Abstract
Although previous research has emphasized the beneficial effects of dopamine (DA) on functions of the prefrontal cortex (PFC), recent studies of animals exposed to mild stress indicate that excessive DA receptor stimulation may be detrimental to the spatial working memory functions of the PFC (Arnsten and Goldman-Rakic, 1990; Murphy et al., 1994, 1996a,b, 1997). In particular, these studies have suggested that supranormal stimulation of D1 receptors may contribute to the detrimental actions of DA in the PFC (Murphy et al., 1994, 1996a). The current study directly tested this hypothesis by examining the effects of infusing a full D1 receptor agonist, SKF 81297, into the PFC of rats performing a spatial working memory task, delayed alternation. SKF 81297 produced a dose-related impairment in delayed-alternation performance. The impairment was reversed by pretreatment with a D1 receptor antagonist, SCH 23390, consistent with drug actions at D1 receptors. SCH 23390 by itself had no effect on performance, although slightly higher doses impaired performance (Murphy et al., 1994, 1996a). There was a significant relationship between infusion location and drug efficacy; animals with cannulae anterior to the PFC were not impaired by SKF 81297 infusions. Taken together, these results demonstrate that supranormal D1 receptor stimulation in the PFC is sufficient to impair PFC working memory function. These cognitive data are consistent with recent electrophysiological studies of D1 receptor mechanisms affecting the PFC (Williams and Goldman-Rakic, 1995; Yang and Seamans, 1996). Increased D1receptor stimulation during stress may serve to take the PFC “off-line” to allow posterior cortical and subcortical structures to regulate behavior, but may contribute to the vulnerability of the PFC in many neuropsychiatric disorders.
Keywords: dopamine, D1 receptor, prefrontal cortex, working memory, stress, schizophrenia
For many years it has been appreciated that dopamine (DA) has a powerful beneficial influence on the spatial working memory functions of the prefrontal cortex (PFC) in monkeys (Brozoski et al., 1979) and rats (Simon, 1981; Bubser and Schmidt, 1990). Later studies identified the importance of D1 DA receptor mechanisms in this response. Infusions of the D1 receptor antagonists SCH 23390 or SCH 39166 into the PFC of monkeys (Sawaguchi and Goldman-Rakic, 1991) or rats (Seamans et al., 1995) produced a delay-related impairment in spatial working memory performance, whereas comparable infusions of D2 DA receptor antagonists were without effect. A similar pattern of response was observed after systemic administration of the D1receptor antagonist SCH 23390 in young adult monkeys (Arnsten et al., 1994), indicating that the impairments after intracortical infusions were not simply the result of local anesthetic actions but rather drug actions at D1 receptors. The importance of D1receptor mechanisms has been corroborated by electrophysiological studies of PFC pyramidal cells in monkeys (Sawaguchi et al., 1988;Sawaguchi and Kubota, 1996) and rats (Yang and Seamans, 1996). This basic research has had a major impact on theories of PFC dysfunction in schizophrenia and other disorders that may involve altered DA transmission (Daniel et al., 1991; Davis et al., 1991; Grace, 1993).
Although there is a body of work upholding the beneficial influence of D1 receptor mechanisms in the PFC, more recent studies suggest an inverted “U” relationship whereby excessive as well as insufficient D1 DA receptor stimulation impairs PFC cognitive function (Arnsten and Goldman-Rakic, 1986, 1990; Arnsten et al., 1994; Murphy et al., 1994, 1996a,b, 1997; Williams and Goldman-Rakic, 1995; Verma and Moghaddam, 1996). Biochemical studies in rodents have shown that mild stressors preferentially increase DA turnover in the PFC compared with other DA terminal fields (Thierry et al., 1976; for review, see Deutch and Roth, 1990). Mild stress exposure in monkeys or rats produced working memory deficits that could be blocked by agents that prevent the rise in DA turnover in rodents (Arnsten and Goldman-Rakic, 1986; Murphy et al., 1996b) or that block DA receptors (i.e., haloperidol, SCH 23390, or clozapine; Arnsten and Goldman-Rakic, 1990; Murphy et al., 1994, 1996a, 1997). Cognitive impairment in rodents correlated with increased DA turnover in the PFC (Murphy et al., 1994, 1996a), consistent with a hyperdopaminergic mechanism. The efficacy of the selective D1 receptor antagonist SCH 23390 in this paradigm suggests that excessive D1 DA receptor stimulation may underlie the PFC deficits induced by stress. Consistent with this hypothesis, systemic administration of D1 receptor agonists (dihydrexidine, SKF 81297, and A 77636) to aged monkeys produces inverted U-shaped dose–response curves, with higher doses impairing delayed-response performance through a D1 receptor mechanism (Arnsten et al., 1994; Cai and Arnsten, 1997).
Although these studies suggest that excessive DA D1receptor stimulation impairs PFC cognitive function, the results are not conclusive. For example, studies of D1 agonists in monkeys (Arnsten et al., 1994; Cai and Arnsten, 1997) used systemic administration, and thus conclusions cannot be drawn regarding actions in the PFC. Stress has widespread effects in the nervous system and, even within the PFC, alters the release of many neurotransmitters (e.g., norepinephrine) in addition to DA (e.g., Goldstein et al., 1996). Thus it is not known whether increased DA receptor stimulation in the PFC is sufficient to produce PFC cognitive deficits. In particular, a role for D1 receptor mechanisms was not supported in a recent study that used the noncompetitive NMDA receptor antagonist ketamine to increase DA release in the PFC in rats (Verma and Moghaddam, 1996). Ketamine induced delayed-alternation deficits that were reversed by D2, but not D1, receptor antagonists (Verma and Moghaddam, 1996).
The current study directly tested the hypothesis that supranormal D1 receptor stimulation in the PFC is sufficient to impair the spatial working memory functions of the PFC. These experiments used SKF 81297, a full D1 receptor agonist (activity comparable with DA itself; Andersen and Jansen, 1990). Rats were tested on the delayed-alternation task, the test of spatial working memory most associated with the PFC in rodents (Larsen and Divac, 1978; van Haaren et al., 1985). Experiment 1 examined the effects of intra-PFC infusions of SKF 81297 on delayed-alternation performance. In experiment 2, the role of D1 receptor mechanisms was confirmed by blocking the SKF 81297 response with the D1 receptor antagonist SCH 23390. Finally, the efficacy of the SKF 81297 response was related to anatomical localization of the cannulae and to the delay intervals used during delayed-alternation testing.
MATERIALS AND METHODS
Subjects. Male Sprague Dawley CAMM rats (n = 6 per experiment) weighing 240–280 gm were paired and housed in filter frame cages. The rats were kept on a 12 hr light/dark cycle, and the experiments were conducted during the light phase. The animals were fed a diet of autoclaved Purina rat chow (17 gm/rat per day) immediately after behavioral testing. Water was available ad libitum. Rats were weighed weekly, and weights were maintained at ∼400–450 gm. Food rewards during cognitive testing were highly palatable miniature chocolate chips, thus minimizing the need for dietary regulation. Rats were assigned a single experimenter who handled them extensively before behavioral testing. The experimenter testing the animal was blind to the drug treatment conditions.
Delayed alternation. The delayed-alternation task was selected for comparison with previous studies of (1) PFC DA depletion and (2) stress, which similarly used this paradigm. The delayed-alternation task uses a number of processes associated with PFC function: (1) spatial working memory (Goldman-Rakic, 1987), (2) egocentric spatial processing (Kesner et al., 1989), and (3) inhibition of proactive interference and inappropriate motor responses (Mishkin, 1964; Kolb, 1990), and is thus a good task for detecting altered PFC function. Cognitive testing methods were similar to those developed previously in this laboratory by Murphy and Arnsten to examine the effects of stress on spatial working memory in rats (Murphy et al., 1994, 1996a,b). Rats were initially habituated to a T-maze (dimensions, 90 × 65 cm) for 5 d until they were readily eating chocolate chips placed in the food wells at the end of each arm. After habituation, rats were trained on the delayed-alternation task. On the first trial, animals were rewarded for entering either arm. Thereafter, for a total of 10 trials per session, rats were rewarded only if they entered the maze arm that was not chosen previously. Between trials, the choice point was wiped with alcohol to remove any olfactory clues. The delay between trials was “0” sec during initial training. After approximately five training sessions, animals underwent surgery to implant indwelling guide cannulae directed at the PFC. Testing on the delayed-alternation task was reinstated only after the implant had healed completely, ∼2 weeks after surgery. For each animal, delays were adjusted to produce performance levels stabilized at ∼80% correct. Delays averaged 12.2 ± 3.1 (range, 5–30) sec. This baseline level of performance allowed for the detection of either improvement or impairment with drug administration.
The response to SKF 81297 was characterized further by analyzing the pattern of errors on the delayed-alternation task. A perseverative pattern of response is consistent with dysfunction of the PFC (Kolb, 1990). Perseverative responding was assessed using two methods: (1) the absolute difference between left and right arm responses, and (2) the greatest number of consecutive entries into a single arm. Run times were measured to detect any changes in motor performance, and rats were observed for any gross changes in behavior.
Behavioral ratings. Special attention was paid to anxiety-related behaviors (e.g., piloerection, freezing, urination, and defecation) that had been observed previously after pharmacological stress. Rats were rated on a four point scale whereby 0 = normal behavior and 1 = slight, 2 = moderate, and 3 = severe evidence of anxiety-related behaviors.
Cannula implantation. After training on the delayed-alternation task, rats underwent stereotaxic implantation of chronic guide cannulae. Surgery was performed under Equithesin (pentobarbital plus chloral hydrate, 4.32 mg/kg) anesthesia using aseptic methods. Guide cannulae consisted of 9.0 mm of 23 ga stainless steel directed immediately dorsal to the medial PFC [prelimbic (PL) PFC; stereotaxic coordinates: anteroposterior, +3.2 mm; mediolateral, ±0.75 mm; dorsoventral, −3.0 mm]. Cannulae (Plastics Products) were affixed to the skull using dental cement secured with sterile stainless steel screws. A sterile stylette was screwed into place in each guide cannula to prevent occlusion. Stylets were changed on a regular basis to maintain patency.
Great care was taken to minimize pain and infection after the operation to decrease stress to the animal. Rats were monitored on a daily basis for signs of distress or infection and were initially treated with Buprenex (0.01 mg/kg) to decrease pain. Rats were housed singly during the period after the operation and were returned to paired housing after the implant had healed.
Infusion procedure. Animals were initially adapted to a mock infusion protocol to minimize any stress associated with the procedure. Rats were gently restrained while the stylets were removed and replaced with 30 ga sterile infusion needles that extended 1 mm below the guide cannulae. The rats received bilateral infusions of SKF 81297 at a concentration of either 0 (vehicle), 0.01, or 0.1 μg in 0.5 μl sterile saline. Infusions were driven by a Harvard Apparatus syringe pump set at a flow rate of 0.225 μl/min using 25 μl Hamilton syringes for an infusion time of 2 min and 13 sec. Needles remained in place for 2 min after the completion of the infusion. Stylettes were inserted back into the cannulae, and behavioral testing began immediately after the infusion procedure. SKF 81297 hydrobromide was purchased from Research Biochemicals (Natick, MA).
Injection procedure. Rats in experiment 2 were initially adapted to intraperitoneal injections of saline to minimize the stress of injections. Before the infusion study, animals were tested with SCH 23390 (0.035, 0.03, and 0.01 mg/kg) to identify the highest dose that did not induce cognitive or motor impairment. The 0.035 mg/kg dose had been used in previous studies from this laboratory (Murphy et al., 1994, 1996a). This dose produced a small but significant impairment in delayed-alternation performance (Murphy et al., 1994, 1996a; see Fig.6). During test days, rats received intraperitoneal injections of sterile saline or SCH 23390 (0.01 or 0.03 mg/kg) 1 hr before intra-PFC infusions. SCH 23390 was diluted in sterile saline and injected in a concentration of 0.01 mg/ml. SCH 23390 HCL was purchased from Research Biochemicals.
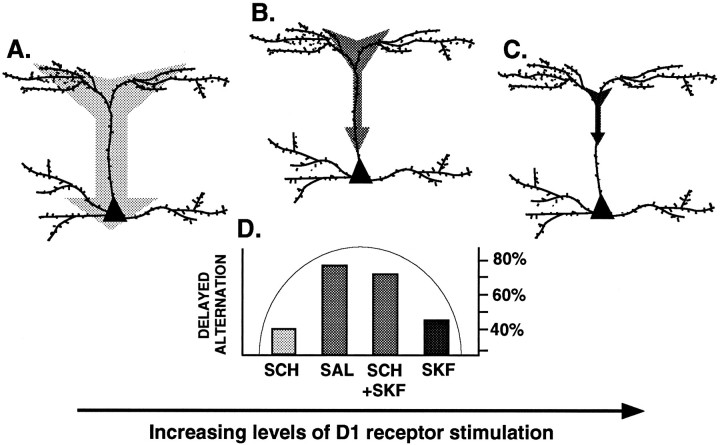
Fig. 6.
Highly schematized representation of a model describing D1 receptor mechanisms influencing PFC function, based on the electrophysiological findings of Yang and Seamans (1996)and the cognitive data discussed in the current paper. Yang and Seamans have shown that D1 receptor stimulation alters signal transfer from apical dendrites to the soma by attenuating the high threshold calcium spikes that propagate signals along the dendrite. These D1 actions are particularly prominent along the apical dendritic stem (i.e., primary dendritic branch). They have proposed that with insufficient D1 receptor stimulation (A), signals are unfocused temporally and spatially, whereas with optimal levels of D1 receptor stimulation (B), signals are sharpened for optimal transfer to the soma [based on Yang and Seamans (1996), their Fig. 9A,B]. However, with increasing levels of D1 receptor stimulation, signals are oversharpened and would not reach the soma because of abolition of high threshold calcium spikes (C). This inverted U dose–response curve is also observed at the behavioral level in studies of PFC cognitive function (D; results from current study shown as mean percent correct). Thus, either insufficient D1 receptor stimulation (0.035 mg/kg SCH 23390, i.p.; SCH) or excessive D1 receptor stimulation (0.1 μg of SKF 81297 intra-PFC infusion; SKF) results in impaired delayed-alternation performance, whereas optimal levels of D1 receptor stimulation (saline, SAL; or 0.03 mg/kg SCH 23390 + 0.1 μg of SKF 81297 intra-PFC infusion,SCH+SKF) result in superior cognitive performance.
Histology. At the completion of the experiment, rats were killed by overdose with barbiturate. Dye was infused into the cannulae premortem in a subset of animals to aid visualization of cannula placement. Brains were stored in formalin, sectioned, and analyzed for histological verification of cannula placement. Only rats with correctly placed cannulae were used in the data analysis.
Data analysis. Because of within-subjects comparisons, the data were analyzed with repeated-measures designs. In experiment 1, the effects of increasing the dose of SKF 81297 were evaluated using a one-way repeated-measures ANOVA (1-ANOVA-R) with planned comparisons (test of effects; Systat). In experiment 2, the effects of SCH 23390 pretreatment on the SKF 81297 response were evaluated using a two-way repeated analysis (2-ANOVA-R) with planned comparisons (test of effects; Systat). Paired comparisons were evaluated using a pairedt test, T-dependent (Tdep). Nonparametric tests were used to assess behavioral ratings. A paired nonparametric Wilcoxon analysis was used for comparing the effects of saline versus SKF 81297 on behavioral ratings, whereas a Spearman correlation test was used to examine possible relationships between cognitive impairment and behavioral ratings. The Pearson test was used to examine the correlation between drug efficacy and either cannula placement or delay interval. Statistical analyses were performed on a Macintosh LC computer using Systat software.
RESULTS
Figure 1 shows the position of the cannula tips immediately dorsal to the rat PFC (PL region). All animals had cannulae within the appropriate area of the PFC with the exception of two animals (one from experiment 1 and the other from experiment 2), with cannulae placed 1 mm anterior to the targeted region at +4.2 mm. The data from these two animals were excluded from analysis, thus reducing the number of animals to n = 5 for experiments 1 and 2. However, these two animals were included in the final correlation between drug efficacy and cannula placement (Correlation of cannula position or delay interval with drug efficacy below).
Fig. 1.
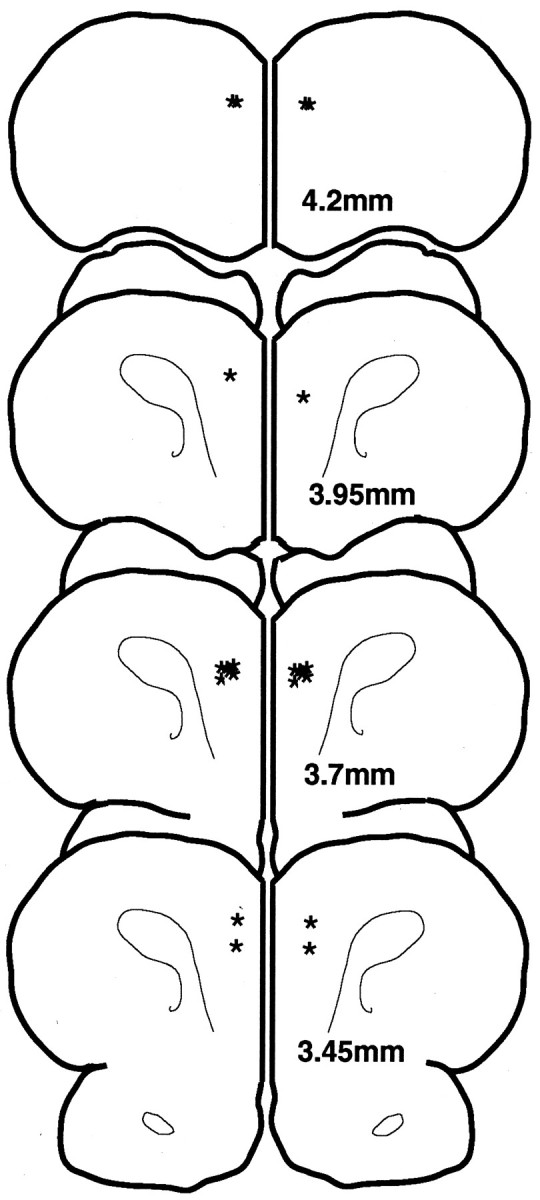
Location of the ventral tips (stars) of the guide cannulae in the rat brains used in this study. Sections indicate millimeters anterior to bregma.
Experiment 1: effects of intra-PFC infusions of SKF 81297 on delayed-alternation performance
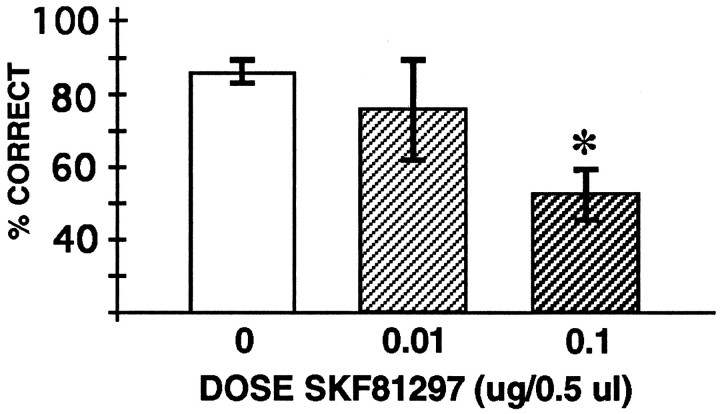
In this experiment, 0.5 μl of saline or 0.01 or 0.1 μg of SKF 81297 in 0.5 μl of saline was infused into the PFC in counterbalanced order. Saline infusions had no significant effect on performance compared with the baseline performance without infusions (Tdep = 2.24; df = 4; p = 0.09). SKF 81297 infusion into the PFC produced a dose-related reduction in delayed-alternation performance (1-ANOVA-R: significant effect of SKF 81297 dose [F(2,8) = 4.93; p = 0.04]). Tests of effects showed that infusion of 0.1 μg [F(1,4) = 13.44; p = 0.02], but not of 0.01 μg [F(1,4) = 0.63;p = 0.47], significantly impaired performance compared with saline infusion (Fig. 2). Impairment in performance induced by SKF 81297 was temporary; performance returned to normal levels of responding by the next testing session, with a mean of 87.5% correct for the four animals tested the day after infusion of 0.1 μg of SKF 81297 (not significantly different from baseline performance; Tdep = 1.21; df = 3; p = 0.31).
Fig. 2.
The effects of bilateral intra-PFC infusions of the D1 DA full agonist SKF 81297 (0, 0.01, and 0.1 μg/0.5 μl) on accuracy of performance on the delayed-alternation task. Results represent mean percent correct ± SEM;n = 5 rats in experiment 1; *significantly different from saline (0 μg) infusion performance.
The SKF 81297 data were further analyzed for qualitative changes in response. Analysis of the errors after infusion of 0.1 μg of SKF 81297 showed a perseverative pattern of response (Fig.3A). Thus, SKF 81297 significantly increased the absolute difference between left and right arm responses (saline, 1.6 ± 0.8; SKF, 3.6 ± 0.8; Tdep = 3.16; df = 4; p = 0.034) and the greatest number of consecutive entries to a single arm (saline, 1.2 ± 0.22; SKF, 3.2 ± 0.89; Tdep = 2.83; df = 4; p = 0.047; Fig. 3A). Response (run) times were not affected by infusion of 0.1 μg of SKF 81297. As can be seen in Figure3B, there was no significant difference between response times when animals were infused with saline versus 0.1 μg of SKF 81297 (Tdep = 0.45; df = 4; p = 0.68). Thus, gross motor function seemed unaffected by drug treatment. There were also no significant changes in anxiety-related behavioral ratings after infusion of 0.1 μg of SKF 81297 into the PFC [median score saline, 0 (all scores, 0); median score SKF 81297, 0 (range, 0–2); Wilcoxonp = 0.18). Drug-induced changes in behavioral ratings did not correlate with changes in performance on the delayed-alternation task (Spearman = 0.574; p > 0.1). Thus, the impairment in delayed-alternation performance occurred independently of gross changes in locomotor or affective responses. There were no significant changes in any of these measures for the 0.01 μg dosage (all p > 0.14).
Fig. 3.
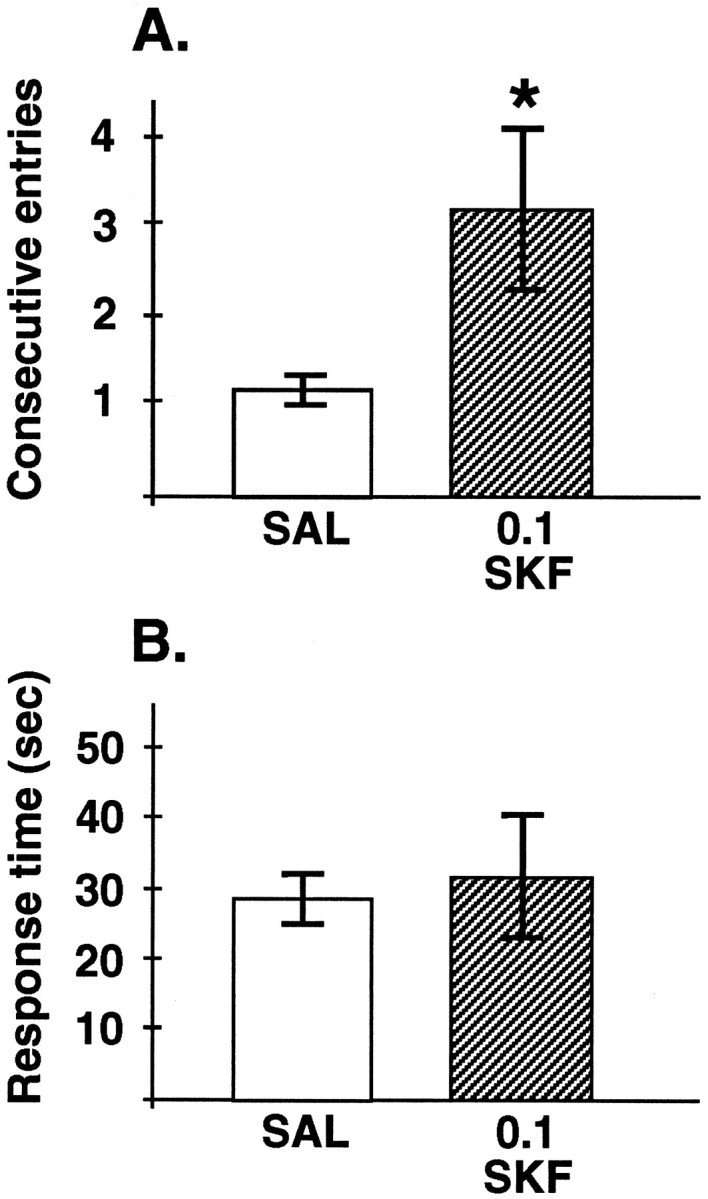
Further characterization of the SKF 81297 (0.1 μg) response. A, The effects of bilateral intra-PFC infusions of the D1 DA full agonist SKF 81297 (0.1 μg) versus saline on a measure of perseverative responding: the greatest number of consecutive entries into a single arm during delayed-alternation performance. Results represent mean number of entries ± SEM; n = 5 rats. SKF 81297 significantly increased measures of perseverative responding, including the absolute difference between entries into the left versus right arm (see Results); *significantly different from saline infusions.B, The effects of bilateral intra-PFC infusions of the D1 DA full agonist SKF 81297 (0.1 μg) versus saline on response times during delayed-alternation performance. Results represent mean response time (sec) ± SEM; n = 5 rats.
Experiment 2: reversal of the SKF 81297 response with a D1 receptor antagonist
The role of D1 DA receptors in the response of 0.1 μg of SKF 81297 was tested in a second group of animals by challenging SKF 81297 with D1 receptor antagonist pretreatment. These animals received four drug treatments in counterbalanced order: (1) systemic saline plus intra-PFC saline infusion, (2) systemic saline plus intra-PFC infusion of 0.1 μg of SKF 81297, (3) systemic SCH 23390 plus intra-PFC saline infusion, or (4) systemic SCH 23390 plus intra-PFC infusion of 0.1 μg of SKF 81297. A dose of SCH 23390 (0.03 mg/kg) was selected that had no effect on delayed-alternation performance in pilot experiments. However, two animals showed motor deficits with this dose alone; in these animals the dose was lowered to 0.01 mg/kg.
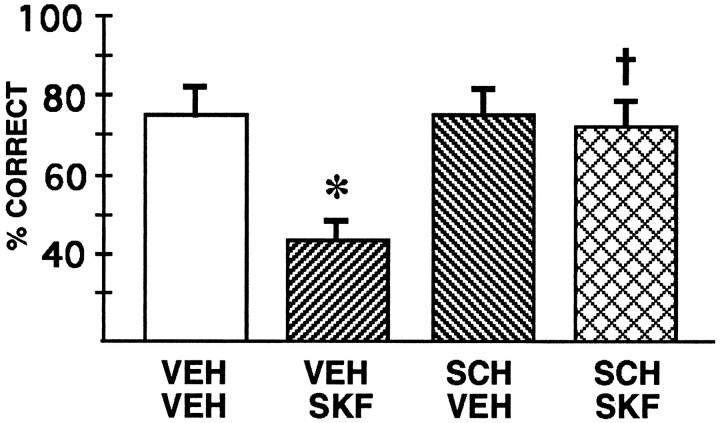
The data from this experiment can be seen in Figure4. Again, performance after saline infusion did not differ from baseline performance without infusions (Tdep = 0.3; df = 4; p = 0.78). 2-ANOVA-R showed a significant effect of SKF 81297 infusion [F(1,4) = 12.86; p = 0.023], a significant effect of SCH 23390 injection [F(1,4) = 9.17; p = 0.039], and a significant interaction between SKF 81297 and SCH 23390 treatment [F(1,4) = 26.00; p = 0.007]. As in experiment 1, planned comparisons (test of effects; Systat) revealed that infusion of 0.1 μg of SKF 81297 significantly impaired performance compared with saline infusion when animals were pretreated with saline [VEH VEH vs VEH SKF: F(1,4) = 18.00;p = 0.013]. Pretreatment with SCH 23390 significantly blocked the response to SKF 81297 infusions [VEH SKFvs SCH SKF: F(1,4) = 23.06;p = 0.009]. SCH 23390 pretreatment alone had no effect in saline-infused animals [VEH VEH vs SCH VEH: F(1,4) = 0.29; p= 0.62]. These findings are consistent with SKF 81297 impairing delayed-alternation performance through actions at D1 DA receptors. As seen in experiment 1, impairment in performance induced by SKF 81297 was temporary; performance returned to normal levels of responding by the next testing session, with a mean of 75.0% correct for the four animals tested the day after infusion of 0.1 μg of SKF 81297 (not significantly different from baseline performance; Tdep = 0.52; df = 3; p = 0.64).
Fig. 4.
The effects of SCH 23390 pretreatment on the cognitive deficits induced by bilateral intra-PFC SKF 81297 infusions in experiment 2. Results represent mean percent correct ± SEM;n = 5 rats. VEH VEH, Both systemic and intra-PFC administration of saline vehicle; VEH SKF, systemic administration of saline vehicle and intra-PFC infusion of 0.1 μg of SKF 81297; SCH VEH, systemic administration of SCH 23390 and intra-PFC infusion of saline vehicle; and SCH SKF, systemic administration of SCH 23390 and intra-PFC infusion of 0.1 μg of SKF 81297. *Significantly different fromVEH VEH performance; †significantly different from VEH SKF performance.
A qualitative analysis of the SKF 81297 response replicated the findings observed in experiment 1. As shown in Table1, SKF 81297 induced a perseverative pattern of responding as measured either by the greatest number of consecutive entries into a single arm or by the absolute difference in responses to the left versus right arm. These perseverative effects were ameliorated by SCH 23390 pretreatment. Thus, a 2-ANOVA-R of the consecutive entry data showed a significant effect of SKF 81297 infusion [F(1,4) = 12.74; p = 0.023], no significant effect of SCH 23390 injection [F(1,4) = 2.31; p = 0.2], and a significant interaction between SKF 81297 and SCH 23390 treatment [F(1,4) = 10.00; p = 0.03]. Similar results were observed with the absolute difference in responses to the left versus right arm, in which there was a significant effect of SKF 81297 infusion [F(1,4) = 8.34;p = 0.045], no significant effect of SCH 23390 injection [F(1,4) = 2.11; p = 0.22], and a significant interaction between SKF 81297 and SCH 23390 treatment [F(1,4) = 8.34; p = 0.045]. There was no significant effect of either drug treatment on run time: no significant effect of SKF 81297 infusion [F(1,4) = 0; p = 1.0], no significant effect of SCH 23390 injection [F(1,4) = 2.6; p = 0.18], and no significant interaction between SKF 81297 and SCH 23390 treatment [F(1,4) = 0.01; p = 0.92]. Some animals showed an increase in response time with SCH 23390 treatment preceding either saline or SKF 81297 infusions; however, the large variation in response did not yield significant effects.
Table 1.
Qualitative analysis of the effects of SCH 23390 (SCH) on the SKF 81297 (SKF) response
| VEH + VEH | VEH + SKF | SCH + VEH | SCH + SKF | |
|---|---|---|---|---|
| Consecutive entries | 2.0 ± 0.3 | 3.8 ± 0.7* | 2.0 ± 0.0 | 2.2 ± 0.2 |
| Absolute difference in arms | 0.8 ± 0.5 | 3.6 ± 0.6* | 1.2 ± 0.5 | 1.2 ± 0.5 |
| Run time (sec) | 48.6 ± 25.9 | 51.2 ± 15.3 | 139.0 ± 60.9 | 136.4 ± 79.5 |
VEH = saline; mean ± SEM; n = 5.
Significantly different from VEH + VEH.
Correlation of cannula position or delay interval with drug efficacy
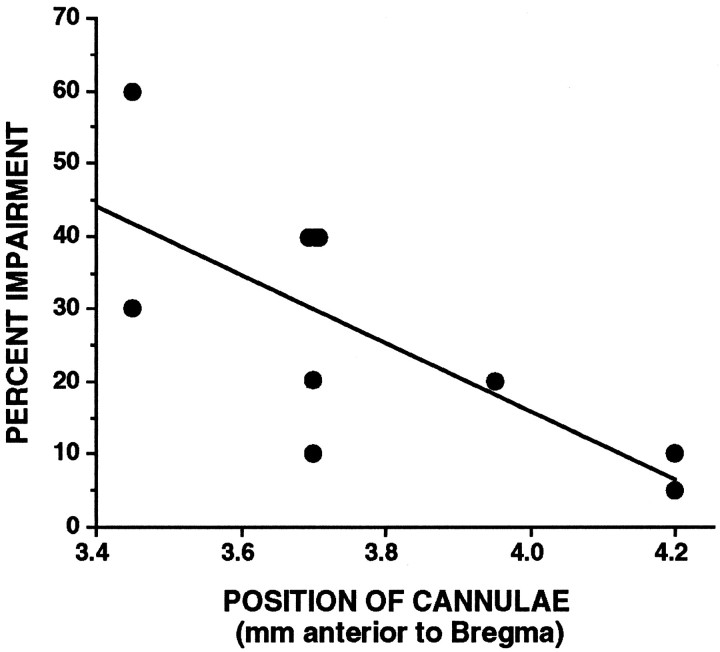
The relationship between drug efficacy and cannula position was examined by correlating the percent change in performance produced by 0.1 μg of SKF 81297 with the estimated position of the guide cannulae as drawn in Figure 1. This analysis used the data from rats in both experiments 1 and 2, including the two animals with cannulae placed too anterior at 4.2 mm. There was a significant correlation between location of the cannulae and drug efficacy (Pearson r = 0.735; p < 0.02; Fig.5). Thus, with increasing distance from the targeted site, SKF 81297 infusions became less efficacious. The two rats with cannulae placed 4.2 mm anterior to bregma showed little or no impairment after SKF 81297 infusion.
Fig. 5.
The correlation between cannula location (millimeters anterior to bregma) and efficacy of the SKF 81297 response (percent impairment relative to saline infusion). Data include the animals from experiments 1 and 2 with cannulae 4.2 mm anterior to bregma.
The relationship between drug efficacy and delay interval used during delayed-alternation testing was examined in the animals from experiments 1 and 2. Delays ranged from 5 to 30 sec; however, there was no relationship between delay interval and SKF 81297 efficacy (Pearsonr = 0.14, NS). Thus, the drug was effective irrespective of delays used during testing.
DISCUSSION
Supranormal D1 receptor stimulation in the PFC impairs delayed-alternation performance
The finding that SKF 81297 infusion into the PFC produces a dose-related, SCH 23390-reversible impairment in spatial working memory performance demonstrates that supranormal D1 receptor stimulation in the PFC is sufficient to induce PFC cognitive dysfunction. SKF 81297 is highly selective for the D1family of DA receptors (Andersen and Jansen, 1990). Reversal of the SKF 81297 response with the D1 receptor antagonist SCH 23390 confirms actions at D1 receptors rather than nonspecific drug actions. These findings are consistent with previous results showing that SCH 23390 blocked the cognitive impairment induced by stress exposure in rats and monkeys (Murphy et al., 1994, 1996a) (A. F. T. Arnsten and P. S. Goldman-Rakic, unpublished observations). However, the finding that D1 receptor stimulation alone is sufficient to induce PFC dysfunction does not rule out an additional role for D2 receptors. Cognitive deficits induced by either stress exposure (B. L. Murphy, unpublished observations) or ketamine (Verma and Moghaddam, 1996) can be blocked by selective D2 receptor antagonists. These findings suggest that both D1 and D2 receptor families contribute to the detrimental actions of DA in the PFC and that the two may synergize to take the PFC “off-line” during stress. In contrast to DA receptor antagonists, the β-adrenergic receptor antagonist propranolol (Murphy et al., 1996b), the serotonin reuptake blocker fluoxetine (Murphy et al., 1996a), and the muscarinic antagonist scopolamine (J. D. Jentsch, unpublished observations) have been ineffective in reversing the effects of pharmacological stress.
The importance of D1 receptor mechanisms is underscored by the finding that D1 agonists, including SKF 81297, produce an inverted U dose–response curve in aged monkeys with naturally occurring DA depletion. Thus, very low doses (e.g., 0.0001 mg/kg) improve spatial working memory performance, whereas higher doses induce delay-related impairments in working memory performance (Arnsten et al., 1994; Cai and Arnsten, 1997). Both improvement and impairment were reversed by SCH 23390 pretreatment, consistent with D1receptor mechanisms (Arnsten et al., 1994; Cai and Arnsten, 1997). A similar inverted U can be observed in data from rat experiments. Thus, blocking D1 receptors in the PFC with SCH 23390 locally (Seamans et al., 1995) or systemically (Murphy et al., 1996a; current study, see Fig. 6) impairs spatial working memory, whereas excessive D1 receptor stimulation similarly impairs performance (current study). It is possible that intra-PFC infusion of a much lower dose of SKF 81297 (e.g., 0.0001 μg) might produce supranormal performance in rats; alternatively, physiological levels of DA stimulation may be optimal in these animals, and any further increase in D1 receptor stimulation might be detrimental to performance.
SKF 81297 seems to impair spatial working memory through actions in the PFC. There was a significant relationship between cannula location and SKF 81297 efficacy; infusions became decreasingly effective as they moved anterior to the targeted site PL PFC. [Infusions may also have diffused to the infralimbic (IL) PFC, because this region is situated immediately ventral to the PL region.] This interpretation is consistent with the previous finding that cognitive impairment correlated with increased DA turnover in the same PL/IL PFC region (Murphy et al., 1996a). It is noteworthy that this region is the most responsive to stress in biochemical studies (Deutch et al., 1993). Furthermore, the perseverative pattern of errors produced by SKF 81297 infusions (current study) and pharmacological stress (Murphy et al., 1996a) is consistent with PFC dysfunction (for review, see Kolb, 1990). The finding that SKF 81297 was effective after either short (e.g., 5 sec) or long (e.g., 30 sec) delays is also consistent with a PFC mechanism, because PFC circuits are thought to be engaged at both short and long delays (Goldman-Rakic, 1987), whereas hippocampal and medial temporal cortex circuits are thought to be needed only after the longer delay lengths (Zola-Morgan et al., 1989). Future research could dissect the types of PFC processes affected by SKF 81297 treatment (e.g., behavioral inhibition, egocentric spatial processing) and determine whether there are differences between DA agonist versus antagonist treatments. Interestingly, D1 receptor blockade in the PFC does not seem to result in a perseverative pattern of response (J. K. Seamans, unpublished observations), suggesting a qualitative difference between insufficient and excessive DA D1receptor stimulation in the PFC.
The SKF 81297 response seems to have selectively altered cognitive performance without affecting motor or affective responses. The absence of drug effects on locomotor performance is consistent with previous studies showing no effects of FG 7142 pharmacological stress on automated measures of locomotor activity (Murphy et al., 1996a) and with previous studies showing that stress does not alter performance of control tasks with similar motor and motivational demands that do not depend on the PFC (Arnsten and Goldman-Rakic, 1990; Murphy et al., 1996a). However, more sensitive measures of anxiety-related behaviors (e.g., plus maze) need to be examined to determine whether increased DA D1 receptor stimulation in the PFC contributes to the affective response to stress. This issue is particularly important given the connections of the medial PFC with limbic and autonomic structures (for review, see Neafsey, 1990). However, affective changes do not seem to underlie the changes in delayed-alternation performance. The majority of animals showed marked cognitive impairment and no change in behavioral ratings (score of 0) after SKF 81297 infusions, and one animal that exhibited anxiety-related behaviors showed only minor cognitive impairment.
Relationship to electrophysiological studies
The inverted U dose response observed after D1receptor manipulations in cognitive studies relates well to electrophysiological studies of PFC pyramidal cells (Fig.6). Intracellular studies of rodent PFC slices have demonstrated that DA or D1 receptor agonists “sharpen” NMDA-mediated and other depolarizing synaptic signals arriving on apical dendrites by attenuating high threshold calcium spikes that amplify signals that propagate along the dendrite to reach the soma (Yang and Seamans, 1996; Seamans et al., 1997). This DA mechanism has recently been localized to the apical dendritic stem (C. R. Yang, unpublished observations), as schematically represented in Figure 6. Yang and Seamans have proposed that suboptimal levels of D1 receptor stimulation would result in unfocused signals (schematically represented in Fig. 6A), whereas optimal levels of D1 receptor stimulation would focus signals and thus promote signal transfer from dendrite to soma (Fig. 6B; Yang, unpublished observations). However, higher concentrations of agonists such as SKF 81297 would abolish high threshold calcium spikes and thus prevent NMDA-mediated and other depolarizing synaptic signals from reaching the soma (Fig.6C). Thus, D1 receptor mechanisms have a critical modulatory influence on incoming depolarizing signals. These electrophysiological findings are in accordance with results from cognitive studies, as summarized in Figure 6D. Thus, insufficient D1 receptor stimulation (0.035 mg/kg SCH 23390) impairs spatial working memory performance, normal levels of D1 receptor stimulation (saline or SKF 81297 + SCH 23390) produce optimal levels of responding, and supranormal D1receptor stimulation (0.1 μg of SKF 81297) again impairs performance. Similar inverted U dose–response curves have been observed when stress exposure was used to increase endogenous stimulation of D1receptors (Arnsten and Goldman-Rakic, 1990; Murphy et al., 1994,1996a,b; Arnsten and Goldman-Rakic, unpublished observations). This model is also consistent with the study of Williams and Goldman-Rakic (1995) who found that iontophoresis of very low concentrations of D1 receptor antagonist enhanced delay-related firing in PFC pyramidal cells of monkeys performing an oculomotor-delayed response task. Presumably, these animals had excessive DA release in their PFC because of the challenging nature of the task, and low levels of D1 receptor antagonist restored a more optimal level of D1 receptor stimulation for signal transfer.
The model shown in Figure 6 proposes further that D1-mediated actions may only be apparent under conditions in which NMDA signals are activated. Thus, the failure to reverse cognitive deficits with SCH 23390 in the Verma and Moghaddam (1996)study may have been the result of using the NMDA receptor antagonist ketamine to increase DA release. Similarly, electrophysiological studies of PFC cells in anesthetized (Thierry et al., 1986) or transected (Sesack and Bunney, 1989) rats may have observed negative results with D1 receptor antagonists, because there was little or no signal mediation in these preparations. All of these studies observed D2 receptor actions, suggesting that D2 inhibitory effects on pyramidal cells can occur indirectly, for example, via activation of GABAergic interneurons (Retaux et al., 1991). High levels of D1 and D2receptor stimulation during stress may synergize to take the PFC “off-line,” thus permitting more primary cortical and subcortical structures to control behavior.
Clinical relevance
The model depicted in Figure 6 may have relevance to a number of cognitive disorders associated with PFC dysfunction. Exposure to stress is known to exacerbate or precipitate symptoms in many neuropsychiatric disorders, for example, schizophrenia (Breier et al., 1991; Bebbington et al., 1993; Dohrenwend et al., 1995). The results from the present study suggest that increased D1 receptor stimulation during stress might contribute to PFC cognitive deficits observed in patients. This speculation is supported by the finding that schizophrenic patients, like the rats in the current study, exhibit an inverted U dose–response curve to DA treatments when performing a PFC word fluency task (Bilder et al., 1992). Thus, treatment with either high doses of neuroleptic medication (insufficient DA receptor stimulation) or methylphenidate (excessive DA receptor stimulation) impaired performance, whereas the combined treatment optimized performance (Bilder et al., 1992). Interestingly, methylphenidate treatment increased perseverative responding, reminiscent of the perseverative responses observed in the current study with SKF 81297 intra-PFC infusions.
It is appreciated that most theories regarding PFC dysfunction and schizophrenia speculate that cognitive deficits arise from insufficient DA receptor stimulation in the PFC (e.g., Daniel et al., 1991; Davis et al., 1991; Grace, 1993). Although the current study examined the effects of supranormal D1 receptor stimulation on cognitive functioning, the model depicted in Figure 6 implies that detrimental D1 receptor actions may also be found under conditions of low DA receptor stimulation, when incoming signals on apical dendrites are eroded, e.g., because of changes in dendritic morphology. Thus, the signal “sharpening” associated with even low levels of D1 receptor stimulation may be detrimental in individuals in whom incoming signals are attenuated previously because of loss of dendritic spines or distal dendritic branches (Arnsten, 1997). This model may explain the seemingly contradictory finding that DA agonists can often impair cognitive function in subjects with presumed DA depletion. For example, aged monkeys have naturally occurring DA depletion from the PFC, yet they are readily impaired by D1agonist treatment (Arnsten et al., 1994). This sensitivity to D1 detrimental actions may arise from the loss of dendritic spines (Uemura, 1980) and distal dendritic branches (Cupp and Uemura, 1980) in the monkey PFC with normal aging, a phenomenon also observed in aged human cortex (Schierhorn, 1981).
Interestingly, there have been recent reports of decreased dendritic spines in the PFC of schizophrenic brains (Garey et al., 1995; Glantz and Lewis, 1995). The model proposed in the current paper suggests that these dendritic changes may render patients especially vulnerable to the detrimental actions of DA at D1 receptors, even under conditions of relatively low DA receptor stimulation. Consistent with this view, recent positron emission tomography (PET) imaging studies have shown that a low dose of apomorphine can ameliorate “hypofrontality” in unmedicated schizophrenic patients (Fletcher et al., 1996). This low dose may have acted presynaptically to decrease DA release and reduce detrimental DA actions in the frontal lobe (Fletcher et al., 1996).
In summary, the results from the current study provide the first definitive evidence that excessive D1 receptor stimulation is sufficient to produce marked PFC dysfunction. These detrimental DA actions need to be considered in theories regarding DA mechanisms and neuropsychiatric illness.
Footnotes
This work was supported by Public Health Service Grant AG06036 to A.F.T.A. We gratefully acknowledge the technical expertise of Tracy White and Lisa Ciavarella and the inspiring discussions with Dr. Charles Yang.
Correspondence should be addressed to Dr. Amy F. T. Arnsten, Section of Neurobiology, Yale Medical School, P.O. Box 208001, New Haven, CT 06520-8001.
REFERENCES
- 1.Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten AFT, Goldman-Rakic PS. Reversal of stress-induced delayed response deficits in rhesus monkeys by clonidine and naloxone. Soc Neurosci Abstr. 1986;12:1464. [Google Scholar]
- 4.Arnsten AFT, Goldman-Rakic PS. Stress impairs prefrontal cortex cognitive function in monkeys: role of dopamine. Soc Neurosci Abstr. 1990;16:164. [Google Scholar]
- 5.Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 6.Bebbington P, Wilkins S, Jones P, Foerster A, Murray R, Toone B, Shon L. Life events and psychosis. Initial results from the Camberwell collaborative psychosis study. Br J Psychol. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- 7.Bilder RM, Lieberman JA, Kim Y, Alvir JM, Reiter G. Methylphenidate and neuroleptic effects on oral word production in schizophrenia. Neuropsychiatr Neuropsychol Behav Neurol. 1992;5:262–271. [Google Scholar]
- 8.Breier A, Wolkowitz OM, Pickar D. Stress and schizophrenia. Advances in neuropsychiatry and psychopharmacology. In: Tamminga CA, Schult SC, editors. Schizophrenia research, Vol 1. Raven; New York: 1991. [Google Scholar]
- 9.Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 10.Bubser M, Schmidt WJ. 6-OHDA lesions of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- 11.Cai JX, Arnsten AFT. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;282:1–7. [PubMed] [Google Scholar]
- 12.Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol. 1980;69:143–163. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- 13.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 15.Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- 16.Deutch AY, Zahm DS, Bourdelais AJ. The nucleus accumbens core and shell: delineation of cortico-striatal circuits and their functional attributes. In: Kalivas PW, Barnes CD, editors. Limbic motor circuits and neuropsychology. CRC; Boca Raton, FL: 1993. pp. 45–88. [Google Scholar]
- 17.Dohrenwend BP, Shrout PE, Link BG, Skodol AE, Stueve A. Life events and other possible psychosocial risk factors for episodes of schizophrenia and major depression: a case-control study. In: Mazure CM, editor. Progress in psychiatry, Vol 46, Does stress cause psychiatric illness? American Psychiatric; Washington, DC: 1995. pp. 43–65. [Google Scholar]
- 18.Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ. A regionally specific and distributed effect of dopamine on the cognitive anatomy of acute unmedicated schizophrenia. J Neurosci. 1996;16:7055–7062. doi: 10.1523/JNEUROSCI.16-21-07055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Hornstein C, Bauer M. Reduction in dendritic spine number on cortical pyramidal neurons in schizophrenia. Soc Neurosci Abstr. 1995;21:237. [Google Scholar]
- 20.Glantz LA, Lewis DA. Assessment of spine density on layer III pyramidal cells in the prefrontal cortex of schizophrenic subjects. Soc Neurosci Abstr. 1995;21:239. [Google Scholar]
- 21.Goldman-Rakic PS. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology, The nervous system, Higher functions of the brain, Sec 1, Vol V, Pt 1. American Physiological Society; Bethesda, MD: 1987. pp. 373–417. [Google Scholar]
- 22.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grace AA. Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Transm. 1993;91:111–134. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- 24.Kesner RP, Farnsworth G, Di Mattia BV. Double dissociation of egocentric and allocentric space following medial prefrontal and parietal cortex lesions in the rat. Behav Neurosci. 1989;103:956–619. doi: 10.1037//0735-7044.103.5.956. [DOI] [PubMed] [Google Scholar]
- 25.Kolb B. Prefrontal cortex. In: Kolb B, Tees RC, editors. The cerebral cortex of the rat. MIT; Cambridge, MA: 1990. pp. 437–458. [Google Scholar]
- 26.Larsen JK, Divac I. Selective ablations within the prefrontal cortex of the rat and performance of delayed alternation. Physiol Psychol. 1978;6:15–17. [Google Scholar]
- 27.Mishkin M. Perseveration of central sets after frontal lesions in monkeys. In: Warren JM, Akert K, editors. The frontal granular cortex and behavior. McGraw-Hill; New York: 1964. pp. 219–241. [Google Scholar]
- 28.Murphy BL, Roth RH, Arnsten AFT. The effects of FG7142 on prefrontal cortical dopamine and spatial working memory in rat and monkey. Soc Neurosci Abstr. 1994;20:1018. [Google Scholar]
- 29.Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996a;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy BL, Arnsten AFT, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996b;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy BL, Roth RH, Arnsten AFT. Clozapine reverses the spatial working memory deficits induced by FG7142 in monkeys. Neuropsychopharmacology. 1997;16:433–437. doi: 10.1016/S0893-133X(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 32.Neafsey EJ. Prefrontal control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- 33.Retaux S, Besson MJ, Penit-Soria J. Opposing effects of dopamine D2 receptor stimulation on the spontaneous and electrically evoked release of 3[H]GABA on rat PFC slices. Neuroscience. 1991;42:61–71. doi: 10.1016/0306-4522(91)90150-m. [DOI] [PubMed] [Google Scholar]
- 34.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 35.Sawaguchi T, Kubota K. Modulatory role of D1-dopamine receptors in mnemonic coding of monkey prefrontal cortical neurons. Soc Neurosci Abstr. 1996;22:417. [Google Scholar]
- 36.Sawaguchi T, Michikazu M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci Res. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- 37.Schierhorn H. Strukturwandel neokortikaler Pyramidenneurone des Menschen wahrend des 5. bis 9. Dezenniums. Psychiatr Neurol Med Psychol. 1981;33:664–673. [PubMed] [Google Scholar]
- 38.Seamans JK, Floresco SB, Phillips AG. Selective impairment on a delayed radial arm task following local administration of a selective D1, but not a D2, antagonist into the prefrontal cortex. Soc Neurosci Abstr. 1995;21:1942. [Google Scholar]
- 39.Seamans JK, Gorelova N, Yang CR. Contributions of voltage-gated Ca2+ channels in the proximal versus distal dendrites to synaptic integration in prefrontal cortical neurons. J Neurosci. 1997;17:5936–5948. doi: 10.1523/JNEUROSCI.17-15-05936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sesack SR, Bunney BS. Pharmacological characterization of the receptor mediating electrophysiological responses to dopamine in the rat medial prefrontal cortex: a microiontophoretic study. J Pharmacol Exp Ther. 1989;248:1323–1333. [PubMed] [Google Scholar]
- 41.Simon H. Dopaminergic A10 neurons and the frontal system. J Physiol (Paris) 1981;77:81–95. [PubMed] [Google Scholar]
- 42.Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of the mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 43.Thierry AM, Le Douarin C, Penit J, Ferron A, Glowinski J. Variation in the ability of neuroleptics to block the inhibitory influence of dopaminergic neurons on the activity of cells in the rat prefrontal cortex. Brain Res Bull. 1986;16:155–160. doi: 10.1016/0361-9230(86)90027-4. [DOI] [PubMed] [Google Scholar]
- 44.Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: synaptic density. Exp Neurol. 1980;69:164–172. doi: 10.1016/0014-4886(80)90151-x. [DOI] [PubMed] [Google Scholar]
- 45.van Haaren F, de Bruin JPC, Heinsbroek RPW, van de Poll NE. Delayed spatial response alternation: effects of delay interval duration and lesions of the medial prefrontal cortex on response accuracy of male and female Wistar rats. Behav Brain Res. 1985;18:41–49. doi: 10.1016/0166-4328(85)90167-6. [DOI] [PubMed] [Google Scholar]
- 46.Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams GV, Goldman-Rakic PS. Blockade of dopamine D1 receptors enhances memory fields of prefrontal neurons in primate cerebral cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 48.Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zola-Morgan S, Squire LS, Amaral DG. Lesions of the hippocampal formation but not lesions of the fornix or the mammillary nuclei produce longlasting memory impairment in monkeys. J Neurosci. 1989;9:898–913. doi: 10.1523/JNEUROSCI.09-03-00898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]