Abstract
Limited studies have reported on outcomes for lymphoid malignancy patients receiving alternative donor allogeneic stem cell transplants. We have previously described combining CD34-selected haploidentical grafts with umbilical cord blood (haplo-cord) to accelerate neutrophil and platelet engraftment. Here, we examine the outcome of patients with lymphoid malignancies undergoing haplo-cord transplantation at the University of Chicago and Weill Cornell Medical College.
We analyzed 42 lymphoma and CLL patients who underwent haplo-cord allogeneic stem cell transplantation. Patients underwent transplant for Hodgkin’s lymphoma (n=9, 21%), CLL (n=5, 12%) and non-Hodgkin lymphomas (n=28, 67%), including 13 T-cell lymphoma. Twenty four patients (52%) had 3 or more lines of therapies. Six (14%) and one (2%) patients had prior autologous and allogeneic stem cell transplant, respectively. At the time of transplant, 12 patients (29%) were in complete remission (CR), 18 had chemotherapy-sensitive disease and 12 patients had chemotherapy-resistant disease. Seven (17%), 11 (26%) and 24 (57%) patients had low, intermediate and high disease risk index (DRI) prior to transplant. Comorbidity index was evenly distributed among 3 groups with 13 (31%), 14 (33%) and 15(36%) patients scored 0, 1–2 and ≥3. Median age for the cohort was 49 years (23–71). All patients received fludarabine/melphalan/ATG conditioning regimen and post-transplant GVHD prophylaxis with tacrolimus and mycophenolate mofetil (MMF). The median time to neutrophil engraftment was 11 days (9–60) and to platelet engraftment was 19.5 days (11–88).
Cumulative Incidence of non-relapse mortality (NRM) was 11.6% at 100 days and 19 % at one year. Cumulative incidence of relapse was 9.3% at 100 days and 19% at one year. With a median follow up of survivors of 42 months, the three-year GVHD/Progression Free Survival (GPFS), progression free survival (PFS) and overall survival (OS) was 53%, 62%, and 65% respectively for these patients. Only 8% of the survivors have chronic GVHD.
In conclusion, haplo-cord transplantation offers a transplant alternative for patients with recurrent or refractory lymphoid malignancies who lack matching donors. Both neutrophil and platelet count recovery is rapid, non-relapse mortality is limited, excellent disease control can be achieved and the incidence of chronic GVHD is limited. Thus, haplo-cord achieves high rates of engraftment and encouraging results.
Introduction
Recent therapeutic advances resulted in progressively better outcomes for patients with both Hodgkin and non-Hodgkin lymphomas, including patients with high-risk features,1, Still, a minority of patients are refractory or may not qualify for an autologous stem cell transplant. Allogeneic stem cell transplantation (SCT) is increasingly used for such high-risk patients.
With improvement in pre-transplant evaluation, use of reduced intensity conditioning regimens and better supportive care, allogeneic SCT has been offered to an older patient population that was previously excluded from such treatment.2, 3,4 For patients who may benefit from allogeneic SCT, but lack suitable HLA-matched sibling or unrelated donors, umbilical cord blood (UCB) is an alternative graft source. Several studies showed similar progression free survival (PFS) and overall survival (OS) to sibling- or unrelated donor transplant, but the delayed neutrophil and platelet recovery associated with cord blood transplant results in prolonged hospitalization, higher costs, increased morbidity and early mortality.5, 6,7,8,9 To improve the neutrophil and platelet engraftment and take advantage of the low incidence of graft-versus-host-disease (GVHD), we and others previously reported combining haploidentical donor grafts with cord blood stem cells.10–14 The haploidentical graft provides a “myeloid bridge” that allows for rapid neutrophil and platelet recovery and that –with rare exceptions15 - is eventually replaced by the cord blood cells. We and other have previously reported on patterns of chimerism,14, 16–18 its consequences and of immune reconstitution19, 20 after this procedure. In a comparative study with double cord transplantation, we have shown improved count recovery and improved GVHD and relapse-free survival.21 In comparison with haplo-identical transplant with post-transplant cyclophosphamide, Kwon et al reported that haplo-cord transplant had faster neutrophil recovery, similar survival rates and lower rates of acute and chronic GVHD.22, 23 Here we report our experience with haplo-cord transplant for high-risk or relapsed and/or refractory lymphoma/CLL patients.
Material and methods
Study design and patient population
Outcomes of all consecutive patients with lymphoma or CLL, treated on the haplo-cord protocols conducted at the University of Chicago between 2007 to Jan 2016 and at Weill Cornell Medical College between 2012 and 2016 were reviewed.14 Details have been previously reported.10 Briefly, all patients received fludarabine 30mg/m2 IV from days −7 through −3, melphalan 140mg/m2 IV on day −2 and rabbit anti-thymocyte globulin 1.5mg/kg on days −5, −3 and −1 as the conditioning regimen. Some early patients received an additional dose of ATG on day −7. Tacrolimus and mycophenolate mofetil (MMF 1g TID) were used for GVHD prophylaxis. Tacrolimus dose was adjusted to maintain plasma level between 5 – 15 ng/ml until day 180 before being tapered off when appropriate. MMF was given until day 28 (or until day 60 for the early patients). Some patients at Weill Cornell Medical Center also received 400cG TBI as part of conditioning regimen.24 One patient with T-cell lymphoma relapsed and received a second haplo-cord transplant two years after the initial one. A different conditioning regimen was used and data on the second transplant are not included.
For haploidentical donors, relatives were preferred. When there were no suitable relatives, HLA-haploidentical unrelated donors were used.25 G-CSF was used for stem cell mobilization. For the haploidentical grafts, CD34+- cell selection was performed, using the Miltenyi cliniMACS® CD34 Reagent System (Miltenyi, Germany) device, attaining less than 1 × 104 CD3+ T cells/kg of the recipient body weight. Prior to April 2010, Isolex 300i device was used for the CD34 cell selection. For umbilical cord blood units, a minimum requirement for HLA matching was 4/8 –using high resolution HLA- typing for HLA-A,B,C and DR, and minimum cell dose was 1.2 × 107/kg total nucleated blood cells (TNC). We prioritized the cord blood HLA-match over the nucleated cell dose as long as CBU dose exceeded 1.2 ×107/kg TNC at the time of cryopreservation.
Chimerism
Chimerism reported as percentage recipient and donor was analyzed using short tandem repeat (STR) analysis using the PowerPlex 16 HS System (Promega, Madison, WI). Multiplex PCR reaction that amplifies 16 STR loci of the recipient- and donor-specific loci were analyzed.26 The sensitivity of the chimerism test was 1%. Both peripheral blood and bone marrow chimerism studies were included.
Definitions
Neutrophil engraftment was defined as the time from the date of stem cell infusion to the first of 3 consecutive days with an absolute neutrophil count of 0.5×109 per liter or higher. Platelet engraftment was defined as the time from the date of stem cell infusion to the first of 7 consecutive days with a platelet count of 20×109 per liter or higher without platelet transfusion. Acute GVHD and chronic GVHD were diagnosed and graded according to consensus criteria.27 Non-relapse mortality (NRM) is defined as death without evidence of relapse/progression of malignancy. Progression Free Survival is defined as the time from transplant until disease progression, whichever happens first. Overall survival is defined as the time from transplant until death.
Statistical methods
Probabilities of TRM, relapse, acute and chronic GVHD, neutrophil recovery and platelet recovery were generated using cumulative incidence estimates to accommodate competing risks. Cumulative incidence of relapse of the original disease, non-relapse mortality and GVHD were calculated using R statistics (http://cran.R-project.org).28 Cmprsk package was used to perform subdistribution analysis of competing risk and CumIncidence R functions were used for the cumulative incidence curve fit.
Probability of OS and PFS were calculated using the Kaplan-Meier estimator, with the variance estimated by Greenwood’s formula. GraphPad Prism 7 was used to create survival curves using the method of Kaplan and Meier and calculates the 95% confidence interval for fractional survival at any particular time. For PFS, subjects were considered treatment failures at the time of relapse or progression or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. Similarly the probability of GVHD-free and relapse free survival (GRFS) was summarized by defining events to include grade 3–4 acute GVHD, any cGVHD required systemic therapy, relapse, or death.
Results
Patient Characteristics
Forty-two patients with lymphoid malignancies underwent haplo-cord transplantation at the University of Chicago or at Weill Cornell Medical Center. Patient characteristics are detailed in Table 1. The median age was 49 years (23–71). Minorities accounted for 38% (n=16) of our patient population. T-cell lymphomas (n=13; 31%) represented the largest group of lymphoma patients, followed by Hodgkin lymphoma (n=9; 21%).
Table 1.
Patient Characteristics
| Characteristics | ||
|---|---|---|
| Number of Patients | N=42 | |
| Median Age | 49yr (22–71) | |
| Gender | male | 28 (67%) |
| female | 14 (33%) | |
| Ethnicity | Caucasian | 26 (62%) |
| Black | 8 (19%) | |
| Hispanic | 6 (14%) | |
| Asian | 2 (5%) | |
| Lymphoma Histology | T cell lymphoma† | 13 (31%) |
| Hodgkin lymphoma | 9 (21%) | |
| CLL | 5 (12%) | |
| Mantle cell lymphoma | 5 (12%) | |
| DLBCL | 7 (17%) | |
| Follicular lymphoma | 3 (7%) | |
| Previous autologous transplant | 6 (14%) | |
| Previous therapy | 1 regimen | 2 |
| 2 regimens | 16 | |
| 3 regimens | 14 | |
| >3 regimens (4–10) | 10 | |
| Disease Status prior to transplant | Complete remission
|
12 (29%) 6 (at least 2 lines, except 1 pt) 4 2 |
| Partial remission (PR) | 2 (5%) | |
| Relapse -sensitive | 4 (9%) | |
| Relapse - resistant | 6 (14%) | |
| Primary induction failure (PIF)-sensitive | 11 (26%) | |
| Primary induction failure (PIF)-resistant | 5 (12%) | |
| No response/stable disease | 2 (5%) | |
| HTC-CI | 0 | 13 (31%) |
| 1–2 | 14 (33%) | |
| ≥3 | 15 (36%) | |
| DRI | low | 7 (17%) |
| Intermediate | 11 (26%) | |
| High | 20 (48%) | |
| Very High | 4 (9%) | |
| Conditioning regimen | Flu/Mel/ATG | 34 (81%) |
| Flu/Mel/ATG + radiation | 8 (19%) | |
| Cord blood HLA match | 4/8 | 2 (5%) |
| 5/8 | 15 (36%) | |
| 6/8 | 14 (33%) | |
| 7/8 | 9 (21%) | |
| 8/8 | 2 (5%) | |
| Haploidentical donor | sibling | 19 (45%) |
| children | 13 (31%) | |
| parents | 8 (19%) | |
| unrelated | 1 (2%) | |
| Cell dose | ||
| Haploidentical graft | CD34+ cells, × 106/kg | 4.99 × 106 (1.51– 5.97) |
| T cells in the selected CD34+ | 0.029 × 104/kg (0.0 –1.7) | |
| Cord blood graft | TNC cells, × 106/kg | 18.5 × 106/kg (10.5 – 46.95) |
| CD34+ cells, × 105/kg | 0.615 × 105 (0.2 – 5.8) | |
| CMV status | ||
| Recipient | positive | 30 (71%) |
| negative | 12 (29%) | |
| D¥/R* (+/+) | 8 (19%) | |
| D/R(±/-) | 10 (24%) | |
| D/R (±/+) | 22 (54%) | |
| D/R (−/−) | 2 (5%) |
T cell lymphomas included cutaneous Peripheral T cell lymphoma (n=6), hepatosplenic lymphoma (n=4), angioimmunoblastic lymphoma (n=2), ALK+ anaplastic T cell lymphoma (n=2 ) and T-PLL(n=1).
haplo-identical donor and cord blood donor;
R: recipient
At the time of haplo-cord transplantation, 12 patients (29%) were in complete remission (CR), including 6 CR1, 4 CR2 and 2 CR3. With the exception of 1 patient, 5 of the 6 patients achieved CR1 only after at least 2 lines of therapies. The remaining 30 patients (71%) had active disease, including one with T-cell lymphoma, who was transplanted in partial remission after initial treatment. Among the latter group, 12 patients were considered chemotherapy-resistant, which included 9 patients with refractory disease upon relapse and 3 patients with primary induction failure – no response or stable disease; and 18 had chemotherapy-sensitive disease. Six (14%) of the 42 patients had undergone a prior autologous stem cell transplant.
Twenty-four (57%) patients had a high/very-high-risk disease risk index (DRI).29 Eleven (26%) and 7 (17%) patients had an intermediate and low-risk DRI respectively. The seven patients with low-risk DRI comprised 3 CLL patients in CR (n=1) and PR (n=2) after multiple therapies, 2 mantle cell lymphoma patients in CR2, 1 Hodgkin lymphoma patient in CR3+ and a grade-3a follicular lymphoma patient in CR2.
Donor characteristics
Sixty nine percent (n=29) of the cord blood units infused were either 5/8 or 6/8 HLA-matched. The rest of the cord grafts were 7/8 (21% n=9) and 8/8 HLA-matched (5%, n=2). Only two patients were 4/8 matched (Table 1). The median number of cord TNC infused was 18.5 × 106/kg (11.2–46.9) and median CD34+ stem cell number infused was 0.61 × 105/kg (0.02–5.8).
The most common haplo-identical donors were siblings, followed by children and parents. The median number of haplo-identical CD34+ donor cells infused were 4.9 × 106/kg (1.5–6.0). The median T-cell dose infused after depletion was 0.03 × 104/kg (0.0–1.7) (Table 1).
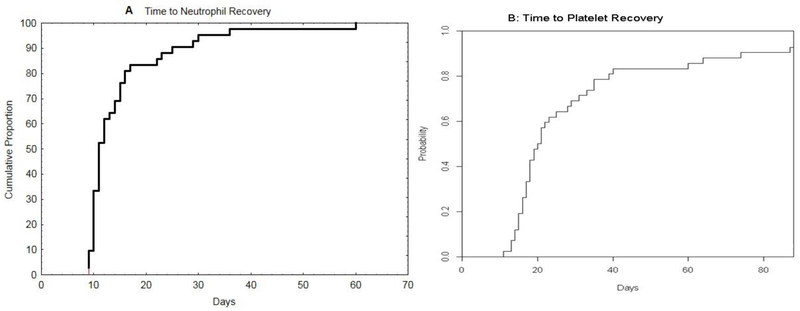
Engraftment
The median time to neutrophil engraftment was 11 days (9–60). The median time to platelet engraftment was 19.5 days (11–88). By day 30, 95% and 74% patients had neutrophil and platelet engraftment. Neutrophil and platelet engraftments were 100% and 91% respectively by day 60. (Figure 1). Three patients with failure of the haplo-bridge were among those with delayed recovery of neutrophils and platelets. One patient, after initial neutrophil recovery on day 36 and evidence of CBU engraftment, died from graft failure on day 48. Day 100 CD3 chimerism data are available for 31 of the 32 patients who were alive and disease-free by day 100 and is summarized in Table 2. CBU chimerism accounted for all, or the large majority of CD3 cells in 21 (67%). Mixed patterns were present in 7 (22%). One patient had haplo-identical engraftment without evidence of CBU engraftment. This patient, with Hodgkin’s lymphoma continues to do well, now three years after transplant. And one HIV positive patient had autologous reconstitution – he later relapsed. (see below).
Figure 1:
Cumulative Incidence of Neutrophil Recovery ( A) and of Platelet Recovery (B)
Table 2.
Day 100 Chimerism
| CD3 | N=31* |
|---|---|
| 100% CBU | 17 |
| CBU >80% | 4 |
| CBU <80% + Haplo | 2 |
| CBU <80% + Host | 1 |
| CBU <80% + Haplo + Host | 4 |
| Haplo 100% | 1+ |
| Host 100% | 1++ |
| CD33 | N= 20** |
| 100% CBU | 6 |
| CBU >90% | 3 |
| CBU <90% + haplo | 6 |
| CBU<90%+ haplo + host | 3 |
| Haplo 100% | 2+ |
| Host 100% | 1++ |
32 patients alive and in remission on day 100 – data available on 31
Data only for WCMC patients ‒20 alive and in remission on day 100
one patient with failure of sustained engraftment of CBU unit and one patient with myeloid CBU engraftment, but no T-lymphoid engraftment.
one patient with autologous count recovery.
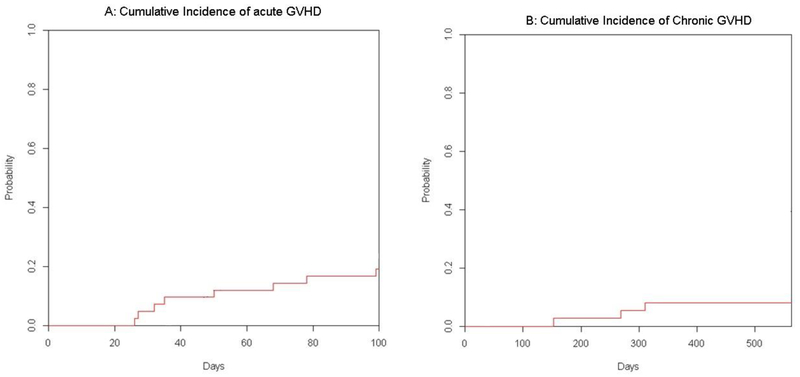
Graft vs host disease
Nine patients developed acute GVHD, including five with grade I/II and four with grade III/IV aGVHD. There were three cases of skin- and six of GI-GVHD. The median time to the development of acute GVHD was 50 days (26–130). The cumulative incidence of acute GVHD at day 100 was 20% % (Figure 2A ). Four patients developed mild (n=3) or moderate (n=1) chronic skin GVHD, only two of whom required systemic treatment. The cumulative incidence of chronic GVHD at 1-year was 8% (Figure 2 B).
Figure 2.
Cumulative Incidence of Acute (A) and of Chronic (B) Graft versus Host Disease.
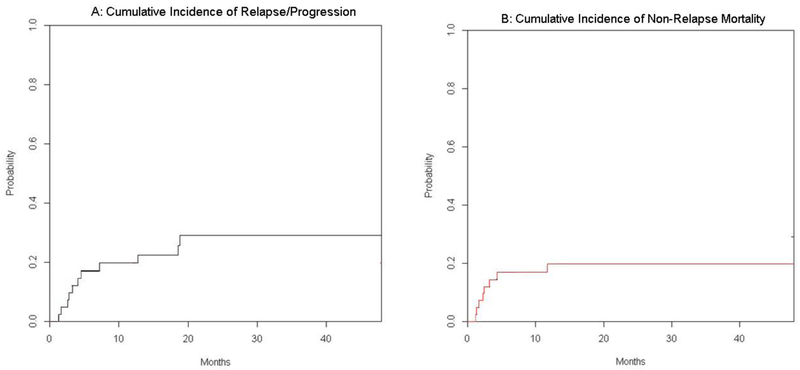
Relapse, Non-Relapse mortality and infectious complications.
Twelve patients had disease progression or relapse post-transplant. Median time to progression was 112 days. Nine of the 12 patients had chemo-resistant disease at the time of transplant. The cumulative incidence of relapse was 12% at day 100 and 19.5% at 1 year (Figure 3A).
Figure 3.
Cumulative Incidence of Relapse/Progression (A) and of Non-relapse Mortality (B).
Thirteen patients have died at the time of analysis, with 6 patients dying from progressive disease and 7 from non-relapse causes, including GVHD (1), PTLD (1), disseminated adenovirus (1), renal failure (1), atypical hemolytic uremic syndrome (1), non-infectious pulmonary syndrome (1), Sinusoidal obstruction syndrome (SOS/VOD) (1). The cumulative incidence of non-relapse mortality was 14 % at day 100 and 19% at one year (Figure 3).
The rate of EBV reactivation was 31% (n=13). Ten of the thirteen patients received rituximab treatments. Two of these patients also received chemotherapy and EBV-directed T cell therapy. One death was attributed to PTLD. The rate of CMV reactivation was 36% (n=15) with no deaths attributed to CMV
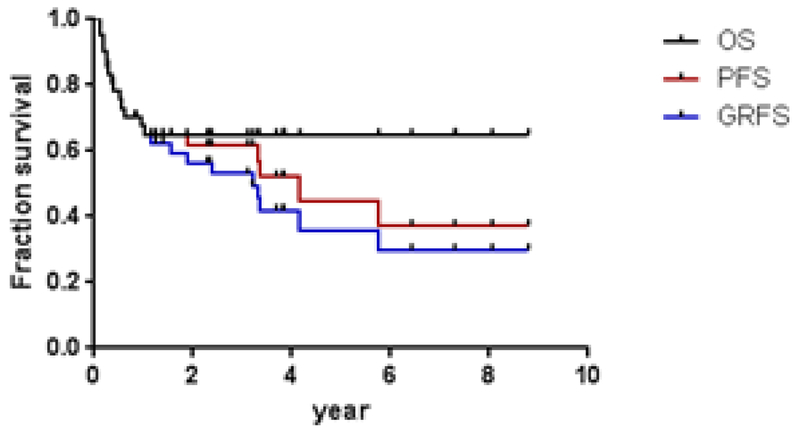
Survival
With a median survivor follow-up of 42 months, the estimated 3-yr GRFS, PFS and OS were, 53% (95% CI 36–68), 62% (95% CI 44–75) and 65% (95% CI 48–78) (Figure 4). The outcomes vary somewhat depending on subtype of lymphoma. All four patients with CLL/SLL and all three patients with follicular lymphoma remain alive and in remission. Of nine patients with Hodgkin’s lymphoma, six remain alive and in remission. One died of PTLD, one patient with Hodgkin’s lymphoma and HIV failed to engraft, had autologous count recovery and died of progressive disease and one is alive with disease. Three of six patients with mantle cell lymphoma remain in remission. Three died of disease recurrence. Two patients with DLBCL remain in remission. Two died of disease recurrence and two of complications. Of thirteen patients with T-cell lymphoma, five remain in remission, four have died of complications and four have relapsed.
Figure 4. Overall Survival and Progression Free Survival (PFS) and GVHD/Progression Free Survival (GPFS) for all lymphoid malignancy patients.
Three-year GPFS, PFS and OS was 53% (95% CI 36–68), 62% (95% CI 44–75) and 65% (95% CI 48–78). (N=42)
Disease risk index was associated with outcome. Three year survival for patients with low, intermediate, high and very high risk DRI was 83%, 72%,61% and 25% respectively (PP=0.03) Gender, older age, CBU dose, CBU match or haplo-graft match were not associated with outcome.
Discussion
Allogeneic transplantation is assuming a larger role in the management of advanced lymphomas. Reported results with reduced intensity conditioning and matched related or unrelated donor transplant show 3 year PFS and OS of 33–51% and 43–58%, respectively.30, 31 For those lacking HLA identical unrelated donors, cord blood transplantation has been employed.9, 32–35,36–38 In the two largest studies, from EBMTR and CIBMTR respectively, outcomes were comparable to those of unrelated donor transplantation.33, 34 Cord blood transplant thus represents a good alternative option for patients without a HLA-matched donor donors, which does not compromise disease control, and has a low incidence of GVHD. Its main drawback remains the unpredictable and often prolonged time to blood cell count recovery, which adds morbidity and expense. By supporting a single cord blood graft with third party CD34-selected progenitors for lymphoma patients, we demonstrated accelerated engraftment, with a median time to neutrophil and platelet engraftment of 11 and 20 days, respectively. By day 30, 95% and 76% of the patients achieved neutrophil and platelet engraftment respectively. The patterns of chimerism by day 100, are similar to what has been previously described by our group and others.15, 20, 39 The cumulative incidence of grade II/IV acute GVHD at day 100 was 9.3% and chronic GVHD at 2 years was only 8%. The incidence of both acute and chronic GVHD is lower than that of double cord blood transplant, as we have previously shown.21 This may be due to ex vivo T cell depletion of the haplo-identical donors, the use of ATG in conditioning regimen, and prioritization of HLA match over the cell dose for cord blood grafts. CMV reactivation, though frequent, was readily controlled and no cases of CMV disease were observed. EBV reactivation was more problematic, occurring in thirty percent of patients and in part related to our use of ATG, required for success of the haplo-myeloid bridge.17 Without it, early rejection of the haplo-identical graft and a second nadir commonly ensues. The use of ATG in double cord blood transplantation has become controversial in cord blood transplantation, because its association with EBV reactivation and in some studies with increased mortality.40, 41 But in a recent large registry analysis, it was associated with reduced incidence of GVHD and had no detrimental impact on long-term outcomes.42 The differences in outcomes may be attributable in part to differences in ATG dosing and formulation.43 With rigorous monitoring and early intervention with rituximab, we too found that fatal PTLD could be mostly prevented. Only two patients required additional treatments beyond rituximab and PTLD was fatal in only one patient.44 Overall, the benefits of a successful myeloid bridge, rapid engraftment and extremely low rates of GVD, outweigh the risk of EBV reactivation.21 Nevertheless, further reduction in EBV reactivation is an aim of ongoing studies and we have partially addressed this by reducing the dose of ATG from 6 mg/kg to 4.5 mg/kg and by pre-transplant administration of rituximab.45
Our cohort included nine heavily pre-treated patients with Hodgkin’s lymphoma. The majority of them had at least 3 lines of prior treatment, but rates of disease recurrence were quite low and they reached 1-year PFS of 73% and OS of 86%, suggesting they may uniquely benefit from this approach. Similarly the small group of patients with advanced follicular lymphoma or CLL/SLL had excellent outcomes. The largest cohort consisted of patients with relapsed and refractory peripheral T-cell lymphoma - a heterogeneous group of patients with poor prognosis.46 Their 1-year PFS and OS was 49% and 64%.
Despite low incidence of GVHD, rates of long-term disease control were excellent. Fifty five percent of our patients had high and very high-risk disease by DRI.29 With a median follow-up of 42 months for survivors, 3 year GPFS, PFS and OS were 53%, 62% and 65% respectively. Although our sample size is small, this compares favorably to both Eurocord/EBMT32 and CIBMTR34 studies, which showed estimated 1 year PFS and OS around 40–44% and 48–54% in similar patient populations.
For patients lacking HLA matched donors, haplo-identical transplant has also emerged as a promising alternative, often utilizing post-transplant cyclophosphamide. The latter procedure is readily implemented and has lower graft acquisition costs. Castagna et recently showed that the 3-year OS, PFS, relapse rates and 1-year non-relapse mortality (NRM) were 63%, 59%, 21% and 20%, respectively for advanced Hodgkin lymphoma patients who underwent haplo-identical transplant.47 Incidence of GVHD, time to count recovery and rates of graft rejection and of disease control were in the same range, but need to be compared to our outcomes in larger patient groups.47,48 Similar results were also reported by Brammer et al, in 22 patients with non-Hodgkin’s lymphoma.49
In conclusion, combined haplo-identical-cord transplant provides rapid neutrophil and platelet engraftment, low incidence of GVHD, and offers excellent disease control for patients with high-risk or relapsed/refractory lymphoid malignancies. It appears superior to double cord blood transplantation in time to engraftment and incidence of GVHD and has similar to possibly superior long-term outcomes.21 Also, in our experience, approximately 25% of adult patients lack suitable first or second degree relatives. For them, haplo-identical transplantation is not an option, but substitution of the haplo-identical donor by CD34 cells from mismatched unrelated donor of other third party donors, allows haplo-cord transplantation to be a near universal donor source.48
Acknowledgments
Jingmei Hsu is supported by Grant from Empire Clinical Research Investigator Program (ECRIP).
Footnotes
Conflict of Interest and Financial disclosure
Koen Van Besien and Andrew Artz: Research funding from Miltenyi Biotec
References
- 1.Geyer MB, Brentjens RJ. Review: Current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy 2016; 18(11): 1393–1409. doi: 10.1016/j.jcyt.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.William BM, de Lima M. Advances in conditioning regimens for older adults undergoing allogeneic stem cell transplantation to treat hematologic malignancies. Drugs Aging 2013; 30(6): 373–381. doi: 10.1007/s40266-013-0076-x [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc Hematol Educ Program 2016; 2016(1): 366–378. doi: 10.1182/asheducation-2016.1.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagna DBaL. Do different conditioning regimens really make a difference? Hematology Am Soc Hematol Educ Program. 2012: 237–245. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010; 11(7): 653–660. doi: 10.1016/S1470-2045(10)70127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004; 351(22): 2276–2285. doi: 10.1056/NEJMoa041469 [DOI] [PubMed] [Google Scholar]
- 7.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004; 351(22): 2265–2275. doi: 10.1056/NEJMoa041276 [DOI] [PubMed] [Google Scholar]
- 8.Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant 2012; 18(5): 805–812. doi: 10.1016/j.bbmt.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 9.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011; 118(2): 282–288. doi: 10.1182/blood-2011-03-344853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood 2011; 118(24): 6438–6445. doi: 10.1182/blood-2011-08-372508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez MN, Regidor C, Cabrera R, Garcia-Marco JA, Fores R, Sanjuan I et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol 2003; 31(6): 535–544. [DOI] [PubMed] [Google Scholar]
- 12.Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Serrano D, Anguita J et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant 2014; 20(12): 2015–2022. doi: 10.1016/j.bbmt.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 13.Kwon M, Balsalobre P, Serrano D, Perez Corral A, Buno I, Anguita J et al. Single cord blood combined with HLA-mismatched third party donor cells: comparable results to matched unrelated donor transplantation in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant 2013; 19(1): 143–149. doi: 10.1016/j.bbmt.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 14.van Besien K, Childs R. Haploidentical cord transplantation-The best of both worlds. Semin Hematol 2016; 53(4): 257–266. doi: 10.1053/j.seminhematol.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 15.Tsai SB, Liu H, Shore T, Fan Y, Bishop M, Cushing MM et al. Frequency and Risk Factors Associated with Cord Graft Failure (CGF) after Transplant with Single Unit Umbilical Cord Cells Supplemented by Haploidentical Cells (Haplo-Cord) with Reduced-Intensity Conditioning . Biol. Blood Marrow Transplant 2016. doi: S1083-8791(16)00115-4 [pii];10.1016/j.bbmt.2016.02.010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemans CA, van Besien K. Topping it up: methods to improve cord blood transplantation outcomes by increasing the number of CD34+ cells. Cytotherapy 2015; 17(6): 723–729. doi: S1465-3249(15)00069-9 [pii];10.1016/j.jcyt.2015.02.005 [doi] [DOI] [PubMed] [Google Scholar]
- 17.Lindemans CA, Te Boome LC, Admiraal R, EC J-vdZ, Wensing AM, Versluijs AB et al. Sufficient Immunosuppression with Thymoglobulin Is Essential for a Successful Haplo-Myeloid Bridge in Haploidentical-Cord Blood Transplantation . Biol. Blood Marrow Transplant 2015; 21(10): 1839–1845. doi: S1083-8791(15)00403-6 [pii];10.1016/j.bbmt.2015.06.001 [doi] [DOI] [PubMed] [Google Scholar]
- 18.van Besien K, Liu H, Jain N, Stock W, Artz A. Umbilical Cord Blood Transplantation Supported by Third-Party Donor Cells: Rationale, Results, and Applications. Biol. Blood Marrow Transplant 2013; 19(5): 682–691. doi: S1083-8791(12)00463-6 [pii];10.1016/j.bbmt.2012.11.001 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain N, Liu H-T, Artz AS, Anastasi J, Odenike O, Godley LA et al. Immune reconstitution after combined haplo-identical and umbilical cord blood transplantation. Leukemia & Lymphoma 2012; 54: 1242–1249. [DOI] [PubMed] [Google Scholar]
- 20.Kwon M, Martinez-Laperche C, Balsalobre P, Serrano D, Anguita J, Gayoso J et al. Early peripheral blood and T-cell chimerism dynamics after umbilical cord blood transplantation supported with haploidentical cells. Bone Marrow Transplant 2013. doi: bmt2013177 [pii];10.1038/bmt.2013.177 [doi] [DOI] [PubMed] [Google Scholar]
- 21.van Besien K, Hari P, Zhang MJ, Liu HT, Stock W, Godley L et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and graft-versus-host disease-free, relapse-free survival. Haematologica 2016; 101(5): 634–643. doi: 10.3324/haematol.2015.138594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Montesinos P, Bermudez A et al. Haplo-Cord transplantation compared to haploidentical transplantation with post-transplant cyclophosphamide in patients with AML. Bone Marrow Transplant 2017; 52(8): 1138–1143. doi: 10.1038/bmt.2017.36 [DOI] [PubMed] [Google Scholar]
- 23.Choe HK, van Besien K. Against the odds: haplo-cord grafts protect from GvHD and relapse. Bone Marrow Transplant 2017. doi: 10.1038/bmt.2017.102 [DOI] [PubMed] [Google Scholar]
- 24.Choe HK, Gergis U, Mayer SA, Nagar H, Phillips AA, Shore TB et al. The Addition of Low-Dose Total Body Irradiation to Fludarabine and Melphalan Conditioning in Haplocord Transplantation for High-Risk Hematological Malignancies. Transplantation 2017; 101(1): e34–e38. doi: 10.1097/TP.0000000000001538 [DOI] [PubMed] [Google Scholar]
- 25.Van Besien KH, JingMei; Gergis, Usama; Phillips, Adrienne; Mayer, Sebastian; Shore, Tsiporah. Cord Blood Transplantation Supported By Unrelated Donor Progenitor Cells for Patients without Haplo Identical Relatives . EBMT 2017; A177. [Google Scholar]
- 26.Rennert H, Leonard DG, Cushing M, Azurin C, Shore T. Avoiding pitfalls in bone marrow engraftment analysis: a case study highlighting the weakness of using buccal cells for determining a patient’s constitutional genotype after hematopoietic stem cell transplantation. Cytotherapy 2013; 15(3): 391–395. doi: 10.1016/j.jcyt.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 27.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015; 21(3): 389–401 e381. doi: 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007; 40(4): 381–387. doi: 1705727 [pii];10.1038/sj.bmt.1705727 [doi] [DOI] [PubMed] [Google Scholar]
- 29.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014; 123(23): 3664–3671. doi: 10.1182/blood-2014-01-552984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armand P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant 2008; 14(4): 418–425. doi: 10.1016/j.bbmt.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraverty R, Mackinnon S. Allogeneic transplantation for lymphoma. J Clin Oncol 2011; 29(14): 1855–1863. doi: 10.1200/JCO.2010.32.8419 [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 2009; 27(2): 256–263. doi: 10.1200/JCO.2007.15.8865 [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues CA, Rocha V, Dreger P, Brunstein C, Sengeloev H, Finke J et al. Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica 2014; 99(2): 370–377. doi: 10.3324/haematol.2013.088997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachanova V, Burns LJ, Wang T, Carreras J, Gale RP, Wiernik PH et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant 2015; 50(2): 197–203. doi: 10.1038/bmt.2014.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010; 116(22): 4693–4699. doi: 10.1182/blood-2010-05-285304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mogul MJ. Unrelated cord blood transplantation vs matched unrelated donor bone marrow transplantation: the risks and benefits of each choice . Bone Marrow Transplant 2000; 25 Suppl 2: S58–60. [DOI] [PubMed] [Google Scholar]
- 37.Messer M, Steinzen A, Vervolgyi E, Lerch C, Richter B, Dreger P et al. Unrelated and alternative donor allogeneic stem cell transplant in patients with relapsed or refractory Hodgkin lymphoma: a systematic review. Leuk Lymphoma 2014; 55(2): 296–306. doi: 10.3109/10428194.2013.802780 [DOI] [PubMed] [Google Scholar]
- 38.Munoz J, Shah N, Rezvani K, Hosing C, Bollard CM, Oran B et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med 2014; 3(12): 1435–1443. doi: 10.5966/sctm.2014-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Besien K, Koshy N, Gergis U, Mayer S, Cushing M, Rennert H et al. Cord Blood Chimerism And Relapse After Haplo-Cord Transplantation. Leuk Lymphoma 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunstein CG, Weisdorf DJ, Defor T, Barker JN, Tolar J, van Burik JA et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006; 108(8): 2874–2880. doi: blood-2006-03-011791 [pii];10.1182/blood-2006-03-011791 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascal L, Tucunduva L, Ruggeri A, Blaise D, Ceballos P, Chevallier P et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood 2015; 126(8): 1027–1032. doi: blood-2014-09-599241 [pii];10.1182/blood-2014-09-599241 [doi] [DOI] [PubMed] [Google Scholar]
- 42.Chen YB, Wang T, Hemmer MT, Brady C, Couriel DR, Alousi A et al. GvHD after umbilical cord blood transplantation for acute leukemia: an analysis of risk factors and effect on outcomes. Bone Marrow Transplant 2017; 52(3): 400–408. doi: 10.1038/bmt.2016.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segovia J, van Besien K. Antithymocyte globulin for graft-versus-host disease prophylaxis: mistakenly maligned. Leuk. Lymphoma 2015: 841–842. doi: 10.3109/10428194.2014.994180 [doi] [DOI] [PubMed] [Google Scholar]
- 44.Bachier L, Shore TB, Gergis U, Hsu J-M, Phillips A, Mayer SA et al. Novel Risk Factors for Epstein-Barr Virus (EBV) reactivation and Post-Transplant Lymphoproliferative Disorder (PTLD) after Allogeneic Hematopoietic Stem Cell Transplant. In: Biology of Blood & Marrow Transplantation, 2017. [Google Scholar]
- 45.Burns DM, Rana S, Martin E, Nagra S, Ward J, Osman H et al. Greatly reduced risk of EBV reactivation in rituximab-experienced recipients of alemtuzumab-conditioned allogeneic HSCT. Bone Marrow Transplant 2016; 51(6): 825–832. doi: 10.1038/bmt.2016.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitz N, Wu HS, Glass B. Allogeneic transplantation in T-cell lymphomas. Semin Hematol 2014; 51(1): 67–72. doi: 10.1053/j.seminhematol.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 47.Castagna L, Bramanti S, Devillier R, Sarina B, Crocchiolo R, Furst S et al. Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone Marrow Transplant 2017; 52(5): 797. doi: 10.1038/bmt.2017.26 [DOI] [PubMed] [Google Scholar]
- 48.Ghosh N, Karmali R, Rocha V, Ahn KW, DiGilio A, Hari PN et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis . J Clin Oncol 2016; 34(26): 3141–3149. doi: 10.1200/JCO.2015.66.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brammer JE, Khouri I, Gaballa S, Anderlini P, Tomuleasa C, Ahmed S et al. Outcomes of Haploidentical Stem Cell Transplantation for Lymphoma with Melphalan-Based Conditioning . Biol Blood Marrow Transplant 2016; 22(3): 493–498. doi: 10.1016/j.bbmt.2015.10.015 [DOI] [PubMed] [Google Scholar]