Abstract
Patients with Huntington's disease (HD) exhibit movement disorders, psychiatric disturbance and cognitive impairments as the disease progresses. Abnormal sleep/wake cycles are common among HD patients with reports of delayed sleep onset, fatigue during the day, and a delayed pattern of melatonin secretion all of which suggest circadian dysfunction. Mouse models of HD confirm disrupted circadian rhythms with pathophysiology found in the central circadian clock (suprachiasmatic nucleus). Importantly, circadian dysfunction manifests early in disease, even before the classic motor symptoms, in both patients and mouse models. Therefore, we hypothesize that the circadian dysfunction may interact with the disease pathology and exacerbate the HD symptoms. If correct, early intervention may benefit patients and delay disease progression. One test of this hypothesis is to determine whether light therapy designed to strengthen this intrinsic timing system can delay the disease progression in mouse models. Therefore, we determined the impact of blue wavelength-enriched light on two HD models: the BACHD and Q175 mice. Both models received 6 h of blue-light at the beginning of their daily light cycle for 3 months. After treatment, both genotypes showed improvements in their locomotor activity rhythm without significant change to their sleep behavior. Critically, treated mice of both lines exhibited improved motor performance compared to untreated controls. Focusing on the Q175 genotype, we sought to determine whether the treatment altered signaling pathways in brain regions known to be impacted by HD using NanoString gene expression assays. We found that the expression of several HD relevant markers was altered in the striatum and cortex of the treated mice. Our study demonstrates that strengthening the circadian system can delay the progression of HD in pre-clinical models. This work suggests that lighting conditions should be considered when managing treatment of HD and other neurodegenerative disorders.
Abbreviations: BACHD, bacterial artificial chromosome mouse model of HD; HD, Huntington's disease; HTT, Huntingtin protein; Htt, huntingtin gene; ipRGCs, intrinsically photoreceptive retinal ganglion cells; KI, knock in; SCN, suprachiasmatic nucleus; UCLA, University of California, Los Angeles; ZT, Zeitgeber time
Keywords: BACHD, Blue light therapy, Circadian rhythms, Huntington's disease, Photic therapy, Q175
Highlights
-
•
Blue-enriched lighting improves locomotor activity rhythms in mouse models of HD.
-
•
Blue-enriched lighting improves some measures of motor performance in the HD mice.
-
•
Blue-enriched lighting alters the expression of HD relevant markers in the cortex and striatum.
-
•
Findings suggest that environmental lighting conditions should be considered in the management of HD.
1. Introduction
Huntington's disease (HD) patients suffer from progressive neurodegeneration that inflicts cognitive, psychiatric, cardiovascular and motor dysfunction (Margolis and Ross, 2003, Bourne et al., 2006, Grimbergen et al., 2008, Fisher et al., 2014). HD is caused by a CAG repeat expansion within the first exon of the Huntingtin (Htt) gene and when translated, produces a polyglutamine repeat that leads to protein misfolding, soluble aggregates, and inclusion bodies detected throughout the body (Saft et al., 2005, Ciammola et al., 2006). The mutated HTT protein leads to dysfunction of a large range of cellular processes, including cytoskeletal organization, protein folding, metabolism and transcriptional activities. HD symptoms start at a range of ages, with an average onset at 40 years of age. Generally, the longer the CAG repeat, the earlier the age of onset and the greater the severity of the symptoms (Duyao et al., 1993, Langbehn et al., 2010). Still, even among patients with the same CAG repeat length, there is considerable range in the onset of symptoms (around a decade) and their severity (Wexler et al., 2004, Gusella et al., 2014). This variability raises the possibility of environmental modifiers to the disease and suggests that optimal disease management can increase the health span of the patients. This possibility is important to pursue as there are no known cures for HD.
Sleep disorders are extremely common in HD and have detrimental effects on daily functioning and the quality of life of patients and their caregivers (Cuturic et al., 2009, Aziz et al., 2010, Goodman et al., 2011). Disruptions in the timing of sleep are common and often become apparent years before the onset of motor symptoms. Mouse models of HD also exhibit a progressive and rapid breakdown of the circadian rest/activity cycle that mimics the condition observed in human patients typified by loss of consolidated sleep, increased wakeful activity during the sleep phase, and more sleep during the active/awake phase (Morton et al., 2005, Kudo et al., 2011, Loh et al., 2013). Disorganized circadian timing causes a number of undesirable effects throughout the body (Colwell 2015) altering the function of key organ systems including the heart, pancreas, liver and lungs as well as the brain. This body of work supports the hypothesis that circadian dysfunctions might interact with HD disease pathology and exacerbate the symptoms. If this hypothesis is correct, we would expect to modify the HD disease progression by employing treatments that improve the sleep/wake cycle.
Light is a powerful regulator of our physiology and behavior. Besides visually driven behaviors, there are also a number of non-visual light responses including those involved in the regulation of circadian behaviors (Duffy and Wright, 2005, Roenneberg et al., 2013). The importance of the intrinsically photoreceptive retinal ganglion cells (ipRGCs) that make use of melanopsin as a photopigment has become clear (Rollag et al., 2003, Schmidt et al., 2011). These ipRGCs underlie circadian light detection as well as the impact of light on mood and perhaps cognition (LeGates et al., 2014). Melanopsin phototransduction is maximally sensitive to blue wavelength light (Panda et al., 2005). However, ipRGCs can also respond to rod- and cone-driven signals (Dacey et al., 2005). The ipRGCs integrate this light information (Lucas et al., 2012) and send a direct projection to the central circadian clock (suprachiasmatic nucleus, SCN) where this signal has a profound impact on electrical activity and gene expression (Colwell 2011). Clinically, timed exposure to bright white light has previously been applied to treat the sleep/wake disturbances in aging and neurodegenerative disease with somewhat mixed results (Willis and Turner, 2007, Riemersma-van der Lek et al., 2008, McCurry et al., 2011, Zhou et al., 2012). More recent work has turned to utilizing blue light of lower intensity to improve the sleep/wake cycle in human subjects (Royer et al., 2012, Gabel et al., 2013, Figueiro et al., 2014, Sloane et al., 2015). Unfortunately, there has been little work applying blue light-therapy to pre-clinical disease models to optimize treatments or explore underlying mechanisms.
We previously established an age-dependent progression of circadian and motor symptoms for two mouse modes of HD: BACHD and Q175 (Kudo et al., 2011, Loh et al., 2013, Kuljis et al., 2016). In the present study, we sought to determine whether blue-enhanced lighting conditions can alter this disease trajectory by applying this light treatment for 3 months (mo) starting just prior to the genotypic-specific decline in activity rhythms (BACHD, 3 mo; Heterozygote Q175, 6 mo). For nocturnal animals, light initiates sleep through a melanopsin-dependent mechanism (Lupi et al., 2008). In order to suppress undesired day-time activity and reinforce photic cues to the circadian timing system of the HD mutants, we applied blue-enriched light treatment during the first 6 h (h) of the light cycle. After the treatment, we assessed the impact of blue-enriched lighting on HD-related decline by comparing locomotor activity, sleep behavior, and motor performance with their age-matched controls. At the end of the study, we collected cortex and striatum tissue samples from the Q175 line for analysis of the expression of 100 previously identified HD transcriptional markers (Langfelder et al., 2016).
2. Material and methods
All experimental protocols used in this study were approved by the University of California, Los Angeles (UCLA) Animal Research Committee (ARC 2009-022). Every effort was made to minimize pain and discomfort. Experiments followed the UCLA Division of Laboratory Animal Medicine recommendations for animal use and welfare, as well as National Institutes of Health guidelines.
2.1. Animals
All mice used in this study were males on the C57BL6/J background. BACHD mice that we employed for this study express a transgenic copy of the full length human mutant huntingtin gene encoding 97 glutamine repeats under the control of endogenous regulatory machinery (Gray et al., 2008). The Q175 mice arose from a spontaneous expansion of the CAG repeat in the CAG140 transgenic knock-in line (Menalled et al., 2012). The Q175 mice have previously been shown to have around 175 CAG repeats and we used mice heterozygous (Het) for the Q175 allele. Mutant mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) from a colony managed by the CHDI Foundation. To confirm previous findings of daily rhythm and motor performance decline in both HD models, we also examined WT mice at 3, 6, and 9 mo of age (Supplemental Table 1).
2.2. Housing conditions
The animals were singly housed within light-tight chambers with independently controlled lighting conditions: 12 h of light followed by 12 h of dark (12:12 LD). The chambers were in the same animal housing facility with controlled temperature and humidity, and each chamber held 8 cages of mice, grouped together by lighting treatment. All animals received cotton nestlets, and rodent chow and water were made available ad libitum. Animals were assessed for cage activity, sleep behavior, and motor performance under these baseline conditions prior to beginning treatments. After baseline measurements were collected, the animals were housed under one of two different lighting conditions for 3 months: the control group was maintained under 12:12 LD using white light, while the blue-enriched light-treated group was also maintained under the 12:12 LD and received additional exposure to blue light during the first half of the light phase (Zeitgeber time, ZT 0–6, where ZT 0 refers to the start of the light cycle). We have previously shown that the BACHD line exhibits abnormally high activity during the beginning of the light period (Kudo et al., 2011). Both control and blue-enriched light chambers were lit from the top of the cabinet using white fluorescent lamps providing approximately 350 lx of full spectrum light. The blue LED lamps (peak at 470 nm wavelength) were vertically positioned on the top of each single cage at the same height as the white lamps providing an additional 150 lx. These readings were obtained when wheel running cages were present. The spectral irradiance was measured using a spectrophotometer (International Light Technologies, Peabody, Massachusetts; Supplemental Fig. 1) while photopic illuminance (lux) intensity was measured by using a light meter (BK precision, Yorba Linda, CA). These measurements were made without the cages in the chamber and thus represent the maximum irradiance to which the mice could have been exposed.
All motor tests were performed in the middle of the night, during ZT 18–22, under dim red light (3 lx). The behavioral tests were performed before the start of treatment to ensure that there were no differences between the untreated mutants and then again after 3 mo of treatment. These final tests were performed while the animals were still under blue-enriched lighting conditions.
2.3. Cage activity
Locomotor activity was recorded using infrared sensors and analyzed using the El Temps (A. Diez-Nogura, Barcelona, Spain; http://www.el-temps.com/principal.html) and ClockLab programs (Actimetrics, Wilmette, IL). Activity levels with the infrared sensors are reported as arbitratry units (a.u.). Animals were entrained for 2 weeks before data collection. Cage activity was recorded in 3 min bins, and 10 days of data were averaged for analysis. We used the 10 days of activity data collected just prior to the motor performance tests performed during baseline conditions and during the final 2 weeks of treatment. The data were analyzed to determine the period and rhythmic strength as previously described (Kudo et al., 2011, Loh et al., 2013). The periodogram analysis used a χ2 test with a threshold of 0.001 significance, from which the amplitude of the periodicities was determined at the circadian harmonic to obtain the rhythm power. The amount of cage activity over a 24 h period was averaged over 10 days and reported here as cage activity/hr. Fragmentation was determined using Clocklab and defined by bouts/day, where each bout was counted when activity was separated by a gap of 21 min or more (max gap setting of 21 min) (Loh et al., 2013; Kudo et al., 2013).
2.4. Video measurement of immobility-defined sleep
Mice were habituated to see-through plastic cages containing bedding, but without the addition of nesting material, for a minimum of 3 days prior to video recording of behavior. A side-on view of each cage was obtained, with minimal occlusion by the food bin or water bottle, both of which were top-mounted. Cages were side-lit using infra-red LED lights. Video capture was accomplished using surveillance cameras with visible light filters (Gadspot Inc., City of Industry, CA) connected to the video-capture card (Adlink Technology Inc., Irvine, CA) on a Dell Optiplex computer system. ANY-maze software (Stoelting Co., Wood Dale, IL) was used to track the animals as described by Fisher and colleagues (2012), who found 99% correlation between immobility-defined and EEG-defined sleep using an immobility detection threshold set to 95% of the area of the animal immobile for 40 s. Immobility-defined sleep in this study is thus defined as 95% immobility recorded in the tracked animal for a minimum of 40 s. Continuous recording and tracking of the mice was performed for a minimum of 3 days, with randomized visits (1/day) by the experimenter to confirm mouse health and video recording. We used data collected from days 2 and 3 for further analysis. Immobility-defined sleep data were exported in 1 min bins, and total sleep was determined by summing the duration of sleep in the rest phase (ZT 0–12) or active phase (ZT 12–24). An average waveform of hourly sleep from both days was produced per genotype per age group.
2.5. Motor tests
Accelerating rotarod and challenging beam tests were applied to determine the progression of motor dysfunction in HD mouse models. A two-day protocol for the accelerating rotarod tests was used. On the first day, the mice were trained on the rotarod (Ugo Basile, Varese, Italy) over 5 trials. The rotarod started at 5 rpm and accelerated to a maximum of 38 rpm. The maximum length of each trial was 600 s, and mice were allowed to rest for a minimum of 60 s between trials. On the second day, mice were tested on the rotarod and the latency to fall from the rotarod was recorded from 5 trials. Mice were again allowed to rest for a minimum of 60 sec between trials. Data from each mouse were analyzed after averaging the times from all 5 trials.
The challenging beam test used is a modified version of the beam traversal test previously described (Carter et al., 1999, Goldberg et al., 2003), and used to characterize the motor deficits of BACHD and Q175 mutant mice in previous studies (Loh et al., 2013, Kuljis et al., 2016). The beam narrows in 4 intervals from 33 mm>24 mm>18 mm>6 mm, with each segment spanning 253 mm in length. Apparatus and methods used are similar to those described by Fleming et al. (2013). The home cage of each mouse was put on the end of the beam as the motivating factor. Animals were trained on the beam for 5 consecutive trials on two consecutive days. During each trial, each mouse was placed on the widest end of the beam and allowed to cross with minimal handling by the experimenter. On the testing day, a metal grid (10×10 mm spacing, formed using 19-gauge wire) was overlaid on the beam. This overlaid grid increases the difficulty of the beam traversal task, and provides a visual indicator of foot slips made while crossing the grid. Each mouse was subjected to 5 consecutive trials, which were recorded by a camcorder under dim red light conditions (3 lx), supplemented with infrared lighting for video recording. The videos were scored post-hoc by two independent observers for the number of missteps (errors) made by each mouse. An error was scored when any foot dipped below the grid. The number of errors was averaged across the 5 trials per mouse to give the final reported values.
2.6. Photic masking of wheel running activity
A separate cohort of BACHD mice (3 mo of age, n=9), Q175 mice (6 mo of age, n=3), and age-matched WT mice were housed under a 12:12 LD cycle (300 lx full spectrum light) to determine the effects of light at night on masking of nocturnal activity. Animals were individually housed in cages with running wheels in light-tight chambers with the same housing conditions as the treatment conditions. Their wheel-running activity was recorded as revolutions (rev) per 3 min interval. The animals were habituated to the wheel-running cages and entrained to the LD cycle before the photic masking treatments. We monitored the wheel running activity to confirm entrainment. After habituation, a minimum of one day of baseline wheel running activity was recorded prior to the first photic manipulation. For the first photic manipulation, the animals were subjected to one hour of blue light (500 lx) at ZT 14 (2 h after lights off, when animals are typically nocturnally active). The number of wheel revolutions during this pulse of white light was compared to the number of wheel revolutions during the equivalent hour on the preceding day (ZT 14 to 15). The number of wheel revolutions during this pulse of blue light was compared to baseline values. In addition to reporting the average wheel running values during the dark and blue light exposures, we also report the percentage decrease in wheel running: [(rev during blue light) – (rev during dark baseline)]/(rev during dark baseline) %. No other behavioral assays were performed on these animals.
2.7. Photic induction of cFos
A separate cohort of BACHD (4 mo), Het Q175 (6 mo) and their age-matched WT controls (n=3/genotype) were housed in constant dark (DD) conditions with access to a running wheel. The mice were exposed to white light (50 lx, 10 min) at CT 16 (4 circadian hrs after activity onset). 60 min after the beginning of the light treatment, mice were anesthetized with isoflurane and perfused intracardially with 4% paraformaldehyde (PFA). Brains were dissected out, post-fixed in 4% PFA at 4 °C overnight, and cryoprotected in 15% sucrose. Sequential coronal sections (50 μm) containing the SCN were collected. Immunolabelling of frozen sections was performed as previously described (Ghiani et al., 2011). Briefly, sections were blocked in carrier solution (1% BSA and 0.3% Triton X-100) containing 10% normal goat serum for 1 h and incubated for 24 h at 4 °C with primary antibody against cFos (Ab-2 rabbit polyclonal, 1:500, EMD Millipore, Temecula, CA) diluted in carrier solution containing 5% normal donkey serum, followed by a donkey anti-rabbit secondary antibody conjugated to Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA). Immunostained sections were mounted in Vectashield mounting medium with DAPI (49,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA), and visualized on a Zeiss Axio Imager 2 equipped with an AxioCam MRm and the ApoTome imaging system (Zeiss, Thornwood NY). Images were acquired using the Zen software (Zeiss, Thornwood NY), using a 10X objective to visualize both the left and right SCN, which were traced using the Axiovision software (Zeiss, Thornwood NY). Cells immunopositive for cFos were counted with the aid of the cell counter plugin of the NIH Image J software (http://imagej.nih.gov//ij) by a masked observer in two consecutive sections at the level of the mid-SCN. Values from both the left and right SCN were averaged.
2.8. NanoString analysis of gene expression
Four weeks after the final behavioral tests were performed, the Q175 mutants were anesthetized with isoflurane prior to dissection of the cortex and striatum at ZT 15. The brain tissue samples were flash frozen and stored at −80 °C prior to nanostring analysis. The NanoString analysis was performed by LabCorp (Seattle, WA) using a custom CodeSet designed to interrogate 100 transcripts previously implicated in transcriptional changes in the striatum and cortex of Q175 mice (Langfelder et al. 2016). The signal intensity of individual genes was normalized by adjusting to internal positive standards within each sample. Eight housekeeping genes were included in the CodeSet: Gins1, Myh15, Pank2, Poc1b, Pum2, Slc25a15, Ssrp1, Utp3. The expression levels for each probe within a sample were scaled using the geometric mean of the eight housekeeping genes for each sample. Each mouse was an individual sample as tissue did not need to be pooled. The fold change of signal intensity was derived by comparing the normalized means between the control group and blue light-treated group. The Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) platform was used to annotate gene function and pathways (Huang et al., 2008, Huang et al., 2009) of altered gene expression in the cortex.
2.9. Statistical methods
To access the impact of blue-enhanced lighting on activity and sleep rhythms as well as motor performance in each model, we employed 2-way ANOVA with age and treatment as factors (Table 1, Table 2, Table 3, Table 5). To determine the impact of the treatment on errors made in each beam of the challenging beam test, we used a repeated-measures 2-way ANOVA with beam and treatment as factors (Table 3). The impact of the treatments on body weight was evaluated with a repeated-measures 2-way ANOVA with age and treatment as factors (Table 4, Table 5). The results of the t-tests determining the impact of blue light treatment on the measured parameters is reported (Table 6). To determine the impact of masking, we first ran a t-test to determine if the blue light significantly reduced activity then a 2-way ANOVA with treatment and genotype as factors. Finally, a t-test was used to detect the differences in cortical and striatal mRNA expression between Q175 mice housed under control and treated conditions. The cohort of mice housed under normal LD conditions are referred to as the control group, while the mice housed under blue-enriched lighting conditions are referred to as the treated group. SigmaPlot (version 12.5, SYSTAT Software, San Jose, CA) was used to run statistical analyses. Between-group differences were deemed significant if P<0.05. All values are reported as group mean±standard error of the mean (SEM).
Table 1.
Cage activity rhythms of BACHD (3 mo) and Q175 mutants (6 mo) at baseline (LD) and after 3 mo in control LD (control cohort) or blue-enriched LD (treated cohort). Data was analyzed with a 2-way ANOVA with age and treatment as factors. Values are shown as mean±SEM. Age-related changes within a cohort are indicated with #(P<0.05) and age-matched between-cohort differences are indicated with *(P<0.05).
|
BACHD (n=8/group) |
Q175 (n=8/group) |
|||
|---|---|---|---|---|
| Control cohort | Treated cohort | Control cohort | Treated cohort | |
| Rhythm power (V%) | ||||
| Baseline | 22.1±1.3 | 25.0±1.9 | 40.7±3.0 | 36.3±1.6 |
| +3 mo | 18.4±1.8# | 31.0 ±2.2* | 32.1±2.2# | 43.5±2.0#,* |
| Cage activity (units/hr) | ||||
| Baseline | 180.8±14.1 | 172.9±19.2 | 99.3±15.0 | 78.8±11.0 |
| +3 mo | 103.0±10.3# | 160.1±15.9* | 75.3±5.9 | 172.3±21.3#,* |
| Fragmentation (# bouts) | ||||
| Baseline | 9.6±0.9 | 8.3±1.0 | 9.1±0.7 | 8.3±0.6 |
| +3 mo | 11.8±0.6 | 10.1±0.7 | 10.8±0.9# | 9.0±0.6 |
Table 2.
Immobility-defined sleep in BACHD (3 mo) and Q175 mutants (6 mo) at baseline and at the end of 3 mo under LD (control cohort) or blue-enriched lighting (treated cohort). Data was analyzed with a 2-way ANOVA with age and treatment as factors. Values are shown as mean±SEM. Age-related changes within a cohort are indicated with #(P<0.05).
|
BACHD (n=8/group) |
Q175 (n=8/group) |
|||
|---|---|---|---|---|
| Control cohort | Treated cohort | Control cohort | Treated cohort | |
| Rest phase sleep (min) | ||||
| Baseline | 460.9±17.0 | 482.7±19.1 | 498.3±20.0 | 521.6±12.2 |
| +3 mo | 443.7±14.6 | 479.6±16.6 | 515.2±16.7# | 515.8±10.4 |
| Active phase sleep (min) | ||||
| Baseline | 139.1±20.4 | 161.2±7.1 | 178.1±21.6 | 214.9±13.5 |
| +3 mo | 203.5±38.1 | 150.4±23.0 | 207.3±21.3 | 191.0±16.2 |
| Sleep onset (ZT h) | ||||
| Baseline | 0.8±0.3 | 1.3±0.4 | 23.9±0.2 | 22.8±0.2 |
| +3 mo | 1.6±0.4 | 24.3±0.5 | 23.8±0.3 | 23.0±0.3 |
| Sleep offset (ZT h) | ||||
| Baseline | 11.7±0.2 | 11.6±0.2 | 12.1±0.2 | 11.9±0.2 |
| +3 mo | 12.7±0.3# | 13.3±0.3# | 12.6±0.1 | 13.0±0.3# |
Table 3.
Motor behavior assays in BACHD and Q175 mutants at baseline and after treatment. Values are shown as mean±SEM. Data was analyzed with a 2-way ANOVA with age and treatment as factors. Age-related changes within a cohort are indicated with #(P<0.05) and age-matched between-cohort differences are indicated with *(P<0.05).
|
BACHD (n=8/group) |
Q175 (n=8/group) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control cohort | Treated cohort | Control cohort | Treated cohort | |||||||||
| Accelerating rotarod test | ||||||||||||
| Latency to fall (sec) | ||||||||||||
| Baseline | 238.3±33.1 | 197.2±18.5 | 215.0±44.6 | 209.7±42.4 | ||||||||
| +3 mo | 102.6±21.8# | 94.6±20.3# | 256.0±30.4 | 371.2±32.5#,* | ||||||||
| Challenge beam test | ||||||||||||
| Total step errors | ||||||||||||
| Baseline | 3.9±0.3 | 4.2±0.6 | 5.3±0.7 | 5.0±0.5 | ||||||||
| +3 mo | 9.6±0.3# | 5.1±0.6 | 7.4±0.5# | 4.1±0.2* | ||||||||
| Step errors in each beam (early disease age) | ||||||||||||
| Beam1 (widest) | 1.2±0.1 | 0.79±0.17 | 0.83±0.13 | 0.56±0.12 | ||||||||
| Beam2 | 1.9±0.20 | 0.91±0.15* | 1.65±0.15 | 1.09±0.16* | ||||||||
| Beam3 | 2.7±0.20 | 1.48±0.21* | 1.55±0.17 | 1.11±1.19 | ||||||||
| Beam4 (narrowest) | 3.9±0.30 | 1.93±0.38* | 3.41±0.46 | 1.31±0.12* | ||||||||
Table 5.
Comparisons of blue enriched light to untreated controls in BACHD and Q175 mice. The results of the 2-way ANOVA are reported. P values of <0.05 were considered significant.
|
BACHD mutants (n=8/group) |
Post hoc Tukey tests |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age |
Treatment |
Age x Treatment |
Age |
Treatment |
||||||
| F | P | F | P | F | P | Within Control, P | Within Treated, P | Within 3 mo, P | Within 6 mo, P | |
| Rhythm Power (%V) | 0.2 | 0.69 | 5.5 | 0.03 | 12.9 | 0.003 | 0.04 | 0.01 | n.s. | 0.002 |
| Cage activity (au) | 26.7 | <0.001 | 0.3 | 0.59 | 10.6 | 0.006 | <0.001 | n.s. | n.s. | 0.001 |
| Fragmentation (bouts/day) | 10.6 | 0.006 | 1.8 | 0.2 | <0.1 | 0.97 | 0.04 | 0.04 | n.s. | n.s. |
| 24 h sleep (hr) | 0.8 | 0.39 | 0.3 | 0.6 | 2.7 | 0.13 | n.s. | n.s. | n.s. | n.s. |
| Day sleep (min) | 0.4 | 0.55 | 2.9 | 0.11 | 0.2 | 0.68 | n.s. | n.s. | n.s. | n.s. |
| Night sleep (min) | 1.5 | 0.25 | 0.3 | 0.58 | 2.9 | 0.11 | n.s. | n.s. | n.s. | n.s. |
| Sleep onset (ZT) | 0.1 | 0.82 | 1.7 | 0.21 | 3.5 | 0.08 | n.s. | n.s. | n.s. | n.s. |
| Sleep offset (ZT) | 58.9 | <0.001 | 0.9 | 0.36 | 3.4 | 0.09 | 0.001 | <0.001 | n.s. | n.s. |
| Rotarod latency to fall (sec) | 28.7 | <0.001 | 0.8 | 0.4 | 0.7 | 0.4 | <0.001 | 0.007 | n.s. | n.s. |
| Challenge beam errors | 50.1 | <0.001 | 16.4 | 0.001 | 25.8 | <0.001 | <0.001 | n.s. | n.s. | <0.001 |
| Body weight (g) | <0.1 | 0.99 | 0.1 | 0.74 | 2.8 | 0.12 | n.s. | n.s. | n.s. | n.s. |
| Q175 Mutants (n=8/group) | Post hoc Tukey tests | |||||||||
| Rhythm Power (%V) | 1.3 | 0.27 | 4.6 | 0.05 | 13.2 | 0.003 | 0.005 | 0.05 | n.s. | <0.001 |
| Cage activity (au) | 5.8 | 0.03 | 9.1 | 0.009 | 14.2 | 0.002 | n.s. | <0.001 | n.s. | <0.001 |
| Fragmentation (bouts/day) | 10.3 | 0.006 | 2.0 | 0.2 | 1.8 | 0.2 | n.s. | n.s. | n.s. | n.s. |
| 24 h sleep (hr) | 0.2 | 0.6 | 0.4 | 0.5 | 4.8 | 0.05 | n.s. | n.s. | n.s. | n.s. |
| Day sleep (min) | 0.1 | 0.7 | 0.7 | 0.4 | 0.5 | 0.5 | n.s. | n.s. | n.s. | n.s. |
| Night sleep (min) | <0.1 | 0.8 | 0.2 | 0.7 | 5.9 | 0.03 | n.s. | n.s. | n.s. | n.s. |
| Sleep onset (ZT) | <0.1 | 0.9 | 9.2 | 0.009 | 0.8 | 0.4 | n.s. | n.s. | n.s. | n.s. |
| Sleep offset (ZT) | 15.1 | 0.002 | 0.2 | 0.7 | 1.8 | 0.2 | n.s. | 0.003 | n.s. | n.s. |
| Rotarod latency to fall (sec) | 8.9 | 0.01 | 1.7 | 0.2 | 3.2 | 0.1 | n.s. | 0.005 | n.s. | 0.04 |
| Challenge beam errors | 1.5 | 0.2 | 10.3 | 0.006 | 8.3 | 0.01 | 0.01 | n.s. | n.s. | <0.001 |
| Body weight (g) | 4.9 | 0.04 | 1.2 | 0.3 | 0.7 | 0.4 | 0.05 | n.s. | n.s. | n.s. |
Table 4.
Weekly monitored body weights of BACHD and Q175 mutants from baseline to the time of the final tests. Week 0 measurements were made before treatment. All weights in grams. Values are shown as mean±SEM.
|
BACHD (n=8/group) |
Q175 (n=8/group) |
|||
|---|---|---|---|---|
| Control cohort | Treated cohort | Control cohort | Treated cohort | |
| Week 0 | 27.8±1.3 | 28.6±0.9 | 25.9±0.4 | 26.4±0.7 |
| Week 4 | 27.7±1.1 | 25.0±3.1 | 25.9±0.4 | 26.5±0.6 |
| Week 8 | 29.4±1.4 | 27.6±0.7 | 25.0±0.4 | 25.4±0.6 |
| Week 12 | 29.1±1.7 | 27.2±0.6 | 25.0±0.4 | 25.9±0.6 |
Table 6.
Comparisons of blue-enriched LD to control LD conditions in BACHD and Q175 mice. The results of t-tests are reported if data passed normality tests. For parameters that did not pass normality tests, the Mann Whitney rank-sum test was run and the U statistic reported. P values<0.05 were considered significant.
|
BACHD Mutants (n=8/group) |
Q175 Mutants (n=8/group) |
|||||
|---|---|---|---|---|---|---|
| Difference | t | P value | Difference | t | P value | |
| Rhythm power (%V) | 10.5 | 3.1 | <0.001 | 14.0 | 4.2 | <0.001 |
| Cage activity (au) | 42.0 | 1.8 | 0.09 | 99.6 | 4.4 | <0.001 |
| Fragmentation (bouts/day) | 1.2 | 16U | 0.10 | 1.9 | 1.9 | 0.11 |
| 24 h sleep (h) | 0.1 | 27U | 0.65 | -0.3 | -0.5 | 0.7 |
| Day sleep (min) | 35.9 | 1.6 | 0.12 | 0.6 | 0.0 | 0.9 |
| Night sleep (min) | -53.1 | -1.2 | 0.885 | -16.4 | -0.6 | 0.6 |
| Sleep onset (ZT) | -1.4 | -1.9 | 0.07 | -0.8 | -1.8 | 0.09 |
| Sleep offset (ZT) | 0.6 | 1.6 | 0.14 | 0.4 | 1.3 | 0.2 |
| Rotarod latency to fall (s) | -4.2 | 0.1 | 0.9 | 115.2 | 3.6 | 0.02 |
| Challenge beam errors | -4.5 | -5.9 | <0.001 | -3.4 | -5.6 | <0.001 |
| Body weight (g) | -1.6 | 27U | 0.6 | 1.0 | 1.4 | 0.2 |
3. Results
3.1. Locomotor activity rhythms were improved by the blue light treatment
Compared to untreated, age-matched (6 mo) controls, the BACHD mice exposed to blue-enriched lighting conditions exhibited more robust locomotor activity rhythms (Fig. 1A, C). As BACHD mice aged from 3 to 6 mo, the untreated group housed under the normal 12:12 LD cycle showed decreased locomotor activity rhythm power (P=0.038, Fig. 2A, Table 1), similar to previous findings of an age-related decline in this model (Kudo et al., 2011, Kuljis et al., 2016). Cage activity in untreated 6 mo BACHD mice also decreased significantly with age (P<0.001, Fig. 2C). Fragmentation was increased with age in this untreated BACHD cohort (P=0.04, Fig. 2E). On the other hand, BACHD mice housed under blue-enriched lighting conditions did not show an age-related decrease in either activity rhythm power, activity amount or fragmentation (Table 5). Compared to untreated mutants, the blue-enhanced lighting increased the power of the rhythm (P<0.002, Table 1). These findings demonstrate that blue-enriched lighting significantly improves activity rhythms in the BACHD line.
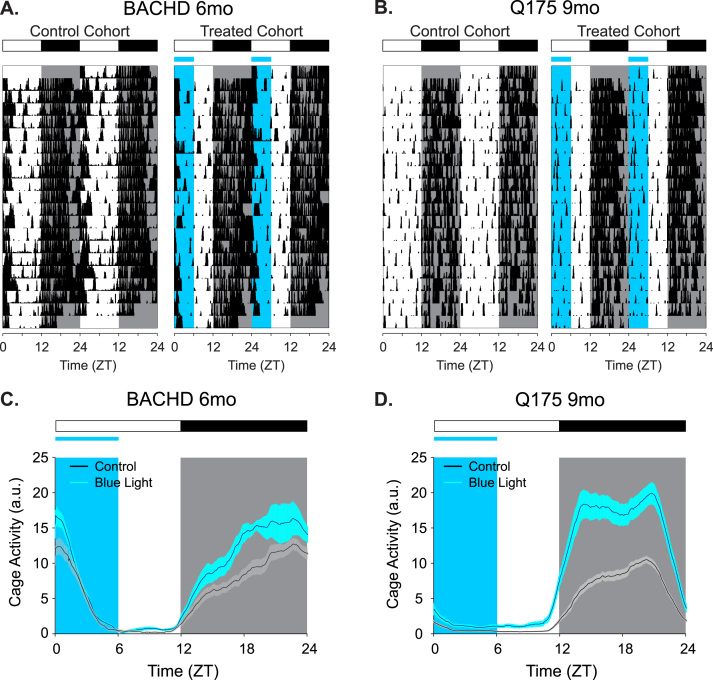
Fig. 1.
Examples of cage activity rhythms recorded from BACHD (A, C) and Q175 (B, D) under control and blue-enriched lighting conditions. Representative double-plotted actograms of cage activity from BACHD (A, 6 mo) and Q175 (B, 9 mo) under control LD and blue-enriched LD conditions. The activity levels in the actograms were normalized to the same scale (85% of the maximum of the most active individual). Each row represents two consecutive days, and the second day is repeated at the beginning of the next row. (C, D) Average waveforms from 10 days of cage activity, using a 3-min smoothing window (n=8/genotyp/treatment), are shown and standard errors across animals are indicated. The white/black bar on the top indicates the 12:12 h LD cycle, and blue shading in the waveforms indicates the time of blue light exposure.
Fig. 2.
Locomotor activity rhythms were improved by the blue-enriched light treatment. Quantification of the locomotor activity rhythms of BACHD (A, C, E) and Q175 (B, D, F) under control LD and blue-enriched LD conditions. Plots represent the first and third quartile (box), group medians (middle line) and data range (whiskers). Gray boxes represent the untreated controls (mice under normal LD) and blue boxes represent mice under blue-enriched lighting. Data were analyzed using a 2-way ANOVA with age and treatment as factors. Significant within-cohort age-related differences are indicated by # (P<0.05). Significant age-matched between-cohort differences treatments are highlighted with * (P<0.05). (A, B) The strength of the activity rhythm is indicated by the power (%V) of the χ2 periodogram analysis. (C, D) An hourly average of cage activity over the 10 days is reported. (E, F) The number of bouts of activity per day are reported as the amount of fragmentation of the daily activity cycle.
Blue-enriched lighting also had a robust impact on the amplitude of diurnal rhythms in the Q175 line compared to untreated, age-matched (9 mo) controls (Fig. 1B, D). Under control LD conditions, Q175 mice exhibited a decrease in rhythm power (P=0.006, Fig. 2B) as they aged from 6 to 9 mo. However, the treated Q175 mice showed an increase in rhythm power after 3 mo in blue-enriched lighting conditions (P<0.001, Table 1, Fig. 2B). The amount of cage activity was also increased under blue-enriched lighting in Q175 line (P<0.001, Figs. 1D, 2D). These increases in rhythm power and activity amount were detected without significant change in fragmentation in the treated cohort (P=0.2, Fig. 1F). Taken together, these findings demonstrate that blue-enriched lighting significantly improves activity rhythms in Q175 line.
Compared with 6 mo WT (Supplemental Table 1), the 6 mo control BACHD mice had significantly reduced rhythm power (P<0.001) whereas rhythm power in 6 mo blue-enriched light-treated BACHD mice was not significantly different from WT (P=0.4). Amount of activity and fragmentation were not significantly different between age-matched WT and either control or treated BACHD mice at 6 mo (P=0.2, P=0.9 respectively). Compared with 9 mo WT (Supplemental Table 1), the 9 mo blue-enriched light-treated Q175 mice had significantly increased rhythm power compared to age-matched WT mice (P<0.001). This increase in rhythm power in the 9 mo treated Q175 line is independent of the amount of cage activity, which remains reduced compared to age-matched WT mice (P=0.02). The 9 mo control Q175 mice showed more activity fragmentation compared to age-matched WT mice (P=0.02). Blue-enriched lighting reduced this fragmentation in the Q175 mice to levels that were not significantly different from WT (P=0.4). The comparisons of the blue-enriched treatment cohorts to age-matched WT mice suggest that the activity rhythm deficits in HD mice can be treated with blue-light therapy, raising rhythm strength to near-normal levels for their respective ages.
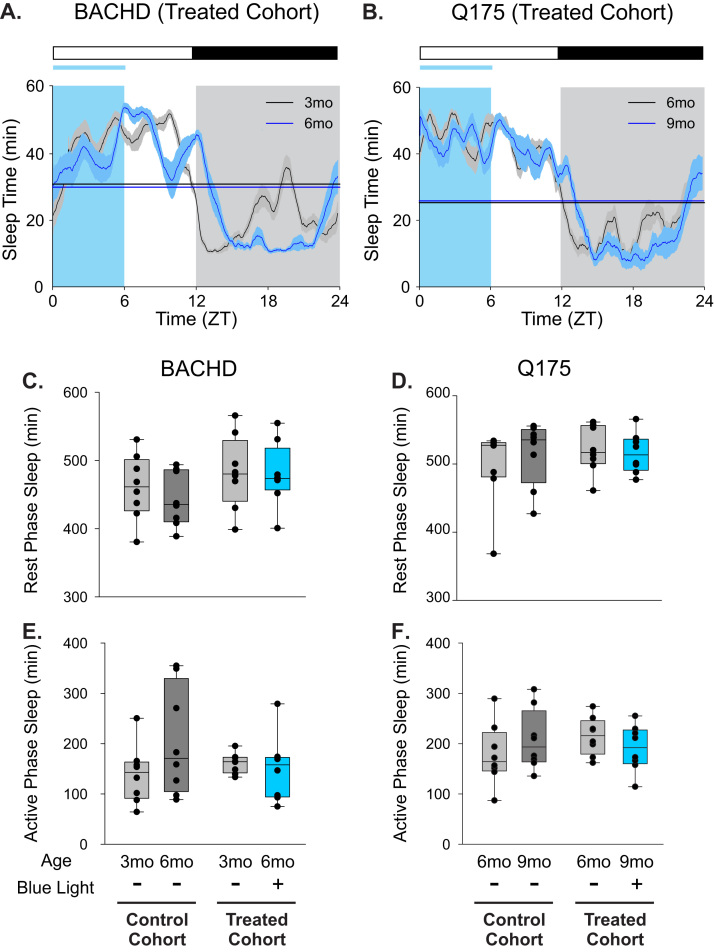
3.2. Sleep behavior was largely unaffected by the blue light treatment
Video recording in combination with automated mouse tracking analysis software was used to measure immobility-defined sleep behavior (Loh et al., 2013, Li et al., 2015). Blue-enriched lighting conditions had minimal impact on sleep behavior of the BACHD line (Fig. 3A, C, E). No significant changes were detected in the amount of sleep behavior over a 24-hr cycle displayed by control BACHD mice as they aged from 3 to 6 mo (P=0.1). The temporal distribution of sleep was also unaltered (day sleep %, P=0.1), where the amount of daytime (rest phase) sleep and nighttime (active phase) sleep were unchanged as mice aged (Pday=0.4 and Pnight=0.1, respectively). The timing of sleep onset was also unaltered with age (P=0.3), but sleep offset was delayed by 1 h (P=0.001). Housing BACHD mice in blue-enriched lighting did not significantly change most of the sleep parameters that we measured (Fig. 3A, C, E; Table 2). While sleep onset was unaltered by age (P=0.19), sleep offset was delayed by 1.4 h (P<0.001). Overall, the blue-enriched lighting environment did not lead to major changes in sleep amount or timing in the BACHD line.
Fig. 3.
Blue-enriched light did not significantly alter the timing or the amount of sleep in either HD model. Video recording in combination with automated mouse tracking analysis software was used to measure immobility-defined sleep. (A, B) Running averages (1 h window) of immobility-defined sleep in BACHD (A) and Q175 (B) mutants are plotted. Data were analyzed using a 2-way ANOVA with age and treatment as factors. The blue light treatment did not significantly alter the onset or offset of sleep. The half-maximum of sleep (min/h) of each group are shown as flat lines. The amount of immobility-defined sleep is not significantly altered by blue-enriched lighting during the day (C, D) or night (E, F) in both HD models.
Similarly, blue-enriched lighting conditions had minimal impact on sleep behavior of the Q175 line (Fig. 3B, D, F). The total amount of sleep behavior exhibited by control Q175 mice remained unchanged as they aged from 6 to 9 mo. However, the temporal distribution (day sleep %, P=0.4) and day or night amounts of sleep (Pday=0.5 and Pnight=0.1) were not altered in the control Q175. The time of sleep onset and offset were also unchanged by age in control Q175 mice (Pon=0.5 and Poff=0.08). Again, housing the Q175 mice in blue-enriched lighting did not significantly change most of the sleep parameters that we measured (Fig. 3B, D, F; Table 2). Although sleep onset was unchanged (P=0.5), sleep offset was delayed by 1 h (P=0.003). Overall, blue-enriched lighting environment did not lead to major changes in sleep amount or timing in the Q175 line.
3.3. Motor performance was improved by the blue light treatment
One of the defining symptoms of HD is movement disorders, where patients have involuntary movements and difficulties coordinating voluntary movements. We hypothesize that sleep-wake and circadian rhythm disruption may contribute to the severity of disease symptoms and that treating these disruptions may delay disease progression. On that premise, we assessed motor performance in the HD models treated with blue-enriched lighting conditions. We used two tests that have been previously shown to detect motor coordination deficits in BACHD (Gray et al., 2008, Kuljis et al., 2016) and Q175 mice (Menalled et al. 2012, Loh et al. 2013): the accelerating rotarod and challenging beam tests.
As previously demonstrated, BACHD mice housed under control LD conditions show an age-related decrease in their latency to fall on the accelerating rotarod test (P<0.001, Fig. 4A; Table 3). BACHD mice housed under blue-enriched lighting also showed an age-related decrease in rotarod performance (P=0.007). There was no difference in rotarod performance between the BACHD control and treated mice (6 mo, P=0.9). Both the control and treated cohorts of BACHD mice fell off the rotarod significantly earlier than age-matched WT mice (P<0.001). In the challenging beam test, control BACHD mice made more errors as they aged from 3 to 6 mo (P<0.001, Fig. 4C). In contrast, the 6 mo BACHD mice housed in blue-enriched lighting were not any more error-prone than when they were 3 mo old (P=0.3). In all, the treated BACHD mice made significantly fewer errors than the control BACHD mice (P<0.001). A closer look reveals that the treated BACHD mice made significantly fewer errors in each beam with the most dramatic differences at the narrowest, and most difficult, beam (Fig. 4E). Compared to 6 mo WT mice, the control BACHD mice made significantly more errors (P<0.001) on the challenging beam, and the blue-enriched treatment group was not significantly different from WT (P=0.06). These results demonstrate that the blue-enriched lighting treatment had a mixed impact on motor performance in the BACHD line resulting in improved performance on the challenging beam but not the rotarod test.
Fig. 4.
Housing mice in blue-enriched lighting improved motor performance in the BACHD (A, C, E) and Q175 (B, D, F) HD models. Significant within-cohort age-related changes are denoted by # (P<0.05), and significant age-matched between-cohort differences are denoted by *(P<0.05,). (A, B) The latency to fall (s) off an accelerating rotarod is plotted. (C, D) Group averages of the total number of errors made while crossing the challenging beam are plotted. Data were analyzed using a 2-way ANOVA with age and treatment as factors. Significant within-cohort age-related differences are indicated by # (P<0.05). Significant age-matched between-cohort differences treatments are highlighted with *(P<0.05). (E, F) Errors made on the individual beams of increasingly narrow widths are shown from 6 mo BACHD mice (E) and 9 mo Q175 mice (F). Beam decrease in width per segment, starting at 33 mm (Beam 1) to 6 mm (Beam 4), and are not drawn to scale. Data were analyzed using a 2-way repeated-measures ANOVA with beam segment and treatment were factors, and the errors were the data.
As previously demonstrated, Q175 mice housed under control LD conditions did not show a significant change in rotarod performance between 6 and 9 mo of with age (P=0.5). Intriguingly, the Q175 mice under blue enriched lighting treated Q175 mice showed improvements in rotarod performance compared to baseline (P=0.005, Fig. 4B). The treated Q175 mice even stayed on the rotarod for longer than age-matched WT mice (P=0.002; Supplemental Table 1). Control Q175 mice showed an age-related decline in challenge beam performance, as they make more errors at 9 mo than 6 mo of age (P=0.01, Fig. 4D). On the other hand, the blue-light treated Q175 mice made significantly fewer errors while crossing the beam compared to control Q175 mice (P<0.001, Fig. 4D). Breaking down the errors made by beam width revealed that the main difference between treated and control Q175 mice (9 mo) were the errors in the narrowest beam (Fig. 4F). Compared with age-matched WT mice, control Q175 mice made more errors than WT (P<0.001), while treated Q175 mice performed at similar levels as WT controls (P=0.55). Thus, the blue-enriched lighting improved the performance of the Q175 mutant mice on both the rotarod and challenging beam tests.
As rotarod performance could be due to changes in body weight, we tracked the body weights of each group of mice over the duration of the treatment. No significant changes were detected within each group of mice (Table 4), suggesting that neither weight loss nor gain played a significant role in these results.
3.4. Reduced activity response to light-suppressing effect in BACHD line
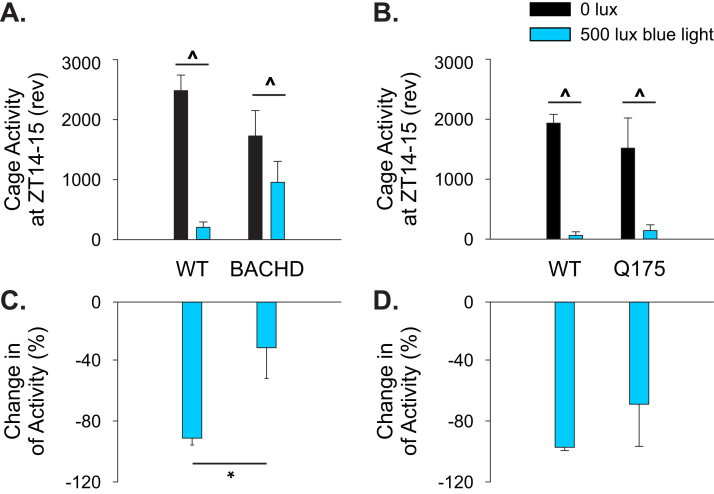
The difference in motor performance outcomes between BACHD and Q175 mice might have been due to differences in the effectiveness of the photic response between the two HD models. As seen by the average waveforms of cage activity (Fig. 1C), the blue light treatment was ineffective in reducing locomotor activity in the beginning of the day in BACHD mice. For example, at ZT 0–1, the untreated BACHD exhibited an average cage activity level of 233±27 a.u. and the addition of blue light had no significant effect on locomotor activity (216±30 a.u.). Therefore, we sought to determine if the BACHD mice exhibited deficits in the blue light to acutely suppress (or mask) locomotor activity in a separate cohort of mice.
In this light-masking experiment, 3 mo BACHD and age-matched WT controls were housed under normal LD conditions to determine a baseline activity rhythm profile per animal. Subsequently, the mice were exposed to blue light (500 lx, 60 min duration) close to the start of the dark phase (ZT 14), and the amount of activity during this hour of light was compared to the preceding day when the mice were under a normal LD cycle. WT mice (n=6) showed an 82% reduction in activity during the blue light exposure at ZT 14 with all the mice exhibiting reduced activity during treatment (t-test, P<0.001; Fig. 5A, C). BACHD mice (n=9) subjected to the same blue light treatment at ZT 14 exhibited a 32% reduction in activity with 2 of the mutant mice exhibiting an increase in activity during the light exposure (t-test, P=0.042; Fig. 5A, C). An analysis of the data with a two-way ANOVA (treatment and genotype as factors) indicated that the WT mice exhibited significant masking (P<0.001) while the BACHD did not (P=0.1). As expected, the Q175 mice (6 mo, n=3) displayed normal blue light suppression of activity (Fig. 5B, D). These light masking experiments revealed that BACHD mice have a decreased behavioral response to light exposure, which could explain the different degree of efficacy of the blue-enriched light treatment.
Fig. 5.
Photic masking of wheel running behavior in the HD mutants. In order to determine if masking behavior was deficient in the BACHD line, BACHD (3 mo; n=9) and age-matched WT (n=6) mice were housed under 12:12 LD were given a 1 h exposure to blue light (500 lx) at ZT 14. (A) Quantification of the amount of wheel running revolutions exhibited by the mice during ZT 14 to 15 are shown, comparing the revolutions during complete darkness (0 lx) and blue light (500 lx). (B) Data from Q175 (6 mo; n=3) is shown for comparison. (C) Blue light-induced masking of locomotor activity is expressed as a % change compared to baseline activity under complete darkness. (D) Data from Q175 shown for comparison. Mean±SEM are plotted. Raw locomotor activity values from WT and mutant mice were first analyzed by t-test to determine if the light significantly suppressed activity. In addition, data were analyzed with treatment and genotype as factors. ^ (P<0.05) denotes significant difference between cage activity level in darkness and in blue light treatment. Between-cohort differences are indicated by * (P<0.05).
3.5. Expression of multiple HD markers in the cortex and striatum were altered by the blue light treatment
To identify pathways that may be responsible for the effect of blue-enriched lighting on HD mutants, we assessed changes in the expression of HD markers in the cortex and striatum. We focused on the Q175 mutants as this line consistently responded better to the blue light treatment. At the end of the study, the same Q175 mice that underwent activity monitoring and behavioral tests were allowed to recover for 4 weeks from manipulations, after which we analyzed mRNA expression in cortex and striatum using NanoString Technology. In each region, we examined the expression levels of 100 genes that have been previously identified as being altered in Q175 mutants (Langfelder et al. 2016; Supplemental Table 2). In the cortex, we found that Q175 mice housed under blue-enriched lighting showed altered expression of 10 of the genes examined compared to the mice housed under control LD (Table 7). In the striatum, the blue light treatment changed the expression of 4 genes (Table 7). Interestingly, our protocol influenced the expression of a different set of genes in each region. Functional clustering revealed transcriptional changes in pathways involved in the response to oxidative stress (increased Hmox1, Sod1, Gclc), locomotor behavior (decreased Drd2, Penk), and extracellular signaling (decreased C3, Penk, Tac1). Hence, the blue-enriched light treatment significantly altered the patterns of gene expression in a tissue-specific manner.
Table 7.
Expression of HD markers in the striatum and cortex of Q175 that are altered by blue light treatment. P value of the t-test comparison with Q175 housed under control LD conditions are shown. *indicates HD markers that are changed in both the striatum and cortex. Transcripts increased by the treatment (fold change>1) are shown in red and those decreased by the treatment (fold change<1) in blue. Full data set in Supplemental Table 2.
 |
4. Discussion
It is well established that melanopsin expressing ipRGCs mediate the effects of light on the circadian system and that this photopigment is maximally sensitive to blue wavelength light (Rollag et al., 2003, Schmidt et al., 2011). Therefore, we sought to determine the impact of 6 h of blue-enriched photic entrainment on locomotor activity rhythms of two distinct mouse models of HD (BACHD and Q175 Hets). Blue wavelength light (at ~470 nm) has been suggested to be as effective as bright light therapy (Gordijn et al., 2012). As such, we used a relatively modest light intensity of treatment. In mice, the maximum absorption of melanopsin peaks around 480 nm (Lucas et al., 2001). This blue-enriched light treatment increased the power of the rhythms in both lines of HD mice (Fig. 1). It is worth pointing out that it is not the acute effect of blue light that is driving the improved rhythms. This can be seen clearly with the average waveforms of the Q175 line (Fig. 2B) in which the activity is increased during the night several hours after the mice were exposed to light.
Recent work has shown that blue light exposure (470 nm, ZT 14) acutely causes arousal and elevates corticosterone through a melanopsin-dependent mechanism (Pilorz et al., 2016). Although we did not measure corticosterone in this study, this is a plausible mechanism by which blue-enriched lighting causes increased arousal during the active phase of the treated animals. The blue-enriched lighting may not have significantly altered sleep amount in the HD mice, but it did cause a phase delay in the onset of wake (Fig. 3). These findings suggest that the timing of blue light application will be critical to avoid unwanted phase shifts.
The hallmark symptoms of HD are motor deficits and both the BACHD (Gray et al., 2008, Kudo et al., 2011, Kuljis et al., 2016) and Q175 (Menalled et al., 2012, Loh et al., 2013) lines exhibit a motor phenotype that progressively worsens with age. Prior work using the R6/2 model of HD has shown that bright white light (10,000 lx) with restricted wheel access improved the strength of locomotor activity as well as reduced inappropriate daytime activity (Cuesta et al., 2014). Under blue-enriched light housing, both the BACHD and Q175 lines demonstrated at least one measure of improved motor performance, making fewer errors while crossing the challenging beam test (Fig. 4). In contrast, there was a divergence in the performance on the rotarod test, where the Q175 Hets showed markedly improved performance, whereas the BACHD mice continued to show age-related decline in latency to fall on the rotarod. This test is particularly sensitive to body weight (Kudwa et al., 2013), but there were no differences in body weight between the treated and untreated BACHD cohorts (Table 4). Another possible reason for the differences between the lines could be due in part to the disparity in the degree of disease progression in the two models. For example, the control BACHD mice (6 mo old) were deficient on the rotarod test compared to WT controls, whereas the control Q175 (at 9 mo old) were not different from WT. The cause of the difference in the performance of these two HD models is not known.
Prior work has shown that the BACHD line exhibits reduced magnitude in the light-induced phase shift of the circadian system when exposed to light at CT 16 (Kudo et al., 2011). Consistent with this finding is the lack of negative masking behavior in the BACHD as seen by continued activity even in the presence of strong light while the Q175 show very little activity during light exposure (Fig. 2C, D). We addressed this issue by introducing 1 h of light exposure at a time when the mice were highly active (ZT 14). While we documented normal light-suppressing effects on activity in WT and Q175 mice, the BACHD mice failed to show significant activity reduction (Fig. 5). It is possible that the reduced effect of blue-enriched light on BACHD could be due to reduced photic input to the circadian system. However, light-induction of cFos was robust in all of the genotypes (Supplemental Fig. 2) so the light information appears to be reaching the SCN. Neural activity within the SCN is known to be compromised in the BACHD line (Kudo et al., 2011) and this pathophysiology may well underlie the reduced light response.
The observation that the benefits of blue light on both circadian rhythms and motor performance are stronger in the Q175 line provides some support for the contention that the stronger rhythms are responsible for the improved motor performance. Still we cannot exclude the possibility that the blue light treatment could be producing benefits on motor performance independent of the circadian system. At this point we do not know the mechanisms by which blue light treatment improves motor performance. A variety of studies have demonstrated that several distinct mouse models of HD all exhibit a progressive and rapid breakdown of the circadian rest/activity cycle that mimics the condition observed in human patients typified by loss of consolidated sleep, increased wakeful activity during the sleep phase, and more sleep during the active/waking phase (Morton et al., 2005, Bode et al., 2009, Kudo et al., 2011, Oakeshott et al., 2011, Loh et al., 2013). There is now evidence that improving the sleep/wake cycle with sleep-inducing drugs (Pallier et al., 2007), bright light and restricted wheel access (Cuesta et al., 2014) and blue light (Fig. 4) can treat HD symptoms. This body of work supports our general hypothesis that improved circadian rhythms will broadly improve disease symptoms in HD.
The alterations in striatal and cortical gene expression pathways suggest that blue-enriched lighting can result in selective neurobiological changes in the HD brain. While we cannot know if the blue enhanced lighting directly or indirectly altered gene expression, our findings provide a starting point to examine mechanisms known to be involved in the pathology of HD. For example, changes in dopamine receptor D1a (Drd1a) and dopamine receptor D2 (Drd2) suggest altered dopaminergic signaling, which modulates locomotor performance. Centrally, DA levels are modulated by monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B), which are key enzymes that regulate the catabolism of several different monoamine neurotransmitters (Shih and Chen 2004). Importantly, MAO-A appears to be a clock-controlled gene. A mutation in the circadian clock gene Period-2 in mice leads to reduced expression and activity of MAO-A in the mesolimbic dopaminergic system, which results in increased DA levels and changes in electrical activity in the striatum (Hampp et al., 2008). The striatum also exhibits the rhythmic expression of clock genes (Cai et al., 2009) and there is some evidence that disrupted striatal clocks can alter motor function (Ruby et al., 2014). Interestingly, methamphetamine treatment can even restore robust circadian activity in rats whose SCN had been electrolytically lesioned (Honma et al., 1987) demonstrating that dopaminergic drugs can modulate the circadian system even without a functional central circadian clock in the SCN. However, we have to consider that HD pathology alters the circadian response to methamphetamine, at least in the R6/2 model (Cuesta et al., 2012). Taken together, it seems possible that the circadian regulation of DA plays a role in the improved motor function observed in the present study.
In addition to the likely impact on neurotransmitter systems, our data indicate that the blue light treatment changes the transcriptional environment in two brain regions intimately involved in HD i.e. the cortex and striatum. Our transcriptional analysis of selected HD markers indicates that blue light treatment might be beneficial by decreasing oxidative stress and thus delaying disease progression. Our data indicate that the blue-enhanced lighting upregulated glutamate-cysteine ligase catalytic subunit (Gclc) in the striatum and cortex as well as superoxide dismutase 2 (Sod1) in the cortex of the Q175 mutants. GCLC plays a key role in glutathione (GSH) synthesis as the rate limiting enzyme (Lu 2013). GSH functions to protect tissue from oxidative stress (Forman et al., 2009). In short, oxidative stress has been considered a pathophysiological mechanism associated with HD (Browne et al., 1999), and the upregulation of the two key antioxidant enzymes suggest that blue light treatment may provide CNS protection through this mechanism.
Light is a strong environmental regulator of circadian timing and a number of studies have examined the impact of “bright light” (2000 to 10,000 lx) therapy on disease symptoms. In treating neurodegenerative disorders, prior studies have applied bright light therapy to patients with dementia and Parkinson's disease (PD). Broadly these studies show some benefits from the bright light treatments (Riemersma-van der Lek et al., 2008, McCurry et al., 2011, Zhou et al., 2012). Another study has found evidence that light therapy has a positive influence on motor function in PD (Willis and Turner 2007). Still it seems at this point, more studies are needed to confirm the effect of light therapy on patients with neurodegenerative diseases. One of the problems with bright light therapy is that bright light itself may be aversive (Kogan and Guilford 1998) which complicates the interpretation of these studies as well as creating problems of compliance.
The growth of our understanding of melanopsin (Rollag et al., 2003, Schmidt et al., 2011) coupled with the technological advances in LED lighting allows more targeted photic interventions. Like the rodent, the human circadian system is sensitive to blue wavelength light (Lockley et al., 2006, Gooley et al., 2010, Rüger et al., 2013). For instance, when treating seasonal affective disorder, 750 lx of blue light has been shown to be equally effective as 10,000 lx standard light therapy (Gordijn et al., 2012). Targeted light exposure shows promise in treating patients (Royer et al., 2012, Gabel et al., 2013, Figueiro et al., 2014) as well as caregivers (Sloane et al., 2015). Highlighting the independence of the melanopsin pathway from visual circuitry, blue light treatment provides cognitive benefits to visually blind individuals (Vandewalle et al., 2013). The treatment that we used in this study (6 h of blue light) is simple to implement but does come with interpretive complications. The light intensity as measured in lux was effectively doubled (375 to 885 lx). However, the maximal absorption of melanopsin is around 480 nm (Lucas et al., 2001) and our spectral analysis indicates that the irradiance at that wavelength increased from 0.3 to 14 µW/cm2 (Sup. Fig. 1). So, our treatment dramatically increased the irradiance at the key wavelength for the circadian system. One of the limitations of the present study is that we could not completely exclude the effects of other dimensions of our blue light treatment. Another limitation is that we cannot rule out the possibility that the gene expression changes are due to the improved locomotor behavior, which may feedback to the central nervous system. While the issue of whether the benefits of bright light or blue light therapy on the patient populations works through the circadian system remains to be resolved, a number of studies are demonstrating a number of benefits to these photic treatments. More pre-clinical work is needed to optimize the treatment strategies as well as develop an understanding of the underlying mechanisms.
5. Conclusion
Sleep disorders are extremely common in HD and have major detrimental effects on daily functioning and the quality of life of patients and their caregivers (Cuturic et al., 2009, Aziz et al., 2010, Goodman et al., 2011). Disruptions in the timing of sleep are common and often become apparent years before the onset of motor symptoms. Our study and others (Pallier et al., 2007, Cuesta et al., 2014) demonstrate that strengthening the circadian system can improve key HD symptoms in pre-clinical models.
Conflicts of interest
All authors are affiliated with the University of California, Los Angeles. We have no conflicts of interest involving this submission.
Acknowledgements
We would like to thank Anahit Aschyan, Laura Gad, Richard Flores, Collette Kokikian, and Olivia Valdes for their careful help in behavioral scoring. In addition, we are appreciative of Donna Crandall's able assistance with graphics. Finally, we thank John Parker for his expert help with the design and construction of our LED lighting system.
This work was supported by the CHDI Foundation [A-7293].
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.nbscr.2016.12.002.
Contributor Information
Dawn H. Loh, Email: lohdhw@gmail.com.
Christopher S. Colwell, Email: ccolwell@mednet.ucla.edu.
Appendix A. Supplementary material
Supplementary material
.
.
References
- Aziz N.A., Anguelova G.V., Marinus J., Lammers G.J., Roos R.A. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington’s disease. Park. Relat. Disord. 2010;16:345–350. doi: 10.1016/j.parkreldis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Bode F.J., Stephan M., Wiehager S., Nguyen H.P., Björkqvist M., von Hörsten S., Bauer A., Petersén Å. Increased numbers of motor activity peaks during light cycle are associated with reductions in adrenergic α2-receptor levels in a transgenic Huntington’s disease rat model. Behav. Brain Res. 2009;205:175–182. doi: 10.1016/j.bbr.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Bourne C., Clayton C., Murch A., Grant J. Cognitive impairment and behavioural difficulties in patients with Huntington’s disease. Nurs. Stand. 2006;20:41–44. doi: 10.7748/ns2006.05.20.35.41.c4146. [DOI] [PubMed] [Google Scholar]
- Browne S.E., Ferrante R.J., Beal M.F. Oxidative stress in Huntington’s disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Liu S., Li N., Xu S., Zhang Y., Chan P. Postnatal ontogenesis of molecular clock in mouse striatum. Brain Res. 2009;1264:33–38. doi: 10.1016/j.brainres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Carter R.J., Lione L.A., Humby T., Mangiarini L., Mahal A., Bates G.P., Dunnett S.B., Morton A.J. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J. Neurosci. 1999;19(8):3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciammola A., Sassone J., Alberti L., Meola G., Mancinelli E., Russo M.A., Squitieri F., Silani V. Increased apoptosis, huntingtin inclusions and altered differentiation in muscle cell cultures from Huntington’s disease subjects. Cell Death Differ. 2006;13:2068–2078. doi: 10.1038/sj.cdd.4401967. [DOI] [PubMed] [Google Scholar]
- Colwell C.S. Linking neural activity and molecular oscillations in the SCN. Nat. Rev. Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell C.S. John Wiley & Sons, Inc.; Hoboken, New Jersey: 2015. Circadian Medicine. [Google Scholar]
- Cuesta M., Aungier J., Morton A.J. The methamphetamine-sensitive circadian oscillator is dysfunctional in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 2012;45:145–155. doi: 10.1016/j.nbd.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Cuesta M., Aungier J., Morton A.J. Behavioral therapy reverses circadian deficits in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 2014;63:85–91. doi: 10.1016/j.nbd.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Cuturic M., Abramson R.K., Vallini D., Frank E.M., Shamsnia M. Sleep patterns in patients with Huntington’s disease and their unaffected first-degree relatives: a brief report. Behav. Sleep Med. 2009;7:245–254. doi: 10.1080/15402000903190215. [DOI] [PubMed] [Google Scholar]
- Dacey D.M., Liao H.-W., Peterson B.B., Robinson F.R., Smith V.C., Pokorny J., Yau K.-W., Gamlin P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Duffy J.F., Wright K.P. Entrainment of the Human circadian system by light. J. Biol. Rhythm. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M., Gray J., Conneally P., Young A., Penney J., Hollingsworth Z., Shoulson I., Lazzarini A., Falek A., Koroshetz W., Sax D., Bird E., Vonsattel J., Bonilla E., Alvir J., Bickham Conde J., Cha J.H., Dure L., Gomez F., Ramos M., Sanchez-Ramos J., Snodgrass S., de Young M., Wexler N., Moscowitz C., Penchaszadeh G., MacFarlane H., Anderson M., Jenkins B., Srinidhi J., Barnes G., Gusella J., MacDonald M. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat. Genet. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Figueiro M.G., Plitnick B.A., Lok A., Jones G.E., Higgins P., Hornick T.R., Rea M.S. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer's disease and related dementia living in long-term care facilities. Clin. Interv. Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C.A., Sewell K., Brown A., Churchyard A. Aggression in Huntington’s disease: a systematic review of rates of aggression and treatment methods. J. Huntingtons Dis. 2014;3:319–332. doi: 10.3233/JHD-140127. [DOI] [PubMed] [Google Scholar]
- Fleming S.M., Ekhator O.R., Ghisays V. Assessment of sensorimotor function in mouse models of Parkinson’s disease. J. Vis. Exp.: JoVE. 2013:50303. doi: 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel V., Maire M., Reichert C.F., Chellappa S.L., Schmidt C., Hommes V., Viola A.U., Cajochen C. Effects of artificial dawn and morning blue light on daytime cognitive performance, well-being, cortisol and melatonin levels. Chronobiol. Int. 2013;30:988–997. doi: 10.3109/07420528.2013.793196. [DOI] [PubMed] [Google Scholar]
- Ghiani C.A., Mattan N.S., Nobuta H., Malvar J.S., Boles J., Ross M.G., Waschek J.A., Carpenter E.M., Fisher R.S., de Vellis J. Early effects of lipopolysaccharide-induced inflammation on foetal brain development in rat. ASN Neuro. 2011;3:e00068. doi: 10.1042/AN20110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M.S., Fleming S.M., Palacino J.J., Cepeda C., Lam H.A., Bhatnagar A., Meloni E.G., Wu N., Ackerson L.C., Klapstein G.J., Gajendiran M., Roth B.L., Chesselet M.-F., Maidment N.T., Levine M.S., Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Goodman A.O.G., Rogers L., Pilsworth S., McAllister C.J., Shneerson J.M., Morton A.J., Barker R.A. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington's disease. Curr. Neurol. Neurosci. Rep. 2011;11:211–217. doi: 10.1007/s11910-010-0163-x. [DOI] [PubMed] [Google Scholar]
- Gooley J.J., Rajaratnam S.M.W., Brainard G.C., Kronauer R.E., Czeisler C.A., Lockley S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000741. (31ra33-31ra33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordijn M.C.M., t Mannetje D., Meesters Y. The effects of blue-enriched light treatment compared to standard light treatment in seasonal affective disorder. J. Affect. Disord. 2012;136:72–80. doi: 10.1016/j.jad.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Gray M., Shirasaki D.I., Cepeda C., Andre V.M., Wilburn B., Lu X.H., Tao J., Yamazaki I., Li S.H., Sun Y.E., Li X.J., Levine M.S., Yang X.W. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J. Neurosci. 2008;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbergen Y.A.M., Knol M.J., Bloem B.R., Kremer B.P.H., Roos R.A.C., Munneke M. Falls and gait disturbances in Huntington’s disease. Mov. Disord. 2008;23:970–976. doi: 10.1002/mds.22003. [DOI] [PubMed] [Google Scholar]
- Gusella J.F., MacDonald M.E., Lee J.-M. Genetic modifiers of Huntington’s disease. Mov. Disord. 2014;29:1359–1365. doi: 10.1002/mds.26001. [DOI] [PubMed] [Google Scholar]
- Hampp G., Ripperger J.A., Houben T., Schmutz I., Blex C., Perreau-Lenz S., Brunk I., Spanagel R., Ahnert-Hilger G., Meijer J.H., Albrecht U. Regulation of monoamine oxidase a by circadian-clock components implies clock influence on mood. Curr. Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Honma K., Honma S., Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methamphetamine. Physiol. Behav. 1987;40:767–774. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan A.O., Guilford P.M. Side effects of short-term 10,000-lux light therapy. Am. J. Psychiatry. 1998;155:293–294. doi: 10.1176/ajp.155.2.293. [DOI] [PubMed] [Google Scholar]
- Kudo T., Schroeder A., Loh D.H., Kuljis D., Jordan M.C., Roos K.P., Colwell C.S. Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp. Neurol. 2011;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa A.E., Menalled L.B., Oakeshott S., Murphy C., Mushlin R., Fitzpatrick J., Miller S.F., McConnell K., Port R., Torello J., Howland D., Ramboz S., Brunner D. Increased body weight of the BAC HD transgenic mouse model of Huntington's disease accounts for some but not all of the observed HD-like motor deficits. PLoS Curr. 2013 doi: 10.1371/currents.hd.0ab4f3645aff523c56ecc8ccbe41a198. pii: ecurrents.hd.0ab4f3645aff523c56ecc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis D.A., Gad L., Loh D.H., MacDowell Kaswan Z., Hitchcock O.N., Ghiani C.A., Colwell C.S. Sex differences in circadian dysfunction in the BACHD mouse model of Huntington’s disease. PLoS One. 2016;11:e0147583. doi: 10.1371/journal.pone.0147583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbehn D.R., Hayden M., Paulsen J.S., the P.-H.D.IotH.S.G. CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am. J. Med. Genet. Part B Neuropsychiatr. Genet.: Off. Publ. Int. Soc. Psychiatr. Genet. 2010;153B:397–408. doi: 10.1002/ajmg.b.30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Cantle J.P., Chatzopoulou D., Wang N., Gao F., Al-Ramahi I., Lu X.-H., Ramos E.M., El-Zein K., Zhao Y., Deverasetty S., Tebbe A., Schaab C., Lavery D.J., Howland D., Kwak S., Botas J., Aaronson J.S., Rosinski J., Coppola G., Horvath S., Yang X.W. Integrated genomics and proteomics define huntingtin CAG length-dependent networks in mice. Nat. Neurosci. 2016;19:623–633. doi: 10.1038/nn.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates T.A., Fernandez D.C., Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Loh D.H., Kudo T., Truong D., Derakhshesh M., Kaswan Z.M., Ghiani C.A., Tsoa R., Cheng Y., Sun Y.E., Colwell C.S. Circadian rhythm disruption in a mouse model of Rett syndrome circadian disruption in RTT. Neurobiol. Dis. 2015;77:155–164. doi: 10.1016/j.nbd.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Lockley S.W., Evans E.E., Scheer F.A., Brainard G.C., Czeisler C.A., Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Loh D.H., Kudo T., Truong D., Wu Y., Colwell C.S. The Q175 mouse model of Huntington’s disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS One. 2013;8:e69993. doi: 10.1371/journal.pone.0069993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta (BBA) - General. Subj. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R.J., Douglas R.H., Foster R.G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas R.J., Lall G.S., Allen A.E., Brown T.M. Chapter 1 - how rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog. Brain Res. 2012;199:1–18. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- Lupi D., Oster H., Thompson S., Foster R.G. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat. Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Margolis R.L., Ross C.A. Diagnosis of Huntington disease. Clin. Chem. 2003;49:1726–1732. doi: 10.1373/49.10.1726. [DOI] [PubMed] [Google Scholar]
- McCurry S.M., Pike K.C., Vitiello M.V., Logsdon R.G., Larson E.B., Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with alzheimer’s disease: results of a randomized, controlled trial. J. Am. Geriatr. Soc. 2011;59:1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled L.B., Kudwa A.E., Miller S., Fitzpatrick J., Watson-Johnson J., Keating N., Ruiz M., Mushlin R., Alosio W., McConnell K., Connor D., Murphy C., Oakeshott S., Kwan M., Beltran J., Ghavami A., Brunner D., Park L.C., Ramboz S., Howland D. Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington’s disease: zq175. PLoS One. 2012;7:e49838. doi: 10.1371/journal.pone.0049838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton A.J., Wood N.I., Hastings M.H., Hurelbrink C., Barker R.A., Maywood E.S. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J. Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeshott S., Balci F., Filippov I., Murphy C., Port R., Connor D., Paintdakhi A., Lesauter J., Menalled L., Ramboz S., Kwak S., Howland D., Silver R., Brunner D. Circadian abnormalities in motor activity in a BAC transgenic mouse model of Huntington's disease. PLoS Curr. 2011;3:RRN1225. doi: 10.1371/currents.RRN1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier P.N., Maywood E.S., Zheng Z., Chesham J.E., Inyushkin A.N., Dyball R., Hastings M.H., Morton A.J. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2007;27:7869–7878. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Nayak S.K., Campo B., Walker J.R., Hogenesch J.B., Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Pilorz V., Tam S.K.E., Hughes S., Pothecary C.A., Jagannath A., Hankins M.W., Bannerman D.M., Lightman S.L., Vyazovskiy V.V., Nolan P.M., Foster R.G., Peirson S.N. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 2016;14:e1002482. doi: 10.1371/journal.pbio.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma-van der Lek R.F., Swaab D.F., Twisk J., Hol E.M., Hoogendijk W.G., Van Someren E.W. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- Roenneberg T., Kantermann T., Juda M., Vetter C., Allebrandt K.V. Light and the human circadian clock. In: Kramer A., Merrow M., editors. Circadian Clocks. Springer Berlin Heidelberg; Berlin, Heidelberg: 2013. pp. 311–331. [DOI] [PubMed] [Google Scholar]
- Rollag M.D., Berson D.M., Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J. Biol. Rhythm. 2003;18:227–234. doi: 10.1177/0748730403018003005. [DOI] [PubMed] [Google Scholar]
- Royer M., Ballentine N.H., Eslinger P.J., Houser K., Mistrick R., Behr R., Rakos K. Light therapy for seniors in long term care. J. Am. Med. Dir. Assoc. 2012;13:100–102. doi: 10.1016/j.jamda.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Ruby C.L., Vadnie C.A., Hinton D.J., Abulseoud O.A., Walker D.L., O'Connor K.M., Noterman M.F., Choi D.-S. Adenosinergic regulation of striatal clock gene expression and ethanol intake during constant light. Neuropsychopharmacology. 2014;39:2432–2440. doi: 10.1038/npp.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger M., St Hilaire M.A., Brainard G.C., Khalsa S.-B.S., Kronauer R.E., Czeisler C.A., Lockley S.W. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J. Physiol. 2013;591:353–363. doi: 10.1113/jphysiol.2012.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saft C., Zange J., Andrich J., Müller K., Lindenberg K., Landwehrmeyer B., Vorgerd M., Kraus P.H., Przuntek H., Schöls L. Mitochondrial impairment in patients and asymptomatic mutation carriers of Huntington’s disease. Mov. Disord. 2005;20:674–679. doi: 10.1002/mds.20373. [DOI] [PubMed] [Google Scholar]
- Schmidt T.M., Chen S.K., Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J.C., Chen K. Regulation of MAO-A and MAO-B gene expression. Curr. Med. Chem. 2004;11:1995–2005. doi: 10.2174/0929867043364757. [DOI] [PubMed] [Google Scholar]
- Sloane P.D., Figueiro M., Garg S., Cohen L.W., Reed D., Williams C.S., Preisser J., Zimmerman S. Effect of home-based light treatment on persons with dementia and their caregivers. Light. Res. Technol. 2015;47:161–176. doi: 10.1177/1477153513517255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle G., Collignon O., Hull J.T., Daneault V., Albouy G., Lepore F., Phillips C., Doyon J., Czeisler C.A., Dumont M., Lockley S.W., Carrier J. Blue light stimulates cognitive brain activity in visually blind individuals. J. Cogn. Neurosci. 2013;25:2072–2085. doi: 10.1162/jocn_a_00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler N.S., Lorimer J., Porter J., Gomez F., Moskowitz C., Shackell E., Marder K., Penchaszadeh G., Roberts S.A., Gayan J., Brocklebank D., Cherny S.S., Cardon L.R., Gray J., Dlouhy S.R., Wiktorski S., Hodes M.E., Conneally P.M., Penney J.B., Gusella J., Cha J.H., Irizarry M., Rosas D., Hersch S., Hollingsworth Z., MacDonald M., Young A.B., Andresen J.M., Housman D.E., De Young M.M., Bonilla E., Stillings T., Negrette A., Snodgrass S.R., Martinez-Jaurrieta M.D., Ramos-Arroyo M.A., Bickham J., Ramos J.S., Marshall F., Shoulson I., Rey G.J., Feigin A., Arnheim N., Acevedo-Cruz A., Acosta L., Alvir J., Fischbeck K., Thompson L.M., Young A., Dure L., O'Brien C.J., Paulsen J., Brickman A., Krch D., Peery S., Hogarth P., Higgins D.S., Jr., Landwehrmeyer B., Project U.S.-V.C.R. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA. 2004;101:3498–3503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis G.L., Turner E.J.D. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol. Int. 2007;24:521–537. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- Zhou Q.P., Jung L., Richards K.C. The management of sleep and circadian disturbance in patients with dementia. Curr. Neurol. Neurosci. Rep. 2012;12:193–204. doi: 10.1007/s11910-012-0249-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material