Significance
PSD-95 is a key scaffolding protein that is localized to the postsynaptic density of excitatory synapses where it regulates a wide variety of receptors, channels, and signaling molecules. However, the role of PSD-95 binding to the cell adhesion molecules, neuroligins, has been less clear. We now report that PSD-95 specifically regulates NLGN1 among the various neuroligin isoforms. In addition, we characterize a dynamic interaction between NLGN1 and PSD-95 that is regulated by the phosphorylation of NLGN1 by PKA. Phosphorylation on S839 of NLGN1 blocks PSD-95 binding, reduces surface and synaptic expression of NLGN1, and inhibits NLGN1-mediated enhancement of excitatory synaptic transmission.
Keywords: PKA, NLGN1, phosphorylation, PSD-95
Abstract
PSD-95 is a scaffolding protein that regulates the synaptic localization of many receptors, channels, and signaling proteins. The NLGN gene family encodes single-pass transmembrane postsynaptic cell adhesion molecules that are important for synapse assembly and function. At excitatory synapses, NLGN1 mediates transsynaptic binding with neurexin, a presynaptic cell adhesion molecule, and also binds to PSD-95, although the relevance of the PSD-95 interaction is not clear. We now show that disruption of the NLGN1 and PSD-95 interaction decreases surface expression of NLGN1 in cultured neurons. Furthermore, PKA phosphorylates NLGN1 on S839, near the PDZ ligand, and dynamically regulates PSD-95 binding. A phosphomimetic mutation of NLGN1 S839 significantly reduced PSD-95 binding. Impaired NLGN1/PSD-95 binding diminished synaptic NLGN1 expression and NLGN1-mediated synaptic enhancement. Our results establish a phosphorylation-dependent molecular mechanism that regulates NLGN1 and PSD-95 binding and provides insights into excitatory synaptic development and function.
PSD-95, a member of membrane-associated guanylate kinases (MAGUK) protein family, is a major constituent of glutamatergic excitatory synapses and is specifically enriched at the postsynaptic density (PSD) (1). A large number of channels, receptors, and adhesion molecules bind to the PSD-95/Discs large/ZO-1 (PDZ), Src-homology-3, and guanylate kinase (GK) domains of PSD-95 (1, 2). As a scaffolding protein at excitatory synapses, PSD-95 has been intensively studied vis a vis the organization of glutamatergic postsynaptic signaling. In fact, synaptic expression and transmission of N-methyl-d-aspartate receptors (NMDARs) and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) are regulated by PSD-95 via direct physical interaction or coordination with auxiliary proteins (3–6). Numerous studies have demonstrated critical roles of PSD-95 in the formation and maintenance of dendritic spines, and recent reports have focused on PSD-95 and the molecular mechanisms underlying synapse maturation and plasticity.
Neuroligins (NLGNs) are type I membrane proteins and postsynaptic cell adhesion molecules. NLGNs were initially reported as endogenous neurexin (NRXN) ligands (7, 8). NLGNs and NRXNs form a transsynaptic interaction, which is important for spinogenesis and functional synapse formation (9, 10). Five NLGN isoforms (NLGN1, NLGN2, NLGN3, NLGN4X, and NLGN4Y) are identified in humans (7, 11). In fact, all identified NLGN isoforms show high similarity in amino acid sequence. In addition, the defined protein binding domains in the cytoplasmic region, including the PDZ ligand, are well conserved in all NLGN isoforms (11, 12). However, isoform-specific mechanisms regulating synaptic localization are not fully understood.
Because NLGN1 and PSD-95 are both localized to excitatory synapses, and their direct interaction via third PDZ domain of PSD-95 was identified in an early study (13), PSD-95 and NLGN1 have been investigated together in many studies for their physiological roles in the glutamatergic signaling pathway. For example, PSD-95 can restrict NLGN1 localization to excitatory synapses (14), and PSD-95 and NLGN1 are known to be necessary for maintaining presynaptic release probability (15). Meanwhile, synaptic targeting of NLGN1 is known to be independent of PSD-95, as PSD-95 is recruited to the plasma membrane after NLGN1 (13, 16, 17). In addition, non-PDZ ligand-dependent NLGN1 and NLGN3 functions have been investigated (18), suggesting a complicated physiological interplay between NLGNs and PSD-95.
Posttranslational modifications have been reported for several NLGN isoforms and are postulated to confer distinct properties (11, 12). For example, phosphorylation of the cytoplasmic region of NLGN1 has recently emerged as a mechanism for isoform-specific function (19, 20). Here we identified a phosphorylation site on NLGN1 near the PDZ ligand. We show that serine 839 (S839) of NLGN1 is phosphorylated by cAMP-dependent protein kinase (PKA) and observed that phosphorylation reduces NLGN1 and PSD-95 binding. Additionally, a phosphomimetic NLGN1 mutant shows reduced surface expression and impaired trafficking. Together, these results demonstrate an NLGN1 regulatory mechanism consisting of PKA phosphorylation, which modulates NLGN1 binding to PSD-95, consequently affecting NLGN1 localization and function at excitatory synapses.
Results
PSD-95 Regulates NLGN1 Surface Expression.
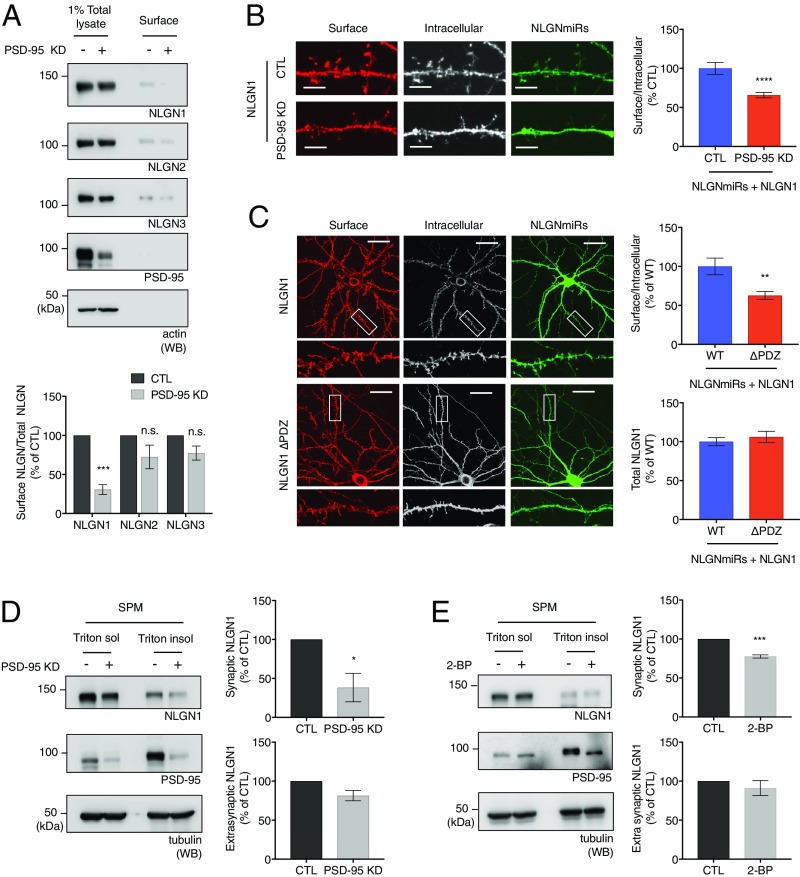
Does PSD-95 regulate NLGN1 surface expression? To answer this question, we examined NLGN1 surface levels in cultured neurons under PSD-95 knockdown conditions. We transduced shPSD-95 lentivirus in cultured cortical neurons and performed surface biotinylation assays as described previously. In neurons with PSD-95 knockdown, the surface NLGN1 level was significantly decreased (Fig. 1A), whereas other NLGN isoforms were not significantly affected. These data indicate PSD-95 is required for efficient NLGN1 surface expression and show specificity for NLGN1 among the NLGN isoforms.
Fig. 1.
NLGN1 and PSD-95 binding regulates NLGN1 surface levels. (A) Cultured cortical neurons were transduced with shPSD-95 lentivirus at DIV 12 to 15. The surface biotinylation assays were performed 7 d after viral transduction. Surface proteins were analyzed by immunoblotting. Graph indicates mean ± SEM (n = 3). ***P = 0.0002 using one-way ANOVA Bonferroni’s multiple comparison test. n.s., not significant. (B) PSD-95 knockdown plasmid was cotransfected with NLGN miRs and HA-NLGN1 WT in cultured hippocampal neurons (n = 14 for CTL and n = 17 for PSD-95 knockdown). (Scale bar, 5 µm.) (C) HA-NLGN1 or HA-NLGN1 ∆PDZ were cotransfected with NLGN miRs in cultured hippocampal neurons (n = 13 for WT and n = 15 for ∆PDZ). (Scale bar, 25 µm.) (B and C) Surface labeling assays were performed as described in Materials and Methods. Regions from three dendrites per each neuron were collected for analysis. Graph indicates mean ± SEM **P = 0.0015 and ****P = 0.000032 using unpaired t test. (D and E) Cultured cortical neurons were transduced with shPSD-95 lentivirus or treated with 100 μM 2-BP for 20 h. The Triton-soluble extrasynaptic region and Triton-insoluble synaptic region were fractionated from the neurons. The fractions were analyzed by immunoblotting. The NLGN1/tubulin ratio was analyzed. The graph indicates mean ± SEM (n = 3). *P = 0.028 and ***P = 0.0004 using unpaired t test.
We also used immunofluorescence confocal microscopy to evaluate NLGN1 surface expression upon PSD-95 knockdown. We cotransfected the PSD-95 knockdown plasmid along with HA-NLGN1 WT and NLGN microRNAs (miRs) in cultured hippocampal neurons. As with our previous experiments (19), we performed these analyses on a triple NLGN knockdown condition (NLGNmiRs) to prevent dimerization of our HA-NLGN1 with endogenous WT NLGNs (18, 19). Consistently, PSD-95 knockdown also reduced surface HA-NLGN1 WT levels (Fig. 1B). These immunocytochemistry data indicate PSD-95 affects HA-NLGN1 WT surface expression. We next examined the effects of deleting the PDZ ligand. We transfected cultured hippocampal neurons with NLGN miRs and HA-NLGN1 WT or HA-NLGN1 ∆PDZ. We observed that HA-NLGN1 ∆PDZ is able to go to the surface, but to a significantly lesser extent than HA-NLGN1 WT (Fig. 1C).
To better understand how PSD-95 regulates NLGN1, we transduced cultured cortical neurons with shPSD-95 lentivirus to knockdown PSD-95 or treated with 2-BP (2-bromopalmitate; palmitoylation inhibitor) to inhibit palmitoylation, which is necessary for synaptic localization of PSD-95. As NLGN1 mostly exerts its function at postsynaptic sites, the extrasynaptic regions (Triton X-100−soluble) and synaptic regions (Triton X-100−insoluble) were fractionated from the neurons. We observed reduced NLGN1 levels in the synaptic fraction with PSD-95 knockdown or 2-BP treatment compared with control (Fig. 1 D and E), whereas NLGN1 levels in the extrasynaptic fraction were comparable. These results indicate synaptic NLGN1 is particularly sensitive to PSD-95 modulation.
PKA Phosphorylates NLGN1 on S839.
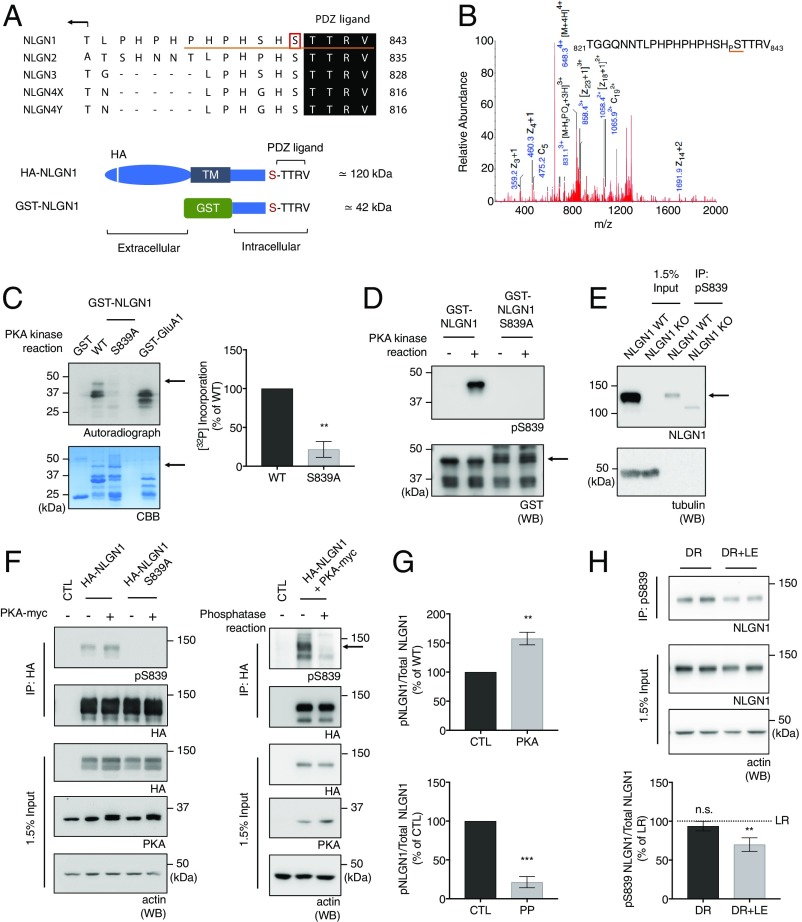
NLGNs are regulated by various intracellular signaling molecules on their cytoplasmic tails. In particular, PKAs have been shown to target various NLGNs. PKA is known to directly phosphorylate many synaptic proteins, including receptors, channels, and scaffolding proteins (21–24). To determine whether PKA is able to phosphorylate NLGNs, we incubated GST-NLGN C-tail fusion proteins with purified PKA and adenosine 5′-triphosphate (ATP) in vitro. We analyzed the samples using liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) and found NLGN1 was phosphorylated by PKA on S839 (Fig. 2B), which is located adjacent to the PDZ ligand (Fig. 2A). When S839 is mutated to alanine, a phosphodeficient mutation, PKA phosphorylation assessed by incorporation of radioactive ATP was markedly reduced in an in vitro kinase assay (Fig. 2C), confirming that this residue is a PKA phosphorylation site. GST-GluA1 was used as a positive control for PKA phosphorylation.
Fig. 2.
PKA phosphorylates NLGN1 on S839. (A) Sequence alignment for the C-tails of the NLGN isoforms. The pS839 antibody epitope is underlined in red. Schematic figures denote motifs within HA-NLGN1 and GST-NLGN1. (B) MS/MS spectrum of the phosphorylated NLGN1 peptide found in GST-NLGN1 fusion proteins incubated with ATP and purified PKA. Samples were digested with trypsin and analyzed using the LC/MS/MS method. (C) Autoradiography analysis of GST fusion proteins that were incubated with purified PKA and [γ-32P] ATP. Quantitative graph represents mean ± SEM (n = 3). **P = 0.0016 using an unpaired t test. (D) Immunoblot analysis of GST-NLGN1 WT and S839A fusion proteins that were phosphorylated in vitro with purified PKA and probed with pS839 antibody. Arrows in C and D denote GST-NLGN1 position in blots. (E) Endogenous phospho-S839 NLGN1 was enriched from WT mouse brain P2 lysates by immunoprecipitation using pS839 antibody with NLGN1 KO mouse brain as control. The immunoprecipitates were analyzed by immunoblotting; β-tubulin was used as a protein loading control for the input. (F) (Left) Immunoblot analysis of HA-NLGN1 WT and S839A transfected or cotransfected with constitutively active PKA in HEK293 cells. Arrows in E and F denote the NLGN1-specific band. (G) (Top) Quantitative graph represents mean ± SEM (n = 3). **P = 0.0017 using an unpaired t test. (F) (Right) PP assays were performed on the HA antibody immunoprecipitates from HEK293 cells expressing HA-NLGN1 WT and constitutively active PKA. The phosphatase assay samples were analyzed by immunoblotting. (G) (Bottom) Quantitative graph represents mean ± SEM (n = 3). ***P = 0.0004 using unpaired t test. (H) The primary visual cortex was macrodissected from a mouse dark-rearing paradigm as described in Materials and Methods. Endogenous phospho-S839 NLGN1 was enriched from P2 lysates by immunoprecipitation using pS839 antibody. Graph indicates mean ± SEM (n = 6). The statistical significance between the mean of LR and the mean of each condition was calculated using one-way ANOVA with Tukey’s multiple comparison test. **P = 0.0098 (LR vs. DR+LE). n.s., not significant.
To characterize the phosphorylation of NLGN1 S839 further, we generated a phosphorylation state-specific antibody, pS839 antibody, targeted against the NLGN1 C-terminal residues 833 to 843 (Fig. 2A). We verified the specificity of pS839 antibody by incubating GST-NLGN1 and GST-NLGN1 S839A with PKA enzyme and ATP in vitro. Samples were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) and analyzed by immunoblotting with pS839 antibody. We observed significantly diminished immunoreactivity for GST-NLGN1 S839A (Fig. 2D). To verify the specificity of pS839 antibody, crude synaptosomal fractions (P2 pellets) were prepared from WT and NLGN1 KO mouse brains, and phosphorylated NLGN1 was immunoprecipitated using the pS839 antibody. The immunoprecipitates were analyzed by immunoblotting with NLGN1 antibody, and a band corresponding to the predicted molecular weight of endogenous NLGN1 was enriched only in the immunoprecipitates from WT mouse brain lysates, whereas a nonspecific uncharacterized band was observed for NLGN1 KO mouse brain lysates (Fig. 2E). All these data show the specificity of the pS839 antibody for NLGN1 S839 phosphorylation.
To determine whether full-length NLGN1 can be phosphorylated by PKA, we cotransfected HA-NLGN1 WT or HA-NLGN1 S839A and the constitutively active PKA catalytic subunit (PKA T198E) in HEK293 cells. HA-NLGN1 WT and HA-NLGN1 S839A were immunoprecipitated, and the immunoprecipitates were analyzed by immunoblotting with pS839 antibody (Fig. 2 F, Left and Fig. 2 G, Top). The immunoblots showed increased immunoreactivity for NLGN1 WT upon PKA cotransfection, which was not the case for the NLGN1 S839A mutant. Additionally, we cotransfected HA-NLGN1 WT and the constitutively active PKA catalytic subunit in HEK293 cells. HA-NLGN1 were immunoprecipitated, and the immunoprecipitates were incubated with or without lambda protein phosphatase (PP). The immunoprecipitates were analyzed by immunoblotting with pS839 antibody, and the immunoblots showed eliminated reactivity of pS839 antibody upon the phosphatase treatment (Fig. 2 F, Right and Fig. 2 G, Bottom). These results indicate that PKA phosphorylates NLGN1 S839 in situ.
To investigate the dynamic regulation of NLGN1 S839 phosphorylation in various conditions, we assessed NLGN1 S839 phosphorylation upon physiological stimulation and in different developmental stages. We used a dark rearing paradigm to activate neurons in vivo as described in Materials and Methods. The primary visual cortex was macrodissected and the P2 pellet (crude synaptosomal fraction) was prepared for analysis as described previously. Intriguingly, neuronal activation in vivo reduced S839 phosphorylation in vivo (Fig. 2H). Furthermore, we also observed a decrease in NLGN1 S839 phosphorylation upon KCl or glutamate treatment on cultured cortical neurons (SI Appendix, Fig. S3 A and B), although we didn’t observe robust changes of the phosphorylation in the mouse brain tissues from the different developmental stages (SI Appendix, Fig. S3C). All these results indicate that NLGN1 S839 phosphorylation can be dynamically regulated by various forms of neuronal activity.
NLGN1 S839 Phosphorylation Attenuates PSD-95 Binding.
Multiple papers have shown that PDZ interactions are regulated by phosphorylation near the PDZ ligand in several proteins such as stargazin, β1-adrenergic receptors, Kir5.1 channels, and the NR2 subunit of NMDARs (24–27). Because S839 is close to the NLGN1 PDZ ligand, we tested whether S839 phosphorylation affects PSD-95 binding with NLGN1.
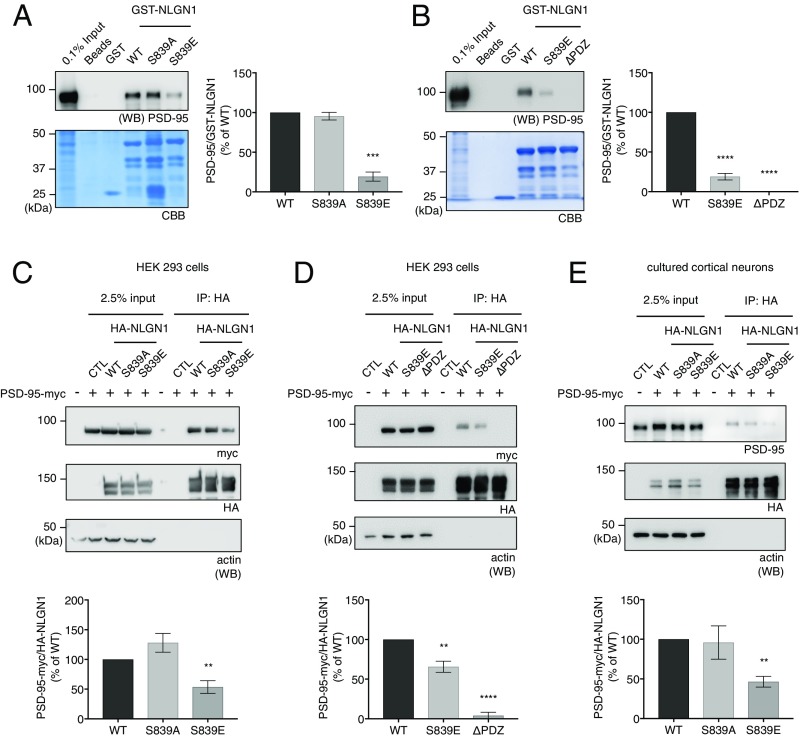
GST-NLGN1 WT, S839A, S839E, and ∆PDZ C-terminal fusion proteins were incubated with rat brain P2 fraction lysates. The isolated beads were resolved by SDS/PAGE and analyzed by immunoblotting. The amount of GST fusion proteins was visualized by Coomassie Brilliant Blue staining in the same gel. As expected, GST-NLGN1 WT showed strong binding with PSD-95 (Fig. 3A), whereas GST-NLGN1 ∆PDZ did not bind to PSD-95 (Fig. 3B). Interestingly, the GST-NLGN1 S839A mutant showed comparable PSD-95 binding to WT. Meanwhile, GST-NLGN1 S839E, a phosphomimetic mutant, showed significantly reduced binding with PSD-95, comparable to that of GST-NLGN1 ∆PDZ (Fig. 3 A and B). These results indicate S839 is a critical regulatory site for NLGN1 and PSD-95 binding.
Fig. 3.
NLGN1 S839 phosphorylation attenuates PSD-95 binding. (A and B) GST-NLGN1 fusion proteins were incubated with adult rat brain P2 fraction lysates for GST pulldown experiments. PSD-95/GST-NLGN1 fusion proteins were analyzed. Graph indicates mean ± SEM (n = 3 to 4). ***P = 0.0002 and ****P = 0.0001. (C and D) HA-NLGN1 (WT, S839A, S839E, or ∆PDZ) and PSD-95-myc were transfected in HEK293 cells as indicated in the figures. PSD-95-myc in the immunoprecipitates with HA antibody were analyzed by immunoblotting. The ratio of coimmunoprecipitated PSD-95-myc/immunoprecipitated NLGN1 was analyzed. Graph indicates mean ± SEM (n = 3 to 5). **P = 0.001824 for C. **P = 0.003735 and ****P = 0.0001 for D. (E) HA-NLGN1 (WT, S839A, or S839E) and PSD-95-myc were transfected in cultured cortical neurons. Graph indicates mean ± SEM (n = 3). **P = 0.005729. The statistical significance between the mean of WT and the mean of each condition was calculated using one-way ANOVA with Dunnett’s multiple comparison test.
To reexamine the pull-down results using coimmunoprecipitation in intact cells, we cotransfected full-length HA-NLGN1 WT, S839A, S839E, or ∆PDZ and PSD-95-myc in HEK293 cells. The cells were lysed, full-length NLGNs were immunoprecipitated and resolved by SDS/PAGE, and coimmunoprecipitated PSD-95-myc was analyzed by immunoblotting. As with the pull-down results, HA-NLGN1 S839E showed significantly reduced binding to PSD-95-myc compared with NLGN1 WT and the S839A mutant (Fig. 3C), whereas HA-NLGN1 ∆PDZ showed impaired binding with PSD-95-myc (Fig. 3D). We also confirmed the specific regulation of S839 phosphorylation on NLGN1 and PSD-95 binding. We cotransfected full-length HA-NLGN1 WT, S839A, or S839E and gephyrin-myc in HEK293 cells. The cells were lysed, full-length NLGNs were immunoprecipitated and resolved by SDS/PAGE, and coimmunoprecipitated gephyrin-myc was analyzed by immunoblotting. The result showed that NLGN1 S839 phosphorylation does not affect NLGN1 and gephyrin binding (SI Appendix, Fig. S1).
We also cotransfected full-length HA-NLGN1 WT, S839A, or S839E and PSD-95-myc in cultured cortical neurons. Crude membranes were prepared and lysed for coimmunoprecipitation. As with the results in HEK293 cells, HA-NLGN1 S839E displayed reduced binding to PSD-95-myc in cultured neurons (Fig. 3E). Together, all of these data show that NLGN1 S839 phosphorylation regulates the NLGN1 and PSD-95 interaction.
NLGN1 S839 Phosphorylation and PSD-95 Binding Regulate NLGN1 Surface Levels.
We showed that PSD-95 knockdown affected NLGN1 surface expression (Fig. 1 A, B, and D). PSD-95 plays important roles in organizing and stabilizing postsynaptic channels, receptors, and membrane proteins at synapses, and, in many cases, phosphorylation is a dynamic regulator of PSD-95 effects (1). Our data showing the regulation of NLGN1 and PSD-95 binding by S839 phosphorylation led us to investigate the role of S839 phosphorylation in NLGN1 trafficking and synaptic colocalization.
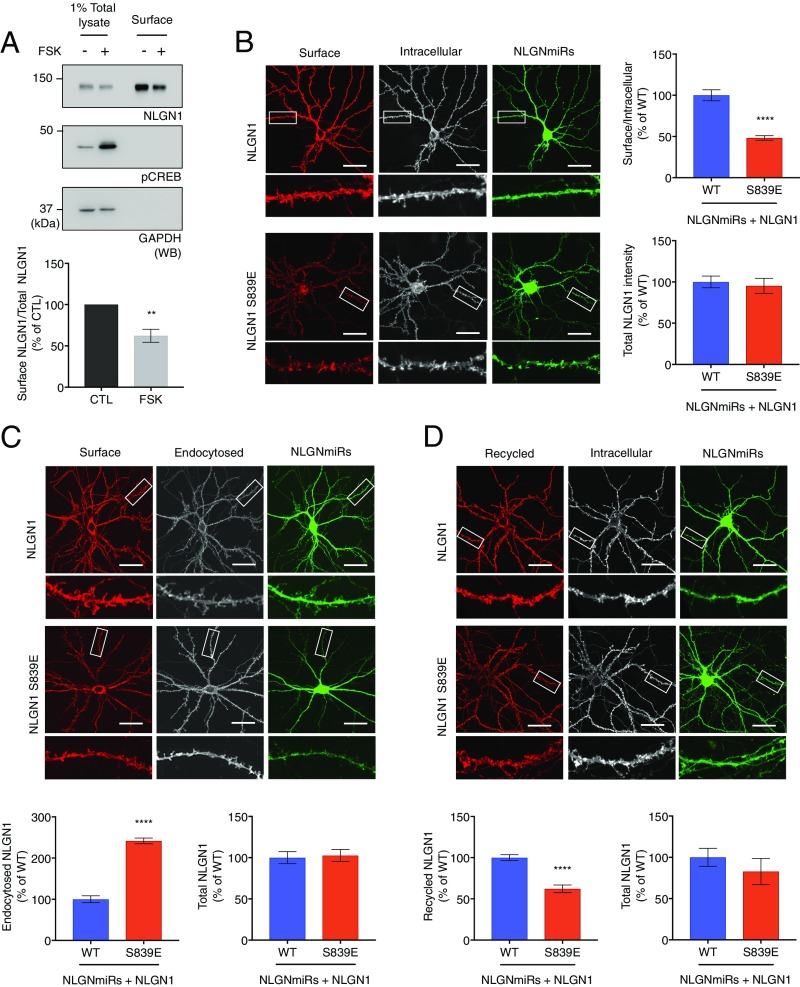
To examine the effect of PKA activity on NLGN1 surface levels, we treated cultured cortical neurons with forskolin to activate PKA and performed surface biotinylation assays. Interestingly, we observed a decrease in the surface expression of NLGN1 in neurons treated with forskolin (Fig. 4A), indicating NLGN1 surface expression is specifically susceptible to PKA activity.
Fig. 4.
NLGN1 S839 phosphorylation state regulates NLGN1 surface levels. (A) Cultured cortical neurons were treated with forskolin and surface biotinylation assays were performed as described in Materials and Methods. Proteins were resolved by SDS/PAGE and analyzed by immunoblotting. Surface NLGN1/total NLGN1 was analyzed. Graph indicates mean ± SEM (n = 4). **P = 0.0031 using unpaired t test. (B−D) NLGN miRs (green) and HA-NLGN1 (WT or S839E) were coexpressed in cultured hippocampal neurons. Enlarged images of the boxed regions are shown below each panel. Regions from three dendrites per each neuron were collected for analysis. HA-NLGN1 S839E was normalized to HANLGN1 WT. (Scale bar, 25 μm.) Graph indicates mean ± SEM ****P < 0.0001 using unpaired t test. (B) Surface HA-NLGN1 was labeled with anti-HA and Alexa 555-conjugated secondary antibody (red). After fixation and permeabilization, intracellular HA-NLGN1 was stained with anti-HA and Alexa 647-conjugated secondary antibody (white, n = 16 for WT and n = 14 for S839E). (C) For antibody-feeding assays, the neurons were incubated with HA antibody in the media at 37 °C for 30 min to feed antibody. Surface HA-NLGN1 (red) and endocytosed HA-NLGN1 (white) were visualized as above (n = 23 for WT and S839E). (D) For recycling assays, the transfected neurons were incubated with anti-HA antibody at RT and allowed internalization at 37 °C for 30 min. Excess amounts of anti-IgG were added to remove surface bound anti-HA antibody. The neurons were transferred back to 37 °C for 60 min for recycling. Recycled HA-NLGN1 (red) and internal HA-NLGN1 (white) were visualized as above (n = 18 for WT and S839E).
We next examined the surface levels of HA-NLGN1 expressed in cultured rat hippocampal neurons with immunofluorescence confocal microscopy. We transfected cultured hippocampal neurons with HA-NLGN1 (WT or S839E) and NLGN miRs for NLGNs knockdown at day in vitro (DIV) 17. Surface and intracellular HA-NLGN1 were labeled at DIV 22, and the dendritic regions were imaged for analysis. Notably, the phosphomimetic NLGN1 S839E mutation greatly reduced surface expression of NLGN1 (Fig. 4B), indicating that S839 phosphorylation is important for the regulation of NLGN1 surface levels. All these data support an important role for PKA phosphorylation of NLGN1 S839 in NLGN1 surface expression.
NLGN1 S839 Phosphorylation Affects NLGN1 Trafficking.
Reduced NLGN1 surface levels could be a consequence of decreased NLGN1 protein levels or impaired NLGN1 trafficking. We didn’t observe any evidence of protein degradation of NLGN1 upon PSD-95 modulation or PKA activation in the biochemical and immunocytochemistry results. Therefore, to examine the effect of S839 phosphorylation on NLGN1 trafficking, we performed an antibody-feeding and recycling assay.
Cultured hippocampal neurons were transfected with NLGN miRs and HA-NLGN1 (WT or S839E), and incubated with anti-HA antibody in the media at 37 °C for 30 min to allow antibody binding and HA-NLGN1 endocytosis. Surface HA-NLGN1 and endocytosed HA-NLGN1 were labeled as described in Materials and Methods. For analysis, dendrites of transfected neurons were imaged and analyzed. Although total HA-NLGN1 fluorescence intensities were comparable, we observed that fluorescence intensity of endocytosed S839E mutant was significantly increased compared with WT (Fig. 4C). This result suggests S839-phosphorylated NLGN1 undergoes robust endocytosis.
We also examined whether S839 phosphorylation affects the NLGN1 recycling process. Briefly, neurons were prepared as above and incubated with anti-HA antibody at room temperature (RT) and allowed HA-NLGN1 internalization at 37 °C. An excess amount of anti-IgG was added later at RT to block any anti-HA antibody bound to surface HA-NLGN1. The neurons were then transferred to 37 °C for 60 min to allow HA-NLGN1 recycling. Interestingly, we found that the HA-NLGN1 S839E mutant is deficient in recycling to the surface compared with WT (Fig. 4D). This result indicates S839 phosphorylation also diminishes NLGN1 recycling to the surface. Together, these data demonstrate that S839 affects both NLGN1 endocytosis and recycling.
NLGN1 S839 Phosphorylation Reduces NLGN1-Dependent Synaptic Enhancement.
Synaptogenic properties of NLGN1 have been well characterized in cell culture systems (28, 29). We next examined whether NLGN1-mediated synaptogenesis is regulated by S839 phosphorylation.
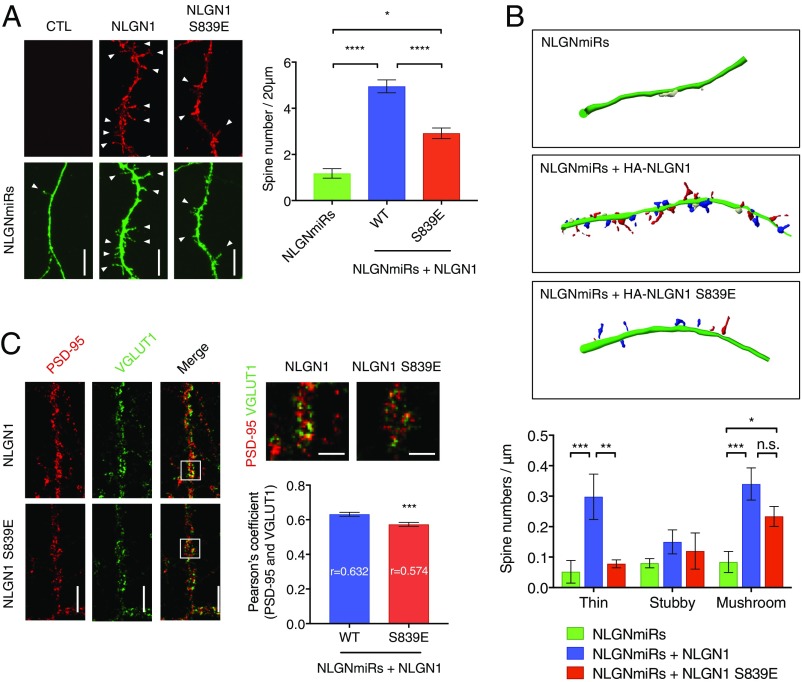
Enhanced spine formation has been shown in young cultured neurons (DIV 8 to 12) upon NLGN1 overexpression (30, 31). The cultured hippocampal neurons were transfected with HA-NLGN1 (WT or S839E) and NLGN miRs at DIV 5 and prepared at DIV 12 to count spine numbers. We observed HA-NLGN1 WT increases spine numbers, whereas HA-NLGN1 S839E has diminished increases in spine numbers compared with WT (Fig. 5A). Together with the previous biochemical results, these data support the importance of the NLGN1 and PSD-95 interaction for NLGN1 synaptic localization and spinogenesis during neuronal development.
Fig. 5.
NLGN1 S839 phosphorylation reduces synaptic enhancement. (A) Cultured hippocampal neurons were transfected with NLGN miRs and HA-NLGN1 (WT or S839E) at DIV 5. After fixation and permeabilization, HA-NLGN1 was stained with anti-HA and Alexa 555-conjugated secondary antibody (red). Regions from secondary dendrites per each neuron were collected for spine number counting. Arrow heads indicate spines. (Scale bar, 5 µm.) Graph indicates mean ± SEM (n = 18). The statistical significance between every condition was calculated using one-way ANOVA with Tukey’s multiple comparison test. *P = 0.024866, and ****P < 0.000001. (B) Regions from secondary dendrites for each neuron were collected to categorize spine morphology using Neurolucida software. Thin (red), mushroom (blue), and stubby (white) spines are indicated. Graph indicates mean ± SEM (n = 6). The statistical significance between every condition was calculated using two-way ANOVA with Tukey’s multiple comparison test. **P = 0.0032 and ***P = 0.0010 for thin spines. *P = 0.0439 and ***P = 0.0006 for mushroom spines. n.s., not significant. (C) NLGN miRs and HA-NLGN1 (WT or S839E) were coexpressed in cultured hippocampal neurons at DIV 12. Endogenous PSD-95 were labeled with anti−PSD-95 and Alexa 555-conjugated secondary antibody (red), and endogenous VGLUT1 were labeled with anti-VGLUT1 and Alexa 647-conjugated secondary antibody (green). (Scale bar, 5 µm.) The selected regions in the merged image were enlarged. (Scale bar, 2.5 µm.) Regions from three dendrites per each neuron were analyzed for Pearson’s coefficient. Graph indicates mean ± SEM (n = 23). ***P = 0.0007 using unpaired t test.
We further categorized spines based on morphology into thin, stubby, and mushroom subtypes (Fig. 5B). Both HA-NLGN1 WT and HA-NLGN1 S839E restored mushroom-type spines on NLGNmiRs conditions, although the HA-NLGN1 S839E rescue was to a lesser extent. Intriguingly, thin spines were further recovered by HA-NLGN1 WT. Thin spines are immature and have structural flexibility for AMPAR insertion and PSD-95 recruitment, which gives a greater potential for synaptic strengthening and plasticity (32, 33). These results indicate that HA-NLGN1 expression induces greater synaptic activity.
Next, we examined whether mutation of S839 would affect functional synapse formation, in addition to the spine formation. Hippocampal cultured neurons were transfected with NLGN1 (WT or S839E) as described previously and stained for endogenous PSD-95 and VGLUT1 to mark postsynaptic and presynaptic sites, respectively. The dendritic regions of transfected neurons were imaged and analyzed for colocalization of PSD-95 and VGLUT1. Interestingly, hippocampal cultured neurons expressing NLGN1 S839E displayed diminished colocalization of PSD-95 and VGLUT1, indicating reduced functional synapse number (Fig. 5C).
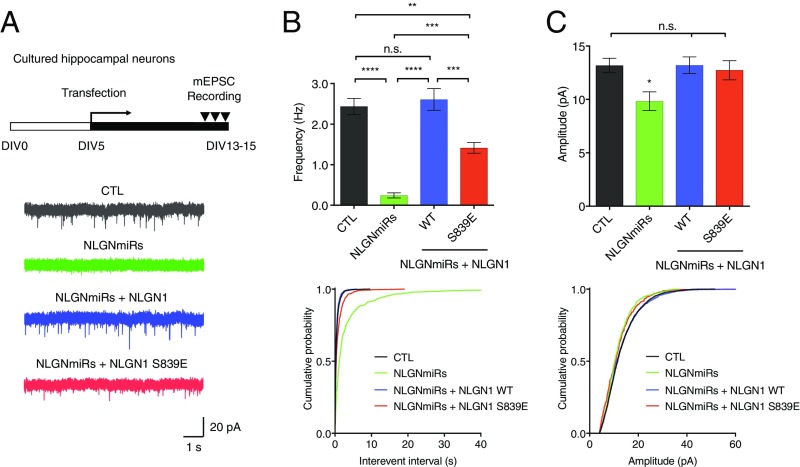
To investigate further any effect of S839 phosphorylation on synaptic transmission, we measured AMPAR miniature excitatory postsynaptic currents (mEPSC) in hippocampal cultured neurons expressing either NLGN miRs alone or NLGN miRs and HA-NLGN1 (WT or S839E). Knockdown of endogenous NLGNs induced a reduction in both mEPSC frequency and amplitude (Fig. 6). HA-NLGN1 fully rescued the reduced mEPSC frequency, but HA-NLGN1 S839E was not able to rescue the mEPSC frequency as efficiently as HA-NLGN1 WT (Fig. 6B). Meanwhile, both HA-NLGN1 WT and S839E rescued the reduced mEPSC amplitude (Fig. 6C). Thus, these findings suggest that NLGN1 S839 phosphorylation decreases excitatory synaptic transmission.
Fig. 6.
NLGN1 S839 phosphorylation reduces synaptic transmission. (A) Representative AMPAR mEPSC traces recorded in cultured hippocampal neurons expressing either NLGN miRs or NLGN miRs and HA-NLGN1 (WT or S839E). (B) Spontaneous AMPAR mEPSC mean frequency and cumulative probability. **P = 0.001525, ***P = 0.000235, and ****P < 0.000001. (C) Spontaneous AMPAR mEPSC mean amplitude and cumulative probability. *P = 0.024275. (B and C) Bar graph indicates mean ± SEM (n = 14 to 15). The statistical significance between every condition was calculated using one-way ANOVA with Tukey’s multiple comparison test. n.s., not significant.
All these results indicate S839 phosphorylation has an important role in the synaptic enhancement by NLGN1. S839 phosphorylation diminishes spine numbers (Fig. 5 A and B) and functional synapse number (Fig. 5C), consequently reducing excitatory synaptic transmission (Fig. 6).
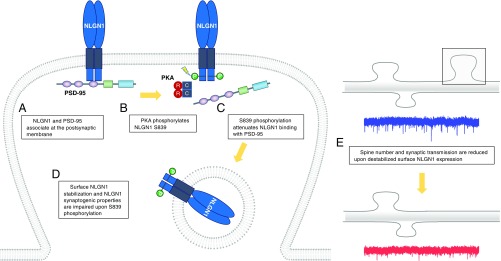
Overall, our findings support a model in which PKA phosphorylates S839 in the C-tail of NLGN1 and the phosphorylation attenuates PSD-95 binding to NLGN1 (Fig. 7 A–C). As illustrated in the model, S839 phosphorylation further regulates NLGN1 surface expression and trafficking (Fig. 7D). Ultimately, S839 phosphorylation affects NLGN1-mediated spinogenesis and excitatory synaptic transmission (Fig. 7E).
Fig. 7.
Model of PKA regulation of NLGN1. (A) NLGN1 and PSD-95 associate at synaptic regions and form a stable complex. (B) PKA phosphorylates S839 in the cytoplasmic region of NLGN1. (C) S839 phosphorylation disrupts the binding between NLGN1 and PSD-95, (D) which results in reduced surface levels of NLGN1. (E) Consequently, spine number and excitatory synaptic transmission are impaired upon destabilized NLGN1 surface expression.
Discussion
PDZ-mediated interactions are fundamental to the organization and signaling of the PSD. NLGN1 contains a conserved PDZ ligand; however, its function and regulation have remained largely elusive. Many studies have investigated aspects of PSD-95 regulation of NLGN1. For example, NLGN1 and PSD-95 can mutually enhance the synaptic localization of each other (14, 30, 34), which indicates a supportive role of PSD-95 in NLGN1 trafficking and stabilization at synapses. Nevertheless, it has also been suggested that the association of NLGN1 and PSD-95 with the dynein motor complex is involved in the retrograde transport and deprivation of surface NLGN1 (35). Other studies have reported that the membrane targeting of NLGN1 is independent of PSD-95 binding altogether (16, 36), and a dendritic sorting motif in the cytoplasmic region of NLGN1 may be sufficient to target NLGN1 to dendrites, but not to synapses (37). These previous studies reveal a controversial role for PSD-95 in its ability to regulate NLGN1 trafficking, which may vary according to different experimental conditions. In particular, most studies have used ectopic protein expression, with fewer studies precisely investigating endogenous NLGN trafficking. To directly assess the effect of PSD-95 on endogenous NLGN trafficking, we evaluated endogenous NLGN1 upon knockdown of PSD-95 in cultured neurons and found a decrease in surface and synaptic NLGN1 (Fig. 1 A and D). Indeed, our results show that PSD-95 is specifically required for stabilizing synaptic NLGN1 expression, emphasizing a critical role for PSD-95 in the trafficking of NLGN1. Furthermore, we show that NLGN1 and PSD-95 binding is under the control of PKA-regulated S839 phosphorylation, affecting NLGN1 trafficking (Fig. 4).
NLGN1 is a key component of the postsynaptic complex of proteins that regulate the assembly of excitatory synapses and mediate excitatory synaptic transmission. NLGN1 is known to control synaptic incorporation and retention of NMDARs by direct binding via its extracellular domain, which is important for NMDAR-mediated transmission in the CA1 region of hippocampus (38). Impairment of long-term potential (LTP) in hippocampus and amygdala following NLGN1 manipulation has been reported (39–41), indicating the requirement of NLGN1 for excitatory synaptic plasticity. It also has been shown that NLGN1 and PSD-95 coordinate AMPAR clusters and AMPAR-mediated mEPSCs for synapse maturation (42). A recent study reported that the NLGN1 and PSD-95 interaction might also be important in aligning presynaptic release sites and AMPAR nanodomains required for AMPAR-mediated synaptic transmission (43). The authors observed that both NLGN1-∆Cter [lacking the last 72 amino acids, but harboring the critical residue identified by Shipman et al. (18)] or biomimetic ligands of 15 C-terminal amino acids of NLGN1 impair synaptic currents. Similarly, Letellier et al. (44) also showed that NLGN1-∆5 (lacking the last five amino acids) displays impaired AMPAR-dependent synaptic transmission and reduced spine density. In our current study, we observed a defect in AMPAR-mediated mEPSC frequency by the NLGN1 S839E mutant compared with WT (Fig. 6 B and C). NLGN1 S839E does colocalize with PSD-95 (Fig. 5C) and can bind to PSD-95 (Fig. 3 B and D), although this is significantly reduced compared with WT NLGN1. All of these studies indicate that NLGN1 expression and its coordination with PSD-95 at synapses are important for glutamate receptor signaling and synaptic plasticity.
We previously showed that CaMKII-mediated phosphorylation on NLGN1 T739 enhances NLGN1 surface expression (19). In the current study, we reveal another precise molecular mechanism that regulates NLGN1 surface expression, in this case, via PKA phosphorylation. Synaptic PKA activity has been reported to be regulated by LTP, long-term depression, and homeostatic scaling (45, 46). Since we show that NLGN1 S839 phosphorylation is dynamically regulated upon neuronal activation in vivo and in cultured neurons (Fig. 2H and SI Appendix, Fig. S3), it is possible that NLGN1 trafficking can be dynamically regulated by NLGN1 S839 phosphorylation in response to a variety of neuronal stimuli. Meanwhile, Schapitz et al. (35) showed that chemical LTP using forskolin increases surface NLGN1 in acute hippocampal slices and cultured hippocampal neurons. This discrepancy could be the result of the different experimental conditions including different neuronal types, stage of neuronal maturation, or specific preparation. It is possible that the NLGN1 S839 phosphorylation mainly regulates NLGN1 surface levels during synaptic recycling, whereas the NLGN1 and PSD-95 interaction is required for retrieving NLGN1 by the dynein-mediated dendritic retrograde transport. Although both studies emphasize that the NLGN1 C-tail binding to PSD-95 is involved in the regulation of NLGN1 surface expression, further molecular study to dissect the balance between the synaptic recycling and dendritic retrograde transport to modulate surface NLGN1 in response to various physiological conditions will be required.
NLGN-mediated spinogenesis and synapse formation have been widely studied in vivo or in cell lines by expressing WT or mutant exogenous NLGNs. However, it is not always clear whether reduced spine density and impaired glutamate currents following manipulations of NLGNs are the result of altered NLGN surface expression. For example, Shipman et al. (18) showed that a single residue in the critical region of the C-tail of NLGNs is required for the enhancement of AMPAR or NMDAR currents and spine density by NLGNs, whereas surface expression of NLGN3 was not affected. Our previous work showed that phosphorylation on NLGN4X T707 affects excitatory synaptic currents and spine number, but doesn’t affect surface expression of NLGN4X (47). These studies indicate that NLGN1 intrinsic properties for spinogenesis and NLGN1 surface expression can be regulated independently. Moreover, Kwon et al. (48) showed that spine density and NMDAR-mediated currents in a single cell are affected by the difference in NLGN1 expression levels relative to neighboring cells, which reveals the complications in deciphering the functional correlation between NLGN surface levels and spinogenesis in various NLGNs expression backgrounds. In this study, we showed that a phosphomimetic mutant of the PKA site, NLGN1 S839E, displays reduced surface expression, reduced spine number, and impaired mEPSC currents (Figs. 4–6). Our results support the model whereby PKA phosphorylation decreases NLGN1 surface expression and thereby impairs spinogenesis and excitatory synaptic transmission.
In addition to PSD-95, other trafficking machinery has been implicated in the regulation of NLGN1 trafficking. Other members of the MAGUK family, such as SAP97, SAP102, and PSD-93, and the MAGUK with inverted orientation (MAGI) family, such as S-SCAM, Magi1, and Magi3, are also known to interact with NLGN1 via the PDZ ligand, although their functional relevance has not been elucidated (36, 49, 50). It is likely that NLGN1 dynamically switches its interactors during its intracellular trafficking itinerary and following endocytosis. The promiscuous interaction of these PDZ domain proteins to NLGN1 in different assays has made it difficult to definitively identify the precise functional significance of these interactions. It is conceivable that phosphorylation of NLGN1 at S839 not only breaks the interaction with PSD-95 but may facilitate a subsequent interaction with other PDZ domain proteins. Further intensive molecular studies will be required to identify the physiological relevance of NLGN1 S839 phosphorylation for additional PDZ protein interactions.
Phosphorylation of PDZ ligands is a common conserved mechanism for regulating synaptic protein interactions with MAGUKs. For example, CK2 phosphorylation of GluN2B regulates NMDAR binding to PSD-95 and stabilization at synapses (27). Interestingly, this is exquisitely subunit-specific, and GluN2A is not phosphorylated by CK2 even though the PDZ ligand is conserved in GluN2A and GluN2B. In addition, K channels are phosphorylated by PKA in the PDZ ligand to disrupt binding to PSD-95 (25). In these other cases of dynamic regulation of PSD-95 binding by receptor/channel phosphorylation, the phosphorylated residue is within the PDZ ligand at the −2 position. Our current findings support a similar role for PKA regulation, but at a residue slightly upstream, for regulating NLGN1 interaction with PSD-95.
Although the C-tails of all NLGNs show high similarity in amino acid sequence, isoform-specific phosphorylation has emerged as a key regulator of isoform specificity. For example, our laboratory revealed CaMKII-mediated phosphorylation on NLGN1 T739 and its importance for NLGN1 surface expression and glutamate currents, and this CaMKII phosphorylation event is not conserved in other isoforms (19). Moreover, phosphorylation on NLGN1 Y782, a site conserved in the gephyrin-binding domain of all NLGNs, inhibits binding with gephyrin, thus inhibiting NLGN1 localization at inhibitory synapses (20). Phosphorylation on NLGN2 S714, which is also conserved in all NLGNs, negatively regulates binding with gephyrin by recruiting Pin1 (51). Although some of these residues are conserved in the other NLGN isoforms, these studies suggest the possibility that phosphorylation of distinct NLGNs can exert NLGN isoform-specific regulation. In the same way, although the NLGN1 S839 residue is conserved in all NLGN isoforms and the analogous S839 site in GST-NLGN3 can be phosphorylated by PKA in vitro (SI Appendix, Fig. S2), S839 phosphorylation has a profound effect on the PSD-95 and NLGN1 interaction. Furthermore, knockdown of endogenous PSD-95 has a much more robust effect on NLGN1 expression compared with NLGN2 or NLGN3, revealing a high level of specificity.
In this study, we demonstrate that PSD-95 regulates NLGN1 trafficking and synaptogenic properties. We identified a PKA phosphorylation site, S839, adjacent to the PDZ ligand of NLGN1, which we show controls the interaction with PSD-95. Phosphorylation of this key residue attenuates NLGN1 binding with PSD-95, thereby reducing its surface expression and diminishing NLGN1-dependent enhancement of excitatory synapses. Our findings confirm an indispensable role of PSD-95 in the NLGN1 physiology and provide a molecular mechanism that can dynamically regulate the NLGN1 and PSD-95 interaction. This study identifies a direct regulation of the PDZ ligand in NLGN1 by phosphorylation. Our present results provide a way of dynamically regulating NLGN1 at synapses, and pave the way for future research in the NLGN field. The specificity of NLGN isoforms and compensation by other MAGUK family proteins have not been fully dissected. Understanding the dynamic regulation of NLGNs and synaptic potentiation will allow a new avenue to be explored for treating psychiatric dysfunction.
Materials and Methods
In Vitro Kinase Assay and GST Pulldown.
GST fusion proteins were phosphorylated in 10 mM Hepes, (pH 7.0), 20 mM MgCl2, 50 μM ATP, and 1 pmol of [γ-32P] ATP (3,000 Ci mmol−1) with 50 ng of purified PKA catalytic subunit (Promega). In vitro kinase assays were performed at 30 °C for 30 min. Proteins were eluted from the glutathione-Sepharose resin and resolved by SDS/PAGE and analyzed by immunoblotting.
For GST pulldown, P2 pellet (crude synaptosomal fraction) was prepared from adult rat brain as described previously and lysed in hypoosmotic buffer [20 mM Tris⋅HCl, pH 8.8, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% sodium deoxycholate (DOC)]. GST fusion proteins were incubated with the P2 lysate at 4 °C overnight. The samples were resolved by SDS/PAGE and analyzed by immunoblotting
Lentiviral Production.
HEK293 cells were transfected with the lentiviral vector FHUGW (FUGW variant) containing the short hairpin RNA sequence against rat PSD-95 or NLGN miRs or HA-NLGN1 (WT or S839A or S839E) and the packaging vector dsPAX2, and the vesicular stomatitis virus G envelope glycoprotein vector by using FUGENE HD (Promega, E2311) in UltraCULTURE medium (Lonza, 12-725F) containing 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.075% sodium bicarbonate. Culture media containing lentiviral particles were harvested and centrifuged at 100,000 × g for 2 h at 4 °C. The lentiviral particles were resuspended in phosphate-buffered saline (PBS), aliquoted, and kept at −80 °C.
Subcellular Fractionation of Brain Tissue and Cultured Neurons.
Biochemical fractionation was carried out as described in our previous studies (4, 19, 27). Briefly, mouse or rat brain tissue or cultured neurons were homogenized in ice-cold Tris/EDTA/vanadate/phosphatase (TEVP) buffer [320 mM sucrose, 10 mM Tris⋅HCl (pH 7.5), 5 mM EDTA, 1× protease inhibitor mixture (Roche, 11697498001), and phosphatase inhibitor mixture II (Sigma, P5726) and III (Sigma, P0044)]. Homogenates were centrifuged at 1,000 × g for 10 min at 4 °C. The supernatant was centrifuged at 10,000 × g for 20 min at 4 °C to get P2 pellet (crude synaptosomal fraction). The P2 pellet was lysed in an appropriate buffer for analysis or resuspended with ice-cold TEVP buffer [35.6 mM sucrose, 10 mM Tris⋅HCl (pH 7.5), 5 mM EDTA, 1× protease inhibitor mixture, and phosphatase inhibitor mixture II and III] and centrifuged at 25,000 × g for 20 min to get synaptic plasma membrane (SPM). The SPM pellet was lysed in ice-cold TEVP buffer [1% Triton X-100, 10 mM Tris⋅HCl (pH 7.5), 5 mM EDTA, 1× protease inhibitor mixture, and phosphatase inhibitor mixture II and III]. The lysates were centrifuged at 33,000 × g for 30 min at 4 °C to obtain the synaptic regions (Triton X-100−insoluble) and extrasynaptic regions (Triton X-100−soluble).
Surface Biotinylation.
Cultured cortical neurons at DIV 17 to 20 were washed three times with cold PBS (including 2 mM CaCl2 and 1 mM MgCl2). Neurons were incubated with 1 mg/mL biotin (EZLink Sulfo-NHS-LC-Biotin, Thermo Fisher Scientific, #21335) in PBS (including 2 mM CaCl2 and 1 mM MgCl2) for 30 min at 4 °C and subsequently quenched free biotin by incubating neurons with PBS containing 100 mM glycine for 20 min. After washing, neurons were lysed with RIPA buffer (150 mM NaCl, 50 mM Tris⋅HCl, pH 7.8, 1 mM EDTA, 1% Triton X-100, 0.5% deoxycholic acid, 0.5% SDS). The lysates were vigorously sonicated, and supernatants were incubated with NeutrAvidin agarose resin (Thermo Fisher Scientific, #29202) for 1 h to 2 h. Proteins were eluted from the agarose resin and resolved by SDS/PAGE, and analyzed by immunoblotting.
Dark-Rearing Experiments.
Male and female C57BL/6J littermates were maintained as a previously published protocol (19, 52). Mice were housed in a normal light and dark cycle (12 h light and 12 h dark) from P0 to P26 (light-reared, LR), were relocated to complete darkness from P21 to P26 (dark-reared, DR), or were kept in complete darkness from P21 to P26 but shifted back to light for 2 h before killing (DR plus light-exposed, DR+LE). The primary visual cortex was macrodissected in the dark for the DR condition or in the light for the LR and DR+LR conditions. P2 pellet (crude synaptosomal fraction) was prepared for analysis as described previously. The National Institute of Neurological Disorders and Stroke (NINDS) Animal Care and Use Committee approved our use of experimental animals (protocol #1171). All animals were handled and the experiments were performed according to these guidelines.
Immunocytochemistry.
Cultured hippocampal neurons were grown on glass coverslips precoated with poly-d-lysine (Sigma). Neurons were cotransfected with pCAG-NLGN miRs-GFP (18) and pCAG-HA-NLGN1 (WT, S839A, or S839E) plasmids with Lipofectamine2000 at DIV 12 to 15 and prepared for analysis at DIV 19 to 21, if not described otherwise.
To label surface HA-NLGN1, transfected neurons were labeled with rat anti-HA antibody for 15 min at RT. Neurons were washed with PBS and fixed with 4% paraformaldehyde and 4% sucrose in PBS for 10 min. The cells were incubated with Alexa 555-conjugated anti-rat secondary antibody (Molecular Probes). After surface labeling, the neurons were permeabilized with 0.25% Triton X-100 in PBS and blocked with 10% goat serum. Intracellular HA-NLGN1 was labeled with rabbit anti-HA antibody and Alexa 647-conjugated anti-rabbit secondary antibody.
For the antibody-feeding assay, the neurons were incubated with rat anti-HA antibody in the media at 37 °C for 30 min to allow antibody uptake. Neurons were washed with PBS and fixed with 4% paraformaldehyde and 4% sucrose in PBS for 10 min. After surface labeling of HA-NLGN1, the neurons were permeabilized with 0.25% Triton X-100 in PBS and blocked with 10% goat serum. Intracellular HA-NLGN1 was labeled as above.
Recycling assays were performed as described in our previous work (53). Briefly, neurons were incubated with anti-HA antibody at RT for 10 min, and surface proteins were allowed to internalize at 37 °C for 30 min. Excess amount of anti-rabbit IgG (1 μg per 100 μL) was added later at RT for 20 min and washed out with Neurobasal Media. The neurons were transferred back to 37 °C for 60 min for recycling. Recycled HA-NLGN1 and intracellular HA-NLGN1 were labeled with appropriate antibodies as above.
Neurons were cotransfected with NLGN miRs and HA-NLGN1 at DIV 5 to 6 and prepared at DIV 12 to count spine number. Total HA-NLGN1 was labeled with rat anti-HA antibody and Alexa 555-conjugated anti-rat secondary antibody.
For endogenous PSD-95 and VGLUT1 staining, neurons were prepared as above, then labeled with anti−PSD-95 (NeuroMab, clone K28/43) and anti-VGLUT1 (Millipore, AB5905).
For analysis, regions from three dendrites per each neuron were collected and quantified by the fluorescence intensity of target proteins. All images were captured with a 63× objective on a Zeiss LSM 510 confocal microscope and analyzed with the Image J and MetaMorph Version 7.
Spine Morphology Analysis.
Neurons were cotransfected with NLGN miRs and HA-NLGN1 at DIV 5–6 and prepared at DIV 12 as described previously. For spine analysis, regions from three dendrites per each neuron were collected, and spine morphology was analyzed with the Neurolucida 360 software (MBF Bioscience).
Electrophysiological Recording.
AMPAR mEPSCs recordings were performed in dissociated rat hippocampal primary cultures (DIV 13 to 15). Recordings were done in artificial cerebrospinal fluid (ACSF) containing 119 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 1 mM Na2PO4, 11 mM glucose, 2.5 mM CaCl2, and 1.3 mM MgCl2; 0.1 mM Picrotoxin 0.1 and 0.5 μM TTX were added to the ACSF before recording. The intracellular solution for mEPSC recording contained 135 mM CsMeSO4, 8 mM NaCl, 10 mM Hepes, 0.3 mM Na3GTP, 4 mM MgATP, 0.3 mM EGTA, 5 mM QX-314, and 0.1 mM spermine. Osmolality of the solutions were adjusted to 285 mOsm to 290 mOsm, and pH was buffered at 7.25 to 7.35. AMPAR mEPSCs were recorded at −70 mV, and the analysis of the mEPSCs was done semiautomatically, using in-house software Igor Pro (Wavemetrics) developed in Roger Nicoll’s laboratory at University of California, San Francisco (UCSF). All events were visually inspected to ensure they were mEPSCs during analysis, and those nonmEPSC traces were discarded. Series resistance was monitored and not compensated, and cells in which series resistance varied by 25% during a recording session were discarded. Synaptic responses were collected with a Multiclamp 700B amplifier (Axon Instruments), filtered at 2 kHz, and digitized at 10 kHz. All pharmacological reagents were purchased from Abcam, and other chemicals were purchased from Sigma.
Statistical Analysis.
Data analysis was carried out in Image Lab Software (Bio-Rad) or GraphPad Prism (GraphPad Software).
Supplementary Material
Acknowledgments
We thank Michael A. Bemben (UCSF) and all other lab colleagues for discussion and assistance. We thank the NINDS light imaging facility for technical assistance. This research was supported by the NINDS Intramural Research Program (J.J., S.P., W.L., and K.W.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821775116/-/DCSupplemental.
References
- 1.Kim E., Sheng M., PDZ domain proteins of synapses. Nat. Rev. Neurosci. 5, 771–781 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Won S., Levy J. M., Nicoll R. A., Roche K. W., MAGUKs: Multifaceted synaptic organizers. Curr. Opin. Neurobiol. 43, 94–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X., et al. , PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl. Acad. Sci. U.S.A. 112, E6983–E6992 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Won S., Incontro S., Nicoll R. A., Roche K. W., PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc. Natl. Acad. Sci. U.S.A. 113, E4736–E4744 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornau H. C., Schenker L. T., Kennedy M. B., Seeburg P. H., Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269, 1737–1740 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Bats C., Groc L., Choquet D., The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Ichtchenko K., et al. , Neuroligin 1: A splice site-specific ligand for beta-neurexins. Cell 81, 435–443 (1995). [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T., Südhof T. C., Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J. Biol. Chem. 272, 26032–26039 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Varoqueaux F., et al. , Neuroligins determine synapse maturation and function. Neuron 51, 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Chubykin A. A., et al. , Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54, 919–931 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bemben M. A., Shipman S. L., Nicoll R. A., Roche K. W., The cellular and molecular landscape of neuroligins. Trends Neurosci. 38, 496–505 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong J., Paskus J. D., Roche K. W., Posttranslational modifications of neuroligins regulate neuronal and glial signaling. Curr. Opin. Neurobiol. 45, 130–138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irie M., et al. , Binding of neuroligins to PSD-95. Science 277, 1511–1515 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Prange O., Wong T. P., Gerrow K., Wang Y. T., El-Husseini A., A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. U.S.A. 101, 13915–13920 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futai K., et al. , Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat. Neurosci. 10, 186–195 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dresbach T., Neeb A., Meyer G., Gundelfinger E. D., Brose N., Synaptic targeting of neuroligin is independent of neurexin and SAP90/PSD95 binding. Mol. Cell. Neurosci. 27, 227–235 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Barrow S. L., et al. , Neuroligin1: A cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 4, 17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shipman S. L., et al. , Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nat. Neurosci. 14, 718–726 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bemben M. A., et al. , CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat. Neurosci. 17, 56–64 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannone G., et al. , Neurexin-1β binding to neuroligin-1 triggers the preferential recruitment of PSD-95 versus gephyrin through tyrosine phosphorylation of neuroligin-1. Cell Rep. 3, 1996–2007 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Esteban J. A., et al. , PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 6, 136–143 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Raman I. M., Tong G., Jahr C. E., Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron 16, 415–421 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Hoffman D. A., Johnston D., Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J. Neurosci. 18, 3521–3528 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J., et al. , Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J. Biol. Chem. 277, 12359–12363 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Tanemoto M., Fujita A., Higashi K., Kurachi Y., PSD-95 mediates formation of a functional homomeric Kir5.1 channel in the brain. Neuron 34, 387–397 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Chetkovich D. M., Chen L., Stocker T. J., Nicoll R. A., Bredt D. S., Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J. Neurosci. 22, 5791–5796 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz-Clemente A., Matta J. A., Isaac J. T., Roche K. W., Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67, 984–996 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheiffele P., Fan J., Choih J., Fetter R., Serafini T., Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Fu Z., Washbourne P., Ortinski P., Vicini S., Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J. Neurophysiol. 90, 3950–3957 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Chih B., Engelman H., Scheiffele P., Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Levinson J. N., et al. , Neuroligins mediate excitatory and inhibitory synapse formation: Involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J. Biol. Chem. 280, 17312–17319 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Berry K. P., Nedivi E., Spine dynamics: Are they all the same? Neuron 96, 43–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourne J., Harris K. M., Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 17, 381–386 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Graf E. R., Zhang X., Jin S. X., Linhoff M. W., Craig A. M., Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119, 1013–1026 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schapitz I. U., et al. , Neuroligin 1 is dynamically exchanged at postsynaptic sites. J. Neurosci. 30, 12733–12744 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iida J., Hirabayashi S., Sato Y., Hata Y., Synaptic scaffolding molecule is involved in the synaptic clustering of neuroligin. Mol. Cell. Neurosci. 27, 497–508 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Rosales C. R., Osborne K. D., Zuccarino G. V., Scheiffele P., Silverman M. A., A cytoplasmic motif targets neuroligin-1 exclusively to dendrites of cultured hippocampal neurons. Eur. J. Neurosci. 22, 2381–2386 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Budreck E. C., et al. , Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc. Natl. Acad. Sci. U.S.A. 110, 725–730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., et al. , Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc. Natl. Acad. Sci. U.S.A. 105, 9087–9092 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung S. Y., et al. , Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 4710–4715 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shipman S. L., Nicoll R. A., A subtype-specific function for the extracellular domain of neuroligin 1 in hippocampal LTP. Neuron 76, 309–316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nam C. I., Chen L., Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc. Natl. Acad. Sci. U.S.A. 102, 6137–6142 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas K. T., et al. , Pre-post synaptic alignment through neuroligin-1 tunes synaptic transmission efficiency. eLife 7, e31755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letellier M., et al. , A unique intracellular tyrosine in neuroligin-1 regulates AMPA receptor recruitment during synapse differentiation and potentiation. Nat. Commun. 9, 3979 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diering G. H., Gustina A. S., Huganir R. L., PKA-GluA1 coupling via AKAP5 controls AMPA receptor phosphorylation and cell-surface targeting during bidirectional homeostatic plasticity. Neuron 84, 790–805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colledge M., et al. , Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27, 107–119 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Bemben M. A., et al. , Autism-associated mutation inhibits protein kinase C-mediated neuroligin-4X enhancement of excitatory synapses. Proc. Natl. Acad. Sci. U.S.A. 112, 2551–2556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon H. B., et al. , Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat. Neurosci. 15, 1667–1674 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer G., Varoqueaux F., Neeb A., Oschlies M., Brose N., The complexity of PDZ domain-mediated interactions at glutamatergic synapses: A case study on neuroligin. Neuropharmacology 47, 724–733 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Lim I. A., Hall D. D., Hell J. W., Selectivity and promiscuity of the first and second PDZ domains of PSD-95 and synapse-associated protein 102. J. Biol. Chem. 277, 21697–21711 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Antonelli R., et al. , Pin1-dependent signalling negatively affects GABAergic transmission by modulating neuroligin2/gephyrin interaction. Nat. Commun. 5, 5066 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philpot B. D., Sekhar A. K., Shouval H. Z., Bear M. F., Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29, 157–169 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Suh Y. H., et al. , A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat. Neurosci. 13, 338–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.