Significance
Many animals use elastic-energy storage and recoil to produce extremely rapid motions. However, all previously studied systems use anatomical structures loaded with a single cycle of muscular contraction. Here, we show that the spider Hyptiotes uses its web like a catapult, loading multiple cycles of muscular contraction and then flinging its own body and the web forward to entrap prey. This is the only known case of a nonhuman utilizing an external device for power amplification. This finding reveals an underappreciated function of spider silk and expands our understanding of how power amplification is used in natural systems, showing remarkable convergence with human-made power-amplifying tools.
Keywords: elastic energy, spider silk properties, power amplification, tool use, prey capture
Abstract
Power amplification allows animals to produce movements that exceed the physiological limits of muscle power and speed, such as the mantis shrimp’s ultrafast predatory strike and the flea’s jump. However, all known examples of nonhuman, muscle-driven power amplification involve anatomical structures that store energy from a single cycle of muscular contraction. Here, we describe a nonhuman example of external power amplification using a constructed device: the web of the triangle-weaver spider, Hyptiotes cavatus, which uses energy stored in the silk threads to actively tangle prey from afar. Hyptiotes stretches its web by tightening a separate anchor line over multiple cycles of limb motion, and then releases its hold on the anchor line when insects strike the web. Both spider and web spring forward 2 to 3 cm with a peak acceleration of up to 772.85 m/s2 so that up to four additional adhesive capture threads contact the prey while jerking caused by the spider’s sudden stop subsequently wraps silk around the prey from all directions. Using webs as external “tools” to store energy offers substantial mechanical advantages over internal tissue-based power amplification due to the ability of Hyptiotes to load the web over multiple cycles of muscular contraction and thus release more stored energy during prey capture than would be possible with muscle-driven anatomical elastic-energy systems. Elastic power amplification is an underappreciated component of silk’s function in webs and shows remarkable convergence to the fundamental mechanical advantages that led humans to engineer power-amplifying devices such as catapults and ballistae.
Power amplification involves the slow storage of energy followed by its rapid release to enable movement speeds and power outputs that exceed the intrinsic physiological limitations of muscles. Humans routinely store muscular work in a variety of tools such as bows, slingshots, and ballistae. However, all other examples of muscle-driven power amplification in nature rely on the organisms’ own anatomical structures to store energy in elastic materials such as tendon or resilin, often in conjunction with catch mechanisms (1–3). Classic examples include the jumping mechanisms of fleas (4), froghopper insects (1), and frogs (5); the strike of the mantis shrimp (6); and the tongue projection of chameleons (7). External power amplification, as exemplified in human tools, offers many advantages. Most notably, elastic external structures can be loaded over multiple cycles of muscular contraction, whereas muscle-driven elastic anatomical systems in animals can store only the work done by a single muscular contraction. This allows a tremendous increase in the maximum energy stored in external devices per unit muscle mass. External power amplification further offers the advantage of tools that are easily customized, rebuilt, modified, and discarded on short time scales. Here, we demonstrate that the triangle-weaver spider Hyptiotes uses its web as a “tool” for external power amplification, producing rapid movements of its web to entangle insect prey—the only known example of external power amplification outside of human tools.
In contrast to the static webs of most orb-weaving spiders, Hyptiotes appears to actively generate and release tension in its web while hunting (8, 9). Hyptiotes waits for prey in the corner of its triangular web where the radial threads converge, its body acting as a bridge between two separate pieces of the web: the anchor line connected to the substrate and the trap line connected to the main web triangle (8, 10) (Fig. 1 A–E). In preparation for hunting, the spider hauls backward along the anchor line in a “leg-over-leg” motion (11), pulling the web taut through multiple loading cycles of muscular contractions. When stimulated by either prey contacting the web (8, 12) or physical attack on its body (13), Hyptiotes releases its hold on the anchor line, and the spider and web rapidly move forward several centimeters (Fig. 1F) (9). When prey are present in the web, the rapid movement of the web causes several extra lines of cribellate capture silk to hit the prey, further tangling it in the web (9). This forward movement ends once slack is removed from the anchor line, likely caused by the spider squeezing the internal valves of its spinnerets to ensure that no new silk is produced (14–16). Hyptiotes can repeat this movement multiple times, walking backward to tighten the line and releasing again to further entangle prey (8, 9).
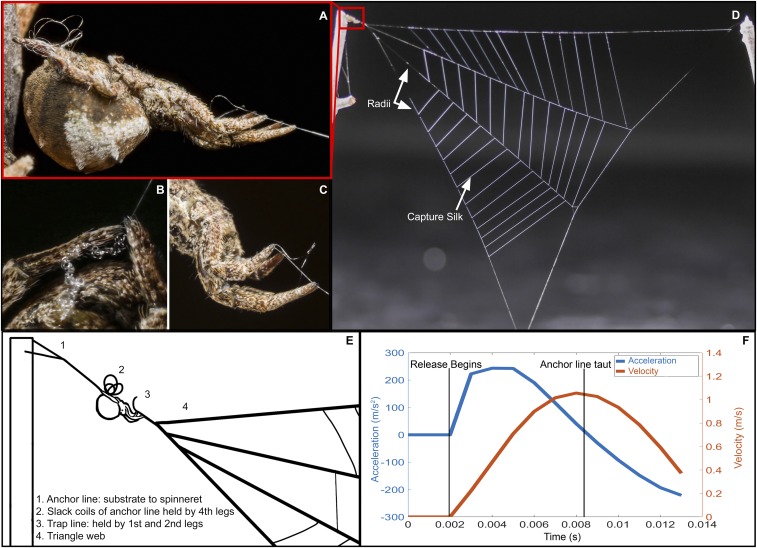
Fig. 1.
Hyptiotes springs forward by releasing its grip on the anchor line, with the spider’s abrupt halt causing large web oscillations. (A) Hyptiotes using its body to bridge two separate silk lines: anchor line and trap line. (B) Anchor line attached to spinnerets held by hind legs; excess silk is gathered during the leg-over-leg tightening of the web. (C) Front legs holding trap line, which is clearly not contiguous with the anchor line. (D) Complete web with labeled radial and capture silk (spider visible Upper Left). (E) Diagram of different silk lines. (F) Spider body acceleration and velocity during the initial release until just before the spider begins to jerk backward.
Based upon these prior observations, we suggest the hypothesis that elastic energy stored in the silk of the web provides propulsive force in this system (as opposed to the spider jumping or moving the web with its legs), and that release of the tension in the silk generates power exceeding muscular capacity alone. To test this hypothesis, we recorded high-speed kinematics of spiders and webs during predatory and experimenter-induced behaviors. Furthermore, we tested whether this behavior was beneficial for prey capture relative to inert webs by comparing capture rates when the spiders performed this behavior versus not. Finally, we investigated the importance of body shape and mechanism of energy storage/release (external, internal, or muscle power alone) on the overall kinematics of the web using a mathematical model, to determine how the unusually rounded opisthosoma (rear body division) (17) of Hyptiotes influences the web and whether the observed propulsion mechanism offers superior performance to alternatives.
Results
To determine the kinematics of motion in the Hyptiotes web, we recorded high-speed video of 19 release movements of six Hyptiotes spiders in response to disturbance by the experimenters. During release trials, up to 3.4 mN (mean ± SD of 2 ± 0.86 mN) of tension causes the spider’s body to accelerate up to 772.85 m/s2 (370.79 ± 158.39 m/s2), reaching velocities of up to 2.15 m/s (1.22 ± 0.42 m/s) (SI Appendix, Table S1) and moving the web forward by as much as 3.18 cm (1.78 ± 0.73 cm) (Fig. 1). The anchor line then tenses, rapidly decelerating the spider and causing both the spider and web to oscillate (Movie S1). The abrupt stop is facilitated by the short length and high elastic modulus (10.7 ± 0.5 GPa) (18) of the Hyptiotes spider’s anchor line as it reaches high tensile loads over a short distance. Despite the rapid motion of the web after release, the web does not tangle with itself unless prey are present in the web, suggesting that the tangling function of the motion is contingent on the presence of prey, eliminating a potential penalty for “misfires” or use of the web for predator evasion. Hyptiotes can hold its web in tension for hours while waiting for prey. There is no obvious catch mechanism in the external morphology of the spider, and the front legs appear to be flexed slightly. Thus, how Hyptiotes can efficiently hold the web in place is an intriguing question for future research.
To determine if Hyptiotes uses external power amplification, we first identified that, after release of the hind tarsal claw, Hyptiotes maintained a fixed body posture. From this observation, we concluded that all of the kinetic energy imparted to the web and spider must come from the stored elastic energy in the silk. Second, we calculated the power generated using the mass, acceleration, and velocity of the spider after release and found that it significantly exceeds the potential output of muscle alone. Web release generated up to 0.0473 W (0.0282 ± 0.0245 W). The highest recorded power output of any arthropod muscle is 326 W/kg of muscle (19). Thus, in the case of the trial with the maximum power, Hyptiotes would need at least 145 mg of muscle mass to generate the power observed during web release using their own bodies to generate propulsion (e.g., if jumping), vastly exceeding their actual ∼7 mg of total body mass. The Hyptiotes triangle web, therefore, is a demonstrated example of nonhuman external power amplification.
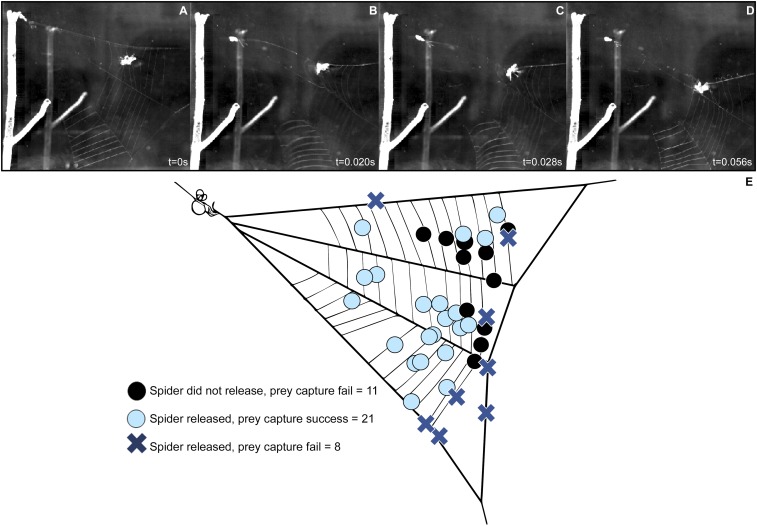
To test the efficacy of the triangle-weaver spider’s hunting strategy, we observed 13 Hyptiotes spiders during 40 prey capture events using flies. Hyptiotes triggered its trap rapidly after the fly contacted the web, with half of all events within 2 s and with times as short as 0.13 s (Movies S2 and S3). Without web release, prey always escaped (n = 11), but when web release occurred (n = 29), prey were captured in 72% of cases (n = 21), indicating that the web release is necessary for prey capture in Hyptiotes. Web release only failed to capture prey (n = 8) when insects were intercepted near the perimeter of the web, due to the prey being flung free during the high acceleration and jerk of the web (Fig. 2E).
Fig. 2.
(A–D) Prey capture sequence: from initial release to subsequent tangling. (A) Prey contacts the web at time 0 s and is adhered to three strands of capture silk; Hyptiotes releases hold on the anchor line. (B) Forward motion of spider ceases at time 0.020 s as the anchor line becomes taut and Hyptiotes begins to move backward; the prey is further wrapped in the silk above it and the adjacent capture silk to the left of the prey is now in line with the prey. (C) Spider jerks backward at time 0.028 s, pulling the web toward its body; the upper section of the web begins to collapse around the prey. (D) Midway through the second oscillation at time 0.056 s, forward motion of Hyptiotes ceases as the anchor line again tightens. Additional capture silk from the left of the prey and the upper section of the web is fully collapsed around the insect. (E) Effect of web release on prey capture. Circles and Xs indicate locations where prey contacted the web, and color indicates spider behavior and outcome: web release with successful prey capture (light blue circles), web release with prey escape (blue X’s), and no release with prey escape (black circles) (n = 40). Without release, all of the prey escaped.
External power amplification facilitates Hyptiotes prey capture in several ways. The initial rapid forward acceleration of the web (relative to the prey) brings additional strands of capture silk into contact with the prey in as little as 4 ms (32 ± 23 ms) (SI Appendix, Fig. S1). Second, the prey is further entangled as two to five subsequent oscillations of the web, over 94 ms, wrap silk from all directions around the prey (Movie S4) so that after a single release (total time, 143 ± 56 ms), up to four (2.2 ± 1.1) additional strands of capture silk contact the prey (Fig. 2 A–D). This sequence of behaviors (loading, release, and oscillations) can be repeated multiple times. The anchor line lengthens after each release such that the web begins to sag due to the mass of the prey, which causes further tangling. Finally, the spider shifts behavior and continuously releases anchor line until the web is fully collapsed around the prey, allowing the spider to wrap the prey (Movie S5). Insect flight behaviors vary immensely in terms of speed, momentum, and maneuverability, and future work should examine how web release influences capture of diverse prey species. However, we hypothesize that web release may be particularly effective in capturing larger insects because the larger inertial differences between prey and web maximize the tangling motions.
To better understand how power amplification and spider body shape influence web kinematics, we constructed a mathematical model in MATLAB. We focused on maximizing acceleration and jerk (the rate of change of acceleration) because these are likely the primary factors influencing the prey’s contact with additional capture strands and subsequent tangling. The model compared our Hyptiotes external power-amplification system to hypothetical alternative systems in which the spiders jumped forward either without power amplification (as in a jumping spider) (20) or utilizing a classic internal power-amplification mechanism storing a single cycle of work from muscular contraction (as in the flea’s jump) (21) (Fig. 3A). The simulation behaved qualitatively similarly to the observed spiders, but direct comparison was prevented due to many unknowns of web properties and individual web variability. In all cases, increased input energy resulted in increased web acceleration and jerk. However, external power amplification via webs clearly resulted in higher acceleration and jerk of the web than either muscle alone or internal power amplification (Fig. 3A). Web-based power amplification showed rapid increases in jerk as more energy was stored in the silk, whereas internal power-amplification jumps showed a slower rate of increase and muscle-powered jumps were unable to produce either high energies or high web jerk (Fig. 3A). Use of an internal mechanism required anatomically unrealistic relative muscle mass (∼20%) to achieve even a fraction of the performance seen in web-based power amplification (Fig. 3A). Thus, external loading of the silk in the triangle web allows high performance without investing in metabolically costly large muscle mass.
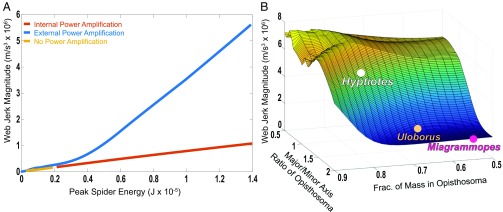
Fig. 3.
Effect of alternative launch mechanisms (A) and body shape (B) on web jerk. (A) Comparison of jerk as a function of release energy resulting from muscle alone (no power amplification, yellow), internal amplification (orange), and external amplification through the web (blue). External power amplification (Hyptiotes web) outperforms internal power amplification across all energies. Muscle-powered jumps were limited to 0.2 × 10−5 J. In the real system, Hyptiotes generates much larger velocities, and therefore energies, than could have been generated by muscle power alone. (B) Influence of body shape and distribution of mass on the jerk of the web. Hyptiotes (white circle) lies at the edge of the plateau; closely related species are plotted as well (Uloborus, Miagrammopes) but lie far from the optimum. Thus, Hyptiotes body shape causes about 4 times more web jerk compared with the other closely related spiders. Jerk and acceleration results are qualitatively the same.
Compared with other uloborids, Hyptiotes can exert more absolute force on silk threads (22, 23), likely due to greater tracheal and mitochondrial supply in the spider’s legs (24, 25). While this morphology is likely advantageous for pulling the web taut and creating the tension to store energy within the web, there may be further morphological modifications of Hyptiotes that improve web kinematics or endurance. The body shape of Hyptiotes likely plays a key role in how triangle webs operate by altering the position of the center of mass relative to the web tension vector and the spider’s rotational moment of inertia. When Hyptiotes launches, the web tension first causes the spider to pitch upwards, followed by a sudden pitch downward when the spider comes to an abrupt halt as the anchor line tenses (SI Appendix, Fig. S2). This tensing jerks the spider’s body forward and backward, tugging on the silk lines and causing the web to oscillate around the prey. Hyptiotes displays a notably rounder and heavier opisthosoma than close relatives in the Uloboridae family (17) (Fig. 3B). Our simulation showed that web acceleration varied twofold and that web jerk varied threefold across a broad range of body shapes (0.5 to 0.9 of mass in the opisthosoma, and major/minor axis ratios of the opisthosoma of 0.5 to 2.0). The body shape of Hyptiotes lies on the edge of plateau for the contribution of both of these characteristics to web motion, and changes in shape associated with feeding (increased mass and roundness of the opisthosoma) will further improve performance (Fig. 3B).
Discussion
Many orb spiders manipulate silk tension during web construction or by pulling their legs inward while sitting in their webs (26), which primarily influences signal transduction through the silk radii (27). Orb spiders also actively maneuver on their webs to attack and subdue prey that otherwise escape quickly (28–30). The Hyptiotes triangle webs combine these functions to a spectacular degree for a novel outcome: using stored energy in silk threads to actively wrap webs around prey from afar. While orb webs are justifiably renowned for their capacity to dissipate kinetic energy of their flying insect prey, Hyptiotes is likely not the only spider to utilize stored energy in silk for prey capture. The ray spider Theridiosoma pulls its web into a cone, releasing the web to entangle prey insects in a mechanism that may be similar to Hyptiotes (31), and Miagrammopes releases tensioned threads to partially collapse webs while hunting (32). Additionally, the most species-rich family of web-hunting spiders, theridiid cobweb spiders, construct sticky gumfooted threads that are held under tension and can sometimes lift prey into the web through springy scaffolding silk (33, 34). The Hyptiotes triangle web, therefore, not only reveals the importance of energy storage as an underappreciated functional property for spider silks in prey capture, it also is a remarkable example of a nonhuman animal using external structures for power amplification.
Using an external tool for power amplification during prey capture offers many advantages over internal mechanisms. First, the leg-over-leg loading of the web by the spider over repeated cycles of muscular contraction allows the spider to store very large amounts of elastic energy per unit muscle mass, escaping classic physiological limits of muscle strain and length–tension relationships. The system thus operates in a manner analogous to the ion and water pump-driven systems that power the carnivorous traps of bladderworts and stinging nematocyst cells of cnidarians (35, 36), but at a much larger scale. The iterative loading of the system offers a second advantage in allowing the spider to regulate the web tension and stored energy as needed so that the system is neither too compliant nor too stiff, enabling the rapid accelerations and range of motion that jerk silk around the prey. Using a constructed device as a weapon in prey capture offers even more advantages. The spider itself is largely freed from the necessity of evolving specialized anatomy to generate and store energy. Furthermore, the web allows Hyptiotes to interact with prey from afar, reducing bodily damage from potentially dangerous insects and allowing the tangling process to begin immediately after prey contact silk. Finally, the web is a renewable system, one that the spider can easily repair or replace by simply spinning more silk. Thus, many of the fundamental advantages that led humans to engineer power-amplifying weapons such as catapults and ballistae are also found in the webs of spiders, opening up energy storage as an underappreciated functional property of spider silks.
Materials and Methods
Spider Collection and Care.
We collected Hyptiotes cavatus spiders from The University of Akron Field Station, located at the Bath Nature Preserve (Bath, OH). All spiders were housed individually in the laboratory in two differently sized terrariums: 25.5 × 15 × 19 cm and 31.5 × 17.5 × 26 cm, inverted onto Styrofoam bases. Wooden dowel rods were embedded vertically in the Styrofoam 12 to 15 cm apart to provide a web-building substrate. Some spiders were given naturally formed Y-shaped twigs to build on instead of the wooden rods, to better simulate field conditions. Hyptiotes spiders were fed several wingless Drosophila melanogaster flies once a week, and misted with water daily. Cages were checked daily for newly constructed webs. Experimental testing began once a new web was found.
Release Mechanism and Prey Capture Experiments.
To study web kinematics, we initiated web release by either throwing wingless D. melanogaster into the web (n = 3) or touching the spider lightly with human hair (n = 16). The web kinematics were then recorded using a Photron Fastcam SA4 (Photron) at 1,000 or 2,000 fps. The videos were then analyzed using ProAnalyst (Xcitex) to quantify the position of the spider and web with respect to time. The position data were then smoothed using MATLAB’s spline tool. The smoothed position data were then used to calculate the velocity, acceleration, and jerk of the tracked locations.
We also studied the effects of web release on prey capture by allowing house flies (Muscidae) (n = 40) to fly into the Hyptiotes web (n = 13). This prey capture experiment was accomplished by inverting a terrarium over the Hyptiotes web and releasing a house fly into the enclosed space. The prey capture event was recorded at 250 or 500 fps to allow longer recording times. The recorded videos were then analyzed for where the house fly contacted the web, the number of sticky threads in contact before and after release, and whether or not the prey was captured.
Computer Simulation.
We constructed a simplified model of the spider-web system in MATLAB to assess the influence of spider morphology and energy-storage/release strategy on the overall movement of the web after release (Movie S6). The spider was modeled as two ellipsoid objects connected at one point (opisthosoma and prosoma). The shape and mass of the ellipsoid objects were varied to assess the morphological impact on web kinematics. The spider was then connected to the anchor line and web line via legs such that the two strands of silk were in line. To assess how different energy-storage/release strategies influence web kinematics, we set up three different situations: external energy storage, internal energy storage, and muscle-powered jump. For the web case (external energy storage), the spider began at rest and was accelerated due to the web forces. For the catch (internal energy storage) and jump (muscle power) cases, the spider was assumed to jump before the beginning of the simulation such that the spider had an initial velocity rather than starting from rest. Additionally, we modeled the silk as a hysteretic polymer. The force-displacement curve of the loaded silk followed the square of the displacement, and during unloading, it followed displacement to the fourth power, resulting in a 50% hysteretic energy loss over a complete cycle of loading and unloading (37). To account for incomplete unloading, we implemented a deformation offset such that the force on the silk reached zero before the deformation of the system reached zero—a common strategy for hysteretic materials (38). Finally, the spider could rotate around its center of mass more than 180° at very low energies, so we terminated the simulation if the spider’s pitch exceeded 51°.
In the first set of simulations, we varied the initial energy stored in the web or generated internally. This was done by keeping the web deformation constant and varying either the web compliance for the web case, or the muscle mass for the jump and the catch cases, assuming that muscle generated 50 J/kg during elastic loading and all energy was recovered during recoil. Takeoff velocity of muscular jumps was calculated based on a constant isotonic power output of 500 W/kg across a spider-leg length of 2.82 mm based on measurements of Hyptiotes. The simulation was run with time steps of 1 μs for a duration of 500 ms. During the simulation, the translational and rotational jerk, acceleration, and velocity of the spider were measured; trends for all variables were similar, so we focused on jerk. We report the mean peak values of these quantities over 10 periods of oscillation.
In the second simulation, we fixed the initial energy of the system and varied the morphological parameters of the opisthosoma and prosoma. We varied the ellipsoidal shape of the opisthosoma, and the mass distribution between the opisthosoma and the prosoma (using a constant density of 1.05 g/cm3 and a total mass of 7 mg for the spider). This resulted in moving the center of mass and changing the rotational moment of inertia of the entire spider.
Data and Materials Availability.
Motion-tracking coordinate data are available on Figshare (39) (https://dx.doi.org/10.6084/m9.figshare.7067960); the MATLAB code is available on Figshare (40) (https://doi.org/10.6084/m9.figshare.7068401.v1).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Motion-tracking coordinate data are available on Figshare (https://dx.doi.org/10.6084/m9.figshare.7067960); and MATLAB code is available on Figshare (https://doi.org/10.6084/m9.figshare.7068401.v1).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821419116/-/DCSupplemental.
References
- 1.Burrows M, Shaw SR, Sutton GP (2008) Resilin and chitinous cuticle form a composite structure for energy storage in jumping by froghopper insects. BMC Biol 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGowan CP, Baudinette RV, Usherwood JR, Biewener AA (2005) The mechanics of jumping versus steady hopping in yellow-footed rock wallabies. J Exp Biol 208:2741–2751. [DOI] [PubMed] [Google Scholar]
- 3.Patek SN. (2015) The most powerful movements in biology. Am Sci 103:330. [Google Scholar]
- 4.Sutton GP, Burrows M (2011) Biomechanics of jumping in the flea. J Exp Biol 214:836–847. [DOI] [PubMed] [Google Scholar]
- 5.Peplowski MM, Marsh RL (1997) Work and power output in the hindlimb muscles of Cuban tree frogs Osteopilus septentrionalis during jumping. J Exp Biol 200:2861–2870. [DOI] [PubMed] [Google Scholar]
- 6.Patek SN, Nowroozi BN, Baio JE, Caldwell RL, Summers AP (2007) Linkage mechanics and power amplification of the mantis shrimp’s strike. J Exp Biol 210:3677–3688. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright PC, Kraklau DM, Bennett AF (1991) Kinematics of tongue projection in Chamaeleo oustaleti. J Exp Biol 159:109–133. [Google Scholar]
- 8.Marples MJ, Marples BJ (1937) Notes on the spiders Hyptiotes paradoxus and Cyclosa conica. Proc Zool Soc London A107:213–221. [Google Scholar]
- 9.Wilder BG. (1875) The triangle spider. Popular Sci Mon 6:1–15. [Google Scholar]
- 10.Fritzén NR. (2002) Hyptiotes paradoxus (Araneae: Uloboridae) found on the Aland Islands—A species new to Finland. Memoranda Societas Pro Fauna et Flora 78:3–7. [Google Scholar]
- 11.Wilder BG. (1874) The nets of Epeira, Nephila and Hyptiotes (Mithras). Proc Am Assoc Adv Sci 22:264–274. [Google Scholar]
- 12.Opell BD. (1982) Post-hatching development and web production of Hyptiotes cavatus (Hentz) (Araneae, Uloboridae). J Arachnol 10:185–191. [Google Scholar]
- 13.McCook HC. (1890) American Spiders and Their Spinningwork: A Natural History of the Orbweaving Spiders of the United States with Special Regard to Their Industry and Habits (Academy of Natural Sciences of Philadelphia, Philadelphia: ). [Google Scholar]
- 14.Madsen B, Vollrath F (2000) Mechanics and morphology of silk drawn from anesthetized spiders. Naturwissenschaften 87:148–153. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS. (1969) Control of drag-line spinning in certain spiders. Am Zool 9:103–111. [Google Scholar]
- 16.Vollrath F, Knight DP (1999) Structure and function of the silk production pathway in the spider Nephila edulis. Int J Biol Macromol 24:243–249. [DOI] [PubMed] [Google Scholar]
- 17.Opell BD. (1989) Centers of mass and weight distribution in spiders of the family Uloboridae. J Morphol 202:351–359. [DOI] [PubMed] [Google Scholar]
- 18.Blackledge TA, Hayashi CY (2006) Unraveling the mechanical properties of composite silk threads spun by cribellate orb-weaving spiders. J Exp Biol 209:3131–3140. [DOI] [PubMed] [Google Scholar]
- 19.Josephson RK. (1993) Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol 55:527–546. [DOI] [PubMed] [Google Scholar]
- 20.Parry DA, Brown RHJ (1959) The jumping mechanism of salticid spiders. J Exp Biol 36:654–664. [Google Scholar]
- 21.Bennet-Clark HC, Lucey EC (1967) The jump of the flea: A study of the energetics and a model of the mechanism. J Exp Biol 47:59–67. [DOI] [PubMed] [Google Scholar]
- 22.Opell BD. (1985) Web-monitoring forces exerted by orb-web and triangle-web spiders of the family Uloboridae. Can J Zool 63:580–583. [Google Scholar]
- 23.Opell BD. (1987) Changes in web-monitoring forces associated with web reduction in the spider family Uloboridae. Can J Zool 65:1028–1034. [Google Scholar]
- 24.Opell BD, Konur DC (1992) Influence of web-monitoring tactics on the density of mitochondria in leg muscles of the spider family Uloboridae. J Morphol 213:341–347. [DOI] [PubMed] [Google Scholar]
- 25.Opell BD. (1987) The influence of web monitoring tactics on the tracheal systems of spiders in the family Uloboridae (Arachnida, Araneida). Zoomorphology 107:255–259. [Google Scholar]
- 26.Nakata K. (2010) Attention focusing in a sit-and-wait forager: A spider controls its prey-detection ability in different web sectors by adjusting thread tension. Proc Biol Sci 277:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frohlich C, Buskirk RE (1982) Transmission and attenuation of vibration in orb spider webs. J Theor Biol 95:13–36. [Google Scholar]
- 28.Eberhard WG. (1989) Effects of orb web orientation and spider size on prey retention. Bull Br Arachnol Soc 8:45–48. [Google Scholar]
- 29.Nakata K, Zschokke S (2010) Upside-down spiders build upside-down orb webs: Web asymmetry, spider orientation and running speed in Cyclosa. Proc Biol Sci 277:3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zschokke S, Nakata K (2010) Spider orientation and hub position in orb webs. Naturwissenschaften 97:43–52. [DOI] [PubMed] [Google Scholar]
- 31.McCook HC. (1881) The snare of the ray spider (Epeira radiosa), a new form of orb-web. Proc Acad Nat Sci Philadelphia 33:163–175. [Google Scholar]
- 32.Lubin YD, Eberhard WG, Montgomery GG (1978) Webs of Miagrammopes (Araneae: Uloboridae) in the neotropics. Psyche (Stuttg) 85:1–23. [Google Scholar]
- 33.Argintean S, Chen J, Kim M, Moore A (2006) Resilient silk captures prey in black widow cobwebs. Appl Phys A Mater Sci Process 82:235–241. [Google Scholar]
- 34.Blackledge TA, Swindeman JE, Hayashi CY (2005) Quasistatic and continuous dynamic characterization of the mechanical properties of silk from the cobweb of the black widow spider Latrodectus hesperus. J Exp Biol 208:1937–1949. [DOI] [PubMed] [Google Scholar]
- 35.Lubbock R, Amos WB (1981) Removal of bound calcium from nematocyst contents causes discharge. Nature 290:500–501. [DOI] [PubMed] [Google Scholar]
- 36.Sydenham PH, Findlay GP (1975) Transport of solutes and water by resetting bladders of Utricularia. Funct Plant Biol 2:335–351. [Google Scholar]
- 37.Kelly SP, Sensenig A, Lorentz KA, Blackledge TA (2011) Damping capacity is evolutionarily conserved in the radial silk of orb-weaving spiders. Zoology (Jena) 114:233–238. [DOI] [PubMed] [Google Scholar]
- 38.Masing G. (1926) Eigenspannungen und verfestigung beim messing. Proceedings of the 2nd International Congress of Applied Mechanics (Zurich), 332–335. German.
- 39.Han SI, Astley HC, Maksuta DM, Blackledge TA (2019) Motion tracking coordinate data of Hyptiotes release movement. Figshare. Available at 10.6084/m9.figshare.7067960. Deposited November 9th, 2018. [DOI] [PMC free article] [PubMed]
- 40.Han SI, Astley HC, Maksuta DM, Blackledge TA (2019) Hyptiotes MATLAB simulation code. Figshare. Available at 10.6084/m9.figshare.7068401.v1. Deposited November 9th, 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.