Significance
Fragile X syndrome (FXS) is caused by loss of expression of fragile X mental retardation protein (FMRP), an RNA-binding protein that has been proposed to regulate local stimulus-dependent protein synthesis. However, how FMRP loss affects distributions of newly synthesized proteins remains unclear. Here, we report that local BDNF application promotes persistent accumulation of new PSD95 at stimulated synapses and dendrites, and that this accumulation is absent in FMRP-deficient mouse neurons. We further identify signaling pathways important for the protein synthesis abnormalities. These results provide direct evidence for deficits in local protein synthesis and accumulation in response to local stimulation in FXS, and support mTORC1-S6K1 pathway inhibition as a potential therapeutic approach for FXS.
Keywords: FMRP, fragile X syndrome, PSD95, BDNF, mTORC1
Abstract
Fragile X syndrome (FXS) is the leading monogenic cause of autism and intellectual disability. FXS is caused by loss of expression of fragile X mental retardation protein (FMRP), an RNA-binding protein that regulates translation of numerous mRNA targets, some of which are present at synapses. While protein synthesis deficits have long been postulated as an etiology of FXS, how FMRP loss affects distributions of newly synthesized proteins is unknown. Here we investigated the role of FMRP in regulating expression of new copies of the synaptic protein PSD95 in an in vitro model of synaptic plasticity. We find that local BDNF application promotes persistent accumulation of new PSD95 at stimulated synapses and dendrites of cultured neurons, and that this accumulation is absent in FMRP-deficient mouse neurons. New PSD95 accumulation at sites of BDNF stimulation does not require known mechanisms regulating FMRP–mRNA interactions but instead requires the PI3K-mTORC1-S6K1 pathway. Surprisingly, in FMRP-deficient neurons, BDNF induction of new PSD95 accumulation can be restored by mTORC1-S6K1 blockade, suggesting that constitutively high mTORC1-S6K1 activity occludes PSD95 regulation by BDNF and that alternative pathways exist to mediate induction when mTORC1-S6K1 is inhibited. This study provides direct evidence for deficits in local protein synthesis and accumulation of newly synthesized protein in response to local stimulation in FXS, and supports mTORC1-S6K1 pathway inhibition as a potential therapeutic approach for FXS.
Fragile X syndrome (FXS), the most common monogenic cause of autism and mental retardation, is caused by loss of function of the fragile X mental retardation protein (FMRP) encoded by the Fmr1 gene. FMRP-deficient mice show abnormal spatial learning and social behavior, serving as a model for FXS (1–3). Dendritic spines of FXS patients and of FMRP-deficient mice exhibit abnormal morphology resembling immature spines during neuronal differentiation, suggesting impaired spine maturation (4).
Deficits in activity-induced protein synthesis near synapses are likely to contribute to the pathology of FXS. FMRP is an RNA-binding protein localized to dendrites in nucleoprotein particles containing specific mRNAs and ribosome subunits (4). FMRP has roles in both mRNA transport and translational repression, and is required for translational induction of a subset of mRNAs in response to neuronal activity (4, 5). A potential target of FMRP is the mRNA for PSD95, which is the core protein of the postsynaptic density and directly anchors neurotransmitter receptors at the synapse (6). PSD95 protein levels increase in spines that persistently enlarge after long-term potentiation (LTP) but not in spines that only transiently enlarge, suggesting a role for long-term PSD95 accumulation in activity-dependent spine growth (7). FMRP binds to the 3′ untranslated region (UTR) of the PSD95 mRNA, increasing its stability (8) and repressing its translation (9–11). A recent study found that FMRP loss abolished rapid translational induction of a yellow fluorescent protein (YFP)-coding sequence flanked by the 5′ and 3′ UTRs of the PSD95 mRNA by metabotropic glutamate receptor stimulation, providing evidence for a role of FMRP in acute regulation of PSD95 mRNA translation (9). However, how this rapid translational regulation relates to long-term changes in synaptic protein expression or turnover remained unclear.
In this study, we show that FMRP is required for brain-derived neurotrophic factor (BDNF)-induced local dendritic expression of new PSD95 in a cell-autonomous manner. Unexpectedly, a constitutively repressing mutant of FMRP can substitute for wild-type protein, suggesting that BDNF can regulate PSD95 synthesis without acute inhibition of FMRP. Inhibition of the mTORC1-S6K1 pathway also rescues the FMRP-deficient phenotype, suggesting that hyperactivity of this pathway in the absence of FMRP occludes PSD95 induction. Lastly, we observed that ERK signaling is required for the rescue of PSD95 induction by mTORC1-S6K1 pathway inhibition. Interestingly, in contrast, wild-type (WT) neurons require the mTORC1-S6K1 pathway but not ERK for PSD95 regulation, indicating that FMRP loss induces a switch in signaling pathway function. These results provide evidence that mTORC1-S6K1 pathway inhibition may be useful for correcting protein synthesis deficits during synaptic plasticity in FXS.
Results
Expression of New PSD95 in BDNF-Stimulated Dendritic Regions Is Absent in FMRP-Deficient Neurons.
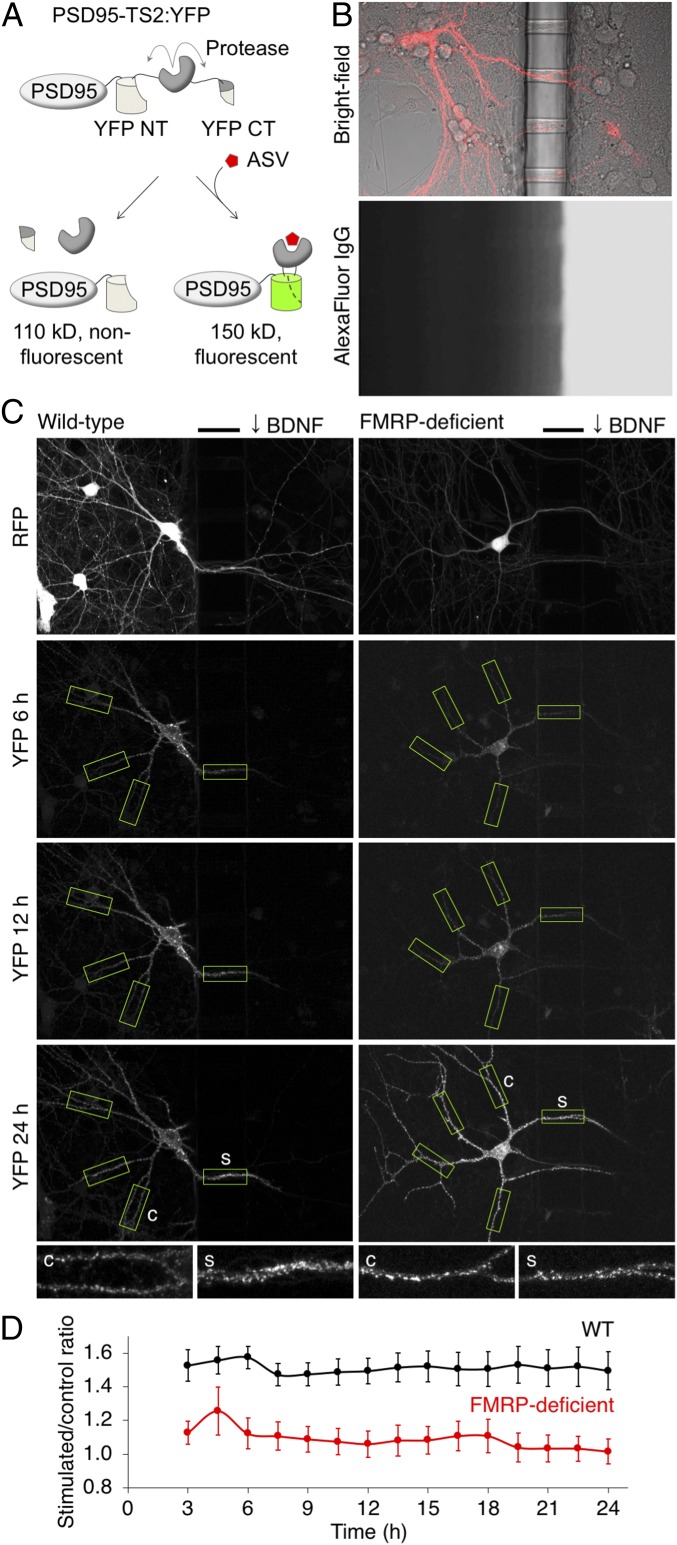
To visualize newly synthesized PSD95 in living neurons, we fused a TimeSTAMP2:YFP (TS2:YFP)-coding sequence to the 3′ end of the mouse PSD95-coding sequence followed by the full-length 3′ UTR. TS2:YFP consists of YFP with a loop insertion of a hepatitis C virus (HCV) NS3 protease domain flanked by cognate cleavage sites (12). By default, the NS3 protease removes itself from YFP immediately after folding, splitting YFP into two fragments and preventing chromophore maturation (12). However, in the presence of an HCV NS3 protease inhibitor such as asunaprevir (ASV), linkage is preserved and the YFP fluorophore matures (Fig. 1A). PSD95 protein copies produced after addition of ASV can thus be distinguished from preexisting copies by protein size on immunoblots or by fluorescence in live neurons (12).
Fig. 1.
Local expression of new PSD95 in BDNF-stimulated dendritic regions is absent in FMRP-deficient neurons. (A) Diagram of the PSD95-TS2:YFP reporter. After asunaprevir is added, YFP fluorescence is preserved on new PSD95 copies. CT, C terminus; NT, N terminus. (B) Compartmentalized culture system. (B, Top) Superimposed fluorescence and bright-field images showing a transfected neuron extending dendrites through tunnels in the barrier. (B, Bottom) Dye added to one side for 24 h demonstrates separation between compartments and dye diffusion down the tunnels. Barrier width is 50 μm. (C) New PSD95 in WT or FMRP-deficient neurons after local BDNF stimulation. The bars mark the location of the 50-μm barrier. BDNF was added to the right chamber. Boxes indicate regions identified and analyzed by the automated algorithm. Stimulated segments were defined as the 50 μm in the tunnel, and then control segments were defined in the unstimulated compartment at an equidistant path length from the cell body. (C, Insets) Stimulated (s) and control (c) segments. (D) Stimulated/control intensity ratios in FMRP-deficient neurons were significantly lower than in WT neurons at all times (P = 0.03 by mixed-effect repeated-measures ANOVA; n = 31 WT and 24 FMRP-deficient neurons). Error bars represent SEM.
As we had previously observed that bath stimulation by BDNF increases global levels of new PSD95 in cultured rat neurons (12), we first asked whether this response depended on FMRP. We quantified new PSD95-TS2:YFP produced after BDNF stimulation in WT or FMRP-deficient mouse neurons. Similar to previous observations in rat neurons, bath stimulation with BDNF for 24 h induced new PSD95 protein in WT mouse neurons, detected as a slower-migrating species by immunoblot. Interestingly, BDNF also induced new PSD95 proteins in FMRP-deficient neurons (SI Appendix, Fig. S1), indicating that FMRP is not required for global induction of new PSD95 over long timescales after BDNF stimulation.
We next explored whether FMRP might instead be required for local accumulation of new PSD95 in response to dendritic BDNF stimulation. To perform dendritic stimulations, we used microfabricated culture chambers in which two compartments are separated by a 50-μm-thick barrier traversed by 7-μm-wide tunnels (12). Neurons transfected with PSD95-TS:YFP reporters were plated in one compartment and allowed to extend neurites through the tunnels to the opposite compartment, making contacts with untransfected neurons on both sides (Fig. 1B). With ASV added to both compartments to visualize new PSD95 distributions, BDNF can be added to the side opposite the transfected cell bodies to stimulate a portion of dendrite. Entrance of factors down the tunnel also allows stimulation of dendritic segments within the tunnels (12). To quantify new PSD95 expression in stimulated regions in an unbiased manner, we performed experiments blinded to genotype and utilized an automated pipeline for image analysis. We wrote custom Matlab algorithms to compare TS:YFP signal density in stimulated dendritic segments to unstimulated segments equidistant from the cell body (SI Appendix, Fig. S2A). To enable the automated neurite tracing required for accurate TS:YFP signal quantitation, we also constructed a bidirectional expression plasmid (SI Appendix, Fig. S2B) to coexpress the PSD95-TS2:YFP reporter with an RFP transfection marker. This allowed us to avoid using IRES or P2A elements, whose interactions with the ribosome might affect FMRP regulation or whose presence on the PSD95-TS:YFP mRNA might interfere with the function of the PSD95 3′ UTR. Thus, the experimental procedure was carefully designed to assess new protein distributions in a robust and unbiased manner.
We had previously observed that local dendritic stimulation by BDNF induced preferential expression of new PSD95 protein at synapses in the stimulated regions (12). We now asked whether this response depends on FMRP. In WT hippocampal neurons, local BDNF stimulation induced expression of new PSD95 in stimulated regions as early as 3 h and persisting to 24 h, with punctate expression patterns suggestive of synaptic localization (Fig. 1C and Movie S1), as previously observed (12). Intensities of new PSD95 in the 50-μm segment of dendrites within the tunnels, which experience a BDNF gradient, were ∼50% higher than in unstimulated control segments equidistant from the cell body throughout the entire imaging period (Fig. 1D). Preferential expression of new PSD95 in dendritic segments located completely within the stimulated compartment, relative to unstimulated control regions equidistant from the cell body, could also be observed, although appearance of signal was delayed compared with the more proximal segments within the tunnels (SI Appendix, Fig. S2 C and D). In parallel with WT neurons, we also imaged neurons from FMRP-deficient mice. Strikingly, in FMRP-deficient neurons, levels of new PSD95 were similar in stimulated and unstimulated control dendritic segments throughout the 24-h imaging period (Fig. 1 C and D and Movie S2). Amounts of new PSD95 in stimulated regions of FMRP-deficient neurons were significantly lower than in stimulated regions of wild-type neurons at all time points for which YFP signal could be detected (Fig. 1D). These results indicate that FMRP is required for local dendritic expression of new PSD95 in response to BDNF stimulation.
FMRP Is Required Cell-Autonomously for BDNF-Induced Local New PSD95 Expression.
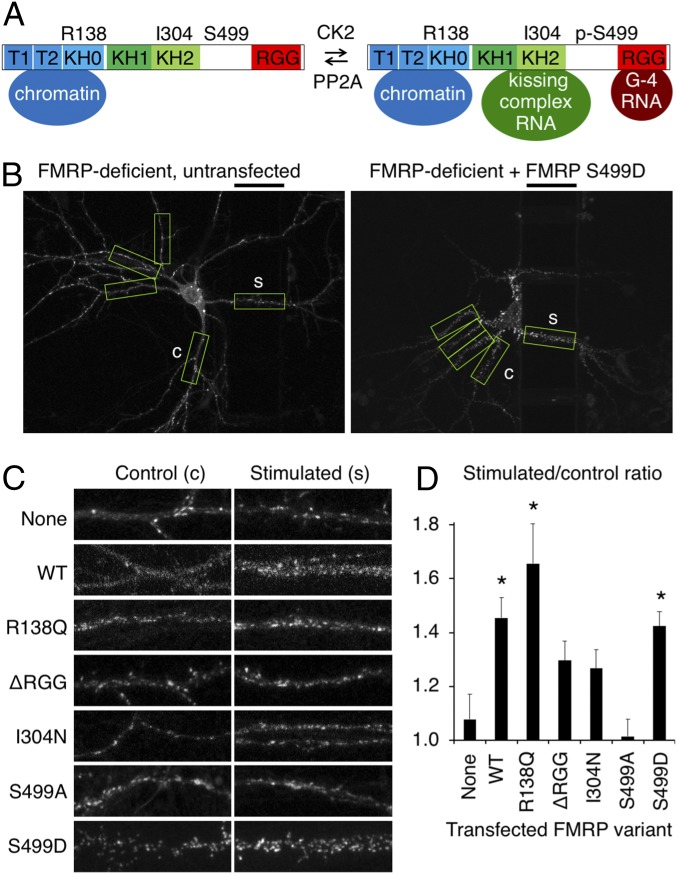
We asked whether FMRP is required directly for local BDNF-induced preferential PSD95 expression, or if the deficit seen in FMRP-deficient neurons is a consequence of indirect developmental deficits, such as excitability changes in the neuronal network (13–16). To address this question, we introduced FMRP-IRES-RFP and our PSD95-TS2:YFP reporter into a sparse subset of FMRP-deficient neurons. FMRP expression restored the ratio of new PSD95 intensity in stimulated dendritic regions versus unstimulated dendritic regions to levels similar to WT neurons (Fig. 2 C and D and SI Appendix, Fig. S3). This indicates that FMRP regulates BDNF-induced local new PSD95 expression in a cell-autonomous manner and the deficits we observe in new PSD95 expression are not due to a network effect.
Fig. 2.
Translational repression by FMRP is required cell-autonomously for BDNF-induced local expression of new PSD95. (A) Diagram of FMRP protein interactions disrupted by mutations. KH, heterogeneous nuclear ribonucleoprotein K homology domain; T, Tudor domain. (B) Representative images of new PSD95 in FMRP-deficient neurons expressing RFP only (Left) or FMRP S499D (Right) 21–24 h after local BDNF stimulation. The black bars above the images indicate the location of the barrier and provide a 50-μm scale bar. BDNF was applied to the right of the barrier. Boxes indicate stimulated and equidistant control regions identified and analyzed by the automated algorithm. (C) Representative stimulated and control regions for each transfected FMRP variant. Whole-neuron images are in SI Appendix, Fig. S3. (D) Relative intensities of stimulated versus unstimulated control dendritic segments. Error bars represent SEM. Untransfected, n = 10 neurons; WT, n = 11 neurons, *P = 0.008; R138Q, n = 14 neurons, *P = 0.01; ΔRGG, n = 21 neurons, P = 0.11; I304N, n = 17 neurons, P = 0.18; S499A, n = 13 neurons, P = 0.58; S499D, n = 15 neurons, *P = 0.005 [each condition vs. untransfected by Student’s t test with Tukey’s correction, after ANOVA test with F (6, 95) = 5.77, P = 3.7 × 10−5].
FMRP has multiple important functional regions, including an N-terminal protein–protein interaction region and two major RNA-binding regions (17, 18). To identify which aspects of FMRP function might be critical for BDNF-induced local new PSD95 expression, we tested the ability of FMRP variants containing different function-altering mutations (Fig. 2A) to rescue BDNF-induced local new PSD95 expression in KO neurons. Function was fully rescued with an R138Q mutant deficient in nucleosome binding (19) (Fig. 2 C and D and SI Appendix, Fig. S3), indicating that nucleosome interaction is not required for the ability of neurons to locally regulate new PSD95 expression and consistent with previous findings that R138Q mainly affects presynaptic FMRP function (20). In contrast, function was not fully rescued by FMRP with a deletion of the RGG box that eliminates binding to RNAs with a G-quadruplex structure and reduces polyribosome association (21), or by FMRP with an I304N mutation in the KH2 domain that impedes association with actively translating polyribosomes (22, 23), suggesting that FMRP binding to RNAs or polyribosomes is important (Fig. 2 C and D and SI Appendix, Fig. S3).
A simple model of FMRP function would be that PSD95 mRNA is repressed by binding to FMRP and then derepressed in response to BDNF. FMRP is constitutively phosphorylated at Ser499, likely by casein kinase II (24), and when phosphorylated represses the translation of mRNA cargoes (25, 26). Synaptic activity is believed to relieve this repression via dephosphorylation of Ser499 by PP2A (27), which also induces destabilization of FMRP (28). To test the possibility that BDNF induces release of PSD95 mRNA from FMRP repression, we attempted to rescue new PSD95 induction in FMRP-deficient neurons with S499D or S499A mutants of FMRP, which lock FMRP into translation-repressing and translation-permissive states, respectively (25, 27). If dephosphorylation of FMRP were the primary mechanism of BDNF-mediated new PSD95 expression, then neither mutant would restore BDNF-mediated new PSD95 expression. FMRP S499A was indeed unable to rescue BDNF-induced local new PSD95 expression in FMRP-deficient neurons (Fig. 2 C and D and SI Appendix, Fig. S3). Surprisingly, however, FMRP S499D restored local new PSD95 expression (Fig. 2 B–D and SI Appendix, Fig. S3). As FMRP S499D cannot be induced to release mRNA targets by dephosphorylation (10, 25), our results suggest that acute dissociation of FMRP from PSD95 mRNA may not be required for BDNF-induced local long-term expression of new PSD95.
BDNF-Induced New PSD95 Expression Requires the PI3K-mTORC1-S6K1 Pathway.
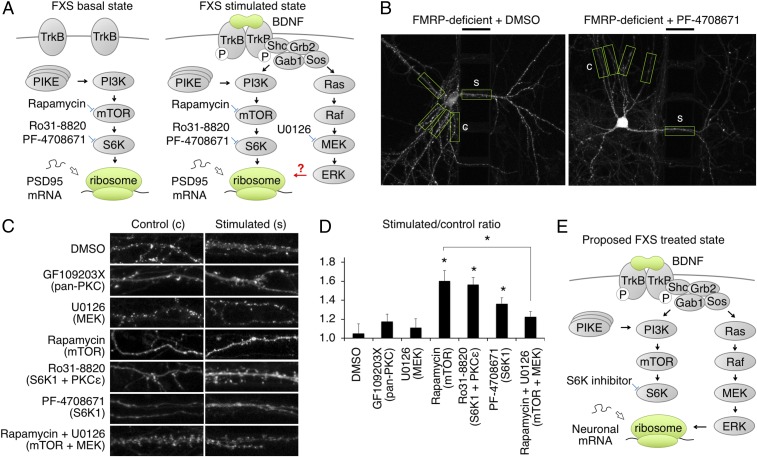
The BDNF receptor TrkB activates a variety of intracellular signaling proteins (SI Appendix, Fig. S4A), including the phosphatase PP2A (27), extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K) (29, 30), and protein kinase C (PKC) (31). To understand which signaling pathway is involved in BDNF-induced local expression of new PSD95, inhibitors of these proteins or their upstream activators were added together with ASV to WT hippocampal neurons expressing the PSD95-TS2:YFP reporter and locally stimulated with BDNF. The PP2A inhibitor okadaic acid did not impair local new PSD95 expression (SI Appendix, Fig. S4 C and J). This is consistent with the earlier finding that phosphomimetic FMRP S499D restores BDNF-induced local expression of new PSD95 in FMRP-deficient neurons, and further confirms that dephosphorylation of FMRP upon BDNF stimulation is not required for new PSD95 expression. New PSD95 expression was also not affected by the pan-PKC inhibitor GF109203X (SI Appendix, Fig. S4 D and J) or the MAPK/Erk kinase (MEK) inhibitor U0126 (SI Appendix, Fig. S4 E and J). Interestingly, however, multiple inhibitors of the PI3K-mTORC1-S6K1 pathway blocked the effect of local BDNF. The PI3K inhibitor LY294002 reduced the degree of new PSD95 expression by about 66% (SI Appendix, Fig. S4 F and J). A similar impairment was observed with the mTOR inhibitor rapamycin (SI Appendix, Fig. S4 G and J), the S6K1 and PKC inhibitor Ro31-8820 (SI Appendix, Fig. S4 H and J), and the selective S6K1 inhibitor PF-4708671 (SI Appendix, Fig. S4 I and J). Together, these results suggest that BDNF regulates new PSD95 expression in wild-type neurons via the PI3K-mTORC1-S6K1 pathway but not via PP2A, ERK, or PKC.
Blockade of mTOR or S6K1 Restores Local New PSD95 Expression in FMRP-Deficient Neurons.
Multiple studies have established that the mTORC1-S6K1 pathway is hyperactive in the FMRP-deficient mouse (5, 32, 33). Given the importance of the mTORC1-S6K1 pathway in local BDNF-induced new PSD95 expression in wild-type neurons, we asked if hyperactive mTORC1-S6K1 signaling occludes localized responses to BDNF in FMRP-deficient neurons (Fig. 3A). To test this hypothesis, we applied the mTOR inhibitor rapamycin, the S6K1/PKC inhibitor Ro31-8820, or the S6K1 inhibitor PF-4708671 to the dendrites of FMRP-deficient neurons during BDNF stimulation. Interestingly, we found that these drugs indeed restored new PSD95 expression (Fig. 3 B–D and SI Appendix, Fig. S5). In contrast, application of the pan-PKC inhibitor GF109203X or MEK inhibitor U0126 had no effect on BDNF-induced local expression of new PSD95 (Fig. 3 C and D and SI Appendix, Fig. S5), demonstrating that the observed rescue is specific to mTORC1-S6K1 inhibition. These findings support the hypothesis that hyperactive mTORC1-S6K1 signaling in FMRP-deficient neurons causes occlusion of BDNF responses required for local expression of new PSD95. They also identify PSD95 as a synaptic protein whose dysregulation in FMRP-deficient neurons can be corrected by pharmacological inhibition of the mTORC1-S6K1 pathway.
Fig. 3.
Inhibition of mTOR or S6K1 rescues BDNF-induced new PSD95 accumulation in FMRP-deficient neurons. (A) Proposed model of mTORC1-S6K1 pathway hyperactivity in FMRP-deficient neurons, occluding induction of local protein synthesis by stimuli. (B) Representative images of new PSD95 in FMRP-deficient neurons 21–24 h after local BDNF stimulation with DMSO (Left) or 10 μM S6K1 inhibitor PF-4708671 (Right). The black bars above the images indicate the location of the barrier and provide a 50-μm scale bar. BDNF was applied to the right of the barrier. Boxes indicate stimulated and equidistant control regions identified and analyzed by the automated algorithm. (C) Representative stimulated and control regions of neurons with DMSO, 2 μM pan-PKC inhibitor GF109203X, 20 μM MEK inhibitor U0126, 10 μM mTORC1 inhibitor rapamycin, 2 μM S6K1/PKC inhibitor Ro31-8820, 10 μM S6K1 inhibitor PF-4708671, or 10 μM rapamycin + 20 μM U0126 added to the stimulated dendrites together with BDNF. (D) Relative intensities of stimulated versus unstimulated control dendritic segments. Error bars represent SEM. DMSO, n = 12 neurons; GF109203X, n = 10 neurons, P = 0.35; U0126, n = 13 neurons, P = 0.67; rapamycin, n = 16 neurons, *P = 0.001; Ro31-8820, n = 12 neurons, *P = 0.0005; PF-4708671, n = 11 neurons, *P = 0.02; rapamycin + U0126, n = 13 neurons, P = 0.13 [each condition vs. DMSO by Student’s t test with Tukey’s correction, after ANOVA test with F (6, 81) = 5.67, P = 5.9 × 10−5]. The difference between rapamycin and rapamycin + U0126 was also statistically significant (*P = 0.01). (E) Proposed treatment model for FXS.
The ability of mTORC1-S6K1 inhibition to rescue local BDNF-induced expression of new PSD95 in FMRP-deficient neurons suggests there are alternative pathways that transduce BDNF signals to local PSD95 synthesis or accumulation. We thus investigated which pathway is responsible for BDNF-induced PSD95 expression in rapamycin-treated FMRP-deficient neurons. Because the MEK-ERK pathway regulates protein synthesis in response to other signals such as mGluR5 stimulation (34), we hypothesized that it might become important for BDNF induction of PSD95 in rapamycin-treated FMRP-deficient neurons, in contrast to its dispensability in wild-type neurons. To test this hypothesis, we treated FMRP-deficient neurons during BDNF stimulation and rapamycin treatment with U0126. Indeed, U1026 blocked the rescue of local BDNF-induced PSD95 accumulation by rapamycin in FMRP-deficient neurons (Fig. 3 C and D and SI Appendix, Fig. S5). These results indicate that the MEK-ERK pathway is required for rapamycin-rescued local BDNF-induced PSD95 accumulation in FMRP-deficient neurons (Fig. 3E).
To obtain further evidence that the MEK-ERK pathway can mediate BDNF-induced new PSD95 accumulation in FMRP-deficient neurons, we also examined its involvement in induction of pan-cellular PSD95 by globally applied BDNF. As demonstrated earlier, in contrast to the situation in dendrites, new PSD95 levels in cell lysates are increased by global BDNF in FMRP-deficient neurons even without inhibition of the TOR-S6K1 pathway (SI Appendix, Fig. S1), suggesting that a substantial fraction of PSD95 mRNA in the cell is not being translated at maximal rates but can be induced. This fraction likely resides in the cell body, as the majority of new PSD95 is found in the cell body (Fig. 1C). We found that, while global BDNF stimulation of FMRP-deficient neurons again robustly increased whole-cell levels of new PSD95 in the absence of pathway inhibition, this failed to occur when MEK or ERK was blocked (SI Appendix, Fig. S6). These findings provide further evidence that the MEK-ERK pathway can mediate BDNF-induced expression of new PSD95 in FMRP-deficient neurons when induction is not occluded by high basal translation rates.
Discussion
In the nervous system, protein synthesis is induced by neuronal activity and is required for memory consolidation in animals (35). Inhibition of protein synthesis blocks late-phase long-term potentiation of synaptic transmission (36), a synaptic mechanism for long-term memory (34), and at least some of the proteins critical for LTP are synthesized locally (37). BDNF is necessary and sufficient to induce long-lasting synaptic plasticity in brain slices (38, 39), and thus BDNF stimulation serves as a useful model for synaptic plasticity. In this study, using the TimeSTAMP method to specifically visualize newly synthesized copies of PSD95, we find that FMRP is required in a cell-autonomous manner for long-term dendritic expression of new PSD95 following local BDNF stimulation. Furthermore, we find that BDNF induction of local new PSD95 occurs via mTORC1-S6K1 signaling in WT neurons, and that mTORC1-S6K1 inhibition can restore the ability of FMRP-deficient neurons to respond to BDNF by increasing local expression of new PSD95.
Unexpectedly, known mechanisms for rapid regulation of FMRP function do not appear to mediate BDNF-induced local expression of new PSD95. A phosphomimetic S499D mutant of FMRP restores the response of new PSD95 to local BDNF stimulation, indicating that FMRP dephosphorylation at Ser499 is not required. PP2A, which dephosphorylates Ser499 (27, 40, 41), is also not required for BDNF-induced local expression of new PSD95 in WT neurons. However, it is possible that TrkB regulates FMRP via other mechanisms. For instance, FMRP–mRNA interactions are also regulated by arginine methylation, and FMRP subcellular localization can be influenced by synaptic activity (42, 43).
Interestingly, FMRP appears to be required for dendritic expression of new PSD95 following local BDNF stimulation, while global expression of new PSD95 in response to bath BDNF stimulation is unaffected by FMRP loss. These contrasting findings with global vs. local BDNF stimulation underscore the utility of visualizing distributions of newly synthesized proteins. There are several possible explanations for why PSD95 induction could be occluded in dendrites but not cell bodies of FMRP-deficient neurons. For example, up-regulated mTORC1-S6K1 pathway components could be preferentially localized to dendrites, or negative regulators could be more effective in cell bodies. Alternatively, mTORC1-S6K1 hyperactivity may not saturate downstream responses in the cell body due to higher substrate abundance. Indeed, in the brain, S6K1 is predominantly expressed in cell bodies and nearly undetectable in tissue regions composed of dendrites (44, 45).
mTORC1/S6K1 Inhibition as a Potential Therapeutic Approach for FXS.
Hyperactivity of the PI3K-mTORC1-S6K1 pathway has been linked to excessive translation in FXS (33, 46). Interestingly, genetic elimination of S6K1 ameliorated synaptic and behavioral phenotypes in FMRP-deficient mice, suggesting that S6K1 inhibition may be useful for treating FXS (32). In the course of this work, pharmacological S6K1 inhibition was found to reverse social and behavioral phenotypes of FMRP-deficient mice as well (47). Our finding that blockade of either mTORC1 or S6K1 restores local activity-dependent new protein synthesis in FMRP-deficient neurons provides a possible mechanistic explanation for the benefit of S6K1 inhibition in FXS.
The utility of mTORC1 suppression has been questioned in a recent finding that 40 d of rapamycin treatment did not ameliorate behavioral phenotypes of FMRP-deficient mice (48). However, chronic administration of rapamycin can be expected to have deleterious effects in vivo, as mTORC1 regulates a variety of cellular processes that are required for ongoing neuronal health including transcription, autophagy, protein degradation, and lipid production (49). In addition, chronic rapamycin treatment can inhibit mTORC2, which is required for normal cytoskeletal regulation and learning (49). These various effects of rapamycin could thus counteract any benefits from reducing dendritic protein synthesis. In contrast, treatment with S6K1 inhibition may be more beneficial by specifically targeting abnormal protein synthesis (47). Another recent study found that inhibition of the specific PI3K isoform p110β can ameliorate FXS behavioral symptoms (50), suggesting that specific inhibition of p110β-driven mTOR activity could also be more beneficial than inhibiting all mTOR signaling.
How is the MEK-ERK pathway able to link TrkB to PSD95 synthesis in FMRP-deficient neurons when mTOR or S6K1 is inhibited? FMRP-deficient mice exhibited more sensitive ERK activation by TrkB (51), and higher phosphorylation of ribosomal protein S6 by ERK (52). Thus, stronger coupling of TrkB to the translational machinery could enable BDNF induction of new PSD95 synthesis, once excessive basal translation from mTORC1-S6K1 hyperactivity is corrected.
Improving and Extending Visualization of New Proteins.
A limitation of the current experiments is that the new copies of PSD95 being visualized derive from an introduced PSD95-TS2:YFP reporter gene. This gene contains the complete sequence of the most common PSD95 mRNA isoform, including the 5′ and 3′ untranslated regions, so that its translation should be representative of endogenous PSD95 mRNA. The introduced reporter increases the total amount of PSD95 mRNA, which could alter either basal or induced rates of PSD95 synthesis. However, the observation of local induction of new PSD95-TS2:YFP reporter protein by BDNF in wild-type neurons suggests that any changes are not substantial enough to occlude stimulus-dependent translational induction. Nevertheless, we are currently producing mice in which the TS2:YFP tag will be recombined in-frame at the 3′ end of the endogenous PSD95 gene; this will allow time-specific visualization of new PSD95 proteins while preserving native levels of PSD95 mRNA and protein.
Finally, our results raise the possibility that the molecular phenotype of deficient local protein expression may be a useful assay for identifying potential therapies for FXS. As a synaptic protein whose dysregulation in FMRP-deficient neurons can be corrected by small-molecule drugs, PSD95 may be a useful indicator of functioning local protein synthesis pathways. It will be interesting to determine if other dendritically synthesized proteins are also regulated locally by BDNF and dysregulated in FMRP-deficient neurons, and if mTORC1 or S6K1 inhibition can also rescue these defects in FMRP-deficient neurons.
Materials and Methods
Neuronal Cultures.
For cultures of neurons for local BDNF stimulation, compartmentalized chambers were fabricated as previously described (12). A P0 rat glia feeder layer was plated into the chambers, followed 1–3 d later by P0 neurons from WT mice (C57BL/6J; 000664; Jackson Laboratory) or FMRP-deficient mice (B6.129P2-Fmr1tm1Cgr/J; 003025; Jackson Laboratory) electroporated with a PSD95-TS2:YFP reporter construct. Animal procedures were approved by the Stanford Institutional Animal Care and Use Committee. More details of the chambers, animals, and culture procedures are provided in SI Appendix.
Microscopy and Immunoblotting.
Experiments were performed at 14–18 d in vitro. For live microscopy, cultures were imaged at 33 °C by confocal microscopy in a CO2-independent medium during incubation in ASV to preserve YFP fluorescence on new copies of PSD95, with stimulation by BDNF in one chamber, without or with pharmacological inhibitors of signaling pathways. Images were acquired blinded and analyzed in an automated manner. For immunoblotting, asunaprevir, pharmacological inhibitors, and BDNF were applied to neuronal cultures in 12- or 24-well plates, and cultures were maintained in neuronal culture media under 5% CO2 in a tissue-culture incubator until lysis. More details of time-lapse microscopy, automated analysis, statistical plan, chemicals, and immunoblotting are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Ms. Lan Xiang Liu for expert technical support, and other members of the M.Z.L. laboratory for useful discussions. This work was supported by a Walter V. and Idun Berry postdoctoral fellowship (to Y.Y.), a Lucille Packard Children’s Hospital Pediatric Research Fund postdoctoral grant (to Y.G.), National Research Foundation of Korea Grant NRF-2018R1A2A1A05019550 (to H.J.K. and N.L.J.), and NIH Grant R01NS076860 (to M.Z.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812056116/-/DCSupplemental.
References
- 1.Dahlhaus R., Of men and mice: Modeling the fragile X syndrome. Front. Mol. Neurosci. 11, 41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazdoba T. M., Leach P. T., Silverman J. L., Crawley J. N., Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis. Res. 3, 118–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kooy R. F., Of mice and the fragile X syndrome. Trends Genet. 19, 148–154 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Bassell G. J., Warren S. T., Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 60, 201–214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter J. D., Bassell G. J., Klann E., Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nat. Rev. Neurosci. 16, 595–605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keith D., El-Husseini A., Excitation control: Balancing PSD-95 function at the synapse. Front. Mol. Neurosci. 1, 4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer D., Bonhoeffer T., Scheuss V., Balance and stability of synaptic structures during synaptic plasticity. Neuron 82, 430–443 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Zalfa F., et al. , A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat. Neurosci. 10, 578–587 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ifrim M. F., Williams K. R., Bassell G. J., Single-molecule imaging of PSD-95 mRNA translation in dendrites and its dysregulation in a mouse model of fragile X syndrome. J. Neurosci. 35, 7116–7130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muddashetty R. S., et al. , Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell 42, 673–688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd P. K., Mack K. J., Malter J. S., The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc. Natl. Acad. Sci. U.S.A. 100, 14374–14378 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butko M. T., et al. , Fluorescent and photo-oxidizing TimeSTAMP tags track protein fates in light and electron microscopy. Nat. Neurosci. 15, 1742–1751 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cea-Del Rio C. A., Huntsman M. M., The contribution of inhibitory interneurons to circuit dysfunction in fragile X syndrome. Front. Cell. Neurosci. 8, 245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contractor A., Klyachko V. A., Portera-Cailliau C., Altered neuronal and circuit excitability in fragile X syndrome. Neuron 87, 699–715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Hulst C., et al. , Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 1121, 238–245 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Huber K. M., Gallagher S. M., Warren S. T., Bear M. F., Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U.S.A. 99, 7746–7750 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myrick L. K., Hashimoto H., Cheng X., Warren S. T., Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Hum. Mol. Genet. 24, 1733–1740 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Annessa I., Cicconardi F., Di Marino D., Handling FMRP and its molecular partners: Structural insights into fragile X syndrome. Prog. Biophys. Mol. Biol. 141, 3–14 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Alpatov R., et al. , A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response. Cell 157, 869–881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myrick L. K., et al. , Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc. Natl. Acad. Sci. U.S.A. 112, 949–956 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwell E., Zhang X., Ceman S., Arginines of the RGG box regulate FMRP association with polyribosomes and mRNA. Hum. Mol. Genet. 19, 1314–1323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darnell J. C., et al. , Kissing complex RNAs mediate interaction between the fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 19, 903–918 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y., et al. , FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1, 109–118 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Bartley C. M., et al. , Mammalian FMRP S499 is phosphorylated by CK2 and promotes secondary phosphorylation of FMRP. eNeuro 3, ENEURO.0092-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceman S., et al. , Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum. Mol. Genet. 12, 3295–3305 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Lee H. Y., et al. , Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72, 630–642 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan U., et al. , FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J. Neurosci. 27, 14349–14357 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalavadi V. C., Muddashetty R. S., Gross C., Bassell G. J., Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: A role in immediate early mGluR-stimulated translation. J. Neurosci. 32, 2582–2587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C. C., Huang C. C., Wu M. Y., Hsu K. S., Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J. Biol. Chem. 280, 18543–18550 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Schratt G. M., Nigh E. A., Chen W. G., Hu L., Greenberg M. E., BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J. Neurosci. 24, 7366–7377 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minichiello L., TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 10, 850–860 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya A., et al. , Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 76, 325–337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A., et al. , Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 30, 694–702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhakar A. L., Dölen G., Bear M. F., The pathophysiology of fragile X (and what it teaches us about synapses). Annu. Rev. Neurosci. 35, 417–443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bekinschtein P., et al. , Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron 53, 261–277 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Je H. S., et al. , Chemically inducible inactivation of protein synthesis in genetically targeted neurons. J. Neurosci. 29, 6761–6766 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw K. D., Emptage N. J., Bliss T. V., A role for dendritic protein synthesis in hippocampal late LTP. Eur. J. Neurosci. 18, 3150–3152 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse E. G., Xu B., New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell. Neurosci. 42, 81–89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harward S. C., et al. , Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 538, 99–103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartley C. M., O’Keefe R. A., Bordey A., FMRP S499 is phosphorylated independent of mTORC1-S6K1 activity. PLoS One 9, e96956 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayanan U., et al. , S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 283, 18478–18482 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolzhanskaya N., Merz G., Aletta J. M., Denman R. B., Methylation regulates the intracellular protein-protein and protein-RNA interactions of FMRP. J. Cell Sci. 119, 1933–1946 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Ferrari F., et al. , The fragile X mental retardation protein-RNP granules show an mGluR-dependent localization in the post-synaptic spines. Mol. Cell. Neurosci. 34, 343–354 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Nihonmatsu I., Ohkawa N., Saitoh Y., Inokuchi K., Targeting of ribosomal protein S6 to dendritic spines by in vivo high frequency stimulation to induce long-term potentiation in the dentate gyrus. Biol. Open 4, 1387–1394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricciardi S., et al. , Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum. Mol. Genet. 20, 1182–1196 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Hoeffer C. A., et al. , Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 11, 332–341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharya A., et al. , Targeting translation control with p70 S6 kinase 1 inhibitors to reverse phenotypes in fragile X syndrome mice. Neuropsychopharmacology 41, 1991–2000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saré R. M., et al. , Negative effects of chronic rapamycin treatment on behavior in a mouse model of fragile X syndrome. Front. Mol. Neurosci. 10, 452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Switon K., Kotulska K., Janusz-Kaminska A., Zmorzynska J., Jaworski J., Molecular neurobiology of mTOR. Neuroscience 341, 112–153 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Gross C., et al. , Isoform-selective phosphoinositide 3-kinase inhibition ameliorates a broad range of fragile X syndrome-associated deficits in a mouse model. Neuropsychopharmacology 44, 324–333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osterweil E. K., Krueger D. D., Reinhold K., Bear M. F., Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J. Neurosci. 30, 15616–15627 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawicka K., Pyronneau A., Chao M., Bennett M. V., Zukin R. S., Elevated ERK/p90 ribosomal S6 kinase activity underlies audiogenic seizure susceptibility in fragile X mice. Proc. Natl. Acad. Sci. U.S.A. 113, E6290–E6297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.