Significance
Electric lighting has fundamentally altered how the human circadian clock synchronizes to the day/night cycle. Exposure to light after dusk is pervasive in the modern world. We examined group-level sensitivity of the circadian system to evening light and the degree to which sensitivity varies between individuals. We found that, on average, humans are highly sensitive to evening light. Specifically, 50% suppression of melatonin occurred at <30 lux, which is comparable to or lower than typical indoor lighting used at night, as well as light produced by electronic devices. Significantly, there was a >50-fold difference in sensitivity to evening light across individuals. Interindividual differences in light sensitivity may explain differential vulnerability to circadian disruption and subsequent impact on human health.
Keywords: circadian rhythms, light sensitivity, circadian disruption, melatonin suppression, evening light
Abstract
Before the invention of electric lighting, humans were primarily exposed to intense (>300 lux) or dim (<30 lux) environmental light—stimuli at extreme ends of the circadian system’s dose–response curve to light. Today, humans spend hours per day exposed to intermediate light intensities (30–300 lux), particularly in the evening. Interindividual differences in sensitivity to evening light in this intensity range could therefore represent a source of vulnerability to circadian disruption by modern lighting. We characterized individual-level dose–response curves to light-induced melatonin suppression using a within-subjects protocol. Fifty-five participants (aged 18–30) were exposed to a dim control (<1 lux) and a range of experimental light levels (10–2,000 lux for 5 h) in the evening. Melatonin suppression was determined for each light level, and the effective dose for 50% suppression (ED50) was computed at individual and group levels. The group-level fitted ED50 was 24.60 lux, indicating that the circadian system is highly sensitive to evening light at typical indoor levels. Light intensities of 10, 30, and 50 lux resulted in later apparent melatonin onsets by 22, 77, and 109 min, respectively. Individual-level ED50 values ranged by over an order of magnitude (6 lux in the most sensitive individual, 350 lux in the least sensitive individual), with a 26% coefficient of variation. These findings demonstrate that the same evening-light environment is registered by the circadian system very differently between individuals. This interindividual variability may be an important factor for determining the circadian clock’s role in human health and disease.
It was once thought that the human circadian system was relatively insensitive to light, with synchronization of our clocks to the 24-h day achieved primarily through nonphotic cues, such as social interactions (1). It is now widely accepted that light is the primary synchronizing stimulus for the human circadian system (2–4). In a landmark study, Zeitzer et al. (5) measured responses of the human circadian system to nocturnal light as a function of the light’s intensity. The resultant dose–response curves demonstrated that half of the maximum-phase resetting and melatonin-suppression responses are achieved at light levels of only ∼100 lux. Nearly all of the change from no responsiveness to maximal responsiveness occurred between 30 and 300 lux.
Before the invention of electric light, humans would have spent nearly all of their time exposed to light intensities at the minimal and maximal ends of the dose–response curve: natural daylight (>300 lux) or dim sources of light after sunset (<30 lux), including moonlight and small fires (6–11). Today’s light environment differs considerably in the amount of time spent at intermediate light intensities (30–300 lux; i.e., in the steepest region of the dose–response curve). Exposure to electric light after sunset in this range is extremely common in industrialized countries (9, 12–14), and the increasing use of light-emitting devices is a further source of evening light that is potentially disruptive to the circadian system (15, 16).
Interindividual differences in light sensitivity are most likely to be impactful where the dose–response curve to light is steepest: at intermediate light levels. To date, interindividual differences in the dose–response curve to light have not been systematically studied. The dose–response curves measured by Zeitzer et al. (5) were group averages, with each of the 21 data points obtained from a single light exposure in a different participant. Results of other studies suggest that interindividual differences in the dose–response curve to light could be present (17–23), including one study of dose–response curves to light in six individuals (24). Gooley et al. (20) found a range of melatonin-suppression responses (29–93%) to the same <200-lux light stimulus. The present study examined group- and individual-level dose–response curves for the human circadian system to evening light by systematically varying light exposures within individuals for a total of 351 nights of data across 55 individuals. Using highly controlled light-exposure techniques, we found that humans are more sensitive to evening light than previously thought and that there is over an order of magnitude difference in sensitivity across individuals.
Results
A total of 27 healthy men [mean ± SD; body mass index (BMI) of 23.07 ± 2.43 kg/m2] and 29 healthy women (mean ± SD; BMI of 23.46 ± 2.23 kg/m2), aged 18–30 y (mean ± SD; age of 20.8 ± 2.6 y), completed a 6- to 7-wk protocol with structured sleep maintained throughout. The first week ended with a baseline dim-light (<1 lux) control condition, followed by weekly white-light exposures in a group-randomized order, counterbalanced for sex. Light-exposure levels included 10, 30, 50, 100, 200, 400, and 2,000 lux. Light exposures began 4 h before habitual bedtime and ended 1 h after habitual bedtime. Hourly saliva samples were collected and assayed for melatonin by radioimmunoassay. Melatonin suppression was calculated for each light condition as a percentage of baseline melatonin. To control for the circadian timing of light exposure, melatonin suppression was calculated from the baseline dim-light melatonin onset (DLMO) onward for each individual, using either all data after DLMO (overall) or for each of the first 3 h after DLMO (hours 1–3). Baseline DLMO was able to be determined for 55 of 56 participants, resulting in 1 exclusion.
High Group-Level Sensitivity to Evening Light.
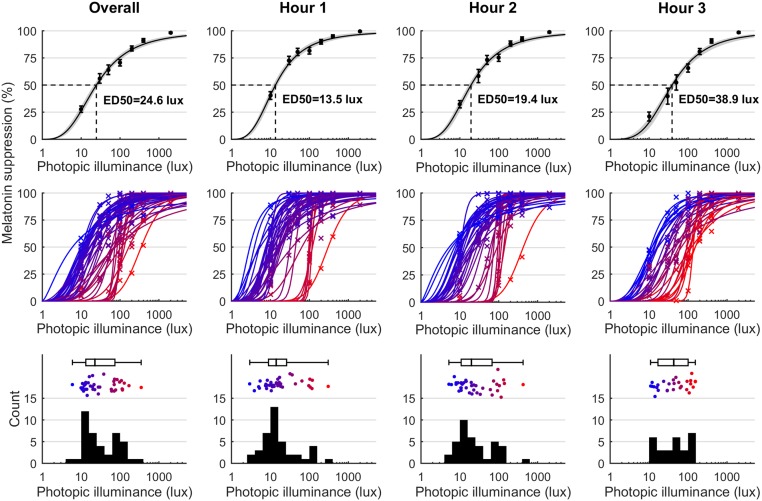
Percentage melatonin suppression was measured for each individual at each time point under each light condition (n = 55 participants after one exclusion, 351 nights of data, and 2,092 valid melatonin samples). Group-level logistic dose–response curves were fit to pooled individual data points for hour 1 (n = 55; 281 suppression values), hour 2 (n = 50; 254 suppression values), and hour 3 (n = 36; 184 suppression values) of light exposure, following DLMO, as well as overall (all data points after DLMO; 281 suppression values). There were fewer data points in later hours due to individuals with later DLMOs relative to bedtime having shorter durations of post-DLMO light. The ED50 values were 24.60 lux (95% CI: 21.33–28.37 lux) for overall, 13.47 lux (95% CI: 11.77–15.42 lux) for hour 1, 19.38 lux (95% CI: 16.61–22.63 lux) for hour 2, and 38.89 lux (95% CI: 32.33–46.77 lux) for hour 3 (Fig. 1). These ED50 values, measured under ecologically relevant light intensities, reflect a high sensitivity of the circadian system to evening light. Melatonin suppression was greatest immediately after DLMO, at a time when exposure to electric lighting is very common.
Fig. 1.
Melatonin suppression depends on light intensity and time of exposure and exhibits large interindividual differences. Columns correspond to overall data (i.e., all data after DLMO) and hours 1–3 after DLMO. Top shows group-level dose–response curves to light. ED50 values are indicated. Error bars represent mean ± SEM. Gray shaded areas in the top row represent 95% CIs for the fitted logistic curve. Middle shows individual-level dose–response curves. Individual curves (solid lines) and corresponding data points (crosses) are colored from blue (lowest ED50) to red (highest ED50). Individual-level curves are shown only for individuals with a reliable ED50 value (95% CI <1 log-unit). Bottom shows histograms of the individual ED50 values, with accompanying box plot and individual data points colored from blue (lowest ED50) to red (highest ED50).
High Interindividual Variability in Evening Light Sensitivity.
Individual-level dose–response curves (Fig. 1) showed high interindividual variability in responsiveness to evening light. Accurate estimates of ED50 (defined as 95% CI <1 log-unit) were obtained for 42 participants for overall, 41 for hour 1, 37 for hour 2, and 29 for hour 3. For the overall fits, individual ED50 values ranged from 6.0 lux (95% CI: 2.1–16.6 lux) to 349.8 lux (95% CI: 187.7–651.9 lux), with a coefficient of variation of 26.0% for the log10-ED50 values (overall data are also expressed in terms of melanopic lux in SI Appendix, Fig. S1). For hourly fits, the individual ED50 values ranged from 2.9 to 303.0 lux for hour 1, 5.2–424.2 lux for hour 2, and 10.9–153.5 lux for hour 3. The ED50 coefficients of variation were 34.7% for hour 1, 33.5% for hour 2, and 21.6% for hour 3. We note that the ED50 range was lower for hour 3 since the least-sensitive individual contributed data only for hours 1–2 due to a late DLMO relative to their habitual bedtime. The ED50 values displayed potentially bimodal distributions (Fig. 1) for overall (kurtosis = 12.8), hour 1 (kurtosis = 15.7), and hour 2 (kurtosis = 18.7), but not for hour 3 (kurtosis = 2.1). Potential bimodality could not be accounted for by any factors we examined [sex, age, habitual bedtime, DLMO, phase angle, morningness–eveningness questionnaire (MEQ), experimental order, or season].
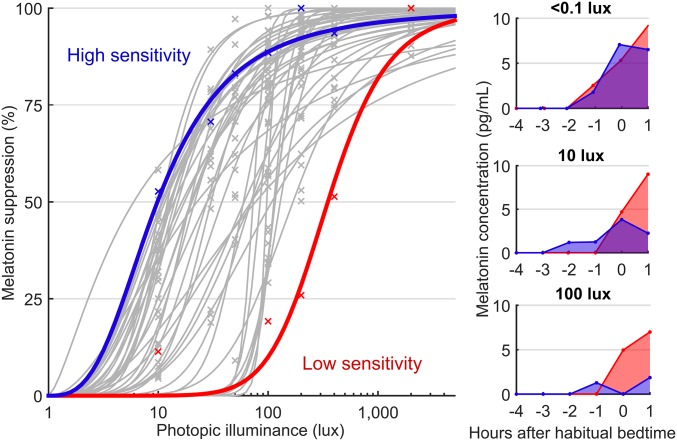
Interindividual differences in the dose–response curve to light were considerable. As illustrated by the low- and high-sensitivity participants in Fig. 2, the individual with low sensitivity responded similarly to 400 lux (51.4% melatonin suppression) as the individual with high sensitivity did to only 10 lux (52.7% melatonin suppression). These two individuals would therefore respond very differently to the same home-lighting environment.
Fig. 2.
Comparison of two individuals with high and low light sensitivity. (Left) Highlights two individual dose–response curves: an individual with high sensitivity (blue) and an individual with low sensitivity (red) and includes individual-level curves for all other participants (gray). Individual data points are shown (crosses). Right show the two individuals’ respective melatonin concentrations across time under the <1-lux (dim control; Top), 10-lux (Middle), and 100-lux (Bottom) conditions, where their differences in responsiveness manifest. The x-axis values are hours relative to habitual bedtime (0).
Contrast analyses for overall ED50, DLMO, and phase angle confirmed no order effects (all P > 0.05). In addition, we found no significant relationship between total melatonin production and individual-level overall ED50 values (r = −0.18, P = 0.18). Nor did we find significant relationships of individual-level overall ED50 values with either DLMO time (r = −0.09, P = 0.53) or the phase angle between DLMO and habitual bedtime (r = −0.01, P = 0.94), which determined the length of experimental light exposure before DLMO. Furthermore, we found no associations between multiple measures of light-exposure history (mean lux, log mean lux, median lux, hours >100 lux, and hours >500 lux) and individual-level overall ED50 values (all P > 0.15; SI Appendix, Table S1). These null findings confirm that the individual differences in ED50 values are not simply due to differing lengths of exposure to light before DLMO or due to differing light-exposure histories.
Melatonin Onset Under Light Depends on Individual-Level Sensitivity.
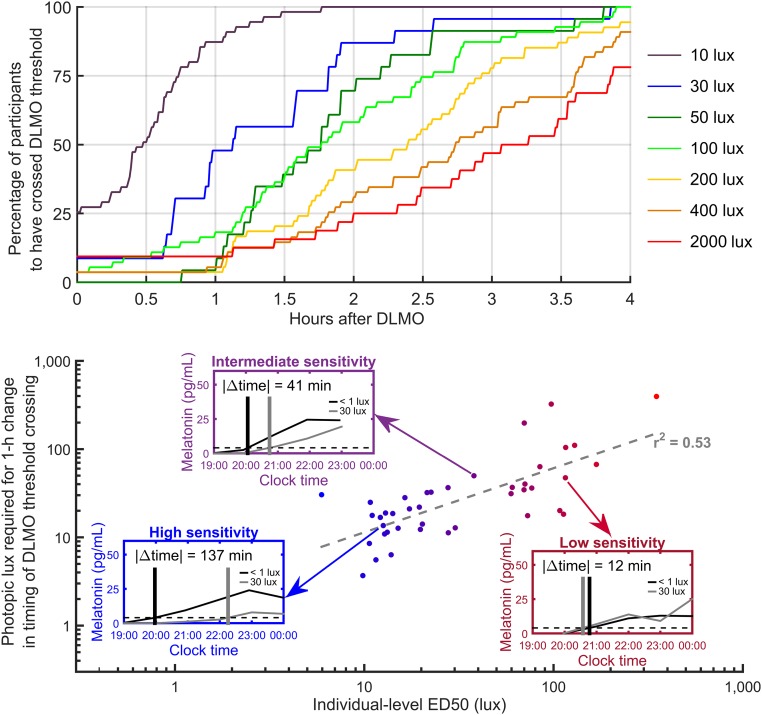
The rise of melatonin signals the beginning of the biological night and promotes sleep onset, but light can affect the timing of this rise. We examined how different levels of evening-light exposure affect the timing of “apparent” melatonin onset, compared with the timing of baseline DLMO (i.e., melatonin onset assessed under truly dim light of <1 lux). A dose–response relationship was observed, with apparent melatonin onset occurring progressively later for progressively higher light levels (Fig. 3). In 10 lux, apparent melatonin onset occurred an average of 22 ± 43 min later than DLMO measured under <1 lux conditions (P < 0.001) and ≥29 min later in 50% of individuals. At 30 lux, apparent melatonin onset occurred an average of 77 ± 56 min later than DLMO (P < 0.000001) and ≥68 min later in 50% of individuals. At 50 lux, apparent melatonin onset occurred an average of 109 ± 46 min later than DLMO (P < 10−10) and ≥106 min later in 50% of individuals. These findings indicate that even relatively dim light has substantial effects on the apparent timing of melatonin onset, compared with assessment under truly dim-light (<1 lux) conditions.
Fig. 3.
The time at which an individual crosses the DLMO threshold has a dose–response relationship with light intensity. Upper shows the percentage of participants who had crossed the 4-pg/mL (DLMO) threshold at a given time after baseline DLMO. Colored curves correspond to different light intensities. Lower shows the relationship between individual-level ED50 values and the estimated level of light required to move the apparent melatonin onset 1 h later than baseline DLMO. The gray dashed line shows a linear regression to the log–log values. Lower, Insets represent three individuals: high sensitivity (blue Inset), intermediate sensitivity (purple Inset), and low sensitivity (red Inset). In each Inset, melatonin concentration is plotted with respect to clock time for baseline (black curve) and at 30 lux (gray curve). Horizontal dashed lines indicate the 4-pg/mL threshold. Vertical lines indicate times at which melatonin concentration crossed the 4-pg/mL threshold. Included are all participants with an accurate ED50 estimate (95% CI < 1 log10-unit).
The change in an individual’s apparent timing of melatonin onset relative to baseline DLMO was associated with their ED50. Specifically, an individual’s ED50 level was significantly associated with the estimated level of light required to move the apparent melatonin onset 1 h later than baseline DLMO (r2 = 0.53, P < 0.0001).
Discussion
Our data demonstrate that the human circadian system is highly responsive to dim evening light. At the group-average level, we observed >50% melatonin suppression at 30 lux. Furthermore, we found substantial interindividual differences in sensitivity to light by generating individual-level dose–response curves to light using a highly controlled within-subjects protocol. The four most-sensitive participants exhibited >50% melatonin suppression in response to dim reading light (10 lux), whereas the least-sensitive participant did not achieve this level of suppression until exposed to bright indoor light of 400 lux.
Our study systematically examined interindividual differences in light sensitivity of the human circadian system in a large sample. The interindividual differences we observed in light sensitivity may underlie interindividual differences in sleep and circadian timing that are known to arise with access to electric light (8, 25, 26), in addition to previously identified physiological factors, including intrinsic period of the circadian clock and sleep homeostatic parameters (27–30). We note that differences in light sensitivity might also have been an important confound in classical circadian studies. In early studies of human circadian rhythms that did not control for exposure to indoor room light, circadian periods exhibited a 30% coefficient of variation (1), similar to the 26% coefficient of variation we observe in ED50 values. In subsequent studies conducted under controlled, dim-light conditions, the coefficient of variation in the human circadian period reduced to <0.6% (4), showing that the human circadian period distribution was in fact similar to that seen in nonhuman animals studied in dark conditions. Thus, the previously high coefficient of variation reported in human period estimates may have been a consequence of the variability in human circadian light sensitivity, which manifested as differences in estimated period due to uncontrolled indoor lighting.
Abnormalities in the sensitivity of the human circadian system to light may contribute to sleep and circadian disruption. Individuals with very high sensitivity (low ED50 values) would be particularly vulnerable to sleep and circadian disruption even by dim indoor light. This is consistent with findings of higher light sensitivity in individuals with delayed sleep–wake-phase disorder compared with healthy controls, based either on measurements of melatonin suppression (31) or phase shifting (22). Increased melatonin suppression has also been observed in patients with bipolar disorder (32–34) and seasonal affective disorder (SAD) (33, 35). Patients with SAD may also exhibit seasonal hyposensitivity to light, suggesting that abnormally high or low light sensitivity may be associated with pathology (35). Methods to efficiently phenotype an individual’s light sensitivity [e.g., by using melatonin suppression or pupillary measures (36)] will likely be needed to develop personalized interventions based on an individual’s circadian physiology.
The large interindividual differences observed in our study may be the result of several different factors. We recently showed that interindividual differences in melatonin suppression relate to functional MRI BOLD activation in the suprachiasmatic area (37). However, it is not clear whether this difference is due to differences at the level of the retina, suprachiasmatic nuclei (SCN) function, or other modulating inputs to the SCN. It has been shown that interindividual differences in the sustained pupil responses to light (likely melanopsin-based) relate to differences in midsleep time (38), the diagnosis of SAD (39), and delayed sleep–wake-phase disorder (22). A recent analysis indicates that melatonin suppression may indeed be driven primarily by melanopsin photoreception (40). It is, however, also possible that interindividual differences exist in the processing of light information at the level of the SCN, independent of differences in retinal signaling (e.g., glutamate or pituitary adenylate cyclase-activating polypeptide receptor function). Furthermore, it is likely that nonretinal input to the SCN can modulate the response to light. As selective serotonin reuptake inhibitors have been found to modulate light sensitivity (41), interindividual variability in raphe input to the SCN is one highly plausible mechanism for the differences we observe in light sensitivity. Further study will be needed to tease apart the many potential mechanisms for interindividual differences in circadian light sensitivity.
Light exposures in this study were designed to occur in the evening (beginning before DLMO) and included intensities that are frequently experienced in the home. This seminaturalistic design is different from that used by Zeitzer et al. (5), who studied later light exposures that were timed to mostly overlap with peak melatonin production (typically beginning after DLMO) and reported far lower group-level sensitivity to light than reported here. The differences in experimental design could account for the reported differences in light sensitivity. Specifically, our pre-DLMO light exposures may have suppressed the rise in melatonin while also phase-delaying the circadian clock and therefore the signal for the rise of melatonin. Although we found higher sensitivity than Zeitzer et al. (5), we did observe larger ED50 values (less sensitivity) progressing from hours 1 to 3 after DLMO, as our light exposure more closely approached the timing used by Zeitzer et al. (5). We note, however, that the Zeitzer et al. (5) study included only three data points in the 10- to 50-lux range and only two data points in the 50- to 100-lux range, meaning that their group-level ED50 estimate depended strongly on the light sensitivities of those five participants. Our findings of a general high sensitivity to light are corroborated by studies employing blue-light exposures (42), including a recent study by Vartanian et al. (43), who found that melatonin suppression can be induced even by very dim blue light. These findings indicate that the rise of melatonin, an important signal for the initiation of sleep that inhibits electrical activity of SCN neurons (44), is extremely sensitive to suppression by evening-light exposure.
The importance of the high sensitivity to evening light extends to the implementation of “dim-light” conditions for studies that measure DLMO—the current gold-standard method for assessing circadian phase. Whereas the Zeitzer et al. (5) dose–response curve for nocturnal light exposure indicates negligible (<10%) melatonin suppression at intensities of 50 lux or less, our findings show that evening-light exposure at intensities of 10, 30, and 50 lux beginning 4 h before bedtime (more typical for DLMO procedures than the timing of exposure in the Zeitzer et al. study) results in later apparent melatonin onsets by 22, 77, and 109 min, respectively. This demonstrates that, on average, clinical and research assessments conducted in light levels 10 lux and above will yield systematically later DLMO timing. Moreover, the degree of inaccuracy will depend on an individual’s light sensitivity. Thus, studies that do not adequately control for evening light when measuring DLMO will be confounded by the large interindividual differences in light sensitivity that we have uncovered.
Since we used broad-spectrum white light for this study, enabling direct comparison with Zeitzer et al. (5), it is important to note that the circadian system receives light via a complex pathway involving contributions from multiple photoreceptors (45, 46). The commonly used measure of photopic illuminance (lux) therefore does not allow accurate comparison of the impact of lights with differing spectral compositions (e.g., white vs. blue light) on the circadian pacemaker (47). While we did report the illuminances of our white-light stimuli for all types of photoreceptors, including melanopic lux, our study did not explicitly examine the role of the spectral composition of light. Future studies should investigate whether interindividual differences in light sensitivity are primarily driven by responses to certain wavelength ranges (or certain durations of light exposure), which in turn could identify specific contributory elements of the photoreceptive pathway. A related avenue of future work would be to examine whether individual differences in melatonin suppression predict individual differences in other outcomes that are known to follow similar dose–response curves at the group-average level, such as the effect of light on alertness (48). Future studies should also attempt to generalize our findings to other ethnicities and a broader age range, since light sensitivity is likely to decrease with age (49).
To distill our key findings, we discovered that even when two healthy young individuals experience the same tightly controlled evening-light environment, their melatonin-suppression response can differ vastly. This interindividual difference is most pronounced across the range of intermediate light intensities that we are most commonly exposed to in the evening in industrialized societies. For typical indoor light (∼50 lux), we found that some individuals respond as if exposed to bright natural daytime light, whereas other individuals respond in the same way as they do to a very dim light source. Under naturalistic lighting conditions in which humans evolved, large interindividual differences in the response to intermediate light levels would likely have been inconsequential, due to minimal time exposed to this narrow range of light levels. In modern industrialized society, however, exposure to intermediate light levels in the evening is virtually ubiquitous, especially due to the proliferation of hand-held blue-enriched light sources to which the circadian system is particularly sensitive. These interindividual differences in sensitivity to light may therefore be an important and previously unappreciated determinant of the circadian clock’s role in human health and disease.
Methods
All procedures were approved by the Monash University Human Research Ethics Committee before commencement. Participants gave written informed consent and were reimbursed for their time.
Participants.
A total of 61 participants were enrolled, of whom 3 were excluded based on actigraphy, and 2 did not complete the study beyond the baseline DLMO. Overall, 56 healthy young Caucasian adults (29 women, 27 men; 20.8 ± 2.6 y of age) completed the study. Participants were free from any medical or psychological conditions, had a BMI of 18–30 kg/m2, and were not taking any medications at the time of the study. Participants had not recently traveled across time zones (1 mo per time zone, up to 3 mo) or engaged in shiftwork in the previous 12 mo. Women were naturally cycling (i.e., free from hormonal contraception) and had a regular menstrual cycle of 21–36 d in duration. Participants were healthy sleepers, reporting no subjective problems or previous diagnoses, and having a regular bedtime before 1 AM. A score of 10 or greater on the Epworth Sleepiness Scale was exclusionary, and participants were predominantly intermediate chronotypes (MEQ score of 52.7 ± 9.2). Participants had an average bedtime and waketime of 23:04 (SD = 44 min) and 07:04 (SD = 44 min), respectively. DLMO occurred on average at 21:05 (SD = 70 min), 2.22 h before bedtime. A total of nine participants wore prescription glasses at each test session. We did not select participants based on their habitual light-exposure history, nor did we instruct them on light-exposure patterns, other than the strict sleep/wake times required for the study. Analysis of light-exposure history is presented in SI Appendix.
Protocol.
Participants completed a total of either six or seven consecutive weekly in-laboratory test sessions. All light exposures commenced 4 h before bedtime and concluded 1 h after. The first session was a dim-light control (<1 lux), with either five or six subsequent experimental light exposures of varying intensities (10; 30; 50; 100; 200; 400; and 2,000 lux; see SI Appendix, Fig. S2 for sample protocol). Participants were randomly allocated to one of six randomly generated light-exposure orders, with each order counterbalanced for sex: (i) 100; 200; 10; 2,000; and 400 lux; (ii) 10; 100; 400; 200; and 2,000 lux; (iii) 2,000; 400; 200; 100; and 10 lux; (iv) 200; 400; 100; 10; 30; and 50 lux; (v) 100; 30; 200; 50; 400; and 10 lux; and (vi) 10; 50; 30; 200; 100; and 400 lux. Of the participants included in the final analysis, 33 were assigned to orders (i)–(iii) and 22 were assigned to orders (iv)–(vi).
Home Sleep Monitoring.
Participants maintained a fixed 16:8-h wake:sleep, light:dark schedule for at least 1 wk before their first test session. Participants self-selected an 8-h scheduled sleep period, based on their habitual sleep–wake habits. Naps were not permitted during the study, and a deviation >30 min in either bed or wake time more than one night a week resulted in exclusion from the study (n = 3). Compliance was verified by wrist-worn actigraphy (Actiwatch-L/2/Plus, Philips Respironics) and sleep diaries collected throughout the study protocol. On light-exposure evenings, when participants had been kept awake an hour after their scheduled bedtime, they were permitted to sleep at a later time (upon arriving home after discharge) and wake 8 h later to ensure an adequate sleep opportunity. They returned to their scheduled bed and wake times the following night.
In-Laboratory Sessions.
Participants underwent six or seven consecutive weekly sessions (depending on assigned light order) at the Monash University Sleep and Circadian Medicine Laboratory. Test sessions were conducted in white, time-free, windowless suites. All equipment was set up out of participants’ line of vision (to eliminate light exposure from electronic-testing equipment), and a researcher remained in the room to monitor participants and confirm compliance. Participants were asked to abstain from caffeine and alcohol for 1 wk before their first laboratory visit and throughout the study. Additionally, abstinence from recreational drugs and medications for 3 wk before admission and throughout the study was required and confirmed before each test session via urine toxicology (SureStep Single Drug Cassette, Innovacon Inc.). Women were also tested for pregnancy (Xcel One-Step Pregnancy Test, Princeton BioMeditech), and a positive test was exclusionary (n = 0). Breath tests were conducted at each session to verify abstinence from alcohol (Enforcer 2 Breathalyzer, Alcolimit).
During light exposures, participants remained awake and seated other than for bathroom breaks (bathroom lighting levels were similar to laboratory lighting levels in all conditions and did not ever exceed laboratory lighting levels). Participants were instructed to direct their gaze toward a specified point that was calibrated to the correct light intensity on a wall for 10-min intervals throughout the experimental light exposures, with alternating 10-min periods of free gaze. This was to ensure intraindividual and interindividual stability of the light exposures by minimizing variability in retinal light exposure across the evening (SI Appendix, Table S2).
Lighting.
Light intensity was measured hourly by using a lux meter (Tektronix J17, Luma Color) at the level of both eyes at horizontal angle gaze during each session (SI Appendix, Table S2). A single LED light source with a peak of 451 nm and a correlated color temperature (CCT) of 4,289 K located at the back of the suite (out of the participants’ line of vision) was used to create the dim condition. Experimental light exposures were achieved by using Philips 4,100-K lamps (Master TL5 HE 28W/840, Philips). These lamps generated broad-spectrum white light with a peak of 545 nm and a CCT of 3,968 K (spectrum in SI Appendix, Fig. S3). To achieve the 10-, 30-, and 50-lux conditions, one-stop neutral density filters were placed over the ceiling mounted bulbs (LEE 209 Neutral Density Filter, Lightmoves). The effective illuminance for human photopigments in the retina across all light-exposure conditions is listed in SI Appendix, Table S3, calculated by using the Irradiance Toolbox (42). Light intensities were measured and tested at least an hour before participants’ arrival at the laboratory and remained stable within and between test sessions.
Melatonin Assays.
Saliva samples were collected hourly by using salivettes (Salimetrics, Inc.). Saliva sampling began 4 h before habitual bedtime and ended 1 h after habitual bedtime. This totaled six samples (−4, −3, −2, −1, 0, and +1 h). Participants were not permitted to clean their teeth during the collection period and were instructed to drink after eating to rinse their mouth. Food and drinks were strictly forbidden during the 20 min before a sample being taken, and participants were instructed to not change their posture for at least 20 min before each sample was collected. Samples were centrifuged at 2,500 rpm for 5 min (Centrifuge 5702R, Eppendorf), before being placed in a −35 °C freezer for storage until assay. Samples were radioimmunoassayed in duplicate with the G280 antibody and the [1251]2-iodomelatonin radioligand to determine melatonin concentration (University of Adelaide). The interassay and intraassay coefficients of variation were <17% and 7%, respectively.
Data Analysis.
Salivary DLMO was determined by using an absolute threshold of 4 pg/mL. Linear interpolation was used to determine the first threshold-crossing time. DLMO values were obtained for 55 of the 56 participants. One participant was excluded from analyses as their DLMO could not be determined, since they did not exceed the threshold on any of the baseline assays.
Melatonin-suppression values were calculated for each participant under each experimental light-exposure condition. Melatonin suppression was calculated by using one of four time intervals: (i) overall: from baseline DLMO to final assay (whichever was earlier of the baseline final assay and experimental final assay); (ii) hour 1: from baseline DLMO to 1 h post-DLMO; (iii) hour 2: from 1 h post-DLMO to 2 h post-DLMO; and (iv) hour 3: from 2 h post-DLMO to 3 h post-DLMO. Linearly interpolated curves were generated for both baseline and experimental light-exposure conditions. Percentage melatonin suppression was the percentage difference in area under the curve between the two interpolated curves within the specified time interval. For the hourly time intervals, we required at least 30 min of overlap where both linearly interpolated curves were defined. Undefined values could occur due to missing assays or due to the final assay occurring within the time interval.
Previous work has demonstrated that melatonin-suppression dose–response curves are best described by a four-parameter logistic model (5). This model was fit to our data:
where y0 is the minimum melatonin-suppression response, a is the difference between the minimum and maximum response, x0 is the ED50, and b is the slope parameter that determines how quickly the curve rises. We made the physiologically reasonable assumptions of 0% suppression at 0 lux and 100% suppression as photopic illuminance approaches infinity (y0 = 0, a = 100). In the dataset, we observed full or near-to-full (>99%) suppression on 61 nights, justifying this assumption. Curves were fit at both the group and individual levels by using the Levenberg–Marquardt residual minimization procedure, implemented in Matlab using the inbuilt function nlinfit. Percentage melatonin-suppression values for all experimental conditions were the y variable; log10-transformed photopic illuminance values for all experimental conditions were the x variable. To ensure convergence of the fit, y values were bounded to a minimum of 0.1% and a maximum of 99.9%. Individual-level dose–response curves were assumed to be monotonically increasing, but we allowed up to a 20% tolerance for experimental error and intraindividual variability (i.e., each melatonin-suppression value on the dose–response curve could be up to 20% less than suppression values at any lower photopic illuminances). Values that did not satisfy this tolerance were excluded (4.3% of suppression values for overall, 4.1% for hour 1, 3.8% for hour 2, and 2.6% for hour 3). We required a minimum of three data points (i.e., valid melatonin-suppression values at three experimental light levels) to perform individual-level fitting. To ensure we obtained accurate individual-level estimates of the ED50, we required that the 95% CI for the individual-level fit of the parameter x0 span less than one log10-unit (i.e., a factor of 10) from minimum to maximum. ED50 distributions were checked for potential bimodality by using kurtosis values.
Contrast analyses were performed to test for possible order effects of experimental light conditions. All pair-wise intergroup comparisons were tested for ED50, DLMO, and phase angle between DLMO and habitual bedtime. In addition, we tested for Spearman correlations between individual-level ED50 values and (i) total baseline melatonin production (using average production after DLMO), (ii) DLMO time, and (iii) phase angle between DLMO and habitual bedtime.
Timing of apparent melatonin onset under experimental light conditions was computed for a 4-pg/mL threshold, with the same method used for computing DLMO. For each experimental light level, we computed the percentage of individuals who had achieved apparent melatonin onset as a function of time after the control-condition DLMO, in 0.1-h steps from 0 up to 4 h after baseline DLMO. For each individual, we estimated the photopic illuminance that would correspond to apparent melatonin onset occurring 1 h after DLMO. This was obtained by log10-transforming illuminance values, performing linear interpolation, and finding the minimum photopic illuminance at which a 1-h change would be predicted.
Supplementary Material
Acknowledgments
We thank the research participants for their dedication and cooperation, as well as the SuperMega research staff and students at the Monash University Sleep and Circadian Medicine Laboratory. We give special thanks to Dr. Melissa St. Hilaire for statistical consultation. This work was supported by National Health and Medical Research Council (NHMRC) Grant NHMRC 1064231 (to S.W.C.). P.V. was supported by a NeuroSleep NHMRC Centre of Research Excellence PhD scholarship. E.M.M. and A.C.B. were supported by Australian Government Research Training Program scholarships.
Footnotes
Conflict of interest statement: C.A. has received a research award/prize from Sanofi-Aventis; contract research support from VicRoads, Rio Tinto Coal Australia, National Transport Commission, and Tontine/Pacific Brands; lecturing fees from Brown Medical School/Rhode Island Hospital, Ausmed, Healthmed, and TEVA Pharmaceuticals; and reimbursements for conference travel expenses from Philips Healthcare. C.A. has served as a consultant to the Rail, Bus, and Tram Union; the Transport Accident Commission; the National Transportation Committee; and Melius Consulting. C.A. has also served as an expert witness and/or consultant in relation to fatigue and drowsy driving. C.A. is a Theme Leader in the Cooperative Research Centre for Alertness, Safety, and Productivity. S.M.W.R. has served as a consultant through his institution to Vanda Pharmaceuticals, Philips Respironics, and Teva Pharma Australia and has, through his institution, received research grants and/or unrestricted educational grants from Vanda Pharmaceuticals, Takeda Pharmaceuticals North America, Philips Lighting, Philips Respironics, Cephalon, and ResMed Foundation, as well as reimbursements for conference travel expenses from Vanda Pharmaceuticals. S.M.W.R. serves as a Program Leader and consultant to the Cooperative Research Centre for Alertness, Safety, and Productivity. S.W.L. has had a number of commercial interests in the last 36 mo (2016–2019). None are directly related to the research reported in this paper but, in the interests of full disclosure, are outlined as follows. S.W.L. has received consulting fees from the Atlanta Falcons, Atlanta Hawks, BHP Billiton, Noble Insights, Slingshot Insights, and Team C Racing; honoraria and/or paid travel from BHP Billiton, DIN, Emory University, IES, Ineos, SLTBR, Solemma, and Teague; has current consulting contracts with Akili Interactive, Apex 2100 Ltd., Consumer Sleep Solutions, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, PlanLED, Six Senses, Stantec, and Wyle Integrated Science and Engineering; has received unrestricted equipment gifts from Bionetics Corporation and F. Lux Software LLC and royalties from Oxford University Press; and has served as a paid expert in legal proceedings related to light, sleep, and health. He holds a patent through Harvard University and Brigham and Women’s Hospital for “Systems and Methods for Determining and/or Controlling Sleep Quality.”
This article is a PNAS Direct Submission. D.B.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901824116/-/DCSupplemental.
References
- 1.Wever R., The Circadian System of Man (Springer, New York, 1979). [Google Scholar]
- 2.Boivin D. B., Duffy J. F., Kronauer R. E., Czeisler C. A., Dose-response relationships for resetting of human circadian clock by light. Nature 379, 540–542 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Klerman E. B., Dijk D. J., Kronauer R. E., Czeisler C. A., Simulations of light effects on the human circadian pacemaker: Implications for assessment of intrinsic period. Am. J. Physiol. 270, R271–R282 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Czeisler C. A., et al. , Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Zeitzer J. M., Dijk D. J., Kronauer R., Brown E., Czeisler C., Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 526, 695–702 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yetish G., et al. , Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 25, 2862–2868 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Iglesia H. O., et al. , Access to electric light is associated with shorter sleep duration in a traditionally hunter-gatherer community. J. Biol. Rhythms 30, 342–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright K. P., Jr, et al. , Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23, 1554–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stothard E. R., et al. , Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr. Biol. 27, 508–513 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson D. R., Crittenden A. N., Mabulla I. A., Mabulla A. Z., Nunn C. L., Hadza sleep biology: Evidence for flexible sleep-wake patterns in hunter-gatherers. Am. J. Phys. Anthropol. 162, 573–582 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Moreno C. R., et al. , Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci. Rep. 5, 14074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace-Guy G. M., et al. , Evening light exposure: Implications for sleep and depression. J. Am. Geriatr. Soc. 50, 738–739 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Goulet G., Mongrain V., Desrosiers C., Paquet J., Dumont M., Daily light exposure in morning-type and evening-type individuals. J. Biol. Rhythms 22, 151–158 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Santhi N., et al. , The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 53, 47–59 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Cajochen C., et al. , Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 110, 1432–1438 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Chang A. M., Aeschbach D., Duffy J. F., Czeisler C. A., Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 112, 1232–1237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bojkowski C. J., et al. , Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm. Metab. Res. 19, 437–440 (1987). [DOI] [PubMed] [Google Scholar]
- 18.Laakso M. L., Porkka-Heiskanen T., Stenberg D., Alila A., “Interindividual differences in the responses of serum and salivary melatonin to light” in Role of Melatonin and Pineal Peptides in Neuroimmunomodulation, Reiter R. J., Fraschini F., Eds. (Springer, Boston, 1991), pp. 307–311. [Google Scholar]
- 19.Higuchi S., et al. , Inter-individual difference in pupil size correlates to suppression of melatonin by exposure to light. Neurosci. Lett. 440, 23–26 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Gooley J. J., et al. , Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 96, E463–E472 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho Mien I., et al. , Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PLoS One 9, e96532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson L. A., et al. , Increased sensitivity of the circadian system to light in delayed sleep-wake phase disorder. J. Physiol. 596, 6249–6261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanifin J. P., et al. , Randomized trial of polychromatic blue-enriched light for circadian phase shifting, melatonin suppression, and alerting responses. Physiol. Behav. 198, 57–66 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Brainard G. C., et al. , Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 454, 212–218 (1988). [DOI] [PubMed] [Google Scholar]
- 25.Skeldon A. C., Phillips A. J. K., Dijk D. J., The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: A modeling approach. Sci. Rep. 7, 45158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swaminathan K., Klerman E. B., Phillips A. J. K., Are individual differences in sleep and circadian timing amplified by use of artificial light sources? J. Biol. Rhythms 32, 165–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taillard J., Philip P., Coste O., Sagaspe P., Bioulac B., The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J. Sleep Res. 12, 275–282 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Mongrain V., Carrier J., Dumont M., Circadian and homeostatic sleep regulation in morningness-eveningness. J. Sleep Res. 15, 162–166 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Phillips A. J. K., Chen P. Y., Robinson P. A., Probing the mechanisms of chronotype using quantitative modeling. J. Biol. Rhythms 25, 217–227 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Duffy J. F., et al. , Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 108 (Suppl 3), 15602–15608 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoki H., Ozeki Y., Yamada N., Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol. Int. 18, 263–271 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Lewy A. J., Sack R. L., Singer C. M., Immediate and delayed effects of bright light on human melatonin production: Shifting “dawn” and “dusk” shifts the dim light melatonin onset (DLMO). Ann. N. Y. Acad. Sci. 453, 253–259 (1985). [DOI] [PubMed] [Google Scholar]
- 33.Nathan P. J., Burrows G. D., Norman T. R., Melatonin sensitivity to dim white light in affective disorders. Neuropsychopharmacology 21, 408–413 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Hallam K. T., Begg D. P., Olver J. S., Norman T. R., Abnormal dose-response melatonin suppression by light in bipolar type I patients compared with healthy adult subjects. Acta Neuropsychiatr. 21, 246–255 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Thompson C., Stinson D., Smith A., Seasonal affective disorder and season-dependent abnormalities of melatonin suppression by light. Lancet 336, 703–706 (1990). [DOI] [PubMed] [Google Scholar]
- 36.McGlashan E. M., et al. , The pupillary light reflex distinguishes between circadian and non-circadian delayed sleep phase disorder (DSPD) phenotypes in young adults. PLoS One 13, e0204621 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGlashan E. M., Poudel G. R., Vidafar P., Drummond S. P. A., Cain S. W., Imaging individual differences in the response of the human suprachiasmatic area to light. Front. Neurol. 9, 1022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Meijden W. P., et al. , Individual differences in sleep timing relate to melanopsin-based phototransduction in healthy adolescents and young adults. Sleep (Basel) 39, 1305–1310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roecklein K., et al. , The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 210, 150–158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prayag A. S., Najjar R. P., Gronfier C., Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal Res. 66, e12562 (2019). [DOI] [PubMed] [Google Scholar]
- 41.McGlashan E. M., et al. , The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacology (Berl.) 235, 3201–3209 (2018). [DOI] [PubMed] [Google Scholar]
- 42.West K. E., et al. , Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J. Appl. Physiol. 110, 619–626 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Vartanian G. V., et al. , Melatonin suppression by light in humans is more sensitive than previously reported. J. Biol. Rhythms 30, 351–354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C., et al. , Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19, 91–102 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Hattar S., et al. , Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gooley J. J., et al. , Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J. Neurosci. 32, 14242–14253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucas R. J., et al. , Measuring and using light in the melanopsin age. Trends Neurosci. 37, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cajochen C., Zeitzer J. M., Czeisler C. A., Dijk D. J., Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 115, 75–83 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Najjar R. P., et al. , Aging of non-visual spectral sensitivity to light in humans: Compensatory mechanisms? PLoS One 9, e85837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.