Abstract
Purpose
Quantification of rotator cuff intramuscular fatty infiltration is important for clinical decision making in patients with rotator cuff tear. The semi-quantitative Goutallier classification system is the most commonly used method, but has limited reliability. Therefore, we sought to test a freely available fuzzy C-means segmentation software program (1) for reliability of quantification of shoulder intramuscular fatty infiltration on T1-weighted MR images and (2) for correlation to fat fraction by 6-point Dixon MRI.
Materials and methods
We performed a prospective cross-sectional study to measure visible intramuscular fat area percentage on oblique sagittal T1 MR images by fuzzy C-means segmentation and fat fraction maps by 6-point Dixon MRI for 42 shoulder muscles. Intra- and inter-observer reliability was determined. Correlative analysis for fuzzy C-means and 6-point Dixon intramuscular fatty infiltration measures was also performed.
Results
We found that inter-observer reliability for quantification of visible intramuscular fat area percentage by fuzzy C-means segmentation and fat fraction by 6-point Dixon MRI was 0.947 and 0.951, respectively. The intra-observer reliability for quantification of visible intramuscular fat area percentage by fuzzy C-means segmentation and fat fraction by 6-point Dixon MRI was 0.871 and 0.979, respectively. We found strong correlation between fuzzy C-means segmentation and 6-point Dixon techniques; r = 0.850, p < 0.001 by individual muscle; and r = 0.977, p < 0.002 by study subject.
Conclusion
Quantification of intramuscular fatty infiltration by fuzzy C-means segmentation on T1-weighted sequences demonstrates excellent reliability and strong correlation to fat fraction by 6-point Dixon MRI. Quantitative fuzzy C-means segmentation is a viable alternative to the semi-quantitative Goutallier classification system.
Keywords: shoulder, rotator cuff, intramuscular fatty infiltration, fuzzy C-means, quantitative, MRI, Dixon
Introduction
Nearly 4.5 million healthcare provider visits occur every year for evaluation of rotator cuff disease in the United States [1]. Rotator cuff (RC) tear is a disease of aging, with 86% of surgical rotator cuff repair (RCR) taking place after the age of 44 years [2]. One out of four adults ≥ 60 years suffers from RC tear in the general population [3]. The prevalence of RC tear is expected to rise with shifting demographics, as the number of older adults in the United States continues to increase.
Shoulder imaging is important in clinical decision making for diagnosis of RC tear and assessment of RC intramuscular fatty infiltration (FI). Many animal and human studies show that FI progression accelerates following RC tear, and that acquired FI does not resolve following RCR [4–8]. Assessment of FI in the setting of RC tear affects clinical decision making, since the presence of 50% or more of FI is a relative contraindication to RCR [9]. In current clinical practice, the semi-quantitative Goutallier classification system is the most often used method to grade FI [9–11]. The Goutallier classification system is graded on a 5-point scale: stage 0, no fat; stage 1, streaks of fat; stage 2, muscle > fat; stage 3, muscle = fat; stage 4, muscle < fat. However, the Goutallier classification system has limitations, despite its ease of use. At best, this subjective grading scheme shows only moderate inter-observer reliability [12]. There is a strong need for reliable quantitative imaging measures of FI.
Quantitative methods offer a more accurate assessment of FI as compared to semi-quantitative methods. Current validated cutting edge techniques for quantification of FI include multi-echo Dixon MRI and proton MR spectroscopy [13–16]. The Dixon technique generates several images including water-only, fat-only, in-phase and out-of-phase images. From these images, measurement of the fat content in each voxel allows for quantitative analysis. However, this technique is relatively new and mostly available only at large academic centers. The MR spectroscopy method takes advantage of the chemical shift difference between fat and water to provide quantitative information at the voxel level. However, this technique, while known for several years, is rarely used in daily clinical practice, as substantive technical expertise is required for its application. Given the wide use of the semi-quantitative Goutallier classification system, a reliable method for quantitative measurement of FI on T1-weighted MR images would be beneficial to aid clinical decision making, and to stratify FI in retrospective research studies when only conventional shoulder MRI sequences are available.
The purpose of our study is to test for correlation between fat fraction estimated by the 6-point Dixon MRI technique and visible intramuscular fat area percentage by fuzzy C-means segmentation on T1-weighted MR images. We hypothesize that quantitative estimation of FI by fuzzy C-means segmentation on T1-weighted images will have a strong correlation to fat fraction by Dixon MRI, and also that both methods will demonstrate strong reliability.
Materials and Methods
Study Population
The study was approved by an institutional review board and complied with Health Insurance Portability and Accountability Act guidelines. During our prospective cross-sectional study, informed consent was obtained. The study population consisted of adults ≥ 18 years. Inclusion criteria: asymptomatic shoulder (volunteer subjects) or painful shoulder with full-thickness rotator cuff tear (subjects referred by an orthopaedic surgeon). Exclusion criteria: history of joint replacement for the ipsilateral shoulder; contraindication to MRI; ipsilateral chronic upper extremity paralysis. Subjects were recruited consecutively from January 2016 to February 2017. Three shoulders from asymptomatic volunteers (mean age, 28.3 years ± 3.2; age range, 26 – 32 years) and four shoulders from subjects (mean age, 59.7 years ± 12.7; age range 45 – 68 years) with confirmed painful full-thickness RC tear by an orthopaedic surgeon were included in the study, allowing for inclusion of 42 shoulder muscles in the analysis.
MR Imaging
All imaging examinations were performed at 3.0T (Magnetom Trio; Siemens Healthcare, Erlangen, Germany) using a four-channel flexible coil. All shoulders with known painful full-thickness RC tear were imaged prior to RCR. The imaging protocol included a standard 2-dimensional (2D) turbo spin-echo (TSE) oblique sagittal T1-weighted sequence (matrix, 448 × 448; repetition time msec (TR)/echo time[s] (TE) msec, 600/22; field of view (FOV), 160 mm; number of acquisitions, 3; slice thickness, 4mm; and axial, oblique sagittal and oblique coronal 2D short tau inversion recovery sequences (matrix, 448 × 448; TR/TE msec, 4420/50; inversion time 180 msec; FOV, 160, number of acquisitions, 1; slice thickness, 4mm). A 3-dimensional 6-point Dixon volumetric sequence was also obtained in the sagittal orientation (matrix, 320 × 320; TR, 9 ms, TE,1.35, 2.65, 3.95, 5.25, 6.55, 7.85 ms; flip angle, 9; FOV 312 × 400 mm; number of acquisitions, 1; slice thickness, 4 mm). From the 6-point Dixon sequence, fat- and water-only images, as well as fat fraction and water fraction maps, were reconstructed. The fat fraction maps were reformatted in the oblique sagittal plane to match the orientation of the T1-weighted MR images.
MRI Evaluation of Rotator Cuff Tendons
A board-certified musculoskeletal radiologist with 8 years of experience determined the presence or absence of a full-thickness RC tear for each shoulder. Rotator cuff evaluation included the supraspinatus, infraspinatus, subscapularis and teres minor tendons. Each rotator cuff tendon was defined as (1) intact or (2) with full-thickness RC tear.
Quantitative MRI Evaluation of Intramuscular Fatty Infiltration
A research coordinator created DICOM image modules for each shoulder included in the study (N = 7). Each module contained a T1-weighted MR image and 6-point Dixon MR fat fraction map image corresponding to the Y-shaped view in the oblique sagittal plane [16–18]. A blinded radiology resident evaluated six muscles (Fig. 1) on MR images in each module with Medical Image Processing, Analysis and Visualization software (MIPAV, version 7, National Institutes of Health, Bethesda, Maryland, USA). MIPAV software is freely available for download at: https://mipav.cit.nih.gov/. The resident independently measured the visible intramuscular fat area percentage of the supraspinatus, infraspinatus, subscapularis, teres minor, deltoid and trapezius muscles in each module on the T1-weighted images with fuzzy C-means by manual segmentation (Fig. 2a). Fuzzy-C means is a pattern recognition clustering mathematical algorithm which allocates each voxel to a pre-defined category based on signal intensity through a process of iterative optimization [19]. Fuzzy-C means is widely used for segmentation in medical imaging and has been applied in clinical scenarios such as separating fat from fibroglandular tissue on breast MRI [19, 20]. The cross-sectional area (CSA) of visually perceptible fat within a muscle was determined by fuzzy C-means segmentation, which parcellated the muscle into two classes using the following parameters: number of desired classes, 2; desired exponent value, 2; end tolerance, 0.01; maximum number of iteration, 200; signal threshold, −1024.0; region, VOI region; segmentation, hard and fuzzy both. The percentage of FI in each ROI was estimated by taking the CSA of visible intramuscular fat segmented by fuzzy C-means segmentation divided by the CSA of the entire ROI. The resident also independently performed a separate analysis, where the mean fat fraction for each muscle on the corresponding Dixon fat fraction map was determined, by taking the average signal from each ROI (Fig. 2b). For both T1-weighted and Dixon MRI analysis, a region of interest was placed at the border of each muscle by manual segmentation, with one exception. A line perpendicular to the inferior scapular border marked the inferior extent of the subscapularis muscle included in the ROI [16]. To determine intra-observer reliability for the 6-point Dixon and T1-weight MR image FI measures, each measurement was repeated 4 weeks later by the same resident. To allow for determination of inter-observer reliability for the 6-point Dixon and T1-weight MR image FI measures, each measurement was also performed once by a blinded board-certified musculoskeletal radiologist. Twenty weeks after the quantitative analyses, the same radiology resident and board-certified musculoskeletal radiologist determined a Goutallier grade for each individual muscle by consensus.
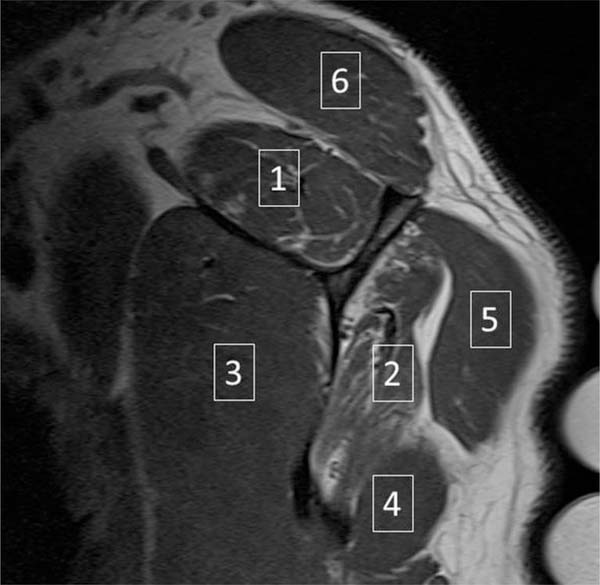
Fig. 1.
Example oblique sagittal T1-weighted MR image of the shoulder. Six skeletal muscles were evaluated: 1 – supraspinatus; 2 – infraspinatus; 3 – subscapularis; 4 – teres minor; 5 – deltoid; 6 - trapezius
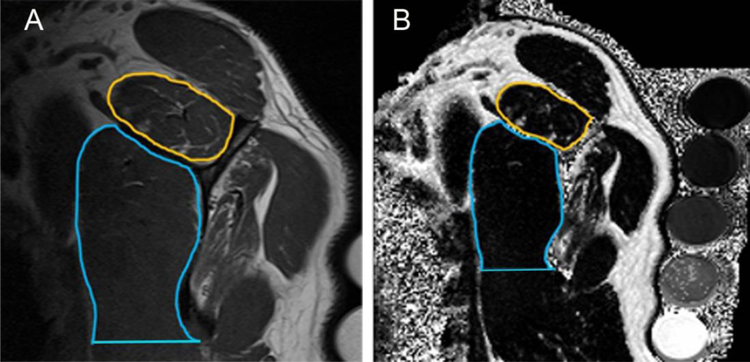
Fig. 2.
Method for calculation of visualized intramuscular fat area percentage and muscle fat fraction on (a) oblique sagittal T1-weighted and (b) corresponding Dixon MR images. An ROI was drawn around the border of every individual muscle (as depicted by the gold ROI on both images for the supraspinatus muscles) in the study with one exception. A line perpendicular to the inferior scapular border marked the inferior extent of the subscapularis muscle included in the ROI (blue ROI).
Statistical analysis
Statistical analysis was performed using Stata statistical software version 14 (StataCorp LP, College Station, Texas). The visible intramuscular fat area percentage on T1-weighted MR images and fat faction on Dixon MR images for each of the muscle groups were obtained for each individual shoulder by the resident. Asymptomatic shoulder group and painful full-thickness RC tear group means were compared by use of the unpaired t test. The mean age of each group were compared with the unpaired t test. Intra-observer and inter-observer reliability were assessed by calculating the intraclass correlation coefficient (ICC), according to the technique proposed by Landis and Koch [21]. The Pearson correlation coefficient (r) was calculated between the visible intramuscular fat area percentage on T1-weighted MR images and fat faction on Dixon MR images for each muscle (n = 42). To correct for any potential clustering, we determined the average mean visible intramuscular fat area percentage on T1-weighted MR images and mean fat faction on Dixon MR images of the supraspinatus, subscapularis, infraspinatus, teres minor, deltoid and trapezius muscles for each individual study subject; and then calculated the r between the study subject mean visible intramuscular fat area percentage on T1-weighted MR images and the mean fat faction on Dixon MR images (n = 7). A p value < 0.05 was considered to indicate a significant difference.
Results
The shoulder MRIs of the asymptomatic volunteers (N = 3) all showed intact rotator cuff tendons. The shoulder MRIs of subjects with painful shoulders (N = 4) all demonstrated the presence of a full-thickness supraspinatus tendon tear, and two also had full-thickness infraspinatus tendon tears. The mean age of the asymptomatic shoulders was younger than those with painful full-thickness RC tear (p = 0.014). As expected, subjects with painful full-thickness RC tear showed a significant difference compared to asymptomatic subjects for supraspinatus muscle visible intramuscular fat area percentage on T1-weighted MR images and fat fraction on Dixon MRI images (Tables 1 and 2). Overall, differences detected in quantification of FI between shoulders with full-thickness RC tear and asymptomatic shoulders were similar for the T1-weighted MRI fuzzy C-means segmentation and Dixon MRI methods for all muscles. The mean Goutallier grades for asymptomatic volunteer muscles were: supraspinatus, 1.0 ± 0.0; subscapularis, 0.7 ± 0.6; infraspinatus, 1.0 ± 0.0; teres minor, 0.0 ± 0.0; deltoid, 0.7 ± 0.6; and trapezius, 0.0 ± 0.0. The mean Goutallier grades for muscles with painful full-thickness RC tear were: supraspinatus, 2.0 ± 0.0; subscapularis, 1.3 ± 0.5; infraspinatus, 1.8 ± 0.5; teres minor 1.3 ± 1.0; deltoid, 1.5 ± 0.6; and trapezius, 2.0 ± 0.0.
Table 1.
Comparison of mean visible intramuscular fat area percentage on T1-weighted MR images in shoulders with painful full-thickness supraspinatus tendon tears and asymptomatic volunteers with intact rotator cuffs.
| Muscle | Full-Thickness Tear mean ± SDA (N = 4) | Intact mean ± SD (N = 3) | P value |
|---|---|---|---|
| Supraspinatus | 16.4 ± 0.7 | 4.6 ± 1.1 | <0.001 |
| Infraspinatus | 12.0 ± 6.2 | 4.4 ± 3.2 | 0.116 |
| Subscapularis | 8.4 ± 5.6 | 5.1 ± 0.9 | 0.370 |
| Teres Minor | 8.4 ± 1.8 | 4.8 ± 2.4 | 0.070 |
| Deltoid | 12.6 ± 3.4 | 7.1 ± 2.3 | 0.064 |
| Trapezius | 13.8 ± 4.5 | 6.6 ± 1.8 | 0.050 |
Standard deviation
Table 2.
Comparison of mean fat fraction on Dixon MR images in shoulders with painful full-thickness supraspinatus tendon tears and asymptomatic volunteers with intact rotator cuffs.
| Muscle | Full-Thickness Tear meanA ± SDB (N=4) | Intact mean ± SD (N=3) | P value |
|---|---|---|---|
| Supraspinatus | 13.2 ± 3.6 | 5.5 ± 2.3 | 0.025 |
| Infraspinatus | 17.2 ± 8.7 | 4.4 ± 1.1 | 0.056 |
| Subscapularis | 8.0 ± 5.0 | 5.2 ± 1.5 | 0.402 |
| Teres Minor | 10.3 ± 4.3 | 4.0 ± 0.6 | 0.059 |
| Deltoid | 10.7 ± 3.5 | 4.3 ± 0.8 | 0.029 |
| Trapezius | 14.8 ± 6.8 | 8.1 ± 1.8 | 0.159 |
fat fraction expressed as a percentage
Standard deviation
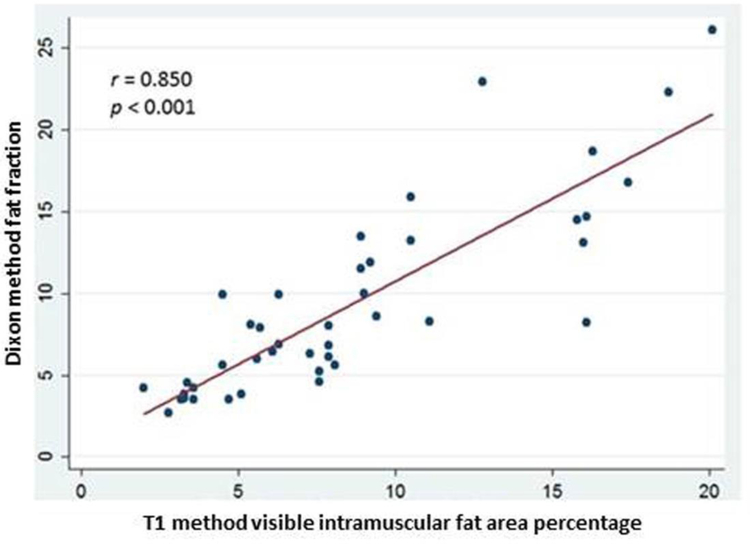
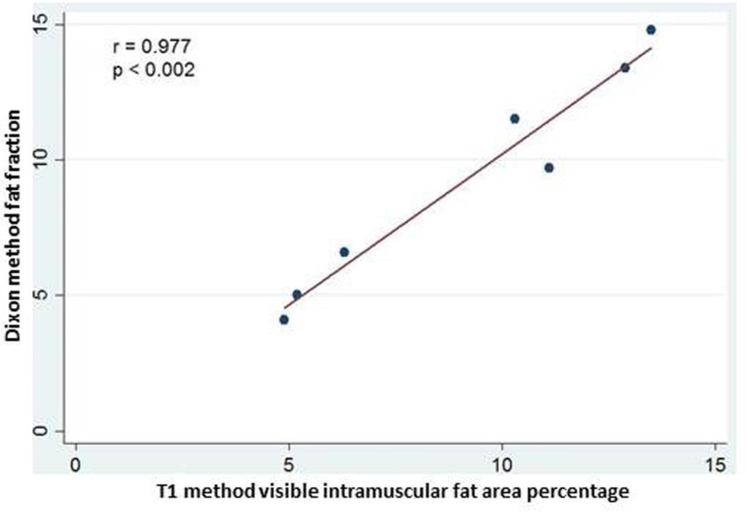
Both intra-observer and inter-observer intraclass correlation was strong for both T1-weighted MRI fuzzy C-means segmentation and Dixon MRI methods (Table 3). The ICC for intra-observer reliability was 0.871 and 0.979 for fuzzy C-means segmentation and Dixon measures of FI, respectively. The ICC for inter-observer reliability for was 0.947 and 0.951 for fuzzy C-means segmentation and Dixon measures of FI, respectively. Strong correlation was found between the T1-weighted MRI fuzzy C-means and the 6-point Dixon MRI measures of FI for all muscles; r = 0.850, p < 0.001 (Figure 3). Strong correlation was also found between shoulder muscle mean T1-weighted MRI fuzzy C-means and the mean 6-point Dixon MRI by study subject; r = 0.977, p < 0.002 (Figure 4).
Table 3.
Intra-observer and inter-observer agreement for all muscles (N = 42) for visible intramuscular fat area percentage on T1-weighted MR images and mean fat fraction on Dixon MR images
| Sequence | Intra-observer ICCA | Inter-observer ICC |
|---|---|---|
| T1 | 0.871 | 0.947 |
| Dixon | 0.979 | 0.951 |
Intraclass correlation coefficient
Fig. 3.
Correlation of fat fraction by the Dixon MRI method and visible intramuscular fat area percentage by the T1-Weighted fuzzy C-means segmentation MRI method for all muscles included in the study (N = 42). Dixon fat fraction is expressed as a percentage.
Fig. 4.
Correlation of study subject shoulder muscle mean fat fraction by the Dixon MRI method and mean visible intramuscular fat area percentage by the T1-Weighted fuzzy C-means segmentation MRI method. Dixon fat fraction is expressed as a percentage.
Discussion
To assess the feasibility and reliability of quantifying shoulder muscle FI on conventional T1-weighted MR images, we compared the freely available MIPAV software fuzzy C-means segmentation to the 6-point Dixon method. Our study shows that quantification of visible intramuscular fat area percentage by T1-weighted MRI fuzzy C-means segmentation is strongly correlated to fat fraction by 6-point Dixon MRI for shoulder muscles. The fuzzy C-means segmentation and Dixon fat fraction are fundamentally two different methods for estimating FI. The fuzzy C-means technique in our study estimates the percentage of visible intramuscular fat present within an ROI, by classifying each voxel as either a fat voxel or non-fat voxel. The 6-point Dixon method is different in that it computes the fat fraction within each voxel and a map of this information is provided as either a fat image or a water image. The fat fraction map estimates the fraction of fat within each voxel. The Dixon method fat fraction estimates FI as an average of all voxels within an ROI.
Reliable quantitative methods for measurement of rotator cuff FI are necessary tools for future research studies and clinical decision making in current practice. Fuzzy C-means segmentation for quantification of FI is a viable alternative to the semi-quantitative Goutallier classification system for measurement of intramuscular fat when only standard imaging sequences are available. Continuous variable grading schemes offers a more reliable and accurate measure of FI than 5-point ordinal scales for RC tear imaging research [22]. Quantitative imaging also offers greater certainty in challenging cases, when there is uncertainty as to whether a rotator cuff muscle is Goutallier grade 2 (muscle > fat) or Goutallier grade 3 (muscle = fat). The fuzzy C-means segmentation algorithm is one of several freely available tools designed to meet the goal of applying quantitative imaging techniques in biomedical research.
Imaging evaluation of rotator cuff muscle fat content is critical for clinical decision making in RC tear, since ≥ 50% FI is a relative contraindication to RCR. There is mounting evidence that FI is predictive of postoperative healing and shoulder function for patients treated with RCR [4, 6, 7, 23]. Therefore, surgeons increasingly advocate for early surgical intervention for treatment of active patients with acute traumatic RC tears. Several animal and human studies show that rotator cuff muscle FI accumulation rapidly progress following acute RC tear, and that early RCR does not reverse FI but instead helps to mitigate the greater accumulation of FI associated with delayed RCR [4, 5, 7, 24–26].
Fuzzy C-means segmentation is appropriate for shoulder muscle FI quantification analysis on T1-weighted MR images, using a similar assumption of the Goutallier classification system that muscle signal is either “fat” or “muscle” signal. Raters assign one of five subjective Goutallier grades to report the perceived degree of FI for a muscle. Our method of fuzzy C-means segmentation operates on a similar premise. The cross-sectional area (CSA) within the region of interest (ROI) (fig. 1a) is dichotomized into two predominant signals, producing a CSA corresponding to an area of fat and a CSA corresponding to an area of muscle. This process allows for the calculation of percentage fat signal within the ROI. Fuzzy C-means segmentation on MIPAV provides accurate quantification of T1-weighted signal on MRI. Prior studies have utilized varied methods for segmentation of skeletal muscle signal on T1-weighted images in the spine, pelvis and lower limb [27–29].
FI is composed of two components on the cellular level: intramyocellular lipid (IMCL) and extramyocellular lipid (EMCL). IMCL is in a dynamic equilibrium and is adjacent to the mitochondria within a myofibril for use during active metabolism, whereas EMCL is deposited between myofibrils for longer-term storage [13, 30]. Multi-echo Dixon MRI techniques are a validated method for quantification of FI in animal and human studies [14–16, 22]. The calculation of fat fraction measured by Dixon MRI includes both IMCL and EMCL. Proton MRI spectroscopy techniques are capable of measuring EMCL and IMCL separately, but are not widely available and require specialized expertise for analysis [13, 14, 30].
Limitations
Fuzzy C-means segmentation of T1-weighted MR images provides quantification of image signal and does measure fat directly. However, our technique for calculation of visible intramuscular fat area percentage is strongly correlated to Dixon fat fraction. Additionally, our method mirrors the assumption underlying classification by the Goutallier classification system, where a human rater assigns a grade based on subjective visible perception of fat and muscle content. During our study we did identify a specific pitfall limiting the use of the fuzzy C-means segmentation on T1-weighed images. Fuzzy C-means segmentation may not be appropriate for evaluation of Goutallier grade 0 (all muscle, with no visible fat). In this circumstance, neither of the two dichotomized signals will represent fat content. We would advocate placement of the ROI abutting the entire border edge of a muscle, and avoid placement of the ROI entirely within the muscle away from the border edge. Additionally, T1-weighted MRI images should be correlated with other standard shoulder imaging sequences to exclude unexpected pathology in a muscle. Potential mimickers of fat signal on T1-weighted images include melanin, protein, blood products and gadolinium.
Conclusion
Quantification of shoulder muscle intramuscular fatty infiltration by fuzzy C-means segmentation on T1-weighted MR images is strongly correlated to fat fraction by the 6-point Dixon technique. Fuzzy C-means segmentation for quantitative measurement of visible intramuscular fat area percentage in rotator cuff muscles is a viable alternative to the semi-quantitative Goutallier classification system for future research studies.
Acknowledgments
Compliance with ethical standards
Conflicts of interest Dr. Derik L. Davis receives partial salary support from the University of Maryland Claude D. Pepper Older Americans Independence Center (NIA 2P30AG028747). He also received a research seed grant in 2016 from the Radiological Society of North America Research & Education Foundation & Hitachi Medical Systems.
Contributor Information
Derik L. Davis, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, Maryland 21201.
Thomas Kesler, Diagnostic radiology resident, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland Medical Center, Baltimore, Maryland
Mohit N. Gilotra, Department of Orthopaedics, University of Maryland School of Medicine, Baltimore, Maryland.
Ranyah Almardawi, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, Baltimore, Maryland.
Syed A. Hasan, Department of Orthopaedics, University of Maryland School of Medicine, Baltimore, Maryland.
Rao P. Gullapalli, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, Baltimore, Maryland.
Jiachen Zhuo, Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, Baltimore, Maryland.
References
- 1.McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA 3rd. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med 2015; 43:491–500 [DOI] [PubMed] [Google Scholar]
- 2.Jain NB, Higgins LD, Losina E, Collins J, Blazar PE, Katz JN. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskelet Disord 2014; 15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 2010; 19:116–120 [DOI] [PubMed] [Google Scholar]
- 4.Liem D, Lichtenberg S, Magosch P, Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joing Surg Am 2007; 89:1770–1776 [DOI] [PubMed] [Google Scholar]
- 5.Uhthoff HK, Coletta E, Trudel G. Effect of timing of surgical SSP tendon repair on muscle alterations. J Orthop Res 2014; 32:1430–1435 [DOI] [PubMed] [Google Scholar]
- 6.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joing Surg Am 2004; 86-A:1973–1982 [DOI] [PubMed] [Google Scholar]
- 7.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007; 35:719–728 [DOI] [PubMed] [Google Scholar]
- 8.Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 2014; 134:985–990 [DOI] [PubMed] [Google Scholar]
- 9.Ashry R, Schweitzer ME, Cunningham P, Cohen J, Babb J, Cantos A. Muscle atrophy as a consequence of rotator cuff tears: should we compare the muscles of the rotator cuff with those of the deltoid? Skeletal Radiol 2007; 36:841–845 [DOI] [PubMed] [Google Scholar]
- 10.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994:78–83 [PubMed] [Google Scholar]
- 11.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999; 8:599–605 [DOI] [PubMed] [Google Scholar]
- 12.Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy 2012; 28:154–159 [DOI] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Schmid MR, Zanetti M, Jost B, Gerber C, Hodler J. Assessment of fat content in supraspinatus muscle with proton MR spectroscopy in asymptomatic volunteers and patients with supraspinatus tendon lesions. Radiology 2004; 232:709–715 [DOI] [PubMed] [Google Scholar]
- 14.Agten CA, Rosskopf AB, Gerber C, Pfirrmann CW. Quantification of early fatty infiltration of the rotator cuff muscles: comparison of multi-echo Dixon with single-voxel MR spectroscopy. Euro Radiol 2016; 26:3719–3727 [DOI] [PubMed] [Google Scholar]
- 15.Gerber C, Meyer DC, Fluck M, Benn MC, von Rechenberg B, Wieser K. Anabolic Steroids Reduce Muscle Degeneration Associated With Rotator Cuff Tendon Release in Sheep. Am J Sports Med 2015; 43:2393–2400 [DOI] [PubMed] [Google Scholar]
- 16.Nozaki T, Tasaki A, Horiuchi S, et al. Predicting Retear after Repair of Full-Thickness Rotator Cuff Tear: Two-Point Dixon MR Imaging Quantification of Fatty Muscle Degeneration-Initial Experience with 1-year Follow-up. Radiology 2016; 280:500–509 [DOI] [PubMed] [Google Scholar]
- 17.Mellado JM, Calmet J, Olona M, et al. Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings. AJR Am J Roentgenol 2005; 184:1456–1463 [DOI] [PubMed] [Google Scholar]
- 18.Yoo JC, Ahn JH, Yang JH, Koh KH, Choi SH, Yoon YC. Correlation of arthroscopic repairability of large to massive rotator cuff tears with preoperative magnetic resonance imaging scans. Arthroscopy 2009; 25:573–582 [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Guo H, Zhang H, et al. A modified fuzzy C-means method for segmenting MR images using non-local information. Technol Health Care 2016; 24 Suppl 2: S785–793 [DOI] [PubMed] [Google Scholar]
- 20.Clendenen TV, Zeleniuch-Jacquotte A, Moy L, Pike MC, Rusinek H, Kim S. Comparison of 3-point Dixon imaging and fuzzy C-means clustering methods for breast density measurement. J Magn Reson Imaging 2013; 38:474–481. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174 [PubMed] [Google Scholar]
- 22.Lee S, Lucas RM, Lansdown DA, et al. Magnetic resonance rotator cuff fat fraction and its relationship with tendon tear severity and subject characteristics. J Shoulder Elbow Surg 2015; 24:1442–1451 [DOI] [PubMed] [Google Scholar]
- 23.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg 2003; 12:550–554 [DOI] [PubMed] [Google Scholar]
- 24.Uhthoff HK, Coletta E, Trudel G. Intramuscular fat accumulation and muscle atrophy in the absence of muscle retraction. Bone Joint Res 2014; 3:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhthoff HK, Matsumoto F, Trudel G, Himori K. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. J Orthop Res 2003; 21:386–392 [DOI] [PubMed] [Google Scholar]
- 26.Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am 2003; 85-A:2391–2402 [DOI] [PubMed] [Google Scholar]
- 27.Sions JM, Coyle PC, Velasco TO, Elliott JM, Hicks GE. Multifidi Muscle Characteristics and Physical Function Among Older Adults With and Without Chronic Low Back Pain. Arch Phys Med Rehabil 2017; 98:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelken F, Wassilew GI, Kohlitz T, et al. Assessment of fatty degeneration of the gluteal muscles in patients with THA using MRI: reliability and accuracy of the Goutallier and quartile classification systems. J Arthroplasty 2014; 29:149–153 [DOI] [PubMed] [Google Scholar]
- 29.Mathur S, Levy RD, Reid WD. Skeletal muscle strength and endurance in recipients of lung transplants. Cardiopulm Phys Ther J 2008; 19:84–93 [PMC free article] [PubMed] [Google Scholar]
- 30.Takashima H, Takebayashi T, Ogon I, et al. Analysis of intra and extramyocellular lipids in the multifidus muscle in patients with chronic low back pain using MR spectroscopy. Br J Radiol 2018:20170536. [DOI] [PMC free article] [PubMed] [Google Scholar]