Abstract
The norepinephrine transporter (NET) is a membrane protein responsible for termination of the action of synaptic norepinephrine and is a site of action of many drugs used to treat major depression. The present study determined whether the binding of [3H]nisoxetine to the NET is altered in the locus coeruleus (LC) in major depression, using brain tissue collected postmortem from subjects diagnosed with major depression and from age-matched normal control subjects. Thirteen of the 15 major depressive subjects studied died by suicide. The distribution of [3H]nisoxetine binding along the rostro-caudal axis of the nucleus was uneven and was paralleled by a similar uneven distribution of neuromelanin-containing cells in both major depressives and psychiatrically normal control subjects. The binding of [3H]nisoxetine to NETs in the midcaudal portion of the LC from major depressive subjects was significantly lower than that from age-matched, normal control subjects. The binding of [3H]nisoxetine to NETs in other regions of the LC was similar in major depressives and control subjects. In contrast to reductions in binding to NETs, there were no significant differences in the number of noradrenergic cells at any particular level of the LC between major depressives and normal control subjects. The decreased binding of [3H]nisoxetine to NETs in the LC in major depression may reflect a compensatory downregulation of this transporter protein in response to an insufficient availability of its substrate (norepinephrine) at the synapse.
Keywords: locus coeruleus, major depression, suicide, norepinephrine transporters, tricyclic antidepressants, norepinephrine, noradrenergic, norepinephrine uptake
The ability of tricyclic antidepressant drugs to enhance the synaptic action of norepinephrine provided early evidence for the hypothesis that the pathophysiology of major depression is characterized, at least in part, by a deficit in brain norepinephrine (Schildkraut, 1965). Despite the apparent importance of the involvement of brain norepinephrine in the actions of these and other drugs used to treat depression, numerous attempts to demonstrate some disruption of noradrenergic transmission directly in depressed patients have produced equivocal results (Post, 1973; Lerner et al., 1978; Halaris and DeMet, 1979; Berger, 1980; Caldecott-Hazard et al., 1991). Recent studies using postmortem brain tissue have demonstrated evidence of neurochemical disruption of central noradrenergic neurons, i.e., locus coeruleus (LC), in victims of suicide relative to normal control subjects (Ordway et al., 1994a,b;Zhu et al., 1995). A similar disruption of noradrenergic chemistry has also been observed in rat models of depression, which can be normalized by tricyclic antidepressant administration (Weiss et al., 1981; Papp et al., 1994).
Tricyclic antidepressants enhance noradrenergic transmission by binding to the norepinephrine transporter (NET), a plasma membrane protein responsible for terminating the action of norepinephrine in the noradrenergic synapse (Iversen, 1975; Graefe and Bonisch, 1988). Blockade of the NET by these drugs results in a prolongation of the action of norepinephrine in the synapse (Graefe and Bonisch, 1988;Barker and Blakely, 1995). Given the importance of the NET to noradrenergic transmission, it is conceivable that regulation of the level of expression of the NET gene in noradrenergic neurons may be a natural mechanism by which noradrenergic transmission can be adjustedin vivo in response to physiological demands placed on this system. Evidence for such a mechanism was first provided by Lee and coworkers (1983), who demonstrated that NETs are upregulated and downregulated in response to enhanced availability or depletion of norepinephrine, respectively. Thus, levels of NETs on brain noradrenergic neurons appear to be regulated in such a way as to maintain “normal” concentrations of norepinephrine in the noradrenergic synapse (Lee et al., 1983).
Given that noradrenergic transmission can be regulated by changes in NET expression, it seems possible that changes in the levels of NETs in the brain may contribute to central noradrenergic dysfunction putatively associated with major depression. To examine this possibility, we measured the binding of [3H]nisoxetine to NETs in the noradrenergic LC from subjects with confirmed premorbid psychiatric diagnoses of major depression and from age-matched, psychiatrically normal control subjects. [3H]Nisoxetine binding to NETs was measured along the rostro-caudal axis of the LC because: (1) NETs are unevenly distributed along the axis of the LC (Ordway et al., 1997); (2) LC neurons are organized topographically with respect to their target areas (Foote et al., 1983; Waterhouse et al., 1983; Loughlin et al., 1986b); and (3) regionally specific alterations in LC cell number have been reported in certain neurological diseases (Chan-Palay and Asan, 1989a; German et al., 1992; Chan-Palay, 1993), in alcoholics (Arango et al., 1994), and in victims of suicide (Arango et al., 1996).
MATERIALS AND METHODS
Study subjects and psychiatric autopsy. Human brain tissue was obtained from subjects at the time of autopsy at the Medical Examiner’s Office of Cuyahoga County, Ohio, in accordance with an approved Institutional Review Board protocol. Subjects were coded to protect their identity. Causes of death were determined by the coroner. All subjects were kept in a refrigerated room before autopsy once arriving at the coroner’s office.
Information on the lifetime and current (within the last month) psychiatric status of subjects was obtained in structured clinical interviews by a trained interviewer with the next of kin for all victims of suicide and for 12 subjects dying of natural causes (2 with and 10 without psychiatric histories). The interview used was a modified Schedule for Affective Disorders and Schizophrenia, lifetime version (SADS-L) (Endicott and Spitzer, 1978) to make diagnoses compatible with the Diagnostic and Statistical Manual of Mental Disorders, revised third edition (DSM-III-R; American Psychiatric Association, 1987). The SADS has obtained adequate validity when comparing the patient report with that of an informant (Andreasen et al., 1977). Evaluation of drug and alcohol abuse and dependency was assessed in the modified SADS-L. Axis I diagnoses were made by a psychiatrist (H.Y.M.) and a clinical psychologist (J.O.), based on data gathered from the structured interview and, when available, supplemented with hospital and doctor’s records. No psychiatric information could be obtained for 9 of the 19 control subjects. Although there was no evidence in the coroner’s records of any history of psychiatric or neurological disease or prescription medications for psychiatric illnesses in these nine control subjects, psychiatric normality could not be verified through structured interviews.
Brain tissues were collected from 15 subjects diagnosed with major depression (summary of subject information is outlined in Table1) and 19 control subjects. The age of subjects ranged from 23 to 83 yr with averages of 55 ± 4 yr for control subjects and 59 ± 5 yr for major depressive subjects. Postmortem delay was 18 ± 1 and 19 ± 2 hr for control subjects and major depressives, respectively. Among the 15 subjects diagnosed with major depression, one was diagnosed with comorbid alcohol dependence, and two were diagnosed with alcohol abuse. One major depressive had been diagnosed with Parkinson’s disease. Subjects in the control group consisted of 6 females and 13 males, and the causes of death in this group were cardiovascular failure (n = 14), gunshot (n = 1), pulmonary embolism (n = 1), aneurism (n = 1), pancreatitis (n = 1), and asphyxia (n = 1). Ten control subjects, who were assessed retrospectively through structured interviews, had no axis I diagnosis (DSM-III-R). One control subject had a history of an episode of adjustment disorder with depressed mood 5 months before death.
Table 1.
Vital data of subjects retrospectively diagnosed with major depression
| Subject | Age (years) | Gender | PMD1-a(hr) | Toxicology | Cause of death | Axis I diagnosis |
|---|---|---|---|---|---|---|
| B | 43 | Male | 21 | NDD1-b | Hanging | Major depression |
| E | 68 | Male | 4 | NDD | CO poisoning | Major depression1-c |
| H | 73 | Male | 18 | Diazepam, codeine | Self-inflicted gunshot | Major depression |
| J | 38 | Female | 12 | Diazepam, lidocaine, temazepam | Drug overdose | Major depression |
| L | 23 | Male | 15 | NDD | Self-inflicted gunshot | Major depression1-d |
| M | 63 | Female | 18 | Lidocaine | Heart disease | Major depression |
| P | 75 | Female | 30 | NDD | CO poisoning | Major depression1-e |
| Q | 62 | Male | 5 | NDD | Hanging | Major depression |
| T | 77 | Female | 32 | Propoxyphene, norpropoxyphene | Heart disease | Major depression |
| U | 39 | Male | 24 | Ethanol | Hanging | Major depression1-d |
| B-1 | 83 | Female | 21 | NDD | Slashed wrists | Major depression |
| D-1 | 74 | Male | 24 | NDD | Hanging | Major depression |
| E-1 | 62 | Male | 20 | NDD | Self-inflicted gunshot | Major depression |
| H-1 | 70 | Male | 23 | Phenytoin-ERf | Self-inflicted gunshot | Major depression |
| I-1 | 42 | Male | 20 | NDD | Self-inflicted gunshot | Major depression |
All subjects died as a result of suicide except for subjects M and T.
Postmortem duration.
No drugs detected.
Also had Parkinson’s disease.
Also had alcohol abuse.
Also had alcohol dependence.
f Phenytoin administered in emergency room.
A toxicology screen on blood and urine from all of the subjects was performed by the county coroner’s office. Qualitative and quantitative assays were used to detect the following compounds or classes of compounds: ethanol, barbiturates, benzodiazepines, sympathomimetic drugs, and many antidepressant and antipsychotic drugs and their metabolites. In the course of collecting tissue for these studies, all subjects with evidence of antidepressant drugs, other psychotherapeutic drugs, or other psychoactive compounds in the toxicology screen were not included in the study. Toxicology results of major depressive subjects are shown in Table 1. The toxicology screen of control subjects revealed the following: one subject had ethanol in the blood, two had chlorpheniramine, one had ephedrine, four had lidocaine, one had codeine and cyclobenzaprine, one had lorazepam, and one had ephedrine and phenylethanolamine. Records collected did indicate antidepressant drug prescriptions within the last 6 months for four of the subjects with major depression. One major depressive suicide victim was found with an empty container of sertraline, an antidepressant selective for the serotonin transporter, at the time of death. At the time of this autopsy, sertraline had just received approval for clinical use and was not routinely analyzed in the toxicological workup. It was ruled likely that this individual had ingested sertraline immediately before the time of death. The binding levels of [3H]nisoxetine to the NET in the LC for this subject were comparable to those of other major depressives.
Dissection. At the time of autopsy, a block of pontine tissue was dissected. The floor of the fourth ventricle and the pons were its dorsal and ventral surfaces, respectively. The rostral surface was formed by a transverse cut immediately caudal to the inferior colliculus (at the frenulum). Tissue lateral to the superior cerebellar peduncles was trimmed away. Particular care was taken in the freezing process to maintain gross morphology. For example, the block of pontine tissue was dissected to form a flat surface on the ventral pontine surface of the LC tissue block. This surface was placed on a hard piece of cardboard, which was then lowered for 10 sec into 2-methylbutane cooled on dry ice to −50°C for quick freezing. Tissue blocks were then placed on powdered dry ice for 10 min and then stored in an ultracold freezer (−83°C).
For experiments examining binding to NETs along the axis of the LC, tissue blocks containing LC were paired (major depressive and age-matched control subject), and the caudal surfaces of each pair were comounted to the specimen chuck of a cryostat microtome (Leica, Cryocut 1800, Reichert-Jung). In this way, a single major depressive–control pair was sectioned simultaneously, and paired sections were mounted on the same microscope slide to be processed concurrently throughout the experiment. This pairing procedure reduced the influence of variables related to experimental techniques that could artifactually contribute to differences between major depressives and normal controls. Another set of experiments examined binding to NET at only two levels of the LC, which were defined anatomically, and tissue blocks from additional control subjects and major depressives for these experiments were sectioned independently. All tissue blocks containing LC were sectioned in a single transverse plane perpendicular to the floor of the fourth ventricle. Tissue sections were cut at −16°C and thaw-mounted onto gelatin-coated microscope slides. The LC was sectioned throughout its entire length sequentially beginning at its rostral end. Two 40-μm-thick sections for morphometry, followed by four 20 μm sections for binding, were cut at each 1 mm interval (except where noted) along the rostro-caudal axis of the LC. The rostral border of the LC was defined by the frenulum (at the caudal edge of the inferior colliculus), and the caudal border was the caudal extent of the LC (at the level of the motor nucleus of the trigeminal nerve), defined as the point at which neuromelanin-containing cells were no longer visible. There are cells of the LC that occur rather diffusely rostral to the frenulum, but this very rostral portion of LC has <5% of the total number of cells (German et al., 1988). The frenulum provides a distinct anatomical reference point from which distances can be measured accurately, facilitating anatomically equivalent comparisons between subjects (German et al., 1988, 1992).
Morphometry of the locus coeruleus. Cell counting of the LC was evaluated in tissue that was frozen, sectioned on a cryostat microtome, and then post-fixed using a standard cresyl violet staining procedure (fixing in xylene). This procedure is different from common morphometric methods that require the tissue to be formalin-fixed and then sectioned. The use of frozen, post-fixed tissue for morphometry was necessary in these studies, because frozen tissue was needed to perform radioligand binding, and because a comparison of receptor binding to LC cell number in the same subjects was sought. The condition of the frozen post-fixed sections was, nevertheless, good, with only minor evidence of freeze-related artifacts. In both control subjects and major depressives, LC cells stained for the Nissl substance (rough endoplasmic reticulum) were visible as characteristically round or oval-shaped somata that contained very dark spheric granules of neuromelanin pigment. The nucleus was visible in most of the cells as a dark spherical spot that was placed eccentrically. These basic morphological characteristics described here for frozen, post-fixed tissue have been described previously for the LC in formalin-fixed tissue (German et al., 1988, Baker et al., 1989;Chan-Palay and Asan, 1989b).
Two adjacent sections for morphometry were dried at room temperature and then stained with cresyl violet. Profiles, as defined by Coggeshall and Lekan (1996), of neuromelanin-pigmented neurons of the LC were counted using a Nikon Optiphot microscope (magnification, 200×) and are referred to as neuromelanin-containing cells throughout this article. Neuromelanin-containing cell counts were estimated by averaging independent counts made by two experimenters who were blind to subject information. For the two experimenters, the number of neuromelanin-containing cells at any particular level of a given subject never differed by >5%. A bilateral neuromelanin-containing cell count at each level was determined from the average of two adjacent sections for each level.
Quantitative autoradiography of [3H]nisoxetine binding to NET. The binding of [3H]nisoxetine to the NET was measured by quantitative autoradiography using the method of Tejani-Butt (1992). Briefly, transverse sections cut through the LC (also containing the caudal extent of the dorsal and median raphe nuclei) were thaw-mounted on gelatin-coated microscope slides. Sections were incubated with 3.0 nm [3H]nisoxetine (82 Ci/mmol; American Radiolabeled Chemicals Inc., St. Louis, MO) in buffer (in mm: 50 Tris, 300 NaCl, and 5 KCl, pH 7.4) at 4°C for 4 hr. Nonspecific binding was defined by 1 μm mazindol.
After incubations, sections were washed in the same buffer three times at 4°C for 5 min and then rinsed briefly (2 sec) in ice-cold water before drying. Sections and brain mash-calibrated 3H standards (American Radiolabeled Chemicals) were apposed to [3H]Ultrofilm (Leica, Nussloch, Germany) and exposed in x-ray cassettes at room temperature for 4 weeks. Films were processed with GBX developer and fixer (Eastman Kodak, Rochester, NY) at 17°C. After autoradiography, the same sections were stained lightly with cresyl violet for aid in the identification of anatomical structures. Densitometric measurements of autoradiograms were made using the Microcomputer-Controlled Imaging Device (M2; Imaging Research Inc., St. Catherines, Ontario, Canada). LC autoradiograms were analyzed by simultaneously overlaying the image of the autoradiogram with the image of the same, histologically stained section. For the LC, the smallest region encompassing all cell bodies containing neuromelanin pigment was outlined. Specific binding was defined as the difference between total and nonspecific binding. The binding of radioligands to the left and right sides of LC cell groups was measured independently. Right and left side binding density was averaged for each sample, because no significant difference in [3H]nisoxetine binding between left and right sides was observed.
Statistics. The uneven distribution of [3H]nisoxetine binding in the LC and differences between groups of subjects were analyzed statistically using ANOVA for repeated measures followed by contrast analyses using a univariateF test (Systat, Inc., Evanston, IL). Linear regression analyses were used to compute correlations between cell numbers and binding and between age or postmortem interval and binding or cell number (GraphPad Prism; GraphPad Software Inc., San Diego, CA). [3H]Nisoxetine binding measured at single rostro-caudal levels in normal controls and major depressives was compared using Student’s t test for independent samples.
RESULTS
The binding of [3H]nisoxetine was measured in transverse sections cut at 1 mm intervals along the rostro-caudal axis of the brainstem containing LC from nine normal control subjects and nine major depressives (Table 1, subjects B, E, H, J, L, M, P, Q, T). As observed previously (Ordway et al., 1997), [3H]nisoxetine binding to NETs was unevenly distributed all along the axis of the human LC, and this pattern of distribution was observed in both controls and major depressives. Likewise, other general features of the binding of [3H]nisoxetine to NETs displayed similar patterns in control and major depressive subjects. For example, at the rostral portion of the LC, the binding of [3H]nisoxetine was less localized to the cellular region of the LC, and moderate amounts of binding were also observed in surrounding areas such as the central gray and the median and dorsal raphe nuclei. The highest amount of [3H]nisoxetine binding was found in the middle portion of the LC in both control and major depressive subjects.
The amount of [3H]nisoxetine binding to NETs in the LC correlates strongly with, and is apparently dependent on, the number of noradrenergic cells at any particular level of the LC (Ordway et al., 1997). Thus, in this study, the number of neuromelanin-containing cells was counted at each level in the same control and major depressive subjects in whom [3H]nisoxetine binding was measured to evaluate the influence of possible psychiatric illness-induced alterations in cell number on NET density.
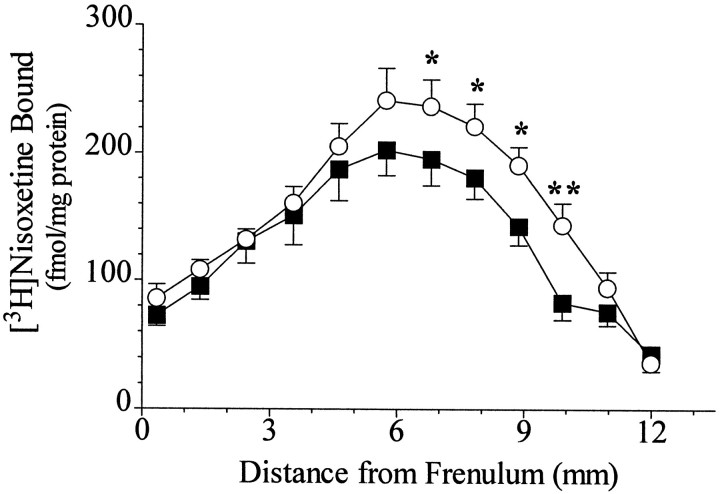
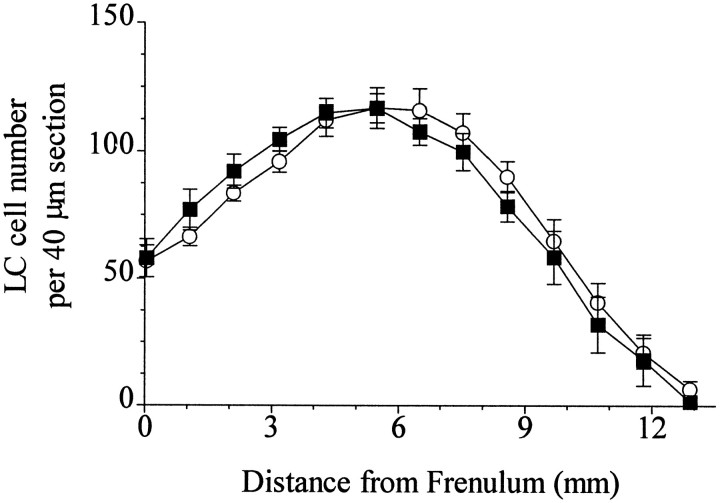
The specific binding of [3H]nisoxetine to NETs in the LC exhibited a significant rostro-caudal gradient in both psychiatrically normal control subjects and subjects diagnosed with major depression (controls, F(8,64) = 14.7;p < 0.001; depressives, F(8,64)= 9.6; p < 0.001) (Figure1). Covariate analysis with age did not show age as a statistically significant variable; therefore a single-factor repeated measure (without covariate) was used to evaluate differences between control and major depressive subjects. There was significantly lower binding of [3H]nisoxetine to NETs in the LC of major depressive subjects compared with normal control subjects. This lower binding in major depressives was limited to the midcaudal extent of LC, in an area from ∼6.5 to 9.5 mm caudal to the frenulum (Figs. 1 and 2). In contrast to differences in NET binding, the number of neuromelanin-containing cells did not differ significantly at any level of LC between the same normal control and major depressive subjects. There was a significant rostro-caudal gradient of the number of neuromelanin-containing cells in the LC for both normal control and major depressive subjects (controls, F(9,72) = 21.08; p < 0.001; depressives,F(9,72) = 17.41; p < 0.001) (Figure 3). This gradient of cell numbers in the LC correlated positively with the specific [3H]nisoxetine binding in both study groups (normal controls, r2 = 0.47;p < 0.001; major depression,r2 = 0.27; p < 0.001) (Fig. 4A,B).
Fig. 1.
The distribution of specific binding of [3H]nisoxetine to NETs along the rostro-caudal axis of the LC from nine subjects diagnosed with major depression (▪) and nine age-matched psychiatrically normal control subjects (○). Theabscissa is the distance from the frenulum in the caudal direction. Values are the average of four estimations (left and right sides in duplicate) for every subject. Asterisksindicate statistically significant differences between groups (*p < 0.05; **p < 0.01).
Fig. 2.
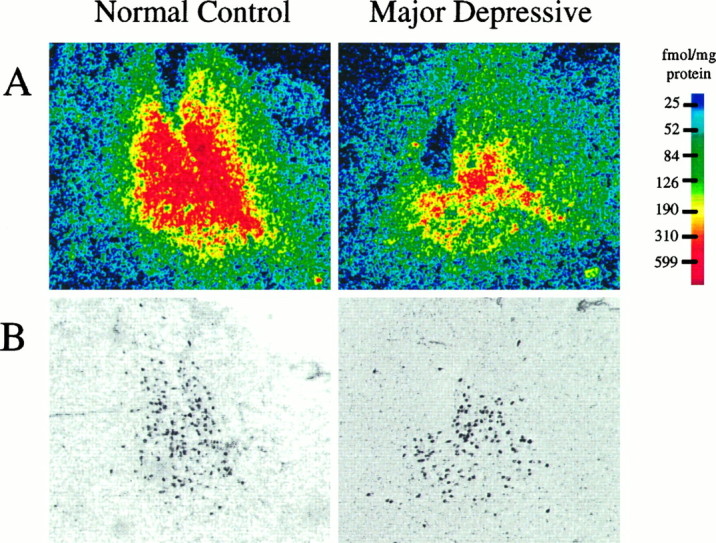
A, Digitized autoradiograms of the specific binding of [3H]nisoxetine to NETs in the LC from a psychiatrically normal control subject and an age-matched subject diagnosed with major depression. Images of [3H]nisoxetine binding to NETs are shown at a transverse level through the LC ∼8 mm caudal to the frenulum. Autoradiographic images were generated by digitally subtracting the image of nonspecific binding of [3H]nisoxetine from the image of the total binding with the aid of a computer.B, Digitized images of tissue sections used to generate the autoradiograms shown in A (sections were stained with cresyl violet). Dark spots are neuromelanin-containing cells of the LC. For densitometric analyses, the smallest region encompassing all LC cell bodies containing neuromelanin pigment was outlined in the histological image, and this outline was projected onto the precisely overlaid image of the autoradiogram.
Fig. 3.
Numbers of neuromelanin-containing cells along rostro-caudal extent of the LC from nine subjects diagnosed with major depression (▪) and nine age-matched psychiatrically normal control subjects (○; same subject pairs as shown in Fig. 1). Theabscissa is the distance from the frenulum in the caudal direction. Values are the average of four estimations (left and right sides in duplicate) for every subject.
Fig. 4.
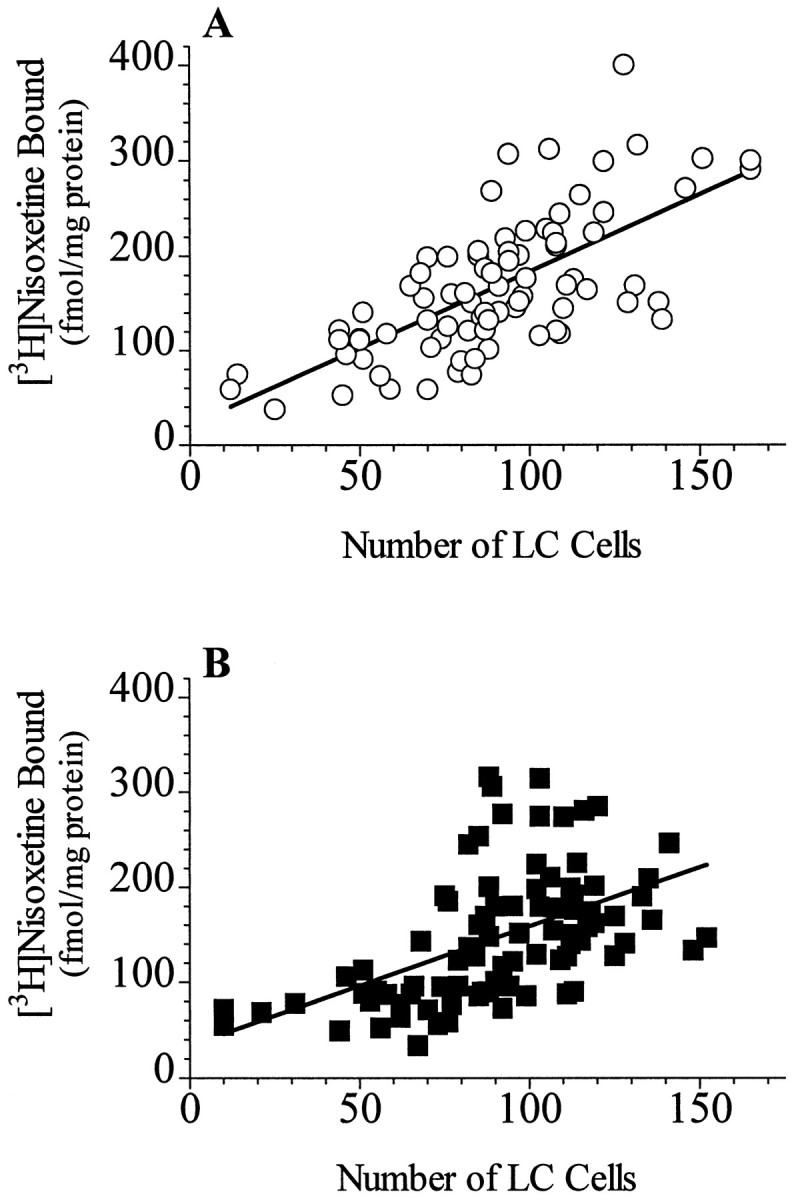
Relationship between neuromelanin-containing cell counts and the binding of [3H]nisoxetine to NETs at all levels of the LC from nine psychiatrically normal control subjects (A; r2 = 0.47; p < 0.001) and from nine subjects diagnosed with major depression (B;r2 = 0.27; p < 0.001).
Because the difference in [3H]nisoxetine binding in major depression was found only in the midcaudal portion of LC, we examined this well defined caudal region and a specific level of the nucleus at its rostral pole in brains from 10 additional control and six additional major depressive (Table 1, subjects U, B-1, D-1, E-1, H-1, I-1) subjects. These additional subjects provided a comparison of these two levels in an additional six age-matched control–major depressive pairs of subjects, for a total of 15 pairs of subjects (including these regions from the nine pairs studied above). For these additional age-matched major depressive–control pairs, different age-matched control subjects were used for rostral LC comparisons than caudal LC comparisons because of limited availability of LC tissue from each additional subject. Nine of the 10 additional control subjects used in this “two-level” study died of natural or accidental causes but were not assessed retrospectively for psychiatric illness (see Materials and Methods). One of these control subjects had no axis I psychiatric diagnosis assessed retrospectively. The binding of [3H]nisoxetine to NETs at the midcaudal level (∼8 mm caudal to the frenulum) of the LC from major depressives was 32% lower than that at the same level from control subjects (p < 0.01) (Fig.5). In contrast, there was no difference in [3H]nisoxetine binding at the rostral pole of the nucleus (∼2.5 mm caudal to the frenulum; Fig. 5). The number of neuromelanin-containing cells per level was not significantly different between the two study groups at either the caudal or rostral level (data not shown).
Fig. 5.
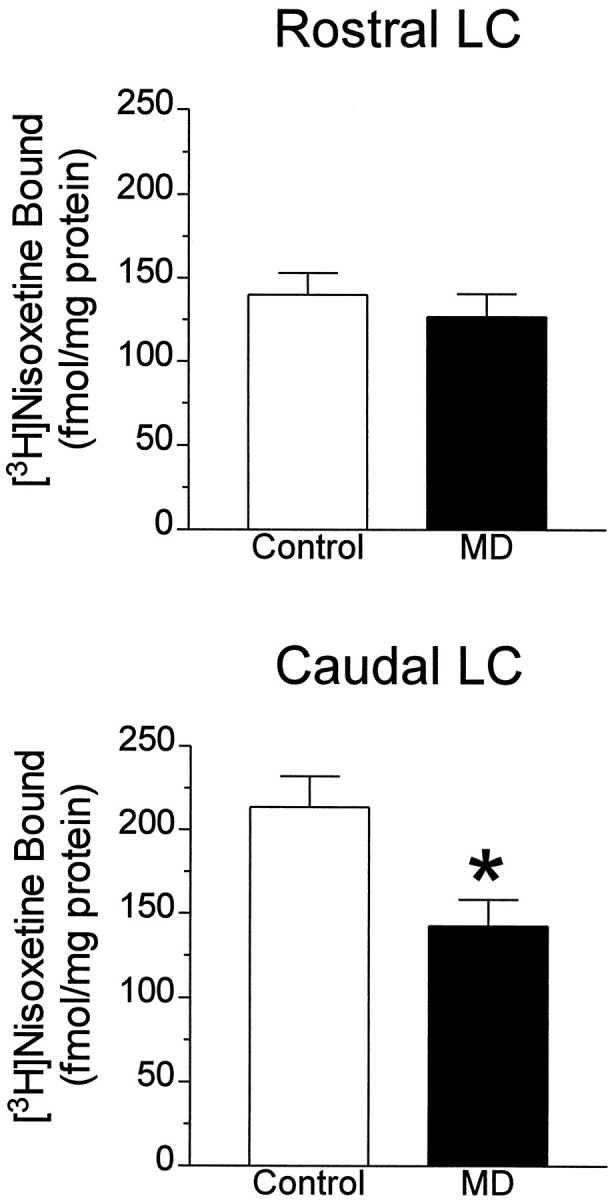
Specific binding of [3H]nisoxetine to NETs at a transverse level of LC cut ∼2.5 mm caudal to the frenulum (Rostral LC) and 8 mm caudal to frenulum (Caudal LC) in 15 age-matched pairs of major depressive (MD) and control subjects (Control). *Significant difference from the control group at p < 0.01.
The median raphe nuclei and the caudal portion of the dorsal raphe nuclei were distinguishable at the level of the rostral pole of the LC. There were moderate to high amounts of binding of [3H]nisoxetine to NETs in both of these subregions of the raphe in normal controls, as has been demonstrated previously (Ordway et al., 1997), as well as in major depressives. The possibility that the binding of [3H]nisoxetine in human raphe nuclei represents binding to serotonin transporters was ruled out previously (Zhu and Ordway, 1997). [3H]Nisoxetine binding in the caudal portion of dorsal raphe and in the median raphe nuclei, measured at ∼0.4, 1.4, and 2.5 mm from the frenulum, showed a trend toward lower amounts in major depressives compared with psychiatrically normal controls, although this difference did not reach statistical significance (Fig. 6). These data indicate that further study of NETs in raphe nuclei from a larger sample size of major depressives is warranted.
Fig. 6.
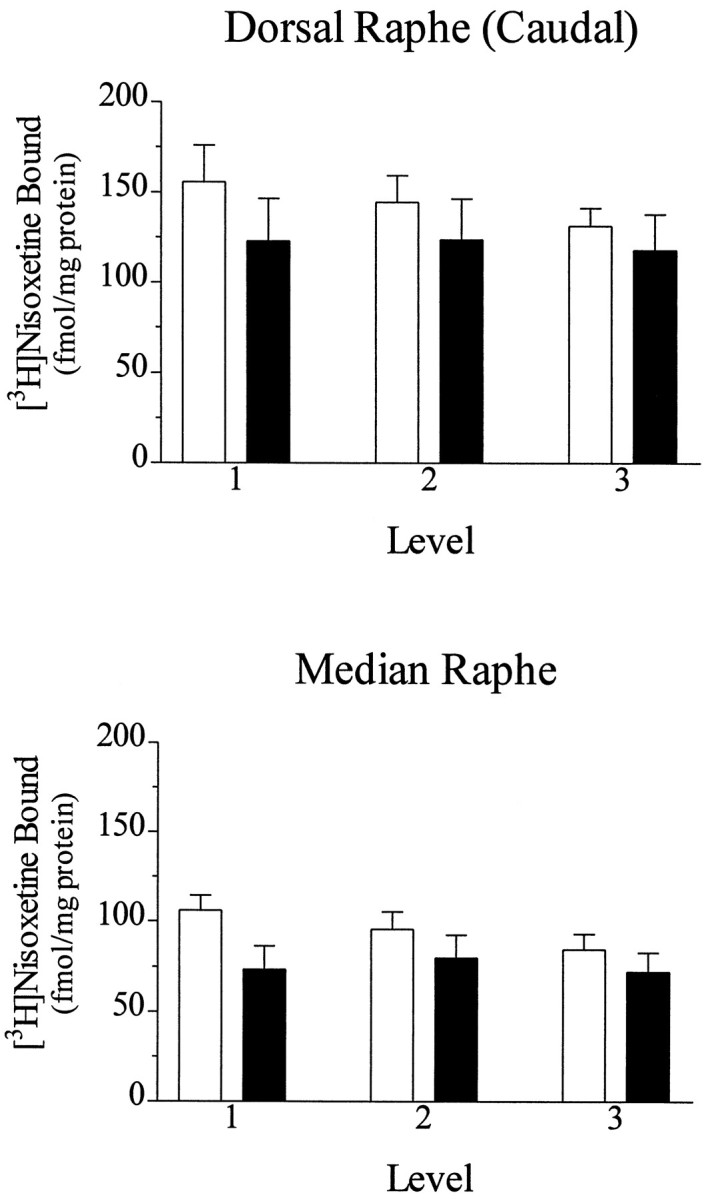
Specific binding of [3H]nisoxetine to NETs in the caudal portion of dorsal raphe and in the median raphe estimated in sections from nine psychiatrically normal control subjects (open bars) and nine subjects diagnosed with major depression (filled bars). Binding was analyzed in sections cut at the rostral portion of LC, ∼0.4 mm (1), 1.4 mm (2), and 2.5 mm (3) caudal to the frenulum. There were no statistically significant differences.
DISCUSSION
The present study measured binding to NETs in human postmortem tissue from psychiatrically characterized subjects using the radioligand [3H]nisoxetine. Furthermore, rather than examining a single cross-section of a brain region, which typically can be complicated by uneven distributions of proteins and/or cell densities, the present study examined radioligand binding along the entire length of the region of interest. The results of this study demonstrate significantly lower amounts of the binding of [3H]nisoxetine to NETs in the LC from subjects diagnosed with major depression compared with age-matched control subjects having no axis I diagnosis at the time of death. The lower [3H]nisoxetine binding in major depressives was restricted to the midcaudal region of the LC. These data imply that the pathophysiology of major depression is characterized, at least in part, by a reduced expression of the NET on noradrenergic neurons of the LC. Because all but two of the major depressives in this study died as a result of suicide, the possibility that differences in [3H]nisoxetine binding to NETs is a result of behaviors other than major depression that contribute to the act of suicide cannot be ruled out at this time.
There is considerable evidence that [3H]nisoxetine binding in the LC as determined in this study represents binding to NETs on noradrenergic neurons. [3H]Nisoxetine has a high affinity for NETs (KD = 0.7 nm) and a low affinity for serotonin transporters (KD > 1 mm) and dopamine transporters (KD > 1 mm) (Tejani-Butt et al., 1990; Tejani-Butt, 1992; Ordway et al., 1997). The regional distribution of [3H]nisoxetine binding sites in rat brain is in close agreement with the distribution of noradrenergic terminals (Tejani-Butt, 1992). In fact, the pharmacological identity of [3H]nisoxetine binding in the human brainstem (LC and raphe nuclei) is characteristic of binding to NETs (Ordway et al., 1997). mRNA encoding the NET is localized solely in noradrenergic cell bodies (Lorang et al., 1994), suggesting that no other neuron or cell type in the brain expresses the NET. A strong correlation between the amount of [3H]nisoxetine binding and the number of neuromelanin-containing cells in the LC found in the present study in both normal control and major depressive subjects is consistent with the contention that [3H]nisoxetine binds to NETs located on soma and/or dendrites of LC neurons. Nevertheless, it should be noted that there are noradrenergic projections to the LC coming from more caudal noradrenergic cell groups (lateral tegmental nuclei) (Foote et al., 1983; Herbert and Saper, 1992; Van Bockstaele and Aston-Jones, 1992). Thus, it cannot be ruled out that at least some NETs measured in the region of the LC may also reside on terminal projections arising from these caudal noradrenergic cells.
The lower [3H]nisoxetine binding to NETs observed only in the midcaudal region of the LC of major depressives could be interpreted as lower numbers of NETs on a regionally specific set of noradrenergic neurons within the LC. However, a number of other possibilities that could explain the data should be considered. First, the binding of [3H]nisoxetine to NETs was measured at a single radioligand concentration. A change in the affinity (KD) or density (Bmax) of the binding of [3H]nisoxetine to NETs could underlie a change in binding at a single ligand concentration. However, changes in the affinity of antagonist radioligands for the NET have not been reported for a variety of treatments that change radioligand binding to NETs in rat brain (Lee et al., 1983; Bauer and Tejani-Butt, 1992) or in cell culture (Zhu and Ordway, 1997). The possibility that a reduction in NET binding in major depressives is a result of residual transporter inhibitors in LC tissue is unlikely, because none of the study subjects had an antidepressant drug (except for the possibility of the serotonin transporter-selective antidepressant sertraline; see Materials and Methods) or a drug of abuse (cocaine or amphetamine) in the toxicology screen. Records indicated that four major depressive subjects had antidepressant drug prescriptions within the last 6 months. The possibility that reduced binding to NETs is secondary to an antidepressant-induced downregulation of NETs in these subjects should be considered, given that undetectable levels of antidepressants could result if the subject ceased taking medication days before committing suicide. Arguing against this possibility is the fact that [3H]nisoxetine binding levels in the LC for these four subjects were comparable to those of the other major depressives. Furthermore, although antidepressant drug exposure can downregulate the NET in rats and in cultured cells (Bauer and Tejani-Butt, 1992; Zhu and Ordway, 1997), recovery of NET levels after desipramine-induced downregulation in vitro is rapid (within 2 d; Zhu and Ordway, 1997).
Another possible interpretation of the data is that reduced [3H]nisoxetine binding in the LC in major depression reflects an increase in the transport of NET protein out to noradrenergic terminals. This interpretation would require that [3H]nisoxetine binds to NETs on the plasma membrane as well as NETs in intracellular compartments that are to be transported to terminals. Whether [3H]nisoxetine binds to both cell surface transporters and transporters located in intracellular pools awaiting trafficking is not known. However, in cell culture, we have found that changes in the binding of [3H]nisoxetine (determined at a single concentration or by saturation analysis) to NETs are paralleled by nearly identical changes (with respect to magnitude) in NET protein as determined by Western blotting (M.-Y. Zhu and G. A. Ordway, unpublished findings) and in the uptake of norepinephrine in intact cells (Zhu and Ordway, 1997). Thus, experiments with cells expressing NETs in culture demonstrate that changes in the binding of [3H]nisoxetine reflect changes in the density of cell surface transporters. However, the possibility that psychiatric disease-related factors could alter [3H]nisoxetine binding to NETs in a manner unrelated to changes in the density of NETs (e.g., affinity change) cannot be ruled out presently.
Another possible explanation of lower binding in major depressives is that there are fewer noradrenergic cells in these subjects. In fact, Arango and coworkers (1996) have reported lower numbers of noradrenergic cells in the LC of a small population of psychiatrically uncharacterized suicide victims (n = 6) relative to control subjects. The lower amount of [3H]nisoxetine binding to NETs in the midcaudal LC from subjects diagnosed with major depression in this study was not associated with any evidence of a lower number of neuromelanin-containing (noradrenergic) cells in the same region. Given that most of the subjects diagnosed with major depression in the present study died of suicide (13 of 15 subjects), it would appear that these data conflict with the findings of Arango and coworkers (1996). However, it should be noted that we made no attempt to compute total cell number as did these authors (Arango et al., 1996), and that the two studies used different subject groups; i.e., the suicide victims studied by Arango and coworkers (1996) were not psychiatrically characterized and therefore could consist of any of a number of psychiatric disorders, including major depression (Rich et al., 1986). Given the dramatic effect of age on the number of LC noradrenergic neurons (Mann and Yates, 1979; Vijayashankar and Brody, 1979; Wree et al., 1980) and on NET binding (Tejani-Butt and Ordway, 1992), major depressives and control subjects were carefully matched by age in the present study. Thus, data here indicate a lower NET number in major depression in the absence of changes in noradrenergic cell number.
The regional specificity of the reduction in [3H]nisoxetine binding to NETs in major depression raises the question of the topographical organization of the LC with respect to projection fields and the possibility that distinct noradrenergic targets in the brain may be affected in major depression. Although the projections of individual neurons can be diffuse (for review, see Foote et al., 1983), there is a loose compartmental organization of the LC neurons with respect to projection fields. For example, the rostral pole of the rat LC projects to the hypothalamus. The middle regions project to the hippocampus, cortex, cerebellum, and spinal cord, depending on the dorsal-ventral location of the neurons, whereas the caudal pole projects to the hippocampus (Loughlin et al., 1986a,b). How these anatomical distinctions made in the rat LC compare with the human LC is difficult to determine, because the overall anatomical shape and orientation within the brainstem of the human LC differs considerably from the rodent. Nevertheless, there is evidence that the LC of the nonhuman primate also displays topographically specific projections (Bowden et al., 1978; Foote et al., 1983). The particular regions of the brain that are targeted by neurons residing in the midcaudal portion of the human LC remain to be determined. Such studies have the capacity to reveal important clues relative to the neurochemical basis of major depression.
The observed reduction of NETs in major depressives may reflect an adaptation to changes in the availability of synaptic norepinephrine, given evidence that the level of brain NET expression is dependent on the concentration of synaptic norepinephrine. The NET removes norepinephrine from the synapse and thereby terminates the action of the neurotransmitter. The capacity or efficiency of this process is dependent on the number of available uptake sites (Iversen, 1975; Zhu and Ordway, 1997). Lee and coworkers (1983) demonstrated that NET density in the cerebral cortex is markedly reduced in rats treated for 5 d with reserpine, a treatment that depletes norepinephrine. Moreover, reserpine administered to rats 24 hr premortem decreases steady-state levels of NET mRNA in the LC (Cubells et al., 1995). Conversely, 18 d of treatment with a monoamine oxidase inhibitor, which would be expected to elevate the synaptic availability of norepinephrine (Benedetti and Dostert, 1989), elevates the density of NET in rat cerebral cortex (Lee et al., 1983). Lee and coworkers (1983) suggest that regulation of NET expression reflects a homeostatic attempt of noradrenergic neurons to normalize norepinephrine transmission after perturbation by these drugs. In the present study, lower transporter expression in the LC in major depression may be a response to lower levels of synaptic norepinephrine. Although norepinephrine levels have not been measured in the LC of major depressive subjects, Weiss and coworkers (1981) have observed robustly low levels of norepinephrine in the LC of rats exposed to an uncontrollable stressor, i.e., in an animal model of depression. Thus, lowered NET expression in major depression could be described as an “auto-antidepressant effect” of noradrenergic neurons attempting to restore noradrenergic neurotransmission.
The possibility that low NET density in the LC reflects reduced synaptic norepinephrine in major depression is an attractive hypothesis consistent with postulates concerning the biology of depression put forth >30 years ago (Prange, 1964; Bunney and Davis, 1965;Schildkraut, 1965). Furthermore, these data are mechanistically consistent with previous findings demonstrating upregulation of tyrosine hydroxylase in the LC from victims of suicide relative to control subjects (Ordway et al., 1994a) and, more recently, in the LC of major depressives relative to psychiatrically normal control subjects (Zhu et al., 1995). Opposite to the NET (Cubells et al., 1995), LC tyrosine hydroxylase is upregulated in response to depletion of norepinephrine in rats (Melia et al., 1992; Cubells et al., 1995). Hence, low NET expression and upregulation of tyrosine hydroxylase are two characteristics of humans with major depression, and both are observed after depletion of norepinephrine in rats.
The present study strengthens growing evidence that disruption of the neurochemistry of the noradrenergic LC is at least one aspect of the pathophysiology of major depression. Further elucidation of the biochemical mechanisms underlying this disorder has the potential to uncover novel and possibly more effective therapeutic interventions.
Footnotes
This research was supported by National Institutes of Health Grants MH46692 and MH45488 and the American Foundation for Suicide Prevention. We gratefully acknowledge the assistance of Dr. Ian A. Paul for advice concerning the statistical analyses of data. The support of Dr. Elizabeth Balraj and the Staff of the Cuyahoga County Coroner’s Office (Cleveland, OH) is appreciated. We also acknowledge Dr. Grazyna Rajkowska for many helpful discussions concerning cell-counting techniques.
Correspondence should be addressed to Dr. Gregory A. Ordway, Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216-4505.
REFERENCES
- 1.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 2.Arango V, Underwood MD, Mann JJ. Fewer pigmented neurons in the locus coeruleus of uncomplicated alcoholics. Brain Res. 1994;650:1–8. doi: 10.1016/0006-8993(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 3.Arango V, Underwood MD, Mann JJ. Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biol Psychiatry. 1996;39:112–120. doi: 10.1016/0006-3223(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 4.Baker KG, Tork I, Hornung JP, Halasz P. The human locus coeruleus complex: an immunohistochemical and three dimensional reconstruction study. Exp Brain Res. 1989;77:257–270. doi: 10.1007/BF00274983. [DOI] [PubMed] [Google Scholar]
- 5.Barker EL, Blakely RD. Norepinephrine and serotonin transporters. Molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. A fourth generation of progress. Raven; New York: 1995. pp. 321–333. [Google Scholar]
- 6.Bauer ME, Tejani-Butt SM. Effects of repeated administration of desipramine or electroconvulsive shock on norepinephrine uptake sites measured by [3H]nisoxetine autoradiography. Brain Res. 1992;582:208–214. doi: 10.1016/0006-8993(92)90134-u. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti MS, Dostert P. Monoamine oxidase, brain ageing and degenerative diseases. Biochem Pharmacol. 1989;38:555–561. doi: 10.1016/0006-2952(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 8.Berger PA. CSF monoamine metabolites in depression and schizophrenia. Am J Psychiatry. 1980;137:174–180. doi: 10.1176/ajp.137.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Bowden DM, German DC, Poynter WD. An autoradiographic, semistereotaxic mapping of major projections from locus coeruleus and adjacent nuclei in Macaca mulatta. Brain Res. 1978;145:257–276. doi: 10.1016/0006-8993(78)90861-2. [DOI] [PubMed] [Google Scholar]
- 10.Bunney WE, Jr, Davis JM. Norepinephrine in depressive reactions: a review. Arch Gen Psychiatry. 1965;13:483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 11.Caldecott-Hazard S, Morgan DG, DeLeon-Jones F, Overstreet DH, Janowsky D. Clinical and biochemical aspects of depressive disorders: II. Transmitter/receptor theories. Synapse. 1991;9:251–301. doi: 10.1002/syn.890090404. [DOI] [PubMed] [Google Scholar]
- 12.Chan-Palay V. Depression and dementia in Parkinson’s disease. Catecholamine changes in the locus ceruleus, a basis for therapy. Adv Neurol. 1993;60:438–446. [PubMed] [Google Scholar]
- 13.Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol. 1989a;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- 14.Chan-Palay V, Asan E. Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J Comp Neurol. 1989b;287:357–372. doi: 10.1002/cne.902870307. [DOI] [PubMed] [Google Scholar]
- 15.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Cubells JF, Kim KS, Baker H, Volpe BT, Chung Y, Houpt TA, Wessel TC, Joh TH. Differential in vivo regulation of mRNA encoding the norepinephrine transporter and tyrosine hydroxylase in rat adrenal medulla and locus ceruleus. J Neurochem. 1995;65:502–509. doi: 10.1046/j.1471-4159.1995.65020502.x. [DOI] [PubMed] [Google Scholar]
- 17.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 18.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 19.German DC, Walker BS, Manaye K, Smith WK, Woodward DJ, North AJ. The human locus coeruleus: computer reconstruction of cellular distribution. J Neurosci. 1988;8:1776–1788. doi: 10.1523/JNEUROSCI.08-05-01776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German DC, Manaye KF, White CLI, Woodward DJ, McIntire DD, Smith WK, Kalaira RN, Mann DMA. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- 21.Graefe K-H, Bonisch H. The transport of amines across the axonal membranes of noradrenergic and dopaminergic neurons. In: Trendelenburg U, Weiner N, editors. Catecholamines I. Springer; Berlin: 1988. pp. 193–245. [Google Scholar]
- 22.Halaris AE, DeMet EM. Studies of norepinephrine metabolism in manic and depressive states. In: Usdin E, Kopin IJ, Barchas J, editors. Catecholamines: basic and clincal frontiers. Raven; New York: 1979. pp. 1866–1868. [Google Scholar]
- 23.Herbert H, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J Comp Neurol. 1992;315:34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- 24.Iversen LL. Uptake processes for biogenic amines. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of psychopharmacology. Plenum; New York: 1975. pp. 381–442. [Google Scholar]
- 25.Lee C-M, Javitch JA, Snyder SH. Recognition sites for norepinephrine uptake: regulation by neurotransmitter. Science. 1983;220:626–629. doi: 10.1126/science.6301013. [DOI] [PubMed] [Google Scholar]
- 26.Lerner P, Goodwin FK, vanKammen DP, Post RM, Major LF, Ballenger JC, Lovenberg W. Dopamine-beta-hydroxylase in the cerebrospinal fluid of psychiatric patients. Biol Psychiatry. 1978;13:685–694. [PubMed] [Google Scholar]
- 27.Lorang D, Amara SG, Simerly RB. Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci. 1994;14:4903–4914. doi: 10.1523/JNEUROSCI.14-08-04903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986a;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 29.Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 1986b;18:307–319. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- 30.Mann DMA, Yates PO. The effects of ageing on the pigmented cells of the human locus coeruleus and substantia nigra. Acta Neuropathol (Berl) 1979;47:93–98. doi: 10.1007/BF00717030. [DOI] [PubMed] [Google Scholar]
- 31.Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 32.Ordway GA, Smith KS, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994a;62:680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- 33.Ordway GA, Widdowson PS, Smith K, Halaris AE. Agonist binding to α2 adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994b;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- 34.Ordway GA, Stockmeier CA, Cason GW, Klimek V. Pharmacology and distribution of norepinephrine transporters in the human locus coeruleus and raphe nuclei. J Neurosci. 1997;17:1710–1719. doi: 10.1523/JNEUROSCI.17-05-01710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papp M, Klimek V, Willner P. Effect of imipramine on serotonergic and beta-adrenergic receptor binding in a realistic animal model of depression. Psychopharmacology. 1994;114:309–314. doi: 10.1007/BF02244853. [DOI] [PubMed] [Google Scholar]
- 36.Post RM., Jr Central norepinephrine metabolism in affective illness: MHPG in CSF. Science. 1973;179:1002–1003. doi: 10.1126/science.179.4077.1002. [DOI] [PubMed] [Google Scholar]
- 37.Prange AJ., Jr The pharmacology and biochemistry of depression. Dis Nerv Syst. 1964;25:217–221. [PubMed] [Google Scholar]
- 38.Rich CL, Young D, Fowler RC. San Diego suicide study. I. Young vs old subjects. Arch Gen Psychiatry. 1986;43:577–582. doi: 10.1001/archpsyc.1986.01800060071009. [DOI] [PubMed] [Google Scholar]
- 39.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 40.Tejani-Butt SM. [3H]Nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- 41.Tejani-Butt SM, Ordway GA. Effect of age on [3H]nisoxetine binding to uptake sites for norepinephrine in the locus coeruleus of humans. Brain Res. 1992;583:312–315. doi: 10.1016/s0006-8993(10)80041-1. [DOI] [PubMed] [Google Scholar]
- 42.Tejani-Butt SM, Brunswick DJ, Frazer A. [3H]Nisoxetine: a new radioligand for norepinephrine uptake sites in brain. Eur J Pharmacol. 1990;191:239–243. doi: 10.1016/0014-2999(90)94155-q. [DOI] [PubMed] [Google Scholar]
- 43.Van Bockstaele EJ, Aston-Jones G. Collateralized projections from neurons in the rostral medulla to the nucleus locus coeruleus, the nucleus of the solitary tract and the periaqueductal gray. Neuroscience. 1992;49:653–668. doi: 10.1016/0306-4522(92)90234-s. [DOI] [PubMed] [Google Scholar]
- 44.Vijayashankar N, Brody H. A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol. 1979;38:490–497. doi: 10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Waterhouse BD, Lin C-S, Burne RA, Woodward DJ. The distribution of neurocortical projection neurons in the locus coeruleus. J Comp Neurol. 1983;217:418–431. doi: 10.1002/cne.902170406. [DOI] [PubMed] [Google Scholar]
- 46.Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res Rev. 1981;3:167–205. [Google Scholar]
- 47.Wree A, Braak H, Schleicher A, Zilles K. Biomathematical analysis of the neuronal loss in the aging human brain of both sexes, demonstrated in pigment preparations of the pars cerebellaris loci coerulie. Anat Embryol. 1980;160:105–119. doi: 10.1007/BF00315653. [DOI] [PubMed] [Google Scholar]
- 48.Zhu M-Y, Ordway GA. Down-regulation of norepinephrine transporters on PC12 cells by transporter inhibitors. J Neurochem. 1997;68:134–141. doi: 10.1046/j.1471-4159.1997.68010134.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhu M-Y, Haycock JW, Klimek V, Luker SN, Stockmeier CA, Dilley G, Meltzer HY, Overholser JC, Ordway GA. Elevation of tyrosine hydroxylase in the locus coeruleus of subjects with major depression. Soc Neurosci Abstr. 1995;21:194. [Google Scholar]