Significance
Although exposure to fine particulate matter (PM) during pregnancy is linked to high risks of adverse pregnancy outcomes and long-term postnatal health, limited mechanistic data exist to assess these impacts under controlled exposure conditions. Here we show that maternal exposure to ultrafine ammonium sulfate aerosols impacts prenatal and postnatal organogenesis in offspring and predisposes metabolic syndrome in adult life. Our animal model reveals increased stillbirths; reduced gestation length and birth weight; increased concentrations of glucose and free fatty acids in plasma; enhanced lipid accumulation in the liver; and decreased endothelium-dependent relaxation of aorta. Understanding the impacts and elucidating the underlying mechanisms for maternal PM exposure are essential in developing strategies to reduce adverse health effects on conceptus/postnatal growth and development.
Keywords: air pollution, pregnancy outcomes, postnatal health, metabolism
Abstract
Exposure to fine particulate matter (PM) during pregnancy is associated with high risks of birth defects/fatality and adverse long-term postnatal health. However, limited mechanistic data are available to assess the detailed impacts of prenatal PM exposure. Here we evaluate fine PM exposure during pregnancy on prenatal/postnatal organogenesis in offspring and in predisposing metabolic syndrome for adult life. Between days 0 and 18 of gestation, two groups of adult female rats (n = 10 for each) were placed in a dual-exposure chamber device, one with clean ambient air (∼3 µg·m−3) and the other with ambient air in the presence of 100 to 200 µg·m−3 of ultrafine aerosols of ammonium sulfate. At birth (postnatal day 0, PND0), four males and four females were selected randomly from each litter to be nursed by dams, whereas tissues were collected from the remaining pups. At PND21, tissues were collected from two males and two females, whereas the remaining pups were fed either a high- or low-fat diet until PND105, when tissues were obtained for biochemical and physiological analyses. Maternal exposure to fine PM increased stillbirths; reduced gestation length and birth weight; increased concentrations of glucose and free fatty acids in plasma; enhanced lipid accumulation in the liver; and decreased endothelium-dependent relaxation of aorta. This lead to altered organogenesis and predisposed progeny to long-term metabolic defects in an age-, organ-, and sex-specific manner. Our results highlight the necessity to develop therapeutic strategies to remedy adverse health effects of maternal PM exposure on conceptus/postnatal growth and development.
With increasing urbanization, industrialization, and economic growth among developing/developed countries worldwide, air pollution has emerged as one of the greatest public health epidemics in the 21st century (1–5). According to the World Health Organization, 9 in 10 people breathe air containing high levels of pollutants, and one in nine of the global deaths is attributed to exposure to air pollution, reaching over total 7 million premature deaths each year (4, 5). Air pollution represents an environmental problem not only in developing countries but also in developed countries. For example, despite major progress made to improve air quality in the United States, ∼111 and 53 million people nationwide still inhabited places with pollutant levels exceeding the National Ambient Air Quality Standards and above the annual and/or 24-h particulate matter (PM) standard, respectively, in 2017 (6). There is accumulating evidence that several critical events in embryonic development during pregnancy are compromised by air pollution. Epidemiological studies have shown that maternal exposure to fine PM (particles with an aerodynamic diameter small than 2.5 μm, PM2.5) is associated with high risks for preterm births, low birth weights, stillbirths, and adverse postnatal health conditions that include both pulmonary and nonpulmonary (e.g., cardiovascular and metabolic) diseases (7–9).
However, large uncertainties exist concerning the detailed impacts of maternal exposure to fine PM and the underlying mechanisms. Noticeably, only limited approaches are available for investigating the health effects of air pollution and for preventing and treating the related health outcomes. While epidemiological studies have been widely adopted to evaluate the health effects of air pollution on humans, such an approach yields little mechanistic results on adverse outcomes and long-term health effects. In addition, direct experimental exposure of humans to high levels of air pollutants is harmful. However, well-controlled exposure experiments using animal models offer an alternative approach to assess the impacts of air pollution and to develop strategies to mitigate the adverse effects on human health (10). Several previous studies investigated the effects of exposure of gestating mice or rats to air pollutants on conceptus growth and postnatal health, including oxidative stress, inflammation, vascular dysfunction, and increased susceptibility to metabolic syndrome including obesity and diabetes (11–17). In addition, a recent study revealed that in utero ultrafine PM exposure leads to offspring pulmonary immunosuppression in offspring (10). However, little is known about the effects of maternal exposure to PM on prenatal and postnatal organogenesis in offspring. In this study, we performed experiments using Sprague-Dawley rats, which have been commonly employed in medical and nutritional research. A dual-exposure chamber apparatus was used in the animal experiments (SI Appendix, Fig. S1), one with clean ambient air (referred to as clean air, CA) with an average PM mass concentration of 3 µg⋅m−3 and the other with ambient air in the presence of 100 to 200 µg⋅m−3 (referred to as polluted air, PA) of aerosols consisting of ammonium sulfate and with a peak diameter of 10 to 20 nm (SI Appendix, Fig. S2). Our animal model replicated several key PM properties (such as the mass concentration, chemical composition, size, hygroscopicity, and acidity) in clean ambient air and during polluted haze episodes in Asia (such as in China and India) and was characterized by well-controlled consumption of food, energy, protein, and other nutrients by rats during exposure. Our work focused on the impacts of maternal PM exposure on prenatal and postnatal organogenesis in offspring and in predisposing long-term metabolic syndrome in adult life (Materials and Methods).
Maternal Parameters
Maternal body weight (BW) increased (P < 0.05) progressively, but food intake and water consumption per kilogram of BW did not change (P > 0.05) during gestation in the CA and PA groups (SI Appendix, Table S1). For both groups of dams, food intake and water consumption per kilogram of BW increased (P < 0.05) progressively during the 21-d period of lactation, whereas maternal BW on day 21 of lactation was less (P < 0.05) than that on day 7 of lactation. Maternal BW, food intake, or water consumption during gestation or lactation did not differ (P > 0.05) between CA and PA dams between days 0 and 18 of gestation (SI Appendix, Table S1). At weaning on postnatal day 21 (PND21), the absolute weights and relative weights of all maternal organs except the lungs were not different (P > 0.05) between the CA and PA groups (SI Appendix, Table S2). However, PM exposure during gestation increased (P < 0.05) the absolute weights and the relative weights of the lungs by 8.4% and 8.9%, respectively.
Pregnancy Outcomes
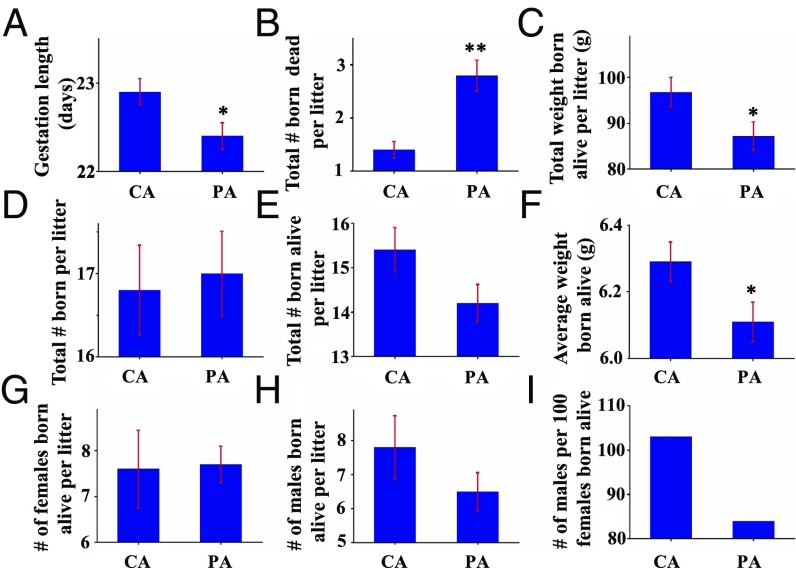
Gestation length decreased (P < 0.05) by 0.5 d, the number of pups born dead increased (P < 0.01) by 1.4 per litter, total birth weight for live-born pups per litter decreased (P < 0.01) by 10%, but the total number of pups born per litter did not differ (P > 0.05) (Fig. 1 A–D and SI Appendix, Table S3) for PM-exposed dams. The number of pups born alive per litter was smaller in the PA group than in the CA group (Fig. 1E), but the difference was statistically insignificant (P = 0.082). The average weight of pups born alive decreased (P < 0.05) by 3% for the PA group compared with the CA group (Fig. 1F). The number of females born alive per litter was comparable in both CA and PA groups, while the number of males born alive per litter was lower in the PA group than in the CA group (Fig. 1 G and H). The secondary sex ratio (i.e., the ratio between the numbers of male and female pups) in the CA group (1.03) was higher than that in the PA group (0.84), although the difference was not of statistical significance (P > 0.05) (Fig. 1I).
Fig. 1.
Adverse pregnancy outcomes in rats. Values are mean ± SEM. *P < 0.05. **P < 0.01. (A) Gestation length (days), (B) total number born dead per litter, (C) total weight born alive per litter (grams), (D) total number born per litter, (E) total number born alive per litter, (F) average weight of pups born alive (g), (G) number of females born alive per litter, (H) number of males born alive per litter, and (I) number of males per 100 females born alive.
BWs and Absolute/Relative Organ Weights of Pups at PND0 and PND21
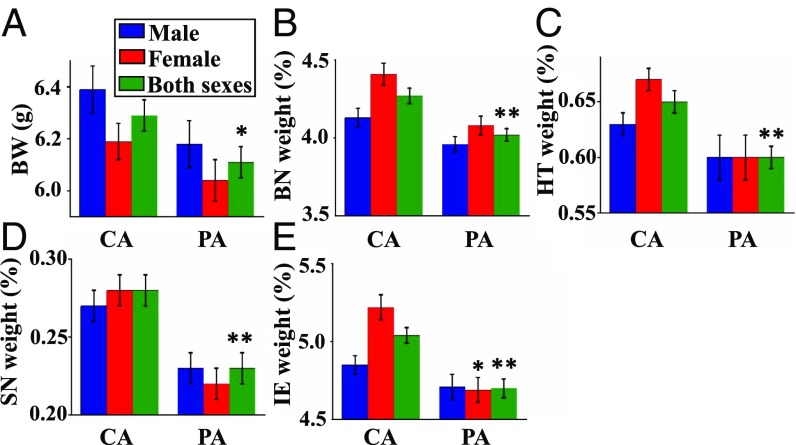
The absolute BWs and relative organ weights of males, females, and both sexes at PND0 and PND21 are displayed in Figs. 2 and 3 (see also SI Appendix, Table S4). The average BWs of males and females exhibited a similar decreasing trend for both sexes (Fig. 1F) when comparing the PA group to the CA group at both PND0 and PND21 (Figs. 2A and 3A). The mean BW of pups at PND 21 was 4.3% lower (P < 0.05) in the PA group than in the CA group, similar to the results at PND0. As evident from the absolute organ weights at PND0 and PND21 (SI Appendix, Table S5), gestational exposure to PM reduced absolute weights of the heart (P < 0.01), spleen (P < 0.01), brain (P < 0.01), pancreas (P < 0.05), testes (P < 0.05), and brown adipose tissue (BAT) (P < 0.05) at birth, compared with the CA group. At PND21, the absolute weights of the liver (P < 0.05), kidneys (P < 0.05), brain (P < 0.05), and lungs (P < 0.01) were lower in the PA group than in the CA group.
Fig. 2.
BWs and relative organ weights of pups at PND0 (birth). Relative organ weights correspond to the percentages of the BW. Values are mean ± SEM. *P < 0.05. **P < 0.01. (A) BW (grams), (B) relative brain weight, (C) relative heart weight, (D) relative spleen weight, and (E) relative intestine weight. BN, brain; HT, heart; SN, spleen; IE, intestine.
Fig. 3.
BWs, relative organ weights of pups, and plasma metabolites at PND21 (weaning). Relative organ weights correspond to the percentage of the BW. Values are mean ± SEM. *P < 0.05. **P < 0.01. (A) BW (grams), (B) relative brain weight, (C) relative lungs weight, (D) relative spleen weight, (E) relative thymus weight, and (F) plasma triacylglycerol concentration. BN, brain; LG, lungs; SN, spleen; TS, thymus.
The relative organ weights of pups at PND0 and PND21 are shown in Figs. 2 and 3, respectively. Compared with the CA group, PM exposure during gestation reduced (P < 0.01) the relative weights of the brain, heart, spleen, and intestine on PND0 (Fig. 2 B–E). At PND21, the relative weights of the spleen and thymus were greater (P < 0.05), but the relative weights of the brains and lungs were lower (P < 0.05) in the PA group than in the CA group (Fig. 3 B–E). The relative weights of other organs at PND0 or PND21 did not differ (P > 0.05) between the CA and PA groups (SI Appendix, Table S6).
Postweaning Growth of Offspring
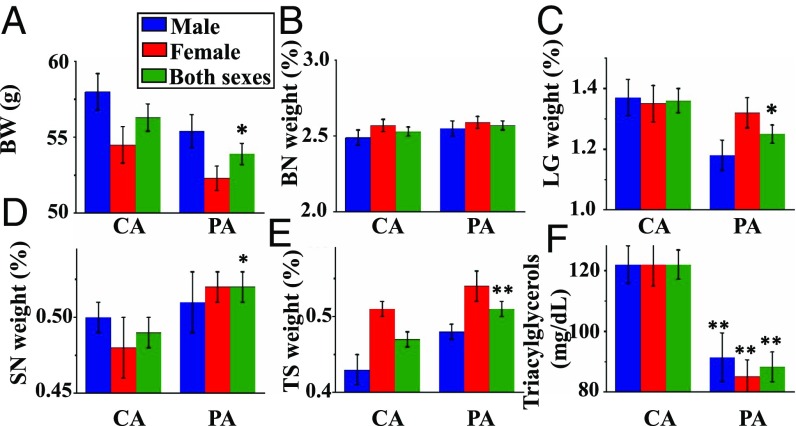
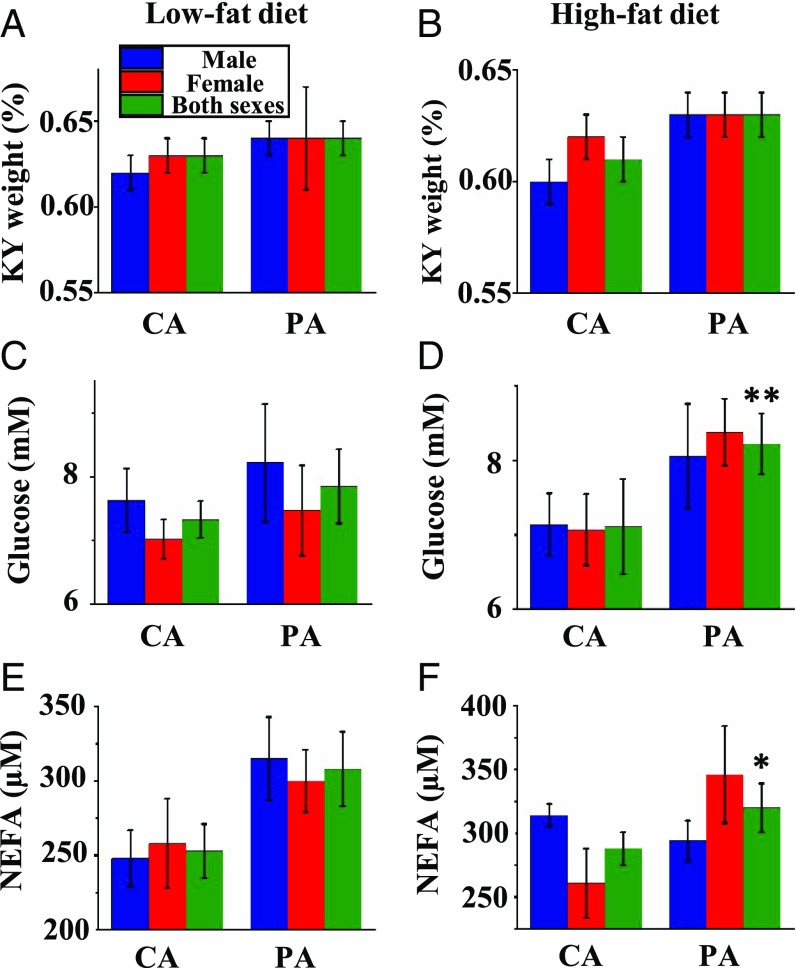
After weaning, all rats were fed either a high-fat or low-fat diet until PND105. Feed intake (SI Appendix, Table S7) and water consumption (SI Appendix, Table S8) for rats per kilogram of BW decreased (P < 0.01) progressively during the postweaning period in both groups. Since the high-fat diet was denser in nutrient content than the low-fat diet, postweaning rats fed the high-fat diet consumed less food, but similar amounts of dietary energy, protein, vitamins, and minerals per kilogram of BW (P > 0.05), compared with those fed the low-fat diet (SI Appendix, Table S7). At PND105, high-fat feeding increased (P < 0.01) BWs, white adipose tissue weight in various fat depots, and mesenteric lymph node weight of offspring in both CA and PA groups (SI Appendix, Table S9). The absolute weights of other organs did not differ (P > 0.05) between the low- and high-fat groups (SI Appendix, Table S10). The weights of the small intestine were greater (P < 0.05) for offspring fed the high-fat diet than for those fed the low-fat diet in the PA group, while the weights of BAT were greater (P < 0.05) for offspring fed the high-fat diet in the CA group (SI Appendix, Fig. S3 and Table S9). The relative weight of kidneys increased (P < 0.01) by 3% (Fig. 4 A and B) for the PA group (0.635 ± 0.005% for PA and 0.616 ± 0.005% for CA, n = 40), but the absolute (SI Appendix, Table S10) or relative (SI Appendix, Table S11) weights of any other organs in offspring fed the low- or high-fat diet did not statistically differ (P > 0.05) between the CA and PA groups.
Fig. 4.
Relative organ weights and plasma metabolites at PND105 (end of study). Concentrations of glucose, triacylglycerols, and NEFAs in plasma of offspring from CA- and PA-exposed female rats at PND105 after consuming a low-fat or high-fat diet between PND21 and PND105. Values are mean ± SEM. *P < 0.05. **P < 0.01. Relative kidney weight (percent) and concentrations of plasma glucose and NEFAs in offspring were measured after consuming a low-fat diet (A, C, and E) or high-fat diet (B, D, and F).
Concentrations of Glucose and Lipids in Plasma at PND21 and PND105
At PND21, the concentrations of glucose and nonesterfied fatty acids (NEFAs) in plasma of male and female offspring did not differ (P > 0.05) between the CA and PA groups (SI Appendix, Table S12). In contrast, prenatal PM exposure decreased (P < 0.01) the concentrations of triacylglycerols in plasma of their 21-d-old male and female pups (Fig. 3F). At PND105, the concentrations of glucose, triacylglycerols, and NEFAs in plasma of male and female offspring did not statistically differ (P > 0.05) between the low- and high-fat groups (SI Appendix, Table S13). The concentrations of triacylglycerols in plasma of offspring did not statistically differ (P > 0.05) between the CA and PA groups, but the concentrations of glucose and NEFAs in plasma of offspring increased by 11% (P < 0.01) and 16% (P < 0.05), respectively, in the PA group compared with the CA group (Fig. 4 C–F and SI Appendix, Table S13).
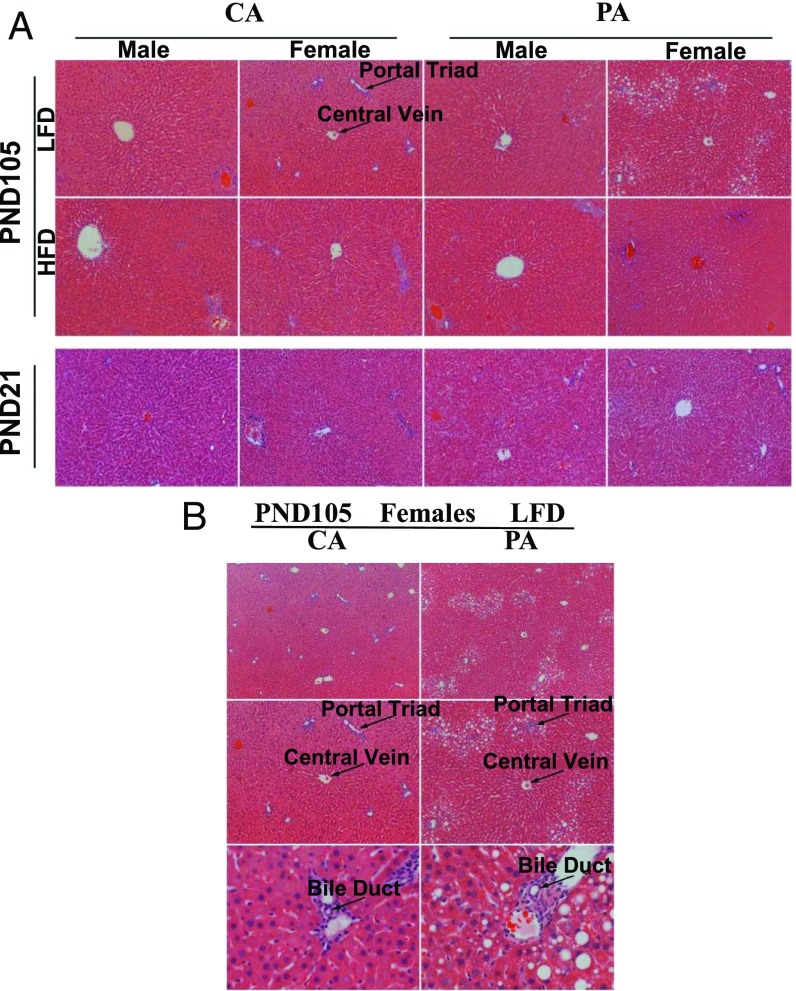
Hepatic Histology and Lipid Concentrations in Offspring
At PND21, histological analysis of the liver indicated no differences between offspring from CA and PA dams (Fig. 5A). However, at PND105, the concentrations of lipids in the liver were greater (P < 0.05) in offspring fed the high-fat diet than in those fed the low-fat diet (SI Appendix, Table S13). Particularly, at PND105, lipid droplets were accumulated near the portal triads of the hepatic lobules for female pups fed the low-fat diet in the PA group (Fig. 5B). Likewise, prenatal PM exposure increased (P < 0.05) the hepatic concentrations of lipids only in females fed the low-fat diet (SI Appendix, Table S13). Interestingly, the hepatic lipid concentrations in livers of male offspring fed the low-fat diet or in either sex of offspring fed the high-fat diet did not statistically differ (P > 0.05) between the CA and PA groups.
Fig. 5.
Female pups of the PA group fed a low-fat diet (LFD) have higher liver lipid content at PND105. (A) H&E staining of livers (width of each field = 890 µm) showed no difference in lipid content at PND21. At PND105, female pups of the PA group showed visible changes in hepatic lipid content in response to postweaning feeding of a high-fat diet (HFD), compared with an LFD. At PND105, female pups of the PA group had an accumulation of lipid droplets near the portal triads, compared with female pups of the CA group, when postweaning offspring were fed the LFD. (B) At PND105, H&E staining of livers of female pups fed the LFD (width of each field in the top, middle, and bottom rows is 1,330, 890, and 230 µm, respectively) revealed that female pups of the PA group accumulated more adipose tissue in livers, compared with those of the CA group.
Hepatic Fabp1 mRNA Levels
Expression of Fabp1 mRNA in livers of pups on PND21 did not differ (P > 0.05) between the CA and PA groups (SI Appendix, Table S14). On PND105, hepatic Fabp1 mRNA levels were reduced (P < 0.05) in female offspring from the PA dams compared with the CA dams (SI Appendix, Table S14). There was no difference (P > 0.05) in hepatic Fabp1 expression either between low-fat and high-fat groups or between male and female offspring.
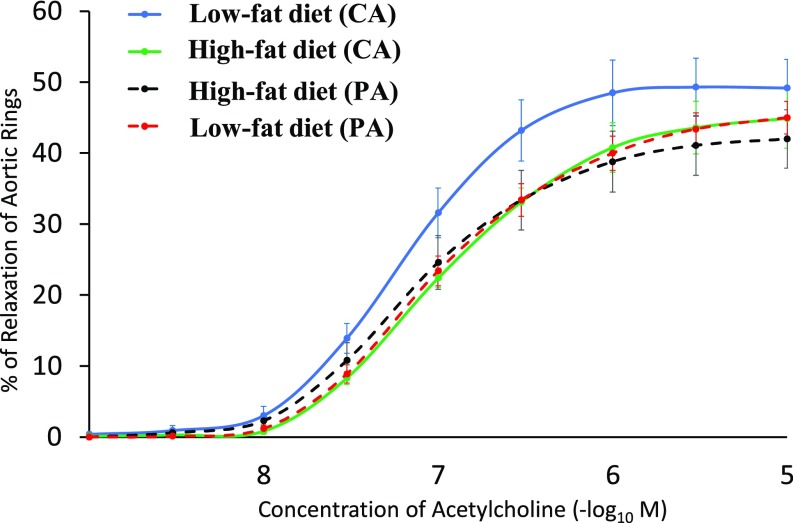
Aortic Vessel Reactivity
At PND105, thoracic aortas from male offspring were analyzed for endothelium-mediated vasoreactivity. Relaxation of aortic vessels from both CA and PA groups exhibited a dose-dependent response (P < 0.01) to extracellular acetylcholine (an inducer of endothelial NO production) ranging from 10−9 M to 10−5 M (Fig. 6). The aortas of offspring from the PA group exhibited a reduction (P < 0.05) in relaxation, compared with offspring from the CA group (Fig. 6). The difference in the relaxation of aortas between the low- and high-fat groups was statistically insignificant (P > 0.05).
Fig. 6.
Impaired relaxation of aortic rings from adult offspring in response to acetylcholine. Aortic rings were obtained on PND105 male offspring that were born to the CA and PA groups and then fed either a low-fat diet or high-fat diet after weaning. Three-way ANOVA analysis indicated that gestational PM exposure reduced (P < 0.05) aortic relaxation in offspring, compared with the CA group. Values are means ± SEM, n = 6 for the low-fat diet (CA) group and n = 7 per group for other treatment groups.
Discussion
Previous epidemiological studies have identified correlations between fine PM inhalation and adverse birth outcomes, including fetal growth restriction, small-for-gestational-age fetuses, and preterm births in humans (7–9). Also, chronic exposure of female mice to fine PM emitted by urban transportation vehicles before and during pregnancy increased postimplantation embryonic death by 70% and reduced birth weights by 20% (11). Exposure of female mice to PM during either early gestation (days 0.5 to 5.5 or 6.5 to 14.5) or early to late gestation (days 0.5 to 16.5) reduced birth weights by the same extent (12). Also, increased susceptibility of offspring to experimentally induced heart failure was identified after female mice were exposed to diesel exhaust PM before conception as well as during gestation and early postnatal life (13). In addition, exposure of gestating mice to PM2.5 during pregnancy impaired development of the cerebral cortex and induced anxiety, depression, and social behavioral changes in offspring (14). Prenatal exposure to diesel exhaust particles between embryonic days 9 and 17 induced neuroinflammation and predisposed offspring to diet-induced obesity in adulthood (15). Furthermore, exposure to ambient unfiltered air in Beijing between days 4 and 18 of gestation and during 0 and 8 wk after birth resulted in greater BWs at 8 wk of age for both female and male offspring (by 10% and 18%, respectively), lung mass (by 12% and 13%, respectively), and mass of other organs (liver, spleen, and heart), compared with a PM-filtered-air group (16). However, because food intake of animals during the experimental periods was not determined (16) or dietary energy intake by offspring was different between the treatment groups (17) in the previous studies, it is unknown whether the observed changes resulted from air pollution or differences in food and energy consumption. Interpretation of the data on metabolic disorders in the previous studies was further complicated since animals were not assigned randomly into control and treatment groups (16). Currently, limited studies under well-controlled exposure conditions are available to assess health effects due to maternal exposure to fine PM and the underlying mechanisms. Furthermore, the health outcomes of maternal PM exposure likely hinge on several PM properties, including particle mass concentration, chemical composition, size, hygroscopicity, and acidity (3, 18), and identification and isolation of these factors during maternal PM exposure represent a highly complex task (3, 18).
In our study, the consumption of food, energy, protein, and other nutrients by rats was well controlled, allowing for unambiguous assessment of the developmental and health effects of prenatal PM exposure. Our results show that exposure of gestating rats to ammonium sulfate PM adversely impacts pregnancy outcomes, as reflected by the increased rates of stillbirths, decreased litter size and pup weights, and shortened gestational length (SI Appendix, Table S3). The high rate of embryonic/fetal mortality in dams due to prenatal PM exposure is consistent with a compromised, inflammatory uterine environment for conceptus development and survival (19). The impaired growth of fetal organs likely resulted from reduced transfer of nutrients from mother to fetus due to placental dysfunction. This view is consistent with the previous finding that maternal exposure to air pollution reduces placental growth and alters placental structure in rats (16). Our exposure conditions replicated the PM properties in clean ambient air and during polluted haze episodes in Asia (such as in China and India), including the particle size, chemical composition, hygroscopicity, and acidity (20, 21). Fine PM consists of an internal mixture of inorganic (such as sulfate and nitrate), organic (primary and secondary), and other (elemental carbon and minerals), and ammonium sulfate typically represents one of the dominant constituents under ambient conditions (1). For example, the average mass fractions of ammonium sulfate in fine PM are about 51% and 31% in Houston and Los Angeles, respectively (1). In addition, when the PM2.5 mass concentration ranges from several hundreds to 1,000 μg⋅m−3 during wintertime haze events in China, fine PM contains a large fraction of ammonium sulfate (up to 40%) and is weakly acidic and strongly hygroscopic (20, 21). Hence, our rat model is suitable for investigating the impacts of gestational exposure to fine PM on fetal and postnatal organogenesis as well as the metabolism of offspring relevant to clean/polluted ambient environments in both developing and developed countries.
One of the key findings from our study is that prenatal PM exposure selectively impairs offspring organogenesis and the offspring exhibit metabolic differences in an age-, organ-, and sex-specific manner (SI Appendix, Fig. S4 and Tables S3, S4, S9, S13, and S14). Such a conclusion is evident from (i) decreases in the relative weights of the brain, heart, small intestine, and spleen at birth and of the brain and lungs at weaning, as well as increases in weights of spleen and thymus at weaning; (ii) hypertrophy of kidneys; (iii) disturbed lipid and glucose homeostasis; (iv) decreased concentrations of triacylglycerols in plasma at weaning, but increased concentrations of glucose and NEFAs as adults; and (v) abnormal elevation of hepatic lipids and reduced expression of the hepatic Fabp1 gene in female, but not male, offspring from the PA group. Sex-specific organogenesis is attributed to differences in hormones, expression of proteins, and concentrations of metabolites between males and females, likely affecting the responses of offspring to environmental changes. However, the various organs are likely affected differently by prenatal PM exposure. The increase in thymus and spleen (two lymphoid organs) reflects immunological challenges in 21-d-old pups. Note that the kidney is the only organ exhibiting a change (i.e., an increase in weight) in adult offspring from the PA group (SI Appendix, Table S15). This is explained by a compensatory mechanism for offspring with prenatal PM exposure or due to a possible reduction in renal function. However, no change in the organ weight does not necessarily indicate no alteration in the organ structure or function. Our findings show that prenatal PM exposure alters hepatic morphology (Fig. 5) and induces endothelial dysfunction in the aortas of adult rats (Fig. 6), although the relative weights of their livers or hearts were similar between CA and PA groups (SI Appendix, Table S4). The differences in the organ weights between the CA and PA offspring were relatively small (SI Appendix, Table S4). However, even small differences in the organ weights likely result in function alterations. Furthermore, our well-controlled animal model reveals that prenatal PM exposure does not predispose rat offspring to be overweight or obese in response to a low-fat or high-fat diet (SI Appendix, Table S9). Therefore, individuals from mothers exposed to PM during gestation may not be overweight or obese in adulthood, in contrast to other previous investigations (16, 17). Apparently, postnatal environmental factors (e.g., nutrition and lifestyle) are likely the major determinants of the current obesity epidemic worldwide.
Epidemiological studies have also linked PM2.5 with increased risk for cardiovascular disease in humans, including hypertension, stroke, heart failure, atherosclerosis, myocardial ischemia, arrhythmias, and enhanced thrombosis (22). In addition, exposure of gestating mice to elevated mass concentrations of PM2.5 during gestation altered cardiac structure (i.e., increased left ventricular end-systolic and diastolic diameters, reduced left ventricular posterior wall thickness, and reduced end-systolic elastance) and blunted contractility (e.g., blunted contractile response to β-adrenergic stimulation) (23). Our study shows that offspring from the PA dams exhibit impairment in the NO-mediated vasodilatory response of their aortas to acetylcholine (Fig. 6). This finding is important for understanding disorders of the circulatory system in individuals from dams subjected to gestational PM exposure, because a hallmark of cardiovascular dysfunction is a reduced bioavailability of NO (a major vasodilator) from endothelial cells (24). The state of endothelial NO deficiency occurs in response to inhalation of PM2.5, as indicated by arterial vasoconstriction and abnormal hemodynamics (25). Oxidants in air also react with NO to yield reactive peroxy species (e.g., peroxynitrite), and this reaction leads to both a NO deficiency and a depletion of reduced glutathione in cells (the major antioxidant in cells), constituting a vicious undesirable cycle (26, 27). Postnatal PM exposure exaggerates inflammation in adipose tissue, whole-body insulin resistance, and impairment of endothelium-dependent relaxation in adult diet-induced obese mice (28). Those conditions suggest possible targets for prevention and treatment of cardiovascular disease in offspring with prenatal PM exposure. In this work, we did not measure any indices of insulin sensitivity in offspring.
The transport of fine PM from air to fetus is not fully understood. Although ultrafine particles contribute only to a small fraction of the total ambient PM mass, they are typically present in high concentrations in the urban atmosphere (3, 29–31) and have a high probability to deposit in the pulmonary system after inhalation than large particles (32, 33). Through blood circulation and endocytosis, ultrafine particles likely enter organs beyond the lungs, such as the reproductive tract, heart, liver, spleen, kidneys, brain, and BAT (34, 35). The large surface area, high redox capacity, and ability to form radical species for ultrafine particles likely induce inflammatory effects, cause cellular DNA damage, and interfere with cell metabolism and function (27, 36). Interestingly, our results indicate that the adverse effects of the exposure of gestating dams to fine PM on offspring are carried over into postnatal life (e.g., weaning and adults), even though offspring are raised in a clean environment after birth (Figs. 5 and 6 and SI Appendix, Table S15). This phenomenon is explained by changes in both epigenetics of the fetal genome and gene expression in offspring (37). Also, gestational PM2.5 exposure increases the activities of DNA methyltransferases 1, 3a, and 3b (regulators of DNA methylation to produce epigenetic marks) in rat tissues (38). In response to high-fat feeding, the hepatic expression of the Fabp1 gene on PND105 is substantially reduced in female offspring from dams with gestational exposure to air pollution, compared with the CA group (SI Appendix, Table S14). Future research is warranted to develop nutritional or pharmaceutical approaches for mitigating cardiovascular, metabolic, and developmental defects in offspring from dams exposed to air pollution during pregnancy. In our study, dams were exposed to ammonium sulfate aerosols with a peak diameter of 10 to 20 nm (SI Appendix, Fig. S2A). Since ambient fine PM typically consists of an internal mixture of inorganic/organic species as well as various toxic components, such as black carbon, future studies using aerosols of different compositions and toxicity are necessary to evaluate the adverse health effects.
In summary, by replicating ambient PM properties and controlling the exposure conditions (including nutrient intakes by the animals), our work improves the understanding of the impacts of gestational PM exposure on organogenesis and metabolism of offspring and elucidates the underlying mechanisms in an age-, organ-, and sex-specific manner. Our results demonstrate that exposure of dams to ultrafine aerosols consisting of ammonium sulfate (i) impairs embryonic/fetal survival and growth; (ii) shortens gestation lengths; (iii) decreases the relative weights of brain, heart, intestine, and spleen of offspring at birth; (iv) reduces the relative weights of the lungs and brain; (v) increases the relative weights of spleen and thymus at weaning; (vi) causes hypertrophy of the kidneys; (vii) disturbs lipid and glucose homeostasis; and (viii) induces endothelial dysfunction in offspring as adults. However, our results suggest that prenatal PM exposure does not necessarily predispose overweight or obesity in adulthood. The results of our animal model are beneficial for development of therapeutic strategies to remedy the adverse effects of gestational PM exposure on conceptus and postnatal growth and development.
Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Animals and Their Exposure to CA or PA.
Female and male Sprague-Dawley rats (60 d old) were obtained from Harlan Laboratories and acclimated to the research facility for 1 wk. Following acclimation, rats were paired with a fertile male and examined each morning for a vaginal plug indicative of mating. Once the vaginal plug was observed (day 0 of gestation), rats were assigned randomly to an airtight chamber (32 × 12 × 8 inches, length × width × height) exposed to either clean ambient air (CA) or clean ambient air plus ammonium sulfate particles (PA), with n = 10 for each group. Both chambers had four subunits (one for each rat) receiving the same air. The chambers were designed to meet several criteria. (i) The PM levels were evenly distributed throughout the chambers (measured at the exit of and several locations inside the chamber) so that the animals were equally exposed. (ii) The individual compartments met requirements set forth by federal regulations. (iii) The enclosure allowed easy access to the animals to provide for their daily needs for water and food. (iv) The airflow generated at least 15 complete air exchanges per hour. (v) The system was reasonably quiet since rats were sensitive to sight, sounds, and smells. (vi) The system was operated and unattended for long periods of time. Air was continuously pumped through the respective chambers through stainless steel tubing attached to the lid and bottom of the chamber. The U-shaped inflow lines, attached to the underside of the lid, had evenly distributed holes over each of the compartments to produce even airflow and exposure in each individual space. The inflow lines bring in ambient room air, which had a mass concentration of less than 5 μg⋅m−3. The outflow line, attached to the bottom of the chamber, had evenly distributed holes under each of the compartments to facilitate uniform removal of air from the chamber. The PA system included an atomizer to produce a steady flow of aerosols, a multitube Nafion drier to remove excess water vapor, and a scanning mobility particle sizer to constantly monitor the size distribution of particles (39–41). A continuous atomizer was positioned between the pump and the polluted chamber to ensure a mass concentration of near 150 μg⋅m−3. The average mass concentration was confirmed to be 153 μg⋅m−3 and typically varied between 100 and 200 μg⋅m−3.
Inside the chambers, rats freely accessed a casein-based diet (42) and drinking water. Once dams were placed into the chambers, cages were cleaned every other day to prevent the buildup of ammonia. On day 18 of gestation, dams were removed from their respective chambers and placed into normal cages until parturition (∼21 d) to prevent direct exposure of pups to PA. Maternal BW, food intake, and water intake were measured on days 6, 12, and 18 of gestation and on the day that they gave birth to pups. At birth (PND 0), pups in each litter were weighed individually, sexed, and culled to eight pups (four males and four females). Within each sex, offspring were selected randomly to be euthanized or killed at later time within the study. All culled pups were euthanized and necropsied to determine weights of organs, including heart, liver, spleen, kidneys, brain, pancreas, intestine, lungs, adrenal glands, BAT, and testes; a sample of each of those tissues was snap-frozen in liquid nitrogen for subsequent analyses. The eight remaining pups were reared on their dams in bedded cages in clean ambient air. At weaning (PND21), four pups (two males and two females) were euthanized and necropsied to determine the weights of organs (i.e., heart, liver, spleen, kidneys, brain, pancreas, small intestine, lungs, adrenal glands, gonads, and thymus), extensor digitorum longus muscle, soleus muscle, BAT, white adipose tissue, and stomach and to collect samples of each of those tissues. Tissue samples were snap-frozen in liquid nitrogen or fixed in paraformaldehyde.
The remaining four pups from each litter were individually housed in bedded cages and placed on either a high-fat (24% fat) or low-fat (4.3% fat) diet (one male and one female on each diet per litter) from PND21 to PND105. The composition of the low-fat and high-fat diets was reported previously (43). At PND105, all pups were euthanized and necropsied to weigh organs and collect tissue samples (43–45). At each necropsy point, plasma was obtained from whole blood that was collected into in EDTA-coated vacutainer tubes via cardiac aspiration after euthanasia (43). In addition, on PND105, the thoracic aortas from male offspring were obtained for the measurement of endothelium-dependent relaxation as an assessment of vascular function (44).
Supplementary Material
Acknowledgments
This work was supported by funds from the Texas A&M University’s Tier One Program. We thank Katherine Kelly and Gayan Nawaratna for technical assistance. R.Z. acknowledges support from the Robert A. Welch Foundation. N.M.J. and R.Z. were supported by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health (R01 ES028866) and a Research Enhancement Development Initiative grant from the Texas A&M School of Public Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902925116/-/DCSupplemental.
References
- 1.Ghio A. J., Soukup J. M., Madden M. C., The toxicology of air pollution predicts its epidemiology. Inhal. Toxicol. 30, 327–334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R., et al. , Formation of urban fine particulate matter. Chem. Rev. 115, 3803–3855 (2015). [DOI] [PubMed] [Google Scholar]
- 3.An Z., et al. , Severe haze in Northern China: A synergy of anthropogenic emissions and atmospheric processes. Proc. Natl. Acad. Sci. U.S.A. 116, 8657–8666 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization , Air pollution (Geneva, Switzerland, 2018). https://www.who.int/airpollution/en/. Accessed 10 January 2019.
- 5.Guo S., et al. , Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. U.S.A. 111, 17373–17378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Environmental Agency (EPA) , Our nation’s air (2018). https://gispub.epa.gov/air/trendsreport/2018/#home. Accessed 10 January 2019.
- 7.Tan Y., et al. , The associations between air pollution and adverse pregnancy outcomes in China. Adv. Exp. Med. Biol. 1017, 181–214 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Klepac P., Locatelli I., Korošec S., Künzli N., Kukec A., Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 167, 144–159 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Melody S. M., Ford J., Wills K., Venn A., Johnston F. H., Maternal exposure to short-to medium-term outdoor air pollution and obstetric and neonatal outcomes: A systematic review. Environ. Pollut. 244, 915–925 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Rychlik K. A., et al. , In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc. Natl. Acad. Sci. U.S.A. 116, 3443–3448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veras M. M., et al. , Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ. Res. 109, 536–543 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Blum J. L., Chen L. C., Zelikoff J. T., Exposure to ambient particulate matter during specific gestational periods produces adverse obstetric consequences in mice. Environ. Health Perspect. 125, 077020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weldy C. S., et al. , In utero and early life exposure to diesel exhaust air pollution increases adult susceptibility to heart failure in mice. Part. Fibre Toxicol. 10, 59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T., et al. , Maternal exposure to PM2.5 during pregnancy induces impaired development of cerebral cortex in mice offspring. Int. J. Mol. Sci. 19, E257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolton J. L., et al. , Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 26, 4743–4754 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Wei Y., et al. , Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 30, 2115–2122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soto S. F., et al. , Exposure to fine particulate matter in the air alters placental structure and the renin-angiotensin system. PLoS One 12, e0183314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamora M. L., “Nucleation, transformation, and impacts of atmospheric aerosols,” PhD dissertation, p. 138, Department of Atmospheric Sciences, Texas A&M University (2015).
- 19.Vadillo-Ortega F., et al. , Air pollution, inflammation and preterm birth: A potential mechanistic link. Med. Hypotheses 82, 219–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue D. L., et al. , Potential contribution of new particle formation to cloud condensation nuclei in Beijing. Atmos. Environ. 45, 6070–6077 (2011). [Google Scholar]
- 21.Wang G., et al. , Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. U.S.A. 113, 13630–13635 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brook R. D., Cardiovascular effects of air pollution. Clin. Sci. (Lond.) 115, 175–187 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Tanwar V., et al. , In utero particulate matter exposure produces heart failure, electrical remodeling, and epigenetic changes at adulthood. J. Am. Heart Assoc. 6, e005796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ignarro L. J., Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 53, 503–514 (2002). [PubMed] [Google Scholar]
- 25.Pope C. A. 3rd, Dockery D. W., Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 56, 709–742 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Pryor W. A., et al. , Free radical biology and medicine: It’s a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R491–R511 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Yang W., Omaye S. T., Air pollutants, oxidative stress and human health. Mutat. Res. 674, 45–54 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Sun Q., et al. , Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119, 538–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R., Khalizov A., Wang L., Hu M., Xu W., Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 112, 1957–2011 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Yue D., et al. , The roles of sulfuric acid in new particle formation and growth in the mega-city of Beijing. Atmos. Chem. Phys. 10, 4953–4960 (2010). [Google Scholar]
- 31.Wang Z. B., et al. , Evaluation on the role of sulfuric acid in the mechanisms of new particle formation for Beijing case. Atmos. Chem. Phys. 11, 12663–12671 (2011). [Google Scholar]
- 32.Stuart B. O., Deposition and clearance of inhaled particles. Environ. Health Perspect. 55, 369–390 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ching J., Kajino M., Aerosol mixing state matters for particles deposition in human respiratory system. Sci. Rep. 8, 8864 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K. H., Kabir E., Kabir S., A review on the human health impact of airborne particulate matter. Environ. Int. 74, 136–143 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Hamanaka R. B., Mutlu G. M., Particulate matter air pollution: Effects on the cardiovascular system. Front. Endocrinol. (Lausanne) 9, 680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grahame T. J., Schlesinger R. B., Health effects of airborne particulate matter: Do we know enough to consider regulating specific particle types or sources? Inhal. Toxicol. 19, 457–481 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Perera F., Herbstman J., Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 31, 363–373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dang S., et al. , PM2.5 exposure during pregnancy induces hypermethylation of estrogen receptor promoter region in rat uterus and declines offspring birth weights. Environ. Pollut. 243, 851–861 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Xue H., Khalizov A. F., Wang L., Zheng J., Zhang R., Effects of coating of dicarboxylic acids on the mass-mobility relationship of soot particles. Environ. Sci. Technol. 43, 2787–2792 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Xue H., Khalizov A. F., Wang L., Zheng J., Zhang R., Effects of dicarboxylic acid coating on the optical properties of soot. Phys. Chem. Chem. Phys. 11, 7869–7875 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Qiu C., Zhang R., Physiochemical properties of alkylaminium sulfates: Hygroscopicity, thermostability, and density. Environ. Sci. Technol. 46, 4474–4480 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Wu G., Flynn N. E., Flynn S. P., Jolly C. A., Davis P. K., Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J. Nutr. 129, 1347–1354 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Jobgen W., et al. , Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. 139, 230–237 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu G., et al. , Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 137, 2680–2685 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Dunlap K. A., et al. , Progesterone and placentation increase secreted phosphoprotein one (SPP1 or osteopontin) in uterine glands and stroma for histotrophic and hematotrophic support of ovine pregnancy. Biol. Reprod. 79, 983–990 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.