Abstract
Cocaine is thought to be addictive because chronic use leads to molecular adaptations within the mesolimbic dopamine (DA) circuitry that affect motivated behavior and emotion. Although the reinforcing effects of cocaine are mediated primarily by blocking DA reuptake into the presynaptic nerve terminal, reciprocal signaling between DA and endogenous opioids has important implications for cocaine dependence. The present study used the opioid antagonist 6 β-[125iodo]-3,14-dihydroxy-17-cyclopropylmethyl-4,5 α-epoxymorphinan ([125I]IOXY) after pretreatment with the site-directed acylating agents 2-(p-ethoxybenzyl)-1-diethylaminoethyl-5-isothiocyanatobenzimidiazole-HCl (μ-selective) andN-phenyl-N-[1-(2-(4-isothiocyanato)-phenethyl)-4-piperidinyl]-propanamide-HCl (δ-selective) to examine the effect of cocaine exposure on the distribution and density of κ2 receptors in autopsy studies of human cocaine fatalities. The selective labeling of the κ2 receptor subtype was demonstrated by competition binding studies, which gave a pharmacological signature (IOXY ≥ (+)-bremazocine ≫ U50,488 ≥ U69,593) distinct from either the κ1 or κ3 receptor subtypes. Visualization of [125I]IOXY labeling revealed that κ2 receptors localize to mesocortical and subcortical limbic areas, including the cingulate, entorhinal, insular, and orbitofrontal cortices and the nucleus accumbens and amygdala. The number of κ2 receptors in the nucleus accumbens and other limbic brain regions from cocaine fatalities was increased twofold as compared with age-matched and drug-free control subjects. Cocaine overdose victims, who experienced paranoia and marked agitation before death, also had elevated densities of κ2 receptors in the amygdala. These findings demonstrate for the first time that κ2 receptor numbers are upregulated by cocaine exposure. The molecular adaptation of κ2 receptor numbers may play a role in the motivational incentive associated with episodes of binge cocaine use and in the dysphoria that follows abrupt cocaine withdrawal.
Keywords: cocaine, human brain, κ opioid receptor, IOXY, delirium, dopamine
Cocaine dependence results from the dysregulation of a number of distinct yet interacting neurochemical systems that act in concert (Nestler et al., 1993). Cocaine enhances dopamine (DA) neurotransmission by interacting with the DA transporter and inhibiting the clearance of extracellular DA (Ritz et al., 1987;Reith et al., 1989; Kuhar et al., 1991). Mesolimbic DA neurotransmission is modulated by endogenous opioids that act at μ and κ receptors to regulate DA release in the striatal reward centers (DiChiara and Imperato, 1988; Spanagel et al., 1990, 1992). Recent studies in animals have provided evidence for a role of the κ opioidergic system in the behavioral effects of cocaine (Shippenberg et al., 1996). κ agonists inhibit cocaine self-administration (Shippenberg et al., 1992; Glick et al., 1995), cocaine-induced place preference (Suzuki et al., 1992; Heidbreder et al., 1993), and the development of sensitization to the behavioral effects of cocaine (Shippenberg and Heidbreder, 1994; Heidbreder et al., 1995; Shippenberg et al., 1996). The mixed partial μ-agonist/κ antagonist buprenorphine reduces cocaine self-administration by rhesus monkeys (Mello et al., 1993) and prevents the reinstatement of cocaine-reinforced responding in rats (Comer et al., 1993). Furthermore, buprenorphine reduces the use of cocaine in dual cocaine- and opiate-dependent men (Mello and Mendelson, 1995).
The potent effects of κ agonists on cocaine administration suggest that κ receptors may be a useful molecular target for the development of pharmacotherapies for cocaine dependence. However, drug development has been hindered by reports that in humans, administration of κ agonists elicits both aversive and psychotomimetic actions (Kumor et al., 1986; Pfeiffer et al., 1986; Herz, 1990). The recent identification of three subtypes of κ receptors with distinct pharmacological and molecular properties (Pfeiffer et al., 1982; Clark et al., 1989; Nishi et al., 1993; Wollemann et al., 1993; Raynor et al., 1994; Rothman, 1994; Pan et al., 1995; Simonin et al., 1995) has lead to the hypothesis that different κ receptor subtypes may mediate distinct actions of κ agonists (Herz, 1990; Rothman et al., 1990; Ni et al., 1993, 1995; Rothman, 1994). The endogenous κ agonist dynorphin A demonstrates 10-fold higher potency for binding to the κ1 receptor (Simonin et al., 1995) as compared with the κ2 receptor (Ni et al., 1993, 1995), whereas truncated fragments of dynorphin A (i.e., dynorphin1–11 and dynorphin1–13) exhibit higher potencies for binding to the κ2 receptor over the κ1 receptor (Nishi et al., 1993; Webster et al., 1993). The molecular characterization of κ receptor subtypes suggests that it may be possible to develop nondysphoric κ-selective drugs as anti-cocaine medications.
The functional significance of each of the κ receptor subtypes in the CNS and their relevance to cocaine dependence is not understood. Most studies have examined the effects of either nonselective or κ1-selective agonists on the behavioral effects of cocaine. The lack of highly selective ligands for the κ2and κ3 receptor subtypes has limited the analysis of the precise role of these subtypes in cocaine dependence. At present, the best available method to measure κ2 receptors is by using nonselective opioid radioligands in the presence of drugs to occlude binding of the radioligand at defined opioid receptor sites. κ2 receptors were originally identified as high-affinity [3H]bremazocine binding sites in the presence of drugs that occluded binding to the κ1, μ, and δ receptors (Webster et al., 1993). Recently, the κ2receptor has been characterized in rat brain using the opioid antagonist 6 β-[125iodo]-3,14-dihydroxy-17-cyclopropylmethyl-4,5 α-epoxymorphinan ([125I]IOXY) in the presence of μ- and δ-selective drug occluders (Ni et al., 1993, 1995). The present study used this approach to visualize the distribution of κ2 receptors in the human brain for the first time. The effect of cocaine exposure on the regulation of [125I]IOXY binding to the κ2receptor was evaluated in subgroups of cocaine overdose victims who had histories of chronic cocaine abuse.
MATERIALS AND METHODS
Materials. IOXY, 2-(p-ethoxybenzyl)-1-diethylaminoethyl-5-isothiocyanatobenzimidiazole-HCl (BIT), andN-phenyl-N-[1-(2-(4-isothiocyanato)-phenethyl)-4-piperidinyl]propanamide-HCl (FIT) were synthesized as described previously (Ni et al., 1993). [125I]IOXY was radiolabeled as described previously (Ni et al., 1993).(±)-Bremazocine,d-Ala2,N-methyl-Phe4, Gly5-ol enkephalin (DAMGO), Leu5-enkephalin, naloxone, naloxonazine, naloxone benzoylhydrazone (NalBZOH), naltrindole,nor-binaltorphimine (nor-BNI),d-Pen2,d-Pen5]enkephalin (DPDPE), (5α,7α,8β)-(+)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4,5]dec-8-yl]benzenacetamide (U69,593), (1S-trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclo hexyl]-benzenacetamide (U50,488) were purchased from Research Biochemicals (Natick, MA). The isomers of pentazocine were provided by the National Institute on Drug Abuse.
Neuropathological tissue specimens. Postmortem neuropathological specimens were obtained during routine autopsy from age-matched and drug-free control subjects. Medicolegal investigations of the deaths were conducted by forensic pathologists. The circumstances of death and toxicology data were reviewed carefully before a death was classified as a cocaine overdose (CO) with or without preterminal excited delirium (ED). Fatal ED victims exhibited an acute onset of bizarre and violent behavior, which was characterized by one or more of the following: aggression, combativeness, hyperactivity, extreme paranoia, demonstration of unexpected strength, or incoherent shouting (Wetli and Fishbain, 1985; Wetli et al., 1996). The syndrome of fatal ED is defined as accidental cocaine toxicity in subjects who exhibited bizarre and violent behavior (as described above) followed by sudden death (Ruttenber et al., 1997). Cases were assigned to the ED subgroup if at least two of the behavioral signs and hyperthermia were present before death. All cases were evaluated for common drugs of abuse and alcohol, and positive urine screens were confirmed by quantitative analysis of blood. Blood cocaine was quantified using gas–liquid chromatography with a nitrogen detector. Frozen brain regions were sampled for quantitation of cocaine and benzoylecgonine using gas chromatography–mass spectroscopy techniques (Hernandez et al., 1994). Drug-free and age-matched control subjects were selected from accidental deaths with no cocaine or metabolites detected in toxicology screens of blood or brain tissue.
Ligand binding assays. Binding of [125I]IOXY was conducted as described previously with minor modifications (Ni et al., 1993, 1995). Briefly, membranes from the human caudate were pretreated to occlude μ and δ receptors with BIT (1 μm) and FIT (1 μm), respectively, in 50 mm potassium phosphate, pH 7.4, 100 mm NaCl. Membranes were washed and resuspended in assay buffer (50 mm Tris HCl, pH 7.4, 10 mm NaCl) containing protease inhibitors (100 μg/ml bacitracin, 1.1 μg/ml leupeptin, 2.75 μg/ml bestatin, 0.55 μg/ml chymostatin, 0.27 μg/ml captopril). Cold saturation analysis was conducted by incubating membranes with increasing concentrations of unlabeled IOXY in the presence of a fixed concentration of [125I]IOXY (0.04 nm) for 4 hr at 4°C. The pharmacological specificity of [125I]IOXY binding was determined by evaluating the potency of various opioid-like drugs in competition binding assays. Nonspecific binding was defined using 10 μm naloxone. Excess radioligand was separated from the bound radioligand using a Brandel Cell Harvester.
In vitro autoradiography. Half-hemisphere slide-mounted sections of brain were prepared from cryopreserved neuropathological specimens. From each coronal block, a series of adjacent cryostat sections were processed for [125I]IOXY autoradiography and for acetylcholinesterase histochemistry and Nissl substance to define cytoarchitectonic boundaries. For [125I]IOXY autoradiography, tissue sections were equilibrated in 10 mm potassium phosphate, pH 7.4, at 4°C before tissue sections were treated with the site-directed acylating agents BIT (1 μm) and FIT (1 μm) in 50 mm potassium phosphate, pH 7.4, 100 mm NaCl to occlude binding to the μ and δ receptors, respectively. Slide-mounted brain tissue sections were washed and incubated with [125I]IOXY (30 pm) in assay buffer containing protease inhibitors at 4°C for 2–3 hr. Nonspecific binding was determined in the presence of 10 μm naloxone. At the end of the incubation, tissue sections were washed in two changes of ice-cold 10 mm Tris HCl, pH 7.4, and dried under a cool stream of air. Autoradiograms were prepared by apposing the slide-mounted tissue sections along with co-placed iodine standards to Hyperfilm for 40–48 hr at −80°C.
Data analysis. For analysis of ligand binding data, binding constants were derived from the saturation data using the iterative, nonlinear curve-fitting program EBDA/LIGAND, (Biosoft, Elsevier). The competition binding data were analyzed using DRUG, with the nonspecific binding defined as the counts per minute bound in the presence of 10 μm naloxone. The best fit to a one- or two-site model was based on the partial F test. For quantitative analysis of [125I]IOXY autoradiograms, films were scanned using a Howtek Scanmaster 3 at 400 dots per inch using a transparency illuminator. The resulting TIFF (tagged image file format for RGB color) files were converted to pseudocolor format in specific activity units using the IMAGE (version 1.44; National Institutes of Health Shareware) and BRAIN (version 1.6; Drexel University) programs. After background subtraction, two-dimensional pseudocolor maps were created to allow radioactivity levels in femtomoles per milligram to be superimposed on the sections (Kuhar et al., 1986). Statistical significance was determined using the Dunnett’s t test.
RESULTS
The CO cases selected for the present study had evidence of a number of surrogate variables of chronic cocaine abuse on the basis of review of previous arrest records and hospital and substance abuse treatment admissions as well as pathological signs determined at autopsy (e.g., perforation of the nasal septum). Cocaine was detected in blood at the time of death for all cocaine overdose victims. No other drugs were detected in urine screens conducted at the time of death. Alcohol was detected in postmortem blood in two of the control subjects and three of the CO victims (blood alcohol concentration < 0.05%). ED deaths are seasonal and tend to cluster during the late summer months, and core body temperatures are markedly elevated in these cases. The demographic and toxicology data for the drug-free and age-matched control subjects and the CO and ED victims are shown in Table 1.
Table 1.
Demographics and clinical characteristics of drug-free and age-matched control subjects and cocaine overdose victims
| Case | Age (years) | Sex | Race | Autolysis (hr) | Cause of death | Toxicology | Temperature (°F) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood Cocaine/BE (mg/l) | Brain Cocaine/BE (mg/kg) | |||||||||
| 1 | 38 | F | W | 11 | Homicide | n.d. | n.d. | n.d. | n.d. | Normal |
| 2 | 20 | F | B | 14.5 | Homicide | n.d. | n.d. | n.d. | n.d. | Normal |
| 3 | 19 | M | W | 9.5 | Homicide | n.d. | n.d. | n.d. | n.d. | Normal |
| 4 | 21 | M | B | 19.5 | Motor vehicle accident | n.d. | n.d. | n.d. | n.d. | Normal |
| 5 | 37 | M | W | 9.5 | Occlusive CA disease | n.d. | n.d. | n.d. | n.d. | Normal |
| 6 | 26 | F | B | 12 | Hypertensive heart disease | n.d. | n.d. | n.d. | n.d. | Normal |
| 7 | 29 | M | W | 11 | Motor vehicle accident | n.d. | n.d. | n.d. | n.d. | Normal |
| 8 | 25 | M | W | 12–18 | Calcific aortic stenosis | n.d. | n.d. | n.d. | n.d. | Normal |
| 9 | 26 | M | W | 13.5 | Homicide | n.d. | n.d. | n.d. | n.d. | Normal |
| 10 | 29 | M | W | 23 | Fibromuscular dysplasia | n.d. | n.d. | n.d. | n.d. | Normal |
| 11 | 26 | M | W | 9 | Cocaine overdose | 2.10 | 10.50 | 6.08 | 5.15 | Normal |
| 12 | 27 | M | W | 16 | Cocaine overdose | 0.94 | 1.40 | 2.24 | 1.80 | Normal |
| 13 | 29 | M | W | 24 | Cocaine overdose | 349.0 | 20.00 | 3.52 | 3.52 | Normal |
| 14 | 38 | M | W | 14 | Cocaine overdose | 8.90 | 7.00 | >20.00 | 3.70 | Normal |
| 15 | 27 | M | W | 21 | Cocaine overdose | 0.36 | 5.8 | 5.40 | 2.98 | Normal |
| 16 | 42 | M | B | 11 | Cocaine overdose | 0.19 | 4.10 | 3.55 | 5.34 | Normal |
| 17 | 29 | M | W | 6.5 | Cocaine overdose | 7.80 | 11.40 | 13.00 | 2.10 | Normal |
| 18 | 26 | M | B | 12.5 | Excited delirium | 0.50 | 0.25 | 1.17 | 0.47 | 105.2 |
| 19 | 25 | M | B | 21 | Excited delirium | 0.40 | 0.05 | 0.37 | 1.51 | 107.2 |
| 20 | 42 | M | W | 14 | Excited delirium | 0.70 | 10.60 | 1.01 | 4.15 | 110.0 |
| 21 | 31 | M | W | 12 | Excited delirium | 1.20 | 6.20 | 1.65 | 2.52 | 105.4 |
| 22 | 30 | M | B | 10 | Excited delirium | 1.00 | 2.30 | 1.96 | 0.84 | 102.4 |
| 23 | 29 | M | B | 6.5 | Excited delirium | 0.26 | 2.20 | 1.48 | 1.31 | 102.5 |
| 24 | 32 | M | B | 6 | Excited delirium | 0.05 | 1.40 | 0.09 | 0.66 | 106.3 |
| 25 | 26 | F | B | 8 | Excited delirium | 0.40 | 1.10 | 3.30 | 0.43 | n.a. |
| 26 | 33 | M | B | 24 | Excited delirium | 0.07 | 1.90 | 0.22 | 0.88 | 108.0 |
Autolysis, Interval between time of death and freezing of the brain; BE, benzoylecgonine; n.d., none detected; n.a., not available.
Binding parameters and pharmacology of IOXY binding in human brain
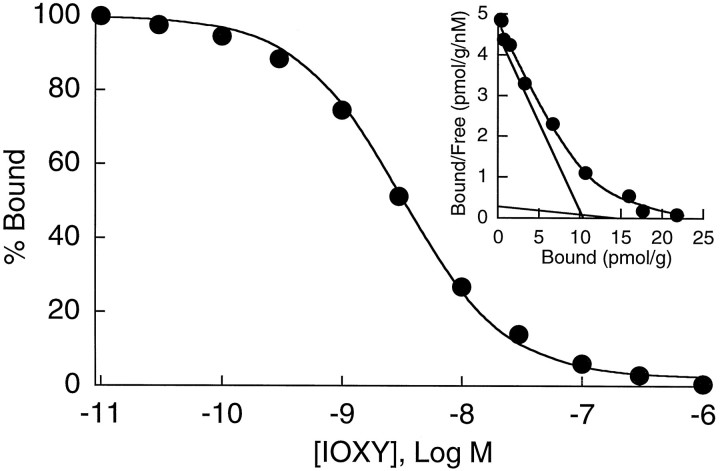
The specificity and parameters for binding of [125I]IOXY to κ2 receptors were assessed in human caudate membranes by saturation and competition binding analysis. Binding of [125I]IOXY was performed using membranes that were pretreated with the acylating agents BIT and FIT to prevent binding to μ and δ receptors, respectively. Analysis of the data as a homologous competition curve resulted in a mean potency value of 3.3 ± 0.2 nm and a Hill slope (nH) of 0.83 ± 0.05 (Fig. 1). Rosenthal transformation of the data revealed a curvilinear relationship, suggesting that [125I]IOXY labeled two binding sites (n = 6) (Fig. 1, inset). The mean dissociation constants (KD values) corresponding to the high and low affinity binding components were 2.6 ± 0.2 and 86.7 ± 15.6 nm, respectively. The density (Bmax) values were 9.6 ± 1.6 and 15.9 ± 3.5 pmol/gm for the high and low affinity sites, respectively.
Fig. 1.
Cold saturation analysis of IOXY binding to human anterior caudate. The mean potency value observed was 3.3 ± 0.2 nm with a Hill slope of 0.83 ± 0.05 (n = 6). Inset, A representative Rosenthal plot of IOXY binding to human caudate membranes from a drug-free control subject.
The pharmacological profile for inhibition of [125I]IOXY binding to the high affinity site in human caudate was determined using various drugs known to bind to μ, κ, and δ opioid receptors (Table 2). Competition binding assays were conducted using a single concentration of [125I]IOXY (40 pm), which labeled ∼97% of the high affinity binding sites. The nonselective κ agonist (±)-bremazocine demonstrated the highest potency, with an IC50 value of 4.7 ± 0.1 nm. In contrast, the κ1-selective agonists U50,488 and U69,593 inhibited [125I]IOXY binding with low micromolar potency values. The putative κ3 antagonist NalBZOH and the nonselective κ antagonist nor-BNI inhibited specific [125I]IOXY binding with nanomolar potency values. The μ-preferring opioid drugs naloxonazine, DAMGO, and Leu5-enkephalin and the δ-preferring opioid drugs naltrindole and DPDPE exhibited lower potency values than those characteristic of their receptor subtype. The stereoisomers of pentazocine demonstrated a rank order of potency characteristic of κ receptors. The high nanomolar potencies observed for IOXY and bremazocine, together with the low micromolar potencies seen for U69,593 and U50,488, confirmed that under the present assay conditions [125I]IOXY primarily labeled the κ2receptor. Similarly, the pharmacological profile for inhibition of [125I]IOXY binding to the amygdala and cingulate cortex was characteristic of the κ2 receptor subtype (Table 2).
Table 2.
Pharmacological profile for inhibition of [125I]IOXY binding in the human caudate, amygdala, and cingulate cortex
| Competitor | IC50, nm | nH |
|---|---|---|
| Caudate | ||
| κ-Preferring | ||
| (±)-Bremazocine | 4.7 ± 0.1 | 0.87 |
| Naloxone benzoylhydrazone | 14.4 ± 0.5 | 0.75 |
| nor-Binaltorphimine | 24.5 ± 6.9 | 0.71 |
| (−)-Pentazocine | 346.5 ± 42.1 | 0.96 |
| U50,488 | 10,600 ± 1800 | 1.08 |
| U69,593 | 32,500 ± 3500 | 1.05 |
| μ-Preferring | ||
| Naloxonazine | 50.1 ± 7.0 | 0.73 |
| DAMGO | 79.8 ± 6.3 | 0.65 |
| Leu-enkephalin | 427.9 ± 135.5 | 0.45 |
| δ-Preferring | ||
| Naltrindole | 111.5 ± 16.8 | 0.96 |
| DPDPE | 40,600 ± 1200 | 1.14 |
| ς-Preferring | ||
| (+) Pentazocine | 4106 ± 688 | 0.96 |
| Amygdala | ||
| κ-Preferring | ||
| IOXY | 0.94 ± 0.32 | 1.02 |
| Bremazocine | 0.85 ± 0.14 | 0.90 |
| U50,488 | 8411.8 ± 1885.1 | 0.91 |
| U69,593 | 11,254.4 ± 1930.9 | 0.59 |
| Cingulate cortex | ||
| κ-Preferring | ||
| IOXY | 1.19 ± 0.26 | 0.90 |
| Bremazocine | 1.08 ± 0.13 | 0.98 |
| U50,488 | 5272.6 ± 1516.6 | 0.56 |
| U69,593 | 8250.6 ± 1098.3 | 0.59 |
The values shown represent the mean ± SD of two independent determinations each performed in triplicate.
Anatomical distribution of [125I]IOXY labeling in human brain
The distribution of [125I]IOXY binding to κ2 receptor in the human brain was visualized usingin vitro autoradiographic techniques and a single concentration of [125I]IOXY (30 pm) to selectively occupy the high affinity binding site. The results demonstrated that κ2 receptors were prevalent throughout most of the subcortical limbic areas, including the amygdala, claustrum, and hypothalamus (Fig. 2,top). The distribution of the κ2 receptor in the amygdala was markedly heterogeneous, with the highest densities visualized over the basolateral nuclei. Moderate to high labeling was seen within the paralimbic belt cortices, including over the orbitofrontal, temporopolar, entorhinal, insular, parahippocampal, and cingulate gyri. Within these cortical sectors, the densest labeling was seen primarily over the deeper laminae (V and VI). Low to moderate labeling was measured in the striatum (Fig. 2C).
Fig. 2.
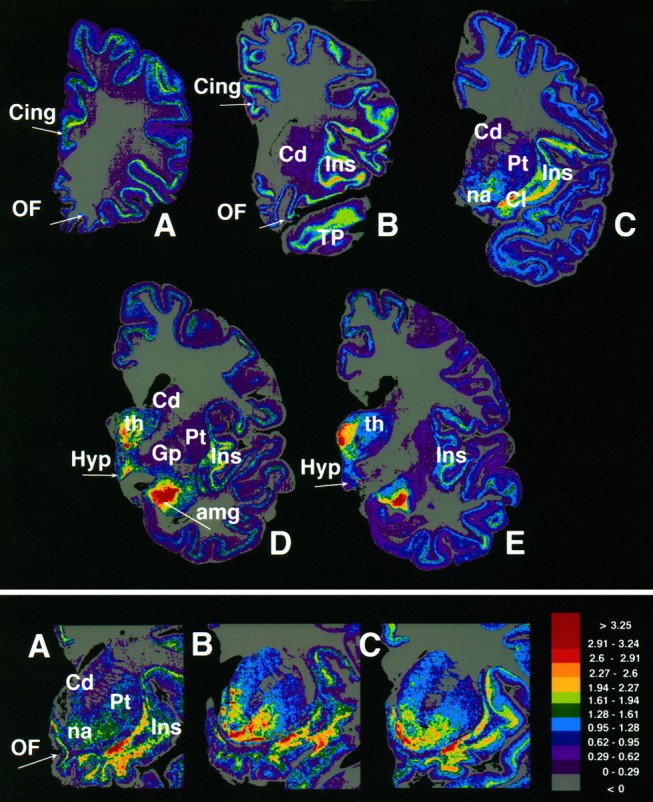
Regional distribution of [125I]IOXY binding to the κ2receptor in the human brain. Top, Computer-generated color coding of the autoradiograms from a series of half-hemisphere coronal sections of the human brain at five different anterior to posterior levels (A–E) is shown.Bottom, Pseudocolor density maps of [125I]IOXY labeling in the anterior striatum of a representative (A) drug-free control subject and (B) CO and (C) ED victim. Note the marked increase in the density of the κ2receptors in the ventral sectors of the anterior striatum in the CO and ED victims as compared with the drug-free control subjects.Cing, Cingulate; amg, amygdala;Cd, caudate nucleus; Cl, claustrum;Hyp, hypothalamus; Ins, insular cortex;na, nucleus accumbens; OF, orbitofrontal cortex; Pt, putamen; th, thalamus;TP, temporopolar cortex; Gp, globus pallidus.
Regulatory effects of cocaine on [125I]IOXY binding to κ2 receptors
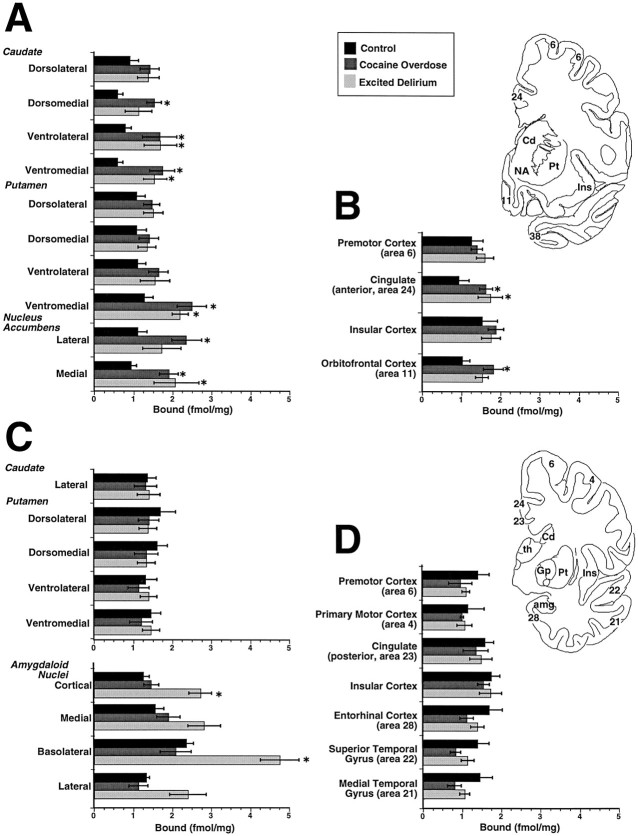
Quantitative region-of-interest measurements of [125I]IOXY binding were taken to assess the regulatory effects of cocaine on the κ2 receptor densities in human brain. Densitometric measurements of [125I]IOXY binding demonstrated a twofold elevation (p < 0.05) in the anterior and ventral sectors of the caudate and putamen and in the nucleus accumbens of the CO and ED victims as compared with drug-free and age-matched control subjects (Figs. 2, bottom, and3). The elevation of striatal labeling was confined to the more anterior sectors of the striatum, which receive the mesolimbic DAergic projections that are implicated in the rewarding effects of psychostimulant drugs (Kuhar et al., 1991). Rosenthal analysis of IOXY saturation binding data demonstrated that there was no significant change in the affinity for [125I]IOXY binding in the striatum of the CO and ED victims as compared with drug-free and age-matched control subjects (data not shown). This observation confirmed that the elevated densities of [125I]IOXY binding in the striatal reward centers of the CO victims was attributable to an increase in binding site densities and not an altered affinity of receptor for the radioligand. Within the cerebral cortex, [125I]IOXY binding was significantly elevated in the anterior cingulate (area 24) and orbitofrontal gyri of the CO victims (p < 0.05). [125I]IOXY binding sites were significantly increased in the cortical and basolateral nuclei of the amygdala in the ED victims (p < 0.05), but not in the CO victims, as compared with drug-free and age-matched control subjects.
Fig. 3.
Summary of the region of interest measurements for [125I]IOXY binding in the drug-free control subjects (n = 9) and CO (n = 6) and ED victims (n = 8). The regional sites sampled throughout the (A) anterior sectors of the striatum and (B) anterior cortical areas, (C) posterior striatum and the amygdaloid nuclei, and (D) the posterior cortical areas are shown. The anterior and posterior levels analyzed are shown in diagrammatic form in the top right corner. Dunnett’st test; *p < 0.05. See legend to Figure 2 for abbreviations.
DISCUSSION
Although the direct activation of DAergic system by cocaine is recognized as the primary substrate mediating the reinforcing properties of cocaine, regulatory adaptations in other neural systems that interact with DA may contribute to the development of cocaine dependence. The present study used the opioid antagonist [125I]IOXY under assay conditions modified to selectively visualize the distribution and densities of the κ2 opioid receptor in human brain and to assess the regulatory effects of cocaine exposure. The major findings are that κ2 receptors primarily localize to mesocortical and limbic brain areas, and that these receptor sites are upregulated in a regionally specific manner by cocaine exposure. Increased densities of [125I]IOXY binding were observed in the limbic sectors of the caudate and putamen, the nucleus accumbens, and the paralimbic belt cortices. Victims of the fatal ED syndrome also exhibited an upregulation of κ2 receptors within certain nuclei of the amygdala, distinguishing this group from other CO deaths. The marked elevation of κ2 receptors in critical brain reward regions of CO and ED victims provides further support for a role of the κ opioidergic system in cocaine dependence.
Pharmacological characterization and distribution of [125I]IOXY binding
Saturation binding studies indicated that when binding of [125I]IOXY to μ and δ receptors was occluded, [125I]IOXY labeled high and a low affinity binding sites. Although the identity of the low affinity site is not known, the competition binding profile (IOXY ≥ (±)-bremazocine > NalBZOH > nor-BNI > naloxonazine > DAMGO > naltrindole > (−)-pentazocine = Leu5- enkephalin > (+)-pentazocine > U50,488 > U69,593 ≥ DPDPE) for inhibition of [125I]IOXY to the high affinity site was similar to that described previously for the κ2 receptor in rat (Ni et al., 1993, 1995) and guinea pig brain (Webster et al., 1993). This unique pharmacological profile is distinct from that described previously for the cloned (Nishi et al., 1993; Simonin et al., 1995) and the native κ1 receptor in guinea pig brain (Pan et al., 1995), calf striatum (Clark et al., 1989), and monkey brain (Emmerson et al., 1994) and the κ3 receptor in rodent (Cheng et al., 1992) and guinea pig brain (Webster et al., 1993) and calf striatum (Clark et al., 1989). Taken together, these findings suggest that under the assay conditions used in the present study, [125I]IOXY labels the putative κ2receptor subtype. In vitro autoradiographic localization of [125I]IOXY in the presence of the selective drugs to occlude binding to μ and δ receptors demonstrated elevated densities of κ2 receptors throughout subcortical limbic areas and over the paralimbic belt cortices of the human brain. Overall, this pattern is similar to the distribution observed previously in human brain using the nonselective opioid agonist [3H]bremazocine (Quirion et al., 1987), the nonselective κ agonist [3H]ethylketocyclazocine (Pfeiffer et al., 1982), and the κ1-preferring receptor agonists [3H]U69,593 (Quirion et al., 1987;Roystin et al., 1991) and [125I]Tyr1-d-Pro10-dynorphin A (Hurd and Herkenham, 1993). [125I]IOXY binding to the κ2 receptor in the human striatum was higher over the ventral sectors. However, this distribution pattern contrasts with that observed previously for [125I]Tyr1-d-Pro10-dynorphin A binding in the human striatum, in which the highest densities of κ receptors were observed in the dorsal caudate, with lower densities seen in the putamen and nucleus accumbens (Hurd and Herkenham, 1993). Because dynorphin A exhibits 10-fold higher affinity for binding to the κ1 receptor (Simonin et al., 1995) as compared with the κ2 receptor (Ni et al., 1993, 1995; Webster et al., 1993), the autoradiographic localization pattern exhibited by [125I]Tyr1-d-Pro10-dynorphin A may reflect binding of the ligand to the κ1 receptor subtype. Therefore, the different dorsal to ventral gradients observed for [125I]Tyr1-d-Pro10-dynorphin A and [125I]IOXY labeling may represent distinct anatomical locations for the κ1 and κ2receptors in human striatal circuits. These findings suggest that drugs targeted to a specific κ receptor subtype may have distinct functions based on their different neuroanatomical locations.
Cocaine-induced adaptations in the κ opioidergic system
The κ2-like pharmacology shown for IOXY in competition binding studies confirms that the elevated receptor densities observed in the CO and ED victims are attributable to specific neuroadaptive changes in the κ2 receptor subtype. These findings are supported by previous studies of the effects of cocaine on κ receptors using radioligands that do not discriminate among the putative receptor subtypes. For example, “binge” cocaine administration (Unterwald et al., 1994) and chronic continuous exposure (Hammer, 1989) in rats caused elevations in [3H]bremazocine and [3H]naloxone binding in the nucleus accumbens. Because these radioligands label both κ1 and κ2 receptors, the observed elevations may reflect regulatory increases in either the κ1 or κ2receptor subtype. Hurd and Herkenham (1993) previously demonstrated elevated numbers of κ binding sites in brains from human cocaine abusers. However, in contrast to the present findings, which demonstrated elevated IOXY binding site densities that were restricted to the ventromedial (limbic) sectors of the striatum, the increased number of κ receptors labeled with [125I]Tyr1-d-Pro10-dynorphin A were marked within the dorsolateral (motor) sectors of the striatum. κ receptors are localized on both pre- and postsynaptic elements in the striatum (Werling et al., 1988; Mansour et al., 1994), with the ventral striatal regions having higher levels of postsynaptic κ receptors (Mansour et al., 1994). One possible explanation for the regional heterogeneity seen in the previous results of Hurd and Herkenham (1993) and those of the present study is that the agonist [125I]Tyr1-d-Pro10-dynorphin A may preferentially label presynaptic sites, whereas the antagonist [125I]IOXY may be a more selective marker of the postsynaptic κ receptor subtype.
Repeated administration of cocaine results in increased tissue levels of immunoreactive dynorphin peptides (Sivam, 1989; Smiley et al., 1990;Spangler et al., 1993) and prodynorphin mRNA (Hurd and Herkenham, 1992;Hurd et al., 1992; Spangler et al., 1993). Chronic treatment with direct or indirect DA agonists increases prodynorphin mRNA and dynorphin peptides in the striatum (Smiley et al., 1990; Spangler et al., 1993). The cocaine-induced increase in dynorphin peptides is prevented by administration of DA receptor antagonists (Sivam, 1989;Smiley et al., 1990). Furthermore, the D1 receptor knockout mouse had significant decreases in striatal levels of dynorphin, indicating that stimulation of the D1 receptor by DA mediates increases in dynorphin (Xu et al., 1994). Previous studies have indicated that dynorphin acts in the striatum to blunt the response of striatonigral neurons to DA input (Steiner and Gerfen, 1996). Sustained elevations in the levels of dynorphin may be expected to cause a compensatory downregulation in the number of κ binding sites. Because both κ binding sites and dynorphin peptides undergo compensatory upregulation, these results suggest that κ2receptor densities may be regulated independent of dynorphin expression by cocaine exposure.
Role of κ opioidergic system in cocaine dependence
Although κ agonists do not generalize to the cocaine cue in drug discrimination paradigms (Broadbent et al., 1995; Ukai et al., 1995), they suppress the stimulus effects of cocaine in monkeys (Spealman and Bergman, 1992). These findings indicate that it is unlikely that κ receptors play a direct role in the reinforcing or euphoric effects of cocaine. Shippenberg and colleagues (1996) have suggested that the conditioned aversive effects related to the hyperactivity of κ opioidergic neurons in the ventral striatum may underlie the motivational incentive to use cocaine. Cocaine dependence is associated with a withdrawal syndrome characterized by dysphoria, anxiety, depression, and intense craving that begins within 30 min after the end of a binge episode and may last for 1–10 weeks. Interestingly, in humans the subjective effects of κ agonists are known to mimic, in part, certain symptoms of cocaine withdrawal. Administration of the nonselective κ agonists ketocyclazocine and cyclazocine to humans caused unpleasant mood and feeling states, distortion of sensory experiences, paranoia, self-reported deficiencies in cognition, and feelings of detachment that may be reversed by administration of naloxone (Kumor et al., 1986; Pfeiffer et al., 1986). The similarity in the subjective effects of κ agonists to the symptoms of cocaine withdrawal suggest that increased activity of the κ opioidergic system may contribute to the dysphoric mood associated with abrupt withdrawal from cocaine.
Cocaine abuse is associated with neuropsychiatric disorders, including acute psychotic episodes, paranoid states, and intoxication delirium. The regionally selective elevation in κ2 receptor densities in the amygdala may play a role in the neuropsychiatric sequelae of the fatal ED syndrome. The amygdaloid complex has long been seen to have a role in the integration and control of emotional and autonomic behaviors (Ben-Ari, 1981). We observed an elevation of κ2 receptors within certain amygdaloid nuclei in the ED cases as compared with control subjects. In contrast, the regional pattern and local densities of κ2 receptors were unchanged in the amygdala in accidental CO deaths. The amygdala is an essential component for the association of the appropriate emotional response with extrapersonal objects and aggressive encounters (Kling et al., 1979). It may be hypothesized that the κ opioid dysfunction within the amygdala may have contributed to the resultant clinical display of aberrant complex emotional behaviors in ED victims.
In summary, the present findings demonstrate that there are high densities of κ2 receptors localized throughout the mesocortical and subcortical limbic circuits in the human brain. Additional studies with in vivo positron emission tomography or single photon emission computer tomography imaging are needed to characterize the time course for changes in κ2 opioid binding after acute and chronic cocaine exposure and in withdrawal. The results of this study suggest that cocaine exposure leads to a neuroadaptive increase in κ2 receptor densities in discrete brain loci, which may underlie in part the dysphoric mood and psychological distress associated with abrupt withdrawal of cocaine. An understanding of the regulatory profiles of opioid synaptic markers that occur with chronic misuse of cocaine may suggest alternative strategies for treating cocaine dependence.
Footnotes
This study was supported by United States Public Health Service Grant DA-06227 (D.C.M.) and the Intramural Research Program of the National Institute on Drug Abuse. We thank Dr. Dorita Matecka for custom synthesizing BIT, FIT, and IOXY. We thank Margaret Basile and Qinjie Ouyang for their expert technical assistance.
Correspondence should be addressed to Dr. Deborah C. Mash, Department of Neurology (D4-5), University of Miami School of Medicine, 1501 N.W. 9th Avenue, Miami, FL 33136.
REFERENCES
- 1.Ben-Ari Y. The amygdaloid complex. Elsevier North-Holland Biomedical Press; Amsterdam: 1981. [Google Scholar]
- 2.Broadbent J, Gaspard TM, Dworkin SI. Assessment of the discriminative stimulus effects of cocaine in the rat: lack of interaction with opioids. Pharmacol Biochem Behav. 1995;51:379–385. doi: 10.1016/0091-3057(94)00408-b. [DOI] [PubMed] [Google Scholar]
- 3.Cheng J, Roques BP, Gacel GA, Huang E, Pasternak GW. Kappa3 opiate receptor binding in the mouse and rat. Eur J Pharmacol. 1992;226:15–20. doi: 10.1016/0922-4106(92)90077-9. [DOI] [PubMed] [Google Scholar]
- 4.Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa1 subtypes and a novel kappa3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- 5.Comer SD, Lac ST, Curtis LK, Carroll ME. Effects of buprenorphine and naltrexone on reinstatement of cocaine-reinforced responding in rats. J Pharmacol Exp Ther. 1993;267:1470–1477. [PubMed] [Google Scholar]
- 6.DiChiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 7.Emmerson PJ, Liu MR, Woods JH, Medzihradshy F. Binding affinity and selectivity of opioids at mu, delta and kappa receptor in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- 8.Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- 9.Hammer RP. Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- 10.Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- 11.Heidbreder CA, Babovic-Vuksanovic D, Shoaib M, Shippenberg TS. Development of behavioral sensitization to cocaine: influence of kappa opioid receptor agonists. J Pharmacol Exp Ther. 1995;275:150–163. [PubMed] [Google Scholar]
- 12.Hernandez A, Andollo W, Hearn WL. Analysis of cocaine and metabolites in brain using solid phase extraction and full-scanning and GC/ion trap mass spectrometry. Forensic Sci Int. 1994;65:149–156. doi: 10.1016/0379-0738(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 13.Herz A. Implications of the multiplicity of opioid receptors for the problem of addiction. Drug Alcohol Depend. 1990;25:125–127. doi: 10.1016/0376-8716(90)90050-o. [DOI] [PubMed] [Google Scholar]
- 14.Hurd YL, Herkenham M. Influence of a single injection of cocaine, amphetamine or GBR 12909 on mRNA expression of striatal neuropeptides. Mol Brain Res. 1992;16:97–104. doi: 10.1016/0169-328x(92)90198-k. [DOI] [PubMed] [Google Scholar]
- 15.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 16.Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- 17.Kling A, Steklis HD, Deutsch S. Radiotelemetered activity from the amygdala during social interactions in the monkey. Exp Neurol. 1979;66:88–96. doi: 10.1016/0014-4886(79)90065-7. [DOI] [PubMed] [Google Scholar]
- 18.Kuhar MJ, DeSouza EB, Unnerstall JR. Neurotransmitter receptor mapping by autoradiography and other methods. Annu Rev Neurosci. 1986;9:27–59. doi: 10.1146/annurev.ne.09.030186.000331. [DOI] [PubMed] [Google Scholar]
- 19.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 20.Kumor KM, Haertzen CA, Johnson RE, Kocher T, Jasinski D. Human psychopharmacology of ketocyclazocine as compared with cyclazocine, morphine and placebo. J Pharmacol Exp Ther. 1986;238:960–968. [PubMed] [Google Scholar]
- 21.Mansour A, Fox CA, Meng H, Akil H, Watson S. κ1 receptor mRNA distribution in the rat CNS: comparison to κ receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- 22.Mello NK, Mendelson JH. Buprenorphine treatment of cocaine and heroin abuse. In: Cowan A, Lewis JL, editors. Buprenorphine, combating drug abuse with a unique opioid. Wiley; New York: 1995. pp. 241–288. [Google Scholar]
- 23.Mello NK, Kamien JB, Lukas SE, Mendelson JH, Drieze JM, Sholar JW. Effects of intermittent buprenorphine administration on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1993;264:530–541. [PubMed] [Google Scholar]
- 24.Nestler EJ, Hope BT, Widnell KL. Drug Addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 25.Ni Q, Xu H, Partilla JS, De Costa BR, Rice KC, Rothman RB. Selective labeling of kappa2 opioid receptors in rat brain by [125I]IOXY: interactions of opioid peptides and other drugs with multiple kappa2a binding sites. Peptides. 1993;14:1279–1293. doi: 10.1016/0196-9781(93)90188-m. [DOI] [PubMed] [Google Scholar]
- 26.Ni Q, Xu H, Partilla JS, De Costa BR, Rice KC, Kayakiri H, Rothman RB. Opioid peptide receptor studies. Interaction of opioid peptides and other drugs with four subtypes of the kappa2 receptor in guinea pig brain. Peptides. 1995;16:1083–1095. doi: 10.1016/0196-9781(95)00091-w. [DOI] [PubMed] [Google Scholar]
- 27.Nishi M, Takeshima H, Fukuda K, Kato S, Mori K. cDNA cloning and pharmacological characterization of an opioid receptor with high affinities for kappa-subtype selective ligands. FEBS Lett. 1993;330:77–80. doi: 10.1016/0014-5793(93)80923-i. [DOI] [PubMed] [Google Scholar]
- 28.Pan Y-X, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW. Cloning and functional characterization through antisense mapping of a kappa3-related opioid receptor. Mol Pharmacol. 1995;47:1180–1188. [PubMed] [Google Scholar]
- 29.Pfeiffer A, Pasi A, Mehraein P, Herz A. A subclassification of kappa-sites in human brain by use of dynorphin 1–17. Neuropeptides. 1981;2:89–97. [Google Scholar]
- 30.Pfeiffer A, Brandt V, Herz A. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer A, Pasi A, Mehraein P, Herz A. Opiate receptor binding sites in human brain. Brain Res. 1982;248:87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- 32.Quirion R, Pilapil C, Magnan J. Localization of kappa opioid receptor binding sites in human forebrain using [3H]U69,593: comparison with [3H]bremazocine. Cell Mol Neurobiol. 1987;7:303–307. doi: 10.1007/BF00711306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 34.Reith MEA, Kramer HK, Sershen H, Lajtha A. Cocaine competitively inhibits catecholamine uptake into brain synaptic vesicles. Res Commun Subst Abuse. 1989;10:205–208. [Google Scholar]
- 35.Ritz MC, Lamb SR, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 36.Rothman RB. Evidence for heterogeneity of kappa-opioid binding sites: a review of data obtained using brain membranes depleted of mu and delta binding sites with irreversible agents. Analgesia. 1994;1:27–49. [Google Scholar]
- 37.Rothman RB, Bykov V, De Costa BR, Jacobson AE, Rice KC, Brady LS. Interaction of endogenous opioid peptide and other drugs with four opioid binding sites with four opioid kappa binding sites in guinea pig brain. Peptides. 1990;11:311–331. doi: 10.1016/0196-9781(90)90088-m. [DOI] [PubMed] [Google Scholar]
- 38.Roystin MC, Slater P, Simpson MDC, Deakin JFW. Analysis of laminar distribution of kappa opiate receptor in human cortex: comparison between schizophrenia and normal. J Neurosci Methods. 1991;36:145–153. doi: 10.1016/0165-0270(91)90040-7. [DOI] [PubMed] [Google Scholar]
- 39.Ruttenber AJ, Lawler-Heavner J, Yin M, Wetli CV, Hearn WL, Mash DC. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Anal Toxicol. 1997;42:25–31. [PubMed] [Google Scholar]
- 40.Shippenberg TS, Heidbreder CH. Kappa opioid receptor agonists prevent sensitization to the rewarding effects of cocaine. NIDA Research Monograph. 1994;153:456. [PubMed] [Google Scholar]
- 41.Shippenberg TS, Herz A, Spanagel R, Bals-Kubik R, Stein C. Conditioning of opioid reinforcement: neuroanatomical and neurochemical substrates. Ann NY Acad Sci. 1992;654:347–356. doi: 10.1111/j.1749-6632.1992.tb25980.x. [DOI] [PubMed] [Google Scholar]
- 42.Shippenberg TS, LeFevour A, Heidbreder C. Kappa opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- 43.Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei M-G, Charron G, Bloch B, Kieffer B. Kappa opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- 45.Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramidal and limbic dynorphin systems. J Pharmacol Exp Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- 46.Spanagel R, Herz A, Shippenberg T. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- 47.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spangler R, Unterwald EM, Kreek MJ. “Binge” cocaine administration induced a sustained increase of prodynorphin mRNA in rat caudate-putamen. Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 49.Spealman RD, Bergman J. Modulation of the discriminative stimulus effects of cocaine by mu and kappa opioids. J Pharmacol Exp Ther. 1992;261:607–615. [PubMed] [Google Scholar]
- 50.Steiner H, Gerfen CR. Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contributions of pre- and postsynaptic mechanisms in dorsal and ventral striatum demonstrated by altered immediate early gene induction. J Comp Neurol. 1996;376:530–541. doi: 10.1002/(SICI)1096-9861(19961223)376:4<530::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- 52.Ukai M, Mori E, Kameyama T. Effects of centrally administered neuropeptides on discriminative stimulus properties of cocaine in the rat. Pharmacol Biochem Behav. 1995;51:705–708. doi: 10.1016/0091-3057(95)00010-t. [DOI] [PubMed] [Google Scholar]
- 53.Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu but not delta opioid receptors. NeuroReport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 54.Webster JL, Polgar WE, Brandt SR, Berzetei-Gurske IP, Toll L. Comparison of kappa2-opioid receptors in guinea pig brain and guinea pig ilieum membranes. Eur J Pharmacol. 1993;231:251–258. doi: 10.1016/0014-2999(93)90457-s. [DOI] [PubMed] [Google Scholar]
- 55.Werling LL, Frattali A, Portoghese PS, Takemori AE, Cox BM. Kappa-receptor regulation of dopamine release from striatum and cortex of rats and guinea pigs. J Pharmacol Exp Ther. 1988;246:282–286. [PubMed] [Google Scholar]
- 56.Wetli CV, Fishbain DA. Cocaine-induced psychosis and sudden death in recreational cocaine users. J Forensic Sci. 1985;30:873–880. [PubMed] [Google Scholar]
- 57.Wetli CV, Mash DC, Karch SB. Cocaine-associated agitated delirium and the neuroleptic malignant syndrome. Am J Emerg Med. 1996;14:425–428. doi: 10.1016/S0735-6757(96)90066-2. [DOI] [PubMed] [Google Scholar]
- 58.Wollemann M, Benhye S, Simon J. The kappa-opioid receptor: evidence for the different subtypes. Life Sci. 1993;52:599–611. doi: 10.1016/0024-3205(93)90451-8. [DOI] [PubMed] [Google Scholar]
- 59.Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]