Abstract
Double Hit (DH) or Double Expresser (DE) diffuse large B-cell lymphoma (DLBCL) are aggressive Non-Hodgkin’s Lymphomas (NHLs) with translocations and/or over-expression of MYC and BCL-2, that are difficult to treat. Aurora kinase (AK) inhibition with alisertib in DH/DE-DLBCL induces cell death in ~30%, while ~70% are aneuploid and senescent cells (AASCs), a mitotic escape mechanism contributing to drug resistance. These AASCs elaborated a high metabolic rate by increased AKT/mTOR and ERK/MAPK activity via BTK signaling through the chronic active B-cell receptor (BCR) pathway. Combinations of alisertib + ibrutinib or alisertib + ibrutinib + rituximab significantly reduced AASCs with enhanced intrinsic cell death. Inhibition of AK + BTK reduced phosphorylation of AKT/mTOR and ERK-1/2, up-regulated phospho-H2A-X and Chk-2 (DNA damage), reduced Bcl-6 and decreased Bcl-2 and Bcl-xL and induction of apoptosis by PARP cleavage. In a DH/DE-DLBCL SCID mouse xenograft model ibrutinib alone was inactive, while alisertib + ibrutinib was additive with a tumor growth inhibition (TGI) rate of ~25%. However, TGI for ibrutinib + rituximab was ~50–60%. In contrast, triple therapy showed a TGI rate of >90%. Kaplan-Meier survival analysis showed 67% of mice were alive at day-89 with triple therapy versus 20% with ibrutinib + rituximab. All treatments were well tolerated with no changes in body weights. A novel triple therapy consisting of alisertib + ibrutinib + rituximab inhibits AASCs induced by AK inhibition in DH/DE-DLBCL leading to a significant anti-proliferative signal, enhanced intrinsic apoptosis and may be of therapeutic potential in these lymphomas.
Keywords: DLBCL, Double hit lymphoma, Apoptosis, Aurora Kinase, Aneuploid and Senescence, DNA damage
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive type of B-NHL. Double hit (DH)-DLBCL is a subgroup with both MYC (t(8;14)(q24;q32)) and BCL-2 (t(14;18)q32;q21)) gene translocation and protein over expression (1). Double-expresser (DE)-DLBCL is a subgroup with both MYC and BCL-2 protein over expression without translocation of these genes (2). Over expression is characterize by ≥40% cells positive for MYC with ≥70% cells positive for BCL-2 by immunohistochemistry (IHC) (3). Approximately 20–25% of newly diagnosed patients with DLBCL have DH/DE-DLBCL (4). Standard therapies with R-CHOP-like regimens have inferior overall and progression-free survival outcomes in patients with DH/DE-DLBCL (2, 5–8). There is clearly an unmet and challenging need for more effective therapies for DH/DE-DLBCL.
MYC is a transcription factor located on chromosome 8q24 that regulates 10–15% of the human genome including aurora kinases (AK) regulating apoptosis, growth, proliferation and cell cycle (1, 9, 10). As direct pharmacologic inhibition of MYC has remained elusive, inhibiting AKs is a novel approach for treating DH/DE-DLBCL (10, 11). AKs are a family of mitotic serine/threonine protein kinases that play important roles in eukaryotic cell division. AK A localizes to centrosomes and functions in centrosome maturation and proper formation of mitotic spindles (12, 13). AK A is able to transform rodent cells and lead to tumor formation in xenograft mice (14, 15). Importantly in humans, AK A is over-expressed in numerous solid and hematological malignancies (16). Several groups have shown AKs to be over-expressed in aggressive B-/T-NHL including DLBCL (17), MCL (18), peripheral T-cell lymphoma (not otherwise specified) (PTCL [NOS]) (19, 20) and are thought to be a key component of the ‘proliferative’signature. In addition, we demonstrated that AK A and B mRNA levels are predictive of a poor prognosis in MCL (21). Xenograft mouse models of both MCL and DLBCL demonstrated that the AK inhibitor alisertib (MLN8237) alone had anti-tumor activity with a tumor growth inhibition (TGI) of ~30% (21–23). A phase II study of alisertib in relapsed/refractory B- and T-cell NHL patients showed a response rate of ~30% (24). AK inhibition is an effective therapy, however, induction of aneuploidy and senescence cells (AASCs) during therapy (1 week ON/ 2 weeks OFF) allows cells to re-enter the cell cycle during the off therapy and proliferate, a drug resistant mechanism. Hence, mitigating AASCs induced by alisertib is key to enhancing its anti-NHL efficacy.
Bruton’s tyrosine kinase (BTK) downstream of the BCR plays key roles in B-cell maturation, activation, proliferation and survival (25–27) and over-expression of BTK prominent in ABC and DH-DLBCL promotes survival and proliferation (28–30). ERK is downstream of ERK/MAPK pathways have critical role in B-cell development, maintenance and progression (31, 32). On the other hand mTOR is down stream of PI3K/AKT pathway and maintains cellular homeostasis by controlling protein synthesis and glycolytic metabolism (33, 34). Both ERK and mTOR expression is regulated by BTK an up-stream regulator of the chronic active BCR.
Here, we demonstrate that alisertib induced AASCs are large in size and are metabolically active. We hypothesized that abrogating alisertib-induced AASCs will be effective if BTK was simultaneously inhibited since alisertib induced AASCs require active ERK/MAPK and PI3K/AKT/mTOR pathways for maintaining high cellular metabolic demand. We show that alisertib + ibrutinib prevents alisertib induced AASC formation and this effect is further amplified by inhibiting CD20 with rituximab, an indirect BCL2 and BCL-xL inhibitor (35–38). We next tested double and triple therapies in an U2932 DE-DLBCL mouse xenograft model and showed that ibrutinib alone was ineffective, however, alisertib + ibrutinib (AI) and rituximab + ibrutinib (RI) had a (TGI) of ~25 and ~60% respectively. Triple therapy (AIR) had a TGI rate of >90% (p<0.001) with a superior Kaplan-Meier survival. Harvested tumors showed loss of Myc and Bcl-2 expression supporting triple therapy efficacy in this DE-DLBCL model.
Materials and Methods
Cells and reagents:
DH/DE-DLBCL cell lines U2932, VAL, OCI-Ly18, and U2904 were maintained in RPMI 1640 medium (Mediatech, VA) supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. Alisertib and Ibrutinib were purchased (Selleck Chemicals, USA). Rituximab was a kind donation from the West Clinic (Memphis, TN) and UACC (Tucson, AZ). The compounds were dissolved in 10mM in DMSO as a stock solution and then further diluted to desired concentrations for in vitro experiments.
Cell proliferation inhibition (MTS assay):
DH/DE-DLBCL cells were seeded at 10,000 per well in 96-well culture plates and allowed to grow for 24-hr followed by the desired treatment with increasing concentrations of the indicated agents for 4 days. Viable cell densities were determined using a CellTiter 96 Cell Proliferation Assay (Promega). Absorbance readings at 490nm were analyzed against the control group for each drug treatment to determine cell viability. The studies were performed in triplicates x 4 and IC50 values were estimated by Calcusyn software (Biosoft, UK). For combination studies of alisertib and ibrutinib an equipotent ratio was calculated to determine a combined graded combination treatment. A control group was established for each drug treatment in six replicates. The effects of the combined treatments were determined by the combination-index (CI) and isobologram methods derived from the median-effect principle of Chou and Talalay (39).
Tissue microarray and Immunohistochemistry analysis:
We evaluated DLBCL samples (n=41) in a tissue microarray from the SWOG S0515(40) trial for aurora A and BTK expression with validated antibodies (aurora A (Calibiochem, Gibbstown, NJ, cat#PC742) and BTK (PA514770, Thermo Fisher Scientific) (IRB approved protocol). TMA was created by punching 1 mm cores (in duplicate) from representative areas of paraffin embedded tissue blocks as identified by pathologist review of the corresponding hematoxylin and eosin stained sections. The cores were then embedded into a single recipient block and cut at 5μm thickness for IHC. Normal ‘reactive’ LN and Tonsil were used as controls. The staining was scored blindly by an expert Hematopathologist (CS) and rated as 1+, 2+ and 3+. IHC was performed using Aurora A rabbit polyclonal antibody diluted 1:40 and BTK antibody. Tissue sections were stained on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc., Tucson, AZ). All steps were performed on this instrument using VMSI validated reagents, including deparaffinization, cell conditioning (antigen retrieval with a borate-EDTA buffer), primary antibody staining, detection and amplification using a biotinylated streptavidin HRP and DAB (diaminobenzidine) system and hematoxylin counterstaining. Aurora A and BTK were detected separately using a goat anti-rabbit secondary antibody. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and cover slipped with mounting medium (Richard Allan, Logan, UT, Cat #4112).
Polyploidy and Senescence Assays:
Flow cytometry-based assays were utilized to quantify polyploidy and senescence in live cells. Polyploidy was based on membrane permeable dye Hoechst-33342 staining and senescence on hydrolysis of a membrane permeable molecule, 5-dodecanoylaminofluorescein di-β-D galactopyranoside (C12FDG) and β-galactosidase enriched in senescent cells. After hydrolysis and laser excitation, the C12FDG emits green fluorescence and is detected by flow cytometry. The overall scheme of the experiment entails that DH/DE-DLBCL cells are seeded 24hr prior to the treatment with alisertib ± targeted agent. On the day of the experiment, cells are incubated with Bafilomycin A1, then with C12FDG followed by Hoechst-33342 and PI, and analyzed on the flow cytometer. The region of interest is determined on the plot FSC versus SSC to exclude dead cells and cellular debris. The untreated cells are run in the flow cytometer and the red cursor is set. Treated cells are run in BD-LSR-II and the senescent cells appear above the red cursor (41) and analyzed with FlowJo software (FlowJo, LLC). In parallel (same sample, same treatment), the cells are stained using the β-galactosidase assay.
Phospho-Kinase Profiling:
U2932 cells were treated with alisertib 3μM for 7 days to optimize polyploidy. Control and treated cell lysates were incubated utilizing the Proteome Profiler Human Phospho-Kinase Array kit, each spotted in duplicate with antibodies against 43 different kinases and 2 related total proteins (R&D Systems). The semi-quantitative experiment was performed twice with 4 data points per p-protein. Image analysis after correction for background and controls estimates the %p-protein pre- and post-alisertib and compared to the averaged value.
TUNEL Assay for DNA breaks detection:
Cells were treated with alisertib, alisertib + ibrutinib and alisertib + ibrutinib + rituximab. Cells were then fixed with 1% (w/v) paraformaldehyde in PBS followed by 70% (v/v) ethanol at −20°C. Cells are treated with terminal deoxynucleotidyl tranferase enzyme (TdT), fluorescein isothiocyanate (FITC) deoxyuridine triphosphate (FITC-dUTP) for labeling DNA breaks and PI/RNase A solution for counterstaining total DNA as per APO-DIRECT Kit (TONBO bioscience) and run in BD-LSR-II and analyzed with FlowJo software (FlowJo, LLC).
Apoptosis assays:
Annexin V staining was used to detect apoptosis. Treated cells were harvested and rinsed with cold PBS once. After centrifugation for 5 min, cells were suspended in 500 μl of 1X Annexin V binding buffer (BioVision, Mountain View, CA, Annexin V-FITC Reagent Kit, Cat.#1001–1000) and then 5μl of Annexin V-FITC and 5μl of propidium iodide (BioVision, Annexin V-FITC Reagent Kit) were added. After incubation for 5 min at room temperature in the dark, the samples were analyzed by flow cytometry. All studies are performed in triplicate. PARP-cleavage is utilized to detect apoptosis by immunoblotting (see below).
Immunoblotting:
DH/DE-DLBCL cells are lysed in 1X RIPA buffer and supplemented 1:100 Protease/ Phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA). Protein concentrations are determined using the BioRad protein assay kit (Hercules, CA) and 30μg of protein resolved by electrophoresis on a 10% SDS-PAGE. The proteins are transferred onto a nitrocellulose membrane and nonspecific binding is blocked by incubating with 5% nonfat milk in TBS-T buffer (0.01 M Tris–Cl, 0.15 M NaCl, 0.5% Tween-20, pH 8.0) at room temperature for 1h. The membrane is subjected to the indicated antibodies and fluorescence detected using a LI-COR Odyssey Infrared Imaging System. Anti-phospho-Btk (Tyr223) (AB #68217) was purchased from Abcam (Cambridge, MA). C-Myc (SC# 40), Anti-Bcl-2 (SC# 783) antibody was purchased from Santa Cruz Biotechnology, Inc (Dallas, TX). Anti-aurora A (CST #14475) and anti-aurora B (CST #3094), Anti-phospho-aurora A (Thr288) (CST #3079), anti-Akt (CST# 4691), anti-phospho-Akt (Ser473) (CST #4060), anti-phospho-Bcl-2 (CST #anti-2827), Bcl-6 (CST #5650), anti-Bcl-xL (CST # 2762), anti-Btk (CST# 8547), anti-ERK1/2 (CST# 4695), anti-phospho-ERK1/2 (CST# 4370), anti-phospho-Histone H2A.X (CST #5438), anti-PARP (CST #9542) and anti-GAPDH (14C10) (CST #2118), anti-β-Actin (CST# 3700) antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Mouse xenograft model:
Animal care and treatment were performed at the University of Arizona (Experimental mouse shared resource, EMSR). SCID mice were injected with 1X107 U2932 cells in matrigel subcutaneously into the right hind flank. When tumors reached a volume of ~200 mm3, mice were divided randomly (pair-matched) into different groups with 12 mice per cohort. The mice were treated with alisertib [A, 30 mg/kg PO, QD for 3 weeks], rituximab [R, 10 mg/kg IV, once/week, X4] and ibrutinib [I, 10 mg/kg PO, QD for 3 weeks] alone at indicated dosages or different combinations [AI, IR, AIR] with the above doses and schedules. The length (L) and width (W) of the subcutaneous tumors were measured by calipers and the tumor volume (TV) was calculated as: TV = (LxW2)/2. Mice were sacrificed at the end of treatment (3 mice per cohort), end of study (all remaining mice) or if tumor volume reached ~2000 mm3 at any time during the study. Excised tumors (end of the treatment) were either fixed in paraffin for IHC analysis or snap frozen for Western blotting and expression profiling studies.
Statistical analysis:
In vitro experiments were performed in triplicate. The data were expressed as mean ±S.D. The difference between two mean values was evaluated using the Student’s t-test and considered to be statistically significant when p≤0.05. Statistical analysis of the mouse xenograft model data was performed by estimating the tumor growth for each mouse by fitting the least squares regression line of the tumor volume by day. The cube root of the observed tumor volumes was used to induce linearity in the raw data values. The slope of the regression line measures the tumor growth rate. Analysis of variance was used to test for the overall treatment effects on tumor growth inhibition (TGI). Tukey’s studentized range test was used to assess the significance of pair-wise differences between the groups adjusted for multiple comparisons. Survival of the mice was measured from the date of pair matching to sacrifice (event) or end of study (censored). The Kaplan-Meier method was used to estimate survival. The log rank test was used to compare survival between the respective treatment groups. P-value adjustments were made for multiple comparisons using SidakSD multiple comparison test. Analysis was performed using Prism (Graphpad, La Jolla, CA). All p-values≤0.05 were considered statistically significant.
Results
AK-A and BTK are co-up expressed in patient samples and in DH/DE-DLBCL cell lines
Forty-one CD20+ DLBCL samples in a TMA from the SWOG S0515 trial (40) were evaluated for expression of AK-A and BTK with a ‘reactive’ lymph node control. AK-A expression had reactivity with 2+ staining (nuclear) in ~25% samples, whereas BTK showed both membranous and cytoplasmic positivity with 2+ staining in ~37% (Fig 1A) and is co-expressed with AK-A. DH/DE-DLBCL cell lines U2932, U2904, VAL, OCI-Ly18 co-express AK-A and BTK as well as c-Myc and Bcl-2 indicative of DH/DE-DLBCL (Fig 1B).
Figure 1. AK-A and BTK are co-expressed in patient samples and in DH/DE-DLBCL cell lines.
(A) IHC shows Aurora-A and BTK are co-expressed in DLBCL patients (S0515), (B) Western blotting shows c-Myc, Aurora-A, BCL2 and BTK over expressed in DH/DE-DLBCL cell lines.
Aneuploidy and senescent cells (AASCs) are responsible for treatment failure with single agent AK-A inhibition
Inhibition of AK-A results in apoptosis of ~30% of B-NHL cells (Fig 2A) which is similar to response rates observed in lymphoma trials (24). ~70% cells escape death in mitosis which is responsible for treatment failure or drug resistance. In order determine the reason(s) for treatment failure, DH/DE-DLBCL cell lines were treated with 50nM alisertib (IC50) and investigated for metabolic activity of treated cells which increased with time: 1.5-fold (day 2), 2.3-fold (day 4) and 3.7-fold (day 6), and corresponded to increased size of treated cells versus control which correlated with increased number of aneuploid cells (Fig 2B). Unusually high metabolic rates of polyploid cells require high mTOR and/or ERK activity. We determined that cells that survived alisertib therapy are predominantly aneuploid compared to cells undergoing apoptosis, which had 2n status (Fig 2C). In order to quantify the fraction of senescent cells, we utilized the C12-FDG flow assay (41) which demonstrated ~25% of alisertib treated cells enter senescence. Further investigation showed these senescent cells were aneuploid with respect to DNA content (Fig 2D). These findings show that AASCs may be responsible for alisertib treatment failure or resistance and can be mitigated by deciphering the underlying mechanisms of aneuploidy and senescence.
Figure 2. Aurora inhibition results in aneuploidy and senescent cells (AASCs) responsible for treatment failure.
U2932 cells are treated with 50nM alisertib for 4 days and analyzed by flow cytometry show (A). Alisertib as a single agent induces cell death in ~30% of cells. (B) Aneuploid cell have an increase in cell size and are metabolically active. (C). Aneuploid cells form the major proportion of cells that avoid cell death. (D) Aneuploid cells are also representative of senescence.
Aurora inhibition induces a DNA damage response promoting senescence
To examine underlying mechanisms of alisertib induced AASCs, we performed phospho-kinase profiling in U2932 cells. This semi-quantitative experiment was performed twice with 4 data points per p-protein. The p-protein response to alisertib showed increased phospho-p53 (S-15) and phospho-Chk-2 (Fig 3A) indicating AK A modulates p53 activity (42) and promotes p53 phosphorylation inducing a DNA damage response guiding cells towards apoptosis likely mediated by Chk-2 (43). Phosphorylation of p38α decreased which is indicative of cells exiting the cell cycle thereby enhancing a senescence phenotype (44) (Fig 3A). Phosphorylation of Hck and Akt increased (Fig 3B) which are directly downstream of BCR signaling (45) indicating AK A inhibition induces a DNA damage response but BCR signaling overcomes the aurora inhibitory effect shown by Western blotting (Fig 3C).
Figure 3. Aurora inhibition induces a DNA damage response promoting senescence.
U2932 cells were treated with 3.0μM alisertib for 7 days to optimize aneuploidy followed by phospho kinase profiling shows (A) Up regulation of a DNA damage response (B) Activation of BCR signaling. (C). U2932 cells treated with 50nM alisertib for 4 days followed by Western blotting shows that the BCR signaling pathways active in alisertib treated cells. (D). The activated BCR pathway is inhibited by ibrutinib, a BTK inhibitor.
IgM cross-linking of the BCR leads to activation of downstream signaling of p-BTK/BTK and p-AKT/AKT. Addition of ibrutinib inhibits activation of BTK, and AKT in a dose dependent manner (Fig 3D). Total BTK and AKT protein level are unchanged indicating decreased pTyr223 is due to inhibition of phosphorylation and not to BTK degradation or down-regulation. Moreover, HCK is a target of the BTK inhibitor ibrutinib (29, 46) providing a strong rationale for combination therapy of alisertib with ibrutinib to inhibit BCR signaling.
Triple therapy with alisertib + ibrutinib + rituximab mitigates alisertib induced AASCs in DH/DE-DLBCL and induces apoptotic cell death through DNA damage
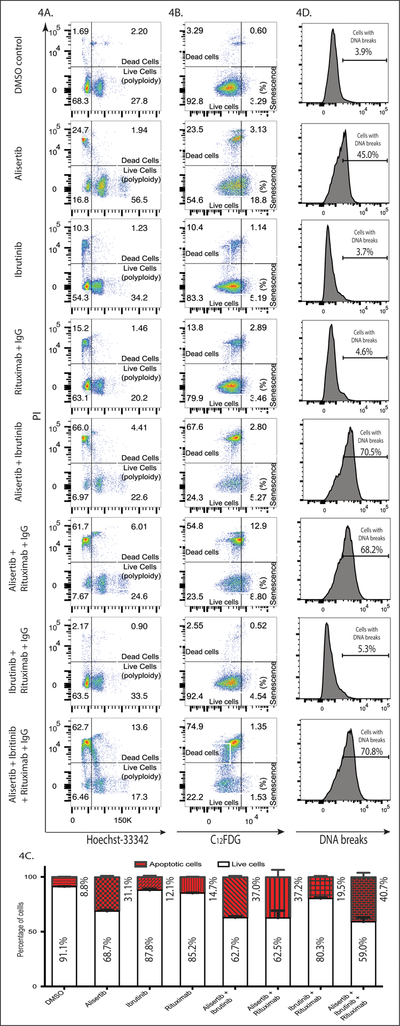
To determine the effect of combination of treatment DH/DE-DLBCL cell lines were treated with alisertib, ibrutinib, rituximab and combinations with respective IC50 concentrations. Triple therapy significantly increased the dead cell population (>75%) compared to single or double therapies. In addition, triple therapy was significantly effective in reducing the aneuploidy cell population compared to single agent alisertib (30.90% vs. 58.44%) (Fig 4A). In contrast, triple therapy significantly lowered the senescence population (2.88%) compared to single agent alisertib (21.93%) (Fig 4B). Together, our data shows that triple therapy prevent AASCs thereby mitigating alisertib failure.
Figure 4. Triple therapy with alisertib + ibrutinib + rituximab mitigates alisertib induced AASCs in DH/DE-DLBCL and induces apoptotic cell death through DNA damage.
U2932 cells were treated with 50nM alisertib, 2.0μM ibrutinib, 10μg/ml cross-linked rituximab and their combination for 4 days. Flow cytometry data shows that with triple therapy (A) The aneuploid population is reduced (B) The senescent population is also reduced (C) Induces significant apoptosis and (D) DNA breaks evident by TUNEL assay.
To investigate cell death mechanisms, each treatment modalities were evaluated for cellular necrosis vs. apoptosis. It was evident that apoptosis is the cause of cell death. Triple therapy induces a significantly higher percentage of apoptosis compared to single therapy or double therapies (Fig 4C). TUNEL assay further showed that triple therapy induces DNA breaks in ~70% of cells which is significantly higher than other drug combinations (Fig 4D). These finding led us to further investigate pathways responsible for inducing apoptosis with triple therapy.
Aurora kinase plus BTK blockade inhibits the chronic active BCR pathway and induces apoptosis in DH/DE-DLBCL
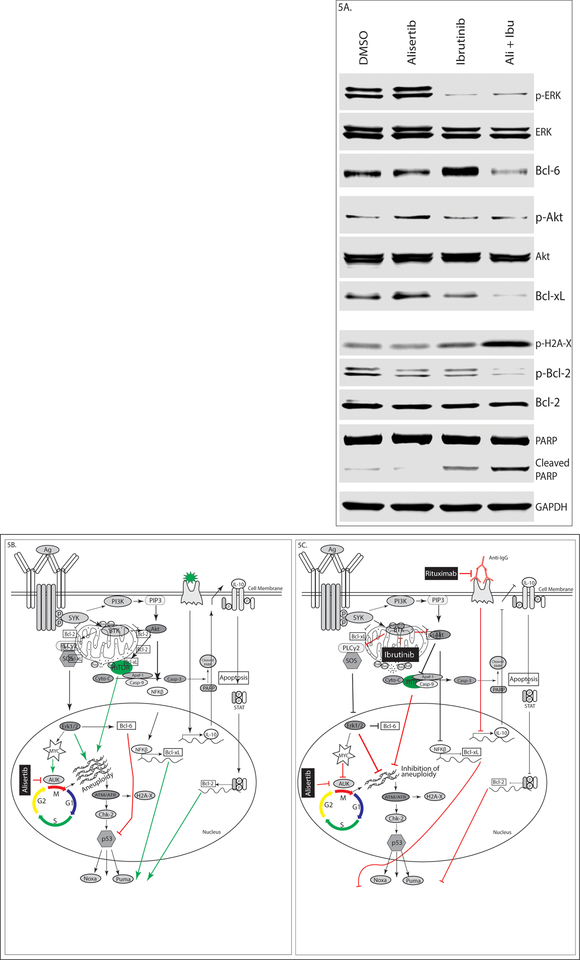
AK is a direct target of the ERK/MAPK pathway and its chronic up regulation is induced by hyper-activation of this pathway (47). Inhibiting AK-A as a single induces DNA damage which is not sufficient for inducing significant apoptosis due to mutated p53 and over expression Bcl-6 which inhibit p53 in U2932 cells. On the other hand, anti-apoptotic proteins Bcl-2 and Bcl-xL over-expressed in U2932 cells that retain mitochondrial outer-membrane potential. In addition, up regulated BTK constitutively activates mTOR/AKT and ERK/MAPK pathways, which ultimately results in metabolically active aneuploid cells during AK-A inhibition (Fig 5B). But concurrent inhibition of AK-A plus BTK reduces the phosphorylation of ERK-1/2 and AKT/mTOR, preventing aneuploidy with attendant increased DNA breaks demonstrated by up- regulation of phospho-H2A-X. Double combination also reduces the expression of Bcl-6 and anti-apoptotic Bcl-2, Bcl-xL that ultimately results in a compromised mitochondrial outer-membrane potential evident by PARP cleavage (endo apoptotic pathway) (Fig 5A & 5C). Adding rituximab in vivo further exacerbates apoptosis (see mouse model).
Figure 5. Aurora kinase plus BTK blockade inhibits the chronic active BCR pathway and induces apoptosis in DH/DE-DLBCL.
U2932 cells were treated with 50nM alisertib, 2.0μM ibrutinib and their combination for 4 days. Western blotting shows (A) Down regulation of the MAPK and PI3K/AKT pathways but up regulation of DNA damage response, (B) Single agent alisertib induces aneuploidy due to active mTOR/AKT and ERK/MAPK pathways. But combination therapy able to overcome aneuploidy and senescence illustrated in (C).
Triple therapy is safe and effective in a DH/DE-DLBCL mouse xenograft model
Based on our in vitro data, we conducted a mouse xenograft model with the U2932 cell line to evaluate triple therapy (alisertib + ibrutinib + rituximab) versus single agent or other combinations. We excluded single agent alisertib and rituximab as they were evaluated in the same mouse model from a previous study (23). In the U2932 mouse model ibrutinib alone was not active and alisertib alone had a TGI of ~10% [27]. However, Alisertib + ibrutinib was additive with a TGI ~25%. In contrast, ibrutinib + rituximab showed a TGI ~50–60% over ibrutinib alone indicating blocking BTK and CD20 is an effective therapeutic modality in DH/DE-DLBCL. However, alisertib + ibrutinib + rituximab showed a TGI of >90% indicating significant synergy (p<0.001) (Fig 6A). All treatments were well tolerated with stable body weights (<5% body weight loss). Kaplan-Meier survival showed 67% of mice alive at day 89 in the triple therapy group versus 20% for ibrutinib + rituximab. No mice from the other treatment groups survived past day 52 (Fig 6B). Western blotting of tumors harvested at the end of treatment showed that triple therapy abolished MYC and BCL-2 expression. In addition, p-Akt was also significantly reduced (Fig 6C). Concurrent targeting of AKA, BTK and CD20 is safe and effective in DH/DE-DLBCL.
Figure 6. Triple therapy is safe and effective in a DH/DE-DLBCL mouse xenograft model.
U2932 xenograft SCID mice (n = 12/cohort) treated with saline (control), ibrutinib 12.5 mg/kg), MLN8237 (30 mg/kg), rituximab (10 mg/kg) and their combination. MLN8237 or ibrutinib was given once daily × 3 weeks PO, rituximab once every week × 4 weeks IV. Tumors grow to ~200 mm3 prior to initiating treatment. The arrows point to start and end of treatments. (A) Tumor burden (volume) represented as mean ± SEM. (B) Kaplan-Meier survival curve (C) Western blotting of harvested mouse tumors show triple therapy effectively reduce Myc and Bcl-2 protein levels.
Discussion
The current standard of care for de novo DLBCL is rituximab (Rituxan) plus CHOP-like (cyclophosphamide, doxorubicin, vincristine, prednisone) [R-CHOP] therapies irrespective of cell-of-origin or genetic heterogeneity (48). AKs [A and B] are important drivers of proliferation in NHLs (49) and are amenable to targeted therapy. Pre-clinical studies (19–23) and clinical trials (24, 50) with a specific AK-A inhibitor alisertib demonstrated significant anti-tumor activity with a good safety profile. However, response to single agent alisertib is not sustained due to alisertib-induced aneuploidy and senescence cells (AASCs) that escape mitotic apoptosis. The maximum tolerated dose of alisertib is 50 mg PO BID 1 week ON and 2 weeks OFF (24). During the off 2 weeks of alisertib these AASCs re-enter the cell cycle and resume proliferation and/or remain senescent, a common mechanism of drug failure attributable to many chemotherapeutic agents.
We explored how alisertib-induced AASCs could be inhibited by selectively targeting BTK and CD20 thus promoting apoptosis in DH/DE-DLBCL. We show that AK inhibition with alisertib induces a DNA damage response. Ibrutinib an irreversible BTK inhibitor alone is able to inhibit p-BTK and p-AKT thereby ERK and mTOR respectively. This provided the rationale for combining alisertib with ibrutinib to inhibit alisertib induced AASCs by inhibiting the chronic BCR pathway. In addition, since BTK and AK-A are both co-expressed in DLBCL (patient samples and cell lines), concurrent treatment with ibrutinib plus alisertib, inhibited AASC formation, inactivated p-AKT, p-ERK and p-BTK and promoted apoptosis through the intrinsic pathway. Together, the data indicate that in DH/DE-DLBCL alisertib-induced AASCs, a phenotypic drug resistant state that can be abrogated by inhibiting BTK and CD20 by activating the intrinsic apoptotic pathway.
Phospho-proteomic analysis of alisertib treated U2932 cells which harbors a pathogenic TP53 mutation showed that AK inhibition promotes p53 phosphorylation at pS15 inducing a DNA damage response that up regulates Chk-2 (43) promoting senescence. AK-A, a direct target of the ERK/MAPK pathway, decreased pMAPK14 (p38α) when treated with alisertib (47). In addition, HCK is concurrently activated and is a direct target of ibrutinib that supports an alisertib-induced AASC phenotype.
A DH/DE-DLBCL mouse xenograft model of U2932 evaluated ibrutinib versus alisertib + ibrutinib ± rituximab. In this model Ibrutinib alone was inactive. Alisertib + ibrutinib was additive with a TGI ~25% versus alisertib ~10% TGI (23). In contrast, Ibrutinib + rituximab showed a TGI ~50–60% over ibrutinib alone indicating blocking BTK and CD20 is an effective therapeutic modality in DH/DE-DLBCL. However, alisertib + ibrutinib + rituximab showed a TGI of >90% indicating significant synergy (p<0.001). All treatments were well tolerated with stable body weights (<5% body weight loss). Kaplan-Meier survival showed 67% of mice alive at day 89 in the triple therapy group versus 20% for ibrutinib + rituximab. No mice from the other groups survived beyond day 52. Together the data indicate that although rituximab + ibrutinib is more active than alisertib + ibrutinib, the addition of alisertib to rituximab + ibrutinib is highly synergistic emphasizing the role of alisertib as an important anti-lymphoma drug in DH/DE-DLBCL. The mechanistic implications are that targeting the chronic active BCR pathway and CD20 critical to cell survival and proliferation can be targeted to eliminate AASCs induced by alisertib.
We have demonstrated that single agent alisertib treatment leads to AASCs, a mechanism that blunts its anti-DLBCL response. Previously, we showed alisertib sensitized and synergized DH/DE-DLBCL cells to vincristine plus rituximab (23). Here, we demonstrate that alisertib synergizes with ibrutinib plus rituximab by eliminating alisertib induced AASCs. Hence, we propose that alisertib plus vincristine plus ibrutinib when combined with rituximab would be an optimal therapeutic strategy in DH/DE-DLBCL patients relapsing after R-CHOP-like therapies. A clinical trial concept has been written for an investigator-initiated clinical trial in relapsed and refractory aggressive DLBCL based on the successfully completed alisertib + vincristine + rituximab study (23) which shows that this combination was well tolerated and had anti-B-NHL activity.
Perspective:
A major barrier to successful therapy in double hit (DH) and double expresser (DE) diffuse large B-cell lymphoma (DLBCL) is drug induced aneuploidy and senescence. We have investigated novel-novel targeted drug combinations based on aurora inhibition that disrupts aneuploidy and senescence in DH/DE- DLBCL.
Acknowledgements:
We wish to thank The Hope Foundation (SWOG), Lymphoma SPORE and the West Clinic/UTHSC for funding. The mouse xenograft studies were conducted by the UACC experimental mouse shared resources (EMSR).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References:
- 1.Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. 2015;16(15):e555–67. [DOI] [PubMed] [Google Scholar]
- 2.Burotto M, Berkovits A, Dunleavy K. Double hit lymphoma: from biology to therapeutic implications. Expert Rev Hematol. 2016;9(7):669–78. [DOI] [PubMed] [Google Scholar]
- 3.Sweetenham DMSJW. Clinical Controversies of Double-Hit Lymphoma. ajhocom. 2015. [Google Scholar]
- 4.Dunleavy K Optimal Management of Double-Hit Lymphoma. J Oncol Pract. 2016;12(3):241–2. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–31; quiz 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253–63. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(28):3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(28):3460–7. [DOI] [PubMed] [Google Scholar]
- 9.den Hollander J, Rimpi S, Doherty JR, Rudelius M, Buck A, Hoellein A, et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116(9):1498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi Y, Sheridan P, Niida A, Sawada G, Uchi R, Mizuno H, et al. The AURKA/TPX2 axis drives colon tumorigenesis cooperatively with MYC. Ann Oncol. 2015;26(5):935–42. [DOI] [PubMed] [Google Scholar]
- 11.Stine ZE, Dang CV. Splicing and Dicing MYC-Mediated Synthetic Lethality. Cancer cell. 2015;28(4):405–6. [DOI] [PubMed] [Google Scholar]
- 12.Terada Y, Uetake Y, Kuriyama R. Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J Cell Biol. 2003;162(5):757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kufer TA, Nigg EA, Sillje HH. Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma. 2003;112(4):159–63. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20(2):189–93. [DOI] [PubMed] [Google Scholar]
- 15.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link ? Trends in cell biology. 2005;15(5):241–50. [DOI] [PubMed] [Google Scholar]
- 16.Mortlock AA, Keen NJ, Jung FH, Heron NM, Foote KM, Wilkinson RW, et al. Progress in the development of selective inhibitors of aurora kinases. Curr Top Med Chem. 2005;5(8):807–21. [DOI] [PubMed] [Google Scholar]
- 17.Hamada M, Yakushijin Y, Ohtsuka M, Kakimoto M, Yasukawa M, Fujita S. Aurora2/BTAK/STK15 is involved in cell cycle checkpoint and cell survival of aggressive non-Hodgkin’s lymphoma. British journal of haematology. 2003;121(3):439–47. [DOI] [PubMed] [Google Scholar]
- 18.Camacho E, Bea S, Salaverria I, Lopez-Guillermo A, Puig X, Benavente Y, et al. Analysis of Aurora-A and hMPS1 mitotic kinases in mantle cell lymphoma. International journal of cancer Journal international du cancer. 2006;118(2):357–63. [DOI] [PubMed] [Google Scholar]
- 19.Mahadevan D, Spier C, Della Croce K, Miller S, George B, Riley C, et al. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Molecular cancer therapeutics. 2005;4(12):1867–79. [DOI] [PubMed] [Google Scholar]
- 20.Qi W, Spier C, Liu X, Agarwal A, Cooke LS, Persky DO, et al. Alisertib (MLN8237) an investigational agent suppresses Aurora A and B activity, inhibits proliferation, promotes endo-reduplication and induces apoptosis in T-NHL cell lines supporting its importance in PTCL treatment. Leukemia research. 2013;37(4):434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi W, Cooke LS, Liu X, Rimsza L, Roe DJ, Manziolli A, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochemical pharmacology. 2011;81(7):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahadevan D, Stejskal A, Cooke LS, Manziello A, Morales C, Persky DO, et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(8):2210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahadevan D, Morales C, Cooke LS, Manziello A, Mount DW, Persky DO, et al. Alisertib added to rituximab and vincristine is synthetic lethal and potentially curative in mice with aggressive DLBCL co-overexpressing MYC and BCL2. PloS one. 2014;9(6):e95184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedberg JW, Mahadevan D, Cebula E, Persky D, Lossos I, Agarwal AB, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middendorp S, Dingjan GM, Maas A, Dahlenborg K, Hendriks RW. Function of Bruton’s tyrosine kinase during B cell development is partially independent of its catalytic activity. J Immunol. 2003;171(11):5988–96. [DOI] [PubMed] [Google Scholar]
- 26.Aoki Y, Isselbacher KJ, Pillai S. Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(22):10606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saouaf SJ, Mahajan S, Rowley RB, Kut SA, Fargnoli J, Burkhardt AL, et al. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(20):9524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103(12):4389–95. [DOI] [PubMed] [Google Scholar]
- 29.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28(4):499–508. [DOI] [PubMed] [Google Scholar]
- 32.Mandal R, Becker S, Strebhardt K. Stamping out RAF and MEK1/2 to inhibit the ERK1/2 pathway: an emerging threat to anticancer therapy. Oncogene. 2016;35(20):2547–61. [DOI] [PubMed] [Google Scholar]
- 33.Eltschinger S, Loewith R. TOR Complexes and the Maintenance of Cellular Homeostasis. Trends in cell biology. 2016;26(2):148–59. [DOI] [PubMed] [Google Scholar]
- 34.Liu LL, Long ZJ, Wang LX, Zheng FM, Fang ZG, Yan M, et al. Inhibition of mTOR pathway sensitizes acute myeloid leukemia cells to aurora inhibitors by suppression of glycolytic metabolism. Molecular cancer research : MCR. 2013;11(11):1326–36. [DOI] [PubMed] [Google Scholar]
- 35.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99(3):1038–43. [DOI] [PubMed] [Google Scholar]
- 36.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology. 2002;107(2):176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alas S, Ng CP, Bonavida B. Rituximab modifies the cisplatin-mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-Hodgkin’s lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(3):836–45. [PubMed] [Google Scholar]
- 38.Stolz C, Hess G, Hahnel PS, Grabellus F, Hoffarth S, Schmid KW, et al. Targeting Bcl-2 family proteins modulates the sensitivity of B-cell lymphoma to rituximab-induced apoptosis. Blood. 2008;112(8):3312–21. [DOI] [PubMed] [Google Scholar]
- 39.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70(2):440–6. [DOI] [PubMed] [Google Scholar]
- 40.Stopeck AT, Unger JM, Rimsza LM, LeBlanc M, Farnsworth B, Iannone M, et al. A phase 2 trial of standard-dose cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) and rituximab plus bevacizumab for patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: SWOG 0515. Blood. 2012;120(6):1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahu J, Sola B. A sensitive method to quantify senescent cancer cells. J Vis Exp. 2013(78). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warnock LJ, Raines SA, Milner J. Aurora A mediates cross-talk between N- and C-terminal post-translational modifications of p53. Cancer Biol Ther. 2011;12(12):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 44.Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, et al. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 2005;169(1):105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, et al. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110(7):2511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang G, Buhrlage SJ, Tan L, Liu X, Chen J, Xu L, et al. HCK is a survival determinant transactivated by mutated MYD88, and a direct target of ibrutinib. Blood. 2016;127(25):3237–52. [DOI] [PubMed] [Google Scholar]
- 47.Bonet C, Giuliano S, Ohanna M, Bille K, Allegra M, Lacour JP, et al. Aurora B is regulated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and is a valuable potential target in melanoma cells. The Journal of biological chemistry. 2012;287(35):29887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahadevan D, Fisher RI. Novel therapeutics for aggressive non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(14):1876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green MR, Woolery JE, Mahadevan D. Update on Aurora Kinase Targeted Therapeutics in Oncology. Expert opinion on drug discovery. 2011;6(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kevin R Kelly DOP, Daruka Mahadevan, Miller Thomas P., Puvvada Soham D., Kevin McDonagh, John Hayslip, Park Steven I., Jia Ruan, Rosen Peter J., Swaminathan Padmanabhan Iyer, Alexandra Stefanovic, Bin Zhang, Xiaofei Zhou, Claudio Dansky Ullmann, Leonard E. Jane, Friedberg Jonathan W.. Phase 1 Study Of Investigational Agent MLN8237 (Alisertib) + Rituximab ± Vincristine In Patients (Pts) With Relapsed/Refractory (Rel/Ref) Aggressive B-Cell Lymphomas. Blood. 2013;122(21):3027. [Google Scholar]