Abstract
Previous studies have demonstrated that mean activity levels in the hippocampus oscillate on a circadian timescale, both at the single neuron and EEG level. This oscillation is also entrained by the availability of food, suggesting that the circadian modulation of hippocampal activity might comprise part of the recently discovered food-entrainable circadian oscillator (FEO). In order to determine whether the circadian oscillation in hippocampal activity is linked to activity in other brain regions, we recorded field-potential EEG from hippocampus and two cortical regions known to connect to hippocampus; the anterior cingulate cortex and the agranular insular cortex. These latter regions are involved in executive control (cingulate) and gustatory feedback (insula) and so are in a position where they could usefully contribute to, or benefit from, hippocampal memorial information in order to undertake task-related processing. We recorded EEG from these three regions for 20 m every hour for 58 consecutive hours in one continuous exposure to the recording environment. We found that there are regular and distinct increases in magnitude coherence between hippocampus and both cortical regions for EEG in both theta (6–12 Hz) and gamma (30–48 Hz) bands. These periods of increased coherence are spaced approximately one solar day apart, appear not to be specifically light-entrained, and are most apparent for gamma frequency activity. The gamma association between the two cortical regions shows the same temporal pattern of coherence peaks as the hippocampal-cortical coherences. We propose that these peaks in coherence represent the transient synchronization of temporally tagged memorial information between the hippocampus and other brain regions for which this information may be relevant. These findings suggest that the FEO involves coordinated activity across a number of brain regions and may underlie a mechanism via which an organism can store and recall salient gustatory events on a circadian timescale.
Keywords: Hippocampus, Cingulate, Insula, Theta, Gamma, Coherence, Circadian
1. Introduction
Circadian organisation of function is ubiquitous in the mammalian brain. Since the discovery of the light-entrained master circadian oscillator in the suprachiasmatic nucleus (Stephan and Zucker, 1972), circadian oscillations have been shown to occur in structures as diverse as the liver (Wuarin and Schibler, 1990), heart (Huikuri et al., 1990), and intestine (Edwards et al., 1972). The circadian rhythms expressed in peripheral tissues, however, are often entrained to the presentation of food, and not strictly by light. Since these findings, many studies have demonstrated that a similar, food-entrainable oscillator (FEO) exists in the central nervous system (Angeles-Castellanos et al., 2005; Davidson et al., 2001a, 2001b, 2003; Davidson and Stephan, 1999)) Importantly, this oscillator appears to function outside of the traditional light-entrained oscillator in the suprachiasmatic nucleus (SCN) (Girotti et al., 2009, Stephan, 2002). Although it has been proposed that the FEO is located in the dorsomedial hypothalamus (Gooley et al., 2006, Mieda et al., 2006), ablation of either ventromedial (Mistlberger and Rechtschaffen, 1984) or dorsomedial hypothalamus appear not to abolish circadian food anticipation (Landry et al., 2011, Landry et al., 2006, Landry et al., 2007), suggesting, at the least, that other brain regions are involved. More recently a consensus has begun to emerge that the FEO may not be located in a single structure, but may instead depend on combined signals from several, possibly independent, oscillators; many of which may respond to food availability as an entraining stimulus (Carneiro and Araujo, 2009, Feillet et al., 2008, Girotti et al., 2009, Mendoza, 2010).
One brain region that may contribute to FEO operation is the hippocampus. This structure has a key role in the formation of episodic memories, and it is possible that these memories are organised with respect to a circadian timescale. “Place cells” in the hippocampus code for space (O’Keefe and Dostrovsky, 1971) and context (Broadbent et al., 2010, Hammond et al., 2004, Hok et al., 2007), and the region has also been shown to code for time over timescales on the order of minutes (Barker et al., 2007, Hoge and Kesner, 2007, MacDonald et al., 2011). New evidence is emerging that the hippocampus might code for information on longer timescales such as hours to days (Eckel-Mahan, 2012, Mankin et al., 2012). Our previous work has shown explicitly that place cells in the hippocampus are potentially capable of incorporating coding for circadian time by alterations in their firing rates that occur over a period of approximately 24 h (Munn and Bilkey, 2012). This circadian variation in firing was not entrained to the absolute solar time (as one might expect from a signal inherited from the master SCN oscillator) but to a variable that occurred coincident with the start of recording; either the change in environmental context, the novel availability of palatable food, or some other salient stimulus (Munn and Bilkey, 2012). Our previous work has also demonstrated that the frequency of hippocampal EEG in the theta band also varies in a quasi-sinusoidal fashion over a circadian period (Munn et al., 2015). These experiments also clarified whether the novel availability of food, or the novelty of being moved from a home cage to a recording chamber entrains this variation. Leveraging a paradigm in which theta can be artificially driven, we were able to disambiguate food availability from the start of recording. This experiment showed that food availability, and not environmental novelty, was the stimulus that entrained the circadian variation in hippocampal activity suggesting that this rhythm is not simply inherited from the light-entrained master circadian oscillator in the SCN. Recent work is consistent with a hypothesis that hippocampal function is modulated on a circadian cycle that is food entrained. For example, feeding on a circadian cycle can affect memory (Loh et al., 2015). In these experiments, feeding was either aligned with normal waking hours, or misaligned (i.e. it occurred during normal sleeping hours). Misaligned feeding caused deficits in memory that were associated with reduced LTP and CREB phosphorylation (both thought to be markers of memory formation). Importantly Loh et al. (2015) demonstrate that misaligned feeding caused a phase shift in the expression of the circadian-related protein PER2 in hippocampus, while the master oscillator in SCN was unperturbed. These findings strengthen the notion that the hippocampus uses food-entrained circadian information as part of a memorial mechanism, and that this can operate independently from the light-synchronized circadian master clock in SCN (Table 1).
Table 1.
Recording start times of each of the nine dataseries.
| Animal I.D | Run 1 Start Time | Run 2 Start Time |
|---|---|---|
| RM1 | 1200 | 0000 |
| RM3 | 1500 | 0300 |
| RM4 | 0800 | 2000 |
| RM8 | 1900 | 0700 |
| RM9 | 1500 | N/A |
A functional hippocampal memory system requires, however, communication with other brain regions, such as those involved in the evaluation of reward, as well as regions involved in taste perception and gustatory feedback (McDonald and White, 1993). Previous studies have shown that such communication is often reflected in periods of increased coherence between the EEG signals of the regions of interest. In particular, considerable research focus has been applied to coherence in the gamma (30–100 Hz) range, which is typically thought to represent local communication and binding within cortical regions, while coherence at theta frequencies has been associated with longer range communication and performance in memory tasks, especially tasks requiring working memory (Benchenane et al., 2010, Weiss and Rappelsberger, 2000).
Two regions that may have a role in the evaluation of reward are the anterior cingulate (ACC) and insular cortex. The anterior cingulate is a frontal cortical region that has long been thought to be involved in the processing of emotion. However, it has also been shown to serve an error-checking function (Bush et al., 2000) and as an evaluator of reward (Bush et al., 2002, Shidara and Richmond, 2002). The anterior cingulate has also been assigned an executive role in decision making, being implicated in motivation (Carter et al., 1999, Hillman and Bilkey, 2010, Hillman and Bilkey, 2012, Kennerley et al., 2006, Kesner and Churchwell, 2011). Interactions between hippocampus and anterior cingulate are thought to mediate effortful choice behavior, and spectral coherence between ACC and hippocampus in the theta band increases when animals engage in a decision making task that recruits working memory (Jones and Wilson, 2005; Remondes and Wilson, 2013).
Insular cortex has been implicated in assessing aversive risk (Clark et al., 2008, Sridharan et al., 2008), interoceptive awareness (Critchley et al., 2004) and in biofeedback processes (Yasui et al., 1991), especially as this pertains to gustation (Cascino and Karnes, 1990, Hanamori et al., 1998, Pritchard et al., 1999) and cardiovascular function (Oppenheimer et al., 1992). Anterior cingulate cortex is anatomically closely associated with the hippocampus (Pandya et al., 1981, Varela et al., 2014, Vogt and Miller, 1983), and with insular cortex (Devinsky et al., 1995), while insular cortex has connectivity to the hippocampus via perirhinal cortex (Burwell and Amaral, 1998).
If the previously observed circadian phasic oscillation in place cell unit firing rates and theta frequency signals time relative to a salient event (food availability), then this information is likely to depend on communication with regions involved in reward evaluation and executive control, such as anterior cingulate, and gustation and interoceptive awareness, such as insular cortex. As a result we predict that these regions may display changes in coherence with each other on a circadian timescale. In order to examine this possibility, we recorded simultaneous field potential EEG from hippocampus, anterior cingulate and agranular insular cortices over very long exposures to a single environment as described in previous studies (Munn et al., 2015, Munn and Bilkey, 2012). We then examined hourly recordings obtained from each of the three regions, hippocampus, anterior cingulate, and insular cortex for spectral coherence in the theta and low gamma bands that might indicate increased information trafficking between them.
2. Materials and methods
The procedures described in these experiments were approved by the University of Otago committee on the care and use of laboratory animals, and all experiments complied with the University of Otago code of ethical conduct.
2.1. Subjects and surgery
The subjects in this experiment were five male Sprague-Dawley rats that were aged between 3 and 6 months old over the course of the experiments and were approximately 4 months old and weighing between 300–500 g at the time of surgery. All animals were implanted with bipolar LFP recording electrodes (twisted-pair Silver Teflon coated .005” diameter) in area CA1 of the hippocampus (3.8 mm posterior to bregma, 2.5 mm lateral from the midline, 2.5 mm below dura), anterior cingulate cortex (0.2 mm anterior to bregma and 0.3 lateral; 2.8 mm below dura), and agranular insular cortex (3.7 mm anterior and 3.5mm lateral, 5 mm below dura). These placements are illustrated in Fig. 1B. All animals were housed in group cages of four animals prior to surgery, and were transferred to individual cages immediately after surgery. The animal housing room was maintained at a thermostatically controlled 22 °C, and was kept in artificial 12:12 L:D conditions coincident with true daylight (lights on at 0700 and off at 1900). The homeroom was otherwise blacked out. As in our previous experiments (Munn et al., 2015, Munn and Bilkey, 2012); post-surgery and for the duration of the experiment animals had ad libitum access to water, and were fed once daily when in their home room at 1600 hours. The amount of food given to animals varied such as to keep their weight at 85% of their pre-surgery free feeding weight. Animals were therefore given a variable weight of food (usually approx. 12–16 g depending on the animal's weight history) each day, and had access to this food until it was totally consumed. Typically, this occurred in the first hour after the food was dispensed.
Fig. 1.
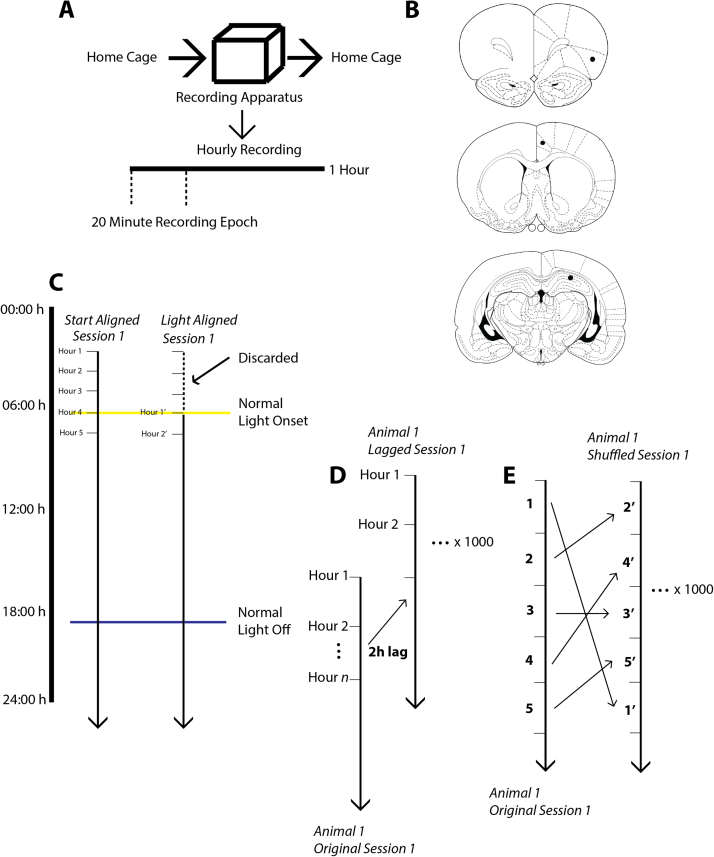
(A) The recording procedure for each of the long recording sessions. Animals are taken directly from their home cage and placed into the recording chamber where they remain for the next 60 h. In each of the 60 h, there is a 20 m epoch in which EEG data is acquired and the animal is encouraged to explore the environment through the random scattering of food pellets via the automatic pellet feeder. (B) Schematic coronal sections of rat brain illustrating the placement of electrodes in, from top to bottom, insular cortex, anterior cingulate cortex (ACC), and hippocampus. (C) Diagrammatic illustration of the light alignment procedure. In this example, a long recording session begins at 03:00. In the non-shifted start-aligned version, hour 1 of this recording session corresponds to 03:00. In the light-shifted version of this session, hour 1 is realigned to light onset and the four hours of recording that occurred before light onset are discarded. (D) Diagrammatic illustration of the data lagging procedure used to generate 95% confidence intervals in Fig. 2, Fig. 3. Lag magnitude varied between 0 and 58. Each dataseries was lagged 1,000 times individually. The lag magnitudes were drawn from a uniform distribution. This shuffling paradigm preserves the internal structure of each dataseries. (E) Diagrammatic illustration of the shuffling procedure used to generate the 95% confidence interval autocorrelation envelopes in Fig. 5, Fig. 6. Each of the 1–60 h is randomly reassigned to a new ordinal position in each of the 1000 shuffled dataseries. As in D), this shuffling procedure was performed 1,000 times on each of the dataseries.
Animals were not fed on this schedule when undergoing their long recording sessions; all food during this period was distributed as described in the apparatus section.
2.2. Apparatus
The recording apparatus was largely as described in Munn and Bilkey (2012). Animals were connected to a headstage that was routed through an 8-channel commutator, which was in turn connected to an Axona DACQUSB recording system (Axona Ltd.). This system acquired and digitally amplified the recordings. The three EEG channels were low-pass (0–500 Hz) filtered in combination with a notch filter at 50 Hz to eliminate electrical noise and sampled at 4.8 kHz. During recordings, animals were free to explore the recording chamber at will. The chamber itself was a black-painted wooden box of dimensions 61.5 × 61.5 × 60 cm. One of the walls was marked with a white cue card (7 × 5.5 cm). The lighting in the recording room was provided by a 60 W hooded lamp in a corner of the room most distal from the recording chamber. This light, in addition to being hooded, was aimed at a corner of the ceiling and wall; the majority of lighting was therefore indirect. This lamp therefore provided constant dim illumination. Each of the hourly 20-min recordings was triggered by a custom-written program that sent the appropriate keypresses to DACQUSB. A custom DACQUSB script also ran during each recording that activated the motor of a mechanical feeder that was connected to the DACQUSB system unit during recording. The feeder was loaded with 45 mg Reliance Stock Pellets, and the script activated the motor for 1.5 s every 60 s. This activation typically released at least one pellet, with minimum of 0 and a maximum of 3 pellets, per activation to give a total of between 15–25 pellets per hourly recording session). This level of feeder activation provided approximately 1 g of pellets/hour, leading to a daily food availability of approximately 24 g. The feeder was mounted over the recording chamber and had a plastic hemisphere suspended under the feeding aperture in order to randomly scatter pellets throughout the environment. Animals were not otherwise fed during the experiment, but had access to water ad libitum from a water bottle rigged on the outside of the recording chamber; the spout of which protruded through the wall of the recording chamber to which the white cue was attached.
2.3. Procedure
Each long exposure session consisted of hourly recordings of 20 min duration made over very long (24–58 h) continuous exposures to the same environment. At the beginning of each long exposure session, animals were connected to the cable headstage and commutator and placed into the recording environment. The script controlling the recording was run, and the first 20 min recording was initiated. Animals thereafter experienced a 20 min recording every hour on the hour for between 24 and 58 h. Animals remained in the recording chamber for the duration of the long exposure session (24–52 hours in the same environment). Animals were free to behave in any way during their long exposure sessions, without intervention from the experimenter, who left the recording room after putting the animal into the recording environment and did not re-enter the recording environment until the conclusion of the long exposure session. Animals were monitored remotely from an adjacent room in the lab to ensure data were being captured. The commutator reliably prevented the cable from becoming excessively wound. This protocol is illustrated diagrammatically in Fig. 1A. At the conclusion of the long exposure session, animals were disconnected from the headstage, replaced in their home environment, and returned to the animal colony room. At least four days (96 h) elapsed between an individual animal's first long exposure session and the next. Animals began their long exposure sessions at a range of different start times (0300–2300). The two sessions from each animal (if present) were separated by 12 h (e.g. If an animal had its first session begin at 0900, the second would begin at 2100).
2.4. Data collection and analysis
Raw EEG data was processed using MATLAB (v2013b). A custom-written script broke the data into five frequency bands (delta (0.5–4 Hz), theta, (6–12 Hz), alpha (8–13 Hz), beta (13–20 Hz) and low gamma (30–48 Hz)). Coherence between pairs of signals over the full 20-minute recording was then calculated for the regions (e.g. between hippocampus and insula, hippocampus and cingulate and cingulate and insula) in each of the frequency bands for each hourly recording using MATLAB's mscohere function. The animals were position tracked throughout each 20 min recording from head-mounted LEDs, and their movement was binned into second-long bins. An average running speed was thereby computed for each hourly recording. In order to assure the data were recorded from freely-moving, non-sleeping animals, individual recording episodes were rejected if, in any given 20 min recording, an animal failed to explore less than 70% of the environment, or if the animal spent more than 20% (4 min) of the recording in the 0–2 cm/s movement bin. In general, this caused increased rejection of data points toward the end of recording, and therefore a commensurate increase in the standard error of these data points as more of the terminal sessions were rejected.
2.5. Data shuffling
2.5.1. Pseudo-random lagging
In order to determine if peaks in coherence between the regions were generated by a small number of large values against a quiet background, for each coherence pair (e.g. coherence between hippocampus and anterior cingulate in the theta band) each individual dataseries from each long recording session (i.e nine individual dataseries for each long exposure for each of the three coherence pairs) was lagged pseudo-randomly against every other dataseries, and a new mean hourly coherence value was computed. For example, there were nine individual long dataseries for each coherence pair. Each of these individual series was lagged against its starting position by a pseudo-randomly selected (from 0–58) number of hours, which produced a new “lagged” hourly mean coherence value. This procedure was repeated 1,000 times, creating 1,000 randomly lagged mean dataseries for each regional coherence pair (e.g. between hippocampus and anterior cingulate in the theta band) for each recording. Dataseries were not looped, resulting in a variable number of coherence values in each relative hour of recording. This analysis produced a shuffling across hours of recording, while preserving the internal structure of each 58-hour long dataseries. This approach maintained the internal structure of each animal's long recording session while the shuffling ensured that no one dataseries was driving the structure of the mean coherence. The 1,000 shuffled series were used to calculate a 5–95% confidence envelope for each regional coherence pair (i.e between hippocampus and insula, hippocampus and cingulate and between the cortical regions) in each frequency band (delta, theta, alpha, beta, and gamma). This analysis was carried out using a custom-written MATLAB script. This procedure is diagrammatically illustrated in Fig. 1D.
2.5.2. Random shuffling and autocorrelation
Rhythmicity in each dataset (i.e. in each of the frequency bands between regions) was computed by partial autocorrelation of each mean dataseries. The expected autocorrelation at each lag was computed by randomly sampling values from each dataseries without replacement 1,000 times, and computing an hourly average from these random samples (i.e. 1000 identical datasets were derived by randomly sampling coherence values from the long recording sessions), producing 1,000 randomly sampled mean dataseries. This procedure is shown in Fig. 1E. These 1,000 shuffled dataseries were then individually autocorrelated, and from these we derived the mean, 5th and 95th percentile correlation values for each lag. This analysis enabled us to assess whether peaks in the autocorrelation of the coherence data were above that expected from random sampling.
2.5.3. Shifting data relative to absolute solar time
In order to determine whether the patterns of coherence observed between regions were under the control of a light-on zeitgeber, data from each individual recording session was shifted relative to every other session such that hour 1 in each of the shifted dataseries corresponded to either the most recent light-on or light-off stimulus (as determined from the fixed lighting schedule in the animal room). In order to determine whether any of the deviations in coherence were above chance, a 95% confidence envelope was calculated in the same manner as described previously. The shifting paradigm is shown schematically in Fig. 1C, and the shifted data are illustrated in Figs. 3A–F.
Fig. 3.
The mean spectral coherence (black lines) in the theta and gamma band when data are aligned to the established external solar time. Black bars above the data illustrate the times that the homeroom light (not the recording room light, which was always on) was off according to this alignment; the homeroom light was on at all other times. (A) and (B) show the theta and gamma band coherence between hippocampus and insula, (C) and (D) show the same between hippocampus and anterior cingulate, (E) and (F) show the same between the two cortical regions. As in Fig. 1. the grey envelope represents the 5th and 95th percentile extents of the distribution of values produced by randomly lagging each dataseries against each other dataseries 1000 times.
2.6. Histology
At the end of the experiments, animals were deeply anesthetized using Halothane, and were then perfused transcardially with solutions of 0.9% saline followed by 10% formalin. Animals were then decapitated, and the brain was removed and stored in 10% formalin solution. Prior to sectioning, brains were transferred into a 30% sucrose solution for 72 h or until they sunk in the solution. Frozen brains were sectioned at 60 μm thickness, and the slices were stained with thionin. Where possible, electrode position was determined by microscopic examination of the slides. Fig. 1B indicates the targeted locations of the electrodes.
3. Results
In total, 522 hourly recordings were made across 5 animals. 4 of these animals contributed two sessions each, the start times of which were separated by 12 h within animal (e.g. if an animal's first session started at 0900, the second started at 2100). One animal contributed only one long exposure session. 84 hourly recordings were rejected from the final analysis according to our previously described criteria regarding lack of coverage or inactivity (less than 70% coverage or more than 20% of the session immobile). This left 438 recordings in the final analysis from 9 sessions.
3.1. The hippocampus and insular cortex are periodically associated
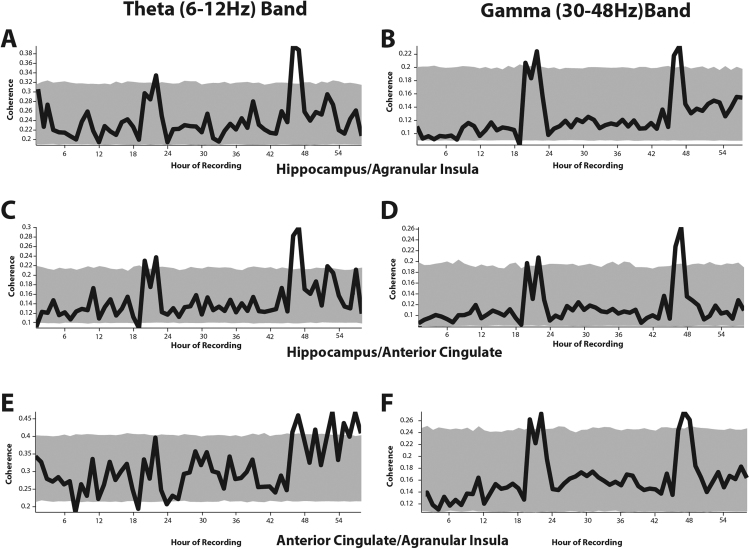
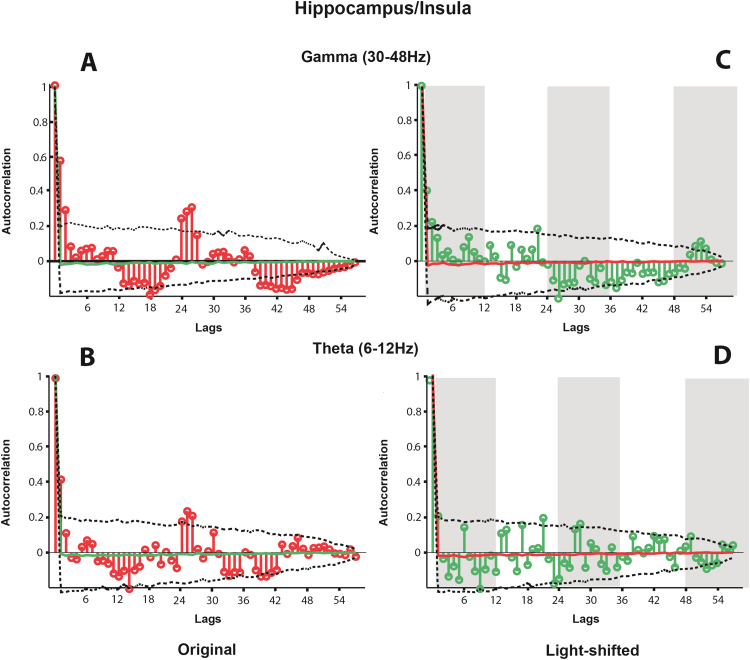
Fig. 2A and B illustrate the mean spectral coherence between the hippocampus and agranular insular cortex in the theta (6–12 Hz) and low gamma (30–48 Hz) bands in each hour of recording. There are marked peaks in spectral coherence at hours 20–24 and 46–48 against a relatively consistent baseline in both the theta and gamma bands. In order to examine whether these peaks in coherence are artefactual or are being driven by one animal, the individual series of data were lagged against each other by a pseudo-random amount and a new mean coherence in each hour of recording was calculated. 1,000 of these lagged average dataseries were produced and from these the 5th and 95th percentile of coherence scores in each hour of recording were derived; these are indicated by the grey envelope in Fig. 2A–B. The peaks in coherence in both the theta and gamma bands are above this envelope. Autocorrelations of these datasets (Fig. 4A; gamma, 4B; theta) demonstrate the periodicity of these data. In both cases there is a clear region of high autocorrelation at between 25–28 h that corresponds to the periodic burst of coherence observed in the data. Autocorrelation of 1000 randomly sampled shuffles of the datasets produced a distribution of autocorrelation values at each lag. The peak in autocorrelation is consistently higher than the 95th percentile of the shuffled correlations at lags between 25–27 h. Kuiper analysis confirms that the coherence between hippocampus and insular cortex is not uniformly distributed in either the theta (V = 0.345, p < 0.001) or gamma (V = 0.556, p < 0.001) bands.
Fig. 2.
The mean spectral coherence (black lines) between the hippocampus and agranular insular cortex in the theta (A) and gamma (B) band in each hour of recording. (C) and (D) show the spectral coherence between hippocampus and anterior cingulate cortex in theta and gamma, respectively. (E) and (F) show the spectral coherence between the cortical regions in the theta (E) and gamma (F) bands. The grey envelope in all cases illustrates the 5th and 95th percentile coherence values in each hour of recording produced from lagging each dataseries by a random amount 1000 times.
Fig. 4.
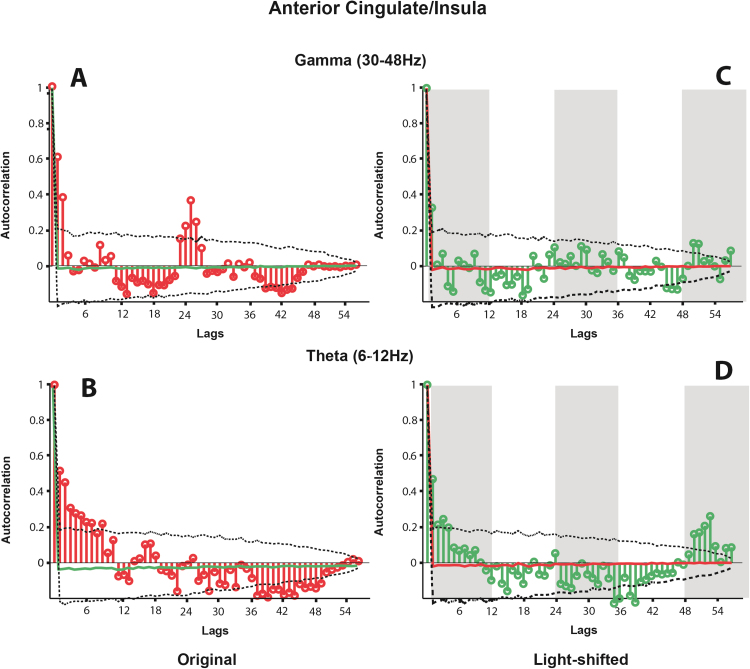
The autocorrelation of the mean spectral coherence between hippocampus and insula in the gamma (A) and theta (B) bands, and the autocorrelation of the same coherence values when shifted relative to light (C & D). In (A) and (B) the autocorrelations at each lag are shown in red. In (C) and (D) these are shown in green. In all cases, the dotted lines indicate the extent of the 5th and 95th percentile of the distribution of autocorrelations produced from randomly resampling each dataseries 1,000 times. In (C) and (D) the normal light cycle is represented by grey bars (lights off). The mean autocorrelation at each lag over these 1,000 shuffles is shown as a green (A and B) or red (C and D) line.
3.2. . The hippocampus and anterior cingulate are periodically associated
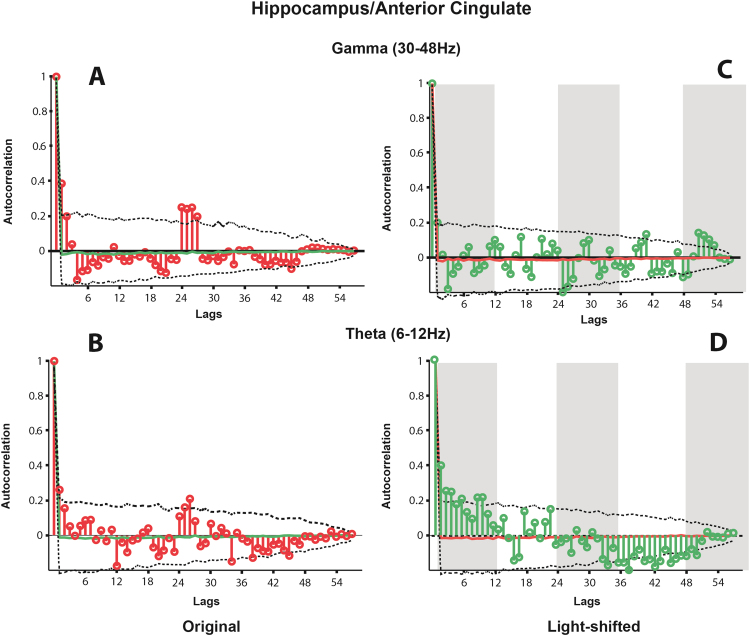
The coherence between hippocampus and anterior cingulate in both the theta and gamma bands, as with the coherence between hippocampus and insula, has clear peaks in coherence in both bands at 20–24 and 46–48 h (Figs. 2C and D) against a relatively stable background of low coherence. The 5th and 95th percentile coherence calculated from the mean hourly coherence of 1000 random lags of the dataseries are shown as a grey envelope. Both of the regions of coherence in both the theta and gamma bands are above this envelope. Fig. 5 illustrates the autocorrelation of the dataseries at lags up to 57 h. Both the theta (5A) and gamma (5B) bands show marked correlations at 24–27 h. Both of these peaks in autocorrelation are above the 95th percentile correlation derived from the autocorrelation distribution of 1000 randomly sampled shuffles of the datasets. Kuiper analysis demonstrates that the coherence between hippocampus and anterior cingulate is not uniformly distributed (theta, V = 0.372, p < 0.001; gamma, V = 0.426, p < 0.001).
Fig. 5.
The autocorrelation of the mean spectral coherence between hippocampus and anterior cingulate in the gamma (A) and theta (B) bands, and the autocorrelation of the same coherence values when shifted relative to light (C & D). In (A) and (B) the autocorrelations at each lag are shown in red. In (C) and (D) these are shown in green. In all cases, the dotted lines indicate the extent of the 5th and 95th percentile of the distribution of autocorrelations produced from randomly resampling each dataseries 1000 times. In (C) and (D) the normal light cycle is represented by grey bars (lights off). The mean autocorrelation at each lag over these 1,000 shuffles is shown as a green (A and B) or red (C and D) line.
3.3. The insula and anterior cortex are only associated in the gamma band
Unlike the periodic coherence observed between the hippocampus and both cortical regions, coherence between the insula and anterior cingulate is only periodic in the low gamma band. As Fig. 2E demonstrates, there is no peak in coherence between the two cortical regions at 20–25 h as occurred between the hippocampus and both of these regions. There is, however, a general trend of increasing coherence between the insula and anterior cingulate toward the end of the recordings, and possibly a conservation of the peak in coherence at 48 h. In the gamma band, however, the two cortical regions are strongly coherent on a periodic basis. Fig. 2F shows that the peaks in coherence at 20–25 h and 45–50 h that we observe between the hippocampus and both cortical regions are also clearly present between both cortical regions. Examination of the autocorrelation of these datasets supports this observation (Fig. 6). Fig. 6A shows a clear peak in correlation at lags of 25–28 h in the gamma band, however this is not true of the autocorrelation in the theta band shown in Fig. 6B. Kuiper analysis supports this observation; the coherence values between insula and anterior cingulate are not uniformly distributed in the gamma band (V = 0.312, p < 0.001) but are not significantly different from a uniform distribution in the theta band (V = 0.222, p = 0.056, n.s).
Fig. 6.
The autocorrelation of the mean spectral coherence between the insula and anterior cingulate in the gamma (A) and theta (B) bands, and the autocorrelation of the same coherence values when shifted relative to light (C & D). In (A) and (B) the autocorrelations at each lag are shown in red. In (C) and (D) these are shown in green. In all cases, the dotted lines indicate the extent of the 5th and 95th percentile of the distribution of autocorrelations produced from randomly resampling each dataseries 1000 times. In (C) and (D) the normal light cycle is represented by grey bars (lights off). The mean autocorrelation at each lag over these 1000 shuffles is shown as a green (A and B) or red (C and D) line.
3.4. The associations between the regions are not entrained by light
The mean hourly coherence between regions that occurs when the data are shifted relative to light-on time (such that hour 0 is the time of light onset for each session) is illustrated in Fig. 3. These figures also illustrate the confidence interval generated from 1000 lags of the dataseries against each other as for the original data. There is no obvious periodicity between hippocampus and insula (3A and 3B), hippocampus and anterior cingulate (3C and 3D) or between the two cortical regions (3E and 3F) in either theta or gamma bands. Toward the end of the dataseries there is a tendency for the mean coherence to take extremely low and high values. This is an artefact produced by the necessity of shifting the individual recordings relative to light onset. This shifting tended to produce relatively more values in the early and middle hours, but data from the end of each series is representative of fewer than four recordings from hour 50 onward.
The autocorrelations of the coherence between these regions in both frequency bands is illustrated in Fig. 4C and D (hippocampus and insula) Fig. 5C and D (hippocampus and anterior cingulate) and Fig. 6C and D (insula and anterior cingulate). Unlike the non-shifted dataseries, there is no periodicity evident in the data.
Kuiper analysis demonstrated that, when shifted relative to light onset, the distribution of coherence values between hippocampus and insular cortex are uniformly distributed in both theta (V = 0.143, p = 0.47, n.s) and gamma (V = 0.137, p = 0.53, n.s). The same is true of the coherence between hippocampus and anterior cingulate (theta, V = 0.192, p = 0.075, n.s; gamma, V = 0.125, p = 0.69, n.s) and is also true of the coherence between the two cortical regions (theta, V = 0.182, p = 0.12, n.s; gamma, V = 0.170, p = 0.19, n.s).
3.5. Coherence is not associated with running speed
One possible explanation for the increased coherence observed between regions during some recordings is that animals tended to run faster during these particular periods. To examine this possibility, the mean running speed (in cm/s) in each hour of recording was correlated against the mean spectral coherence in each of the frequency bands in each hour of recording. No association was found between running speed and spectral coherence between hippocampus and insula in either theta (r = 0.155, p = 0.246, n.s) or gamma (r = −.101, p = 0.451, n.s) bands. This was also true of the association between running speed and coherence between hippocampus and anterior cingulate in either band (theta, r = 0.041, p = 0.760, n.s; gamma, r = −0.020, p = 0.833, n.s) and between the cortical regions (theta, r = 0.212, p = 0.109, n.s; gamma, r = −0.119, p = 0.375, n.s).
3.6. Spectral bands other than theta and gamma are not periodically coherent
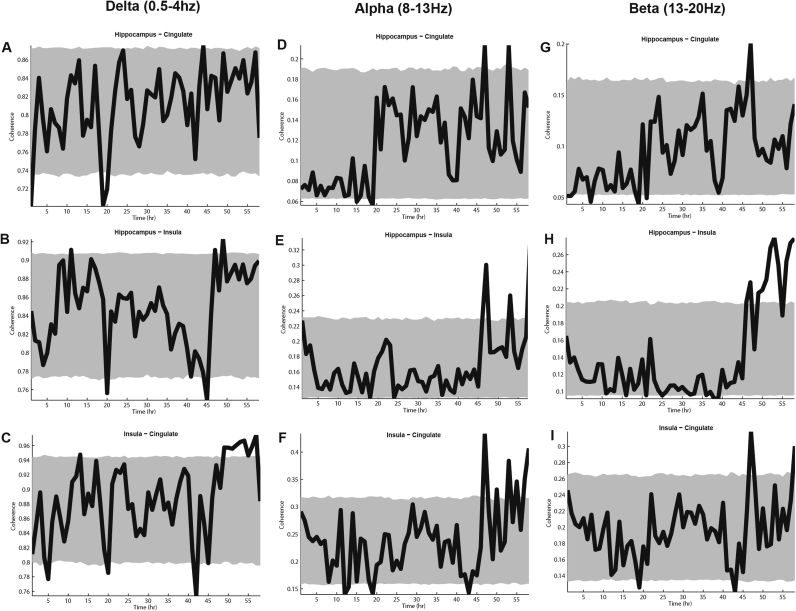
We examined the hypothesis that spectral components other than theta and gamma are periodically coherent. We examined coherence between regions in the Delta (0.5–4 Hz), Alpha (8–13 Hz), and Beta (13–20 Hz) bands. These data are illustrated in Fig. 7. We determined that there is no systematic variation in the coherence between hippocampus and either of the cortical regions, or between cortical regions in any of the frequency bands. Kuiper analysis demonstrates that, in the delta band, the coherence between hippocampus and insula (V = 0.651, p = 0.194, n.s), hippocampus and cingulate (V = 0.500, p = 0.629, n.s), and between insula and cingulate (V = 0.206, p = 0.972, n.s) are uniformly distributed. This was also true of the coherence values between all regions in the Alpha band (hippocampus – insula; V = 0.296, p = 0.597, n.s; hippocampus – cingulate; V = 0.268, p = 0.690, n.s; insula – cingulate; V = 0.240, p = 0.876, n.s) and Beta band (hippocampus – insula; V = 0.256, p = 0.846, n.s; hippocampus – cingulate; V = 0.249, p = 0.840, n.s; insula – cingulate; V = 0.250, p = 0.870, n.s). Autocorrelation of these data also demonstrated no circadian variation in coherence.
Fig. 7.
The mean hourly spectral coherence (solid black lines) between hippocampus and cingulate cortex in the delta (A), alpha (D), and beta (G) bands; between hippocampus and insula in the delta (B), alpha (E), and beta (H) bands; and between the cingulate and insula in the delta (C), alpha (F), and delta (I) bands. The grey envelope in all cases represents the 5th and 95th percentile values produced from lagging each long recording dataseries against each other long recording dataseries by a randomly determined number of hours n, where n takes the value of 0–58.
4. Discussion
In general, we find that the hippocampus has relatively constant low-level coherence in the theta and gamma bands with both the anterior cingulate and agranular insular cortices, and that there is a somewhat larger and more variable baseline of coherence in the theta band between the two cortical regions. Between the hippocampus and both cortical regions, there are, however, striking periodic bursts of markedly increased coherence that are evident in both the theta and gamma bands, that occur approximately 20–24 h after initial exposure to the recording environment and then repeat approximately 24 h later. These bursts in coherence are significantly above the baseline coherence, and persist for several hours. The bursts of coherence operate on a circadian timescale, but are demonstrably absent when the data is aligned relative to light onset/offset as opposed to recording start time. In frequencies other than the theta and gamma bands, which are thought to be critical for long and short range communication respectively, there was no observable periodicity in coherence between any structures. Together, these findings suggest that entry into a novel environment coincident with the availability of novel palatable food is sufficient to drive bursts of coherence between hippocampus and structures involved with gustation and executive function in a circadian pattern, suggesting that there is some daily communication among these structures entrained to these stimuli.
Circadian rhythmicity within hippocampus has been suggested to be a contributor to memory consolidation (Eckel-Mahan and Storm, 2009) We have previously shown (Munn and Bilkey (2012), (2015)) that the circadian modulation of the firing rate of individual neurons, and the frequency of the local EEG in the theta band takes a pseudo-sinusoidal form across a 25 hour period. In contrast, the brief bursts of coherence observed here that last for only 2–3 hours appear to be almost binary in nature. This difference suggests that in the current task a brief period of information transfer between hippocampus and associated cortical regions occurs over a relatively limited relevant time, perhaps triggered by the crossing of some neural-activity threshold.
In contrast to the bursts of coherence observed between hippocampus and the two cortical regions, theta-band coherence between cingulate and insular cortex is much more variable and shows no distinct circadian-period peaks. In the gamma band, the relationship between insula and cingulate is much clearer. Gamma-band coherence was relatively constant throughout the entire 58-h recording epoch, with the exception of two marked bursts of coherence at 22–24 h and 46–48 h. This pattern of coherence mirrors the pattern seen between hippocampus and each of the cortical regions in the theta and gamma bands. That insula and cingulate display peaks of coherence only in the gamma band is consistent with the observation that binding between cortical regions often relies on coherence in the gamma band (Eckhorn et al., 1988, Gregoriou et al., 2009). That the hippocampus is coherent with both cortical regions in the theta band is consistent with findings that theta band coherence is often correlated with performance on memory-based tasks, especially those involving working memory (Sarnthein et al., 1998, Sauseng et al., 2005). When we examined coherence between the hippocampus and both the cingulate and insula cortex in frequency bands outside the theta and gamma range, we saw no discernible periodicity in the data. The same was true for coherence between the two cortical regions. These observations support the proposal that the periodic bursts of coherence that we observed represent a functional, meaningful transfer of information rather than being an aberration caused by abnormal behavior or some more generalized phenomenon.
Our previous work (Munn et al., 2015, Munn and Bilkey, 2012) demonstrated that the circadian oscillations in hippocampus do not seem to be entrained to light, as one might expect from a circadian zeitgeber driven by the SCN. Rather they appeared to be entrained to the start of recording session. In the current experiments, we controlled for light entrainment in much the same way as in our previous work; the recording sessions all began at various times of day, which uncoupled light-onset time from the start of recording, and enabled us to artificially re-align the recordings post-hoc with the onset of light. We found much the same pattern of results as we had previously observed within the hippocampus; the distinct peaks in coherence between the hippocampus and the cortical regions in theta and gamma, and between the cortical regions in the gamma band were only apparent when the data were aligned with the start of recording (i.e. unshifted). When the data were shifted relative to the light onset, there was no apparent pattern in the coherence between any of the regions in either frequency band, demonstrating the periodicity in coherence is not driven by light. It is therefore possible that the theta-band coherence between hippocampus and both cingulate and insula represent a recall or strengthening of information about the entry of the animal into the recording environment and the concurrent novel availability of food, triggered on a roughly 24 h period such as to allow circadian-based prediction of food availability. Similarly, the strong gamma-band coherence between cingulate and insula may represent information binding between these two regions in expectation of a novel stimulus.
Our previous work demonstrates an intrahippocampal circadian response in both the firing rate of single neurons and the frequency of theta frequency (Munn et al., 2015, Munn and Bilkey, 2012) The current study demonstrates that bursts of coherence occur between hippocampus and other structures at times that are close to the previously described peak of single unit firing rate and theta frequency. When these findings are considered alongside data demonstrating that the hippocampus has the clock “machinery” (i.e. expression of clock genes such as PER, CRY, BMAL, and CLOCK) necessary to encode a time signal on a circadian scale (Jilg et al., 2010, Wang et al., 2009) and that the expression of these genes may be regulated by food availability (Girotti et al., 2009, Wakamatsu et al., 2001), they suggest that the hippocampus is influenced by, and/or is a component of, the putative circadian FEO. One potential role for the hippocampus in this system is through the modulation of memory processes. In support of this idea, long term potentiation in the hippocampus; a process thought to be critical to its memorial role is modulated on a circadian period (Chaudhury et al., 2005), and changing the alignment of feeding with the circadian activity cycle both disrupts memory and impairs the expression of the plasticity related protein CREB (Loh et al., 2015).
Unlike the light-entrained SCN oscillator (which entrains slowly; on the order of one hour delta/day), we observe a rapid re-entrainment of both hippocampal activity (in our previous work) and in coherence between hippocampus and cortex in the present data. Interestingly, the FEO also appears to be capable of large-magnitude shifts in short order. For example; Mendoza et al. (2012), Feillet et al. (2008). Flôres et al. (2016b) show behavioral evidence of entrainment to food in mice just 48 h after initial exposure. Flôres et al. (2016a) and Feillet et al. (2008) in particular demonstrate that entrainment appears occur within 24 h; the same timescale as the entrainment in coherence observed in the present study. Feillet et al. (2008) demonstrate that reliable circadian patterns of wheel running are rapidly overlaid by robust food-anticipatory activity on exposure to restricted timed feeding. It is also possible that the neural entrainment might occur in advance of substantial, measurable changes in behavior.
Coherent oscillations among groups of neurons have long been proposed as a mechanism by which neurons can selectively communicate with one another (Fries, 2005, Jensen, 2001). Specifically, rhythmic EEG activity, such as occurs at both theta and gamma frequencies, has been proposed as a mechanism for facilitating synchronization between separate brain regions (Singer, 1999, von der Malsburg, 1999). One measure of this synchronization is the coherence between brain regions; for example, successful memory encoding has been linked with theta coherence (Benchenane et al., 2010, Weiss and Rappelsberger, 2000) and theta coherence between hippocampus and striatum has been correlated with acquisition of a navigation task (DeCoteau et al., 2007); likewise associative learning is correlated with gamma coherence (Miltner et al., 1999). In this vein, the circadian modulation of coherence observed in the current study, likely represents modulation of communication functions.
The finding that the hippocampus, cingulate and insular cortices are involved in food-entrainable, circadian-period transfer of information suggests that all these regions may be part of the proposed food-entrainable oscillator (FEO). This is consistent with previous suggestions that the FEO is not confined to a single structure, but is instead instantiated across a distributed network of brain structures (Carneiro and Araujo, 2009). In this distributed model, one role of the hippocampus may be creating a memorial “tag” of a food-related experience that is projected out of the hippocampus at a time that is most appropriate for generating predictive behavior, in this case perhaps an evolution-driven ‘assumption’ that food that is available at a particular time of day may also be available at that time on subsequent days. The clock-gene machinery to represent this circadian information is present in both the hippocampus (Jilg et al., 2010) and cortex (Masubuchi et al., 2000).
We present here clear evidence that the hippocampus periodically increases coherence with at least two cortical regions that are involved in decision making and gustation. Moreover, the periodic increases in coherence are behaviorally (and not light) entrained, and are only apparent in those frequency bands thought to support memory encoding and long-range information exchange, and are absent in other frequency bands. These findings are consistent with emerging data suggesting a special link between salient cues such as food and mechanisms of memory (e.g. Loh et al. (2015)). Future research will be required to elucidate whether there is a special role for food in these mechanisms (and therefore that this is a true “Food-entrainable” oscillator) or whether the same mechanisms that underlie the FEO are able to be recruited by any stimulus considered appetitive (or otherwise meaningful) and are therefore more broadly generalizable and constitute a general independent timekeeping mechanism.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Robert G.K Munn was supported by a fellowship from the Neurological Foundation of New Zealand.
References
- Angeles-Castellanos M., Mendoza J., Diaz-Munoz M., Escobar C. Food entrainment modifies the c-Fos expression pattern in brain stem nuclei of rats. Am. J Physiol. Regul. Integr. Comp. Physiol. 2005;288(3):R678–R684. doi: 10.1152/ajpregu.00590.2004. [DOI] [PubMed] [Google Scholar]
- Barker G.R.I., Bird F., Alexander V., Warburton E.C. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci.: Off. J. Soc. Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K., Peyrache A., Khamassi M., Tierney P.L., Gioanni Y., Battaglia F.P., Wiener S.I. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66(6):921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Broadbent N.J., Gaskin S., Squire L.R., Clark R.E. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell R.D., Amaral D.G. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J. Comp. Neurol. 1998;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G., Vogt B.A., Holmes J., Dale A.M., Greve D., Jenike M.A., Rosen B.R. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro B.T., Araujo J.F. The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals? Chronobiol. Int. 2009;26(7):1273–1289. doi: 10.3109/07420520903404480. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Botvinick M.M., Cohen J.D. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev. Neurosci. 1999;10(1):49–58. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Cascino G.D., Karnes W.E. Gustatory and second sensory seizures associated with lesions in the insular cortex seen on magnetic resonance imaging. J. Epilepsy. 1990;3(4):185–187. [Google Scholar]
- Chaudhury D., Wang L.M., Colwell C.S. Circadian regulation of hippocampal long-term potentiation. J. Biol. Rhythm. 2005;20(3):225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J.A., Brown C.A., Jones A.K., El-Deredy W. Dissociating nociceptive modulation by the duration of pain anticipation from unpredictability in the timing of pain. Clin. Neurophysiol. 2008;119(12):2870–2878. doi: 10.1016/j.clinph.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Öhman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davidson A.J., Aragona B.J., Houpt T.A., Stephan F.K. Persistence of meal-entrained circadian rhythms following area postrema lesions in the rat. Physiol. Behav. 2001;74(3):349–354. doi: 10.1016/s0031-9384(01)00567-4. [DOI] [PubMed] [Google Scholar]
- Davidson A.J., Aragona B.J., Werner R.M., Schroeder E., Smith J.C., Stephan F.K. Food-anticipatory activity persists after olfactory bulb ablation in the rat. Physiol. Behav. 2001;72(1–2):231–235. doi: 10.1016/s0031-9384(00)00417-0. [DOI] [PubMed] [Google Scholar]
- Davidson A.J., Poole A.S., Yamazaki S., Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2(1):32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Davidson A.J., Stephan F.K. Feeding-entrained circadian rhythms in hypophysectomized rats with suprachiasmatic nucleus lesions. Am. J Physiol. 1999;277(5 Pt 2):R1376–R1384. doi: 10.1152/ajpregu.1999.277.5.R1376. [DOI] [PubMed] [Google Scholar]
- DeCoteau W.E., Thorn C., Gibson D.J., Courtemanche R., Mitra P., Kubota Y., Graybiel A.M. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc. Natl. Acad. Sci. 2007;104(13):5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K.L. Circadian oscillations within the hippocampus support hippocampus-dependent memory processing. Front. Mol. Neurosci. 2012:5. doi: 10.3389/fnmol.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan K.L., Storm D.R. Circadian rhythms and memory: not so simple as cogs and gears. EMBO Rep. 2009;10:584–591. doi: 10.1038/embor.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. Coherent oscillations: a mechanism of feature linking in the visual cortex? Biol. Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Edwards P.A., Muroya H., Gould R.G. In vivo demonstration of the circadian rhythm of cholesterol biosynthesis in the liver and intestine of the rat. J. Lipid Res. 1972;13(3):396–401. [PubMed] [Google Scholar]
- Feillet C.A., Mendoza J., Albrecht U., Pevet P., Challet E. Forebrain oscillators ticking with different clock hands. Mol. Cell Neurosci. 2008;37(2):209–221. doi: 10.1016/j.mcn.2007.09.010. [DOI] [PubMed] [Google Scholar]
- DEFL Flôres, Bettilyon C.N., Jia L., Yamazaki S. The running wheel enhances food anticipatory activity: an exploratory study. Front. Behav. Neurosci. 2016;10:143. doi: 10.3389/fnbeh.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFL Flôres, Bettilyon C.N., Yamazaki S. Period-independent novel circadian oscillators revealed by timed exercise and palatable meals. Sci. Rep. 2016;6:21945. doi: 10.1038/srep21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Girotti M., Weinberg M.S., Spencer R.L. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am. J. Physiol. Endocrinol. Metab. 2009;296(4):E888–E897. doi: 10.1152/ajpendo.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley J.J., Schomer A., Saper C.B. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 2006;9(3):398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Gregoriou G.G., Gotts S.J., Zhou H., Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R.S., Tull L.E., Stackman R.W. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Mem. 2004;82(1):26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hanamori T., Kunitake T., Kato K., Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J. Neurophysiol. 1998;79(5):2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- Hillman K.L., Bilkey D.K. Neurons in the rat anterior cingulate cortex dynamically encode cost-benefit in a spatial decision-making task. J. Neurosci. 2010;30(22):7705–7713. doi: 10.1523/JNEUROSCI.1273-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman K.L., Bilkey D.K. Neural encoding of competitive effort in the anterior cingulate cortex. Nat. Neurosci. 2012;15(9):1290–1297. doi: 10.1038/nn.3187. [DOI] [PubMed] [Google Scholar]
- Hoge J., Kesner R.P. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol. Learn. Mem. 2007;88(2):225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V., Lenck-Santini P.P., Roux S., Save E., Muller R.U., Poucet B. Goal-related activity in hippocampal place cells. J. Neurosci. 2007;27(3):472–482. doi: 10.1523/JNEUROSCI.2864-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huikuri H.V., Kessler K.M., Terracall E., Castellanos A., Linnaluoto M.K., Myerburg R.J. Reproducibility and circadian rhythm of heart rate variability in healthy subjects. Am. J. Cardiol. 1990;65(5):391–393. doi: 10.1016/0002-9149(90)90308-n. [DOI] [PubMed] [Google Scholar]
- Jensen O. Information transfer between rhythmically coupled networks: reading the hippocampal phase code. Neural Comput. 2001;13(12):2743–2761. doi: 10.1162/089976601317098510. [DOI] [PubMed] [Google Scholar]
- Jilg A., Lesny S., Peruzki N., Schwegler H., Selbach O., Dehghani F., Stehle J.H. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010;20(3):377–388. doi: 10.1002/hipo.20637. [DOI] [PubMed] [Google Scholar]
- Jones M.W., Wilson M.A. Theta rhythms coordinate hippocampal–prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley S.W., Walton M.E., Behrens T.E., Buckley M.J., Rushworth M.F. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 2006;9(7):940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kesner R.P., Churchwell J.C. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol. Learn. Mem. 2011;96(3):417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Landry G.J., Kent B.A., Patton D.F., Jaholkowski M., Marchant E.G., Mistlberger R.E. Evidence for time-of-day dependent effect of neurotoxic dorsomedial hypothalamic lesions on food anticipatory circadian rhythms in rats. PLoS One. 2011;6:9. doi: 10.1371/journal.pone.0024187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry G.J., Simon M.M., Webb I.C., Mistlberger R.E. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2006;290(6):R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry G.J., Yamakawa G.R.S., Mistlberger R.E. Robust food anticipatory circadian rhythms in rats with complete ablation of the thalamic paraventricular nucleus. Brain Res. 2007;1141(1):108–118. doi: 10.1016/j.brainres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Loh D.H., Jami S.A., Flores R.E., Truong D., Ghiani C.A., O’Dell T.J., Colwell C.S. Misaligned Feed. Impair. memories. eLife. 2015:4. doi: 10.7554/eLife.09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C., Lepage K., Eden U., Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin E.A., Sparks F.T., Slayyeh B., Sutherland R.J., Leutgeb S., Leutgeb J.K. Neuronal code for extended time in the hippocampus. Proc. Natl. Acad. Sci. USA. 2012;109(47):19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi S., Honma S., Abe H., Ishizaki K., Namihira M., Ikeda M., Ki Honma. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur. J. Neurosci. 2000;12(12):4206–4214. [PubMed] [Google Scholar]
- McDonald R.J., White N.M. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993;107(1):3. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Mendoza, 2010. Food-reward signalling in the suprachiasmatic clock. (vol 112, pg 1489, 2010). Journal of Neurochemistry 113(5):1365. [DOI] [PubMed]
- Mendoza J., Gourmelen S., Dumont S., Sage-Ciocca D., Pevet P., Challet E. Setting the main circadian clock of a diurnal mammal by hypocaloric feeding. J. Physiol. 2012;590(Pt 13):3155–3168. doi: 10.1113/jphysiol.2012.230300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M., Williams S.C., Richardson J.A., Tanaka K., Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Natl. Acad. Sci. USA. 2006;103(32):12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner W.H.R., Braun C., ArnoldMatthias, Witte H., Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397(6718):434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Mistlberger R.E., Rechtschaffen A. Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol. Behav. 1984;33(2):227–235. doi: 10.1016/0031-9384(84)90104-5. [DOI] [PubMed] [Google Scholar]
- Munn R.G., Tyree S.M., McNaughton N., Bilkey D.K. The frequency of hippocampal theta rhythm is modulated on a circadian period and is entrained by food availability. Front Behav. Neurosci. 2015;9:61. doi: 10.3389/fnbeh.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn R.G.K., Bilkey D.K. The firing rate of hippocampal CA1 place cells is modulated with a circadian period. Hippocampus. 2012;22(6):1325–1337. doi: 10.1002/hipo.20969. [DOI] [PubMed] [Google Scholar]
- O’Keefe J., Dostrovsky J. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971 doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.M., Gelb A., Girvin J.P., Hachinski V.C. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9) doi: 10.1212/wnl.42.9.1727. (1727–1727) [DOI] [PubMed] [Google Scholar]
- Pandya D., Van Hoesen G., Mesulam M.-M. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp. Brain Res. 1981;42(3-4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Pritchard T.C., Macaluso D.A., Eslinger P.J. Taste perception in patients with insular cortex lesions. Behav. Neurosci. 1999;113(4):663. [PubMed] [Google Scholar]
- Remondes M., Wilson Matthew A. Cingulate-hippocampus coherence and trajectory coding in a sequential choice task. Neuron. 2013;80(5):1277–1289. doi: 10.1016/j.neuron.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J., Petsche H., Rappelsberger P., Shaw G., Von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. 1998;95(12):7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Schabus M., Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005;57(2):97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Shidara M., Richmond B.J. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296(5573):1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the defination of relations? Neuron. 1999;24(1):49–65. doi: 10.1016/s0896-6273(00)80821-1. (111-25) [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan F.K. The “other” circadian system: food as a zeitgeber. J. Biol. Rhythm. 2002;17(4):284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan F.K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C., Kumar S., Yang J., Wilson M. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct. Funct. 2014;219(3):911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Miller M.W. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J. Comp. Neurol. 1983;216(2):192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C. The what and why of inding: the Modeler's perspective. Neuron. 1999;24(1):95–104. doi: 10.1016/s0896-6273(00)80825-9. [DOI] [PubMed] [Google Scholar]
- Wakamatsu H., Yoshinobu Y., Aida R., Moriya T., Akiyama M., Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur. J. Neurosci. 2001;13(6):1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- Wang L.M.-C., Dragich J.M., Kudo T., Odom I.H., Welsh D.K., O’Dell T.J., Colwell C.S. Expression of the circadian clock gene period2 in the ippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1:3. doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Rappelsberger P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Cogn. Brain Res. 2000;9(3):299–312. doi: 10.1016/s0926-6410(00)00011-2. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990;63(6):1257–1266. doi: 10.1016/0092-8674(90)90421-a. [DOI] [PubMed] [Google Scholar]
- Yasui Y., Breder C.D., Safer C.B., Cechetto D.F. Autonomic responses and efferent pathways from the insular cortex in the rat. J. Comp. Neurol. 1991;303(3):355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]