Significance
Sociality provides various demographic and ecological benefits to animals. However, social systems can be greatly disrupted if populations experience an additive mortality event. Here, we investigated how the social structure of a highly social species, the killer whale, was impacted by a human-induced mortality event (lethal interactions with illegal fisheries), and how these social impacts affected the survival of individuals. Using a unique long-term dataset, we showed that the loss of individuals in social groups resulted in weaker associations among surviving individuals, which may have modified their fitness during and after the additive mortality event. This study therefore highlights the importance of sociality in the resilience of populations to demographic stress and has major implications for the conservation of highly social species.
Keywords: sociality, survival, social structure, anthropogenic disturbances, killer whales
Abstract
In highly social top predators, group living is an ecological strategy that enhances individual fitness, primarily through increased foraging success. Additive mortality events across multiple social groups in populations may affect the social structure, and therefore the fitness, of surviving individuals. This hypothesis was examined in a killer whale (Orcinus orca) population that experienced a 7-y period of severe additive mortality due to lethal interactions with illegal fishing vessels. Using both social and demographic analyses conducted on a unique long-term dataset encompassing periods before, during, and after this event, results indicated a decrease in both the number and the mean strength of associations of surviving individuals during the additive mortality period. A positive significant correlation between association strength and apparent survival suggested that the fitness of surviving individuals was impacted by the additive mortality event. After this event, individuals responded to the loss of relatives in their social groups by associating with a greater number of other social groups, likely to maintain a functional group size that maximized their foraging success. However, these associations were loose; individuals did not reassociate in highly stable social groups, and their survival remained low years after the mortality event. These findings demonstrate how the disruption of social structure in killer whales may lead to prolonged negative effects of demographic stress beyond an additive mortality event. More importantly, this study shows that sociality has a key role in the resilience of populations to human-induced mortality; this has major implications for the conservation of highly social and long-lived species.
Sociality, or group living, is widespread in mammals. To persist, this propensity to engage with conspecifics must enhance individual fitness (1–6). Group living may benefit individuals through food sharing, cooperative foraging, defense against predators, and caring for juveniles; thus sociality has an adaptive value, enhancing survival and other demographic parameters such as reproductive success (7–14).
Natural selection for group living should maximize individual fitness and result in an optimal group size which maximizes the net average energy gain per individual. Optimal group size can depend on the quality of the environment (resource availability, type of prey), on demographic parameters like the numbers of potential partners, or on the abundance of predators (1, 6, 8, 12, 15–17). In social mammals, sociality usually enhances the survival of group members (4, 6, 18–23), and individuals try to maintain the optimal size of the group (8, 16, 17). However, anthropogenic activities can dramatically alter the social organization of group-living species and thus counterbalance the benefits of group living; this might affect the fitness of individuals (24–26). The loss of individuals within groups caused by hunting or harvesting can indirectly affect the survival of remaining members (21, 26–29). For example, the poaching-related mortality of African elephants (Loxodonta africana), a highly social species with a matrilineal social system, greatly disrupted association patterns, increasing calf mortality and male reproductive skew, ultimately leading to local population extinctions (24, 30).
In the marine environment, social species such as odontocetes (toothed whales) may experience additive mortality events caused by anthropogenic activities, primarily through harvesting or lethal interactions with fisheries. These interactions involve incidental bycatch or lethal responses from fishers when odontocetes directly remove and consume fish caught on fishing gear (31–36). Such behavior, known as depredation, may cause fishers to use firearms or explosives to repel odontocetes from fishing gear. This type of response has been primarily documented in poorly regulated legal fisheries and illegal unregulated unreported fisheries (37, 38), generating severe additive mortality of individuals within depredating populations (39, 40). While the effects of additive mortality events have been primarily examined with respect to the demography of odontocete populations, little is known about the consequences of these events for the social organization of individuals, or about how a disrupted social organization may further impact demography after these events.
These two questions were here investigated on the killer whales (Orcinus orca) of the Crozet Islands (South Indian Ocean; 46°S, 51°E), which have been monitored since the 1970s. The killer whale is a highly social odontocete species, with individuals organized into stable social groups (41–43). This social system involves alloparental care and cultural inheritance of ecological knowledge, foraging preferences, and hunting strategies (42, 44–48). Killer whales often forage in groups, and in some populations, group size was shown to determine the success of prey capture and the size of the prey (11, 16, 49). The killer whale is also one of the species most involved in depredation interactions with fisheries worldwide (50). At the Crozet Islands, early studies initiated in the 1970s and 1980s showed that killer whales associated in stable social groups with limited dispersal from these units. The social groups’ structure was assumed to be matrilineal based on long-term observations (42, 43). These studies described the social groups as composed of two to seven individuals, with a generalist diet including seals, birds, cetaceans, and fish, and using unique group hunting strategies such as intentional stranding, which was shown to be socially transmitted from old females to offspring (11, 42, 47, 49). Later, these killer whales were reported to depredate Patagonian toothfish (Dissostichus eleginoides) from the local commercial longline fisheries that began in the mid-1990s, with a high level of interactions with fishing vessels (42 ± 14% of longline sets on average) (37, 38, 51–53). From 1996 to 2002, the Crozet killer whale population experienced substantial additive mortality, with the death of individuals from multiple social groups, mainly caused by lethal responses from illegal fishing vessels that used firearms and explosives to deter killer whales during depredation interaction events (37–40, 54).
Leveraging a unique long-term photoidentification dataset, the present study elucidates the impact of additive mortality events on the social structure of a long-lived, highly social species using longitudinal individual life histories. Using social network analyses, we investigated association patterns among individuals over time to assess how a period of severe demographic stress impacted the survival and sociality of Crozet killer whales. Under the assumption of optimal group size, we hypothesized that the rapid loss of individuals due to lethal responses of illegal fishers decreased the survival of remaining individuals and forced these individuals to interact with other social groups. The acute fisheries-induced additive mortality that took place at the Crozet Islands provides an opportunity to test this hypothesis and to assess the importance of sociality on the survival of a highly social predator.
Methods
Photoidentification Data.
Individual photoidentification data have been collected since 1964 from two zones: Possession Island (46°24′S, 51°46′E) in the Crozet Archipelago and aboard longliners operating in the Crozet Exclusive Economic Zone (55). Photographs were checked for quality, georeferenced, and archived with an observation number. An observation corresponded to a period of continuous presence of killer whales in the same area paired with photoidentification effort, which ended either because the whales left or because photoidentification was stopped (e.g., due to bad weather). Individual killer whales were identified from unique fin shapes, saddle patches, and the presence of any natural marks (56). Photographs were assigned a quality index (Q) ranging from 0 (low) to 2 (high) based on distance, focus, lighting, and angle criteria. Individuals were assigned a distinctiveness index (M), ranging from 0 (not distinctive) to 2 (highly distinctive), depending on the number and the size of marks. Only photographs with Q ≥ 1 and M ≥ 1 were used to avoid bias due to uncertain identifications (40, 51). However, for each observation, the total number of photographs taken was used as an index of the photographic effort, which may influence the proportion of individuals identified out of all individuals present during that observation. Individuals photographed only once during the study were excluded. The number of usable individual identifications varied between years, primarily because the photographic effort was opportunistic. As a result, years with negligible photographic effort (<10 photographs, i.e., all years before 1987, and years 1991, 1992, and 1995) were excluded. In total, 6,087 observations between 1987 and 2014 were analyzed.
Social Analyses.
To investigate social relationships among individuals, we used the half weight index (HWI), which is buffered against bias caused by undetected individuals during an observation period (57–59). The HWI quantifies the association frequency between a pair of individuals and ranges from 0 (never together) to 1 (always together). Two individuals were considered associated when they were identified in the same observation. Permutation tests (with 20,000 permutations of group membership within sampling periods) were used to assess the statistical significance of association (random, preferred, or avoided) (58). This procedure was repeated thrice to ensure the stability of the P value. When the SD of the observed HWI was significantly higher than that of the permutation HWI, associations were preferred (58). The mean HWI (the sum of the HWI of an individual divided by the number of individuals it had associated with) and the mean sum of the associations (equivalent to an index of gregariousness) were calculated for each individual over the study period (1987–2014; 221 identified killer whales) and for three subperiods depending on periods of presence of illegal vessels fishing Patagonian toothfish in Crozet. These subperiods are: (i) preillegal fishing (1987–94; 71 identified killer whales); (ii) illegal fishing (1996–2002; 57 identified killer whales); and (iii) postillegal fishing (2002–14; 147 identified killer whales). Social analyses were performed using the software SOCPROG 2.7 (60).
Generalized additive mixed models (GAMM) were used to investigate changes in the mean HWI over the study period with a beta likelihood, which is adapted for continuous variables bounded between 0 and 1 (61). Photographic effort was included in all models with an interaction with the year (62). Year was added as a fixed variable to test for difference over time, and individual identity was included as a random variable. Periods (preillegal fishing, illegal fishing, and postillegal fishing) were also added as a fixed variable. GAMMs were fitted with package mgcv version 1.8–22 in the software R 3.4.2 (63, 64). The best model was then determined by Akaike Information Criterion (65).
Demographic Analyses.
To investigate the effects of social relationships, social group composition, and size on survival probability we used capture–mark–recapture (CMR) models. Apparent survival rates (i.e., the complement of mortality and emigration out of the study area) and recapture probabilities of marked individuals over the whole study period (1987–2014) were estimated using the Cormack–Jolly–Seber model (CJS) (66). Sampling occasions were defined as years (n = 25) and an individual was considered captured in a sampling occasion if it was photoidentified during at least one observation conducted in the corresponding year. The CMR dataset included 221 individuals. The CJS model Φ(t)p(t) is a time-dependent one, where the survival probability (Φ) and the recapture probability (p) vary over time. The underlying assumptions of the CJS model are (67, 68): (i) recapture probability (p) is the same for each individual at occasion t; (ii) survival probability (Φ) is identical for all of the marked individuals between occasion t and t + 1; (iii) individual markings do not change and are not overlooked; (iv) observations (capture occasions) are short compared with the time interval between successive observations (capture occasions); (v) emigration is permanent; and (vi) individuals are independent regarding survival and capturability.
Accurate inferences from the CJS model require that these underlying assumptions hold. Assumptions (iii) and (iv) were considered validated because of the photoidentification protocol. Temporary emigration is documented in the Crozet population, but these events are believed to be short compared with the intervals between capture occasions. Furthermore, only six individuals have been sighted in another population and resighted in the Crozet population, representing 2.7% of the total population studied. Therefore, assumption (v) was considered validated and analyses were not considered biased by temporary emigration (69). Goodness-of-fit tests were used to check assumptions (i) and (ii). These tests were performed on the CJS model Φ(t)p(t) with the program U-CARE 2.3.4 (70) and did not reveal transience effects (Test 3.SR: χ2 = 13.72, df = 16, P = 0.620), indicating that assumption (ii) was fulfilled. Goodness-of-fit tests, however, revealed a trap-dependence effect (Test 2.CT: χ2 = 44.09, df = 18, P < 0.001), suggesting violation of assumption (i): animals were more likely to be resighted if they have already been sighted once. This “trap happiness” was accounted for in our starting model by differentiating the recapture probabilities of individuals captured at time t − 1 from those not captured at time t − 1.
Since killer whales live in social groups and form clusters, individuals cannot be assumed independent, and assumption (vi) was potentially violated. To model this dependence within social groups, we investigated the dependence of fate (apparent survival and recapture probabilities) between members of social groups using social groups as a random effect (71). To quantify between-social groups variation in survival or recapture probabilities, we considered the following model: , where is the mean survival or recapture probability on the logit scale and the s are independent and identically distributed as a univariate normal distribution , where is the variance of the social group random effect.
Because capture probability may be effort dependent, we added annual photographic effort as a time-varying covariate on capture probability. We tested for the effects of association frequency between pairs of individuals (HWI) and social group size on apparent survival. For the effect of HWI, we built a model where survival between 1987 and 2014 was modeled as a function of HWI using a logit link function: , where is an intercept, is a slope, and is the HWI for individual i calculated between 1987 and 2014. When or , the covariate HWI has a negative or positive effect, respectively, on survival probability. For the effect of social group size, we built a model where social group size was included as a group effect since social group size was an integer variable ranging from 1 to 9. The inclusion of fixed (observation effort, HWI, and social group size) and random effects (time and group ID) was assessed using a Bayesian model selection approach and the widely applicable information criterion (WAIC). All models (see complete model list in Table 2) were fitted with Jags version 4–8 and package rjags (jags code in SI Appendix; ref. 72). We used weakly informative priors: normal priors centered on zero with scale set to 1.5 for intercept parameters and scale set to 0.5 for slope parameters. Half-normal priors with scale set to 0.5 were used for SD parameters. Three chains were run with a warmup of 10,000 iterations, followed by another 10,000 iterations (with a thinning factor of 10). Parameter convergence was assessed with Gelman–Rubin statistics. Posterior inferences were based on the pooled sample of 3,000 values (1,000 per chain).
Table 2.
Model selection for the survival and recapture of Crozet killer whales using data between 1987 and 2014
| Model | Survival | Recapture | k (SE) | WAIC (SE) | Slope (SE) |
| M0 | I | t * m | 59.8 (2.8) | 1,449.8 (46.4) | |
| M1 | I | t + m | 48.9 (2.7) | 1,437.4 (47.6) | |
| M2 | I | effort + m | 20.7 (1.0) | 1,852.9 (45.2) | |
| M3 | HWI | t + m | 45.4 (2.7) | 1,370.2 (46.7) | +0.774 (0.100) |
| M4 | Size | t + m | 46.4 (2.7) | 1,393.3 (46.6) | |
| M5 | r(socialgroup) | t + m | 57.8 (3.0) | 1,377.2 (47.6) | |
| M6 | HWI + r(socialgroup) | t + m | 53.0 (2.8) | 1,340.6 (46.8) | +0.925 (0.133) |
| M7 | size + r(socialgroup) | t + m | 55.5 (2.9) | 1,362.0 (47.1) | |
| M8 | I | t + m + r(socialgroup) | 94.9 (4.3) | 1,314.1 (45.2) | |
| M9 | HWI | t + m + r(socialgroup) | 81.8 (3.9) | 1,261.8 (45.3) | +0.731 (0.100) |
| M10 | HWI + size | t + m + r(socialgroup) | 80.0 (3.8) | 1,252.7 (45.2) | +0.645 (0.103) |
Fixed effects considered were: i, constant parameter; t, time effect; m, trap-dependence effect; HWI, association index effect; size, social group size effect. r(socialgroup) represents the social group random effect; k is the estimated number of parameters in the model; + is an additive effect; * is an interaction effect.
Data Availability.
The datasets analyzed in this study are available in Figshare at https://figshare.com/s/f1147c49815b57963144.
Results
Social Analyses.
Permutation tests indicated that associations between individuals were not random (P < 0.0001). The generalized additive mixed model including the period dummy variable (three levels: preillegal fishing, illegal fishing, and postillegal fishing) had a lower AIC (AIC = −3,432.84) than the model including the year (AIC = −3427.16). Both the mean HWI and the mean sum of associations decreased from the preillegal fishing period (0.10 ± 0.05 and 7.80 ± 3.46, respectively) to the illegal fishing period (0.05 ± 0.03 and 4.14 ± 1.88 respectively; Table 1). While the mean HWI decreased further during the postillegal fishing period (0.04 ± 0.02), the mean sum of associations during the postillegal fishing period (7.54 ± 3.21) returned to values close to the preillegal fishing period level (7.80 ± 3.46).
Table 1.
Social parameters of the Crozet Islands killer whale population before, during, and after illegal fishing
| Period | HWI | Sum of the associations |
| Preillegal fishing (1987–94) | 0.10 ± 0.05 | 7.80 ± 3.46 |
| Illegal fishing (1996–2002) | 0.05 ± 0.03 | 4.14 ± 1.88 |
| Postillegal fishing (2003–14) | 0.04 ± 0.02 | 7.54 ± 3.21 |
Values are mean ± SD
Demographic Analyses.
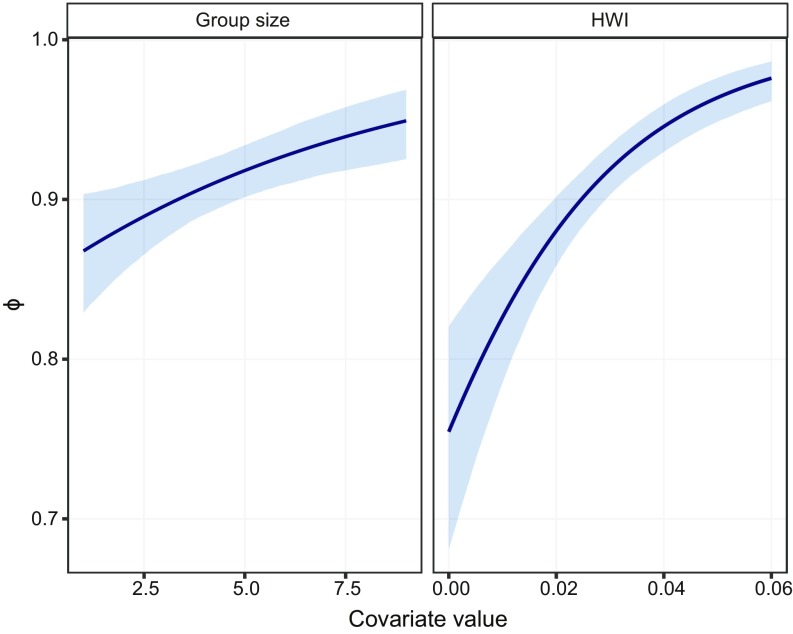
Recapture probability was time dependent, and trap dependence was best modeled as an additive effect (Table 2). There was no effect of photographic effort on recapture probability (Table 2). Survival declined from 0.959 ± 0.032 during the preillegal fishing period down to 0.868 ± 0.034 during the illegal fishing period. This corresponded to a threefold increase in mortality rate (from 0.041 to 0.132). Since the killer whale population during the preillegal fishing period was estimated at 98 individuals (95% confidence interval: 70–156; 39), this corresponded to the disappearance of 61 (44–98) killer whales during the period of illegal fishing. Survival probability was positively related to HWI (Fig. 1 and Table 2). Social group size positively affected survival (standardized effect size: 0.29 [0.11–0.48]), but variation in survival was best explained by HWI (standardized effect size: 0.65 [0.44–0.85]; Table 2). Model with a social group random effect on survival was not supported by WAIC: survival probabilities of individuals associated in social groups could be assumed independent once the effect of HWI was considered. For recapture, there was high heterogeneity in recapture rates between social groups (estimated group-level SD: 1.26 [0.92–1.68]).
Fig. 1.
Relationship between apparent survival probability of Crozet killer whales and group size or the HWI over 25 y. The light blue area represents the 95% confidence interval.
Discussion
This study shows the significant consequences of an additive mortality event on the social structure of a highly social species. The rapid loss of individuals across multiple social groups resulted in a lower survival of the remaining individuals paired with a disruption of the existing social organization. Together, these findings demonstrated the important link between demographic parameters and sociality, and how the disruption of social systems may lead to prolonged negative effects for years after a period of demographic stress.
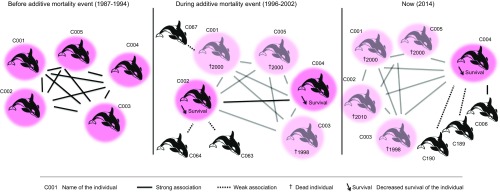
The additive mortality generated through lethal responses during interactions of killer whales with illegal fishing vessels in Crozet waters resulted in the decrease of two key sociality parameters in killer whales. After this additive mortality event, the surviving individuals had a low HWI (an index of associations among individuals), but a high sum of associations (an index of gregariousness). These results suggest that the surviving Crozet killer whales may have increased their number of associations with other individuals opportunistically (i.e., through weaker bonds) in response to losing individuals from their social groups. For example, before illegal fishing took place, the C001 social group was composed of five individuals, including four females and one male (Fig. 2). One of the oldest females (C003) of the group died in 1998 during the illegal fishing period. This event was followed by remaining individuals creating new, but weak, bonds with individuals from other social groups. Toward the end of the illegal fishing period in 2000, male C001 and female C005 died. In 2010, C002, the second oldest female of the initial unit C001, died too, leaving C004 as the only individual remaining in that unit. In recent years, C004 associated with others new individuals, through strong bonds with C006 and weak bonds with C189 and C190 (Fig. 2).
Fig. 2.
Graphical summary of the findings: consequences of an additive mortality event on the social structure and survival of killer whales (example of the C001 social unit). Individuals are named with the code “CXXX”. The social unit is represented with pink color; full lines represent strong associations, whereas dashed lines represent weak associations. Dead individuals are represented in faded color with a cross and the year of their death. An arrow labeled with “Survival” next to an individual indicates a decrease in the survival of that individual.
This behavioral response of increased interactions with outgroup individuals, which was reported in other social mammals such as baboons (Papio hamadryas ursinus) (73), may be explained by in-group individuals trying to maintain a functional group size. As reported in many social predators, group size is a critical driver of foraging success, net energetic gain, and therefore survival. In group-foraging African wild dogs (Lycaon pictus), increased group size increased both prey size and the success of prey capture attempts (74). False killer whales (Pseudorca crassidens) are social animals whose cooperative hunting techniques may fail to sustain a high enough foraging success following a social disruption such as an additive mortality event (75, 76). Cooperative hunting techniques are also found in killer whales, which often forage in groups using various techniques including coordinated attacks (9, 11, 16, 49, 77–79). Group foraging is common among the Crozet killer whales, whose diet includes large prey such as southern elephant seals (Mirounga leonina) and baleen whales (42, 43).
However, in the present study, group size was not the primary determinant of adult killer whale survival. Models indicated that the strength of associations was a better predictor of survival, suggesting a major influence of other sociality factors. Among these, alloparental care is assumed to play an important role in the survival of juveniles. In group-living mammals like meerkats (Suricata suricatta), the growth of pups is positively correlated to the number of carers per pup, demonstrating the importance of a sufficient group size (22). In sperm whales (Physeter macrocephalus), sociality was shown to be primarily driven and maintained by kinship selection and the alloparental care function provided by closely related adult females to calves within matrilineal females/immatures social units (80, 81). The likely alteration of this female sociality caused by the rapid loss of individuals was proposed as one of the factors potentially contributing further to the decline of some populations (80, 82, 83). Female killer whales are also known to take care of their kin, protect them during attacks, transmit ecological knowledge like hunting techniques, and share prey with them (9, 47, 84–87). These factors may have driven the remaining Crozet killer whales to try to restore an efficient group size.
While individuals may have increased their interactions with individuals from other groups to overcome reduced group size, these interactions were loose and individuals in the social groups most impacted by illegal fishing did not reassociate in stable groups. The positive correlation between HWI and survival suggests that the inability of these individuals to rebuild long-term high association levels with others after the additive mortality event decreased their survival, likely through a decrease in their foraging success. We speculate that the surviving individuals did not reassociate in stable groups for two reasons. First, reassociating with other social groups may disrupt an already-optimal group size and increase costs such as intraspecific competition (8, 17) or disease transmission (88). Second, in a kin-based social system such as a matrilineal system, kin selection—which implies enhancing fitness of relatives (5)—may prevent new individuals from permanently joining other groups with which they share low relatedness. Such kin selection processes were reported in killer whales and translated into group-specific dialects and apprenticeship or prey sharing behaviors directed toward relatives only (45, 49, 88–94).
Consequently, the decreased survival of lone individuals, or those in reduced groups, that were unable to reassociate in stable groups, may have contributed to the low overall adult apparent survival of the Crozet population after illegal fishing (40). Survival may have been further decreased by the loss of individuals with key roles in the demographic performance and functionality of groups (28, 29). For instance, in matrilineal social systems of African elephants, the leadership and ecological knowledge of matriarchs influences the survival of other group members (24, 28, 30). In killer whales, leadership by postreproductive females was found to positively affect the fitness of kin, probably through the transfer of ecological knowledge, and this effect was especially prominent during periods of food shortage (44, 46). The age of most female killer whales that died during the period of illegal fishing at Crozet and the role of these females in groups are not known. However, with a 60% decline in killer whale abundance from 1988 to 2000 (39), it is likely that some groups did lose key individuals, further decreasing the fitness of surviving individuals in the Crozet population. Additionally, factors such as age, sex, body size, dominance, or presence/absence of close kin may also have played a role in the variation in HWI and survival during the study period. However, we did not have sufficient information on these factors to allow their inclusion in the analyses.
In summary, this study (i) highlighted the critical role of social structure in the response of highly social species to additional mortality events; and (ii) stressed the importance of stable associations and group size in social animals. The disruption of the latter two features directly affected the survival of individuals, likely through a decrease of foraging success. Together, these results have major conservation implications as they indicate that the recovery of small populations of highly social species is conditioned on both the severity of an additive mortality event and the extent to which social units are disrupted.
Supplementary Material
Acknowledgments
We thank the fieldworkers of Possession Island, the fishery observers, and crews of fishing vessels for their help in collecting photo-ID data. We are also grateful to the fishery observers who collected sighting data from fishing vessels and to the Museum National d’Histoire Naturelle (Prof. Guy Duhamel, Nicolas Gasco, and Charlotte Chazeau) for facilitating access to these data. This work was conducted as part of the 109 program (Principal Investigator, Henri Weimerskirch) with the Institut Polaire Francais. Funding and logistic support were provided by the Terres Australes et Antarctiques Francaises, the Reserve Naturelle des Terres Australes, and the Reunion Island Fisheries Companies as part of the ORCADEPRED research program. R.R.R. and C.G. are supported by the International Whaling Commission’s Southern Ocean Research Partnership (Agence National de la Recherche, Fondation d’Entreprise des Mers Australes, Direction des Pêches Maritimes et de l'Aquaculture). P.T. is supported by the Australian Research Council (Linkage Project 160100329).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The datasets analyzed in this study have been deposited in Figshare at https://figshare.com/s/f1147c49815b57963144.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817174116/-/DCSupplemental.
References
- 1.Alexander RD. (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383. [Google Scholar]
- 2.Axelrod R, Hamilton WD (1981) The evolution of cooperation. Science 211:1390–1396. [DOI] [PubMed] [Google Scholar]
- 3.Choe JC, Crespi BJ (1997) The Evolution of Social Behaviour in Insects and Arachnids (Cambridge Univ Press, Cambridge, UK: ). [Google Scholar]
- 4.Ellis S, et al. (2017) Mortality risk and social network position in resident killer whales: Sex differences and the importance of resource abundance. Proc R Soc B 284:20171313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton WD. (1964) The genetical evolution of social behaviour. II. J Theor Biol 7:17–52. [DOI] [PubMed] [Google Scholar]
- 6.Silk JB. (2007) The adaptive value of sociality in mammalian groups. Philos Trans R Soc Lond B Biol Sci 362:539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberts SC. (2019) Social influences on survival and reproduction: Insights from a long-term study of wild baboons. J Anim Ecol 88:47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JL. (1982) Optimal group size in territorial animals. J Theor Biol 95:793–810. [Google Scholar]
- 9.Ford JK, Ellis GM (2006) Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Mar Ecol Prog Ser 316:185–199. [Google Scholar]
- 10.Guimarães PR Jr, et al. (2007) Vulnerability of a killer whale social network to disease outbreaks. Phys Rev E Stat Nonlin Soft Matter Phys 76:042901. [DOI] [PubMed] [Google Scholar]
- 11.Guinet C, Barrett-Lennard LG, Loyer B (2000) Co-ordinated attack behavior and prey sharing by killer whales at Crozet Archipelago: Strategies for feeding on negatively-buoyant prey. Mar Mammal Sci 16:829–834. [Google Scholar]
- 12.Macdonald DW. (1983) The ecology of carnivore social behaviour. Nature 301:379–384. [Google Scholar]
- 13.Terhune JM, Brillant SW (1996) Harbour seal vigilance decreases over time since haul out. Anim Behav 51:757–763. [Google Scholar]
- 14.Wilkinson GS. (1990) Food sharing in vampire bats. Sci Am 262:76–83. [Google Scholar]
- 15.Archetti M. (2009) The volunteer’s dilemma and the optimal size of a social group. J Theor Biol 261:475–480. [DOI] [PubMed] [Google Scholar]
- 16.Baird RW, Dill LM (1996) Ecological and social determinants of group size in transient killer whales. Behav Ecol 7:408–416. [Google Scholar]
- 17.Caraco T, Wolf LL (1975) Ecological determinants of group sizes of foraging lions. Am Nat 109:343–352. [Google Scholar]
- 18.Cohen S. (2004) Social relationships and health. Am Psychol 59:676–684. [DOI] [PubMed] [Google Scholar]
- 19.Durant SM, Kelly M, Caro TM (2004) Factors affecting life and death in Serengeti cheetahs: Environment, age, and sociality. Behav Ecol 15:11–22. [Google Scholar]
- 20.House JS, Landis KR, Umberson D (1988) Social relationships and health. Science 241:540–545. [DOI] [PubMed] [Google Scholar]
- 21.Nuñez CM, Adelman JS, Rubenstein DI (2014) Sociality increases juvenile survival after a catastrophic event in the feral horse (Equus caballus). Behav Ecol 26:138–147. [Google Scholar]
- 22.Russell AF, et al. (2002) Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J Anim Ecol 71:700–709. [Google Scholar]
- 23.Silk JB, Alberts SC, Altmann J (2003) Social bonds of female baboons enhance infant survival. Science 302:1231–1234. [DOI] [PubMed] [Google Scholar]
- 24.Archie EA, Chiyo PI (2012) Elephant behaviour and conservation: Social relationships, the effects of poaching, and genetic tools for management. Mol Ecol 21:765–778. [DOI] [PubMed] [Google Scholar]
- 25.Jackson JB, et al. (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–637. [DOI] [PubMed] [Google Scholar]
- 26.Lemieux AM, Clarke RV (2009) The international ban on ivory sales and its effects on elephant poaching in Africa. Br J Criminol 49:451–471. [Google Scholar]
- 27.Poole JH, Thomsen JB (1989) Elephant are not beetles: Implications of the ivory trade for the survival of the African elephant. Oryx 23:188–198. [Google Scholar]
- 28.McComb K, Moss C, Durant SM, Baker L, Sayialel S (2001) Matriarchs as repositories of social knowledge in African elephants. Science 292:491–494. [DOI] [PubMed] [Google Scholar]
- 29.Williams R, Lusseau D (2006) A killer whale social network is vulnerable to targeted removals. Biol Lett 2:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittemyer G, Douglas-Hamilton I, Getz WM (2005) The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim Behav 69:1357–1371. [Google Scholar]
- 31.Azevedo AF, et al. (2017) The first confirmed decline of a delphinid population from Brazilian waters: 2000–2015 abundance of Sotalia guianensis in Guanabara Bay, south-eastern Brazil. Ecol Indic 79:1–10. [Google Scholar]
- 32.Avila IC, Kaschner K, Dormann CF (2018) Current global risks to marine mammals: Taking stock of the threats. Biol Conserv 221:44–58. [Google Scholar]
- 33.Dans SL, Koen Alonso M, Pedraza SN, Crespo EA (2003) Incidental catch of dolphins in trawling fisheries off Patagonia, Argentina: Can populations persist? Ecol Appl 13:754–762. [Google Scholar]
- 34.Fraker MA. (2013) Killer whale (Orcinus orca) deaths in Prince William Sound, Alaska, 1985-1990. Hum Ecol Risk Assess 19:28–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewison RL, Crowder LB, Read AJ, Freeman SA (2004) Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol 19:598–604. [Google Scholar]
- 36.Moore JE, et al. (2009) A review of marine mammal, sea turtle and seabird bycatch in USA fisheries and the role of policy in shaping management. Mar Policy 33:435–451. [Google Scholar]
- 37.Roche C, Guinet C, Gasco N, Duhamel G (2007) Marine mammals and demersal longline fishery interactions in Crozet and Kerguelen exclusive economic zones: An assessment of depredation levels. CCAMLR Sci 14:67–82. [Google Scholar]
- 38.Tixier P, et al. (2010) Interactions of Patagonian toothfish fisheries with killer and sperm whales in the Crozet islands exclusive economic zone: An assessment of depredation levels and insights on possible mitigation strategies. CCAMLR Sci 17:179–195. [Google Scholar]
- 39.Poncelet É, Barbraud C, Guinet C (2010) Population dynamics of killer whales (Orcinus orca) in the Crozet Archipelago, southern Indian ocean: A mark–recapture study from 1977 to 2002. J Cetacean Res Manage 11:41–48. [Google Scholar]
- 40.Tixier P, et al. (2017) Demographic consequences of fisheries interaction within a killer whale (Orcinus orca) population. Mar Biol 164:170. [Google Scholar]
- 41.Bigg MA, Olesiuk PF, Ellis GM, Ford JKB, Balcomb KC (1990) Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington state. Rep Int Whaling Comm 12:383–405. [Google Scholar]
- 42.Guinet C. (1991) L’orque (Orcinus orca) autour de l’Archipel Crozet comparaison avec d’autres localités. Rev Ecol 46:321–337. [Google Scholar]
- 43.Tixier P. (2012) Déprédation par les orques (Orcinus orca) et les cachalots (Physeter macrocephalus) sur les palangriers à la légine australe dans la ZEE de l’archipel de Crozet. PhD thesis (Aix-Marseille).
- 44.Brent LJN, et al. (2015) Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr Biol 25:746–750. [DOI] [PubMed] [Google Scholar]
- 45.Ford JK. (1991) Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Can J Zool 69:1454–1483. [Google Scholar]
- 46.Foster EA, et al. (2012) Adaptive prolonged postreproductive life span in killer whales. Science 337:1313. [DOI] [PubMed] [Google Scholar]
- 47.Guinet C, Bouvier J (1995) Development of intentional stranding hunting techniques in killer whale (Orcinus orca) calves at Crozet Archipelago. Can J Zool 73:27–33. [Google Scholar]
- 48.Rendell L, Whitehead H (2001) Culture in whales and dolphins. Behav Brain Sci 24:309–324, discussion 324–382. [DOI] [PubMed] [Google Scholar]
- 49.Guinet C. (1992) Comportement de chasse des orques (Orcinus orca) autour des îles Crozet. Can J Zool 70:1656–1667. [Google Scholar]
- 50.Hamer DJ, Childerhouse SJ, Gales NJ (2012) Odontocete bycatch and depredation in longline fisheries: A review of available literature and of potential solutions. Mar Mammal Sci 28:345–374. [Google Scholar]
- 51.Tixier P, Gasco N, Duhamel G, Guinet C (2016) Depredation of Patagonian toothfish (Dissostichus eleginoides) by two sympatrically occurring killer whale (Orcinus orca) ecotypes: Insights on the behavior of the rarely observed type D killer whales. Mar Mammal Sci 32:983–1003. [Google Scholar]
- 52.Guinet C, Tixier P, Gasco N, Duhamel G (2014) Long-term studies of Crozet Island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian toothfish fishery. ICES J Mar Sci 72:1587–1597. [Google Scholar]
- 53.Tixier P, et al. (2019) Importance of toothfish in the diet of generalist subantarctic killer whales: Implications for fisheries interactions. Mar Ecol Prog Ser 613:197–210. [Google Scholar]
- 54.Tixier P, Authier M, Gasco N, Guinet C (2015) Influence of artificial food provisioning from fisheries on killer whale reproductive output. Anim Conserv 18:207–218. [Google Scholar]
- 55.Tixier P, Gasco N, Guinet C (2014) Killer whales of the Crozet Islands: Photoidentification catalogue 2014. (Centre d’Etudes Biologiques de Chizé-CNRS, Villiers en Bois:). [Google Scholar]
- 56.Bigg MA. (1987) Killer Whales: A Study of Their Identification, Genealogy, and Natural History in British Columbia and Washington State (Phantom, Nanaimo, BC: ). [Google Scholar]
- 57.Cairns SJ, Schwager SJ (1987) A comparison of association indices. Anim Behav 35:1454–1469. [Google Scholar]
- 58.Whitehead H. (2008) Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis (Univ Chicago Press, Chicago, IL: ). [Google Scholar]
- 59.Whitehead H. (1997) Analysing animal social structure. Anim Behav 53:1053–1067. [Google Scholar]
- 60.Whitehead H. (2009) SOCPROG programs: Analysing animal social structures. Behav Ecol Sociobiol 63:765–778. [Google Scholar]
- 61.Cribari-Neto F, Zeileis A (2009) Beta regression in R. Available at http://epub.wu.ac.at/726/. Accessed March 6, 2017.
- 62.Bearzi G, Agazzi S, Bonizzoni S, Costa M, Azzellino A (2008) Dolphins in a bottle: Abundance, residency patterns and conservation of bottlenose dolphins Tursiops truncatus in the semi-closed eutrophic Amvrakikos Gulf, Greece. Aquat Conserv 18:130–146. [Google Scholar]
- 63.Team RC (2018) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.4. 2. Released September 28, 2017.
- 64.Wood S, Wood MS (2015) Package ‘mgcv.’ R package version 1:29. Available at https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed October 8, 2018.
- 65.Hurvich CM, Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76:297–307. [Google Scholar]
- 66.Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol Monogr 62:67–118. [Google Scholar]
- 67.Pollock KH, Nichols JD, Brownie C, Hines JE (1990) Statistical inference for capture-recapture experiments. Wildl Monogr, 3–97. [Google Scholar]
- 68.Williams BK, Nichols JD, Conroy MJ (2002) Analysis and Management of Animal Populations (Academic Press, San Diego, CA: ). [Google Scholar]
- 69.Burnham KP. (1993) A theory for combined analysis of ring recovery and recapture data. Marked Individuals in the Study of Bird Population, pp 199–213. [Google Scholar]
- 70.Choquet R, Lebreton J-D, Gimenez O, Reboulet A-M, Pradel R (2009) U-CARE: Utilities for performing goodness of fit tests and manipulating CApture–REcapture data. Ecography 32:1071–1074. [Google Scholar]
- 71.Choquet R, et al. (2013) Estimating demographic parameters from capture–Recapture data with dependence among individuals within clusters. Methods Ecol Evol 4:474–482. [Google Scholar]
- 72.Plummer M. (2018) rjags: Bayesian Graphical Models using MCMC. R package version 4–8. Available at https://cran.r-project.org/web/packages/rjags/rjags.pdf. Accessed February 18, 2019.
- 73.Engh AL, et al. (2006) Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc Biol Sci 273:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Creel S, Creel NM (1995) Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim Behav 50:1325–1339. [Google Scholar]
- 75.Baird RW, et al. (2008) False killer whales (Pseudorca crassidens) around the main Hawaiian Islands: Long-term site fidelity, inter-island movements, and association patterns. Mar Mammal Sci 24:591–612. [Google Scholar]
- 76.Porter JW. (1977) Pseudorca stranding. Oceans 10:8–16. [Google Scholar]
- 77.Ford JK, et al. (2005) Killer whale attacks on minke whales: Prey capture and antipredator tactics. Mar Mammal Sci 21:603–618. [Google Scholar]
- 78.Hoelzel AR. (1991) Killer whale predation on marine mammals at Punta Norte, Argentina; Food sharing, provisioning and foraging strategy. Behav Ecol Sociobiol 29:197–204. [Google Scholar]
- 79.Similä T, Ugarte F (1993) Surface and underwater observations of cooperatively feeding killer whales in northern Norway. Can J Zool 71:1494–1499. [Google Scholar]
- 80.Konrad CM, Gero S, Frasier T, Whitehead H (2018) Kinship influences sperm whale social organization within, but generally not among, social units. R Soc Open Sci 5:180914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gero S, Gordon J, Whitehead H (2013) Calves as social hubs: Dynamics of the social network within sperm whale units. Proc Biol Sci 280:20131113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gero S, Whitehead H (2016) Critical decline of the Eastern Caribbean sperm whale population. PLoS One 11:e0162019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitehead H, et al. (2012) Multilevel societies of female sperm whales (Physeter macrocephalus) in the Atlantic and Pacific: Why are they so different? Int J Primatol 33:1142–1164. [Google Scholar]
- 84.Baird RW, Whitehead H (2000) Social organization of mammal-eating killer whales: Group stability and dispersal patterns. Can J Zool 78:2096–2105. [Google Scholar]
- 85.McAuliffe K, Whitehead H (2005) Eusociality, menopause and information in matrilineal whales. Trends Ecol Evol 20:650. [DOI] [PubMed] [Google Scholar]
- 86.Ward EJ, Parsons K, Holmes EE, Balcomb KC 3rd, Ford JK (2009) The role of menopause and reproductive senescence in a long-lived social mammal. Front Zool 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wright BM, Stredulinsky EH, Ellis GM, Ford JK (2016) Kin-directed food sharing promotes lifetime natal philopatry of both sexes in a population of fish-eating killer whales, Orcinus orca. Anim Behav 115:81–95. [Google Scholar]
- 88.Wald ER, Dashefsky B, Byers C, Guerra N, Taylor F (1988) Frequency and severity of infections in day care. J Pediatr 112:540–546. [DOI] [PubMed] [Google Scholar]
- 89.Ford JK. (1989) Acoustic behaviour of resident killer whales (Orcinus orca) off Vancouver Island, British Columbia. Can J Zool 67:727–745. [DOI] [PubMed] [Google Scholar]
- 90.Ford JK, Fisher HD (1983) Group-specific dialects of killer whales (Orcinus orca) in British Columbia. Commun Behav Whales 76:129–161. [Google Scholar]
- 91.Hoelzel AR, Osborne RW (1986) Killer whale call characteristics: Implications for cooperative foraging strategies. Behavioral Biology of Killer Whales, eds Kirkevold BC, Lockhard JS (Alan R. Liss, New York, NY), pp 373–403. [Google Scholar]
- 92.Weiss BM, Ladich F, Spong P, Symonds H (2006) Vocal behavior of resident killer whale matrilines with newborn calves: The role of family signatures. J Acoust Soc Am 119:627–635. [DOI] [PubMed] [Google Scholar]
- 93.Reisinger RR, Hoelzel AR, de Bruyn PJ (2017) Kinship and association in a highly social apex predator population, killer whales at Marion Island. Behav Ecol 28:750–759. [Google Scholar]
- 94.Yurk H, Barrett-Lennard L, Ford JKB, Matkin CO (2002) Cultural transmission within maternal lineages: Vocal clans in resident killer whales in southern Alaska. Anim Behav 63:1103–1119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are available in Figshare at https://figshare.com/s/f1147c49815b57963144.