Abstract
The behavior of growing thalamic axons was studied in an organotypic coculture of the lateral geniculate nucleus (LGN) with the visual cortex (VC) to reveal cellular interactions that underlie the formation of lamina-specific thalamocortical connections. The LGN explant was placed at the ventral side, pial surface, or lateral edge of the VC explant, and fluorescent dye-labeled LGN axons were observed by confocal microscopy in fixed and living tissue. The axonal projection pattern in fixed cocultures after 1 week in vitro demonstrated that, in all three configurations, LGN axons formed primitive branches mainly in layer 4. A time-lapse study further examined axonal growth and branch formation in the living cortical explant. The majority of branches emerged within layer 4 behind the axonal tip, regardless of the direction of axonal entry. In addition, most axons entering from the ventral or pial side of the VC exhibited a transient or persistent stop of axonal growth in and around layer 4, whereas those entering from the lateral edge of the VC traveled along layer 4 without exhibiting stop behavior. The axonal stop often was accompanied by growth cone collapse and a slight retraction. These results suggest the existence of branch and stop cues in layer 4 of the cortex that are recognized by LGN axons.
Keywords: axonal branch, target recognition, neocortex, thalamus, growth cone, collapse, time-lapse study, organotypic culture
Neocortical neurons receive lamina-specific afferent inputs from subcortical and other cortical structures (Jones, 1981). Thalamocortical projections from the lateral geniculate nucleus (LGN) to the visual cortex (VC) are among the most prominent examples of lamina-specific afferent connections. Most LGN axons form extensive branches in layer 4 and collaterals in layer 6, although some project to layer 1 (Gilbert, 1983).
The question of how LGN axons find their way to the VC and form axonal arborizations in appropriate layers has been investigated morphologically at various developmental stages in the mammalian cortex. These studies revealed that geniculate axons extend through the intermediate zone underneath nonvisual cortical areas (Ghosh and Shatz, 1992) and accumulate at the VC subplate zone until the maturation of the cortical plate (Lund and Mustari, 1977; Rakic, 1977; Shatz and Luskin, 1986; Molnár and Blakemore, 1995). They then grow toward the pial surface. Finally, LGN axons form branches and synapses in appropriate layers (Kageyama and Robertson, 1993; Miller et al., 1993), although some axons overshoot the target layer (Lund and Mustari, 1977;Ghosh and Shatz, 1992). A remarkable feature in development is that LGN axons may be guided to the target by subplate axons (Ghosh et al., 1990; Ghosh and Shatz, 1992). There may be a specific affinity between LGN axons and subplate neurons or a preferable substratum for LGN axons in the pathway (Bicknese et al., 1994).
In addition to such pathway guidance, in vitro studies indicate that LGN fibers recognize the target cell layers in the cortex. In organotypic cocultures that are composed of LGN and VC explants, thalamocortical connections form in vitro with essentially the same laminar specificity as that found in situ (Yamamoto et al., 1989, 1992a; Molnár and Blakemore, 1991; Bolz et al., 1992). However, the cellular interactions by which thalamic axons find their target layers and form arbors still remain unknown. Revealing the details of axon–target interactions can shed light on the molecular mechanisms that are common to the formation of axonal projection patterns throughout the CNS.
A powerful approach to resolve this issue is to directly observe the growth of living axons. Indeed, time-lapse studies of axonal growth have been applied in several systems and have demonstrated some consistent features of target-finding, such as a reduction of axonal growth rate in the target zone, emergence of interstitial branches, or a change of axonal tip morphology (Harris et al., 1987; O’Rourke and Fraser, 1990; Kaethner and Stuermer, 1992; Myers and Bastiani, 1993; Sretavan and Reichart, 1993; Godement et al., 1994; Halloran and Kalil, 1994; Bastmeyer and O’Leary, 1996). The present study aims to uncover the dynamic behavior of ingrowing afferents using time-lapse imaging of geniculate axons, which should, in turn, reveal the nature of target-derived signals for regulation of afferent growth.
Part of this study has been reported in a preliminary form (Yamamoto et al., 1992b).
MATERIALS AND METHODS
Culture. Timed-pregnant Harlan Sprague Dawley rats were used in the present study. The day of vaginal plug detection was designated as embryonic day (E) 0, and the first 24 hr after birth was referred to as postnatal day (P) 0. A cortical slice was dissected from P1–P3 rats, and a block of the LGN was dissected from E15 rat embryos. An organotypic coculture of the LGN and VC was prepared as reported previously (Yamamoto et al., 1992a). In brief, the VC and LGN explants were cocultured on collagen-coated membrane in serum-free, hormone-supplemented medium. The LGN explant was placed at either the ventral, pial, or lateral side of the VC explant to observe LGN axons entering the cortical explant at these orientations. The cultures were maintained at 37°C in an environment of humidified 95% air and 5% CO2.
Bromodeoxyuridine labeling. To determine the location of layer 4, 5′-bromodeoxyuridine (BrdU, 20–30 mg/kg) was injected into timed-pregnant mothers 17 d after conception. After the birth, cortical slices were dissected and cocultured with the LGN block as described above. The cultures were fixed for 6–18 hr and processed by a method described previously (delRio and Soriano, 1989;Yamamoto et al., 1992a). Briefly, the cultures were cut into sections (50 μm) and immersed in 2 m HCl. Then they were incubated with anti-BrdU (1:5) in PBS containing 0.5% Tween 20 and visualized by the indirect immunofluorescence method.
Axonal labeling and time-lapse imaging with a confocal microscope. After 2–6 d in culture, a small crystal of a fluorescent lipophilic dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) was inserted into the LGN explant with a tungsten needle under a dissecting microscope (Honig and Hume, 1986). After a few days of incubation, the explants growing on the culture membrane were put onto a small piece of lens paper and cut out together with the paper. The explants were then transferred to a special chamber on a microscopic stage (Fig.1). This chamber was composed of a medium container and a piece of membrane (Millipore, Bedford, MA; omnipore filter) on which the explants were placed. To make the same environment as in the culture incubator, the chamber was sealed with a coverslip and supplied with humidified 95% air and 5% CO2. In addition, the space including the chamber and the objective lens was surrounded by an acrylic box, and the inside temperature was maintained at 37°C with a heating apparatus (Kokensha Engineering). To prevent the formation of drops inside the coverslip that sealed the chamber, a U-shaped wire heater was attached to the top of the coverslip.
Fig. 1.
Experimental setup for the time-lapse study. Explants (closed) were placed in a chamber, which produced conditions similar to a CO2 incubator (see Materials and Methods).
Time-lapse imaging was performed using a laser scanning confocal microscope (Nikon, Optiphoto; Bio-Rad, MRC-500). An objective lens with long working distance (20× or 40×; working distance, 5–6 mm) was used for the observation of the culture from above the coverslip. The confocal microscope was equipped with an argon laser (excitation wavelength, 514 nm) and a filter set for DiI. A neutral density filter (1–5%) was placed in the path of the laser to reduce the intensity of excitation and minimize photodynamic damage produced by the excitation beam. The cultures in which dye spread over the cortical explant or in which labeling was too weak were discarded. Axons that could be traced back to the LGN explant were further selected for the time-lapse study. A single optical section was sampled when LGN axons grew exclusively within the plane of the optical section. Otherwise, a series of images was collected at different depths (5–10 μm interval) and superimposed. Each image was averaged 3 or 4 times to increase the signal-to-noise ratio. The focus of the microscope was often adjusted manually to maintain a sharp image of the axons. The time-lapse imaging was conducted for 10–40 hr until the stained axons became faint. Some of the axons whose staining become too weak during the observation were excluded from the analysis.
The interval for imaging was selected to minimize photodynamic damage (Harris et al., 1987). In a preliminary experiment, short interval (<5 min) exposure reduced the growth rate considerably, but the effect was undetectable with an interval of 30 min or more. With such longer intervals, the axonal speed was typically equivalent to the growth rate of callosal axons traveling in the cortical slices under normal fluorescent illumination (Halloran and Kalil, 1994) and comparable to the highest rates of thalamic axons growing on cortical membrane fractions under phase-contrast optics (Hübener et al., 1995). Therefore, the images were taken at an interval of 30 min to 2 hr. At the end of the experiment, images were taken at lower magnification (4× or 10× objective) to determine the locations of axonal tips in the cortical explant.
All of the superimposed images were stored on magneto-optical disks for later analysis. The growth rate of LGN axons was determined from two successive images as the distance between axonal tip position divided by the time interval. The distance was measured with analysis software (Bio-Rad).
Other cultures were fixed with 4% paraformaldehyde in 0.1m phosphate buffer to observe DiI-labeled axons more clearly, because labeling intensity gradually decreased and injected DiI spread out during time-lapse experiments. These fixed cultures were mounted in the fixative and observed by confocal microscopy. The cultures in which individual axons were not distinguishable were discarded.
Histology. The cultures were fixed with 4% paraformaldehyde in 0.1 m phosphate buffer after the time-lapse studies and kept in 30% sucrose in PBS. Then, cultures were placed on an agar block (4% agar in PBS), cut into 30 μm frozen sections, and stained with cresyl violet to observe the cytoarchitecture of the cortical explants.
RESULTS
DiI-labeled LGN axons were observed after 5–8 d in culture, whereas LGN axons were growing in the cortical explant. A total of 263 cocultures were prepared for the present study. Thirty-one cultures were used for the analyses of axonal projection pattern with DiI under fixed conditions (n = 25) and cortical cytoarchitecture with BrdU (n = 6). The remaining 232 cocultures were used in the time-lapse study, but in many of them labeling intensity was weak or became much lower during the experiment. In addition, the dye often spread over the cortical explant. These cultures were discarded. As a result, only 29 cocultures were used for the time-lapse analysis.
Laminar profile of the cortical explant after 1 weekin vitro
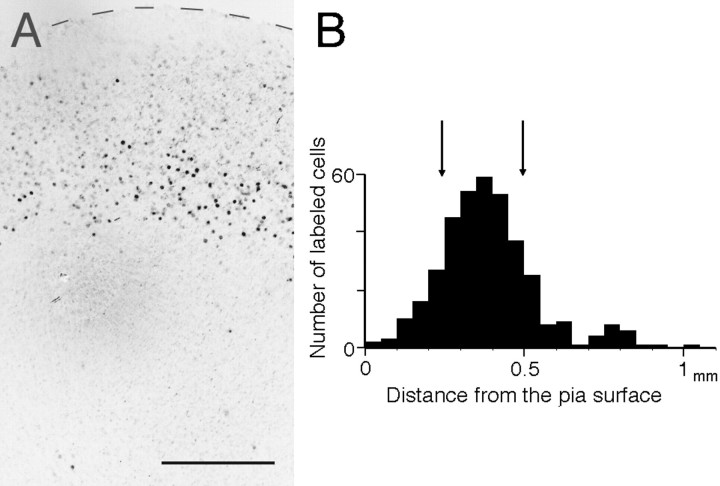
It was difficult to identify layer 4 based on Nissl staining at 5–8 d in vitro. Therefore, a standard layer 4 location was determined by injecting BrdU when layer 4 cells were being born at E17. This injection allows the cells destined for layer 4 to be labeled effectively, although the adjacent cell layers are partly labeled (Berry and Rogers, 1965; Brückner et al., 1976). In all of the cocultures (n = 6), heavily labeled cells were concentrated in the middle part of the cortical explant (Fig.2A, black dots). In the distribution histogram plotting the number of labeled cells by cortical depth (the distance from the pial surface) for the six cocultures (Fig.2B), layer 4 was determined as the zone containing more than 70% (within 1 SD from the mean) of the total number of labeled cells. In the cocultures, layer 4 extended a few hundred micrometers across the middle part of the cortical plate (0.22–0.52 mm from the cortical surface). This laminar distribution is similar to that obtained from cortical explants after a few weeks in culture (Götz and Bolz, 1992; Yamamoto et al., 1992a), indicating that the basic layer organization is already formed after 1 week in vitro.
Fig. 2.
Laminar distribution of BrdU-labeled cells in the cortical explant after 1 week in vitro.A, Cortical explant where BrdU was injected at E17. Note that heavily labeled cells (black dots) are distributed in the middle layer. Contrast is inverted. A dashed lineindicates the pial surface of the cortical explant. Scale bar, 200 μm. B, Distribution histogram plotting the number of labeled cells against the distance from the pial surface for the six cocultures. Arrows indicate the laminar locations in which 70% of cells are labeled.
Projection patterns of LGN axons
The early projection pattern of LGN axons was investigated in fixed cocultures after 1 week in vitro. First, the axonal projections were studied in cocultures in which the LGN explant was placed at the ventral side of the VC. This arrangement mimics the normal entry of LGN axons. DiI placement in the LGN explant demonstrated that labeled axons extended radially toward the pial surface, although axonal invasion did not occur so synchronously as in normal development. A fraction of LGN axons formed a few branches (Fig.3A,B) or reached the superficial layers (Fig.3C), but many axons were still growing in the deep layers (Fig. 3C) and the white matter.
Fig. 3.

Axonal projections from the LGN in the ventral coculture. A, DiI-labeled axons forming branches at 1 week in vitro. B, Another LGN axon similar to A. White arrows represent branching points. C, Axons without branches reach layer 1 or grow toward the pial surface with a growth cone. White arrowheads indicate the growing axons in the middle or deep layer. D, Axonal branching after 2 weeks in culture. Scale bar (shown in A), 250 μm in A–D.E, The distribution of branching points.Arrows indicate the putative layer 4 borders.
A total of 41 individually distinguishable labeled axons, excluding axons that terminated in the middle or deep layers, were collected from 12 cocultures to analyze the laminar profile of the initial axonal projections. Thirteen of the 41 axons had one or more branches (average, 2.9 branches), whereas the remaining axons extended to the superficial layers without branching. The laminar distribution of these branching points (n = 38) demonstrated that approximately one-half of the branches (47%) were formed at 250–500 μm from the pial surface, corresponding to the putative layer 4 (Fig.3E). This laminar distribution was similar to previous results obtained from cocultures after a few weeks in vitro(Yamamoto et al., 1992a). However, the number of branches per axon was much smaller after 1 week in culture than after 2–3 weeks in culture (compare Fig. 3A–C with Fig. 3D). In addition, the proportion (28 of 41) that extended to the superficial layers without branching was much larger than the fraction (20%) of overshooting axons found after a few weeks in vitro(Yamamoto et al., 1992a).
Branching of LGN axons in layer 4 may be attributable to the presence of specific branching cues in layer 4, to the order of layers encountered by ingrowing axons, or to LGN axons simply branching after growing a specific distance into the cortex. These possibilities were tested in LGN axons entering cortical explant from the pial surface. The distance that geniculate axons must travel to reach layer 4 is much shorter in the pial arrangement than in the ventral arrangement (approximate distance, 300 vs 1000 μm).
In the analysis of 51 single-labeled axons from eight cocultures in the pial arrangement, a large number (39 of 51) did not possess any branches, but a significant fraction (12 of 51) formed branches in the cortical explant (Fig. 4A,B). The distribution histogram showed that most of the branching points (15 of 17) were located in the putative layer 4 (Fig. 4D, black columns). Therefore, it is unlikely that LGN axons start to form branches as a result of growing a particular distance or passing through the deep layers.
Fig. 4.
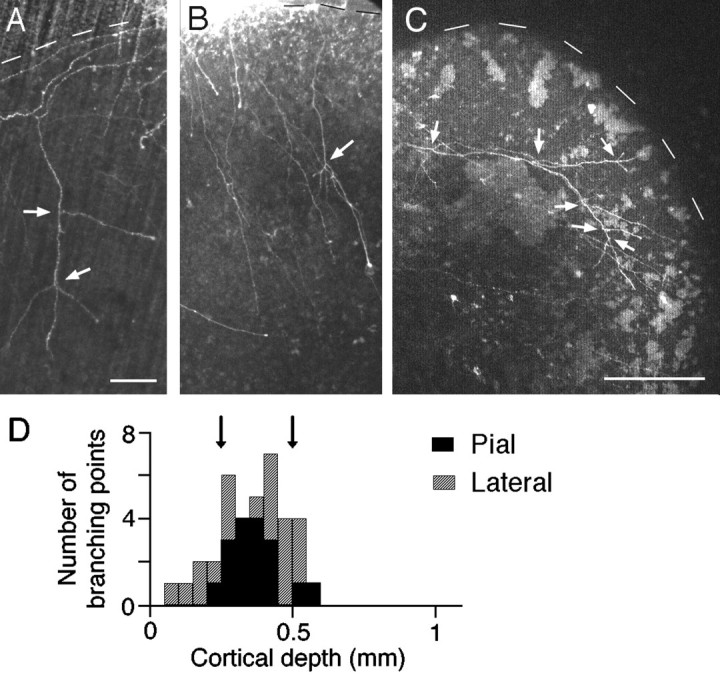
Axonal projections in the pial and lateral cocultures. Axons are shown forming branches in the pial (A and B) and lateral (C) arrangements. White arrows represent branching points. Scale bars: 100 μm in A and B; 250 μm in C. D, The distribution of branching points of axons entering at the pial surface (black columns) or lateral edge (gray columns) of the VC explant. Arrows indicate the putative layer 4 borders.
The analysis conducted in the lateral cocultures (n = 5) in which axons from the LGN explant grew into the cortical explant along an axis parallel to the cortical layers also supports this view. In agreement with previous results in long-term cultures (after 2–3 weeks in vitro), axons in the lateral arrangement traveled along the target layer or gradually extended from inappropriate layers to the target layer (Yamamoto et al., 1992a). The analysis of 19 single-labeled axons showed that more than one-half of the branching points (12 of 20) were present in the putative layer 4 (Fig.4D, gray columns). Interestingly, there was an extremely large variation in the distance between branch point and the point of entry into the cortical explant (from 300 to 2100 μm). In particular, several branches were found to emerge at different points from a single parent axon traveling within layer 4 (Fig.4C).
Dynamic growth patterns of LGN axons
The above findings indicate that after 1 week in vitro, LGN axons are still growing into the cortical explant and begin to form terminal branches in layer 4, regardless of the direction of axonal ingrowth. To analyze LGN axon behavior, including branch formation, a time-lapse study was conducted in coculture preparations at approximately 1 week in vitro. A total of 40 single-labeled axons that were individually distinguishable were imaged in ventral, pial, and lateral cocultures.
LGN axons entering from the ventral side of the VC
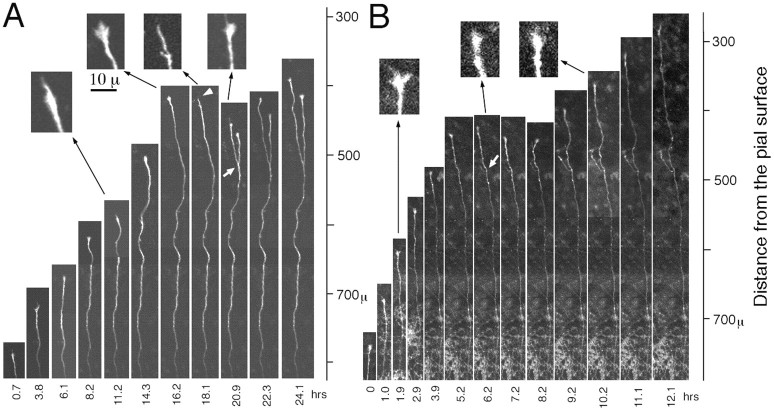
Axons forming branches. Twenty-two axons were followed in a time-lapse study of 13 ventral cocultures. Ten of these axons exhibited branch formation (Table 1). An interesting feature was that a stop of axonal growth was observed before branching. Figure 5A shows time-lapse images of a typical example of this branching preceded by a stop of axonal extension. The growth pattern was quantitatively studied by plotting the positions and growth rates of the axon tip (Fig.6A). This axon grew for 16 hr (20 μm/hr) through the deep layers toward the cortical surface, led by a typical growth cone at the tip. After arriving at the middle of the explant (400 μm from the pial surface), it suddenly stopped growing and even slightly retracted for the next 4 hr. The stop in axonal growth and the retraction were accompanied by a collapse of the growth cone (Fig. 5A, insets). During the stop phase, a branch appeared 70 μm behind the axonal tip. Finally, both the parent axon and axonal branch started to grow again tipped with growth cones. Figure 5B shows another example that exhibits a transient axonal stop (∼2 hr) and subsequent branching behind the axon tip, although the axon traveled quickly (>50 μm/hr) in the deep layers (Fig. 6B). In this case, the tip of the parent axon shrank slightly during the axonal stop but did not seem to collapse (Fig. 5B, insets). In the ventral arrangement, many (7 of 10) of the axons forming branches exhibited a similar stop behavior before branching (Table 1).
Table 1.
Branch and stop of LGN axons
| Branch | No branch | Total | |||
|---|---|---|---|---|---|
| Stop | No stop | Stop | No stop | ||
| Ventral | 7 | 3 | 6 | 6 | 22 |
| Pial | 1 | 1 | 4 | 4 | 10 |
| Lateral | 0 | 4 | 0 | 4 | 8 |
| Total | 8 | 8 | 10 | 14 | 40 |
Each row represents the number of axons that were classified according to the branch and stop behaviors. Ventral, pial, and lateral represent the location of the LGN explant relative to the VC explant.
Fig. 5.
Axonal growth of DiI-labeled LGN axons forming a branch in the ventral arrangement. A, The axon was followed at the intervals of 1–3 hr. B, Similar toA but imaged at 30 min intervals. Every other sampled image is shown in A and B.Insets show a higher magnification of the axonal tip. The images are montaged. To show the entire course of LGN axons, past images were used for the proximal part of the axon, because only the images around the axonal tip were followed at high magnification.Arrows indicate branching points.Arrowhead represents axonal tip with collapse. The distance from the pial surface is indicated to theright.
Fig. 6.
Quantitative analysis of axonal growth in the ventral cocultures. The top and bottomgraphs show the distance from the pia (cortical depth) and the axonal growth rate, respectively. P, Parent axon;B or B1, primary branch;B2, secondary branch. A,B, The growth pattern of the axons shown in Figure 5,A and B, respectively. C, Axons forming a branch without stopping. D, E, Axons without branching and stopping. D, Analysis of the axon shown in Figure 7. F, This axon exhibits stop behavior but no branching. After the stop in the middle layer, this axon changes direction and travels along the layer.
However, a fraction of the fibers (3 of 10) formed branches with no sign of a stop in axonal growth (Table 1). The axon shown in Figure6C extended at a moderate growth rate (30–40 μm/hr) through all cortical layers up to the pial surface, forming a collateral branch and a bifurcating branch in the deep layers. Likewise, the other two axons formed branches in the middle and superficial layers.
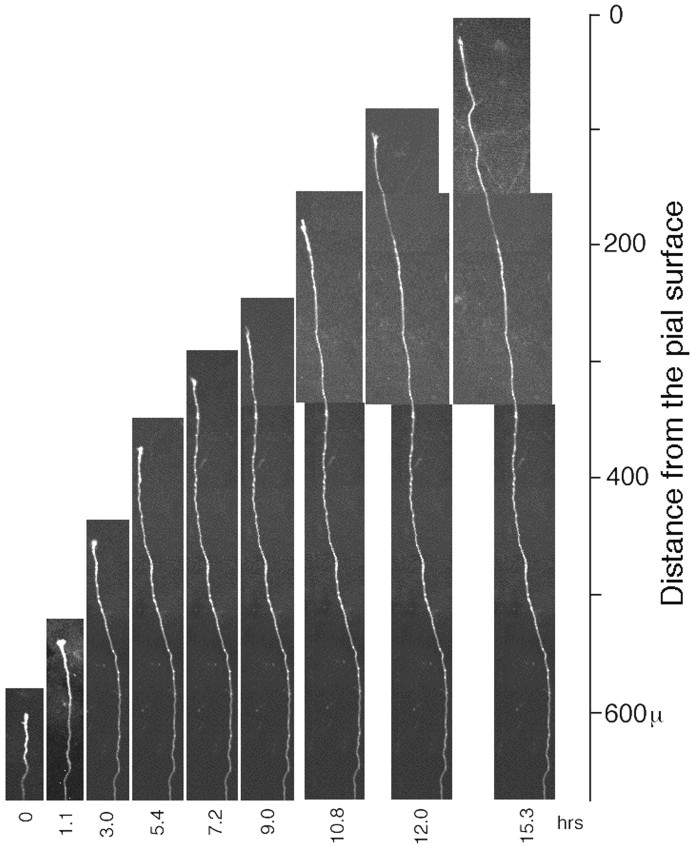
Axons without branches. Twelve of the sampled axons (12 of 22) did not show branch formation (Table 1) except that a short branch appeared transiently (<3 hr). Half of the 12 axons traveled up to the cortical surface without stopping or branching (see Fig.7). The velocity of axonal growth was between 20 and 60 μm/hr (Fig. 6D,E), but was not much different from that of axons forming branches.
Fig. 7.
Axonal growth of DiI-labeled LGN axons without a branch in the ventral arrangement. This axon was followed at an interval of 2 hr for 24 hr. The images are montaged in the same way as in Figure 5.
The remaining six axons exhibited stop behavior but without forming any branches (Table 1). These axons traveled continuously in the deep layers (30–50 μm/hr) but stopped growing in the putative layer 4, or slightly more superficially. Growth cone collapse was clearly evident during the stop in several cases (4 of 6). Four axons stalled for the entire period of the time-lapse study. The remaining two axons (2 of 6) restarted to grow after transiently stopping. One of them kept growing up to the pial surface. The other made a turn and grew along layer 4 (Fig. 6F).
Axons entering from the pial side or the lateral edge of the VC
The growth pattern was further studied in pial and lateral cocultures to examine whether the orientation or distance of axon growth could influence axon behavior.
A total of 10 axons was sampled in the pial cocultures. Two axons formed a persistent branch (Table 1). One of them exhibited stop behavior and subsequent branching. As shown in Figure8A, this axon traveled toward the deep layers at a moderate speed (20–40 μm/hr), but stopped and retracted in the putative layer 4 (400 μm from the pial surface). During the retraction, an axonal collapse was clearly observed. Two hours after the initiation of the stop, a branch bud emerged behind the axonal tip. Another axon extended from the upper to deep layers exhibiting a well formed growth cone the entire time, and then branched interstitially in the deep layers.
Fig. 8.
Quantitative analysis of axonal growth in the pial (A–C) and lateral (D andE) cocultures. The top andbottom graphs show the distance from the pial surface and growth rate, respectively. P, Parent axon;B, primary branch.
However, most of the axons (8 of 10) obtained from the pial cocultures did not show branch formation (Table 1). These axons grew in the superficial layers at a speed of 20–40 μm/hr. Half of them (4 of 8) slowed down as they approached layer 4 and ceased to grow for more than a few hours, accompanied by an axonal collapse (Fig.8B). In contrast, the remaining four axons extended into the deep cortical layers with no sign of reducing speed (Fig.8C). Thus, branching and stopping behaviors in the pial coculture were similar to those found in the ventral arrangement, although the population exhibiting branch formation was smaller (Table1).
On the other hand, none of the LGN axons (n = 8) entering from the lateral edge of the VC showed the stop behavior, but a persistent branch emerged in half of these cases (Table 1). Figure8D shows an axon forming a branch, which traveled a long distance through layer 4 at a speed of 20–40 μm/hr and made an interstitial branch. Similar growth along the length of layer 4 and branch formation were observed in two other axons, but another that entered from the superficial layer gradually steered toward the deeper layers and finally started to branch in layer 4 (Fig.8E). All of the branches appeared as an interstitial branch behind the growth cone. Of the axons forming no branches (4 of 8), all elongated along layer 4 except for one, which extended in the most superficial layer.
Laminar location of branching and stopping
Altogether, 40% of the axons (16 of 40) observed in all three types of cocultures formed persistent branches for the entire period of the time-lapse study (Table 1). The laminar locations of branch points were analyzed for the 16 axons forming one (n = 10) or more (n = 6) branches. In addition to the persistent branches, a transient appearance of small branches (lasting <3 hr) was found in 15 axons. These transient branches usually appeared during a growing phase rather than a stopping phase.
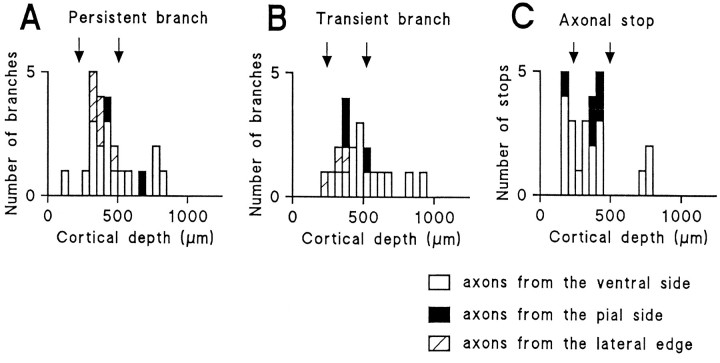
In the ventral arrangement, the majority of the persistent and transient branching points (10 of 16 and 8 of 14, respectively) were located in the putative layer 4 (250–500 μm from the pial surface), although a few were present in the upper and deep layers (Fig.9A,B, open columns). Similar laminar localization was also observed in the axons that entered from the pial and lateral edges. As shown in Figure 9, A andB, most of the branching points, including transient branches (3 of 5 in the pial arrangement and 7 of 8 in the lateral arrangement), were located in layer 4. Therefore, it is unlikely that the laminar restriction of branch points was affected by the direction of axon entry. In the pial arrangement, however, the number of axons forming persistent branches is less (2 of 10) than in other types of cocultures.
Fig. 9.
Laminar distribution of branching and stopping. The distribution of branching points of persistent (A) and transient (B) branches and stopping points (C) are plotted against the distance from the pial surface (cortical depth). Axons entering at the ventral side, pial side, and lateral edge are represented by open,filled, and hatched columns, respectively. Arrows indicate the putative layer 4 borders.
Frequently, a branch bud including transient branches (33 of 43) emerged from behind the axonal tip, although the axonal tip appeared to split in a fraction (10 of 43) of cases. This bifurcation often resulted in the emergence of secondary or tertiary branches.
Axonal stopping behavior (<5 μm advance for 1 hr) was also investigated for 18 axons (Table 1). Six axons exhibited a stop twice during the period of observation. The laminar distribution of all stop points in the ventral cocultures demonstrated that the stop behavior occurred in slightly more superficial layers compared with branching locations, but approximately half of the stopping points (9 of 19) were located in layer 4 (Fig. 9C, open columns). In the pial arrangement, a similar laminar distribution was observed (Fig.9C, filled columns). In contrast, axons entering at the lateral edge of the VC never showed the stop behavior.
The shape of the axonal tip seemed to change according to its behavioral state. Growing axons were tipped by a growth cone, whereas such a structure disappeared during stop or retraction. At least 11 of the 18 axons exhibited such a collapse of growth cones (Fig. 3A, insets), although it was difficult to describe the changes of growth cones quantitatively, given the limited spatial resolution in the present experiment.
DISCUSSION
The analysis of the projection patterns and the time-lapse study in all three types of cocultures demonstrates that LGN axons begin to form branches mostly in layer 4, regardless of their direction of entry into the cortical explant. On the other hand, the majority of LGN axons that traveled perpendicularly to the cortical layers exhibited a persistent or transient stop approximately in layer 4, whereas axons elongating parallel along layer 4 did not show the stop behavior. These findings suggest that the stop behavior may be produced by a relative difference in molecular signals between layer 4 and the adjacent layers, whereas the branch behavior seems attributable to an absolute value of signals localized in the target layer.
Branch formation
In the ventral arrangement, LGN axonal branches mainly formed in layer 4 from the onset. This laminar pattern of branch formation is consistent with that of thalamocortical projections observed in vivo in early developmental stages of the mammalian visual (Lund and Mustari, 1977; Ghosh and Shatz, 1992; Kageyama and Robertson, 1993;Miller et al., 1993) and somatosensory systems (Agmon et al., 1993;Catalano et al., 1996). In the pial and lateral arrangements, branching also occurred mostly in layer 4. This finding not only confirmed previous in vitro studies (Bolz et al., 1992; Yamamoto et al., 1992a), but also demonstrated that initial branches appeared in the target layer regardless of the orientation and distance of ingrowth. However, the fact that the axons from the pial surface showed branching less frequently in analyses of both the projection and growth patterns may reflect some effect of laminar order.
It also must be pointed out that many geniculate axons reached the most superficial layer even in the ventral arrangement. These axons might represent thalamic axons that project to layer 1 in early stages (Lund and Mustari, 1977; Ghosh and Shatz, 1992) and are possibly eliminated later (Kurotani et al., 1993). In support of this view, a smaller population of LGN axons reached layer 1 with little branching after 2–3 weeks in vitro (Yamamoto et al., 1992a). The possibility also remains that the overshooting axons contain the population that projects to layer 1 in the adult VC (Peters and Feldman, 1976) or originate from the thalamic nuclei neighboring the LGN (Herkenham, 1980; Yamamoto et al., 1992a).
A prominent feature in branch formation that we observed is that branching mostly occurred at some distance behind the tip of the parent axons. Such interstitial branching was reported in the frog retinotectal projections in vivo (Harris et al., 1987), in corticocortical projections in acute slice preparations (Halloran and Kalil, 1994), and in corticofugal projections in vitro andin vivo (O’Leary and Terashima, 1988; Sato et al., 1994;Bastmeyer and O’Leary, 1996). It appears to be a common process in branch formation.
One problem in the identification of branching is that what we describe as axonal branching might be simple defasciculation. Indeed, the possibility cannot be excluded entirely because an axon with a thin growth cone might not have been possible to distinguish from an axon on which it was growing. However, it was possible to directly observe fasciculation in some cases in the form of lamellipodia of a growth cone extending on another axonal stem.
Stop signal
One of the striking findings in this study is that many LGN axons stop transiently or persistently after arrival in layer 4 in the ventral and pial arrangements. This finding provides more convincing evidence for the existence of the stop signal, which has been postulated based on the in vitro observation that axonal tips of LGN fibers accumulate within layer 4 (Molnár and Blakemore, 1991). Moreover, an interesting feature is that laterally approaching axons did not exhibit the stop. This suggests that some difference between layer 4 and the layer above or below it may act as the stop signal.
Similar stop behavior has been demonstrated in other nervous systems. In cocultures of dissociated cerebellar granular cells with pontine nucleus explants, axons from the explant do not grow beyond granular cells (Baird et al., 1992). A time-lapse study of retinal axons demonstrated that growth speed is significantly decreased after they invade the tectum from the optic tract (Harris et al., 1987). These findings are consistent with the present result, in that axon growth is tempered by their targets. Axons may also stop when confronted with decision regions. Indeed, a similar stop of retinal axons has been shown to take place in the optic chiasm (Sretavan and Reichart, 1993;Godement et al., 1994).
To date, a time-lapse experiment of callosal axons has demonstrated that relatively short pauses (<30 min) of axonal growth occurred frequently below the target cortical area, by imaging at intervals of a few minutes (Halloran and Kalil, 1994). Whether thalamic axons exhibit similar pauses is unknown, since we could not follow axonal behavior using such short imaging intervals because of potential photodynamic damage. The axonal stopping observed in this study was usually maintained for over a few hours and may be attributable to the repetition of such brief pauses. Alternatively, growth of LGN axons may be suppressed for the entire prolonged period. In any case, it is likely that the stop behavior is associated with a target-finding process.
Coincidence between stop and branch behaviors
The majority of the observed axons demonstrated either branching or stopping in all types of cocultures, suggesting that the stop and branching are separable processes. Nonetheless, these behaviors occasionally occurred conjointly, except in the case of the lateral arrangement. In particular, conjoint occurrence was observed most frequently in the ventrally approaching axons. This may be attributable simply to colocalization of branching and stopping signals, because in this arrangement each behavior occurred around layer 4, with a high probability. In addition, the stop signal may induce branching because a primary branch always followed the axonal stop in the group showing both behaviors.
Possible nature of stop and branch signals
There are several possible molecular mechanisms that could underlie the axonal stop in layer 4. First, a lack of growth-promoting factors may cause LGN axons to stop growing. In agreement with this idea, the membrane-bound growth-promoting activity for thalamic neurons has been found to be lower in layer 4 than in the deep layers (Götz et al., 1992). It also has been indicated that an adhesive factor that could control thalamic axonal growth is expressed in the deep layers (Emerling and Lander, 1994). A recent study of the retinotectal projection reported that the reduction of the growth speed of retinal axons in the tectum is associated with a lower concentration of fibroblast growth factor (McFarlane et al., 1995). A second possibility is that the stop is attributable to factors that inhibit axonal growth (Schwab et al., 1993; Luo and Raper, 1994). In fact, growth cone collapse often accompanied axonal stop behavior. However, neither the absence of growth-promoting factors nor the presence of inhibitory factors may explain axonal elongation and accompanying branch extension within layer 4. Some target-derived factor might contribute to restricting afferent fibers to the appropriate layer. In the neuromuscular junction, the synaptic basal lamina proteins s-laminin and agrin have been suggested to cause motor axons to arrest outgrowth at specific sites on muscle cells (Campagna et al., 1995;Porter et al., 1995). In addition, the mechanisms underlying the stop behavior may also involve the detection (while approaching the target layer) by LGN axons of relative changes in the local concentrations of some molecule or in the types of molecules encountered.
Conversely, it is likely that the branching behavior basically reflects the absolute concentration of the branch factor, because thalamic axons traveling from any orientation formed branches in layer 4. Moreover, the fact that branching points are well localized in the target layer suggests that the responsible molecule may act locally. In agreement with this view, a recent in vitro study suggested that membrane-bound components are involved in target-dependent branch formation by demonstrating that retinal axons form arbors in appropriate layers on fixed tectal tissues (Yamagata and Sanes, 1995). In the neuromuscular junction, the amount of polysialic acid bound to neural cell adhesion molecules has been shown to regulate axon fasciculation and branching (Landmesser et al., 1990).
Several investigators have indicated that some members of the neurotrophin family promote axonal and dendritic arborization in the CNS, including the neocortex (Cabelli et al., 1995; Cohen-Cory and Fraser, 1995; McAllister et al., 1995). However, the fact that the laminar localization of axonal innervation is not altered even after neurotrophin application (Cabelli et al., 1995) indicates that these factors may control growth and elaboration of branches rather than the restriction of branching to their laminar targets. Indeed, in our coculture preparations, transient short branches were often found to emerge in layer 4 and eventually disappear, consistent with the view that some local cues in the target induce branching, whereas other factors are required for growth and stabilization of branches.
Footnotes
This work was supported by grants-in-aid for Scientific Research Projects 04770081, 05780623, 05277215, and 06270217 from the Japanese Ministry of Education and Culture and by a research grant from the Human Frontier Science Program Organization. We thank Drs. F. Murakami, H. Katsumaru, R. Shirasaki, and E. Ruthazer for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Nobuhiko Yamamoto, Department of Biophysical Engineering, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560, Japan.
REFERENCES
- 1.Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird DH, Hatten ME, Mason CA. Cerebellar target neurons provide a stop signal for afferent neurite extension in vitro. J Neurosci. 1992;12:619–634. doi: 10.1523/JNEUROSCI.12-02-00619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastmeyer M, O’Leary DDM. Dynamics of target recognition by interstitial axon branching along developing cortical axons. J Neurosci. 1996;16:1450–1459. doi: 10.1523/JNEUROSCI.16-04-01450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965;99:691–709. [PMC free article] [PubMed] [Google Scholar]
- 5.Bicknese AR, Sheppard AM, O’Leary DDM, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolz J, Novak N, Staiger V. Formation of specific afferent connections in organotypic slice cultures from rat visual cortex with lateral geniculate nucleus. J Neurosci. 1992;12:3054–3070. doi: 10.1523/JNEUROSCI.12-08-03054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brückner G, Mares V, Biesold D. Neurogenesis in the visual system of the rat. An autoradiographic investigation. J Comp Neurol. 1976;166:245–256. doi: 10.1002/cne.901660208. [DOI] [PubMed] [Google Scholar]
- 8.Cabelli RJ, Horn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 9.Campagna JA, Ruegg MA, Bixby JL. Agrin is a differential-inducing “stop signal” for motoneurons in vitro. Neuron. 1995;15:1365–1374. doi: 10.1016/0896-6273(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 10.Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;366:36–53. doi: 10.1002/(SICI)1096-9861(19960325)367:1<36::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Cory S, Fraser S. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 12.delRio JA, Soriano E. Immunocytochemical detection of 5′-bromodeoxyuridine incorporation in the central nervous system of the mouse. Dev Brain Res. 1989;49:311–317. doi: 10.1016/0165-3806(89)90033-3. [DOI] [PubMed] [Google Scholar]
- 13.Emerling DE, Lander AD. Laminar specific attachment and neurite outgrowth of thalamic neurons on cultured slices of developing cerebral cortex. Development. 1994;120:2811–2822. doi: 10.1242/dev.120.10.2811. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Shatz CJ. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992;12:39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- 17.Godement P, Wang L-C, Mason CA. Retinal axon divergence in the optic chiasm: dynamics of growth cone behavior at the midline. J Neurosci. 1994;14:7024–7039. doi: 10.1523/JNEUROSCI.14-11-07024.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Götz M, Bolz J. Formation and preservation of cortical layers in slice cultures. J Neurobiol. 1992;23:783–802. doi: 10.1002/neu.480230702. [DOI] [PubMed] [Google Scholar]
- 19.Götz M, Novak N, Bastmeyer M, Bolz J. Membrane-bound molecules in rat cerebral cortex regulate thalamic innervation. Development. 1992;116:507–519. [Google Scholar]
- 20.Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14:2161–2177. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing on the tectum of Xenopus embryos: a time-lapse study of single fibers in vivo. Development. 1987;101:123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- 22.Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- 23.Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hübener M, Götz M, Klostermann S, Bolz J. Guidance of thalamocortical axons by growth-promoting molecules in developing rat cerebral cortex. Eur J Neurosci. 1995;7:1963–1972. doi: 10.1111/j.1460-9568.1995.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones EG. Anatomy of cerebral cortex: columnar input-output organization. In: Schmitt FO, Worden FG, Adelman G, Dennis SG, editors. The organization of the cerebral cortex. MIT; Cambridge, MA, London: 1981. pp. 199–235. [Google Scholar]
- 26.Kaethner RJ, Stuermer CAO. Dynamics of terminal arbor formation and target approach of retinotectal axons in living zebrafish embryos: a time-lapse study of single axons. J Neurosci. 1992;12:3257–3271. doi: 10.1523/JNEUROSCI.12-08-03257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kageyama GH, Robertson RT. Development of geniculocortical projections to visual cortex in rat. J Comp Neurol. 1993;335:123–148. doi: 10.1002/cne.903350109. [DOI] [PubMed] [Google Scholar]
- 28.Kurotani T, Yamamoto N, Toyama K. Development of neural connections between visual cortex and transplanted lateral geniculate nucleus in rats. Dev Brain Res. 1993;71:151–168. doi: 10.1016/0165-3806(93)90168-a. [DOI] [PubMed] [Google Scholar]
- 29.Landmesser L, Dahm L, Tang J, Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron. 1990;4:655–667. doi: 10.1016/0896-6273(90)90193-j. [DOI] [PubMed] [Google Scholar]
- 30.Lund RD, Mustari MJ. Development of geniculocortical pathway in rats. J Comp Neurol. 1977;173:289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y, Raper JA. Inhibitory factors controlling growth cone motility and guidance. Curr Opin Neurobiol. 1994;4:648–654. doi: 10.1016/0959-4388(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 32.McAllister KA, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 33.McFarlane S, McNeill L, Holt CE. FGF signaling and target recognition in the developing Xenopus visual system. Neuron. 1995;15:1017–1028. doi: 10.1016/0896-6273(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 34.Miller B, Chou L, Finlay B. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993;335:16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- 35.Molnár Z, Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in coculture. Nature. 1991;351:475–477. doi: 10.1038/351475a0. [DOI] [PubMed] [Google Scholar]
- 36.Molnár Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- 37.Myers PZ, Bastiani MJ. Growth cone dynamics during the migration of an identified commissural growth cone. J Neurosci. 1993;13:127–143. doi: 10.1523/JNEUROSCI.13-01-00127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Leary DDM, Terashima T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and “waiting periods.”. Neuron. 1988;1:901–910. doi: 10.1016/0896-6273(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 39.O’Rourke N, Fraser SE. Dynamic changes in optic fiber terminal arbors lead to retinotopic map formation: an in vivo confocal microscopic study. Neuron. 1990;5:159–171. doi: 10.1016/0896-6273(90)90306-z. [DOI] [PubMed] [Google Scholar]
- 40.Peters A, Feldman M. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. I. General description. J Neurocytol. 1976;5:63–84. doi: 10.1007/BF01176183. [DOI] [PubMed] [Google Scholar]
- 41.Porter BE, Weis J, Sanes JR. A motoneuron-selective stop signal in the synaptic protein s-laminin. Neuron. 1995;14:549–559. doi: 10.1016/0896-6273(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 42.Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond Biol. 1977;278:245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- 43.Sato M, Lopez-Mascaraque L, Heffner CD, O’Leary DDM. Action of a diffusible target-derived chemoattractant on cortical axon branch induction and directed growth. Neuron. 1994;13:791–803. doi: 10.1016/0896-6273(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 44.Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 45.Shatz CJ, Luskin MB. The relationship between the geniculocortical afferents and their cortical target cells during development of the cat’s primary visual cortex. J Neurosci. 1986;6:3655–3668. doi: 10.1523/JNEUROSCI.06-12-03655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sretavan DW, Reichart LF. Time-lapse video analysis of retinal ganglion cell axon path finding at the mammalian optic chiasm: growth cone guidance using intrinsic chiasm cues. Neuron. 1993;10:761–777. doi: 10.1016/0896-6273(93)90176-r. [DOI] [PubMed] [Google Scholar]
- 47.Yamagata M, Sanes JR. Lamina-specific cues guide outgrowth and arborization of retinal axons in the optic tectum. Development. 1995;121:189–200. doi: 10.1242/dev.121.1.189. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989;245:192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto N, Yamada K, Kurotani T, Toyama K. Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron. 1992a;9:217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto N, Higashi S, Sugihara H, Toyama K. Axonal growth and branch formation of geniculate fibers in visual cortex studied in coculture preparations. Soc Neurosci Abstr. 1992b;18:224. [Google Scholar]