In addition to the normal set of chromosomes, eukaryote genomes sometimes also contain chromosomes that do not follow the Mendelian law of inheritance. These chromosomes, called B chromosomes, were detected in the early 20th century (1) and are believed to consist of selfish genetic elements that have parasitized the genome (2, 3). B chromosomes are typically supernumerary, derived from ordinary chromosomes, and often vary in numbers both among individuals and among cells within individuals (3, 4). B chromosomes can be tissue-specific, and one type of B chromosome (or B-like chromosome) is restricted to germline cells and, thus, consistently absent from somatic (body) cells, which makes them easily overlooked in cytogenetic and genomic studies. Such germline-restricted chromosomes (GRCs) have previously been reported from several eukaryotes (5), including nematodes (6), lampreys (7), and 2 closely related species of songbirds—the zebra finch and the Bengalese finch (8–10). The finch GRC is similar in size to a large avian autosome, is transmitted via oocytes, and is eliminated from somatic cells and spermatids (8–10) (Fig. 1A). In PNAS, Torgasheva et al. (11) now reveal that avian GRCs are not exclusive to finches but are widely occurring in songbirds, a large clade consisting of over 4,000 species.
Fig. 1.
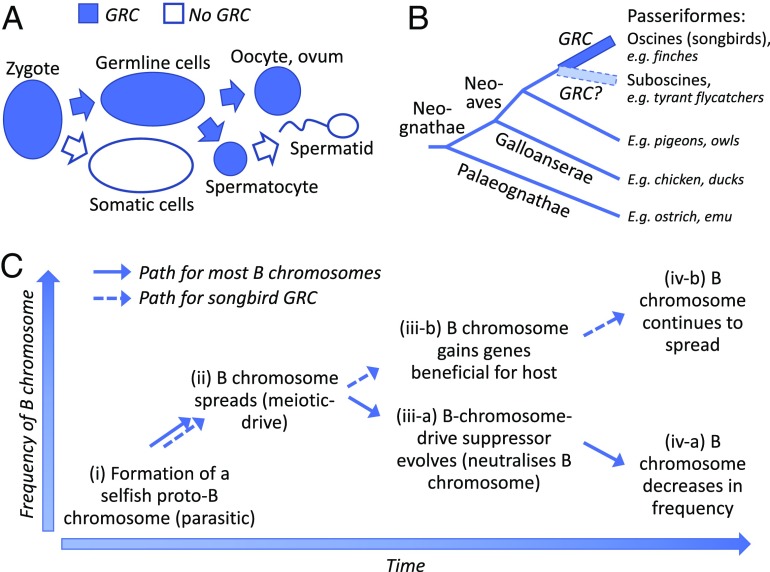
(A) Schematic outline of the life cycle of a GRC that is transmitted via oocytes and eliminated from somatic cells and spermatids (8–10). (B) A phylogeny of birds indicating the GRC of songbirds (oscine passerines) (11). (C) A possible scenario for the evolutionary dynamics of B chromosomes in general and of the songbird GRC in particular (3, 4, 11). (i) Formation of a proto-B chromosome, i.e., a harmful selfish genetic element that possesses a meiotic-drive mechanism. (ii) The B chromosome increases in frequency in the population due to the drive. (iii-a and iv-a) Selection favors the evolution of B chromosome-drive suppressors that neutralize the B chromosome (iii-a), and the B chromosome is eventually lost from the population by genetic drift (iv-a). (iii-b and vi-b) The B chromosome gains genes that are beneficial for the host (genes involved in germ-cell development) (iii-b) and increases further in frequency and eventually becomes fixed (iv-b).
Torgasheva et al. (11) use comparative cytogenetics to search for GRCs in 16 species representing 9 families of oscine passerines (Passeri; Passeriformes), i.e., songbirds, and in 8 species representing 7 nonpasserine orders. Their results are striking: A GRC was present in all the songbirds examined, but in none of the nonpasserines (Fig. 1B). The finding of taxonomically widespread GRCs in songbirds provides strong support for a monophyletic origin of the GRC at the time when the oscines were formed ∼35 million y ago (12). Whether GRCs occur also in suboscine passerines, a clade of over 1,000 species that is the sister group to songbirds, remains to be determined (11). Interestingly, the GRC was found to vary dramatically in size among the 16 songbird species and appeared both as a micro- and a macrochromosome, and there was no phylogenetic clustering of the GRC by size. Even within families, both small and large GRCs occurred. Nevertheless, cross-species fluorescent in situ hybridization analysis showed that the GRCs of closely related species share part of their genetic content (11). These results reveal a highly dynamic nature of the songbird GRC, with various gains and losses of genetic material over relatively brief evolutionary time frames within and between passerine families.
The evolution of B chromosomes is thought to reflect the outcome of an ongoing conflict between parts of the genome with different interests, and the effect of a B chromosome on its carriers may shift from parasitic to neutral and possibly beneficial (3, 4) (Fig. 1C). B chromosomes are believed to start as harmful selfish genetic elements that increase in frequency due to the possession of meiotic-drive mechanisms. As the B chromosome becomes more common in the population, selection will increasingly favor the evolution of B chromosome-drive suppressors and tolerance to the B chromosome effects and/or less harmful or possibly beneficial B chromosome variants. Most commonly, this evolutionary arms race will neutralize the B chromosome, which, when no longer gaining the advantage of drive, eventually will become lost from the population by genetic drift. Therefore, most B chromosomes are ephemeral by nature or occur at low frequencies. In contrast, the songbird GRC is evolutionarily old and has become a fully established part of the host’s genome, although restricted to the germline. This is interesting, as it suggests that the GRC became beneficial or even indispensable for its carriers early in the history of passerines.
As suggested by Torgasheva et al. (11), a possible scenario is that a proto-GRC was formed in a passerine or songbird ancestor by a whole-chromosome duplication of an autosomal microchromosome that spread like a parasitic, selfish genetic element and increased in frequency. It is further possible that the duplicated microchromosome contained some genes involved in germ-cell development or that such genes were translocated to the proto-GRC soon after its formation. This could have given the proto-GRC a selective advantage (release from the evolutionary arms race by being beneficial to the host) that increased its frequency in the population and eventually led to its fixation (Fig. 1C). B chromosomes sometimes carry host-beneficial genes, as is known from, for example, oats (13) and fungi (14).
Recent work by Biederman et al. (15) and Kinsella et al. (16) brings important clues regarding the functional role of the songbird GRC by showing that the macro-GRC of the zebra finch contains genes that are transcribed and translated in gonads, have gonad-development gene ontologies, and show sex-biased expression and signs of positive selection. These results provide strong support for the idea that the GRC is beneficial for gonad development in passerines. If the proto-GRC had similar properties, this may explain how it became integrated in the avian germline genome. However, because large chromosome segments and many genes have been translocated to the finch GRC relatively recently (11, 16), and since the number of GRC genes must differ substantially between species as indicated by the pronounced interspecific variation in the size of the GRC (11), conclusions from the macro-GRC of the zebra finch may not necessarily be transferable to the proto-GRC. Enrichment of genes with gonad functions may have occurred after the GRC became an integrated part of the songbirds’ germline genome.
The GRC lacks recombination, except for its end parts in female meiosis (11). It is therefore expected to accumulate deleterious mutations and transposable elements, degenerate functionally, and lose genes over most of its length through processes analogous to Muller’s ratchet on sex-limited, nonrecombining sex chromosomes (e.g., Y chromosomes) (17, 18). Thus, any ancestral gene that has remained functional (and resisted degeneration) since the formation of the proto-GRC must have been under continuous and strong purifying selection, suggesting that such genes are functionally essential. In contrast, more recently translocated genes to the GRC that will occur only in a part of the songbird phylogeny are expected to be more dispensable. Thus, the expectation regarding the age of genes and their essentiality is similar for GRCs and sex-limited chromosomes—old sex chromosomes have few but functionally indispensable genes (19, 20)—and identifying old and thus possibly essential genes may be key to understanding the evolutionary dynamics of the songbird GRC.
In PNAS, Torgasheva et al. now reveal that avian GRCs are not exclusive to finches but are widely occurring in songbirds, a large clade consisting of over 4,000 species.
The unique transmission and tissue-specific elimination process of the songbird GRC is peculiar. It transmits only through oocytes—and thus exhibits uniparental, female-specific inheritance—and is eliminated from somatic cells in both sexes and from spermatids (8–10). In finches, the GRC is euchromatic in oocytes but heterochromatic and presumably inactive in spermatocytes (9, 11). The mechanisms for elimination of the GRC in songbirds are not yet fully understood (9, 10), but results from nematodes and the sea lamprey, where ∼0.5 Gb is eliminated from somatic cells, suggest that selection has favored somatic elimination of genes that contribute to the development of germ cells but are potentially deleterious if misexpressed in somatic cells (6, 7). Because the finch macro-GRC contains genes with germline-specific functions and expression (15, 16), it is possible that somatic elimination has evolved as a mechanism to minimize genetic conflicts between germline and soma also in songbirds.
Torgasheva et al.’s (11) discovery of a widely occurring GRC in songbirds points to an essential function in the germline to where it is restricted. Although it still remains to be determined whether GRCs occur also in suboscine passerines, its widespread occurrence in oscine passerines enables broad phylogenetic studies of shared and lineage-specific GRC sequences to identify genes that were present on the proto-GRC in the common ancestor of songbirds. This will shed light on the essential properties of the GRC and help to understand the selective regimes that led to the fixation of the GRC among present-day songbirds.
Acknowledgments
This work was supported by the Swedish Research Council (Consolidator Grant 2016-00689).
Footnotes
The author declares no conflict of interest.
See companion article on page 11845.
References
- 1.Wilson E. B., The supernumerary chromosomes of Hemiptera. Science 26, 870–871 (1907). [Google Scholar]
- 2.Werren J. H., Nur U., Eickbush D., An extrachromosomal factor causing loss of paternal chromosomes. Nature 327, 75–76 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Houben A., Banaei-Moghaddam A. M., Klemme S., Timmis J. N., Evolution and biology of supernumerary B chromosomes. Cell. Mol. Life Sci. 71, 467–478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camacho J. P., Sharbel T. F., Beukeboom L. W., B-chromosome evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 163–178 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Davis R. E., Programmed DNA elimination in multicellular organisms. Curr. Opin. Genet. Dev. 27, 26–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., et al. , Comparative genome analysis of programmed DNA elimination in nematodes. Genome Res. 27, 2001–2014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J. J., et al. , The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat. Genet. 50, 270–277 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pigozzi M. I., Solari A. J., Germ cell restriction and regular transmission of an accessory chromosome that mimics a sex body in the zebra finch, Taeniopygia guttata. Chromosome Res. 6, 105–113 (1998). [DOI] [PubMed] [Google Scholar]
- 9.del Priore L., Pigozzi M. I., Histone modifications related to chromosome silencing and elimination during male meiosis in Bengalese finch. Chromosoma 123, 293–302 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Schoenmakers S., Wassenaar E., Laven J. S., Grootegoed J. A., Baarends W. M., Meiotic silencing and fragmentation of the male germline restricted chromosome in zebra finch. Chromosoma 119, 311–324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torgasheva A. A., et al. , Germline-restricted chromosome (GRC) is widespread among songbirds. Proc. Natl. Acad. Sci. U.S.A. 116, 11845–11850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvatti A. P., Gonzaga L. P., Russo C. A. D., A Paleogene origin for crown passerines and the diversification of the Oscines in the New World. Mol. Phylogenet. Evol. 88, 1–15 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Dherawattana A., Sadanaga K., Cytogenetics of a crown rust-resistant hexaploid oat with 42 + 2 fragment chromosomes. Crop Sci. 13, 591–594 (1973). [Google Scholar]
- 14.Miao V. P., Covert S. F., VanEtten H. D., A fungal gene for antibiotic resistance on a dispensable (“B”) chromosome. Science 254, 1773–1776 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Biederman M. K., et al. , Discovery of the first germline-restricted gene by subtractive transcriptomic analysis in the zebra finch, Taeniopygia guttata. Curr. Biol. 28, 1620–1627.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsella C. M., et al. , Programmed DNA elimination of germline development genes in songbirds. 10.1101/444364 (22 December 2018). [DOI] [PMC free article] [PubMed]
- 17.Wright A. E., Dean R., Zimmer F., Mank J. E., How to make a sex chromosome. Nat. Commun. 7, 12087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponnikas S., Sigeman H., Abbott J. K., Hansson B., Why do sex chromosomes stop recombining? Trends Genet. 34, 492–503 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Bellott D. W., et al. , Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 508, 494–499 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellott D. W., et al. , Avian W and mammalian Y chromosomes convergently retained dosage-sensitive regulators. Nat. Genet. 49, 387–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]