Abstract
Emotional processing is particularly sensitive to sleep deprivation, but research on the topic has been limited and prior studies have generally evaluated only a circumscribed subset of emotion categories. Here, we evaluated the effects of one night of sleep deprivation and a night of subsequent recovery sleep on the ability to identify the six most widely agreed upon basic emotion categories (happiness, surprise, fear, sadness, disgust, anger). Healthy adults (29 males; 25 females) classified a series of 120 standard facial expressions that were computer morphed with their most highly confusable expression counterparts to create continua of expressions that differed in discriminability between emotion categories (e.g., combining 70% happiness+30% surprise; 90% surprise+10% fear). Accuracy at identifying the dominant emotion for each morph was assessed after a normal night of sleep, again following a night of total sleep deprivation, and finally after a night of recovery sleep. Sleep deprivation was associated with significantly reduced accuracy for identifying the expressions of happiness and sadness in the morphed faces. Gender differences in accuracy were not observed and none of the other emotions showed significant changes as a function of sleep loss. Accuracy returned to baseline after recovery sleep. Findings suggest that sleep deprivation adversely affects the recognition of subtle facial cues of happiness and sadness, the two emotions that are most relevant to highly evolved prosocial interpersonal interactions involving affiliation and empathy, while the recognition of other more primitive survival-oriented emotional face cues may be relatively robust against sleep loss.
Keywords: Sleep deprivation, Emotion recognition, Facial expression, Perception
Highlights
-
•
Recognition of facial expression of emotion has been shown to be affected by sleep deprivation.
-
•
Sleep deprivation affected recognition of prosocial emotional expressions (happy and sad).
-
•
Recognition of primitive survival oriented facial expressions were unaffected by sleep loss.
1. Introduction
There is an emerging consensus that sleep plays a vital role in recalibrating the emotional functioning of the brain (Walker and Van Der Helm, 2009; Walker, 2009). Without sufficient sleep, there appears to be a reduction in emotional regulation capacities and a loss of perceptual sensitivity to cues that provide critical emotional information about the external environment and internal milieu (Goldstein-Piekarski et al., 2015). Notably, Yoo and colleagues showed that compared to the sleep-rested state, sleep deprivation was associated with increased amygdala responses to negatively valenced visual images (e.g., mutilated bodies; unsanitary conditions; aggressive scenes), and reduced functional connectivity between the top down emotion regulating regions of the medial prefrontal cortex and the emotionally responsive amygdala (Yoo et al., 2007). The findings suggest that without sleep, there is a weakening of the ability of higher order brain regions to exert regulatory control over more primitive threat detection systems, leading to greater emotional reactivity. In a parallel study from the same lab, Gujar and colleagues demonstrated that sleep deprivation produced similar increases in limbic and paralimbic regions to positively valenced images as well (Gujar et al., 2011), suggesting that sleep loss increases emotional reactivity to both positive and negative stimuli. This has led to the suggestion that sleep deprivation may globally lower the threshold for emotional activation, regardless of valence, thus increasing overall sensitivity to emotional stimuli (Simon et al., 2015).
The effects of sleep deprivation are not limited to emotionally evocative scenes, but also affect how the human brain responds to facial expressions of emotion. Huck and colleagues conducted one of the earliest investigations of the effects of sleep deprivation and stimulant countermeasures on the ability to accurately identify emotional displays depicted in photographs of facial expressions (Huck et al., 2008). In that study, participants completed two tasks, one involving categorization of simple photographs of six basic emotions, and the other involving categorization of complex blended images created by morphing pairs of highly confusable emotions (e.g., fear+surprise) from the same set of six primary emotions. While accuracy for simple emotion perception was not affected by sleep deprivation or stimulants, the ability to accurately identify the dominant emotion within complex emotional blends was adversely affected by sleep deprivation and was restored by stimulant medications (Huck et al., 2008). Whereas Huck and colleagues did not examine the effects of sleep loss on accuracy for the six specific emotions, a subsequent study by van der Helm and colleagues focused on recognition of three separate emotions, including happy, sad, and angry facial expressions (Van Der Helm et al., 2010). They presented participants with a set of morphed face photographs that ranged in intensity from neutral to full strength for each prototypical emotion. The authors found that sleep deprivation led to a significant impairment of recognition for angry and happy expressions, but only in the middle range of intensity—an effect that was most prominent in female participants. More recently, Cote and colleagues examined a broader range of facial affects, including happy, sad, angry, and fear and found that sleep deprivation impaired recognition of sadness in simple full face expressions as well as faces morphed to a moderate level of intensity (Cote et al., 2014). Finally, Goldstein-Piekarski and colleagues presented participants with a series of computer generated faces differing on a continuum from safe to highly threatening in appearance and found that sleep deprivation led to a bias toward overestimating the threat in faces, a finding that was associated with altered viscerosensory brain activation (Goldstein-Piekarski et al., 2015). Thus, it is clear that sleep deprivation leads to an impairment in recognition of some aspects of facial affect, particularly when there is some ambiguity in the expressions, but there is no consensus regarding the specific emotions that are most sensitive to these effects.
To provide additional insights into this topic, here we provide a further analysis of the data presented in our previous article (Huck et al., 2008). In that paper, we only reported total recognition accuracy scores that were collapsed across all six expressions. However, given the current interest in the topic, we believe that it would be informative to provide additional unpublished data regarding the effects of sleep deprivation and recovery sleep on accuracy for recognizing the dominant emotional expressions in morphed blends of highly confusable emotions based on the six universal facial expressions, including happiness, sadness, surprise, fear, disgust, and anger. Based on the aforementioned literature, we hypothesized that sleep deprivation would lead to sustained accuracy for ambiguous blends of confusable faces with predominantly high threat relevance (i.e., Anger, Fear, Surprise), whereas morphed expressions with predominantly social/affiliative relevance (i.e., Happiness, Sadness) would be most susceptible to degraded recognition from sleep loss.
2. Method
2.1. Participants
A total of 54 (29 male; 25 female) healthy young adults (Mean Age=23.5, SD=4.0) volunteered for a larger study of the effects of sleep deprivation and stimulant countermeasures on various aspects of cognitive functioning. While related findings from this dataset have been reported elsewhere (Huck et al., 2008), here we present previously unpublished findings and re-analysis of those data regarding the effects of sleep deprivation on the ability to recognize the six universal basic emotions. All participants underwent a physical examination prior to entry into the study and were deemed to be physically healthy by the examining physician. Exclusionary criteria included any history of sleep disorder, psychiatric illness, drug or alcohol abuse, cardiac problems, current pregnancy, or other health issue that would pose a risk for participating in a sleep deprivation study. Volunteers were also excluded for any history of tobacco use in the preceding three years or daily caffeine intake in excess of 400 mg/day. Participants were required to abstain from alcohol, stimulants, or other psychoactive drugs for 48 h prior to participation. Abstinence from stimulants was verified via urine drug screen at time of entry into the study and every 24 h thereafter. Written informed consent was obtained prior to enrollment and all participants were compensated for their time in the lab and were also offered a performance bonus for demonstrated effort on all study tasks. The protocol for this study was approved by the Walter Reed Army Institute of Research Human Use Review Committee and the U.S. Army Human Subjects Research Review Board. This material has been reviewed by the Walter Reed Army Institute of Research and there is no objection to its presentation and/or publication.
2.2. Materials and procedure
As part of the larger study, participants underwent a four night continuous in-residence laboratory study, which consisted of a baseline acclimation night involving 8-h enforced time in bed from 2300 to 0700, a 61-h period of monitored continuous wakefulness during which time participants completed a variety of cognitive and performance tasks, a 12-h enforced recovery sleep opportunity from 2000 to 0800, and a post-recovery day involving additional cognitive and performance tasks. The present analysis is focused primarily on the outcome of the Ekman Hexagon Test (EHT; Thames Valley Test Company, Suffolk, UK), which was administered several times during the course of the study. The EHT is a computer-administered task that required the participant to classify each of a series of displayed facial expression photographs according to one of six different emotion labels (happiness, surprise, fear, sadness, disgust, anger). As depicted in Fig. 1, each face was displayed on the screen with six different emotion labels located below the face. Label order was randomized at each presentation. The participant used a mouse to click on the label that best represented the displayed emotion for each trial. Each facial photograph was displayed for up to five seconds, after which the photograph disappeared but the labels remained until a response was made. There was no time limit for responses. The EHT comprised 150 trials and all photographs were of the same male individual poser.
Fig. 1.
Example of the Emotion Hexagon Test. Participants were shown a series of 150 facial expressions that comprised morphed blends of pairs of highly confusable emotions (e.g., 70% disgust+30% sadness) for five seconds and used the computer cursor to select the most accurate label for each expression.
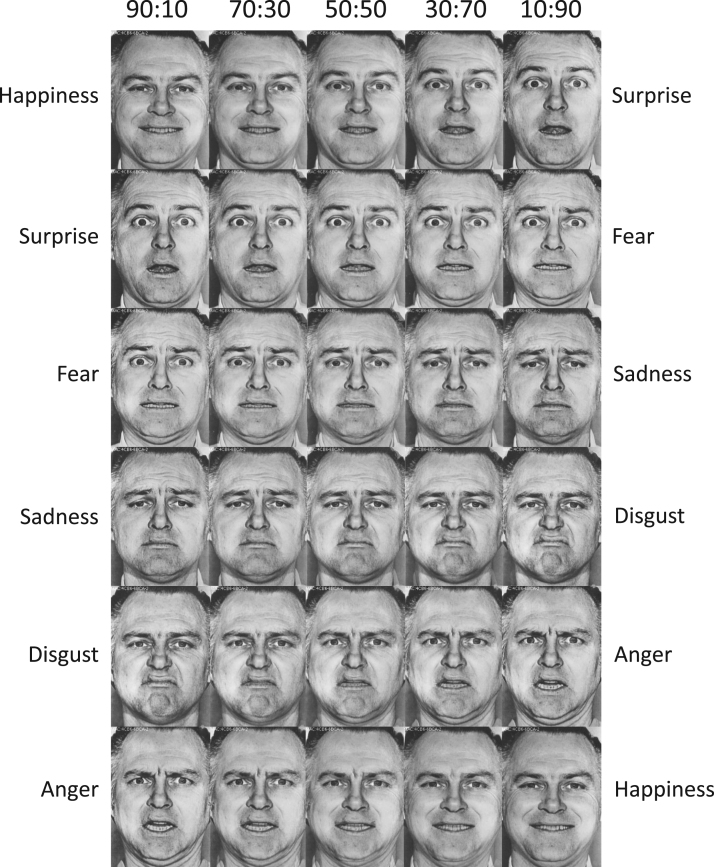
To increase the emotion processing demands of the task, the face stimuli were previously morphed to produce a continuum of facial blends. Briefly, as described in the EHT manual, each basic emotion photograph was computer morphed with its two most similar appearing and frequently confused counterpart emotions (e.g., fear was morphed with surprise and fear was morphed with sadness to create a continuum of expressions from surprise to fear to sadness). For example, two face images would be combined to create a new image depicting 70% fear and 30% surprise. Each morphed image was created in by combining two prototype images according to the following ratios: 90:10; 70:30; 50:50; 30:70; 10:90. As depicted in Fig. 2, this process yielded 30 combinations of facial expressions (6 emotions×5 blend levels), that comprised a continuum of emotional blends, with those closest to the 50:50 ratio being most difficult to discriminate. Although the 50:50 images were presented during the EHT, they were not calculated in the final scores. Since there is no objectively correct response for the 50:50 faces (i.e., either response is correct), those stimuli will not be discussed further here. Thus 120 blended images were presented (20 for each emotion) and recorded as correct or incorrect for each response.
Fig. 2.
The continuum of faces used for the Emotion Hexagon Test. A total of 30 morphed faces of the same male poser were used. Each face was a morph between two neighboring faces at varying ratios (e.g., 90% happiness+10% surprise). The blended expressions are listed along the sides of the continua and the proportions of each expression included in the face are listed along the top axis.
The EHT was administered at 1230 on the baseline day (i.e., after 5.5 h of wakefulness), at 0630 on the first morning following one night of sleep deprivation (i.e., after 23.5 h of wakefulness), again at 0540 on the second morning of sleep deprivation (i.e., after 46.7 h of wakefulness and stimulant medication administration), and finally at 1200 on the final day, following 12 h of recovery sleep (i.e., after 4 h of wakefulness). Data from the 46.7-h post-stimulant session were reported in great detail in a previous publication (Huck et al., 2008) and will not be further analyzed or discussed in the present article. Thus, we report here data for baseline, one night of sleep deprivation (no stimulants), and recovery.
2.3. Analysis
Data were analyzed in IBM SPSS 20. The raw number of correct items was re-calculated in terms of percent correct for each category and entered into a 3 (session)×6 (emotion category)×2 (sex) repeated measures analysis of variance (ANOVA). Because the primary effect of interest was whether sleep deprivation would lead to a significant change in emotion recognition accuracy, planned comparisons examined the effect of sleep deprivation relative to the pre-sleep deprivation and post-recovery performances within each of the specific emotion categories using polynomial planned contrasts (i.e., predicting a quadratic effect of reduced performance during SD compared to baseline and recovery). For those showing a significant quadratic effect, planned simple effects paired comparisons between the SD and pre- and post-recovery means were conducted. Reaction time (RT) data from the PVT were converted to a metric of psychomotor speed (i.e., 1/RT×1000). All significance tests were evaluated at α=.05.
3. Results
The multivariate tests for the repeated measures ANOVA revealed a significant main effect of session, F(2,51)=8.33, p=.001, a significant main effect of emotion type, F(5,48)=23.15, p<.0001, and a significant session×emotion type interaction, F(10,43)=3.69, p=001. Because these findings were not significantly affected by the sex of the participant, as indicated by a non-significant sex×session×emotion type interaction, F(10,43)=0.83, p=.60, data were combined for the sample as a whole for subsequent analyses.
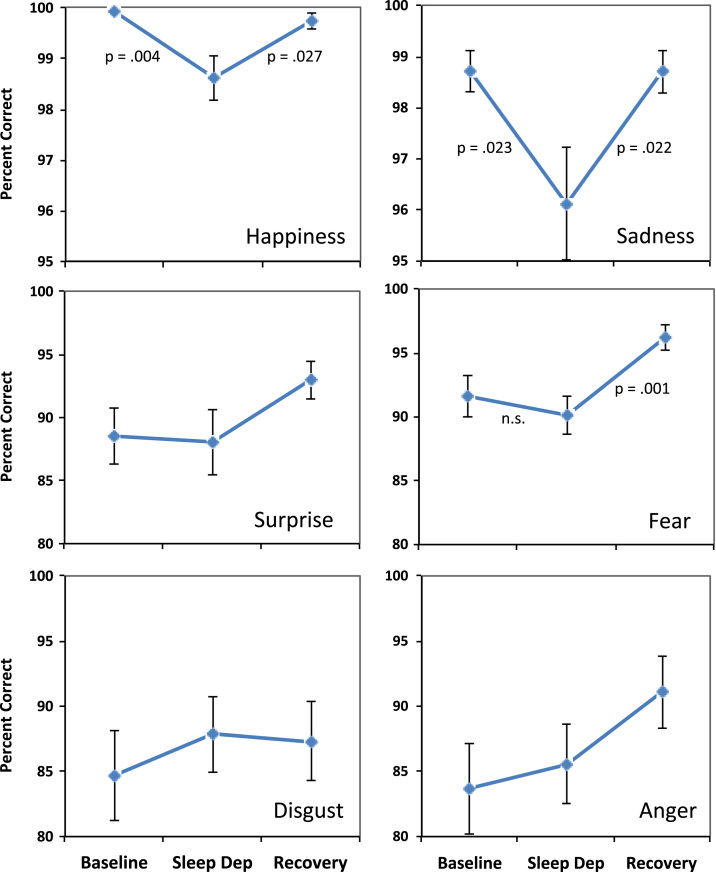
Fig. 3 shows the plots for the individual emotion categories separately. Planned comparisons showed that for happiness, there was a significant quadratic effect, F(1,52)=7.12, p=.01, suggesting an effect of sleep deprivation on accuracy judgments. This was confirmed by planned simple effects comparisons showing a decrease in accuracy from baseline to sleep deprivation (p=.004) and an increase from sleep deprivation to post-recovery (p=.027). Similarly, for sadness, there was a significant quadratic effect, F(1,52)=5.85, p=.019, and further planned comparisons suggested that this was indeed due to a significant decline in accuracy from baseline to sleep deprivation (p=.023) and a significant increase from SD to post-recovery (p=.022). On the other hand, for surprise, there was no significant quadratic effect, F(1,52)=1.73, p=.19, suggesting no effect of sleep deprivation. While there was a significant quadratic effect for fear, F(1,52)=5.92, p=.018, there was no significant decline from baseline to sleep deprivation (p=.39), although there was an increase from sleep deprivation to post-recovery (p=.001). No significant quadratic effect was observed for either disgust, F(1,52)=0.98, p=.33, or anger, F(1,52)=.68, p=.41, suggesting no effect of sleep deprivation on the accuracy of these emotional judgments.
Fig. 3.
The figures show the accuracy of recognition performance for each blended emotion at baseline, 23.5-h of sleep deprivation, and again following a 12-h opportunity for recovery sleep. The top panels show that sleep deprivation was associated with a significant decline in the percent of correct responses for faces with dominant expressions of happiness or sadness. None of the other emotional expressions showed significant declines in perception accuracy with sleep deprivation.
4. Discussion
Human survival has long depended on the ability to accurately read and infer the emotional states of others. Because the face communicates the physical and affective condition, motivation, and potential intentions of those in close proximity, it provides a crucial source of information about factors that could affect survival and wellbeing, including the presence or absence of danger, the availability of resources, the needs of others, and the potential for social inclusion and affiliation (Blair, 2003). We examined the effect of one night of sleep deprivation on the ability to accurately identify the six most broadly accepted basic human facial expressions of emotion (Ekman, 1992) under conditions of varying ambiguity (i.e., each target emotional expression was partially blended with another emotional expression with which it is often confused). Consistent with our hypothesis, we found that sleep deprivation adversely affected the recognition of target expressions involving happiness and sadness, emotions that often relate most strongly to social and affiliative behaviors, while having no discernable effect on the ability to recognize target facial expressions communicating the potential for immediate threat, peril, or danger (i.e., expressions of surprise, fear, disgust, and anger). Together, these findings suggest that during periods of compromised cognitive-emotional capacity induced by sleep loss, the brain may preserve emotional recognition resources necessary for responding to threat-relevant stimuli at the expense of cognitive resources available for sustaining less urgent socio-emotional recognition processes that play a role in empathy, social closeness, and affiliative behavior.
Our findings contribute to a rapidly emerging literature on the effects of sleep deprivation on emotional processing. Notably, a small number of recent studies have examined the effects of sleep deprivation specifically on the accuracy and perceived intensity of emotional face perception. Evidence suggests that sleep deprivation adversely affects the perception of facial emotion, particularly when facial cues are somewhat ambiguous due to morphing or blending of features (Huck et al., 2008). Most studies that have found effects of sleep deprivation on emotion perception have utilized some sort of computer morphing technique to scale images to varying levels of ambiguity or intensity. Applying such techniques, van der Helm and colleagues found that a night of sleep deprivation reduced accuracy for recognizing angry and happy faces, but only in the middle ranges of intensity and only for females (Van Der Helm et al., 2010). A similar study by Cote and colleagues found that sleep deprivation reduced classification accuracy only for sad faces, particularly when the faces were more ambiguous due to morphing (Cote et al., 2014). Our findings are partially consistent with each of these studies, as we found that sleep loss affected recognition of happy faces (as found by van der Helm and colleagues) and sad faces (as found by Cote and colleagues), while other blended affects were recognized at baseline levels. While it is clear that sleep deprivation affects recognition of emotion, it is not clear why the specific emotions that were affected differed between studies. Notably, our study focused on accuracy data, while the others focused more specifically on neurophysiological methods. Our sample size was larger and included more emotion categories than either of the two preceding studies, which could account for some of the differences. The stimuli for the three studies also differ in terms of the faces used and degree of morphing employed. However, the most likely explanation is that the previous two studies focused on morphed expressions designed to differ on the dimension of intensity (i.e., from neutral to strongly emotional) within each emotion category, whereas our approach used expressions that were more ambiguous because they were morphed at full intensity across emotion categories with their nearest confusable expressions. Prior work suggests that ambiguity in expressions may activate threat-detection systems (Blasi et al., 2009, Cote et al., 2014), which may explain why the impairments were restricted to only non-threat (i.e., social-affiliative) emotions.
It should also be noted that although sleep deprivation significantly impaired the perception of happy faces, the absolute magnitude of the decline was quite small and there was an overall ceiling effect for perception of happy faces. This is not entirely surprising, as happy faces are well known to be the easiest and most rapidly identified of all expressions (Alves et al., 2009, Wells et al., 2016). This is partially due to the fact that there are more negative emotional categories to choose from than positive ones. In our study, we had five negative emotions and one positive one, which likely made it fairly easy to discriminate happy from all of the other emotions. We acknowledge that in a “real world” setting, emotion perception may be considerably more complex, especially when there may be multiple faces in view, all dynamically changing, and all competing with other attentional demands. Some evidence suggests that dynamic facial displays may engage additional brain processes compared to static expressions. Future work in this area might benefit from the inclusion of more complex types of facial stimuli, including dynamic expressions embedded with other competing stimuli.
Recent work has also examined similar effects in patients with sleep disorders. For instance, our findings are highly similar to a recent study that showed that patients with either insomnia or sleep apnea showed reduced accuracy for recognizing happy and sad faces, but not for other emotions such as anger, anxiety, fear, or disgust (Cronlein et al., 2016). This raises the possibility that chronic sleep problems may lead to alterations in emotional processing that are similar to that produced by experimental sleep deprivation and could have implications for many social interactions and work contexts. However, in a separate study, patients with psychophysiological insomnia showed no difference from healthy controls in their ability to accurately classify expressions of fear, anger, sadness, and happiness, although they did show lower intensity ratings for sad and fear expressions (Kyle et al., 2014). However, with a much smaller sample size in the latter study, it is possible that the failure to find differences between insomnia and controls may have been due to lack of power. Further research will be needed to clarify this issue in patient populations.
Here, we showed that while social-affiliative emotional expressions were adversely affected by sleep loss, those expressions communicating potentially hazardous conditions (i.e., anger, fear, surprise, and disgust) were sustained. These findings are consistent with a large literature suggesting that the human brain is hardwired to respond to emotional face stimuli and is particularly sensitive to facial cues communicating threat (Green and Phillips, 2004, Killgore et al., 2013, West et al., 2011). From the standpoint of short-term survival, it would make sense that crucial threat detection systems would be more robust against temporary sleep loss than those involved in social affiliation. While information about social and affiliative conditions is important for long-term survival and reproduction, it is probably less critical to the immediate survival of the individual during acute periods of potential danger. Sleep deprivation may place a person at heightened risk when functioning in a threatening environment because it degrades reaction time, cognitive processing, and physical capacities (Durmer and Dinges, 2005, Killgore, 2010). When compromised by sleep loss, survival would be most assured if an individual was able to sustain accurate or enhanced recognition of cues reflecting potential danger (e.g., a face expressing anger, fear, surprise, or disgust). In fact, during periods when physical and mental capacities have been impaired by sleep deprivation, it might actually be advantageous to err on the side of caution with regard to interpreting the meaning of emotional expressions, especially non-threat-related social emotions such as happiness and sadness. These emotional expressions essentially communicate that it is acceptable to lower one's defensive posture because it is safe to affiliate or socially appropriate to show empathy. However, it is not inconceivable that during periods of sleep deprivation, it may actually confer a survival advantage to misinterpret social/affiliative facial expressions of happiness or sadness as something a bit more ominous—just to be on the safe side. In other words, during situations when one's cognitive performance is compromised through sleep loss, the consequences of misreading a safe face as threatening are probably far less grave than misreading a threatening face as safe. Such a perspective is also consistent with other evidence suggesting that sleep deprived people are more likely to violate their own moral personal beliefs (Killgore et al., 2007), report lower levels of empathy (Killgore et al., 2008), and are more likely to feel anxious and suspicious (Kahn-Greene et al., 2007) than when not sleep deprived.
The aforementioned perspective is consistent with recent findings showing that sleep deprivation increases the general tendency to perceive facial expressions as “threatening” in appearance (Goldstein-Piekarski et al., 2015). All things being equal, a sleep-deprived individual shows a lower threshold for interpreting a face as potentially threatening than when normally rested. Moreover, this sleep-loss induced drop in the threshold for threat perception corresponds with changes in brain activation within a visero-sensory network comprising the dorsal anterior cingulate cortex and insula (Goldstein-Piekarski et al., 2015). These findings suggest that sleep deprivation lowers the threshold of interoceptive sensitivity of brain homeostatic systems, enhancing the threat-response system and affecting the interpretation of pro-social and antisocial cues (Goldstein-Piekarski et al., 2015, Simon et al., 2015). Findings from the animal literature are also supportive of this model, suggesting that deprivation of rapid eye movement sleep in pregnant rats increases defensive aggression and reduces the threshold for responding to potentially hostile stimuli (Pires et al., 2015). Overall, our findings are consistent with these data in animals and humans, suggesting that the accuracy of detecting facial displays that communicate clear and present danger may be more robust against sleep deprivation than those involved in detecting social and affiliative emotions.
Several limitations should be considered when interpreting these findings. First, we did not collect data on emotional intensity ratings in this study and it is possible that participants perceived the threat related stimuli as more arousing overall, and such increased arousal may have in turn led to greater accuracy in responding to the threat-related faces during periods of decreased alertness and vigilance. However, several studies have shown that sleep deprivation generally leads to a decrease rather than increase in the perceived intensity of emotional ratings on faces (Kyle et al., 2014; Van Der Helm et al., 2010), suggesting that increased arousal is an unlikely explanation. Second, we did not compare our data to a non-sleep deprived control group over the same time period, so it is possible that similar effects would emerge following repeated testing with the stimuli. It is therefore impossible to rule out effects of learning or carry-over effects due to repeated exposure to the stimuli across multiple sessions. The effects of learning and repeated exposure to the stimuli may have offset deficits in accurate perception of some faces, such as surprise, fear, and anger. This alternate explanation might even suggest that the threat-related emotions are more easily learned than the affiliative emotions, making them somewhat resistant to degradation by sleep deprivation. Furthermore, the fact that accuracy for fear faces was improved after the recovery night, suggests that learning/consolidation for fearful expressions may have been suppressed during sleep deprivation but re-emerged following a night of recovery sleep. In order to fully clarify the combined effects of sleep deprivation and learning, future work will need to compare repeated administrations of these stimuli to a non-sleep deprived control group. Third, the version of the task we used allowed the faces to be displayed for up to five seconds awaiting a response. Thus, there was no significant time pressure and it is possible that different results would have been obtained if the stimuli had been shown for only a fraction of a second or if a specific time pressure had been imposed. Similarly, our methods only allowed collection of accuracy data (i.e., no reaction time data were collected). However, accuracy data may not be sufficient to understand the effects of sleep deprivation on face processing, as it is well established that sleep loss significantly slows general reaction time. These questions will need to be addressed in future research. Fourth, while we utilized a set of standard basic emotional faces that have been well validated (Ekman and Friesen, 1976), it is possible that different effects would have been obtained if a more comprehensive set of emotions or expression stimuli were tested. For instance, the use of a single poser (a middle aged Caucasian male) reduces the generalizability of the findings and may not reveal the complexities of emotional face processing that would emerge with a more diverse set of stimuli in terms of age, gender, and race. At present, this study reflects the largest set of discrete emotional expressions yet tested in a laboratory sleep deprivation experiment, but more remains to be done. While our study and most others have only included a single emotion expression of happiness, emerging research suggests that there may be a number of potential positive emotions with corresponding facial displays (e.g., amusement, awe, pride, etc.) that have been relatively unexplored until recently (Keltner and Shiota, 2003; Mortillaro et al., 2011; Shiota et al., 2003). Future work should examine the effects of insufficient sleep on the perception of these other positive emotions as well. Fifth, the time of day for the testing sessions could have affected the results. Specifically, baseline and post-recovery sessions just after noon, whereas the sleep deprived session occurred early in the morning at around 0630. While this was done to maximize potential deficits in order to determine the practical effects on real-life performance, it makes it impossible to disentangle the individual effects of sleep deprivation and the circadian rhythm of performance. Future work will need to explore this effect under conditions that allow these two influences to be disambiguated. Finally, the present findings may be limited in ecological validity, as they involved fairly artificial two-dimensional displays of black and white static photographs of morphed faces. It will be important to investigate whether similar findings are also observed with more externally valid stimuli, such as dynamic displays of emotion, more diverse ethnic identities, and the incorporation of body posture and contextual cues. Despite the aforementioned limitations, the present study provides the largest sample using the broadest range of emotional facial expressions published to date to examine the effects of sleep deprivation on emotional recognition accuracy. Our findings are consistent with an adaptive survival model suggesting that during periods of sleep deprivation the brain preserves those cognitive-affective processes most relevant to threat detection at the expense of those that do not contribute to short-term survival.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This research was supported in part by an appointment to the Knowledge Preservation Program at the Walter Reed Army Institute of Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, the U.S. Government, or any of the institutions with which the authors are affiliated.
References
- Alves N.T., Aznar-Casanova J.A., Fukusima S.S. Patterns of brain asymmetry in the perception of positive and negative facial expressions. Laterality. 2009;14:256–272. doi: 10.1080/13576500802362927. [DOI] [PubMed] [Google Scholar]
- Blair R.J. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2003;358:561–572. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G., Hariri A.R., Alce G. Preferential amygdala reactivity to the negative assessment of neutral faces. Biol. Psychiatry. 2009;66:847–853. doi: 10.1016/j.biopsych.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote K.A., Mondloch C.J., Sergeeva V., Taylor M., Semplonius T. Impact of total sleep deprivation on behavioural neural processing of emotionally expressive faces. Exp. Brain Res. 2014;232:1429–1442. doi: 10.1007/s00221-013-3780-1. [DOI] [PubMed] [Google Scholar]
- Cronlein T., Langguth B., Eichhammer P., Busch V. Impaired recognition of facially expressed emotions in different groups of patients with sleep disorders. PLoS One. 2016;11:e0152754. doi: 10.1371/journal.pone.0152754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer J.S., Dinges D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Ekman P. Are there basic emotions? Psychol. Rev. 1992;99:550–553. doi: 10.1037/0033-295x.99.3.550. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Consulting Psychologists Press; Palo Alto, CA: 1976. Pictures of Facial Affect. [Google Scholar]
- Goldstein-Piekarski A.N., Greer S.M., Saletin J.M., Walker M.P. Sleep deprivation impairs the human central and peripheral nervous system discrimination of social threat. J. Neurosci. 2015;35:10135–10145. doi: 10.1523/JNEUROSCI.5254-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.J., Phillips M.L. Social threat perception and the evolution of paranoia. Neurosci. Biobehav. Rev. 2004;28:333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Gujar N., Yoo S.S., Hu P., Walker M.P. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck N.O., Mcbride S.A., Kendall A.P., Grugle N.L., Killgore W.D.S. The effects of modafinil, caffeine, and dextroamphetamine on judgments of simple versus complex emotional expressions following sleep deprivation. Int. J. Neurosci. 2008;118:487–502. doi: 10.1080/00207450601125907. [DOI] [PubMed] [Google Scholar]
- Kahn-Greene E.T., Killgore D.B., Kamimori G.H., Balkin T.J., Killgore W.D.S. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–221. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Keltner D., Shiota M.N. New displays and new emotions: a commentary on Rozin and Cohen. Emotion. 2003;3:86–91. doi: 10.1037/1528-3542.3.1.86. (discussion 92-96) [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Kahn-Greene E.T., Lipizzi E.L., Newman R.A., Kamimori G.H., Balkin T.J. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9:517–526. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Killgore D.B., Day L.M., Li C., Kamimori G.H., Balkin T.J. The effects of 53 h of sleep deprivation on moral judgment. Sleep. 2007;30:345–352. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Schwab Z.J., Tkachenko O. Emotional intelligence correlates with functional responses to dynamic changes in facial trustworthiness. Soc. Neurosci. 2013;8:334–346. doi: 10.1080/17470919.2013.807300. [DOI] [PubMed] [Google Scholar]
- Kyle S.D., Beattie L., Spiegelhalder K., Rogers Z., Espie C.A. Altered emotion perception in insomnia disorder. Sleep. 2014;37:775–783. doi: 10.5665/sleep.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortillaro M., Mehu M., Scherer K. Subtly different positive emotions can be distinguished by their facial expressions. Social. Psychol. Pers. Sci. 2011;2:262–271. [Google Scholar]
- Pires G.N., Tufik S., Andersen M.L. Effects of REM sleep restriction during pregnancy on rodent maternal behavior. Rev. Bras. Psiquiatr. 2015;37:303–309. doi: 10.1590/1516-4446-2014-1629. [DOI] [PubMed] [Google Scholar]
- Shiota M.N., Campos B., Keltner D. The faces of positive emotion: prototype displays of awe, amusement, and pride. Ann. N. Y. Acad. Sci. 2003;1000:296–299. doi: 10.1196/annals.1280.029. [DOI] [PubMed] [Google Scholar]
- Simon E.B., Oren N., Sharon H. Losing neutrality: the neural basis of impaired emotional control without sleep. J. Neurosci. 2015;35:13194–13205. doi: 10.1523/JNEUROSCI.1314-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Helm E., Gujar N., Walker M.P. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33:335–342. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.P. The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker M.P., Van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol. Bull. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L.J., Gillespie S.M., Rotshtein P. Identification of emotional facial expressions: effects of expression, intensity, and sex on eye gaze. PLoS One. 2016;11:e0168307. doi: 10.1371/journal.pone.0168307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G.L., Anderson A.A., Ferber S., Pratt J. Electrophysiological evidence for biased competition in V1 for fear expressions. J. Cogn. Neurosci. 2011;23:3410–3418. doi: 10.1162/jocn.2011.21605. [DOI] [PubMed] [Google Scholar]
- Yoo S.-S., Gujar N., Hu P., Jolesz F.A., Walker M.P. The human emotional brain without sleep - a prefrontal amygdala disconnect. Curr. Biol.: CB. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]