Abstract
Disorders of sleep and wakefulness occur in the majority of individuals who have experienced traumatic brain injury (TBI), with increased sleep need and excessive daytime sleepiness often reported. Behavioral and pharmacological therapies have limited efficacy, in part, because the etiology of post-TBI sleep disturbances is not well understood. Severity of injuries resulting from head trauma in humans is highly variable, and as a consequence so are their sequelae. Here, we use a controlled laboratory model to investigate the effects of TBI on sleep-wake behavior and on candidate neurotransmitter systems as potential mediators. We focus on hypocretin and melanin-concentrating hormone (MCH), hypothalamic neuropeptides important for regulating sleep and wakefulness, and two potential downstream effectors of hypocretin actions, histamine and acetylcholine. Adult male C57BL/6 mice (n=6–10/group) were implanted with EEG recording electrodes and baseline recordings were obtained. After baseline recordings, controlled cortical impact was used to induce mild or moderate TBI. EEG recordings were obtained from the same animals at 7 and 15 days post-surgery. Separate groups of animals (n=6–8/group) were used to determine effects of TBI on the numbers of hypocretin and MCH-producing neurons in the hypothalamus, histaminergic neurons in the tuberomammillary nucleus, and cholinergic neurons in the basal forebrain. At 15 days post-TBI, wakefulness was decreased and NREM sleep was increased during the dark period in moderately injured animals. There were no differences between groups in REM sleep time, nor were there differences between groups in sleep during the light period. TBI effects on hypocretin and cholinergic neurons were such that more severe injury resulted in fewer cells. Numbers of MCH neurons and histaminergic neurons were not altered under the conditions of this study. Thus, we conclude that moderate TBI in mice reduces wakefulness and increases NREM sleep during the dark period, effects that may be mediated by hypocretin-producing neurons and/or downstream cholinergic effectors in the basal forebrain.
Keywords: Controlled cortical impact, Orexin, Melanin-concentrating hormone, Histamine, Acetylcholine, Inflammation, Trauma
Highlights
-
•
Effects of TBI on mouse sleep-wake behavior and selected neurotransmitters were determined.
-
•
Moderate TBI decreases wakefulness and increases NREM sleep during the dark period.
-
•
Responses to moderate TBI in this model persist for at least 15 days.
-
•
Reduced wakefulness may be due to hypocretinergic and/or cholinergic dysfunction.
1. Introduction
Traumatic brain injury (TBI) is a major public health problem that is considered a silent epidemic because of the long-term cognitive deficits and behavioral and medical complications experienced by survivors. In the United States alone, more than 5.3 million individuals currently suffer from a TBI-related disability (Chew and Zafonte, 2009). The neuropsychiatric consequences of TBI may include sleep disorders, mood disorders, personality changes, and cognitive impairment (Bhalerao et al., 2013). Chronic sleep-wake disturbance is highly prevalent, affecting the majority of individuals who have sustained a TBI (Kempf et al., 2010, Rao and Rollings, 2002). Many TBI patients report regular daytime napping and increased sleep need (Ponsford et al., 2013, Ponsford and Sinclair, 2014), and excessive daytime sleepiness (EDS) occurs in approximately 25–42% of individuals who have suffered TBI (Ponsford and Sinclair, 2014, Sommerauer et al., 2013). The alterations in sleep-wake behavior after TBI may be prolonged, and evident for years after the trauma (Kempf et al., 2010). Despite the debilitating effects of post-TBI sleep-wake disturbances, their etiology is not well understood. Furthermore, current behavioral and pharmacological therapies targeting post-TBI sleep-wake disturbances have only limited efficacy (Chew and Zafonte, 2009, Ouellet et al., 2015, Ponsford and Sinclair, 2014, Rue-Evans et al., 2013, Sheng et al., 2013).
Post-TBI sleep-wake disturbances may be due, in part, to altered neurotransmitter systems that regulate sleep and wakefulness. Neurotransmitter systems implicated in arousal include, among others, hypocretin (a.k.a. orexin) neurons of the lateral hypothalamus (Adamantidis et al., 2007, Brisbare-Roch et al., 2007), cholinergic neurons of the basal forebrain (Arrigoni et al., 2010, Irmak and de Lecea, 2014), and histaminergic neurons in the tuberomammillary nucleus (TMN) (Brown et al., 2001, Brown et al., 2012, Parmentier et al., 2002). Of importance to the etiology of post-TBI disturbances, hypocretin promotes wakefulness and stabilizes the sleep-wake cycle (Kilduff and Peyron, 2000, Krystal et al., 2013, Mochizuki et al., 2004, Taheri et al., 2002, Zeitzer et al., 2006). Activation of hypocretin neurons increases transitions from sleep to wakefulness (Adamantidis et al., 2007) and antagonizing hypocretin induces somnolence (Brisbare-Roch et al., 2007, Hoever et al., 2012, Morairty et al., 2014).
In contrast, melanin-concentrating hormone (MCH) neurons are sleep-promoting (Peyron et al., 2009): intracerebroventricular injection of MCH (Verret et al., 2003) or optogenetic stimulation of MCH neurons increases NREM sleep and REM sleep (Jego et al., 2013, Konadhode et al., 2013), whereas MCH deficient mice sleep less (Tsunematsu et al., 2014, Willie et al., 2008). MCH neurons are intermingled with hypocretin neurons in the lateral hypothalamus, and as such, damage to the hypocretin and/or MCH neurons of the lateral hypothalamus could alter sleep-wake behavior after TBI. Indeed, hypocretin is reduced in the hypothalamus of mice (Willie et al., 2012) and in cerebrospinal fluid of human patients (Baumann et al., 2005) after TBI. Furthermore, the number of hypocretin neurons are reduced in post-mortem brains of patients who died from TBI (Baumann et al., 2009). In cases of fatal TBI in humans, one study found a significant reduction in MCH neurons (Valko et al., 2015), whereas another found that MCH neurons were not affected (Baumann et al., 2009). To our knowledge, numbers of hypocretin or MCH neurons have not been investigated in cases of nonfatal TBI in humans.
Although post-TBI alterations in sleep may be mediated, in part, by direct actions of hypocretin, these changes in arousal state could also be due to actions of modulatory systems downstream of hypocretin. Hypocretinergic neurons project to many brain regions. For example, the histaminergic neurons of the TMN and the cholinergic neurons of the basal forebrain are both strong promoters of wakefulness (Haas et al., 2008, Han et al., 2014), and these brain regions are densely innervated by hypocretinergic projections [reviewed in Arrigoni et al., 2010, Sundvik and Panula, 2015)]. Importantly, these systems may also be perturbed by TBI. Fatal TBI in humans causes a dramatic reduction in numbers of histaminergic neurons (Valko et al., 2015) and a reduction in activity and immunoreactivity of choline acetyltransferase (ChAT), an enzyme essential for acetylcholine synthesis (Dewar and Graham, 1996, Murdoch et al., 1998, Murdoch et al., 2002). To our knowledge, histaminergic and cholinergic neuronal populations have not been studied within the context of sleep-wake disturbance after experimental TBI.
The primary goal of the present study was to determine the effects of TBI on sleep-wake behavior and hypocretin/MCH cell numbers and their downstream targets in mice. We used the controlled cortical impact (CCI) model to induce mild or moderate TBI and determined the time course of effects on these and other outcome measures. We report that sleep is altered and hypocretin and basal forebrain cholinergic cell numbers are reduced in an injury severity-dependent manner. Cell counts for MCH and histamine neurons were not altered by TBI under the conditions of this study. Collectively, these data suggest that the effects of TBI on sleep may be mediated by hypocretinergic and cholinergic mechanisms.
2. Methods
2.1. Animals
Adult male C57BL/6J mice (~3–4 months old at time of use; Jackson Laboratory, Bar Harbor, ME) were group housed until baseline testing or surgery, after which they were single housed. Mice were housed under a 12:12 light:dark cycle at 29±1 °C with food and water provided ad libitum. All procedures involving the use of animals were approved by the University of Washington IACUC in accordance with the US Department of Agriculture Animal Welfare Act and the National Institutes of Health policy on Humane Care and Use of Laboratory Animals.
2.2. Recording apparatus
Sleep-wake behavior of mice was determined based on the electroencephalogram (EEG) and cage activity patterns. EEG signals were amplified, filtered, and recorded for offline processing using custom software written in LabView for Windows (ICELUS, M. Opp, University of Washington; National Instruments, Austin, TX) as previously described (Baracchi and Opp, 2008, Ingiosi et al., 2015). EEG and cage activity records were visually scored in 10-s epochs. Raw EEG signals were subjected to fast Fourier transformation, yielding power spectra between 0.5 and 30 Hz in 0.5-Hz frequency bins. Arousal states were determined as previously described and classified as non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, or wakefulness (WAKE) based upon published criteria [e.g., Baracchi and Opp, 2008; Ingiosi et al., 2015; Sutton and Opp, 2014].
2.3. Experimental design
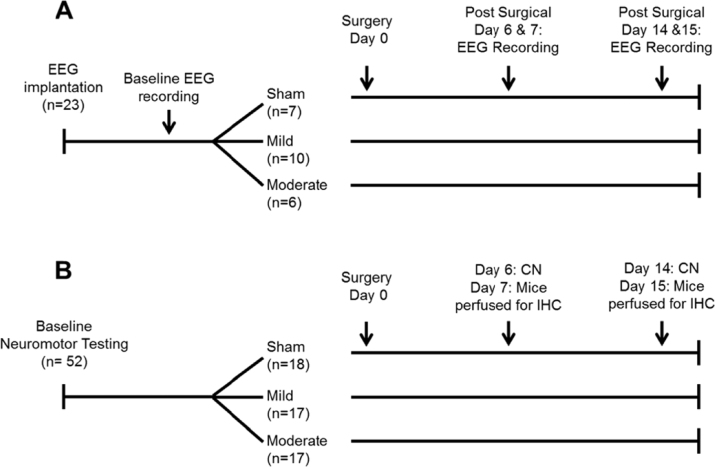
A schematic of the protocols used in Experiments 1–3 is presented in Fig. 1.
Fig. 1.
Schematic representation of the protocols used in Experiments 1, 2 and 3. (A) In Experiment 1, 48 h baseline electroencephalogram (EEG) recordings were obtained from undisturbed mice. Mice were then randomized into one of three surgical conditions: control (sham) surgeries; mild traumatic brain injury (TBI; 0.5 mm controlled cortical impact depth); moderate TBI (1.0 mm controlled cortical impact depth). Recordings of the EEG were obtained from the same animals one and two weeks post-surgery. (B) Animals in Experiments 2 and 3 were used to determine the impact of TBI on neuromotor function (CN: composite neuroscore; Experiment 2) and to provide brain tissue for immunohistochemical assessment of TBI effects on selected neurotransmitters (Experiment 3). After baseline neuromotor testing, animals were randomized into either a sham surgical group (control), mild TBI group or moderate TBI group as in Experiment 1. Composite neuroscores were obtained one and two weeks post-surgery. After each neuromotor testing session, a subset of mice was sacrificed and brains removed for immunohistochemistry (IHC).
2.3.1. Experiment 1: effects of TBI on mouse sleep-wake behavior
For Experiment 1, EEG electrodes were surgically implanted into the skull under isoflurane anesthesia. The leads from the screw electrodes were soldered to the pins of a plastic connector (Digi-Key, ED85100-ND) to allow coupling to the recording system. Dental acrylic (Integrity Caulk, Dentsply) covered the electrodes and formed a headpiece to which the flexible recording tether could be connected. The section of the skull over the left parietal cortex was not covered with dental acrylic at this time. The incision was closed with sutures, and a subcutaneous injection of an analgesic (0.5 mg/kg buprenorphine) was given at the end of the surgery. Mice were allowed 7 days to recover before they were attached to a flexible tether for habituation to the recording system. After 3 days of habituation to the tether and recording environment, 48-h undisturbed baseline recordings were obtained.
After the 48-h baseline recordings, mice were randomized to sham surgeries (n=7; control mice), or to controlled cortical impact (CCI; n=16) to induce TBI as previously described (Febinger et al., 2015). In both groups, a 5-mm diameter craniotomy using a trephine was made over the left parietal cortex, approximately −2 mm relative to bregma and 2.5 mm lateral to the midline. A unilateral impact between lambda and bregma is routinely used in protocols using CCI to induce TBI (Boulet et al., 2013, Boychuk et al., 2016, Febinger et al., 2015, Miller et al., 2014). The skull fragment was removed without disrupting the underlying dura, and TBI was induced in the experimental group. Mice in the experimental group were subjected to CCI using the Leica Impact One system (Richmond, IL) equipped with an electrically-driven 3-mm diameter metal piston controlled by a linear velocity displacement transducer. CCI parameters were: 5.0 m/s impact velocity; 100 ms dwell time; and impact depth of 0.5 mm (mild TBI; n=10) or 1.0 mm (moderate TBI; n=6). Sham (control) animals received identical anesthesia and craniotomy without the CCI injury. A sterilized disc created from a polystyrene weighing boat was placed over the craniotomy and covered with dental acrylic. We (Febinger et al., 2015) and others (Miller et al., 2014) have used this or a similar technique to protect the brain after craniotomy. The incision was closed with sutures and mice were returned to their home cages. All mice received a subcutaneous injection of analgesic (0.5 mg/kg buprenorphine) at the end of the surgery. Animals were closely monitored after surgery and none displayed overt signs of infection.
Additional 48 h recordings were obtained from all mice on days 6–7, and 14–15 post-surgery. As such, a within-subjects protocol was used in which pre- and post-surgery recordings were obtained from each animal. Sleep-wake state was determined and the EEG subjected to fast Fourier transformation to produce power spectra between 0.5 and 30 Hz in 0.5 Hz bins as described previously (Baracchi and Opp, 2008). Power in the delta (0.5–4.5 Hz) frequency band was normalized to the total state-specific power (NREM sleep) summed across all frequency bins from 0.5 to 30 Hz for the light and dark periods and this value was expressed as a percent of total power [see Ingiosi et al. (2015)].
2.3.2. Experiment 2: effects of TBI on neuromotor function
A separate cohort of mice was used to to determine effects of TBI on neuromotor function or neuronal populations. Neuromotor testing performed during the light period disrupts the normal sleep-wake patterns of mice. Because we wanted to determine the effect of TBI on sleep-wake behavior and neuromotor performance at the same time points (i.e. 7 and 15 days post-injury), it was necessary to use different groups of animals. We, and others (Rowe et al., 2014b, Sabir et al., 2015) have used this approach of separate cohorts manipulated in parallel.
The impact of TBI on neuromotor function was determined by calculating a composite neuroscore from neuromotor tests performed during baseline evaluations prior to surgery, and at 7 and 15 days post-surgery. The composite neuroscore was calculated for each mouse, and was derived from measures of forelimb and hindlimb flexion, lateral pulsion reaction, and inclined plane strength/coordination (Fujimoto et al., 2004). Briefly, measures of flexion and lateral pulsion reaction are derived from rodent's reflexes to reach and grasp when lifted or to resist lateral pressure by coordinating movements of all limbs. For the flexion and lateral pulsion reaction tasks, assessments were performed on right and left sides and the ability of the mouse was rated on a scale of 0 (severely impaired) to 4 (no impairment). The inclined angle board consists of a flat acrylic plane that is adjustable from 0° to 90°. The surface is covered with a mat with grooves oriented in the vertical plane so there is traction for the mouse. A mouse must freely stand on the plane for 5 s to successfully complete the assessment at that angle. Baseline testing starts with the plane at an angle of 40°. The angle of the board increases in 2.5° increments until the mouse can no longer stand unassisted. After TBI or sham surgery, assessment of each mouse starts 10° below the lowest baseline angle value for that animal. The maximum angle at which the mouse remains on the angle board is recorded. The post-surgical maximum angle is subtracted from the baseline maximum angle, and a score of 4 recorded if there is no change, 3 for a 2.5° decrease from baseline, 2 for a 5° decrease from the baseline, a 1 for a 7.5° decrease, and 0 for a 10° or more difference from baseline. Larger differences in angle indicate reduced strength and/or motor coordination.
Scores on all components (forelimb and hindlimb flexion; lateral pulsion reaction; angle board) were summed to calculate the composite neuroscore, with the maximum possible score being 28 points. Larger composite neuroscores indicate better performance/less impaired neuromotor skills. The investigator assessing neuromotor function was unaware of the surgical manipulation (sham, CCI) of the animal being tested.
Five days prior to surgery, baseline neuromotor testing was performed. Sham surgeries (n=18) and mild (n=17) or moderate (n=17) CCI surgeries were performed on mice as described in Experiment 1, and animals were placed back into their home cages for recovery. On post-surgical days 6 or 14, mice were again evaluated for CN measures. After the completion of neuromotor testing, animals were returned to their home cages and were sacrificed the next day (7- or 15 days post-TBI). At approximately 6 h after light onset, animals were deeply anesthetized with isoflurane and transcardially perfused with 20 mL chilled phosphate buffered saline, followed by 15 mL chilled 4% paraformaldehyde. The brains were removed and post-fixed in 4% paraformaldehyde for 24 h at 4 °C, and then transferred to a 30% sucrose solution until sectioning and staining. Neuromotor behavior was analyzed in all animals at baseline, at 7 days post-surgery [sham (n=8), mild TBI (n=8), moderate TBI (n=8)], and at 15 days post-surgery [sham (n=10), mild TBI (n=9), moderate TBI (n=9)].
2.3.3. Experiment 3: effects of TBI on numbers of hypocretin and MCH neurons
A subset of mice used in Experiment 2 was randomly selected for use in Experiment 3. Mice were perfused and brains removed for immunohistochemical assessment of TBI effects on selected neurotransmitter systems. Some mice were perfused 7 days post-surgery [sham (n=7), mild TBI (n=8), moderate TBI (n=8)] and some 15 days post-surgery [sham (n=6), mild TBI (n=8), moderate TBI (n=8)].
Brains were sectioned on a Leica cryostat at 40 μm. Sections were stored in cryoprotectant until immunohistochemical staining for hypocretin or MCH. Free-floating sections in a 1:3 series were processed for hypocretin-1 (rabbit anti-mouse orexin-A; H-003-30; Phoenix Pharmaceuticals, Inc.; 1:10,000 dilution) and MCH (rabbit anti-mouse MCH; H-070-47; Phoenix Pharmaceuticals, Inc.; 1:20,000 dilution) as described in Willie et al. (2012).
2.3.4. Experiment 4: impact of TBI on tuberomammillary histaminergic neurons and basal forebrain cholinergic neurons
Two additional groups of mice were used to determine effects of TBI on histaminergic neurons in the tuberomammillary nucleus and cholinergic neurons in the basal forebrain. Based upon the injury severity and time course of TBI effects on hypocretin neurons as determined in Experiment 3, mice (n=7/condition) were subjected to either sham surgery or moderate TBI (1.0 mm controlled cortical impact depth) as previously described. Animals were perfused at 15 days post-surgery and brains removed and sectioned as previously described. IHC for histidine decarboxylase (HDC) and choline acetyltransferase (ChAT) was used to identify histaminergic and cholinergic neurons, respectively. The protocol for HDC was that used in the laboratory of Dr. Thomas Scammell (Beth Israel Deaconess Medical Center/Harvard Medical School). Briefly, free-floating sections in a 1:2 series were processed with rabbit anti-HDC (1: 5000, American Research Products, 03-16045), then incubated with donkey anti-rabbit conjugated to AlexaFluor 555 (Invitrogen; A31572; 1:500 dilution).
IHC for ChAT was performed in a similar fashion as the stains for hypocretin and MCH and similar to previously published protocols (Schmidt and Grady, 1995): free-floating sections in a 1:3 series were processed for ChAT (Abcam; ab18736; 1:2000 dilution). Sections were incubated in biotinylated secondary antibody (Abcam; ab97123; 1:500 dilution), then an avidin-biotin complex, and developed with diaminobenzidine.
2.4. Estimating cell numbers
Cell numbers were estimated using quantitative methods for unbiased stereology (West et al., 1991). Briefly, positively stained cells were visualized on an Olympus BX-51 fluorescent stereoscope using Stereo Investigator 10 (MBF Biosciences, Williston, VT). Colorimetric IHC-processed tissue (stains for hypocretin, MCH, and ChAT) was visualized using brightfield microscopy, whereas fluorescent IHC-processed tissue (stain for HDC) was visualized with fluorescent microscopy.
Hypocretin cell number estimates were obtained from 7 sections spanning approximately −1.20 mm to −2.10 mm from bregma (Paxinos and Franklin, 2001). Estimates of MCH cell numbers were obtained from 11 sections spanning approximately −1.00 mm to −2.30 mm relative to bregma. The contour for the perifornical-lateral hypothalamic region was outlined using a 4x objective. Cells were then counted using the 60x objective and optical fractionator, with a counting frame of 50×50 μm and a grid size of 100×100 μm. ChAT-positive cells were counted in two basal forebrain nuclei using the alternative nomenclature (areas Ch1&2 and Ch3&4) as described by others (Boutros et al., 2015, Mesulam et al., 1983). Areas Ch1&2 and Ch3&4 were outlined using a 4x objective, and then cells were counted using the 60x objective and optical fractionator, with a counting frame of 50×50 μm and a grid size of 100×100 μm. Acetylcholine cell number estimates for Ch1&2 were obtained from 5 sections spanning approximately 1.00 mm to 0.3 mm relative to bregma. Acetylcholine cell number estimates for Ch3&4 were obtained from 11 sections spanning approximately 1.00 mm to −0.70 mm relative to bregma. For HDC-positive cells, the tuberomammillary nucleus was outlined using a 4x objective, then cells were counted as described above. HDC cell number estimates were obtained from 7 sections spanning approximately −2.20 mm to −2.80 mm relative to bregma. All cell counts were obtained from the hemisphere ipsilateral to injury. TBI-induced changes in cellular and tissue outcomes (cell death, inflammatory cytokine expression, presence of immune cells, etc.) are typically most severe in the hemisphere ipsilateral to injury (Hall et al., 2005, Timaru-Kast et al., 2012), and previous studies using unilateral CCI have examined hypocretin neuron number and function in the hypothalamus ipsilateral to injury (Willie et al., 2012).
2.5. Statistical analyses
Two types of statistical analyses were used in this study. We first determined the impact of TBI on outcome measures across time (baseline, 7 days, 15 days), and as such these analyses were designed to reveal differences within each group relative to pre-surgery baseline values. The second type of statistical analysis was used to determine differences between outcome measures with respect to the impact of injury severity (sham, mild TBI, moderate TBI). All analyses were performed using SPSS for Windows (IBM Corporation, Armonk, NY). Data are presented as mean ± SEM, unless otherwise indicated. An alpha value of p<0.05 was accepted as indicating a significant difference between or among groups, whereas an alpha value of 0.05<p<0.1 was considered a trend.
Percent time spent in WAKE, NREM sleep, and REM sleep was evaluated within manipulation (injury severity) in 4 h blocks using a repeated measures ANOVA across three time points (baseline, 7 days, 15 days). Sphericity (an assumption of a repeated measures ANOVA) was tested with Mauchly's test of sphericity. If the assumption of sphericity was violated, the Greenhouse-Geisser correction was used. If significant time effects were detected, post-hoc tests using the Bonferroni correction were used to determine differences between timepoints.
To determine the impact of injury severity on sleep-wake behavior, difference scores were calculated for each parameter by subtracting baseline values from those obtained 7- or 15 days post-surgery. These difference scores were evaluated independently for the 12 h light and dark periods within timepoint (7 days, 15 days) using a one-way ANOVA with injury severity (sham, mild, or moderate) as the between-subjects factor. If significant effects of injury severity were detected, post-hoc comparisons were made using Tukey's HSD to determine differences between injury severity groups.
Normalized NREM delta power was evaluated separately for the 12 h light and dark periods within injury severity group (sham, mild TBI, moderate TBI) using a repeated measures ANOVA across the three time points (baseline, 7 days, 15 days). Assumptions of sphericity were evaluated with Mauchly's test, and the Greenhouse-Geisser correction used if necessary. If significant time effects were detected, post-hoc tests using the Bonferroni correction were used to determine differences between timepoints.
Composite neuroscores (CN) were evaluated using a one-way ANOVA within timepoint (7 days, 15 days) with injury severity (sham, mild TBI, moderate TBI) as the independent variable. If significant effects of injury severity were detected, post-hoc comparisons were made using Tukey's HSD to determine differences between injury severity groups.
Estimated numbers of hypocretin- or MCH-positive cells were evaluated with a two way ANOVA with timepoint (7 days, 15 days) and injury severity (sham, mild TBI, moderate TBI) as factors. If significant effects of timepoint or injury severity were detected, post-hoc comparisons by Tukey's HSD were used to determine differences between groups. Statistical evaluations of estimated numbers of histamine and acetylcholine neurons were made using one-way ANOVA with injury severity (sham, moderate) as the independent variable.
3. Results
3.1. Experiment 1: effects of TBI on mouse sleep-wake behavior
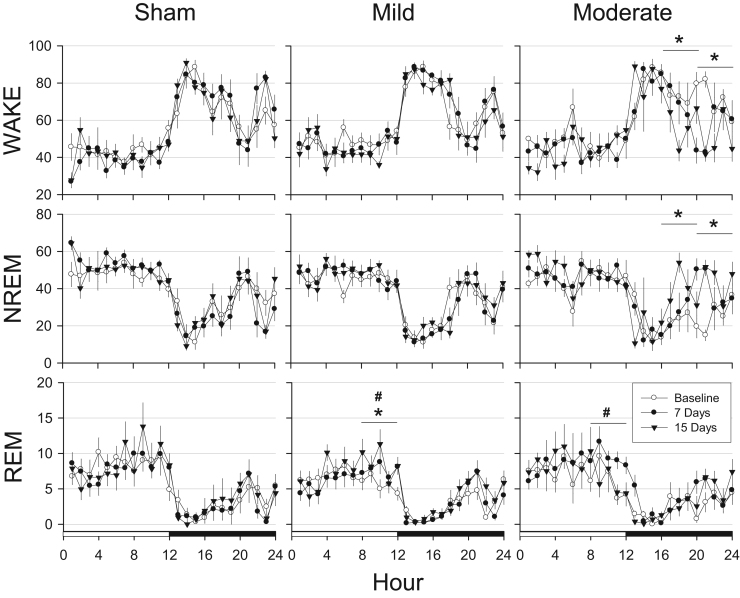
To determine the time course of responses to TBI, sleep-wake behavior was evaluated in 4 h time blocks across 24 h recording periods with a repeated measures ANOVA. Time spent in NREM sleep, REM sleep, and wakefulness was not altered in control mice subjected to sham surgery under the conditions of this study (Fig. 2). Sleep-wake behavior was not substantively altered in mice subjected to mild TBI (0.5 mm controlled cortical impact depth; Fig. 2), although there were some modest but statistically significant differences in REM sleep [hours 9–12 [F(2,78)=7.248, p=.001] and 13–16 [F(2,78)=3.839, p=0.026] after mild TBI (Fig. 2). In contrast, sleep-wake behavior of mice subjected to moderate TBI (1.0 mm controlled cortical impact depth) was dramatically altered (Fig. 2). Mice subjected to moderate TBI spent less time spent in wakefulness during the dark period [hours 17–20: F(2,46)=3.384, p=0.043; hours 21–24: F(2,46)=6.497, p=0.003]. For both of these 4-h time blocks, the reduction in wakefulness at 15 days post-surgery differed significantly from the same time blocks during baseline (hours 17–20, p=0.034; hours 21–24, and 0.004). These mice had corresponding increases in NREM sleep during the same periods [hours 17–20: F(2,46)=4.142, p=0.022; hours 21–24: F(2,46)=6.194, p=0.004], which was due to significant differences between baseline and fifteen days post-surgery (hours 17–20, p=0.019; hours 21–24, p=0.005). REM sleep of mice subjected to moderate TBI increased during one 4-h time block [hours 9–12: F(2,46)=6.361, p=0.004], and post-hoc tests revealed these differences were due to values obtained 7 days post-surgery.
Fig. 2.
Traumatic brain injury alters sleep in an injury-dependent manner. Values are mean (±SEM) percent recording time spent in wakefulness (WAKE), non-rapid eye movement (NREM) sleep, or rapid eye movement (REM) sleep by control mice (sham surgeries, n=7), or those subjected to mild (0.5 mm controlled cortical impact depth, n=10) or moderate (1.0 mm controlled cortical impact depth, n=6) traumatic brain injury. Baseline recordings were obtained from undisturbed animals prior to sham surgeries and surgical procedures to induce injury. The open and filled bars on the X-axis indicate light and dark periods, respectively. Statistical analyses were performed on 4 h time blocks. Statistically significant differences are indicated as: #=p<0.05 baseline vs. 7 days post-surgery; *=p<0.05 baseline vs. 15 d days post-surgery.
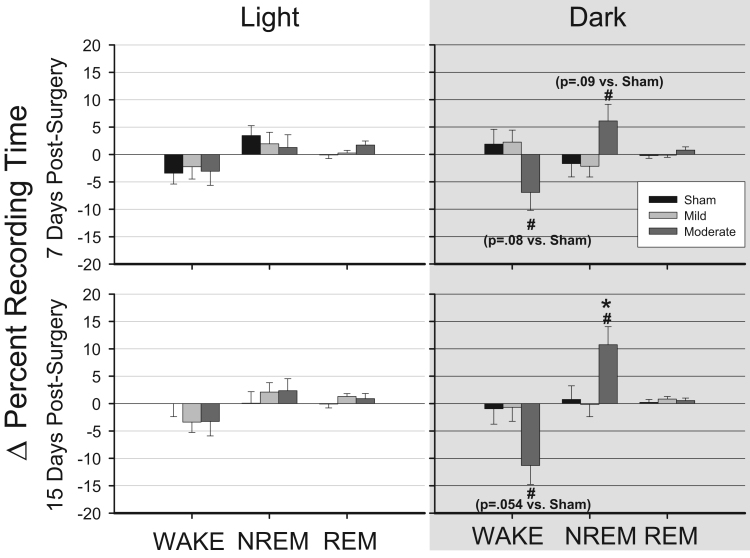
To determine the impact of increasing injury severity on sleep-wake behavior, we directly compared differences between animals subjected to mild TBI and those subjected to moderate TBI. We first calculated hourly difference scores for each animal by subtracting values obtained after surgery (sham, mild TBI, moderate TBI) from corresponding baseline (pre-surgery) values. These hourly difference scores were compared among conditions independently for the 12 h light periods and 12 h dark periods (Fig. 3). No differences with respect to the impact of TBI on sleep were revealed among any of the conditions during the light period. At 7 days post-surgery, there were significant differences among groups during the dark period in time spent awake [F(2, 273)=3.389, p=0.035] and in NREM sleep [F(2, 273)=3.275, p=0.039] (Fig. 3). Post hoc tests indicated that mice subjected to moderate TBI spent less time in wakefulness than did mice subjected to mild TBI (p=0.041), and they tended to spend less time awake than did mice that had sham surgeries (p=0.079). Similarly, mice subjected to moderate TBI spent more time in NREM sleep than mice subjected to mild TBI (p=0.044). Significant differences in sleep-wake behavior among groups persisted for at least 15 days, specifically in time spent awake [F(2, 273)=3.831, p=0.023] and in NREM sleep [F(2, 273)=4.739, p=0.009] during the dark period. Post hoc tests revealed that moderately injured animals spent less time awake than did mildly injured animals (p=0.029), and there was a trend less time awake compared to sham animals (p=0.054). Mice subjected to moderate TBI spent more time in NREM sleep compared to those subjected to mild TBI (p=0.011) or to sham surgeries (p=0.036). No significant differences among groups were observed in number of transitions between sleep-wake states at either of the post-surgical time points (data not shown).
Fig. 3.
Traumatic brain injury decreases wakefulness and increases non-rapid eye movement sleep. Percentage of time spent in wakefulness (WAKE), non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) was determined during the 12 h light and 12 h dark period 7 days and 15 days after control surgeries (sham, n=7), or after mild (0.5 mm controlled cortical impact depth, n=10), and moderate (1 mm controlled cortical impact depth, n=6) traumatic brain injury. Values are means (±SEM) expressed as change in percent recording time relative to undisturbed baseline (represented as the zero line). Statistically significant differences are indicated as: *=p<0.05 vs. sham (control); #=p<0.05 vs. mild TBI. Statistical trends for differences between moderate TBI and sham (control) are given in parentheses.
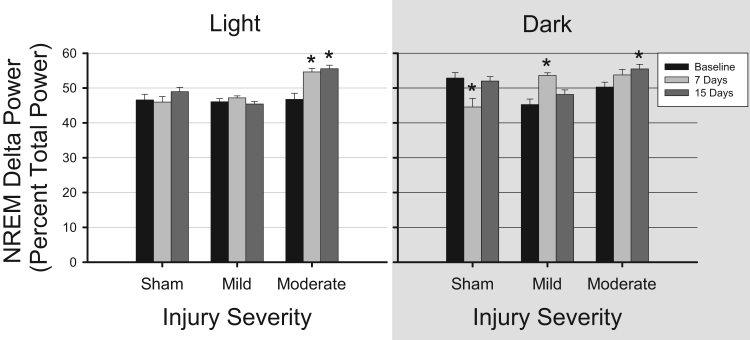
NREM delta power (0.5–4.5 Hz) was normalized by expressing each 0.5 Hz frequency bin as a percentage of total power. These values were then evaluated during the 12 h light and dark periods (Fig. 4). There were no differences in this measure of spectral characteristics during the light period in EEGs obtained from mice subjected to sham surgery or mild TBI. However, NREM delta power during the light period significantly increased in animals subjected to moderate TBI (1.0 mm cortical impact depth) [F(1.572, 99.058)=14.15, p<0.001]. Post-hoc tests revealed that NREM delta power significantly increased 7 days (p=0.002) and 15 days (p<0.001) post-surgery compared to baseline. During the dark period, NREM delta power was significantly reduced in mice subjected to sham surgeries [F(2,106)=8.955, p<0.001) due to differences between baseline and 7 days (p=0.003). Spectral analysis of the EEG of mice subjected to mild TBI indicated increased NREM delta power [F(1.723, 91.3)=16.204, p<0.001], due to changes that occurred 7 days post-surgery (p=0.01, Fig. 4). NREM delta power increased in mice subjected to moderate TBI [F(2, 96)=4.943, p=0.009], an effect due to changes 15 days post-surgery (p=0.015).
Fig. 4.
Traumatic brain injury increases delta power during non-rapid eye movement sleep. Power in the electroencephalogram (EEG) delta frequency band (0.5–4.5 Hz) was determined during the light period and dark period from artifact-free state-specific epochs. Recordings were obtained during pre-surgery baseline conditions, and 7 days and 15 days after control surgeries (sham, n=7), mild (0.5 mm controlled cortical impact depth, n=10), and moderate (1.0 mm controlled cortical impact depth, n=6) traumatic brain injury. Delta power was normalized as the percent of the total power and is plotted as mean±SEM. Statistically significant differences are depicted as: *p<0.05 vs. baseline within the same condition.
3.2. Experiment 2: effects of TBI on neuromotor function
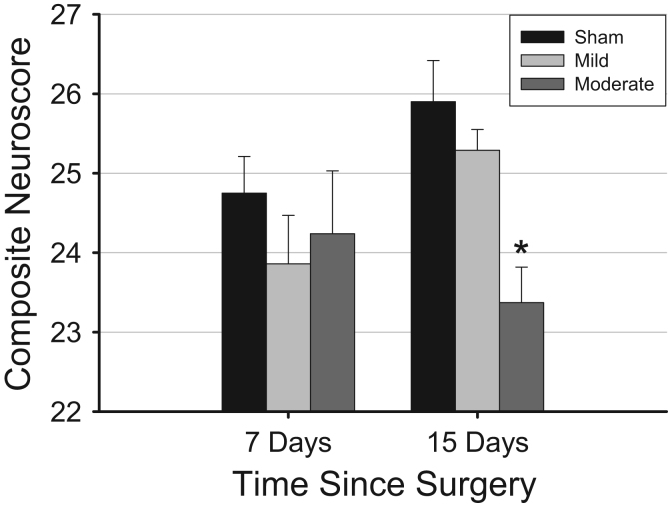
Neuromotor function did not differ, based on composite neuroscores, among groups 7 days post-injury (Fig. 5). However, by 15 days post-injury, one-way ANOVA revealed a small but significant difference in composite neuroscore values among groups [F(2,25)=9.266, p=0.001], with neuromotor function of animals subjected to moderate TBI being worse than that of mice subjected to sham surgeries (Tukey's post-hoc comparison; p=0.001).
Fig. 5.
Traumatic brain injury impairs neuromotor function. Neuromotor function, as quantified by composite neuroscores, is impaired in animals subjected to moderate traumatic brain injury during the chronic post-injury phase. Values are means±SEM obtained from control mice (sham surgeries, 7 days, n=8, 15 days, n=10), or mice subjected to mild (0.5 mm controlled cortical impact depth, 7 days, n=8, 15 days, n=9) or moderate (1.0 mm controlled cortical impact depth, 7 days, n=8, 15 days, n=9) traumatic brain injury. Statistically significant difference are indicated as: *=p<0.05 vs. values from control (sham) mice at same time point.
3.3. Experiment 3: effects of TBI on numbers of hypocretin and MCH neurons
3.3.1. Hypocretin
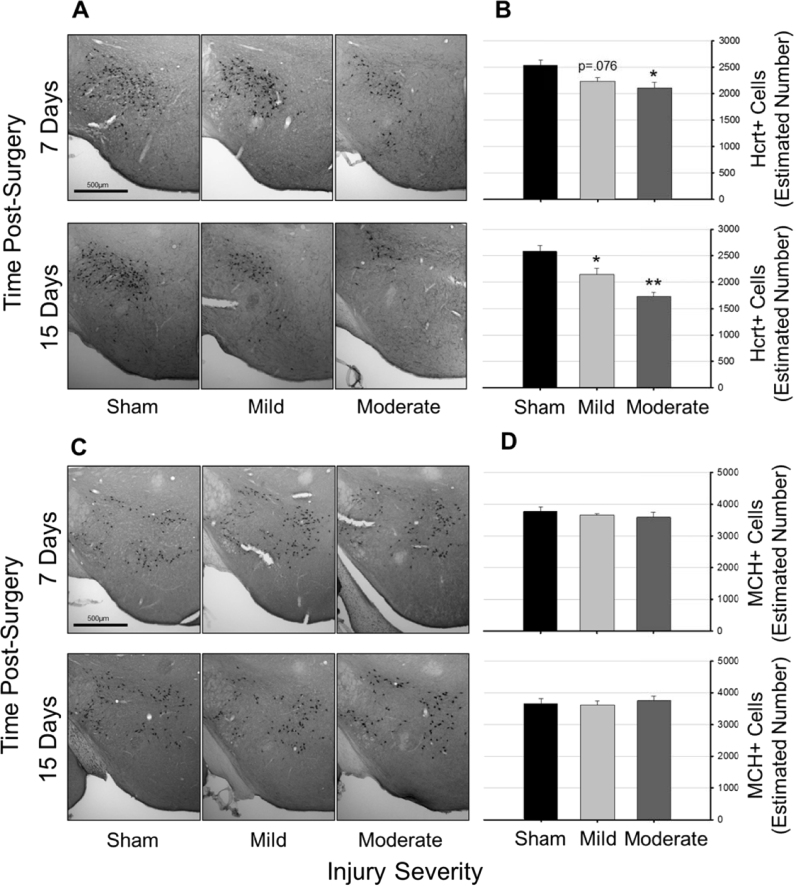
Hypocretin immunoreactivity differed among groups of mice in an injury-dependent manner (Fig. 6), as revealed by two-way ANOVA [F(5,39)=9.911, p<0.001]. There was a main effect of injury severity on number of hypocretin-positive cells [F(2,39)=20.653, p<0.001; Fig. 6A and B], a trend towards a main effect of time since surgery [F(1,39)=3.05, p=0.089], but no interaction effect [F(2,39)=2.33, p=0.11]. Post hoc tests indicated that relative to sham tissue, tissue obtained after mild and moderate TBI contained incrementally fewer hypocretin-positive cells. Even though a two way ANOVA showed only a trend toward a main effect for time, when analyses are restricted to tissue obtained from mice subjected to moderate TBI, an independent t-test revealed there were significantly fewer hypocretin-positive cells present at 15 days than at 7 days [t(14)=2.788, p=0.015]. Numbers of hypocretin-producing neurons were fewest in hypothalami of mice subjected to moderate TBI and sacrificed 15 days post-surgery.
Fig. 6.
Traumatic brain injury reduces numbers of hypocretin neurons, but not melanin-concentrating hormone neurons. Representative photomicrographs of hypocretin- (Panel A) and melanin-concentrating hormone (MCH) positive cells (Panel C) in tissue obtained from control (sham) mice, and mice subjected to mild (0.5 mm controlled cortical impact depth) or moderate (1.0 mm controlled cortical impact depth) traumatic brain injury. Numbers of hypocretin (Panel B) and MCH (Panel D) cells in the perifornical-lateral region of the hypothalamus ipsilateral to the injury site were estimated using unbiased stereology and the optical fractionator method. Values in panels B and D are means±SEM obtained from control mice (sham surgeries, 7 days, n=7, 15 days, n=6), or mice subjected to mild (0.5 mm controlled cortical impact depth, 7 days, n=8, 15 days, n=8) or moderate (1.0 mm controlled cortical impact depth, 7 days, n=8, 15 days, n=8) traumatic brain injury. Statistical differences relative to sham (control) animals are depicted by: *=p<0.05, **=p<0.01.
3.3.2. MCH
Numbers of MCH-positive cells did not differ among groups irrespective of injury severity or time post manipulation as revealed by a two-way ANOVA [F(5,39)=0.312, p=0.903]; (Fig. 6C and D).
3.4. Experiment 4: impact of TBI on tuberomammillary histamine neurons and basal forebrain cholinergic neurons
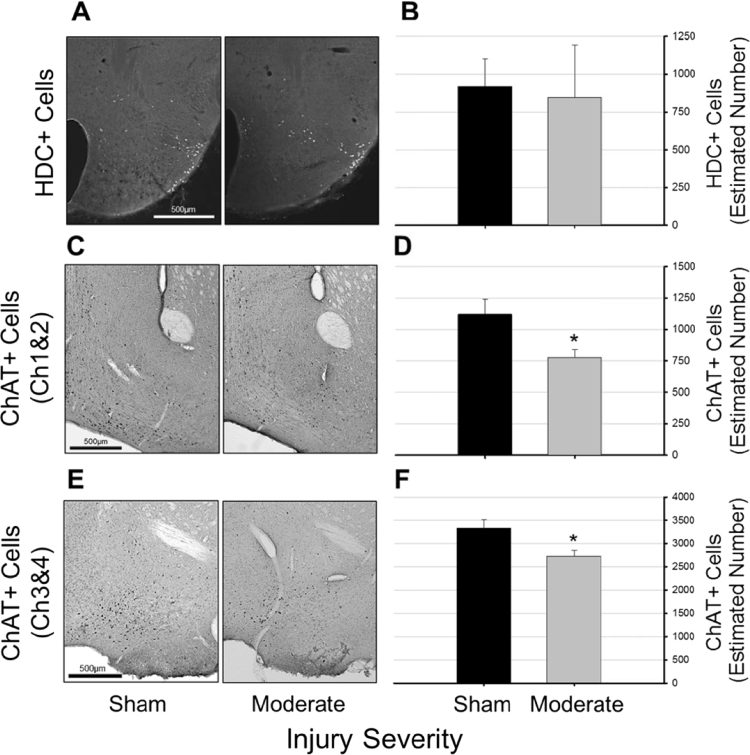
There was no significant difference between numbers of histamine neurons in the ventral TMN between the sham and 1 mm depth impact groups at 15 days post-injury [F(1,12)=0.231, p=0.639; Fig. 7A and B]. However, there were significantly fewer ChAT-positive neurons in both Ch1&2 [F(1,12)=6.089, p=0.03; Fig. 7C and D] and Ch3&4 [F(1,12)=7.12, p=0.02; Fig. 7E and F] in tissue obtained from mice subjected to moderate TBI.
Fig. 7.
Traumatic brain injury reduces numbers of cholinergic neurons in the basal forebrain, but not histaminergic neurons in the tuberomammillary nucleus. Representative photomicrographs of histidine decarboxylase (HDC) (Panel A) and choline acetyltransferase (ChAT) (Panels C and E) positive cells in tissue obtained from control (sham, n=7) mice, and from mice subjected to moderate (1.0 mm controlled cortical impact depth, n=7) traumatic brain injury. Tissue was obtained at 15 days post-surgery. Numbers of HDC- (Panel B) and ChAT-positive cells (Panels D and F) ipsilateral to the injury site were estimated using unbiased stereology and the optional fractionator method. Statistically significant differences relative to cell numbers determined from control (sham) mice are indicated as *=p<0.05. Data in panels B, D, and F are presented as means±SEM.
4. Discussion
Sleep-wake disturbances are frequently reported in individuals suffering from TBI (Baumann et al., 2007, Ponsford et al., 2013, Ponsford and Sinclair, 2014, Sommerauer et al., 2013). Post-TBI sleep-wake disturbances may negatively impact functional and cognitive recovery and are associated with increased anxiety, depression, and pain (Cantor et al., 2008, Chan and Feinstein, 2015, Chaput et al., 2009, Chiu et al., 2014, Rao et al., 2014). However, injuries resulting from trauma are highly variable, and as a consequence so are their sequelae. Controlled laboratory studies provide a means of standardizing injury severity, and although variability exists between and among animals, results may more readily provide insightful information with respect to potential mechanisms by which TBI alters sleep. The current study aimed to characterize the effects of injury severity and time course on aspects of sleep after mild or moderate TBI. By determining the impact on candidate neurotransmitter systems, we sought to elucidate potential mechanistic substrates that may be therapeutic targets for intervention after TBI.
4.1. Effects of TBI on mouse sleep-wake behavior
Our data indicate that in this TBI model wakefulness is reduced and NREM sleep is increased during the period comparable to daytime in humans, i.e., the mouse dark period. We did not observe changes in REM sleep of mice after TBI under the conditions of this study. Collectively, these results are consistent with those of human studies that report high rates of excessive daytime sleepiness (EDS) and daytime napping post-TBI (Castriotta et al., 2007, Imbach et al., 2015, Kempf et al., 2010, Ponsford et al., 2013, Ponsford and Sinclair, 2014, Sommerauer et al., 2013). Just as our study found no consistent effects, human polysomnographic studies often (although not always) fail to reveal changes in REM sleep after TBI (Baumann et al., 2007, Imbach et al., 2015, Sommerauer et al., 2013).
Results of pre-clinical studies using rodents also demonstrate acute (Rowe et al., 2014b, Sabir et al., 2015, Willie et al., 2012) and chronic (Lim et al., 2013, Skopin et al., 2015) changes in sleep. Deficits in wakefulness or difficulty maintaining long periods of wakefulness in rodents after experimental TBI are most robust during the dark (active) period (Lim et al., 2013, Skopin et al., 2015). We are aware of two studies that did not demonstrate chronic changes in rodent sleep-wake behavior after TBI. Noain and colleagues (Buchele et al., 2015) restricted their determination of sleep-wake behavior to the light period, the period during which sleep was not consistently altered after TBI in our present study. One study by Lifshitz and colleagues (Rowe et al., 2014a) did not find any persistent sleep-wake changes during the light or dark periods. Several factors may account for differences between our study and that of Lifshitz, including the manner in which sleep-wake behavior was inferred (EEG recordings in the present study vs. piezoelectric detection of movements), the method to induce TBI (CCI in our study vs. fluid percussion), and brain injury location (lateral in our study vs. midline).
Some pre-clinical and clinical studies demonstrate fragmented sleep after TBI (Hazra et al., 2014, Lim et al., 2013, Shekleton et al., 2010) whereas others do not (Baumann et al., 2007, Imbach et al., 2015, Sommerauer et al., 2013). Sleep of mice in our study using the CCI model to induce mild to moderate TBI is not fragmented. Reasons for differences in the literature with respect to this aspect of sleep are not clear, but could be due to aforementioned differences in the model used and severity and location of injury in animal studies. Clinical studies report effects on patients who have been subjected to head trauma from a variety of sources, and the extent of damage based upon assessment of neurocognitive function is highly variable. This diversity in injury and patient characteristics is important because severity of injury, presence of co-morbid factors such as intracranial hemorrhage, certain polymorphisms, and patient brain characteristics appear to impact the development of sleep-wake disturbance after TBI (Hong et al., 2015, Imbach et al., 2015, Yaeger et al., 2014).
In addition to changes in time spent in wakefulness and NREM sleep, spectral characteristics of the EEG (particularly delta power) are often altered after TBI. In humans, NREM delta power may increase (Imbach et al., 2015) or decrease (Rao et al., 2011) after TBI. At least one rodent study demonstrates increases in EEG delta power during wakefulness after TBI (Sabir et al., 2015), which may be a correlate of subjective daytime sleepiness or fatigue in humans (D’Rozario et al., 2013, Lal and Craig, 2002). Data in this present study demonstrate that moderate TBI increases NREM delta power during the light period at all post-injury time points determined, and during the dark period 15 days post-injury. Because NREM delta power is accepted as an indication of the depth or intensity of sleep (Borbely, 1982, Dijk et al., 1990), these data suggest that mice subjected to moderate TBI sleep more deeply, which may indicate that this pathology causes sleep pressure to build more quickly during wakefulness. Definitive experiments to test this hypothesis remain to be conducted.
4.2. Impact of TBI on arousal-promoting neurotransmitter systems
Changes in sleep-wake behavior after TBI are likely due, at least in part, to changes in neuronal systems implicated in regulating this complex behavior. Although multiple neurochemical systems are involved in regulating arousal state (Brown et al., 2012, Jones, 2008), in this study we focus on the hypocretinergic system and downstream projection targets, specifically the histaminergic tuberomammillary nucleus and the cholinergic basal forebrain. Hypocretin is essential for the maintenance of wakefulness. Hypocretin neurons discharge at their maximum during active wakefulness, especially during exploration (Estabrooke et al., 2001, Lee et al., 2005b, Mileykovskiy et al., 2005); intracerebroventricular injection of hypocretin increases wakefulness (Piper et al., 2000); and optogenetic stimulation of hypocretin neurons increases transitions from sleep to wake (Adamantidis et al., 2007). Conversely, antagonizing the hypocretinergic system promotes sleep (Brisbare-Roch et al., 2007, Hoever et al., 2012, Morairty et al., 2014) and loss of hypocretinergic signaling results in narcolepsy (Liblau et al., 2015). Hypocretin neurons are few in number, tightly clustered in the lateral hypothalamus, and project diffusely to multiple brain regions (Date et al., 1999, Peyron et al., 1998). As such, damage to hypocretin neurons could have far-ranging effects on sleep-wake behavior either directly or by affecting downstream mediators.
Hypocretin signaling is altered during acute and chronic phases of TBI. In vivo microdialysis studies in mice demonstrate reduced extracellular hypocretin three days after injury (Willie et al., 2012), and hypocretin is reduced in cerebrospinal fluid one to four days after injury in humans (Baumann et al., 2005). In cases of fatal TBI in humans, hypocretin cell numbers are reduced (Baumann et al., 2009), and human survivors of TBI with excessive daytime sleepiness have low levels of hypocretin in cerebrospinal fluid for at least six months after injury (Baumann et al., 2007).
Two studies in mice found that TBI impairs hypocretin cell function, but does not alter the number of hypocretin-producing neurons (Lim et al., 2013, Willie et al., 2012). There are several potential reasons for the apparent discrepancy in these previous studies and our current one. Willie and colleagues determined hypocretin cell numbers at only one time point, which was 3 days after injury (Willie et al., 2012). Our data are consistent with those of Willie et al., in that hypocretin cell numbers after TBI do not differ from control until 7–15 days post-injury. Collectively, these data suggest that the impact of mild to moderate TBI on hypocretin cell numbers takes longer than 3 days to develop. Similarly, Lim and colleagues did not observe decreased hypocretin cell numbers after TBI (Lim et al., 2013), but they used a midline fluid percussion model and random sampling of cells rather than unbiased stereology. Lim and colleagues also used 5-7 week old mice, which are considered adolescents in some models of TBI (Lopez-Rodriguez et al., 2015); inflammatory and cellular responses in brain to injury are highly affected by age (Kumar et al., 2013, McPherson et al., 2011, Timaru-Kast et al., 2012).
Nevertheless, our results demonstrating reduced numbers of hypocretin-producing neurons are in agreement with the majority of pre-clinical and clinical observations of persistent hypocretin dysfunction after TBI. While the present study found a loss of hypocretin neurons, others have also observed impairments in hypocretin neuron activity (Lim et al., 2013, Willie et al., 2012); thus neuronal loss and functional impairment both may play a role in post-TBI sleep-wake disturbance. The altered sleep-wake behavior observed in this study is consistent with reduction and/or dysfunction of hypocretin-producing neurons. Hypocretin neurons discharge at their highest rates during an animal's active period (Taheri et al., 2002), and hypocretin peaks during the latter part of the night in nocturnal rodents (Fujiki et al., 2001) and the latter part of the day in diurnal monkeys (Zeitzer et al., 2003) and humans (Salomon et al., 2003). It has been hypothesized that hypocretin is a reactive homeostatic signal needed to maintain wakefulness when sleep pressure increases (Zeitzer et al., 2003), which in humans is highest during the latter part of the day. Thus, in our present study increased sleep during the latter part of the mouse active period is consistent with decreased hypocretin signaling.
Melanin-concentrating hormone (MCH) neurons are intermingled with hypocretin neurons in the lateral hypothalamus (Hassani et al., 2009, Jones and Hassani, 2013, Torterolo et al., 2011). MCH neurons are implicated in the regulation of REM sleep and under some conditions NREM sleep. For example, MCH knockout mice spend significantly more time awake than their wild type counterparts (Willie et al., 2008). MCH neurons fire at a slow rate during NREM sleep and maximally during REM sleep (Hassani et al., 2009). Persistent optogenetic stimulation of MCH neurons increases NREM and REM sleep (Konadhode et al., 2013), but selective stimulation during NREM sleep increases transitions from NREM to REM (Jego et al., 2013, Tsunematsu et al., 2014). Although MCH promotes REM, ablation of MCH neurons does not appear to affect REM sleep, indicating that this peptide may not be necessary for REM to occur (Tsunematsu et al., 2014)
Because MCH neurons are intermingled with hypocretin neurons and generally act in a reciprocal manner with respect to arousal state, we also determined effects of TBI on this neuronal population. MCH neurons are variably reported to be affected by TBI. Some human postmortem studies report that MCH neuron numbers are reduced (Valko et al., 2015) or not significantly affected (Baumann et al., 2009) in cases of fatal TBI. Little pre-clinical research has focused on a role for MCH neurons in response to TBI; the only animal study of which we are aware that quantified changes in this cell type did not reveal changes in cell numbers after TBI (Willie et al., 2012). In the present study, we also found that TBI does not affect MCH cell number.
Hypocretin likely promotes wakefulness through several mechanisms. Although hypocretin-producing neurons are found only in the hypothalamus, they have diffuse projections with terminals in nuclei/brain regions characterized by diverse transmitter systems. For example, hypocretin neurons project to dopaminergic cells in the ventral tegmental area, noradrenergic cells in the locus coeruleus, and serotonergic cells in the dorsal raphe nucleus, in addition to direct excitatory projections to the cortex; each of these populations are important for the promotion of wakefulness [reviewed in (Krystal et al., 2013; Peyron et al., 1998)]. In the present study, we restricted our focus to the effects of TBI on two neuronal populations downstream of hypocretin: the cholinergic neurons of the basal forebrain and the histaminergic neurons of the tuberomammillary nucleus.
Cholinergic neurons fire maximally during wakefulness and REM sleep (Lee et al., 2005a) and promote cortical activation during these states (Jones, 2008). Enhancement of cholinergic signaling with acetylcholinesterase inhibitors increases wakefulness at the expense of NREM sleep and REM sleep (Jung et al., 2012). Cholinergic neurons in the basal forebrain project to the cortex and hippocampus where they promote low-voltage, high frequency EEG activity, which is characteristic of wakefulness and REM sleep (Brown et al., 2012, Shin and Dixon, 2015). As briefly mentioned, cholinergic neurons of the basal forebrain receive direct excitatory projections from hypocretin neurons in the hypothalamus (Fadel and Burk, 2010) and hypocretinergic signaling to the basal forebrain is an important modulator of sleep-wake behavior (Vazquez-DeRose et al., 2016). Cholinergic and hypocretinergic neurons project directly to the cortex, where they may work together to promote wakefulness and a state of attention, although in some cases cortical hypocretin can compensate for deficiencies in cholinergic signaling (Zajo et al., 2015). Although some research has focused on basal forebrain cholinergic neurons as mediators of hypocretin signaling to the cortex, cholinergic neurons do have sparse projections to the hypothalamus (Henny and Jones, 2006), and a subset of hypocretinergic neurons increase their firing rate in response to acetylcholine administration (Zhou et al., 2015). Thus, reciprocal interactions between these two neurotransmitter populations may play a role in consolidating wakefulness, although the functional significance of this reciprocity is not fully understood.
Interactions between hypocretinergic and cholinergic systems may play an important role in post-TBI sleep-wake disturbance. Just as the hypocretinergic system undergoes changes after TBI, the cholinergic system is also altered. During the acute phase after TBI there is a significant upregulation of activity in the cholinergic system, which may contribute to acute excitotoxic processes (Saija et al., 1988, Shin and Dixon, 2015). However, during chronic post-TBI periods, the cholinergic system is hypoactive (Shin and Dixon, 2015) and ChAT enzyme activity (Dewar and Graham, 1996, Murdoch et al., 1998) and immunoreactivity (Murdoch et al., 2002) are reduced in humans. Our results are consistent with these and other (Schmidt and Grady, 1995) observations insofar as we report reduced numbers of ChAT positive neurons in two areas of the basal forebrain. Thus, deficiencies in cholinergic signaling may also contribute to post-TBI sleep-wake disturbances.
Finally, we examined the effects of TBI on tuberomammillary histaminergic neurons. Histamine neurons fire maximally during wakefulness, and antagonizing histamine receptors increases sleep (Brown et al., 2001). Mice lacking brain histamine are unable to stay awake in response to behavioral challenge or environmental stimuli (Parmentier et al., 2002). Although hypocretin neurons project to the histaminergic cells of the tuberomammillary nucleus, the relationship between these two neuronal populations during pathology is not completely understood. For example, although histaminergic neurons receive substantial excitatory input from hypocretin neurons (Sundvik and Panula, 2015), a decrease in hypocretin is not always paralleled by a decrease in histamine: narcoleptics have little to no hypocretin, but greater numbers of histaminergic neurons (John et al., 2013, Valko et al., 2013). We are aware of only one study that has examined numbers of histaminergic neurons after TBI; histaminergic neurons in the tuberomammillary nucleus are reduced in post-mortem tissue after fatal TBI in humans (Valko et al., 2015). Our mouse model induces moderate injury with no mortality. Whether histaminergic neuron numbers would be reduced in pre-clinical models that result in more severe injury is not known. Histaminergic processes may still be important for decreases in wakefulness after TBI, but they may be mediated by changes in receptor density rather than by changes in neuron number (Shimada et al., 2012).
4.3. Inflammation as a mechanism underlying neuronal loss
The mechanisms by which TBI causes the semi-selective loss of hypocretinergic and cholinergic neurons are not known. TBI induces a state of robust neuroinflammation, which includes activation of microglia (Febinger et al., 2015, Harish et al., 2015, Hernandez-Ontiveros et al., 2013), astrogliosis (Harish et al., 2015, Hazra et al., 2014), and upregulation of inflammatory cytokines (Senol et al., 2014, Ziebell and Morganti-Kossmann, 2010). Inflammation is a key factor contributing to secondary injury after TBI (Corps et al., 2015, Ziebell and Morganti-Kossmann, 2010) and may cause hypocretinergic and cholinergic dysfunction.
Hypocretinergic and cholinergic neurons are sensitive to inflammation. For example, sterile inflammation induced by bolus injections of lipopolysaccharide, which also activates microglia and induces an inflammatory response (Camara et al., 2015, Thomson et al., 2014), reduces hypocretin in cerebrospinal fluid (Grossberg et al., 2011, Qin et al., 2005, Vasconcelos et al., 2014). Of relevance to this present study, chronic inflammation induced by repetitive doses of lipopolysaccharide reduces numbers of hypocretin-producing cells, but not MCH cells, indicating that hypocretin neurons are more sensitive to chronic inflammation than are MCH neurons (Palomba et al., 2014). As such, effects of inflammation induced by administration of lipopolysaccharide in the absence of injury on hypocretin and MCH producing cells of the hypothalamus are similar in some respects to those of TBI. Cholinergic neurons in the basal forebrain also are reduced during inflammation induced by chronic administration of lipopolysaccharide (Willard et al., 2000) or the inflammatory cytokine tumor necrosis factor α (Zassler et al., 2003). Therefore, although additional experiments must be conducted to determine if this is indeed the case, one potential mechanism mediating the semi-selective loss of hypocretin and acetylcholine neurons, and subsequent effects on sleep-wake behavior after TBI, is the neuroinflammatory response to this insult.
4.4. Conclusions
The present findings demonstrate that moderate CCI can be used in mice to effectively model some aspects of human sleep-wake disturbance after TBI. Furthermore, results reveal reduced numbers of hypocretinergic neurons in the hypothalamus and cholinergic basal forebrain neurons after TBI, suggesting a role for deficiency in these transmitter systems as contributing factors to post-TBI changes in sleep-wake behavior. If low levels of hypocretin or acetylcholine indeed cause somnolence and excessive daytime sleepiness, clinical research could perhaps lead to new hypocretin- or acetylcholine-based pharmacotherapies to the benefit of the millions affected by chronic TBI.
Conflicts of interest
The authors declare they have no conflicts of interest.
Acknowledgments
The technical assistance of Ms. Jenna Grillo and Mr. Chris Rumer is greatly appreciated. We thank Dr. Thomas Scammell for providing the HDC immunohistochemistry protocol. This study was supported, in part, by the Department of Anesthesiology & Pain Medicine and the Graduate Program in Neuroscience of the University of Washington.
References
- Adamantidis A.R., Zhang F., Aravanis A.M., Deisseroth K., de L.L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni E., Mochizuki T., Scammell T.E. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol. (Oxf.) 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracchi F., Opp M.R. Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav. Immun. 2008;22:982–993. doi: 10.1016/j.bbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C.R., Werth E., Stocker R., Ludwig S., Bassetti C.L. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- Baumann C.R., Stocker R., Imhof H.G., Trentz O., Hersberger M., Mignot E., Bassetti C.L. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- Baumann C.R., Bassetti C.L., Valko P.O., Haybaeck J., Keller M., Clark E., Stocker R., Tolnay M., Scammell T.E. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 2009;66:555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao S.U., Geurtjens C., Thomas G.R., Kitamura C.R., Zhou C., Marlborough M. Understanding the neuropsychiatric consequences associated with significant traumatic brain injury. Brain Inj. 2013;27:767–774. doi: 10.3109/02699052.2013.793396. [DOI] [PubMed] [Google Scholar]
- Borbely A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Boulet T., Kelso M.L., Othman S.F. Long-term in vivo imaging of viscoelastic properties of the mouse brain after controlled cortical impact. J. Neurotrauma. 2013;30:1512–1520. doi: 10.1089/neu.2012.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N., Semenova S., Liu W., Crews F.T., Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int. J. Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk J.A., Butler C.R., Halmos K.C., Smith B.N. Enduring changes in tonic GABAA receptor signaling in dentate granule cells after controlled cortical impact brain injury in mice. Exp. Neurol. 2016;277:178–189. doi: 10.1016/j.expneurol.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C., Dingemanse J., Koberstein R., Hoever P., Aissaoui H., Flores S., Mueller C., Nayler O., van G.J., de Haas S.L., Hess P., Qiu C., Buchmann S., Scherz M., Weller T., Fischli W., Clozel M., Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat. Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Brown R.E., Stevens D.R., Haas H.L. The physiology of brain histamine. Prog. Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Brown R.E., Basheer R., McKenna J.T., Strecker R.E., McCarley R.W. Control of sleep and wakefulness. Physiol. Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchele F., Morawska M.M., Schreglmann S.R., Penner M., Muser M., Baumann C.R., Noain D. Novel rat model of weight drop-induced closed diffuse traumatic brain injury compatible with electrophysiological recordings of vigilance states. J. Neurotrauma. 2015 doi: 10.1089/neu.2015.4001. [DOI] [PubMed] [Google Scholar]
- Camara M.L., Corrigan F., Jaehne E.J., Jawahar M.C., Anscomb H., Baune B.T. Effects of centrally administered etanercept on behavior, microglia, and astrocytes in mice following a peripheral immune challenge. Neuropsychopharmacology. 2015;40:502–512. doi: 10.1038/npp.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J.B., Ashman T., Gordon W., Ginsberg A., Engmann C., Egan M., Spielman L., Dijkers M., Flanagan S. Fatigue after traumatic brain injury and its impact on participation and quality of life. J. Head Trauma Rehabil. 2008;23:41–51. doi: 10.1097/01.HTR.0000308720.70288.af. [DOI] [PubMed] [Google Scholar]
- Castriotta R.J., Wilde M.C., Lai J.M., Atanasov S., Masel B.E., Kuna S.T. Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep Med. 2007;3:349–356. [PMC free article] [PubMed] [Google Scholar]
- Chan L.G., Feinstein A. Persistent sleep disturbances independently predict poorer functional and social outcomes 1 year after mild traumatic brain injury. J. Head Trauma Rehabil. 2015;30:E67–E75. doi: 10.1097/HTR.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Chaput G., Giguere J.F., Chauny J.M., Denis R., Lavigne G. Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 2009;10:713–716. doi: 10.1016/j.sleep.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Chew E., Zafonte R.D. Pharmacological management of neurobehavioral disorders following traumatic brain injury – a state-of-the-art review. J. Rehabil. Res. Dev. 2009;46:851–879. doi: 10.1682/jrrd.2008.09.0120. [DOI] [PubMed] [Google Scholar]
- Chiu H.Y., Lo W.C., Chiang Y.H., Tsai P.S. The effects of sleep on the relationship between brain injury severity and recovery of cognitive function: a prospective study. Int. J. Nurs. Stud. 2014;51:892–899. doi: 10.1016/j.ijnurstu.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Corps K.N., Roth T.L., McGavern D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Rozario A.L., Kim J.W., Wong K.K., Bartlett D.J., Marshall N.S., Dijk D.J., Robinson P.A., Grunstein R.R. A new EEG biomarker of neurobehavioural impairment and sleepiness in sleep apnea patients and controls during extended wakefulness. Clin. Neurophysiol. 2013;124:1605–1614. doi: 10.1016/j.clinph.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Date Y., Ueta Y., Yamashita H., Yamaguchi H., Matsukura S., Kangawa K., Sakurai T., Yanagisawa M., Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D., Graham D.I. Depletion of choline acetyltransferase activity but preservation of M1 and M2 muscarinic receptor binding sites in temporal cortex following head injury: a preliminary human postmortem study. J. Neurotrauma. 1996;13:181–187. doi: 10.1089/neu.1996.13.181. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Brunner D.P., Beersma D.G., Borbely A.A. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–440. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- Estabrooke, McCarthy M.T., Ko E., Chou T.C., Chemelli R.M., Yanagisawa M., Saper C.B., Scammell T.E. Fos expression in orexin neurons varies with behavioral state. J. Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J., Burk J.A. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febinger H.Y., Thomasy H.E., Pavlova M.N., Ringgold K.M., Barf P.R., George A.M., Grillo J.N., Bachstetter A.D., Garcia J.A., Cardona A.E., Opp M.R., Gemma C. Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J. Neuroinflamm. 2015;12:154. doi: 10.1186/s12974-015-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki N., Yoshida Y., Ripley B., Honda K., Mignot E., Nishino S. Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 h and in response to food deprivation. Neuroreport. 2001;12:993–997. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- Fujimoto S.T., Longhi L., Saatman K.E., Conte V., Stocchetti N., McIntosh T.K. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Grossberg A.J., Zhu X., Leinninger G.M., Levasseur P.R., Braun T.P., Myers M.G., Jr., Marks D.L. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J. Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H.L., Sergeeva O.A., Selbach O. Histamine in the nervous system. Physiol. Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., Scheff S.W. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Han Y., Shi Y.F., Xi W., Zhou R., Tan Z.B., Wang H., Li X.M., Chen Z., Feng G., Luo M., Huang Z.L., Duan S., Yu Y.Q. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr. Biol. 2014;24:693–698. doi: 10.1016/j.cub.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Harish G., Mahadevan A., Pruthi N., Sreenivasamurthy S.K., Puttamallesh V.N., Keshava Prasad T.S., Shankar S.K., Srinivas Bharath M.M. Characterization of traumatic brain injury in human brains reveals distinct cellular and molecular changes in contusion and pericontusion. J. Neurochem. 2015;134:156–172. doi: 10.1111/jnc.13082. [DOI] [PubMed] [Google Scholar]
- Hassani O.K., Lee M.G., Jones B.E. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc. Natl. Acad. Sci. USA. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra A., Macolino C., Elliott M.B., Chin J. Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J. Neurosci. Res. 2014;92:1434–1445. doi: 10.1002/jnr.23430. [DOI] [PubMed] [Google Scholar]
- Henny P., Jones B.E. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J. Comp. Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ontiveros D.G., Tajiri N., Acosta S., Giunta B., Tan J., Borlongan C.V. Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever P., de Haas S.L., Dorffner G., Chiossi E., van Gerven J.M., Dingemanse J. Orexin receptor antagonism: an ascending multiple-dose study with almorexant. J. Psychopharmacol. 2012;26:1071–1080. doi: 10.1177/0269881112448946. [DOI] [PubMed] [Google Scholar]
- Hong C.T., Wong C.S., Ma H.P., Wu D., Huang Y.H., Wu C.C., Lin C.M., Su Y.K., Liao K.H., Ou J.C., Hu C.J. PERIOD3 polymorphism is associated with sleep quality recovery after a mild traumatic brain injury. J. Neurol. Sci. 2015;358:385–389. doi: 10.1016/j.jns.2015.09.376. [DOI] [PubMed] [Google Scholar]
- Imbach L.L., Valko P.O., Li T., Maric A., Symeonidou E.R., Stover J.F., Bassetti C.L., Mica L., Werth E., Baumann C.R. Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain. 2015;138:726–735. doi: 10.1093/brain/awu391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingiosi A.M., Raymond R.M., Jr., Pavlova M.N., Opp M.R. Selective contributions of neuronal and astroglial interleukin-1 receptor 1 to the regulation of sleep. Brain Behav. Immun. 2015;48:244–257. doi: 10.1016/j.bbi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Irmak S.O., de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37:1941–1951. doi: 10.5665/sleep.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S., Glasgow S.D., Herrera C.G., Ekstrand M., Reed S.J., Boyce R., Friedman J., Burdakov D., Adamantidis A.R. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J., Thannickal T.C., McGregor R., Ramanathan L., Ohtsu H., Nishino S., Sakai N., Yamanaka A., Stone C., Cornford M., Siegel J.M. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 2013;74:786–793. doi: 10.1002/ana.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.E. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann. N.Y. Acad. Sci. 2008;1129:26–34. doi: 10.1196/annals.1417.026. [DOI] [PubMed] [Google Scholar]
- Jones B.E., Hassani O.K. The role of Hcrt/Orx and MCH neurons in sleep-wake state regulation. Sleep. 2013;36:1769–1772. doi: 10.5665/sleep.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.Y., Roh M., Ko K.K., Jang H.S., Lee S.R., Ha J.H., Jang I.S., Lee H.W., Lee M.G. Effects of single treatment of anti-dementia drugs on sleep-wake patterns in rats. Korean J. Physiol. Pharmacol. 2012;16:231–236. doi: 10.4196/kjpp.2012.16.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf J., Werth E., Kaiser P.R., Bassetti C.L., Baumann C.R. Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2010;81:1402–1405. doi: 10.1136/jnnp.2009.201913. [DOI] [PubMed] [Google Scholar]
- Kilduff T.S., Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Konadhode R.R., Pelluru D., Blanco-Centurion C., Zayachkivsky A., Liu M., Uhde T., Glen W.B., Jr., van Den Pol A.N., Mulholland P.J., Shiromani P.J. Optogenetic stimulation of MCH neurons increases sleep. J. Neurosci. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal A.D., Benca R.M., Kilduff T.S. Understanding the sleep-wake cycle: sleep, insomnia, and the orexin system. J. Clin. Psychiatry. 2013;74(Suppl 1):S3–S20. doi: 10.4088/JCP.13011su1c. [DOI] [PubMed] [Google Scholar]
- Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., Loane D.J. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S.K., Craig A. Driver fatigue: electroencephalography and psychological assessment. Psychophysiology. 2002;39:313–321. doi: 10.1017/s0048577201393095. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Hassani O.K., Jones B.E. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.G., Hassani O.K., Alonso A., Jones B.E. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau R.S., Vassalli A., Seifinejad A., Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 2015;14:318–328. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- Lim M.M., Elkind J., Xiong G., Galante R., Zhu J., Zhang L., Lian J., Rodin J., Kuzma N.N., Pack A.I., Cohen A.S. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3007092. 215ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez A.B., Acaz-Fonseca E., Viveros M.P., Garcia-Segura L.M. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10:e0128782. doi: 10.1371/journal.pone.0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson C.A., Aoyama M., Harry G.J. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav. Immun. 2011;25:850–862. doi: 10.1016/j.bbi.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M., Mufson E.J., Wainer B.H., Levey A.I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy B.Y., Kiyashchenko L.I., Siegel J.M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.M., Wang J.A., Buchanan A.K., Hall E.D. Temporal and spatial dynamics of nrf2-antioxidant response elements mediated gene targets in cortex and hippocampus after controlled cortical impact traumatic brain injury in mice. J. Neurotrauma. 2014;31:1194–1201. doi: 10.1089/neu.2013.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Crocker A., McCormack S., Yanagisawa M., Sakurai T., Scammell T.E. Behavioral state instability in orexin knock-out mice. J. Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty S.R., Wilk A.J., Lincoln W.U., Neylan T.C., Kilduff T.S. The hypocretin/orexin antagonist almorexant promotes sleep without impairment of performance in rats. Front. Neurosci. 2014;8:3. doi: 10.3389/fnins.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch I., Nicoll J.A., Graham D.I., Dewar D. Nucleus basalis of Meynert pathology in the human brain after fatal head injury. J. Neurotrauma. 2002;19:279–284. doi: 10.1089/08977150252807018. [DOI] [PubMed] [Google Scholar]
- Murdoch I., Perry E.K., Court J.A., Graham D.I., Dewar D. Cortical cholinergic dysfunction after human head injury. J. Neurotrauma. 1998;15:295–305. doi: 10.1089/neu.1998.15.295. [DOI] [PubMed] [Google Scholar]
- Ouellet M.C., Beaulieu-Bonneau S., Morin C.M. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015;14:746–757. doi: 10.1016/S1474-4422(15)00068-X. [DOI] [PubMed] [Google Scholar]
- Palomba M., Seke Etet P.F., Veronesi C. Effect of inflammatory challenge on hypothalamic neurons expressing orexinergic and melanin-concentrating hormone. Neurosci. Lett. 2014;570:47–52. doi: 10.1016/j.neulet.2014.03.069. [DOI] [PubMed] [Google Scholar]
- Parmentier R., Ohtsu H., Djebbara-Hannas Z., Valatx J.L., Watanabe T., Lin J.S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; United States: 2001. [Google Scholar]
- Peyron C., Sapin E., Leger L., Luppi P.H., Fort P. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides. 2009;30:2052–2059. doi: 10.1016/j.peptides.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Peyron C., Tighe D.K., van Den Pol A.N., de L.L., Heller H.C., Sutcliffe J.G., Kilduff T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper D.C., Upton N., Smith M.I., Hunter A.J. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur. J. Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Ponsford J.L., Sinclair K.L. Sleep and fatigue following traumatic brain injury. Psychiatr. Clin. N. Am. 2014;37:77–89. doi: 10.1016/j.psc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Ponsford J.L., Parcell D.L., Sinclair K.L., Roper M., Rajaratnam S.M. Changes in sleep patterns following traumatic brain injury: a controlled study. Neurorehabil. Neural Repair. 2013;27:613–621. doi: 10.1177/1545968313481283. [DOI] [PubMed] [Google Scholar]
- Qin L., Li G., Qian X., Liu Y., Wu X., Liu B., Hong J.S., Block M.L. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- Rao V., Rollings P. Sleep disturbances following traumatic brain injury. Curr. Treat. Options Neurol. 2002;4:77–87. doi: 10.1007/s11940-002-0006-4. [DOI] [PubMed] [Google Scholar]
- Rao V., Bergey A., Hill H., Efron D., McCann U. Sleep disturbance after mild traumatic brain injury: indicator of injury? J. Neuropsychiatry Clin. Neurosci. 2011;23:201–205. doi: 10.1176/jnp.23.2.jnp201. [DOI] [PubMed] [Google Scholar]
- Rao V., McCann U., Han D., Bergey A., Smith M.T. Does acute TBI-related sleep disturbance predict subsequent neuropsychiatric disturbances? Brain Inj. 2014;28:20–26. doi: 10.3109/02699052.2013.847210. [DOI] [PubMed] [Google Scholar]
- Rowe R.K., Harrison J.L., O’Hara B.F., Lifshitz J. Diffuse brain injury does not affect chronic sleep patterns in the mouse. Brain Inj. 2014;28:504–510. doi: 10.3109/02699052.2014.888768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.K., Striz M., Bachstetter A.D., Van Eldik L.J., Donohue K.D., O’Hara B.F., Lifshitz J. Diffuse brain injury induces acute post-traumatic sleep. PLoS One. 2014;9:e82507. doi: 10.1371/journal.pone.0082507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rue-Evans L., Nesbitt K., Oka R.K. Sleep hygiene program implementation in patients with traumatic brain injury. Rehabil. Nurs. 2013;38:2–10. doi: 10.1002/rnj.66. [DOI] [PubMed] [Google Scholar]
- Sabir M., Gaudreault P.O., Freyburger M., Massart R., Blanchet-Cohen A., Jaber M., Gosselin N., Mongrain V. Impact of traumatic brain injury on sleep structure, electrocorticographic activity and transcriptome in mice. Brain Behav. Immun. 2015;47:118–130. doi: 10.1016/j.bbi.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Saija A., Hayes R.L., Lyeth B.G., Dixon C.E., Yamamoto T., Robinson S.E. The effect of concussive head injury on central cholinergic neurons. Brain Res. 1988;452:303–311. doi: 10.1016/0006-8993(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Salomon R.M., Ripley B., Kennedy J.S., Johnson B., Schmidt D., Zeitzer J.M., Nishino S., Mignot E. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol. Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Schmidt R.H., Grady M.S. Loss of forebrain cholinergic neurons following fluid-percussion injury: implications for cognitive impairment in closed head injury. J. Neurosurg. 1995;83:496–502. doi: 10.3171/jns.1995.83.3.0496. [DOI] [PubMed] [Google Scholar]
- Senol N., Naziroglu M., Yuruker V. N-acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem. Res. 2014;39:685–692. doi: 10.1007/s11064-014-1255-9. [DOI] [PubMed] [Google Scholar]
- Shekleton J.A., Parcell D.L., Redman J.R., Phipps-Nelson J., Ponsford J.L., Rajaratnam S.M. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010;74:1732–1738. doi: 10.1212/WNL.0b013e3181e0438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng P., Hou L., Wang X., Wang X., Huang C., Yu M., Han X., Dong Y. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS One. 2013;8:e81802. doi: 10.1371/journal.pone.0081802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada R., Nakao K., Furutani R., Kibayashi K. A rat model of changes in dural mast cells and brain histamine receptor H3 expression following traumatic brain injury. J. Clin. Neurosci. 2012;19:447–451. doi: 10.1016/j.jocn.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Shin S.S., Dixon C.E. Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after traumatic brain injury. J. Neurotrauma. 2015;32:1429–1440. doi: 10.1089/neu.2014.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopin M.D., Kabadi S.V., Viechweg S.S., Mong J.A., Faden A.I. Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury. J. Neurotrauma. 2015;32:289–296. doi: 10.1089/neu.2014.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerauer M., Valko P.O., Werth E., Baumann C.R. Excessive sleep need following traumatic brain injury: a case-control study of 36 patients. J. Sleep Res. 2013;22:634–639. doi: 10.1111/jsr.12068. [DOI] [PubMed] [Google Scholar]
- Sundvik M., Panula P. Interactions of the orexin/hypocretin neurones and the histaminergic system. Acta Physiol. (Oxf.) 2015;213:321–333. doi: 10.1111/apha.12432. [DOI] [PubMed] [Google Scholar]
- Sutton B.C., Opp M.R. Musculoskeletal sensitization and sleep: chronic muscle pain fragments sleep of mice without altering its duration. Sleep. 2014;37:505–513. doi: 10.5665/sleep.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S., Zeitzer J.M., Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu. Rev. Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Thomson C.A., McColl A., Cavanagh J., Graham G.J. Peripheral inflammation is associated with remote global gene expression changes in the brain. J. Neuroinflamm. 2014;11:73. doi: 10.1186/1742-2094-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timaru-Kast R., Luh C., Gotthardt P., Huang C., Schafer M.K., Engelhard K., Thal S.C. Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One. 2012;7:e43829. doi: 10.1371/journal.pone.0043829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P., Lagos P., Monti J.M. Melanin-concentrating hormone: a new sleep factor? Front. Neurol. 2011;2:14. doi: 10.3389/fneur.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T., Ueno T., Tabuchi S., Inutsuka A., Tanaka K.F., Hasuwa H., Kilduff T.S., Terao A., Yamanaka A. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J. Neurosci. 2014;34:6896–6909. doi: 10.1523/JNEUROSCI.5344-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko P.O., Gavrilov Y.V., Yamamoto M., Reddy H., Haybaeck J., Mignot E., Baumann C.R., Scammell T.E. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol. 2013;74:794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- Valko P.O., Gavrilov Y.V., Yamamoto M., Finn K., Reddy H., Haybaeck J., Weis S., Scammell T.E., Baumann C.R. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann. Neurol. 2015;77:177–182. doi: 10.1002/ana.24298. [DOI] [PubMed] [Google Scholar]
- Vasconcelos A.R., Yshii L.M., Viel T.A., Buck H.S., Mattson M.P., Scavone C., Kawamoto E.M. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J. Neuroinflamm. 2014;11:85. doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-DeRose J., Schwartz M.D., Nguyen A.T., Warrier D.R., Gulati S., Mathew T.K., Neylan T.C., Kilduff T.S. Hypocretin/orexin antagonism enhances sleep-related adenosine and GABA neurotransmission in rat basal forebrain. Brain Struct. Funct. 2016;221:923–940. doi: 10.1007/s00429-014-0946-y. [DOI] [PubMed] [Google Scholar]
- Verret L., Goutagny R., Fort P., Cagnon L., Salvert D., Leger L., Boissard R., Salin P., Peyron C., Luppi P.H. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M.J., Slomianka L., Gundersen H.J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Willard L.B., Hauss-Wegrzyniak B., Danysz W., Wenk G.L. The cytotoxicity of chronic neuroinflammation upon basal forebrain cholinergic neurons of rats can be attenuated by glutamatergic antagonism or cyclooxygenase-2 inhibition. Exp. Brain Res. 2000;134:58–65. doi: 10.1007/s002210000446. [DOI] [PubMed] [Google Scholar]
- Willie J.T., Sinton C.M., Maratos-Flier E., Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie J.T., Lim M.M., Bennett R.E., Azarion A.A., Schwetye K.E., Brody D.L. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J. Neurotrauma. 2012;29:1908–1921. doi: 10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger K., Alhilali L., Fakhran S. Evaluation of tentorial length and angle in sleep-wake disturbances after mild traumatic brain injury. AJR Am. J. Roentgenol. 2014;202:614–618. doi: 10.2214/AJR.13.11091. [DOI] [PubMed] [Google Scholar]
- Zajo K.N., Fadel J.R., Burk J.A. Orexin A-induced enhancement of attentional processing in rats: role of basal forebrain neurons. Psychopharmacology (Berl.) 2015 doi: 10.1007/s00213-015-4139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zassler B., Weis C., Humpel C. Tumor necrosis factor-alpha triggers cell death of sensitized potassium chloride-stimulated cholinergic neurons. Brain Res. Mol. Brain Res. 2003;113:78–85. doi: 10.1016/s0169-328x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Zeitzer J.M., Nishino S., Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol. Sci. 2006;27:368–374. doi: 10.1016/j.tips.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Zeitzer J.M., Buckmaster C.L., Parker K.J., Hauck C.M., Lyons D.M., Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J. Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.L., Gao X.B., Picciotto M.R. Acetylcholine acts through nicotinic receptors to enhance the firing rate of a subset of hypocretin neurons in the mouse hypothalamus through distinct presynaptic and postsynaptic mechanisms. eNeuro. 2015:2. doi: 10.1523/ENEURO.0052-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell J.M., Morganti-Kossmann M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]