Abstract
Neuropeptide Y (NPY) agonists inhibit glutamate release by a presynaptic action at the CA3–CA1 synapse of rat hippocampus. We have examined the relationship between [Capre]tvia presynaptic, voltage-dependent calcium channels (VDCCs), measured optically by using the fluorescent calcium indicator fura-2, and transmitter release, measured electrophysiologically. Activation of presynaptic NPY Y2 receptors reduced [Capre]t and thereby inhibited synaptic transmission. Multiple calcium channels are involved in synaptic transmission at this synapse. Activation of Y2 receptors inhibits N-type, P/Q-type, and unidentified presynaptic VDCCs. The inhibition of each of these calcium channel types contributes to the reduction of [Capre]t by Y2receptors. Activation of adenosine receptors fully occluded the inhibition of presynaptic calcium influx by Y2 receptors but not the inhibition by GABAB receptors, suggesting a convergent action for Y2 and adenosine receptors, probably by coupling to the same G-protein.
Keywords: hippocampus, presynaptic calcium, synaptic transmission, neuropeptide Y, Y2 receptor, fura-2
Neuropeptide Y (NPY) is expressed in interneurons in the rat hippocampus (Milner and Veznedaroglu, 1992;Pickel et al., 1995), and its receptors are concentrated in this region (Dumont et al., 1993). Application of NPY selectively inhibits excitatory synaptic transmission in area CA1 of rat hippocampusin vitro (Colmers et al., 1987, 1988; Klapstein and Colmers, 1992). The site of action is presumably presynaptic, because not the postsynaptic membrane properties, the postsynaptic response to exogenously applied glutamate, nor the excitability of presynaptic fibers is affected by NPY (Colmers et al., 1987, 1988; McQuiston and Colmers, 1996). Consistent with this, NPY inhibits the frequency of spontaneous (i.e., Ca2+-dependent) but not miniature (Ca2+-independent) synaptic currents in rat CA3 pyramidal neurons of hippocampal slices from adult rats (McQuiston and Colmers, 1996), suggesting indirectly that NPY also may reduce Ca2+ in presynaptic terminals of this region.
In the rat hippocampus, the involvement of at least two types of high-threshold voltage-dependent calcium channels (VDCC) in synaptic transmission has been demonstrated (Wheeler et al., 1994a; Dunlap et al., 1995). These are ω-conotoxin GVIA-sensitive (N-type) and ω-agatoxin-IVA-sensitive (P/Q-type) VDCCs, respectively. N- and P/Q-type VDCCs in neuronal somata are inhibited by the activation of G-protein-coupled receptors for numerous neurotransmitters (Campbell et al., 1995). Measurements of Ca2+ influx into presynaptic terminals of sympathetic neurons filled with a Ca2+ indicator by means of a patch pipette revealed that the presynaptic action of NPY in this system was attributable entirely to the inhibition of Ca2+ entry through N-type channels at these terminals (Toth et al., 1993). Inhibitory modulation of presynaptic VDCCs by neurotransmitters also was observed at presynaptic terminals from CNS, including cerebellar parallel fibers (Dittman and Regehr, 1996), hippocampal CA3 to CA1 axons (Wu and Saggau, 1994a, 1995; Qian and Saggau, 1997), and the giant calyx of Held in the medial nucleus of the trapezoid body (Takahashi et al., 1996). We hypothesize that the activation of presynaptic NPY receptors directly inhibits presynaptic VDCCs, thereby reducing synaptic transmission at the CA3–CA1 synapse of hippocampus.
However, the activation of presynaptic K+conductances, also a well established response to neurotransmitters (Nicoll, 1988), also could result in presynaptic inhibition. Hyperpolarization of presynaptic terminals because of increased K+ conductance would shorten action potentials, resulting in a decreased presynaptic calcium influx and consequent reduction of synaptic transmission.
At the CA3–CA1 pyramidal cell synapse in adult rat hippocampus, receptors for at least five different neurotransmitters can inhibit synaptic transmission (for review, see Thompson et al., 1993; Wu and Saggau, 1997). Of these, NPY is the only transmitter with no postsynaptic action to complicate the interpretation of changes in synaptic responses (Colmers et al., 1988; Thompson et al., 1993). Here, we have taken advantage of this to test the hypothesis that NPY acts by presynaptic inhibition of VDCCs.
MATERIALS AND METHODS
Recording of the field EPSP (fEPSP) and the presynaptic [Capre]t in hippocampal slices.Transverse hippocampal slices (300–350 μm) were prepared from male Sprague Dawley rats (4 weeks of age) and incubated at 30°C in artificial cerebrospinal fluid composed of (in mm): 124 NaCl, 3 KCl, 2.5 CaCl2, 2 MgCl2, 22 NaHCO3, 1.25 NaH2PO4, and 10 d-glucose, gassed with 95% O2/5% CO2 to maintain a constant pH of 7.4. The dentate gyrus and part of CA3 were removed routinely. The procedure for loading calcium indicator into presynaptic terminals of CA3–CA1 synapses has been described in detail elsewhere (Wu and Saggau, 1994a). Briefly, a small amount of 1 mmfura-2 AM or furaptra AM (Molecular Probes, Eugene, OR) dissolved in DMSO solution (80% DMSO plus 20% pluronic acid) was pressure-injected into brain slices in the stratum radiatum (SR) of area CA1, where it was taken up locally into CA3 axons (see Fig. 1A). Approximately 2 hr after injection, an area with a diameter of 200 μm in SR, ∼800 μm away from the injection site, was illuminated at a single excitation wavelength (380 nm). Fluorescence was collected by a 50× objective lens (numerical aperture, 0.9), filtered by a long-pass filter (495 nm), and converted into an electrical signal by a single photodiode.
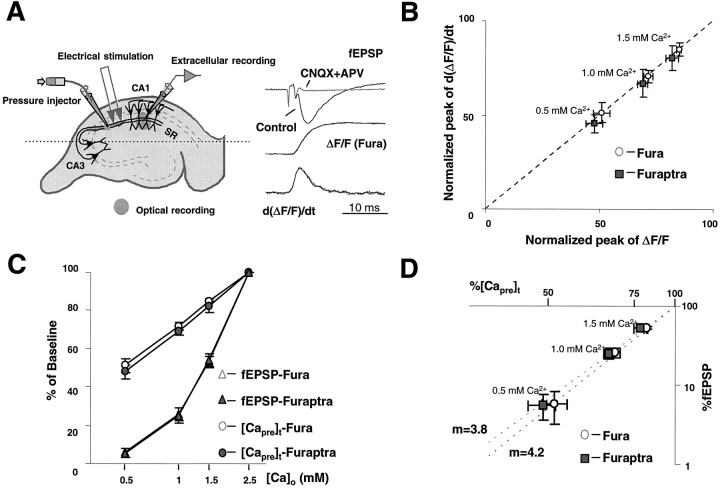
Fig. 1.
A nonlinear relationship exists between [Capre]t and fEPSP. A, Schematic diagram for loading calcium indicators into presynaptic terminals. Esterified (membrane-permeant) forms of fura-2 or furaptra were pressure-injected into stratum radiatum (SR), where they were taken up into CA3 axons, trapped by the action of esterases, and diffused into the remote presynaptic terminals onto CA1 neurons. Typical traces of the electrically recorded field EPSP (fEPSP), normalized transients of calcium indicator-related fluorescence (Fura), and their first derivative are shown under control conditions and during blocked synaptic transmission (CNQX+APV).B, Comparison between the peak of ΔF/Fand the peak of the first derivative of ΔF/F.C, Semi-log plot of normalized synaptic transmission and [Capre]t versus extracellular calcium concentration, [Ca2+]o. [Capre]t was a logarithmic function of [Ca2+]o. D, Double-log plot of normalized fEPSP versus [Capre]t for each tested [Ca2+]o. The regression line has a slope of m = 4.2 (r2 = 0.99) for fura-2 measurement and 3.8 (r2 = 0.99) for furaptra, respectively.
A bipolar tungsten electrode was positioned in SR of area CA1 to stimulate afferent inputs to CA1 neurons. An extracellular glass microelectrode (1–5 MΩ, filled with 2 m NaCl) was used to record fEPSPs in SR of area CA1. Slices were stimulated every 20 sec to elicit a submaximal response, and stimulation-induced presynaptic calcium transient ([Capre]t) and fEPSPs were sampled simultaneously at 10 kHz. Three successive traces were averaged to improve signal-to-noise ratio. The amplitude of calcium transients (ΔF) was measured as the difference between peak and resting fluorescence (F). Signals were corrected for dye bleaching and diffusion by forming the ratio ΔF/F. [Capre]t is assumed to be proportional to the normalized change in fluorescence of the Ca2+ indicator, ΔF/F. Autofluorescence of the brain slice was measured and subtracted from the total fluorescence signal. When testing for changes in presynaptic resting [Ca2+], we performed ratio measurements at excitation wavelengths of 360 and 380 nm. The selective presynaptic loading of the Ca2+ indicator was verified by applying the ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm) andd-amino-phosphonovalerate (d-APV; 25 μm), which did not alter ΔF/F while completely blocking the fEPSP (Fig. 1A). The maximal slope of the fEPSP was taken as the measure of synaptic transmission.
In those experiments in which ω-Aga IVA was used, slices were cut at 300 μm, and 0.1% cytochrome c was added to the bath solution to reduce nonspecific binding of the toxin. An active C-terminal fragment of human PYY (hPYY3–36, a potent agonist at the NPY Y2 receptor; Dumont et al., 1996) was dissolved in water and stocked at 1 mm. Data in each experiment were normalized to baseline before any drug application and then pooled and expressed as the mean ± SD.
Drugs. PYY was a gift from Dr. Yvan Dumont (Children’s Hospital Center, Verdun, Québec, Canada); CNQX andd-APV were purchased from Tocris Cookson (Bristol, UK); ω-CgTx GVIA and ω-CgTx MVIIC were purchased from Bachem (Torrance, CA); ω-Aga IVA was a gift from Pfizer (Groton, CT).
RESULTS
A nonlinear relationship exists between presynaptic calcium influx and synaptic transmission
The Ca2+ indicators, fura-2 and furaptra, were loaded selectively into the presynaptic terminals of the CA3–CA1 synapse at hippocampus, as described by Wu and Saggau (1994b) (Fig.1A). This allowed us to record optically the presynaptic Ca2+ influx ([Capre]t), represented by the ratioΔF/F or the first derivative of this ratio (d(ΔF/F)/dt). The stimulation-evoked [Capre]t was detected simultaneously with the corresponding fEPSP. The extracellular Ca2+concentration ([Ca2+]o) was reduced systematically from 2.5 mm (control) to 0.5 mm, whereas the [Mg2+]owas kept constant. No significant changes in the presynaptic fiber volley were observed. [Capre]t and fEPSP slope from individual experiments were normalized and used to calculate the relationship between presynaptic Ca2+ influx and synaptic transmission in different [Ca2+]o. Figure 1 compares measurements of [Capre]t obtained with the Ca2+ indicators fura-2 and furaptra. In both cases, the peak of ΔF/F was found to maintain a linear relationship with the peak of d(ΔF/F)/dt, as shown in Figure 1B (slope = 0.98). Therefore, the peak ofΔF/F was chosen as the measure of [Capre]t, because it is less noisy than the first derivative of ΔF/F. As indicated by Figure1C, [Capre]t was not proportional to [Ca2+]o but was fairly linear with the logarithm of [Ca2+]o. The nonlinearity between [Capre]t and [Ca2+]o is not attributable to the saturation of Ca2+ indicators, because even in those experiments in which furaptra, an indicator with much lower Ca2+ affinity (KD = 50 μm) than fura-2 (KD = 200 nm), was loaded into terminals, [Capre]t also showed a similar relationship with [Ca2+]o. The mean reduction of [Capre]t measured with fura-2 was slightly less than that with furaptra for the same [Ca2+]o. This might be attributable to slight saturation of the fura-2 signal. However, a major advantage of fura-2 signals was their much higher signal-to-noise ratio, as compared with those obtained with furaptra; therefore, we used fura-2 for all further experiments, except where indicated.
Synaptic transmission also was shown to be steeply dependent on [Capre]t, revealing close to a fourth power relationship between presynaptic Ca2+ influx and transmitter release in both fura-2 and furaptra measurements. The calculated power numbers were 3.9 ± 0.5 (n = 8), 4.1 ± 0.5 (n = 8), and 4.3 ± 0.5 (n = 8) for 1.5, 1.0, and 0.5 mm[Ca2+]o, respectively, in the experiments with fura-2. With furaptra, the power numbers were 3.2 ± 0.6 (n = 10), 3.7 ± 0.4 (n = 10), and 4.0 ± 0.6 (n = 10) for 1.5, 1.0, and 0.5 mm [Ca2+]o, respectively. The average power numbers 4.2 (fura-2) and 3.8 (furaptra) were estimated from the slope of the regression lines, as revealed in a double logarithmic plot (Fig. 1D). Synaptic transmission was not significantly different between fura-2 and furaptra measurements, suggesting at most a minor interference of the Ca2+ indicators with the release process under our conditions. It is interesting to note that, with 0.5 mm[Ca2+]o, synaptic transmission was eliminated almost completely, whereas a residual [Capre]t, ∼50% of control, still could be detected (Fig. 1C).
Neuropeptide Y presynaptically inhibits synaptic transmission at the CA3–CA1 synapse
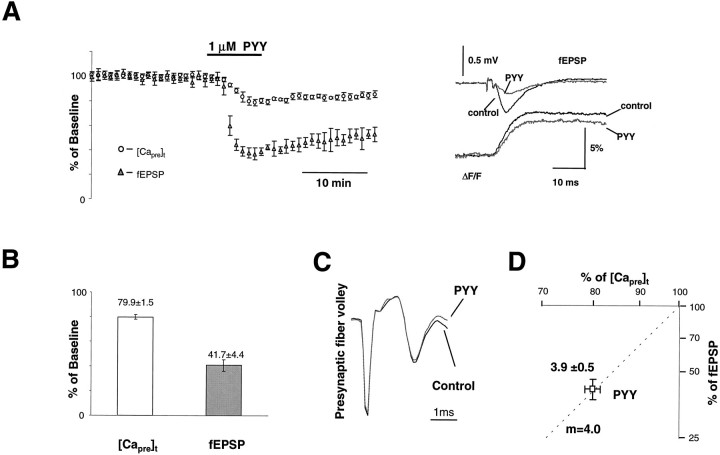
Application of hPYY3–36 (a C-terminal fragment of human PYY, which is a potent agonist at NPY Y2 receptors, abbreviated as PYY throughout) at 1 μm inhibited both [Capre]t and synaptic transmission. Figure2A shows the time course of both [Capre]t and the fEPSP of five experiments. On average, the [Capre]t was inhibited by 20.1 ± 1.5% (n = 8), whereas the corresponding fEPSP slope was reduced by 58.3 ± 4.4%. NPY (1 μm) produced similar, but slightly less potent, effects than PYY (data not shown). The power number calculated from the peak effects of PYY on [Capre]t and fEPSP wasm = 3.9 ± 0.5 (n = 8; Fig.2D). There was no significant change in either the size of the presynaptic fiber volley (Fig. 2C) or resting calcium concentration during the application of PYY (data not shown). The similarity in power numbers obtained by applying PYY or reducing [Ca]o are consistent with the hypothesis that inhibition of synaptic transmission by PYY is attributable mainly to a reduction of [Capre]t.
Fig. 2.
The activation of presynaptic Y2receptors inhibits [Capre]t and fEPSP.A, Group data (n = 5) showing the time course of normalized [Capre]t and fEPSP during the application of 1 μm PYY. Sample traces taken at control and during maximal action of PYY are shown in theinset. B, Summary data for eight experiments. Approximately 20% of [Capre]twas inhibited by application of 1 μm PYY. The synaptic transmission was reduced by ∼60%. C, The presynaptic volley did not show a significant change during the application of PYY.D, Double-log plot of normalized fEPSP and [Capre]t during the peak action of PYY. Estimated power number for the inhibition of synaptic transmission by PYY was 3.9 ± 0.5 (n = 8). Similar power numbers were observed in this preparation for the action of PYY and the reduction of [Ca2+]o.
Effects of calcium channel toxins on the inhibition of [Capre]t by PYY
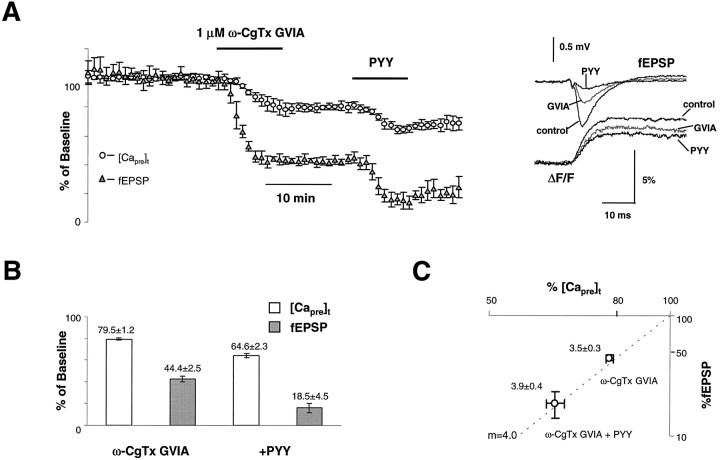
To determine which types of calcium channels are inhibited by Y2 receptors, we used ω-CgTx GVIA (N-type channel toxin) and ω-Aga IVA (P/Q-type channel toxin). If Y2 receptors act exclusively on N-type or P/Q-type calcium channels, these toxins would occlude the effects of PYY completely. Figure3A shows grouped data for six experiments. On average, 1 μm ω-CgTx GVIA inhibited [Capre]t and fEPSP to 79.5 ± 1.2% and 44.4 ± 2.5% of baseline, respectively (n = 7). The calculated power number for the inhibition of synaptic transmission by ω-CgTx GVIA was m = 3.5 ± 0.3. After preapplication of the toxin, PYY further reduced [Capre]t and fEPSP to 64.6 ± 2.3% and 18.5 ± 4.5% of baseline, respectively (n = 7). The power number for the combination of ω-CgTx GVIA and PYY wasm = 3.9 ± 0.4 (Fig. 3C). Compared with the inhibition of [Capre]t under control conditions (20.1%), 14.9% (79.5 − 64.6%) of baseline [Capre]t was inhibited by PYY after preapplication of ω-CgTx GVIA. Thus, ∼5.2% (20.1 − 14.9%) inhibition of [Capre]t by PYY was occluded by ω-CgTx GVIA. Normalized by the fraction of presynaptic N-type channels, the relative inhibition of N-type VDCCs by PYY was ∼25% (5.2%/20.5%).
Fig. 3.
ω-CgTx GVIA does not abolish the effects of PYY.A, Group data (n = 6) showing the time course of normalized [Capre]t and fEPSP during the application of ω-CgTx GVIA (1 μm) and PYY (1 μm). Sample traces taken at control, after application of ω-CgTx GVIA, and during the peak effect of PYY are shown in theinset. B, Summary data for seven experiments. After preapplication of ω-CgTx GVIA, PYY could still inhibit [Capre]t but to a lesser extent (∼15% of control [Capre]t), whereas fEPSP was decreased to 80% of control. C, Double-log plot of normalized fEPSP and [Capre]t during application of ω-CgTx GVIA and ω-CgTx GVIA+PYY. Estimated power numbers were m = 3.5 ± 0.3 (n = 7) for the inhibition by ω-CgTx GVIA alone and m = 3.9 ± 0.4 (n = 7) for the combination of ω-CgTx GVIA and PYY. The peak responses of ω-CgTx GVIA and PYY were used to calculate the power numbers.
Similarly, ω-Aga IVA was applied to test the possible involvement of P/Q-type calcium channels in the modulation of synaptic transmission by PYY. ω-Aga IVA (500 nm) reduced [Capre]t and fEPSP to 66.6 ± 1.8% and 18.1 ± 1.6% of control, respectively (n = 2), which resulted in a power number of m = 4.2 ± 0.1. Like ω-CgTx GVIA, ω-Aga IVA could not block the effect of PYY fully. After blockade of P/Q-type calcium channels, application of PYY still produced an inhibition of ∼15.2 ± 1.7% of control [Capre]t (n = 2; Fig.4A,C). Therefore, the percentage of inhibition of P/Q-type channels was estimated to be ∼15% [(20.1 − 15.2%)/33.4%].
Fig. 4.
ω-Aga IVA and ω-CgTx MVIIC do not abolish the effects of PYY. A, Group data showing the time course of normalized [Capre]t and fEPSP during the action of ω-Aga IVA (500 nm) alone and together with 1 μm PYY. Sample traces taken at control, after application of the calcium channel toxin, and during the peak effect of PYY are shown in the inset. B, Group data showing the time course of normalized [Capre]t and fEPSP during the action of PYY together with ω-CgTx MVIIC (1 μm). Sample traces taken at control, after application of calcium channel toxins, and during peak effect of PYY are shown in theinset. C, Summary data forA. [Capre]t and synaptic transmission were reduced irreversibly by ∼34 and 80%, respectively, after application of ω-Aga IVA. PYY still inhibited [Capre]t by ∼15% of control [Capre]t after blockade of P/Q-type calcium channels, and synaptic transmission also was decreased further.D, Approximately 11% inhibition of [Capre]t was observed even after application of ω-CgTx MVIIC to stop synaptic transmission almost completely.
Neither ω-CgTx GVIA nor ω-Aga IVA alone fully blocked the inhibition of [Capre]t by PYY. This indicates that PYY did not selectively affect either N-type or P/Q-type channels and suggests that multiple types of VDCCs were involved. To confirm this, we used another conotoxin, MVIIC, to block both N- and P/Q-type channels (Turner et al., 1995) to test whether this occluded the inhibition of [Capre]t by PYY. ω-CgTx MVIIC (1 μm) almost completely abolished synaptic transmission, whereas [Capre]t was reduced to ∼56% of control (n = 2; Fig. 4B). Application of PYY after ω-CgTx MVIIC elicited ∼11.1% inhibition of the total [Capre]t. Thus, ω-CgTx MVIIC occluded the inhibition of [Capre]t by PYY (9% = 20.1 − 11.1%) more than the application of either ω-CgTx GVIA (5.2% = 20.1 − 14.9%) or ω-Aga IVA (4.9% = 20.1 − 15.2%). This is consistent with an inhibition of both N- and P/Q-type calcium channels by activation of presynaptic NPY receptors.
It should be noted that the effect of ω-CgTx MVIIC might not have reached steady-state. This toxin should block both N-type (20.5%) and P/Q-type (33.4%) VDCCs and reduce the [Capre]t by ∼54% (20.5% + 33.4%). However, ω-CgTx MVIIC blocked only 44% of the [Capre]t, leaving ∼10% of the total [Capre]t, mediated by N- and P/Q-type, unblocked. This unblocked fraction potentially could contribute to the effect of PYY we observed with ω-CgTx MVIIC. Because the relative percentage of inhibition of N- and P/Q-type channels by PYY was estimated to be ∼25 and 15%, respectively, it is unreasonable to assume that the remaining 11.1% inhibition of total [Capre]t by PYY after application of ω-CgTx MVIIC was entirely attributable to the inhibition of unblocked N- and P/Q-type VDCCs. Instead, the inhibition of Ca2+channels other than N- and P/Q-type significantly contributed to the reduction of [Capre]t by PYY after the application of ω-CgTx MVIIC.
Actions of Y2 and adenosine receptors occlude one another in the inhibition of [Capre]t
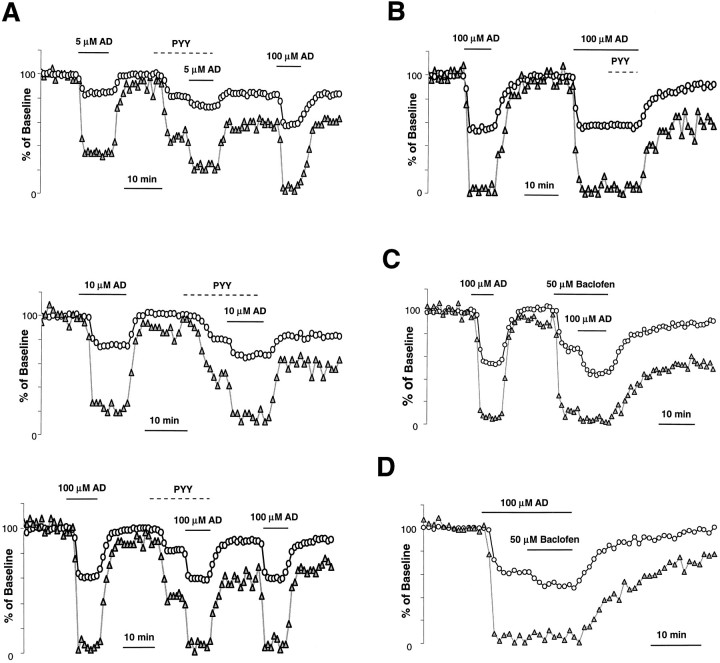
Similar to the effects of other neuromodulators, activation of NPY receptors has been shown to inhibit the [Capre]t. We have reported previously that adenosine and the muscarinic receptor agonist carbachol (CCh) mutually occlude one another in the inhibition of [Capre]t at this synapse (Qian and Saggau, 1997), suggesting a convergent action of different neuromodulators. A common type of G-protein may be the point of convergence in the inhibitory pathway from adenosine and muscarinic receptors to presynaptic VDCCs. We were interested to learn whether NPY receptors shared the same presynaptic transduction pathway as do receptors of other neuromodulators such as adenosine. We choose adenosine for this purpose because its inhibition of presynaptic calcium channels has been demonstrated at both the parallel fiber synapse of cerebellum (Dittman and Regehr, 1996) and the CA3–CA1 synapse of hippocampus, the present experimental preparation (Wu and Saggau, 1994a). Another advantage of using adenosine is its rapid and complete washout, which allows for multiple application of the drug. Results of experiments with coapplication of adenosine and PYY are shown in Figure5, A and B. PYY partially occluded the effect of adenosine in the inhibition of [Capre]t, regardless of the adenosine concentration. The inhibition of [Capre]t by adenosine and PYY was not additive. The occluding effect became more prominent when high does of adenosine were used: PYY had no detectable effect on [Capre]t in the presence of a saturating concentration (100 μm) of adenosine (Fig.5B). Such a complete occlusion strongly suggests convergence of the NPY pathway with the adenosine pathway. However, when baclofen, a GABAB receptor agonist, was coapplied with 100 μm adenosine, only a partial occlusion of [Capre]t inhibition was observed (Fig.5C,D). Because a saturating concentration of adenosine did not occlude the effect of baclofen completely, this indicates that only a partially shared pathway for these two neuromodulators exists at this synapse.
Fig. 5.
Activation of Y2 receptors occludes the inhibition of [Capre]t by adenosine.A, Time course of normalized [Capre]t and fEPSP illustrates the occlusion of inhibition of [Capre]t between activation of Y2 and adenosine (AD) receptors. The inhibition of [Capre]t by adenosine in the presence of PYY (1 μm) was always smaller than that without activation of Y2 receptors, regardless of the concentrations of adenosine (5, 10, or 100 μm).B, A saturating concentration of AD (100 μm) completely abolished the inhibition of [Capre]t by PYY. C,D, Time course of normalized [Capre]t and fEPSP illustrates the partial occlusion between the activation of AD receptors and the activation of GABAB receptors by baclofen (50 μm).
However, if the activation of Y2 receptors were to inhibit calcium channels via an unknown mechanism that depended on Ca2+ influx for its effect, then the reduction in Ca2+ influx caused by the activation of adenosine receptors in the above experiments also might prevent a further inhibition of [Capre]t by PYY. To rule out this possibility, we applied PYY after reducing [Capre]t by lowering [Ca]o to mimic the reduction in presynaptic Ca2+ influx caused by adenosine in the occlusion experiments above. The application of PYY in reduced [Ca2+]o elicited a similar percentage of inhibition of [Capre]tas in control conditions (23.5%; Fig.6), although presynaptic calcium influx was reduced to ∼56% of control. This is inconsistent with the Ca2+-dependent mechanism postulated.
Fig. 6.
PYY inhibits the same percentage of [Capre]t in reduced [Ca2+]o. Shown is the time course of normalized [Capre]t and fEPSP in reduced [Ca2+]o (0.7 mm). Application of PYY still elicited a 23.5% inhibition of [Capre]t under these conditions. Theinset shows the sample traces taken under control conditions (2.5 mm[Ca2+]o), in the presence of 0.7 mm [Ca2+]o, and during the peak effect of PYY.
DISCUSSION
Nonlinear relationship between presynaptic [Capre]t and synaptic transmission
Synaptic transmission is a nonlinear function of presynaptic calcium influx. This has been observed in many preparations, including the squid giant synapse (Katz and Miledi, 1970; Augustine and Charlton, 1986), the guinea pig hippocampal CA3–CA1 synapse (Wu and Saggau, 1994b), the cerebellar parallel fiber synapse (Mintz et al., 1995) and the calyx of Held (Borst et al., 1996). Postsynaptic responses can be described best by a power function of measured [Capre]t. A power number of 4 has been observed in most cases, whereas a power number of 2.5 has been reported at the parallel fiber synapse of rat cerebellum (Mintz et al., 1995) and a retinotectal synapse in the frog (Feller et al., 1996). This estimated power number indicates that the release of neurotransmitter is a process that involves multiple calcium ions. Whether or not the estimated apparent power number can be inferred to the number of calcium binding sites depends on how precisely the calcium concentration near the release sites is proportional to the total calcium influx into the presynaptic terminal that leads to the measured [Capre]t. The relative location of calcium channels to the calcium sensor of release machinery can greatly influence the apparent power number that was measured. This might be the reason for the reported difference in power number between N- and P/Q-type channels in the cerebellum (Mintz et al., 1995). Comparative experimental and modeling studies at the same synapse indicate that the difference in the affinity of the calcium indicator used to measure [Capre]t cannot explain the large difference in apparent power numbers found at the above synapses (Sinha et al., 1997). The present observations from the rat CA3–CA1 synapse confirmed those results, although furaptra gave a slightly smaller power number than fura-2 (see Fig. 1D). Although the [Capre]t measured by Ca2+indicators represents a population average in our preparation, a power number of ∼4 still was observed when [Capre]t was varied by the reduction of [Ca2+]o, the application of calcium channel toxins, or the activation of Y2 receptors. This is similar to the effects of activating presynaptic adenosine, GABAB, or muscarinic receptors, as reported earlier (Wu and Saggau, 1994a, 1995; Qian and Saggau, 1997) (for review, see Wu and Saggau, 1997).
Multiple types of calcium channels are involved in the synaptic transmission at the CA3–CA1 synapse of hippocampus
ω-CgTx GVIA-sensitive (N-type) and ω-Aga IVA-sensitive (P/Q-type) calcium channels are involved in synaptic transmission at the CA3–CA1 synapse (Wheeler et al., 1994b; Wu and Saggau, 1994c). Based on the observation of their supra-additive block of synaptic transmission, a presynaptic colocalization of N- and P/Q-type calcium channels was proposed (Wu and Saggau, 1994c). Our results are consistent with those observations and also suggest that, besides N- and P/Q-type channels, unidentified types of calcium channels, resistant to both ω-CgTx GVIA and ω-Aga IVA, also are significantly involved in the process of transmitter release. We observed a fourth power relationship between [Capre]t and fEPSP by reducing [Ca2+]o, which decreases calcium influx equally for all channel types (N-, P/Q-, and those resistant to toxins). If the toxin-resistant types were not involved in transmitter release, then the power numbers for both N- and P/Q-type would be significantly lower than 4. On the other hand, 0.5 mm [Ca2+]o practically abolished transmitter release, while ∼50% [Capre]t remained. A similar amount of [Capre]t (56%) remained after the application of ω-CgTx MVIIC, which completely inhibited transmitter release, although the toxin specifically blocks only N- and P/Q-type channels. Moreover, we show here that the inhibition of transmitter release by PYY is attributable to the reduction of [Capre]t, and PYY also was shown to inhibit ω-CgTx MVIIC-insensitive Ca2+ channels. This suggests that ω-CgTx MVIIC-insensitive Ca2+channels also are involved in triggering transmitter release.
The hypothesis that other types of calcium channels are involved in triggering transmitter release, besides the N- and P/Q-types, cannot be tested directly, because channel blockers specific for those ω-CgTx GVIA- and ω-Aga IVA-resistant VDCCs are not available. The K+-channel blocker, 4-AP, which is presumed to prolong the presynaptic action potential and thus to increase [Capre]t, recently was used to assess this hypothesis indirectly (Wheeler et al., 1996). In these experiments 4-AP was shown to potentiate synaptic transmission and to shift the transmission curve in the direction of a reduced [Ca2+]o, i.e., lower [Ca2+]o is required to maintain the same amount of transmitter release in the presence of 4-AP. The effective blockade of synaptic transmission by the combined application of ω-CgTx GVIA and ω-Aga IVA even in the presence of 4-AP led these authors to the conclusion that the possible involvement of VDCCs other than N- and P/Q-type to play a role in triggering transmitter release is restricted and that crucial tests have to wait until better tools become available to study these toxin-insensitive channel types separately. Our findings, however, suggest that a simple linear extrapolation of [Capre]t from [Ca2+]o might overestimate the amount of increase in [Capre]t because of the nonlinear relationship between [Capre]t and [Ca2+]o, especially under the conditions of high [Ca2+]o (see Fig.1C). Thus, it is necessary to measure the [Capre]t directly to prove that 4-AP can compensate sufficiently for the reduction of [Capre]t caused by the blockade of N- and P/Q-type channels. If 4-AP could not compensate for this reduction of [Capre]t to values above threshold for transmitter release, then a block of synaptic transmission by the combination of ω-CgTx GVIA and ω-Aga IVA would be anticipated, even in the presence of 4-AP.
Modulation of calcium channels by activation of presynaptic Y2 receptors
Activation of presynaptic Y2 receptors reduced both [Capre]t and synaptic transmission. During application of PYY the power relationship between [Capre]t and fEPSP was 3.9, compared with 4.2 when [Ca2+]o was reduced. This suggests that the inhibition of synaptic transmission by PYY is attributable mainly to the reduction of [Capre]t, and there is no evidence to support the involvement of an additional mechanism downstream of Ca2+ entry. Were the latter the case, then the power number observed would be greater than that obtained by manipulating [Capre]t. Not ω-CgTx GVIA, ω-Aga IVA, nor ω-CgTx MVIIC could abolish fully the inhibition of [Capre]t by PYY. The sum of the inhibitions by PYY after application of ω-CgTx GVIA and ω-Aga IVA, respectively, was larger than the inhibition of [Capre]t by PYY observed without any toxins. These data suggest that other types of VDCCs besides N-type and P/Q-type contribute to the inhibition of [Capre]t by PYY. The estimated percentages of inhibitions are 25, 15, and 21% for N-type, P/Q-type, and unidentified calcium channels, respectively.
Several lines of evidence suggest that PYY inhibits calcium channels rather than activates K+ channels in presynaptic terminals. First, the presynaptic fiber volley did not change significantly during application of PYY. Second, the differential inhibition for different types of calcium channels observed in our experiments is inconsistent with a reduction in duration of the presynaptic action potential that would accompany an activation of K+ channels. Such a shortening of the depolarization of presynaptic terminals would be expected to affect all types of VDCCs equally (Wheeler et al., 1996). Third, the occlusion of PYY-induced inhibition by adenosine that we observed is also inconsistent with an involvement of K+ channels. Furthermore, if activation of Y2 receptors were to open K+ channels and thus shorten the action potential, this should reduce the [Capre]t regardless of the type or number of VDCCs inhibited by the previous application of adenosine. This was not observed when a saturating concentration of adenosine was used. Previous studies revealed no evidence for adenosine action on presynaptic K+ channels at this synapse (Wu and Saggau, 1994a). Finally, the involvement of K+ channels in the modulation of synaptic transmission by NPY was tested previously at this synapse by Klapstein and Colmers (1992). Their observation that 4-AP could not reduce the inhibition of synaptic transmission by NPY is consistent with our conclusion that PYY acts on calcium channels and not on K+ channels.
A common G-protein may be the convergent point of inhibition of [Capre]t by Y2 and adenosine receptors
Y2 and adenosine receptors occlude each other’s inhibition of [Capre]t, as shown in Figure 5. A similar mutual occlusion between activation of muscarinic and adenosine receptors also has been observed at this synapse (Qian and Saggau, 1997). In sympathetic neurons it has been proposed that adenosine, NPY, and muscarinic receptors all act convergently on the same G-protein, Go, to inhibit N-type calcium channels in these cells (Hille, 1994). The mutual occlusion of their inhibition of [Capre]t that we observed at the CA3–CA1 synapse would be consistent with this hypothesis and further suggests that a similar mechanism may underlie the inhibition of presynaptic VDCCs by adenosine and muscarinic and NPY receptors in the CNS. This is in contrast to baclofen, a GABAB receptor agonist, which, when applied at a saturating concentration together with a saturating concentration of adenosine, resulted in a nonoccluding inhibition of [Capre]t (Fig.5C,D). This indicates that the complete occlusion of inhibition of [Capre]t among adenosine, NPY, and muscarinic receptors is not attributable to either a limitation of experimental methods or a saturation of the inhibitory process at the presynaptic terminal (such as insufficient available G-proteins). Rather, it suggests that inhibition of presynaptic VDCCs by GABAB receptors is attributable at least in part to a mechanism different from the one activated by adenosine and muscarinic and NPY receptors.
Studies of presynaptic inhibition by NPY and the other neuromodulators suggest that modulation of presynaptic Ca2+ channels by G-protein-coupled receptors acts as a fast, short-term mechanism to control transmitter release. Our results support the hypothesis that a common mechanism underlies the inhibitory effect of different neuromodulators at the presynaptic site. Such a common mechanism would greatly improve the efficiency of intracellular signaling pathway in controlling transmitter release.
Footnotes
This work was supported by National Institutes of Health Grant NS-33147 to P.S. and Medical Research Council (Canada) Grant MT-10520 to W.F.C. We acknowledge Dr. Yvan Dumont for the gift of PYY. We also thank Dr. N. A. Saccomano at Pfizer Incorporated, Groton, CT, for generously supplying samples of ω-Aga IVA. The computer software for data acquisition and analysis was developed by Dr. S. S. Patel. We thank S. Sinha for critical and helpful comments on this manuscript.
Correspondence should be addressed to Dr. Peter Saggau, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030.
REFERENCES
- 1.Augustine GJ, Charlton MP. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol (Lond) 1986;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 3.Campbell V, Berrow TS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G-protein G(o) with calcium channels by the calcium channel beta-subunit in rat neurones. J Physiol (Lond) 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colmers WF, Lukowiak K, Pittman QJ. Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampal slice. J Physiol (Lond) 1987;383:285–299. doi: 10.1113/jphysiol.1987.sp016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y action in the rat hippocampal slices: site and mechanism of presynaptic inhibition. J Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont Y, Fournier A, St-Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont Y, Fournier A, St-Pierre S, Quirion R. Autoradiographic distribution of [125I][Leu31,Pro34] PYY and [125I]PYY3–36 binding sites in the rat brain evaluated with two newly developed Y1 and Y2 receptor radioligands. Synapse. 1996;22:139–158. doi: 10.1002/(SICI)1098-2396(199602)22:2<139::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Dunlap K, Luebke J, Turner T. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 10.Feller MB, Delaney KR, Tank DW. Presynaptic calcium dynamics at the frog retinotectal synapse. J Neurophysiol. 1996;76:381–400. doi: 10.1152/jn.1996.76.1.381. [DOI] [PubMed] [Google Scholar]
- 11.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 12.Katz B, Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol (Lond) 1970;207:789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klapstein GJ, Colmers WF. 4-Aminopyridine and low Ca2+ differentiate presynaptic inhibition mediated by neuropeptide-Y, baclofen, and 2-chloroadenosine in rat hippocampal CA1 in vitro. Br J Pharmacol. 1992;105:470–474. doi: 10.1111/j.1476-5381.1992.tb14277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuiston AR, Colmers WF. Neuropeptide Y-2 receptors inhibit the frequency of spontaneous but not miniature EPSCs in CA3 pyramidal cells of rat hippocampus. J Neurophysiol. 1996;76:3159–3168. doi: 10.1152/jn.1996.76.5.3159. [DOI] [PubMed] [Google Scholar]
- 15.Milner TA, Veznedaroglu E. Ultrastructure localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus. 1992;2:107–126. doi: 10.1002/hipo.450020204. [DOI] [PubMed] [Google Scholar]
- 16.Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 17.Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988;241:545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- 18.Pickel VM, Chan J, Veznedaroglu E, Milner TA. Neuropeptide Y and dynorphin-immunoreactive large dense-core vesicles are strategically localized for presynaptic modulation in the hippocampal formation and substantia nigra. Synapse. 1995;19:160–169. doi: 10.1002/syn.890190303. [DOI] [PubMed] [Google Scholar]
- 19.Qian J, Saggau P. Presynaptic inhibition of evoked synaptic transmission in the rat hippocampus by activation of muscarinic receptors: involvement of presynaptic calcium influx. Br J Pharmacol. 1997;122:511–519. doi: 10.1038/sj.bjp.0701400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha SR, Wu LG, Saggau P. Presynaptic calcium dynamics and transmitter release evoked by single action potentials at mammalian central synapses. Biophys J. 1997;72:637–651. doi: 10.1016/s0006-3495(97)78702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, Forsythe ID, Tsujimoto T, Barnes Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 23.Toth PT, Bindokas V, Bleakman D, Colmers WF, Miller RJ. Mechanism of presynaptic inhibition by neuropeptide Y at sympathetic nerve terminals. Nature. 1993;364:635–639. doi: 10.1038/364635a0. [DOI] [PubMed] [Google Scholar]
- 24.Turner TJ, Lampe RA, Dunlap K. Characterization of presynaptic calcium channels with omega-conotoxin MVIIC and omega-grammotoxin SIA: role for a resistant calcium channel type in neurosecretion. Mol Pharmacol. 1995;47:348–353. [PubMed] [Google Scholar]
- 25.Wheeler DB, Sather WA, Randall A, Tsien RW. Distinctive properties of a neuronal calcium channel and its contribution to excitatory synaptic transmission in the central nervous system. Adv Second Messenger Phosphoprotein Res. 1994a;29:155–171. doi: 10.1016/s1040-7952(06)80014-5. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994b;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994a;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 29.Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994b;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu LG, Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3–CA1 synapses of the hippocampus. J Neurosci. 1994c;14:5613–5622. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J Physiol (Lond) 1995;485:649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]