Abstract
We have recently reported that the expression of a tight junction protein, claudin-1, is increased during colon carcinogenesis and particularly metastatic colorectal cancer. Manipulation of claudin-1 levels in colon cancer cells showed a positive correlation between claudin-1 expression and tumor growth and metastasis. However, the mechanisms underlying the increased claudin-1 expression in colorectal cancer remains unknown. The tumor suppressor Smad4 is a central intracellular signal transduction component of the transforming growth factor-(β (TGF-β) family of cytokines. Loss of Smad4 protein expression is correlated with poor prognosis and is frequently observed in invasive and metastatic colorectal carcinoma. In the present study, we report an inverse relationship between Smad4 and claudin-1 expression in human colorectal carcinoma tumor samples and in human colon cancer cell lines. We found that the expression of Smad4 in Smad4-deficient but claudin-1-positive SW480 or HT29 colon cancer cell lines down-regulates claudin-1 expression through transcriptional repression by modulating (β-catenin/ T-cell factor/lymphocyte enhancer factor activity. Furthermore, this Smad4-dependent inhibition of claudin-1 expression is independent of TGF-β(3signaling because Smad4 expression alone is insufficient to restore TGF-β signaling in the SW480 cells, and the selective TGF-β receptor kinase inhibitor LY364947 did not prevent the Smad4 suppression of claudin-1 protein expression in either SW480 or HT29 cells. Taken together, these findings suggest a novel mechanism underlying Smad4 tumor-suppressive function through regulation of a potential metastatic modulator, claudin-1, in a TGF-B-independent manner.
Introduction
Disruption of the cell-cell junctions with concomitant changes in the expression of junctional proteins is a critical event in invasive and metastatic progression in cancer. Claudins are integral to the structure and function of tight junctions, and the altered expression of claudins in a tissue-specific manner has been detected in several types of cancers. Furthermore, dysregulated expression/localization of claudins, including claudin-1, has been implicated in tumor progression and metastasis (1). For example, the reduced expression of claudin-7 at the invasive front of esophageal squamous cell carcinoma has been correlated with tumor progression and lymph node metastasis (2), and claudin-4 is shown to be a potent inhibitor of the invasiveness and metastatic phenotype of pancreatic cancer cells (3). Conversely, claudin-3–and claudin-4–expressing ovarian epithelial cells were shown to exhibit increased invasion and have increased matrix metalloproteinase-2 (MMP-2) activity, indicating that claudin-mediated increased invasion might be mediated through the activation of MMP proteins (4). In addition, Oku et al. showed that claudin-1 causes increased cancer cell invasion through the activation of MT1-MMP and MMP-2 in oral squamous cell carcinoma (5). We have also recently shown that claudin-1 expression is progressively increased in human colon carcinogenesis, with the highest expression in metastatic samples and cell lines derived from metastatic sources (1). Further manipulation of claudin-1 levels in colon cancer cells showed a role of claudin-1 in colon tumor progression and metastasis. However, mechanisms underlying the dysregulated expression/localization of claudin-1 in colon cancer are not known.
The transforming growth factor-β (TGF-β) family of cytokines is composed of TGF-βs, activins, and bone morphogenetic proteins (6). Smad4 has a central role in transducing receptor-mediated and Smad-dependent signaling to the nucleus by all members of the TGF-β family. Loss of Smad4 function either due to loss of its expression or genetic mutation has been reported in a significant proportion of colon and pancreatic cancer (7–9). Frequent loss of Smad4 expression in tumors that have acquired invasive and metastatic phenotype strongly implicates tumor-suppressive functions of Smad4 during cancer progression (10). Although Smad4 is a critical mediator of TGF-β–induced growth inhibitory responses (11), recent evidence has suggested additional molecular mechanisms underlying the tumor suppressor effects of Smad4. For example, Smad4 restoration in the Smad4-deficient SW480 colon carcinoma cells resulted in loss of tumorigenicity of these cells in nude mice that was accompanied by the reestablishment of a more epithelioid phenotype and increased expression of E-cadherin and P-cadherin (12). Interestingly, Smad4 restoration did not restore in vitro growth inhibition of these cells in response to TGF-β, and this lack of response was attributed to low levels of type II TGF-β receptor resulting in deficient TGF-β signaling (12). These data suggest that Smad4 may elicit distinct tumor-suppressive responses independently of TGF-β signaling.
In the present study, we report an inverse relationship between the expression of claudin-1, a metastasis-promoting protein, and Smad4, a tumor suppressor protein, in colon cancer cell lines and in human colon cancer tissue samples. We further show that Smad4 expression in Smad4-deficient colon cancer cells inhibits claudin-1 expression through transcriptional regulation. Further analysis suggests the important role of β-catenin/T-cell factor (TCF)/lymphocyte enhancer factor (Lef) activity in the Smad4- dependent regulation of claudin-1 expression. In addition, the inhibition of claudin-1 expression contributes to the ability of Smad4 to inhibit invasion in colon cancer cells. Lastly, we find that Smad4-dependent inhibition of claudin-1 expression is a direct effect of Smad4 expression and not due to modulation of TGF-β signaling.
Materials and Methods
Cell cultures and reagents
Rat intestinal epithelial cells, a kind gift from Dr. Ken Brown (Babraham Institute, Babraham, Cambridge, United Kingdom), and the HCT 15, HCT 116, SW480, SW620, and HT29 cells from the American Type Culture Collection (Manassas, VA) were cultured in the RPMI 1640 media supplemented with 10% fetal bovine serum. TGF-β was purchased from R&D Systems (Minneapolis, MN). The pharmacologic selective inhibitor of TGF-β type I receptor kinase (LY364947) was kindly provided by Eli Lilly (Indianapolis, IN; ref. 13). Stable SW480 clones were obtained by transfection with the pCMV-Script Smad4 expression vector, followed by selection in 1 mg/mL G418 media. The p3TP-Lux reporter was a generous gift from Dr. Joan Massague (HHMI, Memorial Sloan-Kettering Cancer Center, New York, NY; ref. 14).
Plasmid construction
To construct the luciferase reporter plasmid under the control of the claudin-1 promoter, the −1160 to +160 upstream area of the human claudin-1 gene was amplified by PCR using human genomic DNA and a set of forward and reverse primers containing custom MluI and XhoI restriction enzyme sites, respectively. The sequences of the primers are 5′-TCGACGCGTACTCGCACCACACACAAAAA-3′ (forward primer with MluI site underlined) and 5′-CCGCTCGAGGTGCAGAAGGCG- GAGAGTT-3′ (reverse primer with Xho I site underlined). The PCR product was cloned into the promoterless luciferase vector, pGL3-Basic (Promega, Madison, WI) and the sequence of the PCR product was confirmed by sequencing analyses. A constitutively active β-catenin (S33Y) mutant was used for transfection studies, which is insensitive to GSK-3β–mediated phosphorylation and proteasomal degradation.
Overexpression of Smad4 by adenoviral infection
To amplify adenoviruses, the packaging 293T cells cultured in 5% serum DMEM at 80% confluence on P100 plates were infected with adenoviruses in 1 mL fresh 5% serum medium with rocking. Infected cells were allowed to grow for 3 days before being trypsinized and collected in 1 mL of 5% fetal bovine serum medium and subject to three freeze/thaw cycles at − 20°C/37°C. Adenovirus-containing supernatant was obtained from the cell suspension. The relative values of multiplicity of infection of 10 to 20 were determined by examining the cytopathic effect in 293T cells 3 days postinfection. To perform adenoviral-mediated overexpression of Smad4, 70% to 80% confluent cells were washed with PBS once and infected with adenovirus for 3 h, and then returned to regular serum media. Lysates were harvested 48 to 72 h postinfection.
Immunoblot analysis
Cells were lysed in radioimmunoprecipitation assay buffer, and Western blotting was carried out as previously described (1), with antibodies to claudin-1, claudin-4, claudin-7 (Upstate Biotechnology, Inc., Lake Placid, NY), Smad4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), actin antibody (Sigma-Aldrich, Inc., St. Louis, MO), and E-cadherin and β-catenin antibodies (BD Biosciences, San Jose, CA).
RNA isolation and semiquantitative reverse transcription-PCR
Total RNA was extracted from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with RNase-free DNase. One microgram of total RNA was reverse transcribed using RETROscript Kit (Ambion, Austin, TX) according to the manufacturer’s instructions. PCR and Northern blots were done by standard protocols as described previously (1, 15).
Transient transfection and promoter study
Cells were seeded on 12- well plates in triplicate and allowed to grow overnight to reach 50% to 70% confluence. The following day, cells were cotransfected with a firefly luciferase reporter construct and a reference construct that contains Renilla reniformis luciferase, phRL-TK (Promega), using LipofectAMINE Plus reagents (Invitrogen). Forty-eight hours later, luciferase activities were measured using the Dual Luciferase Reporter Assay System kit (Promega) in an Optocomp II Luminometer (MGM Instruments, Inc., Hamden, CT). Firefly luciferase activity was normalized to R. reniformis luciferase activity and plotted as mean ± SD from three independent experiments. The activated β-catenin and dominant negative (dn) TCF-4 cDNA vectors were kindly provided by Dr. K.W. Kinzler (Johns Hopkins, Baltimore, MD; ref. 16) and Dr. H. Clevers (Netherlands Institute for Developmental Biology, Upsalalaan, Utrecht, the Netherlands; ref. 17).
Invasion assay Matrigel
Transwells (12 μm pore size, 12 mm in diameter) from Costar (Cambridge, MA) were coated with 100 μL of 2.5 mg/ mL Matrigel and then left in an incubator for 2 h. Cells were trypsinized, washed with PBS, resuspended in 0.2% bovine serum albumin serum-free medium, and then seeded in Transwells (100,000 cells per Transwell). Cells were allowed to grow on Transwells in the presence of 10% fetal bovine serum medium in the lower chamber of the Transwells for 72 h. Cells remaining inside the inserts were removed with cotton swabs, and the cells that had traversed to the reverse side of the inserts were rinsed with PBS, fixed in 4% formaldehyde for 30 min at room temperature, and stained with 1% crystal violet for 1 hour to overnight at room temperature. Cells were counted under a light microscope (at 200× power), and invasive cell numbers were the averages of those from five areas on each insert. Each invasion assay was done in triplicate and repeated thrice.
Results
Claudin-1 and Smad4 expressions are inversely correlated in human colorectal carcinoma samples and cell lines
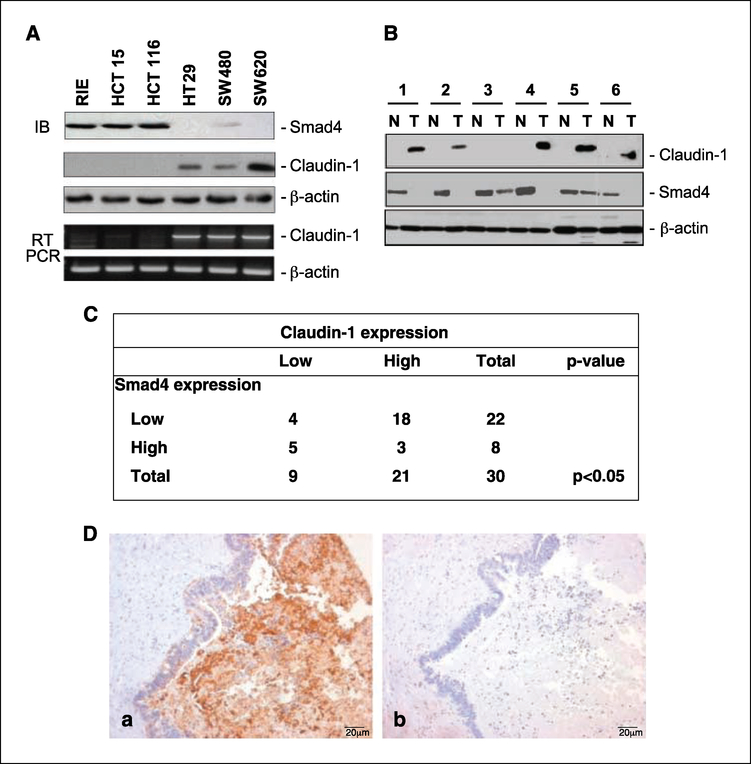
Loss of Smad4 protein expression is associated with advanced stages of colon tumorigenesis and is frequently observed in metastatic samples (18). In contrast, claudin-1 expression positively correlates with tumor progression and metastasis in colon cancer (1). Therefore, we investigated a possible correlation between Smad4 and claudin-1 expressions in colon cancer. A panel of colorectal cancer samples and intestinal epithelial cell lines were examined for claudin-1 and Smad4 expressions. We noted that claudin-1 protein expression was detected only among the cell lines that do not express Smad4 (SW480, SW620, and HT29). In contrast, claudin-1 expression was undetectable at either the protein or mRNA level in Smad4-expressing cell lines (rat intestinal epithelial, HCT 116, and HCT 15; Fig. 1A). We further examined matched human colon samples for a similar inverse relationship between claudin-1 and Smad4. In four out of six sample sets, an inverse relationship between claudin-1 and Smad4 was observed (Fig. 1B). As reported earlier, claudin-1 expression was minimal in normal colon tissues and was increased in tumors. Smad4 was abundantly expressed in normal samples, and expression was often decreased or was undetectable in tumors (Fig. 1B). Based on these observations, we further explored the potential correlation between claudin-1 and Smad4 expressions in a larger population of tissue samples. Immunohistochemical analysis was done for claudin-1 and Smad4 in custom colorectal cancer tissue arrays (tissue microarrays developed at Vanderbilt University by M.K. Washington), which contain surgical specimens from 30 primary and paired metastatic fresh frozen human colorectal cancer samples. As shown in Fig. 1C and D, a significant inverse correlation (P < 0.05) between claudin-1 and Smad4 expressions was observed in 23:30 (76%) metastatic samples, suggesting a reciprocal function of Smad4 and claudin-1 in colorectal cancer. Taken together, these data suggest an inverse correlation between the expressions of Smad4 and claudin-1 in colorectal carcinomas and cell lines.
Figure 1.
Inverse Smad4 and claudin-1 protein expression in matched human colorectal carcinoma samples and cell lines. A, protein lysates and total RNA from the indicated cell lines were obtained as described in Materials and Methods and subjected to immunoblotting (IB) and semiquantitative reverse transcription-PCR (RT-PCR) for claudin-1, Smad4, and β-actin. B, the tissue samples were obtained from six patients with colon cancer; N, normal mucosa; T,primary colon cancer. Protein lysates were prepared from tissues homogenized in a lysis buffer followed with clearance by centrifugation. Equal amounts of lysates were used in immunoblotting assays for claudin-1 and Smad4. Same blots were stripped and reprobed with β-actin to assess equal protein loading. C, correlation between claudin-1 and Smad4 expressions in colon cancer. Data from custom colorectal cancer tissue arrays (tissue microarrays developed at Vanderbilt) that contain surgical specimens from 30 primary and paired metastatic fresβ-frozen human colorectal cancer samples are shown. A significant inverse correlation between claudin-1 and Smad4 expression was observed (P < 0.05) using χ2 approximation in metastatic patient samples. The staining was designated low or high based on staining intensity and the number of cells stained. D, representative staining for claudin-1 (a) and Smad4 (b) for a metastatic patient sample. Sections were counterstained with hematoxylin to determine the structural details.
Restoration of Smad4 expression decreases claudin-1 expression
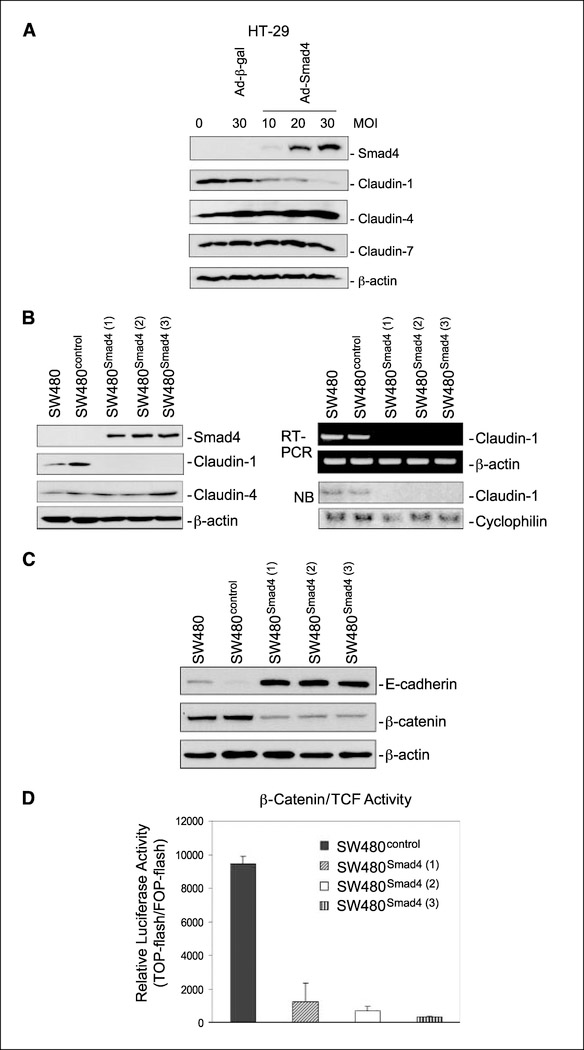
To determine whether the inverse relationship between Smad4 and claudin-1 is causally related, we expressed Smad4 using adenoviral infection in the Smad4-deficient but claudin-1-positive HT29 colon carcinoma cell line. Immunoblotting confirmed the expression of Smad4 only in the Ad-Smad4–infected HT29 cells (Fig. 2A). Claudin-1 expression was decreased in the Smad4-expressing HT29 cells in a dose-dependent manner reaching to its nadir with the highest level of Smad4 expression (Fig. 2A), suggesting a causal role for Smad4 in the inhibition of claudin-1 expression.
Figure 2.
Smad4 reexpression in Smad4-deficient colon cancer cell lines inhibited claudin-1 protein expression. A, HT29 cells were infected with β-galactosidase (Ad-β-Gal) or Smad4 (Ad-Smad4) adenoviruses at the indicated multiplicity of infection (MOI). Seventy-two hours later, cells were lysed, and the lysates were subjected to immunoblotting assays for the indicated proteins.B, protein lysates and total RNA from SW480, SW480control, and three SW480Smad4 clones were obtained and subjected to immunoblotting for the indicated proteins, semiquantitative reverse transcription-PCR for claudin-1 and β-actin expression, and Northern blotting (NB) for claudin-1 and cyclophilin expression. C, Smad4 reexpression restored E-cadherin expression and decreased β-catenin levels. SW480, SW480control, and three SW480Smad4 clones grown on plates were lysed in a lysis buffer, and the whole cell lysates were subjected to immunoblotting for E-cadherin, β-catenin, and β-actin D, effect of overexpression of Smad4 on β-catenin/TCF/ Lef activity. SW480control and three different clones of SW480smad4 cells were transiently cotransfected with TOPflash or FOPflash reporter constructs and a reference reporter construct as internal control. TCF-mediated gene transcription was determined by the ratio of pTOPflash/pFOPflash luciferase activity. Transfections were done in triplicate; columns, mean of three experiments; bars, SD.
To further confirm these findings and also to ensure that the observed suppression of claudin-1 expression upon Smad4 expression in HT29 cells is not a cell-specific phenomenon, a full-length Smad4 cDNA subcloned in the mammalian expression vector (pCMVscript) was stably expressed in the SW480 human colon cancer cell line. The SW480 cells express very low levels of Smad4 and modest levels of claudin-1. Immunoblotting analysis using three different clones of Smad4-positive SW480 cells (SW480Smad4) confirmed the observations from HT29 cells that Smad4 expression inhibits claudin-1 expression. Further analysis, using reverse transcription-PCR and Northern blot analysis confirmed that the inhibition of claudin-1 expression, upon restoration of Smad4 expression, was due to suppression of claudin-1 mRNA (Fig. 2B). This effect of Smad4 upon claudin-1 expression was specific because the expression of another claudin family member, claudin-4, was not affected by the restoration of Smad4 expression (Fig. 2B).
Of interest, expression of Smad4 in SW480 cells resulted in a robust increase in E-cadherin levels and a decrease in steady-state levels of β-catenin (Fig. 2C). We observed a shift in the cellular localization of β-catenin in the SW480Smad4 cells from the nucleus and cytoplasm to the membrane (data not shown). We further asked whether the decrease in abundance of β-catenin and its shift from nuclear/cytosolic location to the cell membrane was correlated with any decrease in Wnt signaling as reflected by β-catenin/TCF/Lef transcriptional activity. Claudin-1 is a known target molecule of the TCF/Lef signaling pathway (19) and β-catenin/TCF elements (−1021,−670) are present within the −1160 to +160 region of the claudin-1 promoter. To explore this possibility, we analyzed β-catenin/TCF/Lef signaling in SW480control and three SW480Smad4 clones by transiently transfecting TCF and mutant TCF reporter plasmids (TOPflash and FOPflash, respectively). TCF/Lef luciferase reporter assay is a well-established assay for measuring transcriptional activation due to activated β-catenin signaling (20). Relative luciferase activities were calculated as described in Materials and Methods. As shown in Fig. 2D, the relative transcriptional activity of the β-catenin/Lef complex was markedly inhibited in SW480Smad4 cells as compared with SW480control cells. These data show that the Smad4-mediated alterations of E-cadherin and β-catenin have the functional effect of suppressing Wnt signaling.
Smad4 expression suppresses claudin-1 promoter activity in SW480 and SW620 cells
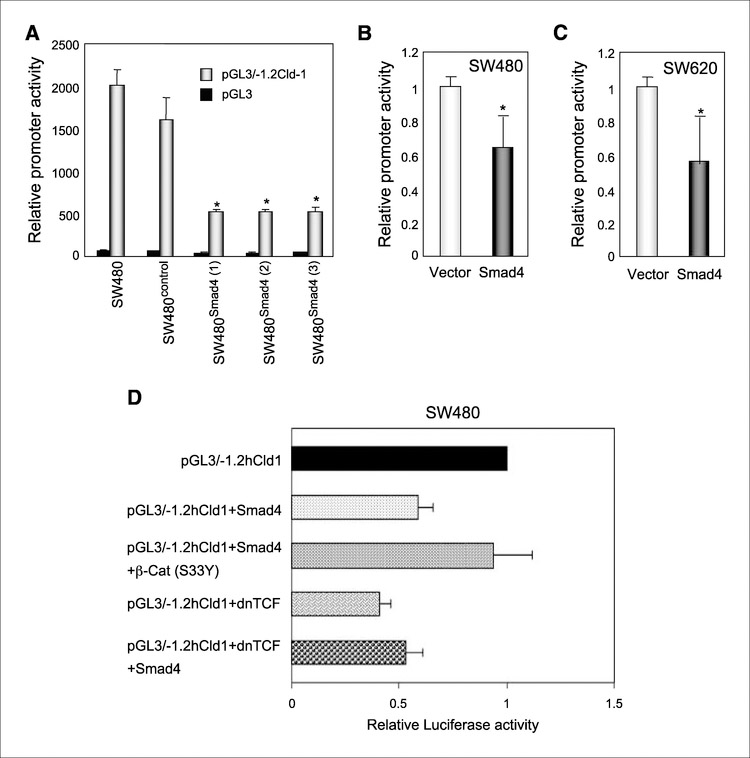
The absence of detectable steady-state claudin-1 mRNA associated with the loss of claudin protein expression suggested possible transcriptional down-regulation of claudin-1 expression upon the restoration of Smad4 expression (Figs. 1B and 2B). To confirm such a possibility, luciferase reporter assay using human claudin-1 promoter was done. A human claudin-1 promoter (−1160 to +160)‒based luciferase reporter plasmid construct was generated as described in Materials and Methods and is referred to as pGL3/−1.2Cld-1. SW480, SW480control, and SW480Smad4 cells were transiently transfected with pGL3/− 1.2Cld-1 or pGL3-Basic (vector alone), along with a reference reporter plasmid. The luciferase activity of pGL3/−1.2Cld-1 was normalized to that of pGL3-Basic in addition to the reference vector. A 60% to 65% reduction in claudin-1 promoter activity was observed in SW480Smad4 compared with SW480 or SW480control cells (Fig. 3A). This inhibitory effect of Smad4 expression on claudin-1 promoter activity was further confirmed by transient cotransfection experiments in SW480 and SW620 cells, where the pCMV-Smad4 plasmid construct and pGL3/−1.2Cld-1 luciferase reporter construct were cotransfected simultaneously (Fig. 3B and C). These findings gain further support from the fact that a canonical Smad binding element (−200) and β-catenin/TCF binding elements are located within this region of claudin-1 promoter (−1160 to +160). Thus, it is possible that changes in TCF/ Lef signaling and/or direct regulation of the claudin-1 promoter by Smad4 mediate the Smad4-dependent inhibition of claudin-1 expression in colon cancer cells.
Figure 3.
Effect of overexpression of Smad4 on claudin-1 promoter activity.A, SW480, SW480contro1, and three SW480Smad4 clones on 12-well plates were transiently transfected in triplicate with 0.2 Ag of pGL3/−1.2Cld-1 or pGL3-Basic (backbone of pGL3/−1.2Cld-1), along with 0.04 μg of a reference reporter (phRL-TK). Forty-eight hours later, cells were lysed, and luciferase activity in lysates was measured. Columns, mean of three independent experiments; bars, SD. *, P < 0.05. Normalized luciferase activity (pGL3/−1.2Cld-1 versus pGL3-Basic) in each individual clone is presented in percentage (100% in SW480 parental cells). B and C, SW480 and SW620 cells were transiently cotransfected with 0.05 μg of pGL3/−1.2Cld-1,0.04 Ag of a reference reporter (phRL-TK) and 0.15 μg of a control or a Smad4 expression vector. Reporter assay was done 72 h after transfection. *, P < 0.05. D, SW480 parental cells were transiently cotransfected with pGL3/−1.2Cld-1, Smad4, constitutively active β-catenin [β-cat (S33Y)], or dnTCF expression constructs as indicated along with a reference reporter construct (phRL-TK). Forty-eight hours later, cells were lysed, and luciferase activity in lysates was measured. Columns, mean of three independent experiments and shown in fold change to the pGL3/− 1.2Cld-1-transfected group; bars, SD.
Smad4-mediated repression of claudin-1 transcription is through modulation of (β-catenin/TCF/Lef activity
To determine a possible involvement of β-catenin/TCF/Lef activity in the Smad4-dependent transcriptional regulation of claudin-1, we used cotransfection experiments of Smad4 with activating S33Y point mutant of β-catenin or dnTCF-4 mutant, and the effect on claudin-1 promoter (pGL3/−1.2Cld-1) activity was determined. Transient transfections were done in SW480 cells, and luciferase activity of pGL3/−1.2Cld-1 was normalized to that of pGL3-Basic in addition to the reference vector. A 40% reduction in claudin-1 promoter activity was observed upon cotransfection with Smad4 (Fig. 3D). This inhibitory effect of Smad4 expression on claudin-1 promoter activity was rescued when activated β-catenin was cotransfected with Smad4, suggesting that the effect of Smad4 on claudin-1 expression is mediated through changes in β-catenin/TCF/Lef activity. Cotransfection of pGL3/−1.2Cld-1 with dnTCF inhibited claudin-1 promoter activity by 60%, further supporting the above notion. Furthermore, cotransfection of Smad4 with dnTCF did not result in a greater degree of inhibition of claudin-1 promoter activity compared with Smad4 or dnTCF alone, ruling out possible additive effects between these two molecules in the regulation of claudin-1 expression. Taken together, our data suggest that the modulation of β-catenin/TCF/Lef activity plays a central role in the Smad4-mediated regulation of claudin-1 expression.
Modulation of claudin-1 expression contributes to the invasion-suppressive effects of Smad4
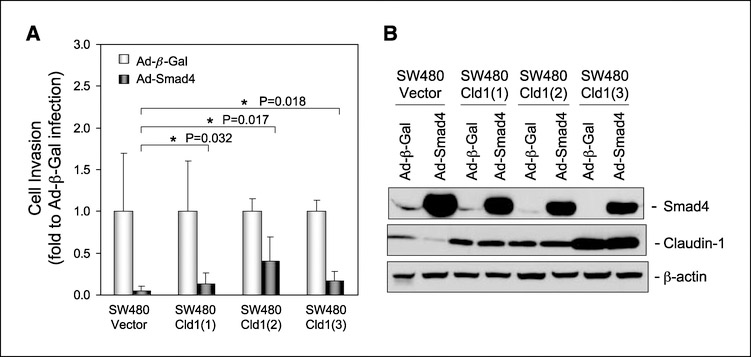
Smad4 restoration in the Smad4-deficient SW480 colon carcinoma cells results in the loss of tumorigenicity by these cells in nude mice (21). Furthermore, Smad4 gene mutation or loss of its expression has been correlated with increased invasiveness or metastatic ability of colon tumors. Because we have previously shown that claudin-1 plays an important role in tumor progression, invasion, and metastasis (1), we tested whether Smad4-dependent inhibition of claudin-1 expression has a causal role for the invasion-suppressive effects of Smad4. In SW480 cells, Smad4 overexpression not only completely inhibited the expression of claudin-1 but also inhibited cell invasion by 90%. To further examine a possible correlation between Smad4-dependent inhibition of claudin-1 expression and cell invasion, we repeated the invasion assay using SW480control (expressing empty vector) and SW480claudin−1 (overexpressing claudin-1; three independent clones) cells. Smad4 was overexpressed in these cells by infection with adenoviral Smad4. As expected in SW480control cells, claudin-1 expression was suppressed upon overexpression of Smad4; however, no major differences due to Smad4 overexpression were observed on claudin-1 expression controlled by a heterologous promoter in SW480claudin−1 cells (Fig. 4). The Matrigel-based invasion assay showed a 95% inhibition of invasion in SW480control cells infected with adenoviral-Smad4. However, the suppressive effect of Smad4 expression on invasion was partially reduced in the claudin-1–overexpressing cells to 80%, 50%, and 75% in three separate clones compared with the control cells. Taken together, the above data suggest that the inhibition of claudin-1 expression contributes to the tumor-suppressive effects of Smad4 expression, but that Smad4 also inhibits invasion through mechanisms that are independent of claudin-1.
Figure 4.
The effect of overexpression of claudin-1 on Smad4-mediated tumor-suppressive function in SW480 cells. A, cells grown on six-well plate to 50% to 60% confluency were infected with β-galactosidase or Smad4 adenovirus at a multiplicity of infection of 20 as described in Materials and Methods. Forty-eight hours after infection, cells were plated in Matrigel-coated Transwells (100,000 cells per Transwell) and allowed to grow for 3 days. Invasive cell numbers were scored as described in Materials and Methods, and the results are presented as fold change to β-galactosidase adenoviral infection. Columns, mean fold change; bars, SD. *, P < 0.05 compared with control. The P value was determined using Student’s t test. B, cells were infected with β-galactosidase or Smad4 adenovirus at a multiplicity of infection of 20. Seventy-two hours after infection, cells were lysed, and the lysates were subjected to immunoblotting for Smad4, claudin-1, and β-actin.
TGF-β signaling is not recovered upon Smad4 restoration and is not required for Smad4-dependent suppression of claudin-1 in SW480 cells
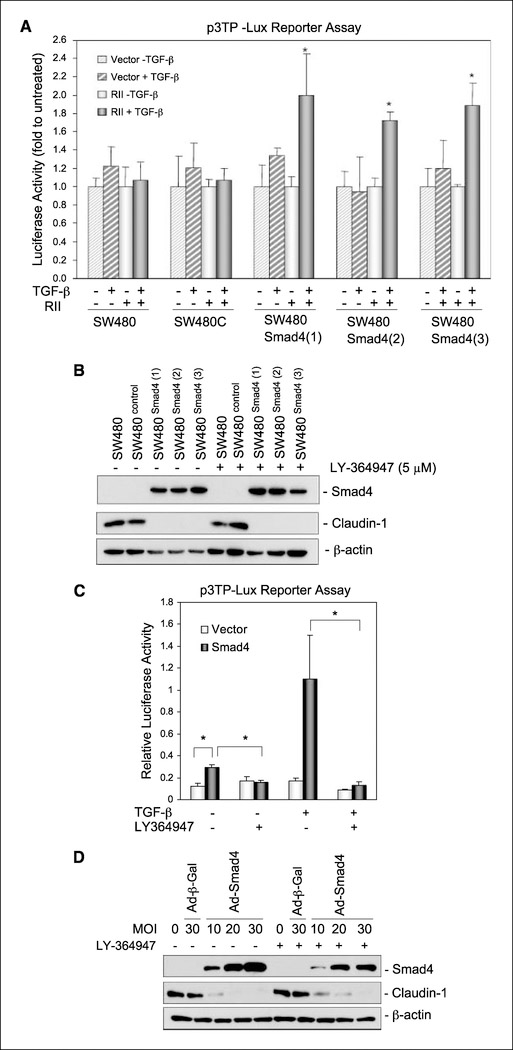
Smad4 is known to play a key role in the TGF-β signaling. SW480 cells secrete endogenous TGF-β (22); however, these cells express very low levels of the TGF-β type II receptor (23) and, therefore, are described as cells that are not responsive to TGF-β signaling. We asked whether the observed inhibitory effects of Smad4 expression on claudin-1 expression were due to the restoration of TGF-β responsiveness (autocrine and/or paracrine TGF-β signaling). To address this, we first sought evidence for TGF-β signaling in SW480Smad4 cells using promoter activation of p3TP-Lux (a luciferase reporter construct that is highly responsive to TGF-β). However, transient transfections showed no induction of p3TP-Lux activity upon TGF-β treatment in SW480Smad4 cells compared with the SW480 or SW480control cells, suggesting that reexpression of Smad4 did not restore TGF-β responsiveness (Fig. 5A). This hypothesis was further supported by our observation that TGF-β–induced 3TP-Lux activation response was restored in SW480Smad4 cells when TGF-β type II receptor was coexpressed with Smad4 in these cells (Fig. 5A).
Figure 5.
TGF-β signaling was not restored and not required for Smad4 suppression of claudin-1 in SW480 cells. A, TGF-β signaling was not restored by Smad4 expression, but was restored by coexpression of Smad4 and wild-type TGF-β type II receptor (Rll) in Smad4-deficient SW480 cells. SW480, SW480control, and SW480Smad4 clones were transiently cotransfected with 0.05 μg p3TP-Lux, 0.04 phRL-TK, and 0.15 μg of a control or a type II receptor expression vector. Sixteen hours after transfection, cells were treated with 3 ng/mL TGF-β and allowed to grow for 48 h before luciferase reporter assay. Columns, luciferase activity mean of three independent experiments and represented in folds (TGF-β treatment versus vehicle treatment) in each individual line; bars, SD. *, P < 0.05. B, the effect of the inhibitor of the TGF-β receptor kinase, LY364947, on Smad4 inhibition of claudin-1 in SW480 cells. SW480, SW480control, and three SW480Smad4 clones were grown in the presence of vehicle or 5 Amol/L of LY364947 for 48 h and then lysed in a lysis buffer. Equal amounts of cell lysates were used for immunoblotting for Smad4, claudin-1, and β-actin. C, TGF-β signaling pathway was not required for Smad4 suppression of claudin-1 in HT29 cells. Smad4 expression restored the autocrine and paracrine TGF-β response, which could be blocked by the inhibitor of TGF-β receptor kinase LY364947. Cells were transiently cotransfected with 0.1 Ag p3TP-Lux, 0.01 Ag phRL-TK, and 0.4 Ag of a control vector or a Smad4 expression vector. Sixteen hours later, cells were treated, as indicated, with or without 5 Amol/L LY364947 and 3 ng/mL TGF-β and cultured for another 48 h before reporter assays. Bars, SD. *, P < 0.05. D, the inhibitor of TGF-β receptor kinase, LY364947, did not abrogate Smad4 suppression of claudin-1 in HT29 cells. Cells were infected with β-galactosidase or Smad4 adenoviruses at the indicated multiplicity of infection for 4 h and then cultured for 72 h in the presence or absence of 5 Amol/L LY364947 before being lysed for immunoblotting for Smad4, claudin-1, and β-actin.
To further examine the effect of autocrine TGF-β effect on these cells, a TGF-β receptor kinase inhibitor, LY364947, was used. LY364947 is a potent ATP competitive inhibitor of the TGF-β type I receptor and inhibits the phosphorylation of Smad2 and Smad3 in response to TGF-β signaling (13). Immunoblotting results show that LY364947 treatment at concentrations sufficient to completely suppress TGF-β-induced phosphorylation of Smad2 (13) and to inhibit TGF-β-induced transcriptional activation (Fig. 5C) did not prevent the inhibition of claudin-1 expression in SW480Smad4 cells (Fig. 5B ). Taken together, these data suggest that Smad4 expression was not sufficient to establish autocrine TGF-β signaling nor did it restore responses to exogenous TGF-β unless the TGF-β type II receptor was coexpressed in SW480 cells. Therefore, TGF-β signaling seems not be required for the Smad4 effect on claudin-1 expression in these cells.
In contrast to SW480 cells, HT29 cells express sufficient levels of functional TGF-β receptors to transduce TGF-β-mediated Smad2 phosphorylation (24). The p3TP-Lux promoter activation in response to TGF-β was observed when p3TP-Lux was cotransfected with Smad4 but not with the control vector (Fig. 5C). It is notable that Smad4 cotransfection enhanced basal p3TP-Lux promoter activity in the HT29 cells, suggesting the establishment of autocrine TGF-β signaling by Smad4 expression (Fig. 5C). LY364947 inhibited basal and TGF-β-induced p3TP-Lux promoter activation in Smad4- cotransfected cells (Fig. 5D). However, despite the presence of LY364947, adenovirus-mediated overexpression of Smad4 still suppressed claudin-1 expression (Fig. 5D), confirming that the effect of Smad4 on claudin-1 expression was independent of TGF-β signaling.
Discussion
Smad4 is a known tumor suppressor, and frequent mutations in Smad4 gene or loss of its expression have been correlated with increased invasiveness or metastatic ability of colon tumors (7, 9, 25). We have recently reported a positive correlation with claudin-1 expression with colon tumor progression and metastasis (1). Because our initial data from different colon cancer cell lines suggested a possible inverse correlation between Smad4 and claudin-1 expressions, this study was undertaken to confirm such a possibility and to understand the possible mechanism underlying such a correlation. Through several independent approaches in human primary tumor samples and human colon cancer cell lines, we confirmed that there is an inverse relationship between Smad4 and claudin-1 expressions in colon cancer. Further analysis confirmed that Smad4-dependent regulation of claudin-1 expression is through transcriptional regulation of the claudin-1 promoter, and that these effects were independent of TGF-β signaling.
Previous studies have shown that Smad4 restoration in the Smad4-deficient SW480 colon carcinoma cells results in loss of tumorigenicity by these cells in nude mice and is accompanied with the reestablishment of a more epithelioid phenotype as well as increased expression of E-cadherin and P-cadherin (23). Our findings from Smad4 overexpression in these cells both supported and extended these previously reported findings in that SW480Smad4 cells exhibited increased expression of E-cadherin along with the translocation of β-catenin from the cytoplasm and nucleus to the cell membrane with a concomitant decrease in β-catenin/TCF transcriptional activity. Most importantly, we observed a marked inhibition of claudin-1 expression at both protein and mRNA levels. Our observations were further confirmed in HT29 cells, another Smad4-deficient but claudin-1–positive group of cells, suggesting that Smad4 regulates claudin-1 at the mRNA level in colon cancer cells.
Inhibition of mRNA expression could result either from the inhibition of mRNA transcription and/or stability. Smad4 is a known transcription factor that binds to DNA with rather low sequence specificity and affinity, and interactions with other DNA binding partners are typically required for Smad-dependent transcriptional responses (26,27). Smad4 has been shown to inhibit gene expression by suppressing promoter activity in association with transcriptional corepressors, such as Nkx3.2, transforming growtβ-interacting factor, and or histone deacetylases in response to TGF-β and/or bone morphogenetic protein (28–30). In our search for possible Smad4 binding site(s) in claudin-1 promoter, we identified a canonical Smad binding element (−200) and β-catenin/TCF binding elements (−1021,−670) within the region (−1160 to +160), which we have used in these studies. In addition, in a previous study, Miwa et al. have provided suggestive evidence that claudin-1 gene is a target of β-catenin signaling (19). Therefore, a possible interplay among Smad4 and β-catenin signaling in the regulation of claudin-1 expression could be postulated. To explore such a possibility, we cotransfected constitutively active β-catenin with Smad4 and pGL3/−1.2hCld-1 or dnTCF-4 with pGL3/−1.2hCld-1. Outcomes from these studies suggested a predominant role for the β-catenin signaling in the Smad4-dependent regulation of claudin-1 expression because activated β-catenin could rescue the Smad4-mediated repression of claudin-1 promoter activity. Conversely, expression of dnTCF-4 repressed claudin-1 promoter activity to the levels similar to that caused by Smad4 expression alone. Taken together, our data suggest that modulation/crosstalk of β-catenin/TCF/Lef activity plays a predominant role in the Smad4-mediated regulation of claudin-1. Such a hypothesis gains support from the recent reports suggesting that Smad4 and β-catenin/Lef-1 (or TCF) complex interact and may converge in regulating some common target(s) (31, 32). The mouse gastrin promoter was recently shown to be regulated synergistically by TGF-β/Smads and β-catenin/TCF (33). However, the question remains: whether the observed effects in our promoter-based studies are effects of interactions between Smad4 and TCF/Lef or simply due to the fact that Smad4 expression depletes β-catenin from cytoplasm/nucleus, thus inhibiting TCF/Lef activation. We are currently investigating such possibilities.
As mentioned above, functional inactivation of the Smad4 gene occurs frequently in advanced colon carcinomas. It is important to note that we have recently shown that claudin-1 expression in colon cancer is positively related with invasion and metastasis where inhibition of claudin-1 expression inhibits cell invasion or metastases to significant levels, whereas its overexpression induces metastatic ability in a cell line that normally does not metastasize when injected in mice (1). Other recent reports have identified a similar correlation between claudin-1 expression and invasion/metastases in oral squamous cell carcinoma (5). Therefore, we sought to determine whether inhibition of claudin-1 by Smad4 expression is causally related to the invasion-suppressive effects of Smad4 expression. We used an in vitro invasion assay to test such a hypothesis using adenoviral-based expression of Smad4 in SW480 cells overexpressing claudin-1. We have found this assay to be predictive of in vivo metastatic potential (1). The fact that adenoviral overexpression of Smad4 in cells stably transfected to overexpress claudin-1 from a heterologous promoter did not show a major alteration in claudin-1 expression further supported our data that Smad4 regulates expression at the level of transcription from the claudin-1 promoter. However, inhibition of invasion due to Smad4 expression in the control cells by 90% was partially reduced in claudin-1–overexpressing cells. These experimental outcomes not only support our previous observation that claudin-1 expression increases cell invasion but also suggests that inhibition of claudin-1 expression is causally related to some of the tumor- suppressive effects of Smad4 expression. The fact that the claudin-1 overexpression was insufficient to completely abrogate the anti-invasive effects of Smad4 suggests that these effects are also dependent on mechanisms independent of claudin-1.
Smad4 is an intracellular mediator of TGF-β family signals, and its tumor suppressor function is presumed to reside in its capacity to mediate TGF-β and bone morphogenetic protein-induced growth inhibition (34, 35). However, there is accumulating evidence that this hypothesis may be too simplistic. The roles of TGF-β in carcinogenesis are complex and also comprise tumor-promoting functions particularly in late-stage carcinogenesis (36). Importantly, functional inactivation of Smad4 in colon carcinomas frequently occurs at late stages when tumors acquire invasive and metastatic capabilities (25). Recently, several studies have shown a role of Smad4 as a tumor suppressor that seems to be independent of TGF-β signaling. Angiotensin II has been shown to activate the Smad signaling system in vascular cells in vivo and in vitro. Smad proteins are involved in angiotensin II-induced connective tissue growth factor and extracellular matrix protein overexpression independently of TGF-β (37). Also, Muller et al. showed that stable reexpression of Smad4 in SW480 human colon carcinoma cells was adequate to suppress tumor growth in nude mice and also induce the expression of invasion suppressor E-cadherin (23). However, Smad4 overexpression did not affect cell growth in vitro, nor did it restore TGF-β responsiveness. In line with previous studies, we show a novel TGF-β–independent role of Smad4 in the regulation of claudin-1 expression in colon cancer cells.
Overall, these data show that Smad4 may play an important invasion-suppressive role in colon cancer that is independent of TGF-β signaling. Further understanding of the mechanisms by which Smad4 functions as a tumor suppressor may provide opportunities for therapeutic intervention in cancer. Taken together, our findings show a novel TGF-β–independent mechanism for the tumor/invasion-suppressive functions of Smad4, in part through the inhibition of Wnt/β-catenin/TCF signaling and claudin-1 expression.
Acknowledgments
Grant support: NIH grant CA119005, ACS (IRG-58–009) (P. Dhawan), DK52334, CA69457 (R.D. Beauchamp), GI cancer spore (P50 CA95103) and CA95195 (P.K. Datta).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We would also like to thank Jonathan M. Yingling, Lily Research Laboratories, Indianapolis, IN, USA for kindly providing us with inhibitor of TGF-β type I and II receptor kinases (LY364947).
References
- 1.Dhawan P, Singh AB, Deane NG, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest 2005;115:1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usami Y, Chiba H, Nakayama F, et al. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol 2006;37:569–77. [DOI] [PubMed] [Google Scholar]
- 3.Michl P, Barth C, Buchholz M, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res 2003;63:6265–71. [PubMed] [Google Scholar]
- 4.Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res 2005;65:7378–85. [DOI] [PubMed] [Google Scholar]
- 5.Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 g2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res 2006;66:5251–7. [DOI] [PubMed] [Google Scholar]
- 6.Massague J, Blain SW, Lo RS. TGFh signaling in growth control, cancer, and heritable disorders. Cell 2000;103: 295–309. [DOI] [PubMed] [Google Scholar]
- 7.Miyaki TI, Konishi M, Sakai K, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene 1999;18:3098–103. [DOI] [PubMed] [Google Scholar]
- 8.Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun 2003;306:799–804. [DOI] [PubMed] [Google Scholar]
- 9.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996;271:350–3. [DOI] [PubMed] [Google Scholar]
- 10.Anke Reinacher-Schick SEB, Romdhana B, Landsberg S, et al. Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol 2004;202:412–20. [DOI] [PubMed] [Google Scholar]
- 11.Schwarte-Waldhoff I, Volpert OV, Bouck NP, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci U S A 2000;97:9624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicole Muller AR-S, Baldus S, van Hengel J, et al. Smad4 induces the tumor suppressor E-cadherin and P-cadherin in colon carcinoma cells. Oncogene 2002;21: 6049–58. [DOI] [PubMed] [Google Scholar]
- 13.Peng S-B, Yan L, Xia X, et al. Kinetic characterization of novel pyrazole TGF-I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry 2005;44:2293–304. [DOI] [PubMed] [Google Scholar]
- 14.Wrana JL, Attisano L, Carcamo J, et al. TGF β signals through a heteromeric protein kinase receptor complex. Cell 1992;71:1003–14. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Sheng H, Shao J, Beauchamp RD, DuBois RN. Posttranscriptional regulation of cyclooxygenase-2 in rat intestinal epithelial cells. Neoplasia 2000;2:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morin PJ, Sparks AB, Korinek V, et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997;275:1787–90. [DOI] [PubMed] [Google Scholar]
- 17.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC—/— colon carcinoma. Science 1997;275:1784–7. [DOI] [PubMed] [Google Scholar]
- 18.Reinacher-Schick A, Baldus SE, Romdhana B, et al. Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol 2004;202:412–20. [DOI] [PubMed] [Google Scholar]
- 19.Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the β-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res 2000;12: 469–76. [DOI] [PubMed] [Google Scholar]
- 20.Willert K, Nusse R. β-Catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 1998;8:95–102. [DOI] [PubMed] [Google Scholar]
- 21.Schwarte-Waldhoff I, Klein S, Blass-Kampmann S, et al. DPC4/SMAD4 mediated tumor suppression of colon carcinoma cells is associated with reduced urokinase expression. Oncogene 1999;18:3152–8. [DOI] [PubMed] [Google Scholar]
- 22.Coffey RJ Jr., Goustin AS, Soderquist AM, et al. Transforming growth factor a and h expression in human colon cancer lines: implications for an autocrine model. Cancer Res 1987;47:4590–4. [PubMed] [Google Scholar]
- 23.Muller N, Reinacher-Schick A, Baldus S, et al. Smad4 induces the tumor suppressor E-cadherin and P- cadherin in colon carcinoma cells. Oncogene 2002;21: 6049–58. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B, Zhang J-S, Du J, Urrutia R, Barnard J. Growth inhibitory signalling by TGFh is blocked in Ras-transformed intestinal epithelial cells at a postreceptor locus. Cell Signal 2003;15:699–708. [DOI] [PubMed] [Google Scholar]
- 25.Miyaki M, Iijima T, Konishi M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene 1999;18:3098–103. [DOI] [PubMed] [Google Scholar]
- 26.Massague J, Chen YG. Controlling TGF-β signaling. Genes Dev 2000;14:627–44. [PubMed] [Google Scholar]
- 27.Peter Ten Dijke M-JG, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol 2002;191: 1–16. [DOI] [PubMed] [Google Scholar]
- 28.Wotton DMJ. Smad transcriptional corepressors in TGF β family signaling. Curr Top Microbiol Immunol 2001;254:145–64. [PubMed] [Google Scholar]
- 29.Wotton D, Lo RS, Swaby L-AC, Massague J. Multiple modes of repression by the Smad transcriptional corepressor TGIF. J Biol Chem 1999;274: 37105–10. [DOI] [PubMed] [Google Scholar]
- 30.Wotton D, Lo RS, Lee S, Massague J. A Smad transcriptional corepressor. Cell 1999;97:29–39. [DOI] [PubMed] [Google Scholar]
- 31.Chakladar A, Dubeykovskiy A, Wojtukiewicz LJ, Pratap J, Lei S, Wang TC. Synergistic activation of the murine gastrin promoter by oncogenic Ras and β-catenin involves SMAD recruitment. Biochem Biophys Res Commun 2005;336:190–6. [DOI] [PubMed] [Google Scholar]
- 32.Hussein SM, Duff EK, Sirard C. Smad4 and β-catenin co-activators functionally interact with lymphoid enhancing factor to regulate graded expression of Msx2. J Biol Chem 2003;278:48805–14. [DOI] [PubMed] [Google Scholar]
- 33.Lei S, Dubeykovskiy A, Chakladar A, Wojtukiewicz L, Wang TC. The murine gastrin promoter is synergistically activated by transforming growth factor-β/Smad and Wnt signaling pathways. J Biol Chem 2004;279: 42492–502. [DOI] [PubMed] [Google Scholar]
- 34.Pardali K, Kowanetz M, Heldin C-H, et al. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21. J Cell Physiol 2005;204:260–72. [DOI] [PubMed] [Google Scholar]
- 35.Kowanetz M, Valcourt U, Bergstrom R, Heldin C-H, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor h and bone morphogenetic protein. Mol Cell Biol 2004;24:4241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deckers M, van Dinther M, Buijs J, et al. The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res 2006;66:2202–9. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-β-independent mechanism. Circulation 2005;111:2509–17. [DOI] [PubMed] [Google Scholar]