Abstract
The differential expression and association of cytoplasmic β-subunits with pore-forming α-subunits may contribute significantly to the complexity and heterogeneity of voltage-gated K+ channels in excitable cells. Here we examined the association and colocalization of two mammalian β-subunits, Kvβ1 and Kvβ2, with the K+ channel α-subunits Kv1.1, Kv1.2, Kv1.4, Kv1.6, and Kv2.1 in adult rat brain. Reciprocal coimmunoprecipitation experiments using subunit-specific antibodies indicated that Kvβ1 and Kvβ2 associate with all the Kv1 α-subunits examined, and with each other, but not with Kv2.1. A much larger portion of the total brain pool of Kv1-containing channel complexes was found associated with Kvβ2 than with Kvβ1. Single- and multiple-label immunohistochemical staining indicated that Kvβ1 codistributes extensively with Kv1.1 and Kv1.4 in cortical interneurons, in the hippocampal perforant path and mossy fiber pathways, and in the globus pallidus and substantia nigra. Kvβ2 codistributes extensively with Kv1.1 and Kv1.2 in all brain regions examined and was strikingly colocalized with these α-subunits in the juxtaparanodal region of nodes of Ranvier as well as in the axons and terminals of cerebellar basket cells. Taken together, these data provide a direct demonstration that Kvβ1 and Kvβ2 associate and colocalize with Kv1 α-subunits in native tissues and provide a biochemical and neuroanatomical basis for the differential contribution of Kv1 α- and β-subunits to electrophysiologically diverse neuronal K+ currents.
Keywords: ion channel, central nervous system, cerebellum, striatum, immunoprecipitation, immunohistochemistry, immunofluorescence, epilepsy
All excitable cells express voltage-gated K+ channels. These channels are critical for a wide variety of processes, including action potential propagation and lymphocyte activation, and play an essential role in neurons where they regulate the resting membrane potential, impact dendritic excitability, control the frequency and duration of action potentials, and modulate neurotransmitter release (Hille, 1992). Recent work in several laboratories has determined that K+channels are integral membrane, hetero-oligomeric glycoprotein complexes composed of four pore-forming α-subunits and four cytoplasmic β-subunit polypeptides (for review, see Pongs, 1995; Jan and Jan, 1997). Although it has been appreciated that the association of functionally distinct α-subunits contributes to the tremendous diversity of K+ channel types observed in native cells (Sheng et al., 1993; Wang et al., 1993; Scott et al., 1994a), the recent discovery and characterization of a family of β-subunits (Rettig et al., 1994; Scott et al., 1994b; England et al., 1995a,b;Heinemann et al., 1995, 1996; Majumder et al., 1995; McCormack et al., 1995; Morales et al., 1995; Rhodes et al., 1995, 1996; Fink et al., 1996; Nakahira et al., 1996; Shi et al., 1996) indicate that these cytoplasmic polypeptides also make a significant contribution to channel diversity. In particular, the Kvβ1 β-subunit has dramatic effects on the inactivation of Shaker-related or Kv1 subfamily K+ channel α-subunits, although Kvβ2 does not (Rettig et al., 1994; Heinemann et al., 1996). Moreover, it is clear from these data that the association and localization of both α- and β-subunits must be considered to ascribe electrophysiologically observed currents to channels of specific subunit composition.
We described previously the expression and distribution of two K+ channel β-subunits, Kvβ1 (also referred to as Kvβ1a or Kvβ1.1) and Kvβ2, in rat brain (Rhodes et al., 1996). We also demonstrated that, in transfected cells, these β-subunits associated with all Kv1 α-subunits, but not with Kv2.1 (Nakahira et al., 1996). α- and β-Subunit interaction is mediated by domains in the cytoplasmic N terminus of the α-subunit (Sewing et al., 1996; Yu et al., 1996), near the domain responsible for mediating Kv1 subfamily-specific α-subunit oligomerization (for review, seeScannevin and Trimmer, 1997). In addition to the effects on inactivation mediated by Kvβ1, both Kvβ1 and Kvβ2 have been found to exert chaperone-like effects on the surface expression of Kv1 subfamily α-subunits expressed in transfected cells (Shi et al., 1996), through an interaction occurring early in channel biosynthesis (Shi et al., 1996; Nagaya et al., 1997).
The specific contribution of distinct β-subunit isoforms to K+ channel complexes in mammalian brain and the specific combinations of α- and β-subunit polypeptides that form these complexes are important for correlating the channel subunits identified in molecular cloning studies with neuronal K+ currents. A combined biochemical and neuroanatomical approach, as has been used to study the α-subunit composition of hetero-oligomeric K+ channels in mammalian brain (Sheng et al., 1993; Wang et al., 1993), is warranted but requires the availability of subunit-specific antibodies to the component α- and β-subunit polypeptides. Previously, we used a pan-β-subunit antibody and antibodies against the Kv1.2, Kv1.4, and Kv2.1 α-subunits to begin to address the association and colocalization of α- and β-subunit polypeptides in mammalian brain (Rhodes et al., 1995). We recently generated subtype-specific antibodies to Kvβ1 and Kvβ2 (Rhodes et al., 1996) and a panel of polyclonal and monoclonal antibodies to various K+channel α-subunits (Bekele-Arcuri et al., 1996; Shi et al., 1996). Here we use these antibodies to immunopurify rat brain K+ channel complexes and to determine the constituent subunit composition by immunoblotting. We also use these antibodies in single- and multiple-label immunohistochemical and immunofluorescence analyses to examine the cellular and subcellular colocalization of these channel polypeptides. Taken together, these studies provide an important biochemical and neuroanatomical foundation for increased understanding of the contribution of specific K+ channel α- and β-subunits to multiple, functionally distinct K+ currents in the brain.
MATERIALS AND METHODS
Materials. All reagents were molecular biology grade from Sigma (St. Louis, MO) or Boehringer-Mannheim (Indianapolis, IN), except where noted otherwise.
Production of antibodies. The production of the anti-α- and anti-β-subunit-specific monoclonal and affinity-purified polyclonal antibodies is described in detail elsewhere (Trimmer, 1991;Rhodes et al., 1995, 1996; Bekele-Arcuri et al., 1996; Shi et al., 1996). In brief, these antibodies were raised using synthetic peptides or fusion proteins as the immunogen. Each antibody was examined for specificity on immunoblots of rat brain membranes, on immunoblots of membranes prepared from COS-1 cells transiently transfected with a broad panel of K+ channel cDNAs, and by immunofluorescence staining of transiently transfected cells (Bekele-Arcuri et al., 1996). Each antibody recognized only the appropriate protein on these immunoblots and stained only cells transfected with the appropriate cDNA. Moreover, immunoreactivity was completely eliminated by previous incubation with the corresponding peptide or fusion protein immunogen.
Immunoprecipitation. A crude adult rat brain synaptosomal membrane fraction was prepared as described previously (Trimmer, 1991;Rhodes et al., 1995). Immunoprecipitation reactions were performed at 4°C using detergent lysates of these membranes as described previously (Rhodes et al., 1995, 1996). In brief, membranes (1 mg of membrane protein/tube) were solubilized to 1 ml final volume per tube in lysis buffer [1% Triton X-100 and (in mm) 150 NaCl, 1 EDTA, 10 sodium azide, and 10 Tris-HCl, pH 8.0] containing a protease inhibitor mixture (Trimmer, 1991). Affinity-purified antibodies were added, and the volume was adjusted to 1 ml with lysis buffer. Samples were incubated for 2 hr on a rotator, followed by addition of 50 μl of a 50% slurry of protein A–Sepharose and further incubation for 45 min. After incubation, protein A–Sepharose was pelleted by centrifugation at 10,000 × g for 20 sec, and the resulting pellets were washed by resuspension and centrifugation six times with lysis buffer. The final pellets were resuspended in 200 μl of reducing SDS sample buffer.
SDS-polyacrylamide gels and immunoblotting. Products of immunoprecipitation reactions (20 μl, representing the yield from 100 μg of starting crude rat brain membrane protein) were size fractionated on 9% (for analysis of α-subunit polypeptides) or 12% (for analysis of β-subunit polypeptides) SDS-polyacrylamide gels (Maizel, 1971). Sixty micrograms of crude rat brain membrane protein were also resuspended in reducing SDS sample buffer and loaded directly onto each SDS gel. Disulfide bonds were reduced by the addition of 20 mm 2-mercaptoethanol to the sample buffer. Lauryl sulfate (Sigma) was the SDS source used for all SDS-PAGE (Shi et al., 1994). After electrophoretic transfer to nitrocellulose paper, the resulting blots were blocked in TBS containing 4% low-fat milk (Blotto) (Johnson et al., 1984), incubated in affinity-purified antibody diluted 1:50–1:2000 in Blotto for 1 hr or undiluted monoclonal antibody tissue culture supernatants, and washed three times in Blotto for 30 min total. Blots were then incubated in HRP-conjugated secondary antibody (Organon Teknika, West Chester, PA; 1:2000 dilution in Blotto) for 1 hr and then washed in TBS three times for 30 min total. The blots were then incubated in substrate for enhanced chemiluminescence (ECL) for 1 min and autoradiographed on preflashed (to OD545 = 0.15) Kodak (Rochester, NY) XAR-5 film.
Immunohistochemistry. The procedures for single-label light microscopic immunohistochemistry are described in detail elsewhere (Rhodes et al., 1995, 1996; Bekele-Arcuri et al., 1996). Briefly, 35-μm-thick sections of adult rat brain were incubated overnight at 4°C in an antibody vehicle containing affinity-purified rabbit polyclonal or mouse monoclonal antibodies. Detection of antibody–antigen complexes was accomplished using the avidin–biotin ABC procedure (Vector Laboratories, Burlingame, CA) and visualized using a nickel-enhanced diaminobenzidine procedure (Rhodes et al., 1995, 1996).
For multiple-label immunofluorescence, 10-μm-thick frozen sections were mounted on glass slides and incubated for 36 hr at 4°C in an antibody vehicle containing a mixture of affinity-purified rabbit polyclonal and mouse monoclonal antibodies (Bekele-Arcuri et al., 1996). If two mouse monoclonal antibodies were used in the same incubation, they were of distinct isotype. These sections were then incubated in vehicle containing affinity-purified species- and isotype-specific secondary antibodies conjugated to fluorescein (FITC), Texas Red sulfonyl chloride (TRSC), or 7-amino-4-methylcoumarin 3-acetic acid (AMCA). Secondary antibodies conjugated to FITC were used to label the most abundant (or most easily detected) antigen, and antibodies conjugated to TRSC were used to label the least abundant (or least easily detected) antigen. For triple labeling, sections were incubated in antibody vehicle containing a mixture of two isotype-specific mouse monoclonal antibodies plus a rabbit polyclonal antibody. The sections were then incubated in antibody vehicle containing fluorochrome-conjugated, isotype-specific secondary antibodies (Southern Biotechnology, Inc., Atlanta, GA; or Boehringer-Mannheim, Indianapolis, IN), plus an affinity-purified, fluorochrome-conjugated anti-rabbit secondary antibody. For example, for triple labeling of Kv1.1, Kv1.2, and Kvβ2, we used the affinity-purified rabbit anti-Kv1.1 antibody, the mouse K14/16 (IgG2b) anti-Kv1.2 monoclonal antibody, and the mouse K17/70 (IgG1) anti-Kvβ2 monoclonal antibody (Bekele-Arcuri et al., 1996; Shi et al., 1996) to detect the corresponding antigens. We then used an affinity-purified, AMCA-conjugated goat anti-rabbit secondary antibody to label Kv1.1, an FITC-conjugated goat anti-mouse-IgG2b secondary antibody to label Kv1.2, and a TRSC-conjugated goat anti-mouse-IgG1 secondary antibody to label Kvβ2. The sections were then washed in PBS, dried, and coverslipped using ProLong mounting medium (Molecular Probes, Eugene, OR).
Sections processed for immunofluorescence were viewed and analyzed using a computer-based image analysis system (Micro Computer Imaging Device M2; Imaging Research) coupled to a Zeiss Axiovert microscope equipped with epifluorescence illumination as well as appropriate excitation, bandpass, and barrier filter sets (Omega Optical, Brattleboro, VT). This system was equipped with a Sony DXC-97 OMD color CCD camera that, under computer control, was capable of integrating fluorescent signals over a user-defined time interval. This system was also equipped with color imaging hardware that permitted us to view and digitize true color images of immunofluorescent specimens. In some cases in which triple-label immunofluorescence was performed, digitized color images of the three individual fluorochromes were combined (“image fusion”) to generate a single composite image in which the extent of immunofluorescent colocalization could be determined directly. Images obtained using this digital imaging approach were printed directly from the imaging system using a Fuji Pictrographic color printer.
To determine the specificity of the immunohistochemical reactions, some sections were processed either without addition of the primary antibody or using antibodies previously incubated (1 hr) in vehicle containing an excess of the corresponding immunogen (5–25 μg/ml) (Rhodes et al., 1995, 1996; Bekele-Arcuri et al., 1996). Additionally, some sections processed using mouse monoclonal primary antibodies were reacted with fluorochrome-tagged goat anti-rabbit secondary antibodies and vice versa. Finally, each of the isotype-specific anti-mouse secondary antibodies was tested versus primary antibodies of nonmatching isotype and versus the rabbit polyclonal antibodies. We did not observe any specific staining with the primary antibodies after exposure to the appropriate immunogen, nor did we observe cross-species or cross-isotype reactivity of fluorochrome-tagged secondary antibodies. We also did not observe any bleed-through detection of fluorochrome-tagged secondary antibodies using filter sets for the other fluorochromes.
RESULTS
Reciprocal coimmunoprecipitation analyses reveal association of Kvβ1 and Kvβ2 with Kv1 subfamily α-subunits in mammalian brain
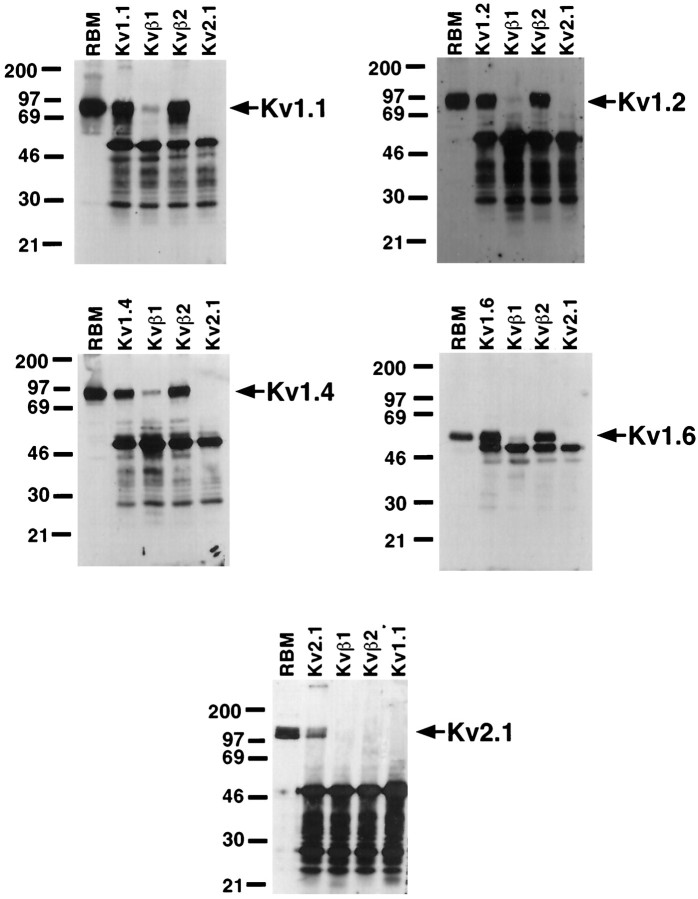
To determine the association of Kvβ1 and Kvβ2 with specific α-subunits in rat brain K+ channel complexes, we performed reciprocal coimmunoprecipitation experiments using anti-α- and anti-β-subunit-specific antibodies. Detergent lysates were prepared from rat brain membranes under conditions previously shown to preserve α- and β-subunit interactions (Rhodes et al., 1995, 1996;Nakahira et al., 1996; Shi et al., 1996). These lysates were then used in immunoprecipitation reactions performed with the anti-Kvβ1 and anti-Kvβ2 polyclonal antibodies (Rhodes et al., 1996) and with isotype-specific antibodies against the Kv1.1, Kv1.2, Kv1.4, Kv1.6, and Kv2.1 α-subunits (Trimmer, 1991; Rhodes et al., 1995, 1996;Bekele-Arcuri et al., 1996; Nakahira et al., 1996; Shi et al., 1996). In each case, immunoprecipitation reactions were performed under conditions of antibody excess, which was verified by subsequent removal and analysis of the depleted supernatant after pelleting of the immunoprecipitation reaction product. In each case all recoverable antigen had been removed by the initial immunoprecipitation reaction. Immunoprecipitation products, representing immunopurified channel complexes, were then subjected to immunoblot analyses to assay for the presence of the specific α- and β-subunit polypeptides.
Immunoprecipitation reactions were first analyzed for the presence of the Kvβ1 β-subunit polypeptide using an affinity-purified rabbit polyclonal antibody raised against the unique N-terminus of the Kvβ1 polypeptide (Rhodes et al., 1996). In coexpression studies, recombinant Kvβ1 has been shown to confer rapid inactivation on all members of the Kv1 subfamily α-subunits tested, with the exception of Kv1.6, suggesting that this β-subunit could be an important modulator of K+ channel complexes containing Kv1 α-subunits (Rettig et al., 1994; Heinemann et al., 1996). As expected from our previous studies (Rhodes et al., 1996), anti-Kvβ2 antibodies quantitatively coimmunoprecipitated the 44 kDa Kvβ1 polypeptide from these rat brain extracts (Fig. 1,top panel), showing that most of the channel complexes that contain Kvβ1 also contain at least one Kvβ2 β-subunit. In addition, all of the Kv1 family members tested (Kv1.1, Kv1.2, Kv1.4, and Kv1.6) were able to coimmunoprecipitate Kvβ1, indicating that a portion of the total pool of each Kv1 α-subunit in the brain is present in complexes that contain Kvβ1. Of these, the immunoprecipitation reaction performed with the anti-Kv1.1 antibody contained the most Kvβ1, followed by the Kv1.2, Kv1.4, and Kv1.6. Antibodies to Kv2.1 did not coimmunoprecipitate any detectable Kvβ1, extending previous results obtained using a pan-β-subunit antibody in rat brain (Rhodes et al., 1995), and on recombinant K+ channel subunits expressed in transfected cells (Nakahira et al., 1996), which suggested a lack of interaction between Kvβ1 and Kv2.1.
Fig. 1.
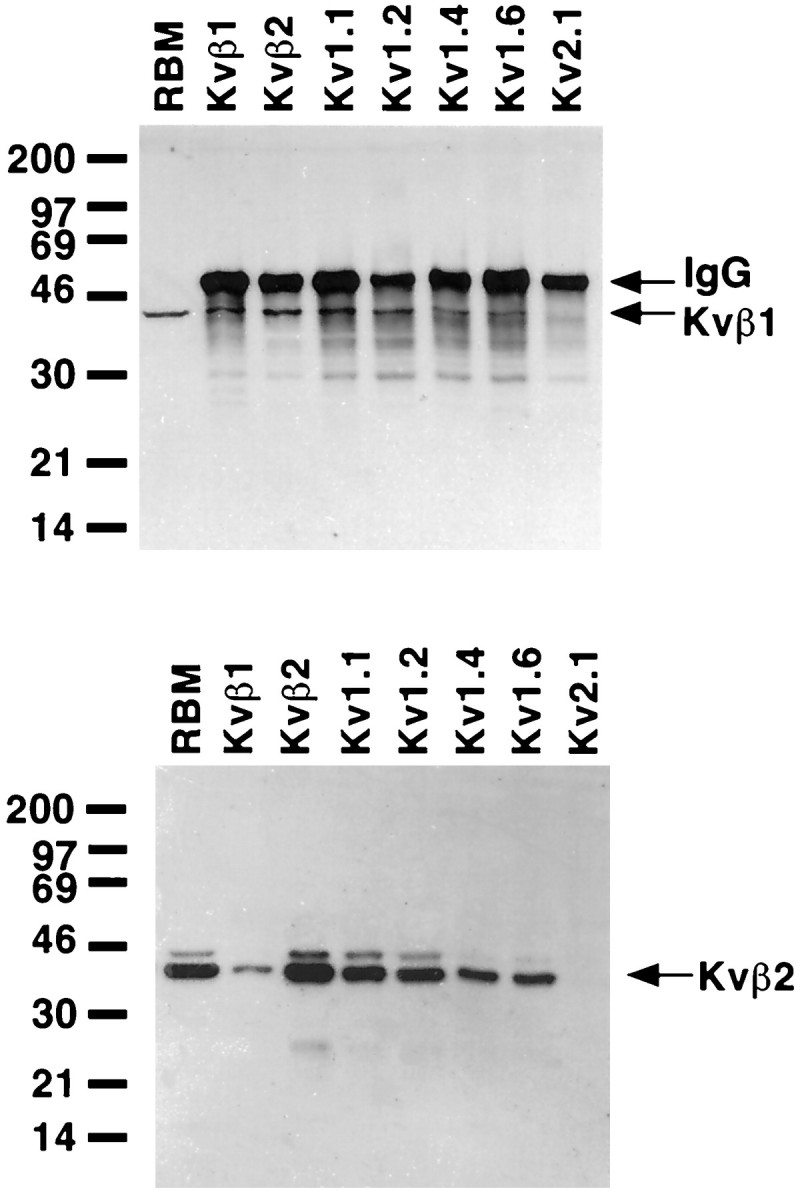
Presence of Kvβ1 and Kvβ2 in rat brain K+ channel complexes. A detergent lysate of adult rat brain membranes (RBM; 60 μg) and aliquots of products of immunoprecipitation reactions from detergent extracts of 100 μg of RBM performed with the indicated rabbit polyclonal antibodies were size-fractionated by 12% SDS-PAGE. Samples were transferred to nitrocellulose and probed with affinity-purified rabbit anti-Kvβ1 polyclonal antibody (top panel) or mouse anti-Kvβ2 monoclonal antibody K17/70 (bottom panel). Bound antibody was detected by ECL and autoradiography for 15 min (top panel) or 5 min (bottom panel). Arrows on thetop panel point to the band resulting from detection of the rabbit IgG used in the immunoprecipitation reactions by the anti-rabbit secondary antibody used for immunoblotting (IgG) and the Kvβ1-specific band (Kvβ1). Rabbit IgG bands are not present in the bottom panel, because the immunoblot was developed with anti-mouse secondary antibodies.Kvβ2 arrow denotes the Kvβ2 β-subunit. Numbers at the left denoteMr values of prestained molecular weight standards.
A similar approach was used to characterize α- and β-subunit polypeptides that associate with Kvβ2, using the Kvβ2-specific monoclonal antibody K17/70 (Bekele-Arcuri et al., 1996) to probe immunopurified K+ channel complexes (Fig. 1,bottom panel). The Kvβ2 subunit pool in rat brain consists of two components of Mr = 38 and 41 kDa. The molecular basis of the size differences between these two polypeptide species that share immunoreactivity with both N-terminally directed Kvβ2-specific monoclonal and polyclonal antibodies (Rhodes et al., 1996) and C-terminally directed pan-β-subunit monoclonal and polyclonal antibodies (Rhodes et al., 1995) is not known. Immunoprecipitation reactions performed with the anti-Kvβ1 antibody yielded only small amounts of Kvβ2, consistent with previous studies (Rhodes et al., 1996) that suggested that Kvβ1 is a minor brain β-subunit relative to Kvβ2, and that very few of the Kvβ2-containing K+ channel complexes in the brain contain Kvβ1. All of the Kv1 family members tested were found to exist in complexes containing Kvβ2, with anti-Kv1.1 and anti-Kv1.2 antibodies the most effective at coimmunoprecipitating Kvβ2 and anti-Kv1.4 and anti-Kv1.6 antibodies less effective (Fig. 1,bottom panel). Kvβ2, like Kvβ1, could not be coprecipitated by antibodies to Kv2.1. It should be noted that each of the anti-β-subunit and anti-Kv1 subfamily α-subunit antibodies tested could coimmunoprecipitate both the 38 and 41 kDa components of the rat brain Kvβ2 pool, although the 41 kDa band is not visible in all samples on the exposure presented here (Fig. 1, bottom panel).
Reciprocal immunoblots were performed using antibodies specific for each of the α-subunit polypeptides. Each of the affinity-purified rabbit polyclonal anti-α-subunit antibodies could directly immunoprecipitate the respective α-subunit from the brain membrane extracts (Fig. 2). Immunoprecipitation reactions performed with the anti-Kvβ2 antibody were quite effective at coimmunoprecipitating the bulk of the Kv1.1, Kv1.2, Kv1.4, and Kv1.6 α-subunits, indicating that the bulk of the K+channel complexes in the brain that contain these α-subunits also contain Kvβ2. Kv1.1 and Kv1.4 and, to a lesser extent, Kv1.2 and Kv1.6 could be detected in K+ channel complexes immunopurified using the anti-Kvβ1 antibody (Fig. 2). However, the amount of these Kv1 subfamily α-subunits that were found associated in complexes containing Kvβ1 was substantially less than those found with Kvβ2, consistent with the overall lower levels of Kvβ1 present in the brain. Neither the anti-Kvβ1 nor anti-Kvβ2 antibody could coimmunoprecipitate the Kv2.1 α-subunit, even though the Kv2.1-specific antibody could efficiently directly precipitate this α-subunit (Fig. 2). Overall, these results suggest that the vast majority of Kv1-containing K+ channel complexes in the brain contain at least one Kvβ2 β-subunit, and that complexes containing Kvβ1 are also present but are far less abundant.
Fig. 2.
Presence of α-subunits in rat brain K+ channel complexes. A detergent lysate of adult rat brain membranes (RBM; 60 μg) and aliquots of products of immunoprecipitation reactions from detergent extracts of 100 μg of RBM performed with polyclonal antibodies specific for the indicated K+ channel α- and β-subunit polypeptides were size-fractionated by 9% SDS-PAGE. Samples were transferred to nitrocellulose and then probed with subunit-specific affinity-purified rabbit antibodies. The panels represent blots probed with the following antibodies and the respective exposure times for ECL and autoradiography: Kv1.1 (top left; 40 sec), Kv1.2 (top right; 5 min), Kv1.4 (middle left; 2 min) Kv1.6 (middle right; 40 sec), and Kv2.1 (bottom, 20 min). Bound antibody was detected by ECL and autoradiography. Arrows point to the respective α-subunit polypeptides; also visible are bands resulting from detection of the rabbit IgG used in the immunoprecipitation reactions by the anti-rabbit secondary antibody used for immunoblotting.Numbers at the left denote Mr values of prestained molecular weight standards.
Immunohistochemical colocalization of α- and β-subunit polypeptides
To examine the distribution and colocalization of Kvβ1 and Kvβ2 with α-subunits of the Kv1 and Kv2 subfamilies, these same antibodies were used in immunohistochemical studies in serial sections of rat brain. Using these sections, we made side-by-side comparisons of the immunohistochemical staining pattern and subcellular distribution of each subunit. But because we cannot conclude from comparisons in adjacent sections that individual subunits are precisely colocalized, here we use the term “codistributed” to refer to instances in which subunits are found in identical cell types and subcellular domains, but in adjacent sections. In some cases, the colocalization of α- and β-subunits was examined directly using dual- or triple-label immunofluorescence in a single tissue section. When these direct, within-section comparisons were made, we use the term “colocalized” to refer to the precise superimposition of fluorochromes identifying two or more distinct subunits. With these distinctions in mind, the salient features of the immunohistochemical staining are described below with emphasis on three areas: the hippocampal formation, the globus pallidus, and the cerebellar cortex.
Hippocampal formation
Low-magnification photomicrographs showing the distribution of immunoreactivity for Kv1.1, Kv1.2, Kv1.4, Kv1.6, Kvβ1, Kvβ2, and Kv2.1 in the hippocampus are shown in Figure3. In the dentate gyrus, there is a close correspondence between the distribution of Kvβ1 and Kvβ2 and the Kv1.1, Kv1.2, and Kv1.4 α-subunits. All five of these subunits are concentrated in a prominent band located in the middle third of the molecular layer. Sections taken through the dentate gyrus processed for triple-label immunofluorescence clearly demonstrate the colocalization of Kv1.1, Kv1.4, and Kvβ1 in the middle third of the molecular layer (Fig. 4). As described previously by us and by others (Sheng et al., 1993; Wang et al., 1993, 1994; Rhodes et al., 1995), this pattern closely matches the termination zone of the medial perforant path (see Rosene and Van Hoesen, 1987) and correlates well with the high density of Kvβ1 and Kvβ2, as well as Kv1.1, Kv1.2, and Kv1.4, mRNA expression in cells located in layer II of the entorhinal cortex (Sheng et al., 1994; Wang et al., 1994; Rhodes et al., 1996) (N. X. Barrezueta, M. M. Monaghan, J. S. Trimmer, and K. J. Rhodes, unpublished observations), suggesting that all five subunits are localized at or near the axon terminals of entorhinal afferents. In inner and outer thirds of the molecular layer of the dentate gyrus, there is also diffuse immunoreactivity for Kv1.2, Kv1.4, Kv1.6, Kvβ1, and Kvβ2. This staining may represent subunit expression in the dendrites of dentate granule cells (see Wang et al., 1994) or in axons of other afferent inputs to the dentate gyrus, e.g., the dentate gyrus association pathway and basal forebrain cholinergic system.
Fig. 3.
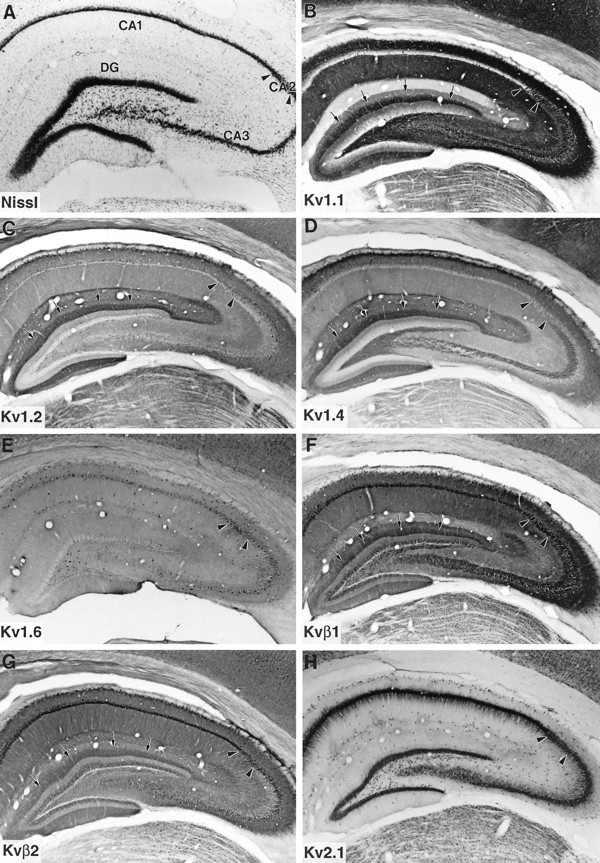
Immunohistochemical localization of K+ channel α- and β-subunits in rat hippocampus.Arrows in B–D, F, and Gpoint to the band of immunoreactivity for Kv1.1, Kv1.2, Kv1.4, Kvβ1, and Kvβ2, respectively, in the middle third of the molecular layer of the dentate gyrus (DG). Arrowheads mark the boundaries between hippocampal subfields.
Fig. 4.

Triple-label immunfluorescence demonstrating the colocalization of Kv1.1 (A), Kv1.4 (B), and Kvβ1 (C) in the dentate gyrus. These three subunits are colocalized in the middle third of the molecular layer, in a pattern that overlaps with terminals of the medial perforant path. gc, Granule cell layer;it, inner third of the molecular layer;mt middle third of the molecular layer;ot, outer third of the molecular layer.
In the CA3 subfield, there is a prominent band of immunoreactivity for Kvβ1, Kv1.1, and Kv1.4 in a narrow zone immediately above stratum pyramidale of CA3 (Figs. 3, 5). The location of this band suggests that these three subunits are associated with mossy fiber axons (also see Wang et al., 1994). Surprisingly, there was not a clear band of immunoreactivity for Kv1.2 or Kvβ2 in the mossy fiber termination zone, indicating that Kv1.2 and Kvβ2 are either not present in these K+ channel complexes or are not detectable by our antibodies. Many medium- to large-sized interneurons located within stratum pyramidale, stratum oriens, and stratum radiatum of CA1–CA3 and in the subiculum were strongly immunoreactive for Kvβ1 and were also immunoreactive for Kv1.1 and Kv1.6. A subpopulation of interneurons located within and immediately adjacent to stratum pyramidale were also immunoreactive for Kv1.4 and Kvβ2, suggesting that these cells may express K+channel complexes containing four different Kv1 α-subunits in addition to both β-subunits. As mentioned above, the coexpression of Kvβ1 with Kv1.1 and Kv1.4 within these cells suggests that they most likely contain a high density of rapidly inactivating, dendrotoxin (DTX)-sensitive channels.
Fig. 5.
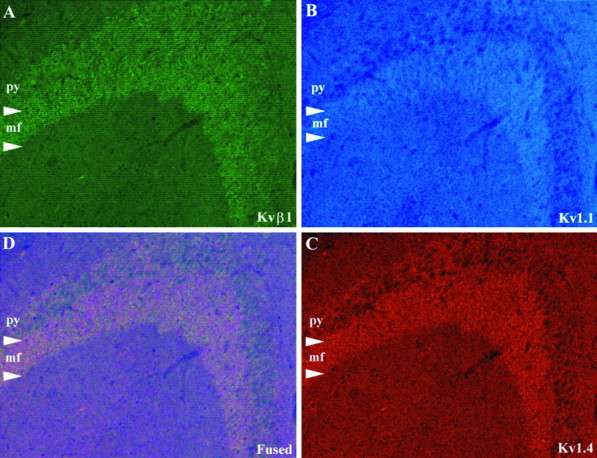
Triple-label immunfluorescence demonstrating the colocalization of KvB1 (A), Kv1.1 (B), and Kv1.4 (C) in the mossy fiber zone of CA3. These three subunits are colocalized at or near the terminals of the mossy fiber pathway. py, Stratum pyramidale; mf, mossy fiber zone.
In stratum radiatum and stratum oriens of CA1–CA3 there is a prominent zone of immunoreactivity for Kv1.1 that corresponds precisely with the termination zone of the Schaffer collateral pathway (Fig. 3). This zone of Kv1.1 immunoreactivity is matched by similar, although much less prominent, patterns of immunoreactivity for Kv1.4, Kvβ1, and Kvβ2. In stratum moleculare of CA1–CA3 there is a band of immunoreactivity for Kv1.2 and Kv1.4 and a weaker, less distinct band of immunoreactivity for Kvβ2 (Fig. 3). Interestingly, the location of this band corresponds precisely to the termination zone of the perforant path. Somewhat surprisingly, this CA component of the perforant path seems to lack Kv1.1, suggesting that the subunit composition of channel complexes located on the Ammonic and dentate components of the perforant path are distinct. Moreover, the inclusion of Kv1.2 in these complexes, together with Kv1.4, would be expected to confer DTX sensitivity to these channels.
As described previously (Rhodes et al., 1995; Bekele-Arcuri et al., 1996), Kv2.1 immunoreactivity is present in many of the same neurons that are immunoreactive for Kv1 α- and β-subunits. However, in these cells, immunoreactivity for Kv2.1 is concentrated in large patches on the somatodendritic membrane (see Scannevin et al., 1996), whereas immunoreactivity for the Kv1 α- and β-subunits is concentrated in axons and terminal fields or is distributed evenly throughout the cell soma and along dendritic processes. These differences between the qualitative appearance of Kv2.1 immunoreactivity from that observed for the other subunits are consistent with our biochemical data and indicate that Kv2.1 does not associate with these subunits despite extensive cellular coexpression.
Caudate nucleus, globus pallidus, and substantia nigra
Moderate to high levels of Kv1.1, Kv1.4, Kvβ1, and Kvβ2 mRNA expression have been observed in rat caudate nucleus (Sheng et al., 1992; Rettig et al., 1994; Wang et al., 1994; Rhodes et al., 1996), with low levels in the globus pallidus and substantia nigra. However, only moderate to low levels of immunoreactivity for these subunits have been observed in the caudate nucleus itself, suggesting that the corresponding polypeptides are likely to be concentrated at or near the axons and terminals of striatal neurons. In fact, there is a moderate to high density of immunoreactivity for Kv1.1, Kv1.4, Kvβ1, and Kvβ2 in both of the major subcortical targets of striatal efferents, the globus pallidus and the pars reticulata of the substantia nigra (Fig. 6). The pattern of immunoreactivity in these latter structures indicates that Kv1.1, Kv1.4, Kvβ1, and Kvβ2 are codistributed at or near the terminal fields of striatal efferents where they may regulate GABAergic or peptidergic (substance P and enkephalin) neurotransmission.
Fig. 6.
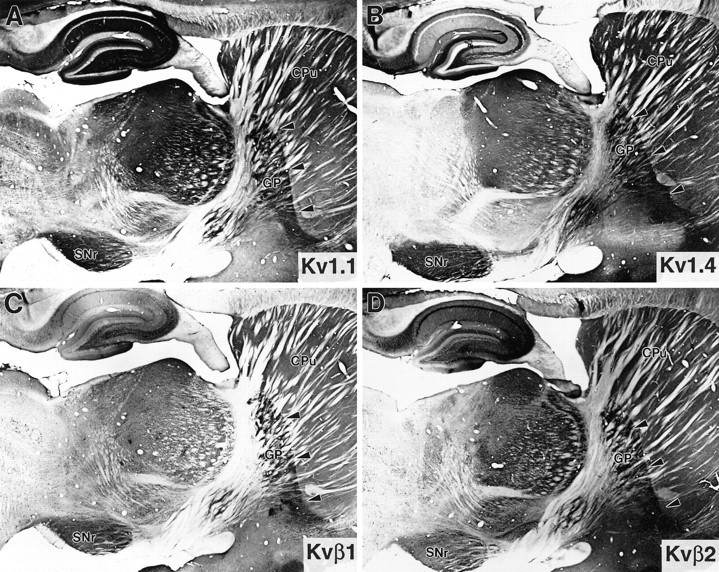
Immunohistochemical localization of Kv1.1, Kv1.4, Kvβ1, and Kvβ2 (A–D, respectively) in the caudate nucleus (CPu), globus pallidus (GP;arrowheads), and pars reticulata of the substantia nigra (SNr). These four subunits are codistributed in the termination zones of neostriatal efferents to the globus pallidus and substantia nigra.
Cerebellar cortex
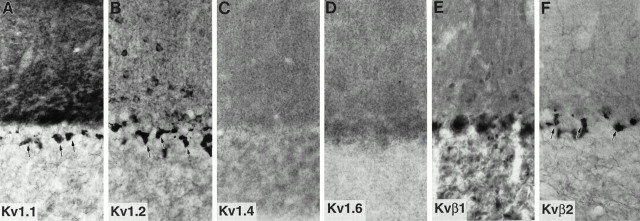
As described previously by us (Rhodes et al., 1995, 1996) and by others (McNamara et al., 1993, 1996; Sheng et al., 1993; Wang et al., 1993, 1994; Scott et al., 1994a; Veh et al., 1995), there is a high density of Kv1.1, Kv1.2, Kvβ1, and Kvβ2, and a much lower density of Kv1.4 and Kv1.6 in the cerebellum (Fig.7). Perhaps the greatest concentration of Kv1.1, Kv1.2, and Kvβ2 in the entire brain is found in the axon terminal plexuses of cerebellar granule cells that surround the initial segment of Purkinje cell axons. When these three subunits are visualized simultaneously in a single tissue section by triple-label immunofluorescence (Fig. 8), it is apparent that these subunits are precisely colocalized in this terminal plexus. Similarly, it is obvious that these three subunits are precisely colocalized in the juxtaparanodal region of virtually all myelinated axons located in the cerebellar white matter (Fig.9). Because neither Kvβ1 nor Kv1.4 is expressed at either the basket cell terminals or at nodes of Ranvier in the cerebellar white matter, Kv1.1, Kv1.2, and Kvβ2 associate here to form what is likely a DTX-sensitive, slowly inactivating, delayed rectifier type channel complex. Interestingly, immunoreactivity for Kv1.1 is also concentrated in the neuropil of the molecular layer of the cerebellar cortex. This pattern of Kv1.1 immunoreactivity is matched by much weaker immunoreactivity for Kv1,4, Kv1.6, Kvβ1, and Kvβ2, suggesting that these subunits may all be localized on afferent inputs to the cerebellar cortex.
Fig. 7.
Immunohistochemical localization of K+ channel α- and β-subunits in the cerebellar cortex. Note the high density of immunoreactivity for Kv1.1, Kv1.2, and Kvβ2 in the terminals of cerebellar basket cells (arrows).
Fig. 8.
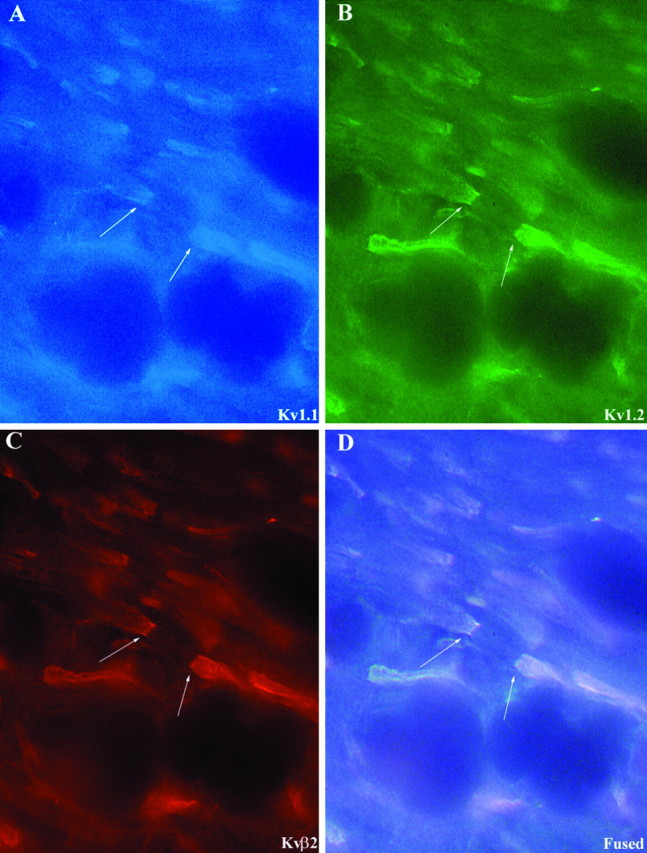
Triple-label immunfluorescence demonstrating the colocalization of Kv1.1 (A), Kv1.2 (B), and Kvβ2 (C) in cerebellar basket cell terminals. Arrows point to the terminal Pinceau of cerebellar basket cells, where all three subunits are colocalized.
Fig. 9.
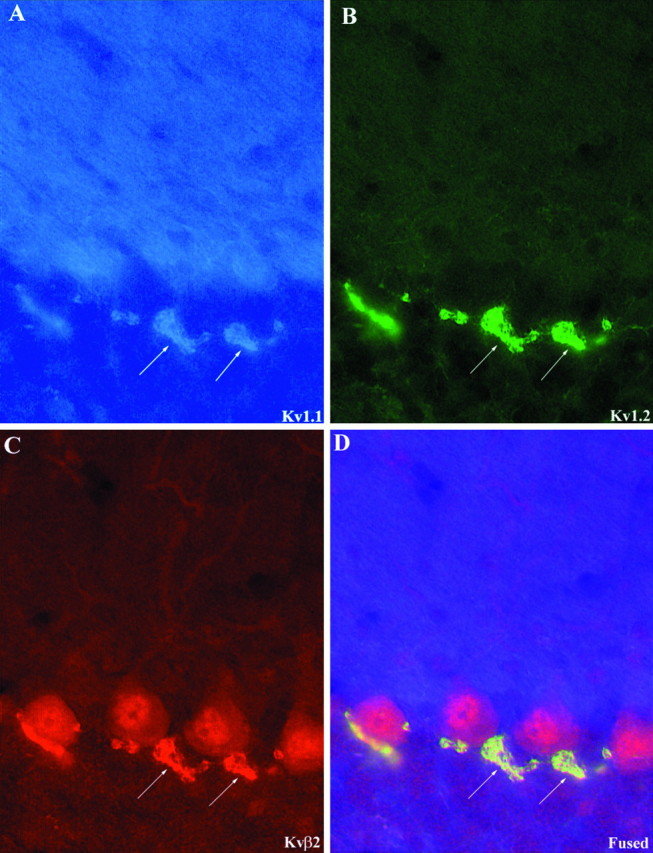
Triple-label immunfluorescence demonstrating the colocalization of Kv1.1 (A), Kv1.2 (B), and Kvβ2 (C) at the juxtaparanodal region of nodes of Ranvier in the cerebellar white matter. Arrows point to the juxtaparanodal membrane of a node of Ranvier.
DISCUSSION
The results of the present study indicate that Kvβ1 and Kvβ2 associate and colocalize with each other and with each of theShaker-related Kv1 α-subunits but not with theShab-related Kv2.1 α-subunit. These data confirm and extend our previous studies performed with a pan-β-subunit antibody that recognizes both Kvβ1 and Kvβ2, which showed β-subunit association and colocalization with Kv1.2 and Kv1.4 but not Kv2.1 (Rhodes et al., 1995). These results are also consistent with our previous studies of recombinant α- and β-subunit polypeptides expressed in transfected cells, in which both Kvβ1 and Kvβ2 were found to associate with all members of the Kv1 subfamily tested but not with Kv2.1 (Nakahira et al., 1996).
Although Kvβ1 and Kvβ2 are found associated with all of the Kv1 subfamily members examined, distinct differences in the levels of specific α-subunit polypeptides that could be coimmunoprecipitated with the different β-subunit-specific antibodies were observed. For example, most if not all of the Kv1.1, Kv1.2, Kv1.4, and Kv1.6 in these brain preparations was present in complexes containing at least one Kvβ2 β-subunit, based on the amount of the α-subunit coimmunoprecipitated by the Kvβ2 antibody relative to that obtained by direct immunoprecipitation with the respective α-subunit-specific antibody. In contrast, only small fractions of the pool of rat brain Kv1.1 and Kv1.4, and even less of Kv1.2 and Kv1.6, were found in K+ channel complexes immunopurified with the anti-Kvβ1 antibody. These data together predict that overall in the brain, most if not all Kv1-containing K+ channel complexes contain Kvβ2, whereas few have Kvβ1 as a constituent subunit. These results are especially intriguing given that Kv1-containing K+ channel complexes are thought to have an α4β4 subunit composition (Parcej et al., 1992). Thus, despite the presence of four β-subunits per K+ channel complex, only a small proportion of rat brain K+ channels have even a single Kvβ1 subunit present. These results are consistent with our previous studies that indicated that Kvβ2 was overall much more abundant than Kvβ1 in the brain, and that most β-subunit-containing K+channel complexes contain Kvβ2 in the absence of Kvβ1 (Rhodes et al., 1996). However, it is important to stress that these data reflect only the overall bulk of all brain Kv1-containing channel complexes and in no way preclude the possibility that important pools of channels in discrete neuronal populations may be under the functional influence of the inactivation-modulating Kvβ1 subunit.
Our immunoprecipitation data show that all of the Kv1 family members tested could be found in association with Kvβ1 and Kvβ2 and vice versa. Recently, members of the mammalian disks large family, such as postsynaptic density protein (PSD)-95 and synapse associated protein (SAP)-97, have been shown to interact with Kv1 family members and to induce their clustering through the formation of large, multiprotein complexes (Kim et al., 1995; Sheng and Kim, 1996). It is formally possible that such interactions between multiple Kv1-containing K+ channel complexes could complicate studies of subunit associations within K+channel complexes. However, immunoblot analyses using antibodies that recognize PSD-95 and SAP-97 could not detect these proteins in any of the anti-α- or anti-β-subunit immunoprecipitation reactions characterized here (Z. Bekele-Arcuri and J. S. Trimmer, unpublished observations). These results suggest that if such supramolecular aggregation of K+ channel α- and β-subunit polypeptides does occur, it apparently does not remain intact under the detergent extraction conditions used here that are designed to maintain subunit interactions within K+channel complexes.
Direct comparisons of the cellular localization of Kvβ1 and Kvβ2 immunoreactivity with staining for the Kv1.1, Kv1.2, Kv1.4, Kv1.6, and Kv2.1 α-subunits in sequential sections confirmed the immunoprecipitation data and indicated that there is extensive overlap in the distribution of Kvβ1 with Kv1.1 and Kv1.4 and extensive overlap in the distribution of Kvβ2 with Kv1.1 and Kv1.2. In addition, triple immunofluorescence labeling of Kv1.1, Kv1.2, and Kvβ2 indicated that these three subunits are precisely colocalized in many brain regions and strikingly so in the juxtaparanodal membrane at nodes of Ranvier and in the terminals of cerebellar basket cells. Triple-label immunofluorescence also revealed that in the hippocampal mossy fiber pathway (Fig. 5) and in the globus pallidus (data not shown), Kv1.1, Kv1.4, and Kvβ1 are precisely colocalized. Together, the immunoprecipitation and immunohistochemical data indicate that although Kvβ1 is widely expressed in the brain, this β-subunit seems to be preferentially expressed in cell types and projection systems that also express a high density of Kv1.1 and Kv1.4. The tendency of Kvβ1 to associate and colocalize with Kv1.1 and Kv1.4 in the brain is intriguing, because Kvβ1 has been shown to dramatically accelerate the kinetics of inactivation of Kv1.1 and Kv1.4 and other Kv1 subfamily members on transient coexpression in Xenopusoocytes (Rettig et al., 1994; Heinemann et al., 1996). Thus it appears that Kvβ1 may be incorporated into native K+channel complexes that produce DTX-sensitive transient K+ currents. This conclusion is based on a number of considerations. First, when the DTX-sensitive, noninactivating Kv1.1 α-subunit is coexpressed with the DTX-insensitive, rapidly inactivating Kv1.4 α-subunit, DTX-sensitive, rapidly inactivating currents indicative of the heteromultimeric channels are obtained (Ruppersberg et al., 1990). Second, coexpression of Kvβ1 confers rapid inactivation on the normally noninactivating Kv1.1 currents and in fact further accelerates the inactivation of Kv1.4 (Rettig et al., 1994; Heinemann et al., 1996). Thus, channels containing Kv1.1, Kv1.4, and Kvβ1 would be expected to have rapid inactivation typical of transient or IA currents and to exhibit sensitivity to block by DTX. This may explain the initial discrepancy that cloned cDNAs that yield transient currents (Kv1.4, Kv4.2, and Kv4.3) in each case are DTX-insensitive, yet many of theIA currents that have been recorded from central neurons are DTX-sensitive (Halliwell, 1990; Harvey, 1997).
Using a similar logic, channels composed of Kv1.1, Kv1.2, and Kvβ 2, such as those seen in juxtaparanodal regions and in basket cell terminals would be predicted to form DTX-sensitive, noninactivating, delayed rectifier channels, giving rise toIK(DTX) currents similar to those that have been described in peripheral neurons (Stansfeld and Feltz, 1988; Safronov et al., 1993). These DTX-sensitive, noninactivating and slowly inactivating currents at peripheral nodes of Ranvier have been studied extensively; however, such currents in central neurons are less well characterized, perhaps attributable to their inaccessibility at nodes and at presynaptic terminals. Thus the specific contribution of these abundant Kv1.1, Kv1.2, and Kvβ2 channel complexes to neuronal K+ currents in the cerebellum and in other regions rich in this channel type remains unclear.
Since the initial cloning of Kvβ1 and Kvβ2 from mammalian brain (Rettig et al., 1994; Scott et al., 1994b), other β-subunit cDNAs have also been isolated. An alternative splice variant of Kvβ1, termed Kvβ1b or Kvβ1.2, has been isolated from human, ferret, and rat heart (England et al., 1995a,b; Majumder et al., 1995; Morales et al., 1995). Effects of this β-subunit on Kv subunits appear to be restricted to Kv1.4 and Kv1.5, with no observed effects on Kv1.1, Kv1.2, and Kv2.1. The expression pattern of Kvβ1b in the brain is not known. As mentioned above, a cDNA arising from a third β-subunit gene, Kvβ3, has also been isolated from rat brain (Heinemann et al., 1995). Effects of this β-subunit are restricted to accelerating the inactivation of the transient Kv1.4 current further; effects on other Kv1 subfamily members were not observed (Heinemann et al., 1995). A β-subunit, Kvβ4, that enhances expression of rat Kv2.2, but has no effects on any of the Kv1 subfamily members tested, has also been recently identified (Fink et al., 1996). Additional studies will be required to determine the subunit associations of these recently identified β-subunits, and others that may arise from alternative splicing of the highly complex Kvβ1 gene (Leicher et al., 1996), to determine their relative contributions to voltage-gated K+ currents in the mammalian CNS.
Footnotes
This work was supported by Wyeth-Ayerst Research (Princeton, NJ) and by National Institutes of Health Grant NS34383 (J.S.T.). This work was done during the tenure of an Established Investigatorship from the American Heart Association (J.S.T.). We thank Drs. John A. Moyer and James E. Barrett for critically reviewing this manuscript.
Correspondence should be addressed to Dr. James S. Trimmer, Department of Biochemistry and Cell Biology, State University of New York, Stony Brook, NY 11794-5215.
REFERENCES
- 1.Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel α- and β-subunit polypeptides. Neuropharmacology. 1996;35:851–865. doi: 10.1016/0028-3908(96)00128-1. [DOI] [PubMed] [Google Scholar]
- 2.England SK, Uebele VN, Kodali J, Bennett PB, Tamkun MM. A novel K+ channel β-subunit (hKvβ1.3) is produced via alternative mRNA splicing. J Biol Chem. 1995a;270:28531–28534. doi: 10.1074/jbc.270.48.28531. [DOI] [PubMed] [Google Scholar]
- 3.England SK, Uebele VN, Shear H, Kodali J, Bennett PB, Tamkun MM. Characterization of a voltage-gated K+ channel β subunit expressed in human heart. Proc Natl Acad Sci USA. 1995b;92:6309–6313. doi: 10.1073/pnas.92.14.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink M, Duprat F, Lesage F, Heurteaux C, Romey G, Barhanin J, Lazdunski M. A new K+ channel β subunit to specifically enhance Kv2.2 (CDRK) expression. J Biol Chem. 1996;271:26341–26348. doi: 10.1074/jbc.271.42.26341. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell JV. K+ channels in the central nervous system. In: Cook NS, editor. Potassium channels. Wiley; New York: 1990. pp. 350–359. [Google Scholar]
- 6.Harvey AL. Recent studies on dendrotoxins and potassium ion channels. Gen Pharmacol. 1997;28:7–12. doi: 10.1016/s0306-3623(96)00173-5. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann SH, Rettig J, Wunder F, Pongs O. Molecular and functional characterization of a rat brain Kvβ3 potassium channel subunit. FEBS Lett. 1995;377:383–389. doi: 10.1016/0014-5793(95)01377-6. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of K-v channel beta-subunits from rat brain. J Physiol (Lond) 1996;493:625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 10.Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DAG, Sportsman JR, Elder JH. Improved technique utilizing nonfat dry milk for analysis of proteins and nucleic acids transferred to nitrocellulose. Gene Anal Tech. 1984;1:3–8. [Google Scholar]
- 12.Kim E, Neithammer M, Rothschild A, Jan YN, Sheng M. Clustering of shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;387:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 13.Leicher T, Roeper J, Weber K, Wang X, Pongs O. Structural and functional characterization of human potassium channel subunit Kvβ1 (KCNA1B). Neuropharmacology. 1996;35:787–796. doi: 10.1016/0028-3908(96)00133-5. [DOI] [PubMed] [Google Scholar]
- 14.Maizel JV. Polyacrylamide gel electrophoresis of viral proteins. Methods Virol. 1971;5:179–246. [Google Scholar]
- 15.Majumder K, De Biasi M, Wang Z, Wible BA. Molecular cloning and functional expression of a novel potassium channel β-subunit from human atrium. FEBS Lett. 1995;361:13–16. doi: 10.1016/0014-5793(95)00120-x. [DOI] [PubMed] [Google Scholar]
- 16.McCormack K, McCormack T, Tanouye M, Rudy B, Stümer W. Alternative splicing of the human Shaker K+ channel β1 gene and functional expression of a β2 gene product. FEBS Lett. 1995;370:32–36. doi: 10.1016/0014-5793(95)00785-8. [DOI] [PubMed] [Google Scholar]
- 17.McNamara NM, Muniz ZM, Wilkin GP, Dolly JO. Prominent location of a K+ channel containing the α subunit Kv1.2 in the basket cell nerve terminals of rat cerebellum. Neuroscience. 1993;57:1039–1045. doi: 10.1016/0306-4522(93)90047-j. [DOI] [PubMed] [Google Scholar]
- 18.McNamara NMC, Averill S, Wilkin GP, Dolly JO, Priestly JV. Ultrastructural localization of a voltage-gated K+ channel α subunit (Kv1.2) in the rat cerebellum. Eur J Neurosci. 1996;8:688–699. doi: 10.1111/j.1460-9568.1996.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 19.Morales MJ, Castellino RC, Crews AL, Rasmussen RL, Strauss HC. A novel β subunit increases the rate of inactivation of specific voltage-gated potassium channel α subunits. J Biol Chem. 1995;270:6272–6277. doi: 10.1074/jbc.270.11.6272. [DOI] [PubMed] [Google Scholar]
- 20.Nagaya N, Papazian DM. Potassium channel α- and β-subunits assemble in the endoplasmic reticulum. J Biol Chem. 1997;272:3022–3027. doi: 10.1074/jbc.272.5.3022. [DOI] [PubMed] [Google Scholar]
- 21.Nakahira K, Shi G, Rhodes KJ, Trimmer JS. Selective interaction of voltage-gated K+ channel β-subunits with α-subunits. J Biol Chem. 1996;271:7084–7089. doi: 10.1074/jbc.271.12.7084. [DOI] [PubMed] [Google Scholar]
- 22.Parcej DN, Scott VES, Dolly JO. Oligomeric properties of α-dendrotoxin-sensitive potassium ion channels purified from bovine brain. Biochemistry. 1992;31:11084–11088. doi: 10.1021/bi00160a018. [DOI] [PubMed] [Google Scholar]
- 23.Pongs O. Regulation of the activity of voltage-gated potassium channels by β subunits. Semin Neurosci. 1995;7:137–146. [Google Scholar]
- 24.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel α- and β-subunit polypeptides in rat brain. J Neurosci. 1995;15:5630–5671. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes KJ, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Voltage-gated K+ channel β-subunit polypeptides: expression and distribution of Kvβ1 and Kvβ2 in adult rat brain. J Neurosci. 1996;16:4846–4860. doi: 10.1523/JNEUROSCI.16-16-04846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosene DL, Van Hoesen GW. The hippocampal formation of the primate brain a review of some comparative aspects of cytoarchitecture and connections. In: Jones EG, Peters A, editors. The cerebral cortex, Vol 6. Plenum; New York: 1987. pp. 345–456. [Google Scholar]
- 28.Ruppersberg JP, Schroter KH, Sakman B, Stocker M, Sewing S, Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990;345:535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- 29.Safronov BV, Kampe K, Vogel W. Single voltage-dependent potassium channels in rat peripheral nerve membrane. J Physiol (Lond) 1993;460:675–691. doi: 10.1113/jphysiol.1993.sp019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scannevin RH, Trimmer JS. Cytoplasmic domains of voltage-sensitive K+ channels involved in mediating protein-protein interactions. Biochem Biophys Res Commun. 1997;232:585–589. doi: 10.1006/bbrc.1997.6333. [DOI] [PubMed] [Google Scholar]
- 31.Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. Identification of a cytoplasmic domain important in the polarized expression and clustering of the Kv2.1 K+ channel. J Cell Biol. 1996;135:1619–1632. doi: 10.1083/jcb.135.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott VE, Muniz ZM, Sewing S, Lichtinghagen R, Parcej DN, Pongs O, Dolly JO. Antibodies specific for distinct Kv subunits unveil a hetero-oligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain. Biochem J. 1994a;33:1617–1623. doi: 10.1021/bi00173a001. [DOI] [PubMed] [Google Scholar]
- 33.Scott VE, Rettig J, Parcej DN, Keen JN, Findlay JB, Pongs O, Dolly JO. Primary structure of a β-subunit of α-dendrotoxin-sensitive K+ channels from bovine brain. Proc Natl Acad Sci USA. 1994b;91:1637–1641. doi: 10.1073/pnas.91.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sewing S, Roeper J, Pongs O. Kvβ1 subunit binding specific for Shaker-related potassium channel α subunits. Neuron. 1996;16:455–463. doi: 10.1016/s0896-6273(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 35.Sheng M, Kim E. Ion channel associated proteins. Curr Opin Neurobiol. 1996;6:602–608. doi: 10.1016/s0959-4388(96)80091-2. [DOI] [PubMed] [Google Scholar]
- 36.Sheng M, Liao YJ, Jan YN, Jan LY. Presynaptic -current based on heteromultimeric K+ channels detected in vivo. Nature. 1993;365:72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- 37.Sheng M, Tsaur ML, Jan YN, Jan LY. Contrasting subcellular localization of the Kv1.2 K+ channel subunits in different neurons of rat brain. J Neurosci. 1994;14:2408–2417. doi: 10.1523/JNEUROSCI.14-04-02408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem. 1994;269:23204–23211. [PubMed] [Google Scholar]
- 39.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. β-subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 40.Stansfeld CE, Feltz A. Dendrotoxin-sensitive K+ channels in dorsal root ganglion cells. Neurosci Lett. 1988;93:49–55. doi: 10.1016/0304-3940(88)90011-0. [DOI] [PubMed] [Google Scholar]
- 41.Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel in rat brain. Proc Natl Acad Sci USA. 1991;88:10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 family subunits: contrasting subcellular locations and neuron-specific co-localization in rat brain. Eur J Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu W, Xu J, Li M. Inactivation of Kv1 potassium channels by the β-subunit, Kvβ1, involves Kvβ1 binding to NAB, a region that mediates subfamily-specific subunit assembly. Neuron. 1996;16:441–453. doi: 10.1016/s0896-6273(00)80062-8. [DOI] [PubMed] [Google Scholar]