surmeier et al., 1995). Ca2+ APs were induced by extracellular application of TEA (10–20 mm) (Kita et al., 1985;Galarraga et al., 1989). SKF 81297 (1 μm) increased the duration of the Ca2+ AP by almost 200 msec (Fig.4A). The maximal effect was seen in ∼15 min (Fig. 4B) and persisted for some time after the agonist was washed. Repolarization of a Ca2+ AP often was followed by a slow depolarization (arrowhead in Fig.4A), which may reflect propagation into the dendrites (Llinás and Sugimori, 1980; Bargas et al., 1991; Jaffe et al., 1992; Amitai et al., 1993; Reuveni et al., 1993; Larkum et al., 1996). D1 receptor agonists increased the action potential duration in nearly all of the neurons tested with SKF 81297 (15 of 16 neurons with a mean of increase of 19 ± 7%; p < 0.01; Wilcoxon’s t test). A similar modulation was observed after the application of dopamine (10 μm,n = 2), SKF 82958 (1–5 μm,n = 3), and SKF 38393 (5 μm,n = 2). The effect of D1 agonists was blocked by D1 receptor antagonist SCH 23390 (1 μm, n = 4), but not by the D2receptor antagonist sulpiride (5 μm, n = 2), arguing that the effect was mediated by D1 receptors. If D1 receptors were acting by stimulating adenylyl cyclase, then the effects of the receptor agonist should be mimicked by cAMP analogs. The cAMP analog dibutryl-cAMP (1 mm) lengthened the Ca2+-mediated action potentials in a manner very similar to D1 receptor agonists (n = 4/4). These results are consistent with our previous finding that activation of D1 class receptors leads to an enhancement of L-type Ca2+ currents and Ca2+-dependent action potentials (Surmeier et al., 1995). If this conclusion is correct, the response to D1 receptor agonists should be occluded by the L-type channel agonist BayK 8644. As shown in Figure 4C and reported previously (Cherubini and Lanfumey, 1987), the addition of BayK 8644 (5 μm) promotes an increase in the Ca2+-dependent AP duration that is similar to that produced by D1 agonists (n = 6). The subsequent addition of SKF 81297 (1 μm) failed to produce any further change in the Ca2+ AP (Fig. 4C;n = 4)—suggesting that D1 receptors and BayK 8644 were acting on the same target.
Fig. 4.
D1 agonists increase the duration of TEA-induced Ca2+-dependent action potentials.A, Superimposed records of Ca2+-dependent action potentials induced by short current steps (bottom) delivered at low frequency (0.1 Hz) in the presence of 20 mm TEA. SKF 81297 (1 μm) increases the duration of these events. Hyperpolarizing current steps were interspersed between the depolarizing stimulus to prevent changes caused by current inactivation. Traces shown were taken at times numbered in B. Note slow depolarization at the end of the fast action potential repolarization (arrowhead). Membrane potential is approximately equal to −70 mV. B, Time course of SKF 81297 action on Ca2+ entry.Bar shows time of D1 agonist in the superfusion. C, The dihydropyridine L-type channel agonist BayK 8644 produced a similar increase in duration of the TEA-induced Ca2+ action potential and occluded the effect of the D1 agonists. Membrane potential is approximately equal to −60 mV. Stimulus is shown at bottom.
D1 receptor agonists enhance subthreshold slow depolarizations dependent on L-type channels
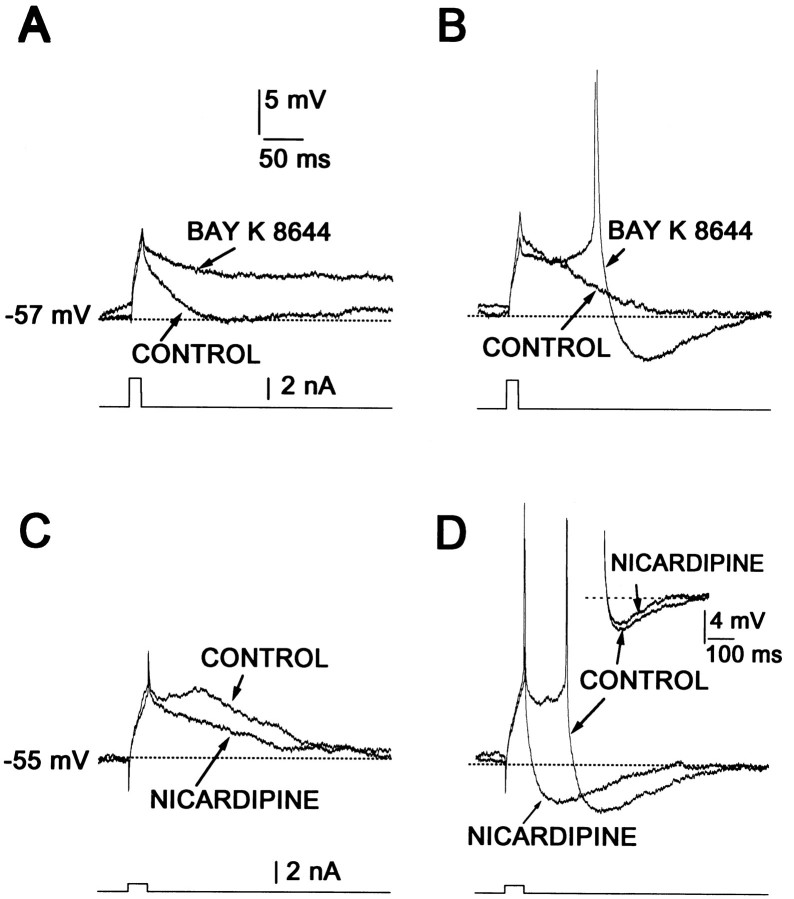
Although D1 class receptors were capable of enhancing L-type currents responsible for the Ca2+-dependent plateau potential, it remained to be determined whether they could enhance subthreshold depolarizations and evoke repetitive discharge at depolarized membrane potentials. When medium spiny neurons are held above −60 mV, brief current steps frequently evoke slow and sustained subthreshold depolarizations (76/95 neurons). Bay K 8644 (1–5 μm) enhanced these slow depolarizations, often leading to the generation of spikes well after the stimulus was terminated (delayed firing, Fig. 5A,B; n= 8). The L-type channel antagonist nicardipine (5 μm) attenuated these depolarizing responses in cases in which the membrane potential remained below spike threshold (Fig. 5C) or even when spikes were evoked (Fig. 5D; n = 4). The L-type channel antagonist calciseptine (1 μm) also mimicked the effects of nicardipine on the slow depolarizations (n = 2). In fact, in the presence of these L-channel blockers, delayed discharge was impossible to obtain.
Fig. 5.
L-type channels participate in firing mechanisms.A, B, Responses to a brief current stimulus may elicit passive (control in A) or small active responses (control in B).BayK 8644 (5 μm) facilitated slow depolarizations that outlasted the stimulus and delayed firing (spike is clipped). Current stimulus is shown at bottom.C, D, Slow subthreshold depolarizations and delayed firing induced by a brief stimulus are abolished bynicardipine (5 μm). Insetshows a small AHP reduction by nicardipine. Current stimulus is shown at bottom.
The slow depolarizations and delayed firing were enhanced significantly by D1 class receptor stimulation. SKF 81297 (1 μm) lengthened the duration of subthreshold depolarizations (Fig. 6A), often leading to a late spike at a time when the membrane potential normally had returned to baseline (Fig. 6B; n= 6). Similar results were obtained with dopamine (10 μm,n = 2) or SKF 82958 (1 μm,n = 2). The D1 class receptor antagonist SCH 23390 (1 μm; n = 4), nicardipine (5 μm; n = 2), and calciseptine (200 nm) prevented D1 class receptor activation from enhancing the slow depolarizations or delayed discharge. The D2 receptor agonist quinpirole (5 μm) had no effect on the slow depolarization (n = 3). As expected of a response triggered by D1 class receptors, the cAMP analog dibutyryl cAMP (500 μm) had effects very similar to the receptor agonists (Fig. 6C,D; n = 4). Last, the K+ current blockers TEA (2–10 mm;n = 4) and Ba2+ (1 mm;n = 2) were able to facilitate slow depolarizations and delayed firing but did not occlude D1 receptor-mediated effects (data not shown).
Fig. 6.
Dopaminergic D1 receptor agonists and cAMP analogs facilitate slow depolarizations and delayed firing.A, B, Superimposed records show that D1agonists (e.g., 1 μmSKF 81297) greatly enhance slow subthreshold depolarizations and promote delayed firing if they are evoked by a brief stimulus (bottom) at approximately −55 mV. C, D, These effects can be mimicked by cAMP analogs (e.g., 500 μmdb-cAMP).
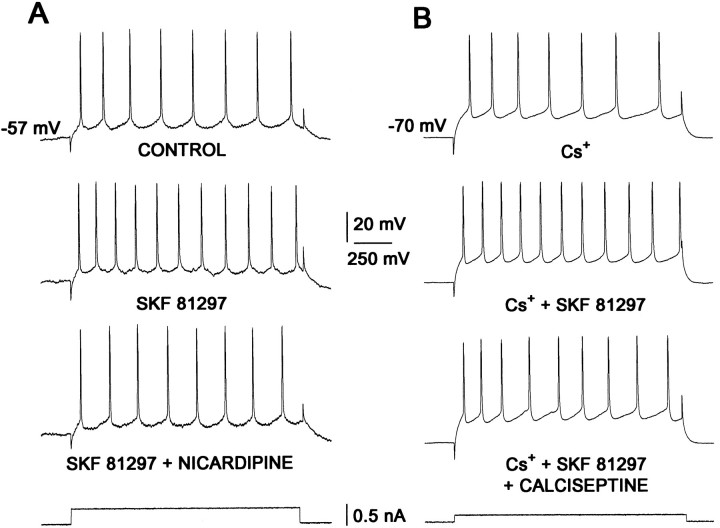
Taken together, these experiments strongly suggest that the dopaminergic D1 receptor enhancement of evoked discharge at depolarized potentials or in the presence of Cs+ is dependent on a potentiation of L-type Ca2+ currents. To test this hypothesis further, we examined the ability of L-channel antagonists to block the dopaminergic effect on evoked discharge. First, neurons were held at depolarized potentials. As shown in Figure7A, SKF 81297 (1 μm) enhanced the discharge evoked by a current step from −57 mV. This effect was blocked by nicardipine (5 μm, n = 6). Next, Cs+ (2 mm) was applied. As shown in Figure 7B, SKF 81297 (1 μm) enhanced the discharge evoked by a current step from −70 mV in this condition. The enhancement was blocked by the L-channel antagonist calciseptine (200 μm, n = 2). These experiments show not only that L-type Ca2+ currents contribute to the regulation of repetitive discharge in medium spiny neurons (see also Galarraga et al., 1989; Bargas et al., 1991; Pineda et al., 1992;Hernández-López et al., 1996a) but that D1class receptors are capable of enhancing evoked activity by augmenting these currents.
Fig. 7.
Firing enhancement induced by D1receptor agonists is blocked by L-type channel antagonists.A, Repetitive firing was evoked at −57 mV (top row, 8 action potentials). In these conditions, addition of 1 μm SKF 81297 to the superfusion increases the firing frequency to the same stimulus (2nd row, 11 action potentials). This effect is reduced by addition of 5 μmnicardipine (3rd row). Bottom row is current stimulus. B, Repetitive firing was evoked at −70 mV in the presence of Cs+ (top row, 7 action potentials). In these conditions, addition of 1 μmSKF 81297 to the superfusion increases the firing frequency to the same stimulus (2nd row, 11 action potentials). This effect is reduced by 0.2 μm calciseptine (3rd row).Bottom row is current stimulus.
DISCUSSION
D1 dopamine receptor activation is capable of enhancing evoked discharge by augmenting L-type Ca2+currents. Our results show that activation of D1 dopamine receptors on medium spiny neurons can have excitatory, as well as inhibitory, effects on evoked discharge (see Fig. 1). The D1 receptor-mediated enhancement of evoked activity was evident when the membrane potential was held above −60 mV—a potential close to that observed in vivo during cortically driven episodic discharge (Wilson, 1993; Wilson and Kawaguchi, 1996). The enhancement in activity also was seen when the membrane potential was held at −80 mV. However, long current steps repeated at relatively high frequency were necessary to reveal the excitatory effect (see Fig.2). The slow, progressive nature of the changes seen with this protocol suggests that the alteration in the D1 receptor effects are likely to be a consequence of the inactivation of slowly inactivating A currents that are prominent in medium spiny neurons (Bargas et al., 1989; Surmeier et al., 1991; Nisenbaum et al., 1996). This conclusion was supported by the ability of extracellular Cs+ to mimic the effects of membrane depolarization insofar as the D1effects were concerned (Bargas et al., 1989; Surmeier et al., 1991;Galarraga et al., 1994; Nisenbaum and Wilson, 1995; Pacheco-Cano et al., 1996) (see Fig. 3). This concentration of Cs+ (2 mm) attenuates prominent K+ currents, probably inwardly rectifying or inactivating conductances (Bargas et al., 1989;Surmeier et al., 1991; Galarraga et al., 1994; Nisenbaum and Wilson, 1995). In addition to blocking A currents, Cs+ at this concentration will block inwardly rectifying K+ currents (Galarraga et al., 1994; Pacheco-Cano et al., 1996). These K+ currents either are closed or are inactivated at depolarized membrane potentials (see also Rutherford et al., 1988). Hence, reducing the availability of a subset of K+-selective conductances unmasks the excitatory consequences of D1 receptor activation on evoked activity.
The mechanism by which D1 receptors enhanced evoked activity did not seem to involve further diminution of K+currents but, rather, the augmentation of inward L-type Ca2+ currents. Several pieces of evidence support this conclusion. One piece of evidence was that D1 agonists lengthened the Ca2+ APs seen in the presence of TEA or Ba2+ (see Fig. 4). These plateau potentials long have been known to be dependent, in part, on L-type Ca2+ channels (Kita et al., 1985; Cherubini and Lanfumey, 1987; Galarraga et al., 1989), as in other cell types (Llinás and Sugimori, 1980;Hounsgaard and Mintz, 1988; Amitai et al., 1993). In agreement with this conclusion, the L-type channel agonist Bay K 8644 mimicked the D1 receptor modulation and occluded any further modulation by receptor activation.
Another observation implicating L-type currents in the excitatory actions of D1 agonists had to do with their role in the generation of slow subthreshold depolarizations (see Fig. 5). These active responses to current injection from depolarized potentials were mimicked by L-type channel agonists (BayK 8644) and blocked by L-type channel antagonists. D1 agonists facilitated these slow depolarizing events (see Fig. 6). L-type Ca2+ channels antagonists blocked the D1 effect. This finding is consistent with voltage-clamp work in medium spiny neurons showing that L-type channels are activated at more negative membrane potentials than other Ca2+ channels (Bargas et al., 1994). It is also consistent with work in hippocampal pyramidal (Avery and Johnston, 1996) and motoneurons (Hounsgaard and Kiehn, 1993) in which L-type currents contribute to low-voltage-activated spikes or slow depolarizations.
The last and most conclusive evidence is that blocking L-type channels prevented D1 agonists from enhancing evoked discharge either from depolarized membrane potentials or from hyperpolarized membrane potentials in the presence of Cs+ (see Fig. 7). All of these findings argue that D1 receptors are capable of initiating a signaling cascade resulting in the enhancement of L-type Ca2+ current. This modulation leads to a potentiation of evoked discharge when the influence of some K+ currents in this process is reduced. This conclusion is in agreement with the observation that D1 receptors in medium spiny neurons are capable of enhancing L-type currents via a protein kinase A signaling cascade (Surmeier et al., 1995). One notable difference with this previous work is that the percentage of neurons in which D1 agonists were capable of enhancing L-type currents was significantly higher here. There are several possible reasons for the apparent discrepancy. One is that whole-cell recordings may compromise signaling via the PKA cascade by dialyzing away critical protein constituents. Another possibility is that sustained D1 receptor stimulation seems to be necessary for expression of the L channel modulation, and agonist application may not have been sufficiently long in our previous study. A third possibility is that the striatonigral neurons may have been sampled preferentially in this study. Combined patch-clamp and single-cell RT-PCR studies (Surmeier et al., 1996) in slices currently are being performed to resolve this question.
Functional implications
The implications of the D1 receptor-mediated modulation are best understood within the context of the natural behavior of medium spiny neurons. In vivo, medium spiny neurons move between two membrane potential ranges, referred to as “down” and “up” states (Wilson, 1993; Wilson and Kawaguchi, 1996). At rest, neurons are in the down-state, near −80 mV. In response to cortically originating excitatory synaptic input, neurons move to and stay in a more depolarized membrane potential (the up-state), near −55 mV, for hundreds of milliseconds or seconds. Although the up-state transition is driven by cortical input, the membrane potential trajectory in achieving the up-state, the mean potential once the up-state is achieved, and the duration of the up-state all are influenced by intrinsic voltage-dependent ion channels (Surmeier et al., 1991; Galarraga et al., 1994; Nisenbaum and Wilson, 1995; Wilson and Kawaguchi, 1996).
Most of the previous work examining the impact of D1receptor stimulation on evoked activity has been performed in striatal slices in which the normal cortical input has been disrupted. Neurons are typically in the down-state in this preparation, with resting membrane potentials near −80 mV. From this resting membrane potential, D1 receptor agonists consistently have been shown to reduce the response to somatic current injection (Akaike et al., 1987;Calabresi et al., 1987; Pacheco-Cano et al., 1996). This has been shown to be a consequence of the apparent enhancement of inward rectification (Pacheco-Cano et al., 1996) and the reduction of depolarizing Na+ currents (Surmeier et al., 1992; Cepeda et al., 1995). As a consequence of these effects, cortically originating excitatory input to medium spiny neurons should be less effective in promoting an up-state transition and discharge.
Once the up-state transition has occurred, however, the situation changes. With the maintained depolarization in the up-state, depolarization-activated Na+ and K+ currents begin to inactivate, and inwardly rectifying K+ currents shut off. At the same time, our results suggest that L-type currents begin to activate, helping to maintain the depolarized state. As predicted by this model, D1 agonists can potentiate the responses of medium spiny neurons to sustained glutamate application (Hu and Wang, 1988; Kiyatkin and Rebec, 1996) (see also Cepeda et al., 1993). So, this constellation of D1 receptor-mediated effects should produce a diminished sensitivity to weak, transitory cortical inputs but an enhanced response to strong, maintained cortical synaptic inputs. This is a type of signal-to-noise enhancement (Chiodo and Berger, 1986; Woodward et al., 1991). A conceptually similar pattern of effects has been described for dendritically originating synaptic events in medium spiny neurons. Here, D1 agonists attenuate fast excitatory synaptic potentials attributable to activation of glutamatergic receptors of the AMPA/KA class and enhance the slower depolarizations attributable to NMDA receptors (Cepeda et al., 1993). These effects may be mediated in part by presynaptic mechanisms (Calabresi et al., 1987; Flores-Hernández et al., 1997), but they definitely have a postsynaptic component (Colwell and Levine, 1995). This postsynaptic component may involve voltage-dependent channels known to be targeted by the D1receptor pathway. D1 receptor-mediated attenuation of Na+ and N-type Ca2+ currents (Surmeier et al., 1992, 1995; Schiffmann et al., 1995) or augmentation of inwardly rectifying K+ currents (Pacheco-Cano et al., 1996) could reduce the amplification of electrotonically remote synaptic AMPA/KA inputs (Amitai et al., 1993; Kim et al., 1993). However, if the depolarization of the dendrites is sustained, NMDA receptors are relieved of their Mg2+ block and become functional. D1 receptor activation and PKA phosphorylation of NMDA channels lead to an augmentation of this component of the cortically evoked response (Cepeda et al., 1993). The collateral enhancement of L-type currents should help support this facilitatory effect and potentially play a role in synaptic plasticity (Magee and Johnston, 1997). Last, the facilitatory effects of D1 receptor agonists found here are also similar to those found in neocortical cells, except that neocortical neurons use other ionic mechanisms (Yang and Seamans, 1996).
What might this mean for cortical control of basal ganglia circuitry? By promoting activity in those neurons receiving sustained, convergent cortical excitatory input and suppressing activity in neurons receiving weak, transient inputs, D1 receptor activation should focus activity effectively in the population of medium spiny neurons processing cortical signals. This modulation should be felt primarily by medium spiny neurons projecting to the globus pallidus and substantia nigra (Kawaguchi et al., 1989), because these neurons express high levels of D1a receptor mRNA and protein (Ariano, 1988; Gerfen, 1992; Surmeier et al., 1992, 1996; Hersch et al., 1995). It long has been suspected that D1 receptor activation in some manner promoted the activity of medium spiny neurons projecting to the substantia nigra (Alexander et al., 1995), but until now it has been unclear how this could happen. Our results give physiological ground to these conjectures for the first time and reconcile them with the bulk of the striatal electrophysiological literature by showing that the effect of D1 dopamine receptor activation is not excitatory or inhibitory—it is both!
Footnotes
This work was partially supported by DGAPA-UNAM Grants IN201094 to E.G., IN201194 to J.B., and National Institutes of Health Grant 34696 to D.J.S. We thank Dagoberto Tapia for his skillful help in anatomical work.
Correspondence should be addressed to Dr. Elvira Galarraga, Departamento de Biofísica, Instituto de Fisiología Celular, Universidad Nacional Autonoma de Mexico, P.O. Box 70-253, México DF, 04510, México.
REFERENCES
- 1.Akaike A, Ohno Y, Sasa M, Takaori S. Excitatory and inhibitory effects of dopamine on neuronal activity of the caudate neurons in vitro. Brain Res. 1987;418:262–272. doi: 10.1016/0006-8993(87)90094-1. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE. Basal ganglia. In: Arbib MA, editor. The handbook of brain theory and neural networks. MIT; Cambridge, MA: 1995. pp. 139–144. [Google Scholar]
- 3.Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative electrical activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993;3:26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Ariano MA. Striatal D1 dopamine receptor distribution following chemical lesion of the nigrostriatal pathway. Brain Res. 1988;443:204–214. doi: 10.1016/0006-8993(88)91614-9. [DOI] [PubMed] [Google Scholar]
- 5.Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargas J, Galarraga E. Ion channels: keys to neuronal specialization. In: Arbib MA, editor. The handbook of brain theory and neural networks. MIT; Cambridge, MA: 1995. pp. 496–501. [Google Scholar]
- 7.Bargas J, Galarraga E, Aceves J. Electrotonic properties of neostriatal neurons are modulated by extracellular potassium. Exp Brain Res. 1988;72:390–398. doi: 10.1007/BF00250260. [DOI] [PubMed] [Google Scholar]
- 8.Bargas J, Galarraga E, Aceves J. An early outward conductance modulates the firing latency and frequency of neostriatal neurons of the rat brain. Exp Brain Res. 1989;75:146–156. doi: 10.1007/BF00248538. [DOI] [PubMed] [Google Scholar]
- 9.Bargas J, Galarraga E, Aceves J. Dendritic activity on neostriatal neurons as inferred from somatic intracellular recordings. Brain Res. 1991;539:159–163. doi: 10.1016/0006-8993(91)90700-6. [DOI] [PubMed] [Google Scholar]
- 10.Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated adult rat neostriatal neurons. J Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional cellular and subcellular variation in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of c-fos-like protein in the striatum. J Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- 13.Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 14.Cenci MA, Campbell K, Wictorin K, Bjorklund A. Striatal c-fos induction by cocaine or apomorphine occurs preferentially in output neurons projecting to the substantia nigra in the rat. Eur J Neurosci. 1992;4:376–380. doi: 10.1111/j.1460-9568.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 15.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cepeda C, Chandler SH, Shumate LW, Levine MS. Persistent Na+ conductance in medium-sized neostriatal neurons: characterization using infrared videomicroscopy and whole-cell patch-clamp recordings. J Neurophysiol. 1995;74:1343–1348. doi: 10.1152/jn.1995.74.3.1343. [DOI] [PubMed] [Google Scholar]
- 17.Cherubini E, Lanfumey L. An inward calcium current underlying regenerative calcium potentials in rat striatal neurons in vitro enhanced by BAY K 8644. Neuroscience. 1987;21:997–1005. doi: 10.1016/0306-4522(87)90054-6. [DOI] [PubMed] [Google Scholar]
- 18.Chiodo LA, Berger TW. Interactions between dopamine and amino acid-induced excitation and inhibition in the striatum. Brain Res. 1986;375:198–203. doi: 10.1016/0006-8993(86)90976-5. [DOI] [PubMed] [Google Scholar]
- 19.Civelli O, Bunzow JR, Grandy DK, Zhou QY, Van Tol HHM. Molecular biology of the dopamine receptor. Eur J Pharmacol. 1991;207:277–286. doi: 10.1016/0922-4106(91)90001-x. [DOI] [PubMed] [Google Scholar]
- 20.Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 21.Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cAMP-dependent mechanisms. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores-Hernández J, Galarraga E, Pineda JC, Bargas J. Patterns of excitatory and inhibitory synaptic transmission in the rat neostriatum as revealed by 4-AP. J Neurophysiol. 1994;72:2246–2256. doi: 10.1152/jn.1994.72.5.2246. [DOI] [PubMed] [Google Scholar]
- 23.Flores-Hernández J, Galarraga E, Bargas J. Dopamine selects glutamatergic inputs to neostriatal neurons. Synapse. 1997;25:185–195. doi: 10.1002/(SICI)1098-2396(199702)25:2<185::AID-SYN9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Galarraga E, Bargas J, Sierra A, Aceves J. The role of calcium in the repetitive firing of neostriatal neurons. Exp Brain Res. 1989;75:157–168. doi: 10.1007/BF00248539. [DOI] [PubMed] [Google Scholar]
- 25.Galarraga E, Pacheco-Cano MT, Flores-Hernández J, Bargas J. Subthreshold rectification in neostriatal spiny projection neurons. Exp Brain Res. 1994;100:239–249. doi: 10.1007/BF00227194. [DOI] [PubMed] [Google Scholar]
- 26.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Vegas JA. Antagonism of dopamine-mediated inhibition in the nigro-striatal pathway: a mode of action of some catatonia-inducing drugs. Brain Res. 1974;80:219–228. doi: 10.1016/0006-8993(74)90686-6. [DOI] [PubMed] [Google Scholar]
- 28.Hernández-López S, Bargas J, Reyes A, Galarraga E. Dopamine modulates the afterhyperpolarization in neostriatal neurons. NeuroReport. 1996a;7:454–456. doi: 10.1097/00001756-199601310-00019. [DOI] [PubMed] [Google Scholar]
- 29.Hernández-López S, Bargas J, Reyes A, Galarraga E. Ion mechanisms for dopaminergic excitation in neostriatal neurons. Soc Neurosci Abstr. 1996b;22:411. [Google Scholar]
- 30.Herrling PL, Hull CD. Iontophoretically applied dopamine depolarizes and hyperpolarizes the membrane of cat caudate neurons. Brain Res. 1980;192:441–462. doi: 10.1016/0006-8993(80)90896-3. [DOI] [PubMed] [Google Scholar]
- 31.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5220–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horikawa A, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- 33.Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol (Lond) 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in turtle. J Physiol (Lond) 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X-T, Wang RY. Comparison of effects of D1 and D2 dopamine receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. J Neurosci. 1988;8:4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu X-T, Watchel SR, Galloway MP, White FJ. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate–putamen neurons and relieve D2 receptors from the necessity of the D1 receptor stimulation. J Neurosci. 1990;10:2318–2329. doi: 10.1523/JNEUROSCI.10-07-02318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN. The spread of Na spikes determines the pattern of dendritic Ca entry into hippocampal neurons. Nature. 1992;357:244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- 38.Kawaguchi Y, Wilson CJ, Emson P. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- 39.Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13:5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kita H, Kita T, Kitai ST. Active membrane properties of rat neostriatal neurons in an in vitro slice preparation. Exp Brain Res. 1985;60:54–62. doi: 10.1007/BF00237018. [DOI] [PubMed] [Google Scholar]
- 41.Kitai ST, Sugimori M, Kocsis JC. Excitatory nature of dopamine in the nigro-caudate pathway. Exp Brain Res. 1976;24:351–363. doi: 10.1007/BF00235003. [DOI] [PubMed] [Google Scholar]
- 42.Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- 43.Larkum ME, Rioult MG, Lüscher H-R. Propagation of action potentials in the dendrites of neurons from spinal cord slices in cultures. J Neurophysiol. 1996;75:154–170. doi: 10.1152/jn.1996.75.1.154. [DOI] [PubMed] [Google Scholar]
- 44.Llinás R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 45.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol (Lond) 1980;354:319–331. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magee JC, Johnston D. A synaptically controlled associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–212. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 47.Mercuri N, Bernardi G, Calabresi P, Cotugno A, Levi G, Stanzione P. Dopamine decreases cell excitability in rat striatal neurons by pre- and postsynaptic mechanisms. Brain Res. 1985;358:110–121. doi: 10.1016/0006-8993(85)90954-0. [DOI] [PubMed] [Google Scholar]
- 48.Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988;241:545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- 49.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 50.Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nisenbaum ES, Wilson CJ, Foehring RC, Surmeier DJ. Isolation and characterization of a persistent potassium current in neostriatal neurons. J Neurophysiol. 1996;76:1180–1194. doi: 10.1152/jn.1996.76.2.1180. [DOI] [PubMed] [Google Scholar]
- 52.Norcross K, Spehlmann R. A quantitative analysis of the excitatory and depressant effects of dopamine on the firing of caudatal neurons: electrophysiological support for the existence of two distinct dopamine-sensitive receptors. Brain Res. 1978;156:168–174. doi: 10.1016/0006-8993(78)90095-1. [DOI] [PubMed] [Google Scholar]
- 53.Pacheco-Cano MT, Bargas J, Hernández-López S, Tapia D, Galarraga E. Inhibitory action of dopamine involves a subthreshold Cs+-sensitive conductance in neostriatal neurons. Exp Brain Res. 1996;110:205–211. doi: 10.1007/BF00228552. [DOI] [PubMed] [Google Scholar]
- 54.Pineda JC, Galarraga E, Bargas J, Cristancho M, Aceves J. Charybdotoxin and apamin sensitivity of the calcium-dependent repolarization and the afterhyperpolarization in neostriatal neurons. J Neurophysiol. 1992;68:287–294. doi: 10.1152/jn.1992.68.1.287. [DOI] [PubMed] [Google Scholar]
- 55.Reuveni I, Friedman A, Amitai Y, Gutnick MJ. Stepwise repolarization from Ca2+ plateaus in neocortical pyramidal cells: evidence for nonhomogeneous distribution of HVA Ca2+ channels in dendrites. J Neurosci. 1993;13:4609–4621. doi: 10.1523/JNEUROSCI.13-11-04609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutherford A, García-Muñoz M, Arbuthnott GW. An after hyperpolarization recorded in striatal cells “in vitro”: effect of dopamine administration. Exp Brain Res. 1988;71:399–405. doi: 10.1007/BF00247499. [DOI] [PubMed] [Google Scholar]
- 57.Schiffmann SN, Lledo PM, Vincent JD. Dopamine D1 receptor modulates the voltage-gated sodium current in rat striatal neurones through protein kinase A. J Physiol (Lond) 1995;483:95–107. doi: 10.1113/jphysiol.1995.sp020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 59.Sibley DR. Molecular biology of dopamine receptors. In: Ariano MA, Surmeier DJ, editors. Molecular and cellular mechanisms of neostriatal function. Landes; Austin, TX: 1995. pp. 255–272. [Google Scholar]
- 60.Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surmeier DJ, Stefani A, Foehring RC, Kitai ST. Developmental regulation of a slowly inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett. 1991;122:41–46. doi: 10.1016/0304-3940(91)90188-y. [DOI] [PubMed] [Google Scholar]
- 62.Surmeier DJ, Eberwine J, Wilson CJ, Stefani A, Kitai ST. Dopamine receptor subtypes co-localize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 64.Surmeier DJ, Song W-J, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uchimura N, Higashi H, Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D1 and D2 receptors on nucleus accumbens neurons. Brain Res. 1986;375:368–372. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- 66.Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- 67.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodward DJ, Moises HC, Waterhouse BD, Yeh HH, Cheun JE. The cerebellar norepinephrine system: inhibition, modulation, and gating. Prog Brain Res. 1991;88:331–341. doi: 10.1016/s0079-6123(08)63820-0. [DOI] [PubMed] [Google Scholar]
- 69.Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic–somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]