Abstract
BACKGROUND
Cold ischemia time (CIT) causes ischemia-reperfusion injury to the mitochondria and detrimentally effects myocardial function and tissue viability. Mitochondrial transplantation replaces damaged mitochondria and enhances myocardial function and tissue viability. Herein, we investigate the efficacy of mitochondrial transplantation in enhancing graft function and viability after prolonged CIT.
METHODS
Heterotopic heart transplantation was performed in C57BL/6J mice. Upon heart harvesting from C57BL/6J donors, 0.5 mL of either mitochondria (1 × 108 in respiration buffer; Mitochondria) or respiration buffer (Vehicle) was delivered antegrade to the coronary arteries via injection to the coronary ostium. The hearts were excised and preserved for 29 ± 0.3 hours in cold saline (4°C). The hearts were heterotopically transplanted. A second injection of either mitochondria (1 × 108) or respiration buffer (Vehicle) was delivered antegrade to the coronary arteries 5 minutes after transplantation. Grafts were analyzed for 24 hours. Beating score, graft function and tissue injury were measured.
RESULTS
Beating score, calculated ejection fraction and shortening fraction were significantly enhanced (P < 0.05), while necrosis and neutrophil infiltration were significantly decreased (P < 0.05) in Mitochondria as compared to Vehicle at 24 hours of reperfusion. Transmission electron microscopy showed the presence of contraction bands in Vehicle but not in Mitochondria grafts.
CONCLUSION
Mitochondrial transplantation prolongs CIT to 29 hours in the murine heart transplantation model and significantly enhances graft function and decreases graft tissue injury. Mitochondrial transplantation may provide a means to reduce graft failure and improve transplantation outcomes after prolonged CIT.
Keywords: Cold ischemia time, heart transplantation, ischemia-reperfusion, mitochondria, mitochondria transplantation
Introduction
Heart transplantation is the preferred treatment strategy for patients suffering from end stage heart failure. This treatment offers the most beneficial survival outcome and improvement of life quality for heart failure patients and each year the number of transplantations performed increases. However, a growing number of patients requiring heart transplantation are indefinitely waitlisted due to the scarcity of donor hearts.1 The latest available Annual Data Report, by the Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients shows that only 50% of the candidates waitlisted throughout the year undergo transplantation, which results in significant mortality of patients awaiting heart transplantation.1 The imbalance between heart donors and heart transplantation demand and the increasing incidence of primary graft dysfunction and early graft failure requires modification of current technical preservation protocols.2,3
Presently, the average cold ischemia time (CIT) in humans is 4 – 6 hours and CIT ≥ 4 hours is associated with considerably lower survival than CIT < 4 hours.3, 4, 5 The mechanism primarily responsible for decreased survival is ischemia-reperfusion (IR) injury.6 Therefore, decreasing IR injury could have significant importance in heart transplantation and could prolong currently acceptable CIT and improve post-transplantation outcomes.
We and others have shown that IR injury induces damage to mitochondrial structure and function. This damage occurs during ischemia and extends into reperfusion, severely compromising heart function and viability.7, 8, 9 In previous studies we have pioneered a novel therapy, mitochondrial transplantation.10, 11, 12, 13, 14, 15 This therapy replaces native mitochondria damaged by IR, with viable, respiration-competent, mitochondria isolated from non-ischemic tissue. Our prior results show that mitochondrial transplantation is safe, and significantly enhances post-ischemic myocardial function and viability.10, 11, 12, 13, 14, 15 In this study, we investigate the effect of mitochondria transplantation to enhance post-transplantation function and heart graft viability in a murine heterotopic heart transplantation model.
We hypothesized that mitochondrial transplantation would enhance heart graft function and decrease tissue injury after prolonged CIT in the murine heart transplantation model. This could reduce heart graft failure in the post-transplantation period and improve transplantation outcomes after prolonged CIT, thereby increasing the heart donor pool and improving organ allocation.
Methods
Animals
Male C57BL/6J mice (7–9 weeks, n = 50; Jackson Laboratory, Bar Harbor, ME) were used. All experiments were approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital and conformed to the National Institutes of Health guidelines on animal care and use.
Mitochondrial isolation
Gastrocnemius muscle from C57BL/6J mice (n = 8) was dissected and used immediately to isolate syngeneic mitochondria. Mitochondria were isolated and the number and viability of the isolated mitochondria was determined as previously described.16, 17 The isolated mitochondria were suspended in 0.5 mL respiration buffer (250 mmol/L sucrose, 20 mmol/L K+-HEPES buffer, pH 7.2, 0.5 mmol/L K+-EGTA, pH 8.0) and used immediately for mitochondrial transplantation.
CIT and heterotopic heart transplantation
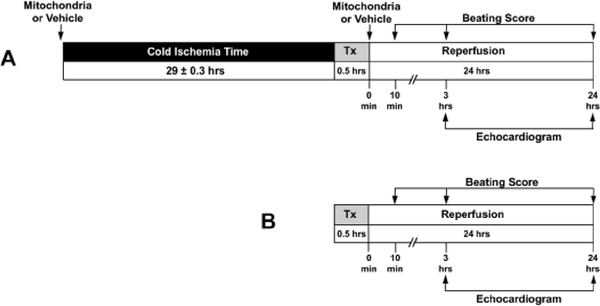
The experimental protocol is shown in Figure 1. Heterotopic heart transplantation was performed according to the procedure described by Corry et al.18 In brief, C57BL/6J donor mice (n = 21) were anesthetized and maintained on 2 – 3% inhaled isoflurane. A middle laparotomy and sternotomy were performed. The ascending aorta was exposed, and either 1 × 108 mitochondria in 0.5 mL respiration buffer (Mitochondria, n = 8) or 0.5 mL respiration buffer (Vehicle, n = 9) was delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle. One mL of heparin (100 IU/mL) was injected via the inferior vena cava and allowed to circulate for 10 min.19 The heart grafts were then excised and stored in normal saline solution (Baxter, Deerfield, IL, USA) containing 1% Penicillin and 1% Streptomycin at 4°C. Following, 29 ± 0.3 hours CIT, the heart grafts were transplanted into the abdomen of C57BL/6J recipients and coronary circulation was re-established.18 Heart grafts received a 2nd injection, 5 minutes after transplantation, of 1 × 108 mitochondria in 0.5 mL respiration buffer (Mitochondria) or 0.5 mL respiration buffer (Vehicle), delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle. Sham control hearts received heparin and following 10 minutes of circulation, were excised and immediately transplanted to recipient mice (Sham, n = 4).
Fig. 1.
Experimental protocol. (A) C57BL/6J male mice (n = 17, 7–9 weeks) were used for all experimental groups. Donor hearts received 1 × 108 mitochondria in 0.5 mL respiration buffer (Mitochondria, n = 8) or 0.5 mL respiration buffer (Vehicle, n = 9) delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle, 10 minutes before organ harvest. Heart grafts were then preserved in normal saline solution containing 1% Penicillin and 1% Streptomycin at 4 °C for 29 ± 0.3 hours prior to heterotopic heart transplantation. Five minutes after transplantation, heart grafts received a second injection of 1 × 108 mitochondria in 0.5 mL respiration buffer (Mitochondria) or 0.5 mL respiration buffer (Vehicle) delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle. Mice were allowed to recover for 24 hours, and tissue was then collected for further analysis. Serial beating score assessment and echocardiography were obtained for analysis. (B) C57BL/6J male mice (n = 8, 7–9 weeks) were used for sham control (Sham, n = 4). Donor mice heart grafts were immediately transplanted with no CIT and no mitochondrial or respiration buffer injection. Recovery and analysis were identical as mentioned above.
Heart graft functional analysis
Beating score of heart grafts was assessed visually after 10 minutes and 24 hours of reperfusion (open laparotomy) and by palpitation at 3 hours of reperfusion (closed laparotomy) using the Stanford cardiac surgery laboratory graft scoring system (0, no contraction; 1, contraction barely visible or palpable; 2, obvious decrease in contraction strength, but still contracting in a coordinated manner; rhythm disturbance; 3, strong, coordinated beat but noticeable decrease in strength or rate; 4, strong contraction of both ventricles, regular rate).20 Transabdominal two-dimensional echocardiographic analysis was performed with an 8–12 MHz ultrasound probe at 3 and 24 hours of reperfusion. Left ventricular end-diastolic diameter (LVIDd), and left ventricular end-systolic diameter (LVIDs) were measured to assess heart graft functional performance. Left ventricular shortening fraction (FS) was determined by (LVIDd-LVIDs)/LVIDd.21 Left ventricular calculated ejection fraction (EF) was determined by (LVIDd3-LVIDs3)/LVIDd3.21
Tissue analysis
After 24 hours of reperfusion heart grafts were collected and fixed in 10% formalin. The tissue was paraffin embedded and sectioned at 5μm thickness. Serial slides were used for hematoxylin and eosin (H&E) staining and myeloperoxidase staining. H&E stained slides were evaluated for the area of necrosis and inflammatory cells infiltration.22, 23 The area of necrosis and inflammatory cells infiltration was determined as previously described and expressed as a percentage of the whole section.22
Neutrophil infiltration was determined by immunohistochemistry using the myeloperoxidase antibody (DAKO M0398 polyclonal, Agilent, Santa Clara, CA) 1:1000 dilution in Leica antibody diluent (Leica, Newcastle Upon Tyne, UK).21 The number of neutrophils was counted in 5 random (20x) visual fields.
TUNEL assay was performed using the ApopTag detection system (EMD Millipore Corporation, Temecula, CA).24 Nuclear staining 4’,6-diamidino-2-phenylindole (DAPI) (Thermo-Fisher, Waltham, MA) was applied according to the manufacturer’s directions.11 All cells were counted in 27 (20x) visual fields per section and the number of TUNEL-positive nuclei was expressed as a percentage of all cardiomyocytes.
Transmission electron microscopy was used to analyze structural damage in the heart grafts as previously described.11
Heart uptake of radiolabeled mitochondria
In separate set of experiments Wistar rats (200–250 g, n = 4, Charles River Laboratories, Worcester, MA) were used for visualization of mitochondrial uptake in the heart. A donor rat (n = 1) was used to isolate syngeneic mitochondria from the gastrocnemius muscle tissue as previously described.16, 17 The isolated mitochondria were labeled with 18F-Rhodamine 6G.25 Following, Wistar rats (n = 3) were anesthetized and maintained on 2 – 3% inhaled isoflurane. A sternotomy was performed and the ascending aorta was exposed. The 18F-Rhodamine 6G labeled mitochondria (1 × 109 in 0.5 mL respiration buffer) were delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 30 G needle. Ten minutes after the injection, the animals were euthanized in a CO2 chamber and examined using positron emission tomography (PET) and microcomputed tomography (μCT).
Statistical analysis
All data were assessed by observers blinded to the treatments. All data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed by non-parametric Mann-Whitney U test. Statistical significance was defined by an exact two-tailed P < 0.05.
Results
Uptake of mitochondria
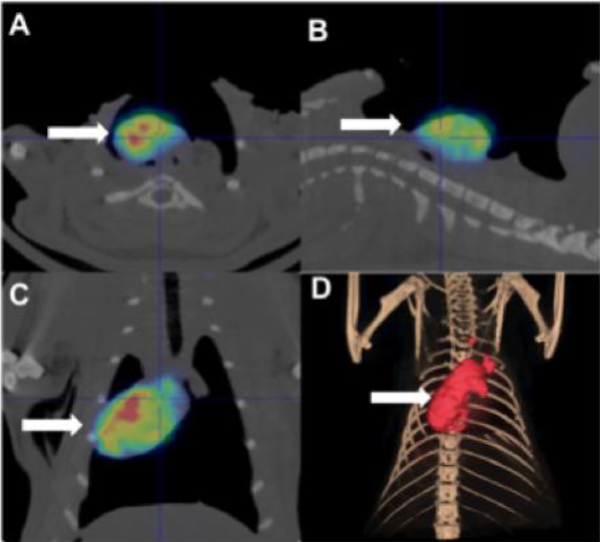
18F-Rhodamine 6G labeled mitochondria demonstrated diffuse distribution throughout the heart (Figure 2). Radiolabeled mitochondria were not detectable in any other organ or region of the body.
Fig. 2.
Uptake and distribution of transplanted mitochondria. 18F-Rhodamine 6G labeled mitochondria (1 × 109 in 0.5 mL respiration) were delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 30 G needle. Ten minutes after the injection animals were euthanized in CO2 chamber and examined using positron emission tomography (PET) and microcomputed tomography (μCT). 18F-Rhodamine 6G labeled mitochondria were distributed throughout the heart and were not detectable in any other region of the body. Images are shown for (A) transverse, (B) sagittal, (C) coronal and (D) 3D reconstructed views.
Beating score
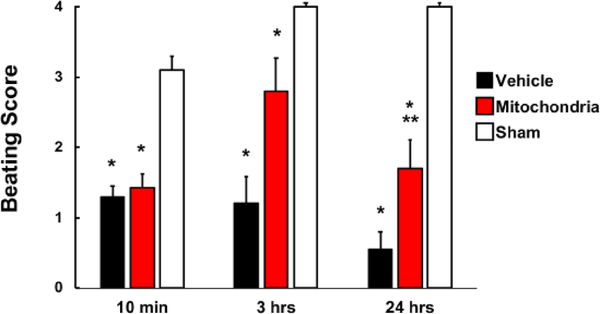
No significant difference was observed in beating score (BS) at 10 minutes of reperfusion between Mitochondria heart grafts,1.4 ± 0.2 and Vehicle heart grafts,1.3 ± 0.2 (P = 0.6, Figure 3). BS at 3 hours of reperfusion was enhanced to 2.8 ± 0.5 in Mitochondria, but not in Vehicle, 1.2 ± 0.4 (P = 0.056). BS at 24 hours of reperfusion in Mitochondria was not significantly different as compared to 3 hours of reperfusion (2.8 ± 0.5 vs 1.7 ± 0.4, respectively for 3 and 24 hours, P = 0.68) and was significantly increased (P < 0.05) when compared to Vehicle heart grafts, 0.6 ± 0.2. All BS for both Mitochondria and Vehicle heart grafts were significantly decreased as compared to Sham heart grafts (P < 0.05; Figure 3).
Fig. 3.
Beating score in heart grafts at 10 minutes, 3 and 24 hours of reperfusion in Vehicle, Mitochondria and Sham groups. Beating score was determined as; 0, no contraction; 1, contraction barely visible or palpable; 2, obvious decrease in contraction strength, but still contracting in a coordinated manner; rhythm disturbance; 3, strong, coordinated beat but noticeable decrease in strength or rate; 4, strong contraction of both ventricles, regular rate. All analysis was performed by a blinded observer. Results show significant difference in BS between Mitochondria and Vehicle heart grafts at 24 hours of reperfusion. All values are mean ± SEM. *P < 0.05 vs. Sham. **P < 0.05 vs. Vehicle. n = 8 for Mitochondria, 9 for Vehicle, 4 for Sham.
Measurement of global function
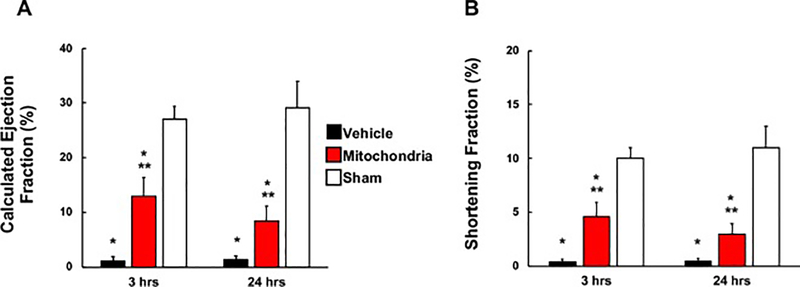
At 3 hours of reperfusion, calculated ejection fraction (EF) and shortening fraction (FS) in Mitochondrial heart grafts (13.03 ± 3.39 % and 4.58 ± 1.32 %, respectively) were significantly increased (P < 0.05 each) as compared to Vehicle heart grafts (1.15 ± 0.75 % and 0.39 ± 0.25 %, respectively) (Figure 4). At 24 hours of reperfusion both EF and FS remained significantly increased (P < 0.05) in Mitochondria heart grafts (8.46 ± 2.67 % and 2.96 ± 0.96 %, respectively) as compared to Vehicle heart grafts (1.38 ± 0.64 % and 0.47 ± 0.22 %, respectively) (Figure 4). There was no significant difference observed in EF or FS in Mitochondria heart grafts (P = 0.72, P = 0.72; respectively) or Vehicle heart grafts (P = 0.83, P = 0.83; respectively) at 3 and 24 hours. All echocardiography results for both Mitochondria and Vehicle heart grafts were significantly decreased as compared to Sham heart grafts (P < 0.05; Figure 4).
Fig. 4.
Echocardiographic analysis in heart grafts. (A) Calculated ejection fraction (EF) and (B) shortening fraction (FS) were measured at 3 and 24 hours of reperfusion in Vehicle, Mitochondria and Sham groups. Results show significant difference in EF and FS between Mitochondria and Vehicle heart grafts at 3 and 24 hours of reperfusion. All values are mean ± SEM. *P < 0.05 vs. Sham. **P < 0.05 vs. Vehicle. n = 8 for Mitochondria, 9 for Vehicle, 4 for Sham.
Myocardial tissue injury and neutrophil infiltration
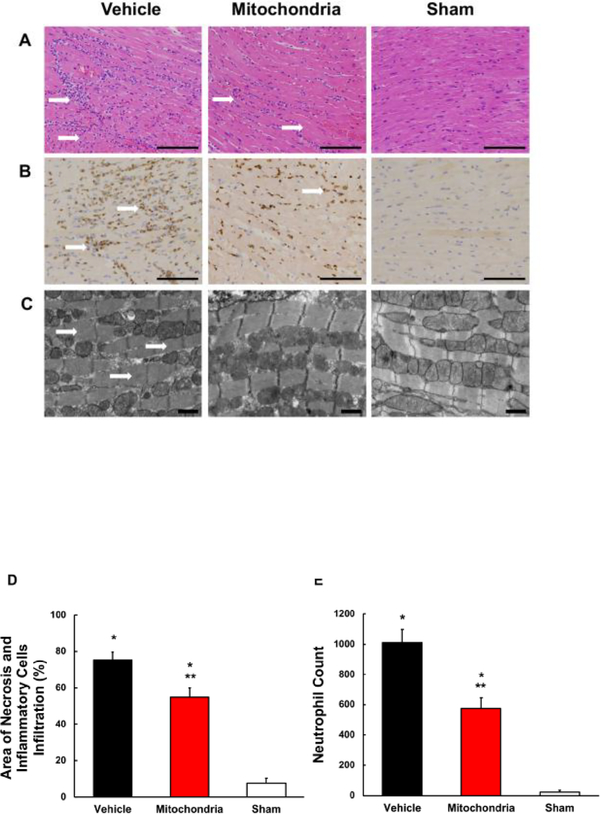
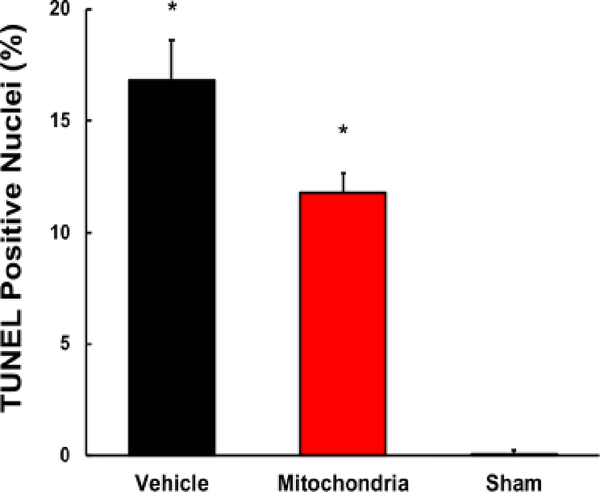
H&E analysis showed significantly less necrosis and inflammatory cell infiltration in Mitochondria heart grafts as compared to Vehicle heart grafts (55.0 ± 5 %, 75.6 ± 4.1 %, respectively; P < 0.05; Figure 5A, D). In heart grafts receiving mitochondria, neutrophil infiltration was significantly lower as compared to Vehicle heart grafts (577 ± 69, 1011 ± 88, respectively; P < 0.05; Figure 5B, E). Neutrophil infiltration was significantly lower in Sham group as compared to Vehicle and Mitochondria heart grafts (P < 0.05, Figure 5B, E). No significant difference in TUNEL positive nuclei was observed between Mitochondria and Vehicle heart grafts (11.5 ± 1.0 %, 16.9 ± 1.9 %, respectively; P > 0.05; Figure 6). Transmission electron micrographs showed contraction bands, indicating myocardial injury, in Vehicle heart grafts. No contraction bands were observed in Mitochondria and Sham heart grafts (Figure 5C).
Fig. 5.
Myocardial tissue injury and neutrophil infiltration at 24 hours of reperfusion. (A) Representative H&E micrographs of heart grafts tissue sections. Tissue sections from Mitochondria heart grafts show significantly less severe necrosis and inflammatory cells infiltration as compared to Vehicle heart grafts. Sham heart grafts show only minimal myocardial injury and inflammatory cells infiltration. Scale bars, 100 μm. (B) Representative micrographs of tissue sections from heart grafts stained for myeloperoxidase content to quantify neutrophil infiltration. Tissue sections from Mitochondria heart grafts show decreased neutrophil infiltration as compared to Vehicle heart grafts. Tissue sections from Sham heart grafts show no neutrophil infiltration. Scale bars, 100 μm. (C) Representative transmission electron micrographs. Transmission electron microscopy analysis shows similar profile in Mitochondria and Sham heart grafts, with no observed contraction bands, while Vehicle heat grafts show contraction bands indicating myocardial injury. Scale bars, 1 μm. (D) Area of necrosis and inflammatory cells infiltration in the entire transversal tissue sections of Vehicle, Mitochondria and Sham heart grafts. Results show significantly less necrosis and inflammatory cells infiltration in Mitochondria heart grafts as compared to Vehicle. (E) Neutrophil count determined by positive myeloperoxidase staining counted in 5 random (20x) visual fields per tissue section. Results show significantly lower neutrophil infiltration in Mitochondria heart grafts as compared to Vehicle. All values are mean ± SEM. *P < 0.05 vs. Sham. **P < 0.05 vs. Vehicle. n = 8 for Mitochondria, 9 for Vehicle, 4 for Sham.
Fig. 6.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay of the heart grafts tissue sections at 24 hours of reperfusion to determine severity of apoptosis in Vehicle, Mitochondria and Sham heart grafts. Twenty-seven visual fields per section at 20x were analyzed. Results show no significant difference in TUNEL positive nuclei between Mitochondria and Vehicle heart grafts. All values are mean ± SEM. *P < 0.05 vs. Sham. **P < 0.05 vs. Vehicle. n = 8 for Mitochondria, 9 for Vehicle, 4 for Sham.
Discussion
IR injury to heart graft plays an important role in post-transplantation morbidity and mortality of transplant recipients.3 Here, we show the efficacy of mitochondrial transplantation in prolonging CIT to 29 hours and enhancing heart graft tissue viability and function for at least 24 hours recovery.
Previous studies investigating IR injury in murine heart transplantation have utilized CIT ranging from 18 to 24 hours.22, 26 The severity of IR injury in these models ranged from mild to more severe neutrophil infiltration and necrosis. No functional data was evaluated in these studies. 22, 27 In our study, we wished to prolong CIT beyond 24 hours and provide both histological and functional data. Preliminary experiments demonstrated that a CIT of >30 hours in Vehicle heart grafts resulted in severe neutrophil infiltration and necrosis, and failure to recover functionally. We therefore chose a CIT of 29 hours to reduce graft damage and to allow for functional recovery in Vehicle heart grafts to allow for comparative analysis with Mitochondria hearts.
To further allow for comparative analysis, we have used normal saline as the preservation media. It has been previously demonstrated that heart graft tissue survival can be prolonged to a maximum of 24 hours CIT using a solution containing antioxidant reagents or a modified histidine-tryptophan ketoglutarate solution.19, 23 The advantages of normal saline solution are that it is readily available and does not enhance recovery.27 This allowed for direct analysis of the efficacy of mitochondrial transplantation. No hearts were given cardioplegia to protect the heart.
To demonstrate the localization and distribution of mitochondria delivered by vascular perfusion we have used 18F-Rhodamine 6G labeled mitochondria. The rat was used as it allows for greater detail for distribution analysis of radiolabeled mitochondria as compared to smaller mouse. We delivered mitochondria antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle. Our results show diffuse distribution of the injected mitochondria throughout the heart with no detectable mitochondria in any other region of the body.
To enhance function in donor hearts we have used 1 × 108 mitochondria. This concentration is considerably higher (5 × 108 per gram wet weight) as compared to the mitochondrial concentration used in our previous studies in porcine heart (2 – 5 × 105 per gram wet weight).10, 11, 12, 13 The increased dosage of mitochondria is based on preliminary investigations showing greater efficacy using higher mitochondrial concentration in the murine transplantation model. Lower mitochondria concentrations 1 × 106 and 1 × 107 (5 × 106 - 5 × 107 per gram wet weight) were not as effective. The increased mitochondria concentration needed in this study is most likely due to the significantly increased metabolic rate and energy requirements in the murine heart, as compared to the porcine heart.30
Mitochondria were delivered to the heart graft at two times. The mitochondria were first delivered just prior to CIT, to allow for protection from ischemia and then again at the beginning of reperfusion. In our preliminary experiments in which mitochondria were delivered either only prior to CIT (n = 2) or both prior and at the beginning of reperfusion (n = 8), we found that addition of a second mitochondrial injection at the beginning of reperfusion enhanced beating score at 10 minutes (0.8 ± 0.2 vs 1.4 ± 0.2, respectively) at 3 hours (1.8 ± 0.3 vs 2.8 ± 0.5, respectively) and at 24 hours (1.0 ± 0.0 vs 1.7 ± 0.4). However, we did not evaluate a single post-CIT injection, because at the time of heart harvest, donor’s muscle tissue is an excellent source of fresh, viable mitochondria, readily available and should therefore be effectively utilized.
Our results demonstrate that mitochondrial transplantation significantly reduces neutrophil infiltration and necrosis. Contraction bands, evidence of severe ischemic injury, were observed only in Vehicle heart grafts. We also show that mitochondrial transplantation provides prolonged functional benefits. Heart graft function, beating score, calculated ejection fraction and shortening fraction were all significantly increased at 24 hours of reperfusion in Mitochondria heart grafts as compared to Vehicle heart grafts.
The relationship between prolonged CIT and impairment of mitochondria and post-CIT myocardial function has been previously noted.12, 30, 31, While the mechanism of mitochondrial transplantation in ameliorating the deleterious effects of CIT is beyond the scope of this paper, we speculate based on our previous studies that the transplanted mitochondria would augment or support the mitochondria damaged during prolonged CIT. We have previously demonstrated that transplanted mitochondria, rapidly taken up by the heart cells by endocytosis, increase tissue ATP content, high energy synthesis, replace damaged mitochondrial DNA, and enhance proteomic pathways for the mitochondrion and the generation of precursor metabolites for energy and cellular respiration.11, 12, 28, 34 These alterations significantly enhance functional recovery and cellular viability.
It should be noted that while we have used syngeneic mitochondria for both mitochondrial injections, the source of the mitochondria is not limited to the donor. We have recently demonstrated that there is no direct or indirect, acute or chronic alloreactivity or allorecognition or DAMPs reaction to single or serial injections of either syngeneic or allogeneic mitochondria.34
In a recent study we have shown that mitochondrial transplantation is safe and efficacious for use in humans.14,15 Mitochondrial isolation is a rapid process taking less than 30 minutes, conveniently done at the bed side. For clinical usage we envisage pre-CIT mitochondrial injection to be isolated from the donor’s muscle and injected via vasculature immediately after cross clamping the donor’s aorta. The post-CIT mitochondrial injection would be isolated from the recipient’s muscle during heart graft implantation process. Mitochondria would be injected after reestablishing the grafts circulation.
A limitation of our study is the use of a robust model of heterotopic heart transplantation in which the heart was preserved in saline for 29 hours. This robust model does not provide for analysis at shorter time points and further study in a less robust model is required using orthotopic heart transplantation with other preservation solutions with physiologic heart perfusion using a loaded heart preparation prior to translation to human transplantation.
In conclusion, mitochondrial transplantation provides a novel approach to prolonging CIT for heart transplantation. Mitochondrial transplantation enhances heart graft function and decreases heart graft tissue injury after prolonged CIT. These early results suggest that mitochondrial transplantation could reduce heart graft failure in the post-transplantation period and improve transplantation outcomes after prolonged CIT thereby increasing the heart donor pool and improving organ allocation. We speculate that mitochondrial transplantation can be used in ex vivo heart perfusion system - both in warm and cold perfusion systems. Mitochondrial transplantation when combined with EVHP could potentially prolong CIT and even warm ischemia in donation from cardiocirculatory death, decreasing ischemic injury to the graft and improving the transplantation outcomes.
Highlights.
Mitochondrial transplantation prolongs cold ischemia time to 29 hours.
Mitochondrial transplantation enhances heart graft function.
Mitochondrial transplantation decreases heart graft tissue injury.
Acknowledgments
This work was supported by Boston Children’s Hospital Anesthesia Foundation, Ryan Family Endowment, the Cardiac Conduction Fund, NIH 5T32HL007734, NIH 5R01HL108107, the Richard A. and Susan F. Smith President’s Innovation Award, Michael B. Klein and Family, The Sidman Family Foundation, The Michael B. Rukin Charitable Foundation, The Kenneth C. Griffin Charitable Research Fund and The Boston Investment Council.
Footnotes
Conflict of Interest statement
Dr. McCully, Dr. Cowan and Dr. del Nido have patents pending for the isolation and usage of mitochondria. There are no other conflicts of interest by any of the authors. The authors attest they had full freedom to explore the data, analyze the results independent from any sponsor and that they had sole authority to make the final decision to submit the material for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 Annual Data Report: Heart. Am J Transplant 2018;18(Suppl 1):291–362. [DOI] [PubMed] [Google Scholar]
- 2.Shah MR, Starling RC, Schwartz Longacre L, Mehra MR, on behalf of the Working Group Participants. NHLBI Working Group: Heart Transplantation Research in the Next Decade-A Goal to Achieving Evidence-Based Outcomes. J Am Coll Cardiol 2012;59:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramaniam K Early graft failure after heart transplantation: prevention and treatment. Int Anesthesiol Clin 2012;50:202–227. [DOI] [PubMed] [Google Scholar]
- 4.Lund LH, Khush KK, Cherikh WS, et al. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037–1046. [DOI] [PubMed] [Google Scholar]
- 5.Weiss ES, Allen JG, Kilic A, et al. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant 2012;31:266–273. [DOI] [PubMed] [Google Scholar]
- 6.Russo MJ, Iribarne A, Hong KN, et al. Factors Associated With Primary Graft Failure After Heart Transplantation. Transplantation 2010;90:444–450. [DOI] [PubMed] [Google Scholar]
- 7.Lesnefsky EJ, Chen Q, Slabe TJ, et al. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol 2004;287:H258–H267. [DOI] [PubMed] [Google Scholar]
- 8.McCully JD, Rousou AJ, Parker RA, Levitsky S. Age- and gender related differences in mitochondrial oxygen consumption and calcium with cardioplegia and diazoxide. Ann Thorac Surg 2007;83:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Opening of mitochondrial KATP enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium accumulation and respiration. Am J Physiol Heart Circ Physiol 2004;287:H1967–H1976. [DOI] [PubMed] [Google Scholar]
- 10.McCully JD, Cowan DB, Pacak CHA, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 2009;296:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuzawa A, Black KM, Pacak CHA, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2013;304:H966–H982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan DB, Yao R, Akurathi V,et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 2016,11(8):e0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaza AK, Wamala I, Friehs I, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg 2017;153:934–943. [DOI] [PubMed] [Google Scholar]
- 14.Emani SM, Piekarski BL, Harrild D, del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2017;154:286–289. [DOI] [PubMed] [Google Scholar]
- 15.Emani SM, McCully JD. Mitochondrial transplantation: applications for pediatric patients with congenital heart disease. Transl Pediatr. 2018;7: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preble JM, Pacak CA, Kondo H, McKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation. J Vis Exp 2014;91:e51682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preble JM, Kondo H, Levitsky S, McCully JD. Quality control parameters for mitochondria transplant in cardiac tissue. JSM Biochem Mol Biol 2014;2:1008. [Google Scholar]
- 18.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 1973;16:343–350. [DOI] [PubMed] [Google Scholar]
- 19.Cai S, Ichimaru N, Zhao M, et al. Prolonged Mouse Cardiac Graft Cold Storage via Attenuating Ischemia-Reperfusion Injury Using a New Antioxidant-Based Preservation Solution. Transplantation 2016;100:1032–1040. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Terry RD, Mokhtari GK, et al. Suppression of graft coronary artery disease by a brief treatment with a selective epsilonPKC activator and a deltaPKC inhibitor in murine cardiac allografts. Circulation 2004;110(11 Suppl 1):II194–199. [DOI] [PubMed] [Google Scholar]
- 21.Scherrer-Crosbie M, Glysing-Jensen T, Fry SJ, et al. Echocardiography Improves Detection of Rejection After Heterotopic Mouse Cardiac Transplantation. J Am Soc Echocardiogr 2002;15:1315–1320. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Moszczynski LA, Liu Q, et al. Over-expression of growth differentiation factor 15 (GDF15) preventing cold ischemia reperfusion (I/R) injury in heart transplantation through Foxo3a signaling. Oncotarget 2017;8:36531–36544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Turk TR, Rauen U, et al. Prolonged cold storage using a new histidine–tryptophan–ketoglutarate-based preservation solution in isogeneic cardiac mouse grafts. European Heart Journal 2011;32:509–516. [DOI] [PubMed] [Google Scholar]
- 24.Stadler B, Phillips J, Toyoda Y, Federman M, Levitsky S, McCully JD. Adenosine-Enhanced Ischemic Preconditioning Modulates Necrosis and Apoptosis: Effects of Stunning and Ischemia–Reperfusion. Ann Thorac Surg 2001;72:555–564. [DOI] [PubMed] [Google Scholar]
- 25.Bartholomä MD, Zhang S, Akurathi V, et al. 18F-Labeled Rhodamines as Potential Myocardial Perfusion Agents: Comparison of Pharmacokinetic Properties of Several Rhodamines. Nucl Med Biol 2015;42:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Zang G, Zhang G, et al. MicroRNA and mRNA Signatures in Ischemia Reperfusion Injury in Heart Transplantation. PLoS ONE 2013;8(11):e79805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanchard JM, Pollak R. Techniques for perfusion and storage of heterotopic heart transplantation in mice. Microsurgery 1985;6:169–174. [DOI] [PubMed] [Google Scholar]
- 28.Cowan DB, Yao R, Thedsanamoorthy JK, Zurakowski D, del Nido PJ, McCully JD. Transit and integration of extracellular mitochondria in human heart cells. Sci Rep 2017;7(1):17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whary MT, Baumgarth N, Fox JG, Barthold SW. Biology and Diseases of Mice In: Anderson LC, Otto G, Pritchett-Corning KR, Whary MT, Fox JG, editors. Laboratory Animal Medicine (Third Edition). San Diego: Elsevier; 2015. p. 43–149. [Google Scholar]
- 30.Kuznetsov AV, Schneeberger S, Seiler R, et al. Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am J Physiol Heart Circ Physiol 2004;286:H1633–H1641. [DOI] [PubMed] [Google Scholar]
- 31.Kay L, Daneshrad Z, Saks VA, Rossi A. Alteration in the control of mitochondrial respiration by outer mitochondrial membrane and creatine during heart preservation. Cardiovascular Research 1997;34:547–556. [DOI] [PubMed] [Google Scholar]
- 32.Kuwabara M, Takenaka H, Maruyama H, Onitsuka T, Hamada M. Effect of prolonged hypothermic ischemia and reperfusion on oxygen consumption and total mechanical energy in rat myocardium: participation of mitochondrial oxidative phosphorylation. Transplantation 1997;64:577–583. [DOI] [PubMed] [Google Scholar]
- 33.Pacak CHA, Preble JM, Kondo H, Seibel P, Levitsky S, del Nido PJ, Cowan DB, McCully JD. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biology Open 2015;4:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Barbieri G, Moskowitzova K, Shin B, et al. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion 2018. 10.1016/j.mito.2018.03.002. [DOI] [PubMed] [Google Scholar]