Abstract
Brain angiotensin II (Ang II) inhibits pituitary prolactin release by an indirect mechanism requiring stimulation of dopamine formation and release. We report that [125I]Sar1–Ang II binding to AT1 receptors and AT1A receptor mRNA expression increase selectively in the dorsomedial arcuate nucleus of 17β-estradiol-primed ovariectomized rats after treatment with progesterone. In hormone-treated rats, arcuate nucleus AT1Areceptor mRNA expression is associated with tyrosine hydroxylase-positive neurons. No AT1A receptor mRNA was detected in tyrosine hydroxylase-positive cells of the arcuate nucleus of intact male rats. Conversely, in the anterior pituitary, where local or circulating Ang II stimulates prolactin release, [125I]Sar1–Ang II binding to AT1 receptors and AT1B receptor mRNA expression are decreased in 17β-estradiol/progesterone-treated ovariectomized rats.
Thus, AT1A receptors in the dorsal arcuate nucleus and AT1B receptors in the anterior pituitary are regulated inversely by estrogen/progesterone treatment, supporting the hypothesis of a dual role for brain and pituitary Ang II on prolactin release. The colocalization of AT1A receptor mRNA and tyrosine hydroxylase in neurons of the arcuate nucleus furthermore indicates that within this area central Ang II acts directly on dopaminergic neurons. These results support the hypothesis that central Ang II inhibits pituitary prolactin release indirectly via modulation of dopaminergic activity in the arcuate nucleus.
Keywords: angiotensin II receptors, catecholamines, tyrosine hydroxylase, in situ hybridization, anterior pituitary, prolactin
Angiotensin II (Ang II) is produced by many tissues, including the brain and pituitary gland, where the peptide plays a role in the control of reproductive hormones (Saavedra, 1992). In the anterior pituitary, Ang II is synthesized locally and stimulates prolactin release (Steele et al., 1981; Aguilera et al., 1982; Schramme and Denef, 1983; Ganong et al., 1989; Jones et al., 1990; Steele and Myers, 1990; Thomas and Sernia, 1990). Ang II also participates in brain mechanisms controlling pituitary prolactin release, but here its role is inhibitory rather than stimulatory (Myers and Steele, 1989, 1991).
There is evidence that the regulation of pituitary and brain Ang II receptors is important in the control of prolactin secretion and that the Ang II receptor expression in specific brain areas and in the anterior pituitary is under the control of reproductive hormones. Treatment of estrogen-primed ovariectomized (OVX) rats with progesterone upregulates Ang II receptors in selective brain areas involved in the inhibition of pituitary prolactin release, whereas estrogen treatment downregulates the expression of Ang II receptors in the anterior pituitary (Chen and Printz, 1983; Carriére et al., 1986; Seltzer et al., 1992, 1993).
Ang II receptors have been classified pharmacologically into two subtypes, AT1 and AT2 receptors (Timmermans et al., 1995). On the basis of quantitative autoradiography with displacement of [125I]Sar1–Ang II binding by AT1 but not AT2receptor-selective ligands, we reported the presence of AT1receptors in the anterior pituitary and in all brain areas related to the central control of pituitary function (Tsutsumi and Saavedra, 1991a,b). AT1 receptor subtypes have been subdivided further by the cloning of AT1A and AT1Breceptors, which are highly homologous and encoded by two distinct genes (Elton et al., 1992; Iwai and Inagami, 1992; Kakar et al., 1992;Sandberg et al., 1992). Because it is not possible to differentiate between AT1A and AT1B receptors by using binding studies (Timmermans et al., 1995), we subcloned fragments from the 3′ noncoding regions of AT1A, AT1B, and AT2 receptor cDNAs to produce receptor subtype-specific riboprobes (Jöhren et al., 1995b). No significant homology exists among the noncoding regions of Ang II receptor cDNAs (Iwai and Inagami, 1992; Inagami et al., 1994). In situ hybridization experiments using these probes showed that in adult rats the AT1A receptor subtype predominates in brain areas involved in pituitary function, whereas the AT1Breceptor subtype is expressed in the anterior pituitary (Jöhren et al., 1995b; Jöhren and Saavedra, 1996).
We asked the question of whether reproductive hormones controlled not only the expression of the Ang II receptor protein but also the expression of receptor mRNA. We studied this question with quantitative receptor autoradiography and in situ hybridization histochemistry in ovariectomized (OVX) rats treated with estrogen and progesterone. Because central Ang II has been proposed to inhibit prolactin secretion by stimulating the synthesis and/or release of dopamine (Steele, 1992), we attempted to clarify how Ang II receptors within the dorsal arcuate nucleus are associated with dopamine-producing neurons.
MATERIALS AND METHODS
Animals and tissue preparation. All animal procedures were approved by the National Institute of Mental Health Animal Care and Use Committee. Intact male (200–250 gm) and ovariectomized (OVX) female (150–200 gm) Sprague Dawley rats were obtained from Zivic-Miller (Zelienople, PA). Rats were provided with standard food and water ad libitum and kept under a 12 hr/12 hr light/dark cycle. Two weeks after surgery female rats were divided into two groups (OVX plus estradiol/progesterone replacement and OVX plus placebo). Hormones were given in the form of slow-release pellets designed to deliver a constant dose of hormone over at least 21 d (Innovative Research of America, Sarasota, FL). In the first group each rat received one pellet of 17β-estradiol (0.05 mg/pellet) at 10:00 A.M. (Day 0) placed subcutaneously in the interscapular region. This replacement dose of estrogen (0.05 mg/pellet) has been shown to result in 17β-estradiol levels of <50 pg/ml (Seltzer et al., 1992). In the second group each rat received one placebo pellet (Innovative Research of America). On day 2 at 10:00 A.M., each rat of the estradiol-treated group received one pellet of progesterone (Innovative Research of America; 50 mg/pellet, 21 d release, s.c.), and each rat of the placebo group received a second placebo pellet. The selected dose for 17β-estradiol and progesterone results in serum hormone levels within the physiological range (Butcher et al., 1974; Barron et al., 1986;Arbogast and Voogt, 1993; Brann et al., 1993; Michels et al., 1993). All pellets were implanted under ketamine anesthesia (150 mg/kg ketamine-HCl and 15 mg/kg azepromazine maleate, i.p.). Rats were decapitated between 3:00 and 4:00 P.M. on day 3. It has been shown before that this timing of hormone administration decreases plasma prolactin levels and increases arcuate nucleus dopamine turnover on the afternoon of day 3 (Rance et al., 1981).
Brains and pituitary glands were removed immediately, frozen by immersion in isopentane at −30°C, and stored at −80°C. For colocalization studies, rats were anesthetized with ketamine and perfused transcardially with 100 ml of saline, followed by 200 ml of 4% paraformaldehyde in PBS. Brains were removed, post-fixed for 24 hr in 4% paraformaldehyde/PBS at 4°C, and incubated overnight at 4°C in PBS containing 18% sucrose. Brains were frozen by immersion in isopentane at −30°C and stored at −80°C.
Consecutive 16-μm-thick coronal sections of brains and pituitaries were cut at −20°C in a cryostat. Alternate sections were collected for receptor binding and in situ hybridization. For receptor autoradiography, sections were thaw-mounted on gelatin-coated glass slides, dried overnight in a desiccator at 4°C, and stored at −80°C. For in situ hybridization and immunohistochemistry, sections were thaw-mounted on silanated glass slides (Digene Diagnostics, Beltsville, MD) and stored at −80°C.
Quantitative receptor autoradiography.Sar1–Ang II (Peninsula Laboratories, Belmont, CA) was iodinated by New England Nuclear (Boston, MA) to a specific activity of 2200 Ci/mmol. Receptor binding was performed as described (Tsutsumi and Saavedra, 1991a). Briefly, sections were preincubated for 15 min at 22°C in 10 mm sodium phosphate buffer, pH 7.4, containing 120 mm NaCl, 5 mmNa2EDTA, 0.005% bacitracin, and 0.2% proteinase-free BSA, and then incubated for 120 min in fresh buffer containing 5 × 10−10m[125I]Sar1–Ang II (total binding). Nonspecific binding was determined by incubation in the presence of 5 × 10−6m unlabeled Ang II. To characterize Ang II receptor subtypes, we incubated consecutive sections with [125I]Sar1–Ang II in the presence of 10−5m losartan (DuPont Merck, Wilmington, DE), an AT1 receptor antagonist, or 10−7m CGP 42112 (Neosystem SA, Strasbourg, France), an AT2 receptor-selective ligand. The number of AT1 receptors was determined as the specific binding displaced by unlabeled losartan. The number of AT2receptors was determined as the specific binding displaced by unlabeled CGP 42112. After incubation, slides were washed four times in ice-cold 50 mm Tris-HCl, pH 7.6, dried, and exposed for several days to Hyperfilm-3H (Amersham, Arlington Heights, IL). [125I]Sar1–Ang II binding was quantified by measuring optical densities on a Macintosh computer, using the public domain National Institutes of Health Image program (developed at National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Optical densities were transformed to corresponding values of femtomoles per milligram of protein (Nazarali et al., 1989).
In situ hybridization histochemistry. We used AT1A, AT1B, and AT2receptor subtype-specific riboprobes (Jöhren et al., 1995b, 1996;Jöhren and Saavedra, 1996). Therefore, fragments from the 3′ noncoding regions of the rat AT1A receptor cDNA (corresponding to nucleotides 1316–1684; Murphy et al., 1991), rat AT1B receptor cDNA (corresponding to nucleotides 1445–1841; Sandberg et al., 1992), and rat AT2 receptor cDNA (corresponding to nucleotides 1467–1838; Kambayashi et al., 1993) were subcloned into the polylinker site of the pBluescript II KS(+) vector (Stratagene, La Jolla, CA).
Antisense and sense (control) probes were labeled to a specific activity of 1.28 × 109 dpm/μg by in vitro transcription, using the TransProbe T Kit (Pharmacia Biotech, Piscataway, NJ). Transcription was performed according to the manufacturer’s protocol in the presence of 200 μCi of [35S]UTP (800 Ci/mmol, Amersham), 1 μg of linearized plasmid, and T3 or T7 RNA polymerase. Labeled riboprobes were separated from unincorporated [35S]UTP by centrifugation through Nick spin columns (Pharmacia).
In situ hybridization was performed as described previously (Jöhren et al., 1996). Sections of rat adrenal gland were included in the hybridization experiments as a positive control, because all antisense riboprobes (AT1A, AT1B, and AT2) used in this study have been shown to hybridize specifically to sections of rat adrenal gland (Jöhren et al., 1995b). Sections were fixed in 4% paraformaldehyde for 10 min, rinsed twice in PBS, acetylated for 10 min in 0.1 m triethanolamine HCl, pH 8.0, containing 0.25% acetic anhydride, dehydrated in alcohols, and air-dried. Each section was covered with 50 μl of hybridization buffer containing 50% formamide, 0.3 m NaCl, 2 mm EDTA, 20 mm Tris, pH 8.0, 1× Denhardt’s solution, 10% dextran sulfate, 100 μg/ml salmon sperm DNA, 250 μg/ml yeast RNA, 250 μg/ml yeast tRNA, 100 mm DTT, 0.1% SDS, and 10 ng/ml sense or antisense probe. After hybridization for 18 hr at 54°C, sections were rinsed several times in 4× SSC (1× SSC is 0.15m sodium chloride and 0.015 m sodium citrate). Nonhybridized probes were digested by incubation with 40 μg/ml RNase A (Sigma, St. Louis, MO) for 30 min. After a final high-stringency wash in 0.1× SSC at 65°C for 60 min, sections were dehydrated in alcohols containing 0.3 m ammonium acetate and air-dried.
Sections were exposed to Hyperfilm-3H (Amersham) for 8–14 d. Films were developed in D-19 developer (Eastman Kodak, Rochester, NY) for 4 min at 0°C and fixed in rapid fixer (Eastman Kodak) for 4 min at 22°C. Slides were dipped in Kodak NTB2 photo emulsion, exposed for 4–6 weeks, developed in Kodak D-19 developer for 3 min at 15°C, fixed for 4 min, and counterstained with toluidine blue. The location of silver grains was analyzed by microscopic examination of the sections. For semiquantitative analysis of placebo-treated OVX rats and estrogen/progesterone-treated OVX rats, silver grains were counted at high (1000×) magnification. A cell was considered positive if, after hybridization with AT1A, AT1B, and AT2 receptor antisense probes, it contained more than two times the number of silver grains than those present over cells after hybridization with nonspecific sense probes (background). The background counted in adjacent sections was less than four silver grains per cell. In the arcuate nucleus, silver grains were counted in all positive neurons present in each section, usually 10–20 neurons. In the anterior pituitary, microscopic fields were preselected randomly within each section at low (25×) magnification. Silver grains over all positive cells within the preselected field were counted at high magnification (1000×). All groups were coded for blind analysis. Comparison of mean values was performed by ANOVA, followed by the Tukey–Kramer multiple comparisons test.
Colocalization of AT1A receptor mRNA and tyrosine hydroxylase. For colocalization studies, in situhybridization for AT1A receptors was combined with immunohistochemical staining for tyrosine hydroxylase, the first and rate-limiting enzyme in the biosynthesis of catecholamine neurotransmitters. Sections of perfusion-fixed brains were washed for 5 min in 2× SSC, incubated for 20 min in 1 μg/ml proteinase K (Boehringer Mannheim, Indianapolis, IN), and post-fixed for 5 min in 4% paraformaldehyde. The labeling of probes and the hybridization procedure were identical to those described above. After the last washing step in 0.1× SSC, sections were processed for immunohistochemistry as described before (Jöhren et al., 1995a).
To detect tyrosine hydroxylase-positive cells, we used a monoclonal antibody (clone TH-2) purchased from Sigma. The primary antibody was diluted 1:1000 in Tris-buffered saline, pH 7.6, containing 0.1% BSA, and sections were incubated with the primary antibody for 60 min at 37°C. Positive staining was detected by the avidin–biotin complex (ABC) method (Hsu et al., 1981) with a biotinylated secondary antibody and peroxidase-conjugated streptavidin, using the DAKO LSAB Kit (DAKO, Carpinteria, CA) according to the manufacturer’s protocol. The chromagen was 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma). As a negative control, sections were processed for immunostaining in the absence of the first antibody. In these control experiments no neuronal staining was observed in the arcuate nucleus (see Fig. 10C). Furthermore, the specificity of the tyrosine hydroxylase antibody was evaluated by the specific localization of tyrosine hydroxylase-positive neurons in catecholaminergic areas like the A1, A2, A11, A12 (arcuate nucleus), and A13 (zona incerta) cell groups (zona incerta is shown in Fig. 9A).
Fig. 10.
Bright-field (A) and dark-field (B) photomicrographs showing tyrosine hydroxylase-like immunoreactivity (arrows) and the absence of AT1A receptor mRNA (B) in neurons of the arcuate nucleus of male rats. Sections were double-labeled for AT1A receptor mRNA and tyrosine hydroxylase-like immunoreactivity. No immunoreactivity was observed in the arcuate nucleus after immunostaining of an adjacent section without the first antibody against tyrosine hydroxylase (C). B is a dark-field view ofA. 3V, Third ventricle. Scale bar, 100 μm (applies to all panels).
Fig. 9.

Photomicrographs showing tyrosine hydroxylase-like immunoreactivity and AT1A receptor mRNA in the A13 cell group (A, B) and the lateral hypothalamus (C, D) of estrogen/progesterone-treated rats. Sections were double-labeled for AT1A receptor mRNA and tyrosine hydroxylase-like immunoreactivity. Note the presence of scattered AT1A receptor mRNA-expressing neurons in the lateral hypothalamus (arrows). B andD are dark-field views of A andC, respectively. ZI, Zona incerta;LH, lateral hypothalamus. Scale bar, 100 μm (applies to all panels).
RESULTS
Regulation of [125I]Sar1–Ang II binding by estrogen/progesterone
Quantitative receptor autoradiography of sections from the hypothalamus and pituitary of placebo-treated OVX rats revealed specific binding of [125I]Sar1–Ang II in the median eminence, lateral hypothalamus (Fig.1), and anterior pituitary (Fig.2). In the dorsomedial arcuate nucleus of placebo-treated OVX rats, binding of [125I]Sar1–Ang II was very low and not detected in other ventral or lateral parts of the arcuate nucleus (Fig. 1A). In all brain areas, as well as in the pituitary gland, specific [125I]Sar1–Ang II binding was totally displaced by the AT1 receptor antagonist losartan (Figs. 1C, 2C), but not by the AT2receptor ligand CGP 42112 (Figs. 1E,2E). Thus, all specific [125I]Sar1–Ang II binding can be assigned to AT1 receptors (Fig.3).
Fig. 1.
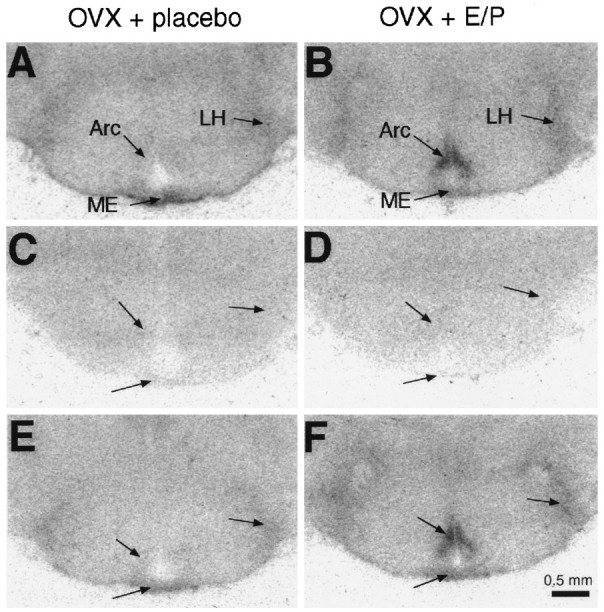
Film autoradiographs of coronal brain sections showing [125I]Sar1–Ang II binding in the arcuate nucleus (Arc), the lateral hypothalamus (LH), and the median eminence (ME) of placebo-treated OVX rats (A, C, E) and estrogen/progesterone-treated OVX rats (B, D, F). Brain sections were incubated with 5 × 10−10m [125I]Sar1–Ang II alone (A, B; total binding), or they were incubated in the presence of 10−5m the AT1 receptor antagonist losartan (C, D) or 10−7m the AT2 receptor ligand CGP 42112 (E, F).
Fig. 2.

Film autoradiographs of sections from pituitary showing [125I]Sar1–Ang II binding in the anterior lobe of the pituitary (APit) of placebo-treated OVX rats (A, C, E) and estrogen/progesterone-treated OVX rats (B, D, F). Sections were incubated with 5 × 10−10m [125I]Sar1–Ang II alone (A, B; total binding), or they were incubated in the presence of 10−5m the AT1 receptor antagonist losartan (C, D) or 10−7m the AT2 receptor ligand CGP 42112 (E, F). PPit, Posterior lobe of the pituitary.
Fig. 3.
Quantitative analysis of Ang II receptor subtypes in the arcuate nucleus, the median eminence, the lateral hypothalamus, and the anterior pituitary of placebo-treated OVX rats and estrogen/progesterone-treated OVX rats. Shown is the mean ± SEM obtained by quantitative autoradiography from six rats per group.specific, Amount of total [125I]Sar1–Ang II binding displaced by 5 × 10−6m unlabeled Ang II (specific binding); AT1, amount of total binding displaced by 10−5m losartan; *p ≤ 0.001 (arcuate nucleus) or p ≤ 0.01 (anterior pituitary).
Estrogen/progesterone treatment of OVX rats produced a fivefold increase in [125I]Sar1–Ang II binding in the dorsomedial arcuate nucleus, when compared with placebo-treated OVX rats (Figs. 1B, 3). As in placebo-treated OVX rats, all [125I]Sar1–Ang II binding in the dorsomedial arcuate nucleus of estrogen/progesterone-treated OVX rats was displaced by losartan (Fig. 1D), but not by CGP 42112 (Fig. 1F). No difference in [125I]Sar1–Ang II binding was detected in the median eminence and the lateral hypothalamus between placebo-treated OVX rats and estrogen/progesterone-treated OVX rats (Fig. 3). In the anterior pituitary, estrogen/progesterone treatment of OVX rats decreased [125I]Sar1–Ang II binding by 60% (Figs. 2B, 3). Again, all [125I]Sar1–Ang II binding in the anterior pituitary of estrogen/progesterone-treated OVX rats was displaced by losartan (Fig. 2D), but not by CGP 42112 (Fig. 2F), and therefore represents binding to AT1 receptors (Fig. 3).
Regulation of AT1 receptor mRNA expression by estrogen/progesterone
To identify the AT1 receptor subtype (AT1Aor AT1B) involved and to address the question of whether estrogen/progesterone treatment affects AT1receptor mRNA levels, we performed in situ hybridization in sections taken from brain and pituitary adjacent to the ones used for the binding experiments. Sections of placebo-treated OVX rats and estrogen/progesterone-treated OVX rats were hybridized with AT1A and AT1B receptor antisense probes or with AT1A receptor sense probes that served as a negative control. In all brain areas studied and in the anterior pituitary, hybridization with AT1A receptor sense probes resulted in a low background signal (Figs.4D,6D). The background signal was usually less than four silver grains per cell (Fig. 5).
Fig. 4.
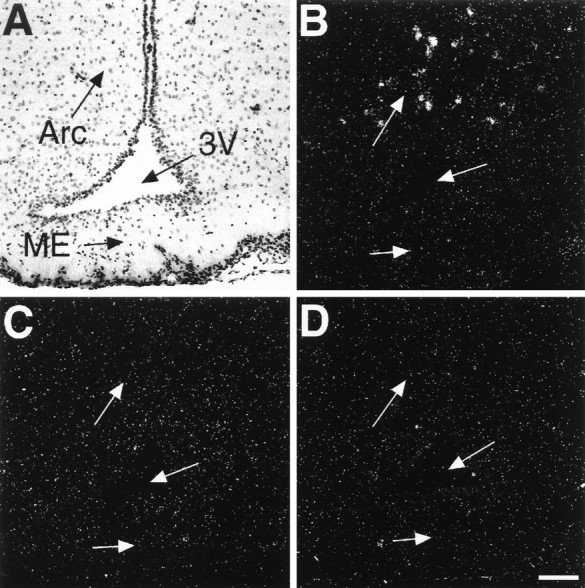
Bright-field (A) and dark-field (B–D) photomicrographs taken from adjacent coronal brain sections through the arcuate nucleus of estrogen/progesterone-treated OVX rats after in situhybridization with AT1A receptor-specific (A, B) and AT1Breceptor-specific (C) antisense probes or with control AT1A receptor sense probes (D). B is a dark-field view ofA. Arc, Arcuate nucleus;ME, median eminence; 3V, third ventricle. Scale bar, 100 μm (applies to all panels).
Fig. 6.
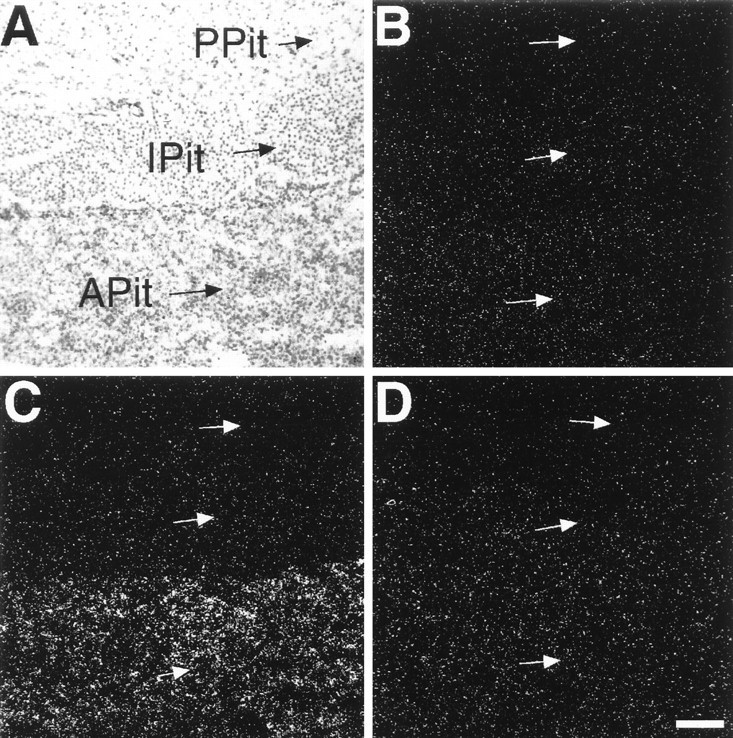
Bright-field (A) and dark-field (B–D) photomicrographs taken from adjacent pituitary sections of placebo-treated OVX rats after in situ hybridization with AT1A(B) and AT1B (A,C) receptor-specific antisense probes or with control AT1A receptor sense probes (D).C is a dark-field view of A.APit, Anterior lobe of the pituitary;IPit, intermediate lobe of the pituitary;PPit, posterior lobe of the pituitary. Scale bar, 100 μm (applies to all panels).
Fig. 5.
Semiquantitative analysis of AT1A and AT1B receptor mRNA levels in the arcuate nucleus, the median eminence, the lateral hypothalamus, and the anterior pituitary of OVX rats and estrogen/progesterone-treated OVX rats. Silver grains were counted over single cells after in situhybridization, using AT1A and AT1Breceptor-specific antisense or control sense probes. Shown is the mean ± SEM of silver grains per cell from six rats per group; *p ≤ 0.001.
In the arcuate nucleus or the median eminence of placebo-treated OVX rats, the hybridization signal obtained with AT1A or AT1B receptor antisense probes was not significantly higher than the signal found after hybridization with nonspecific sense probes (Fig. 5). Significant AT1A receptor mRNA expression, however, was detected in scattered cells of the lateral hypothalamic area (Fig. 5). In the anterior pituitary we detected AT1Breceptor mRNA (Fig. 6C) but no AT1A receptor mRNA (Fig. 6B). Although present in control adrenal sections, no AT2 receptor mRNA expression was detected in the brain areas studied or in the anterior pituitary of placebo-treated OVX rats.
Marked alterations in the expression of both AT1A and AT1B receptor mRNA levels occurred after treatment of OVX rats with estrogen/progesterone. In the arcuate nucleus of estrogen/progesterone-treated OVX rats, in situhybridization with AT1A receptor antisense probes resulted in intense labeling of cells (Fig. 4B), indicating the induction of AT1A receptor gene expression in this area (Fig. 5). Conversely, in the anterior pituitary, estrogen/progesterone treatment decreased AT1B receptor mRNA expression by 70% (Fig. 5). The expression of AT1A receptor mRNA in cells of the lateral hypothalamic area was not affected by hormone treatment (Fig. 5).
As it was the case in placebo-treated OVX rats, no AT1A receptor mRNA expression was detected in the median eminence of estrogen/progesterone-treated OVX rats, and no AT1B receptor mRNA was detected in any of the brain areas studied (Figs. 4C, 5). In the anterior pituitary no significant AT1A receptor mRNA expression over background was detectable in hormone-treated OVX rats (Fig. 5). As in placebo-treated OVX rats, no AT2 receptor mRNA was detected in any brain area studied or in the pituitary of estrogen/progesterone-treated OVX rats.
Colocalization of AT1A receptor mRNA with tyrosine hydroxylase in the arcuate nucleus
To clarify whether the expression of AT1Areceptor mRNA found in cells of the arcuate nucleus of estrogen/progesterone-treated OVX rats was associated with dopaminergic neurons, we double-labeled sections by combining in situhybridization for AT1A receptors with immunological staining of tyrosine hydroxylase-expressing neurons. The localization of AT1A receptor mRNA in cells of the dorsomedial arcuate nucleus of estrogen/progesterone-treated OVX rats correlated well with the localization of tyrosine hydroxylase-immunoreactive neurons (Fig. 7C,D). Although tyrosine hydroxylase-positive neurons were present in the arcuate nucleus of placebo-treated rats, no AT1A receptor mRNA was detected here (Fig. 7A,B). Microscopic examination at high magnification of the arcuate nucleus of estrogen/progesterone-treated OVX rats revealed the cellular colocalization of AT1Areceptor mRNA and tyrosine hydroxylase (Fig.8). Most of the tyrosine hydroxylase-positive neurons in the arcuate nucleus expressed AT1A receptor mRNA. These neurons were characterized by the brown reaction product from the immunological staining in the cytoplasm and by an accumulation of silver grains surrounding and overlaying their nucleus (Fig. 8).
Fig. 7.
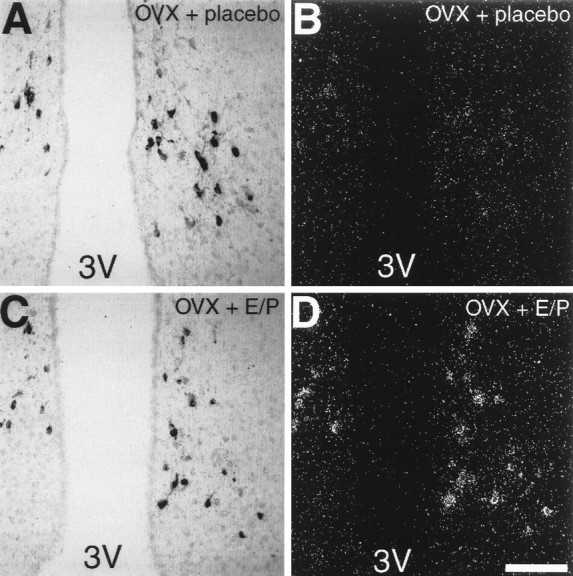
Photomicrographs showing tyrosine hydroxylase-like immunoreactivity (A, C) and AT1A receptor mRNA (B, D) in neurons of the arcuate nucleus of placebo-treated OVX rats (A, B) and estrogen/progesterone-treated OVX rats (C, D). Sections were double-labeled for AT1A receptor mRNA and tyrosine hydroxylase-like immunoreactivity. B andD are dark-field views of A andC, respectively. 3V, Third ventricle. Scale bar, 100 μm (applies to all panels).
Fig. 8.
High-power photomicrographs illustrate the colocalization of AT1A receptor mRNA expression with tyrosine hydroxylase immunoreactivity in neurons of the arcuate nucleus of estrogen/progesterone-treated OVX rats (arrows). B shows a polarized epifluorescence illumination of A to visualize silver grains. Note the absence of silver grains over one tyrosine hydroxylase-positive neuron (arrowhead). Scale bar, 20 μm.
The association of AT1A receptor mRNA with tyrosine hydroxylase-positive neurons was selective for the arcuate nucleus because in the zona incerta of estrogen/progesterone-treated OVX rats tyrosine hydroxylase-positive neurons were present in the A13 cell group (Fig. 9A), but no positive AT1A receptor hybridization signal was detected (Fig. 9B). Conversely, in the lateral hypothalamic area, where AT1A receptor mRNA was found in scattered cells (Fig.9D), no tyrosine hydroxylase-positive cells were detected (Fig. 9C).
Tyrosine hydroxylase and AT1 receptors in the arcuate nucleus of male rats
In intact male rats, tyrosine hydroxylase-positive neurons were present in the arcuate nucleus (Fig.10A). However, we were not able to detect any AT1A receptor mRNA in the arcuate nucleus of intact male rats (Fig. 10B).
DISCUSSION
The regulation of pituitary prolactin release by Ang II has been proposed to be mediated by brain and pituitary mechanisms operating in opposing directions (Steele, 1992). In the brain, Ang II has indirect inhibitory control of prolactin release. Brain Ang II may function to limit the magnitude of the prolactin secretion under certain conditions, such as after concurrent estrogen/progesterone administration to OVX rats (Myers and Steele, 1989) or after stress in male rats (Myers and Steele, 1991). This indirect inhibitory effect of Ang II probably is attributable to its stimulation of dopamine release, the predominant inhibitor of prolactin secretion (Steele et al., 1982;Inoue and Negro-Vilar, 1989).
A likely site for the central inhibitory control of prolactin secretion by Ang II is the arcuate nucleus. The dorsomedial arcuate nucleus contains small tyrosine hydroxylase-immunopositive dopaminergic neurons (Everitt et al., 1992), and Ang II- immunoreactive nerve fibers are in close proximity to these neurons (Mounzih et al., 1994). The dorsal arcuate nucleus of the female rat expresses AT1, but not AT2, receptors (Seltzer et al., 1993). We hypothesized that it is via stimulation of these receptors that brain Ang II exerts its inhibitory effect on prolactin release by stimulating dopamine formation and its release into the portal circulation. Indeed, Ang II has been shown to selectively regulate dopamine levels in the arcuate nucleus (Steele et al., 1982) and to facilitate dopamine release from the hypothalamus (Inoue and Negro-Vilar, 1989). This hypothesis has been supported by the blockade of Ang II-induced inhibition of prolactin secretion by dopamine receptor antagonists (Steele et al., 1982).
We recently have provided additional support for this hypothesis, showing that the expression of AT1 receptors in the dorsomedial arcuate nucleus is high only during the estrus phase of the estrous cycle (Seltzer et al., 1993). Thus, a parallelism between the variations in portal plasma dopamine levels, which reach the highest levels at estrus (Ben-Jonathan et al., 1977), and the expression of AT1 receptors exists during the estrous cycle. We also have shown that estrogen/progesterone replacement in OVX rats results in a marked, and anatomically selective, upregulation of AT1receptors in the arcuate nucleus (Seltzer et al., 1993), a report confirmed here. The timing we used for the estrogen/progesterone treatment was the same as that described by Rance et al. (1981), who have shown that in the afternoon of day 3, 24 hr after progesterone pellet implantation, plasma prolactin concentrations were reduced significantly. The prolactin decrease was accompanied by an increase of dopamine turnover in the arcuate nucleus (Rance et al., 1981). Higher numbers of AT1 receptors could possibly magnify the effect of endogenous Ang II. This may explain the enhancement of the estrogen/progesterone-caused inhibition of prolactin release by intraventricular injection of Ang II (Myers and Steele, 1989). Progesterone increases the secretion of dopamine into the hypophyseal portal blood (Cramer et al., 1979), an effect that could be mediated via a regulation of the number of AT1 receptors in the dorsomedial arcuate nucleus. In support of this hypothesis is the presence of estrogen and progesterone receptors in dopaminergic neurons of the arcuate nucleus (Sar, 1984, 1988; Simerly et al., 1990; Kohama et al., 1992), the colocalization of estrogen and progesterone receptors in this area (Warembourg et al., 1989), and the regulation of progesterone receptors by estrogen (Romano et al., 1989).
Our present in situ hybridization study demonstrates that the increased binding of [125I]Sar1Ang II to AT1 receptors in the arcuate nucleus of estrogen/progesterone-treated OVX rats is paralleled with an expression of AT1A receptor mRNA. Because in the arcuate nucleus AT1A receptor mRNA is expressed in significant amounts only after hormonal treatment, our results suggest an induction of AT1A receptor gene expression in this area by reproductive hormones. Furthermore, our results indicate that the expression of AT1A receptor mRNA in the arcuate nucleus differs between intact male rats and female rats treated with estrogen/progesterone, because no receptor mRNA could be detected in intact male rats. In accordance with this observation, Myers and Steele (1991) found no effect of centrally injected Ang II receptor antagonists on prolactin levels in male rats. Because this is also the case in untreated OVX rats (Myers and Steele, 1989), it is possible that endogenous Ang II affects prolactin release only under certain conditions like estrogen treatment. Indeed, during restraint stress in male rats, when prolactin levels are increased, blocking of central Ang II receptors further arguments the prolactin increase (Myers and Steele, 1991). Thus, central Ang II may function to limit the magnitude of stress-induced prolactin response (Steele, 1992). It is conceivable that stress as well as estrogen/progesterone treatment induces the expression of arcuate nucleus AT1 receptors in male rats.
The hypothesis of a direct regulation of dopamine formation and release by Ang II prompted us to determine whether AT1A receptors were associated with the dopamine-forming, tyrosine hydroxylase-containing neurons. Our present results show that this is indeed the case, because we were able to selectively colocalize AT1A receptor mRNA and tyrosine hydroxylase immunoreactivity in neurons of the arcuate nucleus. It has been shown recently that Ang II can increase tyrosine hydroxylase activity and tyrosine hydroxylase mRNA levels (Yang et al., 1996; Yu et al., 1996). Our present data demonstrate for the first time that tyrosine hydroxylase-producing neurons can express AT1A receptors and support the hypothesis of the involvement of Ang II in the regulation of neuronal catecholamine synthesis. On the basis of our observations, we propose that Ang II acts on AT1A receptors produced by dopaminergic neurons of the arcuate nucleus to stimulate directly dopamine formation and/or its release to the portal circulation.
In the anterior pituitary, Ang II is produced locally in gonadotrophs and acts in a paracrine manner on lactotrophs to stimulate prolactin release (Aguilera et al., 1982; Paglin et al., 1984; Ganong et al., 1989; Thomas and Sernia, 1990), an effect mediated by AT1receptors (Becú-Villalobos et al., 1994; Moreau et al., 1994). Estrogen treatment of OVX rats downregulates the number of AT1 receptors in the anterior pituitary (Chen and Printz, 1983; Carriére et al., 1986; Seltzer et al., 1992). Our present results indicate that a combined estrogen and progesterone treatment of OVX rats also results in a downregulation of AT1 receptors in the anterior pituitary. In addition, our present in situhybridization study shows that estrogen/progesterone replacement in OVX rats decreases the expression of AT1B receptor mRNA and confirms earlier reports on the presence of AT1B, but not AT1A or AT2, receptor mRNA in the anterior pituitary gland of the rat (Kakar et al., 1992;Jöhren and Saavedra, 1996).
In conclusion, we demonstrate for the first time that the expression of the AT1A receptor mRNA is induced in dopaminergic neurons of the arcuate nucleus in OVX rats after estrogen/progesterone treatment. Thus, the effect of reproductive hormones on receptor expression is likely to represent transcriptional events rather than changes in the receptor turnover or availability for binding. Our finding that both brain AT1A receptor and pituitary AT1B receptor expression is inversely regulated by reproductive hormones confirms the hypothesis of a dual role of Ang II on the regulation of prolactin release: brain Ang II inhibits and pituitary Ang II stimulates the release of the hormone. Such regulation can be considered part of delicate feedback mechanisms that fine-tune the hormonal responses and their central regulations in conditions of health and disease.
Footnotes
G.L.S. was supported by Grant 200794/94 from the National Council of Scientific and Technological Development of Brazil. We thank Ricardo Dreyfuss, Medical Arts and Photographic Branch at National Institutes of Health, for the expert microphotography, and we thank the National Institutes of Health Scientific Computer Resource Center for image processing.
Correspondence should be addressed to Dr. Olaf Jöhren, Section on Pharmacology, National Institute of Mental Health, 10 Center Drive, MSC 1514, Building 10, Room 2D-57, Bethesda, MD 20892.
REFERENCES
- 1.Aguilera G, Hyde C, Catt KJ. Angiotensin II receptors and prolactin release in pituitary lactotrophs. Endocrinology. 1982;111:1045–1050. doi: 10.1210/endo-111-4-1045. [DOI] [PubMed] [Google Scholar]
- 2.Arbogast LA, Voogt JL. Progesterone reverses the estradiol-induced decrease in tyrosine hydroxylase mRNA levels in the arcuate nucleus. Neuroendocrinology. 1993;58:501–510. doi: 10.1159/000126583. [DOI] [PubMed] [Google Scholar]
- 3.Barron WM, Schreiber J, Lindheimer MD. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am J Physiol. 1986;250:E352–E361. doi: 10.1152/ajpendo.1986.250.4.E352. [DOI] [PubMed] [Google Scholar]
- 4.Becú-Villalobos D, Lacau-Mengido IM, Thyssen SM, Díaz-Torga GS, Libertun C. Effects of LHRH and Ang II on prolactin stimulation are mediated by hypophysial AT1 receptor subtype. Am J Physiol. 1994;266:E274–E278. doi: 10.1152/ajpendo.1994.266.2.E274. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Jonathan N, Oliver C, Weiner HJ, Mical RS, Porter JC. Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology. 1977;100:452–458. doi: 10.1210/endo-100-2-452. [DOI] [PubMed] [Google Scholar]
- 6.Brann DW, Zamorano PL, Chorich LP, Mahesh VB. Steroid hormone effects on NMDA receptor binding and NMDA receptor mRNA levels in the hypothalamus and cerebral cortex of the adult rat. Neuroendocrinology. 1993;58:666–672. doi: 10.1159/000126607. [DOI] [PubMed] [Google Scholar]
- 7.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone, and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 8.Carrière PD, De Lèan A, Gutkowska J, Genest J, Cantin M. Chronic estradiol treatment decreases angiotensin II receptor density in the anterior pituitary gland and adrenal cortex but not in the mesenteric artery. Neuroendocrinology. 1986;43:49–56. doi: 10.1159/000124508. [DOI] [PubMed] [Google Scholar]
- 9.Chen FM, Printz M. Chronic estrogen treatment reduces angiotensin II receptors in the anterior pituitary. Endocrinology. 1983;113:1503–1510. doi: 10.1210/endo-113-4-1503. [DOI] [PubMed] [Google Scholar]
- 10.Cramer OM, Parker CR, Porter JC. Stimulation of dopamine release into hypophysial portal blood by administration of progesterone. Endocrinology. 1979;105:929–933. doi: 10.1210/endo-105-4-929. [DOI] [PubMed] [Google Scholar]
- 11.Elton TS, Stephan CC, Taylor GR, Kimball MG, Martin MM, Durand JN, Oparil S. Isolation of two distinct type I angiotensin II receptor genes. Biochem Biophys Res Commun. 1992;184:1067–1073. doi: 10.1016/0006-291x(92)90700-u. [DOI] [PubMed] [Google Scholar]
- 12.Everitt BJ, Meister B, Hökfelt T. The organization of monoaminergic neurons in the hypothalamus in relation to neuroendocrine integration. In: Nemeroff CB, editor. Neuroendocrinology. CRC; Boca Raton, FL: 1992. pp. 87–128. [Google Scholar]
- 13.Ganong WF, Deschepper CF, Steele MK, Intebi A. Renin–angiotensin system in the anterior pituitary of the rat. Am J Hypertens. 1989;2:320–322. doi: 10.1093/ajh/2.4.320. [DOI] [PubMed] [Google Scholar]
- 14.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 15.Inagami T, Guo DF, Kitami Y. Molecular biology of angiotensin II receptors: an overview. J Hypertens Suppl. 1994;12:S83–S94. [PubMed] [Google Scholar]
- 16.Inoue T, Negro-Vilar A. Evidence that vasoactive intestinal peptide, angiotensin II, and LHRH modulate the release of prolactin induced by dopamine blockade. Soc Neurosci Abstr. 1989;15:724. [Google Scholar]
- 17.Iwai N, Inagami T. Identification of two subtypes in the rat type I angiotensin II receptor. FEBS Lett. 1992;298:257–260. doi: 10.1016/0014-5793(92)80071-n. [DOI] [PubMed] [Google Scholar]
- 18.Jöhren O, Saavedra JM. Expression of AT1A and AT1B angiotensin II receptor messenger RNA in forebrain of 2-wk-old rats. Am J Physiol. 1996;271:E104–E112. doi: 10.1152/ajpendo.1996.271.1.E104. [DOI] [PubMed] [Google Scholar]
- 19.Jöhren O, Viswanathan M, Saavedra JM. Expression of non-angiotensin II [125I]CGP 42112 binding sites on activated microglia after kainic acid-induced neurodegeneration. Brain Res. 1995a;702:153–161. doi: 10.1016/0006-8993(95)01035-3. [DOI] [PubMed] [Google Scholar]
- 20.Jöhren O, Inagami T, Saavedra JM. AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. NeuroReport. 1995b;6:2549–2552. doi: 10.1097/00001756-199512150-00024. [DOI] [PubMed] [Google Scholar]
- 21.Jöhren O, Inagami T, Saavedra JM. Localization of AT2 angiotensin II receptor gene expression in rat brain by in situ hybridization histochemistry. Mol Brain Res. 1996;37:192–200. doi: 10.1016/0169-328x(95)00309-g. [DOI] [PubMed] [Google Scholar]
- 22.Jones TH, Brown BL, Dobson PRM. Paracrine control of anterior pituitary hormones secretion. J Endocrinol. 1990;127:5–13. doi: 10.1677/joe.0.1270005. [DOI] [PubMed] [Google Scholar]
- 23.Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Biochem Biophys Res Commun. 1992;183:1090–1096. doi: 10.1016/s0006-291x(05)80302-x. [DOI] [PubMed] [Google Scholar]
- 24.Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–24546. [PubMed] [Google Scholar]
- 25.Kohama SG, Freesh F, Bethea CL. Immunocytochemical colocalization of hypothalamic progestin receptors and tyrosine hydroxylase in steroid-treated monkeys. Endocrinology. 1992;131:509–517. doi: 10.1210/endo.131.1.1351839. [DOI] [PubMed] [Google Scholar]
- 26.Michels KM, Lee WH, Seltzer A, Saavedra JM, Bondy CA. Up-regulation of pituitary [125I]insulin-like growth factor-I (IGF-I) binding and IGF binding protein-2 and IGF-I gene expression by estrogen. Endocrinology. 1993;132:23–29. doi: 10.1210/endo.132.1.7678216. [DOI] [PubMed] [Google Scholar]
- 27.Moreau C, Rasolonjanahary R, Audinot V, Kordon C, Enjalbert A. Angiotensin II effects on second messengers involved in prolactin secretion are mediated by AT1 receptor in anterior pituitary cells. Mol Cell Neurosci. 1994;5:597–603. doi: 10.1006/mcne.1994.1073. [DOI] [PubMed] [Google Scholar]
- 28.Mounzih K, Grove KL, Speth RC, Steele MK, Ganong WF. Further studies of the site at which angiotensin II acts in the central nervous system to inhibit the secretion of prolactin. Endocr J. 1994;2:41–45. [Google Scholar]
- 29.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 30.Myers LS, Steele MK. The brain renin–angiotensin system and the regulation of prolactin secretion in female rats: influence of ovarian hormones. J Neuroendocrinol. 1989;1:299–303. doi: 10.1111/j.1365-2826.1989.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 31.Myers LS, Steele MK. The brain renin angiotensin system and prolactin secretion in the male rat. Endocrinology. 1991;129:1744–1748. doi: 10.1210/endo-129-4-1744. [DOI] [PubMed] [Google Scholar]
- 32.Nazarali AJ, Gutkind JS, Saavedra JM. Calibration of [125I]-polymer standards with [125I]-brain paste standards for use in quantitative receptor autoradiography. J Neurosci Methods. 1989;30:247–253. doi: 10.1016/0165-0270(89)90135-0. [DOI] [PubMed] [Google Scholar]
- 33.Paglin S, Stukenbrok H, Jamieson JD. Interaction of angiotensin II with dispersed cells from the anterior pituitary of the male rat. Endocrinology. 1984;114:2284–2292. doi: 10.1210/endo-114-6-2284. [DOI] [PubMed] [Google Scholar]
- 34.Rance N, Wise PM, Barraclough CA. Negative feedback effects of progesterone correlated with changes in hypothalamic norepinephrine and dopamine turnover rates, median eminence luteinizing hormone-releasing hormone, and peripheral plasma gonadotropins. Endocrinology. 1981;108:2194–2199. doi: 10.1210/endo-108-6-2194. [DOI] [PubMed] [Google Scholar]
- 35.Romano GJ, Krust A, Pfaff DW. Expression and estrogen regulation of progesterone receptor mRNA in neurons of the mediobasal hypothalamus: an in situ hybridization study. Mol Endocrinol. 1989;3:1295–1300. doi: 10.1210/mend-3-8-1295. [DOI] [PubMed] [Google Scholar]
- 36.Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- 37.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem. 1992;267:9455–9458. [PubMed] [Google Scholar]
- 38.Sar M. Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science. 1984;223:938–940. doi: 10.1126/science.6141639. [DOI] [PubMed] [Google Scholar]
- 39.Sar M. Distribution of progestin-concentrating cells in rat brain: colocalization of [3H]ORG.2058, a synthetic progestin, and antibodies to tyrosine hydroxylase in hypothalamus by combined autoradiography and immunocytochemistry. Endocrinology. 1988;123:1110–1118. doi: 10.1210/endo-123-2-1110. [DOI] [PubMed] [Google Scholar]
- 40.Schramme C, Denef C. Stimulation of prolactin release by angiotensin II in superfused rat anterior pituitary cell aggregates. Neuroendocrinology. 1983;36:483–485. doi: 10.1159/000123502. [DOI] [PubMed] [Google Scholar]
- 41.Seltzer A, Pinto JEB, Viglione PN, Correa FMA, Libertun C, Tsutsumi K, Steele MK, Saavedra JM. Estrogens regulate angiotensin-converting enzyme and angiotensin receptors in female rat anterior pituitary. Neuroendocrinology. 1992;55:460–467. doi: 10.1159/000126157. [DOI] [PubMed] [Google Scholar]
- 42.Seltzer A, Tsutsumi K, Shigematsu K, Saavedra JM. Reproductive hormones modulate angiotensin II AT1 receptors in the dorsomedial arcuate nucleus of the female rat. Endocrinology. 1993;133:939–941. doi: 10.1210/endo.133.2.8344227. [DOI] [PubMed] [Google Scholar]
- 43.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 44.Steele MK. The role of brain angiotensin II in the regulation of luteinizing hormone and prolactin secretion. Trends Endocrinol Metab. 1992;3:295–301. doi: 10.1016/1043-2760(92)90140-v. [DOI] [PubMed] [Google Scholar]
- 45.Steele MK, Myers LS. In vivo studies on paracrine actions of pituitary angiotensin II in stimulating prolactin release in rats. Am J Physiol. 1990;258:E619–E624. doi: 10.1152/ajpendo.1990.258.4.E619. [DOI] [PubMed] [Google Scholar]
- 46.Steele MK, Negro-Vilar A, McCann SM. Effect of angiotensin II on in vivo and in vitro release of anterior pituitary hormones in the female rat. Endocrinology. 1981;109:893–899. doi: 10.1210/endo-109-3-893. [DOI] [PubMed] [Google Scholar]
- 47.Steele MK, McCann SM, Negro-Vilar A. Modulation by dopamine and estradiol of the central effects of angiotensin on anterior pituitary hormone release. Endocrinology. 1982;111:722–729. doi: 10.1210/endo-111-3-722. [DOI] [PubMed] [Google Scholar]
- 48.Thomas WG, Sernia C. Immunocytochemical localization of angiotensinogen and angiotensin II in the rat pituitary. J Neuroendocrinol. 1990;2:297–304. doi: 10.1111/j.1365-2826.1990.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 49.Timmermans PBMWM, Inagami T, Saavedra JM, Ardaillou R, Rosenfeld CR, Mendelsohn FAO. Angiotensin receptor subtypes and their pharmacology. In: Cuello AC, Collier B, editors. Pharmacological sciences: perspectives for research and therapy in the late 1990s. Birkhäuser Verlag; Basel: 1995. pp. 37–58. [Google Scholar]
- 50.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991a;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 51.Tsutsumi K, Saavedra JM. Angiotensin II receptor subtypes in median eminence and basal forebrain areas involved in regulation of pituitary function. Endocrinology. 1991b;129:3001–3008. doi: 10.1210/endo-129-6-3001. [DOI] [PubMed] [Google Scholar]
- 52.Warembourg M, Jolivet A, Milgrom E. Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- 53.Yang H, Lu D, Yu K, Raizada MK. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras-dependent mitogen-activated protein kinase pathway. J Neurosci. 1996;16:4047–4058. doi: 10.1523/JNEUROSCI.16-13-04047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu K, Lu D, Rowland NE, Raizada MK. Angiotensin II regulation of tyrosine hydroxylase gene expression in the neuronal cultures of normotensive and spontaneously hypertensive rats. Endocrinology. 1996;137:3566–3576. doi: 10.1210/endo.137.8.8754788. [DOI] [PubMed] [Google Scholar]






