Abstract
Dopamine release can regulate striatal acetylcholine effluxin vivo through at least two receptor mechanisms: (1) direct inhibition by dopamine D2 receptors on the cholinergic neurons, and (2) excitation initiated by dopamine D1 receptors. The neuroanatomical locus of the latter population of D1 receptors and the pathway(s) involved in the expression of their influence are controversial issues. We have tested the hypothesis that D1 receptors in substantia nigra pars reticulata are involved in the excitatory component of dopaminergic actions on striatal acetylcholine output. In vivo microdialysis was used in awake rats. Infusion of the selective D1 receptor agonistR(+)-1-Phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine-7,8-diol (SKF 38393) hydrochloride into pars reticulata of substantia nigra elicited a significant increase in striatal acetylcholine efflux. Likewise, d-amphetamine applied into pars reticulata of substantia nigra by reverse dialysis produced an elevation in acetylcholine output measured at a second microdialysis probe in the striatum. Application of d-amphetamine in the striatum by reverse dialysis elicited a decrease in striatal acetylcholine efflux that could be reversed subsequently by local application ofd-amphetamine in substantia nigra pars reticulata. A 2 mg/kg intraperitoneal dose of d-amphetamine, which has no net effect on striatal acetylcholine output under control conditions, elicited a significant decrease in acetylcholine efflux when the D1 receptor antagonistR(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH 23390) hydrochloride was applied simultaneously via a second microdialysis probe in substantia nigra pars reticulata. Thus, an excitatory D1-mediated influence on striatal acetylcholine output is initiated in substantia nigra pars reticulata, and this influence contributes to the effects of indirect dopaminergic agonists such as d-amphetamine on striatal acetylcholine efflux. These results indicate an important role of somatodendritic dopamine release, in addition to nerve terminal dopamine release, in the regulation of activity in basal ganglia circuits.
Keywords: acetylcholine, dopamine, GABA, substantia nigra, striatum, d-amphetamine, dopamine D1 receptor, in vivo microdialysis
Recently, it has become apparent that at least two dopaminergic mechanisms regulate striatal cholinergic activity. Two distinct dopamine receptors, D1 and D2 (Civelli et al., 1993), are known to participate in this regulation. The D2 receptor inhibits striatal acetylcholine (ACh) efflux (Lehmann and Langer, 1983; Stoof et al., 1992), whereas the D1 receptor modulates this variable in an excitatory manner (Fage and Scatton, 1986; Consolo et al. 1987; Damsma et al., 1990; Imperato et al., 1993). Thus, indirect dopamine agonists such as amphetamine have been observed to produce an increase, a decrease, or no net change in striatal ACh efflux depending on the dose administered and according to the methods used in the experiment (Ladinsky et al., 1975; Damsma et al., 1991; DeBoer and Abercrombie, 1996a). Such variability in ACh response to mixed dopaminergic agonists presumably reflects the fact that D2-mediated inhibition or D1-mediated excitation may predominate in determining the level of ACh output in any given circumstance and that the effects of the two influences may even cancel.
It generally is accepted that the dopamine D2 receptor directly inhibits striatal ACh efflux via receptors located on the striatal cholinergic cells (Lehmann and Langer, 1983; DeBoer and Abercrombie, 1996b). However, the precise nature of the mechanism(s) underlying the D1-mediated stimulation of striatal ACh output is a topic of some controversy. Particularly unclear is the extent to which D1 receptors within the striatum versus extrastriatal D1 receptors contribute to this phenomenon. Although virtually all striatal cholinergic interneurons express D2 receptor mRNA in high abundance, only a fraction of these cells are reported to express low levels of D1receptor mRNA (Le Moine et al., 1990, 1991; Weiner et al., 1991). Studies of ACh release using in vitro striatal slice preparations are primarily negative or equivocal, generally reporting no detectable D1 receptor-mediated modulation of ACh output in this situation (Scatton, 1982; Consolo et al., 1987; Dolezal et al., 1992; Tedford et al., 1992). These results suggest an extrastriatal mechanism of D1 receptor regulation of striatal ACh output. On the other hand, a number of in vivo microdialysis studies have reported that the application of D1 receptor-selective compounds into the striatum can reproduce the effects of systemic administration of such compounds on striatal ACh, suggesting an intrastriatal site of action (Ajima et al., 1990; Consolo et al., 1992;Anderson et al., 1994a). This latter conclusion is not definitive, however, because failure to replicate these in vivomicrodialysis findings has been reported in several studies (Damsma et al., 1991; DeBoer et al., 1992; Acquas et al., 1997). Taken together, the available data leave open the distinct possibility that a population of D1 receptors localized outside of the striatum primarily is responsible for the initiation of an indirect dopaminergic excitation of striatal ACh output.
The goal of the present studies was to test the hypothesis that D1 receptors located in substantia nigra pars reticulata participate in a circuit that underlies the D1-mediated stimulatory component of the striatal cholinergic response to indirect dopamine agonists such as amphetamine. The somatodendritic release of dopamine within substantia nigra is well known (Korf et al., 1976;Chéramy et al., 1981; Robertson et al., 1991; Heeringa and Abercrombie, 1995), and the substantia nigra pars reticulata possesses a density of D1 receptors that is among the highest of that of any brain region (Savasta et al., 1986; Dawson et al., 1988). Nigral D1 receptors are localized exclusively on the terminals of the GABAergic striatonigral projection (Porceddu et al., 1986; Yung et al., 1995), and stimulation of these receptors seems to modulate positively the release of GABA from these sites (Floran et al., 1990;Cameron and Williams, 1993). In turn, an enhanced inhibitory drive to the GABAergic nigrothalamic projection neurons could disinhibit thalamocortical activity (Deniau and Chevalier, 1985; Timmerman and Abercrombie, 1996) and increase the level of glutamate input to striatal cholinergic neurons. In support of this model, previous work indicates that somatodendritic dopamine release can elevate a variety of indices of thalamocortical activation (Wilson and Wightman, 1985;Gauchy et al., 1987; Robertson and Robertson, 1987; LaHoste and Marshall, 1990; Yurek and Hipkins, 1993; Timmerman and Abercrombie, 1996). Moreover, reverse dialysis in the striatum of the noncompetitive NMDA receptor antagonist (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK 801) has been reported to attenuate the increase in striatal ACh release produced by systemic administration of the dopamine D1 receptor agonist CY 208-243 (Damsma et al., 1991).
MATERIALS AND METHODS
Animals. Adult male Sprague Dawley rats (Zivic-Miller Laboratories, Pittsburgh, PA) were used. Before microdialysis probe implantation, the rats were housed individually in plastic shoe box cages under conditions of constant temperature (21°C) and humidity (40%) on a 12 hr light/dark cycle (lights on at 7:00 A.M. and off at 7:00 P.M.) with food and water available ad libitum. The rats weighed between 350 and 450 gm at the time of the experiment. During microdialysis experiments, the animals were kept in Plexiglass cylinder cages (Abercrombie and Finlay, 1991). Animal procedures were conducted in accordance with guidelines published in the NIH Guide for Care and Use of Laboratory Animals, and all protocols were approved by the Rutgers University Institutional Animal Care and Use Committee.
Drug treatments. Injection of artificial CSF (aCSF) vehicle orR(+)-1-Phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine-7,8-diol (SKF 38393) hydrochloride (5 μg/0.5 μl) into the substantia nigra pars reticulata was controlled by a microliter infusion pump (Harvard Apparatus, South Natick, MA). A microsyringe (7101; Hamilton Co., Reno, NV) was connected via polyethylene-10 (PE-10) tubing (Clay Adams, Parsippany, NJ) to the 30 ga infusion cannula that extended 1 mm beyond the guide. All infusions were of 5 min duration and were performed at a rate of 0.1 μl/min. The injection cannula remained in place for 5 min before and 5 min after the infusion. Administration ofd-amphetamine was either systemic at a dose of 2 mg/kg intraperitoneally in a volume of 1 ml/kg saline (0.9% NaCl in distilled water) or direct into brain extracellular fluid via a microdialysis probe. For reverse dialysis experiments, compounds were dissolved in aCSF immediately before the beginning of the experiment. Each animal participated in only one treatment condition. Drug doses and concentrations were selected based on previously published work (Consolo et al., 1987; Asin and Montana, 1988; DeBoer et al., 1990;Keefe et al., 1992; DeBoer and Westerink, 1994).
Intranigral infusion cannulae. Seven to 10 d before striatal microdialysis probe implantation, a unilateral guide cannula aimed at the substantia nigra pars reticulata was implanted under chloral hydrate anesthesia (400 mg/kg, i.p.) using stereotaxic technique. Briefly, a burr hole was drilled into the skull, and a stainless steel guide cannula (26 ga; Plastics One Inc., Roanoke, VA) was inserted to a position 0.5 mm above the substantia nigra pars reticulata at the following coordinates: anteroposterior, −5.3 mm, and mediolateral, ±2.2 mm, from bregma; and dorsoventral, −6.7 mm, from dura (Paxinos and Watson, 1997). The guide cannula was secured in place using skull screws and fast-curing dental cement. A dummy cannula (30 ga), which extended 0.5 mm beyond the guide cannula, was inserted. Beginning on the first day after surgery, the rats were gently handled twice daily to habituate them to the intracerebral injection procedures.
Microdialysis probes. The microdialysis probes used in the present investigation were of a vertical, concentric design and incorporated a dialysis membrane with an active length of 2 mm (outer diameter, 250 μm; Spectra/Por; Spectrum, Houston, TX). A piece of PE-20 tubing (Clay Adams) served as the probe inlet, whereas a piece of fused silica capillary tubing (inner diameter, 75 μm; outer diameter, 150 μm; Polymicro Technologies, Phoenix, AZ) served as the outlet (DeBoer and Abercrombie, 1996a).
Microdialysis probe implantation. Microdialysis probes were continuously perfused with aCSF (in mm: NaCl 147, KCl 2.5, CaCl2 1.3, and MgCl2 0.9, pH = 7.4) at a rate of 1.5 μl/min by means of a microliter syringe pump (Harvard Apparatus). The animals were anesthetized with chloral hydrate (400 mg/kg), and a microdialysis probe was implanted into the striatum at the following coordinates (flat skull): anteroposterior, +1.0 mm, and mediolateral, +2.7 mm, relative to bregma; and −6.0 mm below the dura, according to Paxinos and Watson (1997). In some animals, a second microdialysis probe was implanted into the ipsilateral substantia nigra pars reticulata (Heeringa and Abercrombie, 1995). Nigral probes were first set at a lateral angle of 30° and subsequently positioned at the following coordinates: anteroposterior, −5.0 mm, and mediolateral, +5.3 mm, relative to bregma; and dorsoventral, −9.3 mm below dura (Paxinos and Watson, 1997). Microdialysis probes were lowered into the brain, at a speed of ∼400 μm/min, using a hydraulic microdrive (Narishige, Tokyo, Japan) or an electrode carrier (model 1760; David Kopf, Tujunga, CA) and then were secured to the skull with fast-curing dental cement and three skull screws. The inlet and outlet lines of the microdialysis probe were guided along a metal tether that was secured to the skull with fast-curing dental cement at one end and connected to a single-channel fluid swivel (Instech Laboratories, Plymouth Meeting, PA) at the other. Microdialysis experiments were conducted 18–24 hr after probe implantation.
Analysis of dialysate. Dialysate samples were collected every 15 min and injected into a 20 μl sample-loop of an HPLC apparatus. The criterion for baseline was the detection of ACh in three consecutive samples at levels that did not vary by >20% from one another. The acetylcholinesterase inhibitor neostigmine was present in the aCSF microdialysis perfusion solution at a concentration of 10 nm. The neostigmine solution was applied through the microdialysis probe directly into the striatum and was present for the entire duration of the experiment. ACh levels in striatal dialysates are increased two to threefold by the presence of 10 nmneostigmine, and we have shown previously that this neostigmine concentration does not affect the pharmacological responsiveness of the striatal cholinergic system to administration of dopaminergic drugs (DeBoer and Abercrombie, 1996a).
ACh was separated on a cation exchange column prepared by loading a reverse-phase column (100 × 2.0 mm), filled with Chromspher 5C18 packing material (Chrompack, Middelburg, The Netherlands), with a sodium lauryl sulfate ion-pairing solution (Damsma and Westerink, 1991). An enzymatic postcolumn reactor was used for the conversion of ACh to hydrogen peroxide and was prepared by loading a guard column (10 × 1.0 or 10 × 2.0 mm) with Lichrosorb-NH2(Merck, Darmstadt, Germany). After loading, 1 ml of 25% glutaraldehyde solution was pumped through the guard column at a flow rate of 100 μl/min to activate the Lichrosorb-NH2. Immediately after activation, enzymes were covalently immobilized on the activated matrix by passing 500 μl of a solution containing 40 U of choline oxidase and 80 U of acetylcholinesterase in 0.1 m potassium phosphate buffer, pH 8.0, through the guard column at a flow rate of 50 μl/min. The enzyme reactor thus prepared was rinsed with buffer for an additional 30 min at a flow rate of 400 μl/min before use. The amount of hydrogen peroxide generated by the enzyme reactor was quantified using a platinum wall jet electrode (model CU-04-AZ; ANTEC, Leiden, The Netherlands) set at +0.55 V versus Ag/AgCl. The mobile phase was a 0.1 m potassium phosphate buffer, pH 8.0, containing 1.5 mm tetramethylammonium chloride, 1.5 mm sodium octyl sulfate, and 0.1 mm EDTA, and was delivered at a flow rate of 350–450 μl/min by either a Waters Associates model 510 (Milford, MA) or an ESA model 580 (Bedford, MA) HPLC pump. The detection limit of the assay was ∼10 fmol of ACh (DeBoer and Abercrombie, 1996a).
Data analysis. The amount of ACh obtained in the dialysate fractions is expressed as femtomoles per sample. Group values are mean ± SEM. For the analysis of within-group drug effects, one-way ANOVA with repeated measures over time was used (p ≤ 0.05). Possible between-group differences were assessed using two-way ANOVA with repeated measures over time (p ≤ 0.05). When appropriate, ANOVA determinations were followed by Dunn’s post hoc test. The level of significance for all post hoc analyses wasp ≤ 0.01.
Histology. At the conclusion of the nigral injection experiments, 2% pontamine sky blue solution (0.2 μl/2 min) was infused via the injector. After all experiments, rats were killed by an overdose of sodium pentobarbital (100 mg/kg) and decapitated, and the brain was removed. The whole brain was fixed by storage in buffered formalin (10%) for at least 1 week. The correct placement of microdialysis probes and infusion cannulae was verified in 40-μm-thick coronal sections stained with cresyl violet or neutral red. Representative histological sections showing microdialysis probe tracks in the striatum and in substantia nigra pars reticulata are shown in Figure 1.
Fig. 1.
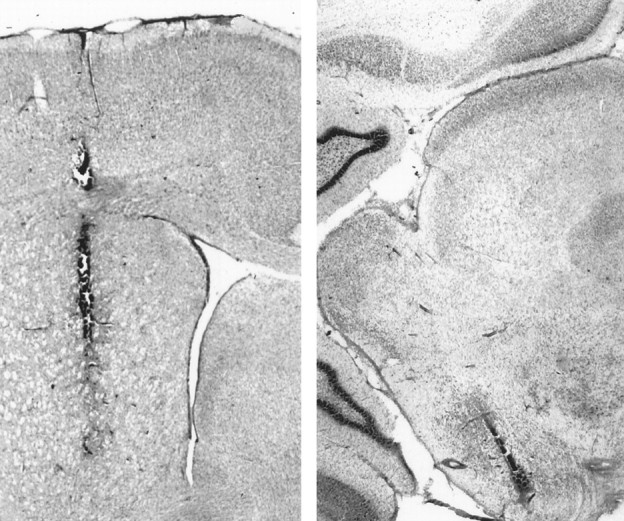
Digitized photographic montages of histological sections showing the localization of microdialysis probes in the striatum (left) and in substantia nigra pars reticulata (right).
Materials. Acetylcholinesterase type VI-S, choline oxidase,d-amphetamine sulfate, and neostigmine bromide were purchased from Sigma (St. Louis, MO). 2-Amino-5-phosphonopentanoic acid (AP-5), (−)-bicuculline methchloride, 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX),R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH 23390) hydrochloride, and R(+)-SKF-38393 hydrochloride were obtained from Research Biochemicals (Natick, MA). All other reagents and chemicals were of analytical grade and were obtained from Fisher Scientific (Springfield, NJ).
RESULTS
Effect of intranigral SKF 38393 injection on striatal ACh efflux
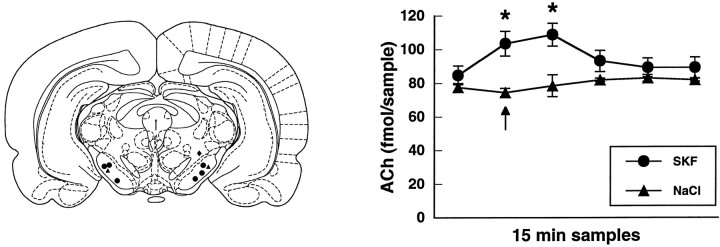
Direct injection into substantia nigra pars reticulata of the D1 agonist SKF 38393 (5 μg/0.5 μl) produced a significant increase in striatal ACh efflux [F(5,25) = 25.8; p < 0.001;n = 6]. No effect on striatal ACh output was observed when the aCSF vehicle was injected (n = 2) or when the infusion cannula tip was located outside of substantia nigra pars reticulata (n = 1). These data are shown in Figure2.
Fig. 2.
Left, Locations of intracerebral injection sites (adapted from Paxinos and Watson, 1997). •, Sites at which injection of the D1 agonist SKF 38393 (0.5 μg/0.5 μl) was effective in increasing striatal ACh efflux. ▴, Injection sites of the aCSF vehicle (0.5 μl). ♦, Site at which injection of SKF 38393 (0.5 μg/0.5 μl) had no significant effect on striatal ACh efflux. Right, Effect of intranigral injection of the D1 agonist SKF 38393 (0.5 μg/0.5 μl;circles) or the aCSF vehicle (0.5 μl;triangles) on the output of striatal ACh. Injection of SKF 38393 or aCSF took place between minutes 5 and 10 of the 15 min dialysis sample (arrow). Data are femtomoles of ACh/20 μl sample of dialysate (mean ± SEM). *p < 0.01, compared with baseline.
Effects of nigral amphetamine application on striatal ACh efflux
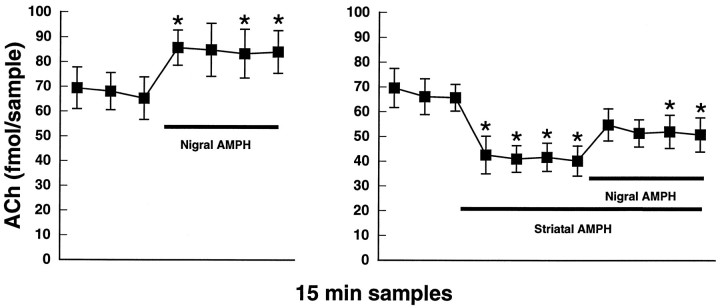
Intranigral application of 100 μmd-amphetamine by reverse dialysis elicited a significant increase in striatal ACh output [F(6,42) = 6.1;p < 0.001; n = 8]. Nigral application of d-amphetamine also was able to reverse significantly the direct inhibitory effect of striatal d-amphetamine application (10 μm) on ACh efflux [F(10,40) = 11.2; p < 0.001;n = 5]. These data are shown in Figure3.
Fig. 3.
Left, Intranigral infusion of amphetamine (AMPH; 100 μm) by reverse dialysis produces a significant increase in striatal ACh output.Right, The inhibitory effect of striatal amphetamine application (10 μm) is reversed by subsequent application of amphetamine (100 μm) into substantia nigra.Black bars represent the indicated drug application intervals. Data are femtomoles of ACh/20 μl sample of dialysate (mean ± SEM). *p < 0.01, compared with the final baseline sample.
Effect of systemic amphetamine administration on striatal ACh efflux
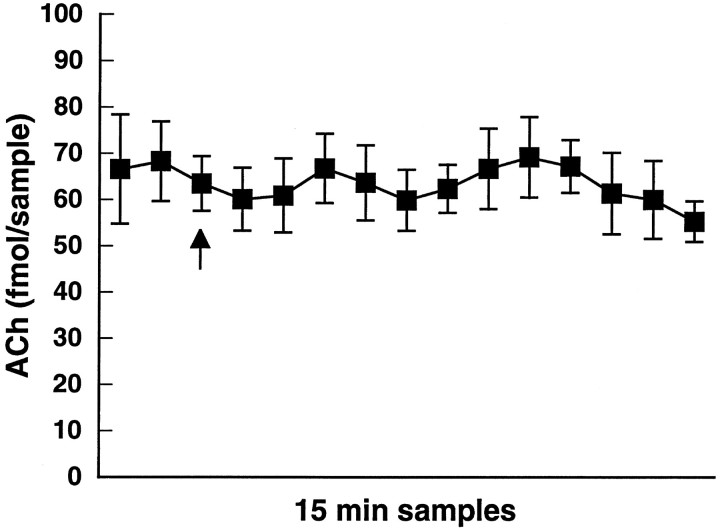
As shown in Figure 4, administration of 2 mg/kg d-amphetamine did not significantly alter the output of striatal ACh examined for 3 hr after administration of the drug [F(6,24) = 0.5; p = 0.79;n = 5]. The lack of effect of this dose of systemicd-amphetamine is attributable to the multiple dopaminergic mechanisms regulating striatal ACh output that summate to yield no net effect under this condition (DeBoer and Abercrombie, 1996a). Subsequent experiments therefore used administration of 2 mg/kgd-amphetamine to examine the ability of various manipulations to disrupt this balance.
Fig. 4.
Systemic administration of amphetamine at 2 mg/kg intraperitoneally (arrow) did not affect striatal ACh output. Data are femtomoles of ACh/20 μl sample of dialysate (mean ± SEM).
Role of dopamine D1 receptors in substantia nigra versus striatum in the dopaminergic regulation of striatal ACh efflux
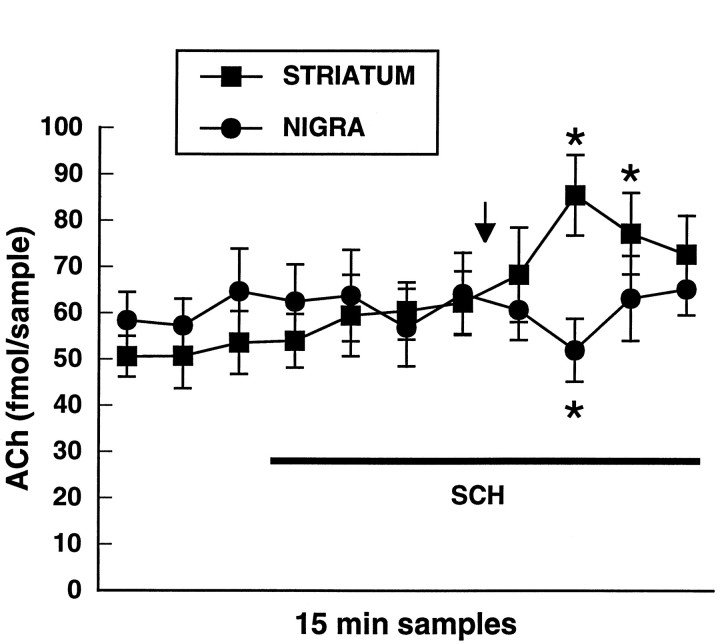
In this experiment, we examined whether local antagonism of dopamine D1 receptors in substantia nigra or in the striatum would alter the release profile of ACh in the striatum obtained in response to 2 mg/kg d-amphetamine. The effect of 2 mg/kg d-amphetamine on striatal ACh efflux was examined during concomitant application of the D1 dopamine receptor antagonist SCH 23390 (100 μm) via a second dialysis probe implanted into substantia nigra pars reticulata or via the striatal dialysis probe (Fig. 5). SCH 23390 did not significantly affect basal ACh output when applied into either structure. Systemic d-amphetamine produced a significant decrease in striatal ACh efflux when SCH 23390 was present in substantia nigra [F(10,40) = 2.2;p < 0.05; n = 5]. In contrast, when SCH 23390 was applied into the striatum, systemicd-amphetamine administration elicited a significant increase in striatal ACh output [F(10,40) = 7.1; p < 0.001; n = 5].
Fig. 5.
Effect of local antagonism of dopamine D1 receptors in the striatum (squares) or in substantia nigra (circles) on the release profile of striatal ACh in response to amphetamine at 2 mg/kg intraperitoneally. The D1 dopamine receptor antagonist SCH 23390 (100 μm) was applied via reverse dialysis into either the striatum or substantia nigra during the interval indicated by theblack bar and systemic amphetamine was administered at the arrow. Data are femtomoles of ACh/20 μl sample of dialysate (mean ± SEM). *p < 0.01, compared with the final baseline sample.
Role of striatal glutamatergic neurotransmission in the dopaminergic regulation of striatal ACh efflux
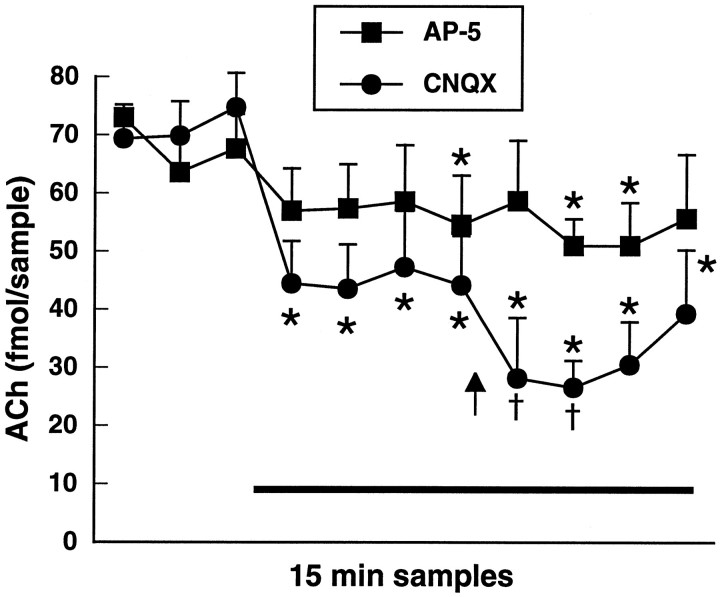
The observation that 2 mg/kg d-amphetamine produced a decrease in striatal ACh efflux when SCH 23390 was present in substantia nigra suggested that a circuit-mediated mechanism is responsible for the stimulation of striatal ACh by dopamine D1 receptors. To determine whether dopaminergic facilitation of striatal ACh release occurs via a final common pathway involving glutamatergic afferents to the striatum, we examined the effect of striatal application of glutamate receptor antagonists on the release of ACh in the striatum in response to 2 mg/kgd-amphetamine. Figure 6 shows the effect of 2 mg/kg d-amphetamine on striatal ACh output during concomitant application of the NMDA receptor antagonist AP-5 (100 μm) or the non-NMDA receptor antagonist CNQX (10 μm). AP-5 application produced a modest decrease in ACh output in the striatum, but subsequent administration ofd-amphetamine failed to elicit a significant further decrease in this variable [F(10,30) = 2.8;p = 0.01; n = 4]. CNQX application significantly decreased basal ACh efflux in the striatum; under this condition, systemic d-amphetamine administration produced a second decrease in ACh output that was significantly greater than the initial decrease observed with CNQX alone [F(10,40) = 10.4; p < 0.001;n = 5].
Fig. 6.
Effect of local antagonism of NMDA receptors with AP-5 (100 μm; squares) or of non-NMDA receptors with CNQX (10 μm; circles) on the release profile of striatal ACh in response to amphetamine at 2 mg/kg intraperitoneally. AP-5 or CNQX was applied into the striatum via reverse dialysis during the interval indicated by the black bar and systemic amphetamine was administered at thearrow. Data are femtomoles of ACh/20 μl sample of dialysate (mean ± SEM). *p < 0.01, compared with the final baseline sample. †p < 0.01, compared with the final preamphetamine sample.
Role of striatal GABAergic neurotransmission in the dopaminergic regulation of striatal ACh efflux
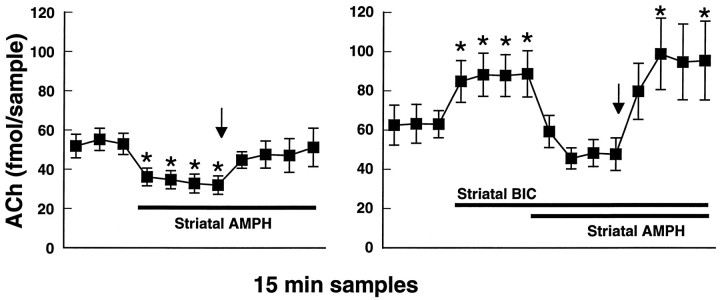
Because 2 mg/kg d-amphetamine produced an increase in striatal ACh output when SCH 23390 was applied locally in the striatum, we hypothesized that direct D1-mediated increases in striatal GABA release might also play a role in the regulation of striatal ACh in this situation. Local application of the GABAA receptor antagonist bicuculline (10 μm) via the microdialysis probe produced a significant increase in the basal level of striatal ACh output (Fig.7). To avoid a possible ceiling effect on striatal ACh output, for this experiment we therefore examined the ability of 2 mg/kg d-amphetamine to reverse the direct inhibitory effect of intrastriatal application of 10 μmd-amphetamine in the absence or presence of bicuculline. In the absence of bicuculline, local d-amphetamine application produced a significant decrease in striatal ACh output that then could be reversed by the systemic administration of d-amphetamine [F(10,40) = 4.5; p < 0.001;n = 5]. In the presence of bicuculline, the addition of 10 μmd-amphetamine to the perfusion solution decreased striatal ACh output to baseline levels. Under this condition, 2 mg/kg d-amphetamine produced a significant increase in striatal ACh release [F(14,56) = 3.9; p < 0.001; n = 5]. The increase in striatal ACh release produced by systemic d-amphetamine was significantly enhanced in the presence of local bicuculline plusd-amphetamine in comparison with locald-amphetamine alone [F(6,48) = 3.6;p < 0.01]. These data are shown in Figure 7.
Fig. 7.
Left, The direct inhibitory effect of striatal amphetamine application (AMPH; 10 μm) is reversed by subsequent administration of amphetamine at 2 mg/kg intraperitoneally (arrow).Right, The experiment was repeated in the presence of the GABAA receptor antagonist bicuculline (BIC; 10 μm) that by itself raised extracellular ACh levels in the striatum. Addition of amphetamine (10 μm) to the perfusion solution decreased striatal ACh output to baseline levels, and subsequent administration of amphetamine at 2 mg/kg intraperitoneally (arrow) reversed this effect. Black bars represent the indicated drug application intervals. Data are femtomoles of ACh/20 μl sample of dialysate (mean ± SEM). *p < 0.01, compared with the final baseline sample.
DISCUSSION
We observed that striatal ACh efflux was significantly increased by injection of the direct dopamine D1 receptor agonist SKF 38393 into substantia nigra pars reticulata. The D1receptors in substantia nigra pars reticulata therefore are likely candidates for mediating the excitatory component of the striatal ACh response to indirect dopamine agonists such as amphetamine. That an increase in somatodendritic dopamine release is sufficient to stimulate striatal ACh output is evidenced not only by the action of intranigral injection of SKF 38393 but also by the observation that nigral application of d-amphetamine via reverse dialysis was able to elicit this effect. Furthermore, nigral application ofd-amphetamine was able to reverse significantly the direct inhibitory effect of striatal d-amphetamine application on ACh efflux. Because striatal d-amphetamine application elicits a large impulse-independent release of dopamine in that structure (Fischer and Cho, 1979; Westerink et al., 1987), these actions of nigral d-amphetamine are not likely to be caused by local autoinhibition of nigrostriatal dopamine neuron activity and subsequent D2-mediated disinhibition of striatal ACh release.
The present data further suggest that nigral D1 receptor stimulation is necessary for the excitatory component of the striatal ACh response to increased endogenous dopamine release after systemic administration of d-amphetamine. By itself, systemicd-amphetamine at the dose used (2 mg/kg) has no net effect on striatal ACh efflux because of offsetting D1- and D2-mediated influences (DeBoer and Abercrombie, 1996a). In contrast, we observed that this dose of systemicd-amphetamine produced an inhibition of striatal ACh efflux when the D1 receptor antagonist SCH 23390 concomitantly was applied in substantia nigra pars reticulata. Although it generally is accepted that SCH 23390 is a selective dopamine D1 receptor antagonist in vivo, this drug does show significant affinity at 5-HT2 receptors (Hyttel, 1984; Bischoff et al., 1988). Because d-amphetamine can act not only as a potent releaser of dopamine but also has weaker activity as a releaser of 5-HT (Raiteri et al., 1975; Kuczenski et al., 1995), there is the possibility that interactions of SCH 23390 and d-amphetamine with nigral 5-HT mechanisms could contribute to the present results. Such interactions are unlikely to be important in the present case, however, because: (1) 5-HT receptors in substantia nigra pars reticulata belong predominantly to the 5-HT1-like class (Appel et al., 1990;Pompeiano et al., 1994), and (2) systemic administration of a 5 mg/kg dose of d-amphetamine has been shown to be ineffective in increasing the extracellular concentration of 5-HT in substantia nigra (Yamamoto et al., 1995). Thus, findings from the present investigation suggest that not only is the initiation site of D1-mediated excitation of striatal ACh efflux an extrastriatal one but also that the substantia nigra pars reticulata is the principal locus of this action. This conclusion is supported further by the present experiments in which striatal ACh output was inhibited by locald-amphetamine application, and this effect subsequently was reversed, to an equivalent extent, by either nigrald-amphetamine application or systemicd-amphetamine administration (see Figs. 2 and 5).
If the excitatory, D1-mediated component of the ACh response to systemic d-amphetamine is mediated via a circuit involving glutamatergic input to the striatum (see introductory remarks; Damsma et al., 1991), then blockade of striatal glutamate receptors should reveal an inhibition of ACh output in response tod-amphetamine challenge. In our experiments, both the NMDA receptor antagonist AP-5 and the non-NMDA receptor antagonist CNQX decreased the basal output of ACh when applied locally into the striatum; CNQX was more effective in this regard. In response to a subsequent injection of d-amphetamine, a further decrease in ACh output occurred only in the presence of CNQX, suggesting that the excitation of cholinergic neurons in this situation primarily is non-NMDA receptor-mediated. In the study of Damsma et al. (1991), however, inclusion of the NMDA receptor antagonist MK 801 in the dialysis perfusion solution attenuated by ∼50% the increase in striatal ACh efflux produced by systemic administration of the direct D1 agonist CY 208-243. The difference between the ability of AP-5 (present study) and MK 801 (Damsma et al., 1991) to antagonize D1-mediated excitation of striatal ACh efflux may be attributable to the use of an indirect versus a direct agonist as the challenge drug and/or the use of a competitive versus a noncompetitive NMDA receptor antagonist, respectively. Thus, the lack of interaction between AP-5 and the cholinergic response to systemicd-amphetamine in the present study does not necessarily preclude a possible contribution of NMDA receptors in the determination of striatal ACh output after d-amphetamine administration. Most likely, both NMDA and non-NMDA receptors participate in the glutamatergic regulation of striatal ACh output, and the relative influence of these receptor types may depend on the conditions of the experiment (Anderson et al., 1994b; Jin and Fredholm, 1994; Giovannini et al., 1995).
Unexpectedly, we observed that the cholinergic response to 2 mg/kgd-amphetamine shifted from no net change to an increase when the D1 receptor antagonist SCH 23390 concomitantly was applied via the striatal microdialysis probe. This outcome indicates the existence of a D1-mediated inhibitory influence on striatal ACh output that is dependent on D1 receptors located within the striatum. We hypothesize that this inhibition of striatal ACh efflux may be exerted via a direct D1-mediated activation of striatal GABA neurons that, in turn, can inhibit the cholinergic interneurons through GABAA receptors (Anderson et al., 1993; DeBoer and Westerink, 1994). In support of this hypothesis, the excitatory component of the cholinergic response to systemic d-amphetamine was enhanced by local application of the GABAA receptor antagonist bicuculline, indicating the removal of an offsetting GABAergic influence that normally is operative in this condition. Interestingly, striatal ACh efflux was inhibited to the same level by local d-amphetamine application in the presence or absence of bicuculline in the striatal perfusate. The latter result may suggest that the inhibition of ACh output in this situation is predominantly direct via D2 receptors. Expression of the hypothesized striatal, D1-mediated inhibition of ACh output via increased GABA release may be dependent on elevated glutamate input to the striatum. Such an influence therefore would not be apparent under basal conditions but would play a role in determining the net effect of systemic d-amphetamine on striatal ACh efflux. A permissive relation between glutamate transmission and D1 activity in regulating neuronal function in the striatum has been observed previously for a number of variables including striatal GABA release, NMDA-mediated excitation of striatal cells, and Fos induction in striatal neurons (Cepeda et al., 1993; Morari et al., 1994; Sharp et al., 1995; Wang and Johnson, 1995). Finally, in the striatum a 2 mg/kg dose of d-amphetamine has been shown to increase dialysate concentrations of dopamine from 28 to ∼700 nm and also to increase dialysate concentrations of 5-HT from 1 to ∼7 nm (Kuczenski et al., 1995). Thus, we cannot exclude the possibility that activity of SCH 23390 at striatal 5-HT2 receptors may contribute to the effect observed (see above).
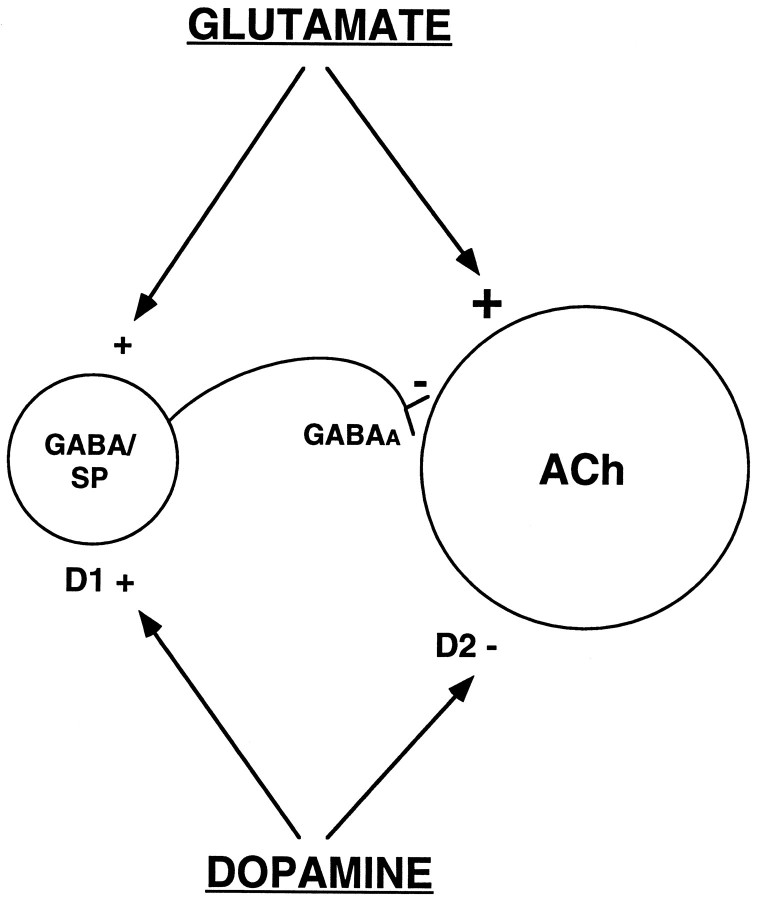
The results of the present investigation clearly are not compatible with a number of previous in vivo studies that suggest D1 receptors localized within the striatum are responsible for the excitation of ACh efflux by dopamine (see introductory remarks;DiChiara et al., 1994). This conclusion is not supported, however, by other studies in which evidence of a striatal D1-mediated facilitation of ACh efflux could not be found (see introductory remarks). Our data indicate that the D1-mediated excitation of striatal ACh release is not mediated by receptors in the striatum; rather, this effect is explained by a circuit mechanism involving substantia nigra D1 receptors and glutamatergic input to the striatum. Indeed, we have obtained evidence that D1receptors in the striatum may regulate ACh release in an inhibitory manner under certain conditions. At present, the basis for the existing discrepancies in the data across laboratories is not clear. We recently have shown that the pharmacological responsiveness of striatal ACh output to dopaminergic manipulations can be qualitatively altered by the experimental conditions, specifically, by the concentration of acetylcholinesterase inhibitor (AChE-I) present in the microdialysis perfusion solution (DeBoer and Abercrombie, 1996a). It is interesting to note that the studies in which a local D1-mediated stimulation of striatal ACh output was found, μmconcentrations of AChE-I were used, whereas studies reporting no local effect of D1 receptors on striatal ACh efflux used AChE-I concentrations in the range of 100–300 nm. In the present study, in which an inhibitory effect of striatal D1receptors on ACh release was indicated, we used a 10 nmconcentration of the AChE-I neostigmine. It is likely that the complex neurochemical interactions that act in concert to determine ACh efflux are altered when extracellular ACh levels are artificially elevated by the presence of a high concentration of AChE-I. For example, in this latter situation, stimulation of D1 receptors on the striatal GABA neurons that contain substance P may preferentially evoke the release of the peptide (Fig. 8; Lucas and Harlan, 1995). Substance P is known to be excitatory to striatal cholinergic neurons, and there is evidence that this peptide may play a stimulatory role under experimental conditions in which local D1-mediated facilitation of striatal ACh output is observed (Anderson et al., 1994a).
Fig. 8.
Schematic diagram illustrating the hypothesized interactions that are involved in determining the striatal ACh response to administration of indirect dopaminergic agonists such as amphetamine. Stimulation of dopamine D1 receptors in substantia nigra elicits an increase in glutamate input to striatal neurons. Within the striatum, dopamine D2 receptor activation directly inhibits the cholinergic interneuron. Stimulation of dopamine D1 receptors on striatal GABA/substance P (SP) neurons may provide an indirect inhibitory influence on the cholinergic interneuron; enhanced glutamate input may play a permissive role in this latter effect.
The present results demonstrate that dopaminergic cells in the substantia nigra can influence extrapyramidal function at both the somatodendritic and the nerve terminal level. In particular, the substantia nigra pars reticulata seems to be a major anatomical locus at which dopamine D1 receptor stimulation initiates an excitatory influence on striatal ACh release. Furthermore, our results support the hypothesis that this action is mediated through a circuit mechanism involving glutamatergic input to the striatum such that an excitation of striatal ACh interneurons is effected. This previously undescribed mechanism is consistent with an important role of somatodendritic dopamine release in regulating thalamocortical activity. The overall influence of dopamine in the regulation of striatal ACh efflux is demonstrably complex, involving both direct and indirect influences (Fig. 8). Depending on the balance of activity among the various components of the basal ganglia circuitry, altered dopamine release may produce an increase, a decrease, or no net change in striatal ACh output. Consideration of this complexity may aid in the design of novel therapeutic approaches to treatment of extrapyramidal-related disorders.
Footnotes
This research was supported by United States Public Health Service Grants DA08086 and NS19608 (E.D.A.) and the Tourette Association Inc. (P.D.B.). E.D.A. is an Alfred P. Sloan Foundation Research Fellow.
Correspondence should be addressed to Dr. Elizabeth D. Abercrombie, Center for Molecular and Behavioral Neuroscience, Rutgers University, 197 University Avenue, Newark, NJ 07102.
Dr. DeBoer’s present address: University Center for Pharmacy, University of Groningen, A. Deusinglaan 2, 9713 AW, Groningen, The Netherlands.
REFERENCES
- 1.Abercrombie ED, Finlay JM. Monitoring extracellular norepinephrine in brain using in vivo microdialysis and HPLC-EC. In: Robinson TE, Justice JB Jr, editors. Microdialysis in the neurosciences. Elsevier; Amsterdam: 1991. pp. 253–274. [Google Scholar]
- 2.Acquas E, Wilson C, Fibiger HC. Nonstriatal dopamine D1 receptors regulate striatal acetylcholine release in vivo. J Pharmacol Exp Ther. 1997;281:360–368. [PubMed] [Google Scholar]
- 3.Ajima A, Yamaguchi T, Kato T. Modulation of acetylcholine release by D1, D2 dopamine receptors in rat striatum under freely moving conditions. Brain Res. 1990;518:193–198. doi: 10.1016/0006-8993(90)90972-e. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JJ, Kuo S, Chase TN, Engber TM. GABAA and GABAB receptors differentially regulate striatal acetylcholine release in vivo. Neurosci Lett. 1993;160:126–130. doi: 10.1016/0304-3940(93)90395-2. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JJ, Kuo S, Chase TN, Engber TM. Dopamine D1 receptor-stimulated release of acetylcholine in rat striatum is mediated indirectly by activation of striatal neurokinin1 receptors. J Pharmacol Exp Ther. 1994a;269:1144–1151. [PubMed] [Google Scholar]
- 6.Anderson JJ, Kuo S, Chase TN. Endogenous excitatory amino acids tonically stimulate striatal acetylcholine release through NMDA but not AMPA receptors. Neurosci Lett. 1994b;176:264–268. doi: 10.1016/0304-3940(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 7.Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, DeSouza EB. Autoradiographic characterization of (±)-1-(2,5-dimethoxy-4-[125I]iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1C receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- 8.Asin KE, Montana WE. Rotation following intranigral injections of a selective D1 or a selective D2 dopamine receptor agonist in rats. Pharmacol Biochem Behav. 1988;29:89–92. doi: 10.1016/0091-3057(88)90279-1. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff S, Heinrich M, Krauss J, Sills MA, Williams M, Vassout A. Interaction of the D1 receptor antagonist SCH 23390 with the central 5-HT system: radioligand binding studies, measurements of biochemical parameters and effects on L-5-HTP syndrome. J Recept Res. 1988;8:107–120. doi: 10.3109/10799898809048981. [DOI] [PubMed] [Google Scholar]
- 10.Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- 11.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chéramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- 13.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;32:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 14.Consolo S, Wu CF, Fusi R. D-1 receptor-linked mechanism modulates cholinergic neurotransmission in rat striatum. J Pharmacol Exp Ther. 1987;242:300–305. [PubMed] [Google Scholar]
- 15.Consolo S, Girotti P, Russi G, DiChiara G. Endogenous dopamine facilitates striatal in vivo acetylcholine release by acting on D1 receptors localized in the striatum. J Neurochem. 1992;59:1555–1557. doi: 10.1111/j.1471-4159.1992.tb08473.x. [DOI] [PubMed] [Google Scholar]
- 16.Damsma G, Westerink BHC. A microdialysis and automated on-line analysis approach to study central cholinergic transmission in vivo. In: Robinson TE, Justice JB Jr, editors. Microdialysis in the neurosciences. Elsevier; Amsterdam: 1991. pp. 237–252. [Google Scholar]
- 17.Damsma G, Tham C-S, Robertson GS, Fibiger HC. Dopamine D1 receptor stimulation increases striatal acetylcholine release in the rat. Eur J Pharmacol. 1990;186:335–338. doi: 10.1016/0014-2999(90)90456-g. [DOI] [PubMed] [Google Scholar]
- 18.Damsma G, Robertson GS, Tham C-S, Fibiger HC. Dopaminergic regulation of striatal acetylcholine release: importance of D1 and N-Methyl-d-aspartate receptors. J Pharmacol Exp Ther. 1991;259:1064–1072. [PubMed] [Google Scholar]
- 19.Dawson TM, Barone P, Sidhu A, Wamsley JK, Chase TN. The D1 dopamine receptor in the rat brain: quantitative autoradiographic localization using an iodinated ligand. Neuroscience. 1988;26:83–100. doi: 10.1016/0306-4522(88)90129-7. [DOI] [PubMed] [Google Scholar]
- 20.DeBoer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996a;277:775–783. [PubMed] [Google Scholar]
- 21.DeBoer P, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol. 1996b;317:257–262. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- 22.DeBoer P, Westerink BHC. GABAergic modulation of striatal cholinergic interneurons: an in vivo microdialysis study. J Neurochem. 1994;62:70–75. doi: 10.1046/j.1471-4159.1994.62010070.x. [DOI] [PubMed] [Google Scholar]
- 23.DeBoer P, Damsma G, Fibiger HC, Timmerman W, DeVries JB, Westerink BHC. Dopaminergic–cholinergic interactions in the striatum: the critical significance of calcium concentrations in brain microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:528–534. doi: 10.1007/BF00169041. [DOI] [PubMed] [Google Scholar]
- 24.DeBoer P, Damsma G, Schram Q, Stoof JC, Zaagsma J, Westerink BHC. The effect of intrastriatal application of directly and indirectly acting dopamine agonists and antagonists on the in vivo release of acetylcholine measured by brain microdialysis. The importance of the post-surgery interval. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:144–152. doi: 10.1007/BF00165729. [DOI] [PubMed] [Google Scholar]
- 25.Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striatonigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res. 1985;334:227–233. doi: 10.1016/0006-8993(85)90214-8. [DOI] [PubMed] [Google Scholar]
- 26.DiChiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 27.Dolezal V, Jackisch R, Hertting G, Allgaier C. Activation of dopamine D1 receptors does not affect D2 receptor-mediated inhibition of acetylcholine release in rabbit striatum. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:16–20. doi: 10.1007/BF00175463. [DOI] [PubMed] [Google Scholar]
- 28.Fage D, Scatton B. Opposing effects of D-1 and D-2 receptor antagonists on acetylcholine levels in the rat striatum. Eur J Pharmacol. 1986;129:359–362. doi: 10.1016/0014-2999(86)90447-4. [DOI] [PubMed] [Google Scholar]
- 29.Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;192:642–653. [PubMed] [Google Scholar]
- 30.Floran B, Aceves J, Sierra A, Martinez-Fong D. Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci Lett. 1990;116:136–140. doi: 10.1016/0304-3940(90)90399-t. [DOI] [PubMed] [Google Scholar]
- 31.Gauchy C, Kemel ML, Desban M, Romo R, Glowinski J, Besson MJ. The role of dopamine released from distal and proximal dendrites of nigrostriatal dopaminergic neurons in the control of GABA transmission in the thalamic nucleus ventralis medialis in the cat. Neuroscience. 1987;22:935–946. doi: 10.1016/0306-4522(87)92971-x. [DOI] [PubMed] [Google Scholar]
- 32.Giovannini MG, Camilli F, Mundula A, Bianchi L, Colivicchi MA, Pepeu G. Differential regulation by N-Methyl-d-aspartate and non-N-Methyl-d-aspartate receptors of acetylcholine release from the rat striatum in vivo. Neuroscience. 1995;65:409–415. doi: 10.1016/0306-4522(94)00503-w. [DOI] [PubMed] [Google Scholar]
- 33.Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- 34.Hyttel J. Functional evidence for selective dopamine D-1 receptor blockade by SCH 23390. Neuropharmacology. 1984;23:1395–1401. doi: 10.1016/0028-3908(84)90079-0. [DOI] [PubMed] [Google Scholar]
- 35.Imperato A, Obinu MC, Casu MA, Mascia MS, Dazzi L, Gessa GL. Evidence that neuroleptics increase striatal acetylcholine release through stimulation of dopamine D1 receptors. J Pharmacol Exp Ther. 1993;266:557–562. [PubMed] [Google Scholar]
- 36.Jin S, Fredholm BB. Role of NMDA, AMPA and kainate receptors in mediating glutamate- and 4-AP-induced dopamine and acetylcholine release from rat striatal slices. Neuropharmacology. 1994;33:1039–1048. doi: 10.1016/0028-3908(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 37.Keefe KA, Zigmond MJ, Abercrombie ED. Extracellular dopamine in striatum: influence of nerve impulse activity in medial forebrain bundle and local glutamatergic input. Neuroscience. 1992;47:325–332. doi: 10.1016/0306-4522(92)90248-z. [DOI] [PubMed] [Google Scholar]
- 38.Korf J, Zieleman M, Westerink BHC. Dopamine release in substantia nigra? Nature. 1976;260:257–258. doi: 10.1038/260257a0. [DOI] [PubMed] [Google Scholar]
- 39.Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ladinsky H, Consolo S, Bianchi S, Samanin R, Ghezzi D. Cholinergic–dopaminergic interaction in the striatum: the effect of 6-hydroxydopamine or pimozide treatment on the increased striatal acetylcholine levels induced by apomorphine, piribedil and d-amphetamine. Brain Res. 1975;84:221–226. doi: 10.1016/0006-8993(75)90977-4. [DOI] [PubMed] [Google Scholar]
- 41.LaHoste GJ, Marshall JF. Nigral D1 and striatal D2 receptors mediate the behavioral effects of dopamine agonists. Behav Brain Res. 1990;38:233–242. doi: 10.1016/0166-4328(90)90178-h. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- 43.Le Moine C, Tison F, Bloch B. D2 dopamine receptor gene expression by cholinergic neurons in the rat striatum. Neurosci Lett. 1990;117:248–252. doi: 10.1016/0304-3940(90)90671-u. [DOI] [PubMed] [Google Scholar]
- 44.Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas LR, Harlan RE. Cholinergic regulation of tachykinin- and enkephalin-gene expression in the rat striatum. Mol Brain Res. 1995;30:181–195. doi: 10.1016/0169-328x(94)00288-p. [DOI] [PubMed] [Google Scholar]
- 46.Morari M, O’Connor WT, Ungerstedt U, Fuxe K. Dopamine D1 and D2 receptor antagonism differentially modulates stimulation of striatal neurotransmitter levels by N-Methyl-d-aspartic acid. Eur J Pharmacol. 1994;256:23–30. doi: 10.1016/0014-2999(94)90611-4. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- 48.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 49.Porceddu ML, Giorgi O, Ongini E, Mele S, Biggio G. 3H-SCH 23390 binding sites in the rat substantia nigra: evidence for a presynaptic localization and innervation by dopamine. Life Sci. 1986;39:321–328. doi: 10.1016/0024-3205(86)90650-8. [DOI] [PubMed] [Google Scholar]
- 50.Raiteri M, Bertollini A, Angelini F, Levi G. d-Amphetamine as a releaser or reuptake inhibitor of biogenic amines in synaptosomes. Eur J Pharmacol. 1975;34:189–195. doi: 10.1016/0014-2999(75)90239-3. [DOI] [PubMed] [Google Scholar]
- 51.Robertson GS, Robertson HA. D1 and D2 dopamine agonist synergism: separate sites of action? Trends Pharmacol Sci. 1987;8:295–299. [Google Scholar]
- 52.Robertson GS, Damsma G, Fibiger HC. Characterization of dopamine release in the substantia nigra by in vivo microdialysis in freely moving rats. J Neurosci. 1991;11:2209–2216. doi: 10.1523/JNEUROSCI.11-07-02209.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- 54.Scatton B. Further evidence for the involvement of D2, but not D1 dopamine receptors in dopaminergic control of striatal cholinergic transmission. Life Sci. 1982;31:2883–2890. doi: 10.1016/0024-3205(82)90679-8. [DOI] [PubMed] [Google Scholar]
- 55.Sharp FR, Liu J, Nickolenko J, Bontempi B. NMDA and D1 receptors mediate induction of c-fos and junB genes in striatum following morphine administration: implications for studies of memory. Behav Brain Res. 1995;66:225–230. doi: 10.1016/0166-4328(94)00146-7. [DOI] [PubMed] [Google Scholar]
- 56.Stoof JC, Drukarch B, DeBoer P, Westerink BHC, Groenewegen HJ. Regulation of the activity of striatal cholinergic neurons by dopamine. Neuroscience. 1992;47:755–770. doi: 10.1016/0306-4522(92)90027-y. [DOI] [PubMed] [Google Scholar]
- 57.Tedford CE, Crosby G, Iorio LC, Chipkin RE. Effect of SCH 39166, a novel dopamine D1 receptor antagonist, on [3H]acetylcholine release in rat striatal slices. Eur J Pharmacol. 1992;211:169–176. doi: 10.1016/0014-2999(92)90525-9. [DOI] [PubMed] [Google Scholar]
- 58.Timmerman W, Abercrombie ED. Amphetamine-induced release of dendritic dopamine in substantia nigra pars reticulata: D1-mediated behavioral and electrophysiological effects. Synapse. 1996;23:280–291. doi: 10.1002/(SICI)1098-2396(199608)23:4<280::AID-SYN6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Johnson KM. Regulation of striatal cyclic-3′,5′-adenosine monophosphate accumulation and GABA release by glutamate metabotropic and dopamine D1 receptors. J Pharmacol Exp Ther. 1995;275:877–884. [PubMed] [Google Scholar]
- 60.Weiner DM, Levey AI, Sunahara RK, Niznik HB, O’Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci USA. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westerink BHC, Tuntler J, Damsma G, Rollema H, DeVries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Arch Pharmacol. 1987;336:502–507. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- 62.Wilson RL, Wightman RM. Systemic and nigral application of amphetamine both cause an increase in extracellular concentration of ascorbate in the caudate nucleus of the rat. Brain Res. 1985;339:219–226. doi: 10.1016/0006-8993(85)90086-1. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto BK, Nash JF, Gudelsky GA. Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J Pharmacol Exp Ther. 1995;273:1063–1070. [PubMed] [Google Scholar]
- 64.Yung KKL, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- 65.Yurek DM, Hipkins SB. Intranigral injections of SCH 23390 inhibit amphetamine-induced rotational behavior. Brain Res. 1993;623:56–64. doi: 10.1016/0006-8993(93)90009-c. [DOI] [PubMed] [Google Scholar]