Abstract
The excitability of spinal neurons that transmit pain is modulated by glutamate and substance P (SP). Glutamate is an excitatory neurotransmitter in the dorsal horn, and its effects are enhanced by SP acting on neurokinin 1 receptors (NK1Rs). We assessed activation of NK1Rs by studying their internalization in spinal cord slices. NK1Rs were localized in sections from the slices by using immunohistochemistry combined with fluorescence and confocal microscopy. Incubating the slices with SP induced internalization in most NK1R-positive neurons in laminae I, IIo, and X and in half of NK1R-positive neurons in laminae III–V. SP-induced internalization was abolished by the specific NK1R antagonist L-703,606 (1 μm). Stimulating the dorsal root with long-duration (0.4 msec) pulses evoked EPSPs in dorsal horn neurons with latencies consistent with the conduction speed of A∂- and C-fibers. High-frequency (100 Hz) stimulation of the dorsal root with these pulses induced NK1R internalization in neurons in laminae I–IIo of the stimulated side of the slice but not in the contralateral side or in other laminae. Stimulation at lower frequencies (1 and 10 Hz) failed to elicit significant internalization, suggesting that the release of SP is frequency-dependent. Internalization produced by the 100 Hz tetanus was mimicked by NMDA and blocked by an NMDA antagonist, 2-amino-5-phosphonopentanoic acid, but not by the AMPA and kainate antagonist CNQX. The NK1R antagonist L-703,606 abolished the internalization produced by 100 Hz stimulation or NMDA. Therefore, the release of SP in the dorsal horn appears to be controlled by NMDA receptors.
Keywords: C-fibers, central sensitization, dorsal horn, glutamate receptor, internalization, LTP, NK1 receptor, NMDA receptor, slices, substance P
Many instances of hyperalgesia seem to be caused by an increase in the excitability of spinal sensory neurons, termed central sensitization, induced by an increased activity of nociceptive afferents (McMahon et al., 1993; Yaksh, 1993;Zieglgänsberger and Tolle, 1993). Phenomena related to central sensitization include “wind-up,” a progressive increase in the number of action potentials evoked per stimulus when afferent fibers are repeatedly stimulated (Mendell, 1966; Dickenson and Sullivan, 1987), and long-term potentiation (LTP) of dorsal horn synapses (Randic et al., 1993; Liu and Sandkühler, 1995). LTP of excitatory synapses in nociceptive pathways may cause hyperalgesia, and its long duration could explain the persistence of certain types of chronic pain.
Substantial evidence suggests that neurokinin and glutamate receptors play an important role in mediating central sensitization (Xu et al., 1992). SP causes a prolonged depolarization of dorsal horn neurons (Murase and Randic, 1984), enhances their responses to C-fiber input, and participates in wind-up (Kellstein et al., 1990), whereas NMDA receptors are required for the induction of both wind-up (Davies and Lodge, 1987; Dickenson and Sullivan, 1987) and LTP (Randic et al., 1993; Liu and Sandkühler, 1995) in the dorsal horn. Numerous observations indicate that glutamate and neurokinins act synergistically in the dorsal horn. Spinal application of SP and glutamate increases the responses of dorsal horn neurons to mechanical stimulation of the skin (Dougherty et al., 1995). Glutamate and SP coexist in primary afferent terminals (Battaglia and Rustioni, 1988; De Biasi and Rustoni, 1988), whereas neurokinin 1 receptors (NK1Rs) and glutamate receptors are coexpressed in dorsal horn neurons, which have responses to glutamate that are enhanced by SP (Womack et al., 1988;Randic et al., 1990; Rusin et al., 1993a,b). Conversely, presynaptic NMDA receptors found in afferent terminals in the dorsal horn may control the release of SP and other neuropeptides (Liu et al., 1994b,1997).
After agonist binding, NK1Rs are internalized (Mantyh et al., 1995;Grady et al., 1996), a process that appears to mediate receptor resensitization (Garland et al., 1996). Recent studies (Mantyh et al., 1995) have used the internalization of NK1Rs in dorsal horn neurons to demonstrate their activation by noxious stimulation in vivo. Spinal cord slices with attached dorsal roots allow controlled application of drugs and electrical stimulation and preserve many of the synapses present in the intact tissue. These characteristics make this preparation suitable to study the internalization of NK1Rs in dorsal horn neurons as a marker of their activation by various stimuli. Here, we have used spinal cord slices (1) to observe NK1R internalization induced in vitro; (2) to determine whether endogenous neurokinins are released in the slices by electrical stimulation of the dorsal root; and (3) to investigate whether NMDA receptors are involved in neurokinin release.
MATERIALS AND METHODS
Reagents. CNQX, 2-amino-5-phosphonopentanoic acid (AP-5), L-703,606, and NMDA were from Research Biochemicals International (Natick, MA). Metofane (methoxyflurane) was from Pitman-Moore (Mundelein, IL). PBS (in mm: 138 NaCl, 2.7 KCl, and 10 Na+ phosphate, pH 7.4) and thiorphan were obtained from Sigma Chemical Co. (St. Louis, MO). SP was from Molecular Research Laboratories (Durham, NC). Slow Fade was from Molecular Probes (Eugene, OR). All other reagents were purchased from standard commercial sources.
Slice preparation. Transverse slices of 400 μm, some with one dorsal root attached, were cut from a lumbar segment (L2–L5) of the spinal cord of a young rat (14–28 d) using a Vibratome (Technical Products International, Inc., St. Louis, MO), as described previously (Randic et al., 1993). Slices were kept in artificial CSF (ACSF; in mm: 124 NaCl, 1.9 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, and 10 glucose, bubbled with 95% O2 and 5% CO2, pH 7.4, 305 mOsm) except during their preparation, when NaCl was iso-osmotically replaced by sucrose (215 mm), and the concentration of KCl was increased to 5 mm. Immediately after cutting, slices were incubated at 35°C for 1–1.5 hr in ACSF containing 5 mmKCl.
Electrophysiology. Intracellular recordings from neurons in laminae I–II were performed using sharp electrodes as described previously (Randic et al., 1993). Recording electrodes (100–200 MΩ) were pulled from aluminosilicate glass tubing (1.0 mm outer diameter, 0.53 mm inner diameter) and filled with 3 m potassium acetate and 10 mm KCl, pH 7.2. Slices were kept submerged in a recording chamber perfused at 3–6 ml/min with ACSF at 35°C. Neurons were impaled by slowly advancing the recording electrode and oscillating the capacity compensation circuit of an Axoprobe 1A amplifier (Axon Instruments, Foster City, CA). Recordings were performed from neurons that had a resting membrane potential negative to −50 mV, action potentials that overshot 0 mV, and membrane resistance >60 MΩ.
EPSPs were evoked by stimulating the whole root distally (3–7 mm) from the slice with a hook platinum bipolar electrode connected to a Grass S-88 stimulator through a stimulus-isolating unit operating in capacity mode. The duration of the electrical pulses was 0.4 msec, and their intensity was adjusted in the range of 5–30 V to yield EPSPs of 5–20 mV without evoking action potentials. Sweeps (200 msec), including the response to a current step (−0.1 to −0.3 nA, 30–50 msec) and the evoked EPSP, were analyzed on-line (Neuropro; RC Electronics, Santa Barbara, CA) to measure baseline membrane potential and resistance, and the latency, amplitude, and slope of the evoked EPSP. Conduction speeds were calculated by dividing the length of the root stimulated by the latency of the evoked EPSPs. LTP or long-term depression (LTD) were induced with a 100 Hz tetanus (three trains of 1 sec every 10 sec, pulses identical to those used to sample EPSPs) after sampling EPSPs at 0.03 Hz for at least 10 min to establish a baseline, and assessed by recording evoked EPSPs for an additional 20–80 min.
Slice treatments. The procedure for electrical stimulation of the dorsal root was similar to that used to evoke EPSPs. One dorsal root was used to stimulate the corresponding dorsal horn, whereas the contralateral dorsal horn served as control. Lack of good contact between the root and the slice usually derives from not cutting the slice precisely at the point of entrance of the root, or from damage to the root at its point of contact with the slice. Slices were inspected for damage to the root using a dissection microscope, and those judged unsatisfactory were discarded. Slices were placed in the recording chamber perfused at 3–6 ml/min with ACSF at 35°C, and the whole dorsal root was stimulated with square pulses of 20 V and 0.4 msec duration. Stimulation consisted of 300 pulses in three trains at 100 Hz (1 sec) or 10 Hz (10 sec) separated by 10 sec or 1 train at 1 Hz (5 min). After stimulation, slices were kept in the chamber for 10–15 min and then fixed. The stimulated side of the slice was marked with a V-shaped cut in the ventral horn. To avoid bias, this mark was only used to identify the stimulated side of the sections after the cells were counted.
Drug treatment was done by either incubating the slices at 35°C in 2–5 ml of ACSF bubbled with 95% O2 and 5% CO2 containing the appropriate concentration of drug or, in experiments in which the root was stimulated, by adding the drug to ACSF perfused through the recording chamber.
Capsaicin injection. In vivo noxious stimulation was performed as described (Mantyh et al., 1995). Male Sprague Dawley rats (2 months old) were anesthetized with enflurane and injected subcutaneously in the hindpaw with 20 μl of capsaicin dissolved (5 mg/ml) in 5% polyoxyethylenesorbitan mono-oleate (Tween 80). After 7 min the rats were deeply anesthetized with sodium pentobarbital (60 mg/kg of body weight) and perfused through the ascending aorta with 100 ml of 0.1 m PBS, pH 7.4, followed by 500 ml of PBS containing 4% paraformaldehyde and saturated picric acid (4°C). The lumbar spinal cord was then removed and processed like the slices.
Immunohistochemistry. The procedure used was similar to that described by Mantyh et al. (1995). Slices were fixed in PBS containing 4% paraformaldehyde and saturated picric acid for 3–16 hr at 4°C and cryoprotected by incubating with 20% sucrose in phosphate buffer (0.1 m, pH 7.4) for 12–24 hr at 4°C. Each slice was then frozen on dry ice and cut into transverse sections (50 μm, six to eight sections per slice). Sections were washed once with PBS and twice with PBS containing 1% normal goat serum and 0.3% Triton X-100. Then, sections were incubated at room temperature for 1 hr, and at 4°C for 36–40 hr, with the anti-NK1R primary antibody diluted 1:3000 in PBS containing 10% normal goat serum and 0.3% Triton X-100. The primary antibody was a polyclonal rabbit antibody (11826-5, a generous gift from Dr. Steve Vigna, Duke University Medical Center, Durham, NC) raised against a 15 amino acid peptide sequence at the C-terminal of the rat NK1R (Vigna et al., 1994; Mantyh et al., 1995). After three washes with PBS, sections were incubated for 2 hr at room temperature with the secondary antibody (goat anti-rabbit IgG FITC, affinity-purified; Cappel, Durham, NC) diluted 1:100 in PBS, 10% normal goat serum, and 0.3% Triton X-100. Sections were washed three more times with PBS and mounted in Slow Fade.
Confocal microscopy. Neurons were studied as described (Grady et al., 1996) using an MRC 1000 confocal microscope, (Bio-Rad, Hercules, CA) equipped with a krypton–argon laser (488, 565, and 647 nm lines) and T1 and T2A filter blocks, attached to a Zeiss Axiovert microscope. Sections were observed with a Plan Apochromat 100× oil immersion objective with a numerical aperture of 1.4 (∞0.7). Images were collected using an aperture of 2–5 mm and zoom of 1–3. Optical sections were taken at 0.5 μm intervals through the tissue. The combination of objective and microscope settings used resulted in a resolution in the x- and y-axis of 170–200 nm and in the z-axis of 230–400 nm. Images of 768 × 420 pixels were obtained and processed using Adobe Photoshop 3.0, (Adobe Systems Inc., Mountain View, CA) and printed using a Fujix Pictography 3000 printer.
Neuron counting. A Leitz Orthoplan (E. Leitz, Inc., Rockleigh, NJ) fluorescence microscope fitted with a 50× oil immersion objective (Leitz NPL Fluotar) was used to count NK1R immunoreactive neuronal somas. Counting was done according to the following procedure: (1) the observation field was moved systematically along each of the Rexed laminae while focusing up and down through the section to view the neurons; (2) neurons were considered to have internalized receptors when ≥10 endosomes were observed inside the soma and proximal dendrites contiguous with it; (3) neurons were grouped in the following regions: laminae I–IIo, laminae III–V, and lamina X; (4) in each section, all NK1R-positive neuronal somas in these regions were counted and classified as having or not having internalized NK1Rs, except that in some experiments only neurons in laminae I–IIo were counted; (5) at least five (and frequently all) of the six to eight sections obtained per slice were examined; (6) neurons in the right and left side of the sections were counted separately; (7) in stimulated slices, the right and left side of each section was labeled as stimulated or contralateral by locating a notch carved in the ventral horn (see Slice treatments); (8) another investigator independently reexamined the sections to verify the counting; (9) data of the sections were added, and results were expressed as percentage of NK1R-positive neurons showing internalization in each region per slice or hemislice (stimulated or contralateral side); and (10) results of three or four slices were averaged to calculate the mean ± SEM.
Prism software (GraphPad Software, San Diego, CA) was used for data processing and statistical analysis. The statistical significance of differences between pairs of values was determined by performing a one-way ANOVA for each lamina followed by Bonferroni’s multiple comparison test.
RESULTS
NK1R immunoreactive neurons in spinal cord slices
The NK1R was localized by immunofluorescence in fixed sections prepared from live transverse spinal cord slices. The NK1R covered most of the somatic and dendritic surface of the neurons. Sections prepared directly from a fixed rat, labeled using the same immunohistochemistry procedure, yielded high-quality images (Fig.1G) similar to those in earlier reports (Mantyh et al., 1995). Sections prepared from the slices also gave images of good quality (Fig.1A–F), although sometimes they had a slightly higher background. We expected to find some variability in the number of NK1R-immunoreactive neurons between the different histological sections obtained from a slice, because neurons in the surface of the slice tend to be damaged when cutting with the Vibratome, whereas cells inside the slice are better protected. Sections superficial to the slice (first and last cut) could be identified because they have rugged, broken edges. These superficial sections did have less neurons and uneven thickness, and they were often discarded after sectioning or excluded from the cell counting. We found little variability in the number of NK1R-positive neurons between the rest of the sections from a slice.
Fig. 1.
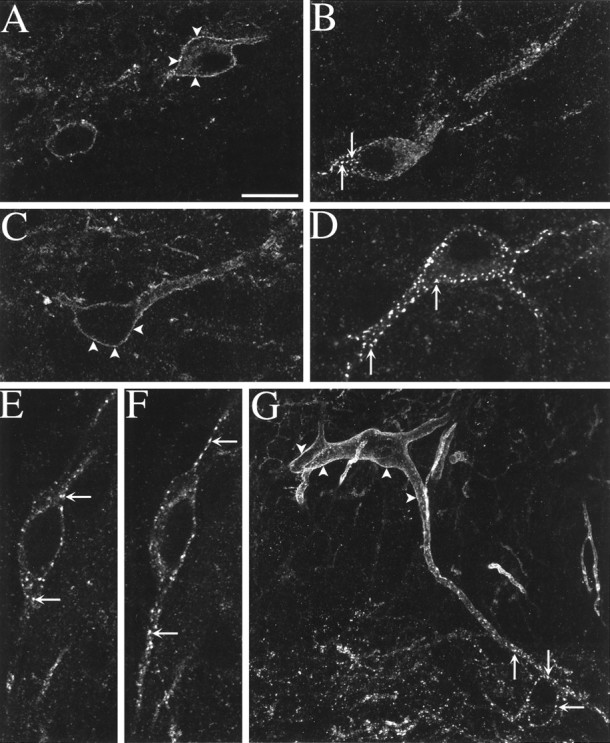
Fluorescent confocal images of NK1R-immunoreactive neurons in the dorsal horn. Arrowheads indicate surface labeling, and arrows indicate NK1Rs internalized in endosomes. A–F, Spinal cord slices were incubated for 10 min at 35°C with ACSF alone (A, C) or containing 100 nm SP (B, D, E, F).A, Lamina I neurons in a control slice (1 optical section). B, Lamina I neuron showing NK1R internalization after incubation with SP (8 optical sections).C, Lamina III neuron in a control slice (1 optical section). D, Lamina III neuron in a slice incubated with SP (5 optical sections). E, F, Lamina I neuron in a slice incubated with SP; single central (E) and superficial (F) optical sections show internalization in the soma and the dendrites, respectively. G, Lamina III neuron with a dendrite projecting dorsally to lamina I (at thebottom) in a section cut from the lumbar (L4) spinal cord of a rat injected with capsaicin (100 μg) in the hindpaw. This panel is a computer-generated juxtaposition of two confocal images, one in lamina III (28 optical sections) and other in laminae I and II (34 optical sections). Scale bar: A, G, 20 μm;B, 13.3 μm; C–F, 11.6 μm.
Earlier reports (Bleazard et al., 1994; Liu et al., 1994a; Brown et al., 1995; Mantyh et al., 1995), indicated that NK1R-positive neurons are found in all of the Rexed laminae of the dorsal horn except lamina II. Our observations confirmed these reports. However, although NK1R-positive neurons were practically absent in the internal portion of lamina II (lamina IIi), some NK1R-positive neurons appear to be present in the outermost portion of lamina II (lamina IIo). The number of NK1R-immunoreactive neurons per slice was as follows: laminae I and IIo, 195 ± 15 (n = 15); laminae III and IV, 238 ± 30 (n = 9); laminae V, 93 ± 13 (n = 9); and lamina X, 28 ± 7 (n= 15). Some large NK1R-positive neurons were found in the lateral spinal nucleus (27 ± 3 neurons per slice; n = 15), i.e., the dorsalmost part of the dorsolateral white matter. Several neurons in laminae III and IV had a large, dorsally directed dendritic arbor that traversed lamina II and spread in lamina I (see Figs. 1G, 5D).
Fig. 5.
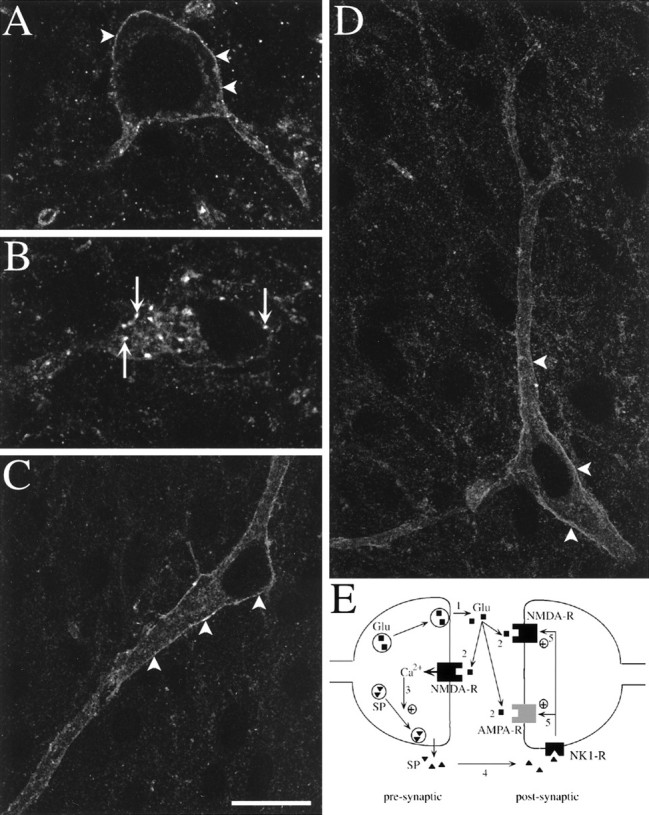
Fluorescent confocal images of NK1R-immunoreactive neurons in slices electrically stimulated. Stimulation consisted of three trains of 1 sec at 100 Hz separated by 10 sec, delivered to one of the dorsal roots. Arrowheads indicate surface labeling, and arrows indicate internalized NK1R.A–C, Single optical sections. A, Lamina I neuron in the contralateral side showing surface immunoreactivity.B, Lamina I neuron in the stimulated side showing extensive NK1R internalization. C, Lamina III neuron in the contralateral side. D, Lamina III neuron in the stimulated side with a dendrite projecting to lamina I (at thetop); this is a computer-generated juxtaposition of two confocal images of four (top) or five (bottom) optical sections. Scale bar: A, B, 10 μm; C, D, 20 μm. E, Model of the synergistic action of SP and glutamate at a dorsal horn synapse. 1, Noxious stimuli produce presynaptic depolarization and glutamate release. 2, Presynaptic NMDA receptors are activated by glutamate and depolarization, letting Ca2+ inside the presynaptic button.3, Increases in presynaptic calcium concentration trigger SP release. 4, SP binds to NK1Rs in surrounding neurons starting the internalization process. 5, Activated NK1Rs increase postsynaptic responses to glutamate.
In untreated slices (control in Figs. 2,4; n = 4 slices), NK1R immunoreactivity was localized to the cell surface in practically all NK1R-positive neurons in laminae III–V (99 ± 0.2%, 224–530 NK1R-positive neurons per slice) or lamina X (98 ± 2%, 9–48 NK1R-positive neurons per slice) and most NK1R-positive neurons in laminae I–IIo (82 ± 2%, 148–294 NK1R-positive neurons per slice). It is unlikely that the baseline internalization in laminae I–IIo was caused by a continuous release of neurokinins, because it was not abolished by incubating the slices for 40 min with the NK1R antagonist L-703,606 (1 μm; Fig. 2). It may be attributable to neurokinin release during preparation of the slices and the inability of a few neurons to recycle the receptor to the membrane during the recovery period. Confocal microscopy confirmed the absence of internalization in untreated slices; surface immunofluorescence was detected in single optical sections taken through the center of neurons in laminae I and III (Fig. 1A,C).
Fig. 2.
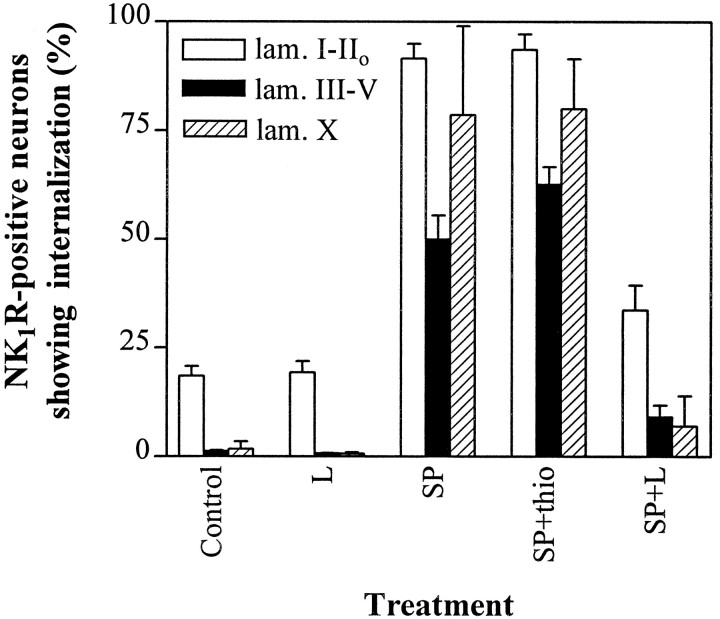
Percentage of NK1R-positive neurons showing internalization after incubation with SP and a NK1R antagonist. Slices were incubated at 35°C in ACSF bubbled with 95% O2 and 5% CO2 as follows: Control, no additions;L, 1 μm L-703,606 for 40 min;SP, 100 nm SP for 10 min;SP+thio, 10 μm thiorphan for 20 min adding 100 nm SP during the last 10 min; SP+L, and 1 μm L-703,606 for 40 min adding 100 nm SP during the last 10 min. Bars represent the percentage of NK1-positive neurons with internalization in laminae I–IIo, III–V, or X. Values are mean ± SEM of three slices, except the control (4 slices). Significant differences (ANOVA, p < 0.001) were found between control and SP, control and SP+thio, SP and L, and SP and SP+L in all three regions. Less pronounced inhibition of internalization was obtained with 100 nm L-703,606 (data not shown).
Fig. 4.
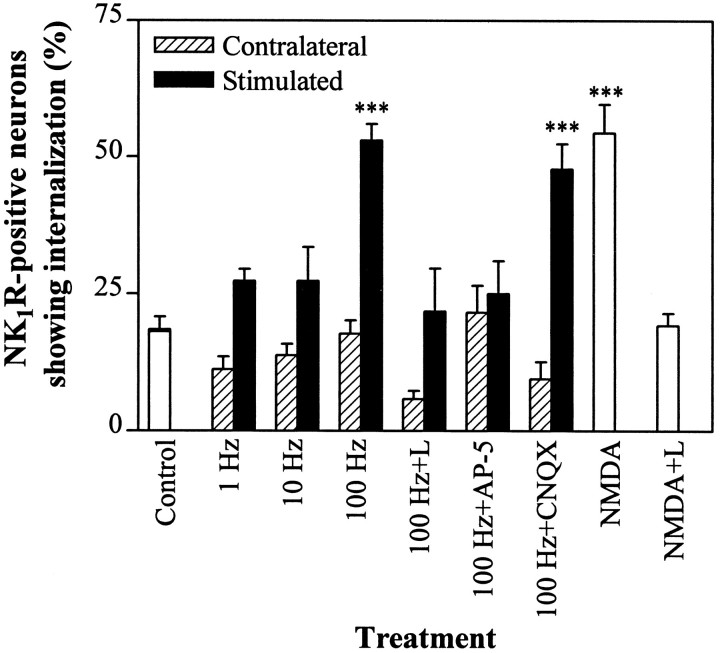
Percentage of NK1R-positive neurons in laminae I–IIo showing internalization after dorsal root stimulation or incubation with NMDA. One dorsal root was used to stimulate the corresponding side of the slice, whereas the contralateral side served as control. Open barsrepresent slices that were not electrically stimulated; filled bars represent the stimulated side of the slices; andhatched bars represent the contralateral side. Stimulation was as follows: Control, no stimulation;1 Hz, one train of 5 min at 1 Hz; 10 Hz, three trains of 10 sec at 10 Hz every 10 sec; 100 Hz, three trains of 1 sec at 100 Hz every 10 sec; 100 Hz+L, 5 μm L-703,606 perfused for 10 min before and after 100 Hz; 100 Hz+AP-5, 50 μm AP-5 perfused for 5 min before and after 100 Hz; 100 Hz+CNQX, 5 μm CNQX perfused for 10 min before and after 100 Hz;NMDA, 100 μm NMDA for 1 min followed by ACSF for 10 min; NMDA+L, 100 μm NMDA for 1 min and 5 μm L-703,606 for 10 min before and after NMDA. Values are mean ± SEM of four slices (control, 100 Hz, 100 Hz+CNQX, NMDA) or three slices (rest). ***Significantly different from control (p < 0.001, ANOVA and Bonferroni’s test); these three bars are also significantly different (p < 0.05) from any other bars in the figure.
NK1R internalization produced by SP application
Incubating spinal cord slices with SP (100 nm) induced NK1R internalization into multiple discrete vesicles, as determined by confocal microscopy (Fig. 1B,D–F). SP was applied for 10 min, the time required for the internalization process to reach its peak (Mantyh et al., 1995). Single confocal optical sections confirmed that the internalization occurred both in the soma (Fig. 1E) and the dendrites (Fig.1F). Internalization occurred in all regions of the dorsal horn (p < 0.001 compared with control slices) and was blocked (p < 0.001 compared with SP alone; Fig. 2) by the specific, nonpeptide NK1R antagonist L-703,606 (1 μm, Cascieri et al., 1992). Hence, NK1Rs and the mechanisms mediating their internalization were functional in the slices.
SP-induced NK1R internalization (Fig. 2, n = 3 slices) was observed in nearly all NK1R-positive neurons in laminae I–IIo (92 ± 3%, 123–196 NK1R-positive neurons per slice), lamina X (79 ± 21%, 4–24 NK1R-positive neurons per slice), and the lateral spinal nucleus (98 ± 2%, 21–27 NK1R-positive neurons per slice), but only in 50 ± 5% of NK1R-positive neurons in laminae III–V (187–204 NK1R-positive neurons per slice). To ensure that SP penetrates the slices, we used it at relatively high concentrations, 100 nm, 30 and 600 times the KD of SP for low- and high-affinity binding sites in the spinal cord, respectively (Routh and Helke, 1995). We wondered whether this difference was because of a greater degradation of SP in laminae III–V by endogenous neutral endopeptidase (NEP, EC3.4.24.11). Combining SP with the NEP inhibitor thiorphan (10 μm) resulted in a small increase (to 63 ± 4%;p < 0.05 compared with SP alone) in the number of neurons with internalized NK1Rs in this area (Fig. 2), which is consistent with reports showing that peptidase inhibitors increase extracellular SP in the deep dorsal horn (Duggan et al., 1992). Thiorphan alone did not increase NK1R internalization in any of the regions studied (p > 0.05, data not shown). In any case, 35% of the neurons in laminae III–V failed to show internalization in the presence of SP combined with thiorphan, suggesting that some neurons in laminae III–V may be unable to internalize the NK1R in their somas. Neurons in laminae III–V consistently internalized NK1R less readily than lamina I neurons in all treatments used in this study, including noxious stimulationin vivo (Fig. 1G) (Mantyh et al., 1995).
NK1R internalization induced by electrical stimulation of the dorsal root
To verify that root stimulation elicited action potentials that reached the dorsal horn, we performed intracellular recordings from neurons in laminae I and II. Electrical pulses (5–30 V, 0.4 msec) delivered to the root evoked EPSPs in 14 of 14 neurons recorded. In agreement with earlier reports (Li and Bak, 1976; Yoshimura and Jessell, 1990; Randic et al., 1993; Liu and Sandkühler, 1995), pulses of relatively long duration (0.4 msec) evoked EPSPs with long latencies consistent with the conduction speed of C- and A∂-fibers. EPSPs in the experiment shown in Figure 3appeared to be monosynaptic, because they had a constant latency of 12 msec. From this latency value and the length of dorsal root stimulated (5 mm), we calculated a conduction speed of 0.4 m/sec, which is consistent with the conduction speed of C-fibers (Li and Bak, 1976;Swett and Bourassa, 1981). High-frequency stimulation of the dorsal root (three trains of 1 sec at 100 Hz, separated 10 sec) potentiated synaptic responses in three of four dorsal horn neurons (Fig. 3). In these three neurons, EPSP amplitudes were increased to 155 ± 10% (n = 3) of baseline for up to 80 min, suggesting that LTP was induced. In the remaining neuron, EPSP amplitude was substantially decreased for 80 min after the tetanus (data not shown), suggesting the induction of LTD. The presence of LTP and LTD in these synapses has been demonstrated by other investigators (Randic et al., 1993; Liu and Sandkühler, 1995).
Fig. 3.
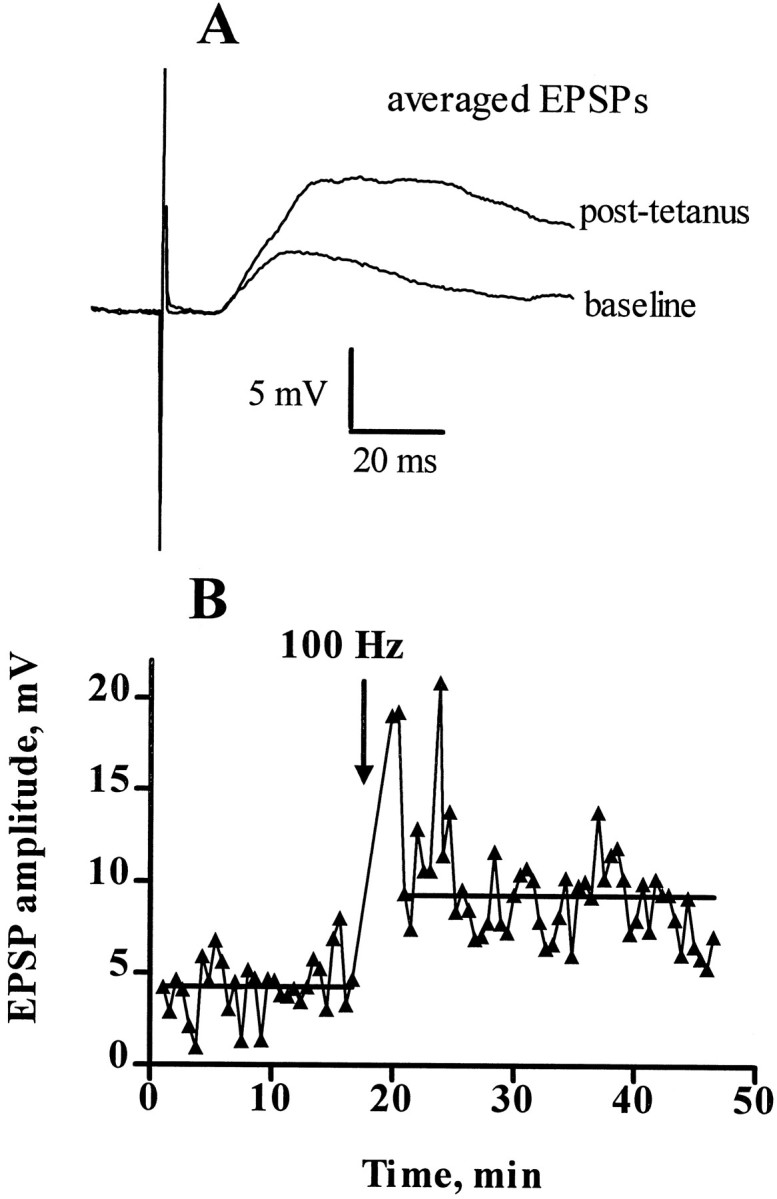
EPSPs and LTP of dorsal horn synapses. Intracellular recording from a dorsal horn neuron (laminae I–II) in a spinal cord slice. The resting membrane potential of the neuron was −63 mV, its input resistance was 160 MΩ, and its time constant was 5 msec. The cell was held at −71 mV by passing current (−20 to −60 pA) through the recording electrode. EPSPs were evoked every 30 sec by stimulating the dorsal root, 5 mm away from the slice, with pulses of 8.8 V and 0.4 msec. Evoked EPSPs had a constant latency of 12 msec. At the time indicated by the arrow, a tetanus was delivered to the root consisting of pulses (8.8 V, 0.4 msec) delivered at 100 Hz in three trains of 1 sec duration separated by 10 sec intervals. Current injection was stopped for 2 min during the tetanus.A, Evoked EPSPs during the baseline (average of 32 sweeps) and after the tetanus (average of 40 sweeps excluding post-tetanic potentiation). B, Horizontal lines represent averages of the amplitude of the evoked EPSPs before (4.4 ± 0.3 mV, n = 32) and after (8.7 ± 0.3 mV, n = 40) the tetanus. The increase in EPSP amplitude after the tetanus was very significant (p < 0.0001, t test). The experiment was repeated three times with similar results.
We then investigated whether electrical stimulation of the dorsal root entering the slice was able to induce NK1R internalization through the release of endogenous SP. Pulses of 20 V and 0.4 msec were delivered using a bipolar electrode stimulating the whole root. After high-frequency stimulation (100 Hz, three trains of 1 sec) of the dorsal root (Fig. 4; n = 4 slices), the percentage of NK1R-positive neurons with internalized receptors was increased in laminae I–IIo of the stimulated side (to 53 ± 3%; p < 0.001; 84–124 NK1R-positive neurons per hemislice), but not of the contralateral side (18 ± 2%; 59–136 NK1R-positive neurons per hemislice). Figure5A,B shows confocal images of representative neurons in the contralateral and stimulated side of the slice, respectively. The increase in internalization produced by high-frequency stimulation was abolished by the NK1R antagonist L-703,606 (5 μm, Fig. 4), perfused for 10 min before and after the tetanus. Hence, this stimulation protocol appears to induce the release of endogenous SP or other neurokinins from primary afferent terminals or secondary dorsal horn neurons. Crisp surface immunoreactivity was found in neuronal somas in deeper laminae of the stimulated side of the slices (Fig. 5C,D); neurons with internalization were 2 ± 1% in laminae III–V (of 80–187 NK1R-positive neurons per slice) and 8 ± 4% in lamina X (of 7–53 NK1R-positive neurons per slice). Nevertheless, some internalization could be detected in dendrites of lamina III neurons projecting into lamina I (Fig. 5D, top), although this was less clear than in sections prepared directly from rats injected with capsaicin (Fig. 1G).
Stimulation with the same number of pulses at lower frequencies (three trains at 10 Hz for 10 sec or one train at 1 Hz for 5 min; Fig. 4) failed to increase NK1R internalization in laminae I–IIoneurons over control levels (p > 0.05). Moreover, laminae I–IIo neurons with internalized NK1Rs were significantly (p < 0.01) more numerous in dorsal horns stimulated at 100 Hz than in dorsal horns stimulated at 1 or 10 Hz, suggesting that the release of SP from afferent terminals is frequency-dependent.
NK1R internalization induced by NMDA receptor activation
It has been suggested that NMDA autoreceptors control the release of neuropeptides in the dorsal horn (Liu et al., 1994b). To test this hypothesis, the NMDA receptor antagonist AP-5 (50 μm) was perfused through the chamber containing the slices for 5 min before and 5 min after the 100 Hz tetanus was delivered to the dorsal root (Fig.4; n = 3 slices). AP-5 significantly decreased (p < 0.001) NK1R internalization in laminae I–IIo of the stimulated side of the slices to 22 ± 5% (90–122 NK1R-positive neurons per hemislice), a value not significantly different from control (p > 0.05), confirming the hypothesis. In contrast, CNQX (5 μm; Fig. 4), an antagonist of AMPA and kainate receptors (Honoré et al., 1988), perfused for 10 min before and 10 min after the tetanus, did not decrease the internalization produced by the 100 Hz tetanus in laminae I–IIo, indicating that non-NMDA glutamate receptors are not involved in the control of SP release.
To investigate the hypothesis further, spinal cord slices were incubated with 100 μm NMDA without electrical stimulation (Fig. 4; n = 4 slices). Exposure to NMDA was kept short (1 min) to minimize possible cell damage caused by neurotoxicity and was followed by a 10 min incubation in the absence of drug to leave time for internalization to occur. This treatment resulted in a significant (p < 0.001) increase in the number of neurons with internalized NK1R in laminae I–IIo (to 54 ± 5%; 144–433 NK1R-positive neurons per slice) but not in laminae III–V (4 ± 2%; p > 0.05; 235–371 NK1R-positive neurons per slice) or lamina X (5 ± 3%;p > 0.05; 19–34 NK1R-positive neurons per slice). The effect of NMDA was abolished by the NK1R antagonist L-703,606 (5 μm; Fig. 4; p < 0.001 compared with NMDA alone). Hence, NMDA receptors do appear to mediate SP release in the dorsal horn, and this effect seems to be restricted to laminae I and II.
DISCUSSION
Frequency dependence of SP release
High-frequency stimulation (100 Hz) of the dorsal root induced internalization of NK1Rs in laminae I–IIo neurons. The internalization was abolished by an SP antagonist, indicating that this stimulation elicited the release of endogenous SP. The same number of pulses delivered at lower frequencies (1 or 10 Hz) failed to produce NK1R internalization. It is possible that 1–10 Hz stimulation elicits some SP release in the dorsal horn but in amounts too low to induce significant NK1R internalization, particularly considering that some SP may leak out of the slices. In any case, our results show that more SP is released at 100 Hz than at 1–10 Hz. There is evidence that many neuropeptides are released more effectively at higher frequencies of stimulation (Duggan et al., 1995, and references therein). However, there is not a consensus on the role of stimulation frequency in controlling SP release from the spinal cord; although initial reports (Go and Yaksh, 1987) showed that SP release was enhanced by increasing the stimulation frequency from 12 to 20 Hz, Duggan et al. (1995) found that SP release was unchanged over a frequency range of 0.5–20 Hz but also reported that a greater release of SP was produced by a series of short bursts at high frequency (three pulses at 50–300 Hz).
Another issue is the type of fibers stimulated by our protocol. Intracellular recordings showed that the pulses used evoked EPSPs with latencies consistent with the conduction speed of A∂- and C-fibers (Li and Bak, 1976; Swett and Bourassa, 1981). However, because C-fibers do not follow 100 Hz (McCarthy and Lawson, 1989; Waddell and Lawson, 1990), at least some of the SP that elicits the internalization may be released from A∂-fibers, which follow 100 Hz and contain SP in 20% of their terminals (McCarthy and Lawson, 1989; Waddell and Lawson, 1990). Alternatively, our stimulation protocol may have produced short bursts of high frequency in C-fibers before they failed to follow the stimulation; these short bursts may be sufficient to elicit substantial SP release from their terminals (Duggan et al., 1995; Ribeiro-da-Silva and Claudio Cuello, 1995). Because SP may diffuse out of the slice, it is possible that release from both A∂- and C-fibers is necessary to produce internalization. Another possibility is that high-frequency stimulation of A∂- and Aβ-fibers decrease presynaptic inhibition of C-fibers. When comparing our observations with in vivoresults, it has to be kept in mind that slices lack bulbospinal control and input from other spinal segments. Nevertheless, slices with attached roots will be a valuable tool in future studies elucidating what type of afferent activity causes SP release.
Role of NMDA receptors in SP release
Our findings are consistent with the hypothesis that NMDA autoreceptors control the release of neuropeptides in the dorsal horn (Liu et al., 1994b). NK1R internalization produced by 100 Hz stimulation of the dorsal root was blocked by the NMDA receptor antagonist AP-5. Moreover, a short incubation with NMDA also elicited NK1R internalization in laminae I–IIo. The internalization produced by both 100 Hz stimulation and NMDA application was abolished by an NK1R antagonist, indicating that this internalization was caused by the release of endogenous SP controlled by NMDA receptors. A recent report (Liu et al., 1997) showed a similar increase in NK1R internalization after intrathecal injection of NMDA, which produced pain behavior.
In contrast with the effect of AP-5, CNQX, an antagonist of AMPA and kainate receptors (Honoré et al., 1988), did not decrease the NK1R internalization produced by 100 Hz stimulation, indicating that non-NMDA glutamate receptors do not mediate the release of SP. Furthermore, CNQX should block synaptic transmission between primary afferents and dorsal horn neurons, because non-NMDA glutamate receptors mediate most of the excitatory synaptic transmission in the dorsal horn (Yoshimura and Jessell, 1990; Randic et al., 1993). Hence, the lack of effect of CNQX suggests that SP is released from primary afferents, which contain half of the neurokinins present in the dorsal horn (Ogawa et al., 1985).
The most plausible explanation for these findings is that the release of SP in the dorsal horn is controlled by presynaptic NMDA receptors in the primary afferents. Alternative explanations are (1) that SP is released by secondary neurons having NMDA receptors postsynaptic to primary afferents; and (2) that postsynaptic NMDA receptors control neurokinin release from the primary afferents by means of a retrograde messenger such as nitric oxide (Schuman and Madison, 1991). The first possibility has to be ruled out, because blocking postsynaptic NMDA receptors does not appreciably decrease synaptic transmission in the dorsal horn (Yoshimura and Jessell, 1990; Randic et al., 1993), whereas all the internalization elicited by dorsal root stimulation appears to be NMDA receptor-dependent (Fig. 4). Moreover, as discussed above, the lack of inhibition of the internalization by CNQX suggests that SP is released from primary afferents.
Although the second explanation cannot be ruled out, there is strong evidence supporting the presence of NMDA autoreceptors in the dorsal horn. NMDA receptors have been detected on the presynaptic terminals of afferent fibers using electron microscopy immunohistochemistry (Liu et al., 1994b). Other reports show that NMDA receptors are expressed in most dorsal root ganglion neurons, including the small cells related to C- and A∂-fibers (Sato et al., 1993). Furthermore, glutamate coexists with SP in primary afferent terminals in the dorsal horn (Battaglia and Rustioni, 1988; De Biasi and Rustoni, 1988). Nevertheless, experiments ruling out the involvement of retrograde messengers in SP release elicited by NMDA application are necessary to confirm that these NMDA receptors are indeed presynaptic.
Sites of release of SP
NMDA and high-frequency stimulation produced internalization exclusively in neurons in laminae I–IIo, although internalization seems to occur also in the dendrites of laminae III–V neurons that extend into lamina I. This is probably attributable to the fact that SP is released in laminae I and II in amounts too small to reach laminae III–V (Liu et al., 1994a; Duggan et al., 1995;Ribeiro-da-Silva and Claudio Cuello, 1995). Alternative explanations are that internalization mechanisms are less active in laminae III–V, as suggested by the limited internalization produced by SP in this region, or that laminae III–V have a higher content of NEP.
Studying SP release in spinal cord slices has two potential problems: (1) released SP may diffuse out of the slices before it can reach deeper laminae; and (2) dorsal root fibers running rostrally before entering the dorsal horn are severed in the slices. Nevertheless, these problems do not invalidate our conclusion that SP release occurs mainly in laminae I and II, because NK1R internalization elicited by NMDA application to the slices or by noxious stimulation in vivo(Fig. 1G) (Mantyh et al., 1995) was also restricted to laminae I and II.
NK1Rs and dorsal horn LTP
LTP of synapses between afferent fibers, including C-fibers, and neurons in the superficial dorsal horn has been described by other investigators (Randic et al., 1993; Liu and Sandkühler, 1995). We have replicated these results here to show that the same 100 Hz tetanus that we used to produce NK1R internalization is able to induce LTP, confirming the integrity of the synapses. Both dorsal horn LTP and NK1R internalization are induced by high-frequency stimulation and require the activation of NMDA receptors. In vivo field potential recordings (Sandkühler et al., 1995) have shown that NK1R antagonists block the induction of dorsal horn LTP, raising the possibility that NK1Rs participate in dorsal horn LTP. The main problem with this idea is that LTP can be induced in half of the neurons in laminae I and II (Randic et al., 1993), whereas NK1R-positive neurons are scarce in lamina II (Liu et al., 1994a) and represent only 5–10% of the neurons in lamina I (Brown et al., 1995). Hence, LTP can be induced in neurons lacking NK1Rs. Further studies are necessary to elucidate whether the similarities between the production of LTP and SP release in the dorsal horn are purely coincidental, or whether neurons with NK1Rs somehow participate in the induction of LTP in other neurons.
Conclusion
Our results are consistent with the following model for the synergistic action of neurokinins and glutamate in the dorsal horn (Fig. 5E). (1) Noxious stimuli induce high-frequency action potentials in A∂- and C-fibers, producing presynaptic depolarization and glutamate release from these terminals. (2) Presynaptic depolarization and extracellular glutamate activate NMDA autoreceptors, which let calcium inside the presynaptic button. (3) This produces a further increase in the intracellular calcium concentration of the synaptic button, triggering SP release from dense core vesicles. (4) SP binds to NK1Rs in surrounding neurons, starting the internalization process. (5) Activated NK1Rs increase postsynaptic responses to glutamate (Randic et al., 1990; Rusin et al., 1993a,b) through signal transduction pathways, leading to an increase in neuronal excitability.
Footnotes
This work was supported by National Science Foundation Grant IBN-9510314 to E.F.G., National Institutes of Health Grants DK40919 and DK48351 to E.A.M, DK43207 and DK39957 to N.W.B, and DK41301 to CURE: Digestive Diseases Research Center. A pilot and feasibility grant to J.C.M. and access to facilities in the Morphology/Imaging Core and Animal Models Core were provided by CURE: Digestive Diseases Research Center. We thank Dr. Mirjana Randic, Dr. Rok Cerne, Dr. Lawrence Kruger, Dr. Thomas O’Dell, Dr. Catia Sternini, and Dr. Nicholas Brecha for their advice and Dr. Steve Vigna for the NK1R antibody.
Correspondence should be addressed to Juan Carlos G. Marvizón, University of California Los Angeles Neuroenteric Disease Program, West Los Angeles Veterans Administration Medical Center, Building 115, 11301 Wilshire Boulevard, Los Angeles, CA 90073.
REFERENCES
- 1.Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988;277:297–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- 2.Bleazard L, Hill RG, Morris R. The correlation between the distribution of the NK1 receptor and the actions of tachykinins agonists in the dorsal horn of the rat indicates that substance P does not have a functional role on substantia gelatinosa (lamina II) neurons. J Neurosci. 1994;14:7655–7664. doi: 10.1523/JNEUROSCI.14-12-07655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. doi: 10.1002/cne.903560302. [DOI] [PubMed] [Google Scholar]
- 4.Cascieri MA, Ber E, Fong TM, Sadoski S, Bansal A, Swain C, Seward E, Frances B, Burns D, Strader CD. Characterization of the binding of a potent, selective, radioiodinated antagonist to the human neurokinin-1 receptor. Mol Pharmacol. 1992;42:458–463. [PubMed] [Google Scholar]
- 5.Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in “wind-up” of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- 6.De Biasi S, Rustoni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA. 1988;85:7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty PM, Palecek J, Paleckova V, Willis W. Infusion of substance P or neurokinin A by microdialysis alters responses of primate spinothalamic tract neurons to cutaneous stimuli and to iontophoretically released excitatory amino acids. Pain. 1995;61:411–425. doi: 10.1016/0304-3959(94)00222-Z. [DOI] [PubMed] [Google Scholar]
- 9.Duggan AW, Schaible H-G, Hope PJ, Lang CW. Effect of peptidase inhibition on the pattern of intraspinally released immunoreactive substance P detected with antibody microprobes. Brain Res. 1992;579:261–269. doi: 10.1016/0006-8993(92)90059-i. [DOI] [PubMed] [Google Scholar]
- 10.Duggan AW, Riley RC, Mark MA, MacMillan SJA, Schaible H-G. Afferent volley patterns and the spinal release of immunoreactive substance P in the dorsal horn of the anesthetized spinal cat. Neuroscience. 1995;65:849–858. doi: 10.1016/0306-4522(94)00541-c. [DOI] [PubMed] [Google Scholar]
- 11.Garland AM, Grady EF, Lovett M, Vigna SR, Frucht MM, Krause JE, Bunnett NW. Mechanisms of desensitization and resensitization of G protein-coupled neurokinin1 and neurokinin2 receptors. Mol Pharmacol. 1996;49:438–446. [PubMed] [Google Scholar]
- 12.Go VLW, Yaksh TL. Release of substance P from the cat spinal cord. J Physiol (Lond) 1987;391:141–167. doi: 10.1113/jphysiol.1987.sp016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grady EF, Gamp PD, Jones E, Baluk P, McDonald DM, Payan DG, Bunnett NW. Endocytosis and recycling of neurokinin receptors in enteric neurons. Neuroscience. 1996;75:1239–1254. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- 14.Honoré T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241:701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- 15.Kellstein DE, Price DD, Hayes RL, Mayer DJ. Evidence that substance P selectively modulates C-fiber-evoked discharges of dorsal horn nociceptive neurons. Brain Res. 1990;526:291–298. doi: 10.1016/0006-8993(90)91234-8. [DOI] [PubMed] [Google Scholar]
- 16.Li CL, Bak A. Excitability characteristics of the A- and C-fibers in a peripheral nerve. Exp Neurol. 1976;50:67–79. doi: 10.1016/0014-4886(76)90236-3. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Brown JL, Jasmin L, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc Natl Acad Sci USA. 1994a;91:1009–1013. doi: 10.1073/pnas.91.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-d-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci USA. 1994b;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 20.Liu X-G, Sandkühler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-d-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- 21.Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, Simone DA. Receptor endocytosis and dendritic reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with substance P-like immunoreactivity. Neuroscience. 1989;28:745–753. doi: 10.1016/0306-4522(89)90019-5. [DOI] [PubMed] [Google Scholar]
- 23.McMahon SB, Lewin GR, Wall PD. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993;3:602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- 24.Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 25.Murase K, Randic M. Actions of substance P on rat spinal dorsal horn neurones. J Physiol (Lond) 1984;346:203–217. doi: 10.1113/jphysiol.1984.sp015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa T, Kanazawa I, Kimura S. Regional distribution of substance P, neurokinin A and neurokinin B in rat spinal cord, nerve roots and dorsal root ganglia, and the effects of dorsal root section or spinal transection. Brain Res. 1985;359:152–157. doi: 10.1016/0006-8993(85)91423-4. [DOI] [PubMed] [Google Scholar]
- 27.Randic M, Hecimovic H, Ryu PD. Substance P modulates glutamate-induced currents in acutely isolated rat spinal dorsal horn neurones. Neurosci Lett. 1990;117:74–80. doi: 10.1016/0304-3940(90)90122-p. [DOI] [PubMed] [Google Scholar]
- 28.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro-da-Silva A, Claudio Cuello A. Organization of peptidergic neurons in the dorsal horn of the spinal cord: anatomical and functional correlates. Prog Brain Res. 1995;104:41–59. doi: 10.1016/s0079-6123(08)61783-5. [DOI] [PubMed] [Google Scholar]
- 30.Routh VH, Helke CJ. Tachykinin receptors in the spinal cord. Prog Brain Res. 1995;104:93–108. doi: 10.1016/s0079-6123(08)61786-0. [DOI] [PubMed] [Google Scholar]
- 31.Rusin KI, Bleakman D, Chard PS, Randic M, Miller RJ. Tachykinins potentiate N-methyl-d-aspartate responses in acutely isolated neurons from the dorsal horn. J Neurochem. 1993a;60:952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 32.Rusin KI, Jiang MC, Cerne R, Randic M. Interactions between excitatory amino acids and tachykinins in the rat spinal dorsal horn. Brain Res Bull. 1993b;30:329–338. doi: 10.1016/0361-9230(93)90261-9. [DOI] [PubMed] [Google Scholar]
- 33.Sandkühler J, Liu X-G, Zimmermann M. N-Methyl-d-aspartate and neurokinin receptors are critical for the induction of long-term potentiation of C-fiber-evoked field potentials in rat spinal dorsal horn. Soc Neurosci Abstr. 1995;21:645.6. [Google Scholar]
- 34.Sato K, Kiyama H, Tae Park H, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. NeuroReport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 36.Swett JE, Bourassa CM. Electrical stimulation of peripheral nerve. In: Patterson MM, Kesner RP, editors. Electrical stimulation research techniques. Academic; New York: 1981. pp. 243–295. [Google Scholar]
- 37.Vigna SR, Bowden JJ, McDonald DM, Fisher J, Okamoto A, McVey DC, Payan DG, Bunnett NW. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994;14:834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waddell PJ, Lawson SN. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience. 1990;36:811–822. doi: 10.1016/0306-4522(90)90024-x. [DOI] [PubMed] [Google Scholar]
- 39.Womack MD, MacDermott AB, Jessell TM. Sensory transmitters regulate intracellular calcium in dorsal horn neurons. Nature. 1988;334:351–353. doi: 10.1038/334351a0. [DOI] [PubMed] [Google Scholar]
- 40.Xu X-J, Dalsgaard C-J, Wiesenfeld-Hallin Z. Spinal substance P and N-methyl-d-aspartate receptors are coactivated in the induction of central sensitization of the nociceptive flexor reflex. Neuroscience. 1992;51:641–648. doi: 10.1016/0306-4522(92)90303-j. [DOI] [PubMed] [Google Scholar]
- 41.Yaksh TL. The spinal pharmacology of facilitation of afferent processing evoked by high-threshold afferent input of the postinjury pain state. Curr Opin Neurol Neurosurg. 1993;6:250–256. [PubMed] [Google Scholar]
- 42.Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol (Lond) 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zieglgänsberger W, Tolle TR. The pharmacology of pain signaling. Curr Opin Neurobiol. 1993;3:611–618. doi: 10.1016/0959-4388(93)90063-5. [DOI] [PubMed] [Google Scholar]