Abstract
Background
In most pregnancies that miscarry, arrest of embryonic or fetal development occurs some time (often weeks) before the miscarriage occurs. Ultrasound examination can reveal abnormal findings during this phase by demonstrating anembryonic pregnancies or embryonic or fetal death. Treatment has traditionally been surgical but medical treatments may be effective, safe, and acceptable, as may be waiting for spontaneous miscarriage. This is an update of a review first published in 2006.
Objectives
To assess, from clinical trials, the effectiveness and safety of different medical treatments for the termination of non‐viable pregnancies.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (24 October 2018) and reference lists of retrieved studies.
Selection criteria
Randomised trials comparing medical treatment with another treatment (e.g. surgical evacuation), or placebo, or no treatment for early pregnancy failure. Quasi‐randomised studies were excluded. Cluster‐randomised trials were eligible for inclusion, as were studies reported in abstract form, if sufficient information was available to assess eligibility.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed the quality of the evidence using the GRADE approach.
Main results
Forty‐three studies (4966 women) were included. The main interventions examined were vaginal, sublingual, oral and buccal misoprostol, mifepristone and vaginal gemeprost. These were compared with surgical management, expectant management, placebo, or different types of medical interventions were compared with each other. The review includes a wide variety of different interventions which have been analysed across 23 different comparisons. Many of the comparisons consist of single studies. We limited the grading of the quality of evidence to two main comparisons: vaginal misoprostol versus placebo and vaginal misoprostol versus surgical evacuation of the uterus. Risk of bias varied widely among the included trials. The quality of the evidence varied between the different comparisons, but was mainly found to be very‐low or low quality.
Vaginal misoprostol versus placebo
Vaginal misoprostol may hasten miscarriage when compared with placebo: e.g. complete miscarriage (5 trials, 305 women, risk ratio (RR) 4.23, 95% confidence interval (CI) 3.01 to 5.94; low‐quality evidence). No trial reported on pelvic infection rate for this comparison. Vaginal misoprostol made little difference to rates of nausea (2 trials, 88 women, RR 1.38, 95% CI 0.43 to 4.40; low‐quality evidence), diarrhoea (2 trials, 88 women, RR 2.21, 95% CI 0.35 to 14.06; low‐quality evidence) or to whether women were satisfied with the acceptability of the method (1 trial, 32 women, RR 1.17, 95% CI 0.83 to 1.64; low‐quality evidence). It is uncertain whether vaginal misoprostol reduces blood loss (haemoglobin difference > 10 g/L) (1 trial, 50 women, RR 1.25, 95% CI 0.38 to 4.12; very‐low quality) or pain (opiate use) (1 trial, 84 women, RR 5.00, 95% CI 0.25 to 101.11; very‐low quality), because the quality of the evidence for these outcomes was found to be very low.
Vaginal misoprostol versus surgical evacuation
Vaginal misoprostol may be less effective in accomplishing a complete miscarriage compared to surgical management (6 trials, 943 women, average RR 0.40, 95% CI 0.32 to 0.50; Heterogeneity: Tau² = 0.03, I² = 46%; low‐quality evidence) and may be associated with more nausea (1 trial, 154 women, RR 21.85, 95% CI 1.31 to 364.37; low‐quality evidence) and diarrhoea (1 trial, 154 women, RR 40.85, 95% CI 2.52 to 662.57; low‐quality evidence). There may be little or no difference between vaginal misoprostol and surgical evacuation for pelvic infection (1 trial, 618 women, RR 0.73, 95% CI 0.39 to 1.37; low‐quality evidence), blood loss (post‐treatment haematocrit (%) (1 trial, 50 women, mean difference (MD) 1.40%, 95% CI ‐3.51 to 0.71; low‐quality evidence), pain relief (1 trial, 154 women, RR 1.42, 95% CI 0.82 to 2.46; low‐quality evidence) or women's satisfaction/acceptability of method (1 trial, 45 women, RR 0.67, 95% CI 0.40 to 1.11; low‐quality evidence).
Other comparisons
Based on findings from a single trial, vaginal misoprostol was more effective at accomplishing complete miscarriage than expectant management (614 women, RR 1.25, 95% CI 1.09 to 1.45). There was little difference between vaginal misoprostol and sublingual misoprostol (5 trials, 513 women, average RR 0.84, 95% CI 0.61 to 1.16; Heterogeneity: Tau² = 0.10, I² = 871%; or between oral and vaginal misoprostol in terms of complete miscarriage at less than 13 weeks (4 trials, 418 women), average RR 0.68, 95% CI 0.45 to 1.03; Heterogeneity: Tau² = 0.13, I² = 90%). However, there was less abdominal pain with vaginal misoprostol in comparison to sublingual (3 trials, 392 women, RR 0.58, 95% CI 0.46 to 0.74). A single study (46 women) found mifepristone to be more effective than placebo: miscarriage complete by day five after treatment (46 women, RR 9.50, 95% CI 2.49 to 36.19). However the quality of this evidence is very low: there is a very serious risk of bias with signs of incomplete data and no proper intention‐to‐treat analysis in the included study; and serious imprecision with wide confidence intervals. Mifepristone did not appear to further hasten miscarriage when added to a misoprostol regimen (3 trials, 447 women, RR 1.18, 95% CI 0.95 to 1.47).
Authors' conclusions
Available evidence from randomised trials suggests that medical treatment with vaginal misoprostol may be an acceptable alternative to surgical evacuation or expectant management. In general, side effects of medical treatment were minor, consisting mainly of nausea and diarrhoea. There were no major differences in effectiveness between different routes of administration. Treatment satisfaction was addressed in only a few studies, in which the majority of women were satisfied with the received intervention. Since the quality of evidence is low or very low for several comparisons, mainly because they included only one or two (small) trials; further research is necessary to assess the effectiveness, safety and side effects, optimal route of administration and dose of different medical treatments for early fetal death.
Plain language summary
Medical treatment for early fetal death (less than 24 weeks)
What is the issue?
A miscarriage is the spontaneous death and/or expulsion of an embryo or fetus from the uterus before it is able to survive on its own. This natural death of an embryo or fetus ('non‐viable pregnancy' or 'intrauterine fetal death', depending on the duration of pregnancy) can be identified by ultrasound before symptoms like blood loss and abdominal pain occur. Sometimes an embryo may not have even developed ('empty sac'). In the past, treatment for a deceived embryo/fetus, has usually been by dilatation and curettage (D&C) surgery, but drugs have now been developed to replace the need for surgery which may be helpful for the expulsion to happen. Misoprostol and gemeprost are synthetic prostaglandin E analogues that can stimulate expulsion of the embryo/fetus from the uterus. Mifepristone blocks the activity of progesterone, a hormone that supports pregnancy. These and similar drugs may be useful in bringing on expulsion in women with a non‐viable pregnancy and can be used before 24 weeks' gestation.
Waiting for spontaneous expulsion is also possible. Women who retain the dead embryo/fetus can experience severe blood loss or develop an infection of the womb. These are rare complications. Gastro‐intestinal side effects such as nausea and diarrhoea, cramping or abdominal pain and fever have been reported with misoprostol.
Why is this important?
Surgical treatment has the disadvantage of requiring anaesthesia. It carries risks of damage to the uterus or cervix and possible development of fibrous tissue in the inner lining of the uterus. These can be avoided if the non‐viable pregnancy is treated with medication, or if the woman is able to wait for a spontaneous expulsion.
We set out to determine if medical treatment is as good as, or better than, surgical treatment or expectant management (waiting for the expulsion to happen). Furthermore, we compared different doses and administration routes in order to detect which regimen most often induces a complete miscarriage with the fewest side effects.
What evidence did we find?
For this updated review, 43 randomised clinical trials involving 4966 women with non‐viable pregnancies at less than 24 weeks' gestation were included. The main interventions examined were vaginal, sublingual, oral and buccal misoprostol, mifepristone and vaginal gemeprost. These were compared with surgical management, expectant management, placebo, or different types of medical interventions were compared with each other. Fourteen comparisons had only one trial. The studies varied in risk of bias. The quality of the evidence ranged from very low or low for most comparisons.
Vaginal misoprostol may hasten miscarriage when compared with placebo but made little difference to rates of nausea, diarrhoea or to whether women were satisfied with the acceptability of the method. It is uncertain whether vaginal misoprostol when compared to placebo reduces blood loss or pain because the quality of the evidence for these outcomes was found to be very low.
Vaginal misoprostol was less effective in accomplishing a complete miscarriage compared to surgical management and may be associated with more nausea and diarrhoea. Vaginal misoprostol made little difference to pelvic infection, blood loss, pain or women's satisfaction/acceptability of method when compared to surgical management.
There was little difference between different routes of giving misoprostol when trials compared the vaginal route with placing it under the tongue or between oral and vaginal misoprostol. Single studies found mifepristone to be more effective than placebo and vaginal misoprostol to be more effective than expectant management. However the quality of this evidence was found to be very low and so we are not convinced of these findings. Mifepristone did not appear to provide any additional benefit when added to misoprostol.
What does this mean?
Using misoprostol as an alternative to surgical treatment may decrease the need for surgery for women with an early fetal death. The use of misoprostol can have some side effects such as nausea and diarrhoea, but risks of severe blood loss or pelvic infection were not higher compared to surgical treatment or expectant management. Further research is needed on drug doses, routes of administration and potential adverse effects, including future fertility, and also on women's views of drug treatment, surgery and waiting for spontaneous miscarriage.
Summary of findings
Background
Miscarriage is the most frequent pregnancy complication with an incidence of at least 10% to 15% of all pregnancies (Grudzinskas 1995; Howie 1995; Simpson 1991). Traditionally, early non‐viable pregnancies (less than 14 weeks) have been terminated by surgical evacuation. However, the use of medical treatment for early non‐viable pregnancies is increasing. Later pregnancies (14 to 24 weeks) have been ended by medical induction of miscarriage (Say 2002).
Description of the condition
A miscarriage is defined as an intrauterine pregnancy demise confirmed by ultrasound or histology up to 13 weeks of gestation. There are different forms of non‐viable pregnancies such as 'anembryonic pregnancies' (formerly called 'blighted ova') if no embryo has developed within the gestational sac, or 'missed abortions' if an embryo or fetus is present, but is dead. When fetal death occurs in later pregnancy (14 to 24 weeks of gestation) it is called intrauterine fetal demise.
The widespread use of ultrasound in early pregnancy for either specific reasons (for example, vaginal bleeding) or as a routine examination (Whitworth 2015) reveals 'non‐viable pregnancies' destined inevitably to miscarry in due course.
Description of the intervention
Traditionally, early non‐viable pregnancies (less than 14 weeks) have been terminated by surgical evacuation. Later pregnancies (14 to 24 weeks) have been ended by medical induction of miscarriage (Say 2002). Although clotting problems occasionally occur in women with prolonged retention of a dead fetus, this is rare and does not usually happen within the first month after fetal death. There are, therefore, not pressing medical reasons to terminate non‐viable pregnancies. Although, anecdotally, many women favour early termination, so‐called 'expectant management' (that is, awaiting spontaneous miscarriage) is a legitimate alternative and this policy should be considered in clinical care and in planning trials (Nanda 2012; Wieringa 2002). More recently, medical treatment is used as an alternative to surgical termination of non‐viable pregnancies. There are various types of medical treatment that could be used as alternatives to surgical treatment; misoprostol, mifepristone, gemeprost, methotrexate or oxytocin. The drug most frequently investigated and now used is misoprostol. This drug can be administered via several different routes; oral, sublingual, vaginal and extra amniotic, and as a single drug therapy or combined with other types of medication such as mifepristone, methotrexate or oxytocin. Furthermore, the optimal dose of misoprostol is not known, and therefore different doses are used ranging from 100 mg up to 800 mg per dose.
How the intervention might work
Misoprostol is a synthetic prostaglandin E1 analogue. It is a type of medication that was first registered as treatment for peptic ulcers. It is also used as medical treatment for terminating an unwanted or non‐viable pregnancy. Misoprostol ripens the cervix and causes uterine contractions. Furthermore, it is cost‐effective (Costa 1993; Graziosi 2005; Norman 1991). Misoprostol could be especially useful in low‐income countries, where transport and storage facilities are inadequate and the availability of uterotonic agents and blood is limited. Its use in obstetrics and gynaecology has been explored, especially to induce first and second trimester abortion (Ashok 1998; Bugalho 1996), for the induction of labour (Alfirevic 2014; Hofmeyr 2010) and for the prevention of postpartum haemorrhage (Tunçalp 2012), despite the fact that it has not been registered for such use. The sensitivity of the uterus for misoprostol increases with the duration of pregnancy. Though the optimal dose for the induction of first or second trimester miscarriage is not known, and remains a subject of interest in the included studies.
Dinoproston is a natural prostaglandin E2. It advances uterine contraction and also ripens the cervix, though its exact mechanism is not known. Other uterotonic drugs include ergometrine (while it acts at alpha‐adrenergic, dopaminergic and serotonin receptors, it exerts on the uterus a stimulant effect) and oxytocin (a synthetic nano peptide, identical to oxytocin produced by the pituitary gland, causing rhythmic contractions of the uterus).
Other uterotonic drugs that could have a role in the induction of miscarriage include ergometrine, oxytocin,
The progesterone antagonist, mifepristone, is of value in terminating early unwanted pregnancies and may be useful in non‐viable pregnancies and spontaneous miscarriage (Baulieu 1986, Kovacs 1984), alone or in combination with prostaglandin (Cameron 1986). Methotrexate has been researched for medical treatment of ectopic pregnancy and might have a place in the treatment of intrauterine non‐viable pregnancies as well.
Why it is important to do this review
The use of medical treatment in termination of non‐viable pregnancies is increasing. Since miscarriage is the most frequent complication of pregnancy it is important to have knowledge about the different types of medical treatment, their (cost) effectiveness and their side effects.
The initial protocol for this review aimed to combine trials of medical treatments for both non‐viable pregnancies and for incomplete miscarriage but on further reflection, this was illogical. Non‐viable pregnancies contain viable trophoblast (placental) tissue, which produces hormones, which may in theory make these pregnancies more susceptible to anti‐hormone therapy and more resistant to uterotonic (stimulating uterine contractions) therapy than pregnancies in which (incomplete) miscarriage has already taken place. This review will therefore focus exclusively on non‐viable pregnancies, before miscarriage. Another review assesses trials of medical treatments after miscarriage has occurred (Kim 2017). A further review compares expectant management with surgical treatment for miscarriage (Nanda 2012).
Our review was first published in 2006. It was last edited and published online on January 21, 2009. Since the publication of the review in 2006, multiple new clinical trials concerning medical treatment of early fetal death have been conducted, and results published. The review therefore needed to be updated.
Objectives
To assess, from clinical trials, the effectiveness and safety of different medical treatments for the termination of non‐viable pregnancies.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials comparing a medical treatment with another treatment (for example, surgical evacuation), or placebo, or no treatment to terminate non‐viable pregnancies. Quasi‐randomised studies were excluded. Cluster‐randomised trials were eligible for inclusion, as were studies reported in abstract form, if sufficient information was available to assess eligibility.
Types of participants
Women with non‐viable pregnancies (i.e. where the embryo or fetus had died in utero, and in whom miscarriage would have happened inevitably in due course) if less than 24 weeks estimated gestational age. If applicable, subgroup analyses were performed for women in first and women in the second trimester (up to 24 weeks of gestational age) of pregnancy. Since different studies might use different cut‐off values to consider a pregnancy in its second trimester (varying between 12 and 15 weeks of gestational age), in the subgroup analysis the exact gestational age that was used in the included studies is mentioned.
Types of interventions
Trials were considered if they compared medical treatment with other methods (for example, expectant management, placebo or any other intervention including surgical evacuation). Comparisons between different routes of administration of medical treatment (for example, oral versus vaginal), or between different drugs or doses of drug, or duration or timing of treatment, were also included if data existed.
Types of outcome measures
Trials were considered if any of the following outcomes were measured.
Primary outcomes
Complete miscarriage (i.e. no pregnancy tissues remaining in uterus ‐ based on clinical findings at surgery or ultrasound examination, or both after a specific period or an uncomplicated follow‐up period, or both without the need for additional surgical intervention).
Death or serious complications (e.g. uterine rupture, uterine perforation, hysterectomy, organ failure, intensive care unit admission).
Secondary outcomes
Blood transfusion.
Haemorrhage.
Blood loss (measured amount of blood, post‐treatment haemoglobin or post‐treatment haematocrit, or both).
Days of bleeding.
Pain (relief) (defined as: 1. differences in pain scores between the different treatment methods and/or 2. the increase or decrease in pain score after a certain treatment) and/or 3. incidence of pain as a complaint and/or 4. the use of pain medication after a certain treatment).
Pelvic infection (defined by the authors as fever most likely caused by pelvic infection or documented pelvic infection, or both).
Cervical damage.
Digestive disorders (nausea or vomiting or diarrhoea).
Hypertensive disorders.
Time to expulsion.
Duration of stay in hospital.
Psychological effects.
Subsequent fertility.
Woman's satisfaction/acceptability of method.
Costs.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (24 October 2018).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (24 October 2018) for unpublished, planned and ongoing trial reports using the search terms given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeNeilson 2006.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. Potential trials were assessed for eligibility according to the criteria described in the ‘Eligibility criteria’ section above. If study eligibility needed to be further clarified we contacted the investigators to request further information. Studies published in abstracts only were assessed in the same way as full‐text papers. If there was sufficient information presented in the abstract to demonstrate that it met the eligibility criteria, it was included in analyses. Otherwise it was excluded with reasons noted in the Characteristics of excluded studies table.
We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
Data were extracted from each relevant publication using a data collection form.
In addition to the main outcome measures listed above, information on the setting of the study (country, type of population, socioeconomic status), the method of randomisation, a detailed description of the regimen used (drug(s), route, dose, frequency), definitions of the outcomes (if provided), and whether or not clinicians and participants were 'blind' to treatment allocated, were collected. Furthermore, any information on completeness of follow‐up was collected as well. Also, we collected the key conclusions of the included studies as reported by their authors.
For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author.
Data were imported in Review Manager software (RevMan 2014), and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number), if during data extraction we found that the trial was quasi‐randomised we excluded it from further analysis;
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the primary and if applicable secondary outcomes for the main comparisons (with a maximum of seven outcomes). The following outcomes were assessed.
Complete miscarriage
Pelvic infection
Nausea
Diarrhoea
Blood loss
Pain (relief)
Woman's satisfaction/acceptability of method
These outcomes were assessed (if applicable) for all 23 comparisons. The most clinically meaningful comparisons are presented in (Table 1; Table 2); these were:
Summary of findings for the main comparison. Vaginal misoprostol compared to placebo for early fetal death (less than 24 weeks).
| Vaginal misoprostol compared to placebo for early fetal death (less than 24 weeks) | ||||||
| Patient or population: early fetal death (less than 24 weeks) Setting: worldwide Intervention: vaginal misoprostol Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with vaginal misoprostol | |||||
| Complete miscarriage | Study population | RR 4.23 (3.01 to 5.94) | 305 women (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | There were differences in timing of outcome measurement: after 24 hours (2 studies), after 48 hours (2 studies) or after 7 days (1 study). | |
| 189 per 1.000 | 800 per 1.000 (569 to 1.000) | |||||
| Pelvic infection | Study population | not estimable | (studies) | ‐ | ||
| 0 per 1.000 | 0 per 1.000 (0 to 0) | |||||
| Nausea | Study population | RR 1.38 (0.43 to 4.40) | 88 women (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 93 per 1.000 | 128 per 1.000 (40 to 409) | |||||
| Diarrhoea | Study population | RR 2.21 (0.35 to 14.06) | 88 women (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 23 per 1.000 | 51 per 1.000 (8 to 327) | |||||
| Blood loss: haemoglobin difference > 10 g/L | Study population | RR 1.25 (0.38 to 4.12) | 50 women (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 6 | ||
| 160 per 1.000 | 200 per 1.000 (61 to 659) | |||||
| Pain (opiate use) | Study population | RR 5.00 (0.25 to 101.11) | 84 women (1 RCT) |
⊕⊝⊝⊝4 6 VERY LOW |
||
| 0 per 1.000 | 0 per 1.000 (0 to 0) | |||||
| Woman’s satisfaction/acceptability of method | Study population | RR 1.17 (0.83 to 1.64) | 32 women (1 RCT) | ⊕⊕⊝⊝ LOW 6 | ||
| 750 per 1.000 | 878 per 1.000 (622 to 1.000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Serious indirectness: differences in medication regimens used between the included studies. However: very strong association; dose‐response relation (‐1).
2 Serious risk of bias: problems with blinding in various studies, downgraded because of limitation in study design (‐1).
3 Serious imprecision: only two studies with relatively few patients (‐1).
4 Serious risk of bias: unclear allocation concealment (‐1).
5 Serious risk of indirect evidence: haematocrit difference was used to estimate the amount of blood loss (‐1).
6 Serious imprecision: only one study included, wide confidence interval (‐2).
Summary of findings 2. Vaginal misoprostol compared to surgical evacuation of uterus for early fetal death (less than 24 weeks).
| Vaginal misoprostol compared to surgical evacuation of uterus for early fetal death (less than 24 weeks) | ||||||
| Patient or population: early fetal death (less than 24 weeks) Setting: worldwide Intervention: vaginal misoprostol Comparison: surgical evacuation of uterus | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with surgical evacuation of uterus | Risk with vaginal misoprostol | |||||
| Complete miscarriage | Study population | RR 0.40 (0.32 to 0.50) | 943 women (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Blinding of patients and treating personnel was impossible due to the nature of the interventions. All studies used the same dosage of misoprostol (800 mcg). | |
| 921 per 1.000 | 368 per 1.000 (295 to 460) | |||||
| Pelvic infection | Study population | RR 0.73 (0.39 to 1.37) | 618 women (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | Only 1 study included but with relatively large patient numbers. | |
| 71 per 1.000 | 52 per 1.000 (28 to 97) | |||||
| Nausea | Study population | RR 21.85 (1.31 to 364.37) | 154 women (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 0 per 1.000 | 22 per 1.000 (1 to 364) | |||||
| Diarrhoea | Study population | RR 40.85 (2.52 to 662.57) | 154 women (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 0 per 1.000 | 41 per 1.000 (3 to 663) | |||||
| Blood loss: post‐treatment haematocrit (%) | The mean blood loss: post‐treatment haematocrit (%) was 35.5 | mean 1.40 lower (3.51 lower to 0.71 higher) | ‐ | 50 women (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | |
| Pain relief | Study population | RR 1.42 (0.82 t0 2.46) | 154 women (1 RCT) |
⊕⊕⊝⊝ LOW 4 5 | ||
| 213 per 1.000 | 303 per 1.000 (175 to 525) | |||||
| Woman’s satisfaction/acceptability of method | Study population | RR 0.67 (0.40 to 1.11) | 45 women (1 RCT) |
⊕⊕⊝⊝ LOW 6 7 | ||
| 800 per 1.000 | 536 per 1.000 (320 to 888) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Serious risk of bias: only one study blinded the outcome assessors. All other studies were not blinded (‐1).
2 Serious inconsistency: varied sampling, different medication regimes, I2 = 46% (‐1).
3 Serious risk of bias: no blinding of outcome assessors (‐1).
4 Serious imprecision: only one study with relatively few patients, small number of events and wide confidence intervals (‐1).
5 Serious risk of bias: high risk of selective reporting and unclear allocation concealment (‐1).
6 Serious risk of bias: high risk of bias for blinding and attrition (‐1).
7 Serious imprecision: only one study with relatively few patients (‐1).
vaginal misoprostol versus placebo;
vaginal misoprostol versus surgical evacuation.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create the ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we use the mean difference if outcomes are measured in the same way between trials. In future updates, if applicable, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
Our protocol stated that we would include cluster‐randomised trials in the analyses along with individually‐randomised trials. We planned to adjust their standard errors using the methods described in the Handbook (Section 16.3.6) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we used ICCs from other sources, we planned to report this and to conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. No cluster‐randomised trials were included in this update.
Cross‐over trials
It is unlikely that cross‐over designs would be a valid study design for this particular review, and so were expected to be excluded. In the unlikely event that cross‐over trials would have a valid design and were eligible for inclusion in the review, we would use specific methods for 'Risk of bias' assessment and analysis as described in the Handbook (Section 16.4).
Other unit of analysis
It was likely that we would identify trials with more than two treatment groups, for example, trials comparing surgical, medical and expectant management of non‐viable pregnancies. If so, we first determined which intervention groups addressed the review objective. If applicable, pair‐wise comparisons of interventions were included in the appropriate analysis.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we tried to explore it by subgroup analysis.
Assessment of reporting biases
We planned to investigate reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If heterogeneity was identified we checked if there were clinical subgroups of interest; and if there were, that would be the main reason to perform subgroup analysis. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
Separate comparisons were made of different drug regimens, grouped where appropriate by number of doses given and the route of administration. Furthermore, subgroup analyses were made for comparisons that included studies with variation in dosages of medication, time in between different administrations and/or time until follow‐up examination; and subgroup analyses of first versus second trimester pregnancies were performed. All of these mentioned differences might influence the chance of successful outcome. For example: in later gestational age (second trimester pregnancies), the prostaglandin receptors are more developed and therefore the outcomes of interventions with a same dosage of misoprostol could differ between first and second trimester. Another example: when different routes of administration are assessed, the dosage and whether repeat dosages are applied might influence the outcome, which means these should be considered as different subgroups of interest.
The primary and secondary outcomes used in subgroup analysis were the same as the outcomes used in the overall analysis.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
See: Figure 1. We retrieved 162 trial reports to assess from the database searching (52 new reports, plus 108 that were already awaiting further classification, and two that were ongoing in the previous version of the review (Neilson 2006). In addition, we found three more published reports from following up clinical trial registry records (we subsequently excluded these trials as it was clear from the full report that they were not eligible). Of the 165 reports we assessed, we included 21 new trials (27 reports), excluded 112 (124 reports) and six are ongoing trials. We also added one new trial report to a previously included study, and seven new reports to previously excluded studies.
1.
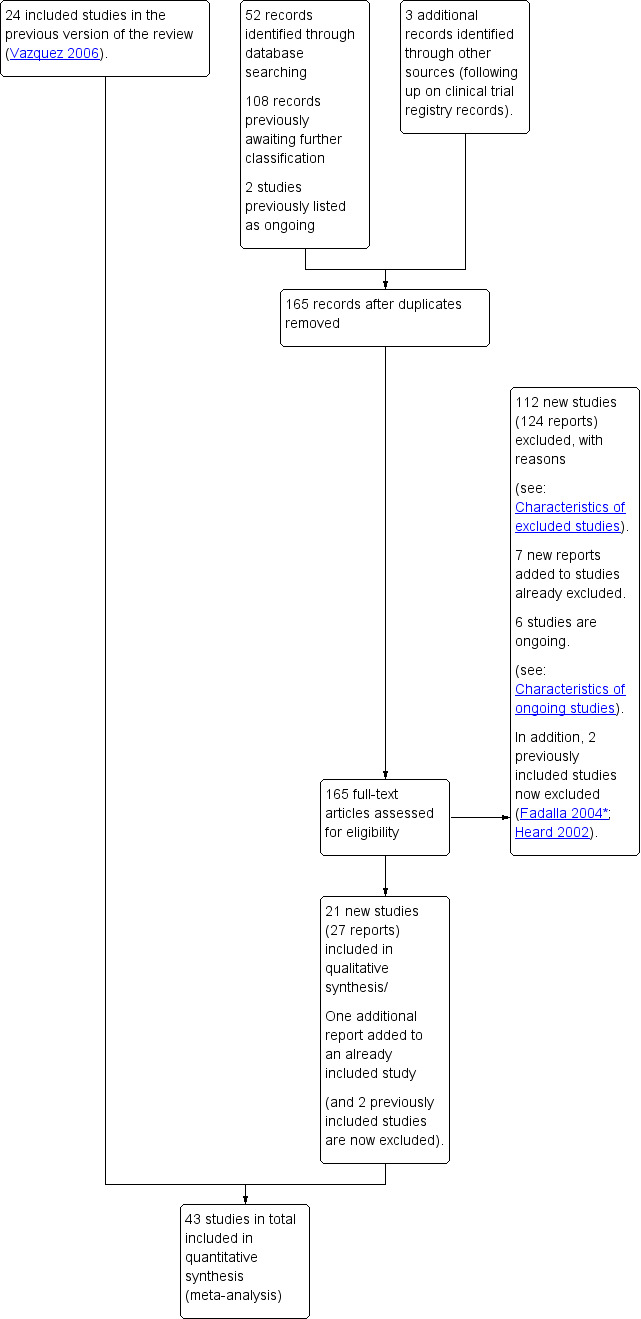
Study flow diagram.
The original review included 24 studies. One of these studies was published as an abstract (Heard 2002). Since there were serious concerns about the methodology and no full‐text article was published, this study was excluded from the updated review. We also reassessed and excluded another previously included study (Fadalla 2004*).
The review now has 43 included studies.
Included studies
This review has included 43 studies comparing vaginal misoprostol versus expectant management (Trinder 2006), placebo (Bagratee 2004; Herabutya 1997; Kovavisarach 2002; Lister 2005; Wood 2002), surgical evacuation (Demetroulis 2001; Fang 2009; Ganguly 2010; Graziosi 2004; Muffley 2002; Trinder 2006), oral or sublingual misoprostol (Chittacharoen 2003*; Creinin 1997; Dehbashi 2016; Marwah 2016; Ngoc 2004; Rita 2006; Shah 2010*; Sonsanoh 2014; Tang 2003; Tanha 2010a), other types of vaginal or intracervical prostaglandin preparation (Al Inizi 2003; Eng 1997*; Kara 1999*); oxytocin (Abediasl 2016*); extra‐amniotic preparations (Mitwaly 2016*); different doses (Kovavisarach 2005; Mizrachi 2017; Niromanesh 2005*; Petersen 2013) and preparations (Gilles 2004) of vaginal misoprostol; the addition to vaginal misoprostol of methotrexate (Autry 1999) or laminaria tents (Jain 1996*). Furthermore, there were studies comparing sublingual misoprostol versus oral misoprostol (Ayudhaya 2006; Kushwah 2009); different doses (Tang 2006) and preparations (Saichua 2009) of sublingual misoprostol; and one study on buccal misoprostol in different doses (Bracken 2014*). Studies using other types of medication other than (only) misoprostol involved mifepristone versus placebo (Lelaidier 1993); mifepristone plus oral misoprostol versus expectant management (Nielsen 1999); mifepristone plus oral misoprostol versus misoprostol alone (Fang 2009Schreiber 2018; Sinha 2018); and vaginal gemeprost versus surgical evacuation (Egarter 1995).
The Bagratee 2004 trial used a comparison of vaginal misoprostol versus placebo to explore comparisons with expectant management (up to seven days) and, therefore, differed in concept from the Herabutya 1997 and Wood 2002 studies in which early surgical intervention occurred after, respectively, 24 and 48 hours.
Eight of the 43 included studies addressed medical treatment of non‐viable pregnancies in the second trimester. The definition of second trimester however varied from gestational age (GA) > 12 weeks to GA > 15 weeks (Abediasl 2016*; Bracken 2014*; Chittacharoen 2003*; Eng 1997*; Jain 1996*; Kara 1999*; Mitwaly 2016*; Niromanesh 2005*). One study (Shah 2010*) included women with non‐viable pregnancies up to a GA of 20 weeks, but made subgroup analyses for first and second trimester pregnancies. These studies are labelled with an asterisk for ease of interpretation.
There are additional trials that included data on women with both non‐viable pregnancies and incomplete miscarriages; or that included women with a GA of more than 24 weeks. We contacted several authors to ask for separated data. Four authors responded but were not able to send us the separated data (Brouns 2010; Eslamian 2007; Hidar 2005; Petrou 2006 (additional report to Trinder 2006); Promwangkwa 2017). One author responded and sent separated data (Bracken 2014*), this study was included in the review. The authors that did not respond are listed under 'Excluded studies'.
Dates of study
Included studies date from 1993 until 2018.
Funding
Among the included studies no information on funding was available in 29 trials. In 12 trials the funding was independent and mainly provided by the university hospital. One trial mentioned not to have received funding at all, and one trial mentioned to having received a donation from a pharmaceutical company for the execution of the trial.
Declaration of interest
Declaration of interest was not mentioned in 28 trials. One trial, of which the authors received a donation from a pharmaceutical company, reported this donation in their declaration of interest. The remaining 14 trials reported not to have any interests to declare.
Excluded studies
The trials that were excluded in the initial review were checked to ensure that no trial has been excluded for non‐reporting of outcomes and that reasons are still valid according to current Cochrane standards. There are 162 excluded studies and these are listed in the reference section under Excluded studies. The table Characteristics of excluded studies states the reasons for exclusion from this review. These reasons mainly include: study not randomised; study including women with ongoing or incomplete miscarriage only; studies assessing medical treatment for fetal demise > 24 weeks of gestational age (GA), and studies including women having termination of pregnancy. We have also excluded studies where we tried to contact the authors for data that separates treatment of non‐viable pregnancies with other types of patients (with either incomplete miscarriage, > 24 weeks, or planned termination of pregnancy), however either the authors did not respond or they were not able to provide suitable data (Behrashi 2008; Biswas 2007; Brouns 2010; Caliskan 2005; Dickinson 1998; Dickinson 2002; Elhassan 2008; El Sokkary 2016; Eppel 2005; Eslamian 2007; Fadalla 2004*Feldman 2003; Ghorab 1998; Gonzalez 2001; Grimes 2004; Herabutya 1997a; Hidar 2001; Hidar 2005; Hogg 2000; Hughes 1996; Imran 2010; Jain 1994; Jain 1999; Kurshid 2010; Kyaw 2015; Makhlouf 2003; Mostafa‐Gharebaghi 2010; Nakintu 2001; Ngai 2001; Niinimaki 2006; Nuutila 1997; Owen 1999; Promwangkwa 2017Ramsey 2004; Tanha 2013; Thavarasah 1986; Thida 2015; Toptas 2011; Torre 2012; Van Mensel 2009; Zhang 2000; Zhang 2005). Eight references turned out to be trial protocols or conference abstracts regarding studies that were also retrieved in our search and were added as additional reports to the reference of the published study results (Bracken 2014*; Lughmani 2008; Mitwaly 2016*; Nassar 2006; Nuthalapaty 2005; Stockheim 2006; Tanha 2013; Torre 2012). Sixteen studies were excluded because only a conference abstract was available and full data publication could not be retrieved (Abdel Fattah 1997; Anderman 2000; Anderson 2009; Ara 2009; Aye 2017; Chowdhury 2012; Heard 2002; Hombalegowda 2015; Linn 2015; Machtinger 2004; Nasreen 2009; Roy 2003; Shaikh 2008; Shobeira 2007; Suchonwanit 1999; Surita 1997). One study was published twice (Kushwah 2009), these two references were grouped together as one study.
Several studies turned out to be secondary analyses (cost‐analyses, follow‐up on fertility outcome of subsequent pregnancies, etcetera) of previous randomised controlled trials and were added as additional reports to the main references (five additional reports to Zhang 2005, two to Trinder 2006, two to Bracken 2014*, and one extra reference to respectively Elami‐Suzin 2013, Niinimaki 2006 and Kovavisarach 2002). These reports however did not provide suitable additional data for meta‐analysis.
Risk of bias in included studies
Please see Figure 2; Figure 3 for a summary of 'Risk of bias' assessments.
2.
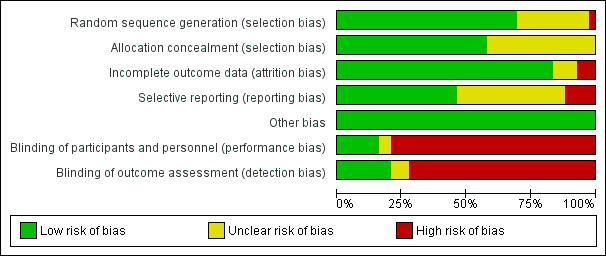
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
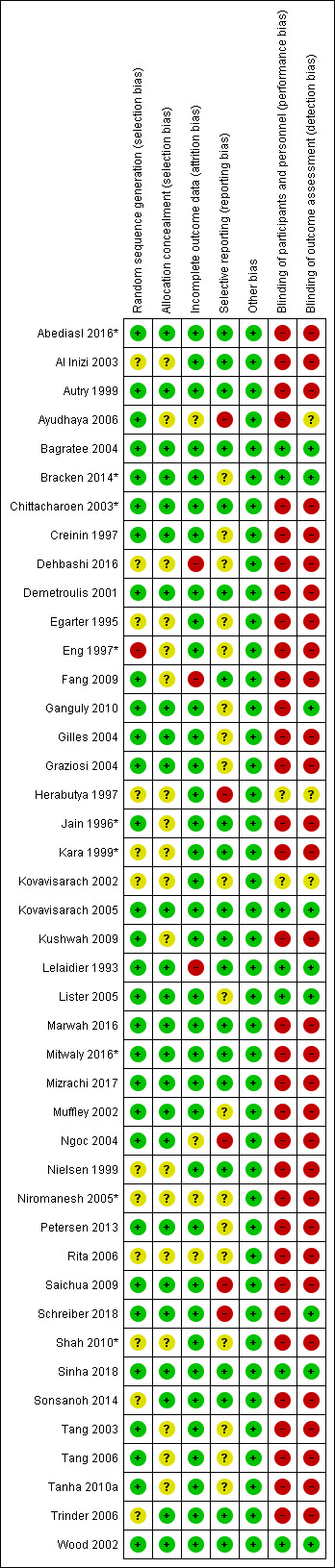
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 30 studies the risk of bias concerning random sequence generation was assessed as being at low risk of bias (Abediasl 2016*; Autry 1999; Ayudhaya 2006; Bagratee 2004; Bracken 2014*; Chittacharoen 2003*; Creinin 1997; Demetroulis 2001; Fang 2009; Ganguly 2010; Gilles 2004; Graziosi 2004; Jain 1996*; Kovavisarach 2005; Kushwah 2009; Lelaidier 1993; Lister 2005; Marwah 2016; Mitwaly 2016*; Mizrachi 2017; Muffley 2002; Ngoc 2004; Petersen 2013; Saichua 2009; Schreiber 2018; Sinha 2018; Tang 2003; Tang 2006; Tanha 2010a; Wood 2002). These studies mainly used (computer‐generated) random number tables. In one study (Eng 1997*) randomisation was carried out by "blindly picking a sealed number from a box and then odd numbers were assigned to group A (misoprostol) and even numbers to group B (gemeprost)", and so although the picking of the number from a box describes a random component to the method of sequence generation, we are unclear about the use of an odd and even number to assign thereafter. We therefore considered this as potentially high risk of bias. In the remaining 12 studies random sequence generation was not (adequately) described (Al Inizi 2003; Dehbashi 2016; Egarter 1995; Herabutya 1997; Kara 1999*; Kovavisarach 2002; Nielsen 1999; Niromanesh 2005*; Rita 2006; Shah 2010*; Sonsanoh 2014; Trinder 2006). Three studies mentioned the use of block randomisation without further description. The risk of bias was for random sequence generation was therefore considered unclear.
Twenty‐five studies used robust methods of allocation concealment. Most studies used sequentially numbered sealed opaque envelopes, or numbered and sealed packets containing study medication. Furthermore, randomisation using a computer program to guarantee allocation concealment was used (Abediasl 2016*; Autry 1999; Bagratee 2004; Bracken 2014*; Chittacharoen 2003*;Creinin 1997; Demetroulis 2001; Ganguly 2010; Gilles 2004; Graziosi 2004; Kovavisarach 2005; Lelaidier 1993; Lister 2005; Marwah 2016; Mitwaly 2016*; Mizrachi 2017; Muffley 2002; Ngoc 2004; Petersen 2013; Saichua 2009; Schreiber 2018; Sinha 2018; Sonsanoh 2014; Trinder 2006; Wood 2002). For these studies the risk of bias was considered low risk for allocation concealment. Sixteen reports failed to describe the process of allocation concealment (Al Inizi 2003; Ayudhaya 2006; Dehbashi 2016; Egarter 1995; Fang 2009; Herabutya 1997; Jain 1996*; Kara 1999*; Kovavisarach 2002; Kushwah 2009; Nielsen 1999; Niromanesh 2005*; Rita 2006; Tang 2003; Tang 2006; Tanha 2010a). In these studies risk of bias for allocation concealment was unclear. This was also the case for two more studies (Eng 1997*; Shah 2010*). In these studies numbers were picked from a box, and depending on the randomness of the sequence, blinding of allocation cannot be guaranteed.
Blinding
Seven studies describe both doctors and women were blinded for the treatment allocation and used matching placebo medication to establish this (Bagratee 2004; Bracken 2014*;Kovavisarach 2005; Lelaidier 1993; Lister 2005; Sinha 2018; Wood 2002). The risk of bias was therefore considered low. Two other studies mention the use of placebo medication (Herabutya 1997; Kovavisarach 2002). It is therefore likely that women were blinded for the intervention. However the authors fail to describe if placebo tablets look similar to medication and therefore it is unsure whether doctors were also blinded for the intervention. Performance bias was unclear for these two studies. In the remaining 33 studies blinding was either not possible (due to the nature of the intervention) or not performed. In these studies performance bias was assessed high.
For the six double‐blind placebo‐controlled trials (Bagratee 2004; Bracken 2014*; Kovavisarach 2005; Lelaidier 1993; Lister 2005; Wood 2002) it was very likely that outcome assessors were blinded for the intervention and detection bias was therefore considered low. This was also the case for two more studies (Ganguly 2010 and Sinha 2018) which described outcome assessors were blinded for the intervention. Two more studies used placebo medication, (Herabutya 1997; Kovavisarach 2002) and it is therefore likelier that outcome assessors were blinded for the intervention. This is however not clearly described. Risk of bias was assessed as unclear for these two studies. One study describes nurses being in charge of the administration of medication (sublingual or oral misoprostol) (Ayudhaya 2006) and since doctors were the outcome assessors it could have been that they were blinded for the intervention. This is however not described. Risk of detection bias was assessed unclear in this case. In the remaining 32 studies blinding of the outcome was either not possible (due to the nature of the intervention) or not described. We considered it to be very unlikely that in these cases outcome assessors were blinded for the intervention. In these remaining 32 studies risk of detection bias was considered high.
Incomplete outcome data
Data were incomplete in at least three studies (Dehbashi 2016; Fang 2009; Lelaidier 1993). In these studies women were allocated to a specific treatment, and then wrongfully excluded from analysis. Risk of bias was considered high for these studies. In four more studies risk of attrition bias was unclear (Ayudhaya 2006; Ngoc 2004; Niromanesh 2005*; Rita 2006). For these studies lost to follow up was < 10%, secondary outcomes were not available for all included women, or failed to report on loss to follow‐up. In the remaining 36 studies risk of attrition bias was considered low, because primary outcomes were available for nearly all included women.
Selective reporting
Five studies (Ayudhaya 2006; Herabutya 1997; Ngoc 2004; Saichua 2009; Schreiber 2018) had inconsistencies in outcome reporting and showed evidence of omission of outcomes in results. Furthermore, there was one study mentioning that several secondary outcomes were not reported in this paper. It was unclear whether these outcomes were reported elsewhere. These studies were considered to have a high risk on reporting bias. For 18 studies (Bracken 2014*; Creinin 1997; Dehbashi 2016; Egarter 1995; Ganguly 2010; Gilles 2004; Graziosi 2004; Kovavisarach 2002; Lister 2005; Muffley 2002; Niromanesh 2005*; Petersen 2013; Rita 2006; Shah 2010*; Tang 2003; Tang 2006; Tanha 2010a) reporting bias was unclear due to the problem that all studies reported on outcomes that were not prespecified in the method section. It was therefore impossible to assess whether all outcomes were reported upon. One study (Egarter 1995) failed to present a clear description of primary and secondary outcomes in the methods section which makes it difficult to give a judgment on selective reporting and the risk was therefore also labelled as unclear. Risk of reporting bias was considered low in 19 studies that reported on all outcomes that were mentioned in their method section.
Other potential sources of bias
For none of the included studies other potential sources of bias were detected.
Effects of interventions
Forty‐three studies, with a total of 4966 women, were included. Twenty‐two of the studies addressed termination of non‐viable pregnancies before 14 weeks. There were few reports of serious adverse effects in the reported trials, but one woman required a bowel resection after uterine perforation at evacuation of the uterus (Egarter 1995).
Subgroup analyses
For a number of comparisons with subgroups of clinical interest, extra subgroup analyses were carried out. These included the following.
For comparison 1: vaginal misoprostol versus placebo; primary outcome complete miscarriage:
complete miscarriage less than one day;
complete miscarriage less than two days;
complete miscarriage less than seven days.
For comparison 6: vaginal misoprostol wet versus dry preparations: primary outcome complete miscarriage:
complete miscarriage less than three days;
complete miscarriage less than eight days;
complete miscarriage less than 15 days;
complete miscarriage less than 30 days.
For comparison 8: vaginal misoprostol plus laminaria tents versus vaginal misoprostol alone: primary outcome complete miscarriage:
complete miscarriage less than one day;
complete miscarriage less than two days.
For comparison 18: buccal misoprostol lower versus higher regimen: primary outcome complete miscarriage 13 to 23 weeks:
complete miscarriage less than one day;
complete miscarriage less than two days.
For comparison 19: mifepristone versus placebo: primary outcome complete miscarriage:
complete miscarriage less than two days;
complete miscarriage less than three days;
complete miscarriage less than four days;
complete miscarriage less than five days.
All results per comparison are mentioned in the following paragraphs.
1. Vaginal misoprostol versus placebo
Primary outcomes
Treatment with vaginal misoprostol hastens miscarriage (passage of products of conception, whether complete or incomplete) when compared with placebo: miscarriage less than 24 hours (2 trials, 138 women, risk ratio (RR) 4.73, 95% confidence interval (CI) 2.70 to 8.28) miscarriage less than 48 hours (2 (other) trials, 84 women, RR 5.74, 95% CI 2.70 to 12.19); complete miscarriage without need for surgical intervention at seven days (1 trial, 83 women, RR 2.99, 95% CI 1.80 to 4.99). For these five studies combined (total of 305 women) RR of successful evacuation with misoprostol compared to placebo was 4.23, 95% CI 3.01 to 5.94; low‐quality evidence; Analysis 1.1. In the GRADE assessment, the risk of bias was considered as serious because several studies lacked (information) on blinding. Furthermore, there was serious indirectness since there were differences in timing of outcome measurement: after 24 hours (two studies), after 48 hours (two studies) or after seven days (one study) which might have influenced the incidence of successful outcome, though effect of the outcome was considered large. The quality of evidence was therefore assessed as low (Table 1). In one study, one women in the placebo group had a uterine perforation after surgical evacuation was performed (1 trial, 84 women, RR 0.33, 95% CI 0.01 to 7.96) (Herabutya 1997) (Analysis 1.2).
1.1. Analysis.
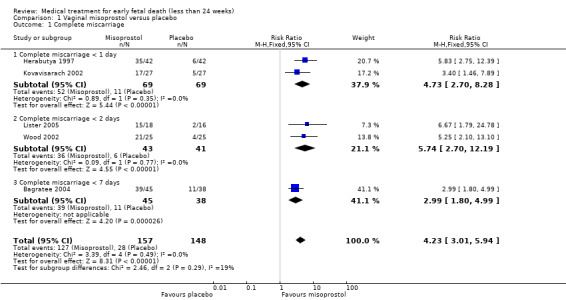
Comparison 1 Vaginal misoprostol versus placebo, Outcome 1 Complete miscarriage.
1.2. Analysis.
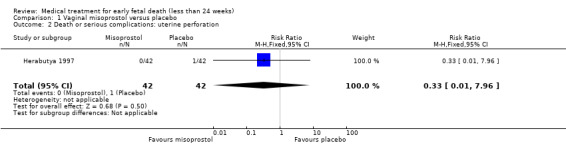
Comparison 1 Vaginal misoprostol versus placebo, Outcome 2 Death or serious complications: uterine perforation.
Secondary outcomes
There was no difference in the need for blood transfusion (1 study, 84 women, RR 0.20, 95% CI 0.01 to 4.04), no difference in haemoglobin level after treatment (1 study, 50 women, RR 1.25, 95% CI 0.38 to 4.12; very‐low quality evidence) or duration of bleeding (in days) (1 study, 32 women, RR 1.00, 95% CI 0.41 to 2.45; Analysis 1.3; Analysis 1.4; Analysis 1.5). There was no increase in adverse effects: nausea (2 trials, 88 women, RR 1.38, 95% CI 0.43 to 4.40; low‐quality evidence), diarrhoea (2 trials, 88 women, RR 2.21, 95% CI 0.35 to 14.06; low‐quality evidence; Analysis 1.6; Analysis 1.7). In one small study (Herabutya 1997), two out of 42 women used opiates for pain relief when treated with misoprostol, compared to 0 out of 42 women in the placebo group (1 trial, 84 women, RR 5.00, 95% CI 0.25 to 101.11; very‐low quality evidence; Analysis 1.8). According to one study a similar number of women (58%) who would choose the same treatment strategy in the future (Graziosi 2004); although more women who had complete miscarriage after misoprostol (76%) would choose this treatment than those who required subsequent curettage (38%) (1 trial, 32 women, RR 1.17, 95% CI 0.83 to 1.64; low‐quality evidence; Analysis 1.9). For all these secondary outcomes there were some limitations in study design, with unclear allocation concealment for some studies, there was evidence of 'imprecision' with small numbers of studies and wide CIs contributing to effect estimates and also some evidence of indirectness for one study (see Table 1).
1.3. Analysis.
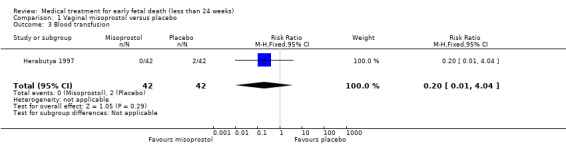
Comparison 1 Vaginal misoprostol versus placebo, Outcome 3 Blood transfusion.
1.4. Analysis.
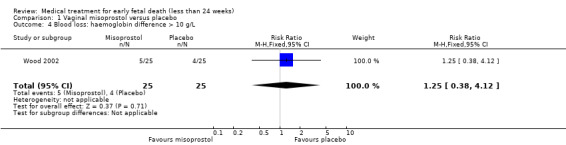
Comparison 1 Vaginal misoprostol versus placebo, Outcome 4 Blood loss: haemoglobin difference > 10 g/L.
1.5. Analysis.
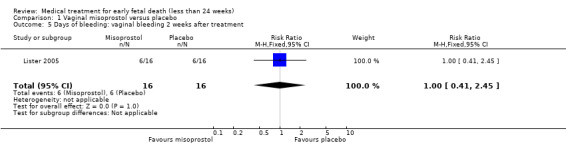
Comparison 1 Vaginal misoprostol versus placebo, Outcome 5 Days of bleeding: vaginal bleeding 2 weeks after treatment.
1.6. Analysis.
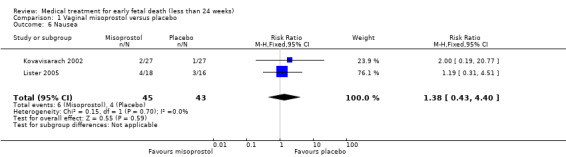
Comparison 1 Vaginal misoprostol versus placebo, Outcome 6 Nausea.
1.7. Analysis.
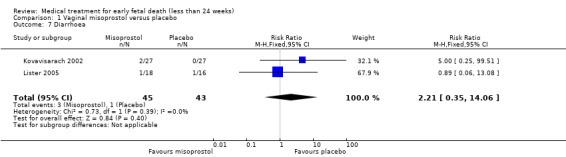
Comparison 1 Vaginal misoprostol versus placebo, Outcome 7 Diarrhoea.
1.8. Analysis.
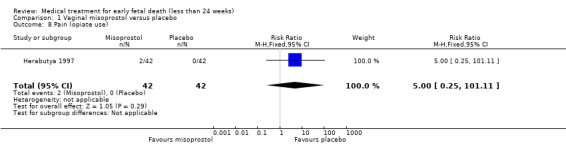
Comparison 1 Vaginal misoprostol versus placebo, Outcome 8 Pain (opiate use).
1.9. Analysis.
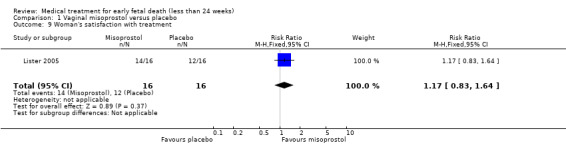
Comparison 1 Vaginal misoprostol versus placebo, Outcome 9 Woman's satisfaction with treatment.
The following secondary outcomes were not reported in the trials for this comparison: haemorrhage; pelvic infection; cervical damage; hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; and costs.
2. Vaginal misoprostol versus expectant management
Primary outcomes
One study was included (614 women); in which a complete miscarriage (described as no need for additional intervention) occurred more often after misoprostol treatment compared to expectant management (RR 1.25, 95% CI 1.09 to 1.45; Analysis 2.1). The quality of this evidence in GRADE assessment was downgraded because of serious risk of bias (only one study included, no blinding performed) and serious imprecision; and was therefore assessed as low.
2.1. Analysis.
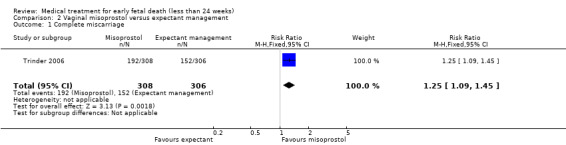
Comparison 2 Vaginal misoprostol versus expectant management, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
Although the total number of events was low, in the misoprostol group more infections occurred within eight weeks after study entry compared to the expectant management group (1 study, 618 women, RR 8.05, 95% CI 1.87 to 34.72; Analysis 2.2); in the included trial (Trinder 2006) infections were defined as two or more of purulent vaginal discharges, pyrexia more than 38.0°C, tenderness over the uterus on abdominal examination, and a white cell count above 15x10^9/L. Risk of bias was considered serious since no blinding was performed, and there was serious imprecision with very wide CIs because of few events in the treatment arms. The GRADE certainty of evidence is therefore considered low.
2.2. Analysis.
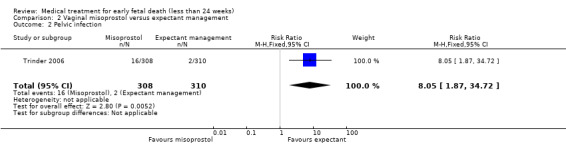
Comparison 2 Vaginal misoprostol versus expectant management, Outcome 2 Pelvic infection.
The following secondary outcomes were not reported in the trials for this comparison: blood transfusion; haemorrhage; blood loss; days of bleeding; pain (relief); cervical damage; digestive disorders (nausea or vomiting or diarrhoea); hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; woman's satisfaction/acceptability of method; and costs.
3. Vaginal misoprostol versus surgical evacuation of uterus
Primary outcomes
Complete miscarriage was lower after initial misoprostol treatment compared to primary surgical treatment (6 studies, 943 women, average RR 0.40, 95% CI 0.32 to 0.50; low‐quality evidence; Heterogeneity: Tau20.03 I2 = 46%; Analysis 3.1). The GRADE certainty of evidence was assessed as low; there was a serious risk of bias with no blinding performed in all studies but one and concerns due to inconsistency, but the effect was large and there were no other serious risks (Table 2). Though in the women who were treated successfully with misoprostol, surgery could be avoided. One study reported on uterine perforation (Graziosi 2004), and occurred in one woman (1 trial, 154 women, RR 0.32, 95% CI 0.01 to 7.65; Analysis 3.2).
3.1. Analysis.
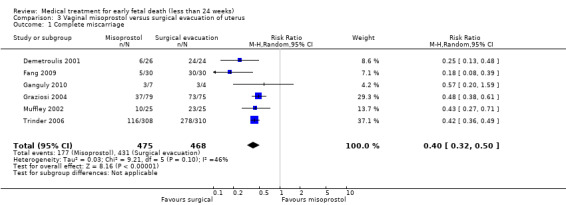
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 1 Complete miscarriage.
3.2. Analysis.
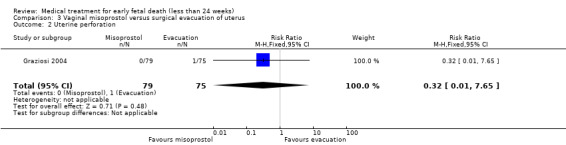
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 2 Uterine perforation.
Secondary outcomes
One study (Muffley 2002) assessed blood loss in women treated with vaginal misoprostol compared to surgical evacuation, and showed no difference in haematocrit level post treatment % (1 study, 50 women mean difference (MD) ‐1.40, 95% CI ‐3.51 to 0.71; low‐quality evidence; Analysis 3.3). The use of pain relief was similar among women treated with vaginal misoprostol and surgical evacuation (1 study, 154 women, RR 1.42, 95% CI 0.82 to 4.46; low‐quality evidence; Analysis 3.4). The rate of infections less than eight weeks after study entry was similar (1 trial, 618 women, RR 0.73, 95% CI 0.39 to 1.37; low‐quality evidence; Analysis 3.5). Misoprostol treatment was associated with more nausea (1 trial, 154 women, RR 21.85, 95% CI 1.31 to 364.37; low‐quality evidence) and diarrhoea (1 trial, 154 women, RR 40.85, 95% CI 2.52 to 662.57; low‐quality evidence; Analysis 3.6; Analysis 3.7). Woman's satisfaction was not better when treated with curettage compared to misoprostol (1 study, 45 women, RR 0.67, 95% CI 0.40 to 1.11; low‐quality evidence; Analysis 3.8). The quality of evidence was low because of serious risk of bias concerns, some inconsistency with varied sampling and different medication regimens and much of the data for outcomes were from single studies with wide CIs (see Table 2). In one trial (Graziosi 2004), one women in the surgical evacuation group developed Asherman syndrome.
3.3. Analysis.
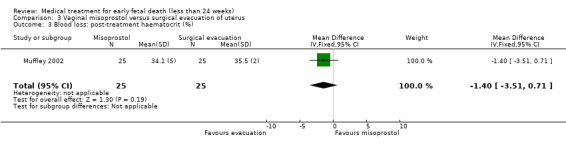
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 3 Blood loss: post‐treatment haematocrit (%).
3.4. Analysis.
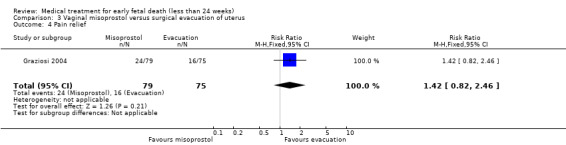
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 4 Pain relief.
3.5. Analysis.
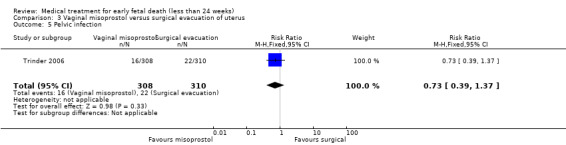
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 5 Pelvic infection.
3.6. Analysis.
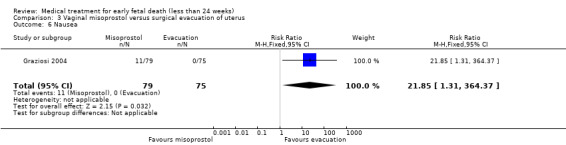
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 6 Nausea.
3.7. Analysis.
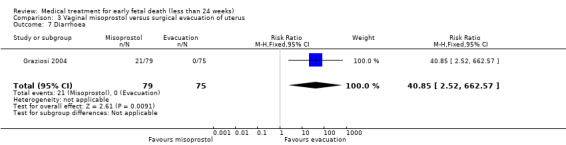
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 7 Diarrhoea.
3.8. Analysis.
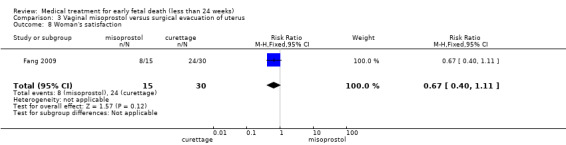
Comparison 3 Vaginal misoprostol versus surgical evacuation of uterus, Outcome 8 Woman's satisfaction.
The following secondary outcomes were not reported in the trials for this comparison: blood transfusion; haemorrhage; cervical damage; hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; and costs.
4. Vaginal misoprostol versus vaginal dinoprostone
Primary outcomes
Vaginal misoprostol is more effective to achieve a complete miscarriage than vaginal dinoprostone for pregnancies < 14 weeks as well as > 14 weeks (2 trials, 125 women, RR 1.83, 95% CI 1.37 to 2.46; Analysis 4.1). However there was a very serious risk of bias with no information on randomisation method in the included studies, no information on allocation concealment, and no blinding. The quality of the evidence was therefore considered very low.
4.1. Analysis.
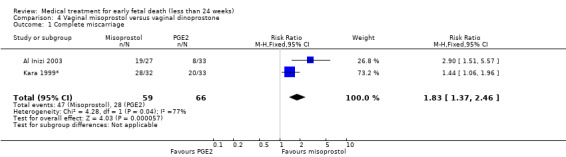
Comparison 4 Vaginal misoprostol versus vaginal dinoprostone, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
In the misoprostol group, two women needed blood transfusion (1 trial, 60 women, RR 6.07, 95% CI 0.30 to 121.33; Analysis 4.2). The incidence of nausea was similar in the one small trial that was included in this comparison (65 women, RR 1.03, 95% CI 0.28 to 3.78; Analysis 4.3). The mean duration of hospital stay in days was lower in the misoprostol group (1 trial, 60 women, MD ‐2.38, 95% CI ‐3.36 to ‐1.40; Analysis 4.4). The GRADE quality of evidence for these outcomes was very low because of very serious risk of bias (no clear randomisation method, no blinding) and serious imprecision (study not powered for this outcome, wide CI).
4.2. Analysis.
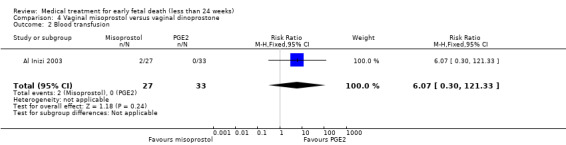
Comparison 4 Vaginal misoprostol versus vaginal dinoprostone, Outcome 2 Blood transfusion.
4.3. Analysis.
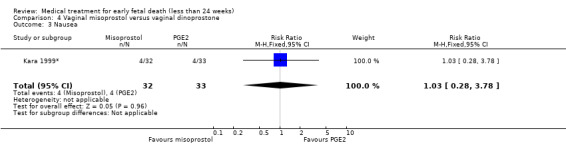
Comparison 4 Vaginal misoprostol versus vaginal dinoprostone, Outcome 3 Nausea.
4.4. Analysis.
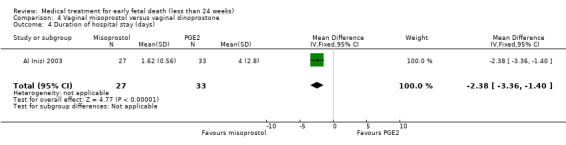
Comparison 4 Vaginal misoprostol versus vaginal dinoprostone, Outcome 4 Duration of hospital stay (days).
The following secondary outcomes were not reported in the trials for this comparison: haemorrhage; blood loss; days of bleeding; pain (relief); pelvic infection; cervical damage; digestive disorders (vomiting or diarrhoea); hypertensive disorders; time to expulsion; psychological effects; subsequent fertility; woman's satisfaction/acceptability of method; and costs.
5. Vaginal misoprostol lower versus higher‐dose regimens
Primary outcomes
Vaginal misoprostol has been administered in doses of 400 mcg, 600 mcg, and 800 mcg in trials: higher‐dose regimens were no more effective in producing miscarriage < 13 weeks, (2 studies, 397 women, average RR 0.82, 95% CI 0.58 to 1.14; Heterogeneity Tau2 = 0.05; I2 = 73%) or 13 to 23 weeks (1 study, 100 women, RR 1.05, 95% CI 0.87 to 1.26; Analysis 5.1; Analysis 5.2). There was risk of bias because lack of proper blinding in two of the included studies (Niromanesh 2005*; Petersen 2013), and there was serious inconsistency between the studies with differences in the gestational age (GA) of included patients (< or > 14 weeks), differences in misoprostol regimen, and differences in time to outcome measurements (24 hours, 48 hours or seven days). There seemed to be a dose‐response gradient, the quality of the evidence was assessed as moderate.
5.1. Analysis.
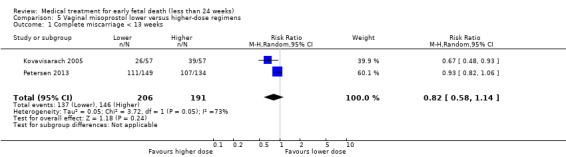
Comparison 5 Vaginal misoprostol lower versus higher‐dose regimens, Outcome 1 Complete miscarriage < 13 weeks.
5.2. Analysis.
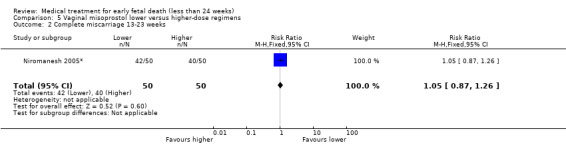
Comparison 5 Vaginal misoprostol lower versus higher‐dose regimens, Outcome 2 Complete miscarriage 13‐23 weeks.
Death or serious complications were not reported in the trial.
Secondary outcomes
There were no differences in nausea (2 trials, 214 women, RR 0.67, 95% CI 0.31 to 1.41) and diarrhoea (2 trials, 214 women, RR 0.54, 95% CI 1015 to 1.91) between higher‐ or lower‐dose regimens (Analysis 5.3; Analysis 5.4). However, because the risk of bias and the inconsistencies described above at the primary outcomes; the quality of the evidence were assessed as low.
5.3. Analysis.
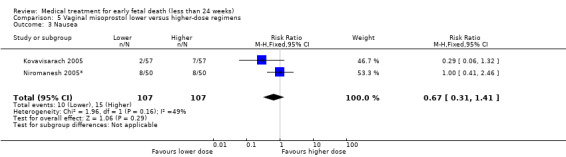
Comparison 5 Vaginal misoprostol lower versus higher‐dose regimens, Outcome 3 Nausea.
5.4. Analysis.
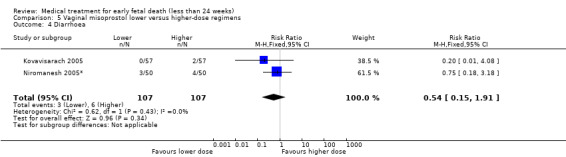
Comparison 5 Vaginal misoprostol lower versus higher‐dose regimens, Outcome 4 Diarrhoea.
The following secondary outcomes were not reported in the trials for this comparison: blood transfusion; haemorrhage; blood loss; days of bleeding; pain (relief); pelvic infection; cervical damage; digestive disorders (nausea or vomiting or diarrhoea); hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; and costs.
6. Vaginal misoprostol wet versus dry vaginal preparations
Primary outcomes
Based on one trial there seems no clear advantage to administering a 'wet' preparation of vaginal misoprostol compared to a 'dry' preparation; miscarriage less than three days (1 trial, 80 women, RR 1.14, 95% CI 0.85 to 1.54; Analysis 6.1). When the outcome complete miscarriage was assessed on day eight, 15 or 30 there was still no clear advantage of a 'wet' preparation compared to a 'dry' preparation. Since there was serious risk of bias with only one study included, no blinding performed, and a small sample size, the quality was assessed as being low‐quality.
6.1. Analysis.
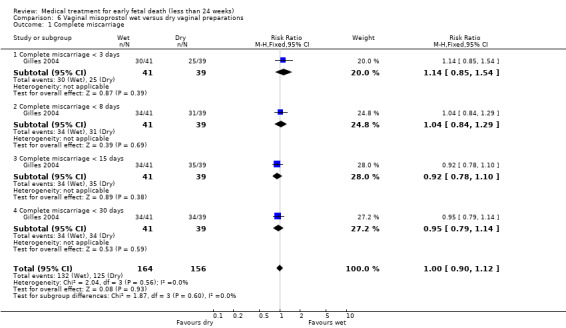
Comparison 6 Vaginal misoprostol wet versus dry vaginal preparations, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
There were no differences in diarrhoea (1 trial, 77 women, RR 1.75, 95% CI 0.89 to 3.42) and vomiting (1 trial, 77 women, RR 0.93, 95% CI 0.33 to 2.62), (Analysis 6.2; Analysis 6.3). Woman's satisfaction, as measured by whether they would wish/probably wish same treatment in the future, suggests no difference between wet and dry vaginal preparations (1 trial, 73 women, RR 1.18, 95% CI 0.93 to 1.49; Analysis 6.4). Again, the quality of the evidence was considered very low.
6.2. Analysis.
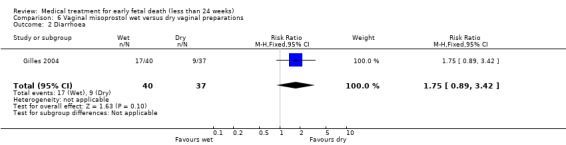
Comparison 6 Vaginal misoprostol wet versus dry vaginal preparations, Outcome 2 Diarrhoea.
6.3. Analysis.
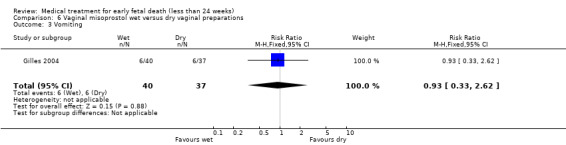
Comparison 6 Vaginal misoprostol wet versus dry vaginal preparations, Outcome 3 Vomiting.
6.4. Analysis.
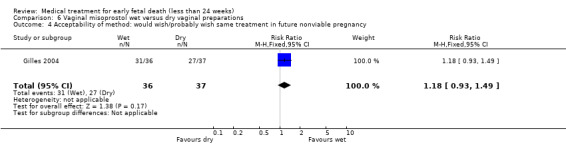
Comparison 6 Vaginal misoprostol wet versus dry vaginal preparations, Outcome 4 Acceptability of method: would wish/probably wish same treatment in future nonviable pregnancy.
The following secondary outcomes were not reported in the trials for this comparison: blood transfusion; haemorrhage; blood loss; days of bleeding; pain (relief); pelvic infection; cervical damage; hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; and costs.
7. Vaginal misoprostol + methotrexate versus vaginal misoprostol
Primary outcomes
Adding methotrexate treatment to vaginal misoprostol has not been demonstrated to be advantageous in the single small trial to address this: complete miscarriage after treatment (21 women, RR 1.13, 95% CI 0.85 to 1.50; Analysis 7.1). The quality of this evidence is very low because of very serious risk of bias (only one small study included, no blinding).
7.1. Analysis.
Comparison 7 Vaginal misoprostol + methotrexate versus vaginal misoprostol alone, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
In the small trial with 21 women that was included in this comparison there were no differences in incidence of haemorrhage (RR 2.31, 95% CI 0.10 to 50.85; Analysis 7.2) and in pain relief (RR 0.75, 95% CI 0.25 to 2.22; Analysis 7.3); but due to the small number of participants and a serious risk of bias (no blinding) the quality of this evidence is very low.
7.2. Analysis.
Comparison 7 Vaginal misoprostol + methotrexate versus vaginal misoprostol alone, Outcome 2 Haemorrhage.
7.3. Analysis.
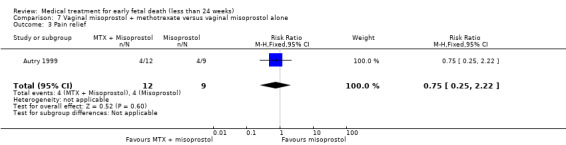
Comparison 7 Vaginal misoprostol + methotrexate versus vaginal misoprostol alone, Outcome 3 Pain relief.
The following secondary outcomes were not reported in the trials for this comparison: blood transfusion; blood loss; days of bleeding; pelvic infection; cervical damage; hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; woman's satisfaction/acceptability of method; and costs.
8. Vaginal misoprostol plus laminaria tents versus vaginal misoprostol alone
Laminaria tents were not proven useful adjuncts to vaginal misoprostol during the second trimester: complete miscarriage less than 24 hours (1 trial, 38 women, RR 0.90, 95% CI 0.65 to 1.25), or 48 hours (Analysis 8.1). GRADE score on quality of the evidence was downgraded two levels because of serious risk of bias (only one small study included, no blinding) and imprecision; and was considered very low.
8.1. Analysis.
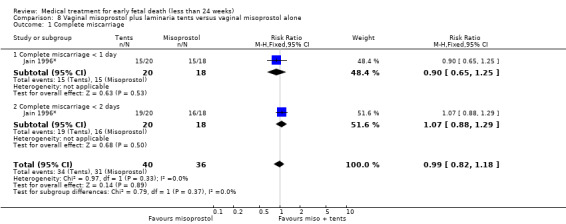
Comparison 8 Vaginal misoprostol plus laminaria tents versus vaginal misoprostol alone, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial. No secondary outcomes were reported.
9. Vaginal misoprostol versus sublingual misoprostol
Primary outcome
No differences in effects were established when comparing vaginal versus sublingual preparations of misoprostol in inducing complete miscarriage < 13 weeks, although the evidence was limited by small sample sizes and heterogeneity of the included trials (five trials, 513 women, random‐effects model average RR 0.84, 95% CI 0.61 to 1.16; heterogeneity Tau² = 0.10; I² = 82%; Analysis 9.1). One trial comparing vaginal misoprostol and sublingual misoprostol for miscarriage 13 to 23 weeks also showed no difference, and also included very limited numbers (9 women, RR 0.50, 95% CI 0.13 to 2.00). The quality of evidence was downgraded because of serious risk of bias (no blinding performed in the including studies) and therefore assessed as low.
9.1. Analysis.
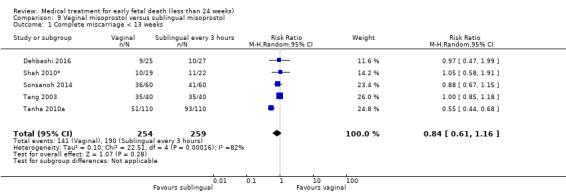
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 1 Complete miscarriage < 13 weeks.
Death or serious complications were not reported in the trial.
Secondary outcomes
Although there seemed to be no differences between vaginal and sublingual misoprostol regarding nausea (4 trials, 302 women, RR 0.42, 95% CI 0.12 to 1.44; Analysis 9.5); vomiting (2 trials, 300 women, RR 0.76, 95% CI 0.46 to 1.26; Analysis 9.6); and excessive blood loss (2 trials, 340 women, RR 0.54, 95% CI 0.15 to 1.89; Analysis 9.3) these results are based on relatively small trials with large heterogeneity. Vaginal misoprostol caused less pain than sublingual misoprostol (3 trials, 392 women, RR 0.58, 95% CI 0.46 to 0.74; Analysis 9.4); and less diarrhoea (4 trials, 472 women, RR 0.71, 95% CI 0.54 to 0.92; Analysis 9.7); because of serious risk of bias described in the primary outcome above the GRADE assessment showed a very low quality of evidence. Quality of evidence was very low because of serious risk of bias, serious imprecision and serious inconsistency with differences in GA and misoprostol regimen of the included studies).
9.5. Analysis.
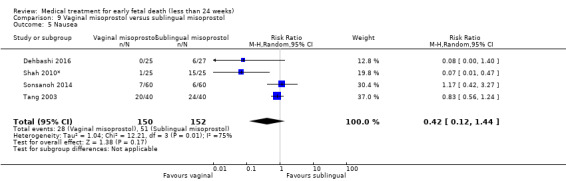
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 5 Nausea.
9.6. Analysis.
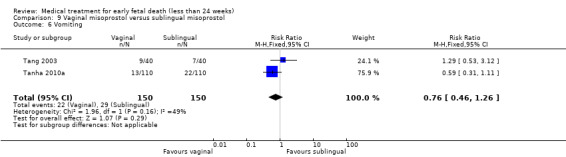
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 6 Vomiting.
9.3. Analysis.
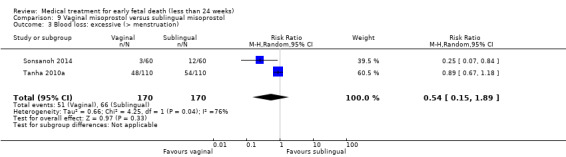
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 3 Blood loss: excessive (> menstruation).
9.4. Analysis.
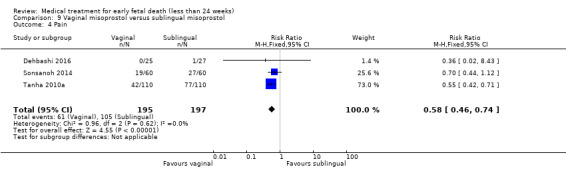
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 4 Pain.
9.7. Analysis.
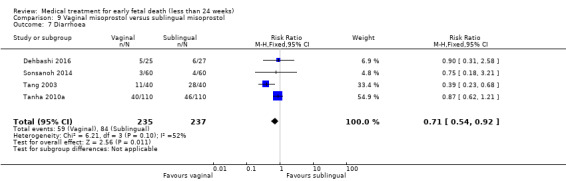
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 7 Diarrhoea.
The following secondary outcomes were not reported in the trials for this comparison: blood transfusion; haemorrhage; days of bleeding; pelvic infection; cervical damage; hypertensive disorders; time to expulsion; duration of stay in hospital; psychological effects; subsequent fertility; woman's satisfaction/acceptability of method; and costs.
10. Vaginal misoprostol versus intravenous oxytocin
Primary outcomes
Misoprostol and oxytocin had similar efficacy in inducing complete evacuation of the uterus in second trimester fetal death (1 trial, 85 women, RR 1.10, 95% CI 0.96 to 1.25; Analysis 10.1). The quality of evidence was assessed as low because of very serious risk of bias (only one small study included, no blinding).
10.1. Analysis.
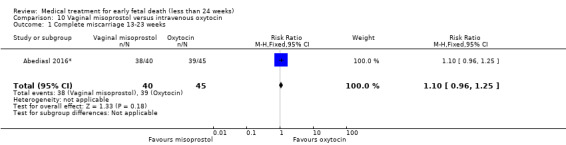
Comparison 10 Vaginal misoprostol versus intravenous oxytocin, Outcome 1 Complete miscarriage 13‐23 weeks.
Death or serious complications were not reported in the trial.
Secondary outcomes
The incidence of excessive blood loss was not different between the groups, however the total number of events was very low (1 trial, 85 women, RR 0.56, 95% CI 0.05 to 5.97), the quality of evidence is low because of the same reasons as described in the primary outcome) (Analysis 10.2).
10.2. Analysis.
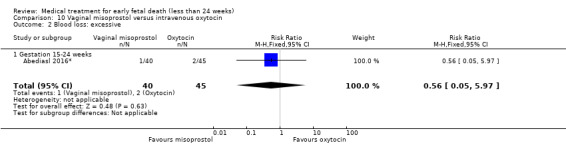
Comparison 10 Vaginal misoprostol versus intravenous oxytocin, Outcome 2 Blood loss: excessive.
No other secondary outcomes were reported.
11. Vaginal misoprostol versus vaginal gemeprost
Primary outcomes
There was no difference between vaginal misoprostol and gemeprost in the induction of miscarriage less than 24 hours for fetal death after 13 weeks (1 trial, 50 women, RR 1.24, 95% CI 0.90 to 1.70; Analysis 11.1). GRADE assessment produced a low quality of evidence because of a very serious risk of bias (no blinding, doubtful randomisation report, and some signs of selective reporting).
11.1. Analysis.
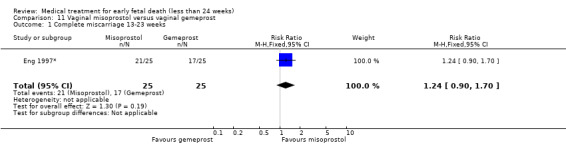
Comparison 11 Vaginal misoprostol versus vaginal gemeprost, Outcome 1 Complete miscarriage 13‐23 weeks.
Death or serious complications were not reported in the trial.
Secondary outcomes
One study reported on the use of opiates for pain relief (Eng 1997*). In this study, none of the women either treated with misoprostol or gemeprost used opiates for pain relief. The incidences of vomiting and diarrhoea did not differ between the misoprostol and the gemeprost group (1 trial, 50 patients, respectively RR 3.00, 95% CI 0.13 to 70.30; RR 0.14, 95% CI 0.01 to 2.63); however the studies were relatively small (Analysis 11.3; Analysis 11.4). The quality of evidence for the outcomes which were assessed were low because of the very serious risk of bias as described previously.
11.3. Analysis.
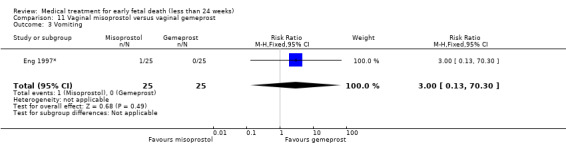
Comparison 11 Vaginal misoprostol versus vaginal gemeprost, Outcome 3 Vomiting.
11.4. Analysis.
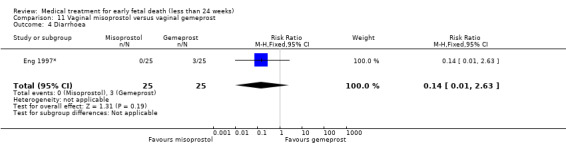
Comparison 11 Vaginal misoprostol versus vaginal gemeprost, Outcome 4 Diarrhoea.
No other secondary outcomes were reported.
12. Sublingual + vaginal misoprostol versus only vaginal misoprostol
Primary outcomes
Sublingual and oral misoprostol did not differ in inducing a complete miscarriage (2 studies, 238 women, RR 1.07, 95% CI 0.88 to 1.30; Analysis 12.1). There was a serious risk of bias in the two included studies (no blinding, no clear allocation concealment) and there were serious inconsistencies (differences between the included studies regarding misoprostol regimen), which was partly compensated by a dose‐response gradient. The GRADE quality of evidence was therefore assessed as moderate.
12.1. Analysis.
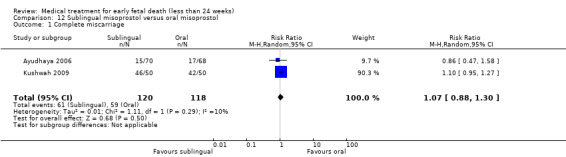
Comparison 12 Sublingual misoprostol versus oral misoprostol, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
There were no differences in abdominal pain, diarrhoea and patients satisfaction (Analysis 12.2; Analysis 12.4; Analysis 12.5). Gastro‐intestinal side effects (nausea, vomiting) occurred less in the sublingual misoprostol group (2 studies, 238 women, RR 0.59, 95% CI 0.41 to 0.85; Analysis 12.3). For fever, nausea, diarrhoea and abdominal pain the quality of evidence is low because of serious risk of bias and serious inconsistencies. For patients satisfaction with treatment the quality was downgraded to very low because only one small study was included at high risk of bias for blinding.
12.2. Analysis.
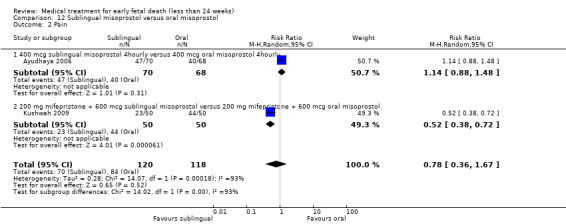
Comparison 12 Sublingual misoprostol versus oral misoprostol, Outcome 2 Pain.
12.4. Analysis.
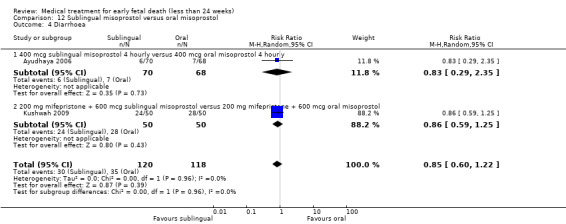
Comparison 12 Sublingual misoprostol versus oral misoprostol, Outcome 4 Diarrhoea.
12.5. Analysis.
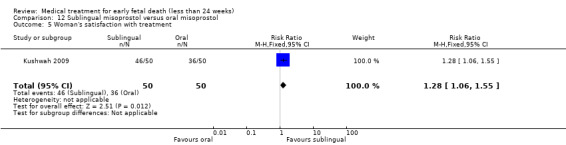
Comparison 12 Sublingual misoprostol versus oral misoprostol, Outcome 5 Woman's satisfaction with treatment.
12.3. Analysis.
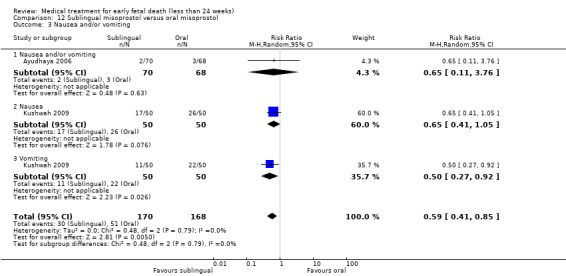
Comparison 12 Sublingual misoprostol versus oral misoprostol, Outcome 3 Nausea and/or vomiting.
No other secondary outcomes were reported.
13. Sublingual powdery versus sublingual compact misoprostol
Primary outcomes
According to the small trial included in this comparison there is no clear advantage of administering a powdery preparation of sublingual misoprostol compared to a compact preparation: complete miscarriage (1 trial, 54 women, RR 0.96, 95% CI 0.66 to 1.41; Analysis 13.1). Since there is only one study included in which no blinding was performed and that showed signs of selective reporting, there was a very serious risk of bias and the quality of evidence is very low.
13.1. Analysis.
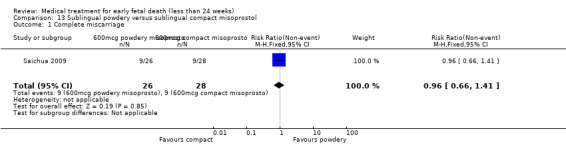
Comparison 13 Sublingual powdery versus sublingual compact misoprostol, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
The incidence of nausea/vomiting and diarrhoea was similar between the groups (Analysis 13.2; Analysis 13.3). Again, the quality of evidence is very low because of the very serious risk of bias as described in the primary outcome.
13.2. Analysis.
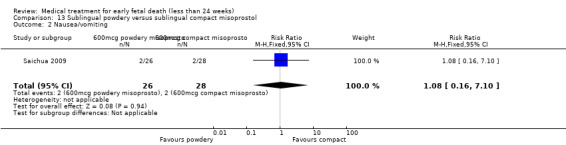
Comparison 13 Sublingual powdery versus sublingual compact misoprostol, Outcome 2 Nausea/vomiting.
13.3. Analysis.
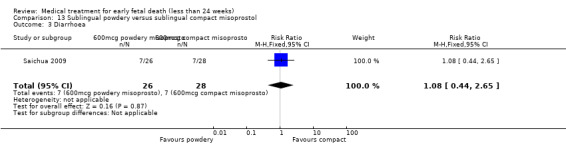
Comparison 13 Sublingual powdery versus sublingual compact misoprostol, Outcome 3 Diarrhoea.
No other secondary outcomes were reported.
14. Sublingual misoprostol with versus without extended course
Primary outcomes
An extended course of daily 400 mcg misoprostol for a week after initial misoprostol treatment does not lead to more cases with complete miscarriage (1 trial, 180 women, RR 1.01, 95% CI 0.93 to 1.10; Analysis 14.1). The quality of evidence was downgraded because of a very serious risk of bias (only one small study included, no blinding performed, signs of selective reporting) which was partly compensated by a dose‐response gradient, and was assessed as low.
14.1. Analysis.
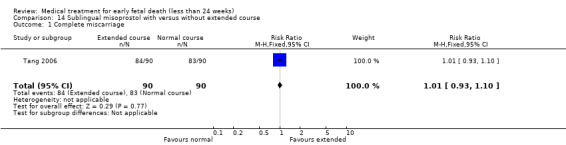
Comparison 14 Sublingual misoprostol with versus without extended course, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
The extended course of misoprostol produces more diarrhoea (1 trial, 180 women, RR 2.00, 95% CI 1.25 to 3.19; Analysis 14.5). The incidence of other side effects like nausea, vomiting and pain was not different (Analysis 14.2; Analysis 14.3; Analysis 14.4; again quality of evidence was assessed as low since these comparisons included the same trial as was described in the primary outcome).
14.5. Analysis.
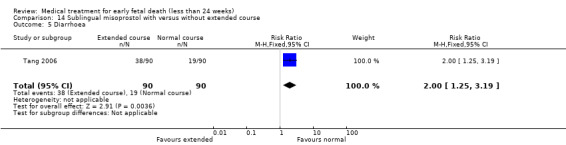
Comparison 14 Sublingual misoprostol with versus without extended course, Outcome 5 Diarrhoea.
14.2. Analysis.
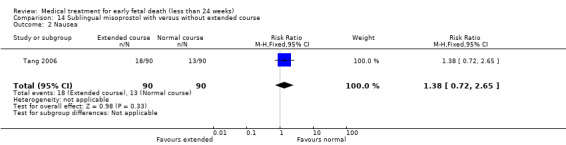
Comparison 14 Sublingual misoprostol with versus without extended course, Outcome 2 Nausea.
14.3. Analysis.
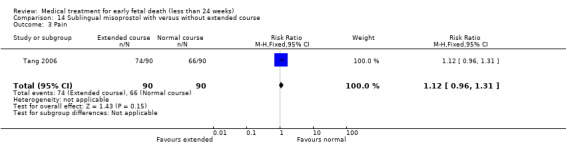
Comparison 14 Sublingual misoprostol with versus without extended course, Outcome 3 Pain.
14.4. Analysis.
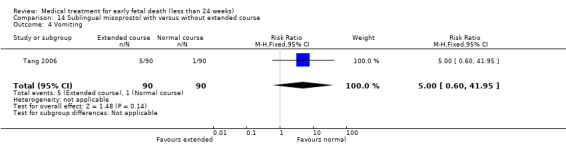
Comparison 14 Sublingual misoprostol with versus without extended course, Outcome 4 Vomiting.
No other secondary outcomes were reported.
15. Sublingual misoprostol versus oral misoprostol
Primary outcomes
Adding sublingual misoprostol to vaginal misoprostol does not lead to more complete miscarriages (1 trial, 80 women, RR 1.00, 95% CI 0.85 to 1.18; Analysis 15.1). In this comparison only one study was included (Tang 2003), with a small number of participants, no blinding and no allocation concealment, which means a very serious risk of bias. The GRADE quality of evidence therefore is very low.
15.1. Analysis.
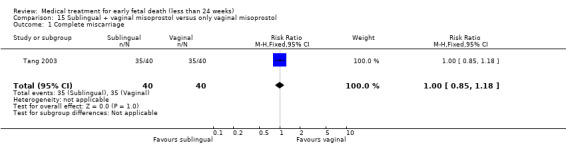
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
While efficacy seemed not to differ, adding sublingual misoprostol to a vaginal misoprostol treatment produces more diarrhoea (1 trial, 80 women, RR 2.55, 95% CI 1.48 to 4.38, quality of evidence very low because of the reasons described above). The incidence of other side effects like nausea, vomiting and pain was no different between the groups (respectively RR 1.20, 95% CI 0.80 to 1.79; RR 0.78, 95% CI 0.32 to 1.88; RR 0.75, 95% CI 0.29 to 1.97; Analysis 15.3; Analysis 15.4; Analysis 15.6). A large proportion of women was satisfied with either sublingual and vaginal misoprostol or vaginal misoprostol alone. There was no difference in women's satisfaction (1 study, 77 women, RR 0.99, 95% CI 0.79 to 1.25; Analysis 15.7). Again, the quality of evidence for these outcomes is very low.
15.3. Analysis.
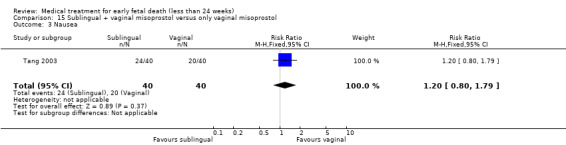
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 3 Nausea.
15.4. Analysis.
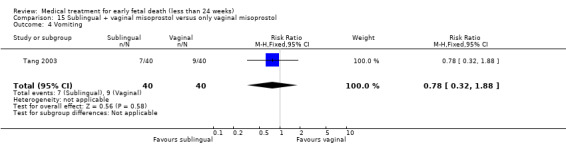
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 4 Vomiting.
15.6. Analysis.
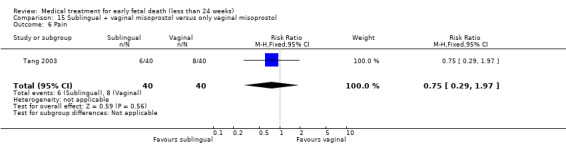
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 6 Pain.
15.7. Analysis.
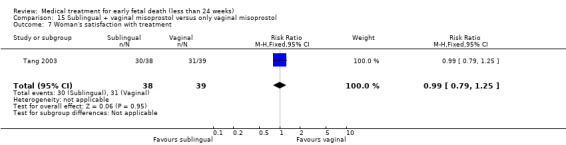
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 7 Woman's satisfaction with treatment.
No other secondary outcomes were reported.
16. Oral misoprostol versus vaginal misoprostol
Primary outcomes
Overall, oral misoprostol seemed to be less effective than vaginal misoprostol in producing complete miscarriage < 13 weeks but this was not clearly shown in the effect estimates (4 studies, 418 women, average RR 0.68, 95% CI 0.45 to 1.03; Heterogeneity Tau2 = 0.13, I2 = 90%). A difference was seen only with the 400 mcg oral versus 800 mcg vaginal dose in first trimester miscarriages (1 trial, 20 women, RR 0.29, 95% CI 0.10 to 0.79) and with the 400 mcg oral versus 600 mcg vaginal dose in first trimester miscarriage (1 trial, 100 women, RR 0.45, 95% CI 0.30 to 0.67; Analysis 16.1). In one trial (Chittacharoen 2003*) all participating women using oral and vaginal misoprostol had a complete miscarriage, thus both regimens were equally effective. GRADE assessment showed serious risk of bias and serious inconsistencies because of differences between the studies regarding GA, misoprostol regimen and timing of outcome measurement; and blinding was performed in non of the included studies. Since a dose‐response gradient could be suspected the quality of evidence was assessed as moderate.
16.1. Analysis.
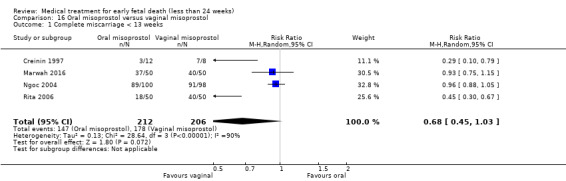
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 1 Complete miscarriage < 13 weeks.
Death or serious complications were not reported in the trial.
Secondary outcomes
There seemed to be no differences in the incidence of vomiting (2 trials, 290 women, random‐effects model average RR 0.73, 95% CI 0.11 to 4.89; Heterogeneity: Tau² = 1.52; I² = 80%), nausea (3 trials, 220 women, RR 1.18, 95% CI 0.93 to 1.48) and diarrhoea (4 trials, 410 women, RR 1.06, 95% CI 0.72 to 1.58; Analysis 16.6; Analysis 16.7; Analysis 16.8). However, oral misoprostol seemed to cause slightly more often pain than vaginal misoprostol (2 trials, 200 women, RR 1.60, 95% CI 1.01 to 2.55; Analysis 16.5). There were high (and similar) levels of satisfaction with treatment (1 trial, 198 women, RR 0.96, 95% CI 0.86 to 1.06, but the quality of evidence for this outcome is very low since only one trial was included).
16.6. Analysis.
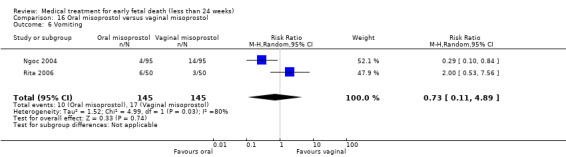
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 6 Vomiting.
16.7. Analysis.
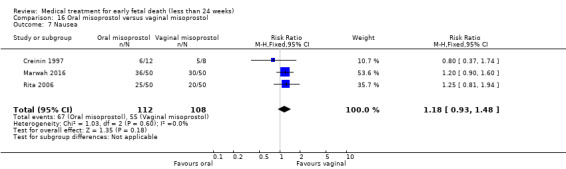
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 7 Nausea.
16.8. Analysis.
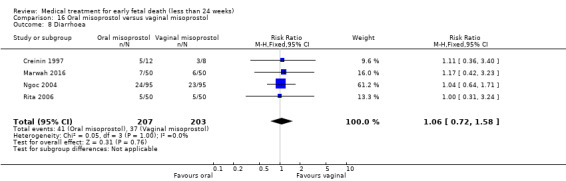
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 8 Diarrhoea.
16.5. Analysis.
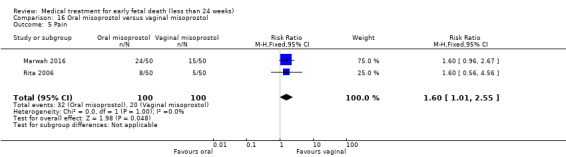
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 5 Pain.
No other secondary outcomes were reported.
17. Oral misoprostol + mifepristone versus expectant management
Primary outcomes
In the single study included in this comparison, there was no difference in medical treatment compared to expectant management for complete miscarriage after five days (1 study, 122 women, RR 1.08, 95% CI 0.90 to 1.30; Analysis 17.1). The quality of evidence is low because of very serious risk of bias (only one study included, no blinding performed).
17.1. Analysis.
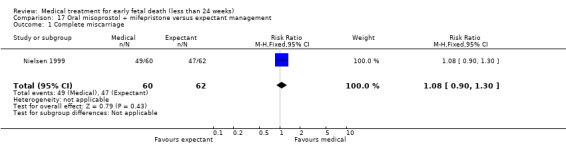
Comparison 17 Oral misoprostol + mifepristone versus expectant management, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
A difference in severe blood loss could not be established between the groups (1 study, 122 women, RR 0.34, 95% CI 0.01 to 8.29) but this was based on one study with large CIs (Analysis 17.2). Furthermore, the incidence of pelvic inflammatory disease (RR 0.52, 95% CI 0.05 to 5.55; Analysis 17.5) did not differ but the total number of events for this outcome was low. Woman's satisfaction was not different for both treatment modalities (1 study, 122 women, MD 3.40, 95% CI ‐5.54 to 12.34; Analysis 17.6). The quality of evidence was calculated as very low in the GRADE assessment because of a very serious risk of bias and serious imprecision (only one study included, wide CIs).
17.2. Analysis.
Comparison 17 Oral misoprostol + mifepristone versus expectant management, Outcome 2 Blood loss (severe).
17.5. Analysis.
Comparison 17 Oral misoprostol + mifepristone versus expectant management, Outcome 5 Pelvic infection.
17.6. Analysis.
Comparison 17 Oral misoprostol + mifepristone versus expectant management, Outcome 6 Woman's satisfaction with treatment (visual analogue scale day 14).
No other secondary outcomes were reported.
18. Buccal misoprostol lower versus higher‐dose regimen
Primary outcomes
The efficacy of a higher‐dose regimen of buccal misoprostol is better than a lower dose: complete miscarriage within two days (1 study, 135 women, RR 0.76, 95% CI 0.60 to 0.96); complete evacuation less than one day (1 study, 135 women, RR 0.64, 95% CI 0.46 to 0.89; Analysis 18.1). The quality of evidence was calculated as low in the GRADE assessment because of a very serious risk of bias (only one small study included, signs of selective reporting).
18.1. Analysis.
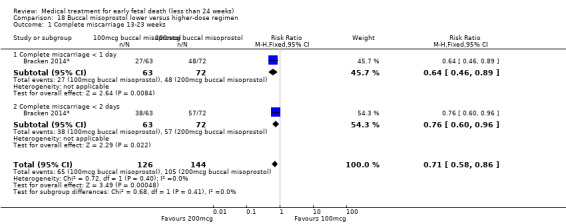
Comparison 18 Buccal misoprostol lower versus higher‐dose regimen, Outcome 1 Complete miscarriage 13‐23 weeks.
Death or serious complications were not reported in the trial.
Secondary outcomes
A higher‐dose regimen caused more vomiting (1 study, 135 women, RR 0.30, 95% CI 0.12 to 0.76) and diarrhoea (RR 0.40, 95% CI 0.19 to 0.82; Analysis 18.3; Analysis 18.4). The incidence of nausea was similar between the groups (RR 0.61, 95% CI 0.28 to 1.34), as well as the incidence of pain (RR 0.96, 95% CI 0.87 to 1.06; Analysis 18.2; Analysis 18.5). The quality of evidence for the secondary outcomes was low because of the reasons described above.
18.3. Analysis.
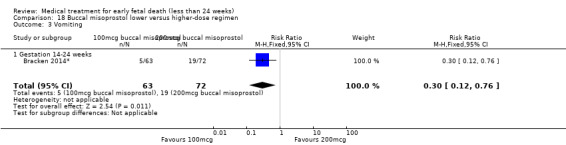
Comparison 18 Buccal misoprostol lower versus higher‐dose regimen, Outcome 3 Vomiting.
18.4. Analysis.
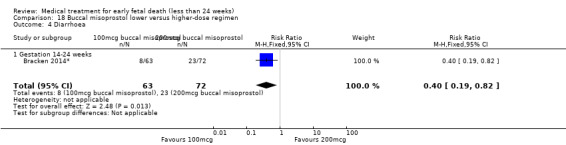
Comparison 18 Buccal misoprostol lower versus higher‐dose regimen, Outcome 4 Diarrhoea.
18.2. Analysis.
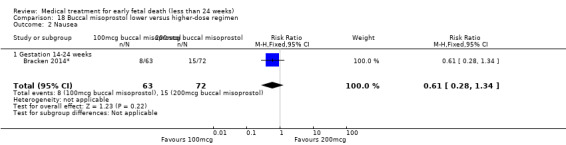
Comparison 18 Buccal misoprostol lower versus higher‐dose regimen, Outcome 2 Nausea.
18.5. Analysis.
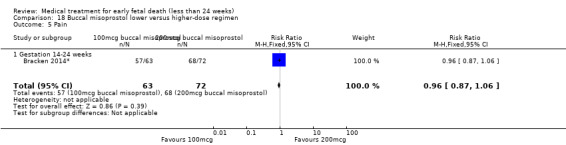
Comparison 18 Buccal misoprostol lower versus higher‐dose regimen, Outcome 5 Pain.
No other secondary outcomes were reported.
19. Mifepristone versus placebo
Primary outcomes
The single study included in this comparison found mifepristone to be more effective than placebo: miscarriage complete by day five after treatment (46 women, RR 9.50, 95% CI 2.49 to 36.19; Analysis 19.1). However the quality of this evidence is very low: there is a very serious risk of bias with signs of incomplete data and no proper intention‐to‐treat analysis in the included study; and serious imprecision with wide confidence intervals.
19.1. Analysis.
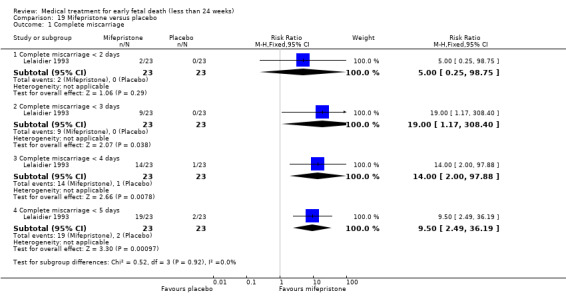
Comparison 19 Mifepristone versus placebo, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
The incidence of vaginal bleeding before day five was higher in the misoprostol group (1 trial, 44 women, RR 3.92, 95% CI 1.89 to 8.10; Analysis 19.2). There were no major differences in the incidence of pain (1 trial, 44 women, RR 2.19, 95% CI 0.93 to 5.17; Analysis 19.3) but again the quality of evidence is very low according to the GRADE assessment, for the same reasons as described in the primary outcome.
19.2. Analysis.
Comparison 19 Mifepristone versus placebo, Outcome 2 Days of bleeding.
19.3. Analysis.
Comparison 19 Mifepristone versus placebo, Outcome 3 Pain.
No other secondary outcomes were reported.
20. Mifepristone + misoprostol versus misoprostol alone
Primary outcomes
Three studies were included in this comparison and showed no additional value of mifepristone for the complete miscarriage rate (3 studies 447 participants, RR 1.18, 95% CI 0.95 to 1.47; Analysis 20.1). This quality of these studies was assessed moderate, two studies were not blinded, though in one of these the outcome assessor was blinded for the intervention.
20.1. Analysis.
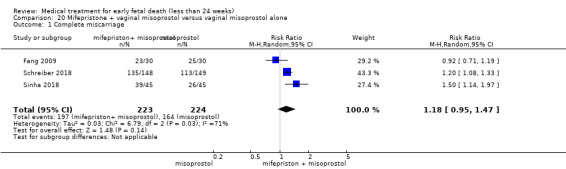
Comparison 20 Mifepristone + vaginal misoprostol versus vaginal misoprostol alone, Outcome 1 Complete miscarriage.
Secondary outcomes
One trial reported on the need for blood transfusion, pelvic infection, nausea and diarrhoea. Incidence of transfusion was low was similar in both groups (300 women, RR 3.04, 95% CI 0.32 to 28.90; Analysis 20.2). Similarly, incidence of pelvic infection was low and equal in both groups (300 women, RR 1.01, 95% CI 0.14 to 7.10; Analysis 20.3). Nausea and diarrhoea were more common side effects, but incidence did not differ between both groups (nausea: 300 women, RR 1.01, 95% CI 0.76 to 1.36; Analysis 20.4 and diarrhoea: 300 women, RR 0.94, 95% CI 0.66 to 1.35).
20.2. Analysis.
Comparison 20 Mifepristone + vaginal misoprostol versus vaginal misoprostol alone, Outcome 2 Blood transfusion.
20.3. Analysis.
Comparison 20 Mifepristone + vaginal misoprostol versus vaginal misoprostol alone, Outcome 3 Pelvic infection.
20.4. Analysis.
Comparison 20 Mifepristone + vaginal misoprostol versus vaginal misoprostol alone, Outcome 4 nausea.
Two trials reported on woman's satisfaction. Women were more satisfied when treated with mifepristone + misoprostol compared to misoprostol alone (two trials, 135 women, RR 1.36, 95% CI 1.06 to 1.75; Analysis 20.6).
20.6. Analysis.
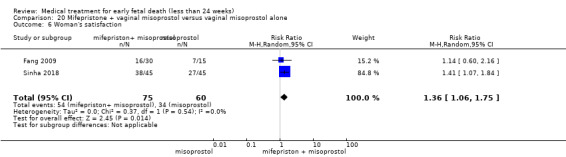
Comparison 20 Mifepristone + vaginal misoprostol versus vaginal misoprostol alone, Outcome 6 Woman's satisfaction.
21. Vaginal gemeprost versus surgical evacuation of uterus
Primary outcomes
In the one study included in this comparison (Egarter 1995), surgical evacuation was more effective than gemeprost treatment (87 women, RR 0.80, 95% CI 0.67 to 0.96; Analysis 21.1). In the surgical group, two of 44 women underwent additional treatment, one because of persistent vaginal bleeding and one because of ambiguous histology results based on what later turned out to be a tubal pregnancy. Two patients in the surgical evacuation group had a perforation of the uterus (RR (Non‐event) 1.05, 95% CI 0.97 to 1.13) (4.5%, Analysis 21.2). In the GRADE assessment, the score was downgraded because of a very serious risk of bias: no clear description of primary and secondary outcomes in the methods of the included study. There is also serious imprecision: only one small study included; especially for the secondary outcomes there are wide CI. The quality of evidence is therefore calculated as very low.
21.1. Analysis.
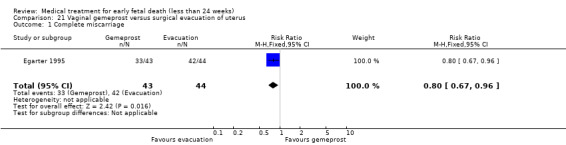
Comparison 21 Vaginal gemeprost versus surgical evacuation of uterus, Outcome 1 Complete miscarriage.
21.2. Analysis.
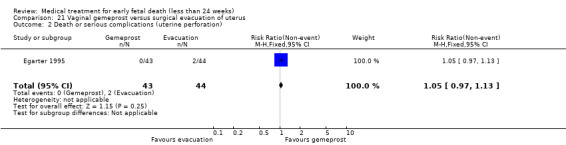
Comparison 21 Vaginal gemeprost versus surgical evacuation of uterus, Outcome 2 Death or serious complications (uterine perforation).
Secondary outcomes
The incidence of nausea was similar in both groups (RR 1.79, 95% CI 0.56 to 5.68; Analysis 21.3), with very low quality of evidence because of the reasons described above.
21.3. Analysis.
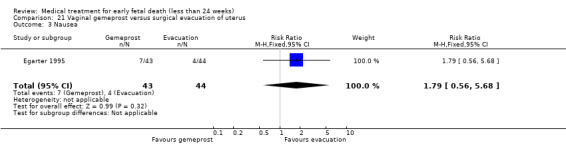
Comparison 21 Vaginal gemeprost versus surgical evacuation of uterus, Outcome 3 Nausea.
No other secondary outcomes were reported.
22. Intravaginal extraamniotic misoprostol versus vaginal misoprostol
Primary outcomes
In the one study included in this comparison (Mitwaly 2016*), women receive either misoprostol dissolved in saline through a Foley catheter (extraamniotic route) or vaginal misoprostol. There seemed to be no differences in inducing complete miscarriage (180 women, RR 1.10, 95% CI 1.00 to 1.22; Analysis 22.1). The quality of evidence was low because of a very serious risk of bias with only one study included; there was no blinding performed and there was very serious imprecision since the study was not powered for the secondary outcomes.
22.1. Analysis.
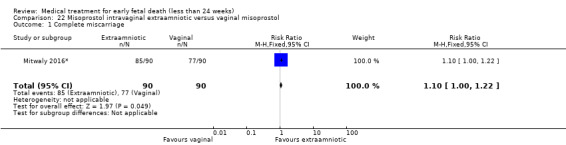
Comparison 22 Misoprostol intravaginal extraamniotic versus vaginal misoprostol, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
The time to expulsion (in hours) was shorter for the extra‐amniotic preparation (MD ‐4.81, 95% CI ‐5.66 to ‐3.96; Analysis 22.6). Although incidences of diarrhoea and vomiting were similar (Analysis 22.3; Analysis 22.4); there were more complaints of nausea in the group receiving vaginal misoprostol (1 trial, 180 women, RR 1.57, 95% CI 1.33 to 1.85; Analysis 22.2). The quality of evidence for the secondary outcomes is assessed as very low because of the very serious risk of bias described in the primary outcome; and imprecision with wide CIs, especially for diarrhoea.
22.6. Analysis.
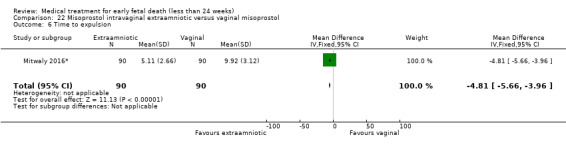
Comparison 22 Misoprostol intravaginal extraamniotic versus vaginal misoprostol, Outcome 6 Time to expulsion.
22.3. Analysis.
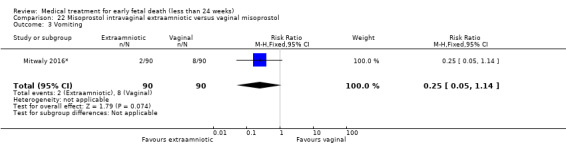
Comparison 22 Misoprostol intravaginal extraamniotic versus vaginal misoprostol, Outcome 3 Vomiting.
22.4. Analysis.
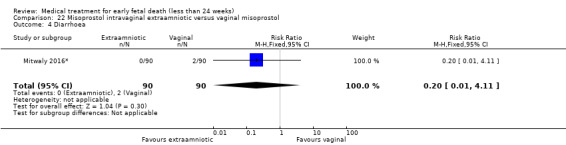
Comparison 22 Misoprostol intravaginal extraamniotic versus vaginal misoprostol, Outcome 4 Diarrhoea.
22.2. Analysis.
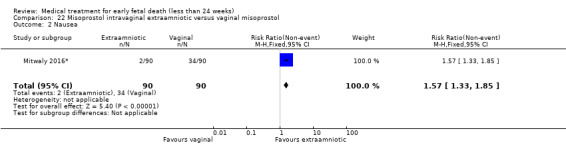
Comparison 22 Misoprostol intravaginal extraamniotic versus vaginal misoprostol, Outcome 2 Nausea.
No other secondary outcomes were reported.
23. Vaginal misoprostol with or without extended course
Primary outcomes
In one study included in this comparison (Mizrachi 2017), women were treated with 800 mcg of vaginal misoprostol either once, or twice with an interval of four days. In the other study included (Tang 2006), women were treated with 600mcg of sublingual misoprostol every three hours on the first day. Half of the women were treated with an extended course of misoprostol: 400mcg of sublingual misoprostol from day two until day eight. There were no differences in inducing complete miscarriage (351 women, RR 1.00, 95% CI 0.92 to 1.09; Analysis 23.1). The quality of evidence is low because of a very serious risk of bias (two studies included, no blinding of outcome assessors performed).
23.1. Analysis.
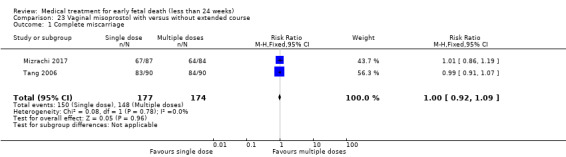
Comparison 23 Vaginal misoprostol with versus without extended course, Outcome 1 Complete miscarriage.
Death or serious complications were not reported in the trial.
Secondary outcomes
There were no differences found in the incidence of nausea, vomiting and diarrhoea (Analysis 23.2; Analysis 23.3; Analysis 23.4). Fewer women required analgesia for pain in the single dose group (171 women, RR 0.84, 95% CI 0.71 to 1.00; Analysis 23.5). Patients satisfaction was similar for both treatment arms, the majority of women would probably choose this treatment again (171 women, RR 1.01, 95% CI 0.84 to 1.22; Analysis 23.6).
23.2. Analysis.
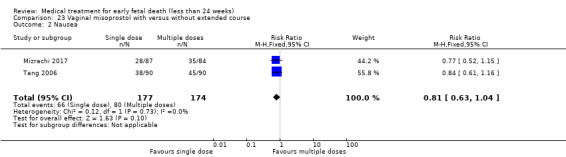
Comparison 23 Vaginal misoprostol with versus without extended course, Outcome 2 Nausea.
23.3. Analysis.
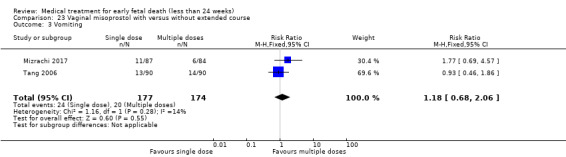
Comparison 23 Vaginal misoprostol with versus without extended course, Outcome 3 Vomiting.
23.4. Analysis.
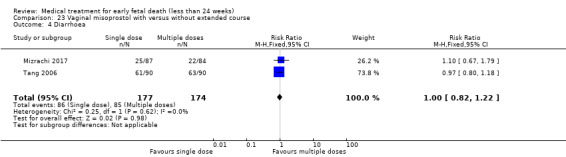
Comparison 23 Vaginal misoprostol with versus without extended course, Outcome 4 Diarrhoea.
23.5. Analysis.
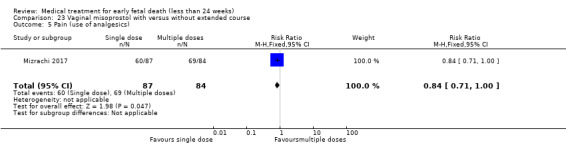
Comparison 23 Vaginal misoprostol with versus without extended course, Outcome 5 Pain (use of analgesics).
23.6. Analysis.
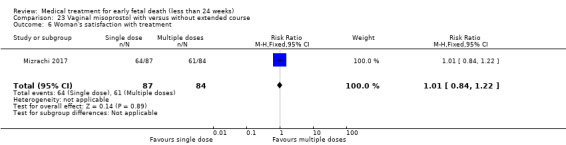
Comparison 23 Vaginal misoprostol with versus without extended course, Outcome 6 Woman's satisfaction with treatment.
No other secondary outcomes were reported.
Sensitivity analyses
We had planned to perform sensitivity analyses, but since too few studies were included in any analysis to carry out meaningful sensitivity analysis, this was not performed.
Discussion
Summary of main results
The majority of included trials (41/43) assessed the use of misoprostol (mainly by vaginal administration). Vaginal misoprostol is an effective treatment option for early fetal death, compared to expectant management or placebo, in effecting a complete miscarriage. Compared to surgical evacuation there are more gastro‐intestinal side effects, such as nausea and diarrhoea. However surgical evacuation may have particular risks that can be avoided by primary medical treatment (for example, perforation of the uterus, lesions of the cervix). Higher‐dose regimens of vaginal misoprostol seemed not to be more effective than lower‐dose regimens with a similar incidence of adverse effects. Furthermore, a repeat dose of vaginal misoprostol after a certain time period seems not to further increase the number of complete miscarriages. For vaginal misoprostol treatment, adding methotrexate or laminaria tents has no advantage. Based on one trial, a wet preparation does not seem to be more effective than dry preparations of misoprostol.
Misoprostol can be administered through different routes. The vaginal route is well studied. Other routes that were assessed in trials were sublingual, oral or buccal administrations. None of the other administration routes were superior to vaginal misoprostol.
The efficacy of vaginally or sublingually administered misoprostol is similar, but sublingual administration seems to cause more pain. Adding sublingual misoprostol to a vaginal course does not improve outcomes while it may have more side effects, especially diarrhoea. Using a powdery sublingual preparation has no advantage compared to compact tablets. Sublingual misoprostol is as effective as oral misoprostol but gives less gastro‐intestinal side effects.
Oral misoprostol seems to be less effective than vaginal misoprostol. For buccal administrations, a higher dose improves complete miscarriage, but may lead to more side effects.
Vaginal misoprostol has equivalent efficacy compared to prostaglandin analogues (dinoprostone, gemeprost) and (in second trimester fetal death) to intravenous oxytocin.
Other medications that were assessed in this review were mifepristone (versus placebo) and vaginal gemeprost (versus surgical evacuation). Mifepristone was more effective than placebo in inducing complete miscarriage. Treatment with vaginal gemeprost was less effective than curettage for complete miscarriage, with a similar incidence of side effects (nausea).
Overall completeness and applicability of evidence
This review comprises 23 comparisons of medication (compared to other medication or to other types of treatment) that can be used for treatment of early fetal death. Several types of preparations, routes of administration and dosages were assessed, especially for misoprostol treatment. This large heterogeneity in medication regimens makes it difficult to present robust statements on the efficacy of misoprostol in general; especially since for several comparisons the level of evidence was low or even very low. For misoprostol treatment alone, 30 different regimens were used in the included trials. Most investigated were 800 mcg of misoprostol vaginally in one dosage (eight trials), 800 mcg of vaginal misoprostol repeated once after 24 hours (six trials) or four days (one trial) and 600 mcg of oral or sublingual misoprostol repeated every three hours with a maximum of four dosages (four trials). Especially for the higher dose of vaginal misoprostol (800 mcg) and the higher dose of sublingual misoprostol (600 mcg) it is safe to say that these are effective treatment options for early fetal death. The other routes of administration and dosages require more investigation to compose more robust results.
Quality of the evidence
There were large differences in quality of evidence among the different comparisons. In 16 comparisons only one trial was included, which meant a downgrade of at least one level in the GRADE assessment for certainty of evidence. In general, the quality of evidence for comparisons that included more trials was higher than comparisons in which only one trial was included.
'Summary of findings' tables are presented for the two comparisons that we considered clinically most meaningful: vaginal misoprostol versus placebo (Table 1) and vaginal misoprostol versus surgical evacuation (Table 2). The first of these comparisons presents the results of (vaginal) medical treatment itself, while the latter presents the results for medical treatment compared to the most applied other treatment option: surgical evacuation of the uterus. Since we found no major differences in effectiveness between different routes of administration; these findings might be generalised to other routes of administration as well.
The GRADE score for vaginal misoprostol compared to placebo, was assessed as low quality for complete miscarriage, nausea and diarrhoea and for treatment satisfaction. For treatment blood loss and pain, the quality of the evidence was assessed as very low. Since no included studies for this comparison reported on pelvic infection, GRADE scores could not be established for this outcome. The main reasons for downgrading the quality of evidence were due to risk of bias concerns with unclear allocation concealment and blinding and also due to concerns regarding imprecision and indirectness of the evidence.
The quality of the evidence for the comparison vaginal misoprostol versus curettage, was assessed as low quality for all outcomes: complete miscarriage; pelvic infection; nausea; diarrhoea; blood loss; pain; and woman's satisfaction. Quality of evidence was downgraded because for these outcomes due to imprecision, with only one trial providing data for these outcomes. Furthermore, blinding was not possible due to the nature of the intervention, and reports were inconsistent.
The risk of bias varied among the included trials (Figure 2). For 12 trials randomisation procedure had an unclear risk of bias due to inadequate description of the random sequence allocation and in 16 trials allocation concealment was unclear. Improper allocation concealment might have influenced the results in these trials, especially in trials where patients and personnel were not blinded for the type of intervention. One trial (Eng 1997*) was considered to have a high risk of bias. In this trial even and odd numbers were used for sequence allocation, and this is of course not random (Figure 3). In several trials blinding would have been very difficult or even impossible due to the nature of the interventions; for example, in trials that compared medical treatment to surgical evacuation. For trials that compared vaginal versus sublingual or oral medication one could argue that the use of placebo would have been possible: one group should receive vaginal medication and oral or sublingual placebo, the other group oral/sublingual medication and vaginal placebo. This is laborious and in some cases more inconvenient for the patients, but it would have been a manner to guarantee proper blinding. Furthermore, even if blinding of patients was impossible, still the outcome assessor could have been blinded. In only eight of the 43 trials blinding of patients, personnel and/or outcome assessors was mentioned (Bagratee 2004; Bracken 2014*; Ganguly 2010; Kovavisarach 2005; Lelaidier 1993; Lister 2005; Sinha 2018; Wood 2002). In most trials outcome was assessed by performing (transvaginal) ultrasound. However, ultrasound after miscarriage might have inter‐ and even intra‐observer variability; without blinding of the outcome assessor this imposes a risk of bias. Other concerns in risk of bias were the reporting of incomplete outcome data in three trials (Dehbashi 2016; Fang 2009; Lelaidier 1993) and signs of selective reporting in five trials (Ayudhaya 2006; Herabutya 1997; Ngoc 2004; Saichua 2009; Schreiber 2018). In 18 other trials it was unclear whether there was selective reporting; in most of these trials the methods section stated that 'adverse effects' or 'side effects' were measured without further specification, while the results section showed detailed outcomes on specific side effects, but it was unclear if that were all the effects that were measured.
Since the quality of evidence is low or very low for several comparisons, mainly because they included only one or two (small) trials; further research is necessary to assess the effectiveness, safety and side effects of medical treatment in different medication regimes.
Potential biases in the review process
We have conducted a thorough investigation but still there could be biases in the review process.
Screening for eligible articles from the updated search and data extraction was performed by at least two review authors using the prespecified set of inclusion and exclusion criteria. There were some discrepancies, but we resolved these through mutual discussion. The inclusion or exclusion of conference abstracts was discussed in detail. We decided to exclude conference abstracts that were not clearly randomised trials or did not present applicable results. Furthermore, we searched for full‐text articles that might have been published on these trials, and in some cases we contacted the authors to ask for full results of their studies. Unfortunately, none of them replied thus, in the end we excluded all conference abstracts.
There were several trials that could have been useful for the review, but that included patients with a gestational age more than 24 weeks as well, or patients with induced abortion/termination of pregnancy (for example, because of congenital malformations). We contacted the authors of these trials to ask for subgroup analyses or individual data. If the authors did not respond immediately, we sent them a reminder. If still they did not respond we excluded their trial. Some articles did not provide contact details for the authors; in which case we searched for other articles from these authors to find contact details, or we tried to find them on social media (Research Gate). For most of these authors we found contact details, but none of them responded to our questions. We believe that this approach was the best in order to obtain as much information as possible; however it did not provide us with extra data except for one trial (Bracken 2014*). Not having included results from these trials (especially in comparisons where currently only one trial is included) could have biased our results in two different ways; either over‐ or underestimating the potential effects.
Assessment of the level of evidence was also performed by two authors, any discrepancies were resolved through discussion. We think our assessment was as thorough and complete as possible.
Agreements and disagreements with other studies or reviews
The reproductive use of misoprostol is considered in other Cochrane Reviews, for indications that include treatment of incomplete miscarriage (Kim 2017), termination of unwanted pregnancies (Kulier 2011; Say 2002), induction of labour (Alfirevic 2014; Hofmeyr 2010; Muzonzini 2004), and prevention and treatment of postpartum haemorrhage (Mousa 2014; Tunçalp 2012).
For treatment of incomplete miscarriage (Kim 2017), there appeared to be a slightly lower incidence of complete miscarriage with misoprostol in comparison to surgical evacuation (average risk ratio (RR) 0.96, 95% confidence interval (CI) 0.94 to 0.98; 15 studies, 3862 women, random‐effects; very low‐quality evidence) but with success rate high for both methods. Overall, there were fewer surgical evacuations with misoprostol (average RR 0.05, 95% CI 0.02 to 0.11; 13 studies, 3070 women, random‐effects; very low‐quality evidence) but more unplanned procedures (average RR 5.03, 95% CI 2.71 to 9.35; 11 studies, 2690 women, random‐effects; low‐quality evidence).
In termination of unwanted pregnancies the rate of abortions not completed with the intended method was higher in the prostaglandin group (RR 2.7, 95% CI 1.1 to 6.8) compared to surgery (Say 2002). This is in line with our finding that misoprostol is less effective than surgery. In incomplete miscarriage the cervical ostium is already open, therefore misoprostol for incomplete miscarriage might be more effective than misoprostol for fetal death. Nonetheless, for all indications misoprostol still reduces the overall number of patients that receive surgical evacuation. Since surgical evacuation has some specific risks (in our review for example, there were patients with uterus perforation or Asherman syndrome), misoprostol would be a good alternative as primary treatment.
The incidence of pelvic infection in our review was comparable to treatment of incomplete miscarriage and termination of unwanted pregnancy. Duration of bleeding was longer for medical treatment compared to surgery in termination of pregnancy (Say 2002). In our review duration of bleeding for this comparison was not assessed, but post treatment haematocrit was comparable between the groups (Analysis 3.3).
Compared to expectant management, in incomplete miscarriage there was no difference identified in the need for surgical evacuation if patients were treated with misoprostol compared to expectant management (average RR 0.62, 95% CI 0.17 to 2.26; 2 studies, 308 women, random‐effects; low‐quality evidence). Furthermore, there was no difference in complete miscarriage (average RR 1.23, 95% CI 0.72 to 2.10; 2 studies, 150 women, random‐effects; very low‐quality evidence) (Kim 2017). On the contrary, in our review misoprostol decreases the need for surgical evacuation in patients with early fetal death. In incomplete miscarriage the mechanism of miscarriage is already in motion, for example, the cervical ostium is dilated and there might be contractions of the uterus. Therefore, expectant management in incomplete miscarriage might be more effective than expectant management in early fetal death, when the ostium is closed and there are no contractions. There was no difference identified in pelvic infection between misoprostol and expectant management in incomplete miscarriage (average RR 2.42, 95% CI 0.59 to 9.98; 3 studies, 333 women; Kim 2017); while in our review incidence of pelvic infection was higher in the misoprostol group.
For termination of unwanted pregnancy, misoprostol administered orally is less effective (more failures) than the vaginal route (RR 3.00, 95% CI 1.44 to 6.24; Kulier 2011), and may be associated with more frequent side effects such as nausea and diarrhoea. This is in line with our findings. Sublingual routes in induced abortion were similarly effective compared to the vaginal route (Kulier 2011), but had higher rates of side effects; while in our review side effects were similar apart from abdominal pain. Both in our review as in the review by Kulier and colleagues, there was large variety in medication regimens; this might have influenced the incidence of side effects: higher dosages that are repeated more often might lead to a higher incidence and more severe side effects.
Authors' conclusions
Implications for practice.
Available evidence from randomised trials supports the use of misoprostol as one possible option for the treatment of non‐viable pregnancies before 24 weeks. In general, side effects of medical treatment were minor. There were no major differences in effectiveness between different routes of administration. Treatment satisfaction was addressed in only a few studies, in which the majority of women were satisfied with the received intervention.
There is intense interest in the reproductive uses of misoprostol because it appears a potent method for pregnancy interruption as well as being cheap and stable at room temperature. Using misoprostol as an alternative to surgical treatment for early fetal death could decrease the number of curettages, thus preventing women from the specific risks that are related to surgical intervention.
Implications for research.
Ultrasound demonstration of early pregnancy failure before 14 weeks is a common problem that merits greater research effort than has occurred to date. Further research to assess the effectiveness, safety and side effects of misoprostol, including optimal route of administration and dose, should focus on the dose regimens that tend to be most effective according to our review results: vaginal or sublingual misoprostol in higher dosages. Women's views about the acceptability of medical treatment, surgical treatment and expectant management could be integral to future research protocols, as could economic assessments. Long‐term outcomes, notably subsequent fertility, deserves further study in appropriately powered randomised controlled studies.
What's new
| Date | Event | Description |
|---|---|---|
| 24 October 2018 | New citation required but conclusions have not changed | This update has added 21 new studies. Two studies previously included have now been excluded (Fadalla 2004*; Heard 2002). Ten new comparisons have been added. The review now includes a total of 23 comparisons including a wide variety of different interventions, mainly consisting of single studies. The available evidence from randomised control trials still supports the use of vaginal misoprostol. |
| 24 October 2018 | New search has been performed | Search updated. 'Summary of findings' tables incorporated. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 8 August 2012 | Amended | Search updated. One hundred reports added to Studies awaiting classification. |
| 18 September 2008 | Amended | Converted to new review format. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
Each line was run separately
fetal death
anembryonic pregnancy
fetal demise
pregnancy loss
non viable pregnancy
Data and analyses
Comparison 1. Vaginal misoprostol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 5 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.23 [3.01, 5.94] |
| 1.1 Complete miscarriage < 1 day | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.73 [2.70, 8.28] |
| 1.2 Complete miscarriage < 2 days | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.74 [2.70, 12.19] |
| 1.3 Complete miscarriage < 7 days | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [1.80, 4.99] |
| 2 Death or serious complications: uterine perforation | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 3 Blood transfusion | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.04] |
| 4 Blood loss: haemoglobin difference > 10 g/L | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.12] |
| 5 Days of bleeding: vaginal bleeding 2 weeks after treatment | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.45] |
| 6 Nausea | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.40] |
| 7 Diarrhoea | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.35, 14.06] |
| 8 Pain (opiate use) | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.11] |
| 9 Woman's satisfaction with treatment | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.83, 1.64] |
Comparison 2. Vaginal misoprostol versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.09, 1.45] |
| 2 Pelvic infection | 1 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.05 [1.87, 34.72] |
Comparison 3. Vaginal misoprostol versus surgical evacuation of uterus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 6 | 943 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.50] |
| 2 Uterine perforation | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.65] |
| 3 Blood loss: post‐treatment haematocrit (%) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐3.51, 0.71] |
| 4 Pain relief | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.82, 2.46] |
| 5 Pelvic infection | 1 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.37] |
| 6 Nausea | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 21.85 [1.31, 364.37] |
| 7 Diarrhoea | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 40.85 [2.52, 662.57] |
| 8 Woman's satisfaction | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.40, 1.11] |
Comparison 4. Vaginal misoprostol versus vaginal dinoprostone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.37, 2.46] |
| 2 Blood transfusion | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [0.30, 121.33] |
| 3 Nausea | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.28, 3.78] |
| 4 Duration of hospital stay (days) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.38 [‐3.36, ‐1.40] |
Comparison 5. Vaginal misoprostol lower versus higher‐dose regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage < 13 weeks | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.14] |
| 2 Complete miscarriage 13‐23 weeks | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.26] |
| 3 Nausea | 2 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.41] |
| 4 Diarrhoea | 2 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.15, 1.91] |
Comparison 6. Vaginal misoprostol wet versus dry vaginal preparations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 1.1 Complete miscarriage < 3 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.54] |
| 1.2 Complete miscarriage < 8 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 1.3 Complete miscarriage < 15 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.10] |
| 1.4 Complete miscarriage < 30 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 2 Diarrhoea | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.89, 3.42] |
| 3 Vomiting | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.33, 2.62] |
| 4 Acceptability of method: would wish/probably wish same treatment in future nonviable pregnancy | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.93, 1.49] |
Comparison 7. Vaginal misoprostol + methotrexate versus vaginal misoprostol alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 2 Haemorrhage | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.10, 50.85] |
| 3 Pain relief | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.25, 2.22] |
Comparison 8. Vaginal misoprostol plus laminaria tents versus vaginal misoprostol alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.18] |
| 1.1 Complete miscarriage < 1 day | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.25] |
| 1.2 Complete miscarriage < 2 days | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.29] |
Comparison 9. Vaginal misoprostol versus sublingual misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage < 13 weeks | 5 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| 2 Complete miscarriage 13‐23 weeks | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 2.00] |
| 3 Blood loss: excessive (> menstruation) | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 1.89] |
| 4 Pain | 3 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.46, 0.74] |
| 5 Nausea | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.12, 1.44] |
| 6 Vomiting | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.26] |
| 7 Diarrhoea | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.92] |
9.2. Analysis.
Comparison 9 Vaginal misoprostol versus sublingual misoprostol, Outcome 2 Complete miscarriage 13‐23 weeks.
Comparison 10. Vaginal misoprostol versus intravenous oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage 13‐23 weeks | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.25] |
| 2 Blood loss: excessive | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 5.97] |
| 2.1 Gestation 15‐24 weeks | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 5.97] |
Comparison 11. Vaginal misoprostol versus vaginal gemeprost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage 13‐23 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.90, 1.70] |
| 2 Opiates for pain relief | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 4 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.63] |
11.2. Analysis.
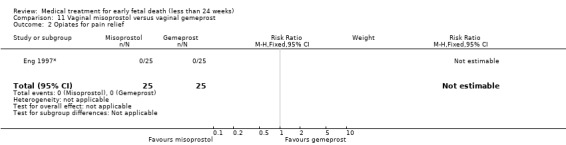
Comparison 11 Vaginal misoprostol versus vaginal gemeprost, Outcome 2 Opiates for pain relief.
Comparison 12. Sublingual misoprostol versus oral misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 2 Pain | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.36, 1.67] |
| 2.1 400 mcg sublingual misoprostol 4hourly versus 400 mcg oral misoprostol 4hourly | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.88, 1.48] |
| 2.2 200 mg mifepristone + 600 mcg sublingual misoprostol versus 200 mg mifepristone + 600 mcg oral misoprostol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.38, 0.72] |
| 3 Nausea and/or vomiting | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.85] |
| 3.1 Nausea and/or vomiting | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.11, 3.76] |
| 3.2 Nausea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.05] |
| 3.3 Vomiting | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.27, 0.92] |
| 4 Diarrhoea | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.22] |
| 4.1 400 mcg sublingual misoprostol 4 hourly versus 400 mcg oral misoprostol 4 hourly | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.29, 2.35] |
| 4.2 200 mg mifepristone + 600 mcg sublingual misoprostol versus 200 mg mifepristone + 600 mcg oral misoprostol | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.25] |
| 5 Woman's satisfaction with treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.06, 1.55] |
Comparison 13. Sublingual powdery versus sublingual compact misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 2 Nausea/vomiting | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.16, 7.10] |
| 3 Diarrhoea | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.44, 2.65] |
Comparison 14. Sublingual misoprostol with versus without extended course.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 2 Nausea | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.72, 2.65] |
| 3 Pain | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.96, 1.31] |
| 4 Vomiting | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.60, 41.95] |
| 5 Diarrhoea | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.25, 3.19] |
Comparison 15. Sublingual + vaginal misoprostol versus only vaginal misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.85, 1.18] |
| 2 Blood loss: haemoglobin level | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
| 3 Nausea | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.80, 1.79] |
| 4 Vomiting | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.88] |
| 5 Diarrhoea | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.48, 4.38] |
| 6 Pain | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.29, 1.97] |
| 7 Woman's satisfaction with treatment | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
15.2. Analysis.
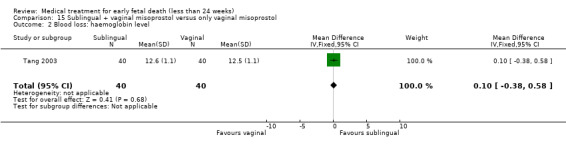
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 2 Blood loss: haemoglobin level.
15.5. Analysis.
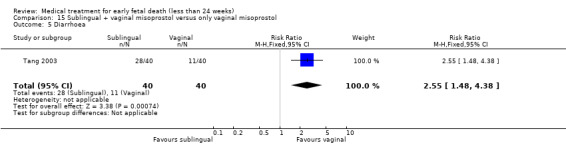
Comparison 15 Sublingual + vaginal misoprostol versus only vaginal misoprostol, Outcome 5 Diarrhoea.
Comparison 16. Oral misoprostol versus vaginal misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage < 13 weeks | 4 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.45, 1.03] |
| 2 Complete miscarriage > 13‐23 weeks | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.92, 1.09] |
| 3 Blood loss: excessive | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.87] |
| 4 Pain (visual analogue scale) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐4.82, 1.02] |
| 5 Pain | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [1.01, 2.55] |
| 6 Vomiting | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.11, 4.89] |
| 7 Nausea | 3 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.93, 1.48] |
| 8 Diarrhoea | 4 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.58] |
| 9 Woman's satisfaction with treatment | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
16.2. Analysis.
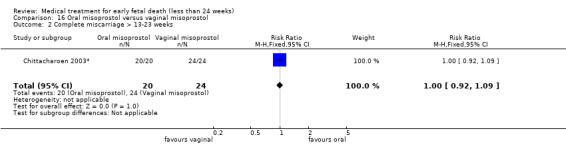
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 2 Complete miscarriage > 13‐23 weeks.
16.3. Analysis.
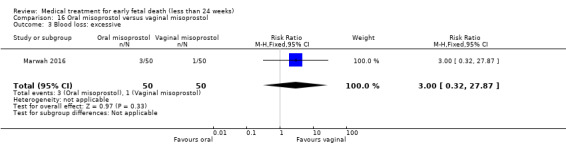
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 3 Blood loss: excessive.
16.4. Analysis.
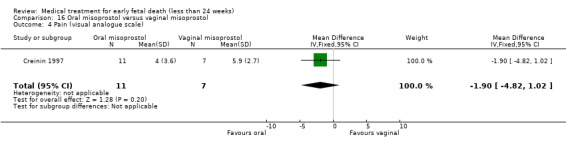
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 4 Pain (visual analogue scale).
16.9. Analysis.
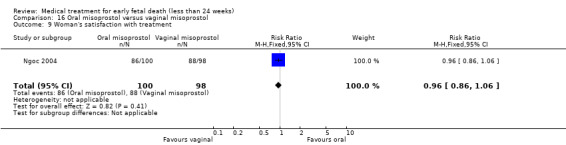
Comparison 16 Oral misoprostol versus vaginal misoprostol, Outcome 9 Woman's satisfaction with treatment.
Comparison 17. Oral misoprostol + mifepristone versus expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 2 Blood loss (severe) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.29] |
| 3 Days of bleeding | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.43, 1.83] |
| 4 Pain | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [‐5.92, 14.12] |
| 5 Pelvic infection | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.55] |
| 6 Woman's satisfaction with treatment (visual analogue scale day 14) | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐5.54, 12.34] |
17.3. Analysis.
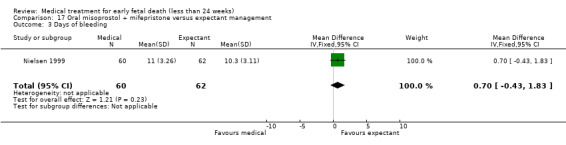
Comparison 17 Oral misoprostol + mifepristone versus expectant management, Outcome 3 Days of bleeding.
17.4. Analysis.
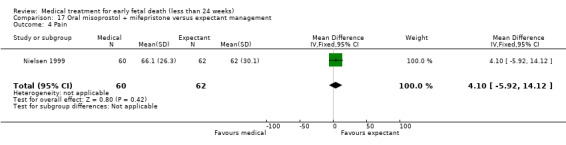
Comparison 17 Oral misoprostol + mifepristone versus expectant management, Outcome 4 Pain.
Comparison 18. Buccal misoprostol lower versus higher‐dose regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage 13‐23 weeks | 1 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.58, 0.86] |
| 1.1 Complete miscarriage < 1 day | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| 1.2 Complete miscarriage < 2 days | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 2 Nausea | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.28, 1.34] |
| 2.1 Gestation 14‐24 weeks | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.28, 1.34] |
| 3 Vomiting | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.76] |
| 3.1 Gestation 14‐24 weeks | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.76] |
| 4 Diarrhoea | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.82] |
| 4.1 Gestation 14‐24 weeks | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.82] |
| 5 Pain | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 5.1 Gestation 14‐24 weeks | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 6 Woman's satisfaction with treatment (satisfied or very satisfied) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.17] |
| 6.1 Gestation 14‐24 weeks | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.17] |
18.6. Analysis.
Comparison 18 Buccal misoprostol lower versus higher‐dose regimen, Outcome 6 Woman's satisfaction with treatment (satisfied or very satisfied).
Comparison 19. Mifepristone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Complete miscarriage < 2 days | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 98.75] |
| 1.2 Complete miscarriage < 3 days | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.0 [1.17, 308.40] |
| 1.3 Complete miscarriage < 4 days | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.0 [2.00, 97.88] |
| 1.4 Complete miscarriage < 5 days | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.5 [2.49, 36.19] |
| 2 Days of bleeding | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [1.89, 8.10] |
| 3 Pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [0.93, 5.17] |
Comparison 20. Mifepristone + vaginal misoprostol versus vaginal misoprostol alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.47] |
| 2 Blood transfusion | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.32, 28.90] |
| 3 Pelvic infection | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.10] |
| 4 nausea | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.36] |
| 5 Diarrhoea | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.35] |
| 6 Woman's satisfaction | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.06, 1.75] |
20.5. Analysis.
Comparison 20 Mifepristone + vaginal misoprostol versus vaginal misoprostol alone, Outcome 5 Diarrhoea.
Comparison 21. Vaginal gemeprost versus surgical evacuation of uterus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.67, 0.96] |
| 2 Death or serious complications (uterine perforation) | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.13] |
| 3 Nausea | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.56, 5.68] |
Comparison 22. Misoprostol intravaginal extraamniotic versus vaginal misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.00, 1.22] |
| 2 Nausea | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.33, 1.85] |
| 3 Vomiting | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.14] |
| 4 Diarrhoea | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.11] |
| 5 Pain (use of analgesics) | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.58] |
| 6 Time to expulsion | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐5.66, ‐3.96] |
22.5. Analysis.
Comparison 22 Misoprostol intravaginal extraamniotic versus vaginal misoprostol, Outcome 5 Pain (use of analgesics).
Comparison 23. Vaginal misoprostol with versus without extended course.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete miscarriage | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 2 Nausea | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.04] |
| 3 Vomiting | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.68, 2.06] |
| 4 Diarrhoea | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.82, 1.22] |
| 5 Pain (use of analgesics) | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.71, 1.00] |
| 6 Woman's satisfaction with treatment | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abediasl 2016*.
| Methods | RCT. Computerised random‐number generator was used for sequence generation. Participants: 85 pregnant women with confirmed IUFD who were admitted for labour induction at Shariati Hospital, Bandar Abbas, Iran, from January 2013 through January 2014. | |
| Participants | The inclusion criteria were: pregnant women with documented IUFD, a gestational age of 15–24 weeks, and a Bishop score < 4. | |
| Interventions | Intervention: the starting dose was 200 mcg misoprostol vaginal tablets. The tablet was wet with a drop of water for injection and inserted into the posterior fornix of the vagina using a speculum and a spatula. After 12 hours, if the conception products were not expelled and the effective uterine contractions (> 3 contractions/10 minutes) were not established, another dose of 200 mcg misoprostol vaginal tablets was inserted, reaching a maximal total dose of 400 mcg (n = 40). Control: oxytocin infusion was given in 500 cm3 of 5% dextrose with the starting oxytocin dose of 6 mU/minute. If no effective uterine contractions were noted, the dose was increased at a rate of 6 mU/minute at 45‐minute intervals to reach a maximal dose of 40 mU/minute (n = 45). |
|
| Outcomes | The primary outcome of the study was the time of induction‐to‐delivery interval. Secondary outcomes were the success rate (evacuation < 24 hours), duration of admission, postpartum haemorrhage and complications of labour induction. | |
| Funding | This research was funded by the Maternal, Fetal and Neonatal Research Center, Tehran University of Medical Sciences and Hormozgan University of Medical Sciences. | |
| Declarations of interest | The authors declare that they have no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerized random‐number generator was used for sequence generation, which was carried out by M.S. Simple randomization was used in this study". |
| Allocation concealment (selection bias) | Low risk | Quote: "We used consecutive opaque envelopes for the concealment of allocation, which was performed by F.K. The envelopes were opaque when held to the light, and opened sequentially and only after the participant’s name and other details". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8 women with induction failure were analysed according an intention‐to‐treat principle. There was no information on lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section are presented in the result section. |
| Other bias | Low risk | No other source of bias could be detected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The implementation of assignments was carried out by Z.A.; which is another person then the persons who performed the randomization". Comment: the article does not further state whether patients and personnel were blinded, however due to the nature of the interventions blinding would be practically impossible. Not blinding of personnel might have had an impact on outcome assessment (see detection bias). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the article does not state whether there was blinding of outcome assessment. If there was no blinding, this might have had an impact on judgment of successful outcome (empty uterus). |
Al Inizi 2003.
| Methods | 'Random allocation'. Details unknown. Study conducted at Tawam Hospital— a teaching hospital tertiary care unit in the United Arab Emirates. Duration of study not mentioned. |
|
| Participants | 60 women with early non‐viable pregnancies diagnosed by ultrasound. | |
| Interventions | Vaginal misoprostol 400 mcg repeated twice a day to maximum of 1600 mcg (n = 27) vs dinoprostone (PGE2) vaginal tablets repeated at 6‐hourly intervals to maximum of 36 mg (n = 33). | |
| Outcomes | Complete miscarriage/need for surgical evacuation. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Authors contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "60 women with a diagnosis of missed abortion were randomly allocated". Comment: no further information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 60 women were randomised; and for all 60 women outcomes were reported (table 1). |
| Selective reporting (reporting bias) | Low risk | Comment: there is no information on how many eligible women were counselled but refused participation. Apart from that there are no signs of selective reporting; all outcome measures mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: there is no information on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no information on blinding of outcome assessment. |
Autry 1999.
| Methods | Randomisation using a random number tables. Allocation concealment was accomplished in sequentially numbered opaque sealed envelopes made available at the time of enrolment in the study. Intention‐to‐treat analysis. Sinai Samaritan Medical Center and the Medical College of Wisconsin; no information on study duration. |
|
| Participants | 21 women diagnosed with a non‐viable first trimester intrauterine pregnancy up to 49 days gestation. Evidence of non‐viability included 1 of the following findings on TVS: 1) mean gestational sac diameter greater than 18 mm and no embryonic pole; 2) embryonic pole 5 mm to 10 mm without cardiac activity; 3) intrauterine gestational sac with abnormal hCG titres. Others entry criteria: 1) 18 years of age or greater; 2) closed cervix on digital exam; 3) no known intolerance or allergy to misoprostol or MTX; 4) haemoglobin of 9 g/dL or greater; 5) platelet count of 100,000/µL or greater; 6) no history of blood clotting disorders; 7) no active liver or renal disease; 8) ability and willingness to comply with visit schedule; 9) hCG less than 40,000 IU/L; and 10) easy access to a telephone and transportation. | |
| Interventions | Combined group (n = 12): IM MTX 50 mg/m2 body surface area (day 1) followed 2 days later (day 3) by vaginal misoprostol 800 mcg (by vaginal placement of 4 200 mcg tablets of misoprostol). If the gestational sac was present vaginal misoprostol was repeated. Misoprostol only group (n = 9): 4 200 mcg tablets placed in the vagina on day 1. The remainder of the follow‐up was similar to that for combined group. | |
| Outcomes | Successful complete abortion: MTX plus misoprostol 12/12 vs misoprostol only 8/9. No blood transfusion or antibiotics. Positive urine pregnancy test at the initial follow‐up appointment: 2/9 vs 7/7. Pain relief: 4/12 vs 4/9. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Wisconsin, Milwaukee, USA. All women received: 1) prescription for 10 tablets acetaminophen with codeine (300 mg/30 mg) and 8 tablets of ibuprofen (600 mg); 2) instruction sheet including phone number to contact physician 24 hours/day; and a diary sheet to record symptoms, side effects, and pain medication use. Data about side effects (headache, nausea and emesis) and women's satisfaction reported as no separate data. Authors conclude that both treatments are effective regimens for the complete evacuation of non‐viable early first trimester pregnancy, and represent a reasonable alternative for women wishing to avoid surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a random number table for each centre". |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was accomplished in sequentially numbered opaque sealed envelopes made available at the time of enrolment in the study". Comment: adequate type of allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: according to the results section outcomes were measured for all 21 included patients, no signs of loss to follow‐up or incomplete data. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting; all outcomes mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information on blinding of participants and personnel, probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessor, probably not done. |
Ayudhaya 2006.
| Methods | Parallel randomised controlled trial. Randomisation according to computer‐generated numbers. Performed in antenatal care clinic of department of Obstetrics and Gynaecology at Ramathibodi Hospital in Bangkok, Thailand. 138 women with diagnosis of early pregnancy failure were included between November 2004–December 2005. | |
| Participants | Pregnant women with gestational age 7‐12 weeks and on ultrasound: 1. intrauterine fetal sac > 2 cm without fetal pole, or 2. presence of fetal pole without cardiac activity, or gestational sac < 2 cm with no interval growth or persistent absence of fetal cardiac pulsation on rescanning after 7‐10 days. |
|
| Interventions | 400 mcg misoprostol sublingually every 4 hours up to 6 doses (n = 70) vs 400 mcg misoprostol orally every 4 hours up to 6 doses (n = 68). | |
| Outcomes | Outcomes 1. Complete abortion, defined as cervical os closed, no bleeding and endometrial thickness < 1 cm; mean induction to abortion interval. 2. Secondary outcome: adverse effects (abdominal pain, diarrhoea, nausea/vomiting, fever, chills). |
|
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: women were randomised according to computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: outcome group: oral misoprostol 68 women were randomised after which 2 women were excluded due to incomplete hospital records. However, table 2 reports of 68 women, and not of 66 women. |
| Selective reporting (reporting bias) | High risk | Comment: the methods section states that primary outcome is induction‐to‐delivery interval; however in the results section also dichotomous success rates are mentioned (complete or incomplete abortion). There were 68 patients in the intervention group, but for only 66 patients outcome is described. Furthermore, the methods section mentions ‘adverse effects’ as secondary outcome without further specification. Therefore is it unclear whether the adverse effects mentioned in the results section are the only ones that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention blinding would be difficult; the only way for blinding both participants and personnel would be to give group A oral misoprostol and sublingual placebo and group B oral placebo and sublingual misoprostol. The article does not state that placebos were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: medication was administered by nurses; outcome assessment was performed by doctors according to the article. The article does not state whether these doctors were blinded for type of intervention. |
Bagratee 2004.
| Methods | Computer‐generated random allocation of study number. Numbered envelopes containing misoprostol or placebo. All women presenting to the Early Pregnancy Assessment Unit (EPAU) at St Mary's Hospital, London, UK, from August 2001 to March 2002. |
|
| Participants | 104 women who attended Early Pregnancy Unit, St Mary's Hospital, with incomplete miscarriage or early pregnancy failure < 13 weeks. | |
| Interventions | 600 mcg misoprostol (n = 52) or placebo [expectant management] (n = 52). Second dose next day unless complete miscarriage had occurred in meantime. Review day 7 and surgical evacuation if miscarriage not complete. Further review at day 14. | |
| Outcomes | Primary: complete miscarriage without need for ERPC by day 7. Secondary outcomes: clinical, side effects, satisfaction and future choices. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Primary outcome reported for both non‐viable pregnancies and incomplete miscarriages, but not for secondary outcomes. These will be added if authors can provide data separately for non‐viable pregnancies and incomplete miscarriages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐based allocation", allocation "according to the random schedule". |
| Allocation concealment (selection bias) | Low risk | Quote: "Three misoprostol or placebo tablets were placed in each of two small envelopes and sealed. These small envelopes were then placed in consecutively numbered larger envelopes according to the random schedule and sealed by staff not involved in the study". Comment: adequate allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The 104 women randomized to the trial attended the scheduled visits as per protocol and completed the trial". Comment: no signs of missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the results section, no signs of selective reporting. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Three misoprostol or placebo tablets were placed in each of two small envelopes and sealed. These small envelopes were then placed in consecutively numbered larger envelopes according to the random schedule and sealed by staff not involved in the study". Comment: this means both patients as well as the doctor randomising the patients were unaware of the type of treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: patients and doctors randomising the patients were blinded for treatment allocation (see blinding of participants and personnel above), assuming the doctor assessing the outcome was the same as the 1 randomising the patients, there was sufficient blinding of outcome assessment. |
Bracken 2014*.
| Methods | Double‐blind randomised trial. Randomised using a simple randomisation sequence generated by computer with blocks of 10. Randomisation was stratified by study site. Montefiore Medical Center, Stanford University, Stroger Hospital, Christiana Health System, the Huong Vuong Hospital in Ho Chi Minh City, Viet Nam; from December 2008 to December 2011 |
|
| Participants | Women who sought medical care for possible fetal demise in pregnancies of between 14 and 28 weeks from December 2008 to December 2011. Confirmation of fetal demise and final gestational age were determined by ultrasound. | |
| Interventions | Intervention: 100 mcg buccal misoprostol (n = 63). Study drug was administered at 6‐hourly intervals, for a maximum of 8 doses. Control: 200 mcg buccal misoprostol (n = 72). Study drug was administered at 6‐hourly intervals, for a maximum of 8 doses. |
|
| Outcomes | The primary outcome was the fetal‐placental delivery rate within 48 hours of misoprostol commencement without any additional intervention. Rates of success were compared across study arms. | |
| Funding | This study was funded by a grant from the Office of Orphan Products Development of the United States Food and Drug Administration. | |
| Declarations of interest | The authors declare no conflicts of interest. | |
| Notes | This study included patients with gestational age > 24 weeks. We contacted the author, who could provided us with subgroup analysis for patients with gestational age < 24 weeks; therefore we were able to include this study in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The groups were created by Gynuity Health Projects using a simple randomization sequence generated by computer with blocks of 10. Randomization was stratified by study site". Comment: this is an adequate type of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Comment: the article states that research assistants created packages of medication but randomisation seems to be done by doctors, there probably was allocation concealment for the doctor randomising the patient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the flowchart shows that discontinuation was < 5%. All patients that were initially randomised were included in the analysis. Since there was no loss to follow‐up and discontinuation was very low, it is likely data outcome data were complete. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the results section presents secondary outcome measures that were not mentioned in the methods section. Unclear whether these were all the outcomes measured, or if other variables were measured but not presented. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A research assistant prepared numbered and sealed randomization packets before beginning enrolment. Each packet contained eight individually labelled dose envelopes. Each woman was administered a randomization envelope containing two tablets' (100 mcg misoprostol tablet + placebo resembling this tablet or 2 tablets of 100 mcg misoprostol)". Comment: probably the packets were handed out to the patients by other personnel than the research assistant preparing them; so there was probably blinding of both patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the care taking physician was blinded for the intervention, and therefore also blinded during assessment of the outcome. |
Chittacharoen 2003*.
| Methods | Parallel randomised controlled trial; computer‐generated random numbers in sealed opaque envelopes. Department of Obstetrics and Gynaecology, Ramathibodi Hospital, Bangkok, Thailand between July 1999 and June 2001. |
|
| Participants | Women at 16–41 weeks’ gestation with intrauterine fetal death; subgroup analysis on gestational age 16‐22 weeks available. | |
| Interventions | Group A (n = 40) 2 tablets of 200 mcg of misoprostol orally. The progression of labour was evaluated by cervical examination before subsequent dosage at 4‐hour intervals until delivery; group B (n = 40) 1 tablet of 200 mcg of misoprostol inserted high in the posterior fornix and a subsequent dose of 200 mcg at 12‐hour intervals until delivery. | |
| Outcomes | Success (complete abortion) within 48 hours. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated random numbers in sealed opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Comment: sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcomes were presented for all 80 patients. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting; al outcomes mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the treatment (medication orally vs vaginally) blinding is difficult; probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of blinding on outcome; no statement that the doctor assessing the outcome was another person than the one randomising the patient. Probably not done. |
Creinin 1997.
| Methods | Sealed, numbered, sequential envelopes containing instructions based on computer‐generated random number table. Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine, Magee‐Womens Hospital, Pittsburgh, Pennsylvania; no information on study duration. | |
| Participants | 20 women with non‐viable pregnancies diagnosed by transvaginal ultrasound; < 9 weeks; closed cervix; no contra‐indication to misoprostol; no heavy bleeding. | |
| Interventions | 400 mcg misoprostol orally, repeated after 24 hours if the pregnancy had not been expelled (n = 12); vaginal misoprostol 800 mcg ‐ repeated after 24 hours if necessary (as above) (n = 8). Surgical evacuation offered to women in both groups after 48 hours if treatment unsuccessful. | |
| Outcomes | Miscarriage; pain (visual analogue scale); side effects. | |
| Funding | Supported by a grant from the Magee Women's Health Foundation. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Pilot study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer‐generated random number table to account for 25 patients". Comment: adequate type of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The group was assigned by opening the next sequentially numbered, sealed, opaque envelope. Randomization and envelope preparation were performed by a person not directly associated with the study". Comment: adequate type of allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there is no description of missing data other than for 2 patients bHCG level was missing (which was not the primary outcome). |
| Selective reporting (reporting bias) | Unclear risk | Quote: 'Two subjects in group 1, upon review, did not appropriately meet the ultrasound criteria for early pregnancy failure'. Comment: apart from these 2 excluded patients; there might have been selective reporting: in the methods section is stated that side effects were measured, but no further specification. In the results section nausea, vomiting and diarrhoea were presented; unclear whether these were all outcomes that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the clinician nor the patient was blinded to the treatment group". Comment: due to the nature of the interventions blinding was practically impossible; but not blinding might have influenced outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Dehbashi 2016.
| Methods | Randomised clinical trial; April 2014‐Nov 2014 in Amiralmomenin hospital in Zabol city (Iran). | |
| Participants | Women in first trimester admitted for pregnancy termination because of fetal IUFD or missed abortion | |
| Interventions | Sublingual misoprostol 400 mcg, repeated every 4 hours, max 5 times (n = 25); vaginal misoprostol 400 mcg, repeated every 4 hours, max 5 times (n = 27) | |
| Outcomes | Complete miscarriage < 24 hours; secondary outcomes: side effects like nausea, diarrhoea | |
| Funding | No information on funding. | |
| Declarations of interest | The authors report no conflict of interest related to this paper. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned, because of small sample size, block randomization was performed according to the time of admission" |
| Allocation concealment (selection bias) | Unclear risk | Quote: ''Single blind allocation and intervention were conducted by a nurse" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: in sublingual group, 1 of the patients did not respond to medical abortion that underwent curettage surgery and was thus excluded. Another one had severe abdominal pain that was also excluded because on intolerability. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "In sublingual group, one of the subjects did not respond to medical abortion that underwent curettage surgery and was thus excluded. Another one had severe abdominal pain that was also excluded because on intolerability" |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding was not performed. Due to the nature of the intervention blinding was difficult; the only way to achieve this would have been to give the 'sublingual misoprostol group' vaginal placebos and vice versa. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessment is not described. |
Demetroulis 2001.
| Methods | Randomisation by opening sealed opaque envelope containing computer‐generated allocation code number. No attempt at masking given the manifest differences between medical and surgical interventions. Newham General Hospital; no information on study duration. |
|
| Participants | 80 women with incomplete miscarriage or anembryonic pregnancy or missed miscarriage < 13 weeks, diagnosed by ultrasound. The data in this review are derived only from the subgroup with non‐viable pregnancies (n = 50) and not those with incomplete miscarriages. Women were reviewed 8‐10 hours after medical treatment; if they had empty uteruses on ultrasound examination they were discharged home; if not, surgical evacuation was arranged. | |
| Interventions | Vaginal misoprostol 800 mcg once only (n = 26) vs surgical evacuation of the uterus (n = 24). | |
| Outcomes | Need for surgical evacuation, symptoms including pain and bleeding, 'satisfaction'. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Authors contacted for information on outcomes according to indication for treatment. Only usable data currently available are on incidence of surgical evacuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated numbers. |
| Allocation concealment (selection bias) | Low risk | Comment: use of sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data, it seems that all patients completed the study. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting. 94 patients were counselled, 14 declined study participation and chose surgical evacuation. All outcome measures mentioned in the methods section were reported in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No attempt was made to conceal the intervention assignment schedule from the patients or clinicians as the treatment methods for the study and control were obviously different (...) No attempt was made to mask the intervention as the study compared a medical treatment with a surgical procedure". Comment: this might have influenced (perception of) outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not performed. Quote: "No attempt was made to mask the intervention as the study compared a medical treatment with a surgical procedure". |
Egarter 1995.
| Methods | Women quote: "randomly assigned"; no details. Department of Gynecology and Obstetrics, University of Vienna; no information on study duration. |
|
| Participants | 87 women in Austria with non‐viable pregnancies between 8 and 12 weeks, diagnosed by ultrasound. | |
| Interventions | Vaginal gemeprost 1 mg every 3 hours up to maximum of 3 mg daily for 2 days (n = 43) vs uterine curettage (n = 44). | |
| Outcomes | Need for surgical curettage. Adverse effects. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information on random sequence generation other than 'patients were randomly assigned'. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients that were randomised completed the study: for all randomised patients outcome was presented. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no clear description of primary and secondary outcomes in methods section; unclear what precise outcome measure was. Several outcomes were presented in the results section, unclear if this was all that was measured. Furthermore, it is unclear how it was measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not mentioned, probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not mentioned, probably not done. |
Eng 1997*.
| Methods | Randomised by quote: "blindly picking a sealed number from a box". Treatment allocation was then based on whether the number was odd or even. Hospital Kuala Lumpur Malaysia; June 1995 to January 1996 | |
| Participants | 50 women with IUFD at 13‐26 weeks of pregnancy. | |
| Interventions | Vaginal misoprostol 200 mcg 3‐hourly up to a maximum dose of 1200 mcg (n = 25) vs vaginal gemeprost 1 mg 3‐hourly up to a maximum dose of 5 mg (n = 25). | |
| Outcomes | Main outcome quote: "treatment failure" defined as failure to miscarry within 24 hours, or side effects severe enough to preclude use of additional dose of drug. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomization was carried out by blindly picking a sealed number from a box. Odd numbers were assigned to group A (misoprostol) and even numbers to group B (gemeprost)". Comment: no information on who put the numbers in the box. |
| Allocation concealment (selection bias) | Unclear risk | Comment: inadequate allocation concealment; a sealed number was picked from a box; not clear if the investigators used opaque envelopes, not clear who put the numbers in the box. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: it seems that all 50 patients completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the methods section states that 'side effects' were measured, without further specification. It is unclear whether the side effects that are mentioned in the results section were all that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information on blinding participants and personnel, probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Fang 2009.
| Methods | Women with IUFD were randomised into 3 groups. Group A (n = 30): vaginal misoprostol (MP) 0.4 mg, 3 hours before vacuum aspiration; group B (n = 30): vaginal MP 0.4 mg every 3 hours, up to 5 doses; group C (n = 30): oral mifepristone (MF) 200 mg 36 to 48 hours before vaginal MP 0.4 mg, MP was given every 3 hours, up to 5 doses. This trial covered women hospitalised for treatment on missed abortion from 2005.09. 01 to 2007.02.28 |
|
| Participants | Patients of missed abortion, identified via ultrasound: a) irregular intrauterine gestation sac in a max diameter > 20 mm, no embryo observed; b) impaired intrauterine gestational sac development > 1 week; c) intrauterine gestational sac > 6 mm in max diameter, embryo visualised without cardiac canal beating. 4) Impaired intrauterine gestational sac development, gestational age < 84 days (12 weeks). | |
| Interventions | Vaginal misoprostol 400 mcg every 3 hours, up to 5 doses vs oral mifepristone 200 mg 36 to 48 hours before vaginal misoprostol 400 mcg every 3 hours up to 5 doses. | |
| Outcomes | Complete miscarriage, women's satisfaction | |
| Funding | No information on funding | |
| Declarations of interest | No information on conflicts of interest | |
| Notes | Outcome was only reported for 15 of 30 women receiving vaginal misoprostol treatment. The other 15 women were excluded from the analysis because emergency curettage was performed due to blood loss. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women enrolled were randomized (computer‐generated random numbers)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome was only reported for 15 of 30 women receiving vaginal misoprostol treatment. The other 15 women were excluded from the analysis because emergency curettage was performed due to blood loss. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre specified were reported |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not mentioned, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not mentioned, probably not done |
Ganguly 2010.
| Methods | Parallel randomised controlled trial. Computer‐generated random number list. The study was conducted at RG Car Medical College and Hospital, Kolkata, India between 1st May 2007 and 30th April 2008. | |
| Participants | Anembryonic gestation or embryonic or fetal death with CRL 5 mm to 40 mm without cardiac activity; inevitable miscarriage with gestational sac < 45 mm or embryonic pole < 40 mm, open cervical os and vaginal bleeding. | |
| Interventions | Intervention: 800 mcg misoprostol vaginally (n = 120). Control: manual vacuum aspiration under iv sedation (n = 60). |
|
| Outcomes | Success rate (complete evacuation at day 8); secondary outcomes: adverse events (haemorrhage, cervical tear/perforation, fever, nausea, diarrhoea, abdominal pain, satisfaction (would use this treatment again). | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Subgroup analyses on fetal death and anembryonic gestation available; therefore the study was included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Comment: opaque sealed envelope. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: for all 180 patients outcome was presented. |
| Selective reporting (reporting bias) | Unclear risk | Comment: in the results section several outcome measures are presented that were not mentioned in the methods section; unclear whether there were more outcome measures. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: there is no information on blinding of participants and personnel. Due to the nature of the intervention blinding would have practically be impossible. However especially the secondary outcomes (experience of pain and satisfaction among the non‐blinded patients) might have been influenced by type of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome assessors of the study were blinded". |
Gilles 2004.
| Methods | Random allocation by computer‐automated telephone response system. Stratification by pregnancy type. Random permuted blocks of size 4 or 8. Participants were recruited from 4 clinical centres between September 2001 and February 2002. |
|
| Participants | 80 women with anembryonic pregnancy < 46 mm sac diameter or embryonic/fetal death with crown‐rump length < 41 mm. 4 centres. | |
| Interventions | Quote: "Wet misoprostol" 800 mcg + 2 mL saline vaginally (n = 41) vs "dry misoprostol" (as above without saline) (n = 39). Second dose given day 3 if no miscarriage. | |
| Outcomes | Primary outcome: miscarriage without need for curettage before 30 days. Secondary outcomes: miscarriage < 3, < 8 and < 15 days; side effects, women's views. | |
| Funding | Supported by National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services under contracts No. N01‐HD‐1‐3321 through 3325. | |
| Declarations of interest | The following persons and institutions participated in the National Institute for Child Health and Human Development Management of Early Pregnancy Failure Trial (principal investigators are indicated by asterisks): J. Zhang* and T. Nansel (National Institute of Child Health and Human Development); C. Westhoff,* A. Davis, and C. Robilotto (Columbia University); J. Gilles,* J. Kang, F. Doyle, and N. Vazquez (University of Miami); K. Barnhart,* T. Bader, and K. Timbers
(University of Pennsylvania); M. Creinin,* B. Harwood, R. Guido, M. Fox, L. Reid (University of Pittsburgh); and M. Frederick,* S. Forman, and X. K. Huang (Clinical Trials and Surveys Corporation). No further information on conflicts of interest. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with a computer automated telephone response system. The subjects were stratified by pregnancy type with the use of random permuted blocks of size 4 or 8. The Data Coordinating Center developed the process for randomization". |
| Allocation concealment (selection bias) | Low risk | Quote: "The enrolment sequence was concealed from investigators". Comment: adequate allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 patients were lost to follow‐up (both in group I) from day 15 (according to table 1); primary outcome was still measured for them before; so for primary outcome there were no incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: some outcomes in the results section (for example, abdominal pain) were not mentioned in the methods section. Unclear how many secondary outcomes were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the investigators nor the subjects were masked because the addition of saline solution made the interventions visibly different". Comment: this might have influenced the (perception of) outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, considering that investigators and subjects were not masked for the intervention this was probably not done. |
Graziosi 2004.
| Methods | Consent for study obtained at time of diagnosis of early pregnancy failure. Randomised after at least 1 week of expectant management. Computer programme with block randomisation sequence. Stratification by previous vaginal birth; gestational age < or > 10 weeks; centre. The study was performed in 3 teaching hospitals in the Netherlands (St Antonius Hospital Nieuwegein, St Elisabeth Hospital Tilburg and Tweesteden Hospital Tilburg) between November 2001 and June 2003 |
|
| Participants | 154 women with ultrasound‐diagnosed early pregnancy failure ‐ either anembryonic pregnancy or fetal death at 6‐14 weeks. 6‐centre study in the Netherlands. | |
| Interventions | Vaginal misoprostol 800 mcg; repeated after 24 hours if ultrasound indicated remaining tissue in the uterus. Curettage after 3 days if miscarriage hadn't occurred or was incomplete (n = 79) or suction curettage within a week of randomisation (n = 75). | |
| Outcomes | Primary: complete evacuation. Secondary: side effects, pain and need for analgesia, intensity/duration of bleeding. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Of 241 eligible women, 87 (36%) declined to participate and chose curettage. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer program with a block randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by their treating gynaecologist using a computer program with a block randomization sequence, thus guaranteeing the concealment of allocation". Comment: adequate type of allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: flowchart (fig 1) shows detailed information on follow‐up of patients. No signs of incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: methods section states that side effects were measured, without further specification. Unclear whether the side effects that are presented in the results are all that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information on blinding of participants and personnel, probably not done considering the type of intervention (medication vs surgical evacuation). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done considering the type of intervention (medication vs surgical evacuation). |
Herabutya 1997.
| Methods | Quote: "Random allocation" but method not discussed in paper. Ramathibodi Hospital between March 1995 and April 1996, Bangkok, Thailand. |
|
| Participants | 84 women with ultrasound confirmation of fetal death with uterine size < 14 weeks, no bleeding, and cervix closed. | |
| Interventions | Misoprostol (200 mcg vaginally) (n = 42) or vaginal placebo (n = 42) on admission to hospital. | |
| Outcomes | Primary outcome was miscarriage within 24 hours of treatment. Some information available on complications. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Much of the outcome data reported describes only the subgroups who did miscarry before surgical evacuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: for all 84 randomised patients outcome was presented. |
| Selective reporting (reporting bias) | High risk | Comment: The methods section states that side effects were registered, but they were not reported in the results. Much of the outcome data reported describes only the subgroups who did miscarry before surgical evacuation. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information on blinding of patients and personnel. Sinces patients received either misoprostol or placebo it is likely that they were blinded, but this is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: there is no information on blinding of outcome assessment. Considering that placebo was used as comparison there might have been blinding of the outcome assessor, assuming this was not the person providing the medication (and thus capable of recognising a placebo if it had another shape than the misoprostol). |
Jain 1996*.
| Methods | "Random number table". From the Department of Obstetrics and Gynecology, University of Southern California School of Medicine; no information on study duration. |
|
| Participants | 70 women in Los Angeles, USA, with either fetal death (n = 40) or medical or genetic indications for termination of pregnancy (n = 30) at 12‐22 weeks. Only data from pregnancies complicated by fetal death included here. | |
| Interventions | Vaginal misoprostol 200 mcg 12‐hourly plus laminaria tents (n = 20) vs vaginal misoprostol 200 mcg 12‐hourly alone (n = 18). | |
| Outcomes | Miscarriage. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Adverse effects are described for the groups as wholes, so are not included here. 2 women excluded from analyses ‐ 1 protocol violation; 1 was found to have interstitial ectopic pregnancy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: use of a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome was described for all 38 patients. 2 patients were excluded before analyses (1 protocol violation and 1 found to have interstitial ectopic pregnancy). |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information on blinding of patients and personnel. Due to the nature of the intervention (misoprostol with or without laminaria tents) it is unlikely that there was blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no information on blinding of outcome assessment. Considering the type of treatment (laminaria tents or not) it is unlikely that there was blinding. |
Kara 1999*.
| Methods | Quote: "Random allocation". No details. Zeynep Kamil Women and Childrens Hospital, Istanbul, Turkey. No information on study duration. |
|
| Participants | 65 women in Istanbul, Turkey, with ultrasound‐diagnosed fetal death in second trimester. | |
| Interventions | Vaginal misoprostol 200 mcg (n = 32) vs intracervical dinoprostone 0.5 mg (n = 33). Intravenous oxytocin started after 6 hours if no 'effective contractions'. | |
| Outcomes | Complete miscarriage. Adverse effects. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Misoprostol dose reported as 200 mg. Assumed to be 200 mcg. Time to miscarriage not included as standard deviations seem incorrect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: quote: "Random allocation". No details. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 65 patients were randomised, for all of them outcomes were presented, there seems to be no missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: misoprostol dose reported as 200 mg. Assumed to be 200 mcg. Other than these findings no signs of selective or unclear reporting. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information on blinding of participants and personnel, considering the type of intervention (different number and shape of tablets used) probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Kovavisarach 2002.
| Methods | Quote: "Random allocation". Method not discussed. Between 1 July 1998 and 31 January 1999 at the gynaecologic clinic at Rajavithi Hospital, Bangkok, Thailand. |
|
| Participants | 54 women with anembryonic pregnancies < 12 weeks diagnosed by TVS. Single centre study in Bangkok, Thailand. | |
| Interventions | Vaginal misoprostol 400 mcg (n = 27) or placebo (n = 27). Reviewed after 24 hours and curettage offered if no or incomplete miscarriage. Further review after 1 week. | |
| Outcomes | Primary: complete miscarriage within 24 hours of treatment. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated". Comment: method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 54 women were recruited in the study, for all of them outcomes were reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: in the results section several side effects (nausea, pain) are reported that were not mentioned in the methods section; unclear if these were the only side effects that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information on blinding of patients and personnel. Sinces patients received either misoprostol or placebo it is likely that they were blinded. Blinding of personnel that might recognise misoprostol if the placebo tablets had another shape remains unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: there is no information on blinding of outcome assessment. Considering that placebo was used as comparison there might have been blinding of the outcome assessor, assuming this was not the person providing the medication (and thus capable of recognising a placebo if it had another shape than the misoprostol). |
Kovavisarach 2005.
| Methods | Random allocation using sealed, sequentially numbered envelopes, prepared using published table of random numbers. Between 25 November 2002 and 31 July 2003, Rajavithi Hospital, Bangkok, Thailand |
|
| Participants | 114 women in Bangkok, Thailand, with non‐viable pregnancies (anembryonic or fetal deaths) at < 12 weeks, diagnosed by TVS. Women with open cervices were not eligible for recruitment. | |
| Interventions | Vaginal misoprostol 600 mcg (n = 57) or 800 mcg (n = 57). If complete miscarriage not effected within 24 hours, or if clinical circumstances dictated (pain, bleeding), uterine curettage was performed. | |
| Outcomes | Primary: complete miscarriage without need for uterine curettage within 24 hours. Secondary: adverse effects. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women were randomly assigned to either dose of misoprostol using sealed sequentially numbered envelopes that had been prepared using a published table of random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "The drugs had been placed in the opaque envelopes by a nurse who was not involved in any of the other study processes". Comment: adequate type of allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No women withdrew from the trial". Comment: no signs of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting, variables that were measured according to the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The drugs had been placed in the opaque envelopes by a nurse who was not involved in any of the other study processes. All other staff and patients were blinded to regimen allocation". Group A received 3 tablets of misoprostol and 1 placebo, group B received 4 tablets of misoprostol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: staff was blinded to regimen allocation. |
Kushwah 2009.
| Methods | Parallel group randomised controlled trial. Patients were randomly assigned to 1 of 2 groups by computer‐generated numbers. The study was conducted from April 2003 to March 2004 with 100 women attending the prenatal clinic of the Department of Obstetrics and Gynecology of Sucheta Kriplani Hospital, Delhi, India. All had early pregnancy failure confirmed by ultrasound between the 7th and 14th week. | |
| Participants | The inclusion criteria were (1) a gestational sac of 25 mm in mean diameter or larger with no embryo present (an anembryonic pregnancy) or (2) the presence of a fetal pole without cardiac pulsations (a missed abortion). |
|
| Interventions | Group 1: 200 mg mifepristone + 600 mcg misoprostol sublingually; with up to 3 supplemental doses of 400 mcg after 12, 15 and 18 hours (if 4 hours after last dose still no expulsion: surgical evacuation) (n = 50). Group 2: 200 mg mifepristone + 600 mcg misoprostol orally; with up to 3 supplemental doses of 400 mcg after 12, 15 and 18 hours (if 4 hours after last dose still no expulsion: surgical evacuation) (n = 50). |
|
| Outcomes | The primary outcome was the mean induction‐to‐evacuation interval, defined as the time between when the first dose of misoprostol was taken and the time when the POC were expelled. The secondary outcome was the incidence of the 5 following adverse effects: blood loss, abdominal pain, nausea, vomiting, diarrhoea, and fever. Regimen acceptability was defined as whether it would be accepted again, if needed. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomly assigned to one of 2 groups by computer generated numbers". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no adequate description of the concealment process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: for all 100 patients outcomes were presented. |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measures that were mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention blinding would be difficult; the only way for blinding both participants and personnel would be to give group A oral misoprostol and sublingual placebo and group B oral placebo and sublingual misoprostol. The article does not state that placebos were used. This might particularly have influenced patients experiences that were assessed as secondary outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Lelaidier 1993.
| Methods | Drug or identical placebo supplied by pharmacy using randomisation list using permutation blocks of 4. Department of Obstetrics and Gynaecology, Hopital A.Beclere, Clamart, France. Study duration 6 months, no further information. |
|
| Participants | 46 women with non‐viable pregnancies diagnosed by ultrasound on 2 examinations separated by 1 week. < 14 weeks. No bleeding or pain. | |
| Interventions | Mifepristone 600 mg orally (n = 23) or placebo (n = 23). All women were reviewed after 5 days and if miscarriage had not occurred, surgical evacuation was performed that day. | |
| Outcomes | Primary outcome was expulsion of the pregnancy. Symptoms also recorded. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | 2 women in the placebo group underwent surgical evacuation by private practitioners before 5th day review. Both were in the process of miscarriage and were classed as expulsion positive; no information available on symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: drug or identical placebo supplied by pharmacy using randomisation list using permutation blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Comment: identical placebo were used, supplied by pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 46 patients included in this trial, two were not included in the results since contradictory advice from private clinicians ended in regular dilatation and aspiration. They both came from the placebo group and were excluded from the denominator when calculating percentages of spontaneous abortion". Comment: this is not an adequate intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting; outcome measures mentioned in the results section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''This study was prospective, randomized and double‐blind". Comment: adequate blinding by use of identical placebo in control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no specific information on blinding of outcome assessment. Considering that blinding of patients and personnel was adequate, this was probably also done. |
Lister 2005.
| Methods | Random allocation ‐ blocked and stratified by physician office and by day of recruitment ‐ day of diagnosis, or after day of diagnosis. Patients were enrolled between February 15,2002, and March 19, 2003 at Riverside Methodist Hospitals, Columbus, USA. |
|
| Participants | 34 women in Columbus, Ohio, USA, with early pregnancy failure (anembryonic pregnancies or early fetal deaths) diagnosed by TVS. | |
| Interventions | Vaginal misoprostol 800 mcg, repeated after 24 hours if sac still present on TVS (n = 18) or placebo (n = 16). | |
| Outcomes | Primary: miscarriage complete at 48 hours. | |
| Funding | Supported by Riverside Methodist Hospital Medical Research Foundation. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Planned sample size 84 but trial stopped after interim analysis of first 36 women. 2 women excluded from analysis ‐ 1 protocol violation; 1 did not meet entry criteria. 2 women did not come for assessment 2 weeks after initial treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study epidemiologist generated the allocation sequence. Randomization to misoprostol or placebo was blocked and stratified by physician office and timing of treatment in relation to diagnosis (on the day of diagnosis or 1‐14 days after diagnosis)". |
| Allocation concealment (selection bias) | Low risk | Comment: opaque randomisation packets with instruction sheets and either misoprostol or matching placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 patients withdrew consent before treatment. These were not included in the analyses. All other 34 patients were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: table 3 shows side effects; these were not mentioned in the methods section. Unclear whether these were all the side affects that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: physicians randomising and treating the patients received opaque randomisation packets containing misoprostol or matching placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: not described; but considering the treating physician was blinded for intervention, blinding of outcome assessment was probably also done. |
Marwah 2016.
| Methods | Governmental multi‐speciality hospital, Chandigarh, India from June 2013‐June 2014 | |
| Participants | Women aged 18‐45, gestational age < 12 weeks, diagnosis of missed abortion on ultrasound, minor vaginal blood loss, but cervical os closed. Haemoglobin level >= 9 mg/dL axillary temperature < 37.5 degree C, no history of inflammatory bowel disease, asthma, liver disease or contraindication to use of misoprostol, place of residence within 100 km from of the hospital, willingness and ability to give informed consent, willingness to abstain from intercourse for first 14 days of study | |
| Interventions | 400 mcg vaginal misoprostol (wet preparation) every 6 hours, max 3 doses (n = 50); 400 mcg oral misoprostol, every 6 hours, max 3 doses (n = 50) | |
| Outcomes | No need for surgical evacuation (AP diameter < 15 mm) < 12 hours after last dose of misoprostol | |
| Funding | Financial interests: none | |
| Declarations of interest | Other competing interests: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned to one of the two regimens, using computer generated sequentially numbered envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially numbered envelopes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data of all included women were presented |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting, data of all included women were presented |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention blinding would have been very difficult and would only be achieve by using oral placebos for the 'vaginal group' and vice versa. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor was not described. |
Mitwaly 2016*.
| Methods | Randomised controlled trial, Assiut women’s health hospital Egypt, 1 Feb 2015‐1 Dec 2015. | |
| Participants | Women 13‐24 weeks of gestation with IUFD confirmed by ultrasound | |
| Interventions | Intrauterine, extra‐amniotic misoprostol 200 mcg in saline dissolute solution, administered through Foley catheter per 4 hours (n = 90); vaginal misoprostol 200 mcg (wet preparation) every 4 hours (n = 90) | |
| Outcomes | Induction to (fetal) expulsion interval. Secondary: dose of misoprostol used, need for analgesics and need for surgical evacuation in cases of retained placenta and occurrence of side effects. |
|
| Funding | No information on funding. | |
| Declarations of interest | The authors declare that they have no conflict of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician prepared a computer generated random table" |
| Allocation concealment (selection bias) | Low risk | Quote: "...placed the allocation data in serially numbered sealed envelopes. The envelopes opened only by the clinician according to the order of attendance of women. Allocation unchanged after opening the closed envelopes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all data on randomised women were available |
| Selective reporting (reporting bias) | Low risk | Comment: there are no signs of selective reporting, all pre described outcomes were presented in results |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention blinding of personnel nor participants was possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor was not described |
Mizrachi 2017.
| Methods | Randomised controlled trial, single university affiliated tertiary medical centre, between August 2015 and June 2016 | |
| Participants | Women diagnosed with early pregnancy loss in the gynaecologic emergency room, either anembryonic gestation or embryonic death, were eligible for inclusion if pregnancy size by TVS was up to 12 weeks’ gestation | |
| Interventions | 800 mcg vaginal misoprostol + 800 mcg vaginal misoprostol on day 4 (n = 84); 800 mcg vaginal misoprostol (n = 87) | |
| Outcomes | The primary outcome was treatment success, defined as no need for surgical intervention up to Day 8. This included emergent and elective surgical interventions. Secondary outcomes were adverse effects, pain level, OTC analgesics use, treatment acceptability and the need for late intervention as reported by the participants by telephone on day 45. | |
| Funding | The authors did not receive funding for this study | |
| Declarations of interest | The authors declare no conflict of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned to either a single‐dose protocol or a repeat‐dose protocol in a 1:1 ratio. A blocked randomization scheme was created using a computer generated list of random numbers. Each block consisted of 30 participants." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was concealed by placing assignments in sequentially numbered opaque envelopes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: flow chart displays all outcome data available. Missing data are explained. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting, all outcomes described are presented in results |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention blinding would be difficult |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor is not described |
Muffley 2002.
| Methods | Computer‐generated random number table with blocked permutations ‐ group assignments recorded in sealed opaque numbered envelopes. This clinical study was conducted between June 1999 and March 2000 at Naval Medical Center Portsmouth. |
|
| Participants | 50 women with non‐viable pregnancies diagnosed by ultrasound (anembryonic or embryonic/fetal deaths) < 12 weeks. Exclusions: excessive bleeding, anaemia, unstable vital signs, coagulopathy, asthma or other contra‐indication to prostaglandin treatment, infection, open cervix. | |
| Interventions | 800 mcg misoprostol vaginally, repeated after 24 hours if ultrasound showed tissue still present in uterus; final review after further 24 hours ‐ if tissue still present, surgical evacuation performed (n = 25). Suction curettage (n = 25). | |
| Outcomes | Primary outcome: miscarriage. | |
| Funding | Supported by the Chief, Navy Bureau of Medicine and Surgery, Washington DC, Clinical Investigation Program (CIP No. 99‐037) | |
| Declarations of interest | The views expressed in this article are those of the authors and do not reflect the official position of the Department of Defense, the Department of the Navy, or the United States Government | |
| Notes | Analysis by intention‐to‐treat. Details about nausea, vomiting, diarrhoea reported only for misoprostol group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: use of computer‐generated random number table with blocked permutations. |
| Allocation concealment (selection bias) | Low risk | Comment: group assignments were recorded in sealed opaque numbered envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: '"Twenty‐five women were placed randomly in the medical arm of the study, and 25 women were placed randomly in the surgical arm. 2 patients in the surgical arm had spontaneous pregnancy loss before their scheduled procedures. All but 2 of the subjects had a complete post procedure evaluation". Comment: this means a loss to follow up of < 10%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: in the results section there are reports about nausea, vomiting and haemorrhage. These side effects were not mentioned in the methods section. Unclear whether these were all the side effects that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: randomisation and envelope preparation was performed by a person not directly associated with the study.However due to the nature of the interventions (medication vs surgical evacuation) blinding would be practically impossible and was probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no information on blinding of outcome assessment. Probably not done. |
Ngoc 2004.
| Methods | Randomised by opening sequentially numbered envelope ‐ prepared by computer‐generated code in blocks of 10. Recruitment took place at Hung Vuong Hospital in Ho Chi Minh City, Vietnam, from January through August 2003. |
|
| Participants | 200 women in Ho Chi Minh City, Vietnam, with non‐viable first trimester pregnancies (anembryonic or early fetal death) diagnosed by ultrasound; cervix closed. | |
| Interventions | Oral misoprostol 800 mcg (n = 100) vs vaginal misoprostol 800 mcg (n = 98). Women reviewed after 48 hours; if retained products present, they were given option of surgical evacuation or further review after another 5 days (when evacuation was performed if there were still products present). | |
| Outcomes | Primary: complete miscarriage without need for surgical evacuation. Secondary: adverse effects. | |
| Funding | Funding by David and Lucile Packard Foundation | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | 2 women lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the randomisation scheme was created by Population Council staff, using a computer‐generated code in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Quote: "The study investigator opened the next sequentially numbered randomized envelope to determine the treatment arm". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Two women in the vaginal group and one in the oral group were lost to follow‐up. One woman in the vaginal group was later reached by telephone". Comment: table 2 shows side effects for 190 patients (not 200 patients), so there are some missing data. This was < 10% of total study population. |
| Selective reporting (reporting bias) | High risk | Comment: in table 2 side effects were presented for 95 patients per treatment arm, which is a sign of missing data. Analyses for these side effects were measured as an percentage of 95 women instead of 100 women. This influences the outcomes. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the investigator nor the woman was blinded to the treatment assignment". Comment: due to the nature of the interventions (oral vs vaginal medication) blinding would have been difficult. Nonetheless this might have influenced (perception of) outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding of outcome assessment. |
Nielsen 1999.
| Methods | Randomisation method not discussed in paper. Sahlgrenska University Hospital, Sweden. No information on study duration. |
|
| Participants | 122 women < 13 weeks with symptoms of threatened miscarriage (bleeding +/‐ pain), a closed cervix, and ultrasound demonstration of pregnancy non‐viability (anembryonic pregnancy n = 44; embryonic/fetal death n = 46; 'complex mass with deformed gestational sac' n = 32). Surgical evacuation at day 5 if transvaginal ultrasound showed retained products > 15 mm diameter. | |
| Interventions | Mifepristone (400 mg orally) followed by oral misoprostol (400 mcg) 48 hours later (n = 60) vs expectant management (n = 62). | |
| Outcomes | Clinical events; routine transvaginal ultrasound at 5 days to identify retained products; visual analogue scale to assess pain at day 5; visual analogue scale to assess satisfaction at day 14. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Seeking clarification from authors if "complex mass with deformed gestational sac" represents missed or incomplete miscarriage. Data included in meantime. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomisation method not discussed in paper. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up, no signs of missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: article does not state that in the expectant management group placebo were used. Therefore there probably was no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Niromanesh 2005*.
| Methods | Randomisation method not discussed in paper. Mirza Khochak khan Hospital, Tehran, Iran. No information on study duration. |
|
| Participants | 100 women in Tehran, Iran, with fetal deaths between 14 and 25 weeks. | |
| Interventions | Vaginal misoprostol: 400 mcg (n = 50) vs 600 mcg (n = 50) ‐ both 12‐hourly for 48 hours. | |
| Outcomes | Miscarriage; surgical evacuation; adverse effects. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomisation method not discussed in paper. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information on loss to follow up, number of eligible patients, etcetera. |
| Selective reporting (reporting bias) | Unclear risk | Comment: side effects mentioned in the results (table 2) were not mentioned in the methods section; unclear if these were all outcomes that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no information on blinding, also no statements on use of placebo. Probaby no blinding (since the difference between 2 or 3 tablets would be clear for both patients as well as personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Petersen 2013.
| Methods | This was a parallel group randomised controlled study performed between September 2005 and July 2010 at 2 hospitals in Australia. Randomisation was performed using a computer‐generated model with a block size of 6 stratified for study site. | |
| Participants | Inclusion criteria Clinically confirmed early pregnancy loss 6 + 0 and 12 + 6 weeks’ gestation. Haemodynamically stable and not requiring emergency treatment. Willingness and consenting to undergo medical management. Ready access to emergency medical care (lives within 30 minutes of hospital). Immediate availability of another responsible adult with a driver's license. Ability to understand spoken English instructions without the need of a translator. |
|
| Interventions | Intervention: 400 mcg (n = 158) vaginal misoprostol; if needed repeated the next day vs 800 mcg (n = 152) vaginal misoprostol; if needed repeated the next day. | |
| Outcomes | Outcomes: the primary outcome was the effectiveness to induce complete miscarriage, evaluated using 2 different methods. 1 Ultrasound criteria: complete = no gestational sac + an endometrial thickness < 30 mm on day 7 scan; incomplete = gestational sac or endometrial thickness > 30 mm3. 2 Clinical criteria: resolution of bleeding and pain, and return to a normal menstrual cycle, without the need for D&C at the completion of follow‐up. Secondary outcomes included patient satisfaction and clinical outcomes – need for second dose; patient‐reported side effects recorded in Study Questionnaire 1; adverse events; unplanned visits to a doctor or hospital Emergency Department; fall in haemoglobin from baseline. |
|
| Funding | Completion of this study was supported in part by a grant from the Toowoomba Hospital Foundation. | |
| Declarations of interest | The authors have nothing to declare. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was performed using a computer‐generated model with a block size of 6 stratified for study site. |
| Allocation concealment (selection bias) | Low risk | Comment: allocation to the study groups was made by opening the next consecutively numbered, sealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data on incomplete follow‐up are provided in figure 2 (participation flow chart). |
| Selective reporting (reporting bias) | Unclear risk | Comment: methods section states that adverse events were measured without further specification. It is unclear if the outcomes mentioned in table 3 were all that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the allocated dose was recorded in the medication chart and administered by the non blinded attending staff. The allocated dose was not revealed to the study population (although they would probably notice the difference between 2 or 4 tablets). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the allocated dose was recorded in the medication chart and administered by the non‐blinded attending staff. This attending staff seems to also have performed the ultrasounds after treatment. |
Rita 2006.
| Methods | Parallel randomised controlled trial, permuted block method randomisation. This study was carried out in the department of obstetrics and gynaecology, SMGS Hospital, Government Medical College, Jammu, J&K in the year 2002‐2003 | |
| Participants | A total of 100 women consented to participate in the study. The specified inclusion criteria were a period of gestation less than 13 weeks, haemodynamically stable women with haemoglobin more than 10gm%, closed cervical os, axillary temperature of less than 37.50 C, no previous history of inflammatory bowel disease or allergy to misoprostol. | |
| Interventions | Intervention: 400 mcg of misoprostol was given orally and repeated every 4 hours for a maximum of 3 doses if required (n = 50). Control: 600 mcg of misoprostol was inserted in posterior vaginal fornix and the second dose was repeated after 4 hours (n = 50). |
|
| Outcomes | The primary outcome evaluated was drug‐induced complete expulsion of the conceptus (within 10‐12 hours). Secondary outcome evaluated were side effects, induction expulsion interval, number of doses required and permeability of cervical canal in those women who required surgical evacuation. |
|
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation other than permuted block method, not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mentioning of missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: methods section states that side effects were measured, without further specification. It is unclear if the outcomes mentioned in the results section were all the outcomes that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention (oral vs vaginal medication) blinding of participants and personnel would be very difficult. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no description of an independent doctor assessing the outcome. |
Saichua 2009.
| Methods | Parallel randomised controlled trial. Randomisation scheme was generated using a random number table. This RCT was performed at Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, from June 2007 to May 2008. | |
| Participants | Pregnant women with·13 weeks of gestation who came to antenatal care clinic or gynaecologic outpatient department, diagnosed with embryonic death or anembryonic pregnancy by transvaginal ultrasound were recruited into the study. Embryonic death was defined as an intrauterine pregnancy with a fetal pole longer than 6 mm without cardiac activity. Anembryonic pregnancy was defined as an intrauterine gestational sac of diameter more than 20 mm without embryonic pole or yolk sac. | |
| Interventions | Intervention: 600 mcg powdery sublingual misoprostol (n = 26). Control: 600 mcg sublingual misoprostol (n = 28). |
|
| Outcomes | The primary outcome measure was complete abortion. The secondary outcome measure was the duration of complete abortion and side effects. |
|
| Funding | No information on funding. | |
| Declarations of interest | 'Conflicts of interest statement: none.' | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization scheme was generated using a random number table. The co investigator generated the allocation sequence, and study staff enrolled participants and assigned participants to their groups. When a woman met the study inclusion criteria, the study staff picked a sequentially numbered opaque envelope which contained a ticket indicating treatment group". |
| Allocation concealment (selection bias) | Low risk | Quote: "The co investigator generated the allocation sequence, and study staff enrolled participants and assigned participants to their groups". Comment: study staff assigned patients to a group by picking a sequentially numbered opaque envelope. Adequate type of allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: according to the flowchart there were no patients lost to follow up, furthermore, there were no patients who discontinued the intervention. |
| Selective reporting (reporting bias) | High risk | Comment: the methods section states that a specific outcome (headache) was measured, however this was not reported in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the provider nor the woman was blinded to the treatment regimens". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, considering the provider was not blinded also outcome assessment was probably not blinded. |
Schreiber 2018.
| Methods | Parallel multi‐centre randomised controlled trial | |
| Participants | Women with an anembryonic pregnancy, embryonic or fetal death with a gestational age between five and 12 weeks | |
| Interventions | 200 mg of mifepristone, administered orally, followed by 800 mcg of misoprostol, administered vaginally (mifepristone‐pretreatment group) or 800 mcg of misoprostol alone, administered vaginally (misoprostol‐alone group) | |
| Outcomes | Treatment success (defined as complete expulsion without the need of additional vacuum aspiration within 30 days after treatment) Secondary outcomes reported were rate of vacuum aspiration, blood transfusion, pelvic infection, side effects of medication such as nausea, diarrhoea, headache and fever. |
|
| Funding | Supported by the National Institute of Child Health and Human Development of the National Institutes of Health (Eunice Kennedy Shriver award number R01‐HD0719‐20 [to Dr. Schreiber] and Women’s Reproductive Health Research award number K12‐HD001265‐18 [to Dr. Sonalkar]). | |
| Declarations of interest | Dr. Creinin reports receiving consulting fees from Danco Laboratories. No other potential conflict of interest relevant to this article was reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned in permuted blocks of two to eight, stratified according to trial site, with the use of Research Electronic Data Capture software". |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomly assigned in permuted blocks of two to eight, stratified according to trial site, with the use of Research Electronic Data Capture software." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 women lost to follow‐up in intervention arm, 1 in the control arm. For 2 women, reasons for lost to follow‐up were not mentioned. In 1 women there was a suspicion of caesarean section scar pregnancy |
| Selective reporting (reporting bias) | High risk | Quote: "assessments of quality of life, costs, and biomarkers that predict complete gestational sac expulsion were performed, but the data are not presented here". It is not mentioned if these outcomes are or will be presented elsewhere. |
| Other bias | Low risk | No other bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo was used, therefore blinding was not possible for both personnel and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the initial follow‐up visit, an investigator who was unaware of the treatment‐group assignments assessed the outcome by means of endovaginal ultrasonography. |
Shah 2010*.
| Methods | This was a prospective randomised open‐labelled trial conducted in the Department of Obstetrics and Gynaecology Unit‐III at Civil Hospital Karachi. No information on study duration. | |
| Participants | The inclusion criteria was an ultrasound diagnosis of missed miscarriage < 20 weeks' gestation. | |
| Interventions | Intervention: (n = 25) 400 mcg of misoprostol sublingually every 3 hours for a maximum of 5 doses. Patients having a gestational age of more than 12 weeks whose uterine size was also more than 12 weeks were given 200 mcg of misoprostol instead of 400 mcg in both sublingual and vaginal groups. Control: (n = 25) 400 mcg of misoprostol vaginally every 3 hours for a maximum of 5 doses. Patients having a gestational age of more than 12 weeks whose uterine size was also more than 12 weeks were given 200 mcg of misoprostol instead of 400 mcg in both sublingual and vaginal groups. |
|
| Outcomes | The primary outcome measures were, complete evacuation of POC, mean induction to delivery time and the occurrence of side effects. | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: article states the study was a randomised controlled trial, however there is no information on type of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation was concealed using sealed envelopes, though depending on the randomness, allocation might have been predictable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcomes are presented for all 50 patients. |
| Selective reporting (reporting bias) | Unclear risk | Comment: there seems to be no loss to follow‐up or incomplete data; outcomes were reported for all 50 patients. Table 3 shows 'side effects' without further specification, unclear which side effects were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: there is no information on blinding in the article. Due to the nature of the interventions (sublingual vs vaginal medication), blinding would be difficult. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome seems not to be assessed by an independent doctor. |
Sinha 2018.
| Methods | This study was a parallel double‐blind RCT conducted at University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, from October 2011 to April 2013 | |
| Participants | Women with early pregnancy failure < 12 weeks of gestation. | |
| Interventions | women were randomised to 200mg of oral mifepristone or placebo. 48 Hours later 800mcg of vaginal misoprostol and if necessary 400 mcg misoprostol were given orally at 3‐hourly interval to a maximum of 2 doses in women < 9 weeks by scan and 4 doses in women > 9 weeks by scan similarly in both groups | |
| Outcomes | Primary outcome was complete expulsion within 14 days after start treatment. Treatment success was defined as not needing any surgical intervention. Secondary outcomes were the need for surgical intervention due to heavy bleeding or incomplete expulsion by day 14. Other secondary outcomes were nausea/vomiting, bleeding and treatment acceptability. |
|
| Funding | no funding was mentioned | |
| Declarations of interest | It was stated there were no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sealed packets were numbered from 1 to 92 by simple randomization using computer generated random tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "The third party used to dispense the coded sealed packet to the treating obstetrician". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was one participant lost to follow‐up in both groups. |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐defined outcome measures were reported in the results section |
| Other bias | Low risk | No other bias could be detected. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: this was a placebo‐controlled double‐blind trial. The placebo consisted of tablets of 500 mg calcium who were similarly looking to the tablets of 200 mg mifepristone. Blinding seems adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the outcome was assessed blinded since both caregiver and participant were blinded for the intervention. |
Sonsanoh 2014.
| Methods | A prospective randomised trial was done to 120 healthy pregnant women with early pregnancy failure from August 2012 to August 2013, at the Department of Obstetrics and Gynecology, Chonburi Hospital, Thailand. | |
| Participants | Women with early pregnancy failure, defined as 1) an intrauterine gestational sac with a mean diameter of 25 mm or greater and no visible embryonic pole; 2) an embryonic pole of 5 mm to14 mm with no cardiac activity; and 3) abnormal growth or persistent absence of fetal cardiac activity on a second scan 7‐10 days later(16). In addition, all participants should be over 18 years old. | |
| Interventions | In Group 1 (n = 60), they were given 4 tablets of 200 mcg misoprostol with 2‐3 drops of normal saline placed in the posterior vaginal fornix by digital insertion. In Group 2 (n = 60), 4 tablets of 200 mcg misoprostol were sublingually given. |
|
| Outcomes | Complete abortion; defined as the termination of pregnancy with the complete expulsion of conceptus without the need for surgical intervention or additional misoprostol dose. If the complete abortion did not occur, the repeated induction in the same route would be done every 6 hours for maximum of 3 doses. The treatment was considered a failure if the pregnancy was still continuing after 48 hours from the third dose of misoprostol. Furthermore, adverse effects were measured. | |
| Funding | No information on funding. | |
| Declarations of interest | The authors do not have any conflict of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We used and assigned blocks of four randomizations to two groups of participants" Comment: This does not state how the randomisation list was created. |
| Allocation concealment (selection bias) | Low risk | Quote:"‘Cards labelled with the assigned route were placed in sealed, opaque envelopes which were filled and labelled in accordance with the list of randomizations. The allocation was concealed by the use of sealed number of treatments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: according to table 2 all 120 patients randomised completed the study. 19 patients did not have complete abortion, therefore in table 3 (time‐to‐delivery interval) only 50 and 51 patients in each group are described. This does make sense. |
| Selective reporting (reporting bias) | Low risk | Comment: there are no signs of selective outcome reporting. All outcomes mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding is not described. Due to the nature of the intervention (vaginal vs sublingual medication) blinding would be difficult; nonetheless this might have influenced (perception of) outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the article does not state that outcome assessors were blinded. |
Tang 2003.
| Methods | Randomisation by quote: "computer‐generated random numbers". Queen Mary Hospital, Hong Kong SAR, China. No information on study duration. |
|
| Participants | 80 women with non‐viable pregnancies diagnosed by ultrasound < 13 weeks. | |
| Interventions | Group 1: 600 mcg misoprostol sublingually every 3 hours for maximum of 3 doses (n = 40); group 2: 600 mcg misoprostol vaginally every 3 hours for maximum of 3 doses (n = 40). Women discharged home after completion of treatment and reassessed day 7 ‐ when surgical evacuation performed if gestation sac still present, or retained POC plus heavy bleeding. | |
| Outcomes | Primary outcome: complete miscarriage (defined as no need for surgical evacuation up until return of menstruation). | |
| Funding | No information on funding. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcomes were presented for all 80 patients that were initially randomised. |
| Selective reporting (reporting bias) | Unclear risk | Comment: table 3 shows several side effects. The methods section states only that 'side effects' were measured without further specification. It is unclear if other side effects than the ones presented in table 3 were also measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. Sublingual misoprostol was taken by the patient itself while vaginal misoprostol was administered by a research nurse. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment. Probably not done. |
Tang 2006.
| Methods | Open parallel RCT. Eligible women were randomised according to computer‐generated random numbers into 2 groups. The study was carried out from July, 2002 to January, 2004; Queen Mary Hospital, Hong Kong SAR, China. | |
| Participants | Women with (i) intrauterine gestational sac with a mean sac diameter of ≥ 2 cm without a fetal pole; (ii) presence of a fetal pole with no cardiac pulsation; (iii) the gestational sac was < 2 cm with no interval growth or persistent absence of fetal cardiac pulsation on rescanning 7–10 days later. | |
| Interventions | Women in both groups (total n = 180) received 600 mcg misoprostol sublingually every 3 hours for a maximum of 3 doses (day 1). Additionally, women in group 2 (n = 90) also received 400 mcg misoprostol sublingually daily for a further week (day 2–8). | |
| Outcomes | The outcome of the study was assessed on day 9. A transvaginal ultrasound examination of the pelvis was performed The primary outcome measure was the complete miscarriage rate. The incidence of side effects, duration of vaginal bleeding and the change in haemoglobin level were also studied. | |
| Funding | The work described in this paper was supported by a grant from the Committee on Research and Conference Grants of The University of Hong Kong of the Hong Kong Special Administrative Region, China. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible women were randomized according to computer‐generated random numbers into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: according to the flowchart, there was no loss to follow up and no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: table 3 shows several side effects. The methods section only states that 'side effects' were measured without further specification. It is unclear if the effects mentioned in table 3 were the only side effects that were measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was an open randomized study and both the subjects and the investigators knew the treatment that the women had received". Comment: due to the nature of the interventions blinding was practically impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no information on blinding of outcome assessment available; it seems that outcome was not assessed by an independent doctor. |
Tanha 2010a.
| Methods | Parallel randomised controlled trial. Randomisation using a computer‐generated code. Recruitment took place at Mirza Kochak Khan Hospital, a premier research and referral facility, in Tehran, Iran, from January 2005 through to February 2007. | |
| Participants | (i) intrauterine gestational sac with a mean sac diameter of < 2 cm without a fetal pole; (ii) presence of a fetal pole with no cardiac activity; and (iii) gestational sac < 2 cm with no interval growth or persistent absence of fetal cardiac pulsation on rescanning 7–10 days later. Additional eligibility criteria included having no known contraindications to misoprostol, general good health and no vaginal bleeding. | |
| Interventions | Intervention: 400 mcg tablets every 6 hours sublingually (n = 110). Control: 400 mcg tablets every 6 hours vaginally (n = 110). |
|
| Outcomes | The primary outcome measure was efficacy of the treatment in inducing complete abortion, which was defined as passing of the POC without needing vacuum aspiration or dilatation or curettage; incomplete abortion as expulsion of the fetus but some POC remaining in the uterus, needing evacuation; and missed abortion as a gestational sac in the uterus without cardiac activity on ultrasound examination, needing emptying of the uterus. Success rate was defined as no need for surgical intervention. If a woman from either group did not bleed within 48 hours after completing the protocol, she was requested for a TVS scan. If a gestational sac was still found on TVS examination, surgical evacuation was performed. Other outcome measures were side effects recorded 1 hour up to 24 hours after every administration of misoprostol at the hospital by women after the treatment, until the first follow‐up visit. Side effects were classified as pregnancy‐related, treatment‐related, and those related to the abortion process itself. |
|
| Funding | No information on funding. | |
| Declarations of interest | This study is Dr Mohadeseh Feizi’s postgraduate thesis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: using a computer‐generated code. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study investigator opened the next sequentially numbered randomized envelope to determine the treatment arm. This randomization scheme was created by Population Council staff, using a computer‐generated code". Comment: it is still unclear who put the randomisation scheme in the envelopes and if the envelopes were opaque. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all 220 patients were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the methods section states that 'side effects' were measured without further specification, it is unclear if the side effects presented in the results are the only ones measured. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the investigator nor the woman was blinded to the treatment assignment". Comment: due to the nature of intervention blinding would be practically impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome was not assessed by independent doctors. |
Trinder 2006.
| Methods | Randomised controlled trial comparing medical and expectant management with surgical management of first trimester miscarriage. This was a multi‐centre trial with 7 participating hospitals, each of which had an early pregnancy clinic. Recruitment started in May 1997 and finished in December 2001. | |
| Participants | Women with a pregnancy of less than 13 weeks’ gestation who had been diagnosed as having either an incomplete miscarriage or early fetal/embryonic demise were eligible. | |
| Interventions | Intervention: in the medical management arm, women with an incomplete miscarriage were admitted to hospital and given a single vaginal dose of 800 mcg misoprostol.1200 women with early fetal or embryonic demise were pre‐treated with a single oral dose of 200 mg mifepristone,21 then admitted to hospital 24‐48 hours later for a single vaginal dose of 800 mcg misoprostol (n = 398). Control: women in the expectant management arm were allowed home with no intervention (n = 399). Control: women in the surgical management arm were admitted for surgical suction curettage under general anaesthesia (n = 403). |
|
| Outcomes | Confirmed gynaecological infection at 14 days and 8 weeks; need for unplanned admission or surgical intervention. | |
| Funding | The MIST study was funded by a South and West NHS Executive research and development grant. A donation of £20 000 was accepted from Exelgyn. Neither the NHS Executive nor Exelgyn had any role in the study design; collection, analysis, or interpretation of data; writing of the report; or the decision to submit the paper for publication. | |
| Declarations of interest | The study group accepted a donation of £20 000 from Exelgyn, the manufacturers of mifepristone. The authors have no other competing interests. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was by a central telephone system at the Clinical Trials Services Unit, Oxford. We used minimisation to ensure comparability between women with respect to participating centre, parity, type of miscarriage, and gestation". Comment: this still does not state how the randomisation scheme was generated. |
| Allocation concealment (selection bias) | Low risk | Comment: use of a central telephone system for randomisation, operated by other persons than the doctors randomising the patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: according to the flowchart loss to follow‐up was < 10%. |
| Selective reporting (reporting bias) | Low risk | Comment: flow chart displays all eligible and recruited women. No signs of selective reporting; all outcomes mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: there is no information on blinding of patients and personnel. However, due to the nature of the interventions, blinding would be practically impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no information on blinding of outcome assessment, probably not done. |
Wood 2002.
| Methods | Computer‐generated random number list in blocks. Pharmacy prepared numbered envelopes. Tablets not identical so placed by nurse in opaque vaginal introducer for physician to insert ‐ to maintain allocation concealment. Department of Obstetrics and Gynecology, University of Calgary, Calgary, Alberta, Canada; between February 1999 and April 2000. |
|
| Participants | 50 women with ultrasound diagnosed non‐viable pregnancies. Gestational age 7‐17 weeks but women not included if fetal size by ultrasound > 12 weeks equivalent. Also excluded from recruitment if experiencing uterine cramping or bleeding. | |
| Interventions | Misoprostol (800 mcg vaginally) (n = 25) or vaginal placebo (n = 25). If complete miscarriage not suspected after 24 hours, treatment was repeated. At 48 hours, if no miscarriage or miscarriage thought to be incomplete, uterine curettage was offered. | |
| Outcomes | Sample size based on reduction of uterine curettage from 50% to 10%. Women's satisfaction also assessed, but are not included in analyses as data not reported from control group. | |
| Funding | This work was supported by a grant from the Office of the Associate Dean of Research, Faculty of Medicine, University of Calgary. | |
| Declarations of interest | No information on conflicts of interest. | |
| Notes | Analysis by intention‐to‐treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated random number list in blocks. |
| Allocation concealment (selection bias) | Low risk | Comment: pharmacy prepared numbered envelopes. Tablets not identical so placed by nurse in opaque vaginal introducer for physician to insert ‐ to maintain allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: it seems that all patients completed the study. Outcomes were presented for all patients. |
| Selective reporting (reporting bias) | Low risk | Comment: no signs of selective reporting. Outcome measures mentioned in the methods section were presented in the results section. |
| Other bias | Low risk | No other source of bias could be detected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: tablets not identical so placed by nurse in opaque vaginal introducer for physician to insert ‐ to maintain allocation concealment. This assures blinding of patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no information on blinding of outcome assessment. Considering that there was blinding of the physician treating the patient (by the use of a opaque vaginal introducer with either misoprostol or placebo) probably the physician was also blinded for outcome assessment. |
AP diameter: anterior‐posterior diameter bHCG: beta human chorionic gonadotrophin CRL: crown‐rump length ERPC: evacuation of retained products of conception hCG: human chorionic gonadotropin IM: intramuscular IU: international units IUFD: intrauterine fetal death mcg: microgram mm: millimetre MTX: methotrexate POC: products of conception RCT: randomised controlled trial TVS: transvaginal sonography vs: versus
µL: microlitre
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbas 2018 | Participants do not meet inclusion criteria (includes women undergoing termination of pregnancy for other reasons than non vital pregnancies, and up to a GA of 27 weeks). |
| Abd‐El‐Maeboud 2012 | Termination of 'viable' pregnancies; the intervention is priming before medical treatment and not the treatment itself. |
| Abdel Fattah 1997 | Conference abstract. No information about GA but, given title, probably includes pregnancies > 24 weeks as well as < 24 weeks. |
| Al‐Bdour 2007 | Quasi‐randomised trial, patients assigned to treatment according to military ID number. |
| Ali 2018 | Different topic, study includes women induced with balloon catheters and not with medication. |
| Almog 2005 | Termination of 'viable' pregnancies ‐ mainly with fetal anomalies. |
| Altaf 2006 | Not a randomised study. No subgroup analysis with only patients with missed abortion and GA < 24 weeks. |
| Amjad 1999 | Other subject; ‘priming’ of cervix while Foley catheter in situ. |
| Anderman 2000 | Conference abstract. Includes pregnancies > 24 weeks as well as < 24 weeks. |
| Anderson 2009 | Conference abstract. Duration of pregnancy unclear. |
| Ara 2009 | Conference abstract. |
| Arellano 2009 | Conference abstract on other subject. Treatment of incomplete abortion. |
| Avila‐Vergara 1997 | Intrauterine deaths mainly third trimester. |
| Aye 2017 | Conference abstract, further results not published. It is not clear if also women with incomplete miscarriage were included in this study. |
| Azra 2007 | Termination of pregnancies for congenital malformations as well as non‐viable pregnancies. No subgroup analyses. |
| Bagratee 2009 | Conference abstract on other subject. Predictive/etiologic study, size of RPOC as predictor of successful treatment. |
| Bani‐Irshaid 2006 | Other subject (TOP); no subgroup analysis of women with GA < 24 weeks. |
| Bartz 2013 | Other subject, randomised trial of 2 methods for dilatation of the cervix before surgical evacuation. |
| Bebbington 2002 | Termination of viable pregnancies. |
| Behrashi 2008 | Includes patients with GA < 24 weeks and > 24 weeks; and patients with 'viable' pregnancies. No subgroup analyses performed. We tried to contact the authors by e‐mail but they could not be reached. |
| Behrashi 2010 | Not a publication of study results but a registration of a RCT in Iranian Trial Register. |
| Ben‐Meir 2009 | RCT comparing priming with misoprostol vs placebo before oxytocin induction. Patients with GA > 24 weeks included. |
| Betstadt 2007 | Registration of trial protocol, no results published. Author was contacted, stated that the trial was stopped prematurely because of a lack of participants. |
| Bique 2007 | Trial concerning treatment of incomplete abortion. |
| Biswas 2007 | Termination of pregnancy because of various reasons, no subgroup analyses on patients with missed miscarriage or early fetal death. We tried to contact the authors but could not reach them. |
| Blohm 2005 | Includes patients with incomplete (ongoing) miscarriage (with gestational residue between 15 mm to 50 mm). |
| Brouns 2010 | Trial also includes patients with legal termination of viable pregnancies. We contacted the authors to ask for subgroup analyses of only patients with non‐viable pregnancies; but the original data were not accessible to them anymore. |
| Cabrol 1990 | Trial of mifepristone for induction of labour after intrauterine death ‐ but mainly late second and third trimester pregnancies. |
| Caliskan 2005 | Includes all patients with indication for termination of pregnancy; but does not state which indications are meant. We tried to contact the authors but could not reach them. |
| Caliskan 2009 | Other subject (termination of pregnancy). |
| Chaudhuri 2015 | Reference of trial registration. Results were published in 2015. Study participants included women with second and third trimester intrauterine fetal death. No subgroup analyses for GA < 24 weeks. |
| Chowdhury 2012 | Conference abstract. No information on GA. |
| Clevin 2001 | Abstract in Danish. A prospective, randomised study carried out to clarify the effect of vaginal administration of a prostaglandin E1 analogue (gemeprost) versus surgical management (curettage) on miscarriages at up to 12 weeks of gestation. 3 groups: 1 (n = 27), 2A (n = 17) and 2B (n = 17), allocated according the endometrial thickness. The measured outcomes were reduction of endometrial thickness, duration of vaginal bleeding and pain, reported in a non‐suitable format for analysis. |
| Dabash 2009 | Conference abstract, other subject (treatment of incomplete abortion). |
| Dao 2007 | Other subject (treatment of incomplete abortion). |
| Das 2014 | Other subject; treatment of incomplete miscarriage. |
| David 2003 | Randomised trial (details of randomisation unclear) of 2 methods to soften the cervix before surgical evacuation for early non‐viable pregnancies. No usable clinical data, given short timescale between treatment and surgery. |
| David 2005 | Other subject (cervical priming before surgical evacuation). |
| Demirezen 2018 | The participants in this study do not meet the inclusion criteria for this review (gestational age up to 28 weeks, and termination of both vital and non vital pregnancies). The intervention studied (induction with different type of balloon catheter) does not meet the inclusion criteria for this review. |
| Dickinson 1998 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 14 and 28 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Dickinson 2002 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 14 and 30 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Dickinson 2003 | Randomised trial comparing oral with vaginal administration of misoprostol to terminate pregnancies with fetal malformations ‐ not non‐viable pregnancies. |
| Diop 2009 | Other subject; treatment of incomplete abortion. |
| El Sokkary 2016 | Unclear up to which GA patients were included and if there were subgroup analyses made for patients with GA eligible for this review. Furthermore, unclear what type of randomisation was used and therefore if this truly was a randomised controlled trial. We tried to contact the author but there was no response. |
| Elami‐Suzin 2013 | Trial included also patients with therapeutic abortion; no subgroup analysis on only missed miscarriage other than 1 remark in text (time until expulsion shorter than therapeutic abortion'; but that is not an outcome in our review). Furthermore, all women underwent curettage after medication, so it would be impossible to draw conclusions about the primary outcome in the review (complete evacuation) because it would be unclear whether the uterus was empty because of the medication or because of the curettage. |
| Elhassan 2008 | Includes patients with GA up to 28 weeks. We e‐mailed the authors to ask for a subgroup analysis of patients with GA < 24 weeks, but they did not respond. |
| Eppel 2005 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 14 and 23 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Eslamian 2007 | Study group also includes patients with maternal medical disorders, TOP because of congenital malformations and PPROM. We contacted the authors: there were no subgroup analyses of only patients with fetal demise. |
| Fadalla 2004* | Women included in this trial had a GA 13‐28 weeks, no subgroup analysis for GA < 24 weeks was available |
| Feldman 2003 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 14 and 23 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Fernlund 2018 | Includes women with ongoing miscarriage (vaginal blood loss in combination with a sonographically diagnosed non vital first trimester pregnancy). |
| Fiala 2005 | Other subject (pain medication in requested abortion for socio‐economic reasons). |
| Ghorab 1998 | Trial included women with fetal malformations for pregnancy termination, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Gonzalez 2001 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 14 and 23 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Grimes 2004 | Trial included women with other reasons for pregnancy termination, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Gronland 2002 | Not a randomised trial. 3‐centre study of women with non‐viable pregnancies comparing 3 treatment regimens: misoprostol, mifepristone + misoprostol, surgical evacuation ‐ with treatment regimen changing at each hospital every 4 months. |
| Guix 2005 | Trial includes patients seeking termination of pregnancy because of congenital malformations. |
| Halimi 2004 | Trial includes patients with termination of pregnancy because of fetal demise or congenital malformations, up to GA of 28 weeks. No subgroup analyses available. |
| Hassan 2007 | Quasi‐randomised trial; other subject (treatment of incomplete abortion). |
| Hausler 1997 | Prospective RCT evaluating 3 interventions for complete spontaneous abortion. Diagnosis was based on positive pregnant test, vaginal bleeding and/or evacuation of tissue from the vagina, a closed uterine orifice with only slight bleeding on admission and a possible clear sonographic pregnancy diagnosis in the history. Interventions: A) n = 15 curettage; B) n = 20 only controlled and; C) n = 15 additionally treated for 10 days with an oral hormone intake of 2 mg norethisterone acetate and 0.01 mg ethinyl oestradiol 3 x day. Randomisation by sealed unmarked envelopes. 63 patients were included in the study and allocated randomly to each group. 13 women (20.6%) were excluded from the study after randomisation: 10 did not report for the planned follow‐up control, 1 did not report for curettage, in 1 the height of the endometrium was > 8 mm and in 1 an ectopic pregnancy was diagnosed 6 days after the randomisation. The study only presents outcomes, in a non‐suitable format, regarding hCG clearing time and duration of the secondary haemorrhage from the day of randomisation. |
| Heard 2002 | Conference abstract. Unclear what type of randomisation; 12 patients were assigned to group A and 21 to group B which seems odd in cases of 1:1 and even in case of 1:2 randomisation; no further information on methodology. No full article for this trial found. |
| Herabutya 1997a | Includes patients with all GA; no subgroup analyses of only patients with GA < 24 weeks; authors could not be reached for further clarification. |
| Herabutya 2005 | RCT of misoprostol for terminating viable pregnancies. |
| Hidar 2001 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 13 and 29 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Hidar 2005 | Trial includes patients with GA > 29 weeks and patients with TOP because of congenital anomalies or PPROM. We contacted the authors: there were no subgroup analysis available of only patients with intrauterine fetal death. |
| Hill 1991 | Trial includes fetal deaths in both second and third trimesters. |
| Hinshaw 1993 | Henshaw 1995: conference abstract. No subgroup analysis of randomised proportion (trial was partly randomised and partly treatment according to patients preference). Hinshaw 1993: interim results of partially randomised trial; no subgroup analysis on randomised patients, full results in other article. Hinshaw 1995: interim results of partially randomised trial; no subgroup analysis on randomised patients, full results in other article. Rispin 1993: conference abstract concerning study protocol of ongoing study; no results presented. |
| Hogg 2000 | Abstract. Trial included women with other reasons for pregnancy termination, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Hombalegowda 2015 | Conference abstract. No article with full results found. We tried to contact the authors to ask for such an article but could not reach them. |
| Hughes 1996 | Cost‐effectiveness analysis of previous study that included patients with incomplete miscarriage (no subgroup analysis on patients with fetal demise); partly randomised trial. We contacted authors for subgroup analysis on RCT patients with fetal demise, however they did not respond. |
| Imran 2010 | Includes patients with GA > 24 weeks and TOP because of congenital malformations. We tried to contact the authors to ask for subgroup analyses but they did not respond. |
| Islam 2006 | Not randomised; patients were divided in 2 equal groups. Trial included patients seeking TOP because of congenital malformations; no subgroup analysis on patients with fetal demise. |
| Jabir 2009a | Conference abstract. Other subject (cervical dilation before surgical evacuation). |
| Jabir 2009b | Conference abstract. Other subject (cervical preparation 3 hours before surgical evacuation). |
| Jain 1994 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 12 and 22 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Jain 1999 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 12 and 22 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Johnson 1997 | RCT evaluating pain and bleeding and comparing surgical to medical treatment. Surgical arm (n = 12) uterine curettage under general anaesthesia. Medical arm (n = 17) include 3 different participant conditions and treatments: a) no treatment if women had a complete abortion and uterine cavity echo (myometrium) less than 15 mm; b) women with incomplete abortion: 1 mg pessary of gemeprost (Cervagem, May and Baker) and remained in hospital for 4 hours or until they had passed POC; and c) women with intact gestational sac (but non‐viable fetus) 200 mg RU 486 (mifepristone) and then allowed home, readmitted 36‐48 hours later for 1 mg of vaginal Cervagem. Data from each subgroup in the medical arm are not separated. The sample size is too small to detect any difference among such number of groups. |
| Kamal 2005 | Quasi‐experimental study, no RCT. Includes patients with GA > 24 weeks and with TOP because of maternal or fetal reasons. |
| Kanhai 1989 | Includes both second and third trimester fetal deaths. |
| Kapp 2007 | Quasi‐randomised trial; trial includes patients seeking termination of pregnancy, indication for termination unclear. |
| Khosravi 2017 | Trial registration, includes women with termination of first trimester pregnancies for early fetal demise as well as termination on maternal indication. |
| Kong 2013 | Trial includes also patients with incomplete miscarriage. There is 1 sentence in results section that provides success rates for only patients with silent miscarriage ("Focussing on women who were diagnosed to have silent miscarriage at recruitment, complete miscarriage rate after surgical treatment, medical evacuation and expectant management was 97.7%, 63% and 62.5%, respectively"); but when these percentages are used to calculate the number of patients with successful treatment using the number of study participants in each group (49 surgical, 46 medical and 25 expectant management; see table 1) the outcomes are impossible. So it looks like either the percentages are not right, or not all patients with missed miscarriage were analyses. Unfortunately for this group there was no specific information on missing data. |
| Kurshid 2010 | Trial includes patients wit indication for TOP because of IUFD, congential malformations, PPROM. We tried to contact the authors for subgroup analyses on only patients with IUFD, but could not reach them. |
| Kyaw 2015 | Conference abstract. No information on method of randomisation. Authors could not be reached for further clarification. |
| Linn 2015 | Conference abstract. Trial includes patients with GA > 24 weeks; no subgroup analysis of only patients with GA < 24 weeks available. |
| Lippert 1978 | Second and third trimester fetal deaths. Not obviously randomised. |
| Lu 2014 | Article in Chinese, after translation signs of weak methodology: no exact description of dosages of medication. Furthermore no information on type of randomisation. |
| Lughmani 2008 | Conference abstract. Unclear if time span between treatment is too short (looks like surgical evacuation is performed within 12 hours after misoprostol treatment). Authors could not be reached for further clarification. |
| Machtinger 2004 | Abstract. Appears to include both non‐viable pregnancies and miscarriages. |
| Mahjabeen, 2009 | Quasi‐randomised trial. Includes patients with therapeutic TOP, unclear what indication for this TOP was. |
| Makenzius 2017 | Trial that compares miscarriage care by midwife to care by physician; other topic. |
| Makhlouf 2003 | Not clear from paper if all pregnancies complicated by fetal death. Seeking clarification from authors. |
| Martin 1965 | Allocation based on alternation, not randomisation. Alternation violated. |
| Montesinos 2011 | Wrong patient population ‘incomplete abortion’. |
| Moran 2005 | Other topic (treatment of pregnancy of unknown location). |
| Mostafa‐Gharebaghi 2010 | Trial includes patients with termination of pregnancy because of fetal death, congenital malformations, PPROM and 'other causes'. We tried to contact the authors for subgroup analyses on only patients with fetal death, but could not reach them. |
| Mulayim 2009 | Other subject (misoprostol after surgical treatment for miscarriage). |
| Naghshineh 2015 | Trial included women with spontaneous miscarriage (non‐viable pregnancy) < 17 weeks as well as induced abortion. No subgroup analyses for spontaneous miscarriage only. |
| Nakintu 2001 | Both second and third trimester fetal deaths. Seeking separate data from author. |
| Nasreen 2009 | Conference abstract. Trial includes patients with incomplete miscarriage. |
| Nassar 2006 | Reference is trial registration. Trial was ended prematurely because of difficulties in recruitment of patients. |
| NCT02141555 | Reference of trial registration. According to the trial register the current recruitment status is unknown, last updated in 2014. We did not find any published results. |
| NCT02573051 | Reference of trial registration. According to the trial register the current recruitment status is unknown, last update in October 2015. We could not find any published results. |
| Ng 2015 | Wrong patient population‐‘incomplete abortion’. |
| Ngai 2001 | Includes data on women with both non‐viable pregnancies and incomplete miscarriages. If these data can be separated by the researchers, these data may be included in the future. |
| Nguyen 2005 | Other subject; treatment of incomplete abortion. |
| Niinimaki 2006 | Trial also includes patients with incomplete miscarriage. We contacted the authors to ask for subgroup analyses on only patients with missed miscarriage and anembryonic gestation, however they did not respond. |
| Nor 2006 | Other subject (termination of pregnancy; indication unclear), trial includes patients up to GA of 26 weeks; no subgroup analysis on patients with GA < 24 weeks. |
| Nuthalapaty 2005 | Includes patients with induction because of congential malformations or maternal indications. 1 of the outcome measures was live birth rate (?). We tried to contact the authors for further clarification but could not reach them. |
| Nuutila 1997 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 12 and 24 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Owen 1999 | Trial included women with fetal malformations and maternal indications for pregnancy termination between 16 and 24 weeks, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Paraskevaides 1992 | Small study of 16 women "randomised" to surgical evacuation or prostaglandin F2alpha or Trilostane treatment. No details about clinical presentation or ultrasound and clinical findings, but from abstract includes both women with non‐viable pregnancies and incomplete miscarriage. |
| Paritakul 2010 | Wrong patient population‐‘incomplete abortion’. |
| Patua 2013 | Other subject, treatment of incomplete miscarriage. |
| Perry 1999 | Excluded women with fetal deaths. |
| Piotrowski 1979 | Not clear that this was a randomised trial. |
| Pongsatha 2004 | Trial excluded women with fetal deaths. |
| Prasartsakulchai 2004 | Quasi‐randomised: patients could choose for medical, surgical or expectant management. Only patients who chose medical management were further randomised. However patients did not meet inclusion criteria for the review, as they already experienced abdominal pain and vaginal bleeding, e.g. ongoing miscarriage, which is beyond the scope of this review. |
| Promwangkwa 2017 | Participants in this study had a gestational age 14‐24 weeks. Indications for termination of pregnancy included intra uterine fetal demise, but also termination of pregnancy of live fetus for other fetal and maternal indications. No subgroup analyses were made for IUFD up to 20 weeks of gestation. |
| Rahimi‐Sharbaf 2015 | Trial studies women with termination of pregnancy with GA 13‐24 weeks because of congenital of maternal indications. No subgroup analyses were performed for only women with IUFD. |
| Ramadan 2009 | Conference abstract. Other subject, incomplete abortion. |
| Ramsey 2004 | Trial included women with other reasons for pregnancy termination, as well as pregnancies with fetal death. We tried to contact the authors but could not reach them. |
| Reeves 2006 | Other subject (endometrial thickness as predictor for further intervention); no subgroup analyses on only patients with missed abortion. |
| Reeves 2008 | Other subject (endometrial thickness as predictor for further intervention); no subgroup analyses on only patients with missed abortion. |
| Rivero‐Lopez 1998 | Other subject; cervical priming before intervention. |
| Robledo 2007 | Other subject; predictive study (to identify indicators for success of misoprostol treatment). |
| Roy 2003 | Abstract. Not clear if fetal death included as indication for termination. |
| Ruangchainikhom 2006 | Other subject (termination of pregnancy because of obstetric reasons). Full data unavailable. |
| Saeed 2018 | This trial meets all inclusion criteria. However data extraction was not possible. The table presenting the main results contained numbers of unknown origin. It was unclear whether percentages or number of participants were displayed. The numbers in this table did also not correspond with the main text, attributing to further doubt as to what the numbers in the table represent. |
| Salamalekis 1990 | Abstract only. Treatment allocation by alternation, not by randomisation. |
| Salari 2012 | Conference abstract. Other subject (other patient population); therapeutic abortion. |
| Shaheen 2017 | In this trial women were not adequately randomised. The paper describes a quasi randomised trial with women being "divided into two groups". |
| Shaikh 2008 | Conference abstract. No subgroup analysis on missed miscarriage. |
| Shelley 2005 | Other subject; treatment of incomplete or ongoing miscarriage. |
| Shobeira 2007 | Conference abstract. No article with full study results found. Authors could not be reached to ask for such an article. |
| Shochet 2012 | Other subject (incomplete abortion). |
| Shokry 2009 | Other subject, other intervention (reduction of bleeding after surgical evacuation). |
| Shuaib 2013 | The type of randomisation is unclear. It seems that both groups had different types of follow up, especially for the surgically treated group it is unclear if they really all had successful outcome (for example: no information on ultrasound follow‐up). Weak methodology, high risk of bias on all fronts. |
| Shwekerela 2007 | Other subject (reduction of bleeding after surgical evacuation). |
| Smith 2006a | This was a qualitative study. No numeric comparison between the groups. Furthermore, study group includes women with an incomplete miscarriage; no subgroup analyses were performed for only patients with missed miscarriage. |
| Smith 2009 | Study includes also patients with incomplete miscarriage. There was no subgroup analysis available for only patients with a non‐viable pregnancy. |
| Srikhao 2005 | Since patients participating in this study already experienced vaginal blood loss and abdominal pain this is considered ongoing or incomplete miscarriage; therefore this study is not eligible for the review. |
| Sripramote 2000 | Other subject; cervical priming before surgical evacuation. |
| Stockheim 2006 | The data presented in this trial were reciprocal. It is not valid to present reciprocal data for outcomes from trials because they are not reported in the way we have specified the review. This study was therefore not included in this review. |
| Su 2005 | Termination of pregnancy for fetal anomalies, social reasons or maternal disease; not for non‐viable pregnancies. |
| Suchonwanit 1999 | Abstract of residents research paper. No article with full study results found; author could not be reached to ask for such an article. |
| Surita 1997 | Abstract only. May include third trimester fetal deaths. |
| Tam 2005 | Study investigating reproductive outcome after miscarriage treatment; patients were included in a previous trial. This previous trial was not retrieved from the search, but was identified screening the reference list of an excluded study; this trial also included patients with incomplete miscarriage. There were no subgroup analyses available for only patients with a non‐viable pregnancy. |
| Tanha 2013 | Unclear whether all patients meet inclusion criteria for review, it seems like also patients with legal abortion or TOP because of congenital malformations were included. We tried to contact the authors for further clarification but could not reach them. |
| Taylor 2011 | Other subject; treatment of incomplete abortion. |
| Thavarasah 1986 | Unclear from paper but allocation may have been by alternation. We tried to contact the authors but could not reach them. |
| Thida 2015 | Conference abstract. We searched for full study results but could not find them. We tried to contact the authors to ask if there is an article with study results published, but could not reach them. |
| Toppozada 1994 | Includes third trimester fetal deaths. |
| Toptas 2011 | Conference abstract. No subgroup analysis of only patients with termination because of IUFD. Authors could no be reached for further clarification. |
| Torre 2012 | Trial also includes patients with incomplete miscarriage. We tried to contact the authors for subgroup analysis on patients with missed miscarriage, but they did not respond. |
| Van Mensel 2009 | Trial includes patients with GA > 24 weeks. We tried to contact the authors to ask for subgroup analyses on patients with GA < 24 weeks; but they did not respond. |
| Yapar 1996 | Includes indications for termination other than fetal death. High degree of protocol violation (60/400). Results not presented as intention‐to‐treat. |
| Yilmaz 2005 | Other subject; termination of pregnancy because of congenital or chromosomal abnormalities. |
| Yilmaz 2007 | Other subject; termination of pregnancy because of congenital/chromosomal abnormalities. |
| Zanganeh 2012 | Other subject; termination of pregnancy because of fetal or maternal problems. |
| Zhang 2000 | Seems to be a trial about cervical priming before delivery. Outcome measures irrelevant for this review. |
| Zhang 2005 | Includes both non‐viable pregnancies and miscarriages. We tried to contact the authors to retrieve data on non‐viable pregnancies only, but we could not reach them. |
GA: gestational age hCG: human chorionic gonadotropin IUFD: intrauterine fetal death mg: milligram mm: millimetre POC: products of conception PPROM: preterm premature rupture of membranes RCT: randomised controlled trial RPOC: retained products of conception TOP: termination of pregnancy
Characteristics of ongoing studies [ordered by study ID]
ACTRN12615000483550.
| Trial name or title | Buccal versus vaginal (200 microgram) misoprostol for second trimester abortion termination |
| Methods | Clinical randomised trial to compare efficacy and safety of vaginal and buccal misoprostol in second trimester abortion due to intrauterine fetal death |
| Participants |
|
| Interventions | The study had 2 treatment groups: group I received a dose of misoprostol (200 μg) (1 tablet of Misotac 200 μg; Sigma co., Cairo, Egypt) every 4 hours buccally (and the patient was instructed not to swallow it for 1 hour) till expulsion of the fetus for maximum 24 hours. Group II received a dose of moistened misoprostol (200 μg) (1 tablet of Misotac 200 ug; Sigma co., Cairo, Egypt) every 4 hours vaginally (tablet was put into the posterior fornix) till expulsion of the fetus for maximum 24 hours. |
| Outcomes | The primary outcome measure is the induction interval, the time from the initial misoprostol dose until complete fetal expulsion. Incidence of side effects of misoprostol (such as nausea, vomiting, fever, chills, diarrhoea, tachycardia, and headache) Number of misoprostol doses |
| Starting date | 17/07/2012 |
| Contact information | Dr Mohammad Sayed Abdellah; msayed21@yahoo.com |
| Notes | Last patient should have been included in 2013. It seems that the results have not been published (yet); no publications by the mentioned authors regarding this randomised controlled trial were retrieved in our extensive search. |
Ali 2017.
| Trial name or title | Vaginal misoprostol in management of first trimester missed abortion |
| Methods | Randomised, parallel assignment, open‐label trial |
| Participants | Inclusion criteria
|
| Interventions | Vaginal misoprostol (800 µg x 2 doses 3 hours) versus buccal/sublingual misoprostol (200 µg x 6 doses 4 hours) |
| Outcomes | Not specified |
| Starting date | Not yet recruiting |
| Contact information | Dr Mohammed Khairy Ali, Assiut University |
| Notes |
El Shahawy 2016.
| Trial name or title | Sublingual versus vaginal misoprostol in medical treatment of first trimestric missed miscarriage |
| Methods | Single‐blind, randomised, parallel‐assignment trial |
| Participants | Inclusion criteria
|
| Interventions | Sublingual misoprostol versus vaginal misoprostol |
| Outcomes | Primary outcome: completeness of abortion (expulsion of products of conception by visual inspection |
| Starting date | January 2016 |
| Contact information | Ahmed Abdel Shafy El Shahawy, Ains Sham University |
| Notes |
NCT02620904.
| Trial name or title | Mifepristone induction for fetal demise, a randomised control trial |
| Methods | Double‐blinded, randomised controlled trial with 1:1 allocation of mifepristone or placebo at initiation of induction of labour for fetal demise 20 weeks estimated gestational age or greater. |
| Participants | Inclusion criteria
|
| Interventions | Interventional arm: ingest 200 mg tablet of mifepristone orally. Control arm: ingest a placebo tablet orally with similar physical properties. |
| Outcomes | Time to delivery of fetus [time frame: from the initiation of medical therapy for induction to delivery of fetus] |
| Starting date | February 2016 |
| Contact information | Montefiore medical centre, principal investigator: Jessica Atrio, MD, jatrio@montefiore.org |
| Notes |
NCT02633761.
| Trial name or title | Mifepristone and misoprostol versus misoprostol alone for treatment of fetal death at 14‐28 weeks of pregnancy: a randomised, placebo‐controlled double‐blinded trial |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Inclusion criteria
|
| Interventions | Active comparator group 1: 200 mg mifepristone followed in 24 hours by repeated doses of 200 μg: buccal misoprostol given every 3 hours Placebo comparator group 2: placebo followed in 24 hours by 200 μg: buccal misoprostol given every 3 hours. |
| Outcomes | Complete uterine evacuation of the fetus and placenta without surgical intervention [time frame: 48 hours] Complete uterine evacuation of fetus and placenta using study drug alone without recourse to any additional surgical intervention |
| Starting date | April 2015 |
| Contact information | Hillary Bracken, PhD; hbracken@gynuity.org |
| Notes |
NCT03212352 2017.
| Trial name or title | Comparing two medical treatments for early pregnancy failure |
| Methods | Double‐blind placebo‐controlled randomised trial ‐ parallel group assignment |
| Participants | Women with ultrasonographically confirmed early pregnancy failure (6‐14 weeks postmenstrual), managed expectantly for at least 1 week |
| Interventions | Oral mifepristone (600 mg) or oral placebo |
| Outcomes | Primary outcome: complete evacuation 6 weeks after initial treatment (whether or not complete evacuation (total endometrial thickness < 15 mm) will be assessed through ultrasonography |
| Starting date | June 27 2018, estimated primary completion date = January 1 2020 |
| Contact information | Charlotte C Hamel l.hamel@cwz.nl 0031243658750 Marcus P Snijders m.snijders@cwz.nl 0031243658750 Radboud University, The Netherlands Collaborators: Innovatiefonds Zorgverzekeraars, Canisius‐Wilhelmina Hospital |
| Notes |
mg: milligram mm: millimetre μg: microgram
Differences between protocol and review
The protocol for this review aimed to include both trials for treatment of both ultrasound‐diagnosed, non‐viable pregnancies and incomplete miscarriage. For the reasons described in the review, two separate reviews now address these topics ‐ thus, the change in title from 'Medical management for miscarriage' to ‘Medical treatment for early fetal death (less than 24 weeks)’.
In this update, the evidence has been assessed for quality using the GRADE approach and 'Summary of findings' tables have been incorporated.
Several subgroup analyses that were not prespecified have been performed because there were subgroups of clinical interest. These included the following.
For comparison 1: vaginal misoprostol versus placebo; primary outcome complete miscarriage:
complete miscarriage less than one day;
complete miscarriage less than two days;
complete miscarriage less than seven days.
For comparison 6: vaginal misoprostol wet versus dry preparations: primary outcome complete miscarriage:
complete miscarriage less than three days;
complete miscarriage less than eight days;
complete miscarriage less than 15 days;
complete miscarriage less than 30 days.
For comparison 8: vaginal misoprostol plus laminaria tents versus vaginal misoprostol alone: primary outcome complete miscarriage:
complete miscarriage less than one day;
complete miscarriage less than two days.
For comparison 18: buccal misoprostol lower versus higher regimen: primary outcome complete miscarriage 13 to 23 weeks:
complete miscarriage less than one day;
complete miscarriage less than two days.
For comparison 19: mifepristone versus placebo: primary outcome complete miscarriage:
complete miscarriage less than two days;
complete miscarriage less than three days;
complete miscarriage less than four days;
complete miscarriage less than five days.
In the protocol "pain relief" was determined as outcome. However, various articles had different ways to assess pain relief or pain. We therefore added an extra definition to this outcome to further specify.
in the 2018 update, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports.
Contributions of authors
Marike Lemmers: screening for in/exclusion, data extraction, data analyses, quality of evidence assessment, completing first draft, and further revisions of the updated review.
Marianne Verschoor: review update protocol development, screening for in/exclusion, data extraction, data entry, analyses, quality of evidence assessment, assisting first draft of the updated review.
Bobae Kim: data extraction, data entry, quality of evidence assessment, revisions to first draft of the updated review.
Martha Hickey: original protocol development and revisions to the first draft of the original review.
Juan Vazquez: original protocol development and revisions to first draft of the original review.
Ben Willem Mol: supervision of review update protocol development, supervision of data extractions, data entry and analyses, revisions to first draft of the updated review.
Jim Neilson: supervision of original protocol development; completion of first draft of original review. Supervision of total process of preparing the updated review.
Sources of support
Internal sources
America Arias Hospital, Havana, Cuba.
The University of Liverpool, UK.
Academic Medical Centre, Amsterdam, Netherlands.
External sources
HRP/WHO, Geneva, Switzerland.
Declarations of interest
Marike Lemmers: for previous work (the MisoREST trial), which focuses on miscarriage treatment, my institution (AMC) received a ZonMW grant. ZonMW is a Dutch governmental organization for Health Research and Development.
Marianne AC Verschoor: my institution linked received a ZonMW grant for the MisoREST study.
Bobae Veronica Kim: none known.
Martha Hickey: none known.
Juan C Vazquez: none known.
Ben Willem J Mol: my institution and I have received payment for consultancy from ObsEva Geneva. I have received payment for review preparation from Eur J Obste Gynaecol and I have received travel/accommodation/meeting expenses for various non‐commercial scientific meetings.
James P Neilson: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abediasl 2016* {published data only}
- Abediasl Z, Sheikh M, Pooransari P, Farahani Z, Kalani F. Vaginal misoprostol versus intravenous oxytocin for the management of second‐trimester pregnancies with intrauterine fetal death: a randomized clinical trial. Journal of Obstetrics and Gynaecology Research 2016;42(3):246‐51. [DOI] [PubMed] [Google Scholar]
Al Inizi 2003 {published data only}
- Al Inizi SA, Ezimokhai M. Vaginal misoprostol versus dinoprostone for the management of missed abortion. International Journal of Gynecology & Obstetrics 2003;83:73‐4. [DOI] [PubMed] [Google Scholar]
Autry 1999 {published data only}
- Autry A, Jacobson G, Sandhu R, Isbill K. Medical management of non‐viable early first trimester pregnancy. International Journal of Gynecology & Obstetrics 1999;67(1):9‐13. [DOI] [PubMed] [Google Scholar]
Ayudhaya 2006 {published data only}
- Ayudhaya OP, Herabutya Y, Chanrachakul B, Ayuthaya NI, O‐Prasertsawat P. A comparison of the efficacy of sublingual and oral misoprostol 400 microgram in the management of early pregnancy failure: a randomized controlled trial. Journal of the Medical Association of Thailand 2006;89(Suppl 4):S5‐S10. [PubMed] [Google Scholar]
Bagratee 2004 {published data only}
- Bagratee JS, Khullar V, Regan L, Moodley J, Kagoro H. A randomized controlled trial comparing medical and expectant management of first trimester miscarriage. Human Reproduction 2004;19:266‐71. [DOI] [PubMed] [Google Scholar]
Bracken 2014* {published data only}
- Bracken H, Ngoc NT, Banks E, Blumenthal P, Derman R, Patel A, et al. Misoprostol for treatment of intrauterine fetal death at 14‐28 weeks of pregnancy. American Journal of Obstetrics and Gynecology 2013;208 (1Suppl):S62‐3. [DOI: 10.1016/j.contraception.2013.11.014; CRSREF: 3292934] [DOI] [Google Scholar]
- Bracken H, Ngoc NT, Banks E, Blumenthal PD, Derman RJ, Patel A, et al. Buccal misoprostol for treatment of fetal death at 14‐28 weeks of pregnancy: a double‐blind randomized controlled trial. Contraception 2014;89(3):187‐92. [DOI] [PubMed] [Google Scholar]
- Winikoff B. Misoprostol for treatment of fetal death at 14‐28 weeks of pregnancy, inclusive, not accompanied by complete expulsion of the contents of the uterus. clinicaltrials.gov/ct2/show/record/NCT00671060 (first received 29 April, 2008).
Chittacharoen 2003* {published data only}
- Chittacharoen A, Herabutya Y, Punyavachira P. A randomized trial of oral and vaginal misoprostol to manage delivery in cases of fetal death. Obstetrics & Gynecology 2003;101:70‐3. [DOI] [PubMed] [Google Scholar]
Creinin 1997 {published data only}
- Creinin MD, Moyer R, Guido R. Misoprostol for medical evacuation of early pregnancy failure. Obstetrics & Gynecology 1997;89:768‐72. [DOI] [PubMed] [Google Scholar]
Dehbashi 2016 {published data only}
- Dehbashi Z, Moosazadeh M, Afshari M. Comparison between sublingual and vaginal route of misoprostol in management of first trimester miscarriage missing. Materia Socio‐Medica 2016;28(4):271‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demetroulis 2001 {published data only}
- Demetroulis C, Saridogan E, Kunde D, Naftalin AA. A prospective randomized control trial comparing medical and surgical treatment for early pregnancy failure. Human Reproduction 2001;16:365‐9. [DOI] [PubMed] [Google Scholar]
- Demetroulis C, Saridogan E, Kunde D, Naftalin AA. A prospective randomized control trial comparing medical and surgical treatment for early pregnancy failure. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000. [DOI] [PubMed]
Egarter 1995 {published data only}
- Egarter C, Lederhilger J, Kurz C, Karas H, Reisenberger K. Gemeprost for first trimester missed abortion. Archives of Gynecology and Obstetrics 1995;256:29‐32. [DOI] [PubMed] [Google Scholar]
Eng 1997* {published data only}
- Eng NS, Guan AC. Comparative study of intravaginal misoprostol with gemeprost as an abortifacient in second trimester missed abortion. Australian and New Zealand Journal of Obstetrics and Gynaecology 1997;37:331‐4. [DOI] [PubMed] [Google Scholar]
Fang 2009 {published data only}
- Fang AH, Chen QF, Zheng W, Li YH, Chen RY. Termination of missed abortion in a combined procedure: a randomized controlled trial. Journal of Reproduction and Contraception 2009;20(1):45‐9. [Google Scholar]
Ganguly 2010 {published data only}
- Ganguly RP, Mukhopadhyay S, Burman SK, Patra KK, Jha T, Mukherji J. A randomized trial of misoprostol compared with manual vacuum aspiration for early pregnancy failure. Nepal Journal of Obstetrics and Gynaecology 2010;5(2):8‐13. [Google Scholar]
Gilles 2004 {published data only}
- Barnhart KT, Bader T, Huang X, Frederick MM, Timbers KA, Zhang JJ. Hormone pattern after misoprostol administration for a nonviable first‐trimester gestation. Fertility and Sterility 2004;81:1099‐105. [DOI] [PubMed] [Google Scholar]
- Creinin MD, Harwood B, Guido RS, Fox MC, Zhang J, NICHD Early Pregnancy Failure Trial. Endometrial thickness after misoprostol use for early pregnancy failure. International Journal of Gynecology & Obstetrics 2004;86:22‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AR, Robilotto CM, Westhoff CL, Forman S, Zhang J, NICHD Management of Early Pregnancy Failure Trial. Bleeding patterns after vaginal misoprostol for treatment of early pregnancy failure. Human Reproduction 2004;19:1655‐8. [DOI] [PubMed] [Google Scholar]
- Gilles J, Creinin MM, Barnhart KT, Westhoff C, Frederick MM, Zang J, et al. Wet versus dry misoprostol application for treatment of early pregnancy failure. Fertility and Sterility 2002;78:S64‐S65. [Google Scholar]
- Gilles JM, Creinin MD, Barnhart K, Westhoff C, Frederick MM, Zhang J. A randomized trial of saline solution‐moistened misoprostol versus dry misoprostol for the first‐trimester pregnancy failure. American Journal of Obstetrics and Gynecology 2004;190:389‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graziosi 2004 {published data only}
- Graziosi GC, Bruinse HW, Reuwer PJ, Teteringen O, Mol BW. Fertility outcome after a randomized trial comparing curettage with misoprostol for treatment of early pregnancy failure. Human Reproduction 2005;20:1749‐50. [DOI] [PubMed] [Google Scholar]
- Graziosi GC, Bruinse HW, Reuwer PJ, Kessel PH, Westerweel PE, Mol BW. Misoprostol versus curettage in women with early pregnancy failure: impact on women's health‐related quality of life. A randomized controlled trial. Human Reproduction 2005;20:2340‐7. [DOI] [PubMed] [Google Scholar]
- Graziosi GC, Mol BW, Reuwer PJ, Drogtrop A, Bruinse HW. Misoprostol versus curettage in women with early pregnancy failure after initial expectant management: a randomized trial. Human Reproduction 2004;19:1894‐9. [DOI] [PubMed] [Google Scholar]
- Graziosi GC, Steeg JW, Reuwer PH, Drogtrop AP, Bruinse HW, Mol BW. Economic evaluation of misoprostol in the treatment of early pregnancy failure compared to curettage after an expectant management. Human Reproduction 2005;20:1067‐71. [DOI] [PubMed] [Google Scholar]
Herabutya 1997 {published data only}
- Herabutya Y, O‐Prasertsawat P. Misoprostol in the management of missed abortion. International Journal of Gynecology & Obstetrics 1997;56:263‐6. [DOI] [PubMed] [Google Scholar]
Jain 1996* {published data only}
- Jain JK, Mishell DR. A comparison of misoprostol with and without laminaria tents for induction of second‐trimester abortion. American Journal of Obstetrics and Gynecology 1996;175:173‐7. [DOI] [PubMed] [Google Scholar]
Kara 1999* {published data only}
- Kara M, Ozden S, Eroglu M, Cetin A, Arioglu P. Comparison of misoprostol and dinoproston administration for the induction of labour in second trimester pregnancies in cases of intrauterine fetal loss. Italian Journal of Gynecology and Obstetrics 1999;1:13‐6. [Google Scholar]
Kovavisarach 2002 {published data only}
- Kovavisarach E, Sathapanachai U. Intravaginal 400 micrograms misoprostol for pregnancy termination in cases of blighted ovum: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2002;42:161‐3. [DOI] [PubMed] [Google Scholar]
- Sathapanachai U. Intravaginal 400 micrograms misoprostol for pregnancy termination in cases of blighted ovum. Thai Journal of Obstetrics and Gynaecology 2000;12(4):363. [DOI] [PubMed] [Google Scholar]
Kovavisarach 2005 {published data only}
- Kovavisarach E, Jamnansiri C. Intravaginal misoprostol 600mcg and 800mcg for the treatment of early pregnancy failure. International Journal of Gynecology & Obstetrics 2005;90:208‐12. [DOI] [PubMed] [Google Scholar]
Kushwah 2009 {published data only}
- Kushwah B, Singh A. Sublingual versus oral misoprostol for uterine evacuation following early pregnancy failure. International Journal of Gynecology & Obstetrics 2009;106(1):43‐5. [DOI] [PubMed] [Google Scholar]
- Kushwah DS, Kushwah B, Salman MT, Verma VK. Acceptability and safety profile of oral and sublingual misoprostol for uterine evacuation following early fetal demise. Indian Journal of Pharmacology 2011;43(3):306‐10. [DOI: 10.4103/0253-7613.81513; CRSREF: 3293000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lelaidier 1993 {published data only}
- Lelaidier C, Baton‐Saint‐Mleux C, Fernandez H, Bourget P, Frydman R. Mifepristone (RU 486) induces embryo expulsion in first trimester non‐developing pregnancies: a prospective randomized trial. Human Reproduction 1993;8:492‐5. [DOI] [PubMed] [Google Scholar]
Lister 2005 {published data only}
- Lister MS, Shaffer LE, Bell JG, Lutter KQ, Moorma KH. Randomized, double‐blind, placebo‐controlled trial of vaginal misoprostol for management of early pregnancy failures. American Journal of Obstetrics and Gynecology 2005;193:1338‐43. [DOI] [PubMed] [Google Scholar]
Marwah 2016 {published data only}
- Marwah S, Gupta S, Batra NP, Bhasin V, Sarna V, Kaur N. A comparative study to evaluate the efficacy of vaginal vs oral prostaglandin E1 analogue (misoprostol) in management of first trimester missed abortion. Journal of Clinical and Diagnostic Research 2016;10(5):QC14‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitwaly 2016* {published data only}
- Mitwally AB. Intra uterine extra amniotic (200 µg) versus vaginal (200 µg) misoprostol for second trimester pregnancy termination: randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02669420 (first received 1 February 2016).
- Mitwaly AB, Abbas AM, Abdellah MS. Intra uterine extra‐amniotic versus vaginal misoprostol for termination of second trimester miscarriage: a randomized controlled trial. International Journal of Reproductive Biomedicine 2016;14(10):643‐8. [PMC free article] [PubMed] [Google Scholar]
Mizrachi 2017 {published data only}
- Mizrachi Y, Dekalo A, Gluck O, Miremberg H, Dafna L, Feldstein O, et al. Single versus repeat doses of misoprostol for treatment of early pregnancy loss‐a randomized clinical trial. Human Reproduction 2017;32(6):1202‐7. [DOI] [PubMed] [Google Scholar]
Muffley 2002 {published data only}
- Muffley PE, Stitely ML, Gherman RB. Early intrauterine pregnancy failure: a randomized trial of medical versus surgical treatment. American Journal of Obstetrics and Gynecology 2002;187:321‐6. [DOI] [PubMed] [Google Scholar]
Ngoc 2004 {published data only}
- Ngoc NT, Blum J, Westheimer E, Quan TTV, Winikoff B. Medical treatment of missed abortion using misoprostol. International Journal of Gynecology & Obstetrics 2004;87:138‐42. [DOI] [PubMed] [Google Scholar]
Nielsen 1999 {published data only}
- Nielsen S, Hahlin M, Platz‐Christensen J. Expectant management or pharmacological treatment for first trimester spontaneous abortion: a randomised trial. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167:2):77. [Google Scholar]
- Nielsen S, Hahlin M, Platz‐Christensen J. Randomised trial comparing expectant with medical management for first trimester miscarriages. British Journal of Obstetrics and Gynaecology 1999;106:804‐7. [DOI] [PubMed] [Google Scholar]
Niromanesh 2005* {published data only}
- Niromanesh S, Hashemi‐Feasharaki M, Mosavi‐Jarrahi A. Second trimester abortion using intravaginal misoprostol. International Journal of Gynecology & Obstetrics 2005;89:276‐7. [DOI] [PubMed] [Google Scholar]
Petersen 2013 {published data only}
- Petersen SG, Perkins A, Gibbons K, Bertolone J, Devenish‐Meares P, Cave D, et al. Can we use a lower intravaginal dose of misoprostol in the medical management of miscarriage? A randomised controlled study. Australian & New Zealand Journal of Obstetrics & Gynaecology 2013;53(1):64‐73. [DOI] [PubMed] [Google Scholar]
Rita 2006 {published data only}
- Rita, Gupta S, Kumar S. A randomised comparison of oral and vaginal misoprostol for medical management of first trimester missed abortion. JK Science 2006;8(1):35‐8. [Google Scholar]
Saichua 2009 {published data only}
- Saichua C, Phupong V. A randomized controlled trial comparing powdery sublingual misoprostol and sublingual misoprostol tablet for management of embryonic death or anembryonic pregnancy. Archives of Gynecology and Obstetrics 2009;280(3):431‐5. [DOI] [PubMed] [Google Scholar]
Schreiber 2018 {published data only}
- Schreiber CA, Sonalkar S, Barnhart KT, Ratcliffe SJ, Creinin MD, Atrio J. Mifepristone pretreatment for the medical management of early pregnancy loss. New England Journal of Medicine 2018;378(23):2161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2010* {published data only}
- Shah N, Azam SI, Khan NH. Sublingual versus vaginal misoprostol in the management of missed miscarriage. JPMA ‐ Journal of the Pakistan Medical Association 2010;60(2):113‐6. [PubMed] [Google Scholar]
Sinha 2018 {published data only}
- Sinha P, Suneja A, Guleria K, Aggarwal R, Vaid NB. Comparison of mifepristone followed by misoprostol with misoprostol alone for treatment of early pregnancy failure: a randomized double‐blind placebo‐controlled trial. Journal of Obstetrics and Gynecology of India 2018;68(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonsanoh 2014 {published data only}
- Sonsanoh A, Chullapram T. Comparison of sublingual and vaginal misoprostol for termination of early pregnancy failure: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 2014;22(3):128‐36. [Google Scholar]
Tang 2003 {published data only}
- Tang OS, Lau WN, Ng EHY, Lee SW, Ho PC. A prospective randomized study to compare the use of repeated doses of vaginal with sublingual misoprostol in the management of first trimester silent miscarriage. Human Reproduction 2003;18:176‐81. [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang OS, Ong CY, Tse KY, Ng EH, Lee SW, Ho PC. A randomized trial to compare the use of sublingual misoprostol with or without an additional 1 week course for the management of first trimester silent miscarriage. Human Reproduction 2006;21(1):189‐92. [DOI] [PubMed] [Google Scholar]
Tanha 2010a {published data only}
- Tanha FD, Feizi M, Shariat M. Sublingual versus vaginal misoprostol for the management of missed abortion. Journal of Obstetrics and Gynaecology Research 2010;36(3):525‐32. [DOI] [PubMed] [Google Scholar]
Trinder 2006 {published data only}
- Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of first‐trimester miscarriage based on results from the MIST trial. BJOG: An International Journal of Obstetrics & Gynaecology 2006;113(8):879‐89. [DOI: 10.1111/j.1471-0528.2006.00998.x; CRSREF: 3293040] [DOI] [PubMed] [Google Scholar]
- Smith L. Extension to: randomised controlled trial of expectant, medical and surgical management of early miscarriage. Research Findings Register (www.refer.nhs.uk) (accessed 7 March 2006) 2006.
- Trinder J, Brocklehurst P, Porter R, Read M, Vyas S, Smith L. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial). BMJ 2006;332(7552):1235‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2002 {published data only}
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstetrics & Gynecology 2002;99:563‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbas 2018 {published data only}
- Abbas AM, NCT03584698. The effect of adding vaginal evening primrose oil to misoprostol during induction of second‐trimester missed miscarriage; a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03584698 (12 July 2018).
Abdel Fattah 1997 {published data only}
- Abdel Fattah IH. PGE1 analogue for the induction of midtrimester abortion in cases of intrauterine fetal death. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167:2):26. [Google Scholar]
Abd‐El‐Maeboud 2012 {published data only}
- Abd‐El‐Maeboud KH, Ghazy A, Ibrahim A, Hassan N, El‐Bohoty A, Gamal‐El‐Din I. Vaginal acidity enhancement with a 3% acetic acid gel prior to misoprostol treatment for pregnancy termination in the midtrimester. International Journal of Gynecology and Obstetrics 2012;119(3):248‐52. [DOI] [PubMed] [Google Scholar]
Al‐Bdour 2007 {published data only}
- Al‐Bdour AN, Akasheh H, Al‐Jayousi T. Missed abortion: termination using single‐dose versus two doses of vaginal misoprostol tablets. Pakistan Journal of Medical Sciences 2007;23(6):920‐3. [Google Scholar]
Ali 2018 {published data only}
Almog 2005 {published data only}
- Almog B, Levin I, Winkler N, Fainaru O, Pauzner D, Lessing JB, et al. The contribution of laminaria placement for cervical ripening in second trimester termination of pregnancy induced by intra‐amniotic injection of prostaglandin F2alpha followed by concentrated oxytocin infusion. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;118:32‐5. [DOI] [PubMed] [Google Scholar]
Altaf 2006 {published data only}
- Altaf F, Sultana N, Iqbal N. Therapeutic abortions; efficacy of intra‐vaginal misoprostol in comparison to extra amniotically administered prostaglandin f2a. Professional Medical Journal 2006;13(3):417‐22. [Google Scholar]
Amjad 1999 {published data only}
- Amjad T, Akhtar S. Termination of pregnancy with foetal death in second trimester: Foley's catheter versus extra amniotic prostaglandins. Journal of College of Physicians & Surgeons Pakistan 1999;9(9):403‐5. [Google Scholar]
Anderman 2000 {published data only}
- Anderman S, Jaschevatzky OE, Ballas S. Comparison between a double balloon device and the foley catheter in extraamniotic prostaglandin F2a infusion for termination of midtrimester missed abortion. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000:162.
Anderson 2009 {published data only}
- Anderson J, Gouk E, Young L, Turnbull L, Sayeed G, Elattar A, et al. A randomised controlled trial of oral versus vaginal misoprostol for medical management of early fetal demise. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S533. [Google Scholar]
Ara 2009 {published data only}
- Ara G, Nargis S, Khatun R, Saha A. Vaginal misoprostol as a medical management in early pregnancy loss. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S533‐S534. [Google Scholar]
Arellano 2009 {published data only}
- Arellano M, Durocher J, Leon W, Montesinos R, Pena M, Winikoff B. Introduction of misoprostol for incomplete abortion care in Latin America: evidence from Ecuador. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S49. [Google Scholar]
Avila‐Vergara 1997 {published data only}
- Avila‐Vergara MA, Morgan‐Ortiz F, Fragoza‐Sosa O, Haro‐Garcia L. Cervical labor induction with prostaglandin E2 in patients with fetal death [Maduracion cervical con prostaglandina E2 en pacientes con feto muerto]. Ginecologia y Obstetricia de Mexico 1997;65:155‐8. [PubMed] [Google Scholar]
Aye 2017 {published data only}
- Aye TT, Aung KL, Myint SS. A comparative study on effect of sublingual versus vaginal misoprostol in management of first trimester miscarriage in Magway Teaching Hospital. Journal of Obstetrics and Gynaecology Research 2017;43:185‐6, Abstract no: 9020. [Google Scholar]
Azra 2007 {published data only}
- Azra B, Shakeel S, Nilofer M. A comparison of two protocols of intra vaginal misoprostol for second trimester medical termination of pregnancy. Pakistan Armed Forces Medical Journal 2007;57(1):61‐5. [Google Scholar]
Bagratee 2009 {published data only}
- Bagratee J, Regan L, Khullar V, Moodley J, Connolly C. Does the volume of retained products of conception and hormonal parameters influence the success of conservative methods of management of first trimester miscarriage?. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S116. [Google Scholar]
Bani‐Irshaid 2006 {published data only}
- Bani‐Irshaid I, Athamneh TZ, Bani‐Khaled D, Al‐Momani M, Dahamsheh H. Termination of second and early third trimester pregnancy: comparison of 3 methods. Eastern Mediterranean Health Journal 2006;12(5):605‐9. [PubMed] [Google Scholar]
Bartz 2013 {published data only}
- Bartz D, Maurer R, Allen RH, Fortin J, Kuang B, Goldberg AB. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstetrics and Gynecology 2013;122(1):57‐63. [DOI] [PubMed] [Google Scholar]
Bebbington 2002 {published data only}
- Bebbington MW, Kent N, Lim K, Gagnon A, Delisle MF, Tessier F, et al. A randomized controlled trial comparing two protocols for the use of misoprostol in midtrimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;187:853‐7. [DOI] [PubMed] [Google Scholar]
Behrashi 2008 {published data only}
- Behrashi M, Mahdian M. Vaginal versus oral misoprostol for second‐trimester pregnancy termination: a randomized trial. Pakistan Journal of Biological Sciences 2008;11(21):2505‐8. [DOI] [PubMed] [Google Scholar]
Behrashi 2010 {published data only}
- Behrashi M. Comparison between the oral and vaginal misoprostol effects on pregnancy termination in second trimester. irct.ir/trial/129 (first received 29 August, 2008).
Ben‐Meir 2009 {published data only}
- Ben‐Meir A, Erez Y, Feigenberg T, Hamani Y, Laufer N, Rojansky N. Mifepristone followed by high‐dose oxytocin drip for second‐trimester abortion: a randomized, double‐blind, placebo‐controlled, pilot study. Journal of Reproductive Medicine 2009;54(8):511‐6. [PubMed] [Google Scholar]
Betstadt 2007 {published data only}
- Betstadt SJ. MiMi: a randomized trial of mifepristone and misoprostol for treatment of early pregnancy failure. clinicaltrials.gov/ct2/show/NCT00468299 (first received 1 May, 2007).
Bique 2007 {published data only}
- Bique C, Usta M, Debora B, Chong E, Westheimer E, Winikoff B. Comparison of misoprostol and manual vacuum aspiration for the treatment of incomplete abortion. International Journal of Gynecology & Obstetrics 2007;98(3):222‐6. [DOI] [PubMed] [Google Scholar]
Biswas 2007 {published data only}
- Biswas SC, Dey R, Jana R, Chattopadhyay N. Comparative study of intravaginal misoprostol and extra amniotic ethacridine lactate instillation for mid trimester pregnancy termination. Journal of Obstetrics and Gynaecology of India 2007;57(3):211‐3. [Google Scholar]
Blohm 2005 {published data only}
- Blohm F, Friden BE, Milsom I, Platz‐Christensen JJ, Nielsen S. A randomised double blind trial comparing misoprostol or placebo in the management of early miscarriage. BJOG: an international journal of obstetrics and gynaecology 2005;112(8):1090‐5. [DOI] [PubMed] [Google Scholar]
Brouns 2010 {published data only}
- Brouns JF, Wely M, Burger MP, Wijngaarden WJ. Comparison of two dose regimens of misoprostol for second‐trimester pregnancy termination. Contraception 2010;82(3):266‐75. [DOI] [PubMed] [Google Scholar]
Cabrol 1990 {published data only}
- Cabrol D, Dubois C, Cronje H, Gonnet JM, Guillot M, Maria B, et al. Induction of labour with mifepristone (RU 486) in intrauterine fetal death. American Journal of Obstetrics and Gynecology 1990;163:540‐2. [DOI] [PubMed] [Google Scholar]
Caliskan 2005 {published data only}
- Caliskan E, Dilbaz S, Doger E, Ozeren S, Dilbaz B. Randomized comparison of 3 misoprostol protocols for abortion induction at 13‐20 weeks of gestation. Journal of Reproductive Medicine 2005;50(3):173‐80. [PubMed] [Google Scholar]
Caliskan 2009 {published data only}
- Caliskan E, Doger E, Cakiroglu Y, Corakci A, Yucesoy I. Sublingual misoprostol 100 microgram versus 200 microgram for second trimester abortion: a randomised trial. European Journal of Contraception & Reproductive Health Care 2009;14(1):55‐60. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2015 {published data only}
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. Journal Obstetrics and Gynaecology Research 2015;41(12):1884‐90. [DOI] [PubMed] [Google Scholar]
- Datta S. A randomized double‐blind study to compare efficacy of mifepristone and misoprostol versus misoprostol alone for induction of labour in intrauterine foetal death. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=7823 (first received 21 March 2014).
Chowdhury 2012 {published data only}
- Chowdhury S, Uddin AW. Misoprostol in the management of second trimester missed abortion: a randomized controlled trial. International Journal of Gynaecology and Obstetrics 2012;119(Suppl 3):S310. [Google Scholar]
Clevin 2001 {published data only}
- Clevin L, Munk T, Hansen TR. Spontaneous abortion. Drug treatment versus surgery [Spontan abort. Medicinsk versus kirurgisk behandling]. Ugeskrift for Laeger 2001;163(15):2136‐9. [PubMed] [Google Scholar]
Dabash 2009 {published data only}
- Dabash R, Cherine M, Darwish E, Blum J, Hassanein N, Abdel Daiem T, et al. Bleeding following surgical (MVA) and medical (400 ug sublingual misoprostol) treatment of incomplete abortion. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S150‐S151. [Google Scholar]
Dao 2007 {published data only}
- Dao B, Blum J, Thieba B, Raghavan S, Ouedraego M, Lankoande J, et al. Is misoprostol a safe, effective and acceptable alternative to manual vacuum aspiration for postabortion care? Results from a randomised trial in Burkina Faso, West Africa. BJOG: an international journal of obstetrics and gynaecology 2007;114(11):1368‐75. [DOI] [PubMed] [Google Scholar]
Das 2014 {published data only}
- Das CM, Sharma M, Pardeep K, Khurshid F. To compare the safety and efficacy of manual vacuum aspiration with misoprostol (st mom) 600mg in incomplete miscarriage. Journal of the Liaquat University of Medical and Health Sciences 2014; Vol. 13, issue 3:93‐6.
David 2003 {published data only}
- David M, Chen FC, Lichtenegger W. NO‐donor nitroglycerin versus the prostaglandin gemeprost for cervical ripening in first trimester missed abortion. International Journal of Gynecology & Obstetrics 2003;83:71‐2. [DOI] [PubMed] [Google Scholar]
David 2005 {published data only}
- David M, Chen FC. Comparison of isosorbide mononitrate (Mono Mack) and misoprostol (Cytotec) for cervical ripening in the first trimester missed abortion. Archives of Gynecology and Obstetrics 2005;273(3):144‐5. [DOI] [PubMed] [Google Scholar]
Demirezen 2018 {published data only}
- Demirezen G, Aslan Cetin B, Aydogan Mathyk B, Koroglu N, Yildirim G. Efficiency of the Foley catheter versus the double balloon catheter during the induction of second trimester pregnancy terminations: a randomized controlled trial. Archives of Gynecology and Obstetrics 2018;298(5):881–7. [DOI] [PubMed] [Google Scholar]
Dickinson 1998 {published data only}
- Dickinson JE, Godfrey M, Evans SF. Efficacy of intravaginal misoprostol in second‐trimester pregnancy termination: a randomized controlled trial. Journal of Maternal‐Fetal Medicine 1998;7:115‐9. [DOI] [PubMed] [Google Scholar]
Dickinson 2002 {published data only}
- Dickinson JE, Evans SF. The optimization of intravaginal misoprostol dosing schedules in second‐trimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;186:470‐4. [DOI] [PubMed] [Google Scholar]
Dickinson 2003 {published data only}
- Dickinson JE, Evans SF. A comparison of oral misoprostol with vaginal misoprostol administration in second‐trimester pregnancy termination for fetal abnormality. Obstetrics & Gynecology 2003;101:1294‐9. [DOI] [PubMed] [Google Scholar]
Diop 2009 {published data only}
- Diop A, Raghavan S, Rakotovao JP, Comendant R, Blumenthal PD, Winikoff B. Two routes of administration for misoprostol in the treatment of incomplete abortion: a randomized clinical trial. Contraception 2009;79(6):456‐62. [DOI] [PubMed] [Google Scholar]
Elami‐Suzin 2013 {published data only}
- Elami‐Suzin M, Freeman MD, Porat N, Rojansky N, Laufer N, Ben‐Meir A. Mifepristone followed by misoprostol or oxytocin for second‐trimester abortion: a randomized controlled trial. Obstetrics and Gynecology 2013;122(4):815‐20. [DOI] [PubMed] [Google Scholar]
- Freeman MD, Porat N, Rojansky N, Elami‐Suzin M, Winograd O, Ben‐Meir A. Physical symptoms and emotional responses among women undergoing induced abortion protocols during the second trimester. International Journal of Gynaecology and Obstetrics 2016;135(2):154‐7. [DOI: 10.1016/j.ijgo.2016.05.008] [DOI] [PubMed] [Google Scholar]
Elhassan 2008 {published data only}
- Elhassan EM, Abubaker MS, Adam I. Sublingual compared with oral and vaginal misoprostol for termination of pregnancy with second‐trimester fetal demise. International Journal of Gynecology & Obstetrics 2008;100(1):82‐3. [DOI] [PubMed] [Google Scholar]
El Sokkary 2016 {published data only}
- Sokkary HH. Comparison between sublingual and vaginal administration of misoprostol in management of missed abortion. Journal of Obstetrics and Gynaecology of India 2016;66(S1):S24‐S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eppel 2005 {published data only}
- Eppel W, Facchinetti F, Schleussner E, Piccinini F, Pizzi C, Gruber DM, et al. Second trimester abortion using isosorbide mononitrate in addition to gemeprost compared with gemeprost alone: a double blind randomized, placebo‐controlled multicenter trial. American Journal of Obstetrics and Gynecology 2005;192:856‐61. [DOI] [PubMed] [Google Scholar]
Eslamian 2007 {published data only}
- Eslamian L, Gosili R, Jamal A, Alyassin A. A prospective randomized controlled trial of two regimens of vaginal misoprostol in second trimester termination of pregnancy. Acta Medica Iranica 2007;45(6):497‐500. [Google Scholar]
Fadalla 2004* {published data only}
- Fadalla FA, Mirghani OA, Adam I. Oral misoprostol vs vaginal misoprostol for termination of pregnancy with intrauterine fetal demise in the second‐trimester. International Journal of Gynecology & Obstetrics 2004;86:52‐3. [DOI] [PubMed] [Google Scholar]
Feldman 2003 {published data only}
- Feldman DM, Borgida AF, Rodis JF, Leo MV, Campbell WA. A randomized comparison of two regimens of misoprostol for second‐trimester pregnancy termination. American Journal of Obstetrics and Gynecology 2003;189:710‐3. [DOI] [PubMed] [Google Scholar]
Fernlund 2018 {published data only}
- Fernlund A, Jokubkiene L, Sladkevicius P, Valentin L. A randomised controlled trial comparing misoprostol to expectant care in early pregnancy failure. Ultrasound in Obstetrics & Gynecology 2017;50(Suppl 1):15, Abstract no: OC08.03. [Google Scholar]
- Fernlund A, Jokubkiene L, Sladkevicius P, Valentin L. Misoprostol treatment vs expectant management in women with early non‐viable pregnancy and vaginal bleeding: a pragmatic randomized controlled trial. Ultrasound in Obstetrics & Gynecology 2018;51(1):24‐32. [DOI] [PubMed] [Google Scholar]
Fiala 2005 {published data only}
- Fiala C, Swahn ML, Stephansson O, Gemzell‐Danielsson K. The effect of non‐steroidal anti‐inflammatory drugs on medical abortion with mifepristone and misoprostol at 13‐22 weeks gestation. Human Reproduction 2005;20(11):3072‐7. [DOI] [PubMed] [Google Scholar]
Ghorab 1998 {published data only}
- Ghorab MN, Helw BA. Second‐trimester termination of pregnancy by extra‐amniotic prostaglandin F2alpha or endocervical misoprostol. Acta Obstetricia et Gynecologica Scandinavica 1998;77:429‐32. [PubMed] [Google Scholar]
Gonzalez 2001 {published data only}
- Gonzalez JA, Carlan SJ, Alverson MW. Outpatient second trimester pregnancy termination. Contraception 2001;63:89‐93. [DOI] [PubMed] [Google Scholar]
Grimes 2004 {published data only}
- Grimes DA, Smith MS, Witham AD. Mifepristone and misoprostol versus dilatation and evacuation for midtrimester abortion: a pilot randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2004;111:148‐53. [DOI] [PubMed] [Google Scholar]
Gronland 2002 {published data only}
- Gronland A, Gronland L, Clevin L, Andersen B, Palmegren N, Lidegaard O. Management of missed abortion: comparison of medical treatment with either mifepristone + misoprostol or misoprostol alone with surgical evacuation. A multi‐center trial in Copenhagen county, Denmark. Acta Obstetricia et Gynecologica Scandinavica 2002;81:1060‐5. [PubMed] [Google Scholar]
Guix 2005 {published data only}
- Guix C, Palacio M, Figueras F, Bennasar M, Zamora L, Coll O, et al. Efficacy of two regimens of misoprostol for early second‐trimester pregnancy termination. Fetal Diagnosis and Therapy 2005;20(6):544‐8. [DOI] [PubMed] [Google Scholar]
Halimi 2004 {published data only}
- Halimi M. Therapeutic termination of second trimester pregnancy: a comparison of extra‐amniotic foley`s catheter balloon alone with the combined use of foley`s catheter balloon and extra‐amniotic instillation of prostaglandin f2‐alpha. Journal of Postgraduate Medical Institute 2004;18(3):408‐18. [Google Scholar]
Hassan 2007 {published data only}
- Hassan FI, Mostapha MK, Sattar MA, Marouf E, Azim SA. Oral versus rectal route of misoprostol administration: a randomized controlled trial. Middle East Fertility Society Journal 2007;12(1):53‐6. [Google Scholar]
Hausler 1997 {published data only}
- Häusler MC, Koroschetz F, Tamussino K, Walcher W. Is a curettage after spontaneous abortion still relevant. A prospective randomised study [Ist eine Curettage nach Abortus completus noch zeitgemäb?. Eine prospektiv randomisierte Studie]. Geburtshilfe und Frauenheilkunde 1997;57:396‐9. [Google Scholar]
Heard 2002 {published data only}
- Heard MJ, Stewart GM, Buster JE, Carson SA, Miller HJ. Outpatient management of missed abortion with vaginal misoprostol [abstract]. Obstetrics & Gynecology 2002;99(4 Suppl):20S. [Google Scholar]
Herabutya 1997a {published data only}
- Herabutya Y, O‐Prasertsawat P. A comparison of intravaginal misoprostol with intracervical prostaglandin E2 gel for the management of dead fetus in utero. Thai Journal of Obstetrics and Gynaecology 1997;9(2):95‐8. [DOI] [PubMed] [Google Scholar]
Herabutya 2005 {published data only}
- Herabutya Y, Chanrachakul B, Punyavachira P. A randomised controlled trial of 6 and 12 hourly administration of vaginal misoprostol for second trimester pregnancy termination. BJOG: an international journal of obstetrics and gynaecology 2005;112:1297‐301. [DOI] [PubMed] [Google Scholar]
Hidar 2001 {published data only}
- Hidar S, Fekih M, Chaieb A, Bibi M, Mellouli R, Khairi H. Oxytocin and misoprostol administered intravaginally for termination of pregnancy at 13‐29 weeks of amenorrhea. A prospective randomized trial [Apport de l'association d'ocytocine au misoprostol administre en intravaginal au cours des interruptions de grossesses entre 13 et 29 semaines d'amenorrhee. Essai clinique prospectif randomise]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30:439‐43. [PubMed] [Google Scholar]
Hidar 2005 {published data only}
- Hidar S, Bouddebous M, Chaieb A, Jerbi M, Bibi M, Khairi H. Randomized controlled trial of vaginal misoprostol versus vaginal misoprostol and isosorbide dinitrate for termination of pregnancy at 13‐29 weeks. Archives of Gynecology and Obstetrics 2005;273(3):157‐60. [DOI] [PubMed] [Google Scholar]
Hill 1991 {published data only}
- Hill NC, Selinger M, Ferguson J, MacKenzie IZ. Management of intra‐uterine fetal death with vaginal administration of gemeprost or prostaglandin E2: a random allocation controlled trial. Journal of Obstetrics and Gynaecology 1991;11:422‐6. [Google Scholar]
Hinshaw 1993 {published data only}
- Henshaw RC, Hinshaw K, Smith NC, Templeton AA. The medical management of miscarriage. Fertility Society of Australia / Australian Gynaecological Endoscopy Society; 1995 November 19‐25; Melbourne, Australia. 1995:FSA 75.
- Hinshaw K, Rispin N, Smith N, Templeton A. Medical versus surgical management in first trimester miscarriage: a prospective, pragmatic random allocation trial. Journal of Obstetrics and Gynaecology 1993;13:404‐5. [Google Scholar]
- Hinshaw K, Rispin R, Henshaw R, Smith N, Templeton A. Medical versus surgical uterine evacuation in first trimester miscarriage: a prospective, pragmatic randomised trial. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin. 1995:4.
- Rispin R, Hinshaw K, Henshaw R, Smith N, Templeton A. New aspects of care in the management of miscarriage. Proceedings of Research in Midwifery Conference; 1993 September 14; Birmingham, UK. 1993.
Hogg 2000 {published data only}
- Hogg B, Owen J. Laminaria versus extraamniotic saline infusion (EASI) for cervical ripening and mid‐trimester labor induction. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S135. [DOI] [PubMed] [Google Scholar]
Hombalegowda 2015 {published data only}
- Hombalegowda RB, Samapthkumar S, Vana H, Jogi P, Ramaiah R. A randomized controlled trial comparing different doses of intravaginal misoprostol for early pregnancy failure. Contraception 2015;92(4):364‐5. [Google Scholar]
Hughes 1996 {published data only}
- Hughes J, Ryan M, Hinshaw K, Henshaw R, Rispin R, Templeton A. The costs of treating miscarriage: a comparison of medical and surgical management. British Journal of Obstetrics and Gynaecology 1996;103(12):1217‐21. [DOI] [PubMed] [Google Scholar]
Imran 2010 {published data only}
- Imran F, Anser A, Danish N, Fatima N. Misoprostol for the purpose of mid‐trimester termination of pregnancy: a comparative study with prostaglandin F2 alpha. Journal of Ayub Medical College, Abbottabad: JAMC 2010;22(4):87‐91. [PubMed] [Google Scholar]
Islam 2006 {published data only}
- Islam A, Abbasi AN, Sarwar I. Use of Foley's catheter and prostaglandin F‐2 alpha in second trimester termination of pregnancy. Journal of Ayub Medical College, Abbottabad 2006;18(3):35‐9. [PubMed] [Google Scholar]
Jabir 2009a {published data only}
- Jabir M, Smeet RI. Comparison of oral and vaginal misoprostol for cervical ripening before evacuation of first trimester missed miscarriage. Saudi Medical Journal 2009;30(1):82‐7. [PubMed] [Google Scholar]
Jabir 2009b {published data only}
- Jabir M, Smeet R. Comparison of oral and vaginal misoprostol for cervical ripening before evacuation of first trimester missed miscarriage. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S209. [PubMed] [Google Scholar]
Jain 1994 {published data only}
- Jain JK, Mishell DR. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of second‐trimester pregnancy. New England Journal of Medicine 1994;331:290‐3. [DOI] [PubMed] [Google Scholar]
Jain 1999 {published data only}
- Jain JK, Kuo J, Mishell DR. A comparison of two dosing regimens of intravaginal misoprostol for second‐trimester pregnancy termination. Obstetrics & Gynecology 1999;93:571‐5. [DOI] [PubMed] [Google Scholar]
Johnson 1997 {published data only}
- Johnson N, Priestnall M, Marsay T, Ballard P, Watters J. A randomised trial evaluating pain and bleeding after a first trimester miscarriage treated surgically or medically. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;72(2):213‐5. [DOI] [PubMed] [Google Scholar]
Kamal 2005 {published data only}
- Kamal R, Parveeen F, Mazhar SB. Role of misoprostol in vaginal versus double oro‐vaginal route for termination of pregnancy in mid trimester pregnancy. Annals of Pakistan Institute of Medical Sciences 2005;1(4):196‐200. [Google Scholar]
Kanhai 1989 {published data only}
- Kanhai HH, Keirse MJ. Induction of labour after fetal death: a randomized controlled trial of two prostaglandin regimens. British Journal of Obstetrics and Gynaecology 1989;96:1400‐4. [DOI] [PubMed] [Google Scholar]
- Kanhai HH, Keirse MJ. Intravenous administration of sulfprostone for the induction of labour after fetal death: a randomised comparison of two dose schedules. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:201‐2.
- Kanhai HH, Keirse MJC. Intravenous administration of sulfprostone for the induction of labour after fetal death: a randomised comparison of two dose schedules. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:45.
Kapp 2007 {published data only}
- Kapp N, Todd CS, Yadgarova KT, Alibayeva G, Nazarova D, Loza O, et al. A randomized comparison of misoprostol to intrauterine instillation of hypertonic saline plus a prostaglandin F2alpha analogue for second‐trimester induction termination in Uzbekistan. Contraception 2007;76(6):461‐6. [DOI] [PubMed] [Google Scholar]
Khosravi 2017 {published data only}
- Khosravi D, IRCT2017040633255N1. Comparison of the effect between two dose (800 µg/day & 400 µg/6) of vaginal misoprostol in the termination of first‐trimester pregnancy: a double‐blinded randomized trial. https://en.irct.ir/trial/25706 (25 June 2017).
Kong 2013 {published data only}
- Kong GW, Lok IH, Yiu AK, Hui AS, Lai BP, Chung TK. Clinical and psychological impact after surgical, medical or expectant management of first‐trimester miscarriage ‐ a randomised controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology 2013;53(2):170‐7. [DOI] [PubMed] [Google Scholar]
Kurshid 2010 {published data only}
- Kurshid R, Ahmed A, Mir S, Ul Shamas I. To assess the efficacy of two regimens of misoprostol for second trimester pregnancy termination‐a randomized comparison. Internet Journal of Gynecology and Obstetrics 2010; Vol. 14, issue 1.
Kyaw 2015 {published data only}
- Kyaw O, Yi KH, Thida M. A comparison of medical evacuation using single dose vaginal misoprostol (800mug) versus surgical evacuation in management of early pregnancy loss in North Okkalapa General and Teaching Hospital, Yangon. Journal of Obstetrics and Gynaecology Research 2015;41:3, Abstract no: YGA O 06. [Google Scholar]
Linn 2015 {published data only}
- Linn TW, Nyunt KK, Ku SK. Effectiveness of low doses vaginal misoprostol in intrauterine fetal death. Journal of Obstetrics and Gynaecology Research 2015;41(Suppl S1):60, Abstract no: FC 10.12. [Google Scholar]
Lippert 1978 {published data only}
- Lippert TH, Luthi A. Induction of labour with prostaglandin E2 gel in cases of intrauterine fetal death. Prostaglandins 1978;15:533‐42. [DOI] [PubMed] [Google Scholar]
Lu 2014 {published data only}
- Lu PH, Lu J, Zou S. Comparisons of the effects of misoprostol by two different application on the treatment of missed abortion. Chinese Journal of Pharmaceutical Biotechnology 2014;21(2):159‐61. [Google Scholar]
Lughmani 2008 {published data only}
- Lughmani ST. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of 1st trimester pregnancy. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):179. [Google Scholar]
- Lughmani ST. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of 1st trimester pregnancy. Double blind randomized trial [abstract]. 31st British International Congress of Obstetrics and Gynaecology. London, UK, 2007, July 4‐6; Vol. 209. [CRSREF: 3293006]
Machtinger 2004 {published data only}
- Machtinger R, Stockheim D, Shulman A, Dulitzki M, Schiff E, Seidman DS. A randomized prospective study comparing the effectiveness of four protocols for treatment of first trimester spontaneous abortion. Fertility and Sterility 2004;82 Suppl 2:S80. [Google Scholar]
Mahjabeen, 2009 {published data only}
- Mahjabeen, Khawaja NP, Rehman R. Comparison of oral versus vaginal misoprostol for mid‐trimester pregnancy termination. Journal of the College of Physicians & Surgeons Pakistan 2009;19(6):359‐62. [PubMed] [Google Scholar]
Makenzius 2017 {published data only}
- Makenzius M, Oguttu MA, Odera T, Klingberg‐Allvin M, Gemzell‐Danielsson K, Faxelid E. Post‐abortion care (PAC) and contraceptive counselling by midwives or physicians: A facility based study in Kisumu, Western Kenya. 31st International Confederation of Midwives Triennial Congress. Midwives ‐ Making a Difference in the World; 2017 June 18‐22; Toronto, Canada. 2017:Abstract no: F14.01.
Makhlouf 2003 {published data only}
- Makhlouf AM, Al‐Hussaini TK, Habib DM, Makarem MH. Second‐trimester pregnancy termination: comparison of three different methods. Journal of Obstetrics and Gynaecology 2003;23:407‐11. [DOI] [PubMed] [Google Scholar]
Martin 1965 {published data only}
- Martin RH, Menzies DN. Oestrogen therapy in missed abortion and labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;62:256‐8. [DOI] [PubMed] [Google Scholar]
Montesinos 2011 {published data only}
- Montesinos R, Durocher J, Leon W, Arellano M, Pena M, Pinto E, et al. Oral misoprostol for the management of incomplete abortion in Ecuador. International Journal of Gynecology & Obstetrics 2011;115(2):135‐9. [DOI] [PubMed] [Google Scholar]
Moran 2005 {published data only}
- Moran T, Deutsch R. Methotrexate/misoprostol vs. a more standard approach for termination of pregnancies of undetermined location: a randomized, controlled trial. Journal of Reproductive Medicine 2005;50(10):784‐92. [PubMed] [Google Scholar]
Mostafa‐Gharebaghi 2010 {published data only}
- Mostafa‐Gharebaghi P, Mansourfar M, Sadeghi‐Bazargani H. Low dose vaginal misoprostol versus prostaglandin E2 suppository for early uterine evacuation: a randomized clinical trial. Pakistan Journal of Biological Sciences 2010;13(19):946‐50. [DOI] [PubMed] [Google Scholar]
Mulayim 2009 {published data only}
- Mulayim B, Celik NY, Onalan G, Zeyneloglu HB, Kuscu E. Sublingual misoprostol after surgical management of early termination of pregnancy. Fertility & Sterility 2009;92(2):678‐81. [DOI] [PubMed] [Google Scholar]
Naghshineh 2015 {published data only}
- Naghshineh E, Allame Z, Farhat F. The effectiveness of using misoprostol with and without letrozole for successful medical abortion: A randomized placebo‐controlled clinical trial. Journal of Research in Medical Sciences 2015;20(6):585‐9. [PUBMED: 26600834] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabestari PS. Comparison the effect of letrozole plus misoprostol and misoprostol alone in termination of nonviable first trimester pregnancies; a single blinded randomized trial. en.search.irct.ir/trial/13912 (first received 14 March, 2014).
Nakintu 2001 {published data only}
- Nakintu N. A comparative study of vaginal misoprostol and intravenous oxytocin for induction of labour in women with intrauterine fetal death in Mulago Hospital, Uganda. African Health Sciences 2001;1:55‐9. [PMC free article] [PubMed] [Google Scholar]
Nasreen 2009 {published data only}
- Nasreen Z. What for early pregnancy failure manual vacuum aspiration (MVA) with small dose misoprostol or misoprostol alone?. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S539‐S540. [Google Scholar]
Nassar 2006 {published data only}
- Nassar AH. A randomized trial of two regimens of misoprostol for second trimester intrauterine fetal death. clinicaltrials.gov/ct2/show/NCT00141895 2006.
NCT02141555 {published data only}
- NCT02141555, Mody S. Comparing buccal and vaginal misoprostol in management of early pregnancy loss: a pilot randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT02141555 (first received 19 May, 2014).
NCT02573051 {published data only}
- NCT02573051. Misoprostol plus isosorbide mononitrate versus misoprostol for termination of anembryonic pregnancy. clinicaltrials.gov/ct2/show/record/NCT02573051 (first received 9 October, 2015).
Ng 2015 {published data only}
- Ng BK, Annamalai R, Lim PS, Aqmar Suraya S, Nur Azurah AG, Muhammad Abdul Jamil MY. Outpatient versus inpatient intravaginal misoprostol for the treatment of first trimester incomplete miscarriage: a randomised controlled trial. Archives of Gynecology and Obstetrics 2015;291(1):105‐13. [DOI] [PubMed] [Google Scholar]
Ngai 2001 {published data only}
- Ngai SW, Chan YM, Tang OS, Ho PC. Vaginal misoprostol as medical treatment for first trimester spontaneous miscarriage. Human Reproduction 2001;16(7):1493‐6. [DOI] [PubMed] [Google Scholar]
Nguyen 2005 {published data only}
- Nguyen TN, Blum J, Durocher J, Quan TT, Winikoff B. A randomized controlled study comparing 600 versus 1,200 micrograms oral misoprostol for medical management of incomplete abortion. Contraception 2005;72(6):438‐42. [DOI] [PubMed] [Google Scholar]
Niinimaki 2006 {published data only}
- Niinimaki M, Jouppila P, Martikainen H, Talvensaari‐Mattila A. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertility and Sterility 2006;86(2):367‐72. [DOI] [PubMed] [Google Scholar]
- Niinimaki M, Karinen P, Hartikainen AL, Pouta A. Treating miscarriages: a randomised study of cost‐effectiveness in medical or surgical choice. BJOG: an international journal of obstetrics and gynaecology 2009;116(7):984‐90. [DOI: 10.1111/j.1471-0528.2009.02161.x] [DOI] [PubMed] [Google Scholar]
Nor 2006 {published data only}
- Nor Azlin MI, Abdullah HS, Zainul Rashid MR, Jamil MA. Misoprostol (alone) in second trimester terminations of pregnancy: as effective as Gemeprost?. Journal of Obstetrics and Gynaecology 2006;26(6):546‐9. [DOI] [PubMed] [Google Scholar]
Nuthalapaty 2005 {published data only}
- Nuthalapaty F, Ramsey P, Biggio J, Owen J. Comparative efficacy of high dose vaginal misoprostol versus concentrated oxytocin + low dose vaginal misoprostol for mid‐trimester labor induction. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuthalapaty FS, Ramsey PS, Biggio JR, Owen J. High‐dose vaginal misoprostol versus concentrated oxytocin plus low‐dose vaginal misoprostol for midtrimester labor induction: a randomized trial. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1065‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuutila 1997 {published data only}
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmaki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics & Gynecology 1997;90:896‐900. [DOI] [PubMed] [Google Scholar]
Owen 1999 {published data only}
- Owen J, Hauth JC. Vaginal misoprostol vs concentrated oxytocin plus low‐dose prostaglandin E2 for second trimester pregnancy termination. Journal of Maternal‐Fetal Medicine 1999;8:48‐50. [DOI] [PubMed] [Google Scholar]
Paraskevaides 1992 {published data only}
- Paraskevaides E, Prendiville W, Stuart B, Scanaill SN, Walsh D, McGuinness N, et al. Medical evacuation of first trimester (twelve weeks gestation) incomplete abortion and missed abortion. Journal of Gynecologic Surgery 1992;8:159‐63. [Google Scholar]
Paritakul 2010 {published data only}
- Paritakul P, Phupong V. Comparative study between oral and sublingual 600 microg misoprostol for the treatment of incomplete abortion. Journal of Obstetrics and Gynaecology Research 2010;36(5):978‐83. [DOI] [PubMed] [Google Scholar]
Patua 2013 {published data only}
- Patua B, Dasgupta M, Bhattacharyya SK, Bhattacharya S, Hasan SH, Saha S. An approach to evaluate the efficacy of vaginal misoprostol administered for a rapid management of first trimester spontaneous onset incomplete abortion, in comparison to surgical curettage. Archives of Gynecology and Obstetrics 2013;288(6):1243‐8. [DOI] [PubMed] [Google Scholar]
Perry 1999 {published data only}
- Perry KG, Rinehart BK, Terrone DA, Martin RW, May WL, Roberts WE. Second‐trimester uterine evacuation: a comparison of intra‐amniotic (15S)‐15‐methyl‐prostaglandin F2alpha and intravaginal misoprostol. American Journal of Obstetrics and Gynecology 1999;181:1057‐61. [DOI] [PubMed] [Google Scholar]
Piotrowski 1979 {published data only}
- Piotrowski J, Basta A, Klimczyk K, Malolepazy A, Dluzniewska M, Splawinski JA. Indomethacin increases abortifacient effect of PGE2 in man. Prostaglandins 1979;17:451‐9. [DOI] [PubMed] [Google Scholar]
Pongsatha 2004 {published data only}
- Pongsatha S, Tongsong T. Intravaginal misoprostol for pregnancy termination. International Journal of Gynecology & Obstetrics 2004;87:176‐7. [DOI] [PubMed] [Google Scholar]
Prasartsakulchai 2004 {published data only}
- Prasartsakulchai C, Tannirandorn Y. A comparison of vaginal misoprostol 800 microg versus 400 microg in early pregnancy failure: a randomized controlled trial. Journal of the Medical Association of Thailand 2004;87 Suppl 3:S18‐23. [PubMed] [Google Scholar]
Promwangkwa 2017 {published data only}
- Promwangkwa K, Puntitpong B, Chirdchim W, Sananpanichkul P. Efficacy of sublingual misoprostol with or without loading vaginal misoprostol in second trimester termination of pregnancy: a randomized controlled trial. Journal of the Medical Association of Thailand 2017;100(10):1050‐5. [Google Scholar]
Rahimi‐Sharbaf 2015 {published data only}
- Rahimi‐Sharbaf F, Adabi K, Valadan M, Shirazi M, Nekuie S, Ghaffari P, et al. The combination route versus sublingual and vaginal misoprostol for the termination of 13 to 24 week pregnancies: a randomized clinical trial. Taiwanese Journal of Obstetrics & Gynecology 2015;54(6):660‐5. [DOI] [PubMed] [Google Scholar]
Ramadan 2009 {published data only}
- Ramadan MC. Misoprostol versus MVA for incomplete abortion: results from a randomized controlled trial in Egypt. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S68‐9. [Google Scholar]
Ramsey 2004 {published data only}
- Ramsey PS, Savage K, Lincoln T, Owen J. Vaginal misoprostol versus concentrated oxytocin and vaginal PGE2 for second‐trimester labor induction. Obstetrics & Gynecology 2004;104:138‐45. [DOI] [PubMed] [Google Scholar]
Reeves 2006 {published data only}
- Reeves MF, Lohr PA, Harwood B, Creinin MD. Sonographic findings after misoprostol or vacuum aspiration for early pregnancy failure. Contraception 2006;74(2):182. [Google Scholar]
Reeves 2008 {published data only}
- Reeves MF, Lohr PA, Harwood BJ, Creinin MD. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstetrics & Gynecology 2008;111(1):106‐12. [DOI] [PubMed] [Google Scholar]
Rivero‐Lopez 1998 {published data only}
- Rivero‐Lopez E, Marquez‐Maraver F, Duenas‐Diez JL, Cabezas‐Sanchez B. Deferred miscarriage: effectiveness of intravaginal misoprostol versus laminaria alone [Aborto diferido: eficacia del misoprostol intravaginal versus la aplicacion de tallos de laminaria]. Progresos de Obstetricia y Ginecologia 1998;41:579‐81. [Google Scholar]
Robledo 2007 {published data only}
- Robledo C, Zhang J, Troendle J, Barnhart K, Creinin MD, Westhoff C, et al. Clinical indicators for success of misoprostol treatment after early pregnancy failure. International Journal of Gynecology & Obstetrics 2007;99(1):46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2003 {published data only}
- Roy G, Ferreira E, Hudon L, Marquette G. The efficacy of oral versus vaginal misoprostol for second‐trimester termination of pregnancy: a double blind, randomized placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2003;189(6):S70. [Google Scholar]
Ruangchainikhom 2006 {published data only}
- Ruangchainikhom W, Phongphissanou E, Bhekasuta J, Sarapak S. Effectiveness of 400 or 600 micrograms of vaginal misoprostol for terminations of early pregnancies. Journal of the Medical Association of Thailand 2006;89(7):928‐33. [PubMed] [Google Scholar]
Saeed 2018 {published data only}
- Saeed S, Manzoor R, Tazion S, Butt F, Badar N. Misoprostol for 1st trimester miscarriage: efficacy of vaginal versus oral misoprostol. Pakistan Journal of Medical and Health Sciences 2018;12(2):849‐52. [Google Scholar]
Salamalekis 1990 {published data only}
- Salamalekis E, Loghis C, Kassanos D, Traka A, Zourlas PA. Comparison of extra‐amniotic prostaglandin F2alpha and dinoprostone use for labor induction after second trimester intrauterine fetal death. Proceedings of 12th European Congress of Perinatal Medicine; 1990; Lyon, France. 1990:228.
Salari 2012 {published data only}
- Salari Z. Comparison of the efficacy of vaginal misoprostol with and without laminaria in second trimester induction abortion in patients referring to afzalipour hospital in 2008‐2009. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S582. [Google Scholar]
Shaheen 2017 {published data only}
- Shaheen H, Khosa MS, Hanif H. Comparison of efficacy of manual vacuum aspiration (MVA) and medical treatment in the management of first trimester missed miscarriage. Pakistan Journal of Medical and Health Sciences 2017;11(1):270‐3. [Google Scholar]
Shaikh 2008 {published data only}
- Shaikh ZA. Comparison between misoprostol alone and misoprostol with manual vacuum aspiration for the treatment of missed and incomplete miscarriage. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):83. [Google Scholar]
Shelley 2005 {published data only}
- Shelley JM, Healy D, Grover S. A randomised trial of surgical, medical and expectant management of first trimester spontaneous miscarriage. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45:122‐7. [DOI] [PubMed] [Google Scholar]
Shobeira 2007 {published data only}
- Shobeira JM, Atashkhoii S. Second trimester pregnancy termination by intravaginal and parenteral form of prostaglandin E2 [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:210.
Shochet 2012 {published data only}
- Shochet T, Diop A, Gaye A, Nayama M, Sall AB, Bukola F, et al. Sublingual misoprostol versus standard surgical care for treatment of incomplete abortion in five sub‐Saharan African countries. BMC Pregnancy and Childbirth 2012;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokry 2009 {published data only}
- Shokry M, Shahin AY, Fathalla MM, Shaaban OM. Oral misoprostol reduces vaginal bleeding following surgical evacuation for first trimester spontaneous abortion. International Journal of Gynecology & Obstetrics 2009;107(2):117‐20. [DOI] [PubMed] [Google Scholar]
Shuaib 2013 {published data only}
- Shuaib AA, Alharazi AH. Medical versus surgical termination of the first trimester missed miscarriage. Alexandria Journal of Medicine 2013;49:13‐6. [Google Scholar]
Shwekerela 2007 {published data only}
- Shwekerela B, Kalumuna R, Kipingili R, Mashaka N, Westheimer E, Clark W, et al. Misoprostol for treatment of incomplete abortion at the regional hospital level: results from Tanzania. BJOG: an international journal of obstetrics and gynaecology 2007;114(11):1363‐7. [DOI] [PubMed] [Google Scholar]
Smith 2006a {published data only}
- Smith LF, Frost J, Levitas R, Bradley H, Garcia J. Women's experiences of three early miscarriage management options: a qualitative study. British Journal of General Practice 2006;56(524):198‐205. [PMC free article] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith LF, Ewings PD, Quinlan C. Incidence of pregnancy after expectant, medical, or surgical management of spontaneous first trimester miscarriage: long term follow‐up of miscarriage treatment (MIST) randomised controlled trial. BMJ 2009;339:b3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Srikhao 2005 {published data only}
- Srikhao N, Tannirandorn Y. A comparison of vaginal misoprostol 800 microg versus 400 microg for anembryonic pregnancy: a randomized comparative trial. Journal of the Medical Association of Thailand 2005;88(Suppl 2):S41‐7. [PubMed] [Google Scholar]
Sripramote 2000 {published data only}
- Sripramote M, Chatsuphang W. A randomized comparison of oral and vaginal misoprostol for cervical priming before uterine curettage in the first trimester of pregnancy. Vajira Medical Journal 2000;44(3):207‐15. [Google Scholar]
Stockheim 2006 {published data only}
- Machtinger R, Stockheim D, Goldenberg M, Soriano D, Atlas M, Seidman DS. A randomized prospective study of misoprostol alone or combined with mifepristone for treatment of first trimester spontaneous abortion. Fertility and Sterility 2002;78(3 Suppl 1):S64. [Google Scholar]
- Stockheim D, Machtinger R, Wiser A, Dulitzky M, Soriano D, Goldenberg M, et al. A randomized prospective study of misoprostol or mifepristone followed by misoprostol when needed for the treatment of women with early pregnancy failure. Fertility and Sterility 2006;86(4):956‐60. [DOI] [PubMed] [Google Scholar]
Su 2005 {published data only}
- Su LL, Biswas A, Choolani M, Kalaichelvan V, Singh K. A prospective randomized comparison of vaginal misoprostol versus intra‐amniotic prostaglandins for midtrimester termination of pregnancy. American Journal of Obstetrics and Gynecology 2005;193:1410‐4. [DOI] [PubMed] [Google Scholar]
Suchonwanit 1999 {published data only}
- Suchonwanit P. Comparative study between vaginal misoprostol 200 mg and 400 mg in first trimester intrauterine fetal death and anembryonic gestation. Thai Journal of Obstetrics and Gynaecology 1999;11(4):263. [Google Scholar]
Surita 1997 {published data only}
- Surita FG, Cecatti JG, Pinto e Silva JL. Misoprostol versus laminaria for cervical ripening in intrauterine fetal death. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167:2):32. [Google Scholar]
Tam 2005 {published data only}
- Tam WH, Tsui MH, Lok IH, Yip SK, Yuen PM, Chung TK. Long‐term reproductive outcome subsequent to medical versus surgical treatment for miscarriage. Human Reproduction 2005;20(12):3355‐9. [DOI] [PubMed] [Google Scholar]
Tanha 2013 {published data only}
- Tanha FD. Comparison of the efficacy of two routes of misoprostol administration (sublingual and vaginal) for termination of second trimester pregnancy. en.irct.ir/trial/2327 2010. [[CRSREF: 3293102]
- Tanha FD, Golgachi T, Niroomand N, Ghajarzadeh M, Nasr R. Sublingual versus vaginal misoprostol for second trimester termination: a randomized clinical trial. Archives of Gynecology and Obstetrics 2013;287(1):65‐9. [DOI] [PubMed] [Google Scholar]
Taylor 2011 {published data only}
- Taylor J, Diop A, Blum J, Dolo O, Winikoff B. Oral misoprostol as an alternative to surgical management for incomplete abortion in Ghana. International Journal of Gynecology & Obstetrics 2011;112(1):40‐4. [DOI] [PubMed] [Google Scholar]
Thavarasah 1986 {published data only}
- Thavarasah AS, Almohdzar SA. Prostaglandin (F2alpha) in missed abortion. Intravenous extra‐amniotic and intramuscular administration ‐ a randomized study. Biological Research in Pregnancy 1986;7:106‐10. [PubMed] [Google Scholar]
Thida 2015 {published data only}
- Thida M, Shwe MM, Htun KT, Maung NM, Khine EP, Win KS, et al. A randomised clinical trial comparing different routes of administration of repeated doses of 400ug misoprostol for management of missed miscarriages and anembryonic gestations in North Okkalapa General Hospital, Yangon, Myanmar. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):26‐7. [Google Scholar]
Toppozada 1994 {published data only}
- Toppozada MK, Shaala SA, Anwar MY, Haiba NA, Abdrabbo S, El‐Absy HM. Termination of pregnancy with fetal death in the second and third trimesters ‐ the double balloon versus extra‐amniotic prostaglandin. International Journal of Gynecology & Obstetrics 1994;45:269‐73. [DOI] [PubMed] [Google Scholar]
Toptas 2011 {published data only}
- Toptas T, Mendilcioglu I, Simsek M, Taskin O. Comparison of intravaginal misoprostol alone to combination of intravaginal misoprostol and extraamniotic Foley catheter for the second trimester of pregnancies. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S126. [Google Scholar]
Torre 2012 {published data only}
- Rosenberg P. Expectant versus immediate medical management for the evacuation of the non evolutive pregnancies before 13 GW. clinicaltrials.gov/ct2/show/NCT00190294 (first received 19 September 2005).
- Torre A, Huchon C, Bussieres L, Machevin E, Camus E, Fauconnier A. Immediate versus delayed medical treatment for first‐trimester miscarriage: a randomized trial. American Journal of Obstetrics & Gynecology 2012;206(3):215.e1‐6. [DOI] [PubMed] [Google Scholar]
Van Mensel 2009 {published data only}
- Mensel K, Claerhout F, Debois P, Keirse MJ, Hanssens M. A randomized controlled trial of misoprostol and sulprostone to end pregnancy after fetal death [Article ID 496320]. Obstetrics and Gynecology International 2009. [DOI] [PMC free article] [PubMed]
Yapar 1996 {published data only}
- Yapar EG, Senoz S, Urkutur M, Batioglu S, Gokmen O. Second trimester pregnancy termination including fetal death: comparison of five different methods. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:97‐102. [DOI] [PubMed] [Google Scholar]
Yilmaz 2005 {published data only}
- Yilmaz B, Kelekci S, Ertas IE, Kahyaoglu S, Ozel M, Sut N, et al. Misoprostol moistened with acetic acid or saline for second trimester pregnancy termination: a randomized prospective double‐blind trial. Human Reproduction 2005;20(11):3067‐71. [DOI] [PubMed] [Google Scholar]
Yilmaz 2007 {published data only}
- Yilmaz B, Kelekci S, Ertas IE, Ozel M, Sut N, Mollamahmutoglu L, et al. Randomized comparison of second trimester pregnancy termination utilizing saline moistened or dry misoprostol. Archives of Gynecology and Obstetrics 2007;276(5):511‐6. [DOI] [PubMed] [Google Scholar]
Zanganeh 2012 {published data only}
- Zanganeh M. Comparing the effects of multiple doses of misoprostol with single dose of misoprostol plus oxitocin in induction of second trimester abortion. en.irct.ir/trial/3910 (first received 12 June 2010).
- Zangeneh M, Malek‐Khosravi S, Veisi F, Rezavand N, Rezaee M, Rajatee M. Multiple‐dose vaginal misoprostol and single‐dose misoprostol plus oxytocin for termination of second‐trimester pregnancy. International Journal of Gynaecology and Obstetrics 2012;117(1):78‐80. [PUBMED: 22261129] [DOI] [PubMed] [Google Scholar]
Zhang 2000 {published data only}
- Zhang C, Cheng W. A contrastive analysis of the efficacy of misoprostol and li fan nuo in intermediate term of pregnancy. Journal of Wuhan University of Science and Technology (Natural Science Edition) 2000;23(4):409‐11. [Google Scholar]
Zhang 2005 {published data only}
- Chen BA, Reeves MF, Creinin MD, Gilles JM, Barnhart K, Westhoff C, et al. Misoprostol for treatment of early pregnancy failure in women with previous uterine surgery. American Journal of Obstetrics & Gynecology 2008;198(6):626.e1‐626.e5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creinin MD, Huang X, Westhoff C, Barnhart K, Gilles JM, Zhang J, et al. Factors related to successful misoprostol treatment for early pregnancy failure. Obstetrics & Gynecology 2006;107(4):901‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AR, Hendlish SK, Westhoff C, Frederick MM, Zhang J, Gilles JM, et al. Bleeding patterns after misoprostol vs surgical treatment of early pregnancy failure: results from a randomized trial. American Journal of Obstetrics and Gynecology 2007;196(1):31. [10.1016/j.ajog.2006.07.053] [DOI] [PubMed] [Google Scholar]
- Harwood B, Nansel T, National Institute of Child Health and Human Development Management of Early Pregnancy Failure Trial. Quality of life and acceptability of medical versus surgical management of early pregnancy failure. BJOG: an international journal of obstetrics and gynaecology 2008;115(4):501‐8. [DOI: 10.1111/j.1471-0528.2007.01632.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch M, Lorch S, Chung K, Frederick M, Zhang J, Barnhart K. A cost‐effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertility and Sterility 2012;97(2):355‐60.e1. [DOI: 10.1016/j.fertnstert.2011.11.044] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. New England Journal of Medicine 2005;353:761‐9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gilles K, Barnhart K, Creinin M, Westhoff C, Frederick M. Medical management with misoprostol for early pregnancy failure: a multicenter, randomized equivalence trial. Fertility and Sterility 2004;82 Suppl 2:S53‐S54. [Google Scholar]
References to ongoing studies
ACTRN12615000483550 {published data only}
- Abdellah AS. Clinical randomized trial to compare efficacy and safety of vaginal and buccal misoprostol in second trimester abortion due to intrauterine fetal death. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367522 (first received 15 May 2015).
Ali 2017 {published data only}
- Ali MK, NCT03148314. Home‐based extended low dose buccal misoprostol versus hospital‐based standard vaginal dose in management of first trimester missed abortion. https://clinicaltrials.gov/ct2/show/NCT03148314 (first received 11 May 2017).
El Shahawy 2016 {published data only}
- Shahawy A, NCT02686840. Sublingual versus vaginal misoprostol in medical treatment of first trimestric missed miscarriage: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT02686840 (first received 22 February 2016).
NCT02620904 {published data only}
- Atrio J. Mifepristone induction for fetal demise, a randomized control trial. clinicaltrials.gov/ct2/show/NCT02620904 (first received 1 December 2015).
NCT02633761 {published data only}
- Bracken H. Mifepristone and misoprostol versus misoprostol alone for treatment of fetal death at 14‐28 weeks of pregnancy: a randomized, placebo‐controlled double‐blinded trial. clinicaltrials.gov/ct2/show/NCT02633761 (first received 17 December 2015).
NCT03212352 2017 {published data only}
- NCT03212352. Comparing two medical treatments for early pregnancy failure. https://clinicaltrials.gov/show/nct03212352 (first received 11 July 2017).
Additional references
Alfirevic 2014
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001338.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashok 1998
- Ashok PW, Penney GC, Flett GM, Templeton A. An effective regimen for early medical abortion: a report of 2000 consecutive cases. Human Reproduction 1998;13:2962‐5. [DOI] [PubMed] [Google Scholar]
Baulieu 1986
- Baulieu E, Ulmann A. Antiprogesterone activity of RU‐486 and its contragestive and other applications. Human Reproduction 1986;1:107‐10. [PUBMED: 3031127] [DOI] [PubMed] [Google Scholar]
Bugalho 1996
- Bugalho A, Faundes A, Jamisse L, Usfa M, Maria E, Bique C. Evaluation of the effectiveness of vaginal misoprostol to induce first trimester abortion. Contraception 1996;53:244‐6. [DOI] [PubMed] [Google Scholar]
Cameron 1986
- Cameron IT, Michie AF, Baird DT. Therapeutic abortion in early pregnancy with antiprogestogen RU486 alone or in combination with prostaglandin analogue (gemeprost). Contraception 1986;34(5):459‐68. [PUBMED: 3816230] [DOI] [PubMed] [Google Scholar]
Costa 1993
- Costa SH, Vessey MP. Misoprostol and illegal abortion in Rio de Janeiro, Brazil. Lancet 1993;341:1258‐61. [PUBMED: 8098402] [DOI] [PubMed] [Google Scholar]
Graziosi 2005
- Graziosi GC, Steeg JW, Reuwer PJ, Drogtrop A, Bruinse HW, Mol BW. Economic evaluation of misoprostol in the treatment of early pregnancy failure compared to curettage after an expectant management. Human Reproduction 2005;20:1067‐71. [DOI] [PubMed] [Google Scholar]
Grudzinskas 1995
- Grudzinskas JG. Endocrinological and metabolical assessment of early pregnancy. In: Chamberlain G editor(s). Turnbull's Obstetrics. London: Pearson Professional Ltd, 1995:185‐93. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2010
- Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howie 1995
- Howie PG. Abortion and ectopic pregnancy. In: Whitfield CR editor(s). Dewhurst's Textbook of Obstetrics and Gynecology for Postgraduates. Oxford: Blackwell Science Ltd, 1995:140‐63. [Google Scholar]
Kim 2017
- Kim C, Barnard S, Neilson JP, Hickey M, Vazquez JC, Dou L. Medical treatments for incomplete miscarriage. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD007223.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovacs 1984
- Kovacs L, Sas M, Resch BA, Ugocsai G, Swahn ML, Bygdeman, et al. Termination of very early pregnancy by RU 486‐‐an antiprogestational compound. Contraception 1984;29(5):399‐410. [DOI] [PubMed] [Google Scholar]
Kulier 2011
- Kulier R, Kapp N, Gulmezoglu AM, Hofmeyr GJ, Cheng LN, Campana A. Medical methods for first trimester abortion. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD002855.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousa 2014
- Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003249.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nanda 2012
- Nanda K, Lopez LM, Grimes DA, Peloggia A, Nanda G. Expectant care versus surgical treatment for miscarriage. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD003518.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrou 2006
- Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of first‐trimester miscarriage based on results from the MIST trial. BJOG: an international journal of obstetrics & gynaecology 2006;113(8):879‐89. [DOI: 10.1111/j.1471-0528.2006.00998.x; CRSREF: 3293040] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2002
- Say L, Kulier R, Gulmezoglu AM, Campana A. Medical versus surgical methods for first trimester termination of pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003037.pub2] [DOI] [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitworth 2015
- Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD007058.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wieringa 2002
- Wieringa‐de Waard M, Vos J, Bonsel GJ, Bindels PJ, Ankum WM. Management of miscarriage: a randomized controlled trial of expectant management versus surgical evacuation. Human Reproduction 2002;17(9):2445‐50. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Neilson 2006
- Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002253.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vazquez 2000
- Vazquez JC, Hickey M, Neilson JP. Medical management for miscarriage. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002253] [DOI] [Google Scholar]
Vazquez 2006
- Vazquez JC, Hickey M, Neilson JP. Medical management for miscarriage. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD002253.pub2] [DOI] [Google Scholar]


