Significance
CD8 T cells are immune cells that protect against viral infections and help fight tumors. Not surprisingly, the regulatory mechanisms that control CD8 T cells have been extensively studied. However, whether a new class of RNA molecules, termed long noncoding RNAs (lncRNAs), regulate CD8 T cells during viral infections remains largely unexplored. Here, we show that in response to potent antiviral cytokines (type I IFNs), the lncRNA Morrbid and the genomic region from where it is produced tightly regulate CD8 T-cell survival and function. Thus, we now provide evidence that a lncRNA locus and the RNA it produces can cooperate to control immune responses mediated by CD8 T cells. Moreover, these results may represent a therapeutic avenue to combat viral infections and tumors.
Keywords: CD8 T cells, lncRNAs, viral infection
Abstract
The transcriptional programs that regulate CD8 T-cell differentiation and function in the context of viral infections or tumor immune surveillance have been extensively studied; yet how long noncoding RNAs (lncRNAs) and the loci that transcribe them contribute to the regulation of CD8 T cells during viral infections remains largely unexplored. Here, we report that transcription of the lncRNA Morrbid is specifically induced by T-cell receptor (TCR) and type I IFN stimulation during the early stages of acute and chronic lymphocytic choriomeningitis virus (LCMV) infection. In response to type I IFN, the Morrbid RNA and its locus control CD8 T cell expansion, survival, and effector function by regulating the expression of the proapoptotic factor, Bcl2l11, and by modulating the strength of the PI3K–AKT signaling pathway. Thus, our results demonstrate that inflammatory cue-responsive lncRNA loci represent fundamental mechanisms by which CD8 T cells are regulated in response to pathogens and potentially cancer.
Long noncoding RNAs (lncRNAs) are a recently described class of RNA defined as RNAs that do not code for protein that are greater than 200 bp in length (1, 2). lncRNAs are typically transcribed by Polymerase II (PolII), capped, spliced, and polyadenylated (3). In recent years, lncRNAs have been shown to play critical roles in regulating gene expression at multiple levels from the epigenetic control of chromatin accessibility, to splicing and posttranscriptional regulation of gene expression (3). Furthermore, recent findings indicate that either the lncRNA or transcription of lncRNA genes can regulate 3D architecture of the genome and gene expression regulation (4, 5). While the roles of lncRNAs in immune cell development and protective immunity have started to be elucidated (6), the function of these enigmatic molecules or transcription of these loci during adaptive immune responses against viral infections remains poorly understood. Addressing this gap in knowledge may reveal therapeutic avenues to boost protective immune responses against both pathogens and cancer.
CD8 T cells play a key role in adaptive immunity and contribute to host defense and antitumor responses. Upon encountering antigen and initial activation, CD8 T cells undergo proliferative expansion and differentiate into effector CD8 T cells (7). The great majority of these effector CD8 T cells will die through apoptosis during the contraction phase following antigen clearance, but a subset of antigen-specific memory precursors survives and differentiates into long-lived memory T cells (8). Memory CD8 T cells have the capacity for long-term host protection as they can undergo robust recall responses upon secondary antigen challenge (9). Due to their significance in protective immunity and tumor immunosurveillance, substantial efforts have been devoted to understanding the cellular, molecular, and transcriptional processes that regulate CD8 T-cell differentiation and function. However, how lncRNAs or the loci from which they are transcribed regulate CD8 T-cell differentiation or function during viral responses remains largely unexplored.
Here, we describe a critical role for the locus of the recently described lncRNA Morrbid in CD8 T-cell responses during lymphocytic choriomeningitis virus (LCMV) infection. While the Morrbid lncRNA was originally identified as a critical regulator of myeloid cells under homeostasic conditions (10), we now show that transcription of Morrbid is induced in CD8 T cells following viral infection in response to T-cell receptor (TCR) and type I IFN stimulation. Furthermore, we show that the Morrbid locus and its RNA are important in the negative regulation of CD8 T-cell expansion and effector function. These results demonstrate that Morrbid, and likely other extracellular cue-responsive lncRNA loci, is critical in finely tuning the protective and pathogenic potential of cytotoxic CD8 T cells.
Results
Morrbid Is Induced in CD8 T Cells During Viral Infection and in Response to TCR and Type I IFN Stimulation.
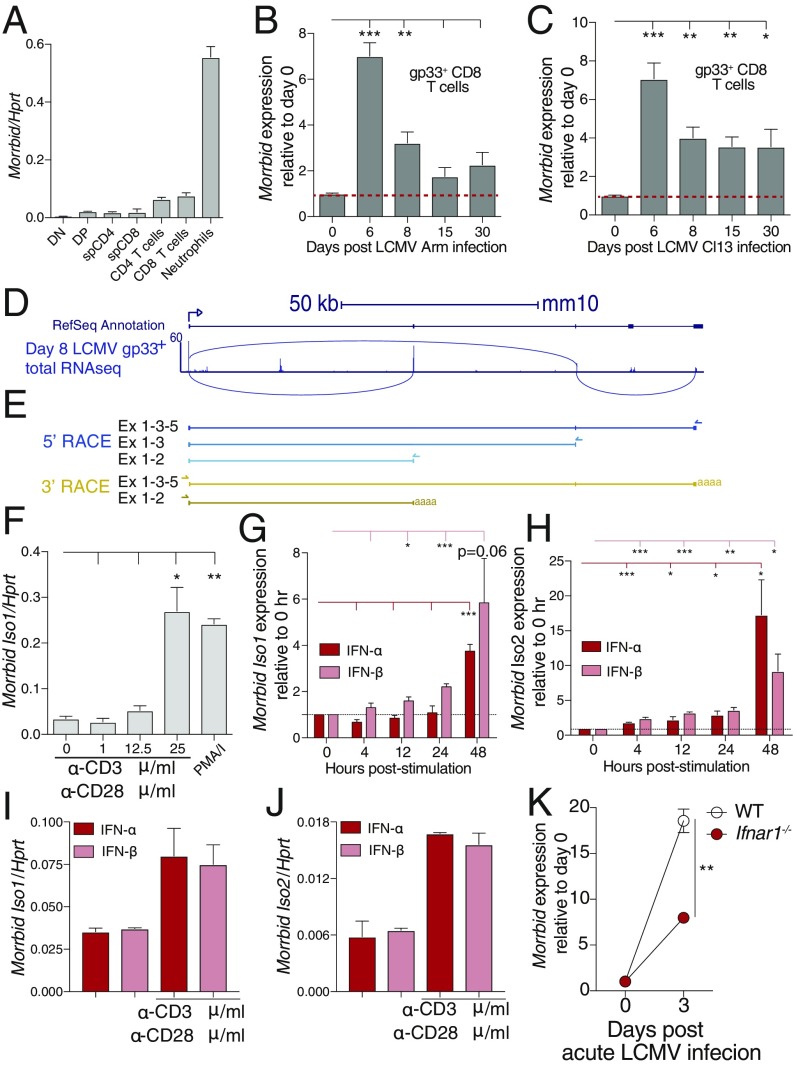
Following a primary infection, naive CD8 T cells are activated by antigen-presenting cells, clonally expand, and differentiate into short-lived effector and long-lived memory cell populations (8). To provide protective immunity and limit immunopathology, proliferation and the life span of antigen-specific CD8 T cells are tightly controlled (8). As we previously demonstrated that the lncRNA Morrbid strictly controls the life span of myeloid cells at homeostasis, we hypothesized that this lncRNA or its locus might regulate the life span of other immune cells under nonhomeostatic conditions, such as CD8 T cells following viral infection. To address this hypothesis, we utilized LCMV Armstrong, a well-characterized model of acute viral infection (11). At homeostasis, Morrbid was lowly expressed by CD8 T cells, in both the thymus and in the periphery (Fig. 1A). However, following infection with LCMV Armstrong (Arm), Morrbid expression was induced by approximately sevenfold in gp33-tetramer specific CD8 T cells at day 6 postinfection, and returned to near baseline following this time point (GSE41867; Fig. 1B). Similar kinetics of induction were observed following infection with LCMV clone 13 (Cl13), a viral strain that establishes persistent infection (Fig. 1C).
Fig. 1.
Morrbid is induced in CD8 T cells during viral infection and in response to TCR and type I IFN stimulation. (A) Morrbid transcript expression was assessed by qPCR in sorted double-negative (DN), double-positive (DP), single-positive (sp) CD4, and sp CD8 T-cell thymocytes, as well as splenic CD4 and CD8 T cells. Sorted neutrophils were used as positive control (n = 3 biological replicates; these data are representative of two independent experiments). (B and C) Morrbid expression in gp33-tetramer–specific CD8 T cells by microarray after (B) LCMV Armstrong (Arm) and (C) LCMV clone 13 (Cl13) infection represented relative to uninfected (GSE41867; n = 3–4 biological replicates). (D, Top) Schematic of the Morrbid locus and its predicted exons. (D, Bottom) Day 8 post-LCMV P14 CD8 T-cell total RNAseq reads that map to the Morrbid locus (GSE88987). Lines indicate reads spanning two locations. (E, Top) 5′- and (Bottom) 3′-rapid amplification of cDNA ends (RACE) of the Morrbid locus from CD8 T cells stimulated with αCD3/αCD28/IFN-β. The arrows indicate gene-specific primers. (F) qPCR of Morrbid transcript expression in sorted splenic CD8 T cells from naive WT spleens stimulated with the indicated doses of plate-bound αCD3 and 1 μg/mL soluble αCD28, or PMA/I for 4 h (n = 3 biological replicates). (G and H) qPCR of (G) Morrbid isoform 1 and (H) isoform 2 in negatively selected splenic CD8 T cells stimulated for indicated time with 1 μg/mL plate-bound αCD3, 1 μg/mL soluble αCD28, and 20 ng/mL IFN-α or IFN-β. Expression is represented as fold change relative to 0 h (n = 3 biological replicates; these data are representative of three independent experiments). (I and J) qPCR of (I) Morrbid isoform 1 and (J) isoform 2 transcript in negatively selected splenic CD8 T cells unstimulated or stimulated for 48 h with IFN-α or IFN-β (n = 3 biological replicates; these data are representative of three independent experiments). (K) Morrbid expression in WT or Ifnar1−/− P14 CD8 T cells by microarray after acute LCMV infection represented relative to uninfected (GSE57355; n = 3 biological replicates). Error bars show SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired two-sided t test, B, C, F–H, and K).
To better understand transcription across the Morrbid locus in CD8 T cells during infection, we utilized a previously published total-RNA transcriptomics dataset of LCMV-specific CD8 T cells following LCMV Armstrong infection (GSE88987). Several regions of the Morrbid locus are transcribed during the effector phase of these cells at day 8 postinfection, including nonexonic regions. Additionally, when examining sequencing reads that align across exons, it became clear that CD8 T cells likely express a second isoform of Morrbid (Fig. 1D). Using 5′- and 3′-rapid amplification of cDNA ends (RACE) in stimulated CD8 T cells, we confirmed the expression of both a short isoform that includes exons 1 and 2 (isoform 1), and a longer second isoform that includes exons 1, 3, and 5 (isoform 2; Fig. 1E). We also confirmed the expression of both isoforms in stimulated CD8 T cells by qPCR using primers spanning exons 1–2 to quantify the short transcript (isoform 1), and primers spanning exons 3–5 to quantify the longer transcript (isoform 2) (SI Appendix, Fig. S1 A and C–E). Neutrophils were used as a positive control and were shown to express both isoform 1 and isoform 2, although they more highly expressed isoform 1 than isoform 2 (SI Appendix, Fig. S1B).
As LCMV Arm is known to be cleared by day 8 postinfection (11), the kinetics of Morrbid expression suggests that its transcription is induced downstream of CD8 T-cell activation. As such, we first stimulated sorted splenic CD8 T cells with varying doses of αCD3 in combination with αCD28 or phorbol 12-myristate 13-acetate (PMA)/ionomcyin (I), a pharmacological surrogate for strong TCR signaling. Interestingly, we found that both isoforms of Morrbid were only induced at maximal nonphysiologic doses of αCD3 or PMA/I stimulation (Fig. 1F and SI Appendix, Fig. S1C), highlighting that additional signals are likely important for induction of Morrbid transcription in vivo.
T cells are activated in the context of several signals including the TCR, surface costimulatory receptors such as CD28, and the inflammatory cytokine milieu (12). As such, we provided TCR and CD28 costimulation in the context of a panel of different cytokines known to be important for CD8 T-cell activation during viral infection. Interestingly, using a lower dose of αCD3/αCD28 that alone did not induce Morrbid expression (Fig. 1F), we consistently observed a strong induction of both isoforms of this lncRNA with the addition of either IFN-α or IFN-β 48 h following stimulation (Fig. 1 G and H and SI Appendix, Fig. S1 D and E), but not other cytokines known to play important roles early during LCMV infection such as IL-7 or IL-2 (8). Importantly, IFN-α and IFN-β alone were unable to similarly induce the transcription of Morrbid, demonstrating that its induction likely requires multiple signals (Fig. 1 I and J). Consistent with the importance of type I IFN for Morrbid induction in vitro, Morrbid expression is reduced by threefold following LCMV infection in Ifnar1-deficient LCMV-specific CD8 T cells relative to their LCMV-specific wild-type (WT) counterparts in vivo (GSE57355; Fig. 1K). These results suggest that, following viral infection in vivo, transcription of Morrbid is induced in part through type I IFN signaling.
Morrbid Is a Negative Regulator of CD8 T Cell Numbers Following Viral Infection.
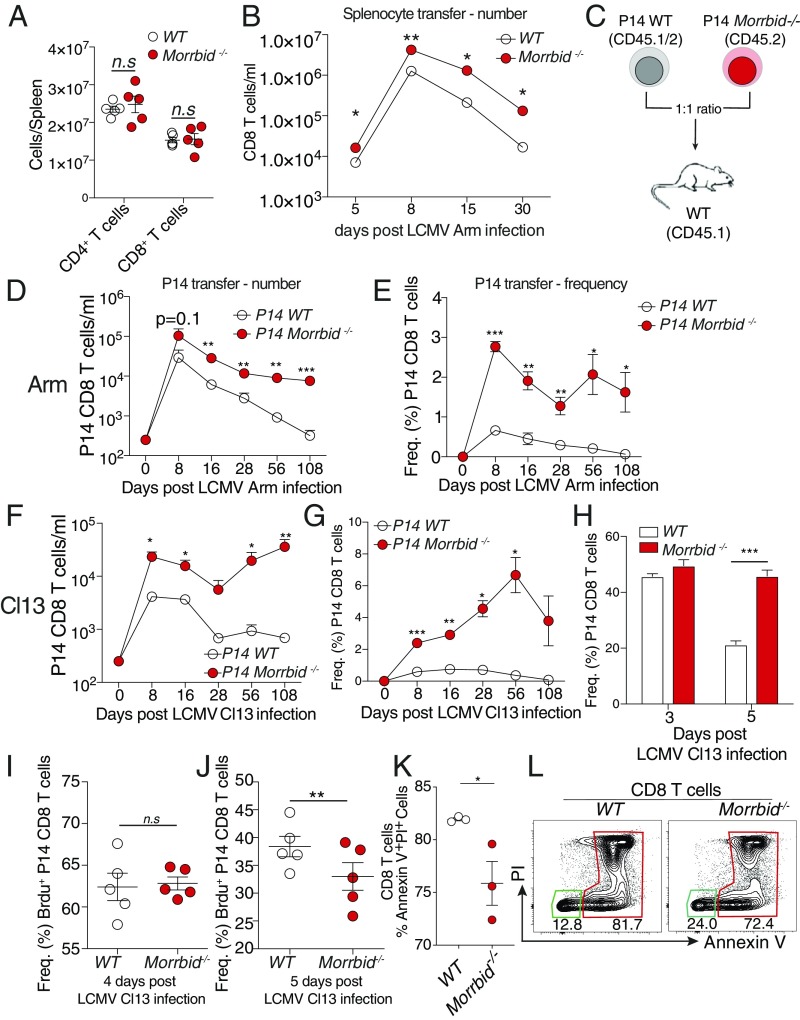
In accordance with the low expression of both isoforms of Morrbid at homeostasis in the thymus and periphery, mice deficient for the Morrbid locus did not demonstrate defects in circulating peripheral CD8 T-cell numbers (Fig. 2A). Additionally, we did not observe gross developmental defects in the thymus, but we did note an increase in thymic cellularity and an increase in mature CD24loTCRβhi CD8 T cells in the absence of Morrbid (SI Appendix, Fig. S1 F and G). We next interrogated whether the Morrbid locus impacts CD8 T-cell function following LCMV Arm infection. First, we independently transferred 20 × 106 WT or Morrbid−/− splenocytes into congenic (CD45.1+) WT hosts, infected these mice with LCMV Arm, and assessed CD8 T-cell numbers over the course of infection in blood. Although the same number of WT and Morrbid−/− CD8 T cells were initially transferred before infection, the Morrbid−/− bulk CD8 T-cell population expanded to greater numbers, and maintained increased numbers following the acute phase of this infection (Fig. 2B). To control for any differences in the TCR repertoires between WT and Morrbid−/− CD8 T cell populations, as well as to assess the antigen-specific response to LCMV, we generated Morrbid−/− P14 mice, which express a transgenic TCR specific for the gp33 epitope of LCMV (13). We subsequently adoptively transferred a 1:1 mix of congenic P14 CD8 T cells from WT (CD45.1+CD45.2+) and Morrbid-deficient (CD45.2+) mice into WT (CD45.1+) hosts and assessed CD8 T-cell frequency and number in blood over time following LCMV Arm infection (Fig. 2C). We observed a greater number and frequency of Morrbid-deficient CD8 T cells compared with WT CD8 T cells at nearly all time points analyzed (Fig. 2 D and E). As Morrbid was also induced following infection with LCMV clone 13 (Cl13) (Fig. 1C), we next used the P14 cotransfer model to assess the role of Morrbid in CD8 T cells during chronic viral infection. Following LCMV Cl13 infection, we observed nearly identical results, with a greater number and frequency of Morrbid-deficient CD8 T cells relative to that of WT at all time points analyzed (Fig. 2 F and G). KLRG1, together with IL-7Rα, have been used as markers to identify effector CD8+ T-cell subsets (8). KLRG1+IL-7Rαlo are well-characterized short-lived effector CD8+ T cells that have a limited potential to become memory cells (8). In contrast, effector CD8+ T cells that do not express KLRG1 (KLRG1loIL-7Rαhi) have been referred to as memory precursor effector T cells, which display increased survival during the contraction phase, and retain the capacity to differentiate into multiple memory cell lineages (8). Interestingly, we observed minimal differences in the expansion of KLRG1loIL-7Rαhi population, and short-lived effector CD8+ T cells (KLRG1hiIL-7Rαlo) both following acute and chronic infection (SI Appendix, Fig. S1 H–K). Additionally, we did not observe a significant difference in the expression of programmed cell death 1 (PD-1), a negative regulator of T-cell activation, in chronic infection (SI Appendix, Fig. S1L). Taking all this together, our results demonstrate that the Morrbid locus negatively regulates CD8 T-cell numbers following viral infection, even when these cells are exposed to the same antigen and cytokine environment.
Fig. 2.
Morrbid is a negative regulator of CD8 T-cell numbers following viral infection. (A) Number of splenic CD4 and CD8 T cells from WT and Morrbid-deficient mice assessed by flow cytometry (n = 5 mice per group; these data are representative of three independent experiments). (B) Number of donor CD8 T cells in blood over time in mice that received 10 × 106 WT or Morrbid-deficient splenocytes followed by LCMV Arm infection (n = 5 mice per group). (C) Schematic of P14 adoptive cotransfer experiments. (D–G) Number and frequency of donor P14 cells assessed by flow cytometry in blood over time in mice that received a 1:1 mix of 250 WT (CD45.1+, CD45.2+) and 250 Morrbid-deficient (CD45.2+) P14 CD8 T cells followed by (D and E) LCMV Arm or (F and G) LCMV Cl13 infection (n = 5 mice per group; these data are representative of three independent experiments). (H) Frequency of donor P14 cells assessed by flow cytometry in spleens of mice that received a 1:1 mix of 0.5 × 106 WT and Morrbid-deficient P14 CD8 T cells followed by LCMV Cl13 infection (n = 5 mice per group; these data are representative of two independent experiments). (I and J) Frequency of BrdU incorporation as measured by flow cytometry in cotransferred Morrbid-deficient and WT P14 CD8 T cells in spleen at (I) day 4 and (J) day 5 following LCMV Cl13 infection. BrdU was injected 12 h before takedown (n = 5 mice per group). (K) Frequency and (L) representative flow cytometry plots of Annexin V and propidium iodide staining of WT and Morrbid-deficient P14 CD8 T cells stimulated with 1 μg/mL plate-bound αCD3 and 1 μg/mL soluble αCD28 for 48 h (n = 3 mice per group; these data are representative of two independent experiments). Error bars show SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired two-sided t test, A, B, and K; paired two-sided t test, D–J).
CD8 T-cell numbers following infection can be influenced by proliferation, cell death, and trafficking. We observed an increase in the frequency and numbers of Morrbid−/− CD8 T cells following LCMV infection in all of the tissues that we examined, including blood, spleen, liver, and lung, suggesting that trafficking was likely not responsible for the increase in circulating CD8 T cells. Cotransferred congenically marked Morrbid−/− P14 CD8 T cells did not demonstrate a competitive advantage over their WT counterparts in vivo until ∼5 d post-LCMV infection (Fig. 2H), a time point after which responding CD8 T cells have completely diluted cytoplasmic dyes such as CFSE (14). To examine T-cell proliferation at these later stages, we pulsed the mice with the thymidine analog bromodeoxyuridine (BrdU) and assessed its incorporation, as well as Ki67, a surrogate of cell cycle entry, 12 h following BrdU injection. BrdU incorporation and Ki67 frequency were similar between cotransferred WT and Morrbid−/− P14 CD8 T cells at day 4 and 5 postinfection (Fig. 2 I and J), suggesting that the competitive advantage of Morrbid−/− CD8 T cells is not likely secondary to increased proliferation. To further analyze T-cell proliferation, we stimulated CFSE-labeled CD8 T cells from WT and Morrbid−/− spleens with αCD3/αCD28 and assessed the number of cells per division after 3 d. Similar to what we observed in vivo, Morrbid-deficient CD8 T-cell proliferation was similar to that of WT CD8 T cells (SI Appendix, Fig. S1M). However, when we stimulated these cells in vitro with αCD3/αCD28 for 48 h and stained with Annexin V and propidium iodine to examine activation-induced cell death, we noted that, relative to that of WT, a greater percentage of Morrbid-deficient CD8 T cells remained alive (Fig. 2 K and L and SI Appendix, Fig. S1N). These data suggest that, following stimulation, Morrbid-deficient CD8 T cells have a survival advantage over WT CD8 T cells.
Morrbid Controls CD8 T Cell Effector Function and Naive Cell Homeostasis in a Cell-Intrinsic Manner.
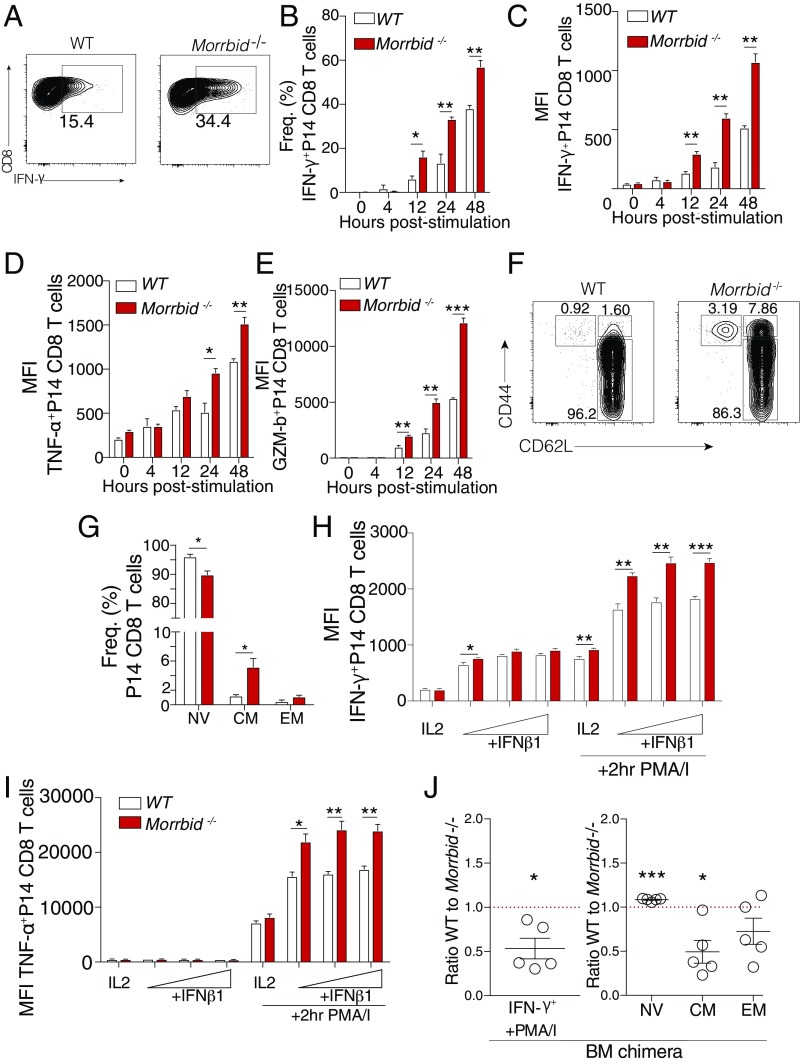
In addition to expanding their numbers, CD8 T cells must also develop appropriate effector functions, such as cytokine production and degranulation, to mount efficient antipathogen responses (15). Thus, we asked whether Morrbid impacts CD8 T-cell effector function. To establish the baseline effector profile of Morrbid−/− relative to WT P14 CD8 T cells, we activated these cells in vitro under conditions that induce Morrbid transcription (Fig. 1 G and H) and compared cytokine production and degranulation by flow cytometry. We observed a significant increase in the frequency of IFN-γ–producing Morrbid-deficient P14 CD8 T cells compared with that of WT P14 CD8 T cells (Fig. 3 A and B). In addition, Morrbid-deficient CD8 T cells also showed a significant increase in the cellular quantity of IFN-γ, TNF-α, and Granzyme B (GzmB), as assessed by mean florescent intensity (MFI) (Fig. 3 C–E), a phenomenon that was recapitulated in non-TCR transgenic TCR Morrbid−/− CD8 T cells (SI Appendix, Fig. S2 A–C). These results indicated that, in addition to cellular expansion, the Morrbid locus also restrains IFN-γ production and other effector functions following stimulation of CD8 T cells.
Fig. 3.
Morrbid controls CD8 T-cell effector function and naive cell homeostasis in a cell-intrinsic manner. (A) Representative flow cytometry plots, (B) frequencies, and (C–E) mean florescent intensity (MFI) of indicated proteins in WT or Morrbid-deficient splenic P14 CD8 T cells stimulated over time with αCD3, αCD28, and IFN-β (n = 3 mice per group; these data are representative of five independent experiments). (F) Representative flow cytometry plots and (G) frequencies of naive (NV) (CD62LhiCD44lo), central memory (CM) (CD62LhiCD44hi), and effector memory (EM) (CD62LloCD44hi) P14 CD8 T cells from blood of WT and Morrbid-deficient mice (n = 3–4 mice per group; these data are representative of five independent experiments). (H and I) Flow cytometry MFI of (H) IFN-γ and (I) TNF-α production in sorted CD62LhiCD44lo splenic WT and Morrbid-deficient CD8 T cells stimulated in vitro with αCD3, αCD28, and IL-2 with varying doses of IFN-β, with or without PMA/I restimulation (n = 3 mice per group; these data are representative of two independent experiments). (J) Flow cytometry analysis of WT and Morrbid-deficient P14 LSK competitive bone marrow chimera mice 9 wk post-reconstitution. (J, Left) Frequency of IFN-γ production 4 h post-PMA/I stimulation. (J, Right) Frequencies of NV, CM, and EM cell compartments. Frequencies are represented as the WT-to-Morrbid-deficient ratio (n = 5 mice per group). Error bars show SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired two-sided t test, B–E and G–I; paired two-sided t test, J).
Given the increased numbers and augmented effector profile of Morrbid−/− CD8 T cells following stimulation, we next investigated whether this hyperactivity impacts the capacity of Morrbid−/− CD8 T cell to maintain a naive phenotype at homeostasis in vivo. Loss of negative regulators of T-cell activation such as tuberous sclerosis component 2 (Tsc1), a negative regulator of mammalian target of rapamycin (mTOR), can result in expansion of memory-like CD44hi CD8 T cells (16). In Morrbid-deficient P14 mice, we observed a minor but significant decrease in naive (CD62LhiCD44lo) CD8 T cells with a concordant increase in the central memory (CM) (CD62LhiCD44hi) compartment (Fig. 3 F and G). In nontransgenic Morrbid-deficient mice, we also observed a decrease in naive CD8 T cells, but with an increase in effector memory (EM) (CD62LloCD44hi) CD8 T cells (SI Appendix, Fig. S2 D and E). Altogether, these results demonstrate that Morrbid is important for maintaining the homeostasis of naive CD8 T cells. As this expansion of memory-phenotype CD8 T cells in Morrbid-deficient mice potentially contributed to the rapid responsiveness and hyperactivity exhibited by these cells, we sorted naive (CD62LhiCD44lo) WT and Morrbid−/− CD8 T cells and stimulated these cells in vitro. Stimulated naive Morrbid−/− CD8 T cells produced more IFN-γ, TNF-α, and GzmB than their stimulated naive WT counterparts (Fig. 3 H and I and SI Appendix, Fig. S2F), demonstrating that Morrbid−/− hyperresponsiveness stems in part from the naive CD8 compartment.
As short-lived myeloid cells are absent in Morrbid-deficient animals (10), we questioned whether the hyperactivity of Morrbid-deficient CD8 T cells occurs in a cell-intrinsic or -extrinsic manner. Although our adoptive transfer studies suggest that the phenotype is cell-intrinsic, we sought to determine whether development in the absence of a full myeloid compartment contributes to Morrbid-deficient CD8 T-cell hyperactivity. First, we transferred WT splenocytes into WT and Morrbid−/− hosts to assess the cell-extrinsic impact of host Morrbid deficiency on CD8 T-cell homeostasis. After 8 wk, we did not observe any difference in IFN-γ production or the memory phenotype of WT cells exposed to either a WT or Morrbid-deficient environment (SI Appendix, Fig. S2G). To more rigorously address this question, we generated competitive bone marrow chimeras using sorted LSK cells from WT P14 and Morrbid-deficient P14 bone marrow mixed 1:1 and transferred into irradiated WT hosts. After allowing the bone marrow to reconstitute for 9 wk, we observed a greater frequency of IFN-γ+ Morrbid-deficient CD8 T cells in response to PMA/I stimulation compared with that of WT CD8 T cells, suggesting that increased production of IFN-γ with Morrbid deficiency occurs in a cell-intrinsic manner in the absence of the Morrbid locus (Fig. 3J). In addition, we observed a similar loss of naive and expansion of central memory CD8 T cells in the Morrbid-deficient P14 populations compared with WT P14 CD8 T cells in the same recipient hosts (Fig. 3J), a phenomenon that was recapitulated when reconstituting mice with non-P14 LSK cells (SI Appendix, Fig. S2H).
We next aimed to establish the functionality of these cells in the context of viral infection in vivo. Thus, we adoptively transferred WT P14 CD8 T cells and Morrbid−/− P14 CD8 T cells into WT hosts and assessed viral titers in the spleens at days 5 and 6 (LCMV-Arm) and in serum at day 8, 15, 21, and 28 (LCMV-Cl13) postinfection. At these doses of virus and number of adoptively transferred P14 CD8 T cells, Morrbid deficiency does not significantly impact primary Arm or Cl13 LCMV viral kinetics (SI Appendix, Fig. S2 I and J). Altogether, our findings suggest that the Morrbid locus and potentially its RNA molecule functions in a cell-intrinsic manner to restrain effector memory phenotype CD8 T cells at steady state, and to control effector cytokine production following CD8 T-cell activation. Moreover, our data indicate that these alterations are largely independent of the defects in the myeloid compartment of Morrbid-deficient mice.
The Morrbid Locus Is Required for the Up-Regulation of Bcl2l11 Expression in CD8 T Cells Following TCR Stimulation.
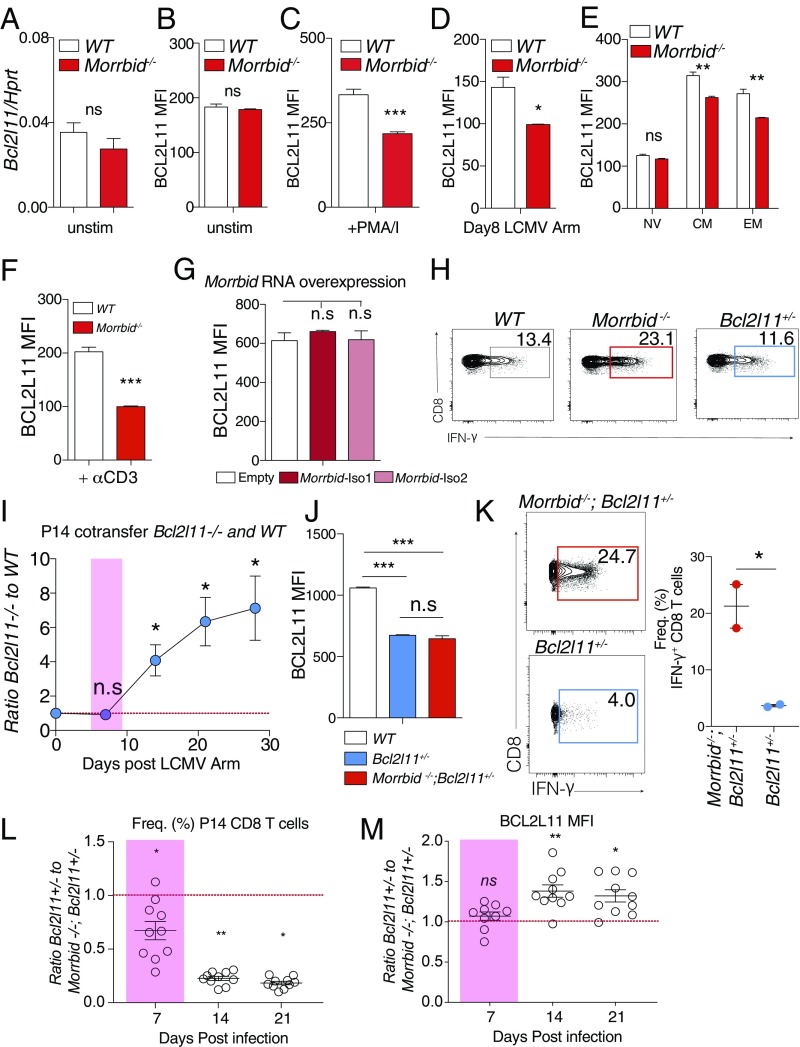
As previously discussed, Bcl2l11, the key downstream target of Morrbid in short-lived myeloid cells, is required for appropriate contraction of CD8 T cells following viral clearance (17–19). CD8 T cells deficient for Bcl2l11 fail to contract at later stages of infection (17–19), which closely mirrors the kinetics of Morrbid-deficient CD8 T cells in the later stages of acute and chronic LCMV infection (Fig. 2 D–G). Moreover, similar alterations in the memory compartment present in Morrbid−/− mice are also observed in the absence of Bcl2l11 (19). However, no published studies have demonstrated that Bcl2l11 regulates CD8 T-cell expansion or effector function. We first aimed to determine whether Morrbid RNA and/or its locus regulate Bcl2l11 expression. At homeostasis, Bcl2l11 expression was not altered in naive Morrbid-deficient CD8 T cells at the transcript or protein levels (Fig. 4 A and B). However, in contrast to short-lived myeloid cells, Bcl2l11 expression was decreased in Morrbid−/− CD8 T cells following stimulation in vitro, in central memory and effector memory CD8 T cells, as well as at day 8 post-LCMV Arm infection in vivo (Fig. 4 C–E and SI Appendix, Fig. S3 A and B). It is important to highlight that BCL2L11 protein expression was decreased in Morrbid-deficient CD8 T cells under stimulation conditions and time points at which we did not previously observe significant Morrbid RNA expression (Fig. 4F and SI Appendix, Fig. S3 A and B), suggesting that the locus, not the RNA, might be primarily responsible for controlling Bcl2l11 expression under these conditions. Thus, to better understand the contribution of the Morrbid locus and its RNA to Bcl2l11 expression in CD8 T cells, we independently overexpressed both isoforms of Morrbid in WT or Morrbid−/− CD8 T cells and determine its contribution to the regulation of Bcl2l11 expression (SI Appendix, Fig. S3C). In WT CD8 T cells, we did not observe an impact of either isoform of Morrbid RNA on Bcl2l11 expression relative to empty vector (Fig. 4G). Interestingly, we observed a small but significant increase in Bcl2l11 expression upon overexpression of Morrbid isoform 2 in Morrbid−/− CD8 T cells (SI Appendix, Fig. S3D), suggesting that through either direct or indirect mechanisms Morbid isoform 2 RNA has the capacity to contribute to the promotion of Bcl2l11 expression. Together, these data indicate that the Morrbid locus is required for promoting Bcl2l11 expression following CD8 T-cell stimulation, and that Morrbid RNA in trans may contribute to the regulation of this proapoptotic gene in these cells. Further studies are required to determine the contribution of Morrbid RNA to Bcl2l11 regulation in cis in this context. Altogether, these results suggest that Morrbid-deficient alterations in CD8 T-cell contraction during LCMV infection and loss of naive homeostasis are likely secondary to decreased Bcl2l11 expression due to loss of the Morrbid locus and potentially Morrbid RNA.
Fig. 4.
Bcl2l11 expression is reduced in the absence of the Morrbid locus in CD8 T cells following stimulation but does not significantly contribute to Morrbid-deficient expansion or effector function. (A) qPCR of Bcl2l11 transcript expression in sorted naive (CD62LhiCD44lo) WT and Morrbid-deficient CD8 T cells (n = 3 mice per group; these data are representative of two independent experiments). (B and C) BCL2L11 protein mean florescent intensity (MFI) measured by flow cytometry in (B) unstimulated or (C) 4-h PMA/I-stimulated WT and Morrbid-deficient mice CD8 T cells (n = 3 mice per group; these data are representative of five independent experiments). (D) BCL2L11 protein MFI by flow cytometry in WT or Morrbid-deficient cotransferred P14 cells 8 d post-LCMV Arm infection (n = 5 mice per group; these data are representative of three independent experiments). (E) BCL2L11 protein MFI by flow cytometry in naive (NV) (CD62LhiCD44lo), central memory (CM) (CD62LhiCD44hi), and effector memory (EM) (CD62LloCD44hi) WT and Morrbid-deficient CD8 T cells from blood (n = 5 mice per group; these data are representative of three independent experiments). (F) BCL2L11 protein MFI by flow cytometry in WT and Morrbid-deficient CD8 T cells stimulated with αCD3/αCD28 for 24 h (n = 5 mice per group; these data are representative of three independent experiments). (G) BCL2L11 protein MFI by flow cytometry in WT CD8 T cells transduced with the empty vector, Morrbid isoform (iso) 1, or Morrbid iso 2 (n = 3 biological replicates; data pooled from three independent experiments). (H) Representative flow cytometry plot of IFN-γ production in WT, Morrbid-deficient, and Bcl2l11-heterozygous CD8 T cells stimulated for 3 h with PMA/I and IFN-β (n = 3 mice per group; these data are representative of four independent experiments). (I) Frequency of donor P14 cells in blood over time in mice that received a 1:1 mix of 250 WT and 250 Bcl2l11-deficient P14 CD8 T cells and infected with LCMV Arm. Represented as the ratio of donor Bcl2l11-deficient to donor WT (n = 5 mice per group; these data are representative of two independent experiments). (J) BCL2L11 protein MFI of CD8 T cells from blood of WT, Bcl2l11+/−, and Morrbid −/−; Bcl2l11+/− mice (n = 4–5 mice per group). (K, Left) Representative flow cytometry plots and (Right) frequency of IFN-γ production in CD8 T cells from Bcl2l11+/−, and Morrbid −/−; Bcl2l11+/− mice 3 h post-PMA/I and IFN-β stimulation (n = 2 mice per group; these data are representative of two independent experiments). (L and M) Frequency of donor P14 cells in blood over time in mice that received a 1:1 mix of 250 Bcl2l11+/− and 250 Morrbid−/−; Bcl2l11+/− and infected with LCMV Arm. (L) Ratio of frequencies of the indicated donor populations. (M) Ratio of BCL2L11 protein MFI of the indicated donor populations. (n = 5 mice per group; these data are representative of two independent experiments). Error bars show SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired two-sided t test, A–G, J, and K; paired two-sided t test, I, L, and M).
Since Bcl2l11 has not been previously associated with alterations in CD8 T-cell expansion or effector function, we next formally tested the impact of Bcl2l11 on these processes. As the reduction in Bcl2l11 expression observed in Morrbid-deficient mice was similar to that observed in Bcl2l11 heterozygous mice (SI Appendix, Fig. S3 E and F), we first stimulated Morrbid−/−, Bcl2l11+/−, and WT CD8 T cells and examined their production of effector molecules. Interestingly and in concordance with previous reports, we found that Bcl2l11+/− CD8 T cells have a nearly identical IFN-γ production profile to that of WT cells (Fig. 4H and SI Appendix, Fig. S3 G and H). We subsequently cotransferred WT and Bcl2l11−/− P14 CD8 T cells into congenic hosts and assayed the number and frequency of these cells following acute LCMV Arm infection. We did not observe differences in expansion between WT and Bcl2l11−/− P14 cells during the early phase following LCMV Arm (Fig. 4I). These results, coupled with the pattern of expression of Morrbid in CD8 T cells in vitro and in vivo, suggest that the Morrbid locus and/or its RNA may contribute to the regulation of CD8 T-cell expansion and effector function in a Bcl2l11-independent manner.
To more thoroughly test whether this lncRNA and/or its locus have Bcl2l11-independent roles in CD8 T cells, we generated P14 transgenic mouse strains with equally reduced levels of Bcl2l11 expression in the presence or absence of Morrbid (Morrbid−/−; Bcl2l11−/+ and Bcl2l11−/+; Fig. 4J). Using these strains normalized for Bcl2l11 expression, we subsequently tested CD8 T-cell effector cytokine production following stimulation, expansion, and contraction upon LCMV infection. Interestingly, following stimulation, Morrbid−/−; Bcl2l11−/+ CD8 T cells express significantly more IFN-γ and TNF-α than their Bcl2l11−/+ counterparts (Fig. 4K and SI Appendix, Fig. S3 I–L), further supporting that Morrbid impacts the effector profile of CD8 T cells in a Bcl2l11-independent manner. Next, we cotransferred Bcl2l11−/+ and Morrbid−/−; Bcl2l11−/+ P14 CD8 T cells into naive recipients, infected these mice with LCMV Arm, and assessed the frequency of these populations at days 7, 14, and 21 postinfection. During the expansion phase at day 7, a time point at which this proapoptotic factor does not seem to play a large role, Morrbid−/−; Bcl2l11−/+ P14 T cells outcompeted their Bcl2l11−/+ counterparts (Fig. 4L), with no significant difference in their expression of Bcl2l11 (Fig. 4M and SI Appendix, Fig. S3M). However, during the contraction phase (days 14 and 21), Morrbid−/−; Bcl2l11−/+ P14 CD8 T cells outcompeted their Bcl2l11−/+ counterparts and expressed significantly less Bcl2l11 (Fig. 4 L and M). Due to the key role that Bcl2l11 plays in the contraction phase of CD8 T-cell responses, these results suggest that the decrease in Bcl2l11 in Morrbid−/−; Bcl2l11−/+ CD8 T cells might be a key contributor to their slower contraction following viral clearance. Altogether, these data strongly support a Bcl2l11-independent role for Morrbid in negatively regulating CD8 T-cell expansion and effector function following acute viral infection.
The Morrbid Locus and Its RNA Represses AKT Signaling in CD8 T Cells Downstream of Type I IFN.
Morrbid transcription in CD8 T cells in the early stages of acute LCMV infection is largely dependent on type I IFN signaling. Thus, we hypothesized that some of the regulatory roles of Morrbid that are independent of Bcl2l11 could be associated with controlling the impact of type I IFN signaling on CD8 T cells. Type I IFNs are well recognized for their potent effects on CD8 T-cell activation, proliferation, differentiation, and survival (20). Although the functional consequences of type I IFNs can be diverse depending on the relative timing of IFN exposure and TCR stimulation, within the LCMV system type I IFNs have been reported to act directly on CD8 T cells to promote their survival and effector function (21–23). To start to address this hypothesis, we first tested whether Morrbid-deficient CD8 T cells have altered surface expression of IFN-alpha/beta receptor 1 (IFNAR1) and found no significant differences in expression between naive WT and Morrbid−/− CD8 T cells (SI Appendix, Fig. S4A). We next asked whether downstream canonical signaling was dysregulated, and tested the phosphorylation of signal transducer and activator 1 (STAT1) and STAT4 in WT and Morrbid−/− CD8 T cells in response to varying doses of IFN-β in combination with αCD3. Morrbid−/− CD8 T cells were similarly sensitive to IFN treatment, with a minor reduction in STAT1 and STAT4 phosphorylation (SI Appendix, Fig. S4 B–D). Although reduced but imbalanced STAT1 to STAT4 signaling may contribute to Morrbid−/− hyperactivity, these results suggest that canonical type I IFN signaling is not grossly dysregulated in Morrbid−/− CD8 T cells.
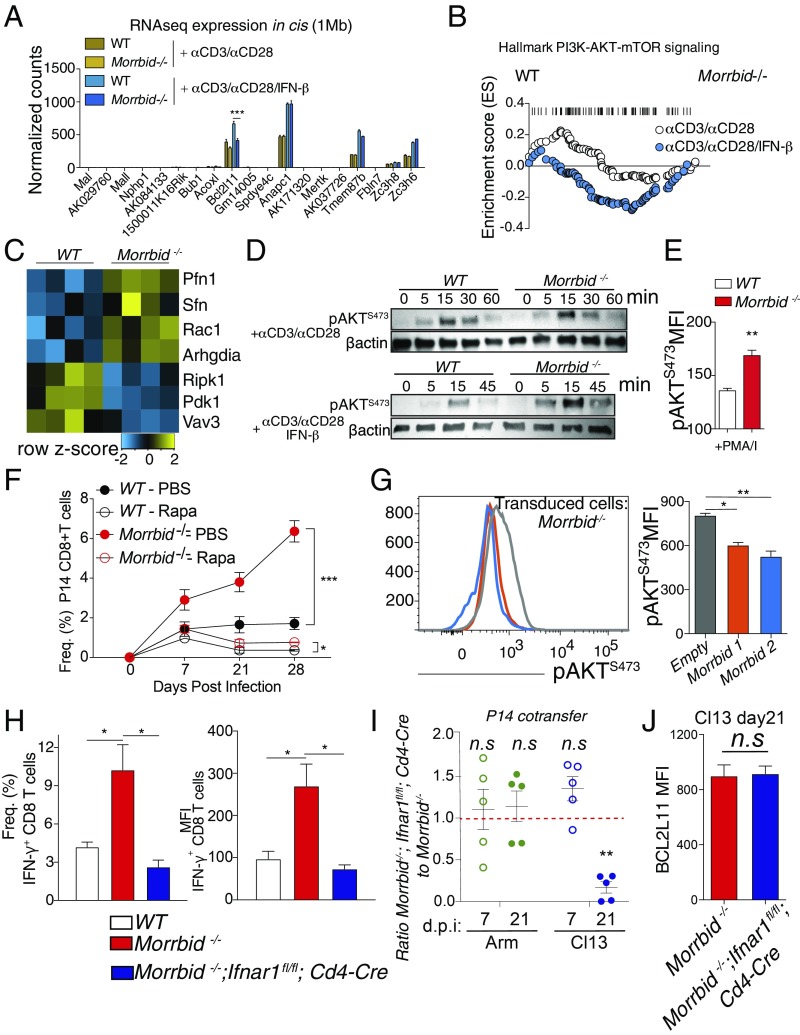
To take an unbiased approach and identify potential pathways regulated by the Morrbid RNA or its locus downstream of type I IFN signaling, which could contribute to the regulation of CD8 T-cell function and expansion, we performed RNA sequencing of sorted naive (CD62LhiCD44lo) WT and Morrbid-deficient splenic CD8 T cells stimulated with αCD3/αCD28 with and without IFN-β, and asked what pathways were altered. Importantly, within 1 Mb of the Morrbid locus, apart from Bcl2l11, we observed no significant differences in cis gene expression between WT and Morrbid-deficient CD8 T cells under any stimulation condition (Fig. 5A), supporting the notion that Morrbid could be acting in trans. Interestingly, using gene set enrichment analysis (GSEA), we found that several noncanonical type I IFN signaling pathways, including PI3K-AKT and NF-κB, were significantly dysregulated in the absence of Morrbid (Fig. 5B and SI Appendix, Fig. S4E). AKT is a well-described key signaling molecule downstream of IFN receptor stimulation that promotes autophagy, glucose uptake, and translation of IFN-stimulated genes (ISGs), as well as the proliferation, survival, and effector function of CD8 T cells (24–27). AKT signaling was of particular interest as it was only enriched in Morrbid−/− CD8 T cells under conditions in which this lncRNA is induced, such as αCD3/αCD28 and IFN-β, but not with αCD3/αCD28 alone (Fig. 5B). This dysregulation of AKT targets was also apparent when visualizing individual targets statistically significant by differential expression (Fig. 5C). Therefore, we hypothesized that, in addition to decreased expression of Bcl2l11, increased PI3K-AKT activity could contribute to the alterations observed in Morrbid-deficient CD8 T cells. To test this possibility, we first asked whether phosphorylation of AKT is enhanced following TCR and IFN stimulation in Morrbid−/− CD8 T cells. Indeed, we observed that under conditions capable of inducing Morrbid expression such as αCD3/αCD28 with IFN-β or PMA/I, but not αCD3/αCD28 alone, there is a more rapid phosphorylation of AKT at S473 in Morrbid−/− CD8 T cells (Fig. 5 D and E). AKT is also a fundamental signaling hub downstream of the TCR and costimulatory receptors (28). Importantly, we did not observe gross alterations in key proximal TCR signaling pathways such as calcium flux and ERK phosphorylation (SI Appendix, Fig. S4 F–H), indicating that increased AKT activity in Morrbid−/− CD8 T cells is restricted to conditions in which CD8 T cells are stimulated with type I IFN and that there is no global dysregulation of TCR signaling in these cells. To determine whether the alteration in Morrbid-deficient CD8 T-cell numbers following viral infection is partially dependent on dysregulated PI3K-AKT-mTOR signaling, we used rapamycin to target this pathway in vivo. WT and Morrbid-deficient P14 CD8 T cells were cotransferred into WT hosts that were subsequently treated with rapamycin from day −1 before LCMV-Cl13 infection to day 27 postinfection. Interestingly, Morrbid-deficient P14 CD8 T cells were more sensitive to rapamycin treatment compared with WT P14 CD8 T cells at all of the time points analyzed (Fig. 5F). These results indicate that the competitive expansion and delayed contraction of Morrbid-deficient CD8 T cells is in part PI3K–AKT–mTOR dependent. Taken together, these results suggest that, in response to type I IFN, the Morrbid locus plays a critical role in controlling the strength of PI3K–AKT signaling.
Fig. 5.
The Morrbid locus and its RNA negatively regulate AKT signaling in CD8 T cells, and Morrbid-deficient effector hyperactivity is type I IFN dependent. (A–C) RNAseq analysis of sorted naive (CD62LhiCD44lo) WT and Morrbid-deficient CD8 T cells stimulated with αCD3/αCD28 with or without IFN-β for 6 h. (A) Expression of genes within 1 Mb of the Morrbid locus. (B) GSEA of hallmark PI3K–AKT–mTOR gene set in WT and Morrbid-deficient CD8 T cells under the indicated stimulation conditions. αCD3/αCD28 (NES 0.88, FDR 0.59) and αCD3/αCD28/IFN-β (NES −1.51, FDR 0.06). (C) Heatmap of genes significantly differentially expressed with FDR < 0.05 following stimulation αCD3/αCD28/IFN-β that are also identified within the hallmark PI3–AKT–mTOR pathway. Reads were normalized using DESeq2 (n = 3–4 biological replicates per genotype per condition). (D) Western blot of pS473 AKT in WT and Morrbid-deficient CD8 T cells at the indicated time points following stimulation with αCD3/αCD28 with or without IFN-β (these data are representative of two independent experiments). (E) Phospho-flow analysis as measured by gMFI of pS473 AKT in CD8 T cells from WT and Morrbid-deficient mice stimulated with PMA/I for 15 min (n = 3 mice per group; these data are representative of two independent experiments). (F) Frequency of donor P14 CD8 T cells in blood at the indicated time points in mice that received a 1:1 mix of 250 WT and 250 Morrbid−/− P14 CD8 T cells and were infected with LCMV Cl13. Mice were treated daily with rapamycin or PBS i.p. from day −1 before LCMV-Cl13 infection to day 27 postinfection (n = 3–5 mice per group; these data are representative of two independent experiments). (G) Phospho-flow analysis as measured by gMFI of pS473 AKT in Morrbid-deficient CD8 T cells that were transduced with empty, Morrbid isoform 1, or Morrbid isoform 2 expressing vectors. Cells were plated in triplicate, and then stimulated with αCD3/αCD28/IFN-β for 15 min (n = 3 replicate per group). (H, Left) Frequency and (Right) MFI of IFN-γ production in WT, Morrbid−/−, and Morrbid−/−; Ifnar1f/f; Cd4-Cre CD8 T cells stimulated with αCD3/αCD28/IFN-β for 24 h (n = 4 mice per group; data pooled from three independent experiments). (I and J) Flow cytometry analysis of cotransferred Morrbid−/− and Morrbid−/−; Ifnar1f/f; Cd4-Cre P14 CD8 T cells following LCMV Arm or Cl13 infection. (I) Frequency of the cotransfer donor P14 cells represented as the ratio of Morrbid−/− to Morrbid−/−; Ifnar1f/f; Cd4-Cre at the indicated time points following infection. (J) BCL2L11 protein MFI in each donor P14 population 21 d post-Cl13 infection (n = 5 mice per group; these data are representative of two independent experiments). Error bars show SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (DESeq2 FDR, A and C; GSEA FDR, B; unpaired two-sided t test, E, G, and H; paired two-sided t test, F, I, and J).
We next assessed the contribution of Morrbid RNA to the regulation of AKT signaling downstream of the type I IFN receptor. To do so, we overexpressed Morrbid RNA in WT or Morrbid locus-deficient CD8 T cells. Strikingly, WT and Morrbid-deficient CD8 T cells overexpressing either isoform of Morrbid demonstrated a dramatic reduction in AKT phosphorylation following stimulation with αCD3/αCD28 and IFN-β relative to their control counterparts (Fig. 5G and SI Appendix, Fig. S4I), effectively rescuing the aberrant increase in AKT phosphorylation observed in the absence of this lncRNA. These results suggest that Morrbid RNA plays a critical role in downregulating the PI3K–AKT pathway downstream of type I IFN signaling in CD8 T cells.
As type I IFN induces Morrbid expression and this lncRNA and its locus contribute to the regulation of noncannonical type I IFN signaling through the PI3K–AKT pathway in CD8 T cells, we next aimed to formally test whether the alterations that we observed in Morrbid−/− CD8 T cells could be explained by enhanced type I IFN-dependent signaling. To address this possibility, we first generated Morrbid-deficient mice that lack the type I IFN signaling receptor IFNAR1 (Morrbid−/−; Ifnar1fl/fl; Cd4-Cre) and determined whether the absence of type I IFN signaling could rescue the hyperactive effector function of Morrbid-deficient CD8 T cells. Indeed, following in vitro stimulation with αCD3/αCD28 and IFN-β, we find that Morrbid−/−; Ifnar1fl/fl; Cd4-Cre CD8 T cells display an effector profile similar to that of WT CD8 T cells (Fig. 5H and SI Appendix, Fig. S4J), demonstrating that Morrbid regulates CD8 T-cell effector function in a type I IFN-dependent manner. Of note, absence of the type I IFN receptor did not rescue decreased BCL2L11 expression in the absence of Morrbid (SI Appendix, Fig. S4K). These data suggest that the Morrbid locus and its RNA function downstream of type I IFN signaling as negative autoregulators of the stimulatory effects of type I IFN on CD8 T cells.
As Morrbid-deficient CD8 T-cell AKT signaling and cytokine effector profile are increased in a type I IFN-dependent manner, we hypothesized that the increased expansion and decreased contraction of Morrbid−/− cells in vivo is also type I IFN dependent. To test this hypothesis, we performed cotransfer experiments in which we could directly compare the kinetics of Morrbid−/− and Morrbid−/−; Ifnar1fl/fl; Cd4-Cre P14 CD8 T cells upon acute and chronic LCMV infection. Interestingly, while we did not observe significant differences in expansion in the context of either acute or chronic LCMV infection, we noted a clear reduction in Morrbid−/−;Ifnar1fl/fl; Cd4-Cre P14 CD8 T cells 21 d following chronic LCMV Cl13 infection (Fig. 5I). As we did not observe a difference in BCL2L11 expression at any of the time points that we examined, including 21 d post-Cl13 infection (Fig. 5J), these results suggest that the impact of Morrbid on Bcl2l11 is independent of type I IFN signaling. More importantly, restoring the kinetics of contraction of Morrbid-deficient CD8 T cells by ablating the type I IFN receptor suggests that regulation of PI3K–AKT signaling strength downstream of this receptor by Morrbid is critical for tuning CD8 T-cell numbers during chronic viral infections. Moreover, it highlights the possibility that a lncRNA and its locus could have independent roles in CD8 T cells during viral infection and that these roles have different degrees of relevance and are highly contextualized.
Discussion
In this study, we show that transcription of the lncRNA Morrbid is specifically induced by type I IFN in CD8 T cells in vitro and during the early stages of acute and chronic LCMV infection in vivo. Moreover, this lncRNA locus and/or its RNA regulate CD8 T-cell expansion, contraction, and acquisition of effector functions in the context of viral infection. We find that the Morrbid locus promotes the expression of the proapoptotic factor BCL2L11, which likely contributes to Morrbid regulation of CD8 T-cell contraction. However, alterations in CD8 T-cell expansion and effector functions are regulated, at least partially, through the capacity of Morrbid RNA to modulate signaling strength through the type I IFN receptor. Thus, our results demonstrate that inflammatory cue-responsive lncRNA loci, through either cis-regulatory elements, RNA transcription, or the RNA molecule itself, provide a key regulatory layer to tune the functions of CD8 T cells during antipathogen responses.
BCL2L11 plays a pivotal role in the contraction of CD8 T-cell numbers during viral infection, yet it does not seem to control CD8 T-cell expansion or acquisition of effector functions in the early stages of LCMV infection. In accordance with BCL2L11’s role in contraction, Morrbid-deficient mice express less BCL2L11 following stimulation and have a striking impairment in CD8 T-cell contraction at later stages of LCMV infection. As Bcl2l11 expression is decreased in CD8 T cells under conditions in which Morrbid is not expressed, such as upon stimulation with low doses of anti-CD3 alone or during the contraction phase of LCMV infection, and Morrbid RNA overexpression in trans seems to have a modest impact in BCL2L11 expression in this cell type, these results suggest that DNA regulatory elements within the Morrbid locus or transcription across the locus are likely key in controlling the expression of this proapoptotic factor in cis. This Morrbid–Bcl2l11 relationship is in stark contrast to that in short-lived myeloid cells, where prosurvival cytokines promote Morrbid expression, which in turn represses Bcl2l11 transcription in cis to control the life span of these cells. As the CRISPR-Cas9–edited Morrbid-deficient mice were backcrossed more than six times to WT mice, there is no predicted homology between the CRISPR guide RNAs and the Bcl2l11 sequence, and littermate controls were used whenever possible, it is unlikely that CRISPR-Cas9 off-target effects in the Bcl2l11 gene contribute to our observed phenotype. It will be critical for future work to determine how DNA elements within the Morrbid locus, transcription across the locus, or the RNA itself contribute to the regulation of Bcl2l11 transcription in CD8 T cells at different stages of infection, and what impact this regulatory circuit has on CD8 T-cell homeostasis and function.
The timing of Morrbid transcription during LCMV infection, and the presence of CD8 T-cell alterations that could not be explained by Bcl2l11 dysregulation, suggest that Morrbid functions through additional mechanisms in this cell type. Our studies indicate that, in response to type I IFN and TCR stimulation, the signaling strength of the PI3K–AKT pathway is increased in Morrbid-deficient CD8 T cells. While the PI3K–AKT pathway was not the only pathway that was altered at the transcriptional level under these conditions, our Morrbid RNA overexpression studies strongly support the notion that this lncRNA contributes to the down-regulation of the PI3K–AKT pathway signaling strength. Interestingly, lncRNAs have previously been shown to regulate the PI3K–AKT signaling pathway by directly interacting with PIP3 to promote AKT activation, or by serving as sponges for microRNAs that target a negative regulator of this pathway, PTEN (29–32). The work presented here establishes Morrbid as an important regulator of this fundamental signaling pathway in CD8 T cells, and implies that this Morrbid–AKT axis is likely important in other contexts and cell types in which this lncRNA is expressed. Furthermore, as the AKT pathway controls multiple fundamental processes in CD8 T cells during viral infection or tumor immune surveillance, it will be important to determine the exact molecular mechanism by which Morrbid regulates this pathway, as it may provide insights into how to manipulate AKT signaling to improve antipathogen and anticancer immunity.
The current study of the Morrbid lncRNA and its locus in CD8 T cells highlights the exciting possibilities that individual lncRNAs may have more than one function, and that the biological significance of each of those functions is dependent on the cellular context in which the lncRNA is expressed and the isoforms being expressed at any given time. For example, our results suggest that Morrbid may control key signaling pathways in addition to regulating Bcl2l11 expression. Of note, in short-lived myeloid cells, Morrbid regulation of Bcl2l11 and survival was striking and dominant; thus, in this context, additional functions of Morrbid were not thoroughly assessed. As such, it will be important to formally determine whether this lncRNA controls additional signaling pathways such as the PI3K–AKT pathway in these innate myeloid cells. Alternatively, it is possible that different cell types express different isoforms of Morrbid, which could have alternative subcellular localizations, binding partners, and therefore functions. Although both isoforms of Morrbid are expressed in short-lived myeloid cells and stimulated CD8 T cells, these isoforms are enriched in the cytoplasm of CD8 T cells. These data differ from the chromatin-enriched predominance in myeloid cells. This dichotomy demonstrates that further work is required to address the function of each Morrbid isoform, and whether these functions vary depending on the cellular context in which they are transcribed.
In summary, our results provide evidence that lncRNA loci, and potentially the RNA that they transcribe, have the capacity to regulate CD8 T-cell function and kinetics during LCMV infection. Moreover, this work suggests that specific lncRNA may have multiple functions depending on the cellular context in which they are expressed.
Materials and Methods
All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility at the University of Pennsylvania. Mice were housed in accordance with the procedures outlined in Guide for the Care and Use of Laboratory Animals (33) under an animal study proposal approved by an institutional animal care and use committee. Morrbid-deficient mice were generated as previously described (10, 34). Samples sizes were estimated based on our preliminary phenotyping of Morrbid-deficient CD8 T cells following viral infection. No animals were excluded from analysis. All experimental and control mice were run in parallel to control for experimental variability and were not randomized. P values were calculated using unpaired two-sided t test, paired two-sided t test, one-way ANOVA with Tukey post hoc analysis, and false-discovery rate (FDR) as indicated. FDR was calculated using DESeq2 or GSEA algorithms. All error bars indicate mean ± SEM.
For fully detailed materials and procedures, please refer to SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Makoto Kurachi, Junko Kurachi, Bertram Bengsch, Shin Ngiow, Kaela Parkhouse, and Scott Hensley for technical support. This work was supported by funds from The Children’s Hospital of Philadelphia, University of Pennsylvania (UPenn) Institute for Immunology and Institute for Diabetes, Obesity, and Metabolism pilot projects, The PEW Charitable Foundation, The Burroughs Welcome Fund, and NIH Grants R21 AI128060, R21 DK111755, and R01 HL136572 (J.H.-M.); J.J.K. by NIH Grant F30 HL138739; F.I. by UPenn/The Children's Hospital of Philadelphia Diversity postdoctoral fellowship; W.K.M. by NIH Grant F31 AI124538; S.P.S. by NIH Grant F30 DK094708; A.W. by Grants R21 AI135221 and R21 AI133440; and E.J.W. by Grants AI105343, AI108545, AI117950, AI082630, and CA210944. E.J.W. is a member of the Parker Institute for Cancer Immunotherapy, which supported the UPenn cancer immunotherapy program.
Footnotes
Conflict of interest statement: E.J.W. has consulting agreements with and/or is on the scientific advisory board for Merck, Roche, Pieris, Elstar, and Surface Oncology. E.J.W. has a patent licensing agreement on the PD-1 pathway with Roche/Genentech.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE129352).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819457116/-/DCSupplemental.
References
- 1.Guttman M., et al. , Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 28, 503–510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derrien T., et al. , The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 22, 1775–1789 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinn J. L., Chang H. Y., Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81, 145–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson K. M., et al. , Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engreitz J. M., et al. , Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mowel W. K., Kotzin J. J., McCright S. J., Neal V. D., Henao-Mejia J., Control of immune cell homeostasis and function by lncRNAs. Trends Immunol. 39, 55–69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang N., Bevan M. J., CD8+ T cells: Foot soldiers of the immune system. Immunity 35, 161–168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaech S. M., Cui W., Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wherry E. J., Ahmed R., Memory CD8 T-cell differentiation during viral infection. J. Virol. 78, 5535–5545 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotzin J. J., et al. , The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R., Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtsinger J. M., Lins D. C., Mescher M. F., Signal 3 determines tolerance versus full activation of naive CD8 T cells: Dissociating proliferation and development of effector function. J. Exp. Med. 197, 1141–1151 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butz E. A., Bevan M. J., Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odorizzi P. M., Pauken K. E., Paley M. A., Sharpe A., Wherry E. J., Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harty J. T., Tvinnereim A. R., White D. W., CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18, 275–308 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Yang K., Neale G., Green D. R., He W., Chi H., The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12, 888–897 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayson J. M., Weant A. E., Holbrook B. C., Hildeman D., Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J. Virol. 80, 8627–8638 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hildeman D. A., et al. , Activated T cell death in vivo mediated by proapoptotic Bcl-2 family member Bim. Immunity 16, 759–767 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Wojciechowski S., et al. , Bim mediates apoptosis of CD127lo effector T cells and limits T cell memory. Eur. J. Immunol. 36, 1694–1706 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crouse J., Kalinke U., Oxenius A., Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 15, 231–242 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Kolumam G. A., Thomas S., Thompson L. J., Sprent J., Murali-Krishna K., Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen K. B., et al. , Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297, 2063–2066 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Wiesel M., et al. , Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur. J. Immunol. 42, 320–329 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Burke J. D., Platanias L. C., Fish E. N., Beta interferon regulation of glucose metabolism is PI3K/Akt dependent and important for antiviral activity against coxsackievirus B3. J. Virol. 88, 3485–3495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur S., et al. , Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl. Acad. Sci. U.S.A. 105, 4808–4813 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleiro D., et al. , Central role of ULK1 in type I interferon signaling. Cell Rep. 11, 605–617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rani M. R. S., Hibbert L., Sizemore N., Stark G. R., Ransohoff R. M., Requirement of phosphoinositide 3-kinase and Akt for interferon-beta-mediated induction of the beta-R1 (SCYB11) gene. J. Biol. Chem. 277, 38456–38461 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Smith-Garvin J. E., Koretzky G. A., Jordan M. S., T cell activation. Annu. Rev. Immunol. 27, 591–619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koirala P., et al. , LncRNA AK023948 is a positive regulator of AKT. Nat. Commun. 8, 14422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin A., et al. , The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat. Cell Biol. 19, 238–251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R., et al. , Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget 8, 26079–26089 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henao-Mejia J., et al. , The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity 38, 984–997 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 34.Henao-Mejia J., et al. , Generation of genetically modified mice using the CRISPR-Cas9 genome-editing system. Cold Spring Harb. Protc. 2016, pdb.prot090704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.