Significance
Adipose tissue inflammation is a key mediator linking obesity to metabolic complications. In obesity, proinflammatory responses are rapidly elevated in visceral adipose tissue, whereas inflammatory responses in subcutaneous adipose tissues are relatively down-regulated. Nonetheless, among the adipose tissue components other than immune cells, the modulators of fat depot-selective inflammatory response is not well understood. Here, we show that γ-aminobutyric acid (GABA) reduces obesity-induced adipose tissue macrophage (ATM) infiltration in subcutaneous inguinal adipose tissues (IAT), but not in visceral epididymal adipose tissue. Our data show that adipose-derived stem cells from IAT respond to GABA to suppress ATM infiltration. Consequently, GABA shows enhanced insulin action in obese IAT, which could be a possible contributor in the regulation of whole-body glucose metabolism.
Keywords: adipose tissue macrophage (ATM), epididymal adipose tissue (EAT), inguinal adipose tissue (IAT), gamma (γ)-aminobutyric acid (GABA), adipose-derived stem cell (ADSC)
Abstract
Accumulating evidence suggests that subcutaneous and visceral adipose tissues are differentially associated with metabolic disorders. In obesity, subcutaneous adipose tissue is beneficial for metabolic homeostasis because of repressed inflammation. However, the underlying mechanism remains unclear. Here, we demonstrate that γ-aminobutyric acid (GABA) sensitivity is crucial in determining fat depot-selective adipose tissue macrophage (ATM) infiltration in obesity. In diet-induced obesity, GABA reduced monocyte migration in subcutaneous inguinal adipose tissue (IAT), but not in visceral epididymal adipose tissue (EAT). Pharmacological modulation of the GABAB receptor affected the levels of ATM infiltration and adipose tissue inflammation in IAT, but not in EAT, and GABA administration ameliorated systemic insulin resistance and enhanced insulin-dependent glucose uptake in IAT, accompanied by lower inflammatory responses. Intriguingly, compared with adipose-derived stem cells (ADSCs) from EAT, IAT-ADSCs played key roles in mediating GABA responses that repressed ATM infiltration in high-fat diet-fed mice. These data suggest that selective GABA responses in IAT contribute to fat depot-selective suppression of inflammatory responses and protection from insulin resistance in obesity.
Adipose tissue, developmentally derived from the mesoderm, is capable of expanding to accommodate excess energy, which distinguishes it from other metabolic tissues (1). Functionally, while white adipose tissue is specialized in storing extra energy sources into unilocular lipid droplet, brown adipose tissue is primarily specialized in generating heat with uncoupling protein-1 (1). Anatomically, white adipose tissue is largely subdivided into visceral adipose tissue and subcutaneous adipose tissue (2, 3). Visceral adipose tissue is found in the abdominal cavity and readily communicates with internal organs. On the other hand, subcutaneous adipose tissue protects organs from physical damage and contributes to the maintenance of body temperature. In obesity, expansion of visceral adipose tissue is closely linked to metabolic diseases, including insulin resistance (4, 5). However, expansion of subcutaneous adipose tissue appears to protect against metabolic complications (6, 7). In accordance herewith, transplantation of subcutaneous adipose tissue into visceral depots improved systemic glucose intolerances upon high-fat diet (HFD) (8). In addition, subcutaneous adipose tissue-transplanted recipients express high levels of antidiabetic adipokines (9, 10), implying that subcutaneous adipose tissue expansion in obesity mediate metabolic benefits by reducing lipotoxicity and insulin resistance. Nonetheless, the mechanisms by which subcutaneous adipose tissue expansion in obesity is less detrimental in causing metabolic complications are yet unclear.
In obesity, adipose tissue undergoes numerous remodeling processes, such as proinflammatory responses, endoplasmic reticulum stress, hypoxia, and mitochondrial dysregulation (2, 11). Among these, inflammatory responses are one of the major risk factors for insulin resistance in obesity (12–14). For example, inflammatory cytokines, such as TNF-α, inhibit insulin signaling by inhibiting phosphorylation of insulin receptor substrate-1 (IRS-1) (15, 16). In addition, inflammatory responses in adipocytes increase free fatty acids, facilitating insulin resistance (17, 18). In obesity, enhanced adipose tissue macrophages (ATMs) play key roles in inflammatory responses in adipose tissue (19–22). ATM depletion or inactivation in mice ameliorates proinflammatory responses as well as insulin resistance, indicating that ATMs might be important for insulin resistance in obesity (23, 24). Furthermore, it has been shown that in obesity, visceral adipose tissue seems to be more susceptible to ATM accumulation and proinflammatory responses than subcutaneous adipose tissue (20, 25), and subcutaneous fat transplantation into visceral fat relieves circulating inflammatory cytokine levels (10). Similarly, obese humans with higher subcutaneous adiposity exhibited lower inflammatory profiles and lower insulin resistance than those with higher visceral adiposity (26). Although compelling evidence suggests that subcutaneous adipose tissues are less inflammatory and are beneficial for energy metabolism, the mechanisms that render subcutaneous adipose tissue less susceptible to ATM accumulation and proinflammatory responses in obesity are still unclear.
In this study, we aimed to understand the molecular mechanisms by which subcutaneous inguinal adipose tissue (IAT) accumulates fewer ATMs than does visceral epididymal adipose tissue (EAT) in obesity. To examine environmental and intrinsic factors that are crucial in the regulation of differential ATM accumulation in obese EAT and obese IAT, we performed fat-tissue transplantation. We used transcriptome analysis to delineate intrinsic differences between EAT and IAT. Moreover, we performed both ex vivo and in vivo fluorescently labeled mononuclear cell (MNC) migration assays to find out the mechanism in explaining fat depot-selective differential ATM infiltration in obesity. Collectively, our data suggest that the γ-aminobutyric acid (GABA) response is an important factor in determining fat depot-selective ATM infiltration and proinflammatory responses in obesity.
Results
IAT Is Less Prone to Recruit ATM in Obesity.
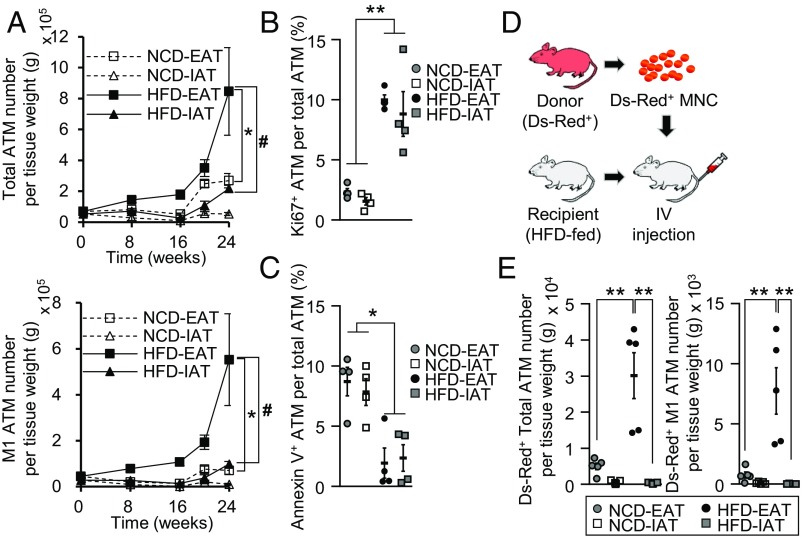
To investigate the extent of inflammatory responses in subcutaneous IAT and visceral EAT, 8-wk-old mice were fed either normal chow diet (NCD) or HFD for 10 wk. As expected, body weights were gradually increased over time upon HFD feeding (SI Appendix, Fig. S1A). As shown in SI Appendix, Fig. S1B, however, EAT and IAT showed different tissue weight-gain patterns upon HFD. When adipocyte size was measured, hypertrophic adipocytes were more abundant in EAT than in IAT upon HFD (SI Appendix, Fig. S1C). Given that proinflammatory responses are elevated in obese adipose tissue, inflammatory gene expression was examined in EAT and IAT. Consistent with previous reports (27, 28), IAT from HFD-fed mice exhibited substantially lower proinflammatory gene expression than EAT from HFD-fed mice (SI Appendix, Fig. S1 D and E). Because increased ATMs have been implicated in obesity-induced adipose tissue inflammation, total ATMs (CD11b+ and F4/80+) and proinflammatory M1-like ATMs (CD11b+, F4/80+, and CD11c+) were counted. Similar to previous findings (20, 29), ATM accumulation was significantly higher in obese EAT than in obese IAT (Fig. 1A and SI Appendix, Fig. S1F). Similarly, different degrees of ATM accumulation in EAT and IAT were also observed in genetically obese and diabetic db/db mice (SI Appendix, Fig. S1G), implying that IAT is less prone to ATM accumulation in obesity. In obese human subjects, the mRNA levels of proinflammatory genes and ATM marker genes were less elevated in subcutaneous adipose tissue than in visceral adipose tissues (SI Appendix, Fig. S1H).
Fig. 1.
ATM infiltration is down-regulated in obese IAT compared with obese EAT. (A) Flow cytometry of (CD11b+ and F4/80+) ATMs (Upper) and (CD11b+, F4/80+, and CD11c+) M1-like ATMs (Lower) of IAT and EAT in the progression of DIO. (B and C) Relative fractions of (B) Ki67+ proliferative and (C) annexin V+ apoptotic ATMs were measured in IAT and EAT from NCD- or HFD-fed mice. (D) An experimental strategy of the in vivo ATM infiltration assay. Recipient mice were intravenously injected with either PBS or Ds-Red+ MNCs isolated from Ds-Red transgenic mice. The mice were rested for 24 h and analyzed for ATM infiltration in IAT and EAT from HFD-fed mice. (E) Flow cytometry of Ds-Red+ ATMs (Left) and M1-like ATMs (Right) in IAT and EAT. Error bars represent means ± SEs of treatment groups. *P < 0.05, #P < 0.05, **P < 0.01. n = 4 or 5 for each experimental group. For B, C, and E, 8-wk-old mice were fed HFD for 10 wk before being killed.
Next, we asked how IAT would reveal less ATM accumulation in diet-induced obesity (DIO). Because ATM accumulation could be determined by proliferation, apoptosis, and infiltration (30–32), we decided to test these processes in HFD-fed mice. In accordance with previous reports (30, 31), the portion of Ki67+ proliferative ATMs was greatly expanded in obese EAT compared with lean EAT (Fig. 1B). On the other hand, the portion of annexin V+ dead ATMs was markedly lower in obese EAT than in lean EAT (Fig. 1C). However, there were no significant differences in proliferative and dead ATM populations between EAT and IAT (Fig. 1 B and C). To assess whether ATM infiltration might be involved, exogenous Ds-Red+ MNCs were injected into recipient HFD-fed mice, and the degree of exogenous ATM infiltration into each fat depot was evaluated (Fig. 1D). As shown in Fig. 1E and SI Appendix, Fig. S1 I and J, the levels of infiltrated Ds-Red+ total ATMs and Ds-Red+ M1-like ATMs were significantly elevated in EAT from HFD-fed mice. In contrast, the numbers of Ds-Red+ ATMs and M1-like ATMs were significantly lower in IAT from HFD-fed mice. Taken together, these data suggest that obese IAT is less susceptible to ATM accumulation due to lower macrophage infiltration rather than proliferation or apoptosis of ATM.
Intrinsic Characters of Adipose Tissue Determine Fat Depot-Selective ATM Infiltration.
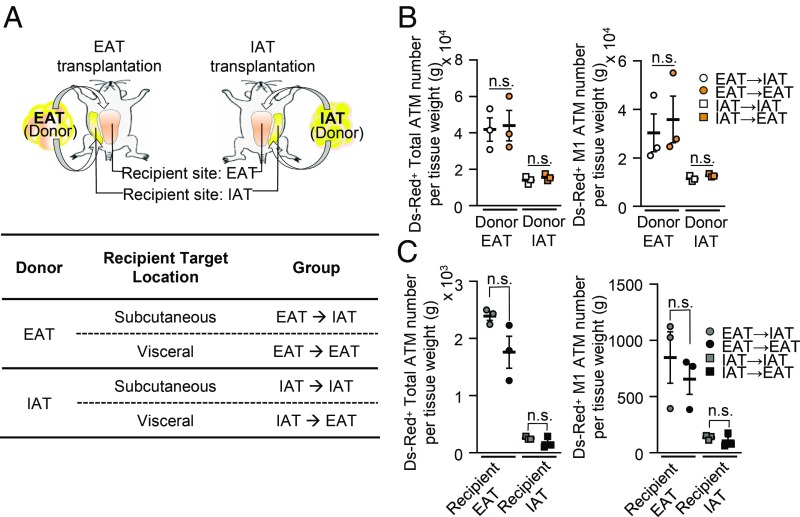
As it has been suggested that in vivo adipogenesis is affected by the adipogenic niche (33, 34), we asked whether fat depot-selective environmental factors might determine ATM infiltration in obesity. To address this, EAT and IAT from HFD-fed donor mice were transplanted into HFD-fed recipient mice. As shown in Fig. 2A, fat tissue transplantation groups were named as “EAT → IAT,” “EAT → EAT,” “IAT → IAT,” and “IAT → EAT.” Consistent with a previous report (8), IAT-transplanted mice showed beneficial effects on metabolic parameters, such as decreased body weight gain and improved glucose tolerance, compared with EAT-transplanted mice (SI Appendix, Fig. S2 A and B). All donor fat tissues were well-vascularized after surgery (SI Appendix, Fig. S2C). In recipient HFD-fed mice, the extent of ATM infiltration was investigated with Ds-Red+ MNCs. As shown in Fig. 2B, the numbers of Ds-Red+ ATMs in donor fat tissues from the EAT → EAT and EAT → IAT groups were greatly higher than those from the IAT → EAT and IAT → IAT groups. When the same donor fat tissue was transplanted into different recipient fat depots, the degree of Ds-Red+ ATM infiltration in each donor fat tissue was similar between EAT → EAT and EAT → IAT, and between IAT → EAT and IAT → IAT. These data implied that environmental factors of EAT or IAT would not be crucial to decide the degree of exogenous Ds-Red+ macrophage infiltration in obesity. When we examined the effects of fat depot transplantation on ATM infiltration in recipient fat tissue, the degrees of ATM infiltration into recipient IAT or EAT were comparable regardless of donor fat tissues (Fig. 2C). Consistently, H&E staining of donor fat tissues from the EAT → EAT and EAT → IAT groups revealed severe fibrosis and crown-like structures compared with the IAT → EAT and IAT → IAT groups (SI Appendix, Fig. S2 D and E). Taken together, these data suggest that fat depot-selective ATM infiltration in DIO results from intrinsic characters of fat tissue rather than environmental or niche effects of host fat tissue.
Fig. 2.
Intrinsic characters of fat depot determine ATM infiltration in DIO. (A) Illustration of adipose tissue transplantation. Each donor sample of obese IAT or obese EAT was transplanted at either a subcutaneous or a visceral location in HFD-fed recipient mice. Nomenclature of transplanted donor adipose tissues from each group is indicated in the table below the illustration. (B) Comparison of the amounts of Ds-Red+ ATM infiltration (Left) and M1-like ATMs (Right) in transplanted donor adipose tissues. (C) Comparison of the number of Ds-Red+ ATMs (Left) and M1-like ATMs (Right) infiltrated in recipient adipose tissues. Error bars represent means ± SEs of treatment groups. n.s., not significant. n = 3 for each experimental group.
GABA Signaling Differs Between EAT and IAT.
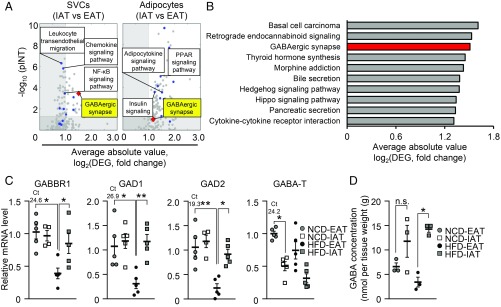
To elucidate which cell types might determine fat depot-selective ATM infiltration, monocyte migration was assessed using conditioned media (CM) from adipocytes or stromal vascular cells (SVCs) from each fat depot (SI Appendix, Fig. S3A). As shown in SI Appendix, Fig. S3B, monocyte migration was prominently down-regulated in CM from SVCs compared with CM from adipocytes. Next, we analyzed the transcriptomes of adipocytes and SVCs from obese IAT and obese EAT (35). As shown in SI Appendix, Fig. S3C, numerous genes were differentially expressed between IAT and EAT. Through comparative Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of adipocytes and SVCs from each fat depot of HFD-fed mice (SI Appendix, Fig. S3D), we found that the “GABAergic synapse” was differentially regulated between SVCs from obese IAT (IAT-SVC) and SVCs from obese EAT (EAT-SVCs) (Fig. 3 A and B). The degree of differential expression of GABAergic synapse between IAT-SVC and EAT-SVC was ranked higher than immune and inflammatory signals (SI Appendix, Fig. S3E). Consistently, the number of differentially expressed genes (DEGs) in GABAergic synapse was higher in SVCs than in adipocytes (SI Appendix, Fig. S3F). G proteins (Gng11 and Gnb4) and GABAB receptor 1 (Gabbr1) were the most strongly increased in IAT-SVCs, whereas adenylyl cyclases (Adcy7 and Adcy8) were among the DEGs showing the lowest decrease in IAT-SVCs (SI Appendix, Fig. S3G). Moreover, a bipartite graph of pathways and genes generated on the basis of functional enrichment analyses showed that GABAergic synapse might be associated with cell adhesion and extracellular matrix receptor interaction, which seems to be related to innate immune cell infiltration (SI Appendix, Fig. S3H).
Fig. 3.
In obesity, GABA signaling is differentially regulated in IAT and EAT. (A) Half-volcano plots showing the degree of differential expression of KEGG pathways in SVCs (Left) and adipocytes (Right) from IAT and EAT. The x axis indicates the average absolute fold-change (log2) of DEGs in each pathway. The y axis indicates the negative log10 P value (pINT) from signaling pathway impact analysis. Circular dots (blue) indicate pathways categorized in immunity, inflammation, and energy metabolism. The rhombus dot (red) indicates the “GABAergic synapse” KEGG pathway. (B) KEGG pathways, the most strongly differentially expressed between IAT-SVC and EAT-SVC from HFD-fed mice. The x axis of the bar plot indicates the score (log2) of average fold-changes of DEGs in a single pathway. (C) qRT-PCR analyses of GABAergic genes in IAT and EAT from NCD- and HFD-fed mice. Threshold cycle (Ct) values of each mRNA from NCD-EAT are denoted. n = 4 or 5 for each experimental group for analysis of RNA-sequencing. (D) GABA concentrations in NCD and HFD-fed mice. n = 3 for each experimental group. Error bars represent means ± SEs of treatment groups *P < 0.05, **P < 0.01, n.s., not significant.
GABA is an inhibitory neurotransmitter synthesized by glutamate decarboxylase 1 (GAD1) and 2 (GAD2) (36). GABA is further converted into succinate by GABA transaminase (GABA-T) or is secreted to activate GABAA or GABAB receptors (37, 38). Because GABA signaling has been primarily studied in the brain, the mRNA levels of GABA receptor genes in fat tissue needed to be examined. mRNA levels of the GABAB receptor, a heterodimer composed of GABBR1 and GABAB receptor 2 (GABBR2), was abundantly expressed in fat tissues, whereas the subunits of GABAA receptor were hardly expressed in most peripheral tissues (SI Appendix, Fig. S4 A and B). In HFD-fed mice, GABBR1, GAD1, GAD2, and GABA-T genes were differentially expressed in IAT and EAT (Fig. 3C). Next, to examine whether differential mRNA levels of GABAergic genes, such as GAD1 and GAD, in IAT and EAT might affect the level of GABA in each fat depot, we measured GABA concentrations in IAT and EAT. In obesity, the level of GABA was higher in IAT (Fig. 3D), implying that differential expression of GAD1 and GAD2 might contribute to different amounts of GABA in the two fat depots. Metabolomic analyses revealed that the circulating level of GABA was significantly decreased in obesity (SI Appendix, Table S1). Accordingly, differential mRNA expression of GABBR1 and GABA-T was also observed in human obese subjects (SI Appendix, Fig. S4 C and D). These results led us to hypothesize that differential GABA responses might be associated with fat depot-selective ATM infiltration in obesity.
GABA Signaling in IAT-SVCs Down-Regulates Monocyte Migration.
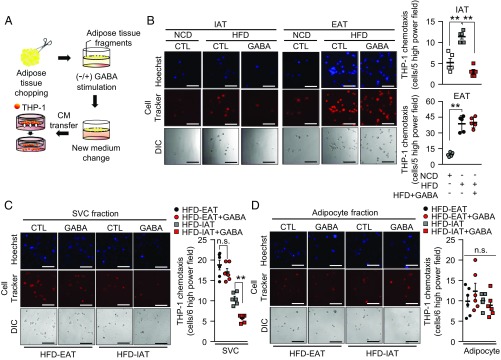
Given that GABA reportedly has antiinflammatory roles in the central nervous system (39), we hypothesized that different GABAergic actions in IAT and EAT might play certain roles in fat depot-selective ATM infiltration in obesity. To test this, IAT or EAT preincubated with GABA and CM collected from each fat tissue type were subjected to monocyte migration assays (Fig. 4A). As shown in Fig. 4B, CM from GABA pretreated IAT reduced the numbers of migrated monocytes. However, such an effect was not observed in CM from GABA pretreated EAT, indicating that the GABA response in IAT might selectively down-regulate ATM infiltration in obesity. When we examined direct effects of GABA on monocyte recruitment, the numbers of migrated monocytes were not modulated by GABA (SI Appendix, Fig. S5A). Then, we examined whether GABA-dependent fat depot-selective monocyte recruitment might be derived from SVCs or adipocytes of each obese fat depot (SI Appendix, Fig. S5B). The degree of monocyte migration was selectively lower in CM from GABA pretreated IAT-SVCs (Fig. 4C), whereas CM from adipocytes with or without GABA pretreatment did not show any difference in monocyte migration (Fig. 4D). In agreement with HFD data (Fig. 4 C and D), IAT-SVC from NCD-fed mice selectively inhibited monocyte migration (SI Appendix, Fig. S5 C and D). Because THP-1 is human monocyte cell line, we decided to determine whether the GABA-treated IAT also effectively suppress migration of mouse primary monocytes. As shown in SI Appendix, Fig. S5E, the number of migrated monocytes was selectively reduced by CM from GABA-treated IAT. Additionally, CM from GABA-treated female IAT exhibited antichemotactic effects on monocyte migration (SI Appendix, Fig. S5F), implying that GABA would suppress monocyte migration in IAT from both male and female mice. These data propose that the lower ATM infiltration in obese IAT would be attributable to GABA responses in IAT-SVCs.
Fig. 4.
In obesity, the GABA response determines selective repression of monocyte migration into IAT. (A) A scheme of monocyte migration assay with or without upon GABA treatment. (B) Monocyte migration in IAT (Left) and EAT (Center). Quantification of monocyte migration from CM of GABA-stimulated IAT (Upper Right) and EAT (Lower Right). (A and B) THP-1 monocytes were incubated in Transwell for 12 h. n = 5 for each experimental group. (Scale bars, 100 μm.) (C and D) From the HFD-fed mice, IAT and EAT were fractionated into SVCs and adipocytes. Measurements of THP-1 monocyte migration in CM of GABA-treated (C) SVC fraction, and (D) adipocyte fraction. THP-1 was incubated in Transwell for 12 h. n = 6 for each experimental group. The number of migrated monocytes is indicated in the right of each figure. (Scale bars, 100 μm.) Error bars represent means ± SEs of treatment groups. **P < 0.01, n.s., not significant.
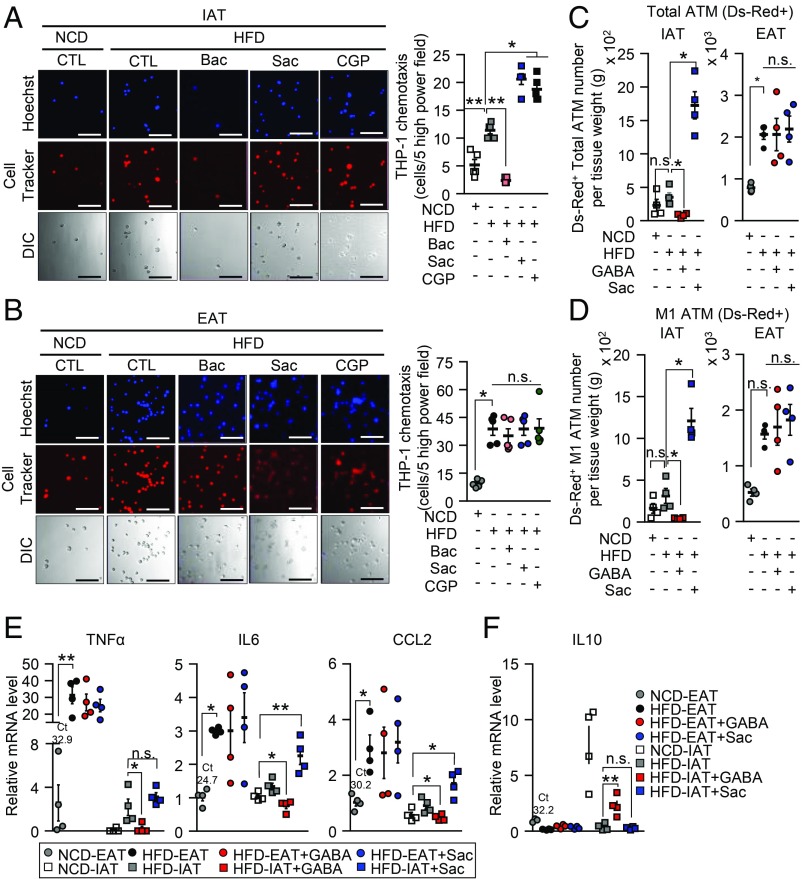
GABAB Receptor Mediates Amelioration of ATM Infiltration and Proinflammatory Responses in Obese IAT.
Because both the GABAA and the GABAB receptor are activated by GABA, we decided to test whether GABA-dependent suppression of ATM recruitment from IAT-SVCs might be mediated by GABAA and the GABAB receptor. To this end, we used baclofen (Bac), a GABAB receptor agonist (40), and saclofen (Sac) and CGP35348 (CGP), which are GABAB receptor antagonists (41, 42). In Bac pretreated IAT, monocyte migration was markedly suppressed (Fig. 5A). Conversely, inhibition of GABAB receptor by Sac or CGP promoted monocyte migration in IAT from HFD-fed mice (Fig. 5A). Such effects were not observed in obese EAT with or without GABAB agonist or antagonists (Fig. 5B). In addition, when we tested the effect of the GABAA receptor on fat depot-selective monocyte migration, bicuculline methiodide (Bicu), a GABAA receptor selective antagonist, did not affect the intrinsic activity of IAT or EAT in terms of monocyte migration (SI Appendix, Fig. S6 A and B). Then, to validate the effect of GABAB receptor signaling on ATM infiltration in obese IAT in vivo, HFD-fed mice were intraperitoneally injected with either GABA or Sac before intravenous injection of Ds-Red+ MNCs (SI Appendix, Fig. S6C). As shown in Fig. 5 C and D, IAT showed selective suppression of total ATM and M1-like ATM infiltration upon GABA stimulation, which was not observed in EAT. In contrast, GABAB receptor inhibition with Sac augmented not only ATM infiltration, but also proinflammatory M1-like ATM infiltration into obese IAT, which were not detected in obese EAT (Fig. 5 C and D). Because elevated ATM infiltration is closely linked to proinflammatory responses in obesity (32), we further hypothesized that GABAB receptor signaling in IAT might affect inflammatory responses as well as ATM infiltration. In obese IAT, mRNA levels of proinflammatory genes, such as CCL2, IL6, and TNFα were markedly reduced by GABA but increased by Sac (Fig. 5E). On the other hand, mRNA expression of an antiinflammatory gene, such as IL10, tended to be elevated in obese IAT upon GABA (Fig. 5F). Accordingly, the phosphorylation levels of JNK and p38, markers for proinflammatory signaling cascade, were enhanced by Sac in obese IAT (SI Appendix, Fig. S6D). Taken together, these data suggest that activation of GABAB receptor signaling would mediate the suppression of ATM infiltration and inflammatory responses in obese IAT.
Fig. 5.
In obese IAT, activation of GABAB receptor signaling suppresses ATM infiltration. (A and B) Activation of GABAB receptor by Bac treatment or inhibition of GABAB receptor signaling using Sac and CGP in THP-1 monocyte migration assays. Each CM sample was collected from either (A) IAT or (B) EAT with or without GABAB receptor agonist or antagonist. THP-1 monocytes were incubated in Transwell for 12 h. (Scale bars, 100 μm.) n = 5 for each experimental group. (C and D) Fat depot-selective (C) ATM and (D) M1-like ATM infiltration in IAT (Left) and EAT (Right). n = 4 for each experimental group. (E and F) mRNA levels of (E) the proinflammatory genes and (F) the antiinflammatory IL-10 were measured with or without GABA and Sac treatment. n = 4 for each experimental group. Average Ct values of NCD-EAT are denoted. Error bars represent means ± SEs of treatment groups. *P < 0.05, **P < 0.01, n.s., not significant.
GABA Improves Insulin Resistance in DIO.
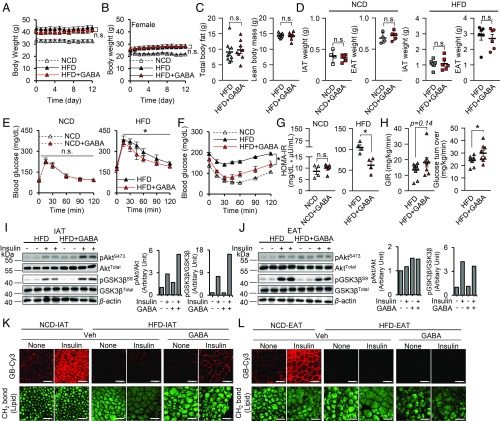
As proinflammatory responses in obese adipose tissue have been implicated in insulin resistance (12), we raised the question of whether GABA stimulation might ameliorate insulin resistance in HFD-fed mice. Two weeks of daily intraperitoneal administration of GABA (50 mg/kg) did not alter adiposity in male and female HFD-fed mice (Fig. 6 A–D). Under the same condition, adipose inflammatory responses and the number of ATMs were selectively reduced in IAT, but not in EAT (SI Appendix, Fig. S7 A–C). On the other hand, blood glucose level was lowered after 3 d of GABA administration (SI Appendix, Fig. S7D). In addition, glucose tolerance tests and insulin tolerance tests revealed that GABA administration improved systemic insulin resistance and glucose clearance in HFD-fed male and female mice, but not in NCD-fed mice (Fig. 6 E–H and SI Appendix, Fig. S7E).
Fig. 6.
In obesity, GABA treatment alleviates systemic insulin resistance and enhances glucose uptake in IAT. HFD-fed mice were injected daily with GABA for 2 wk. Their littermates were given either NCD or HFD and were injected with the vehicle as controls. (A and B) Measurements of body weight from (A) male mice, and (B) female mice. (C) Total body fat composition (Left) and lean body mass (Right). (D) Measurement of IAT weight and EAT weight. (E and F) Measurements of (E) glucose tolerance and (F) insulin tolerance. Mice were intraperitoneally given either (E) glucose or (F) insulin and measured the level of blood glucose in each time point. (G) Measurement of homeostatic model assessment for insulin resistance (HOMA-IR). (H) A hyperinsulinemic-euglycemic clamp study was performed in HFD and HFD+GABA groups to measure glucose infusion rate (GIR) and glucose turnover rate. (I and J) Western blot images (Left) and quantification (Center and Right) with ImageJ (NIH) for insulin signaling cascades in (I) IAT and (J) EAT. Phosphorylation of Akt (pAktS473) (Center) and GSK3β (pGSK3βS9) (Right) was quantified in each adipose tissue. Total Akt, total GSK3β, and β-actin were measured as controls. (K and L) Measurement of insulin-dependent glucose uptake ability. Insulin-dependent glucose uptake was measured in (K) IAT and (L) EAT using Cy3 fluorescence conjugated glucose bioprobe (GB-Cy3). Adipose tissue lipid droplets (green) were visualized by TCS SP8 CARS microscopy. (Scale bars, 100 μm.) Error bars represent means ± SEs of treatment groups. *P < 0.05, **P < 0.01, n.s., not significant. n = 5 or 6 in A, B, and D–G, n = 8 or 9 in C and H.
Previously, it has been reported that GABA would impact the insulin secretion of β-cells (43, 44). Therefore, we examined pancreatic islet size and measured glucose-stimulated insulin secretion with or without GABA using NCD-fed mice to exclude the effects of obesity-induced inflammation. As shown in SI Appendix, Fig. S7F, GABA treatment did not alter in vivo glucose-stimulated insulin secretion nor overall pancreatic islet sizes in NCD-fed mice. These data indicate that alleviation of insulin resistance mediated by GABA in HFD-fed mice may not be attributable to changes in insulin secretion from β-cells. In addition, we examined whether GABA could ameliorate insulin resistance in insulin-sensitive organs, such as IAT, EAT, liver, and muscle. As shown in Fig. 6I, levels of insulin-dependent phosphorylation of Akt and GSK3β were obviously enhanced in GABA-treated IAT from HFD-fed mice. However, the levels of phosphorylation of Akt and GSK3β in liver and muscle, as well as in EAT, were not altered by GABA stimulation (Fig. 6J and SI Appendix, Fig. S7 G and H), indicating that GABA selectively potentiates the insulin signaling cascade in obese IAT, but not in other metabolic organs. To confirm whether improved insulin signaling in IAT enhances glucose uptake, insulin-dependent glucose uptake ability was accessed with either fluorescent glucose bioprobe (GB-Cy3), or [14C] radioisotope labeled 2-deoxyglucose in IAT or EAT. In accordance with the above data, the degree of insulin-dependent glucose uptake was slightly, but significantly up-regulated in GABA-treated obese IAT (Fig. 6K and SI Appendix, Fig. S7I), whereas such an effect was not observed in obese EAT (Fig. 6L and SI Appendix, Fig. S7J). Therefore, these data strongly suggest that GABA-dependent mitigation of ATM recruitment in obese IAT might, at least in part, ameliorate systemic insulin resistance via enhanced glucose uptake in IAT.
GABA-Stimulated Adipose-Derived Stem Cells Decrease ATM Infiltration in Obese IAT.
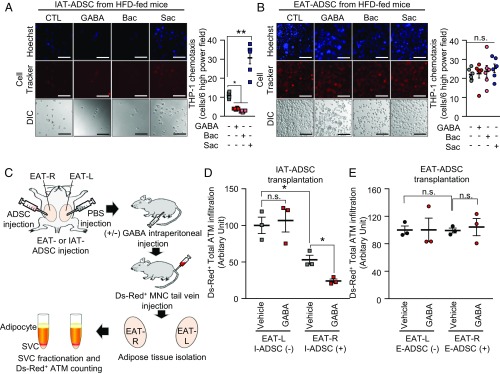
Given that GABA-stimulated IAT-SVCs could reduce monocyte migration in DIO, we decided to investigate which cell types among SVCs were responsible for the suppression of monocyte migration upon GABA. Adipose SVCs comprise various cell types, including ATMs, T cells, B cells, endothelial cells, and adipose-derived stem cells (ADSCs). It has been recently shown that these cells play important roles in the regulation of adipose tissue immune responses and homeostasis (2). Because GABA-responsive cells in SVCs should express GABAB receptors to repress proinflammatory responses, we isolated each SVC cell type and examined mRNA levels of GABAB receptors and GABAergic genes. In HFD-fed mice, GABAergic genes tended to be abundantly expressed in ADSCs compared with other SVC cell types (SI Appendix, Fig. S8A). The mRNA level of GABBR1, a GABA-binding subunit, was even higher in IAT-ADSCs than in EAT-ADSCs. When the numbers of ADSCs in the two fat depots were counted, IAT-ADSCs appeared to be more abundant than EAT-ADSCs (SI Appendix, Fig. S8B). Then, we investigated whether IAT-ADSCs might respond to GABA and affect monocyte migration in HFD-fed mice. As shown in Fig. 7A, IAT-ADSCs down-regulated monocyte migration via GABAB receptor activation with GABA or Bac, which was reversed by Sac. However, EAT-ADSCs did not show any difference in monocyte migration upon GABA activation or inactivation (Fig. 7B). In addition, GABA stimulation in CD4+ T cells did not affect monocyte migration (SI Appendix, Fig. S8C). To assess GABA-dependent suppressive effects of obese IAT-ADSCs on ATM recruitment in vivo, the same numbers of IAT-ADSCs or EAT-ADSCs were transplanted into EAT on the right (EAT-R), while vehicle was injected into EAT on the left (EAT-L) of obese recipient mice in the absence or presence of GABA administration (Fig. 7C). As presented in Fig. 7D, the numbers of Ds-Red+ proinflammatory ATMs were lower in IAT-ADSC–transplanted EAT-R than in vehicle-treated EAT-L. Notably, GABA stimulation further facilitated the suppressive effects of obese IAT-ADSCs on ATM infiltration. However, ATM recruitment was not affected in obese EAT-ADSCs transplanted EAT-R with or without GABA (Fig. 7E). Taken together, these results suggest that obese IAT-ADSCs could be a key cell type in mediating fat depot-selective inflammatory responses in response to GABA in obesity.
Fig. 7.
GABA-stimulated IAT-ADSCs are involved in the repression of ATM infiltration in obese IAT. (A and B) Measurement of THP-1 monocyte migration in CM of ADSCs in the regulation of GABA signaling. THP-1 monocytes were incubated in Transwell for 12 h. In HFD-fed mice, ADSCs isolated from (A) IAT and (B) EAT were pretreated with or without GABA, Bac, or Sac, and each CM was subjected to THP-1 monocyte migration assays. Representative images of migrated monocytes (Left) and quantification (Right). (Scale bars, 100 μm.) n = 6 for each experimental group. (C) An experimental strategy to examine fat depot-selective effects of IAT- or EAT-ADSCs on ATM infiltration in vivo. In two EATs from each recipient mouse, one EAT (EAT-R) was locally injected with either IAT-ADSCs or EAT-ADSCs, whereas the other EAT (EAT-L) was injected with vehicle (PBS). After recovery from surgery, recipient mice were intraperitoneally injected with or without GABA, and then, Ds-Red+ monocytes were intravenously injected. Ds-Red+ ATM were counted in EAT-L and EAT-R by flow cytometry. (D and E) Effects of (D) IAT-ADSCs and (E) EAT-ADSCs on ATM infiltration in obesity. n = 3 for each experimental group. Error bars represent means ± SEs of treatment groups. *P < 0.05, **P < 0.01, n.s., not significant.
Discussion
Adipose tissue plays a key role in the modulation of systemic energy homeostasis in response to physiological stimuli (2). In obese visceral adipose tissue, the increase in proinflammatory response is positively associated with insulin resistance (12). Conversely, metabolic profiles of obese individuals with larger portion of subcutaneous adipose tissue than visceral adipose tissue exhibit normal insulin sensitivity as well as lower inflammatory responses (7, 26). Furthermore, mice transplanted with subcutaneous adipose tissue exhibit improved glucose tolerance and attenuated inflammatory parameters upon HFD (8, 10, 45). However, how subcutaneous adipose tissue plays beneficial roles in energy metabolism compared with visceral adipose tissue has hardly been studied. In this study, we have elucidated a mechanism underlying the selective suppression of ATM infiltration in obese IAT. Fat transplantation and transcriptome analyses revealed that GABA signaling was intrinsically and differentially regulated between obese IAT and obese EAT. Ex vivo and in vivo analyses indicated that the GABA response would be a key factor in mitigating ATM infiltration in IAT, especially in IAT-ADSC. Moreover, we found that GABA ameliorated systemic insulin resistance in HFD-fed mice, concurrently with enhanced insulin-dependent glucose uptake in IAT. Taken together, our data suggest that subcutaneous adipose tissue can suppress adipose inflammatory responses via GABA, eventually leading to augmented insulin sensitivity.
Although it is well established that visceral adipose tissue is rich in ATMs compared with subcutaneous adipose tissue in obesity (20, 29), the process of ATM infiltration in each fat depot is largely unknown. Among the possibilities, such as proliferation, apoptosis, and infiltration that would explain fat depot-selective ATM accumulation in obesity, we found that infiltration of exogenous ATM was greatly higher in obese EAT than that in obese IAT. Proliferative and apoptotic portions of ATM were similar between EAT and IAT in DIO. In response to changes in nutritional status, adipose tissue undergoes dynamic remodeling, and an adipogenic microenvironment appears to be crucial herein (34, 46). As visceral and subcutaneous adipose tissues are present in anatomically different regions, we investigated whether the different environments of these two fat depots might determine differential ATM infiltration in obesity. Surprisingly, fat transplantation experiments revealed that the degree of ATM infiltration in donor EAT was higher than that in donor IAT, regardless of the fat depot in which it was transplanted. These findings suggest that differential susceptibility of ATM infiltration in each fat depot would be mainly determined by the intrinsic characteristics, rather than by the niche or environmental factors.
Adipose tissue largely consists of two cell populations, adipocytes and SVCs. In this study, we showed that SVCs in adipose tissue could be a primary intrinsic source of fat depot-selective ATM infiltration. To identify the intrinsic factors regulating ATM infiltration, RNA-sequencing and metabolomic analyses were conducted. KEGG pathway analysis revealed that GABA signaling was different between SVCs from EAT and IAT. In addition, CM from GABA-treated IAT-SVCs, but not EAT-SVCs, lowered monocyte migration, whereas CM from GABA-treated adipocytes did not affect monocyte migration. Given that SVCs are composed of various cell types, we attempted to identify major GABA-responsive cell types in the regulation of ATM infiltration in obesity. Several lines of evidence suggest that IAT-ADSCs play a major role in reduction of ATM infiltration in obesity. First, adipocytes or SVCs from EAT or IAT were treated with GABA, migrated monocyte was selectively reduced in the presence of CM from GABA-treated IAT-SVCs. Second, mRNA levels of GABBR1 and GABAergic genes were high in IAT-ADSCs. Third, IAT-ADSCs could suppress ATM infiltration upon GABA exposure both ex vivo and in vivo. CM from GABA- and Bac-pretreated IAT-ADSCs lowered monocyte migration. Finally, when ADSCs from each fat depot were transplanted into recipient EAT of HFD-fed mice, ATM infiltration was reduced only in the group with IAT-ADSC–transplanted EAT. Notably, ATM infiltration in IAT-ADSC–transplanted EAT was even further repressed by GABA administration, whereas recipient EAT transplanted with EAT-ADSC was not affected by GABA treatment. Thus, it is plausible to speculate that certain metabolites and cytokines secreted from GABA-stimulated IAT-ADSCs would repress ATM infiltration in obese IAT (SI Appendix, Fig. S9). Our findings are supported by recent findings that stem cells are able to regulate immune responses (47, 48). For example, neuronal precursors repress inflammatory responses via TGF-β2 in a multiple sclerosis mouse model (48). Mesenchymal stem cells reduce proinflammatory responses in corneal wound healing (49). Therefore, our data imply that IAT-ADSCs would be a key fat depot-selective immunomodulatory cell type involved in the regulation of obesity-induced adipose tissue inflammation.
In the brain, GABA is essential for information processing, and neuronal plasticity and activity (50, 51). Thus, GABAergic medications have been utilized to treat psychological disorders, such as epilepsy, anxiety, and depression (52). However, roles of GABA in peripheral tissues have been barely reported. Here, the present study revealed a peripheral role of GABA as a suppressive metabolite of ATM infiltration in obese IAT. Pharmacological modulations of GABA receptor activity revealed that GABAB receptor selectively affected ATM recruitment in obese IAT. Activation of GABAB receptor down-regulated monocyte migration in IAT, whereas inactivation of GABAB receptor up-regulated monocyte migration and in vivo ATM infiltration in IAT, but not in EAT. Together, these data suggest that GABAB receptor signaling in IAT would be one of the crucial factors in fat depot-selective proinflammatory responses in obesity.
It has been recently reported that GABA might be involved in the regulation of obesity and energy metabolism (43, 53–55). In pro-opiomelanocortin neurons, GABAB receptor ablation results in increased obesity and insulin resistance via dysregulation of the appetite (53). Orally administered GABA induces less adiposity and improves insulin resistance in DIO (54). However, it is still unclear whether or not the effect of GABA on energy metabolism depends on adiposity. In this study, we observed that 2-wk intraperitoneal injection of low-dose GABA ameliorated insulin resistance, without affecting adiposity. It is well established that elevation of inflammatory responses in adipose tissue is one of the crucial risk factors for insulin resistance (23). Here, GABA treatment enhanced insulin-dependent glucose uptake in IAT, but not in EAT. Furthermore, GABA selectively reduced inflammatory responses in obese IAT where insulin signaling was potentiated, implying that GABA-mediated suppression of inflammatory responses would ameliorate insulin resistance in obese IAT. Although we cannot rule out the possibilities that GABA would enhance glucose uptake in other peripheral tissues, GABA-mediated mitigation of proinflammatory responses in IAT, at least in part, might contribute to attenuation of obesity-induced insulin resistance. Emerging evidence suggests that obesity is not a sole determinant of insulin resistance and metabolic complications (7, 26, 56). In particular, obese but metabolically healthy individuals exhibit lower proinflammatory features (57). In this aspect, our findings are somewhat supported by a recent report that the GABA was elevated in obese but metabolically healthy subjects (58). Thus, it is feasible to postulate that GABA-mediated suppression of ATM infiltration in IAT might be one of the processes to explain obese but metabolically healthy individuals.
In this work, we isolated IAT-ADSCs and EAT-ADSCs using the same adipogenic precursor surface markers including CD31–, CD34+, and Sca-1+. Nonetheless, the levels of GABA-related gene expression and the degree of GABAB receptor response were enhanced in IAT-ADSCs compared with EAT-ADSCs in the regulation of ATM infiltration. These data imply that IAT-ADSCs and EAT-ADSCs appear to have intrinsically different characteristics, which would be consistent with the data from the fat tissue transplantation experiment. Recently, it has been reported that ADSCs from EAT and IAT seem to be different not only in marker gene expression, but also in their characters (46, 59). In obesity, fibrotic ADSCs are more abundant in EAT than in IAT (60, 61). Moreover, these fibrotic ADSCs strongly express the surface marker CD9 (60). These reports are somewhat consistent with our finding that ADSCs from EAT and IAT might be different not only in their characteristics, but also in their developmental origins. This idea is also supported by previous findings that IAT and EAT are derived from different cell lineages (62, 63). Therefore, it will be important to identify the factors that determine the differences between IAT-ADSCs and EAT-ADSCs in the regulation of fat depot-selective inflammatory responses.
In conclusion, this study provides an important clue to understanding peripheral roles of GABA in the regulation of fat depot-selective inflammatory responses and energy metabolism in obesity. Our data suggest that two white fat depots, subcutaneous and visceral fat tissues, have intrinsic factors that lead to fat depot-selective regulation of ATM infiltration, eventually resulting in differential insulin sensitivity in obesity. We identified IAT-ADSCs as a potentially major cell type in mediating fat depot-selective GABA response to lower ATM infiltration in obesity (SI Appendix, Fig. S9). Taken together, our data propose that modulation of GABA activity in adipose tissue would be an attractive approach to treating obesity-induced metabolic disorders.
Materials and Methods
For fully detailed procedures, please refer to SI Appendix, Materials and Methods.
Animals.
Six-week-old C57BL6/J male and female mice, and C57BLKS/J-Leprdb/Leprdb (db/db) male mice were purchased from Central Lab Animal Inc. Ds-Red+ transgenic mice were generously provided by Gou Young Koh (KAIST, Daejeon, South Korea). The mice were housed in colony cages under 12-h light/12-h dark cycles with free access to food and water. At 8 wk of age, male mice were fed HFD containing 60% kcals of fat (Research Diets) with free access to drinking water. All experiments were approved by the Seoul National University Institutional Animal Care and Use Committees.
Human Samples.
Human adipose tissue samples were obtained from the Obesity Research Center at the College of Medicine, King Saud University, Riyadh, Saudi Arabia. The protocol was approved by the College of Medicine Ethics Committee, King Saud University (approval code 07–602), in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before enrollment in the study.
Monocyte Migration Assay.
Monocyte migration was assayed as previously described (64). Briefly, THP-1 monocytes or mouse monocyte from blood was prestained with 2 μM of Cell Tracker-CMPTX (C34552; Thermo Fisher Scientific) at 37 °C for 1 h and then washed with PBS. CM from each sample were loaded to the lower layer of 8-μm pore-size Transwell insert (CLS3428; Sigma-Aldrich). In each sample group, 3 × 105 THP-1 cells were loaded on the upper surface of upper layer of Transwell insert. After 12 or 48 h of incubation, upper layer was removed. Then the chemotactic cells were stained with Hoechst dye (H3570; Thermo Fisher Scientific) for 30 min. After removal of the dye by washing, 10% FBS high-glucose DMEM was added, and the plates were observed under the LSM700 confocal microscope. In randomized microscope images, cells positive for Cell-Tracker (red) and Hoechst (blue) were counted and quantified.
Supplementary Material
Acknowledgments
This work was supported by the National Creative Research Initiative Program (2013-003395) and by the Ministry of Science, Information and Communications Technology (ICT) & Future Planning (MSIP). I.H. was supported by the BK21 program and Hi Seoul Science (Humanities) Fellowship funded by the Seoul Scholarship Foundation. Part of this study was performed at the National Mouse Metabolic Phenotyping Center at the University of Massachusetts Medical School, funded by National Institutes of Health Grant 5U2C-DK093000 (to J.K.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq information reported in this paper has been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE129665 (accession no.GSE129665).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1822067116/-/DCSupplemental.
References
- 1.Gesta S., Tseng Y. H., Kahn C. R., Developmental origin of fat: Tracking obesity to its source. Cell 131, 242–256 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Choe S. S., Huh J. Y., Hwang I. J., Kim J. I., Kim J. B., Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 7, 30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tchkonia T., et al. , Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouliot M. C., et al. , Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 41, 826–834 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki Y., et al. , Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 283, E1135–E1143 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Kim J. Y., et al. , Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusminski C. M., et al. , MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran T. T., Yamamoto Y., Gesta S., Kahn C. R., Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 7, 410–420 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamauchi T., et al. , The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Hocking S. L., et al. , Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia 58, 1587–1600 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U., et al. , Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Shoelson S. E., Herrero L., Naaz A., Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Olefsky J. M., Glass C. K., Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Park Y. J., et al. , Regulatory roles of invariant natural killer T cells in adipose tissue inflammation: Defenders against obesity-induced metabolic complications. Front. Immunol. 9, 1311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotamisligil G. S., Budavari A., Murray D., Spiegelman B. M., Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Invest. 94, 1543–1549 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rui L., et al. , Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Invest. 107, 181–189 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirosumi J., et al. , A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Dresner A., et al. , Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103, 253–259 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumeng C. N., Bodzin J. L., Saltiel A. R., Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisberg S. P., et al. , Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin K. C., et al. , Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat. Commun. 8, 1087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ham M., et al. , Macrophage glucose-6-phosphate dehydrogenase stimulates proinflammatory responses with oxidative stress. Mol. Cell. Biol. 33, 2425–2435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patsouris D., et al. , Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aouadi M., et al. , Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458, 1180–1184 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartier A., et al. , Visceral obesity and plasma glucose-insulin homeostasis: Contributions of interleukin-6 and tumor necrosis factor-alpha in men. J. Clin. Endocrinol. Metab. 93, 1931–1938 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Klöting N., et al. , Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, E506–E515 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Miller N. E., et al. , Secretion of adipokines by human adipose tissue in vivo: Partitioning between capillary and lymphatic transport. Am. J. Physiol. Endocrinol. Metab. 301, E659–E667 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Montague C. T., et al. , Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 47, 1384–1391 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Michaud A., Drolet R., Noël S., Paris G., Tchernof A., Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism 61, 689–698 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Amano S. U., et al. , Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill A. A., et al. , Activation of NF-κB drives the enhanced survival of adipose tissue macrophages in an obesogenic environment. Mol. Metab. 4, 665–677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumeng C. N., Deyoung S. M., Bodzin J. L., Saltiel A. R., Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Lee Y. H., Petkova A. P., Granneman J. G., Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffery E., et al. , The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metabol. 24, 142–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang I., Jo K., Kim S., Kim J. B., RNA-sequencing of adipocyte and stromal vascular cells (SVCs) of epididymal and inguinal adipose tissue from high-fat diet (HFD)-fed mice. Gene Expression Omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE129665. Deposited 11 April 2019.
- 36.Bu D. F., et al. , Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc. Natl. Acad. Sci. U.S.A. 89, 2115–2119 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macdonald R. L., Olsen R. W., GABAA receptor channels. Annu. Rev. Neurosci. 17, 569–602 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Kerr D. I. B., Ong J., GABAB receptors. Pharmacol. Ther. 67, 187–246 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Bhat R., et al. , Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. U.S.A. 107, 2580–2585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook J. B., Nathan P. W., On the site of action of diazepam in spasticity in man. J. Neurol. Sci. 5, 33–37 (1967). [DOI] [PubMed] [Google Scholar]
- 41.Kerr D. I. B., Ong J., Johnston G. A. R., Abbenante J., Prager R. H., 2-Hydroxy-saclofen: An improved antagonist at central and peripheral GABAB receptors. Neurosci. Lett. 92, 92–96 (1988). [DOI] [PubMed] [Google Scholar]
- 42.Olpe H. R., et al. , CGP 35348: A centrally active blocker of GABAB receptors. Eur. J. Pharmacol. 187, 27–38 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Ben-Othman N., et al. , Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168, 73–85.e11 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Li J., et al. , Artemisinins target GABAA receptor signaling and impair α cell identity. Cell 168, 86–100.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster M. T., et al. , Subcutaneous adipose tissue transplantation in diet-induced obese mice attenuates metabolic dysregulation while removal exacerbates it. Physiol. Rep. 1, e00015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeffery E., Church C. D., Holtrup B., Colman L., Rodeheffer M. S., Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 17, 376–385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernardo M. E., Fibbe W. E., Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 (2013). [DOI] [PubMed] [Google Scholar]
- 48.De Feo D., et al. , Neural precursor cell-secreted TGF-β2 redirects inflammatory monocyte-derived cells in CNS autoimmunity. J. Clin. Invest. 127, 3937–3953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh J. Y., et al. , The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 26, 1047–1055 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Schuler V., et al. , Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31, 47–58 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Magnaghi V., et al. , Altered peripheral myelination in mice lacking GABAB receptors. Mol. Cell. Neurosci. 37, 599–609 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Cryan J. F., Kaupmann K., Don’t worry ‘B’ happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 26, 36–43 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Ito Y., et al. , GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J. Neurosci. 33, 17166–17173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian J., et al. , Oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS One 6, e25338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikegami R., et al. , Gamma-aminobutyric acid signaling in brown adipose tissue promotes systemic metabolic derangement in obesity. Cell Rep. 24, 2827–2837.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Stefan N., et al. , Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 168, 1609–1616 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Karelis A. D., et al. , The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 90, 4145–4150 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Palleja A., et al. , Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 8, 67 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macotela Y., et al. , Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61, 1691–1699 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcelin G., et al. , A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 25, 673–685 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Iwayama T., et al. , PDGFRα signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 29, 1106–1119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chau Y. Y., et al. , Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 16, 367–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto Y., et al. , Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 18, 872–878 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee Y. S., et al. , Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J. Biol. Chem. 285, 22174–22185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.