Abstract
Objective:
To describe one-year cognitive and neurologic outcomes among extracorporeal cardiopulmonary resuscitation (ECPR) survivors enrolled in the Therapeutic Hypothermia after Paediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial; and compare outcomes between survivors who received ECPR, later extracorporeal membrane oxygenation (ECMO), or no ECMO.
Methods:
All children recruited to THAPCA-IH were comatose post-arrest. Neurobehavioral function was assessed by caregivers using the Vineland Adaptive Behaviour Scales, 2nd edition (VABS-II) at pre-arrest baseline and 12 months post-arrest. Age-appropriate cognitive performance measures (Mullen Scales of Early Learning or Wechsler Abbreviated Scale of Intelligence) and neurologic examinations were obtained 12 months post-arrest. VABS-II and cognitive performance measures were transformed to standard scores (mean=100, SD=15) with higher scores representing better performance. Only children with broadly normal pre-arrest function (VABS-II ≥70) were included in this analysis.
Results:
One-year follow-up was attained for 127 survivors with pre-arrest VABS-II ≥70. Of these, 57 received ECPR, 14 received ECMO later in their course, and 56 did not receive ECMO. VABS-II assessments were completed at 12 months for 55 (96.5%) ECPR survivors, cognitive testing for 44 (77.2%) and neurologic examination for 47 (82.5%). At 12 months, 39 (70.9%) ECPR survivors had VABS-II scores ≥70. On cognitive testing, 24 (54.6%) had scores ≥70, and on neurologic examination, 28 (59.5%) had no/minimal to mild impairment. Cognitive and neurologic score distributions were similar between ECPR, later ECMO and no ECMO groups.
Conclusions:
Many ECPR survivors had favourable outcomes although impairments were common. ECPR survivors had similar outcomes to other survivors who were initially comatose post-arrest.
Keywords: Extracorporeal cardiopulmonary resuscitation, Paediatric, Outcome, Neurobehavioral, Cognitive, Neurologic
INTRODUCTION
Extracorporeal cardiopulmonary resuscitation (ECPR) is an advanced rescue therapy increasingly used among children with refractory in-hospital cardiac arrest. During ECPR, an extracorporeal circuit is established to maintain vital organ perfusion until potentially reversible causes of cardiac arrest are identified and treated. Survival to hospital discharge after ECPR is about 40% [1,2]; however, long-term cognitive and neurologic outcomes among survivors are largely unknown [3–6]. Knowledge regarding these outcomes is important for clinical decision-making, counselling parents, and planning rehabilitation and educational services during recovery.
The Therapeutic Hypothermia after Paediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial was a randomized trial comparing two targeted temperature management interventions in children who were comatose after in-hospital arrest [7]. Neurobehavioral function was assessed longitudinally by caregiver report using the Vineland Adaptive Behaviour Scales, 2nd edition (VABS-II) [8]. The primary outcome measure was one-year survival with good/favourable neurobehavioral function defined as VABS-II score ≥70 (population mean=100, SD=15). Results of the THAPCA-IH trial showed the primary outcome did not differ between temperature management groups.
We previously reported that about a third of children receiving ECPR in the context of the THAPCA-IH trial survived with good neurobehavioral function at one year based on caregiver responses to the VABS-II [3]. Benefits of using a caregiver-report measure included the ability to collect data in-person or by telephone, and to obtain a retrospective assessment of each child’s pre-arrest functional status. Despite benefits, caregiver-report measures of functional status may lack the sensitivity of performance-based measures in identifying global and selective impairments. In the THAPCA-IH trial, performance-based cognitive evaluation and neurologic examination were obtained at follow-up in addition to the VABS-II in order to provide complementary, objective outcomes for one-year survivors. The purpose of this study was to describe detailed one-year performance-based cognitive and neurologic outcomes in paediatric ECPR survivors enrolled in the THAPCA-IH trial, and to compare outcomes between survivors who received ECPR, later extracorporeal membrane oxygenation (ECMO), or no ECMO.
METHODS
Study Design
This study is a secondary analysis of the THAPCA-IH trial [7]. Thirty-seven children’s hospitals in the United States, Canada, and the United Kingdom recruited children between September 1, 2009 and February 27, 2015. Details of the trial were previously published [7,9,10]. Institutional review boards at the University of Utah Data Coordinating Centre, the Kennedy Krieger Institute, and all study sites approved the study. Caregiver permission was obtained for all participants.
Study Participants
Children eligible for the THAPCA-IH trial were >48 hours and <18 years of age, had an in-hospital cardiac arrest with chest compressions for ≥2 minutes, and required mechanical ventilation after return of circulation [7]. Major exclusion criteria were a Glasgow Coma Scale motor subscale score of 5 or 6 (i.e., purposeful lateralizing response to painful stimulus) [11], inability to be randomized within 6 hours of return of circulation, and a decision by the clinical team to withhold aggressive treatment. Additional inclusion criteria for this secondary analysis included having broadly normal pre-arrest neurobehavioral function defined as pre-arrest VABS-II ≥70, survival to 12 months, and completion of at least one 12-month follow-up measure. ECPR was defined as ECMO initiation during active chest compressions or before sustained return of spontaneous circulation >20 minutes was achieved [12,13]. Of 329 children recruited to the THAPCA-IH trial, 269 had broadly normal pre-arrest function. Of these 135 survived 12 months; of these, 127 had follow-up. Of those with follow-up, 57 received ECPR, 14 received ECMO later in their hospital course, and 56 did not receive ECMO.
Measures
The VABS-II is a caregiver-report measure of adaptive behaviour defined as performance on daily life activities necessary for personal and social independence [8]. The VABS-II provides age-corrected standard scores (mean=100, SD=15) in four domains (communication, daily living, socialization, and motor skills) and an overall adaptive behaviour composite. Each domain includes subdomains with developmentally sequenced items starting with skills typically observed in infancy. Subdomain raw scores are age-corrected and standardized as v-scores. The VABS-II has a caregiver rating form and a survey interview (using caregiver as informant) that yield comparable scores.
The Mullen Scales of Early Learning (Mullen) is a performance-based measure of cognitive function for young children [14]. The Mullen has 4 scales (visual reception, fine motor, receptive language, expressive language). Normative data are available from birth through age 5 years and 8 months. Age-corrected standardized scores are available for each scale and for an overall early learning composite.
The Wechsler Abbreviated Scale of Intelligence (WASI) measures intellectual or general cognitive functioning and includes verbal and visual reasoning subtests [15]. Normative data are based on a standardization sample highly representative of the English-speaking United States population aged 6–89 years. Age-corrected standardized scores are available for the verbal and visual reasoning subtests. When combined, these subtests yield age-corrected standard scores for general intellectual functioning (Full Scale IQ).
For this report, all standardized scores (VABS-II, Mullen, WASI) were transformed to standard scores (mean=100, SD=15) with higher scores representing better performance. Scores >115 are above average, 85–115 are average, 70–84 are below average, 50–69 are impaired, and <50 are severely impaired.
The Paediatric Resuscitation after Cardiac Arrest (PRCA) form was used to record and score detailed, conventional age-appropriate neurologic examinations [16]. The PRCA was developed as a modification of the Paediatric Stroke Outcome Measure (PROM) [17]. Versions were developed for children ≥3 years old and >3 years, reflecting age-related items for assessing language and cognition. Paediatric neurologists performed detailed neurological examinations and scored neurologic function (0, normal to 3, severe impairment) in 6 domains. The sensorimotor domain was scored independently for each side of the body (so that scores ranged from 0–6). The five other scored domains included other non-lateralizing sensorimotor function (encompassing cranial nerve deficits, movement/tone disorder, global delays), language production, language comprehension, cognition, and behaviour. Total PRCA scores range from 0–21, with 0 indicating no deficits and 21 indicating maximal deficits. Scores were categorized as 0–3 (no/minimal impairment), 4–7 (mild impairment), 8–11 (moderate impairment), 12–16 (severe impairment), and 17–21 (profound impairment).
Paediatric Cerebral Performance Category (PCPC) measures neurologic functioning and Paediatric Overall Performance Category (POPC) measures overall health including neurologic functioning [18]. Both are 6-point scales with lower scores representing better function. PCPC and POPC lack detailed assessment but are often used in studies of cardiac arrest and facilitate comparison with other studies.
Procedures
Caregivers completed pre-arrest VABS-II assessments at the local sites within 24 hours of randomization in the THAPCA-IH trial using the caregiver rating form. Research coordinators assisted caregivers with the pre-arrest VABS-II as needed. Research coordinators also rated PCPC and POPC using medical records or caregiver report, and collected child and cardiac arrest characteristics. Child characteristics included age, sex, race, ethnicity, pre-existing conditions and baseline technology dependence (tracheostomy or percutaneous feeding tube). Cardiac arrest characteristics included primary aetiology of cardiac arrest, initial rhythm at the start of chest compressions, whether the child was post-cardiac surgery at the time of arrest, and duration of chest compressions.
One year after cardiac arrest, an interviewer from the Kennedy Krieger Institute, unaware of treatment group assignment, completed the VABS-II with caregivers by telephone. Subsequently, children underwent cognitive testing and neurologic examination at the local sites. Children were tested with the Mullen up through 5 years, 8 months and 30 days. Children who were >5 years and 9 months but <6 years were tested after their sixth birthday. Children ≥6 years of age completed the WASI. Spanish-speaking caregivers completed 12-month VABS-II interviews in Spanish. Spanish-speaking children were tested by a Spanish-speaking examiner. Paediatric neurologists trained in the use of the PRCA performed the neurologic examinations.
Statistical Analyses
Twelve-month VABS-II composite scores were compared between children who completed 12-month Mullen/WASI and PRCA and those who did not in order to evaluate whether children completing the Mullen/WASI and PRCA were representative of all children included in the study. Wilcoxon rank-sum tests and Fisher’s exact tests were used for these comparisons. Baseline child characteristics and cardiac arrest characteristics were compared between survivors who received ECPR, later ECMO, or no ECMO using Fisher’s exact tests. Change in VABS-II scores was calculated as difference scores between pre-arrest baseline and 12 months post-arrest. Change in VABS-II scores was evaluated using signed-rank tests. Twelve-month outcomes were compared between survivors who received ECPR, later ECMO, or no ECMO using Fisher’s exact tests. Analyses were performed using SAS software, version 9.4 (SAS Institute).
RESULTS
ECPR Survivor Characteristics
Of 57 ECPR survivors, 46 (80.7%) were <6 years old, 37 (64.9%) were male and 26 (45.6%) were White (Table 1). ECPR survivors were more likely to have a cardiovascular event as the primary aetiology of arrest, post-operative cardiac surgery status, and longer duration of chest compressions than survivors who received later ECMO or no ECMO (Table 1). ECPR survivors were more likely to have a pre-existing cardiac condition and less likely to have neurologic conditions, respiratory conditions or technology dependence than other survivors. Twelve-month VABS-II scores were similar between ECPR survivors who completed 12-month Mullen/WASI and PRCA and those who did not (Supplemental Material 1).
Table 1.
Descriptive Characteristics
| ECPR (N = 57) |
Later (N = ECMO14) |
No ECMO Use (N = 56) |
P-value1 | |
|---|---|---|---|---|
| Age at time of 12-month follow-up | 0.378 | |||
| < 3 years | 43 (75.4%) | 9 (64.3%) | 34 (60.7%) | |
| 3 - < 6 years | 3 (5.3%) | 1 (7.1%) | 8 (14.3%) | |
| ≥ 6 years | 11 (19.3%) | 4 (28.6%) | 14 (25.0%) | |
| Sex | 0.153 | |||
| Male | 37 (64.9%) | 6 (42.9%) | 28 (50.0%) | |
| Female | 20 (35.1%) | 8 (57.1%) | 28 (50.0%) | |
| Race | 0.156 | |||
| Asian | 3 (5.3%) | 1 (7.1%) | 0 (0.0%) | |
| Black or African American | 20 (35.1%) | 4 (28.6%) | 13 (23.2%) | |
| White | 26 (45.6%) | 9 (64.3%) | 35 (62.5%) | |
| Other/Unknown | 8 (14.0%) | 0 (0.0%) | 8 (14.3%) | |
| Hispanic or Latino | 9 (15.8%) | 2 (14.3%) | 15 (26.8%) | 0.341 |
| Primary aetiology of cardiac arrest | 0.002 | |||
| Cardiovascular event | 47 (82.5%) | 8 (57.1%) | 29 (51.8%) | |
| Respiratory event | 10 (17.5%) | 6 (42.9%) | 22 (39.3%) | |
| Other | 0 (0.0%) | 0 (0.0%) | 5 (8.9%) | |
| Initial cardiac arrest rhythm | 0.812 | |||
| Asystole | 1 (1.8%) | 1 (7.1%) | 2 (3.6%) | |
| Bradycardia | 28 (49.1%) | 6 (42.9%) | 33 (58.9%) | |
| Pulseless electrical activity | 16 (28.1%) | 5 (35.7%) | 12 (21.4%) | |
| Ventricular fibrillation or tachycardia | 11 (19.3%) | 2 (14.3%) | 8 (14.3%) | |
| Unknown | 1 (1.8%) | 0 (0.0%) | 1 (1.8%) | |
| Duration of chest compressions (minutes) | <.001 | |||
| ≤ 15 | 5 (8.8%) | 12 (85.7%) | 44 (78.6%) | |
| 16–30 | 7 (12.3%) | 1 (7.1%) | 9 (16.1%) | |
| 31–45 | 20 (35.1%) | 1 (7.1%) | 3 (5.4%) | |
| 46–60 | 13 (22.8%) | 0 (0.0%) | 0 (0.0%) | |
| > 60 | 12 (21.1%) | 0 (0.0%) | 0 (0.0%) | |
| Post-operative cardiac surgery | 36 (63.2%) | 3 (21.4%) | 10 (17.9%) | <.001 |
| Technology dependence | 2 (3.5%) | 1 (7.1%) | 11 (19.6%) | 0.019 |
| Any pre-existing condition | 53 (93.0%) | 13 (92.9%) | 47 (83.9%) | 0.279 |
| Pre-existing Conditions | ||||
| Cardiac condition | 50 (87.7%) | 7 (50.0%) | 29 (51.8%) | <.001 |
| Congenital heart disease | 42 (73.7%) | 5 (35.7%) | 27 (48.2%) | 0.004 |
| Single ventricle | 16 (28.1%) | 2 (14.3%) | 9 (16.1%) | 0.257 |
| Acquired heart disease | 7 (12.3%) | 2 (14.3%) | 5 (8.9%) | 0.777 |
| Arrhythmia | 22 (38.6%) | 2 (14.3%) | 8 (14.3%) | 0.008 |
| Pre-existing heart transplant | 5 (8.8%) | 0 (0.0%) | 1 (1.8%) | 0.254 |
| Respiratory condition | 10 (17.5%) | 6 (42.9%) | 22 (39.3%) | 0.022 |
| Neurologic condition | 5 (8.8%) | 3 (21.4%) | 20 (35.7%) | 0.002 |
| Gastrointestinal condition | 10 (17.5%) | 5 (35.7%) | 14 (25.0%) | 0.290 |
| Prenatal condition | 11 (19.3%) | 1 (7.1%) | 19 (33.9%) | 0.065 |
| Pulmonary hypertension | 2 (3.5%) | 0 (0.0%) | 1 (1.8%) | 1.000 |
| Immunocompromised | 7 (12.3%) | 1 (7.1%) | 8 (14.3%) | 0.866 |
| Renal condition | 7 (12.3%) | 2 (14.3%) | 5 (8.9%) | 0.777 |
| Other pre-existing condition | 13 (22.8%) | 3 (21.4%) | 18 (32.1%) | 0.486 |
| Pre-cardiac arrest PCPC | 0.080 | |||
| Normal = 1 | 35 (61.4%) | 11 (78.6%) | 40 (71.4%) | |
| Mild disability = 2 | 18 (31.6%) | 1 (7.1%) | 8 (14.3%) | |
| Moderate disability = 3 | 3 (5.3%) | 2 (14.3%) | 8 (14.3%) | |
| Severe disability = 4 | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | |
| Pre-cardiac arrest POPC | 0.089 | |||
| Good = 1 | 22 (38.6%) | 9 (64.3%) | 25 (44.6%) | |
| Mild disability = 2 | 27 (47.4%) | 2 (14.3%) | 20 (35.7%) | |
| Moderate disability = 3 | 6 (10.5%) | 2 (14.3%) | 11 (19.6%) | |
| Severe disability = 4 | 2 (3.5%) | 1 (7.1%) | 0 (0.0%) |
P-values from Fisher’s Exact Test.
Data are counts and percentages.
Abbreviations: ECPR, extracorporeal cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; PCPC, Paediatric Cerebral Performance Category; POPC, Paediatric Overall Performance Category.
Neurobehavioral Function at One Year for ECPR Survivors
Of 57 ECPR survivors, caregivers of 55 (96.5%) completed 12-month VABS-II adaptive behaviour assessment. Table 2 shows baseline and 12-month VABS-II composite, domain and subdomain scores for ECPR survivors. Mean baseline scores ranged from 93 to 109, mean 12-month scores from 77 to 96, and mean change from −21 to −2. Larger declines were observed in the daily living and motor function domains than the communication or socialization domains.
Table 2.
Vineland Adaptive Behavior Scales, Second Edition, Mean Adaptive Behavior Composite, Domain, and Subdomain Scores for Paediatric Extracorporeal Cardiopulmonary Resuscitation Survivors
| N1 | Baseline | Month 12 | Baseline to month 12 change | P-value2 | |
|---|---|---|---|---|---|
| Adaptive Behavior Composite | 55 | 95 (16) | 82 (19) | -13 (22) | <.001 |
| Communication | 55 | 96 (16) | 86 (19) | -10 (22) | <.001 |
| Receptive | 55 | 100 (13) | 92 (16) | -8 (17) | <.001 |
| Expressive | 55 | 95 (15) | 85 (21) | -9 (24) | 0.006 |
| Written | 13 | 98 (15) | 90 (12) | -8 (11) | 0.035 |
| Daily Living | 55 | 97 (20) | 80 (20) | -18 (26) | <.001 |
| Personal | 55 | 98 (18) | 77 (21) | -21 (25) | <.001 |
| Domestic | 18 | 101 (14) | 93 (10) | -9 (15) | 0.022 |
| Community | 18 | 106 (19) | 89 (14) | -10 (20) | 0.014 |
| Socialization | 55 | 94 (18) | 90 (16) | -4 (23) | 0.139 |
| Interpersonal Relationship | 55 | 93 (16) | 91 (20) | -2 (25) | 0.461 |
| Play and Leisure | 55 | 94 (14) | 88 (14) | -7 (20) | 0.024 |
| Coping Skills | 17 | 109 (13) | 96 (12) | -10 (16) | 0.030 |
| Motor Functioning | 55 | 97 (15) | 79 (21) | -18 (20) | <.001 |
| Gross | 55 | 94 (10) | 77 (19) | -17 (19) | <.001 |
| Fine | 55 | 103 (15) | 87 (23) | -15 (21) | <.001 |
N is the number of children with both baseline and 12-month assessment.
P-Value from the Signed Rank test. Data are means and standard deviations.
Cognitive Performance at One Year for ECPR Survivors
Of 57 ECPR survivors, cognitive performance-based testing was attained for 44 (77.2%) (34 Mullen, 10 WASI). Table 3 shows the cognitive performance composite and domain scores for ECPR survivors. For survivors <6 years old, 1 (2.9%) had a Mullen composite score in the above average range, 8 (23.5%) average, 6 (17.6%) below average, 9 (26.5%) impaired and 10 (29.4%) severely impaired. For survivors ≥6 years old, 8 (80.0%) had WASI Full-Scale IQ in the average range, 1 (10.0%) below average, and 1 (10.0%) impaired. The degree of impairment was similar to the respective composite scores for all Mullen scales and WASI subsets. Figure 1 shows distributions of VABS-II and cognitive performance scores for ECPR survivors.
Table 3.
Cognitive Performance at 12-Month Follow-Up for Children Treated with Extracorporeal Cardiopulmonary Resuscitation
| 3a. Mullen Scales of Early Learning Composite and Domain Scores (Age < 6 years) | |||||
|---|---|---|---|---|---|
| Early Learning Composite | Visual Reception | Fine Motor | Receptive Language | Expressive Language | |
| Score Category (N = 34) | |||||
| < 50 (Severely impaired) | 10 (29.4%) | 10 (29.4%) | 10 (29.4%) | 9 (26.5%) | 10 (29.4%) |
| 50–69 (Impaired) | 9 (26.5%) | 7 (20.6%) | 6 (17.6%) | 7 (20.6%) | 8 (23.5%) |
| 70 – 84 (Below average) | 6 (17.6%) | 4 (11.8%) | 5 (14.7%) | 8 (23.5%) | 5 (14.7%) |
| 85 – 115 (Average) | 8 (23.5%) | 13 (38.2%) | 13 (38.2%) | 9 (26.5%) | 11 (32.4%) |
| > 115 (Above average) | 1 (2.9%) | 0 (0%) | 0 (0%) | 1 (2.9%) | 0 (0%) |
| 3b. Wechsler Abbreviated Scale of Intelligence Full Scale IQ Composite and Subtest Scores (Age ≥ 6 years) | |||
|---|---|---|---|
| Full-scale IQ score | Vocabulary | Matrix Reasoning | |
| Score Category (N = 10) | |||
| 50–69 (Impaired) | 1 (10.0%) | 2 (20.0%) | 1 (10.0%) |
| 70 – 84 (Below average) | 1 (10.0%) | 1 (10.0%) | 1 (10.0%) |
| 85 – 115 (Average) | 8 (80.0%) | 7 (70.0%) | 6 (60.0%) |
| > 115 (Above average) | 0 (0%) | 0 (0%) | 2 (20.0%) |
Scores were transformed to correspond to a scale with mean 100 and standard deviation 15.
Data are counts and percentages.
Figure 1. One Year Adaptive Behaviour and Cognitive Performance in Survivors of Extracorporeal Cardiopulmonary Resuscitation.
Percent of survivors with above average, average, below average, impaired, and severely impaired adaptive behaviour and cognitive performance 12 months after extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest, and expected population norms.
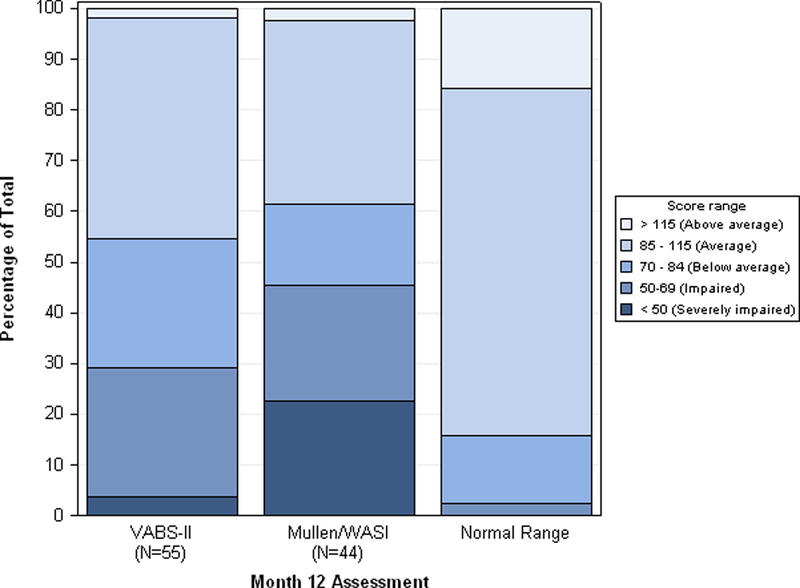
Abbreviations: Mullen, Mullen Scales of Early Learning; WASI, Wechsler Abbreviated Scale of Intelligence
Neurologic Examination at One Year for ECPR Survivors
Of 57 ECPR survivors, neurologic examination was performed in 47 (82.5%). Table 4 shows the PRCA total and domain scores for ECPR survivors. Median total PRCA score was 4.0 (IQR 0.0, 12.0). Impairment was none/minimal for 23 (48.9%), mild for 5 (10.6%), moderate for 5 (10.6%), severe for 9 (19.1%), and profound for 5 (10.6%).
Table 4.
Neurologic Examination: Paediatric Resuscitation after Cardiac Arrest Scores at 12-month Follow-up for Children Treated with Extracorporeal Cardiopulmonary Resuscitation
| Overall (N = 47) |
|
|---|---|
| Global assessment score (range 0–21) | 4.0 [0.0, 12.0] |
| Global assessment score category | |
| 0–3 (None/minimally impaired) | 23 (48.9%) |
| 4–7 (Mildly impaired) | 5 (10.6%) |
| 8–11 (Moderately impaired) | 5 (10.6%) |
| 12–16 (Severely impaired) | 9 (19.1%) |
| 17–21 (Profoundly impaired) | 5 (10.6%) |
| Sensorimotor Deficit (range 0–6) | 2.0 [0.0, 4.0] |
| Other motor or sensory deficits (includes cranial nerve deficits) | 0.0 [0.0, 2.0] |
| (range 0–3) | |
| Language, Cognition, and Behaviour (range 0–12) | 1.0 [0.0, 7.0] |
| Language Deficit - Production (including dysarthria) (range 0–3) | 0.0 [0.0, 2.0] |
| Language Deficit - Comprehension (range 0–3) | 0.0 [0.0, 2.0] |
| Cognitive Deficit (range 0–3) | 0.0 [0.0, 2.0] |
| Behavioural Deficit (range 0–3) | 0.0 [0.0, 1.0] |
| Overall (N = 47) |
Data are counts and percentages or medians and interquartile ranges.
Comparisons of ECPR, later ECMO and no ECMO groups
Table 5 shows VABS-II adaptive behaviour composite, cognitive performance composite and PRCA total scores for ECPR, later ECMO and no ECMO groups. Caregiver-report 12-month VABS-II composite scores were broadly normal (≥70) for 39 (70.9%) ECPR survivors, 10 (71.4%) later ECMO survivors, and 47 (83.9%) no ECMO survivors; 12-month VABS-II scores were not statistically significantly different across groups. Performance-based cognitive evaluation composite scores ≥70 were achieved for 24 (54.5%) ECPR survivors, 8 (72.7%) later ECMO survivors, and 27 (61.4%) no ECMO survivors; cognitive evaluation scores were not statistically significantly different across groups. Neurologic examination scores in the none/minimal impairment to mild impairment range were observed for 28 (59.5%) ECPR survivors, 10 (83.3%) later ECMO survivors, and 33 (73.3%) no ECMO survivors; neurologic examination scores were also not statistically significantly different across groups.
Table 5.
Outcomes at 12-Month Follow-Up by Extracorporeal Membrane Oxygenation Use
| ECPR | Later ECMO | No Use ECMO | P-value1 | |
|---|---|---|---|---|
| Total with Month 12 VABS-II | 55 | 14 | 56 | |
| Adaptive Behaviour Composite | 0.208 | |||
| < 50 (Severely impaired) | 2 (3.6%) | 0 (0.0%) | 1 (1.8%) | |
| 50–69 (Impaired) | 14 (25.5%) | 4 (28.6%) | 8 (14.3%) | |
| 70 – 84 (Below average) | 14 (25.5%) | 1 (7.1%) | 19 (33.9%) | |
| 85 – 115 (Average) | 24 (43.6%) | 8 (57.1%) | 28 (50.0%) | |
| > 115 (Above average) | 1 (1.8%) | 1 (7.1%) | 0 (0.0%) | |
| Total with Month 12 Mullen/WASI | 44 | 11 | 442 | |
| Mullen or WASI score category (all ages combined) | 0.435 | |||
| < 50 (Severely impaired) | 10 (22.7%) | 1 (9.1%) | 5 (11.4%) | |
| 50–69 (Impaired) | 10 (22.7%) | 2 (18.2%) | 12 (27.3%) | |
| 70 – 84 (Below average) | 7 (15.9%) | 5 (45.5%) | 11 (25.0%) | |
| 85 – 115 (Average) | 16 (36.4%) | 2 (18.2%) | 14 (31.8%) | |
| > 115 (Above average) | 1 (2.3%) | 1 (9.1%) | 2 (4.5%) | |
| Total with Month 12 PRCA | 47 | 12 | 45 | |
| Global assessment score category | 0.727 | |||
| 17–21 (Profoundly impaired) | 5 (10.6%) | 1 (8.3%) | 2 (4.4%) | |
| 12–16 (Severely impaired) | 9 (19.1%) | 1 (8.3%) | 5 (11.1%) | |
| 8–11 (Moderately impaired) | 5 (10.6%) | 0 (0.0%) | 5 (11.1%) | |
| 4–7 (Mildly impaired) | 5 (10.6%) | 2 (16.7%) | 9 (20.0%) | |
| 0–3 (None/minimally impaired) | 23 (48.9%) | 8 (66.7%) | 24 (53.3%) |
P-values from Fisher Exact Test.
One child >6 years in the No ECMO Use group had no consistent means of functional communication based on the 12-month VABS-II assessment and did not undergo cognitive testing. Data are counts and percentages.
Abbreviations: VABS-II, Vineland Adaptive Behaviour Scales, Second Edition; Mullen, Mullen Scales of Early Learning; WASI, Wechsler Abbreviated Scale of Intelligence; PRCA, Paediatric Resuscitation after Cardiac Arrest.
DISCUSSION
This is the first multicentre study to report detailed one-year cognitive and neurologic outcomes among paediatric ECPR survivors. All children included in this study had broadly normal pre-arrest neurobehavioral function and were comatose in the early post-arrest period. Among ECPR survivors, 70.9% had caregiver-reported VABS-II adaptive behaviour composite scores ≥70 at 12 months post-arrest. Using performance-based cognitive evaluation, 54.5% ECPR survivors had standardized total scores ≥70. On neurologic examination, 59.5% ECPR survivors had impairment in the range of none/minimal to mild. Overall, these findings suggest that many ECPR survivors in our study had a broadly favourable functional outcome. These findings also suggest that performance-based testing may reveal impairments not readily identified by caregiver report. Outcomes based on caregiver report, performance testing, or neurologic examination were similar among survivors who were comatose post-arrest and who received ECPR, later ECMO, or no ECMO during their hospital course.
Although many ECPR survivors in our study had a favourable functional outcome at one-year follow-up, impairments were common and the range of outcomes was wide. Overall, functional outcomes for our cohort of ECPR survivors were shifted below population norms. Caregiver-reported adaptive behaviour declined from pre-arrest baseline in all domains; declines were greater in daily living and motor functioning than socialization and communication domains. Differential impairment among adaptive behaviour domains may reflect a specific pattern of brain injury. On the other hand, caregivers may more accurately recognize their child’s physical impairments, and tend to minimize deficits in social and communication skills. Greater decline in daily living and motor function domains of adaptive behaviour were also observed in the overall THAPCA-IH study population [19] as well as the Therapeutic Hypothermia after Paediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) population [20].
In spite of broadly normal pre-arrest functioning, 55.9% of ECPR survivors <6 years old had Mullen composite scores in the impaired or severely impaired range (i.e., <70), and 10% of ECPR survivors ≥6 years had WASI composite scores in the impaired range. These findings might suggest that younger ECPR survivors have worse cognitive outcomes than older survivors. However, these findings must be interpreted with caution due to the small number of ECPR survivors ≥6 years old (n=11); only ten underwent performance-based cognitive testing and one declined participation in this particular aspect of follow-up. Younger children enrolled in THAPCA-IH may have been more likely to have complex congenital anomalies and multisystem disorders at baseline that contribute to lower cognitive performance at follow-up. Cognitive performance is also inherently more difficult to assess formally in a single out-patient visit in young children with antecedent prolonged hospitalization.
A small number of single centre studies have reported functional outcomes among ECPR survivors. Guerra et al [4] prospectively evaluated neurocognitive outcomes of 17 paediatric ECPR survivors at 4.5 years of age and at least 6 months after ECPR admission to a single Canadian centre using the Wechsler Preschool and Primary Scales of Intelligence. Paediatric ECPR survivors had a mean (SD) Full Scale IQ score of 76.5 (15.9) with 4 (24%) children having intellectual disability defined as a Full Scale IQ over two standard deviations below the population mean (i.e., <70). In a recent retrospective study, Beshish et al [5] reported functional outcomes of 38 paediatric ECPR survivors from a single US centre using the Functional Status Scale (FSS) [21] assessed at hospital admission and discharge. The FSS assesses function in 6 domains (mental, sensory, communication, motor, feeding and respiratory) with total scores ranging from 6–30; higher scores represent more dysfunction. Beshish et al found that half of ECPR survivors had a new functional morbidity defined as an increase in FSS by ≥3 points from baseline and 68% had a favourable outcome defined as an increase in FSS by <5 points from baseline. Small sample sizes and variation in outcome measures and study designs make comparisons between studies difficult.
ECPR survivors in our study had similar cognitive and neurologic outcomes to in-hospital cardiac arrest survivors who received ECMO later in their course or who did not receive ECMO. Although all children were comatose post-arrest, ECPR survivors were different from other survivors for important pre-arrest and arrest characteristics such as greater likelihood of pre-existing cardiac conditions, post-cardiac surgery status, cardiovascular events as the primary aetiology of arrest, and longer durations of chest compressions. Many ECPR survivors might have died without ECPR as failure to achieve sustained return of spontaneous circulation often prompts ECMO initiation. In a study based on the American Heart Association’s Get with the Guidelines-Resuscitation Registry, Lasa et al [2] evaluated children with in-hospital CPR ≥10 minutes duration using propensity-score matching to compare outcomes from ECPR and conventional CPR. Children receiving ECPR had greater odds of survival to hospital discharge and survival with favourable neurologic status based on PCPC scores. Although the ECPR, later ECMO and no ECMO groups in our study are difficult to compare due to baseline differences and small sample size in each group, our findings and those of Lasa et al suggest that ECPR can result in functional outcomes as favourable as those observed among other in-hospital cardiac arrest survivors. However, small samples in our comparison groups make it possible that differences were missed.
Strengths of our study include the prospective multicentre design and the use of well-validated measures to assess many domains of functioning both by caregiver report and by performance-based testing. Another strength is the inclusion of a broad paediatric age range from >48 hours to <18 years of age. However, important limitations to the generalizability of our findings exist. Children included in this study were those recruited to the THAPCA-IH trial and therefore were subject to all THAPCA-IH inclusion and exclusion criteria. In addition to these known criteria, children whose caregivers agree for their child to participate in a randomized trial may be different from those whose caregivers refuse participation in ways that are unknown. Children recruited to this study had broadly normal caregiver-reported pre-arrest baseline function and were comatose in the early post-arrest period. Thus, this study excludes children recognized by their caregivers as significantly impaired at baseline and children who rapidly regained consciousness following cardiac arrest. Additionally, this study includes only those children who survived in-hospital arrest and participated in at least one form of 12-month follow-up. Poor neurologic prognoses formulated during the hospital course would have led to withdrawal of life support for some children who might otherwise have survived with severe deficits. Detailed cognitive and neurologic outcomes among survivors who did not participate in 12-month follow-up are unknown. However, participation in the follow-up cognitive and neurologic examinations was similar for the ECPR, later ECMO and no ECMO groups: for ECPR 44/57 and 47/57; for later-ECMO 11/14 and 12/14; and for no-ECMO 44/56 and 45/56. Another limitation is our inability to explore age-related differences in cognitive and neurologic outcomes due to the small number of participants ≥6 years old.
CONCLUSION
We conclude that many paediatric ECPR survivors have favourable cognitive and neurologic outcomes one year after in-hospital cardiac arrest. However, impairments are common and the range of deficits is wide. Overall, functional outcomes for our cohort of ECPR survivors were shifted below population norms. ECPR survivors appear to have similar outcomes to other in-hospital cardiac arrest survivors who are comatose in the early post-arrest period.
Supplementary Material
ACKNOWLEDGEMENTS
Hypothermia after Paediatric Cardiac Arrest (THAPCA) trial investigators participated in this study: Frank W. Moler, MD, C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor; Kathleen L. Meert, MD, Children’s Hospital of Michigan, Detroit; Jamie S. Hutchinson, MD, Hospital for Sick Children, Toronto, Ontario, Canada; Christopher J. L. Newth, MD, Children’s Hospital Los Angeles, Los Angeles, California; Kimberly S. Bennett, MD, MPH, Primary Children’s Hospital, Salt Lake City, Utah; John T. Berger, MD, Children’s National Medical Centre, Washington, DC; Alexis A. Topjian, MD, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Jose A. Pineda, MD, Washington University, St Louis, Missouri; Joshua D. Koch, MD, Children’s Medical Centre Dallas, University of Texas Southwestern Medical School; Charles L. Schleien, MD, MBA, Morgan Stanley Children’s Hospital–Columbia University Medical Centre, New York, New York; Heidi J. Dalton, MD, Phoenix Children’s Hospital, Phoenix, Arizona; George Ofori-Amanfo, MB, ChB, Duke Children’s Hospital, Durham, North Carolina; Denise M. Goodman, MD, Anne and Robert Lurie Children’s Hospital of Chicago, Chicago, Illinois; Ericka L. Fink, MD, University of Pittsburgh Medical Centre, Pittsburgh, Pennsylvania; Patrick McQuillen, MD, University of California, San Francisco Benioff Children’s Hospital; Jerry J. Zimmerman, MD, PhD, Seattle Children’s Hospital, Seattle, Washington; Neal J. Thomas, MD, Penn State Children’s Hospital, Hershey, Pennsylvania; Elise W. van der Jagt, MD, MPH, University of Rochester Medical Centre/Golisano Children’s Hospital, Rochester, New York; Melissa B. Porter, MD, Kosair Charities Paediatric Clinical Research Unit, Department of Paediatrics, University of Louisville and the Kosair Children’s Hospital, Louisville, Kentucky; Michael T. Meyer, MD, Medical College of Wisconsin, Milwaukee; Rick Harrison, MD, Mattel Children’s Hospital UCLA (University of California, Los Angeles); Nga Pham, MD, Children’s Healthcare of Atlanta, Atlanta, Georgia; Adam J. Schwarz, MD, Children’s Hospital of Orange County, Orange, California; Jeffrey E. Nowak, MD, Children’s Hospitals and Clinics of Minnesota, Minneapolis; Jeffrey Alten, MD, The Children’s Hospital of Alabama, Birmingham; Derek S. Wheeler, MD, Cincinnati Children’s Hospital, Cincinnati, Ohio; Utpal S. Bhalala, MD, Johns Hopkins Children’s Centre, Baltimore, Maryland; Karen Lidsky, MD, Rainbow Babies and Children’s Hospital, Cleveland, Ohio; Eric Lloyd, MD, Nationwide Children’s Hospital, Columbus, Ohio; Mudit Mathur, MD, Loma Linda University Children’s Hospital, Loma Linda, California; Samir Shah, MD, University of Tennessee Health Science Centre, Memphis; Theodore Wu, MD, University of Texas Health Sciences Centre at San Antonio; Andreas A. Theodorou, MD, Diamond Children’s Medical Centre, Tucson, Arizona; Ronald C. Sanders Jr, MD, Arkansas Children’s Hospital, Little Rock; Faye S. Silverstein, MD, C. S. Mott Children’s Hospital, University of Michigan, Ann Arbor; James R. Christensen, MD, and Beth S. Slomine, PhD, Outcome Centre, Kennedy Krieger Institute, and Johns Hopkins University, School of Medicine, Baltimore, Maryland; Victoria L. Pemberton, RNC, MS, National Heart, Lung, and Blood Institute, Bethesda, Maryland; and Brittan Browning, MS, RD, CCRC, Richard Holubkov, PhD, and J. Michael Dean, MD, MBA, Data Coordinating Centre, University of Utah, Salt Lake City.
ROLE OF FUNDING SOURCE
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Primary support for the conduct of the THAPCA-IH Trial was funding from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute, Bethesda, MD. HL094345 (FWM) and HL094339 (JMD). Additional support from the following federal planning grants contributed to the planning of the THAPCA Trials: NIH, Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), Bethesda, MD. HD044955 (FWM) and HD050531 (FWM). In part support was obtained from the participation of the following research networks: Paediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Paediatric Critical Care Research Network (CPCCRN) from cooperative agreements (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934. At several centres (as indicated below), clinical research support was supplemented by the following grants or Cooperative Agreements: UL1 RR 024986, UL1 TR 000433, U54 HD087011, UL1TR000003, and P30HD040677. The National Emergency Medical Services for Children (EMSC) Data Analysis Resource Centre Demonstration grant U07MC09174 provided for educational study materials.
Contributor Information
Kathleen Meert, Children’s Hospital of Michigan Wayne State University 3901 Beaubien Boulevard Detroit, MI 48201, USA.
Beth S. Slomine, Kennedy Krieger Institute Johns Hopkins University 707 North Broadway Baltimore, MD 21205, USA
Faye S. Silverstein, University of Michigan Room 8301 MSRB3 Ann Arbor, MI 48109-5646, USA
James Christensen, Kennedy Krieger Institute Johns Hopkins University 707 North Broadway Baltimore, MD 21205, USA.
Rebecca Ichord, Children’s Hospital of Philadelphia University of Pennsylvania 3410 Civic Center Boulevard Philadelphia, PA 19104, USA.
Russell Telford, University of Utah 295 Chipeta Way P. O. Box 581289 Salt Lake City, UT 84158, USA.
Richard Holubkov, University of Utah 295 Chipeta Way P. O. Box 581289 Salt Lake City, UT 84158, USA.
J. Michael Dean, University of Utah 295 Chipeta Way P. O. Box 581289 Salt Lake City, UT 84158, USA
Frank W. Moler, Mott Children’s Hospital University of Michigan 1500 East Hospital Drive Ann Arbor, MI 48109-5636, USA for the Therapeutic Hypothermia after Paediatric Cardiac Arrest (THAPCA) Trial Investigators
REFERENCES
- 1.Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, et al. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasa JJ, Rogers RS, Localio R, Shults J, Raymond T, Gaies M, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge. A report from the Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation 2016;133:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meert KL, Guerguerian AM, Barbaro R, Slomine BS, Christensen JR, Berger J, et al. Extracorporeal cardiopulmonary resuscitation: One-year survival and neurobehavioral outcome among infants and children with in-hospital cardiac arrest. Crit Care Med 2018; doi: 10.1097/CCM.0000000000003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra GG, Zorzela L, Robertson CMT, Alton GY, Joffe AR, Moez EK, et al. Survival and neurocognitive outcomes in pediatric extracorporeal-cardiopulmonary resuscitation. Resuscitation 2015;96:208–13. [DOI] [PubMed] [Google Scholar]
- 5.Beshish AG, Baginski MR, Johnson TJ, Deatrick BK, Barbaro RP, Owens GE. Functional status change among children with extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in a pediatric cardiac ICU: A single center report. Pediatr Crit Care Med 2018;19:665–71. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg MJ, Geri G, Wiberg S, Guerguerian AM, Donnino MW, Nolan JP, et al. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: A systematic review. Resuscitation 2018; 131:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med 2017;376:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales 2nd ed. Minneapolis, MN: Pearson Assessment; 2005. [Google Scholar]
- 9.Moler FW, Silverstein FS, Meert KL, Clark AE, Holubkov R, Browning B, et al. Rationale, timeline, study design, and protocol overview of the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Pediatr Crit Care Med 2013;14:e304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holubkov R, Clark AE, Moler FW, Slomine BS, Christensen JR, Silverstein FS, et al. Efficacy outcome selection in the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Pediatr Crit Care Med 2015;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2(7872):81–4. [DOI] [PubMed] [Google Scholar]
- 12.Conrad SA, Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, et al. The Extracorporeal Life Support Organization Maastricht Treaty for nomenclature in extracorporeal life support. A position paper of the Extracorporeal Life Support organization. Am J Respir Crit Care Med 2018;198:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004;63:233–49. [DOI] [PubMed] [Google Scholar]
- 14.Mullen EM. Mullen Scales of Early Learning Circle Pine, MN: American Guidance; 1995. [Google Scholar]
- 15.Wechsler D Wechsler Abbreviated Scale of Intelligence Psychological Corporation; 1999. [Google Scholar]
- 16.Ichord R, Silverstein FS, Slomine BS, Telford R, Christensen J, Holubkov R, et al. Neurologic outcomes in pediatric cardiac arrest survivors enrolled in the THAPCA trials. Neurology 2018;91:e123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchen L, Westmacott R, Friefeld S, MacGregor D, Curtis R, Allen A, et al. The Pediatric Stroke Outcome Measure: A validation and reliability study. Stroke 2012;43:1602–8. [DOI] [PubMed] [Google Scholar]
- 18.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68–74 [DOI] [PubMed] [Google Scholar]
- 19.Slomine BS, Silverstein FS, Christensen JR, Holubkov R, Telford R, Dean JM, et al. Neurobehavioural outcomes in children after in-hospital cardiac arrest. Resuscitation 2018;124:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slomine BS, Silverstein FS, Christensen JR, Holubkov R, Page K, Dean JM, et al. Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics 2016;137:e20153412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack MM, Holubkov R, Glass, Dean JM, Meert KL, Zimmerman J, et al. Functional Status Scale: New pediatric outcome measure. Pediatrics 2009;124:e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


