Abstract
Cholinergic and serotonergic fiber systems invade the developing visual cortex several weeks before eye opening; both transmitters have been implicated in plasticity of neocortical circuits. These transmitters have been presumed to act predominantly through second messenger-coupled receptors, because fast cholinergic or serotonergic neurotransmission has never been observed in neocortex. However, acetylcholine and serotonin also act on ligand-gated ion channels; the nicotinic acetylcholine receptor and the serotonin 5-HT3receptor, respectively. Here, using whole-cell patch-clamp techniques in developing ferret visual cortex, we pharmacologically isolated fast, spontaneous, and evoked cholinergic and serotonergic synaptic events in pyramidal cells and interneurons of all cortical layers. The number of cells receiving such inputs increased with the ingrowth of thalamic afferents, and the frequencies of the spontaneous events increased at eye opening. Thus, both acetylcholine and serotonin can mediate fast synaptic transmission in the visual cortex; the early onset of these mechanisms suggests a role during initial stages of circuit formation and during subsequent experience-dependent remodeling of cortical connections.
Keywords: visual cortex, ferret, whole-cell recording, serotonin, acetylcholine, nicotinic acetylcholine receptor, 5-HT3receptor, development, synaptic current
Beginning at embryonic stages the mammalian neocortex is densely innervated by cholinergic, serotonergic, and noradrenergic brainstem afferents. Their presence suggests roles for these neuromodulators in circuit formation and synaptogenesis. These systems undergo laminar rearrangements during cortical development (D’Amato et al., 1987; Henderson, 1991; Voigt and De Lima, 1991a,b; Shaw et al., 1984, 1986) and, especially in rodents, are associated with the layout of thalamocortical projections in primary sensory areas (D’Amato et al., 1987; Fuchs, 1989; Schlaggar and O’Leary, 1994; Broide et al., 1995). These transmitters have indeed been repeatedly implicated in the plasticity of neocortical circuits, modulating ocular dominance columns (Kasamatsu and Pettigrew, 1976;Bear and Singer, 1986; Imamura and Kasamatsu, 1989; Gu and Singer, 1993, 1995; Liu et al., 1994) and thalamocortical and intracortical synaptic transmission (Broecher et al., 1992; Vidal and Changeux, 1993;Read et al., 1994; Rhoades et al., 1994). Cholinergic transmission in particular may play a critical role during visual system development. Cholinergic synaptic currents, mediated by nicotinic receptors, trigger propagation of spontaneous waves of excitation in developing ferret retinal ganglion cells (Feller et al., 1996), which may shape connectivity in other regions, including visual cortex. Moreover, thalamic fiber ingrowth triggers nicotinic acetylcholine receptor expression in cortical neurons (Broide et al., 1996), suggesting a role in the development of thalamocortical projection patterns.
The classical concept of the function of cholinergic and monoaminergic afferents in the CNS emphasizes their interactions with second messenger coupled receptors (Hasselmo, 1995), and many of their functions in the developing nervous system are indeed mediated via G-protein-coupled postsynaptic receptor types (Gu and Singer, 1993,1995; Liu et al., 1994). However, both acetylcholine and serotonin can also activate fast, ionotropic receptor subtypes. The predominant physiological role of these receptors, the nicotinic acetylcholine receptor and the serotonergic 5-HT3 receptor, is generally considered to be presynaptic modulation of transmitter release (Kawa, 1994; Role and Berg, 1996). Neuronal nicotinic ACh receptors facilitate the release of various neurotransmitters (Role and Berg, 1996) including glutamate in the prefrontal cortex (Vidal and Changeux, 1993); serotonin 5-HT3 receptor activation, on the other hand, increases GABA release in hippocampal interneurons (Kawa, 1994).
Despite the prominent cholinergic and serotonergic fiber systems in neocortex, fast events mediated by these neurotransmitters have not been detected, although there have been occasional observations of fast postsynaptic transmission in other regions of the adult mammalian CNS (Sugita et al., 1992, Zhang et al., 1993). Using whole-cell recording techniques and optical recording of synaptic activity in slices of developing ferret primary visual cortex, we uncovered fast postsynaptic currents mediated by both acetylcholine and serotonin. This adds a new avenue for the action of these neurotransmitters in addition to their second messenger-mediated effects.
MATERIALS AND METHODS
Animals and dissection. Postnatal ferrets [postnatal day 6 (P6)–P57; Marshall Farms, New Rose, NY] were deeply anesthetized with Nembutal (100 mg/kg, i.p.) and decapitated. Coronal slices (400–500 μm thickness) of primary visual cortex were prepared using a Vibratome (Ted Pella, Inc., Redding, CA). Dissections were made in artificial CSF (ACSF; containing, in mm: 248 sucrose, 5 KCl, 5.3 KH2PO4, 1.3 MgSO4, 3.2 CaCl2, 10 dextrose, 25 NaHCO3, and 1 kynurenic acid) oxygenated with a mixture of 95% O2 and 5% CO2, pH 7.4, chilled to 4°C. Slices were maintained in an interface chamber at a temperature of 33°C and in an atmosphere of 95% CO2/5% O2 as described previously (Durack and Katz, 1996). Sucrose ACSF was replaced with standard ACSF (in mm: 125 NaCl, 5 KCl, 5.3 KH2PO4, 1.3 MgSO4, 3.2 CaCl2, 10 dextrose, and 25 NaHCO3) after 1 hr. For patch-clamp and optical recording individual slices were transferred to a recording chamber and continuously superfused with ACSF at room temperature.
Electrophysiology. Electrophysiological recordings were performed using standard whole-cell patch-clamp methods (Blanton et al., 1989). The intracellular solution consisted of (in mm): 110 d-gluconic acid, 110 CsOH, 11 EGTA, 10 CsCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 1.8 GTP, and 3 ATP, pH 7.2, and contained 0.5%N-(2-aminoethyl)biotinamide (Neurobiotin; Molecular Probes, Eugene, OR). Voltage-clamp recordings were conducted using an Axopatch 1D amplifier (Axon Instruments). Unless specified otherwise the holding potential was −60 mV. Recordings were filtered at 1 kHz and digitized at 2.5–4 kHz using a TL-1 analog to digital converter in conjunction with pClamp 5.5.1 software (Axon instruments). Series resistances ranged from 11 to 17 MΩ; a 30–50% compensation was usually achieved using the amplifier adjustments. To exclude nonspecific effects of antagonists, etc., only cells with a stable holding current over the entire recording period were included in the analysis. The frequency of spontaneous synaptic currents was determined from 1–6 min continuous recordings. Cumulative probability plots were used to analyze amplitude and frequency distributions of synaptic currents. The amplitude and interevent interval distributions before and after addition of receptor selective antagonists were compared using a one-way ANOVA.
Synaptic currents were electrically evoked via a bipolar stimulation electrode positioned at the layer 6–white matter border (pulse duration, 100 μsec; amplitude, 130–600 μA). The slice was stimulated at a frequency of 0.1 Hz. Five to 28 synaptic responses were averaged for further analysis. Slices were fixed in 4% paraformaldehyde in PBS, pH 7.4, for subsequent histological processing of neurobiotin-filled cells. Labeled cells were visualized by standard immunoperoxidase staining techniques (Durack and Katz, 1996).
Characterization of postsynaptic responses using photostimulation. Scanning laser photostimulation (Dalva and Katz, 1994) was used for a fast, direct activation of glutamate, GABA, and acetylcholine receptors on recorded neurons. Slices were incubated in the α-carboxy-2-nitrobenzyl (CNB)-caged forms of (in μm): 150 glutamate, 300 GABA, or 200–400 carbamylcholine, all from Molecular Probes. Active neurotransmitters were liberated from the caged precursors by 2–10 msec illumination with UV light (351–364 nm) delivered by a 50 mW continuous-wave argon ion laser (Coherent Enterprise model 622). To prevent synaptic activation, recordings were done in the presence of 3 μmTTX. Usually a small map (500 × 500 μm) of direct activation was recorded around the cell body at a 50 μm spacing (2 sec interval between flashes). The same positions were stimulated under control conditions and after application of receptor-selective antagonists.
RESULTS
The results of our study are presented in two major sections. We first describe the pharmacology and biophysical properties of spontaneous and evoked cholinergic and serotonergic synaptic currents. We then examine the cell type and laminar specificity of these currents and relate developmental changes in their frequencies and in the percentage of cells showing these inputs to particular maturational stages of the ferret visual system.
Acetylcholine and serotonin mediate fast spontaneous synaptic events in the developing ferret visual cortex
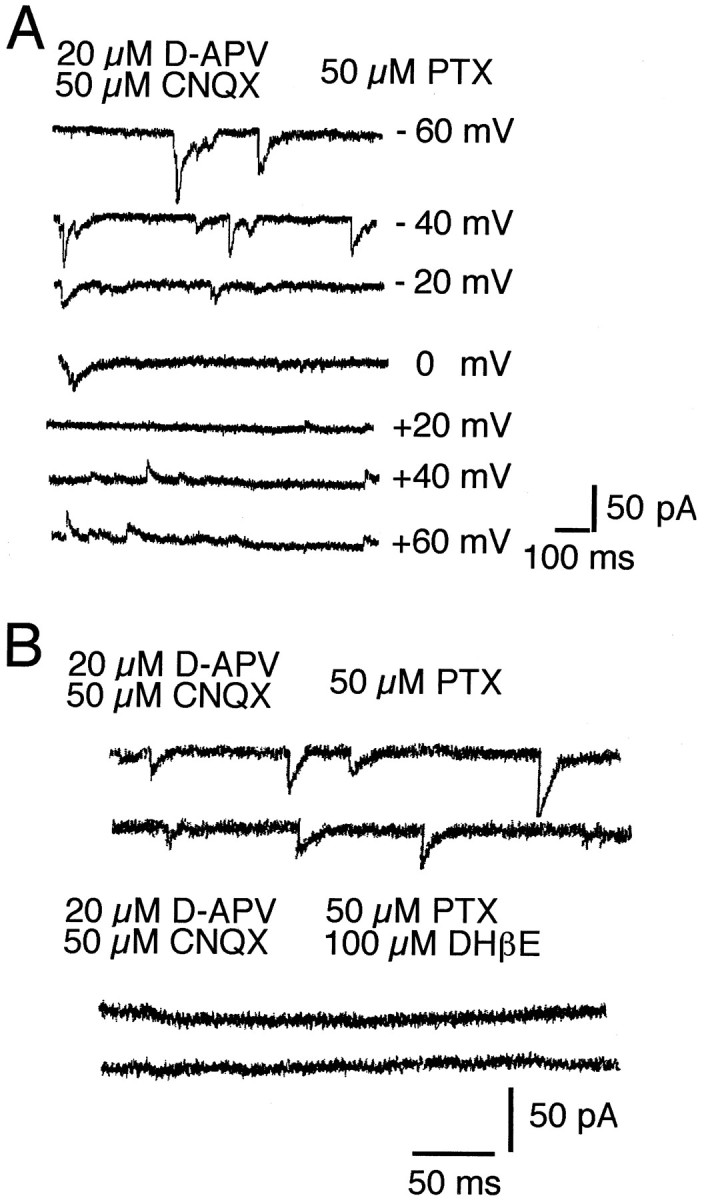
To determine whether acetylcholine and serotonin contribute to fast synaptic transmission in the ferret visual cortex, we used whole-cell and optical recording techniques to monitor synaptic activity in cortical slices. Whole-cell recordings of spontaneous synaptic activity were made from 152 neurons in slices prepared from animals aged between P8 and P37. We used a physiological chloride gradient allowing inhibitory and excitatory synaptic currents to be distinguished by changing the holding potential. Under these conditions inhibitory currents reversed near −30 mV, whereas glutamatergic synaptic events reversed near 0 mV. To isolate nicotinic and serotonergic currents pharmacologically, glutamatergic and GABAergic synaptic transmission were blocked by bath application of the NMDA receptor antagonist d-APV (20 μm, a concentration that has been shown to be more than sufficient to block NMDA receptor-mediated responses both in vitro and in vivo; Davis, 1992), the non-NMDA glutamate receptor antagonist CNQX (50 μm), and the GABAA receptor antagonists picrotoxin (50 μm) and bicuculline methiodide (20–50 μm). Interactions of these antagonists with nicotinic acetylcholine or 5-HT3 receptors have never been observed, which makes them appropriate tools for isolating cholinergic and serotonergic synaptic currents. GABAA receptor-mediated events were completely suppressed by 50 μm picrotoxin. After bath application of these antagonists, residual excitatory spontaneous synaptic activity was recorded in 40% of cortical neurons between P18 and P37 (18 of 45 tested cells). These synaptic currents were completely abolished by 50–100 μmd-tubocurarine, a nicotinic acetylcholine receptor antagonist, suggesting the presence of fast synaptic transmission via neuronal nicotinic acetylcholine receptors in developing ferret visual cortex. The 18 cells displaying tubocurarine-sensitive synaptic events included pyramidal neurons from layer 2/3 (n = 8), layer 5 (n = 7), and layer 6 (n = 2), and one layer 4 stellate cell.
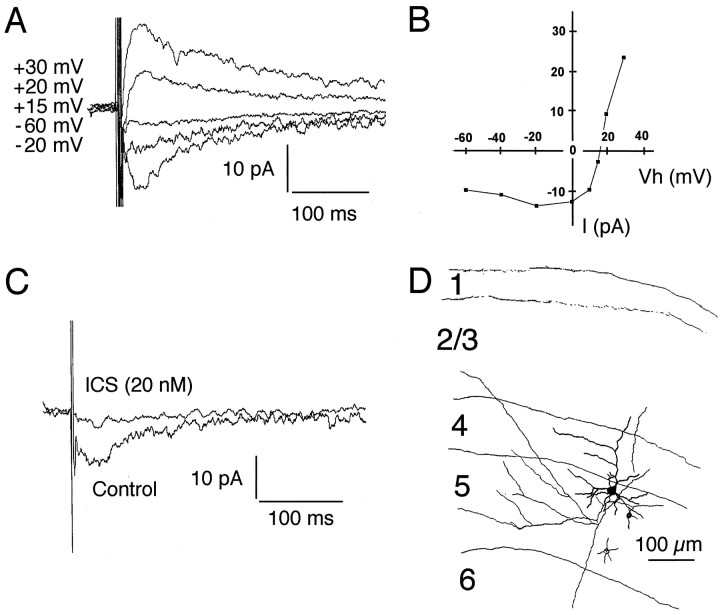
d-Tubocurarine is relatively nonselective, affecting both serotonin 5-HT3 receptors and GABAA and glycine receptors in addition to nACh receptors. Consequently, more selective antagonists were used for analysis of these currents in 107 additional cortical neurons. Between P8 and P12, before ingrowth of thalamic afferents into the developing cortical plate, 23% of tested cells (n = 26) had spontaneous synaptic events suppressed by the selective nACh receptor antagonist dihydro-β-erythroidine (DHβE, 100 μm; Figs. 1,2B). The muscarinic antagonist atropine (100 μm) did not affect these events. Nicotinic events were also blocked by α-cobrotoxin (100–200 nm, n = 7), whereas α-bungarotoxin (100–200 nm, n = 6) had no effect, even if applied for >30 min. This indicates that the nicotinic receptor subtypes expressed by developing cortical neurons are not the α7–9 subunit-containing receptors that are blocked by α-bungarotoxin (Role and Berg, 1996; Weaver and Chiapinelli, 1996). Spontaneous cholinergic synaptic currents reversed between +15 and +40 mV (mean, +23.8 mV; SEM 8.6 mV; n = 4; Fig. 2A), consistent with the prominent Ca2+ permeability described for neuronal nACh receptors (Sargent, 1993). A smaller fraction of the recorded neurons (8%) exhibited spontaneous serotonergic synaptic currents blocked by the 5-HT3 receptor antagonist 3-tropanyl-indole-3-carboxylate-methiodide (ICS) 205-930 (Fig.3A). Serotonergic EPSCs reversed near +10 mV (n = 2). The majority of cells receiving these inputs showed either cholinergic or serotonergic events; however, in a small fraction of neurons (four cells) both types of synaptic currents were observed.
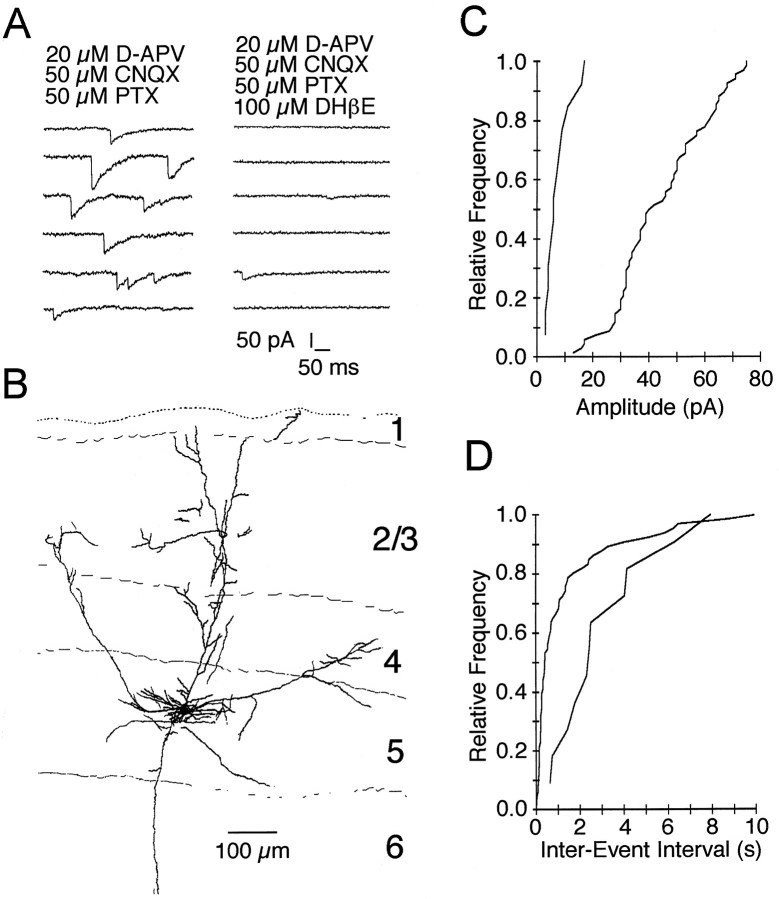
Fig. 1.
Pharmacology of spontaneous synaptic currents mediated by neuronal nicotinic acetylcholine receptors.A, Spontaneous synaptic currents recorded from a P22 layer 5 pyramidal neuron in the presence of GABA and glutamate receptor antagonists. B, These events were abolished by the selective nicotinic acetylcholine receptor antagonist DHβE (100 μm). C, Cumulative amplitude distribution for cholinergic events recorded from the cell shown inB. Addition of DHβE shifted the distribution to the left (p < 0.001, one-way ANOVA; number of events analyzed = 66 and 13), indicating that the few remaining events represent a different type of input. Remaining synaptic activity in the presence of DHβE was abolished by addition of the 5-HT3 receptor antagonist ICS 205-930. D, Cumulative frequency distribution of cholinergic events. Addition of DHβE shifted the distribution toward longer interevent intervals (p < 0.001, one-way ANOVA; number of events analyzed = 66 and 11). PTX, Picrotoxin.
Fig. 2.
A, The amplitude of cholinergic EPSCs depends on holding potential. Cholinergic PSCs reversed between +10 and +20 mV. B, Pharmacologically isolated cholinergic EPSCs recorded from the cell shown in A were abolished by DHβE. PTX, Picrotoxin.
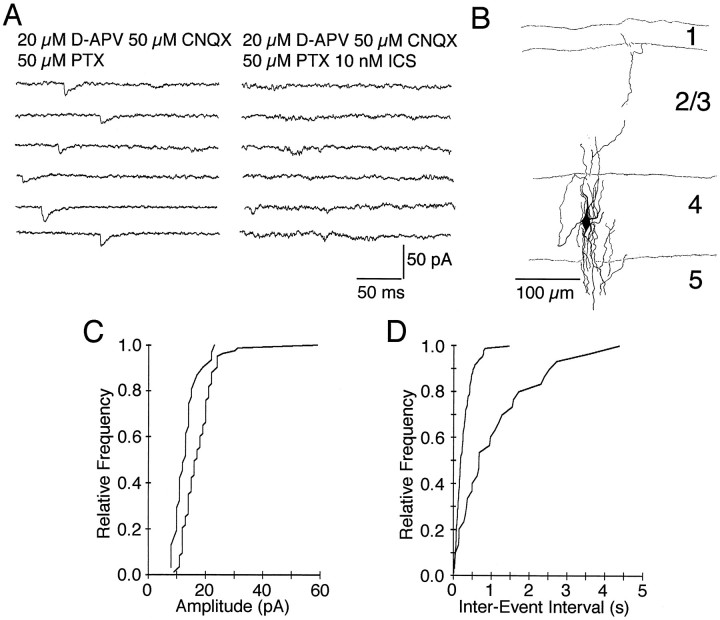
Fig. 3.
Spontaneous serotonergic EPSCs. A, Spontaneous synaptic activity recorded from a layer 4 interneuron.B, Synaptic currents were blocked by bath application of the 5-HT3 receptor antagonist ICS 205-930.C, Cumulative amplitude distribution of serotonergic EPSCs. Addition of the 5-HT3 receptor antagonist ICS 205-930 (10 nm) shifted the distribution to the left (p < 0.001, one-way ANOVA; number of events analyzed = 85 and 31). D, Cumulative frequency distribution of serotonergic events. Addition of the 5-HT3receptor antagonist ICS 205-395 (10 nm) shifted the amplitude distribution toward longer interevent intervals (p < 0.001, one-way ANOVA; number of events analyzed = 85 and 30). PTX, Picrotoxin.
We next examined the magnitude range of these previously undescribed synaptic events (Fig. 4). Amplitudes of cholinergic EPSCs ranged from 5 pA to ∼70 pA, whereas serotonergic events were smaller, ranging between 4 and 30 pA. Thus, cholinergic EPSCs were almost the size of glutamatergic events (∼10–150 pA in P40 ferret cortex), suggesting that they could make a significant contribution to excitatory transmission, whereas the contribution of the smaller, 5-HT3 receptor-mediated events may be less significant. Although cholinergic synaptic currents were slightly larger in older animals, amplitude distributions for both types of synaptic input remained similar between P8 and P37. Bath application of tetrodotoxin (3 μm) abolished both types of synaptic currents, implying that the spontaneous synaptic events were action potential evoked. Miniature EPSCs did not significantly contribute to the spontaneous activity under our recording conditions.
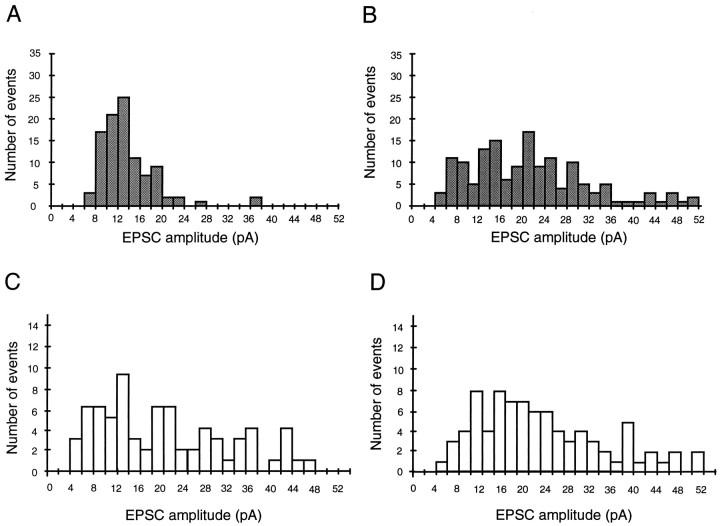
Fig. 4.
Amplitude distribution of spontaneous cholinergic and serotonergic synaptic currents. Spontaneous EPSCs were completely abolished in the presence of TTX (3 μm); distributions thus do not represent miniature synaptic currents but most likely action potential-evoked events. A, Spontaneous serotonergic currents recorded from a P8 interneuron. B, Serotonergic events recorded from a P27 layer 3 pyramidal cell.C, D, Spontaneous nicotinic EPSCs recorded from a P30 layer 5 pyramidal cell (C) and a P15 layer 2/3 pyramidal cell (D). All events were recorded in the presence of glutamate and GABA receptor antagonists and DHβE (100 μm) or ICS (10 nm).
Direct activation of postsynaptic receptors using laser scanning photostimulation
To verify that the concentrations of glutamate and GABA receptor antagonists used to isolate spontaneous cholinergic and serotonergic synaptic currents were sufficient to block all postsynaptic glutamate and GABAA receptors, we directly activated postsynaptic receptors in the presence of TTX (3 μm) using photo-uncaging of CNB-caged glutamate (150 μm) and GABA (300 μm). Photostimulation rapidly evokes postsynaptic responses and reduces the problems associated with desensitization and diffusional access (Nerbonne, 1986).
The direct responses of the recorded neurons were mapped in a 500 × 500 μm area around the recording pipette (Dalva and Katz, 1994). The same stimulation pattern was used in the presence of 20 μmd-APV and 50 μm CNQX or 50 μm picrotoxin in the bath, respectively. Figure5 shows sample recordings from the area of greatest response, which is at or near the cell body. Glutamate receptor-mediated currents were recorded from eight cells (four layer 2/3 pyramidal cells, three layer 5 pyramidal cells, and one layer 4 aspiny stellate cell) in slices from P22–P30 ferrets at a holding potential of −60 mV. Application of 20 μmd-APV considerably reduced the glutamate response in all tested cells (Fig. 5A), consistent with a large NMDA receptor-mediated component of glutamatergic postsynaptic currents during early postnatal development of the neocortex (Carmignoto and Vicini, 1992). Subsequent addition of 50 mm CNQX completely abolished the glutamate response in all recorded neurons (Fig.5A), demonstrating that the concentrations of glutamate receptor antagonists used in this study were sufficient to block all postsynaptic glutamate receptors. Responses to photo-uncaging of CNB–GABA were recorded from six neurons (three layer 2/3 pyramidal cells, two layer 5 pyramidal cells, and one layer 6 pyramidal cell) at a holding potential of −20 mV. GABAA receptor-mediated currents were completely blocked by 50 μm picrotoxin in all tested cells (Fig. 5B). These results confirm that these receptor-selective antagonists block fast glutamatergic and GABAergic synaptic transmission, allowing us to isolate nicotinic and serotonergic synaptic events. Because photostimulation provided us with a fast and reliable assay to test postsynaptic receptor activation, we also used caged carbamylcholine (200–400 μm) to activate nicotinic acetylcholine receptors directly (Denk, 1994; Denk at al., 1994). Small inward currents (5–48 pA) were recorded in 10 cells of 21 tested (five layer 2/3 pyramidal cells, three layer 5 pyramidal cells, one layer 6 pyramidal cell, and one layer 4 spiny stellate cell, ages P24–P31). These responses were blocked by 100 μm DHβE (n = 2; Fig. 5D), demonstrating that they were mediated by postsynaptic neuronal nicotinic receptors.
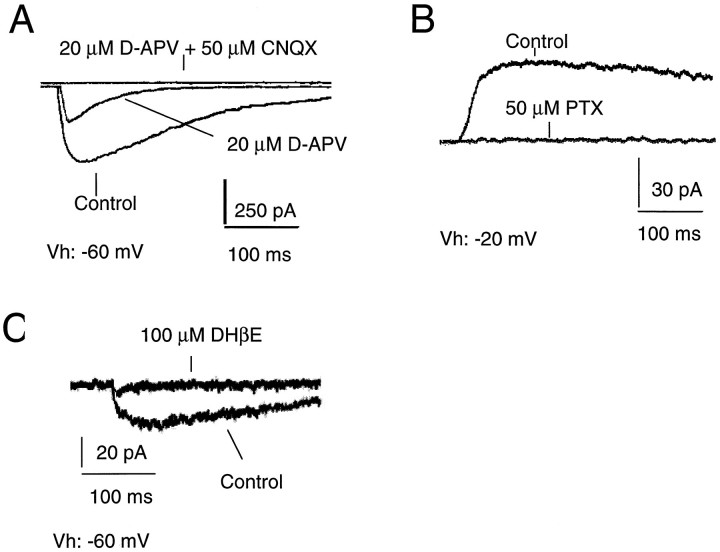
Fig. 5.
Activation of postsynaptic receptors by photo-uncaging of (in μm): 150 CNB-glutamate, 300 GABA, and 400 carbamyl choline. A, Glutamate receptor-mediated currents from a P22 pyramidal cell. In most tested neurons a large fraction of the glutamate response was carried by NMDA receptors. Glutamate receptor-gated currents were completely suppressed by 20 μmd-APV and 50 μm CNQX.B, GABAA receptor-mediated response from a P20 pyramidal cell. GABAA receptor-mediated currents were abolished by 50 μm picrotoxin (PTX). C, Uncaging of CNB-carbamylcholine evoked inward currents that were completely blocked by 50–100 mm DHβE.
Evoked synaptic responses
The spontaneous synaptic currents probably originate from the afferent fiber systems arising from the basal forebrain nucleus magnocellularis and the brainstem raphe nuclei, respectively. The only alternative explanation for the occurrence of spontaneous nicotinic and 5-HT3 receptor-mediated EPSCs would be the presence of spontaneously firing intracortical cholinergic or serotonergic neurons. However, such cells have not been observed in ferret cortex (Henderson, 1991; Voigt and De Lima, 1991a). We therefore assume that the severed fibers retain their ability to fire action potentials and release transmitter under in vitro conditions.
Cholinergic and serotonergic synaptic currents could also be electrically evoked, presumably by activating these fiber pathways in the slice. In 16 of 35 cells tested, electrical stimulation (150–600 μA, 100 μsec pulse duration, 0.1 Hz) of the white matter per layer 6 boundary evoked postsynaptic currents in the presence of glutamate and GABA antagonists (Figs. 6, 7). The stimulation strength was gradually increased in each case, and the maximal stable response was used to average recordings and to conduct pharmacological experiments. In four cells the evoked currents were abolished by the 5-HT3 receptor antagonist ICS 205-930 (Fig. 7C). Electrically evoked serotonergic currents were small (8–25 pA at −60 mV). Amplitudes of isolated cholinergic inputs (recorded in the presence of ICS 205-930) ranged from 5 pA in very young animals (P8–P20) to ∼60 pA in more mature animals (P30–P38). Evoked cholinergic currents were blocked by DHβE (50–100 μm) and α-cobrotoxin (100–200 nm) but were unaffected by α-bungarotoxin (100 nm), a pharmacological profile similar to the spontaneous synaptic currents (Fig. 6).
Fig. 6.
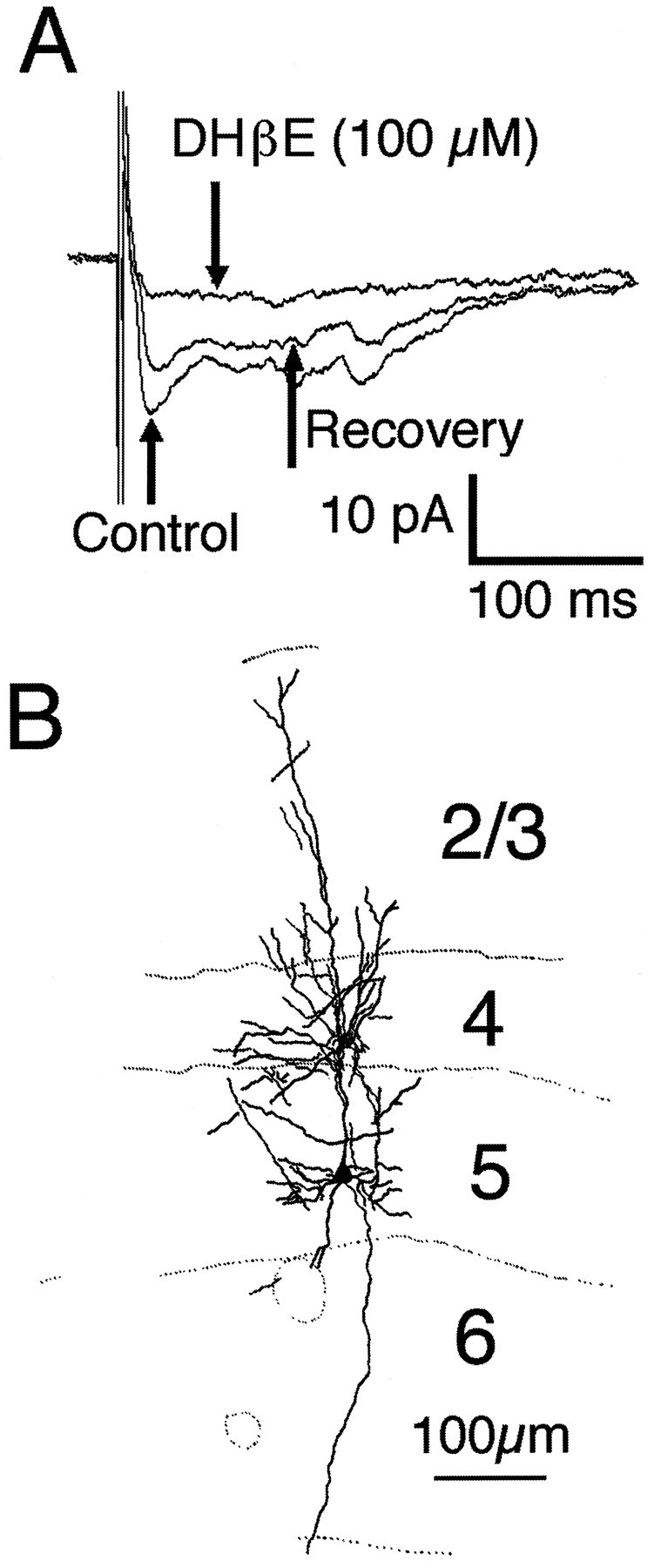
Pharmacology of evoked cholinergic synaptic responses. A, Electrical stimulation of the Layer 6–white matter boundary elicited small synaptic currents that were reversibly reduced by the nicotinic antagonist DHβE (100 μm). Recordings in A were obtained from a P23 pyramidal cell (B) in the presence of 20 μmd-APV, 50 μm CNQX, and 50 μm picrotoxin (PTX). Eachtrace in A represents the average of 14–20 traces. The small peaks occurring with a longer latency than the initial response may represent activation of a different set of fibers or spontaneous synaptic activity.
Fig. 7.
Serotonergic synaptic currents evoked by electrical stimulation. A, Evoked serotonergic responses recorded at different holding potentials from a layer 5 pyramidal neuron (D). B,I–V plot of evoked serotonergic PSCs shown in A. Peak currents were measured to generate theI–V plot. C, Electrically evoked serotonergic responses were also suppressed by ICS 205-930.
Cholinergic and serotonergic synaptic currents occur in both pyramidal cells and interneurons
We next asked whether fast cholinergic or serotonergic synaptic currents showed a cell type and/or laminar specificity. Most recordings were from pyramidal cells located in layers 2/3 or 5/6. However, 13 layer 4 interneurons, mostly spine-free stellate cells, were included in our sample. Spontaneous or evoked cholinergic synaptic currents were observed in pyramidal cells of all layers as well as in stellate cells (Figs. 1, 6). Serotonergic EPSCs were observed in supragranular and infragranular layer pyramidal cells and in layer 5/6 bipolar interneurons (n = 2; Figs. 3, 7). We attribute the apparent absence of fast serotonergic synaptic input in layer 4 to the small sample of layer 4 neurons rather than to an actual lack of 5-HT3 receptor expression or serotonergic terminals in this laminar location.
The developmental time course of nicotinic and serotonergic synaptic currents correlates with distinct events during the development of the ferret visual system
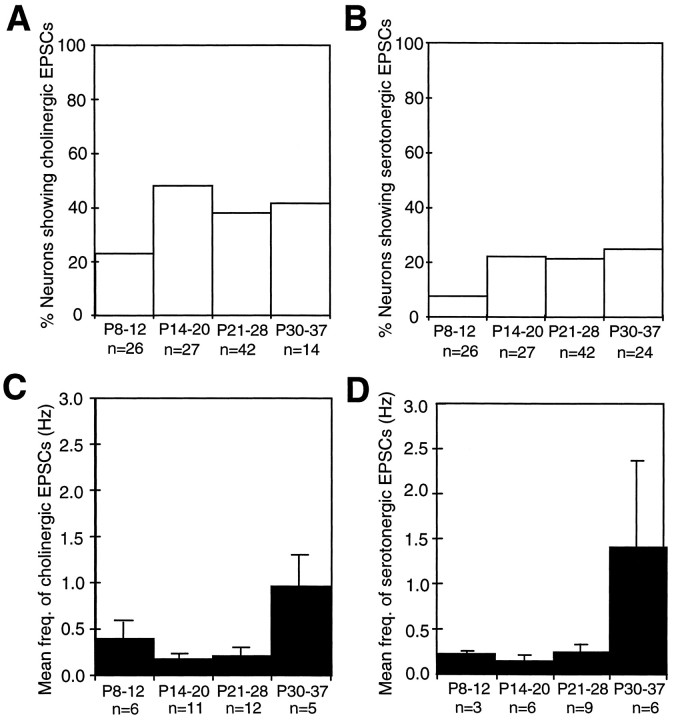
Cholinergic and serotonergic synaptic currents showed similar developmental time courses (Fig. 8, Tables 1,2). The percentage of cells with these synaptic currents increased between P8–P12 and P14–P20 (Fig.8A,B), which is well before eye opening (∼P32 in the ferret). The frequencies of spontaneous cholinergic and serotonergic synaptic currents, on the other hand, increased steeply between P28 and P30–P37 (Fig. 8C,D), which coincides with eye opening. During this period, the mean frequency of cholinergic EPSCs significantly increased over fourfold (from 0.22 ±0.05 Hz;n = 12; between P21 and P28, to 0.96 ±0.27 Hz;n = 5; p = 0.029, two-tailedt test), and the mean frequency of serotonergic synaptic events significantly increased sixfold (from 0.25 ±0.07;n = 9, to 1.41 ±1.11 Hz; n = 6;p < 0.05, two-tailed t test) (Fig.8C,D).
Fig. 8.
The proportion of neurons receiving fast excitatory cholinergic or serotonergic input depends on postnatal age. A larger fraction of tested cells showed cholinergic EPSCs compared with serotonergic events during the entire developmental period investigated. A, B, The percentage of cells showing these events increased after P14, which corresponds to the ingrowth of thalamic afferents. The mean frequencies of both spontaneous cholinergic and serotonergic EPSCs increased after P30 (C, D), i.e., after eye opening. n, Number of cells included; error bars represent SEM. Spontaneous activity was recorded for a period of several minutes in the presence of glutamate and GABA receptor antagonists, and synaptic events were pharmacologically identified by subsequent addition of DHβE and ICS in each cell included in the plots.
Table 1.
Laminar and age distribution of cells showing cholinergic EPSCs
| Age group/layer | 2/3 | 4 | 5 | 6 |
|---|---|---|---|---|
| P8–P12 | 1 | 2 | 3 | |
| P14–P20 | 3 | 4 | 2 | 2 |
| P21–P28 | 4 | 1 | 2 | 2 |
| P30–P37 | 2 | 2 | 1 |
Numbers include pyramidal cells and interneurons.
Table 2.
Laminar and age distribution of neurons showing serotonergic EPSCs
| Age group/layer | 2/3 | 4 | 5 | 6 |
|---|---|---|---|---|
| P8–P12 | 1 | 1 | 1 | |
| P14–P20 | 2 | 2 | 2 | |
| P21–P28 | 4 | 2 | 1 | |
| P30–P37 | 2 | 3 | 1 |
DISCUSSION
Although nicotinic transmission mediated by postsynaptic receptors is well established in peripheral structures (Elgoyen et al., 1994;Role and Berg, 1996; Zhang et al., 1996) as well as in the spinal chord (Belcher and Ryall, 1977) only a few previous studies have demonstrated fast synaptic transmission mediated by acetylcholine or serotonin in CNS regions (Sugita et al., 1992; Zhang et al., 1993; Feller et al., 1996). Thus, the predominant physiological role of nicotinic acetylcholine receptors in the CNS seems to be presynaptic regulation of transmitter release (McGehee et al., 1995; Role and Berg, 1996).
Although nicotinic receptors and serotonin 5-HT3 receptors are expressed in the neocortex (Parkinson et al., 1988; Tecott et al., 1993; Weavers et al., 1994; Bina et al., 1995; Lobron et al., 1995;Nakayama et al., 1995), and widespread cholinergic and serotonergic innervations are found in the majority of cortical areas, no indication of fast transmission mediated by these transmitters has been reported previously. However, using single-cell patch-clamp recordings of spontaneous and evoked activity, we were able to demonstrate that both nicotinic acetylcholine and ionotropic serotonin receptors contribute to fast excitatory synaptic transmission in all layers of the developing ferret visual cortex.
The fact that we detected these currents that have never been observed previously in a cortical slice preparation may seem surprising at first sight, because numerous investigators have failed to observe such synaptic events. One possible explanation is the choice of species, because nicotinic cholinergic mechanisms seem to play an important role in the development of the ferret visual system (Feller et al., 1996). Moreover, few studies have examined nonrodent species, and these events may be more prevalent in carnivores. In addition, these synaptic events occurred in a minority of cells and were typically of small amplitude; the contributions of these types of synaptic transmission in rat or mouse cortical slices might have escaped detection. Another issue is the viability of the severed cholinergic and serotonergic afferents and the release probability of their terminals in a slice preparation; again, species differences and methodological differences could be significant.
Relationship between the development of cholinergic and serotonergic synaptic responses and afferent innervation patterns
Cholinergic fiber density in developing ferret visual cortex increases considerably between P7 and P37, with highest fiber densities in the middle and lower cortical layers (Henderson, 1991). The densest serotonergic innervation, on the other hand, is found in layers 1 and 2/3 in adult ferret area 17 (Voigt and De Lima, 1991b). During early postnatal development, when upper layer neurons are still migrating, the subplate and the lower portion of the cortical plate are most heavily innervated. After cell migration ceases during the third postnatal week, the innervation density is almost uniform across the cortical layers; with further maturation, serotonergic fibers are successively confined to the supragranular layers (Voigt and De Lima, 1991b).
Despite these developmental changes in the morphological pattern of cholinergic and serotonergic input fibers in the ferret cortex (Henderson, 1991; Voigt and De Lima, 1991b), we detected no significant alterations in the laminar distribution of synaptic responses. However, because developmental changes in the contribution of serotonergic and cholinergic inputs to fast synaptic transmission are determined by both changes in afferent fiber density and nicotinic acetylcholine and 5-HT3 receptor expression, respectively, a more detailed knowledge of receptor expression patterns in the ferret visual cortex is required for a final interpretation of our findings. So far there are also no data available on laminar-specific, developmental changes in muscarinic responses or effects of other, G-protein-coupled, serotonin receptors in ferret visual cortex. This leaves open the possibility that effects of these receptor systems may follow the innervation patterns more closely than those of the ionotropic receptors. In addition, the cholinergic and serotonergic innervations of the ferret visual cortex are very dense in layer 1 (Henderson, 1991;Voigt and De Lima, 1991a,b), and serotonergic synapses seem to be preferentially formed on distal dendrites of both interneurons and pyramidal cells (De Lima et al., 1988). Thus the lack of layer specificity may be partially explained by a widespread, distal input to apical dendrites affecting pyramidal neurons of all layers. The cholinergic contribution to fast excitatory transmission is more prominent than the serotonergic component at all ages, which also might be attributed to differences in postsynaptic receptor densities. It has furthermore been suggested that despite its early morphological maturation, cholinergic function of the basal forebrain afferents begins later, because choline acetyltransferase immunoreactivity does not reach mature levels until 3 weeks after birth (Henderson, 1991). However, because we recorded nicotinic synaptic activity as early as P8, cholinergic afferents must be at least partially functional at earlier developmental stages.
Possible functions of ionotropic acetylcholine and serotonin receptors during neocortical development
Nicotinic acetylcholine as well as 5-HT3 receptors have a unique constellation of biophysical properties that might determine their role in the regulation of developing circuits. The 5-HT3 serotonin receptor exhibits voltage-dependent regulation by Mg2+ and Ca2+ ions (Kawa, 1994), giving it properties of an NMDA receptor-like coincidence detector. Depending on subunit composition, nicotinic acetylcholine receptors have calcium permeability greater than that of NMDA receptors (Pugh and Berg, 1994; Rathouz and Berg, 1994; Role and Berg, 1996). Given the many regulatory roles of intracellular calcium elevation, in short- and long-term synaptic plasticity (Ghosh and Greenberg, 1995), regulation of neuronal process outgrowth, and regulation of ion channels, receptors, and enzyme activities as well as transcriptional cascades (Gallin and Greenberg, 1995), nicotinic and serotonergic receptor activation are poised to participate in the regulation of cortical plasticity through diverse pathways. In addition, nACh receptors presynaptically regulate the release of the conventional fast transmitters glutamate and GABA (Role and Berg, 1996), which further implicates them in regulating synaptic function in developing and mature cortex.
Most studies addressing the effects of acetylcholine on cortical plasticity have involved muscarinic mechanisms. Intracortical infusion of nicotinic antagonists in kitten visual cortex did not affect ocular dominance changes in response to monocular deprivation, whereas muscarinic receptor blockade has been reported to reduce ocular dominance plasticity (Gu and Singer, 1993). The receptors involved in the facilitory effects of acetylcholine on visually or electrically evoked responses in vivo remain controversial; in cat visual cortex both exclusively muscarinic effects (Sato et al., 1987) and a nicotinic contribution to the modulation of response properties of cortical neurons have been reported (Parkinson et al., 1988). The nicotinic effect has been attributed to presynaptic nicotinic receptors located on thalamocortical afferents (Parkinson et al., 1988).
Muscarinic modulations of plasticity thresholds have been attributed to a reduction of potassium conductances resulting in increased synaptic activation and a modulation of intracellular Ca2+ levels (Gu and Singer, 1993). Nicotinic receptors, on the other hand, can potentially regulate neocortical circuit functioning both by a presynaptic regulation of transmitter release and by directly activating postsynaptic cells. Our results indicate that the latter function is more significant than believed previously.
The function of ionotropic serotonin receptors in the visual cortex has not been investigated previously. Because serotonergic synaptic currents occurred in both interneurons and pyramidal cells, their functions are likely to be more heterogeneous than in the hippocampus, where 5-HT3 receptor activation exclusively excites interneurons (Kawa, 1994).
Correlation with different stages of visual system development
The number of cortical target neurons in which cholinergic and serotonergic afferents contribute to fast excitatory synaptic transmission increased during the third postnatal week, at which time thalamic fibers invade the differentiating cortical plate. This suggests upregulation of cholinergic and serotonergic transmission by the ingrowing thalamic afferents, probably attributable to induction of receptor expression, as observed previously in rat somatosensory cortex (Broide et al., 1996). A significant frequency increase of cholinergic and serotonergic synaptic currents, on the other hand, occurs later in postnatal development, which coincides with the onset of visual experience. Because we lack any experimental evidence for a causal relationship between these events, we can only describe a temporal correlation here. However, our data suggest that fast postsynaptic transmission mediated by nicotinic acetylcholine receptors and serotonin 5-HT3 receptors might be particularly important during the formation of the thalamocortical projection as well as during the period of experience-dependent circuit remodeling. Both receptor types depolarize both glutamatergic and GABAergic neurons, and nicotinic receptors also presynaptically regulate transmitter release. Thus these systems might provide an additional, fast mechanism to set or adjust excitability levels or modulate the inhibition or excitation ratio that operates on top of the modulatory action of the second messenger-coupled receptor types. Further experimental testing is required to clarify whether this contributes to the selective synapse formation and stabilization that determines physiological response properties such as orientation or direction selectivity.
Footnotes
This work was supported by National Institutes of Health Grant EY07690 (L.C.K.) and a Human Frontier Science Program postdoctoral fellowship (B.R.). We thank Scott Douglas for excellent technical assistance and Julie Kauer, Lori MacMahon, and Michael Weliky for critical comments on this manuscript.
Correspondence should be addressed to Birgit Roerig, Department of Neurobiology, Duke University Medical Center, Box 3209, Durham, NC 27710.
REFERENCES
- 1.Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 2.Belcher G, Ryall RW. Substance P and Renshaw cells, a new concept of inhibitory synaptic interactions. J Physiol (Lond) 1977;272:105–119. doi: 10.1113/jphysiol.1977.sp012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bina KG, Guzman P, Broide RS, Leslie FM, Smith MA, O’Dowd DK. Localization of alpha 7 nicotinic receptor subunit mRNA and alpha-bungarotoxin binding sites in developing mouse somatosensory system. J Comp Neurol. 1995;363:321–332. doi: 10.1002/cne.903630212. [DOI] [PubMed] [Google Scholar]
- 4.Blanton MG, LoTurco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 5.Broecher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- 6.Broide RS, O’Connor LT, Smith MA, Smith JAM, Leslie FM. Developmental expression of a7 neuronal nicotinic receptor messenger RNA in rat sensory cortex and thalamus. Neuroscience. 1995;67:83–94. doi: 10.1016/0306-4522(94)00623-d. [DOI] [PubMed] [Google Scholar]
- 7.Broide RS, Robertson RT, Leslie FM. Regulation of α7 nicotinic acetylcholine receptors in the developing rat somatosensory cortex by thalamocortical afferents. J Neurosci. 1996;16:2956–2971. doi: 10.1523/JNEUROSCI.16-09-02956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 9.Dalva MB, Katz LC. Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science. 1994;265:255–258. doi: 10.1126/science.7912852. [DOI] [PubMed] [Google Scholar]
- 10.D’Amato RJ, Blue ME, Largent DR, Lynch DJ, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci USA. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist d-2-amino-5-phosphonopentanoate (d-APV) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lima AD, Bloom FE, Morrison JH. Synaptic organization of serotonin immunoreactive fibers in primary visual cortex of the macaque monkey. J Comp Neurol. 1988;274:280–294. doi: 10.1002/cne.902740211. [DOI] [PubMed] [Google Scholar]
- 13.Denk W. Two-photon scanning photochemical microscopy: mapping ligand-gated ion channel distributions. Proc Natl Acad Sci USA. 1994;91:6629–6633. doi: 10.1073/pnas.91.14.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denk W, Delaney KR, Gelperin A, Kleinfeld D, Strowbridge BW, Tank DW, Yuste R. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J Neurosci Methods. 1994;54:151–162. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 15.Durack JC, Katz LC. Development of horizontal projections in layer 2/3 of ferret visual cortex. Cereb Cortex. 1996;6:178–183. doi: 10.1093/cercor/6.2.178. [DOI] [PubMed] [Google Scholar]
- 16.Elgoyen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 17.Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1186. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs JL. [125I]α-Bungarotoxin binding marks primary sensory areas of developing rat neocortex. Brain Res. 1989;501:233–234. doi: 10.1016/0006-8993(89)90640-9. [DOI] [PubMed] [Google Scholar]
- 19.Gallin WJ, Greenberg ME. Calcium regulation of gene expression in neurons: the mode of entry matters. Curr Opin Neurobiol. 1995;5:367–374. doi: 10.1016/0959-4388(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 21.Gu Q, Singer W. Effects of intracortical infusion of anticholinergic drugs on neuronal plasticity in kitten striate cortex. Eur J Neurosci. 1993;5:475–485. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 22.Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 23.Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- 24.Henderson Z. Early development of the nucleus basalis-cortical projection but late expression of its cholinergic function. Neuroscience. 1991;44:331–324. doi: 10.1016/0306-4522(91)90056-t. [DOI] [PubMed] [Google Scholar]
- 25.Imamura K, Kasamatsu T. Interaction of noradrenergic and cholinergic systems in regulation of ocular dominance plasticity. Neurosci Res. 1989;6:519–536. doi: 10.1016/0168-0102(89)90042-4. [DOI] [PubMed] [Google Scholar]
- 26.Kasamatsu T, Pettigrew JD. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science. 1976;194:206–209. doi: 10.1126/science.959850. [DOI] [PubMed] [Google Scholar]
- 27.Kawa K. Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: a patch-clamp study. J Neurophysiol. 1994;71:1935–1947. doi: 10.1152/jn.1994.71.5.1935. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Jia W, Gu Q, Cynader M. Involvement of muscarinic acetylcholine receptors in regulation of kitten visual cortex plasticity. Dev Brain Res. 1994;79:63–71. doi: 10.1016/0165-3806(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 29.Lobron C, Wevers A, Damgen K, Jeske A, Rontal D, Birtsch C, Heinemann S, Reinhardt S, Maelicke A, Schroder H. Cellular distribution in the rat telencephalon of mRNAs encoding for the alpha 3 and alpha 4 subunits of the nicotinic acetylcholine receptor. Mol Brain Res. 1995;30:70–76. doi: 10.1016/0169-328x(94)00279-n. [DOI] [PubMed] [Google Scholar]
- 30.McGehee DS, Heath M, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1697. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama H, Shioda S, Okuda H, Nakashima T, Nakai Y. Immunocytochemical localization of nicotinic acetylcholine receptors in rat cerebral cortex. Mol Brain Res. 1995;32:321–328. doi: 10.1016/0169-328x(95)00092-7. [DOI] [PubMed] [Google Scholar]
- 32.Nerbonne JM. Design and application of photolabile intracellular probes. Soc Gen Physiol Ser. 1986;40:417–445. [PubMed] [Google Scholar]
- 33.Parkinson D, Kratz KE, Daw NW. Evidence for a nicotinic component to the actions of acetylcholine in cat visual cortex. Exp Brain Res. 1988;73:553–568. doi: 10.1007/BF00406614. [DOI] [PubMed] [Google Scholar]
- 34.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathouz MM, Berg DK. Synaptic type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. J Neurosci. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Read HL, Beck GB, Dun NJ. Serotonergic suppression of interhemispheric cortical synaptic potentials. Brain Res. 1994;643:17–28. doi: 10.1016/0006-8993(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 37.Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol. 1994;72:2438–2450. doi: 10.1152/jn.1994.72.5.2438. [DOI] [PubMed] [Google Scholar]
- 38.Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 39.Sargent PB. The diversity of neuronal anicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 40.Sato H, Hata HY, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- 41.Schlaggar BL, O’ Leary DDM. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- 42.Shaw C, Needler MC, Cynader M. Alterations in receptor number, affinity and laminar distribution in cat visual cortex during the critical period. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:627–634. doi: 10.1016/0278-5846(84)90025-3. [DOI] [PubMed] [Google Scholar]
- 43.Shaw C, Wilkinson M, Cynader M, Needler MC, Aoki C, Hall SC. The laminar distributions and postnatal development of neurotransmitter and neuromodulator receptors in cat visual cortex. Brain Res Bull. 1986;16:661–671. doi: 10.1016/0361-9230(86)90137-1. [DOI] [PubMed] [Google Scholar]
- 44.Sugita S, Shen KZ, North RA. 5-Hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992;8:199–203. doi: 10.1016/0896-6273(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 45.Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neurosci. 1993;56:23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- 47.Voigt T, De Lima AD. Serotonergic innervation of the ferret cerebral cortex, I, adult pattern. J Comp Neurol. 1991a;314:403–414. doi: 10.1002/cne.903140214. [DOI] [PubMed] [Google Scholar]
- 48.Voigt T, De Lima AD. Serotonergic innervation of the ferret cerebral cortex, II, postnatal development. J Comp Neurol. 1991b;314:415–428. doi: 10.1002/cne.903140215. [DOI] [PubMed] [Google Scholar]
- 49.Weaver WR, Chiapinelli VA. Single-channel recording in brain slices reveals heterogeneity of nicotinic receptors on individual neurons within the chick lateral spiriform nucleus. Brain Res. 1996;724:95–105. doi: 10.1016/0006-8993(96)00391-5. [DOI] [PubMed] [Google Scholar]
- 50.Weavers A, Jeske A, Lobron C, Heinemann S, Maelicke A, Schroder R, Schroder H. Cellular distribution of nicotinic acetylcholine receptor subunit mRNA in the human cerebral cortex as revealed by nonisotopic in situ hybridization. Brain Res. 1994;25:122–128. doi: 10.1016/0169-328x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Wang YT, Vyas DM, Neuman RS, Bieger D. Nicotinic cholinoceptor-mediated excitatory postsynaptic potentials in rat nucleus ambiguus. Exp Brain Res. 1993;96:83–88. doi: 10.1007/BF00230441. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to α-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]