Abstract
Objective:
To determine influence of age and HIV infection on influenza vaccine responses.
Design:
Evaluate serologic response to seasonal trivalent influenza vaccine (TIV) as the immunologic outcome in HIV-infected (HIV+) and age-matched HIV negative (HIV−) adults.
Methods:
During 2013–2016, 151 virologically controlled HIV+ individuals on antiretroviral therapy and 164 HIV− volunteers grouped by age as young (<40 years), middle aged (40–59 years) and old (≥60 years) were administered TIV and investigated for serum antibody response to vaccine antigens.
Results:
At prevaccination (T0) titers were in seroprotective range in more than 90% of participants. Antibody titers increased in all participants postvaccination but frequency of classified vaccine responders to individual or all three vaccine antigens at 3–4 weeks was higher in HIV− than HIV+ adults with the greatest differences manifesting in the young age group. Of the three vaccine strains in TIV, antibody responses at T2 were weakest against H3N2 with those to H1N1 and B antigens dominating. Among the age groups, the titers for H1N1 and B were lowest in old age, with evidence of an age-associated interaction in HIV+ persons with antibody to B antigen.
Conclusion:
Greater frequencies of vaccine nonresponders are seen in HIV+ young compared with HIV− adults and the observed age-associated interaction for B antigen in HIV+ persons are supportive of the concept of premature immune senescence in controlled HIV infection. High-potency influenza vaccination recommended for healthy aging could be considered for HIV+ adults of all ages.
Keywords: aging and HIV, antibody response and aging, HIV, influenza vaccine and HIV
Introduction
Influenza virus infections occur in 5–20% of the US population annually with more than 200 000 hospitalizations and approximately 36 000 deaths; the greatest risk manifesting in individuals of at least 65 years of age [1-3] attributed to waning immunity in old age [1-3]. A patient group that is increasingly joining the ranks of the aged is the HIV infected, resulting from improved survival due to effectiveness of combination antiretroviral therapy (ART) [4,5]. In 2014, 45% of people living with HIV infection (HIV+) in the United States were 50 years or older, and this age group is expected to exceed 70% by 2020 [6]. Importantly, features of inflammation (reviewed in [7,8]) can persist in HIV+ despite virologic control on ART [9-12]. Based on epigenetic modeling, HIV+ were found to age faster than HIV uninfected by an average of 4.9 years with a 19% increased risk of mortality [13], but whether their immune function decline is also accelerated is not well understood.
Due to the increased risk for influenza in the elderly, current recommendations are that people aged at least 65 be given yearly seasonal influenza vaccine [3]. Influenza vaccines currently in use in the United States of America are unadjuvanted and typically consist of a mixture of influenza A and B from three or four viral strains predicted to circulate in the upcoming season [3,14]. However, the effectiveness of this vaccine for clinical protection is variable [15] in HIV uninfected and in HIV infected. The aim of this study was to investigate the impact of age and HIV infection on serologic response to seasonal influenza vaccination. A total of 315 adults ranging in age from 18 to 83 years participated in FLORAH (FLu Responses Of people in relation to Age and HIV) and 257 out of them were reanalyzed in FIND (FInding Novel Determinants of Flu responses) to exclude participants in the upper quartile (>320) of baseline H1N1 titers. Our findings point to impaired vaccine responses in all ages in HIV-infection with responses in young HIV+ participants approximating those of old HIV-uninfected healthy controls.
Materials and methods
Study population
The current study termed FLORAH was conducted during the influenza seasons 2013–2014, 2014–2015 and 2015–2016 with informed consent in participants recruited at the University of Miami clinics and Miami Veteran Affairs Medical Center (MVAMC). HIV-infection status was established as HIV negative (HIV−) or HIV positive (HIV+) by licensed HIV enzyme linked immunoassay. HIV+ participants were on ART with virologic suppression (plasma HIV RNA <20 copies/ml) for at least 1 year prior to study entry. HIV+ and HIV− participants were grouped by age as young (<40 years), middle aged (40–59 years) and old (≥60 years). People on hormone therapy, steroids or immunosuppressant medications or diagnosis of malignancies or other immunodeficiency disorders were excluded. Prevaccination characteristics of study participants are shown in Table 1. Compared with the corresponding HIV− groups, the HIV+ adults showed lower absolute CD4+ cell counts in the middle age and old age groups, with higher absolute CD8+ cell counts and lower CD4+/CD8+ ratio in the young, middle age and old age groups. Among the HIV-infected, young HIV+ showed higher absolute CD4+ cell counts and CD4+/CD8+ ratio compared with middle-age and old-age groups. Peripheral venous blood was collected at prevaccination (T0), and on day 7 (T1), day 21–28 (T2) and month 5–7 (T3) post a single intramuscular dose of trivalent influenza vaccine (TIV) (Seqirus, Cambridge, Massachusetts, USA). Serum and plasma were stored at −80 °C, and peripheral blood mononuclear cells were cryopreserved in liquid N2. This study was approved by the Institutional Review Boards of University of Miami and MVAMC.
Table 1.
Demographic characteristics of the study participants.
| HIV negative |
HIV positive |
|||||
|---|---|---|---|---|---|---|
| Young | Middle | Old | Young | Middle | Old | |
| Number | 48 | 62 | 51 | 34 | 70 | 50 |
| Sex (M/F) | 27/21 | 35/27 | 32/19 | 20/14 | 38/32 | 34/16 |
| Mean age in years (range) | 29.9 (19–39) | 51.4 (41 −59) | 65.7 (60–83) | 30 (19–39) | 51.5 (41–59) | 65 (60–77) |
| Ethnicity, n (%) | ||||||
| Hispanic/Latino | 19 (40) | 21 (34) | 20 (39) | 16 (47) | 17 (25) | 14 (28) |
| Non-Hispanic/non-Latino | 27 (56) | 38 (61) | 27 (53) | 17 (50) | 52 (74) | 32 (64) |
| Not specified | 2 (4) | 3 (5) | 4 (8) | 1 (3) | 1 (1) | 4 (8) |
| Race, n (%) | ||||||
| White | 24 (50) | 28 (45) | 28 (55) | 13 (38) | 18 (26) | 15 (30) |
| Black | 18 (38) | 32 (51) | 18 (35) | 18 (53) | 50 (71) | 35 (70) |
| Asian | 5 (10) | 1 (2) | 5 (10) | 0 | 0 | 0 |
| Not specified | 1 (2) | 1 (2) | 0 | 3 (9) | 2 (3) | 0 |
| Mean absolute counts (cells/μl) | ||||||
| CD45 | 3722 | 2982 | 3441 | 3774 | 3135 | 3119 |
| CD3 | 2557 | 2088 | 2296 | 2645 | 2206 | 2249 |
| CD8+ | 845 | 641 | 805 | 1205a | 1043a | 1167a |
| CD4+ | 1531 | 1308 | 1333 | 1272 | 984a,b | 872a,b |
| CD4+/CD8+ ratio | 1.8 | 2 | 1.6 | 1a | 0.94a,b | 0.74a,b |
| pVL (copies/ml) | ND | ND | ND | <40 | <40 | <40 |
ND, not done; pVL, plasma virus load.
vs. Corresponding HIV− groups.
vs. Young HIV+ group (P<0.05).
Serologic responses to influenza vaccine
antibody response to whole vaccine and individual vaccine antigens H1N1, H3N2 and B (gift from Seqirus, PA) were determined in serum by hemagglutination inhibition (HAI) assay at T0, T1, T2 and T3 as described previously [16,17]. In accordance with guidelines established by the US Food and Drug Administration, a titer of at least 1 : 40 was considered as being seroprotective. This titer, together with an at least four-fold increase from prevaccination at T0 to postvaccination at T2, was taken as the definition for vaccine responders [18]. Participants were further classified as absolute responders if they demonstrated a positive vaccine response to all three vaccine antigens, or ‘other’ if the response was to one or two antigens. All participants who did not fit above definitions were termed vaccine nonresponders (VNR).
Serologic response to cytomegalovirus
Cytomegalovirus (CMV) serostatus was determined at T0 by commercial CMV IgG ELISA (CM027G, Calbiotech), according to the manufacturer’s recommendations and an antibody index of at least 1.1 was considered as positive [19].
Statistical analysis
Differences in antibody titers between groups were determined using two-tailed Student’s t tests or by two-way analysis of variance. Correlation analyses were performed using Pearson and Spearman correlations based on the data distribution. To predict dependent variables [such as antibody titers or fold change antibody titers], multiple univariate linear regression was performed to test the association of independent variables (age or HIV status or CD4+ cell count or CD4+/CD8+ ratio) and their interactions to predict antibody titer or fold change titer for each antigen. Product measures from standardized coefficient and Pearson correlation coefficient were calculated to identify the proportion of response to each antigen contributing to the variance of whole vaccine response. A P value of less than 0.05 was determined significant. Graphpad Prism version 7 (La Jolla, California, USA) and R ‘stats’ package (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Results
Serology and vaccine responsiveness in relation to age and HIV status
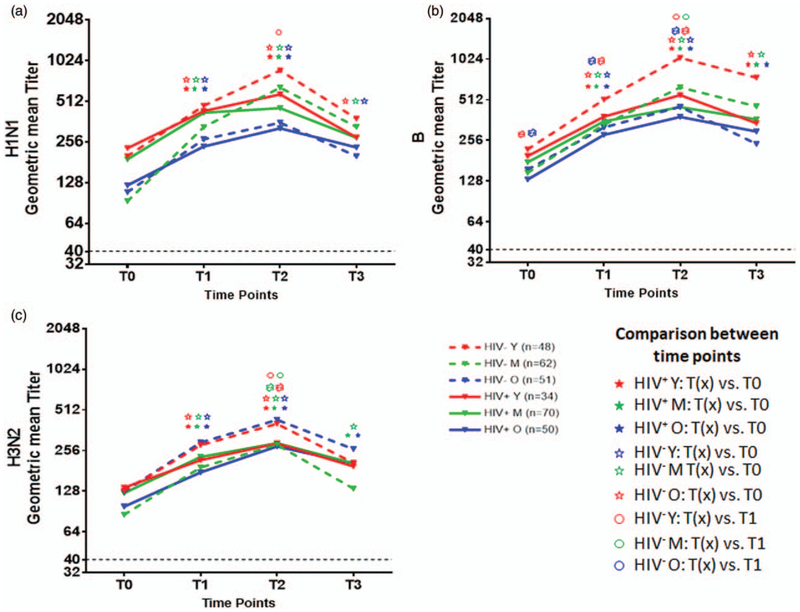
Geometric mean antibody titers for HIV+ and HIV− participants in different age groups at all time points are detailed in Fig. 1 for antigens H1N1 (Fig. 1a); B (Fig. 1b) and H3N2 (Fig. 1c); statistical analysis of antibody responses at different time points in different study groups and differences among the groups are depicted in Tables S1 and S2, http://links.lww.com/QAD/B231, respectively. Prevaccination titers for all antigens ranged from 10 to 5120 with levels at least 1 : 40 in 90–98% of study participants and were equal or greater in the young compared with other age groups. Titers at T0 were similar across the three test seasons in all age groups in HIV+ (Fig. S1A, C, E, http://links.lww.com/QAD/B231) and HIV− (Fig. S1B, D, F, http://links.lww.com/QAD/B231). Figure 1 and Table S1, http://links.lww.com/QAD/B231 show responses at different time points with a variable but significant increase in titers from T0 to T1 and from T0 to T2 for all antigens in all participant groups, but an increase from T1 and T2 occurred only in the HIV−, evident for all antigens in the young and for H1N1 and B antigens in the middle age. Antibody decay based on decrease in titers from T2 to T3 was noted for H1N1 and H3N2 antigen in all the HIV− age groups and only in young HIV+ (Fig. 1a, c and Table S1, http://links.lww.com/QAD/B231). Antibody titers at T0 for H1N1, B and H3N2 correlated inversely with respective fold change in antibody titers at T2 over T0 (T2/T0) in both HIV− and HIV+ groups (Fig. S2, http://links.lww.com/QAD/B231).
Fig. 1. Antibody responses to individual vaccine antigens between study groups.
Antibody titers for H1N1, H3N2 and B were determined before (T0), and day 7 (T1), day 21–28 (T2) and months 5–7 (T3) after vaccination by hemagglutination inhibition assay in HIV− (dotted lines) and HIV+ (solid lines) in young (red), middle aged (green) and old (blue) individuals. Geometric mean titers are shown for each time point. Line graphs depict antibody response to (a); H1N1, (b); B and (c); H3N2 at T0, T1, T2 and T3. P values were calculated with Student’s t test. Each symbol for the statistics represents the comparison between indicated groups with a P less than 0.05. Tables S1 and S2,http://links.lww.com/QAD/B231 provide additional information related to all comparisons including actual P values.
Comparison between HIV+ vs. HIV− in different age groups at each time point (Table S2, http://links.lww.com/QAD/B231) showed that young HIV− compared with young HIV+ had higher titers to B and H3N2 at T2 and for B and H1N1 at T3. In old age, H3N2 titers were higher in HIV− than HIV+ at T1 and T2, but titers for H1N1 and B were not different at any time point. Comparing ages within the HIV+ and HIV− (Table S2, http://links.lww.com/QAD/B231), the HIV− old had lower titers for H1N1 at T0–T3, and for B at T1–T3 compared with young, whereas H3N2 titer was similar to young at all the time points. HIV+ old had lower titers for H1N1 than young at T0–T2, whereas H3N2 and B titers were equivalent at T2 and T3.
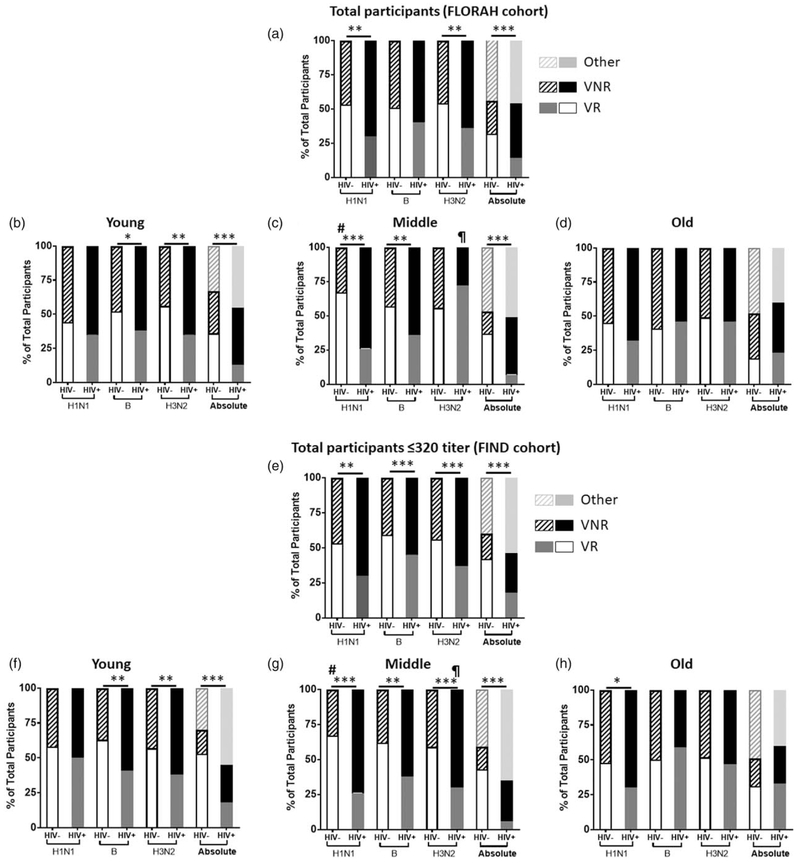
Figure 2 shows status of vaccine responsiveness to each strain, as well as absolute responders. Overall, HIV+ had fewer vaccine responders for H1N1 and H3N2 and fewer absolute responders than the HIV− (Fig. 2a) with the young HIV+ showing the greatest differences from young HIV−, with fewer vaccine responders to B and H3N2 and fewer absolute responders (Fig. 2b). The middle-age HIV+ group had fewer vaccine responders to H1N1 and B and less absolute responders compared with HIV− (Fig. 2c). Old HIV+ vaccine responders frequencies were similar to old HIV− for individual antigens and for absolute responders (Fig. 2d). Comparing age groups within HIV+ and HIV−, young and old HIV− showed fewer vaccine responders to H1N1 compared with middle age HIV− group, whereas young and old HIV+ showed fewer vaccine responders to H3N2 compared with middle-age HIV+. As the T0 titers were high with a negative association to T2/T0 fold change for all the antigens in both HIV+ and HIV−, we also investigated the FIND subgroup which excluded participants with a baseline H1N1 titer of more than 320. As shown in Fig. 2e-h, the result concurred with, and reinforced the findings of the entire cohort. Overall, FIND participants showed higher frequencies of vaccine responders mainly in HIV− groups and the differences in frequencies of vaccine responders and VNR between HIV+ and HIV− were more significant.
Fig. 2. Vaccine responses in HIV− and HIV+ groups.
Bar graphs indicate the vaccine responders and nonresponders for individual vaccine antigens and participants who met criteria for absolute or other (response only to one or two antigens) categories in (a–d); full cohort (flu responses of people in relation to age and HIV) in (a); total participants, (b); young, (c); middle aged and (d); old and (e and f); finding novel determinants of flu responses cohort with baseline titer or less 320 in (e); total participants, (f); young, (g); middle aged and (h); old. Analyzed by chi-squared test. *P < 0.01; **P < 0.001; ***P < 0.0001. #Significant difference compared with Y− and O−; ¶significant compared with Y+ and O+.
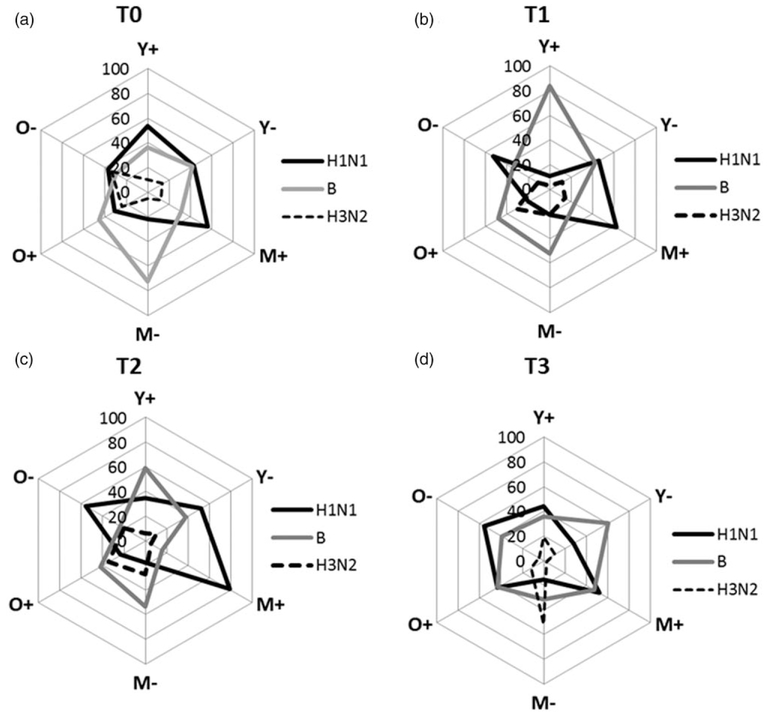
Individual vaccine antigens contribute disproportionately to whole vaccine response in different groups
We adopted a statistical approach in which products from a linear regression analysis for H1N1, H3N2 and B responses were used as explanatory variables to predict the antibody response to whole vaccine at each time point. Contribution of antibody response against each vaccine antigen in relation to the observed whole vaccine response in young, middle-age and old groups are shown in Fig. 3 at T0 (Fig. 3a), T1 (Fig. 3b), T2 (Fig. 3c) and T3 (Fig. 3d). The data are summarized in Supplementary Table S3, http://links.lww.com/QAD/B231 for HIV+ and Table S4, http://links.lww.com/QAD/B231 for HIV− groups. Overall, antibody to B and H1N1 antigens predominated in the whole vaccine response, whereas H3N2 elicited a weak response at all the time points. In young HIV−, antibody to H1N1 and B antigen were nearly equivalent to each other from T0 to T2 with persistence mainly of antibody to B antigen at T3. In young HIV+, H1N1 antibody was greater than HIV−, whereas antibody to B antigen was similar to HIV− at T0, but antibody to B antigen predominated at T1 and T2 constituting 80 and 60%, respectively, of the whole vaccine response; at T3, antibody to B antigen was lower and was similar to T0 levels. In the middle age HIV−, the major contribution of whole vaccine response at T0, T1 and T2 was from antibody to B antigen, whereas H1N1 antibody remained low at 20% at all time points and H3N2 surpassed other antibody at T3. In contrast, in middle-age HIV+, antibody to H1N1 was the major contributor at all time points, followed by antibody to B antigen except at T3 in which they were equivalent. In old HIV−, antibody to all strains were lower at T0, but thereafter, antibody to H1N1 dominated at all time points, whereas in old HIV+, antibody to B antigen dominated at T0 through T2 with lower levels of H1N1 and H3N2 antibody at T1 and T2 and equivalent H1N1 and B antibody at T3.
Fig. 3. Spider plots showing contribution of antibody response to individual antigens to whole vaccine antibody response.
Linear regression analysis performed using different age groups of HIV+ and HIV− at each time point. Product measure from standardized coefficient and Pearson correlation coefficient were calculated to identify the proportion of each antigens contributing to whole vaccine response at T0, T1, T2 and T3, (a–d) Statistical significance of the data is depicted in Supplementary Tables S3 and S4, http://links.lww.com/QAD/B231.
Interaction of HIV infection with age on serum titers against trivalent influenza vaccine
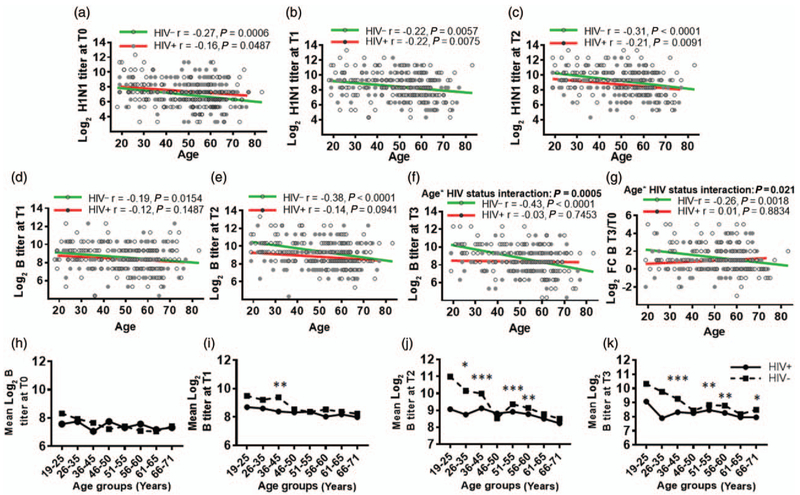
Correlation analyses were performed to determine how antibody responses to individual vaccine antigens associated with age in HIV+ and HIV− (Fig. 4). H1N1 antibody titers at T0 (Fig. 4a), T1 (Fig. 4b) and T2 (Fig. 4c) inversely correlated with age in both HIV+ and HIV−. However, B titers at T1 (Fig. 4d), T2 (Fig. 4e) and T3 (Fig. 4f) and T3/T0 fold change (Fig. 4g) inversely correlated with age in HIV−. Antibody titers against H3N2 or fold change in antibody titer to H1N1 or H3N2 did not correlate with age in either group. Linear regression analysis revealed interaction between titer and T3/T0 fold change for B Ag at T3. Multiple univariate linear regression analysis (Table S5, http://links.lww.com/QAD/B231) revealed influences of age, CD4+ cell counts and CD4+/CD8+ ratio in HIV− on H1N1 response at T0, T1, T2, T3 and on B response at T2, T3, and in HIV+ on H1N1 response at T1 and T2 and on B response at T2. However, absolute CD4+ cell count and CD4+/CD8+ ratio did not interact with each other or with age in predicting H1N1 or B response in HIV+ or HIV−. Further investigation of age and HIV effects on titers to B Ag showed no influence of HIV in any of eight age subgroups at T0 (Fig. 4h), but starting at an age range of 36–45 years, HIV+ and HIV− were significantly different at T1, T2 and T3 and continued in older age subgroups at T2 and T3 (Fig. 4i-k). Time under ART in HIV+ did not influence the antibody response in this model (not shown).
Fig. 4. Correlation of HIV and age with response to H1N1 and B antigens.
Log2 hemagglutination inhibition responses to H1N1 and B were correlated with age in both HIV+ and HIV− by Pearson correlation. Interaction between age and HIV infection on antibody response was tested by univariate multiple linear regression analysis. Significant inverse correlation is evident between age and antibody response to H1N1 at (a) T0, (b) T1, (c) T2, and B at (d) T1, (e) T2, (f) T3, (g) fold change B response at T3. HIV and age interaction for (f), B titer at T3 and (g), fold change B titer at T3. Filled circles indicate HIV+ and open circles indicate HIV−. Red regression line indicates HIV+ and green line for HIV−. (h–k) HIV infection affects the B response starting at the age range of 36–45 years: HIV+ and HIV− groups were divided into eight age groups and mean log2 B titer at each time point was compared between HIV+ and HIV−. Log B titer for HIV+ (solid line) and HIV− (dotted line) at (h) T0, (i), T1, (j), T2 and (k), T3. At each time point, a linear model is fitted to the B titer for each age group. Star indicates the P value of less than 0.05 between HIV+ and HIV−. *P < 0.01; **P < 0.001; ***P < 0.0001.
CMV serostatus did not influence antibody response
CMV seropositivity was higher in the HIV+ compared with HIV− for young, middle age and old age groups (Fig. S3A-C, http://links.lww.com/QAD/B231), although age itself did not affect CMV status. No association was found between CMV serostatus and antibody response to H1N1, H3N2 and B at any time point (not shown).
Discussion
Aging and HIV infection have each been considered to be immune deficient states, but the effect of aging in virologically suppressed HIV+ is not well understood. Influenza vaccination is recommended in old age and in HIV infection but clinical protection is not guaranteed. In the current study, we investigated the serologic response to TIV in relation to aging and HIV. Our observations identified issues that are relevant for understanding the influenza vaccine response in the current period. Almost universally, prevaccination antibody titers of more than 1 : 40 (considered to be seroprotective) were present for all vaccine antigens in TIV in both HIV+ and HIV− of all ages, most likely due to past immunizations [20,21] and infectious episodes. Significant increases in antibody titer from baseline to 1 week and 3—4 weeks postvaccination occurred in all study participant groups but frequency of classified vaccine responders to individual Ag or absolute responders to all three antigens was relatively low, based on response criteria of four-fold increase from baseline titer. Following exclusion of participants with baseline titers of more than 320, absolute responders increased by 6 and 10% in HIV+ and HIV−, respectively. Overall vaccine responders were higher in HIV− than HIV+; significantly, frequencies of VNR in HIV+ compared with HIV− were maximal in young rather than old age groups suggestive of immunosenescence, the effect of which dominates in younger age and is overshadowed by universal immune deficiencies associated with biologic aging.
The high-baseline titers to influenza antigens posed a challenge for designating participants as vaccine responders or VNR based on the current guidelines that require a four-fold increase in titer postvaccination. In the current study, although all participant groups developed a significant increase in their antibody titers to all antigens from T0 to T2, only some were able to achieve a designation of vaccine responders (e.g. vaccine responders to H1N1 were 53% in HIV− and 30% in HIV+). More stringent criteria using absolute responders showed fewer vaccine responders, although the HIV− at 32% were greater than HIV+ at 14%. The frequencies of vaccine responders were increased by 10% in HIV− and 6% in HIV+ in the FIND cohort with 320 or less T0 titer, without altering the landscape of fewer absolute responders in HIV+ (HIV− 42% and HIV+ 18%). We chose an arbitrary cutoff of 320 in the study population that excluded the upper quartile of participants who had the highest baseline titers. For defining vaccine responders, we suggest an alternative or additional definition based on absolute vaccine responders status to incorporate responsiveness to all vaccine antigens. Exclusion of higher prevaccination titers may also assist in defining vaccine responsiveness.
Recent studies raise doubts about clinical protection from influenza infection in vaccinated people despite a HAI titer of 1 : 40 and suggest that current seasonal vaccines are underperforming. The seroprotection threshold of 1 : 40 was based on clinical protection data derived from nearly 24 clinical challenge and field studies performed in the 1970s and 1980s conducted mostly in healthy children and young adults [22,23]. In limited controlled virus challenge studies in adults, this titer was associated with only 50% reduction of infection [24]. The overall seasonal vaccine effectiveness over the past 10 years has ranged from 10 to 50% with a mean of 41% in adults [2,25], not considering populations with greater risk of severe disease from influenza like HIV+ and older individuals [26]. Furthermore, almost every year one or more of the three strains in seasonal influenza vaccines have to be changed as a result of antigenic drift, with an unknown effect on the clinical protection. In practice, the threshold for antibody titers required for clinical protection is unknown [23]. Regardless of uncertainties about the seroprotection threshold, seasonal vaccination has protected vulnerable populations and saved lives and should be continued, whereas research is pursued to improve vaccine efficacy.
A major objective of our study was to ascertain the effect of aging and HIV infection on flu vaccine responses, prompted by our earlier study in a small group of postmenopausal HIV+ women that revealed a lower antibody response to H1N1 antigen compared with HIV− postmenopausal women [16], suggesting an additive deleterious effect of HIV infection on aging. Analysis of the interactions between age and vaccine responses showed that the antibody titer and T3/T0 fold change for the B strain interacted with age in HV+ participants. The main factor driving this interaction was the muted response in young HIV+ compared with HIV−, the difference becoming evident in the 36–45 years age group starting at T1 and becoming more evident at T2 and T3. Absolute CD4+ cell counts and CD4+/CD8+ ratio at baseline [27,28] as well as nadir CD4+ [29] are also known to influence vaccine response in HIV+ virally suppressed patients on ART. We found an independent association of age and absolute CD4+ cell count and CD4+/CD8+ ratio without interactions between them in predicting the H1N1 and B Ag response. We were unable to evaluate the role of nadir CD4+ T cells as we did not have access to this information. Nevertheless, our findings support the concept of premature immunosenescence in HIV infection as corroborated by the interaction of age with B antigen responses, along with lower frequency of absolute responders in the HIV+ compared with HIV− in the young and middle age groups [4,30].
Analysis of antibody responses to individual vaccine antigens revealed an increase post vaccination to all antigens as shown in Fig. 1 and Table S1, http://links.lww.com/QAD/B231. In an attempt to understand how different antigens in the vaccine contribute to the magnitude of the overall whole vaccine response, we found that antibody to B and H1N1 predominated over H3N2, implying that immunodominance of individual antigens in a combination vaccine may differ. TIV contains equal amounts of HA (15 μg) for all antigens, and yet the majority of the influenza infections in the elderly and HIV+ have been caused by H3N2 virus in recent years [31]. During the 2012/13 influenza season, most infections were caused by H3N2 A/Victoria/361/2011, and the seasonal TIV provided protection in only an estimated 9% of elderly (age >60 years) vaccine recipients [32,33]. Low vaccine efficacy to H3N2 in that study was believed to be related to mutations in the egg-adapted H3N2 strain rather than antigenic drift in circulating viruses and underscores the need to monitor vaccine viruses as well as circulating strains to explain vaccine performance [26]. The latest report on the influenza activity in the United States during 2016–2017 indicates vaccine effectiveness in HIV− adults of 34% for H3N2 and 56% for B strain [34]. Adjusting proportions of antigens in combination vaccines could potentially improve responses to less immunogenic strains like H3N2.
The falloff in response observed at T3 might be remarked as germane to the waning efficacy in seasonal influenza vaccination, and it is possible that delaying the time of vaccination to October or November instead of August or September in older adults could offer protection later in the season. As influenza vaccine in the United States does not contain adjuvants, increasing vaccine dose is another strategy for better engagement of immune system for improving the vaccine response. Centers for Disease Control and Prevention offers a ‘high-dose vaccine’ that contains four times the amount of antigen as the regular vaccine as an optional choice for people at least 65 years for improving vaccine efficacy in older population [35]. In a clinical trial, this dose resulted in 24% fewer influenza infections in adults at least 65 years compared with the standard dose [36]. Although the high-dose vaccine has been approved in the US since 2009, it is not in regular use. Several novel adjuvants and newer vaccine approaches (reviewed in [37]) utilizing synthetic peptides, virus-like particles and viral vectors are under investigation or in development. A universal conserved vaccine that protects from antigenic drift and antigenic shift and confers lifelong immunity, circumventing the need for yearly seasonal vaccinations, could prove highly beneficial [37-39]. Based on our findings, we recommend that measures that improve influenza vaccine responses should be considered. For example, high-potency vaccine recommendations for the elderly could be extended to include HIV+ of all ages. Alternatively, booster vaccinations for all vulnerable populations may be worth investigating.
Supplementary Material
Acknowledgements
We thank Miami Center for AIDS Research (Miami CFAR) Laboratory science core for blood processing and Miami CFAR clinical and behavioral science cores for recruitment of participants. The Clinical Research Center at University of Miami assisted in procuring blood samples. Margaret Roach and Maria Pallin provided laboratory assistance. We are grateful to the study participants.
This work was supported by National Institutes of Health Grant: R01AI108472 and P30AI073961 to S.P.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65:1–54. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005–2016. 2016, Available at: http://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. [Accessed 21 April 2017].
- 3.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2017–18 influenza season. MMWR Recomm Rep 2017;66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012;24:501–506. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan-Lewis E, Aberg JA, Lee M. Aging with HIV in the ART era. Semin Diagn Pathol 2017;34:384–397. [DOI] [PubMed] [Google Scholar]
- 6.Siegler EL, Brennan-Ing M. Adapting systems of care for people aging with HIV. J Assoc Nurses AIDS Care 2017;28:698–707. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and antiinflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128:92–105. [DOI] [PubMed] [Google Scholar]
- 8.Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med 2012;13:451–460. [PubMed] [Google Scholar]
- 9.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledwaba L, Tavel JA, Khabo P, Maja P, Qin J, Sangweni P, et al. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012;7:e24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep 2010;7:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrando-Martinez S, Ruiz-Mateos E, Romero-Sanchez MC, Munoz-Fernandez MA, Viciana P, Genebat M, et al. HIV infection-related premature immunosenescence: high rates of immune exhaustion after short time of infection. Curr HIV Res 2011;9:289–294. [DOI] [PubMed] [Google Scholar]
- 13.Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M, et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 2016;62:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe 2015;17:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol 2009;333:43–82. [DOI] [PubMed] [Google Scholar]
- 16.George VK, Pallikkuth S, Parmigiani A, Alcaide M, Fischl M, Arheart KL, et al. HIV infection worsens age-associated defects in antibody responses to influenza vaccine. J Infect Dis 2015; 211:1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One 2013; 8:e79816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074794.htm. [Accessed on November 2017].
- 19.Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 2015; 7:281 ra243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008;197:490–502. [DOI] [PubMed] [Google Scholar]
- 21.Seidman JC, Richard SA, Viboud C, Miller MA. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses 2012;6:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 23.Nauta JJ, Beyer WE, Osterhaus AD. On the relationship between mean antibody level, seroprotection and clinical protection from influenza. Biologicals 2009; 37:216–221. [DOI] [PubMed] [Google Scholar]
- 24.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011;30:1081–1085. [DOI] [PubMed] [Google Scholar]
- 26.Flannery B, Thaker SN, Clippard J, Monto AS, Ohmit SE, Zimmerman RK, et al. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness – United States, February 2014. MMWR Morb Mortal Wkly Rep 2014;63:137–142. [PMC free article] [PubMed] [Google Scholar]
- 27.Chadwick EG, Chang G, Decker MD, Yogev R, Dimichele D, Edwards KM. Serologic response to standard inactivated influenza vaccine in human immunodeficiency virus-infected children. Pediatr Infect Dis J 1994;13:206–211. [DOI] [PubMed] [Google Scholar]
- 28.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 2000; 18:3040–3049. [DOI] [PubMed] [Google Scholar]
- 29.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS 2003; 17:2015–2023. [DOI] [PubMed] [Google Scholar]
- 30.Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, et al. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, et al. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009–2013. PLoS One 2015;10:e0118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannan S, Kossenkov A, Kurupati RK, Xiang JZ, Doyle SA, Schmader KE, et al. A shortened interval between vaccinations with the trivalent inactivated influenza vaccine increases responsiveness in the aged. Aging 2015;7:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC). Influenza activity – United States, 2012–13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep 2013;62:473–479. [PMC free article] [PubMed] [Google Scholar]
- 34.Blanton L, Alabi N, Mustaquim D, Taylor C, Kniss K, Kramer N, et al. Update: influenza activity in the united states during the 2016–17 season and composition of the 2017–18 influenza vaccine. MMWR Morb Mortal Wkly Rep 2017;66:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Fluzone high-dose seasonal influenza vaccine. Available at: https://www.cdc.gov/flu/protect/vaccine/qa_fluzone.htm. [Accessed 9 June 2017].
- 36.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645. [DOI] [PubMed] [Google Scholar]
- 37.Krammer F Novel universal influenza virus vaccine approaches. Curr Opin Virol 2016;17:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He F, Leyrer S, Kwang J. Strategies towards universal pandemic influenza vaccines. Expert Rev Vaccines 2016;15:215–225. [DOI] [PubMed] [Google Scholar]
- 39.Wiersma LC, Rimmelzwaan GF, de Vries RD. Developing universal influenza vaccines: hitting the nail, not just on the head. Vaccines (Basel) 2015;3:239–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.