Abstract
In this concise practitioner protocol, the radiochemical synthesis of [18F]FNDP suitable for human PET studies is described and the results from validation productions are presented. The high specific activity radiotracer product is prepared as a sterile, apyrogenic solution that conforms to current Good Manufacturing Practice (cGMP) requirements.
Introduction
Mammalian soluble epoxide hydrolase (sEH) regulates vascular tone, nociception, angiogenesis and inflammatory response via enzymatic hydrolysis of lipid signaling molecules such as epoxyeicosatrienoic acids. 1–5 Accumulating literature outlining the importance of sEH in regulating various pathophysiological processes, including brain disorders, cardiac hypertrophy, diabetes and hypertension, has suggested sEH as a potential biomarker for development of targeted therapeutics.6–10
We recently reported the first positron emission tomography (PET) radiotracer, N-(3,3-diphenylpropyl)-6-(18F)fluoronicotinamide ([18F]FNDP) (Figure 1), for in vivo imaging of sEH in rodent and baboon brain.11 Results from that study illustrated the applicability of this tracer for non-invasive in vivo assessment of sEH levels in human brain disorders.
Figure 1.
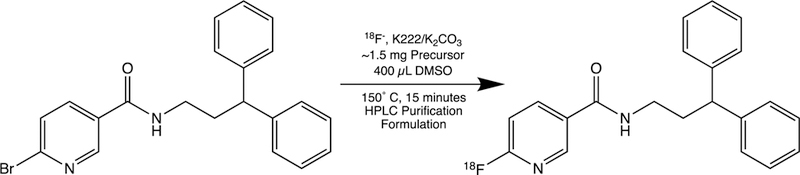
Synthesis of [18F]FNDP via nucleophilic aromatic radiofluorination.
In our previous studies, [18F]FNDP was prepared in a moderate radiochemical yield. This report describes an optimized method of [18F]FNDP radiosynthesis, with full cGMP-compliant quality control specifications and results. This procedure is better suited for future human PET studies than our previous method.11
Synthesis Procedures
Standard Reagent Statement
Reagents and solvents were obtained from Millipore Sigma or Thermo Fisher Scientific in ACS and HPLC grade, respectively, unless otherwise noted. N-(3,3-diphenylpropyl)-6-bromonicotinamide (precursor) and 6-fluoro-N-(3,3-dipropyl)nicotinamide (FDNP) (Figure 1) were synthesized and isolated as described previously.11
The semi-preparative high performance liquid chromatography system consisted of an Agilent 1260 Prep pump with a VICI injector, a Knauer 200 UV detector (254 nm), a Bioscan Hot Cell interface with a diode radioactivity detector. The semi-preparative HPLC column was a Waters XBridge Prep C18 10 µm 10 × 150 mm column eluted with a mixture of 40:60 acetonitrile (MeCN): 57 mM triethylamine adjusted to pH 7.2 with phosphoric acid buffer at flow rate of 10 mL/min. The analytical chromatography system included an Agilent 1260 Infinity System equipped with a quaternary pump, HiP ALS autosampler, and DAD UV detector with a Max-Light flow cell set to 260 nm as well as a Bioscan Flow-Count interface with a NaI radioactivity detector. The analytical HPLC column was an XBridge C18 3.5 µm 4.6 × 100 mm column eluted with a mixture of 45:55 MeCN:pH 7.2 triethylamine/phosphoric acid buffer at a flow rate of 2 mL/min. Chromatographic data were acquired and analyzed with Agilent OpenLAB chromatography data system (Rev. A.04.02).
Radioactivity measurements were made using a Comecer model TALETE HC dose calibrator.
Residual solvent levels were analyzed using an Agilent 7890A gas chromatograph, Agilent OpenLAB chromatography data system for data acquisition and analysis, and a WAX (Polyethyleneglycol phase: USP G16, G20) 30 meters, 0.25 mm ID, 0.25 mm film column.
Production of [18F]Fluoride
[18O]Water (98%, Huayi Isotopes, approx. 1.7 mL) was loaded into the niobium-body, high-yield [18F]fluoride target of a General Electric Medical Systems (GEMS) PETtrace cyclotron. The target was bombarded with a 16 MeV proton beam of 40 – 55 µA for up to 30 min to produce approx. 1.5 Ci (37 GBq) of aqueous [18F]fluoride via the 18O(p,n)18F nuclear reaction.
Preparation of the Radiofluorination Module and Solid Phase Extraction System
The radiochemical synthesis of [18F]FNDP was performed on a custom-made, nucleophilic radiofluorination module as described previously.12 The setup of this module involved attaching a Chromafix 30-PS-HCO3 SPE resin cartridge (ABX GmbH) cartridge for trapping cyclotron-released [18F]fluoride. Vented vials (4 mL, Thermo-Fisher) containing a solution of potassium carbonate (9.5 mg) and Kryptofix[2.2.2] (48 mg) in 50% aqueous MeCN (600 µL), precursor (1.5 mg) in dimethylsulfoxide (DMSO) (400 µL), MeCN (2 vials of 250 µL each), and HPLC-grade water (Milli-Q) (2 mL) were connected to the module. Nitrogen (Matheson UHP) gas was used for evaporation and transfer of all solutions.
A custom-made, syringe pump, solid phase extraction system (SPE) for final production formulation was outfitted with a collection reservoir of HPLC-grade water (50 mL), a Waters Oasis HLB Light LP cartridge, a bottle of absolute ethanol (500 mL), Sodium Chloride Injection, USP (normal saline) (50 mL), and HPLC-grade water (50 mL). The SPE cartridge was conditioned with 10 mL absolute ethanol followed by 10 mL HPLC-grade water and flushed with 10 ml of air.
Azeotropic Drying of [18F]Fluoride
After the preparation of the radiofluorination module, the [18F]fluoride was delivered to the receiving vessel as a solution of [18O]water. The [18F]fluoride was loaded onto the resin cartridge and the [18O]water collected for recycling. The resin cartridge was eluted with the aqueous MeCN solution of potassium carbonate/Kryptofix[2.2.2] into the reaction vessel. The [18F]fluoride, potassium carbonate and Kryptofix mixture was azeotropically dried with sequential acetonitrile additions under nitrogen flow at 110˚C.
Synthesis of [18F]FNDP
After cooling to near room temperature, the solution of the precursor was added to the reaction vessel and heated to 150˚C for 15 minutes.
Purification and Formulation of [18F]FNDP
After cooling to room temperature, the reaction mixture was diluted with HPLC-grade water and remotely injected onto the equilibrated (20 min at 10 ml/min) semi-preparative HPLC column. [18F]FNDP, which eluted at approx. 10.5 min (Figure 2) was collected in the SPE reservoir, passed through the SPE cartridge, washed with 10 mL HPLC water, and eluted with 1 mL absolute ethanol through a sterile 0.22 µ Millex FG filter into a sterile vial containing 4 mL normal saline. The cartridge was further rinsed through the filter with 10 mL of normal saline to ensure complete elution of the final product. [18F]FNDP was isolated with an average radiochemical yield of 24.1 ± 2.8 % (not corrected for decay) (n = 3), from starting [18F]fluoride, in 63.7 ± 1.3 minutes and average specific radioactivity of 3873 ± 888 GBq/µmole (104682 ± 23946 mCi/µmole).
Figure 2.
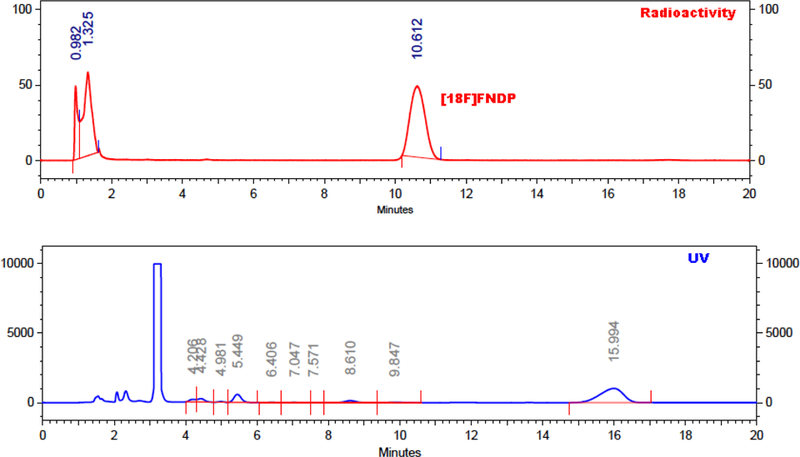
Preparative chromatogram of [18F]FNDP.
Quality Control Procedures
Quality control (QC) procedures for [18F]FNDP performed based upon current requirements for radiotracers set forth in the U.S. Pharmacopeia12 are summarized below. Data for 3 repeat batches of [18F]FNDP produced using the methods disclosed herein are summarized in Table 1. Each of the 3 batches met all of the established QC criteria.
Table 1.
Release and Stability Test Data for Three Qualification Batches of [18F]FNDP Injection Manufactured at the JHU PET Center
| [18F]FNDP Lot Number Date/Time of Manufacture | FNDP-17012301 23 Jan 17; 10:20:41 | FNDP-17012501 25 Jan 17; 09:01:00 | FNDP-17020601 6 Feb 17; 09:16:00 | |
|---|---|---|---|---|
| Test | Specification | |||
| Initial Appearance | Clear, colorless solution, no visible particulate matter | Conforms | Conforms | Conforms |
| Appearance 360 minutes after EOS | Clear, colorless solution, no visible particulate matter | Conforms | Conforms | Conforms |
| Initial Radiochemical Purity, % (t = 0 minutes) |
≥ 95% |
100% | 100% | 100% |
| Expiry Radiochemical Purity, % (t =240 minutes) |
≥ 95% |
100% | 98.3% | 100% |
| pH, Initial | 4.5 – 8.5 | 5.0 | 5.2 | 5.0 |
| pH, Expiry | 4.5 – 8.5 | 5.2 | 5.0 | 5.0 |
| Chemical Purity |
FNDP Precursor ≤ 0.5 µg/mL All others ≤ 0.5 µg/mL |
FNDP: 0.03 µg/mL Precursor: 0.00 µg/mL All others: 0.03 µg/Ml |
FNDP: 0.04 µg/mL Precursor: 0.00 µg/mL All others: 0.02 µg/mL |
FNDP: 0.07 µg/mL Precursor: 0.00 µg/mL All others: 0.11 µg/mL |
| Yield | ≥ 30 mCi [18F]FNDP (referenced to assay recorded at end-of-filtration) |
142 mCi | 235 mCi | 238.5 mCi |
| Specific Activity | ≥ 1,500 mCi/µmole of [18F]FNDP (referenced to end of filtration) |
105641 mCi/µmole | 133519 mCi/µmole | 74887 mCi/µmole |
| Identity (HPLC) |
HPLC Retention Time Matches Reference Standard | Match | Match | Match |
| Radionuclidic Purity | T½ Calc = 105 – 115 min | 107.45 min | 111.20 min | 109.03 min |
| Bubble-Point | ≥ 13 psi | 16 psi | 17 psi | 17 psi |
| Kryptofix Analysis | ≤ 50 µg/mL | Conforms | Conforms | Conforms |
| Residual Solvent Analysis | Ethanol ≤ 10% Acetonitrile ≤ 273 ppm DMSO ≤ 3333 ppm Triethylamine ≤ 280 ppm |
EtOH: 7.4% CH3CN: 0 DMSO: 330 TEA: 0 |
EtOH: 7.4% CH3CN: 0 DMSO: 354 TEA: 0 |
EtOH: 7.5% CH3CN: 9.3 DMSO: 330 TEA: 0 |
| Bacterial Endotoxin | ≤11 Endotoxin Unit per mL | <5 EU/ml | <5 EU/ml | <5 EU/ml |
Visual inspection
Using remote handling equipment and appropriate radiation shielding (leaded glass window or a mirror system), the vial containing the [18F]FNDP product was visually inspected the under bright light. The product met this acceptance specification if it was clear and colorless with no evidence of particulates or foreign matter.
Radiochemical Identity
The radiochemical identity of [18F]FNDP was determined using analytical HPLC. A reference standard solution (1mg/4mL of FNDP in absolute ethanol) was injected to establish the suitability of the system conditions. To determine the radiochemical identity, an aliquot of the final injection matrix of [18F]FNDP was mixed with an aliquot of the reference standard solution. The product met this acceptance specification if the retention time of the reference material, as indicated by the UV detector, was consistent with the retention time of the [18F]FNDP, as determined by the radiation detector, with appropriate correction for the offset between the two detector systems.
Radiochemical Purity
The radiochemical purity of [18F]FNDP was evaluated using the same HPLC system described for the radiochemical identity test. The quantity injected should avoid uncorrected dead-time loss (for main peak) in the radioactive detection system. The percent radiochemical purity of [18F]FNDP was determined by dividing the activity found in the [18F]FNDP peak by total activity found in the chromatogram multiplied by 100. The product met this acceptance specification if the radiochemical purity was ≥95%.
Specific Activity
The specific activity of [18F]FNDP was calculated by dividing the assayed radioactivity of a calibrated aliquot of [18F]FNDP (mCi/mL at end-of-synthesis) by the mass concentration of carrier FNDP measured by HPLC-UV (µmole/mL of FNDP), deduced from the standard mass calibration curve. The product met this acceptance specification if the specific activity was ≥1,500 mCi/µmole.
Figure 3 shows typical chromatograms observed during the determination of the radiochemical identity, radiochemical purity, and specific activity. Chromatogram A displays the analysis of the final product solution. Chromatogram B shows a co-injection with authentic non-radioactive standards of precursor and final product.
Figure 3.
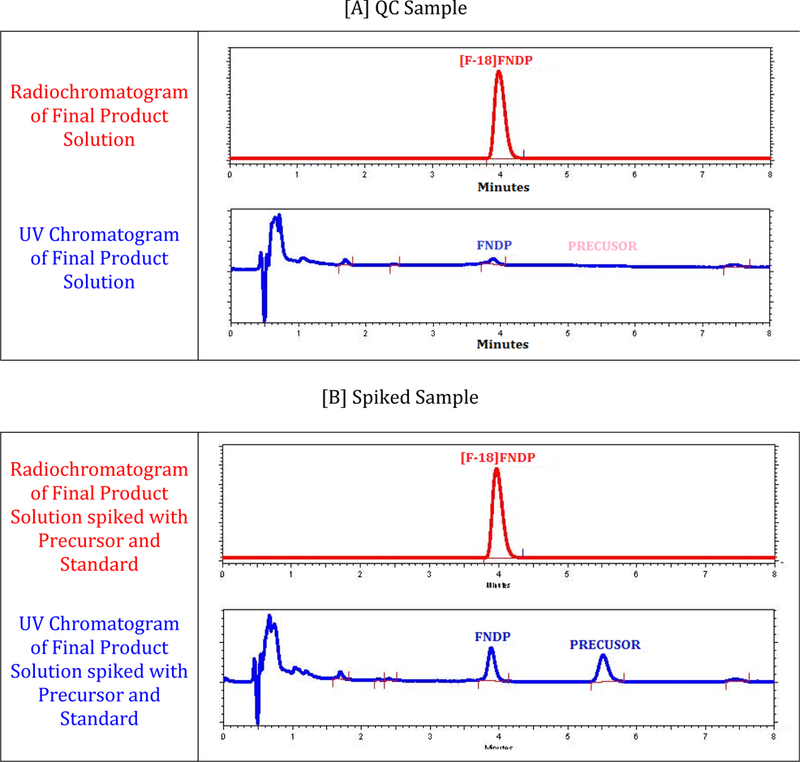
Quality control chromatograms of [18F]FNDP.
Residual Solvent Analysis
An aliquot of a 25 mL standard solution containing 23 mL of HPLC water, 1675 µL of absolute ethanol (6.7%), 12.7 µL of acetonitrile (400 ppm), 13.8 µL of triethylamine (420 ppm), 158 µL acetone (5000 ppm), 23.3 µL of dimethylformamide (880 ppm), 113.6 µL of DMSO (5000 ppm), and 12.4 µL of methylethylketone (400 ppm) was analyzed by gas chromatography to determine system suitability. An aliquot of the final [18F]FNDP product matrix was injected and the levels of residual solvent calculated by comparison to standards. The product met this acceptance specification if the levels of acetonitrile, DMSO, and triethylamine were ≤273, 3333, and 280 ppm, respectively, and the ethanol concentration was ≤10%.14
pH
A drop of the [18F]FNDP final product matrix was applied to pH indicator paper (pH 2–9; sensitivity of 0.3 to 0.5 units). The strip color was matched to an indicator chart. The product met this acceptance specification if the pH was between 4.5 and 8.5.
Residual Kryptofix-[2.2.2] Analysis
Residual Kryptofix[2.2.2] in the [18F]FNDP final product matrix was assayed using an established spot test. On a silica TLC plate (2.5 × 7.5 cm, EMD), 2 µL aliquots of a 5 mg/mL Kryptofix[2.2.2] standard solution in absolute ethanol, a standard solution of 50 µg/mL Kryptofix[2.2.2] standard solution in absolute ethanol, and the [18F]FNDP final product matrix were spotted in separate lanes. The strip was developed in a TLC tank using 9:1 (v:v) methanol:30% ammonium hydroxide after which the TLC plate was dried and stained with iodine vapor. Visualized spots for the 3 samples were compared. The product met this acceptance specification if the residual Kryptofix[2.2.2] level was demonstrated to be ≤50 µg/mL.
Sterile Filter Integrity Test
The sterile microfilter from the [18F]FNDP terminal filtration step was washed with 5 mL of absolute ethanol and attached to a calibrated pressure gauge (Millipore Sigma) and air pressure source. The distal end of the filter was placed in a liquid reservoir and the gas pressure was slowly increased. The product met this acceptance specification for the Millex FG filter if the pressure must be ≥13 psi prior to observing a stream of bubbles.
Radionuclidic Identity
The radioactivity content (mCi) in an aliquot of the [18F]FNDP final product matrix was determined in a Comecer Talete radioisotope dose calibrator at 0 minutes (A) and again 15 minutes later (B). The half-life was calculated using the formula below. The product met this acceptance specification if the calculated half-life was 105 – 115 minutes.
| T1/2=(4.495)/(log A − log B) |
Endotoxin Analysis
Endotoxin levels in batches of the [18F]FNDP final product matrix was analyzed by a Charles River Laboratories EndoSafe® nexgen-PTS™ Portable Testing System. The product met this acceptance specification if the endotoxin level ≤11 USP endotoxin units per mL.
Sterility Testing
In a laminar flow hood, samples of the [18F]FNDP final product matrix (Approx.. 100 µL each) were added to fluid thioglycolate media and soybean casein digest medium. The media were incubated at 32.5±2.5oC and 22.5±2.5oC, respectively, and observed daily for any turbidity indicative of positive growth. The product met this acceptance specification if no growth was observed during the 14-day incubation period.
Conclusion
In summary, a cGMP protocol for the radiosynthesis of [18F]FNDP, a radioligand for imaging soluble epoxide hydrolase, has been developed. The highly reproducible radiosynthesis produced sufficient quantities of [18F]FNDP at high specific radioactivity, chemical and radiochemical purity for use in future human PET studies.
REFERENCES
- 1.Sura P, Sura R, Enayetallah AE, Grant DF Distribution and expression of soluble epoxide hydrolase in human brain. J Histochem Cytochem 2008, 56, 551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enayetallah AE, French RA, Grant DF Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms. J Mol Histol 2006, 37, 133–41. [DOI] [PubMed] [Google Scholar]
- 3.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 2009, 163, 646–61. [DOI] [PubMed] [Google Scholar]
- 4.Newman JW, Morisseau C, Hammock BD Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res 2005, 44, 1–51. [DOI] [PubMed] [Google Scholar]
- 5.Iliff JJ, Wang R, Zeldin DC, Alkayed NJ Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol 2009, 296, H1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martini RP, Ward J, Siler DA, Eastman JM, Nelson JW, Borkar RN, Alkayed NJ, Dogan A, Cetas JS Genetic variation in soluble epoxide hydrolase: association with outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg 2014, 121, 1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, Zeldin DC Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet 2006, 15, 1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, Silbert LC, Grafe MR, Alkayed NJ Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat 2014, 113–115, 30–7. [DOI] [PMC free article] [PubMed]
- 9.Hung YW, Hung SW, Wu YC, Wong LK, Lai MT, Shih YH, Lee TS, Lin YY Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav Immun 2015, 43, 118–29. [DOI] [PubMed] [Google Scholar]
- 10.Qin X, Wu Q, Lin L, Sun A, Liu S, Li X, Cao X, Gao T, Luo P, Zhu X, Wang X Soluble Epoxide Hydrolase Deficiency or Inhibition Attenuates MPTP-Induced Parkinsonism. Mol Neurobiol 2015, 52, 187–95. [DOI] [PubMed] [Google Scholar]
- 11.Horti AG, Wang Y, Minn I, Lan X, Wang J, Koehler RC, Alkayed NJ, Dannals RF, Pomper MG 18F-FNDP for PET Imaging of Soluble Epoxide Hydrolase. J Nucl Med 2016, 57, 1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravert HT, Holt DP, Dannals RF, J Label Compds Radiopharm, 2014, 57, 695. [DOI] [PubMed] [Google Scholar]
- 13. U.S. Pharmacopeia Chapter <823> Radiopharmaceuticals for Positron Emission Tomography – Compounding. USP 41-NF 36, 2017.
- 14. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline Impurities: Guideline for Residual Solvents, 1997.


