Significance
Preterm babies are cared for in neonatal intensive care units (NICU), which are busy places with a lot of mechanical noise increasingly recognized to disrupt normal brain development. NICUs therefore invest in developmental care procedures, with music for example, but neurobiological evidence for these interventions is missing. We present results from a clinical trial to study the effects of a music intervention on preterm infants’ brain development. Based on resting-state fMRI, we provide evidence that music enhanced connectivity in a brain circuitry involving the salience network with regions implicated in sensory and higher-order cognitive functions, previously found to be altered in preterm infants. To our knowledge, this study is unique in observing an impact of music on brain development in preterm newborns.
Keywords: preterm newborns, resting-state fMRI, brain function, salience network, music intervention
Abstract
Neonatal intensive care units are willing to apply environmental enrichment via music for preterm newborns. However, no evidence of an effect of music on preterm brain development has been reported to date. Using resting-state fMRI, we characterized a circuitry of interest consisting of three network modules interconnected by the salience network that displays reduced network coupling in preterm compared with full-term newborns. Interestingly, preterm infants exposed to music in the neonatal intensive care units have significantly increased coupling between brain networks previously shown to be decreased in premature infants: the salience network with the superior frontal, auditory, and sensorimotor networks, and the salience network with the thalamus and precuneus networks. Therefore, music exposure leads to functional brain architectures that are more similar to those of full-term newborns, providing evidence for a beneficial effect of music on the preterm brain.
Music is the art of organizing sounds to generate a sophisticated combination of acoustic frequencies and musical structures that may exert a positive effect on preterm infants who receive care in neonatal intensive care unit (NICU) environments. A number of studies have considered the effects of music listening on preterm infants and have shown stabilizing effects on heart and respiratory rates, reductions in the number of apnea and bradycardia events per day, improved resting-energy expenditure, improved feeding, enhanced weight gain, and more mature sleep patterns; most of these studies report a beneficial effect on at least one of these outcomes (1, 2). Nevertheless, these studies have produced equivocal results. Factors that contribute to these variations are the type of music used (instrument, live or recorded, duration, and so forth), the duration of the exposure (often lasting only few days), various gestational ages (GA) at birth and at the time of the intervention, and diverse outcome measures (essentially behavioral observation, heart rate variability, and so forth) (2, 3). Furthermore, effects of a music intervention on brain network development have not been studied before.
Preterm birth is associated with a high risk of developing structural and functional network alterations in the brain and, consequently, deficits in neurological outcomes (3). Noninvasive neuroimaging tools have provided new insights into the developmental changes occurring in the preterm brain (4). One promising technique for studying development of brain function in newborns is resting-state (RS) fMRI, which allows the identification of large-scale functional networks, termed RS networks (RSNs), showing coordinated blood-oxygen level-dependent (BOLD) signal fluctuations linked to the infant’s spontaneous brain activity (5–8). RS functional connectivity (RS-fc) then measures statistical dependencies between different brain regions, thus providing time-locked spatial patterns of functional connectivity or functional brain networks. RS-fc has been shown to provide information about brain maturity and integrity (9, 10). Furthermore, the spatial localization of networks is similar in preterm and full-term infants (6, 11, 12). However, less complex intranetwork, interhemispheric, internetwork, and thalamo-cortical functional connectivities have been observed in preterm infants scanned at term-equivalent ages (TEAs) than full-term controls (12–14).
Most studies performed to date investigating RS-fc in newborns have examined the main sensory functional networks, while networks implicated in higher-level functions, such as the salience network, have been explored to a much lesser extent. The salience network, which encompasses the anterior insula and dorsal anterior cingulate cortex (ACC) in adults, is thought to facilitate the detection of relevant internal or environmental stimuli and to assist target brain regions in generating appropriate behavioral responses (15). A similar salience network has been defined in the newborn period and the thalamo-salience network connectivity has been the only one to be significantly linked to cognitive function at 2 y of age (16), making it a tremendously relevant brain network in the search for early biomarkers of later neurodevelopment in preterm infants. According to recent empirical evidence, salience networks may allow switching between the central executive network (CEN) and default mode network (DMN) to facilitate attention, behavioral, and cognitive control (17, 18). In preadolescents and adults born prematurely, alterations of RS-fc have been observed between he salience network, the DMN, and the CEN (19, 20). In the newborn period, Toulmin et al. (14) reported decreased connectivity between the thalamus and salience networks that correlated with GA at birth in extremely and very preterm infants at TEAs compared with full-term infants.
The development of these brain functional networks occurs either in utero for full-term newborns or in NICUs for preterm infants, two very different environments in terms of how stimuli are presented, which may have long-lasting effects on RS-fc: for example, salience network development. The identification of NICU interventions for preterm infants that might preserve fc development is therefore an important topic in neonatology. Musical training in children and adults increases RS-fc between visual, motor, and auditory areas, as well as between the thalamus and auditory networks (21, 22). Furthermore, when a salient auditory event occurs in music, the salience network and CEN are activated, whereas the DMN is deactivated (23). This salience network has recently been shown to be enhanced in adults with music training compared with nonmusicians (24–26), indicating that music training changes large-scale brain networks at rest and suggesting potential adaptive, neuroplastic effects of music. Therefore, both music training later in life and simple, repetitive music listening in the newborn period may affect brain development, raising the question of whether an early postnatal musical intervention would enhance brain development in preterm infants. The newborns in the present study listened to recorded music specifically composed by A.V. (http://www.vollenweider.com/) using instruments (i.e., harp, pungi, and bells) that have produced behavioral and brain responses in preterm newborns in a previous study to determine the effects of music listening on the preterm brain (27). The music exposure occurred five times per week from a GA of 33 wk until discharge or TEA. Preterm infants with music exposure and preterm and full-term infants without music exposure underwent fMRI with an RS-fc analysis at TEA to assess functional connectivity in the brain.
Results
The RS-fc analysis was based on a group-level independent component analysis (ICA) repeated 20 times using ICASSO to determine RSNs. RS-fc was assessed by a nonparametric bivariate estimator, accordance, and discordance, reflecting coupling and anticoupling between ICA components, respectively (28). For each subject of the three groups, we derived a RS-fc based on accordance measure defined as circuitry of interest (COI).
The Effect of Prematurity on the Development of fc in the Brain.
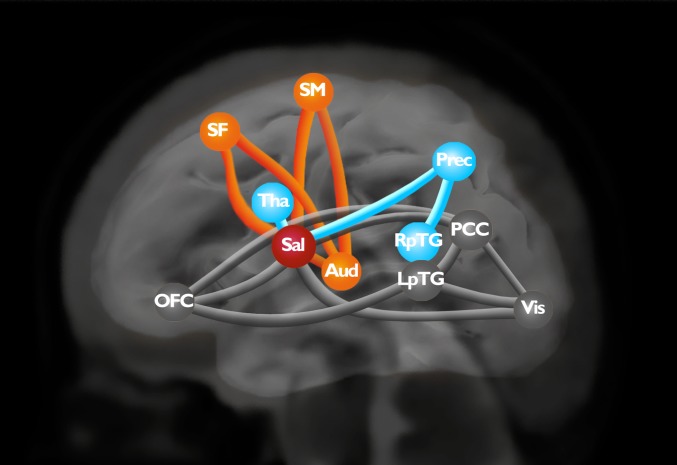
We first assessed brain connectivity characteristics in full-term and preterm controls: that is, infants who had not been exposed to the music intervention. Each connection was compared between these two groups and resulted in a COI, a graph that comprises 16 edges (connections) involving 11 nodes (RSNs). This COI represents lower RS-fc (coupling) in preterm infants than in full-term infants (Bonferroni’s test was used for multiple comparisons, P < 0.05). Using modularity-based decomposition methods, three modules were identified in the COI (Fig. 1). This decomposition has modularity 0.29. The first module (M1) involves medial superior frontal (SF1), auditory, and sensorimotor (SM) networks. The second module (M2) involves the thalamus, the precuneus (Prec), and right posterior temporal gyrus. The third module (M3) involves the orbitofrontal cortex, posterior cingulate cortex (PCC), visual cortex, and left posterior temporal gyrus. The salience network, which is composed of the insula and perigenual ACC, is a hub that connects the three modules and is distinguished as an articulation point in the COI (SI Appendix, Figs. S4 and S5). It has a node degree of eight, which is the highest degree in the COI. Among its connections, five are interconnections between the three modules. Furthermore, removing a salience node from the COI results in an unconnected network with modularity 0.53.
Fig. 1.
COI. Edges represent lower functional connectivity between components in preterm controls than in full-term newborns. Node localization is based on the local maxima of the z-score within each network. Orange: module M1; blue: module M2, black: module M3. Sal, salience network; Aud, auditory cortex; Tha, thalamus; RpTG, right posterior temporal gyrus, Vis, visual cortex; LpTG, left posterior temporal gyrus.
The Effect of an Early Music Intervention on fc in the Brains of Preterm Newborns.
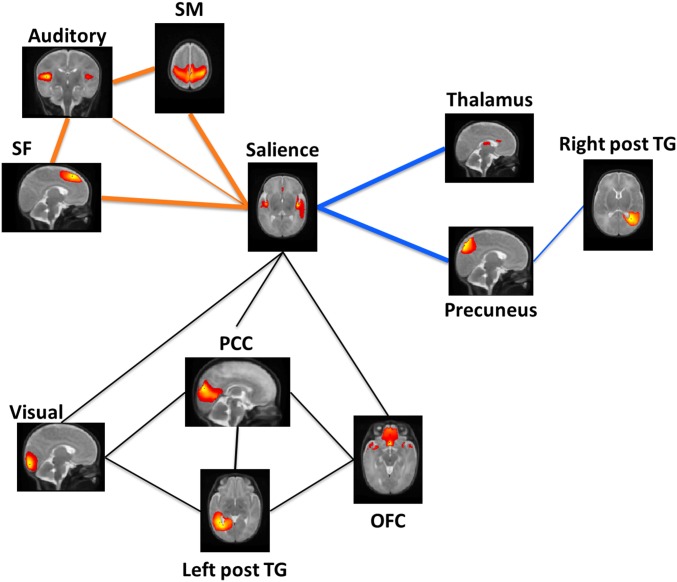
Compared with preterm control infants, the preterm music group displayed significantly higher RS-fc in the following connections: auditory–SM, salience–SM, salience–SF1, auditory–SF1, salience–thalamus, and salience–PCC [false-discovery rate (FDR) < 0.05] (Fig. 2). No connection in module M3 was significantly different between the two groups.
Fig. 2.
Differences in fc between preterm music-exposed and preterm control infants. Bold edges represent significantly higher functional connectivity in the premature infants exposed to music (PM) than in premature control infants (PC) (FDR < 0.05). Orange: module M1; blue: module M2; and black: module M3. Left post TG, left posterior temporal gyrus; Right post TG, right posterior temporal gyrus.
Discussion
This study provides evidence that early environmental enrichment with music improves the development of RS-fc in the brains of preterm infants. The overall decrease in RS-fc observed in our preterm control infants compared with full-term infants was consistent with previous research showing that premature birth has an impact on the development of brain fc (7, 8, 12). The two main tools that were combined here to study RS-fMRI data were an ICA and region-of-interest (ROI) –based analysis. First, we performed an ICA, a data-driven multivariate analysis, to determine components throughout the brain. Then, we used these components as ROIs to compare RS-fc between our three groups. RS-fc is usually studied by calculating Pearson’s or Spearman’s correlation coefficients between pairs of fMRI BOLD time-courses. Here, we used a more robust and consistent bivariate estimator: accordance and discordance (29). This RS-fc estimator exploits extreme BOLD fluctuations, which represent significant activations or deactivations, and has been shown to capture essential information (30). According to Meskaldji et al. (28), this estimator exhibits increased sensitivity for detecting group effects compared with Pearson’s correlation analysis. Recent studies have also shown the effectiveness of the accordance–discordance estimator for analyzing fc in the brain in predictive studies (31, 32). Thus, the methodology of our study provided highly reliable results. Furthermore, in addition to previous studies, the connectivity of the salience network was shown to be distinctly affected by premature birth, and a COI responsible for the altered connectivity observed in preterm infants was identified and consisted of three modules; the salience network was identified as an articulation point (a central hub) that is part of and connects these three modules.
Alterations in the RS-fc of these three modules reflect the three functions of the salience network: detection of the salience of a stimulus, cognitive, and behavioral responses to salient stimuli. By modifying the preterm infants’ environment through the daily introduction of music, we further revealed the effectiveness of this intervention on increasing the RS-fc in the brains of preterm infants, specifically in two modules of the COI that were impaired by premature birth, leading to an RS-fc at TEA in preterm infants exposed to the music intervention that was more similar to that in full-term control newborns.
The first module (M1) showing alterations in internetwork RS-fc in preterm controls compared with full-term newborns was composed of salience, medial superior frontal, auditory, and SM networks. During early postnatal life, the environment of preterm infants (the NICU) is vastly different from that of full-term (in utero) infants, with different solicitations and sensory stimuli (33–35). The immature cortex of preterm infants is exposed to extrinsic and potentially stressful stimuli before the normal time and this condition has been associated with altered brain microstructures, as measured by diffusion imaging, and altered RS-fc, in the temporal lobes (36). Our data provide additional evidence of altered RS-fc between regions implicated in sensory processing (auditory and SM networks) and regions implicated in generating appropriate behavioral responses to these stimuli (SF network). However, RS-fc in this module was also enhanced by an early postnatal music intervention in the present study. According to the results from brain imaging studies, neural activity associated with music listening extends well beyond the auditory cortex, involving a widespread bilateral network of frontal, temporal, parietal, and subcortical areas related to attention, motor functions, and memory (37–39). In adults, the medial superior frontal cortex has been shown to be connected to regions implicated in cognitive and executive control, as well as motor control (40). Increased structural connectivity of the superior frontal gyrus has been observed in children with 2 y of music training (41). Furthermore, long-term musical training increases resting-state RS-fc between motor, SM, and auditory areas, as well as between thalamus and auditory networks (21, 22, 42). In a recent fMRI study with a psychophysiological analysis, an increase in the RS-fc between the auditory cortex and cerebral regions known to be implicated in the processing of tempo was observed when preterm infants at TEA were listening to the music they had previously heard compared with the same music played with a different tempo (27), indicating an auditory learning process. In summary, based on our results, listening to music during a NICU stay affects the development of brain networks in cortical regions implicated in both sensory perception and in the detection and response to salient stimuli.
The second module (M2) displaying altered connectivity in preterm control infants compared with that in full-term infants is composed of salience, thalamus, precuneus and right medial posterior temporal networks. The music intervention improved connectivity between the salience network and thalamus network and the salience network and precuneus network. Correlations between decreased gray matter volume and aberrant intrinsic fc in thalamus and salience networks have been described in adults who were born preterm (43), and altered fc between the thalamus and anterior insula and ACC (salience) has previously been observed in preterm newborns (14). The strength of salience–thalamus fc has further been shown to predict cognitive performance in infancy (16); thus, this circuit is an important contributor to neurodevelopmental outcomes in preterm infants.
Furthermore, our study using a psychophysiological analysis of fMRI data from preterm infants showed increased connectivity between the primary auditory cortex and thalamus in preterm infants who were listening to previously heard music compared with the same music played at a faster tempo. This increased connectivity between the primary auditory cortex and thalamus may be linked to familiarity processing (27). Listening to familiar music during all their stay in the NICU may have also enhanced RS-fc between regions implicated in salience detection (salience network) and regions implicated in familiarity detection (thalamus). In preadolescents, even moderately premature birth has been shown to modify the development of RS-fc between the salience network and DMN (20). Notably, in the present study, we found two networks composed of regions usually included in the DMN in adults: the Prec and PCC. This splitting could be explained by the fact that we used ICA decomposition with number of components estimated using minimum description length (MDL) criteria. It may also reflect an immaturity of the network organization in newborns, as previously described (7, 8). Furthermore, RS-fc in the DMN has been shown to be decreased during sleep in adults which could, at least in part, contribute to our finding of an immature and incomplete DMN (44). In this second module, we observed decreased RS-fc between the salience network and posterior part of the DMN (Prec), and this difference was therefore shown to be present in preterm newborns at TEA. During cognitive tasks, a switch between the CEN and DMN is predicted to facilitate attention, behavioral, and cognitive control in response to salient stimuli, and the salience network has been proposed to play a central role to this switching behavior (17, 18, 45). Thus, our findings from preterm control infants indicate a reduced capacity of the salience network to modulate a part of the DMN (Prec), which therefore might impair the processing of saliency in stimuli. In contrast, we observed an increase in the connectivity between the salience network and this part of the DMN following the early postnatal musical intervention, which is similar to the increases in RS-fc between the salience network and DMN observed in response to passive music listening in adults (23). Furthermore, a higher impact of music listening on salience–Prec connectivity is in line with previous studies in adults showing an increased RS-fc between the Prec and insula in music-trained adults (26). Thus, our results indicate that music exposure in preterm infants modified the RS-fc between the salience network and regions implicated in cognitive processing, and that this alteration can already be observed in preterm infants at TEA.
The third module (M3) included salience, Prec, and PCC, orbitofrontal (OFC), visual, and left medial posterior temporal networks. The OFC network was composed of both the orbitofrontal cortex and bilateral temporal polar regions, both of which are paralimbic cortical regions implicated in socioemotional processing (46). The structure and function of this OFC network has been shown to be affected by prematurity in several studies. Fischi-Gómez et al. (47) reported a relationship between weaker structural connections and decreased social skills in school-age children who were born preterm. Here, the risks of alterations in OFC RS-fc due to prematurity were already observed at TEA. Although the current music intervention did not significantly change the connectivity in this third module, music has been shown to involve limbic and paralimbic regions related to emotional processing in adults (48). Additionally, the temporal polar region has previously been shown to be activated in adults during passive listening to pleasant music (49). Furthermore, increased connectivity within the salience network (insula–ACC) and between the insula and OFC has also been observed in musicians (24), thus implying that salient awareness toward musical stimuli may be increased by early music training. Finally, we did not observe a significant effect of our music intervention on the RS-fc between the salience network and the PCC network, whereas we observed significantly increased connectivity between the salience network and Prec. Higher impact of music listening on salience–Prec connectivity may be because the Prec has been described as an important site of music perception (50–52). Based on these results, we hypothesize that more time spent listening to music during a NICU stay would increase the effect of early music listening on the RS-fc of this third module. The impact of early postnatal music exposure on this third module, which is strongly implicated in affective and emotional processing, thus represents an interesting future research direction.
The salience network has clearly been identified in this study as an articulation point between three brain modules defining a COI affected by premature birth. We observed altered connectivity between the salience network and numerous networks, including sensory networks and networks underlying cognitive functions and behavioral and emotional regulation. These alterations in the RS-fc of the salience network in the newborn period precede previous findings reported in preadolescents (20) and adults (19) and indicate that preterm birth has early, long-lasting effects on salience network connectivity. Thus, in preterm infants, the early postnatal period is a period of increased vulnerability. However, it may also be a window of opportunity. In a previous study, preterm babies with higher spontaneous brain activity in the first 72 h of life showed greater subsequent brain growth to term age (53); therefore, endogenous brain activity clearly provides some potentially protective effect. Musical training has been shown to affect the salience network by increasing RS-fc and functional integration in musicians compared with nonmusicians (25). Additionally, the ACC and insula (composing the salience network) have been shown to have a higher node degree (i.e., number of edges connected to each node when passively listening to music) in adult musicians who started music training earlier than musicians who started training later and nonmusicians (54). Furthermore, even passive music listening for 1 mo has been shown to increase connectivity within the salience network in adult patients with schizophrenia (55). Here, we showed that increased salience network connectivity with regions implicated in sensory and higher-order cognitive functions was already observed in preterm newborns after an early music listening intervention. Together with our data from a previous study using fMRI, where we showed that preterm newborns who listened to familiar music exhibited activation in brain regions implicated in familiar, pleasant, and arousing music processing (27), we conclude that the music heard by our preterm newborns during their NICU stay became familiar and thus more salient to our preterm infants, resulting in increased RS-fc of their salience network. Thus, we propose that an early postnatal music intervention, a salient multimodal stimulation, increases RS-fc of the salience network with both regions implicated in sensory perceptions and regions implicated in salience processing, and generation of an appropriate cognitive, behavioral, and executive response to these relevant stimuli.
This study is unique in observing an impact of music exposure on brain development in preterm newborns. An early music intervention increased functional connectivity in impaired functional networks and thus auditory enrichment of the NICU environment exerted long-lasting effects on brain development. One limitation of this study is that we used the entire RS network component as regions of interest and therefore we explored internetwork connectivity but not intranetwork connectivity between regions that are part of the same component. Previous studies have observed alterations in functional interhemispheric and within-network connectivity in preterm infants compared with full-term infants (12–14). Further studies are therefore needed to assess the effects of a music intervention on interhemispheric and within-network connectivity, as well as moment-to-moment fluctuations in brain activity (56). This study compared music intervention and standard of care in the NICU. We show that this early intervention in preterm newborns leads to functional brain architectures more similar to those of full-term newborns. However, further research is needed to compare music interventions to other sound interventions, such as mother–father speaking, mother–father singing, and so forth, Whole-brain fc in naturally sleeping infants resembles more the RS observed in sleeping adults than in awake adults (44). In the present study, we compared fc in three groups of nonsedated quietly laying infants and found no differences in the number of excluded movement corrupted images between the three groups. Because awake newborns are moving much more, we assume that all three groups were in equivalent quiet states. However, it would be of great interest to assess sleep-state impact on newborns’ fc using EEG-MRI combined techniques in the future. Music is an easy-to-implement and low-cost way to enrich the environment of preterm infants. Further studies are needed to assess the long-term effects of early postnatal music exposure on the cognitive and emotional development of preterm newborns.
Methods
Subjects.
This study, involving human subjects, was approved by the Research and Ethics Committees of the University Hospital of Geneva. Informed consent was obtained from the parents of each newborn before participation in the study. Twenty-four full-term infants (T) and 39 preterm newborns were recruited at Geneva University Hospital between 2013 and 2016. In the preterm infant group, 20 underwent musical intervention (PM), and 19 were allocated to the control group without the music intervention (PC). Inclusion criteria for full-term newborns were birth after a GA of 37 wk and an appropriate height, weight, or head circumference (above the 5th and below the 95th percentiles). Exclusion criteria for all babies were major brain lesions detected on MRI, such as high-grade intraventricular hemorrhage or leukomalacia (one PC and one T were excluded). Three preterm and three full-term infants stopped the study before the MRI (two PM, one PC, and three T) and one was transferred to another hospital (one PM). Three infants were excluded due to insufficient numbers of music intervention/no-intervention sessions (fewer than 15 intervention/no-intervention sessions; two PM and one PC). Furthermore, RS-fMRI data from six babies were not used in subsequent analyses due to high levels of motion (one PM, one PC, and four T) (SI Appendix, Figs. S1 and S2). The final analysis after removal of motion-corrupted MRIs was performed on 16 full-term newborns (9 girls, mean GA: 39.51 ± 1.08 wk) scanned in the first 4 d of life (mean GA at scan: 39.81 ± 1.02 wk); 15 premature babies in the preterm no-intervention/control group (8 girls, mean GA at birth: 28.95 ± 1.84 wk) scanned at TEA (mean GA at scan: 40.50 ± 0.77 wk); and 14 premature babies in the preterm music intervention group (9 girls, mean GA at birth: 28.33 ± 2.06 wk) scanned at TEA (mean GA at scan: 40.41 ± 0.76 wk). No significant differences in gender; GA at birth; weight, height, and head circumference at birth; antenatal steroid exposure; neonatal asphyxia; chorioamnionitis; sepsis (positive blood culture); bronco-pulmonary dysplasia (57); intraventricular hemorrhages grade 1 and 2 (58); and mean number of music/no-music intervention sessions were observed between the two preterm groups. Furthermore, no differences in GA at scan and parental socioeconomic status were observed between the three groups (SI Appendix, Table S1).
Music Intervention.
Preterm infants were randomly assigned to either the PM or the PC group. Parents, music intervention providers, and caregivers were blinded to the group assignment. The music group listened to 8 min of music specifically created by A.V. (http://vollenweider.com/en) that was composed of a soothing background, bells, harp, and punji (charming snake flute) five times per week from a GA of 33 wk until the MRI. Nurses were instructed to put headphones to the baby when waking up or already awake (before or after feeding) but not to a baby who was already sleeping, this to ensure we did not disturb the babies’ sleep. To avoid bias, we performed the intervention only when the baby was lying in the bed (not in parents’ arms and not during clinical care procedures). As the literature has little evidence for the amount and the timing of music intervention, we chose to perform the intervention five times per week starting from a GA of 33 wk, which is in accordance with the auditory pathways maturation until discharge. This music is based on behavioral responses of preterm newborns to the instruments (observed by a nurse specialized in developmental care). Three tracks were created to adapt to the state of wakefulness of the baby: one helped the baby to wake up (Audio File S1), such as before care or feeding; one interacts with an awake baby (Audio File S2); and the last one helps the baby fall asleep (SI Appendix, Fig. S3 and Audio File S3). Readiness for the intervention as well as choice of music track (waking up, falling asleep, be active) was determined by the nurse based on a neonatal behavioral assessment scale (59). Each infant listened to each three tracks an equivalent number of times. The control group had the same handling as the intervention group (namely putting headphones) but the headphones were made open to environmental sounds, as such representing an active control group.
Data Acquisition.
All infants were fed before the MRI. To enable scanning without movement, we gave sufficient time for the baby to get quiet and rest after feeding and the baby was swaddled in a blanket and comfortably positioned inside a vacuum pillow. Infants were monitored (heart rate and oxygen level) during all MRI scans. MR-compatible headphones were used to protect newborns from noise in the MRI. We acquired a run of 8 min of RS-fMRI data (300 volumes) (EPI sequences: TR = 1,600 ms, TE = 30 ms, slice thickness = 3 mm, flip angle = 90°, matrix = 64 × 52) on two Siemens 3T scanners (Siemens): Siemens Trio using a 32-channel head coil (7 PM, 8 PC, 11 T) and Siemens Prisma using 64-channel head coil. We tested scanner/coil effects by ANOVA for each connection (among the 91) within the connectivity matrix. None of the connections showed scanner/coil effects after correction for multiplicity. Furthermore, the distribution of scanner/coils were equally represented in each group. Thus, no significant differences caused by the use the different coils or scanners were observed between the data. A T2*-weighted structural image for anatomical reference was also acquired (113 coronal slices, TR = 4,990 ms, TE = 151 ms, flip angle = 150°, matrix = 256 × 164; voxel size = 0.78 × 0.78 × 1.2 mm3).
Preprocessing.
RS-fMRI data were preprocessed using SPM8 (Wellcome Department of Imaging Neuroscience, University College London, United Kingdom) and included realignment, slice timing, rigid body coregistration with the T2 structural image; normalization of the T2 structural image (1 × 1 × 1 mm) and the EPI (2 × 2 × 2 mm) to a T2 template prepared using data obtained from 20 term and preterm at TEA infants, and smoothing using an isotropic Gaussian kernel (6-mm full width at half maximum). All volumes with a frame-wise displacement (FD) greater than 0.5 mm or with a rate of BOLD signal changes across the entire brain (DVARS) greater than 3% were removed, along with the previous and the two subsequent images (60), to accommodate the high level of motion in infants. The remaining images were included in the analysis. A minimum of 60% of volumes must have remained for inclusion, resulting in the exclusion of one preterm infant subjected to the intervention (PM), one preterm control (PC), and four full-term newborns (T). Significant differences in the number of excluded images were not observed between the three groups (average number of images remaining: T: 251.75 ± 31.40 images, PM: 265.71 ± 37.63, PC: 272.27 ± 29.52).
Region of Interest Selection.
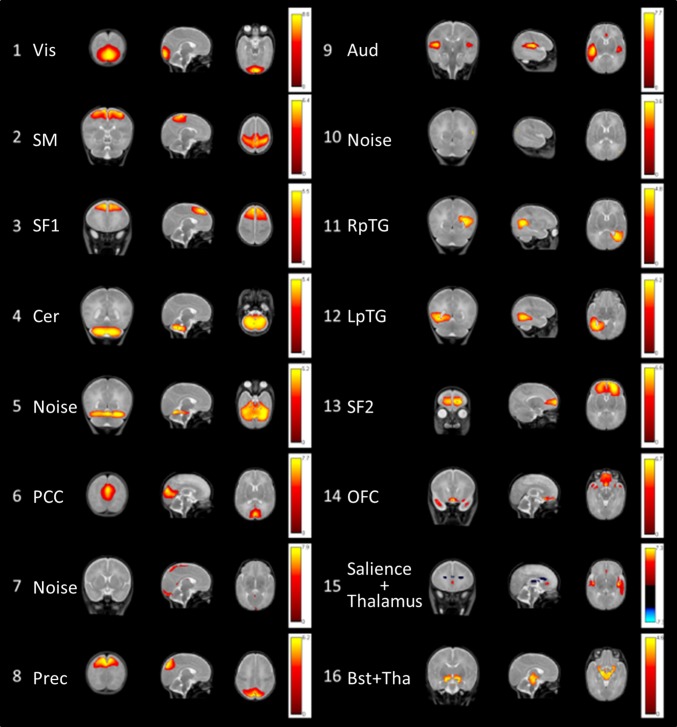
We conducted group-level ICA to extract independent spatial networks, and we used these components with thresholding at a z-score of 3 as ROIs. Data from the three groups were decomposed into 16 relevant components (the number of components was estimated using an MDL algorithm) in a single group-level ICA using the GIFT package (http://mialab.mrn.org/software/gift/index.html). A mask, based on full-term and preterm newborn template segmentation, was used to remove the cerebrospinal fluid, ventricles, eyes, and extracerebral areas. The ICA was repeated 20 times using ICASSO to ensure stability of the decomposition. The results are reported using a z-threshold = 3 for absolute values, and we examined both positive and negative z-score values. The 16 components were identified separately by two experts in neuroanatomy and are illustrated and described in Fig. 3 and SI Appendix, Table S2. Among the 16 components, 3 components reflected motion (nos. 5 and 10) and blood vessels (no. 7) and were excluded from further analyses. Finally, component number 15 was the only one showing two anticorrelated networks. We thus decided to subdivide this component into two networks: one composed of the bilateral thalamus and dorsal ACC (thalamus network, in blue in Fig. 3), and the other composed of the bilateral anterior insula and perigenual ACC (salience network, in red in Fig. 3). The 14 functional RS networks were used as ROIs, and a time-course of each of these networks was extracted using MarsBar (SPM toolbox).
Fig. 3.
Sixteen components obtained from the ICA. Each row shows a coronal, sagittal, and axial view of the components thresholded at a z-score > 3 superimposed on a T2-weighted MR infant brain template. The colored bars show the corresponding z-scores.
Functional Connectivity.
Functional connectivity consists of nodes that correspond to the ROIs (RSNs), and the weighted edges (connections) correspond to the measure of the statistical dependencies between the corresponding ROI time courses. In this study, fc was assessed using a nonparametric estimator called “accordance.” Accordance is a positive measure between 0 and 1 and it reflects coupling between two RSNs. It represents the coactivations and codeactivations between regions, where activations and deactivations are obtained by thresholding the normalized fMRI signal at positive and negative thresholds, respectively. Here, we used the 80% quantile of the normal distribution to specify these thresholds. This measure is robust and consistent compared with the conventional Pearson’s correlation coefficient (28). For each subject in the three groups (T, PC, and PM), we derived a RS-fc based on the accordance measure that was defined as the COI.
Statistical Analysis.
We performed the steps listed below to test our hypothesis that music reverses some effects of prematurity on preterm babies. First, we identified a COI that represents alterations in RS-fc caused by prematurity. This COI was obtained by testing the difference between PC and T fc networks at each individual connection, where the T group was considered the control group. The P values were Bonferroni-corrected and thresholded at an α-level equal to 0.05. The connections that passed the Bonferroni test were identified as the COI. We performed the same method to compare PC and PM groups (SI Appendix, Table S3). For both contrasts, no P value survived the Bonferroni correction, showing no significant differences outside the COI. In the second step, we considered the PC as the control group and tested our hypothesis of the effects of music by restricting our investigation within the COI. We tested the differences between PM and PC groups using an adaptive statistical method that exploits the modular structure of the COI. This method consists of an initial decomposition of the COI into modules (i.e., a group of nodes with dense intraconnectivity compared with interconnectivity). Next, the differences were tested at the level of modules by averaging the connectivity strength of each module. This strategy has the benefit of reducing the number of tests and increasing the signal/noise ratio (61). Third, a t test was performed on each connection, and the connection-wise P values were modified by exploiting the results obtained at the module level. This adaptive method increases the power of testing while controlling the type I error rate (62). We used this adaptive method to compare each connection that belongs to the COI between the PM and PC groups. The P values were corrected for FDR control, as this method is more suitable for exploratory analyses.
Supplementary Material
Acknowledgments
The authors thank all the nurses and all the parents and babies who participated in this study; and the Division of ENT, the Plateforme de Recherche de Pediatrie, and the Centre for Biomedical Imaging of the University Hospital of Geneva for their support. The authors declare no competing interests. This study was supported by grants from the Swiss National Science Foundation (32473B_135817/1), the foundation Prim’enfance, and European Union’s Horizon 2020 research and innovation programme under Grant Agreement 666992.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817536116/-/DCSupplemental.
References
- 1.Anderson D. E., Patel A. D., Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: Might music have an impact? Dev. Med. Child Neurol. 60, 256–266 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Pineda R., et al. , Enhancing sensory experiences for very preterm infants in the NICU: An integrative review. J. Perinatol. 37, 323–332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe J. J. The encephalopathy of prematurity—Brain injury and impaired brain development inextricably intertwined. Semin. Pediatr. Neurol. 16, 167–178 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hüppi P. S., et al. , Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107, 455–460 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Biswal B., Yetkin F. Z., Haughton V. M., Hyde J. S., Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Doria V., et al. , Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U.S.A. 107, 20015–20020 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyser C. D., et al. , Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 20, 2852–2862 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W., Lin W., Grewen K., Gilmore J. H., Functional connectivity of the infant human brain plastic and modifiable. Neuroscientist 23, 169–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dosenbach N. U., et al. , Prediction of individual brain maturity using fMRI. Science 329, 1358–1361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruett J. R., Jr, et al. ; IBIS Network , Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Dev. Cogn. Neurosci. 12, 123–133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damaraju E., et al. , Resting-state functional connectivity differences in premature children. Front. Syst. Neurosci. 4, 23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyser C. D., et al. , Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb. Cortex 26, 322–333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyser C. D., Snyder A. Z., Neil J. J., Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain. Neuroimage 56, 1437–1452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toulmin H., et al. , Specialization and integration of functional thalamocortical connectivity in the human infant. Proc. Natl. Acad. Sci. U.S.A. 112, 6485–6490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeley W. W., et al. , Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcauter S., et al. , Development of thalamocortical connectivity during infancy and its cognitive correlations. J. Neurosci. 34, 9067–9075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon V., Uddin L. Q., Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 214, 655–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uddin L. Q., Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61 (2015). [DOI] [PubMed] [Google Scholar]
- 19.White T. P., et al. , Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 4, 352–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degnan A. J., et al. , Altered structural and functional connectivity in late preterm preadolescence: An anatomic seed-based study of resting state networks related to the posteromedial and lateral parietal cortex. PLoS One 10, e0130686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo C., et al. , Musical training induces functional plasticity in perceptual and motor networks: Insights from resting-state FMRI. PLoS One 7, e36568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S., Kirino E., Reorganization of the thalamocortical network in musicians. Brain Res. 1664, 48–54 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Sridharan D., Levitin D. J., Menon V., A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamorano A. M., Cifre I., Montoya P., Riquelme I., Kleber B., Insula-based networks in professional musicians: Evidence for increased functional connectivity during resting state fMRI. Hum. Brain Mapp. 38, 4834–4849 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo C., et al. , Long-term effects of musical training and functional plasticity in salience system. Neural Plast. 2014, 180138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S., Kirino E., Functional connectivity of the precuneus in female university students with long-term musical training. Front. Hum. Neurosci. 10, 328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lordier L., et al. , Music processing in preterm and full-term newborns: A psychophysiological interaction (PPI) approach in neonatal fMRI. Neuroimage 185, 857–864 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Meskaldji D.-E., et al. , Prediction of long-term memory scores in MCI based on resting-state fMRI. Neuroimage Clin. 12, 785–795 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meskaldji D.-E., Morgenthaler S., Van De Ville D., “New measures of brain functional connectivity by temporal analysis of extreme events” in IEEE 12th International Symposium on Biomedical Imaging (ISBI), (IEEE, New York, NY, 2015), pp. 26–29.
- 30.Tagliazucchi E., Balenzuela P., Fraiman D., Montoya P., Chialvo D. R., Spontaneous BOLD event triggered averages for estimating functional connectivity at resting state. Neurosci. Lett. 488, 158–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo K., et al. , Connectome-based predictive modeling of attention: Comparing different functional connectivity features and prediction methods across datasets. Neuroimage 167, 11–22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Q., et al. , Resting-state functional connectivity predicts cognitive impairment related to Alzheimer’s disease. Front. Aging Neurosci. 10, 94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahav A., Skoe E., An acoustic gap between the NICU and womb: A potential risk for compromised neuroplasticity of the auditory system in preterm infants. Front. Neurosci. 8, 381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahav A. Questionable sound exposure outside of the womb: Frequency analysis of environmental noise in the neonatal intensive care unit. Acta Paediatr. 104, e14–e19 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Vinall J., Grunau R. E., Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr. Res. 75, 584–587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith G. C., et al. , Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541–549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janata P., et al. , The cortical topography of tonal structures underlying Western music. Science 298, 2167–2170 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Peretz I., Zatorre R. J., Brain organization for music processing. Annu. Rev. Psychol. 56, 89–114 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Popescu M., Otsuka A., Ioannides A. A., Dynamics of brain activity in motor and frontal cortical areas during music listening: A magnetoencephalographic study. Neuroimage 21, 1622–1638 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Li W., et al. , Subregions of the human superior frontal gyrus and their connections. Neuroimage 78, 46–58 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Habibi A., Damasio A., Ilari B., Elliott Sachs M., Damasio H., Music training and child development: A review of recent findings from a longitudinal study. Ann. N. Y. Acad. Sci. 10.1111/nyas.13606 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Palomar-García M.-Á., Zatorre R. J., Ventura-Campos N., Bueichekú E., Ávila C., Modulation of functional connectivity in auditory–motor networks in musicians compared with nonmusicians. Cereb. Cortex 27, 2768–2778 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Bäuml J. G., et al. , Correspondence between aberrant intrinsic network connectivity and gray-matter volume in the ventral brain of preterm born adults. Cereb. Cortex 25, 4135–4145 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Mitra A., et al. ; IBIS Network , Resting-state fMRI in sleeping infants more closely resembles adult sleep than adult wakefulness. PLoS One 12, e0188122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raichle M. E., et al. , A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson I. R., Plotzker A., Ezzyat Y., The enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130, 1718–1731 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Fischi-Gómez E., et al. , Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cereb. Cortex 25, 2793–2805 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Koelsch S., Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 15, 170–180 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Brown S., Martinez M. J., Parsons L. M., Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport 15, 2033–2037 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Tabei K., Inferior frontal gyrus activation underlies the perception of emotions, while precuneus activation underlies the feeling of emotions during music listening. Behav. Neurol. 2015, 1–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satoh M., Takeda K., Nagata K., Hatazawa J., Kuzuhara S., Activated brain regions in musicians during an ensemble: A PET study. Brain Res. Cogn. Brain Res. 12, 101–108 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Satoh M., Takeda K., Nagata K., Shimosegawa E., Kuzuhara S., Positron-emission tomography of brain regions activated by recognition of familiar music. AJNR Am. J. Neuroradiol. 27, 1101–1106 (2006). [PMC free article] [PubMed] [Google Scholar]
- 53.Benders M. J., et al. , Early brain activity relates to subsequent brain growth in premature infants. Cereb. Cortex 25, 3014–3024 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Alluri V., et al. , Connectivity patterns during music listening: Evidence for action-based processing in musicians. Hum. Brain Mapp. 38, 2955–2970 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He H., et al. , Music intervention leads to increased insular connectivity and improved clinical symptoms in schizophrenia. Front. Neurosci. 11, 744 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karahanoğlu F. I., Van De Ville D., Dynamics of large-scale fMRI networks: deconstruct brain activity to build better models of brain function. Curr. Opin. Biomed. Eng. 3, 28–36 (2017). [Google Scholar]
- 57.Jobe A. H., Bancalari E., Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Papile L. A., Munsick-Bruno G., Schaefer A., Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J. Pediatr. 103, 273–277 (1983). [DOI] [PubMed] [Google Scholar]
- 59.Martinet M., et al. , Élaboration et validation de contenu d’une grille d’observation du comportement sensorimoteur du nouveau-né à l’usage du personnel soignant. Arch. Pediatr. 20, 137–145 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Power J. D., et al. , Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meskaldji D. E., et al. , Adaptive strategy for the statistical analysis of connectomes. PLoS One 6, e23009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meskaldji D.-E., et al. , Improved statistical evaluation of group differences in connectomes by screening-filtering strategy with application to study maturation of brain connections between childhood and adolescence. Neuroimage 108, 251–264 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.