Abstract
Maturation of electrical excitability during early postnatal development is critical to formation of functional neural circuitry in the mammalian neocortex. Little is known, however, about the changes in gene expression underlying the development of firing properties that characterize different classes of cortical neurons. Here we describe the development of cortical neurons with two distinct firing phenotypes, regular-spiking (RS) and fast-spiking (FS), that appear to emerge from a population of immature multiple-spiking (IMS) neurons during the first two postnatal weeks, both in vivo(within layer IV) and in vitro. We report the expression of a slowly inactivating, 4-AP-sensitive potassium current (K4-AP) at significantly higher density in FS compared with RS neurons. The same current is expressed at intermediate levels in IMS neurons. The kinetic, voltage-dependent, and pharmacological properties of the K4-AP current are similar to those observed by heterologous expression of Kv3.1 potassium channel mRNA. Single-cell RT-PCR analysis demonstrates that PCR products representing Kv3.1 transcripts are amplified more frequently from FS than RS neurons, with an intermediate frequency of Kv3.1 detection in neurons with immature firing properties. Taken together, these data suggest that the Kv3.1 gene encodes the K4-AP current and that expression of this gene is regulated in a cell-specific manner during development. Analysis of the effects of 4-AP on firing properties suggests that the K4-AP current is important for rapid action potential repolarization, fast after-hyperpolarization, brief refractory period, and high firing frequency characteristic of FS GABAergic interneurons.
Keywords: Kv3.1 mRNA, potassium currents, cortical neurons, development, single cell RT-PCR, fast spiking, regular spiking, mouse somatosensory cortex, firing properties
The adult mammalian neocortex is a complex neural network in which regular-spiking (RS) glutamatergic neurons and fast-spiking (FS) GABAergic neurons are principal components (Connors and Gutnick, 1990). Therefore, a determination of the mechanisms that underlie maturation of the characteristic firing properties of these neurons is critical for understanding the development of normal brain function. Previous studies in rodents have reported the appearance of neurons with RS and FS firing properties during the first two postnatal weeks (McCormick and Prince, 1987; Lorenzon and Foehring, 1993; Zhou and Hablitz, 1996); however, the questions of when distinct firing phenotypes are first seen and what cell-specific patterns of neuronal ion channel gene expression are responsible for the appearance of these distinct firing patterns are still largely unanswered.
As a first step toward answering these questions we have characterized the changes in firing properties of neurons in layer IV of the mouse somatosensory cortex during early postnatal development. Previous studies have demonstrated both GABA (Keller and White, 1987; Del Rio et al., 1992) and glutamate (Conti et al., 1987) immunoreactivity within this layer, predicting the presence of neurons with FS and RS firing phenotypes. In this study we demonstrate that layer IV neurons with FS and RS phenotypes emerge from a population of immature multiple-spiking (IMS) neurons during the first two postnatal weeks. In a parallel series of experiments, we observed a similar pattern of electrophysiological maturation in a more heterogenous population of cortical neurons developing in dissociated cell culture. These data are consistent with the possibility that differentiation of neurons with FS and RS firing properties are mediated by similar mechanisms in all cortical layers.
A second series of experiments was performed to identify cell-specific changes in ion channel expression underlying maturation of FS and RS firing phenotypes in cortical neurons. Previous studies have shown that developmental regulation of voltage-gated potassium currents plays an important role in the maturation of neuronal firing properties in a number of systems (Ribera and Spitzer, 1992). Therefore, electrophysiological and pharmacological techniques were used to identify potassium currents that were differentially expressed in cultured neurons with specific firing properties. We were also interested in determining which genes encoded differentially expressed ion channels. Recent studies using single-cell RT-PCR in cortical neurons showed that firing properties can be correlated with specific patterns of ion channel gene expression in neurons from the adult rodent neocortex (Jonas et al., 1994; Lambolez et al., 1996). Using a similar strategy we examined the frequency of expression of the voltage-gated potassium channel gene Kv3.1 in developing cortical neurons. Our results suggest that upregulation in the expression of a 4-AP-sensitive potassium (K4-AP) current encoded by Kv3.1 mRNA plays a role in the development of a number of electrophysiological properties unique to FS GABAergic neurons.
MATERIALS AND METHODS
Acute slice preparation. Neonatal ICR mice between the day of birth (PO) and postnatal day 14 (P14) were used for electrophysiological recordings in acute slice preparations. Pups were anesthetized on ice (<P5) or with halothane (≥P5) before decapitation. Brains were removed in ice-cold artificial cerebral spinal fluid (ACSF) containing (in mm): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 26 NaHCO3, 10 d-glucose, 2.5 CaCl2. Coronal sections (400 μm) were cut through the somatosensory cortex in ACSF using a vibraslicer (Campden Instruments), transferred to a recording chamber, and perfused with oxygenated ACSF at room temperature until recordings were made.
Preparation of cultures. Neuronal cultures were prepared using a procedure modified from Baughman and colleagues (Baughman et al., 1991). P0 pups were anesthetized for ∼1 min on ice before decapitation. Brains were removed in ice-cold balanced salt solution (BSS) containing (in mm): 137 NaCl, 5.3 KCl, 0.15 Na2HPO4, 0.2 KH2PO4, 9.8 HEPES, 33.3 glucose, 43.8 sucrose. Coronal sections (600 μm) were cut through the somatosensory cortex using a vibraslicer. Small pieces of somatosensory cortex (1 mm) from a single mouse were incubated in BSS containing 10 U/ml papain (LS03126, Worthington Biochemical, Freehold, NJ) activated byl-cysteine (1.32 mm) (C-7755, Sigma, St. Louis, MO) along with 5 mmd(−)-2-amino-5-phosphonopentanoic acid (APV), and maintained at 37°C in a humidified incubator for 30 min. The tissue was washed briefly in 5 ml of BSS, twice in 1.5 ml of BSS containing 5 mm APV, 1.0% BSA, 1.0% trypsin inhibitor (1.5 min/wash), and three times in 2.5 ml of BSS containing 5 mm APV, 0.1% BSA, 0.1% trypsin inhibitor (1.5 min/wash). Tissue was rinsed two times in 5 ml of neurobasal medium with B27 supplements (NBM+B27) (Life Technologies, Gaithersburg, MD), mechanically triturated through glass micropipettes, dispersed onto poly-d-lysine-coated glass coverslips, and maintained at 37°C in a humidified 5.0% CO2 incubator overnight. On the following day the coverslips were transferred, cell side up, to dishes containing confluent non-neuronal feeder layers in NBM+B27. Coverslips were subsequently transferred to new feeder cultures every 3–5 d. Neurons preferentially survive and differentiate in NBM+B27, and in all cases the majority of cells on coverslips prepared in this fashion was neuronal, on the basis of morphological and electrophysiological criteria. A small number of non-neuronal cells, however, were present in the older cultures.
Non-neuronal feeder cultures were prepared from whole cortices obtained from mice at P0–P3. Cortices were removed and placed in ice-cold BSS, manually chopped into 2–3 mm pieces with a razor blade, and dissociated in enzyme solution as described for the neuronal cultures. Tissue was washed three times in 5 ml BSS, two times in 5 ml minimal essential medium (MEM) + 10% fetal calf serum (1 min/wash), mechanically dissociated, and plated onto poly-d-lysine-coated plastic tissue-culture dishes. Non-neuronal cultures were fed by replacing the MEM + 10% fetal calf serum every 4 d. In preparation for the neuronal transfers, the MEM + 10% fetal calf serum medium in confluent dishes was replaced with NBM+B27 24 hr before transfer.
Electrophysiological recordings. Electrophysiological recordings were obtained using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) in either current-clamp or voltage-clamp mode. Recording pipettes were unpolished, with open pipette resistances of 2–5 mOhm. The internal pipette solution contained (in mm): 0.1 CaCl2, 2 MgCl2, 1.1 EGTA, 10 HEPES, 120 K+-gluconate, 20 NaCl, pH 7.2. The external solution contained (in mm): 140 NaCl, 4 MgCl2, 5 HEPES, 1 CaCl2, 3 KCl, pH 7.2. The following drugs were added to the external solutions to block specific voltage-gated currents: Na+ currents: 1 μm tetrodotoxin (TTX) (RBI); K4-AP currents: 10–100 μm 4-AP (RBI) or 100–200 μmtetraethylammonium (TEA) (RBI); Ca2+ and KCa2+ currents: 2 mm cobalt. To examine recording stability, leak currents were monitored after each solution change. Voltage-gated currents were analyzed only if leak currents remained unchanged. Data were collected and analyzed using a List EPC-7 patch-clamp amplifier, Dell 386/486 computers, and pCLAMP software (Axon Instruments, v 5.5.1). All recordings were performed at room temperature.
Lucifer yellow fills. To intracellularly label neurons recorded from slices, 0.5% Lucifer yellow/K+-salt was added to the recording pipette. Dye was allowed to fill the cell by passive diffusion. After electrophysiological recording, slices were fixed with 4% paraformaldehyde (in 0.1 m PB) and cleared in methylsalicylate. Filled neurons were imaged using a confocal microscope.
RT-PCR. The presence of Kv3.1 mRNA in RNA isolated from adult mouse brain or cultured somatosensory cortical neurons was determined by RT-PCR. Total RNA was isolated from brain or cultured neurons by the single-step method of Chomczynski and Sacchi (1987). First-strand cDNA was synthesized by random-primed RT of 100–200 ng of total RNA as described previously (O’Dowd et al., 1995), followed by two rounds of amplification using nested primers. Oligonucleotide primer pairs F1/B1 and F2/B2 corresponding to 1422–1445/1647–1626 bp and 1447–1467/1581–1559 bp of the published sequence (Yokoyama et al., 1989) were used to specifically amplify Kv3.1 mRNA. PCR products, labeled by inclusion of ∼5 × 105 cpm of32P-labeled forward primer F2 in the second PCR were separated by electrophoresis on 8% nondenaturing polyacrylamide gels and visualized by film autoradiography or phosphor-imager analysis (Molecular Dynamics, Sunnyvale, CA).
RT-PCR analysis of Kv3.1 mRNA expression was also performed on RNA harvested from single cells as described previously (Smith and O’Dowd, 1994; O’Dowd et al., 1995). Briefly, after acquisition of electrophysiological records using the whole-cell patch-clamp technique, mild suction was used to aspirate the contents of the cell into the tip of the recording electrode that were then expelled into first-strand cDNA synthesis buffer. First-strand cDNA was initiated by the addition of 100 U of Moloney’s murine leukemia virus reverse transcriptase, and the reaction was allowed to proceed for 1 hr at 37°C. After termination of the first-strand reaction, the resulting cDNA was subjected to two rounds of amplification using the nested primers F1/B1 and F2/B2, and the products were analyzed by gel electrophoresis and autoradiography, as described above, for the analysis of Kv3.1 transcripts in RNA isolated from brain or cultured cells. The sequence of the PCR product amplified from RNA harvested from cultured neurons at 6 d in vitro (DIV) was determined using an fmol sequencing kit (Promega, Madison, WI) with an end-labeled primer protocol.
RESULTS
Development of layer IV cortical neurons with FS and RS phenotypes
Our initial experiments focused on a developmental analysis of the firing properties of layer IV neurons in the somatosensory cortex of mouse during the first two postnatal weeks. Whole-cell recordings were obtained, using the blind-patch technique (Blanton et al., 1989), from 95 layer IV neurons in coronal slices through somatosensory cortices prepared from 35 mice, ages P4–P14. Neurons within layer IV were targeted by positioning the pipette over the barrel structures visualized by transillumination of the living slice (Fig.1A). To confirm that this method resulted in recordings from cells in layer IV, Lucifer yellow was included in the intracellular recording solution in some experiments. Subsequent confocal analysis revealed that 16/17 neurons labeled in this manner were located in layer IV, two of which are illustrated in Figure 1B. Although the majority of recordings was from neurons with their cell bodies within layer IV, current clamp recordings from ∼10% of the cells were characterized by small spikes riding on a slow depolarizing wave, consistent with the recording electrode being located on the dendrites (Kim and Connors, 1993). Because it was possible that these were recordings from neurons whose cell bodies were located in other layers, these records were not analyzed further.
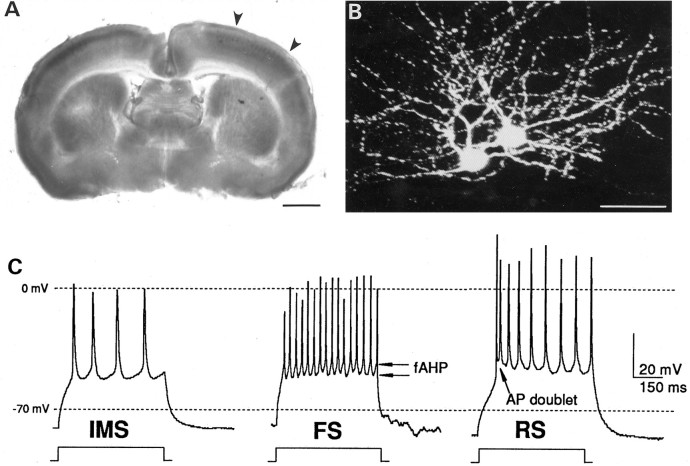
Fig. 1.
A, Layer IV is identified by the presence of barrels visualized by transillumination of a 400-μm-thick living slice through P7 mouse somatosensory cortex. Large arrowheads delineate dorsal and lateral extents of the barrel cortex in this slice. Scale bar, 1 mm. B, Morphology of two stellate neurons located in the same barrel of a P11 slice, visualized with confocal microscopy. The two cells were independently filled with separate whole-cell recording pipettes that contained 0.5% Lucifer yellow in the internal solutions. Pial surface is toward thetop of this collapsed composite photograph (individual images were taken at 3 μm increments throughout a total depth of 78 μm). Scale bar, 25 μm. C, Representative whole-cell recordings illustrating three layer IV neurons with distinct firing phenotypes. The immature multiple-spiking phenotype (IMS) from a neuron in a P5 slice is characteristic of the majority of neurons that can be recorded during the first postnatal week. During the second and third postnatal weeks, both FS neurons (FS) and RS neurons (RS) are observed. Shown here are representative traces from an FS and an RS neuron from a P8 and a P11 slice, respectively. Characteristic fAHP and action potential doublet (AP doublet) are indicated in the traces from the FS and RS neurons, respectively. APs were elicited by a 600 msec depolarizing current pulse.
In the first postnatal week, the majority of neurons fired low-frequency trains of action potentials (APs) at constant interspike intervals, with no evidence of a fast after-hyperpolarization (fAHP) between spikes in the train. These neurons were classified as IMS cells. On the basis of previously established criteria (Connors and Gutnick, 1990), RS and FS phenotypes appeared in the first postnatal week, but the frequency of encountering neurons with these firing patterns increased throughout the second postnatal week. FS neurons fired trains of APs at constant interspike intervals and exhibited a prominent fAHP between spikes in the train (Fig. 1C). RS neurons were characterized by an AP doublet at the beginning of the spike train followed by additional spikes throughout the depolarizing current step at either constant interspike interval or with increasing interspike intervals (Fig. 1C). Thus, two classes of neurons with distinct firing properties (FS and RS) develop within layer IV of the somatosensory cortex during the first two postnatal weeks. During the first week and throughout the second week, neurons that fired only a single AP in response to sustained depolarizing stimuli were encountered, but they were not analyzed further in these studies.
Cortical neurons in cell culture develop FS and RS phenotypes
To examine the developmental changes in voltage-gated currents and in underlying gene expression that give rise to cells with distinct firing properties, we used a primary dissociated cell culture system. Although electrophysiological recordings in the slice were obtained exclusively from layer IV neurons, cultures were prepared from coronal slices spanning the pial-to-white matter boundary and were therefore assumed to contain neurons that would normally reside in all of the cortical laminae, including layer IV. Immediately after dissociation, neurons could be identified as round-phase bright cells with processes of varying lengths (Fig. 2A). As early as 3–5 DIV, individual neurons extended long branching neurites, many of which overlapped extensively (Fig. 2B). During the next 2 weeks in culture, neurites continued to grow in length, often forming thick fascicles. At the later times in culture, neurites formed an intricate mat throughout the culture dish (Fig. 2C,D). Functional synaptic connections that formed between neurons were seen as early as 4–5 DIV (Li et al., 1996) and at all stages in vitro thereafter. At all times throughout development in vitro, individual cell bodies could be visualized for whole-cell patch-clamp recordings and single-cell RT-PCR experiments.
Fig. 2.
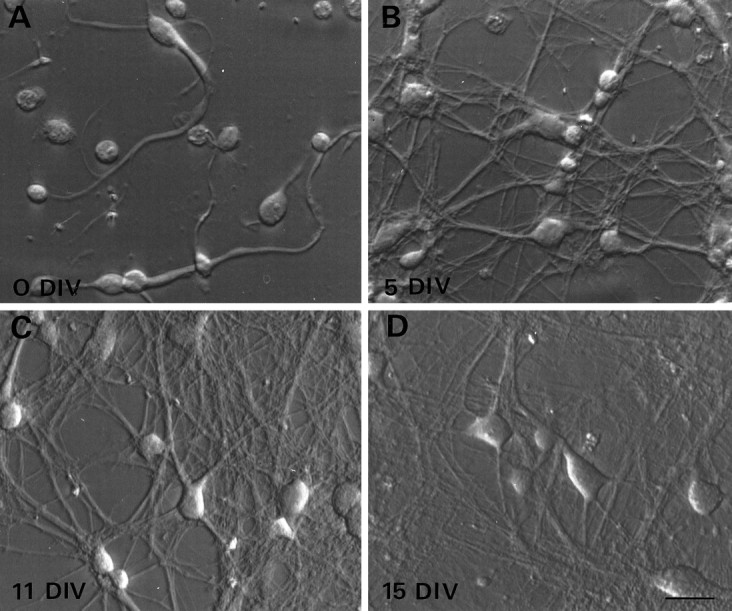
Morphological development of cortical neurons in dissociated cell cultures. Neurons dissociated from somatosensory cortex harvested from newborn mice were plated on poly-d-lysine-coated coverslips in NBM+B27 supplements. Coverslips were transferred into fresh non-neuronal feeder plates on day 1 and every 3–4 d thereafter. A, Immediately after dissociation (0 DIV), the somata of the majority of the cells were spherical or ovoid in shape. Some of the neurons had processes that remained intact throughout the dissociation procedure.B, By 5 DIV the majority of neurons have extended neurites that connect individuals and groups of neurons.C, D, The web of interconnecting processes continues to elaborate throughout the first 3 weeks in vitro. At the plating density used in these cultures, the cell bodies of some of the neurons are physically isolated from each other even at the oldest ages. Scale bar, 20 μm.
Whole-cell recordings were made from 152 neurons in 25 cultures between 3 and 23 DIV. As seen in the slice preparation, neurons that could fire only a single AP in response to sustained depolarizing current injection were encountered at all ages but were not analyzed further; however, the majority of cultured neurons examined exhibited firing properties that could be classified initially as IMS, FS, or RS, on the basis of the same criteria used in the slice preparation. Within each of these groups, firing properties showed considerable variation, as illustrated by the range in firing frequencies observed among IMS, FS, and RS neurons (Fig. 3A). A similar variability was observed in the layer IV neurons recorded in slice (data not shown). Therefore, to test the validity of our classification scheme, a number of electrophysiological measures were examined to determine which, if any, might correlate with firing group assignment. A scatter plot of the maximal firing frequency versus fAHP amplitude reveals that FS and RS neurons fall naturally into two distinct groups, although considerable overlap was evident between IMS and RS cells (Fig. 3B). Better discrimination among the three groups was observed in a plot of firing frequency versus the ratio of the first to second interspike interval, although some overlap occurred between the IMS and both FS and RS groups (Fig. 3B). Table1 shows the mean values for each of these electrophysiological parameters for the three groups. Consistent with the clustering seen in the scatter plots, significant differences were apparent in all five properties shown between FS and RS neurons. These data demonstrate that cortical neurons in dissociated cell culture fall into two distinct firing phenotypes (FS and RS) and that, to some extent, IMS neurons overlap both of these categories depending on compared parameters.
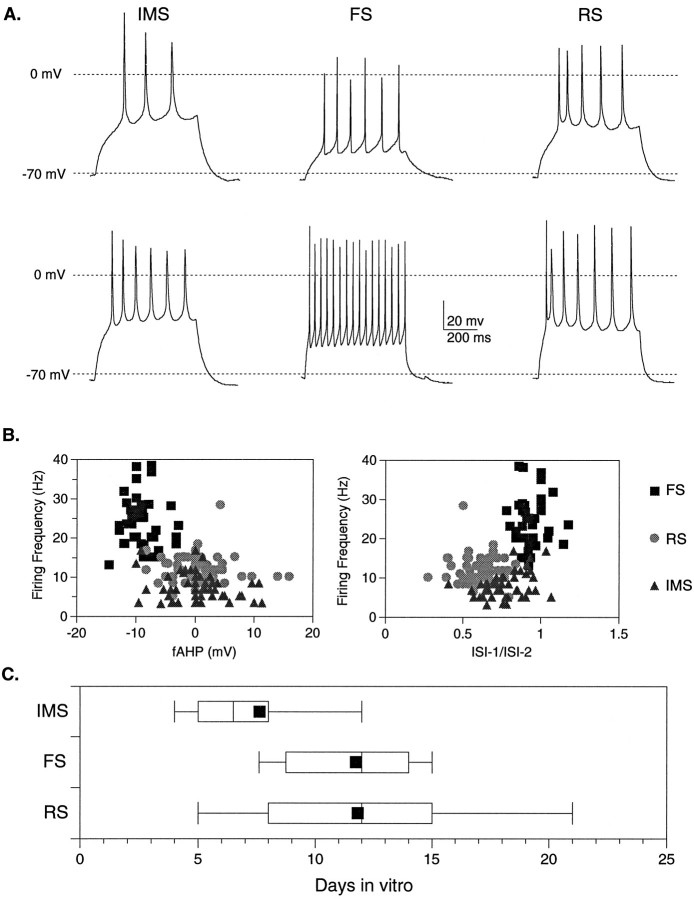
Fig. 3.
Maturation of firing phenotypes in cultured neurons parallels the development of layer IV neurons in vivo. A, Voltage recordings from twoIMS, two FS, and two RSneurons in dissociated cell culture after injection of a 600 msec depolarizing current pulse. The variation in the maximal firing frequencies within each class is illustrated by the topand bottom traces. B, Quantitative analysis demonstrates that when maximal firing frequency and fAHP are plotted for all neurons examined, FS neurons form a distinct group (squares, n = 41), whereas RS (circles,n = 56) and IMS neurons (triangles, n = 47) are not well segregated by this analysis. A scatter plot of firing frequency and the ratio of first to second interspike interval (ISI-1/ISI-2) reveal a better separation among the three groups, although there is still some overlap between the IMS group with both the FS and RS groups. C, A box plotillustrates the age range over which the three firing phenotypes were observed. The 10th and 90th percentiles are indicated by the whiskers, and the 25th, 50th, and 75th percentile boundaries are indicated by thebox for each group. Solid squares denote the means.
Table 1.
Electrophysiological properties of IMS, FS, and RS neurons
| Firing frequency (Hz) | fAHP (mV) | AP duration (msec) | Refractory period (msec) | ISI1/ISI2 | |
|---|---|---|---|---|---|
| IMS | 7.4 ± 0.5 | 0.2 ± 0.7 | 3.5 ± 0.1 | 13.7 ± 1.3 | 0.8 ± 0.02 |
| n = 57 | n = 54 | n = 56 | n = 26 | n = 47 | |
| FS | 24 ± 1.0 | −9.1 ± 0.4 | 1.9 ± 0.1 | 5.9 ± 0.5 | 0.9 ± 0.01 |
| n = 41 | n = 40 | n = 41 | n = 23 | n = 40 | |
| RS | 12.1 ± 0.5 | 0.7 ± 0.6 | 2.6 ± 0.1 | 15.0 ± 1.3 | 0.6 ± 0.02 |
| n = 58 | n = 57 | n = 58 | n = 24 | n = 56 |
The mean firing frequency, fAHP, action potential duration, refractory period, and ratio of first to second interspike interval (ISI1/ISI2) were determined for neurons in each of the three firing categories. The firing frequency was determined from the maximal number of spikes evoked by a suprathreshold 600 msec depolarizing current step. The fAHP was measured from threshold to maximal hyperpolarization, after the first spike in the train. Action potential duration was measured at half amplitude. The refractory period was defined as the interstimulus interval between two identical depolarizing stimuli necessary for a neuron to fire an action potential, during the second pulse, with an amplitude at least 90% of that elicited during the first pulse. To calculate the ISI1/ISI2, the time between the first and second spikes in a train is expressed as a percentage of the time between the second and third spikes in the train. Values presented are mean ± SEM; n indicates total number of neurons examined. All five properties are significantly different between RS and FS neurons (p < 0.01; Student’s t test).
The box plot in Figure 3C illustrates the wide range of ages over which neurons within each of the firing groups were observed. During the first week in culture, however, the majority of the neurons had an IMS phenotype, whereas RS and FS neurons were rarely seen. FS and RS neurons were found predominantly in the second and third weekin vitro, with a median age of 12 d for both groups (Fig. 3C). These findings suggest that RS and FS neurons emerge from a population of IMS neurons, both in vivo andin vitro, over a similar time course.
A K4-AP current is positively correlated with a FS phenotype
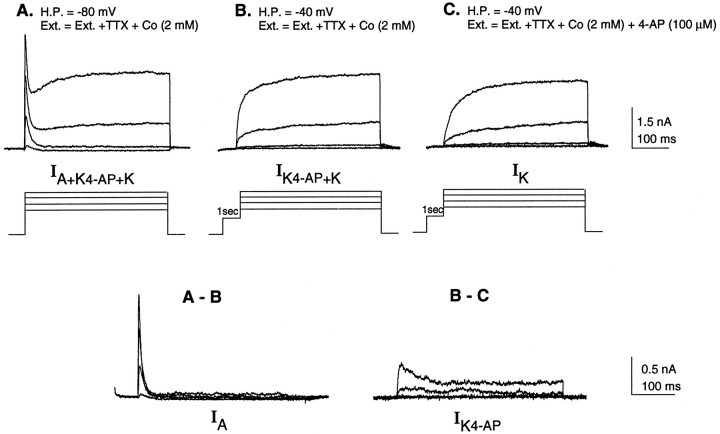
As a first step toward understanding how developmental changes in voltage-gated currents influence the appearance of FS and RS phenotypes, we examined the properties of voltage-gated potassium currents in cultured neurons with identified firing patterns. For each neuron, we initially measured the whole-cell capacitance, resting membrane potential, input resistance, and firing properties using a potassium gluconate-based internal solution and a standard physiological external solution. Subsequently we examined isolated potassium currents using voltage protocols and pharmacological blocking agents similar to those described previously (Storm, 1988; Andreasen and Hablitz, 1992; Wu and Barish, 1992; Foehring and Surmeier, 1993). A family of outward potassium currents evoked in a single cell by a series of depolarizing voltage steps in a normal external solution containing TTX, to block sodium current, and cobalt, to block calcium and calcium-activated potassium currents, is illustrated in Figure4A. Transient A-currents were obtained by digital subtraction of traces in which the A-current was inactivated (−40 mV prepulse) from the total outward potassium current (Fig.4A,B). The K4-AP current was obtained by digital subtraction of traces in which the A-current was inactivated and 4-AP-sensitive current was blocked by 100 μm 4-AP, from traces in which just the A-current was inactivated (Fig.4B,C). The IK was defined as the current activated after a prepulse to −40 mV in the presence of 100 μm 4-AP (Fig. 4C).
Fig. 4.
Isolation of voltage-gated potassium currents in developing cortical neurons. Voltage-gated potassium currents evoked by a series of 400 msec voltage steps to −30, −10, 10, and +30 mV in a single cultured neuron. The whole-cell recording electrode was filled with a potassium gluconate-based internal solution, and in each case the bathing solution contained 1 μm TTX and 2 mm cobalt to block the voltage-gated sodium, calcium, and calcium-activated potassium currents, respectively. A, Total outward current (IA+IK4-AP+IK) is activated from a holding potential of −80 mV. B, The fast transient potassium current (IA) is inactivated by prepulsing the neuron to −40 mV for 1 sec, resulting in isolation ofIK+IK4-AP.C, IK is isolated by prepulsing to −40 mV, thus inactivating IA, and addition of 100 μm 4-AP to the bathing medium to pharmacologically block IK4-AP. A - B, IA is isolated by digitally subtracting traces in B from traces in A.B - C, IK4-AP is isolated by digitally subtracting traces in C from traces inB.
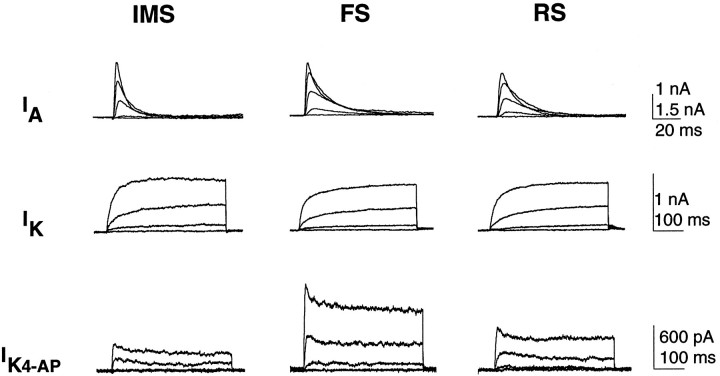
All three potassium currents, IA,IK, and IK4-AP, could be detected in neurons within each of the firing groups (Fig.5). Although there were differences in the average peak current amplitudes and kinetic properties of the isolated potassium currents among individual neurons, the most notable difference among cells in the three groups was amplitude of the K4-APcurrent. The amplitudes of the K4-AP currents in the IMS and RS neurons, at each voltage, were smaller than those in the FS cells (Fig. 5). To control for variation in cell size, we calculated the K4-AP current density in each cell by normalizing the peak current amplitude evoked by a voltage step to +40 mV to the whole-cell capacitance. The K4-AP current density in the population of IMS neurons is intermediate between the RS and FS groups, whereas the density of this current in FS neurons is significantly greater (threefold) than in RS neurons (Fig.6A). This positive correlation between the K4-AP current density and neurons with an FS phenotype suggests that this current contributes to firing properties that are unique to FS neurons.
Fig. 5.
The three voltage-gated potassium currents,IA, IK4-AP, andIK, could be detected in neurons with each of the identified firing phenotypes. Isolated currents were recorded from cultured neurons using the protocols illustrated in Figure 4. IMS records were obtained from three different neurons, 7–8 DIV. FS records were obtained from two different neurons, 8 DIV. RS records were obtained from two different neurons, 10 and 14 DIV. Scale bar for the IA current is 1 nA for the IMS and RS neurons and 1.5 nA for the FS neuron.
Fig. 6.
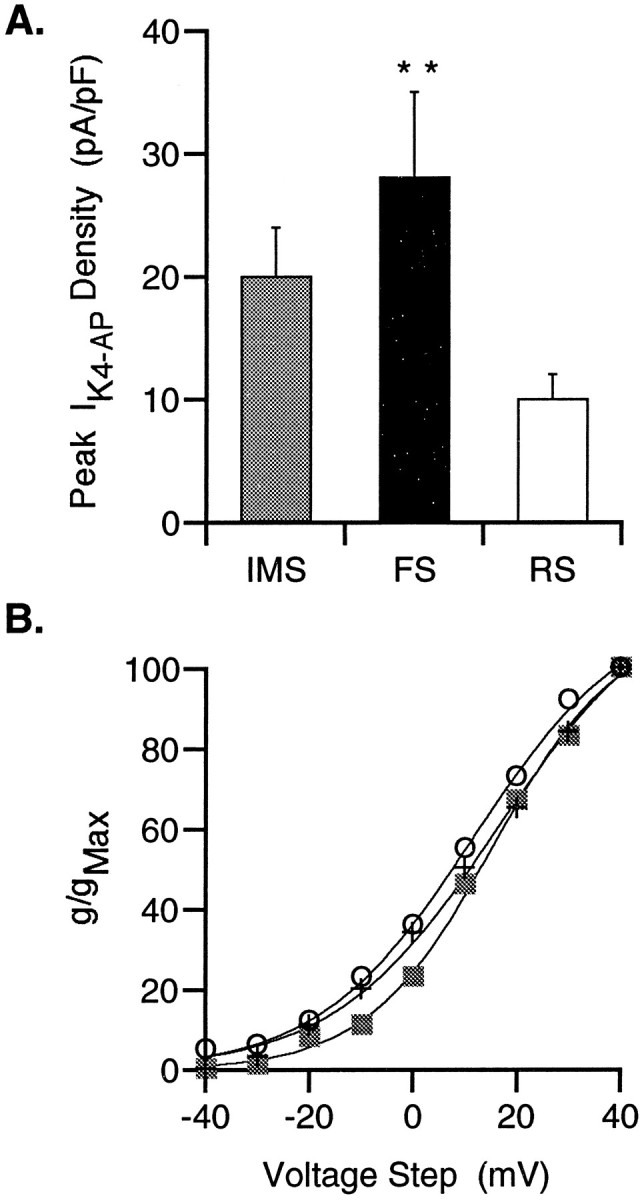
Differential expression of K4-APcurrents among IMS, FS, and RS neurons. A, Because the size of the individual neurons varies with age and firing properties, the K4-AP current density in the three populations of cells, developing in vitro, was examined by normalizing the peak current amplitude (pA) elicited by a voltage step to +40 mV, to the whole-cell capacitance (pF). The K4-AP current density of IMS cells (n = 17) is intermediate between the RS (n = 15) and FS (n = 10) groups. The K4-AP current density of the FS group is significantly greater than that of the RS group (**p < 0.01; Student’s ttest). B, Normalized conductance versus voltage curves were generated for the IMS (squares,n = 15), RS (circles,n = 16), and FS (cross,n = 12) groups. Each of these curves was fit with a Boltzmann’s distribution indicating similarV1/2 values (12–16 mV). SEMs were in all cases smaller than the symbols and therefore were excluded from the graph.
The K4-AP currents are characterized by relatively rapid activation kinetics and a slowly inactivating component at the more depolarized voltages (Fig. 5). Mean conductance–voltage curves for each of the three groups were well fit by Boltzmann’s distributions that indicated a positive (12–16 mV) voltage dependence of activation (Fig. 6B). To investigate further the pharmacological properties of the K4-AP current in FS neurons, a dose–response curve to 4-AP was generated from four FS neurons. These data demonstrate an increase in percentage current blocked with increasing concentration of 4-AP, with a predicted half-maximal blocking concentration of 21 μm (Fig.7A). Currents with a similar voltage dependence, although lacking the slow inactivation at the more depolarized potentials, were also isolated by application of 100 μm TEA (Fig. 7B). The lack of slow inactivation in the TEA-isolated currents could in theory be attributable to 4-AP and TEA blocking the same channels in a kinetically distinct manner; however, currents isolated by combined application of 100 μm 4-AP and 100–200 μmTEA in eight neurons were all similar in time course but consistently slightly larger than those isolated by 4-AP alone (Fig. 7C). These two findings suggest that whereas the K4-AP current is sensitive to TEA, it seems likely that TEA also affects one or more additional currents at this concentration.
Fig. 7.
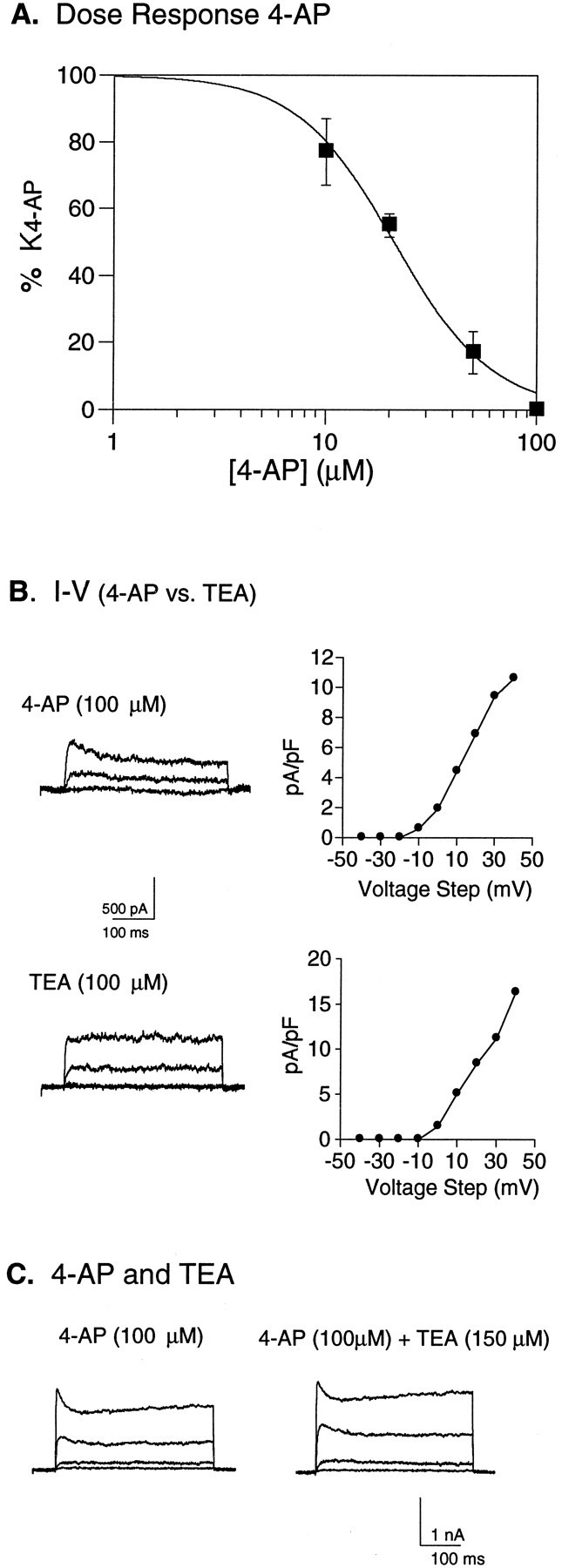
Pharmacological properties ofIK4-AP. A, Average dose–response curve for 4-AP, generated from FS neurons (n = 4) developing in vitro. K4-AP current amplitude was measured after sequential application of 10, 20, 50, and 100 μm 4-AP, and the percentage current (with 100% defined as the current blocked by 100 μm4-AP) was determined at each 4-AP concentration. The average current (% maximal) from the four cells is plotted as a function of drug concentration, and these data are fit by the decreasing logistic equation [y = 1/((xn/EC50n)+1);n = Hill coefficient], with a calculated EC50 of 21 μm. B, Waveforms and current density versus voltage relationship of currents isolated by 100 μm 4-AP in one FS cell and by 100 μmTEA in a second FS cell. C, Current isolated by application of 100 μm 4-AP is compared with the current subsequently isolated from the same cell with 100 μm 4-AP and 150 μm TEA. Currents were evoked by voltage steps between −20 and +40 in steps of 20 mV, from a holding potential of −40 mV.
The K4-AP current contributes to firing properties characteristic of FS neurons
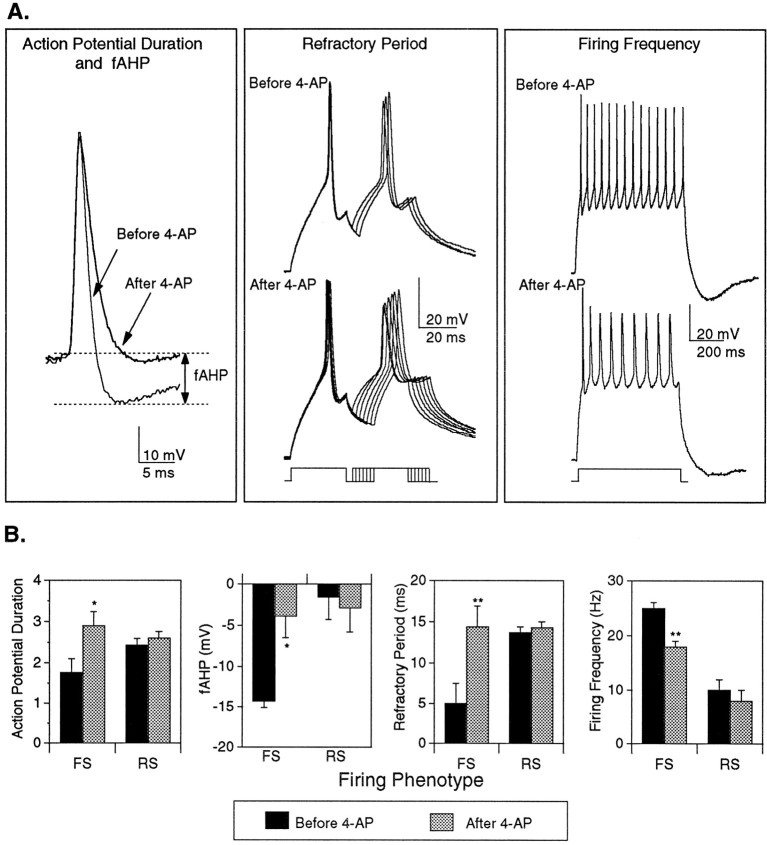
To establish candidate functions for the K4-APcurrent in developing cortical neurons, we looked for firing properties that were differentially affected by 100 μm 4-AP in the three firing groups. Initial studies were performed in the presence of cobalt to rule out the possibility that the effects of 4-AP were mediated either directly or indirectly by Ca2+ and/or KCa2+ currents. In the presence of cobalt, exposure of FS neurons to 4-AP caused an increase in AP duration, a dramatic reduction in the large fAHP amplitude, an increase in refractory period, and a decline in the maximal firing frequency (Fig.8A). Quantitative analyses of the effects of 4-AP in FS and RS neurons are illustrated in Figure8B. Significant changes in AP duration (1.5-fold increase), fAHP amplitude (10-fold decrease), refractory period (threefold slower), and firing frequency (30% decrease) were observed in the FS neurons (Fig. 8B). In contrast, 4-AP treatment did not seem to alter AP duration, refractory period, or firing frequency in RS neurons. The fAHP amplitude of RS neurons was small, both before and after 4-AP, and therefore was not included in the analysis. As is clearly illustrated in Figure 8A, however, the application of 4-AP does not result in repetitive firing properties that are reminiscent of RS neurons (no doublet is present) or IMS neurons (firing frequency is too high). Similar experiments were performed in the absence of cobalt, allowing activation of Ca2+ and/or KCa2+ currents. Under these conditions, similar changes in AP duration, fAHP amplitude, refractory period, and firing frequency were also apparent in FS neurons after treatment with 4-AP. Consistent with the data obtained in the presence of cobalt, 4-AP did not significantly alter the AP duration or firing frequency of RS neurons, although a small increase in the refractory period was noted.
Fig. 8.
Role of the K4-AP current in firing properties characteristic of FS neurons. All of the electrophysiological recordings were obtained from cultured cortical neurons in a standard external solution that included 2 mmcobalt to block both the calcium and calcium-activated potassium currents. A, Exposure of an FS neuron to 100 μm 4-AP results in an increase in AP duration from 1.4 to 2.6 msec. A decrease in the fAHP, from −13.7 to −1.7 mV, is also observed after exposure to 100 μm 4-AP. Examination of the refractory period reveals an increase from 6 msec (top traces) to 15 msec (bottom traces) after exposure of an FS neuron to 4-AP. Exposure to 4-AP also reduces firing frequency, from 23 to 15 Hz in an FS neuron. B, Thebar graphs illustrate the mean values for each of these four properties in seven FS and three RS neurons, before and after 4-AP. Drug treatment caused significant changes in all of the parameters in the FS group (**p < 0.01, *p < 0.02; paired Student’s ttest). No significant changes were noted in the RS neurons. Error bars on histogram indicate SEMs.
These data suggest that the K4-AP current makes a critical contribution to the short AP duration, the large fAHP, the brief refractory period, and the high firing frequency characteristic of neurons that develop an FS phenotype. Because blockade of this current in FS neurons, however, does not result in repetitive firing properties that would be classified clearly as RS or IMS, and the K4-AP current is present in IMS cells and some RS neurons, differences in the numbers, types, or localization of other voltage-gated channels must also contribute to the distinct firing patterns among the three groups.
Kv3.1 mRNA expression is correlated with firing phenotype
Comparison of the electrophysiological and pharmacological properties of the K4-AP currents with those generated by cloned rat potassium channel genes in heterologous expression systems (Chandy and Gutman, 1995) suggested that Kv3.1 was an excellent candidate gene for encoding the K4-AP channels. In rat, the Kv3.1 gene gives rise to two alternatively spliced transcripts, designated Kv3.1a and Kv3.1b (Luneau et al., 1991; Grissmer et al., 1992). Although Kv3.1b has not been described in mouse, sequence data for Kv3.1a, formerly known as NGK2, has been published (Yokoyama et al., 1989). Therefore, to examine Kv3.1 gene expression in developing mouse cortical neurons, primers for RT-PCR were targeted to the 3′ region of the mouse Kv3.1a cDNA in which the sequence diverges from that of other closely related potassium channel genes. Initial studies demonstrated that these primers amplified a single product from RNA extracted from both mouse brain and cortical cultures, which based on its electrophoretic mobility and sequence analysis represents the mouse Kv3.1a transcript.
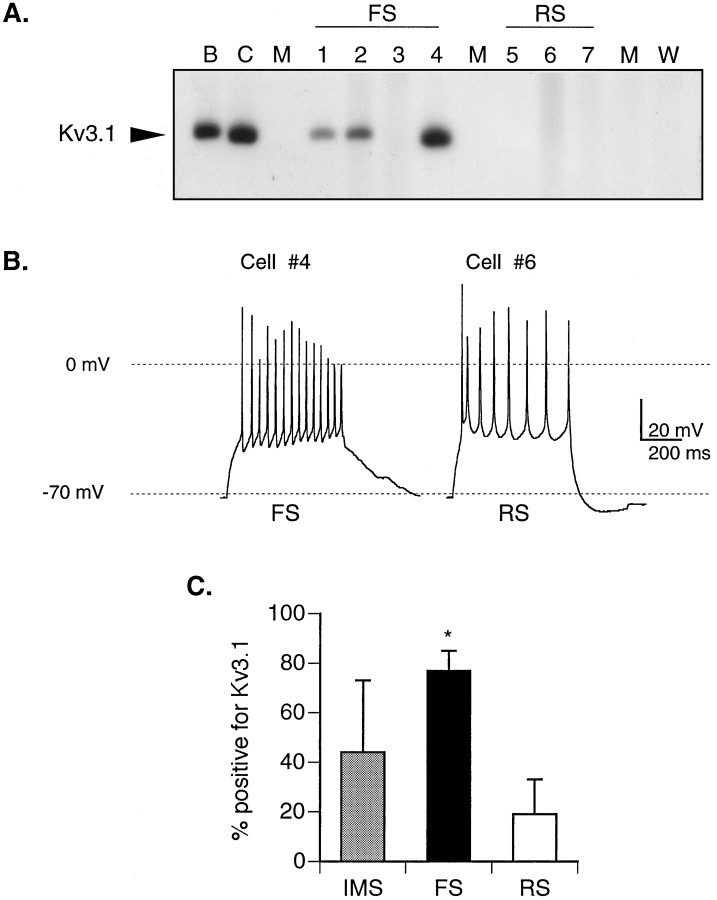
Given the differential expression of the K4-AP current, we hypothesized that FS neurons should have the highest levels of Kv3.1 mRNA. Therefore, in an RT-PCR analysis of RNA harvested from single cultured cortical neurons after characterization of their firing properties, we predicted that the probability of obtaining PCR products in FS neurons, given identical PCR conditions, should be higher than in either IMS or RS neurons. In a typical single-cell RT-PCR experiment, PCR products representing the Kv3.1 gene were obtained in 3/4 FS neurons and 0/3 RS neurons (Fig. 9A,B). The frequency of the Kv3.1 mRNA expression was determined for the distinct firing phenotypes in each of four similar experiments (Fig.9C). The average frequency of amplifying a Kv3.1 product was approximately four times greater in FS versus RS neurons, with an intermediate frequency of expression observed in the IMS cells. The strong positive correlation between the expression of Kv3.1 transcripts and FS neurons supports the hypothesis that this gene encodes the K4-AP current and that levels of expression of this gene are regulated during development in a cell-specific manner.
Fig. 9.
Differential amplification of Kv3.1 transcripts in neurons with distinct firing properties. A, Autoradiogram from a single experiment showing PCR products representing Kv3.1 transcripts amplified from RNA harvested from three of the four identified FS neurons at 12 DIV. In contrast, no PCR products were amplified from the three RS neurons examined. Kv3.1 transcripts amplified from total RNA from adult whole brain (B) and cortical culture at 6 DIV (C) are shown for comparison. Interleaved control lanes in which medium from the recording chamber (M) or water (W) was aspirated into the patch pipette and processed in parallel with other samples are all negative.B, Representative traces from an FS and an RS cell (firing properties corresponding to PCR profile in lanes4 and 6, respectively). C, To determine the average frequency of expression in the different firing classes, we calculated the frequency with which PCR products representing Kv3.1 transcripts were amplified from cultured neurons in the three firing groups as a function of the total number of neurons in each group, in four individual experiments similar to the one illustrated above. A total of 5, 13, and 18 neurons in the IMS, FS, and RS groups, respectively, were examined. The frequency of expression of Kv3.1 was significantly higher in the FS group when compared with the RS group (p < 0.02; Student’st test; n = 4 experiments).
DISCUSSION
Development of FS and RS phenotypes
Our data demonstrate rapid maturation of firing properties of neurons within layer IV during early postnatal development. Neurons that fire low-frequency trains of long-duration APs (IMS) predominate during the first postnatal week, with RS and FS phenotypes seen with increasing frequency throughout the second postnatal week. A similar maturation profile is observed in a more heterogenous population of neurons developing in dissociated cell culture during the first 2 weeksin vitro. As previously demonstrated in cortical neurons from adult rodents (Lambolez et al., 1996), developing neurons with FS and RS phenotypes can also be separated into two distinct groups on the basis of firing frequency and fAHP amplitude; however, the IMS neurons show considerable overlap with the RS neurons in this analysis. The IMS neurons also show overlap with FS neurons when firing frequency and the ratio of the first and second interspike interval are considered. In addition, within all three firing groups there is variation in a number of electrophysiological properties, including firing frequency, that is likely to reflect differences in maturity among neurons within these broad groups. Together these data suggest that cortical neurons, within layer IV and developing in culture, undergo a cell-specific maturation in membrane properties during the first two postnatal weeks such that the RS and FS phenotypes emerge from a population of neurons with an IMS phenotype. In addition, at least within layer IV, specification of distinct firing phenotype seems to occur after laminar differentiation. Although the early firing phenotype of these neurons may be similar, this does not imply that RS and FS neurons necessarily arise from a common progenitor. For example, immunocytochemical studies have indicated the presence of GABA-immunoreactive neurons (Del Rio et al., 1992) within layer IV during early postnatal development, when the IMS phenotype predominates. Thus, other characteristics may distinguish between neurons that will become either FS or RS before differentiation of the firing properties.
Differential expression of the K4-AP current
The similarity in the development of firing phenotypes in vivo and in vitro suggests that there are characteristic developmental changes in ion channel expression that underlie the basic differentiation of cortical neurons from an IMS phenotype to either FS or RS phenotypes. Previous studies have demonstrated changes in sodium, calcium, and potassium currents during early postnatal development of cortical neurons (Huguenard et al., 1988; Hamill et al., 1991; Lorenzon and Foehring, 1995), but the relationship between these changes and maturation of specific firing properties is not well understood. Using a voltage-dependent and pharmacological isolation strategy, we identified three macroscopic currents in the developing cortical neurons similar to those described as A, K, and D in embryonic hippocampal neurons (Wu and Barish, 1992). Consistent with their nomenclature, we classify two of the currents as A and K; however, because the term D-current was first used in an earlier study (Storm, 1988) to describe a 4-AP-sensitive current with voltage-dependent gating properties different from those of the 4-AP-sensitive current described here, we refer to the current as K4-AP.
Our data demonstrate that IA,IK, and IK4-AP were present in neurons within each of the firing classes. This is in contrast to an earlier study that reported the absence ofIA currents in FS neurons (Hamill et al., 1991). Although all of the currents were observed in each class, there were a number of properties such as activation and inactivation kinetics ofIA that varied both among classes (Fig. 5) and within a class. The most striking difference among the three firing phenotypes, however, was the density of the K4-AP current. FS neurons had a significantly higher K4-AP current density than RS neurons. Consistent with the suggestion that both RS and FS neurons arise from neurons that express a similar complement of voltage-gated ion channels that underlie the common firing phenotype, IMS neurons had intermediate K4-AP current density. Although the maturation of firing properties is clearly a product of the coordinate regulation of a number of different ion channels, the differential expression of K4-AP current suggests that developmental upregulation of this current is important in the maturation of firing properties that are unique to FS neurons.
Evidence that the Kv3.1 gene encodes the K4-AP current
Several lines of evidence support the hypothesis that the K4-AP current is encoded by Kv3.1 mRNA. First, currents expressed in mammalian cell lines (Grissmer et al., 1994) andXenopus oocytes (Yokoyama et al., 1989; Luneau et al., 1991) injected with Kv3.1 mRNA exhibit electrophysiological and pharmacological properties that are similar to those of the K4-AP currents described in this study. Second, our single-cell RT-PCR data demonstrate that the frequency of detecting PCR products representing Kv3.1 mRNA is significantly higher in FS neurons that exhibit a high K4-AP current density compared with RS neurons, whereas an intermediate expression frequency is seen in the IMS neurons that express intermediate levels of K4-APcurrent. Because expression of mRNA for several other genes, including agrin and a type II sodium channel, was similar between FS and RS neurons (data not shown), we believe that the low probability of obtaining a Kv3.1 PCR product in RS neurons reflects relatively low levels of Kv3.1 mRNA. Finally, consistent with our finding that the K4-AP current is preferentially expressed in FS neurons, previous studies demonstrate that the Kv3.1 mRNA (Weiser et al., 1994) and protein (Du et al., 1996) are localized in GABAergic neurons in the rodent cortex.
Previous studies in rat have shown that the Kv3.1 gene gives rise to two alternatively spliced variants, Kv3.1a and Kv3.1b, that encode potassium channels with similar biophysical and pharmacological properties (Yokoyama et al., 1989; Luneau et al., 1991; Grissmer et al., 1994). In situ hybridization and other studies have demonstrated further that although both transcripts are present in rat cortical neurons, Kv3.1a is the most abundant of the two isoforms during embryonic and early postnatal development (Perney et al., 1992). The primers used in the present study were specific for the mouse Kv3.1a. Given the pattern of Kv3.1a expression in rat, it is tempting to speculate that many of the developmental changes seen in the K4-AP current are a consequence of changes in Kv3.1a expression; however, although our data are consistent with the Kv3.1 gene encoding 4-AP sensitive channels, we cannot determine the contribution of specific splice variants.
Functional role of the K4-AP current in firing properties
Recent studies have demonstrated that expression of the Kv3.1 protein is localized in GABAergic interneurons in the hippocampus and that exposure of GABAergic neurons to low concentrations of 4-AP affect AP duration, suggesting that a Kv3.1-encoded current influences AP waveform (Du et al., 1996). Our results demonstrating that application of 4-AP differentially altered a number of electrophysiological properties of FS neurons with relatively little effect on neurons with RS phenotypes (does not abolish AP doublet at beginning of spike train) are consistent with this suggestion and also implicate a role for this current in the repetitive firing properties of developing FS neurons. We found that 4-AP significantly increased the AP duration in the cultured neurons, although the average increase in our study was less than that seen in parvalbumin-containing interneurons in the rat hippocampus (Du et al., 1996), which may reflect differences in the relative density of this current. Exposure to 4-AP also significantly decreased the firing frequency in the FS neurons. This is in contrast to the increase in firing frequency reported in embryonic hippocampal neurons under similar conditions (Wu and Barish, 1992). Cell-specific differences in relative numbers and localization of channels mediating K4-AP currents or the expression of other currents that influence firing frequency may underlie these differences. Similar results were obtained in the presence and absence of cobalt, demonstrating that the primary mechanism of 4-AP action is likely to be through specific blockade of the K4-AP current and not mediated through calcium or calcium-activated potassium currents. Interestingly, the prominent fAHP in the FS neurons, also altered by 4-AP, was calcium independent. Although the fAHP reported in hippocampal pyramidal cells seems to be calcium dependent (Storm, 1987), calcium-independent fAHPs have been described previously in motoneurons (Barrett and Barrett, 1976; Yarom et al., 1985) .
These data suggest that upregulation of the K4-APcurrent, either through increased transcription or stability of Kv3.1 mRNA, is important in the development of a number of firing properties characteristic of FS neurons. Blockade of this current in FS neurons, however, does not result in repetitive firing properties that would be clearly classified as RS or IMS. Therefore, additional changes in the numbers, types, or localization of other channel types are clearly important in the development of the mature FS phenotype.
Future studies
Molecular genetic manipulations will be important to test the hypothesis that the Kv3.1 gene encodes the developmentally regulated K4-AP current expressed in developing cortical neurons. This should be possible using a strain of Kv3.1-deficient mice that were generated by homologous recombination (Ho et al., 1997) or with antisense oligonucleotides targeted specifically to Kv3.1 transcripts. We predict that cortical neurons lacking Kv3.1 transcripts will also lack the K4-AP current and that the FS GABAergic neurons should have longer than normal AP durations, little or no fAHP, and longer than normal refractory periods. Changes in the firing properties of FS neurons, resulting from changes in the levels of expression of Kv3.1-encoded currents, may also influence the overall activity of FS GABAergic neurons in developing cortical circuits bothin vivo and in vitro.
Footnotes
This study was supported by National Institutes of Health Grants NS30109 and NS27501 and a Research Career Development Award NS01854 to D.K.O. Additional support was provided by National Institutes of Health Grant NS33213 and National Science Foundation Grant IBN9319355 to M.A.S. We thank Dr. F. Ehlert for helpful discussions and Dr. Ariel Agmon for critical comments on a previous version of this manuscript.
Correspondence should be addressed to Diane K. O’Dowd, Department of Anatomy and Neurobiology, University of California, Irvine, CA 92697-1280.
REFERENCES
- 1.Andreasen M, Hablitz JJ. Kinetic properties of a transient outward current in rat neocortical neurons. J Neurophysiol. 1992;68:1133–1142. doi: 10.1152/jn.1992.68.4.1133. [DOI] [PubMed] [Google Scholar]
- 2.Barrett EF, Barrett JN. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurons. J Physiol (Lond) 1976;255:737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baughman RW, Huettner JE, Jones KA, Khan AA. Cell culture of neocortex and basal forebrain from postnatal rats. In: Bank G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1991. pp. 227–249. [Google Scholar]
- 4.Blanton MS, LoTurco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 5.Chandy KG, Gutman GA. Voltage-gated potassium channel genes. In: North RA, editor. Ligand- and voltage-gated ion channels. CRC; Boca Raton, FL: 1995. pp. 1–71. [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 8.Conti R, Rustioni A, Petrusz P, Towle AC. Glutamate-positive neurons in the somatic sensory cortex of rats and monkey. J Neurosci. 1987;7:1887–1901. doi: 10.1523/JNEUROSCI.07-06-01887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Rio JA, Soriano E, Ferrer I. Development of GABA-immunoreactivity in the neocortex of the mouse. J Comp Neurol. 1992;326:501–526. doi: 10.1002/cne.903260403. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Zhang L, Weiser M, Rudy B, McBain C. Developmental expression and functional characterization of the potassium channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J Neurosci. 1996;12:506–518. doi: 10.1523/JNEUROSCI.16-02-00506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foehring RC, Surmeier DJ. Voltage-gated potassium currents in acutely dissociated rat cortical neurons. J Neurophysiol. 1993;70:51–63. doi: 10.1152/jn.1993.70.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Grissmer S, Ghanshani S, Dethlefs B, McPherson JD, Wasmuth JJ, Gutman GA, Cahalan MD, Chandy K. The Shaw-related potassium channel gene, Kv3.1, on human chromosome 11, encodes the type 1 K+ channel in T cells. J Biol Chem. 1992;267:20971–20979. [PubMed] [Google Scholar]
- 13.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 14.Hamill O, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Hamill OP, Huguenard JR, Prince DA. Patch-clamp studies of voltage-gated currents in identified neurons of the rat cerebral cortex. Cereb Cortex. 1991;1:48–61. doi: 10.1093/cercor/1.1.48. [DOI] [PubMed] [Google Scholar]
- 16.Ho CS, Grange RW, Joho RH (1997) Pleiotropic effects of a disrupted K+ channel gene: reduced body weight, impaired motor skill and muscle contraction, but no seizures. Proc Natl Acad Sci USA, in press. [DOI] [PMC free article] [PubMed]
- 17.Huguenard JR, Hamill OP, Prince DA. Developmental changes in sodium conductances in rat neocortical neurons: appearance of a slowly inactivating component. J Neurophysiol. 1988;59:778–794. doi: 10.1152/jn.1988.59.3.778. [DOI] [PubMed] [Google Scholar]
- 18.Jonas P, Racca C, Sakmann B, Seeberg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 19.Keller A, White EL. Synaptic organization of GABAergic neurons in the mouse SmI cortex. J Comp Neurol. 1987;262:1–12. doi: 10.1002/cne.902620102. [DOI] [PubMed] [Google Scholar]
- 20.Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13:5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambolez B, Ropert N, Perrais D, Rossier J, Hestrin S. Correlation between kinetics and RNA splicing of α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid receptors in neocortical neurons. Proc Natl Acad Sci USA. 1996;93:1797–1802. doi: 10.1073/pnas.93.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Massengill JL, O’Dowd DK, Smith MA (1996) Agrin gene expression in mouse somatosensory cortical neurons during developmentin vivo and in cell culture. Neuroscience, in press. [DOI] [PubMed]
- 23.Lorenzon NM, Foehring RC. The ontogeny of repetitive firing and its modulation by norepinephrine in rat neocortical neurons. Dev Brain Res. 1993;73:213–223. doi: 10.1016/0165-3806(93)90141-v. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzon NM, Foehring RC. Characterization of pharmacologically identified voltage-gated calcium channel currents in acutely isolated rat neocortical neurons. II. Postnatal development. J Neurophysiol. 1995;73:1443–1451. doi: 10.1152/jn.1995.73.4.1443. [DOI] [PubMed] [Google Scholar]
- 25.Luneau CJ, Williams JB, Marshall J, Levitan ES, Oliva C, Smith JS, Antanavage J, Folander K, Stein RB, Swanson R, Kaczmarek LK, Buhrow SA. Alternative splicing contributes to K+ channel diversity in the mammalian central nervous system. Proc Natl Acad Sci USA. 1991;88:3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick DA, Prince DA. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol (Lond) 1987;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Dowd DK, Gee JR, Smith MA. Sodium current density correlates with expression of specific alternatively spliced sodium channel mRNAs in single neurons. J Neurosci. 1995;15:4005–4012. doi: 10.1523/JNEUROSCI.15-05-04005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J Neurophysiol. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- 29.Ribera AB, Spitzer NC. Developmental regulation of potassium channels and the impact on neuronal differentiation. Ion Channels. 1992;3:1–38. doi: 10.1007/978-1-4615-3328-3_1. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, O’Dowd DK. Cell-specific regulation of agrin RNA splicing in the chick ciliary ganglion. Neuron. 1994;12:795–804. doi: 10.1016/0896-6273(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 31.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol (Lond) 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- 33.Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in rat CNS. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu R-L, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarom Y, Sugimori M, Llinas R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985;16:719–737. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama S, Imoto K, Kawamura T, Higashida H, Iwabe N, Miyata T, Numa S. Potassium channels from NG108–15 neuroblastoma-glioma hybrid cells. FEBS Lett. 1989;259:37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F-M, Hablitz JJ. Postnatal development of membrane properties of layer I neurons in rat neocortex. J Neurosci. 1996;16:1131–1139. doi: 10.1523/JNEUROSCI.16-03-01131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]