Abstract
Administration of granulocyte colony-stimulating factor (G-CSF) after autologous peripheral blood stem cell transplantation (PBSCT) is generally recommended to reduce the duration of severe neutropenia; however, there is limited and conflicting data regarding the optimal timing of G-CSFs post-transplant. A retrospective study was performed at NewYork-Presbyterian/Weill Cornell Medical Center (NYP/WC) from November 5, 2013 to August 9, 2016 of adult inpatient autologous PBSCT patients who received G-CSF empirically starting on day +5 (early) versus day +12 only if absolute neutrophil count (ANC) was <0.5×109/L (ANC driven). G-CSF was dosed at 300 mcg for patient weight <75 kg or 480 mcg if ≥75 kg. One hundred consecutive patients underwent autologous PBSCT utilizing either the early (N=50) or ANC driven (N=50) practice. Patient and transplant characteristics were comparable in both groups. In the ANC driven group, 24% (N=12) received G-CSF on day +12 and 60% (N=30) were initiated earlier due to febrile neutropenia or per physician discretion; 6% (N=3) were initiated after day +12 due to physician discretion, and 10% (N=5) did not receive any G-CSF. Median start day of G-CSF in the ANC driven group was day +10 versus day +5 in the early group (p<0.0001). For the primary outcome, median time to neutrophil engraftment was 12 (IQR 11,13) days versus 13 (IQR 12,14) days in the early and ANC driven cohorts, respectively (p=0.07). There were no significant differences in time to platelet engraftment, 1-year relapse rate, or 1-year overall survival. The incidence of febrile neutropenia was 74% in the early group versus 90% in the ANC driven group (p=0.04). However, there was no significant difference in the incidence of positive bacterial cultures or transfer to the intensive care unit. The duration of G-CSF administration until neutrophil engraftment was 6 days in the early group versus 3 days in the ANC driven group (p <0.0001). Median length of post-transplantation hospitalization was 15 (IQR 14,19) days in the early group versus 16 (IQR 15,22) days in the ANC driven group (p=0.28). ANC driven initiation of G-CSF following autologous PBSCT was associated with a similar time to neutrophil engraftment, length of stay post-transplantation, and 1-year overall survival compared to early initiation of G-CSF on day +5.
Keywords: Granulocyte colony-stimulating factor, Engraftment, Autologous, Stem cell transplant
INTRODUCTION
High-dose chemotherapy followed by hematopoietic stem cell transplantation (HSCT) can lead to serious complications such as infection in the setting of prolonged neutropenia. Multiple studies have evaluated the use of granulocyte colony-stimulating factors (G-CSFs) after autologous HSCT, however results vary due to the disparate and relatively small number of patients included, with some showing clinical benefit and others reporting no difference.
Use of G-CSFs post-autologous HSCT is supported by decreased time to engraftment ranging between 1–6 days, with some studies demonstrating savings in duration of hospitalization and overall medical costs while others report mixed results on duration of hospitalization, infections, and survival.1 Evidence based guidelines have differing recommendations on the optimal time to initiate G-CSFs post-autologous HSCT.2–8 Previous studies have been conducted evaluating varying G-CSF initiation strategies post-autologous HSCT including early or delayed approaches and found no differences in neither time to neutrophil engraftment nor safety when initiated up to 10 days post-transplant.9–17 To date there have been only two studies evaluating the use of individualized criteria, based on patient’s absolute neutrophil count (ANC), to determine when to initiate G-CSF, reporting conflicting results.18–19
The limited and conflicting data comparing outcomes with G-CSF use as well as its optimal timing after HSCT necessitates further evaluation to determine appropriate use of G-CSFs in this setting. In addition, given the increased costs of G-CSF agents, it is crucial to understand whether initiation following HSCT confers a clinical benefit. At NewYork-Presbyterian Hospital/Weill Cornell Medical Center in April 2015, standard practice changed from early initiation of G-CSF (day +5) to individualized ANC driven (day +12 for ANC <0.5×109/L) initiation of G-CSF after autologous peripheral blood stem cell transplantation (PBSCT). The goal of the current study was to compare hematologic recovery and transplant related outcomes of early versus ANC driven administration of G-CSF after autologous PBSCT.
MATERIALS AND METHODS
Study design:
This was an institutional review board approved retrospective cohort study conducted at NewYork-Presbyterian/Weill Cornell Medical Center and included adult inpatients (≥18 years) undergoing autologous PBSCT using single agent high-dose melphalan; carmustine-etoposide-cytarabine-melphalan (BEAM); or rituximab-BEAM (RBEAM) conditioning between November 5, 2013 and August 9, 2016. Patients were excluded if they received previous autologous PBSCTs, outpatient autologous PBSCTs, or were discharged prior to engraftment. Patients who were transplanted between November 2013 to April 2015 received filgrastim (Neupogen®, Amgen Inc.), beginning on post-transplant day +5. Patients who were transplanted between April 2015 to August 2016 received tbo-filgrastim (Granix®, Teva Pharmaceuticals Inc.) beginning on post-transplant day +12 only if the ANC was <0.5×109/L. Both groups continued G-CSF until ANC ≥0.5×109/L for 3 days or ≥1.5×109/L for 1 day. Deviation from the protocol based on physician discretion was permitted.
Anti-infective prophylaxis included levofloxacin 500 mg daily, valacyclovir 500 mg twice daily, and fluconazole 400 mg daily for both groups. Transfusion support was administered if indicated per institutional policy.
Outcomes and definitions:
The primary outcome was time to neutrophil engraftment, defined as the first of 3 consecutive days with an ANC ≥0.5×109/L. Secondary outcomes included time to platelet engraftment, defined as the first of 3 consecutive days with a platelet count ≥20×109/L that was maintained without transfusion support for 7 consecutive days; incidence of febrile neutropenia, defined as the occurrence of temperature ≥38°C and ANC <0.5×109/L from day 0 to day of ANC engraftment; incidence of positive blood or urine bacterial cultures during the first 30 days post-transplant, incidence of intensive care unit transfer from day 0 until ANC engraftment, duration of G-CSF administration, duration of hospitalization post-transplant from day +1 until hospital discharge, relapse rate at 1 year, and 1-year overall survival. Based on our institutional policy, G-CSF was dosed at 300 mcg if patients were <75 kg or 480 mcg if ≥75 kg.
Statistical analysis
Fisher’s exact or chi-square test were used to compare categorical variables between groups. Kruskal-Wallis rank-sum test was used to compare continuous variables. Group comparisons were 2-sided with a type I error of <0.05. The Kaplan-Meier method was used for analysis of time to engraftment and length of stay post-transplant.
RESULTS
One hundred patients underwent an autologous PBSCT using either the early (N=50) or ANC driven (N=50) practice. Treatment groups were well balanced with respect to age, sex, disease state, disease status at transplant, Karnofsky performance status, and number of CD34+ cells infused (Table 1). Conditioning chemotherapy was different between the two groups, with the early group receiving RBEAM with rituximab administered on day +1 and day +8, and the ANC driven group receiving RBEAM with rituximab administered prior to stem cell infusion, on day −6 and day −1. In the ANC driven group, 24% (N=12) received G-CSF on day +12 and 60% (N=30) were initiated earlier due to febrile neutropenia or physician discretion; 6% (N=3) were initiated later than day +12 per physician discretion, and 10% (N=5) did not receive any G-CSF. Median start day of G-CSF in the ANC driven group was day +10 versus day +5 in the early group (p<0.0001). The duration of G-CSF administration until neutrophil engraftment was significantly shorter in the ANC driven group compared to the early group (3 days versus 6 days; p<0.0001).
Table 1.
Baseline Characteristics
| Characteristic | Early (N=50) | ANC driven (N=50) | P value |
|---|---|---|---|
| Median age at transplant, years (IQR) | 60 (47,65) | 60 (49,65) | 0.95 |
| Sex, n (%) | 0.53 | ||
| Disease state, n (%) | 0.22 | ||
| Disease status at transplantation, n (%) | 0.86 | ||
| Median Karnofsky performance score (IQR) | 85 (80,90) (n=48) | 80 (70,90) (n=49) | 0.51 |
| Median CD34+ cells infused, ×106 cells/kg (IQR) | 5.11 (4.01,6.35) | 4.58 (3.77,6.42) | 0.47 |
| Conditioning regimen, n (%) | <0.0001 |
IQR indicates interquartile range; NHL, Non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MM, multiple myeloma; CR, complete remission; PR, partial response; SD, stable disease; PD, progressive disease; BEAM, carmustine-etoposide-cytarabine-melphalan; RBEAM, rituximab-carmustine-etoposide-cytarabine-melphalan
Engraftment
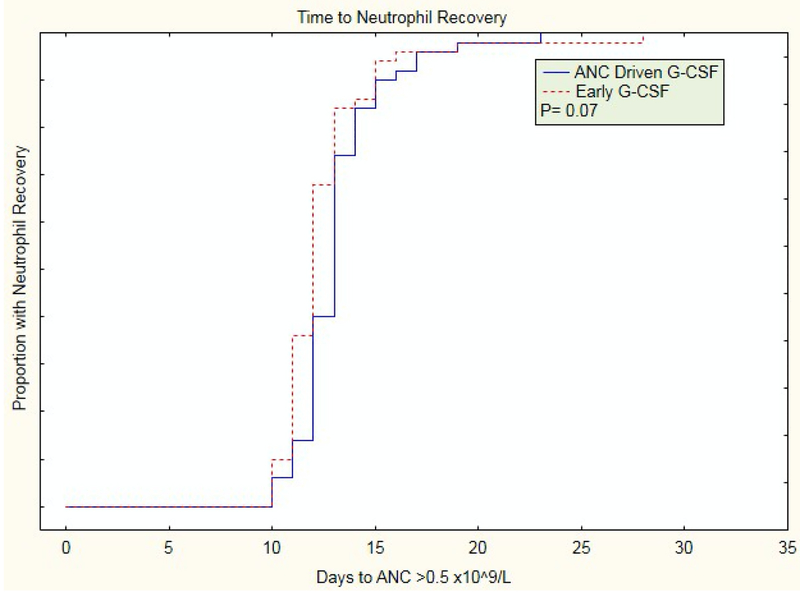
The median time to ANC engraftment in the early group was 12 (IQR 11,13) days compared to 13 (IQR 12,14) days in the ANC driven group (p=0.07); (Table 2; Figure 1).
Table 2.
Outcomes
| Early (N=50) | ANC driven (N=50) | P value | |
|---|---|---|---|
| Median time to neutrophil engraftment, days (IQR) | 12 (11,13) | 13 (12,14) | 0.07 |
| Median time to platelet engraftment, days (IQR) | 20 (15,24) | 20 (16,23) | 0.78 |
| Median start day of G-CSF (IQR) | 5 (5,5) | 10 (8,12) | <0.0001 |
| Median duration of G-CSF administration, days (IQR) | 6 (6,7) | 3 (2,5) | <0.0001 |
| Median duration of hospitalization post-transplant, days (IQR) | 15 (14,19) | 16 (15,22) | 0.28 |
| Febrile neutropenia, n (%) | 37 (74) | 45 (90) | 0.04 |
| ICU transfer, n (%) | 5 (10) | 5 (10) | 1.00 |
| Positive bacterial cultures, n (%) | 10 (20) | 16 (32) | 0.17 |
| Relapse at 1 year, n (%) | 15 (30) | 9 (18) | 0.16 |
| 1-year Overall survival, n (%) | 43 (88) (n=49) | 44 (88) | 0.97 |
Figure 1.
Time to neutrophil engraftment
Duration of hospitalization
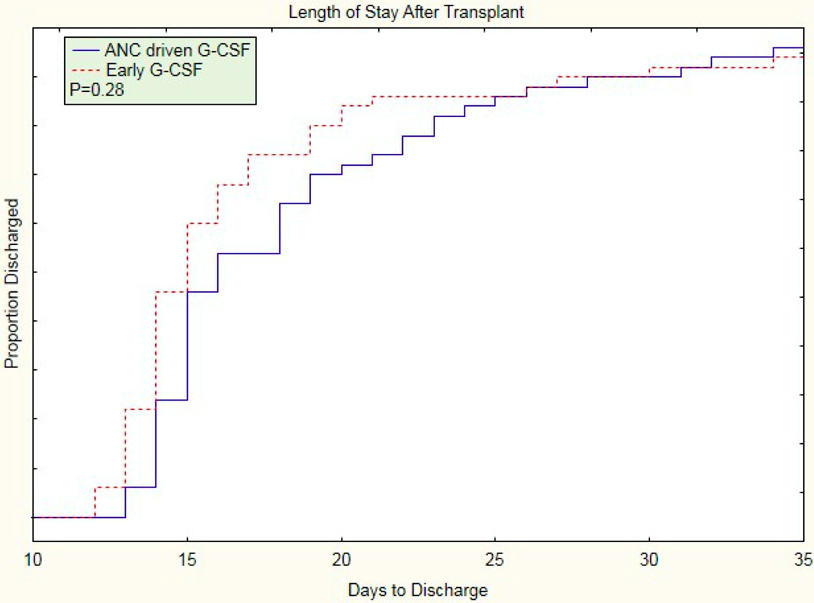
Length of post-transplantation hospitalization was 15 (IQR 14,19) days in the early group compared to 16 (IQR 15,22) days in the ANC driven group (p=0.28); (Table 2; Figure 3).
Figure 3.
Length of stay post-transplantation
Infectious Complications
Febrile neutropenia occurred in 37 (74%) patients in the early group versus 45 (90%) patients in the ANC driven group (p=0.04). There was no difference in the number of patients with positive bacterial cultures or requiring transfer to the intensive care unit between both groups.
Relapse and Survival
The number of patients who relapsed one year post-transplant did not differ (15 patients in the early group versus 9 patients in the ANC driven group; p=0.16). There was no difference in 1-year overall survival between the early and ANC driven groups (88% versus 88%; p=0.97). Six patients died within one year post-transplant in both the early and ANC driven groups. Causes of death in the early group included relapse (n=5) and autologous graft-versus-host disease (n=1). The primary cause of death in the ANC driven group was relapsed disease (n=3).
Cost Analysis
Based on the average wholesale price per prefilled syringe for filgrastim (Neupogen®, Amgen Inc.) and tbo-filgrastim (Granix®, Teva Pharmaceuticals Inc.), along with median duration of G-CSF administration, a cost analysis was performed. ANC driven initiation of G-CSF was associated with cost savings of $1078 to $1168 per patient based on the 300 mcg dose and $1717 to $1859 per patient based on the 480 mcg dose; (Table 3).
Table 3.
Cost Analysis
| Early | ANC driven | Cost-savings | |
|---|---|---|---|
| Cost based on 300 mcg syringe | |||
| Filgrastim | $2335 | $1167 | $1168 |
| Tbo-filgrastim | $2157 | $1079 | $1078 |
| Cost based on 480 mcg syringe | |||
| Filgrastim | $3718 | $1859 | $1859 |
| Tbo-filgrastim | $3435 | $1718 | $1717 |
Cost per patient estimated based on median duration of G-CSF administration (6 days in the early group versus 3 days in the ANC driven group) and average wholesale price per prefilled syringe for filgrastim (Neupogen®, Amgen Inc.) and tbo-filgrastim (Granix®, Teva Pharmaceuticals Inc.)
DISCUSSION
Granulocyte colony-stimulating factors are frequently used after autologous PBSCT to optimize neutrophil recovery, however the literature supporting their impact on clinical outcomes is conflicting and consensus regarding the optimal time to initiate G-CSFs post-transplant is lacking.1 When compared to placebo, G-CSFs have been shown to decrease the duration of neutropenia, length of hospital stay, and number of infections after autologous PBSCT.5–6,20–22 Studies evaluating early versus delayed initiation of G-CSF report conflicting results with some showing early administration decreases time to engraftment, length of stay, and antibiotic use while others report no difference in outcomes when delaying G-CSF administration until day +5, including one study delaying until day +10.9–17,23–24 There has been only two studies evaluating the use of individualized criteria to determine when to initiate G-CSFs post-transplant.18−19 Here, we report our institutional experience with 100 patients over approximately a 3-year period, 50 of whom received G-CSF starting on day +5 and 50 who received G-CSF starting on day +12 for an ANC <0.5×109/L. We found no significant difference in time to neutrophil engraftment (12 days versus 13 days; p=0.07).
Time to platelet engraftment, duration of hospitalization post-transplant, rate of positive blood and urine bacterial cultures, relapse rate at 1 year, and 1-year overall survival were similar between the early and ANC driven groups, indicating that initiation of G-CSF following autologous PBSCT may be based on individualized criteria without compromising engraftment or transplant related outcomes. Our results are similar to those found in a prospective, multicenter, randomized trial by Faber et al.18 One hundred and six patients with lymphoma undergoing an autologous PBSCT following BEAM conditioning were randomized to G-CSF initiated on day +5; G-CSF initiated on day +10 or earlier if ANC ≥0.1×109/L post-nadir; or placebo. Initiation of G-CSF on day +5 was associated with a one day decrease in time to neutrophil engraftment compared to initiation on day +10 or based on ANC cutoffs (median 10 days versus 11 days; p=0.007), with no difference in the incidence of febrile neutropenia, infection, and duration of hospitalization. Since the one day decrease in time to engraftment did not translate into a decrease in duration of hospitalization, the authors conclude that individually determined administration of G-CSF is safe and cost-effective.18 Outcomes from our study which included both lymphoma and multiple myeloma patients also demonstrated a one day difference in time to neutrophil engraftment however this was not significant. While we found an increased incidence of febrile neutropenia in our ANC driven arm (90% versus 74%; p=0.04), there was no difference in infection based on positive bacterial cultures, transfer to the intensive care unit or duration of hospitalization. The reason for this difference in febrile neutropenia rate remains unclear. Cost savings associated with decreased use of G-CSF in the ANC driven arm could be offset by the increased number of patients requiring intravenous antibiotics for febrile neutropenia. Our demonstrated cost benefit with individualized ANC driven administration of G-CSF may not be relevant for patients receiving autologous PBSCTs in the outpatient setting where length of stay is not a factor, however this was not assessed in the current study.
By contrast, a retrospective analysis of multiple myeloma patients undergoing autologous PBSCT, found that G-CSF initiated on day +7 resulted in shorter time to neutrophil engraftment compared to G-CSF initiated when ANC >0.2×109/L but less than 0.5×109/L within 48 hours. Duration of hospitalization was shorter in the day +7 group (17 days versus 19 days; p<0.0001). However, there was no significant difference in the incidence of febrile neutropenia. Our differing results may be due to their longer duration prior to G-CSF initiation for the individualized ANC driven arm. The median day of G-CSF initiation for their ANC driven arm was day +14 and 55% of patients received no G-CSF. Whereas our study deferred G-CSF until day +12 in the ANC driven arm and 10% of patients received no G-CSF.19
Limitations of this study include the retrospective design, therefore our results are subject to the usual restriction and bias of this type of analysis. However, microbiological and laboratory data were collected using electronic medical records, minimizing absent data and under-reporting. A larger sample size would have enabled us to evaluate the statistical significance of the secondary endpoints assessed in this study.
In conclusion, this study, albeit retrospective, represents the first direct comparative analysis of G-CSF initiated on day +5 versus day +12 if the ANC was <0.5×109/L in adult autologous PBSCT inpatients with multiple myeloma and various types of lymphoma. No significant differences in time to neutrophil or platelet engraftment, duration of hospitalization post-transplant, 1-year relapse rate, and 1-year overall survival were found between the two groups. While the incidence of febrile neutropenia was higher in the ANC driven group, there was no significant difference in the incidence of positive bacterial cultures or transfer to the intensive care unit.
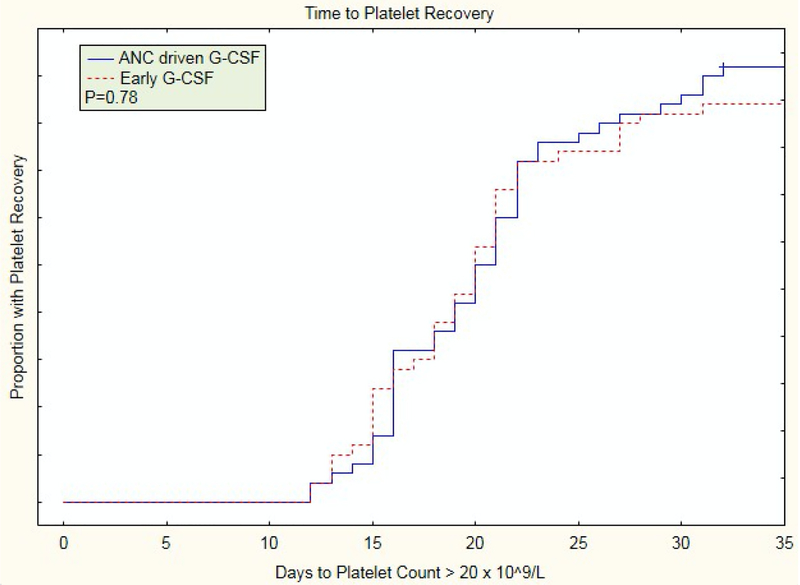
Figure 2.
Time to platelet engraftment
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Financial disclosure:The authors have no financial interests to disclose.
Conflict of interest statement:None declared.
REFERENCES
- 1).Trivedi M, Martinez S, Corringham S et al. Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant. 2009; 43(12):895–908. [DOI] [PubMed] [Google Scholar]
- 2).Smith TJ, Bohlke K, Lyman GH et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015; 33(28):3199–212. [DOI] [PubMed] [Google Scholar]
- 3).Trivedi M, Martinez S, Corringham S et al. Review and revision of clinical practice of using G-CSF after autologous and allogeneic hematopoietic stem cell transplantation at UCSD. J Oncol Pharm Pract. 2011; 17(2):85–90. [DOI] [PubMed] [Google Scholar]
- 4).Linch DC, Milligan DW, Winfield DA et al. G-CSF after peripheral blood stem cell transplantation in lymphoma patients significantly accelerated neutrophil recovery and shortened time in hospital: results of a randomized BNLI trial. Br J Haematol. 1997; 99(4):933–8. [DOI] [PubMed] [Google Scholar]
- 5).Klumpp TR, Mangan KF, Goldberg SL et al. Granulocyte colony-stimulating factor accelerates neutrophil engraftment following peripheral-blood stem-cell transplantation: a prospective, randomized trial. J Clin Oncol. 1995; 13(6):1323–7. [DOI] [PubMed] [Google Scholar]
- 6).Lee SM, Radford JA, Dobson L et al. Recombinant human granulocyte colony-stimulating factor (filgrastim) following high-dose chemotherapy and peripheral blood progenitor cell rescue in high-grade non-Hodgkin’s lymphoma: clinical benefits at no extra cost. Br J Cancer. 1998; 77(8):1294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Spitzer G, Adkins DR, Spencer V et al. Randomized study of growth factors post-peripheral-blood stem-cell transplant: neutrophil recovery is improved with modest clinical benefit. J Clin Oncol. 1994; 12(4):661–70. [DOI] [PubMed] [Google Scholar]
- 8).Kawano Y, Takaue Y, Mimaya J et al. Marginal benefit/disadvantage of granulocyte colony-stimulating factor therapy after autologous blood stem cell transplantation in children: results of a prospective randomized trial. The Japanese Cooperative Study Group of PBSCT. Blood. 1998; 92(11):4040–6. [PubMed] [Google Scholar]
- 9).Demirer T, Ayli M, Dagli M et al. Influence of post-transplant recombinant human granulocyte colony-stimulating factor administration on peritransplant morbidity in patients undergoing autologous stem cell transplantation. Br J Haematol. 2002; 118(4):1104–11. [DOI] [PubMed] [Google Scholar]
- 10).Hornedo J, Solá C, Solano C et al. The role of granulocyte colony-stimulating factor (G-CSF) in the post-transplant period. Bone Marrow Transplant. 2002; 29(9):737–43. [DOI] [PubMed] [Google Scholar]
- 11).Valteau-Couanet D, Faucher C, Aupérin A et al. Cost effectiveness of day 5 G-CSF (Lenograstim) administration after PBSC transplantation: results of a SFGM-TC randomised trial. Bone Marrow Transplant. 2005; 36(6):547–52. [DOI] [PubMed] [Google Scholar]
- 12).Piccirillo N, Sica S, Laurenti L et al. Optimal timing of G-CSF administration after CD34+ immunoselected peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1999; 23(12):1245–50. [DOI] [PubMed] [Google Scholar]
- 13).Bolwell BJ, Pohlman B, Andresen S et al. Delayed G-CSF after autologous progenitor cell transplantation: a prospective randomized trial. Bone Marrow Transplant. 1998; 21(4):369–73. [DOI] [PubMed] [Google Scholar]
- 14).de Azevedo AM, Nucci M, Maiolino A et al. A randomized, multicenter study of G-CSF starting on day +1 vs day +5 after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 2002; 29(9):745–51. [DOI] [PubMed] [Google Scholar]
- 15).Bence-Bruckler I, Bredeson C, Atkins H et al. A randomized trial of granulocyte colony-stimulating factor (Neupogen) starting on day 1 vs day 7 post-autologous stem cell transplantation. Bone Marrow Transplant. 1998; 22(10):965–9. [DOI] [PubMed] [Google Scholar]
- 16).Janusek M, DiGrazia L, Schultz K et al. Neutropenia recovery linked to filgrastim initiation timing after autologous hematopoietic stem-cell transplantation. J Hematol Oncol Pharm. 2016; 6(1):12–6. [Google Scholar]
- 17).Faucher C, Le Corroller AG, Chabannon C et al. Administration of G-CSF can be delayed after transplantation of autologous G-CSF-primed blood stem cells: a randomized study. Bone Marrow Transplant. 1996; 17(4):533–6. [PubMed] [Google Scholar]
- 18).Faber E, Pytlík R, Slabý et al. Individually determined dosing of filgrastim after autologous peripheral stem cell transplantation in patients with malignant lymphoma—results of a prospective multicentre controlled trial. Eur J Haematol. 2006; 77(6):493–500. [DOI] [PubMed] [Google Scholar]
- 19).Cox JE, Campos S, Wu J et al. Efficacy of deferred dosing of granulocyte colony-stimulating factor in autologous hematopoietic transplantation for multiple myeloma. Bone Marrow Transplant. 2014; 49(2):219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Schmitz N, Ljungman P, Cordonnier C et al. Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial. Bone Marrow Transplant. 2004; 34(11):955–62. [DOI] [PubMed] [Google Scholar]
- 21).McQuaker IG, Hunter AE, Pacey S et al. Low-dose filgrastim significantly enhances neutrophil recovery following autologous peripheral-blood stem-cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit. J Clin Oncol. 1997; 15(2):451–7. [DOI] [PubMed] [Google Scholar]
- 22).Tarella C, Castellino C, Locatelli F et al. G-CSF administration following peripheral blood progenitor cell (PBPC) autograft in lymphoid malignancies: evidence for clinical benefits and reduction of treatment costs. Bone Marrow Transplant. 1998; 21(4):401–7. [DOI] [PubMed] [Google Scholar]
- 23).Colby C, McAfee SL, Finkelstein DM et al. Early vs delayed administration of G-CSF following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1998; 21(10):1005–10. [DOI] [PubMed] [Google Scholar]
- 24).Thompson JM, Carlton P, Akard LP et al. Starting granulocyte-colony-stimulating factor (filgrastim) early after autologous peripheral blood progenitor cell transplantation leads to faster engraftment without increased resource utilization. Transfusion. 2009; 49(3):548–54. [DOI] [PubMed] [Google Scholar]