Abstract
The occurrence of collagenous colitis (CC) in patients with pre-existing inflammatory bowel diseases (IBD) is rare, with only seven cases reported in the past. Herein, we report two IBD cases who developed CC after successful treatment of their IBD with two different tumor necrosis factor (TNF)-α inhibitors, which have been previously reported to successfully treat refractory CC. This report highlights the need to do random biopsies of the colon for CC diagnosis in IBD patients with symptoms of diarrhea after complete mucosal healing. The report also reviews plausible mechanisms as to how CC may develop, including the role of multiple medications.
Keywords: Collagenous colitis, inflammatory bowel disease, tumor necrosis factor-α, inhibitors, infliximab, adalimumab
Introduction
Collagenous colitis (CC) and lymphocytic colitis (LC) represent the two main types of microscopic colitis (MC). Patients with CC mostly present with chronic intermittent watery diarrhea [1]. CC mainly affects middle-aged women at an age of approximately 55–63.8 years, with a female-to-male ratio of 3–6.8:1 [1,2]. Macroscopically, during colonoscopy, a patient with CC has normal-appearing colonic mucosa, although some abnormal changes have been reported in the literature, including edema, erythema, abnormal vasculature pattern and diffuse mucosal cloudiness [1,3]. CC is typically diagnosed after microscopic examination, showing a thickened sub-epithelial collagen band ≥10 μm in colorectal biopsies [4]. Surface epithelial damage with inflammatory cell infiltration in both surface epithelium and lamina propria is also present [5].
The cause of CC and its association with other diseases are still unclear. Chutkan et al. in 2000 reported a family of mother, daughter and son who have CC, ulcerative colitis (UC) and Crohn’s disease (CD), respectively, suggesting that CC and inflammatory bowel diseases (IBD) could be related or have a common genetic predisposition [6], but some studies have failed to find any significant association between CC and IBD [2], and yet others have reported the co-occurrence of CC and IBD. In most of the latter studies reporting co-occurrence of the two disease entities, CC was followed by the eventual development of clinical and pathological IBD [7–13]. There are also two reported cases of simultaneous occurrence of CC and IBD [14,15].
Here, we report two cases of IBD who have developed CC years after the onset of IBD in two patients who were in clinical remission. Interestingly, both cases had been successfully treated with tumor necrosis factor (TNF)-α inhibitors. In this report, we also review the seven published cases of CC that developed after pre-existing IBD [16–21]. Our report highlights two important issues: CC development occurs most often in patients who are clinically and endoscopically in remission necessitating random mucosal biopsies during endoscopy before symptoms can be attributed to irritable bowel syndrome (IBS); and CC can develop while the patient is in remission on TNF-α inhibitors, which have been used to treat refractory CC.
Case 1
A 49-year-old white female was diagnosed in 2004 with UC that involved her entire colon (i.e. pan-colitis) at the age of 39. Her symptoms of UC were intermittent bloody diarrhea, fecal urgency, fatigue and abdominal cramping with bowel movements. She was tried on mesalamine which did not control her symptoms; azathioprine and 6-mercaptopurine which gave her abdominal pain and resulted in pancreatitis; and infliximab to which she had a severe infusion reaction. She was then started on adalimumab in March 2008 (before the FDA approval of adalimumab for UC in the US) to which she clinically responded well. She had undergone multiple colonoscopies with surveillance biopsies in 2006, 2009 and 2010. None of these showed any evidence of CC. In 2011, colonoscopy showed minimal to no active disease, presence of multiple pseudo-polyps and no evidence of CC. Her past medical history included diabetes, Hashimoto’s thyroiditis, anxiety, hyperlipidemia, gastroesophageal reflux disease and multiple drug allergies. Her medications at the time of CC diagnosis were adalimumab, rabeprazole, levothyroxine, pravastatin, glimepiride, metformin, ramipril, cetirizine, aspirin (81 mg daily) and multivitamins. She denied any history of smoking and she rarely drank any alcohol.
In 2013, the patient presented to the clinic with complaints of chronic intermittent diarrhea and abdominal pain for the past 3–4 months and she reported that this was very different from what she usually experiences with UC flares. Specifically, she denied any weight loss and blood in the stool. Her physical examination was unremarkable. Her laboratory studies revealed a normal complete blood count (CBC), CMP, erythrocyte sedimentation rate (ESR) and C-reaction protein (CRP). Her transglutaminase Ab (IgA), gliadin Ab (IgG and IgA) and endomysial Ab (IgA) were negative for celiac disease. Her anti-nuclear antibodies (ANA) were also normal. Stool for culture, ova and parasites, and C. difficile toxin were negative. A repeat colonoscopy showed a few 1–4 mm ulcerations around the ileocecal valve and in the sigmoid, and large pseudo-polyps in the left colon, as before. The rest of the colonic mucosa appeared normal, but was scarred and stiff. Histopathology of the surveillance colonic biopsies revealed a new-onset CC, with a thickened sub-epithelial collagen band of > 12 μm to > 15 μm (Figure 1). She was started on cholestyramine and budesonide with resolution of her symptoms of CC. The patient was then maintained on cholestyramine long term to control her CC. A repeat colonoscopy was performed in 2015 for colon cancer surveillance and there was resolution of the histological changes of CC but mildly active patchy histological UC with mild erythema and granularity of the mucosa.
Figure 1.
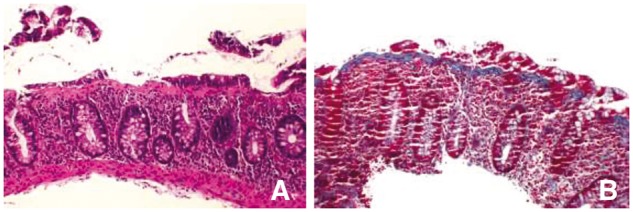
(A) Low-power microphotograph showing colonic mucosa with diffuse chronic colitis and thickened subsurface collagen band (H&E stain, ×100). (B) Trichrome stain showing thickened collagen band (>15 μm).
Case 2
A 61-year-old white Hispanic female with symptoms of CD for 10 years was diagnosed with CD (involving her esophagus, stomach, ileum and colon). She was started on infliximab with great improvement in her disease. She additionally had a past medical history of diabetes, hypothyroidism, diverticulosis and gastroesophageal reflux disease. In addition to infliximab, her medications included levothyroxine, sitagliptin phosphate, metformin, pravastatin, lansoprazole and vitamin D. She actively smoked cigarettes and rarely drank any alcohol. While she was on infliximab, the patient developed dysphagia and an esophagogastroduodenoscopy (EGD) was performed revealing candida esophagitis, which was successfully treated with fluconazole. A year after the start of infliximab in 2014, she presented with an unintentional weight loss of more than 2 kg, fatigue, abdominal pain and chronic intermittent diarrhea with some rectal bleeding. The patient’s laboratory studies revealed a normal CBC, CMP, ESR and CRP. Stool for culture, ova and parasites, and C. difficile toxin PCR were negative for any evidence of infection. She had an EGD with small bowel biopsies that was also negative for celiac disease or other pathology. A surveillance colonoscopy was performed and showed few scattered aphthous ulcerations in the distal terminal ileum and complete mucosal healing of her colon. Histopathology of the colonic biopsies showed inactive CD and a diffuse mild chronic colitis with a thickened collagen band (>25 μm) throughout the colon that is consistent with CC (Figure 2). She was started on cholestyramine and her symptoms of CC completely resolved. She was then maintained on cholestyramine long term to control her CC. Also, in the meantime, the dose of infliximab was reduced in the patient due to recurrent candida esophagitis. A repeat colonoscopy was performed in 2016 for colon cancer surveillance and there was resolution of the histological changes of CC and the CD was inactive in the terminal ileum and colon with complete mucosal healing and inactive colitis on random biopsies.
Figure 2.
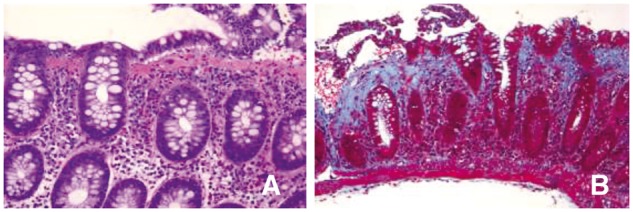
(A) Medium-power microphotograph showing diffuse chronic colitis, thickened collagen band and surface denudation (H&E stain, ×200). (B) Trichrome stain showing thickened collagen band (>25 μm).
Discussion
CC has been linked with IBD, but development of CC after the IBD diagnosis is rare. CC has not been reported to occur in IBD patients in remission or those on TNF-α inhibitors. In fact, TNF-α inhibitors have been reported to successfully treat six out of seven cases of CC that were refractory to treatment [22–24]. Herein, we report two patients who developed CC following successful treatment of their IBD with TNF-α inhibitors, namely infliximab and adalimumab. This finding highlights the importance of taking random biopsies of the colon even if the mucosa appears normal endoscopically in IBD patients. We also highlight that CC in patients with existing IBD can be temporary.
There are seven IBD cases that have been previously reported to develop CC subsequently to their IBD diagnosis and these are shown in Table 1 along with the features and characteristics related to each case, and the two cases from this report are also included in the table. It is important to note that seven out of the nine cases of CC emergence after IBD development occurred in the cases which were in remission. Of the two remaining active cases, one had active perianal disease and the left colon looked otherwise normal [20]. The other had active terminal ileitis and systemic amyloidosis, and the colon also looked normal [17]. These features highlight an important need to keep CC in the differential diagnosis of persistent diarrhea in IBD patients, and to take random mucosal biopsies of the normal-looking colon before symptoms are attributed to IBS or other causes, even after successful treatment. Other causes of diarrhea in IBD have been getting more notice in the context of clinical trials. Until recently, randomized clinical trials of IBD (and especially those in CD) enrolled subjects with persistent diarrhea and symptoms such as abdominal pain and general wellbeing used in composite symptom indices; yet, a significant portion of the patients in such clinical trials had no appreciable endoscopic evidence of inflammation, necessitating a recent recommendation to add objective measures such as CRP/colonoscopy/magnetic resonance elastography to the inclusion criteria of active IBD subjects [25]. This fact points out the importance of looking for additional causes of diarrhea in IBD patients other than the IBD itself, and implies how often the problem of diarrhea in IBD is misinterpreted as active disease. This also highlights a need for literature such as our case report documenting such additional causes of diarrhea in IBD patients and the need for random biopsies of the colon regardless of endoscopic appearances.
Table 1.
Cases in the literature reported to develop CC after an established diagnosis of IBD
| Age at CC diagnosis (years)/gender | IBD type | Part of GI tract involved | Duration of IBD before CC diagnosis (years) | IBD medication at CC diagnosis | Duration of TNFα inhibitor before onset of CC (months) | Active vs remission at CC diagnosis | Other medications | Associated diseases | Medications used to treat CC | Outcome of CC | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 49/Female | UC | Entire colon | 10 | Adalimumab | 66 | Remission | Rabeprazole, levothyroxine, glimepiride, metformin, ramipril, cetirizine, aspirin | Diabetes, Hashimoto’s thyroiditis, anxiety, GERD and multiple drug allergies | Cholestyramine and budesonide | Complete resolution | This report |
| Case 2 | 60/Female | CD | Esophagus, stomach, ileum and colon | 2 | Infliximab | 12 | Remission | Levothyroxine, sitagliptin phosphate/ metformin hydrochloride, pravastatin, lanzoprazole | Diabetes, hypothyroidism, diverticulosis and GERD | Cholestyramine | Complete resolution | This report |
| Case 3 | 35/Male | CD | Terminal ileum | NR | No medications | NA | Active | Indomethacin, ACEI, ARB, IV antibiotics, steroids | Nephrotic syndrome, Amyloidosis | NR | NR | [17] |
| Case 4 | 79/Female | UC | NR | 14 | Mesalamine | NA | Remission | NR | NR | Mesalamine | Complete resolution | [16] |
| Case 5 | 59/Female | UC | Left side of the colon | 13 | Mesalamine | NA | Remission | Ibuprofen (no association with CC symptoms) | NR | Bismuth | Complete resolution | [16] |
| Case 6 | 59/Female | CD | Perianal | NR | Infliximab | NR | Active | Mood stabilizers (in the past) | Depression, h/o acute steroid psychosis | Bismuth subsalicylate and imodium | Complete resolution | [20] |
| Case 7 | 63/Male | UC | Left side of the colon | 8 | Mesalamine | NA | Remission | NR | NR | NR | NR | [18] |
| Case 8 | 57/Female | UC | Entire colon | 5 | Mesalamine and prednisolone | NA | Remission | ACEI, Adriamycin, Cyclophosphamide, Etoposide, Melphalan | Systemic Amyloidosis, autologous stem-cell transplantation | NR | NR | [19] |
| Case 9 | 70/Female | CD | Entire colon | 5 | No medications | NA | Remission | NR | IDDM, Grave’s disease, atrophic gastritis | NR | NR | [21] |
NR, not reported; NA, not applicable; GI, gastrointestinal; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; CC, collagenous colitis; TNFαIs, tumor necrosis factor-α inhibitors; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GERD, gastroesophageal reflux disease; IDDM, insulin-dependent diabetes mellitus.
Why and how CC develops in existing IBD patients is not known. Two patients shown in Table 1 had systemic amyloidosis, suggesting an aberrant immune response as an etiology. Interestingly, however, before the addition of the two cases in this report, six out of the seven previously reported patients were on minimal treatment with mesalamines or were on no medications. There is only one case of CC occurring in a patient with CD who had been successfully treated (also with a TNF-α inhibitor) [20]. This patient had colonic disease in remission, but had active perianal disease with a fistula, when CC developed. The noted collagen deposition in this case was most prominent distally and in the perianal area, which clinically and endoscopicaly looked abnomal, and this led to biopsies of the area and the CC diagnosis. Contrary to this previous case with active perianal disease, both of our cases were in complete clinical remission and had endoscopic colonic healing at the time of development of CC. The temporary occurrence of CC in our IBD patients could suggest some kind of aberrant or exaggerated healing response as a result of an imbalance between inflammatory pathways vs mucosal healing pathways, tipping the balance toward protracted healing. This conjecture fits with the clinical course of disease in our two cases. In Case 1 reported here, interestingly enough, we note disappearance of the CC after reappearance of mild UC. In Case 2, a dose reduction in infliximab treatment corresponds with the disappearance of CC.
Among the proposed theories that initiate the processes leading up to the altered collagen balance seen in CC are immune dysregulation/autoimmunity [2,26]; atypical responses to luminal toxins such as those from bacteria in susceptible patients [27–31] (which also is thought to account for improvement of CC with cholestyramine [32]); and drug idiosyncrasy. The two cases that we report here have preceding autoimmune thyroid disease and diabetes mellitus in addition to IBD, which could have contributed to CC development. In our two cases, despite a response to cholestyramine, we noted no evidence of infectious agents in stool studies. Among the previous medications that have been reported in association with CC and were used in both of our patients are non-steroidal anti-inflammatory drugs (NSAIDs) [33,34], proton pump inhibitors (PPIs) [3,35–37] and statins [34,38,39]. Even though we noted no temporal relationship between the use of these medications and CC onset in our patients, a careful medication history in IBD patients is needed, and it is certainly plausible that one or more of these agents could have led to the development of CC in our two IBD patients.
In conclusion, our cases highlight that CC development in IBD patients could occur despite successful treatment either due to an exaggerated healing response or due to the effects of multiple medications associated with CC; and highlight the need for random biopsies of the colon in IBD patients with new-onset diarrhea after successful treatment, especially in those cases with complete mucosal healing, before symptoms are attributed to IBS. The evidence presented herein is hypothesis-generating, proposing a need for further studies looking at the association between complete mucosal healing and subsequent CC development and its mechanisms.
Acknowledgements
R.E.S. and R.S. acquired and analysed the data and reviewed the literature and wrote the manuscript; S.J. provided the pathology images; E.A.M. conceived the report, wrote and revised the manuscript. A written waiver was provided by Rush University Institutional Review Board. Informed consent to participate is not required for case reports per Rush University Institutional Review Board, if fewer than three cases are being reported.
Conflict of interest
None declared.
References
- 1. Bohr J, Tysk C, Eriksson S. et al. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut 1996;39:846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kao KT, Pedraza BA, McClune AC. et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World J Gastroenterol 2009;15:3122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiba M, Sugawara T, Tozawa H. et al. Lansoprazole-associated collagenous colitis: diffuse mucosal cloudiness mimicking ulcerative colitis. World J Gastroenterol 2009;15:2166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee E, Schiller LR, Vendrell D. et al. Subepithelial collagen table thickness in colon specimens from patients with microscopic colitis and collagenous colitis. Gastroenterology 1992;103:1790–6. [DOI] [PubMed] [Google Scholar]
- 5. Jessurun J, Yardley JH, Giardiello FM. et al. Chronic colitis with thickening of the subepithelial collagen layer (collagenous colitis): histopathologic findings in 15 patients. Hum Pathol 1987;18:839–48. [DOI] [PubMed] [Google Scholar]
- 6. Chutkan R, Sternthal M, Janowitz HD.. A family with collagenous colitis, ulcerative colitis, and Crohn’s disease. Am J Gastroenterol 2000;95:3640–1. [DOI] [PubMed] [Google Scholar]
- 7. Goldstein NS, Gyorfi T.. Focal lymphocytic colitis and collagenous colitis: patterns of Crohn’s colitis? Am J Surg Pathol 1999;23:1075–81. [DOI] [PubMed] [Google Scholar]
- 8. Pokorny CS, Kneale KL, Henderson CJ.. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol 2001;32:435–8. [DOI] [PubMed] [Google Scholar]
- 9. Aqel B, Bishop M, Krishna M. et al. Collagenous colitis evolving into ulcerative colitis: a case report and review of the literature. Dig Dis Sci 2003;48:2323–7. [DOI] [PubMed] [Google Scholar]
- 10. O’Beirne JP, Ireland A.. Progression of collagenous colitis to Crohn’s disease. Eur J Gastroenterol Hepatol 2005;17:573–5. [DOI] [PubMed] [Google Scholar]
- 11. Smith P, Bishop P, Whorwell PJ.. Collagenous colitis, ulcerative colitis, coeliac disease and hyperparathyroidism in one patient: implications for the management of collagenous colitis. Eur J Gastroenterol Hepatol 2005;17:1239–42. [DOI] [PubMed] [Google Scholar]
- 12. Freeman HJ, Berean KW, Nimmo M.. Evolution of collagenous colitis into severe and extensive ulcerative colitis. Can J Gastroenterol 2007;21:315–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giardiello FM, Jackson FW, Lazenby AJ.. Metachronous occurrence of collagenous colitis and ulcerative colitis. Gut 1991;32:447–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chandratre S, Bramble MG, Cooke WM. et al. Simultaneous occurrence of collagenous colitis and Crohn’s disease. Digestion 1987;36:55–60. [DOI] [PubMed] [Google Scholar]
- 15. Nojgaard C, Nielsen PL, Rumessen JJ.. Synchronous onset of collagenous colitis and Crohn disease. Ugeskrift for laeger 2002;164:2299–2300. [PubMed] [Google Scholar]
- 16. Jegadeesan R, Liu X, Pagadala MR. et al. Microscopic colitis: is it a spectrum of inflammatory bowel disease? World J Gastroenterol 2013;19:4252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackmur JP, Chapman FA, Bellamy CO. et al. Anti-TNF-alpha therapy for renal amyloid as a complication of Crohn’s disease. QJM 2014;107:57–9. [DOI] [PubMed] [Google Scholar]
- 18. Haque M, Florin T.. Progression of ulcerative colitis to collagenous colitis: chance, evolution or association? Inflamm Bowel Dis 2007;13:1321. [DOI] [PubMed] [Google Scholar]
- 19. Janczewska I, Mejhert M, Hast R. et al. Primary AL-amyloidosis, ulcerative colitis and collagenous colitis in a 57-year-old woman: a case study. Scand J Gastroenterol 2004;39:1306–9. [DOI] [PubMed] [Google Scholar]
- 20. Malik TA, Peter S, Jhala N. et al. Crohn’s colitis with perianal disease complicated by collagenous colitis: discourse on management options. Digestion 2010;81:142–4. [DOI] [PubMed] [Google Scholar]
- 21. Seguenot D, Marteau P, Lavergne A. et al. [Collagen colitis after Crohn’s disease: a fortuitous association?]. Gastroenterol Clin Biol 1990;14:891–2. [PubMed] [Google Scholar]
- 22. Pola S, Fahmy M, Evans E. et al. Successful use of infliximab in the treatment of corticosteroid dependent collagenous colitis. Am J Gastroenterol 2013;108:857–8. [DOI] [PubMed] [Google Scholar]
- 23. Munch A, Ignatova S, Strom M.. Adalimumab in budesonide and methotrexate refractory collagenous colitis. Scand J Gastroenterol 2012;47:59–63. [DOI] [PubMed] [Google Scholar]
- 24. Esteve M, Mahadevan U, Sainz E. et al. Efficacy of anti-TNF therapies in refractory severe microscopic colitis. J Crohn Colitis 2011;5:612–18. [DOI] [PubMed] [Google Scholar]
- 25. D’Haens G, Feagan B, Colombel JF. et al. Challenges to the design, execution, and analysis of randomized controlled trials for inflammatory bowel disease. Gastroenterology 2012;143:1461–9. [DOI] [PubMed] [Google Scholar]
- 26. Vigren L, Tysk C, Strom M. et al. Celiac disease and other autoimmune diseases in patients with collagenous colitis. Scand J Gastroenterol 2013;48:944–50. [DOI] [PubMed] [Google Scholar]
- 27. Jarnerot G, Tysk C, Bohr J. et al. Collagenous colitis and fecal stream diversion. Gastroenterology 1995;109:449–55. [DOI] [PubMed] [Google Scholar]
- 28. Khan MA, Brunt EM, Longo WE. et al. Persistent Clostridium difficile colitis: a possible etiology for the development of collagenous colitis. Dig Dis Sci 2000;45:998–1001. [DOI] [PubMed] [Google Scholar]
- 29. Vesoulis Z, Lozanski G, Loiudice T.. Synchronous occurrence of collagenous colitis and pseudomembranous colitis. Can J Gastroenterol 2000;14:353–8. [DOI] [PubMed] [Google Scholar]
- 30. Bohr J, Nordfelth R, Jarnerot G. et al. Yersinia species in collagenous colitis: a serologic study. Scand J Gastroenterol 2002;37:711–14. [DOI] [PubMed] [Google Scholar]
- 31. Makinen M, Niemela S, Lehtola J. et al. Collagenous colitis and Yersinia enterocolitica infection. Dig Dis Sci 1998;43:1341–6. [DOI] [PubMed] [Google Scholar]
- 32. Andersen T, Andersen JR, Tvede M. et al. Collagenous colitis: are bacterial cytotoxins responsible? Am J Gastroenterol 1993;88:375–7. [PubMed] [Google Scholar]
- 33. Riddell RH, Tanaka M, Mazzoleni G.. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case-control study. Gut 1992;33:683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez-Banares F, Esteve M, Espinos JC. et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol 2007;102:324–30. [DOI] [PubMed] [Google Scholar]
- 35. Chande N, Driman DK.. Microscopic colitis associated with lansoprazole: report of two cases and a review of the literature. Scand J Gastroenterol 2007;42:530–3. [DOI] [PubMed] [Google Scholar]
- 36. Kitagawa T, Sato K, Yokouchi Y. et al. A case of lansoprazole-associated collagenous colitis with longitudinal ulcer. J Gastrointest Liver Dis 2013;22:9. [PubMed] [Google Scholar]
- 37. Thomson RD, Lestina LS, Bensen SP. et al. Lansoprazole-associated microscopic colitis: a case series. Am J Gastroenterol 2002;97:2908–13. [DOI] [PubMed] [Google Scholar]
- 38. Bonderup OK, Fenger-Gron M, Wigh T. et al. Drug exposure and risk of microscopic colitis: a nationwide Danish case-control study with 5751 cases. Inflamm Bowel Dis 2014;20:1702–7. [DOI] [PubMed] [Google Scholar]
- 39. Pascua MF, Kedia P, Weiner MG. et al. Microscopic colitis and medication use. Clin Med Insights Gastroenterol 2010;2010:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


